
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Ecol. Evol. , 31 March 2022
Sec. Population, Community, and Ecosystem Dynamics
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.857161
This article is part of the Research Topic Living on the Edge: Biodiversity, Adaptation, and Evolution in Extreme Groundwater Habitats View all 7 articles
A new species of stygobiont copepod (Stygocyclopia badinoi sp.nov.) is described from the anchialine environment of Zinzulùsa cave (Castro, Italy). It is the first Pseudocyclopiidae (Copepoda, Calanoida) to be reported from Italian fauna and the second one reported from the Mediterranean area. The current species is characterized by the largest body size among congeners and by a general lacking of spiny fields on the cuticle of Urosome and P5. The genus has representatives in anchialine environments of coastal caves in Australia, New Caledonia, Philippines, Canaries, and Balearic islands, and the present Mediterranean report is a relict station well included in the frame of an ancient and widespread geographic distribution of ancestral Pseudocyclopiidae along the Tethys Ocean coastlines.
Crustacean assemblages of anchialine habitats, although never with abundant populations, are noteworthy for the presence of biogeographic and/or phylogenetic relict species (Iliffe, 1992). The anchialine fauna, in addition, appears as predictable in terms of taxonomic composition, even if spread into geographically distant localities. The particular conditions of the stygologic environment, together with the taxonomic predictability of the assemblage composition and the enormous distance/barriers for the biological connectivity of co-generic species between sites, suggest that vicariance events, more than dispersal ones, are responsible for the current distribution patterns (Stock, 1993; Jaume et al., 2001).
The family Pseudocyclopiidae (Copepoda, Calanoida) has several representatives thriving in anchialine environments. One of its genera, Stygocyclopia Jaume and Boxshall (1995), is exclusively anchialine, and is represented by four species described from Canaries and Balearic islands (Jaume and Boxshall, 1995), Philippines (Jaume et al., 1999), Australia (Jaume et al., 2001), and New Caledonia (reported but not described in Boxshall and Halsey, 2004).
The anchialine habitat of Zinzulùsa (municipality of Castro, south-eastern Italy) has been investigated due to the high biological interest for this karst environment, which hosts a total of 25 species (with two punctuated endemics) (Ciccarese and Pesce, 1999). This high biodiversity allowed the Karst Water Institute (Charleston Town, WV, United States) to insert Zinzulùsa in the list of “the most worthy of protection” among the karst environments of the world (Pesce, 2001).
In this paper, a new species is described based on three adult females and one incomplete male, undoubtedly belonging to Pseudocyclopiidae Stygocyclopia, but showing unique characters which justify the creation of a new species.
Zinzulùsa is a karst cave that opens a few meters above the sea level of the Italian coastline of the Otranto Channel in the neighboring city of Castro (geographical coordinates, 40°00′43″ N, 18°25′50″ E) (Figure 1C). The cave is registered at the Regional Karst Archive with the code 107 Pu, and it has an aerial extension of about 150 m that is intensely visited by tourists. The cave has a karst origin, possibly Pliocene (Tyrrhenian, 5–2 million years). The erosive processes acted on an external, young Eocenic rock (55–34 million years), and an innermost, elder Cretaceous rock (141–65 Million years). The internal portion, prohibited to visitors, starts at 150 m from the entrance and after 20 m, submerges below the water level in an anchialine environment, named Cocìto, for additional 150 m of extension, until a maximum depth of 12 m (Figure 1D). The whole submerged system of Zinzulùsa is inhabited by 25 species of aquatic organisms, with two of them (one Harpacticoida, and one Porifera) unknown in any other site of the world (pointed endemics) (Ciccarese and Pesce, 1999).
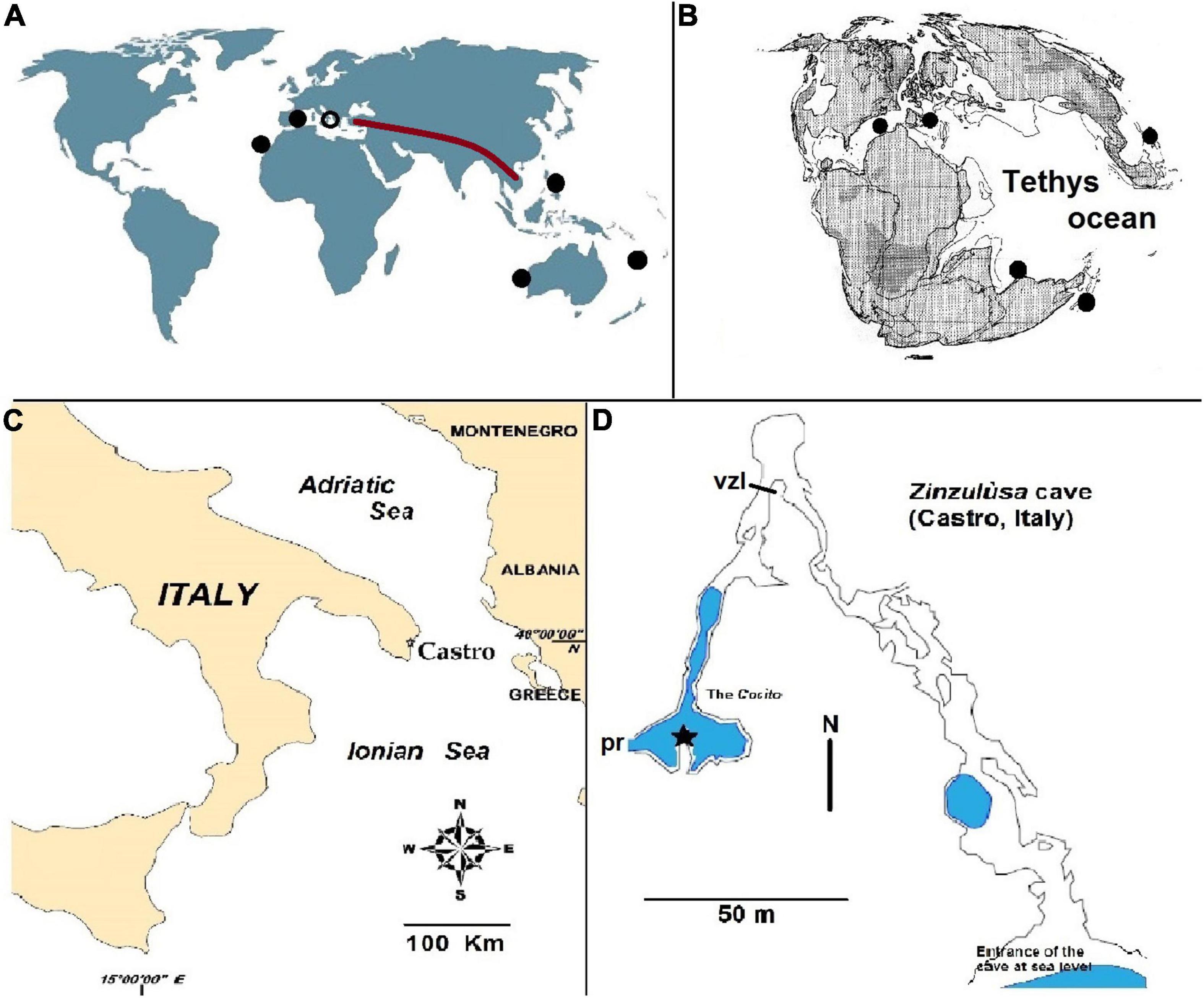
Figure 1. Geographic localization of Zinzulùsa cave. (A) World map with sites hosting copepods of the genus Stygocyclopia (black discks). The position of Zinzulùsa cave is indicated with a black circle. The brown line indicates the ancient Tethyan coastline, uplifted by the Alpine–Himalayan orogenesis. (B) The Tethys ocean geography of 155 Million years ago (Oxfordian) (modified from Smith et al., 1994). (C) Geographic position of Castro (municipality where is the Zinzùlusa cave). (D) Map of the Zinzulùsa cave with an indication of the water level (in blue); black star, position of the collecting device; vzl, visitors zone limit; pr, prosecution of the submerged part (not illustrated).
In recent years, an intense research effort (from 2013 to 2020) has been carried out to describe underwater conditions and collect living organisms from the Cocìto (see Talà et al., 2021). The site, notwithstanding its underground collocation, has appeared variable either in a short time-span (e.g., in the sea level oscillation due to external tide excursions) or over long periods (with a multiannual, intense pH oscillation).
The site has been visited from 2013 to 2020 by speleo scuba divers at distant intervals to avoid excess disturbance to the system, and in the frame of a multidisciplinary study aimed to describe the anchialine part of Zinzulùsa cave (Castro, Otranto Channel, Mediterranean Sea). Each expedition was carried out to collocate a multi-parametric probe in situ, with a battery charge useful for a continuous multi-day monitoring of the environmental parameters. Together with the probe, a modified plankton net (with a mesh size of 200 μm) transformed into a pot was positioned upside down over a hole in the muddy floor from where a water flow comes up in the Cocìto with an intermittent daily cycle. The two instruments were collocated at 35 m from the immersion point at a depth of about 4 m and a distance of 2.5 m from the floor (Figure 1D). Each expedition was concluded with the second visit of the speleo scuba divers after 2–5 days to recover instruments with their log of data and organisms.
The pot–net was also used to collect plankton during the entry and the exit of the speleo scuba divers (along a submerged path of about 35 m) at each visit.
The material collected by the pot–net was filtered in situ at the exit of the Cocìto and rinsed directly with ethanol 90% in a falcon tube (final ethanol concentration, 80–85%).
The samples were analyzed in a laboratory under a compound microscope, and Pseudocyclopiidae specimens were isolated to be studied under a camera lucida acting between 25 and 400 magnifications. For the morphological description, the distinction between body segment and appendage articles has been adopted following Dussart (1967) and Belmonte (1998). The terminology used for body regions follows Dudley (1986). To be compared with other existing Stygocyclopia species, the present description scheme has been arranged following the available literature (Jaume and Boxshall, 1995; Jaume et al., 1999, 2001).
The holotype (an adult female) has been deposited at Naturalis, Leiden, Netherlands (code number RMNH.CRUS.F.4200), and two adult females (paratypes) have been deposited at Marine Biology Museum “Pietro Parenzan” of Porto Cesareo (Lecce, Italy). One additional specimen is a male, damaged. It lacks the terminal part of the URS, the P4, and the terminal parts of Antennules. It was not considered as a reference individual for the species description. However, the P5 is described just for the systematic importance of this morphological detail.
One paratype was dissected to expose the anatomical details of metameric appendages and another paratype has been conserved entirely in 85% ethanol.
SUBCLASS COPEPODA H. MILNE EDWARD, 1830
order Calanoida G.O. Sars, 1903
family Pseudocyclopiidae T. Scott, 1892
genus Stygocyclopia Jaume and Boxshall (1995)
Diagnosis of the genus (modified from Jaume et al., 1999). Rostrum with two apical filaments. Antennules symmetrical, short, and composed of 23 articles (female) or 22 articles (male). Antennule articles 1–4 fused. First antenna endopod not fused with the basis. Maxillary endopod two-articled. Endopod of P1 with five setae. First exopod article of P3 without accessory spine. Male P5 with two unbranched rami, asymmetrical, each ramus bi-pointed at the extremity. Left ramus 5 articled, longer than right. Right ramus 3–4 articled. Female P5 with two unbranched, symmetrical rami, each ramus tri-pointed at the extremity. Genital segment (double somite) of female, symmetrical.
Stygocyclopia badinoi SP. NOV. (Figures 2–4).
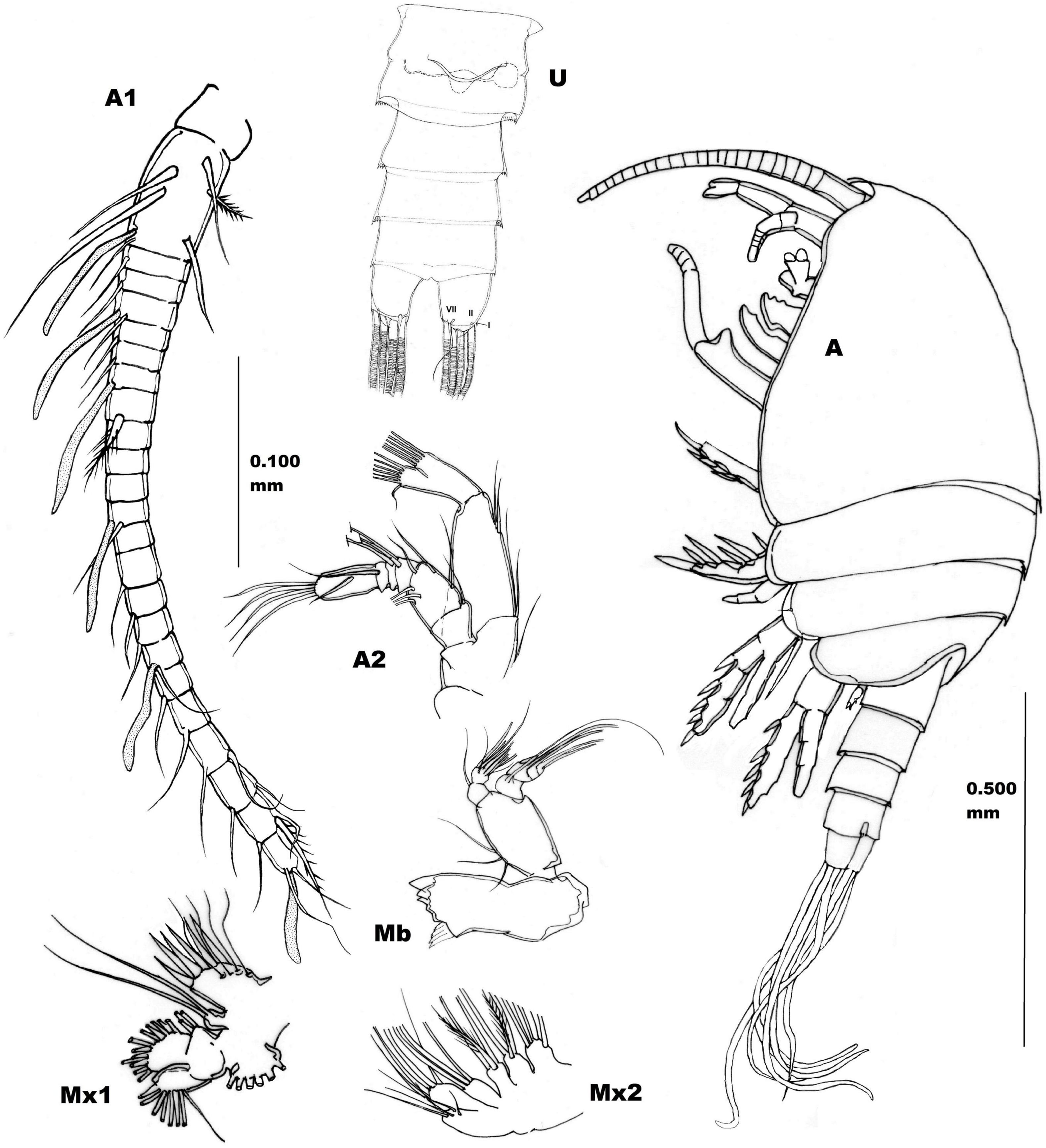
Figure 2. Adult female holotype (RMNH.CRUS.F.4200) of Stygocyclopia badinoi n.sp. Lateral view, left side. Scale bar, 0.500 mm. U, Urosome, ventral view, and Cefalic metameric appendages (from paratypes), scale bar, 0.100 mm. A1, Antennule; A2, Antenna; Mb, Mandible; Mx1, Maxillule; Mx2, Maxilla. Roman numbers I, II, and VII indicate setae of furcal ramus.
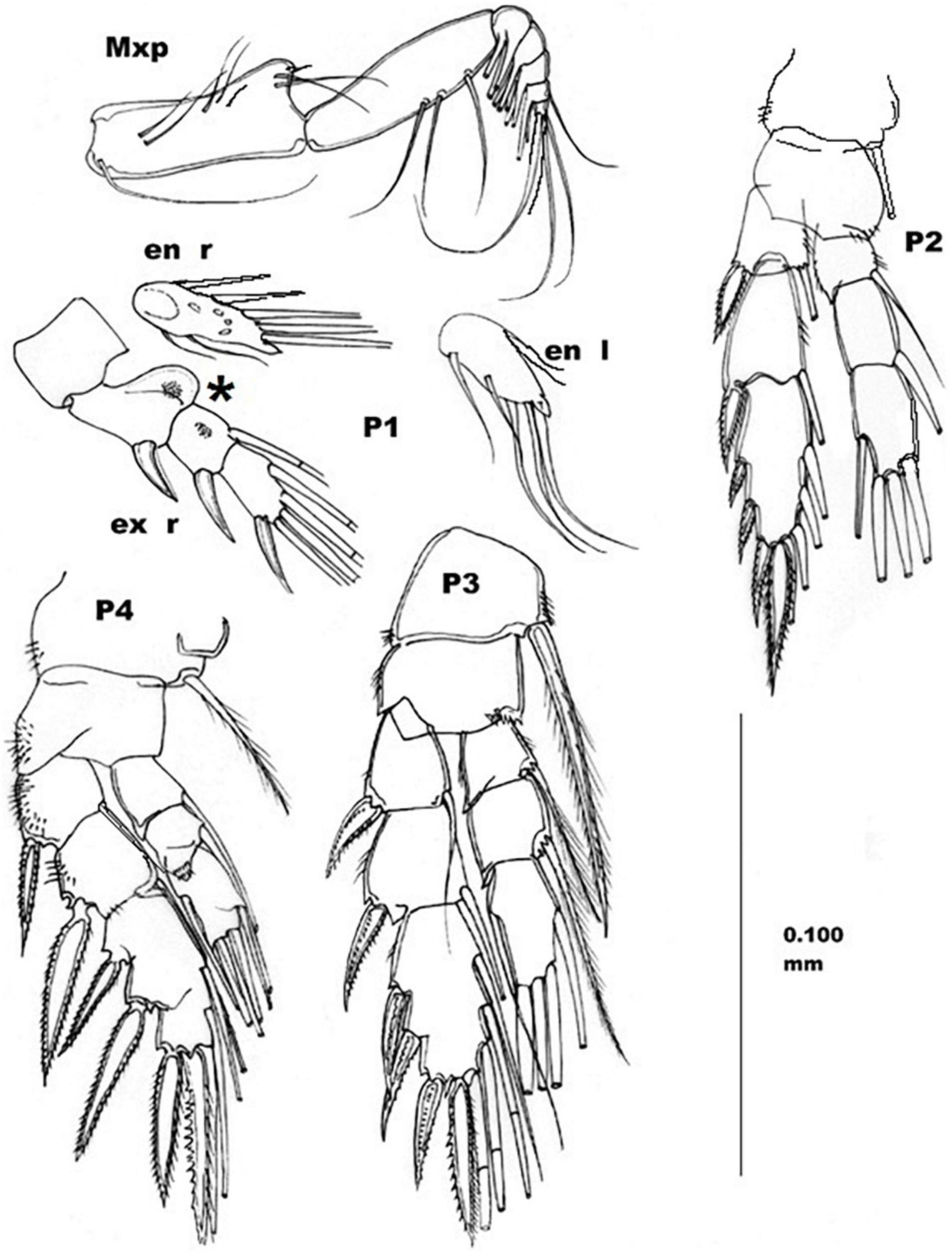
Figure 3. Adult female, Stygocyclopia badinoi n.sp., paratype. Mxp, maxillipede; P1–P4, right swimming legs; en, endopod; ex, exopod; r, right; l, left. Scale bar 0.100 mm. Asterisk indicates the moon shaped body at the level of P1.

Figure 4. Adult female, Stygocyclopia badinoi n.sp., paratype. P5, right ramus. Compared with P5 of other Stygocyclopia species. S.a., (Stygocyclopia australensis); S.b. (Stygocyclopia balearica); S.p. (Stygocyclopia philippensis). Male damaged specimen. P5, both appendages. Scale bar, 0.025 mm.
Collection site: Zinzulùsa cave (Regional Karst code, 107 Pu), the Cocìto (submerged internal part, anchialine habitat) on the Italian coast of the Otranto Channel, Mediterranean Sea, geographic coordinates 40°00′43″ N, 18°25′50″ E (Figures 1C,D).
Holotype: adult female, total length 0.85 mm in 80% ethanol (Naturalis, Leiden, RMNH.CRUS.F.4564). Paratypes: one adult female dissected and mounted in glycerol on three slides (Museum of Marine Biology “Pietro Parenzan,” University of Salento, MBM.CR.SLa-c.0014); another paratype has been conserved entirely in 85% ethanol (Museum of Marine Biology “Pietro Parenzan,” University of Salento, MBM.CR.0014a). Other material: one male, lacking the terminal part of urosome, extremity of Antennulae, and with the absence of right P4 in 85% ethanol (Museum of Marine Biology “Pietro Parenzan,” University of Salento, MBM.CR.0014b). This damaged specimen was not proposed for the species description.
The material has been collected by Raffaele Onorato, Marco Poto, and Michele Onorato (“Apogon” Speleodiver Association) on different dates: March 28, 2017 (male damaged) July 31, 2017 (holotype), and October 24, 2018 (paratypes).
Body compact, with Prosome compressed. Eye absent. Rostrum not articulated, triangular with two filaments at the pointed extremity.
First pedigerous somite fused with the Cephalosome. Second pedigerous somite with rounded postero-lateral margins. A small sensillum is evident in the central position of the lateral margin of the third pedigerous somite (Figure 2 A). Fourth and fifth pedigerous somites fused, with postero-lateral margins rounded without setae. Cephalothorax cuticular surface smooth.
Urosome (length 1/3 of the whole body) 4-segmented, with cuticle without armature. Genital double somite symmetrical with a tiny hyaline frill on the dorsal posterior margin. Seminal receptacle visible on the left side (Figure 2 U). Genital operculum wave-shaped, without armatures. Second and third urosomites with tiny hyaline frill on dorsal posterior margin. Anal somite shorter than the preceding one, without anal operculum.
Furcal rami symmetrical, about as long as wide, without evident cuticular armature. Setae of the furcal rami symmetrically disposed, seta I very reduced, lateral; setae II and VII smooth (not plumose), dorso-laterally and ventro-medially positioned respectively. Seta VII longer than seta II (Figure 2 U). Setae III–VI longer than the URS and flexed (they give the sensation to be internal empty) (Figure 2 A).
Antennules (A1) symmetrical, 23-articulated, short (not longer than the Cephalothorax) stemming from a pedestal (Figure 2 A1). First article longest (fusion of ancestral antennomeres I–IV, according to the interpretation of Jaume et al., 1999), and bearing a total of 5 smooth setae, 1 spiny seta, and 1 aestetask. Each A1 article, but 7th–11th and 13th, provided with at least 1 smooth (not plumose) seta; aestetask present on 1st, 4th, 7th, 12th, 17th, and 23rd articles; a strong and spiny seta is present on 1st, 8th, and 22nd articles (see Figure 2 A1); 9th–11th, and 13th articles (XIII–XV and XVII antennomeres) are naked.
Antenna (A2) (Figure 2 A2) biramous, with a bilobate endopodal extremity and approximately as long as exopod. Coxa with one seta at the distal corner. Basis with two setae at the distal extremity. Exopod distinctly 6-articulated with the following armature formula: 1st art., short with one distal seta; 2nd art., long with three equidistant setae; 3rd art. short, with one seta; 4th art. short, with two setae; 5th art. short, with one seta; 6th art. long, with one medial seta and three distal setae. Endopod with two articles. The 1st art. (proximal) is the longest, with two medio-proximal and two medio-distal setae. 2nd art. (distal) evidently bilobate, medial lobe with eight distal setae (two short, six long), and external lobe with 6 setae (one short, five long).
Mandible (Mb) (Figure 2 Mb) with coxal gnathobasis equipped with five unequal robust teeth; palp with expanded basis with three proximal setae of growing length (from the proximal to the distal one). Mandible palp longer than endopod, indistinctly 4-articulated, with setal formula 1, 1, 1, 2. Endopod 2-articulated, proximal article with one disto-medial seta, and the distal article with a total of 10 setae (9 of the same length plus 1 shorter).
Maxillule (Mx1) (Figure 2 Mx1) prae-coxal arthrite bearing 6 strong spines and 4 setae. Coxal epipodite with 6 plumose setae; coxal endite with two setae; basal endite with four setae; distal endite not well distinguishable from the successive endopodite, which is not articulated with the basis. Both structures with a total of 16 setae. Exopod articulated with the basis, with 8 setae.
Maxilla (Mx2) (Figure 2 Mx2) proximal syncoxal endite with five long spines. Successive (three) syncoxal endites, each with 3 seate. Basal endite with four setae. Endopod short, 2-articulated, with setal formula of 3, 3.
Maxilliped (Mxp) (Figure 3 Mxp) long and evident also in lateral view of the whole specimen (Figure 2 A). Syncoxa long, with 9 unequal setae arranged in 4 different groups according to the formula of 1, 2, 3, 3. Distal extremity of syncoxa with an evident lobe. Basis long, approximately as the syncoxa, with three setae along the medial margin. Endopod 5-articulated, with a total of 21 setae arranged according to the formula of 6, 4, 3, 3 + 1, 4.
First swimming leg (P1) (Figure 3 P1): endopod 1-articulated, with two medio-basal short setae, and three medio-terminal long setae, with a triangular spine at the tip of the endopod article. Two small setae on the lateral margin. Exopod 3-articulated, with one long external smooth spine on each article, and an arrangement of 0, 1, 4 medial setae, respectively. First article of exopod, with a moon-shaped ornament on the postero-medial side. Short spiny setules grouped on the posterior side of the first and second articles of the exopod.
Second swimming leg (P2) (Figure 3 P2): right coxa with a medial plumose seta long until the end of the first exopod article. Left coxa without such a seta. Basis without setae, but with external short spinulae. Endopod 2-articulated in anterior view, and three-articulated in posterior view. Seta formula of 1, 1, 3 + 1. A small triangular tooth on the external corner of the first article. Posterior view of the endopod showing an evident, setulated, and lobed articulation between the second and third articles.
Exopod 3-articulated, with 1, 1, and 3 long lateral spines (the spine on the second exopod article is the longer). The exopod terminates with a long-toothed spine (long as that on the second article, but more robust) which is two-pointed. The spine-seta armature of the exopod is arranged according to the formula: I-1; I-1; III, I, 4. Setae of the third exopod article are plumose, articulated, and flexible. The posterior view of the bfirst and second articles are lightly setulated.
Third swimming leg (P3) (Figure 3 P3): coxae each with a medial plumose long spine reaching the half of the third endopod article. Basis with medio-distal tiny spines and lateral tiny spinules. Endopod 3-articulated with medial long plumose setae arranged according to the formula: 0-1; 0-1; 1,2,2. Small triangular spine on the distal-lateral side of first and second endopod articles. Exopod 3-articulated with strong spines on the lateral side and long setae on the medial side, according to the formula: I-1; I-1; III-I-4. Lateral and terminal spines are secondarily spiny. Setae are plumose and articulated (flexible).
Fourth swimming leg (P4) (Figure 3 P4): basis without spines or setae. Endopod 3-articulated with setae arranged according the formula: 0-1; 0-1; 1, 2, 2. Exopod 3-articulated with lateral spines and medial setae, arranged to according the formula: I-1; I-1; III-I-4.
Fifth leg (P5) (Figure 4 P5): reduced, with unbranched rami, symmetrical, 3-articulated, with tiny and short spines on the distal article. Two terminal spines and one lateral on the extreme article of each ramus, in females (Figure 4). In the male, the P5 right ramus longer, uniramous, 4-articulated. Left ramus shorter, 1-articulated. The right P5 ramus of the male ends with a double slender process, resembling a couple of long and conical spines, not ornamented. The short, left P5 ramus ends with two spines, one very short, and the other long and ornamented with lateral setae.
The species derives its name from Giovanni Badino, a professor of Geophysics at the University of Turin (Italy), and one of the greatest Italian speleologists (Silver medal 1981 for an emergency intervention; President of the Association La Venta; President of Italian Society of Speleology 1994–99; member of directorate of the International Union of Speleology), who passed away on August 8, 2017.
The genus Stygocyclopia contains three species, all found in the anchialine environments of geographically distant locations. S. balearica Jaume and Boxshall (1995) inhabits caves of Balearic island (see also Carola and Razouls, 1996) and Canaries islands (Jaume et al., 1999). S. philippensis Jaume et al. (1999) was recorded in Panglao island (Philippines), and S. australis Jaume et al. (2001) from western Australia. Boxshall and Halsey (2004) and Razouls et al. (2005–2020) report also the existence of a Stygocyclopia from Caledonia, but without a scientific description.
The geographic position of the Cocìto, in the distribution map of the genus Stygocyclopia, conforms with the so called “full Tethyan track” of Stock (1993), typical of many stygobiont Crustacea, also defined as Tethyan relicts (Figures 1A,B). As it is observable from the map (Figures 1A,B), the modern disjuncted geographic distribution of the genus is explained by the disappearance of the coastal Tethys, which today is uplifted in Alpine–Himalayan orogenesis and constitutes mountains from Turkey to Indochina.
The present species is the second to be reported from the Mediterranean area.
Stygocyclopia badinoi sp. nov. shares with all the other species the habitat (anchialine) and the following characteristics of the female: body compressed; rostrum pointed with two filaments; eye absent; short antennulae; 1st–4th antennomeres of antennule fused in a unique, long article; 1st article of the antenna exopod articulated (not fused) with the basis.
As regarding the differences existing with other congeneric species (Table 1), S. balearica, from Balearic Islands (Spain), is easily distinguishable from S. badinoi sp. nov. because it is smaller (0.69 mm) and adorned with a high degree of spinules/micro-setae on different parts of the body, plumose setae on the furca, and a spinulose P5 with three spines positioned side by side at the extremity of each ramus (Figure 4 S.b.). An interesting similarity between S. balearica and S. badinoi sp. nov. is the presence of an evident seminal receptacle only on the left of the genital segment.
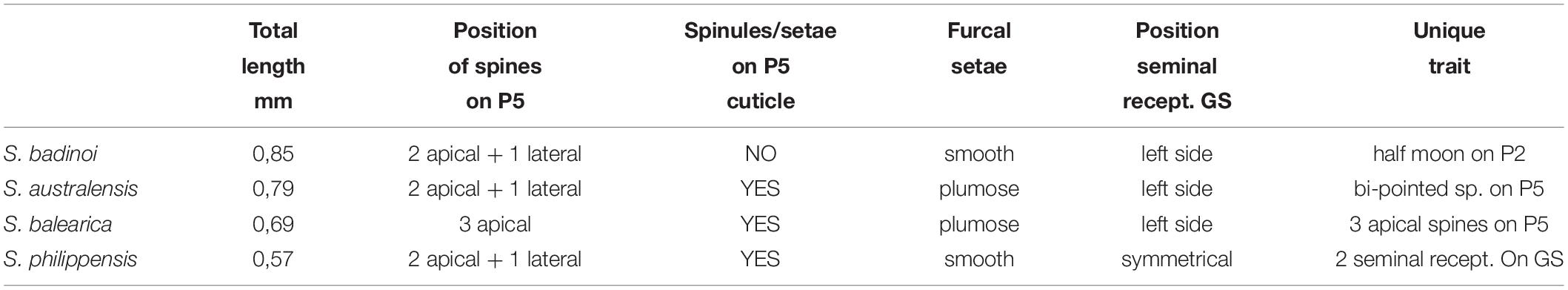
Table 1. Morphological comparison of the main distinguishing features between S. badinoi n.sp. and the other Stygocyclopia species (only females).
S. australis has a body size (0.79 mm) comparable with that of S. badinoi sp.nov., has the same positioning of the three spines on each P5 ramus, which can be observed in S. badinoi sp. nov., but the P5 of S. australis is richly adorned with spinules and the terminal spines are bi-pointed (Figure 4 S.a.).
A similar arrangement of spines on P5 rami is also that of S. philippensis, but the lateral spine is small, not exceeding the size of the other many spines distributed on the appendage. S. philippensis also shows furcal setae similar (not plumose and flexible) to those of S. badinoi sp. nov. The new species, however, appears markedly larger than S. philippensis (0.85 vs. 0.57 mm) and does not show the diffuse presence of spinules and/or micro-setae on legs and urosome, which characterize S. philippensis and all the other Stygocyclopia (Figure 4).
Apart from the evident absence of spinules/micro-setae, the presence of a half-moon-shaped body on the postero-medial side of the first article of P1 exopod (Figure 3) appears as typical of S. badinoi sp. nov.
Also typical, among the co-generic species, appears the asymmetrical presence of a seta on the P2 right coxa (absent on the left). Such a kind of asymmetry in females is not common in morphology descriptions, but it is not surprising because, among Calanoida, adult females can show small differences between the two sides of the body (e.g., Ferrari, 1985; Belmonte, 1998, Bradford-Grieve, 1999).
The small number of specimens did not allow us to discuss intraspecific variability of species characters. This notwithstanding, the observed characters are sufficient to consider the present specimens as belonging to a new species. The distinguishing characters are (1) the absence of micro-setae and spinules on many parts of the body, (2) a very distinguishable P5 in females, and (3) the half-moon-shaped body on the P1. The poorness of Stygocyclopia population in the Cocìto (only four individuals in 18 collections over 8 years) rises problems for the existence of a species in an evolutionary context. This datum, however, could be the byproduct of the sampling method adopted. The genus, in fact, is typically hyperbenthic and of a small size. The pot–net system (suspended at about 2.5 m from the floor) and the mesh size (200 μm) could not be the right device to collect specimens thriving close to the bottom and smaller than the adult female (e.g., males and copepodites), and future attempts will take into consideration these problems in sample collection. Baited traps could be useful in attracting specimens of this copepod, and a narrower mesh size (50 μm) should be able to successfully collect very small specimens (Suarez-Morales et al., 2017).
Finally, it is remarkable that anchialine habitats can be considered as an extension of the hyper benthos from where many new species have been described in the last 30 years. In fact, Ridgewaiidae, Pseudocyclopidae, Pseudocyclopiidae, and Stephidae, typical of hyperbenthos and/or of anchialine habitat, recently produced new species even in geographic areas where Calanoida were very well known, just thanking the attention that researchers have recently dedicated to the hyperbenthic environment (see Belmonte, 2018 for Italian fauna).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
GB was involved in the planning of collection, organization of expeditions, analysis of samples, taxonomical studies, identification, and systematics.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Gaetano Ciccarese (Gruppo Speleologico Salentino “P. De Lorentiis,” Castro) ensured the organization and the logistic support during the entire period of exploration (2013–2020) of Cocìto. Raffaele Onorato, Michele Onorato, and Marco Poto (Scuba Speleodiving Association APOGON Onlus, 73048 Nardò) performed the scuba divings for the positioning of instruments and the sample collections. An elevated number of collaborators (sherpa), first of all Toto De Santis (82 years) brought materials and equipment along the emerged part of the cave to make possible all the expeditions in the Cocìto anchialine environment.
Belmonte, G. (1998). Pteriacartia, A new genus of Acartiidae (Calanoida, Diaptomoidea) for Acartia josephinae Crisafi, 1974. J. Mar. Sys. 15, 359–368. doi: 10.1016/s0924-7963(97)00087-0
Belmonte, G. (2018). Calanoida (Crustacea: Copepoda) of the Italian fauna; a review. Eur. Zool. J. 85, 274–290.
Boxshall, G. A., and Halsey, S. H. (2004). An introduction to copepod diversity, Vol. 2. London: Natural History Museum, 967.
Bradford-Grieve, J. (1999). “Leaflet no. 181. Calanoida Acartiidae. ICES Identification Leaflet for Plankton,” in International Council for the Exploration of the Sea. Plaegade 2-4, DK-1261, (Copenhagen), 19.
Carola, M., and Razouls, C. (1996). Two New Species of Calanoida from a Marine Cave on Minorca Island, Mediterranean Sea: Stephos balearensis New Species (Stephidae) and Paracyclopia gitana New Species (Pseudocyclopiidae). Bull. Mar. Sci. 58, 344–352.
Ciccarese, G., and Pesce, G. L. (1999). La Zinzulusa: 200 anni dopo. Thalassia Salentina 23(Suppl.), 79–88.
Dudley, P. L. (1986). “Aspects of general body shape and development in Copepoda. Sillogeus 58,” in Proceedings of the 2nd International Conference on Copepoda, (Ottawa), 7–25.
Ferrari, F. D. (1985). Postnaupliar development of a looking-glass copepod, Pleuromamma xiphias (Giesbrecht, 1889), with analyses of distributions of sex and asymmetry. Smiths. Contrib. Zool. 420, 1–55. doi: 10.5479/si.00810282.420
Iliffe, T. M. (1992). “Anchialine cave biology,” in The Natural History of Biospeleology. Monografias Museo Nacional de Ciencias Naturales, ed. A. I. Camacho (Madrid: Consejo superior de Investigaciones Cientificas), 614–636.
Jaume, D., and Boxshall, G. A. (1995). Stygocyclopia balearica a new genus and species of calanoid copepod (Pseudocyclopiidae) from anchialine caves in the Balearic islands (Mediterranean). Sarsia 80, 989–1006. doi: 10.1080/00222939600770531
Jaume, D., Boxshall, G. A., and Humphreys, W. F. (2001). New stygobiont copepods (Calanoida, Misophrioida) from Bundera sinkhole, an anchialine cenote in north western Australia. Zool. J. Linn. Soc. 133, 1–24. doi: 10.1111/j.1096-3642.2001.tb00620.x
Jaume, D., Boxshall, G. A., and Iliffe, T. M. (1999). New cave-dwelling Pseudocyclopiids (Copepoda, Calanoida, Pseudocyclopiidae) from the Balearic, Canary, and Philippine archipelagos. Sarsia 84, 391–417. doi: 10.1080/00364827.1999.10807346
Pesce, L. (2001). The Zinzulusa cave: an endangered biodiversity “hot spot” of south Italy. Natura Croatica 10, 207–212.
Razouls, C., de Bovée, F., Kouenberg, J., and Desreumaux, N. (2005-2020). Diversity and Geographic distribution of marine plankton Copepods. Paris: Sorbonne University, CNRS.
Smith, A. G., Smith, D. G., and Funnel, B. M. (1994). Atlas of Mesozoic and Cenozoic Coastlines. Cambridge: Cambridge University Press, 75.
Stock, J. H. (1993). Some remarkable distribution patterns in stygobiont Amphipoda. J. Nat. Hist. 27, 807–819. doi: 10.1080/00222939300770491
Suarez-Morales, E., Cervantes-Martinez, A., Gutiérrez-Aguirre, M. A., and Iliffe, T. M. (2017). A new Speleophria (Copepoda, Misophrioida) from an anchialine cave of the Yucatán Peninsula with comments on the biogeography of the genus. Bull. Mar. Sci. 93:3. doi: 10.5343/bms.2017.1012
Talà, A., Calcagnile, M., Buccolieri, A., Tredici, S. M., Ciccarese, G., Onorato, M., et al. (2021). Profiling of the prokaryotic communities thriving in a submerged coastal cave (Zinzulùsa Cave, Castro, Italy) reveals efficient chemotrophic exploitation of natural organic and mineral nutrient sources. Sci. Tot. Env. 755:142514. doi: 10.1016/j.scitotenv.2020.142514
Keywords: anchialine cave, Stygocyclopia, Mediterranean Sea, stygobiont fauna, relict species
Citation: Belmonte G (2022) A New Species of Pseudocyclopiidae (Crustacea, Copepoda, Calanoida) From an Anchialine Environment of South-Eastern Italy. Front. Ecol. Evol. 10:857161. doi: 10.3389/fevo.2022.857161
Received: 18 January 2022; Accepted: 01 March 2022;
Published: 31 March 2022.
Edited by:
Sanda Iepure, Emil Racovita Institute of Speleology, Romanian Academy, RomaniaReviewed by:
Eduardo Suarez-Morales, The South Border College (ECOSUR), MexicoCopyright © 2022 Belmonte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Genuario Belmonte, Z2VudWFyaW8uYmVsbW9udGVAdW5pc2FsZW50by5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.