- 1Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, ON, Canada
- 2Department of Psychology, University of Toronto Mississauga, Mississauga, ON, Canada
- 3Department of Integrative Biology, University of California, Berkeley, Berkeley, CA, United States
- 4Department of Cell and Systems Biology, University of Toronto, Toronto, ON, Canada
Here we employed the partner preference test (PPT) to examine how naked mole-rat non-breeding individuals of different behavioral phenotypes make social decisions. Naked mole-rats from six colonies were classified into three behavioral phenotypes (soldiers, dispersers, and workers) using a battery of behavioral tests. They then participated in a 3 h long PPT, where they could freely interact with a tethered familiar or tethered unfamiliar conspecific. By comparing the three behavioral phenotypes, we tested the hypothesis that the PPT can be used to interrogate social decision-making in this species, revealing individual differences in behavior that are consistent with discrete social phenotypes. We also tested whether a shorter, 10 min version of the paradigm is sufficient to capture group differences in behavior. Overall, soldiers had higher aggression scores toward unfamiliar conspecifics than both workers and dispersers at the 10 min and 3 h comparison times. At the 10 min comparison time, workers showed a stronger preference for the familiar animal’s chamber, as well as for investigating the familiar conspecific, compared to both dispersers and soldiers. At the 3 h time point, no phenotype differences were seen with chamber or investigation preference scores. Overall, all phenotypes spent more time in chambers with another animal vs. being alone. Use of the PPT in a comparative context has demonstrated that the test identifies species and group differences in affiliative and aggressive behavior toward familiar and unfamiliar animals, revealing individual differences in social decision-making and, importantly, capturing aspects of species-specific social organization seen in nature.
Introduction
Animals show significant variation in social behavior, both within and between species, including selectiveness for interactions with familiar or novel conspecifics. In rodents, some species live in small social groups and show a preference for interactions with familiar group members. For example, prairie voles (Microtus ochrogaster) are socially monogamous, tending to live in mate pairs or small groups with extended family (Carter and Getz, 1993; Getz et al., 1993). Once a pair bond is established, both female (Williams et al., 1992; Carter and Getz, 1993) and male (Blocker and Ophir, 2016) prairie voles prefer their partner to an opposite-sex stranger and show increased aggression toward unfamiliar conspecifics as a form of territorial or mate defense behavior (Carter and Getz, 1993; Young et al., 2011). Other species live in larger, more dynamic groups that vary based on environmental conditions, leading to greater interaction with out-group members (Berdoy and Drickamer, 2007; Ebensperger et al., 2012). For example, degus (Octodon degus) are highly social with significant female peer affiliation, where females have greater fitness in large same-sex groups (Ebensperger et al., 2016; Insel et al., 2020). Due to breeding limitations and environmental risks like predation, these groups will readily accept strangers because breeding females benefit from greater access to mates and communal breeding (Ebensperger et al., 2012; Insel et al., 2020). Thus, how animals make decisions about which individuals to approach vs. avoid and whether to be affiliative vs. aggressive is intimately intertwined with the evolution of their species-specific social organization and also has implications for individual differences in social behavior.
Within rodents, the African mole-rats (family Bathyergidae) provide an excellent opportunity to compare different social structures within closely related species. Among the 30+ species in the family, there are examples of eusocial, social, and solitary living (Faulkes and Bennett, 2021). Our study species is the naked mole-rat (Heterocephalus glaber), which is a eusocial member of the family. Naked mole-rats reside in expansive subterranean colonies with a reproductively active breeder caste consisting of a single queen and 1–3 male consorts, and a non-breeding subordinate caste (Jarvis, 1981; Brett, 1991; Jarvis et al., 1994). Most naked mole-rats exhibit lifelong philopatry by remaining in their natal colony, rarely interacting with foreign conspecifics (Burda et al., 2000). They are xenophobic and sensitive to foreign colony scent, so if an interaction with unfamiliar animals does occur, aggression is common and may result in the intruder’s death (Lacey and Sherman, 1991; O’Riain and Jarvis, 1997). Rapid social decisions during acute interactions are essential and commonplace within a naked mole-rat colony. When meeting in a tunnel, naked mole-rats will make quick decisions for which animal will pass over the other, which is directly related to their social hierarchy (Clarke and Faulkes, 1997, 1999; Toor et al., 2015). Behavioral phenotypes have been described within the non-breeding subordinates, although whether they are discrete phenotypes or continuous traits is still being explored (Mooney et al., 2015; Gilbert et al., 2020; Holmes and Goldman, 2021). In the present study, we classified naked mole-rat subordinates as one of three behavioral phenotypes based on their aggression and motivation to explore. Soldiers were categorized by their high aggression toward novel animals in a one-on-one interaction, dispersers were categorized by their curiosity and willingness to leave the colony as reported previously using the dispersal paradigm, and workers were categorized as non-aggressive and non-exploratory individuals (Lacey and Sherman, 1991; O’Riain et al., 1996; Toor et al., 2020).
Here we employed the partner preference test (PPT) to examine how naked mole-rat non-breeding individuals of different behavioral phenotypes make social decisions. The PPT (Figure 1) is a standardized laboratory social choice paradigm where the experimental animal is placed in a 3 chamber apparatus and has 3 h to explore and interact with a familiar conspecific (e.g., mating partner or sibling) in one chamber and an unfamiliar conspecific in the other (Williams et al., 1992; Beery, 2021). Since naked mole-rats are potentially at risk of losing their colony scent and becoming unrecognizable to colony-mates over time (O’Riain and Jarvis, 1997), we were also interested in investigating if a shorter time in the PPT would be consistent with the full 3 h test. The PPT has commonly been used to study opposite-sex mate preferences in prairie voles but also to test for same-sex alliances in prairie voles, meadow voles (Microtus pennsylvanicus), and degus (DeVries et al., 1997; Beery et al., 2008; Insel et al., 2020). Generally speaking, animals that form selective social relationships with a specific mate or non-mate peers such as prairie voles and meadow voles spend more time with familiar vs. unfamiliar conspecifics (DeVries et al., 1997; Beery et al., 2018; Lee et al., 2019). In contrast, degus—which form flexible, non-kin based social groups—spend more time in social chambers vs. alone, but do not show a preference for familiar or novel conspecifics (Insel et al., 2020). Gregarious species such as rats, mice, and spiny mice show a lack of familiarity preferences and sometimes even show novelty preferences (Moy et al., 2004; Smith et al., 2015; Beery and Shambaugh, 2021; Fricker et al., 2022). By comparing soldier, disperser, and worker naked mole-rats in this paradigm, we tested the hypothesis that the PPT can be used to interrogate social decision-making in this species, revealing individual differences in behavior that are consistent with discrete social phenotypes.

Figure 1. The partner preference test chamber with two tethered stimulus naked mole-rats. One side of the chamber contains the familiar conspecific and the other end contains the unfamiliar conspecific. Tethered animals are blurred in the image because they rarely, if ever, remain still.
Materials and Methods
Animals
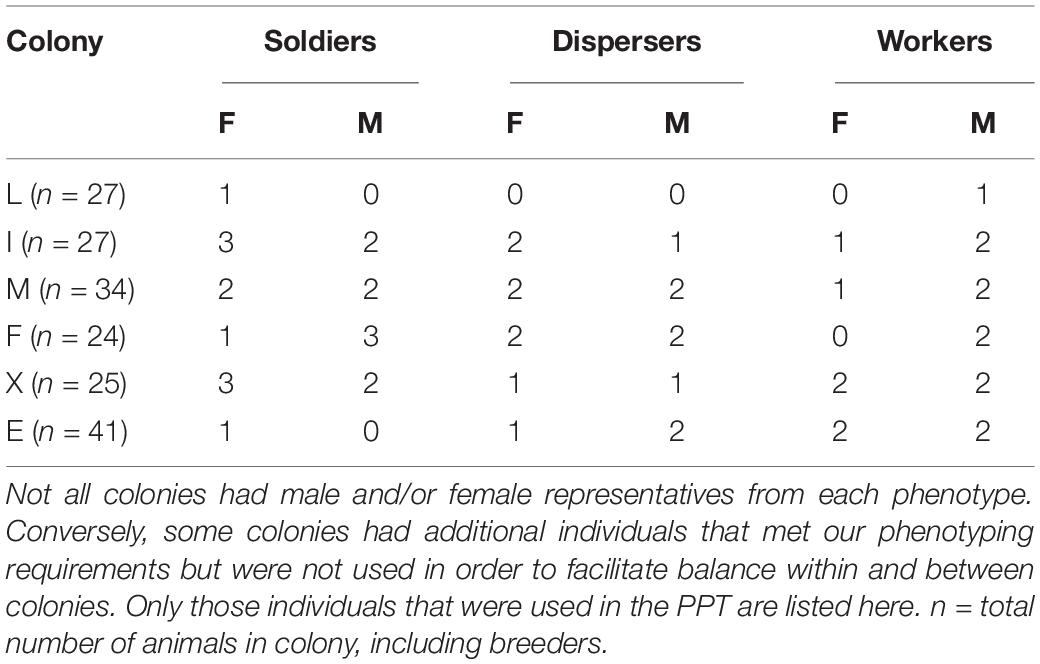
Six captive naked mole-rat colonies (a total of 178 animals) maintained in the University of Toronto Mississauga vivarium were used in this study. Experimental animals ranged from 12 months to 156 months of age. Considering that naked mole-rats reach adulthood within approximately 1 year and can live for over 30 years, all experimental animals were relatively young adults (Buffenstein and Craft, 2021). Each colony was housed in polycarbonate caging comprised of a medium (46 cm × 24 cm × 15 cm high) and small (30 cm × 18 cm × 13 cm high) cage connected via polycarbonate tubing, lined with corncob bedding, crinkle paper, and added tubing within the caging. The habitat size was kept constant for the duration of the study. Animal housing rooms were kept between 27 and 28°C with 50% humidity, and were on a 12 h light:dark cycle with lights on at 7:00 a.m. Animals were fed hydrated sweet potato daily and wet Teklad mouse chow three times a week. At 6 months of age, all animals were implanted with a subcutaneous microchip (Avid, Cat. No. 2,125, 12 mm) for individual identification. Animals were uniquely marked with a permanent marker for every testing session to allow visualization on video recordings. All testing took place between 12:00 P.M. and 5:00 P.M., and all work was approved by the University Animal Care Committee (protocol numbers 20011632 and 20011695). All behavioral scoring (see below) was performed by an experimenter blind to the familiarity status of stimulus animals as well as sex and phenotype, but not colony, of the experimental animals.
Behavioral Phenotype Categorization
A battery of behavioral paradigms was conducted to categorize the phenotypes of all individuals within each colony. First, animals were tested using the dispersal paradigm. This testing procedure was adapted from O’Riain et al. (1996) and Toor et al. (2020). Animals were fed hydrated sweet potato approximately 2 h before testing to minimize hunger as motivation to leave the colony. A single hole was opened on the side of the larger cage, and a plastic platform (22.86 cm x 30.48 cm) was placed directly under the hole so that animals could easily explore the opening and its surrounding area. When an animal fully exited, its identification was recorded, and it was immediately returned to its colony in the cage farthest from the hole. Each trial lasted 30 min, and 3 dispersal trials (one per day for three consecutive days) were performed. Animals who exited 3 or more times during the entire disperser test (combined score across the 3 trials) were considered dispersers unless they showed aggression during the out-pairing test (see below).
Next, an out-pairing test was administered to determine each individual’s aggressive phenotype. The experimental animal was paired for 10 min with an unfamiliar opposite-sex animal from a different colony with similar or less weight. The out-pairing was conducted in a 46 cm × 24 cm × 15 cm high cage (medium size). Interactions in which the focal animal punctured the stimulus animal’s skin were immediately stopped. In rare cases when the stimulus animal was aggressive to the focal animal, the trial was immediately stopped and then repeated with a different stimulus animal on the same day after completing all other remaining trials.
Soldiers were defined as any animal exhibiting aggression in the out-pairing test. Dispersers were defined as any animal who exited a cumulative of three or more times across three trials of the disperser test and was not aggressive during the out-pairing. Individuals who neither exited the colony during the disperser test nor exhibited aggression during the out-pairing test were considered workers. It is important to note that workers were not classified based on working type behaviors but rather the absence of aggression or dispersal-like behavior since working differentiation between phenotypes remains elusive (Gilbert et al., 2020; Toor et al., 2020).
Partner Preference Test
The PPT was conducted to study whether different phenotypes preferentially affiliate with familiar or unfamiliar animals. A 76 cm × 21 cm × 30 cm height cage divided into three equal-sized chambers (21 cm × 25 cm × 30 cm high) lined with corncob bedding was used (see Figure 1). A stimulus subordinate naked mole-rat was tethered using a soft shoelace and a spring stop toggle to either side of the testing apparatus; one stimulus animal came from the same colony as the experimental animal and the other from an unfamiliar colony. Both tethered animals were of the same sex and similar or smaller in size (if possible) to the experimental animal. The tethered stimulus animals were only used once as either a “familiar” or “unfamiliar” individual during the experiment. The stimulus animals were habituated to tethering in the chamber, while the experimental animal was habituated in a separate, empty chamber for 10 min. The experimental animal was then released into the center chamber of the apparatus, and was recorded for 180 min (3 h) using a GoPro Hero 3 camera. At the end of each trial, the corncob bedding was changed, and the apparatus and shoelaces were disinfected. Experimental animals and stimulus animals are generally active for the entire 3 h and never huddle. Therefore, the following behaviors were scored: time spent in each chamber, time spent investigating each stimulus animal, and time spent aggressive toward each stimulus animal. A total of 20 soldiers (9 male, 11 female), 17 workers (10 male, 7 female), and 16 dispersers (8 male, 8 female) were used in this paradigm. Animals that exhibited aggression causing skin punctures were immediately removed, and the trial was ended (3 female soldiers, 4 male soldiers) (Table 1).
Statistical Analysis
Statistical analyses were conducted using SPSS (SPSS Statistics for Windows, v.21.0, I.B.M. Corp., Armonk, NY, United States). Preference scores were calculated as: [(time with familiar) – (time with unfamiliar)]/(total time spent interacting). Preference scores were calculated separately for (1) total time spent within a familiar or unfamiliar individual’s chamber, (2) direct investigative interactions, and (3) aggressive interactions. Changes in behavior across time were investigated by comparing the 0–10 min, 10–30 min, 30 min–1 h, and 1–3 h intervals. For each preference score, a separate Repeated Measures ANCOVA was used to interrogate changes in preference across time with sex and phenotype as the independent variables and with colony as a covariate, using only the individuals who completed the entire session (i.e., were not removed due to aggression; n = 46).
Differences in behavior between phenotype groups were investigated separately at the 10 min and 3 h time points. As behavior was not being compared across time and preference scores represent a proportion of time calculated relative to each individual, we included those animals that had been removed due to aggression prior to test completion (therefore n = 53). For each preference score, a MANCOVA was conducted between the sexes and the three phenotypes with colony as a covariate. Main effects were explored using pairwise comparisons with LSD confidence interval adjustment for between-phenotype comparisons.
Finally, we explored whether (a) naked mole-rats prefer to spend more time in a chamber with a conspecific or alone (neutral chamber) and (b) if there was a general partner preference as revealed by spending more time in either the familiar or unfamiliar animal’s chamber compared to the other animal’s chamber. To do this, we used a separate Repeated Measures ANCOVA for each phenotype as well as for all animals together, followed by pairwise comparisons with LSD confidence interval adjustment for between-chamber comparisons. These analyses were done separately for the 10 min time point and the entire 3 h test.
Results
When exploring changes in behavior across time, no significant effects of time interval [F(3, 37) = 0.394, p = 0.758, Wilks’ Λ = 0.969], time interval × phenotype [F(6, 74) = 1.741, p = 0.123, Wilks’ Λ = 0.768], time interval × sex [F(3, 37) = 1.119, p = 0.354, Wilks’ Λ = 0.917], or time interval × phenotype × sex [F(6, 74) = 0.906, p = 0.495, Wilks’ Λ = 0.868] were found on the preference scores for time spent in chamber (Figure 2A). Similarly, no significant effects of time interval [F(3, 37) = 1.762, p = 0.171, Wilks’ Λ = 0.875], time interval × phenotype [F(6, 74) = 1.031, p = 0.412, Wilks’ Λ = 0.852], time interval × sex [F(3, 37) = 0.024, p = 0.995, Wilks’ Λ = 0.998], or time interval × phenotype × sex [F(6, 74) = 0.387, p = 0.885, Wilks’ Λ = 0.940] were detected on investigative preference scores (Figure 2B). Finally, the same holds true for aggressive preference scores, where no significant effects of time interval [F(3, 37) = 0.513, p = 0.676, Wilks’ Λ = 0.960], time interval × phenotype [F(6, 74) = 1.205, p = 0.313, Wilks’ Λ = 0.830], time interval × sex [F(3, 37) = 0.897, p = 0.452, Wilks’ Λ = 0.932], or time interval × phenotype × sex [F(6, 74) = 0.447, p = 0.845, Wilks’ Λ = 0.931] were detected (Figure 2C).
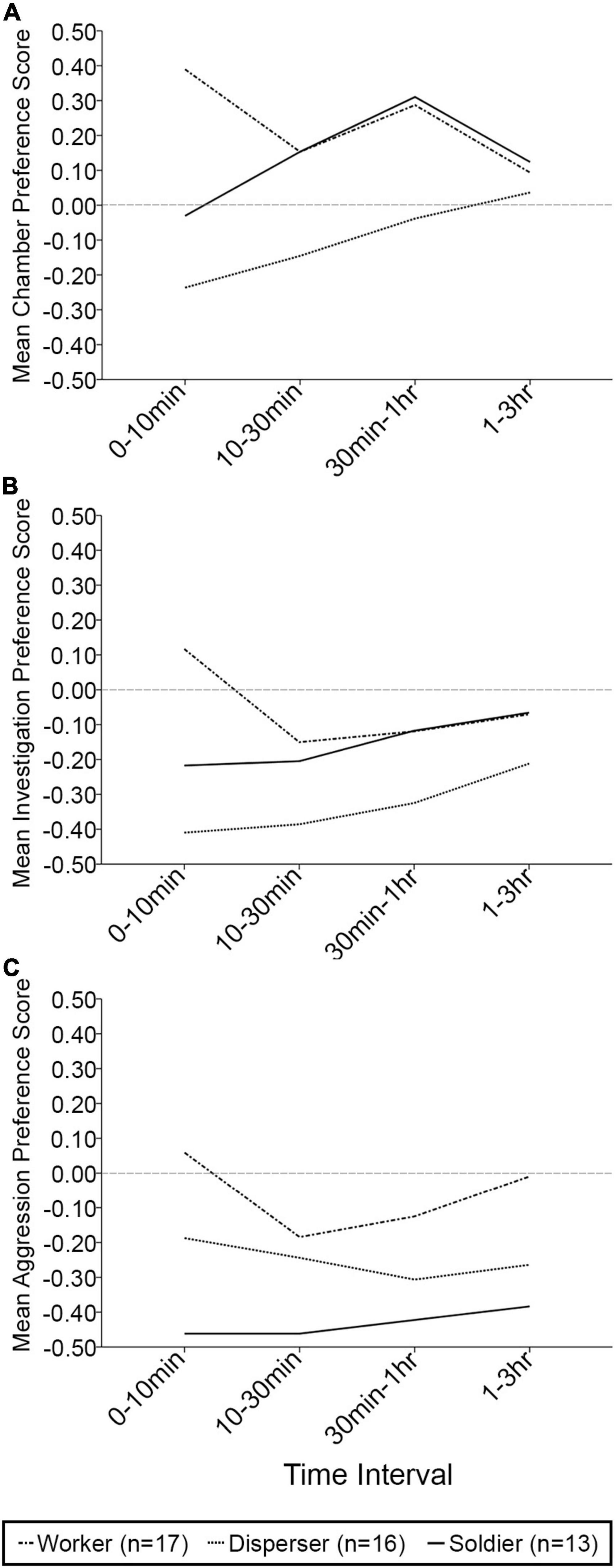
Figure 2. Mean chamber, investigative, and aggressive preference scores over four time intervals in the partner preference test. (A) Mean chamber preference score (positive score indicates preference for time spent in the familiar animal’s chamber). No significant differences were found. (B) Mean investigation preference score (positive score indicates preference for time spent investigating the familiar animal). No significant differences were found. (C) Mean aggression preference score (positive score indicates preference for time spent being aggressive toward the familiar animal). No significant differences were found. n = 17 workers; n = 16 dispersers; n = 13 soldiers and only includes individuals who completed the full 3 h session.
When testing for differences in behavior across phenotype groups, significant effects of phenotype on the chamber, investigative, and aggressive preference scores were detected at the 10 min time point. Phenotype significantly influenced chamber preference score [F(2, 46) = 8.583, p = 0.001], where workers had significantly higher preference scores than soldiers and dispersers (MW = 0.397 ± 0.119, MS = −0.156 ± 0.108, p = 0.001; MD = −0.237 ± 0.119, p < 0.0005) (Figure 3A). Phenotype also influenced investigative preference scores [F(2, 46) = 3.79, p = 0.03], where workers had higher preference scores than soldiers and dispersers (MW = 0.1 ± 0.137, MS = −0.280 ± 0.125, p = 0.048; MD = −0.415 ± 0.138, p = 0.011) (Figure 3C). Furthermore, phenotype influenced aggressive preference scores [F(2, 46) = 7.804, p = 0.001], where soldiers had lower aggressive preference scores than did workers and dispersers (the negative value indicating preference for aggression toward unfamiliar conspecifics) (MS = −0.596 ± 0.112, MW = 0.054 ± 0.123, p < 0.0005; MD = −0.191 ± 0.124, p = 0.019) (Figure 3E). No significant effects of sex were found for chamber preference score [F(1, 46) = 1.995, p = 0.165], investigative preference score [F(1, 46) = 1.231, p = 0.273], or aggressive preference score [F(1, 46) = 0.749, p = 0.391]. Phenotype effects were reduced following the full 3 h test. No significant effects of phenotype [F(2, 46) = 0.648, p = 0.528] or sex [F(1, 46) = 0.404, p = 0.528] were detected for chamber preference score (Figure 3B). Similarly, no significant effects of phenotype [F(2, 46) = 0.443, p = 0.645] or sex [F(1, 46) = 2.282, p = 0.138] were detected for investigative preference score (Figure 3D). Phenotype marginally influenced aggressive preference scores in the full 3 h test [F(2, 46) = 3.110, p = 0.054], where soldiers showed a greater preference for aggression toward unfamiliar animals than did workers (MS = −0.568 ± 0.155, MW = 0.01 ± 0.17, p = 0.017) (Figure 3F). No significant effect of sex on aggressive preference score was detected [F(1, 46) = 1.402, p = 0.243].
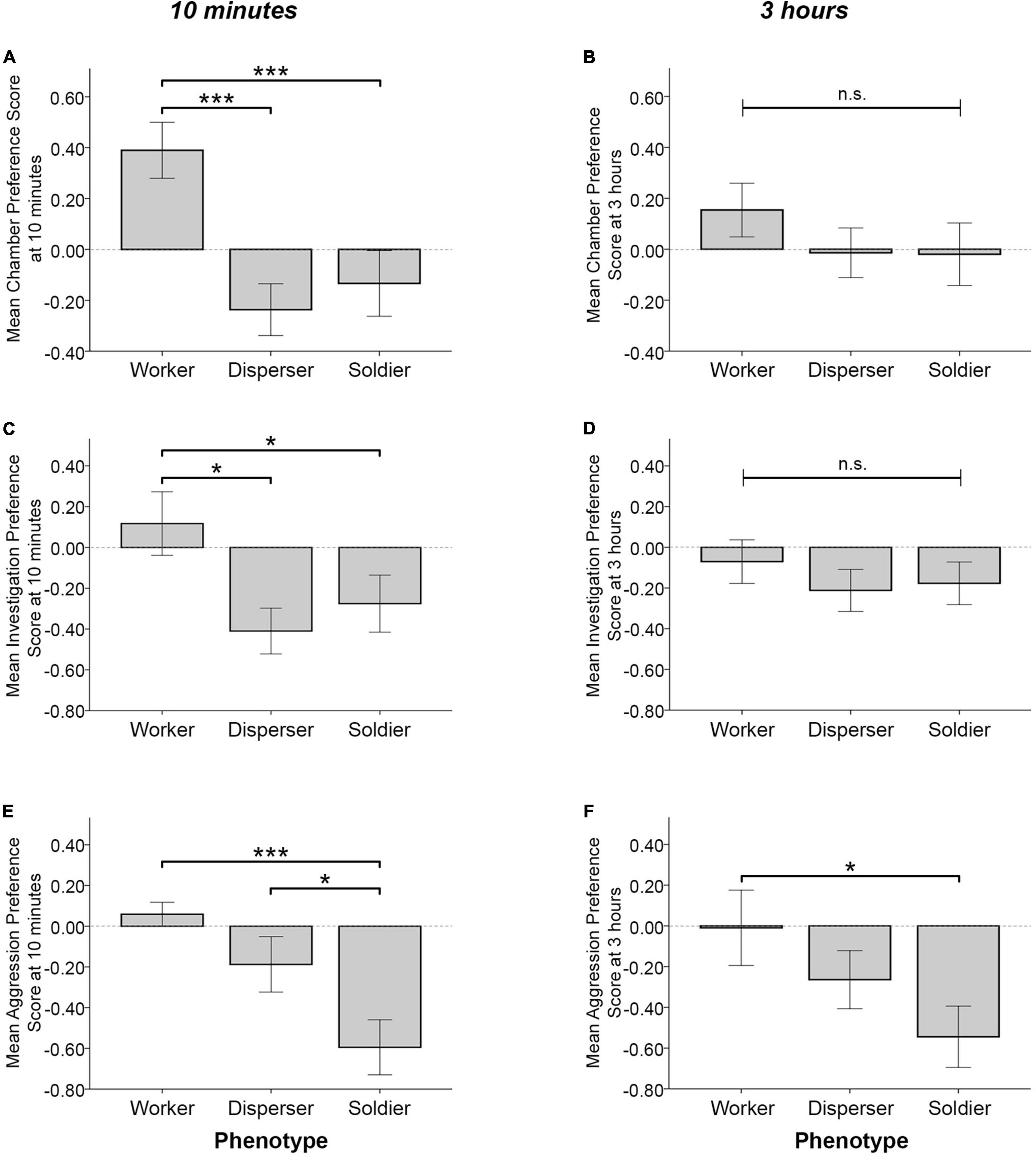
Figure 3. Mean time in chamber, investigative behavior, and aggressive behavior preference scores (± SEM) for the first 10 min (A,C,E) and full 3 h (B,D,F) of the partner preference test. Workers had significantly higher chamber preference scores (positive score indicates preference for time spent in the familiar animal’s chamber) than did dispersers and soldiers at the 10 min time point (A), but not at 3 h (B). Workers (n = 17) had significantly higher investigation preference scores (positive score indicates preference for investigating the familiar animal) than dispersers (n = 16) and soldiers (n = 20) at 10 min (C), but not at 3 h (D). Soldiers had significantly lower aggression preference scores (a negative score indicates preference for aggression toward the unfamiliar animal) than did workers and dispersers at the 10 min time point (E) and following the full 3 h test (F). n.s. = not significant, *p < 0.05, ***p < 0.001 for pairwise comparisons.
For time spent in each type of chamber at the 10 min time point, a marginal effect of chamber type was detected for all animals combined [F(2, 50) = 2.702, p = 0.077, Wilks’ Λ = 0.902]. Significant pairwise effects revealed that animals spent significantly more time in the familiar animal’s chamber than alone in the neutral chamber (MF = 210.039 ± 19.072, MN = 134.230 ± 10.207, p < 0.0005) and also significantly more time in the familiar animal’s chamber than the unfamiliar animal’s chamber (MU = 145.036 ± 13.813, p = 0.015) (Figure 4A). A similar pattern was observed for workers where, although no significant main effect of chamber type was detected [F(2, 14) = 2.314, p = 0.135, Wilks’ Λ = 0.752], significant pairwise effects were found: workers spent significantly more time in the familiar animal’s chamber than alone in the neutral chamber (MF = 306.476 ± 26.694, MN = 158.522 ± 14.749, p = 0.001) and also significantly more time in the familiar animal’s chamber than the unfamiliar animal’s chamber (MU = 134.998 ± 24.565, p = 0.003) (Figure 4C). For dispersers, no significant effect of chamber type was detected [F(2, 13) = 1.468, p = 0.266, Wilks’ Λ = 0.816] though significant pairwise tests revealed dispersers spent significantly more time in the unfamiliar animal’s chamber than in the familiar animal’s chamber (MU = 291.262 ± 32.341, MF = 170.006 ± 23.507, p = 0.045) and alone in the neutral chamber (MN = 138.731 ± 12.821, p = 0.003) (Figure 4E). No significant effect of chamber type was detected for soldiers [F(2, 17) = 0.408, p = 0.671, Wilks’ Λ = 0.954], nor were there any significant pairwise comparisons (Figure 4G).
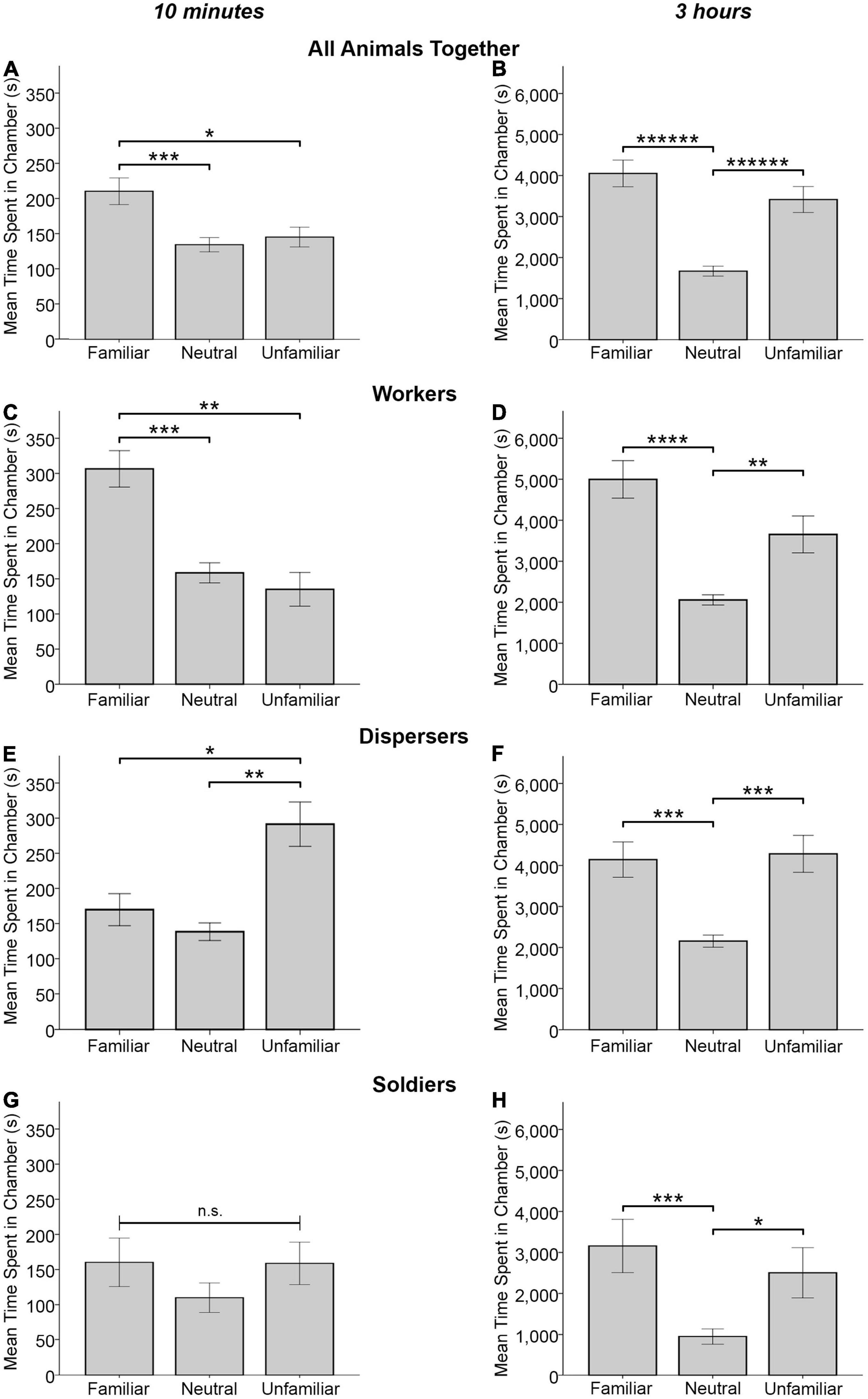
Figure 4. Mean time spent in a chamber (± SEM) with either a familiar animal, unfamiliar animal, or alone in the neutral chamber by all experimental animals (A,B), workers only (n = 17; C,D), dispersers only (n = 16; E,F), and soldiers only (n = 20; G,H). Data are plotted for the 10 min time point (A,C,E,G) and the full 3 h test (B,D,F,H). All animals and all three behavioral phenotypes spent significantly more time in a chamber with another animal vs. being alone in the center chamber for the full 3 h test. At the 10 min time point, dispersers spent more time in the chamber with an unfamiliar animal vs. a familiar animal (E) and workers spent more time in the chamber with a familiar animal vs. an unfamiliar animal (C, also seen for all animals together in A). n.s. = not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ******p < 0.000001 for pairwise comparisons.
For time spent in each type of chamber for the full 3 h test, a significant effect of chamber type was detected for all animals combined [F(2, 50) = 16.609, p < 0.000005, Wilks’ Λ = 0.601] where animals spent more time in the familiar and unfamiliar animal’s chamber than alone in the neutral chamber (MF = 4042.328 ± 328.715, MN = 1666.747 ± 121.301, p < 0.000001; MU = 3307.923 ± 316.385, p < 0.000001) (Figure 4B). For workers, a significant effect of chamber type was detected [F(2, 14) = 14.830, p < 0.0005, Wilks’ Λ = 0.321], where workers spent significantly more time in the familiar and unfamiliar animal’s chamber than in the neutral chamber alone (MF = 4995.890 ± 472.222, MN = 2057.136 ± 127.765, p < 0.00005; MU = 3654.394 ± 464.352, p = 0.007) (Figure 4D). Likewise, a significant effect of chamber type was detected for dispersers [F(2, 13) = 12.718, p = 0.001, Wilks’ Λ = 0.338], where dispersers spent significantly more time in the familiar and unfamiliar animal’s chamber than in the neutral chamber alone (MF = 4136.554 ± 438.357, MN = 2154.663 ± 145.426, p = 0.001; MU = 4277.147 ± 462.692, p = 0.001) (Figure 4F). Although no significant effect of chamber type was detected for soldiers [F(2, 17) = 0.461, p = 0.639, Wilks’ Λ = 0.949], significant pairwise effects were found, where soldiers spent significantly more time in the familiar chamber and unfamiliar conspecific’s chamber than alone in the neutral chamber (MF = 3156.419 ± 644.195, MN = 944.583 ± 193.839, p < 0.0005; MU = 2503.045 ± 624.624, p = 0.017) (Figure 4H).
Discussion
These data reveal that performance on the PPT is influenced by behavioral phenotype in non-breeding naked mole-rats. Compared to workers, soldiers had higher preference scores for aggression toward unfamiliar conspecifics at the 10 min and 3 h comparison times (Figure 3). At the 10 min comparison time, workers showed a stronger chamber preference score, as well as investigative preference score—both indicating a preference for familiar animals—compared to both dispersers and soldiers. At the 3 h time point, no phenotype differences were seen with chamber or investigative preference scores. Overall, all phenotypes spent more time in chambers with another animal vs. being alone (Figure 4). At the 10 min time point, workers spent more time in the chamber with the familiar animal whereas dispersers spent more time in the chamber with the unfamiliar animal (Figure 4). These phenotype specific patterns of time spent in each chamber were no longer present at the end of the 3 h test.
It is not necessarily surprising that soldiers had higher aggressive scores toward unfamiliar animals given they were indeed categorized as soldiers because they showed aggression in the out-pairing paradigm. However, these results do confirm the stability of this trait across the different testing paradigms. Importantly, in the out-pairing paradigm used for phenotyping, animals do not have the opportunity to escape the chamber and thus have reduced opportunity for social decisions. Alternatively, in the PPT, animals have three choices to make: (1) whether to be alone in the center chamber vs. with another animal, (2) whether to be with the familiar animal or the unfamiliar animal, and (3) whether to be affiliative or aggressive. The current data reveal that soldiers actively chose to be aggressive to the unfamiliar animal and they did this more than their worker/disperser siblings. Conversely, after 10 min in the PPT apparatus, worker animals spent more time in the chamber with the familiar animal and had significantly higher chamber and investigative preference scores for the familiar animal than both soldiers and dispersers. Animals classified as dispersers are somewhat intermediate in that they spent more time in the chamber with the unfamiliar animal at the 10 min time point, and had a significantly negative preference score for time in chamber and investigative behavior, indicating a preference for the unfamiliar animal but not as targets of aggression.
The stability of phenotype differences across the 3 h test varied according to behavior. For aggression, the greater preference for unfamiliar animals displayed by soldiers was significant at both 10 min and the end of the 3 h test. For investigative behavior, the greater preference for familiar animals displayed by workers was only significant at the 10 min time point; group differences were no longer significant at the end of the test. Given that naked mole-rats spend the majority of their time in the PPT doing non-social behaviors like digging (mean of total duration: 87.5%), it is possible that social investigation gets overshadowed by general activity after the initial period of investigation. From studies assessing the formation of opposite- and same-sex preferences in prairie voles (Williams et al., 1992; DeVries et al., 1997; Beery et al., 2018; Lee et al., 2019), a 3-h duration allows exploration and habituation to occur and promotes the emergence of huddling behavior toward the latter half of the study period (Beery, 2021). Although naked mole-rats do exhibit huddling behavior with colony members in the wild and laboratory (Withers and Jarvis, 1980; Sherman et al., 1992; Mooney et al., 2014), social contact in the form of huddling was not displayed within the current study. The animals remained active throughout the entire period, never huddling with either stimulus animal. While animals were habituated to the apparatus, it is possible they will not huddle due to the presence of an unfamiliar animal (which would normally disrupt the entire colony). Similarly, the stimulus animals themselves may remain agitated due to the tethering process, preventing them from settling down. Thus, the lack of sustained preferences in naked mole-rats may reflect their agitated state in the apparatus. Furthermore, vocalizations made by the familiar or unfamiliar conspecific might also influence the focal animal’s behavior. Naked mole-rats utilize antiphonal communication and their vocalizations can provide information on animal body size (Yosida et al., 2007; Yosida and Okanoya, 2009). They also produce different vocalizations in prosocial or aggressive interactions (see Barker et al., 2021a for review) and recent evidence reveals colony specific variation in vocalizations, similar to dialects (Barker et al., 2021b). It is therefore likely that vocalizations influence performance, including agitation/general activity, in the partner preference test as the focal animal gains information about familiar vs. unfamiliar and aggressive vs. prosocial individuals. Regardless, the PPT allows insight into the investigative and aggressive behavior of naked mole-rats as they relate to familiar and unfamiliar conspecifics, even if it does not capture huddling, per se.
The current data add to a growing body of work examining same-sex peer relationships using the PPT in rodent species with diverse social organizations. Prairie voles are socially monogamous and will cohabitate with a mate but may also form stable social groups that include extended family members (Carter and Getz, 1993). The formation of stable social groups contributes both to the selectiveness for familiar peers as well as the aggression toward unfamiliar conspecifics, especially following mating (Carter and Getz, 1993; Lee et al., 2019). While there are subtle sex differences in partner preference development, both male and female prairie voles show strong selectivity in their interactions by spending more time with familiar rather than unfamiliar same-sex conspecifics (Brusman et al., 2022). Prairie voles are also often aggressive toward unfamiliar same-sex individuals (Lee et al., 2019; Beery et al., 2021; Vahaba et al., 2021). In meadow voles, a congener species with a promiscuous mating system, animals will form social groups only during the winter season (Madison et al., 1984; Madison and McShea, 1987; Beery, 2019). Meadow voles are selective in their aggression toward strangers in that female voles will exhibit territorial behavior during summer breeding seasons but display more affiliative behavior and engage in social nesting with unfamiliar conspecifics during winter (Madison et al., 1984; Madison and McShea, 1987). In the laboratory, female meadow voles exhibit partner preference for familiar females in the PPT when housed in winter-like short photoperiods (Parker and Lee, 2003; Beery et al., 2009). Finally, in degus, communal nesting occurs in nature with closely related kin and unrelated female conspecifics coming together to form social groups (Ebensperger et al., 2004; Quirici et al., 2011; Davis et al., 2016). The social groups often change in composition across years due to mortality and emigration, but a higher number of females in relatively stable groups confers greater fitness benefits to female degus (Ebensperger et al., 2009, 2016). In the PPT, female degus show minimal aggression toward unfamiliar same-sex conspecifics and predominantly engage in affiliative behaviors, though selective preferences are not formed based on familiarity (Insel et al., 2020). Rats and mice also do not form same-sex familiarity preferences consistent with their gregarious social structure (Beery and Shambaugh, 2021). Similar to naked mole-rats, they do not huddle with either familiar or unfamiliar conspecifics in the PPT. Use of the PPT in a comparative context has demonstrated that the test identifies species and group differences in affiliative and aggressive behavior toward familiar and unfamiliar animals, thus revealing individual differences in social decision-making, as well as capturing aspects of species-specific social organization seen in nature.
Here, we report phenotype differences in social decision-making in non-breeding, subordinate naked mole-rats. Using the PPT, we found that soldiers show a higher preference for aggression toward unfamiliar animals and a reduced preference for investigation of familiar animals compared to workers. Disperser animals were somewhat intermediate with a preference for unfamiliar animals compared to workers but reduced aggression compared to soldiers. These data are consistent with, but not confirmation of, discrete social phenotypes in the non-breeding caste of this eusocial species. Future work will need to examine the stability of these behavioral preferences across weeks and months in addition to tracking the dynamic vocal communication during dyadic/triadic interactions. Also, using the PPT to examine social decision-making in other Bathyergid species will help reveal how performance varies across closely related species with different social organization. This is important beyond the comparative PPT work done to date as it will help reveal effects due to habitat and ecology (e.g., African mole-rats are subterranean) that may be distinct from social organization, per se. While it is essential to study behavior in its natural context and by using ecologically relevant paradigms, using standardized laboratory paradigms across diverse species is an important complementary approach. Doing so will reveal how animals make social decisions and allow more rigorous comparison and translation across species, which will ultimately help us understand the evolution of sociality in mammals.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the University of Toronto Animal Care Committee.
Author Contributions
IT: conceptualizing the experiment, conducting and collecting all data, statistical analyses, writing and editing the manuscript, and figure creation. RM: behavior scoring, and writing and editing the manuscript. XP: behavior scoring, figure processing, and editing the manuscript. AB: conceptualizing the experiment, statistical analyses, and editing the manuscript. MH: conceptualizing the experiment, statistical analyses, and editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Ontario Graduate Scholarship and the Natural Sciences and Engineering Research Council of Canada (NSERC) Postgraduate Scholarship—Doctoral (PGS D) grant to IT and NSERC grants (RGPIN 2018-04780 and RGPAS 2018-522465), CIHR grant (02003PJT-437197), and the Ontario Early Researcher Award to MH.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barker, A. J., Koch, U., Lewin, G. R., and Pyott, S. J. (2021a). “Hearing and vocalizations in the naked mole-rat,” in The Extraordinary Biology of the Naked Mole-Rat, eds R. Buffenstein, T. J. Park, and M. M. Holmes (Cham, Switzerland: Springer), 157–196. doi: 10.1007/978-3-030-65943-1_6
Barker, A. J., Veviurko, G., Bennett, N. C., Hart, D. W., Mograby, L., and Lewin, G. R. (2021b). Cultural transmission of vocal dialect in the naked mole-rat. Science 371, 503–507. doi: 10.1126/science.abc6588
Beery, A., and Shambaugh, K. L. (2021). Comparative assessment of familiarity/novelty preferences in rodents. Front. Behav. Neurosci. 15:648830. doi: 10.3389/fnbeh.2021.648830
Beery, A. K. (2019). Frank Beach award winner: neuroendocrinology of group living. Horm. Behav. 107, 67–75. doi: 10.1016/j.yhbeh.2018.11.002
Beery, A. K. (2021). Familiarity and mate preference assessment with the partner preference test. Curr. Protoc. 1:e173. doi: 10.1002/cpz1.173
Beery, A. K., Christensen, J. D., Lee, N. S., and Blandino, K. L. (2018). Specificity in sociality: mice and prairie voles exhibit different patterns of peer affiliation. Front. Behav. Neurosci. 12:50. doi: 10.3389/fnbeh.2018.00050
Beery, A. K., Loo, T. J., and Zucker, I. (2008). Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Horm. Behav. 54, 153–159. doi: 10.1016/j.yhbeh.2008.02.007
Beery, A. K., Lopez, S. A., Blandino, K. L., Lee, N. S., and Bourdon, N. S. (2021). Social selectivity and social motivation in voles. eLife 10:e72684. doi: 10.7554/eLife.72684
Beery, A. K., Routman, D. M., and Zucker, I. (2009). Same-sex social behavior in meadow voles: multiple and rapid formation of attachments. Physiol. Behav. 97, 52–57. doi: 10.1016/j.physbeh.2009.01.020
Berdoy, M., and Drickamer, L. (2007). “Chapter 32. Comparative Social Organization and Life History of Rattus and Mus,” in Rodent Societies: An Ecological and Evolutionary Perspective, eds J. Wolff and P. Sherman (Chicago, IL: University of Chicago Press), 380–392.
Blocker, T. D., and Ophir, A. G. (2016). A preference to bond? Male prairie voles form pair bonds even in the presence of multiple receptive females. Anim. Behav. 122, 89–97. doi: 10.1016/j.anbehav.2016.10.007
Brett, R. A. (1991). “The population structure of naked mole-rat colonies,” in The Biology of the Naked Mole-Rat, eds P. W. Sherman, J. U. M. Jarvis, and R. D. Alexander (Princeton, NJ: Princeton University Press), 97–136. doi: 10.1515/9781400887132-007
Brusman, L. E., Protter, D. S. W., Fultz, A. C., Paulson, M. U., Chapel, G. D., Elges, I. O., et al. (2022). Emergent intra-pair sex differences and organized behavior in pair bonded prairie voles (Microtus ochrogaster). Genes Brain Behav. 21:e12786. doi: 10.1111/gbb.12786
Buffenstein, R., and Craft, W. (2021). “The idiosyncratic physiological traits of the naked mole-rat; a resilient animal model of aging, longevity, and healthspan,” in The Extraordinary Biology of the Naked Mole-Rat, eds R. Buffenstein, T. J. Park, and M. M. Holmes (Cham, Switzerland: Springer), 221–254. doi: 10.1007/978-3-030-65943-1_8
Burda, H., Honeycutt, R., Begall, S., Locker-Grütjen, O., and Scharff, A. (2000). Are naked and common mole-rats eusocial and if so, why? Behav. Ecol. Sociobiol. 47, 293–303. doi: 10.1007/s002650050669
Carter, C. S., and Getz, L. L. (1993). Monogamy and the prairie vole. Sci. Am. 268, 100–106. doi: 10.1038/scientificamerican0693-100
Clarke, F. M., and Faulkes, C. G. (1997). Dominance and queen succession is captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc. Biol. Sci. 264, 993–1000. doi: 10.1098/rspb.1997.0137
Clarke, F. M., and Faulkes, C. G. (1999). Kin discrimination and female mate choice in the naked mole-rat Heterocephalus glaber. Proc. Biol. Sci. 266, 1995–2002. doi: 10.1098/rspb.1999.0877
Davis, G. T., Vásquez, R. A., Poulin, E., Oda, E., Bazán-León, E. A., Ebensperger, L. A., et al. (2016). Octodon degus kin and social structure. J. Mammal. 97, 361–372. doi: 10.1093/jmammal/gyv182
DeVries, A. C., Johnson, C. L., and Carter, C. S. (1997). Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Can. J. Zool. 75, 295–301. doi: 10.1139/z97-037
Ebensperger, L., Hurtado, M., Soto-Gamboa, M., Lacey, E., and Chang, A. (2004). Communal nesting and kinship in degus (Octodon degus). Naturwissenschaften 91, 391–395. doi: 10.1007/s00114-004-0545-5
Ebensperger, L. A., Chesh, A. S., Castro, R. A., Tolhuysen, L. O., Quirici, V., Burger, J. R., et al. (2009). Instability rules social groups in the communal breeder rodent Octodon degus. Ethology 115, 540–554. doi: 10.1111/j.1439-0310.2009.01635
Ebensperger, L. A., Correa, L. A., León, C., Ramírez-Estrada, J., Abades, S., Villegas, Á, et al. (2016). The modulating role of group stability on fitness effects of group size is different in females and males of a communally rearing rodent. J. Anim. Ecol. 85, 1502–1515. doi: 10.1111/1365-2656.12566
Ebensperger, L. A., Sobrero, R., Quirici, V., Castro, R. A., Tolhuysen, L. O., Vargas, F., et al. (2012). Ecological drivers of group living in two populations of the communally rearing rodent, Octodon degus. Behav. Ecol. Sociobiol. 66, 261–274. doi: 10.1007/s00265-011-1274-3
Faulkes, C. G., and Bennett, N. C. (2021). “Social Evolution in African Mole-Rats – A Comparative Overview”, in The Extraordinary Biology of the Naked Mole-Rat, eds R. Buffenstein, T. J. Park, and M. M. Holmes (Cham, Switzerland: Springer), 1–34. doi: 10.1007/978-3-030-65943-1_1
Fricker, B. A., Seifert, A. W., and Kelly, A. M. (2022). Characterization of social behavior in the spiny mouse, Acomys cahirinus. Ethology 128, 26–40. doi: 10.1111/eth.13234
Getz, L. L., McGuire, B., Pizzuto, T., Hofmann, J. E., and Frase, B. (1993). Social organization of the prairie vole (Microtus ochrogaster). J. Mammal. 74, 44–58. doi: 10.2307/1381904
Gilbert, J. D., Rossiter, S. J., and Faulkes, C. G. (2020). The relationship between individual phenotype and the division of labour in naked mole-rats: it’s complicated. PeerJ 8:e9891. doi: 10.7717/peerj.9891
Holmes, M. M., and Goldman, B. D. (2021). “Social behavior in naked mole-rats: individual differences in phenotype and proximate mechanisms of mammalian eusociality,” in The Extraordinary Biology of the Naked Mole-Rat, eds R. Buffenstein, T. J. Park, and M. M. Holmes (Cham, Switzerland: Springer), 35–58. doi: 10.1007/978-3-030-65943-1_2
Insel, N., Shambaugh, K. L., and Beery, A. K. (2020). Female degus show high sociality but no preference for familiar peers. Behav. Processes 174:104102. doi: 10.1016/j.beproc.2020.104102
Jarvis, J. U. M. (1981). Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science 212, 571–573. doi: 10.1126/science.7209555
Jarvis, J. U. M., O’Riain, M. J., Bennett, N. C., and Sherman, P. W. (1994). Mammalian eusociality: a family affair. Trends Ecol. Evol. 9, 47–51. doi: 10.1016/0169-5347(94)90267-4
Lacey, E. A., and Sherman, P. W. (1991). “Social organization of naked mole-rat colonies: evidence for divisions of labor,” in The Biology of the Naked Mole-Rat, eds P. W. Sherman, J. U. M. Jarvis, and R. D. Alexander (Princeton, NJ: Princeton University Press), 275–336. doi: 10.1515/9781400887132-013
Lee, N. S., Goodwin, N. L., Freitas, K. E., and Beery, A. K. (2019). Affiliation, aggression, and selectivity of peer relationships in meadow and prairie voles. Front. Behav. Neurosci. 13:52. doi: 10.3389/fnbeh.2019.00052
Madison, D. M., FitzGerald, R. W., and McShea, W. J. (1984). Dynamics of social nesting in overwintering meadow voles (Microtus pennsylvanicus): possible consequences for population cycling. Behav. Ecol. Sociobiol. 15, 9–17. doi: 10.1007/bf00310209
Madison, D. M., and McShea, W. J. (1987). Seasonal changes in reproductive tolerance, spacing, and social organization in meadow voles: a microtine model. Am. Zool. 27, 899–908. doi: 10.1093/icb/27.3.899
Mooney, S. J., Douglas, N. R., and Holmes, M. M. (2014). Peripheral administration of oxytocin increases social affiliation in the naked mole-rat (Heterocephalus glaber). Horm. Behav. 65, 380–385. doi: 10.1016/j.yhbeh.2014.02.003
Mooney, S. J., Filice, D. C. S., Douglas, N. R., and Holmes, M. M. (2015). Task specialization and task switching in eusocial mammals. Anim. Behav. 109, 227–233. doi: 10.1016/j.anbehav.2015.08.019
Moy, S. S., Nadler, J. J., Perez, A., Barbaro, R. P., Johns, J. M., Magnuson, T. R., et al. (2004). Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 3, 287–302. doi: 10.1111/j.1601-1848.2004.00076.x
O’Riain, M. J., and Jarvis, J. U. M. (1997). Colony member recognition and xenophobia in the naked mole-rat. Anim. Behav. 53, 487–498. doi: 10.1006/anbe.1996.0299
O’Riain, M. J., Jarvis, J. U. M., and Faulkes, C. G. (1996). A disperser morph in the naked mole-rat. Nature 380, 619–621. doi: 10.1038/380619a0
Parker, K. J., and Lee, T. M. (2003). Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. J. Comp. Psychol. 117, 283–289. doi: 10.1037/0735-7036.117.3.283
Quirici, V., Faugeron, S., Hayes, L. D., and Ebensperger, L. A. (2011). Absence of kin structure in a population of the group-living rodent Octodon degus. Behav. Ecol. 22, 248–254. doi: 10.1093/beheco/arq196
Smith, C. J. W., Wilkins, K. B., Mogavero, J. N., and Veenema, A. H. (2015). Social novelty investigation in the juvenile rat: modulation by the μ-Opioid system. J. Neuroendocrinol. 27, 752–764. doi: 10.1111/jne.12301
Toor, I., Clement, D., Carlson, E. N., and Holmes, M. M. (2015). Olfaction and social cognition in eusocial naked mole-rats, Heterocephalus glaber. Anim. Behav. 107, 175–181. doi: 10.1016/j.anbehav.2015.06.015
Toor, I., Edwards, P. D., Kaka, N., Whitney, R., Ziolkowski, J., Monks, D. A., et al. (2020). Aggression and motivation to disperser in eusocial naked mole-rats, Hetercephalus glaber. Anim. Behav. 168, 45–58. doi: 10.1016/j.anbehav.2020.07.022
Vahaba, D. M., Halstead, E. R., Donaldson, Z. R., Ahern, T. H., and Beery, A. K. (2021). Sex differences in the reward value of familiar mates in prairie voles. Genes Brain Behav. 21:e12790. doi: 10.1111/gbb.12790
Williams, J. R., Catania, K. C., and Carter, C. S. (1992). Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Behav. 26, 339–349. doi: 10.1016/0018-506x(92)90004-f
Withers, P. C., and Jarvis, J. U. M. (1980). The effect of huddling on thermoregulation and oxygen consumption for the naked mole-rat. Comp. Biochem. Physiol. A 66, 215–219. doi: 10.1016/0300-9629(80)90154-1
Yosida, S., Kobayasi, K. I., Ikebuchi, M., Ozaki, R., and Okanoya, K. (2007). Antiphonal vocalization of a subterranean rodent, the naked mole-rat (Heterocephalus glaber). Ethology 113, 703–710. doi: 10.1111/j.1439-0310.2007.01371.x
Yosida, S., and Okanoya, K. (2009). Naked mole-rat is sensitive to social hierarchy encoded in antiphonal vocalization. Ethology 115, 823–831. doi: 10.1111/j.1439-0310.2009.01677.x
Keywords: affiliation, aggression, behavioral phenotype, eusocial, naked mole-rat, partner preference
Citation: Toor I, Maynard R, Peng X, Beery AK and Holmes MM (2022) Naked Mole-Rat Social Phenotypes Vary in Investigative and Aggressive Behavior in a Laboratory Partner Preference Paradigm. Front. Ecol. Evol. 10:860885. doi: 10.3389/fevo.2022.860885
Received: 23 January 2022; Accepted: 04 April 2022;
Published: 28 April 2022.
Edited by:
Stan Braude, Washington University in St. Louis, United StatesReviewed by:
Gary R. Lewin, Max Delbrück Center for Molecular Medicine, Helmholtz Association of German Research Centers (HZ), GermanyMartha Ann Delaney, University of Illinois at Urbana-Champaign, United States
Alison Barker, Max Planck Institute for Brain Research, Germany
Copyright © 2022 Toor, Maynard, Peng, Beery and Holmes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melissa M. Holmes, bWVsaXNzYS5ob2xtZXNAdXRvcm9udG8uY2E=
 Ilapreet Toor
Ilapreet Toor Rashoun Maynard
Rashoun Maynard Xinye Peng
Xinye Peng Annaliese K. Beery
Annaliese K. Beery Melissa M. Holmes
Melissa M. Holmes