- Department of Forestry and Landscape Design, Federal State Budgetary Educational Establishment of Higher Education, Bashkir State Agrarian University, Ufa, Russia
This article examined the honey-bearing potential of Tilia cordata Mill. stands in the Southern Urals using forest management and taxation methods. The studies were conducted in pure and mixed even- and uneven-aged forests with T. cordata Mill. in different natural zones of the Southern Urals on the territory of the Bashkirs. These were forests of the South Ural forest-steppe region, the forest-steppe region of the European part of Russia, the area of coniferous-deciduous (mixed) forests of the European part of Russia. The research employed the method of laying temporary and permanent trial areas. T. cordata Mill. forests on the republic’s territory were divided into zones by forest districts. There is a discrepancy between the territorial localization of “nectar” (honey-bearing) linden and designated forest areas, that is, honey-bearing forest areas on the republic’s territory. It could be due to the lacking methodology for assigning linden to these categories. The increased rotation age of linden trees to 81–90 years for several decades, a ban on final cutting, reduced annual cut of soft-leaved forests by 15% enlarged the stocks of overmature forest to 48%. Depending on the age, the number of flowers on one T. cordata Mill. tree varies from 0 to 60.2 thousand pieces. The maximum amount of nectar is 69.04 kg/ha at 12-day linden flowering. The honey productivity of plants including T. cordata Mill., calculated concerning the linden age, its share in the forest composition, the average number of flowers on the tree, nectar per 1 ha of linden and the flowering period ranged from 252.8 to 662.8 kg/ha.
Introduction
Forests are known to have unique flora and fauna. They are essential in providing numerous ecosystem functions and benefits, including nectar secretion (Bystriakova et al., 2018; Agbelemoge and Adekola, 2020; Cunningham-Minnick and Crist, 2020; Krishnan et al., 2020; Perennes et al., 2021). There are many definitions of ecosystem potential, for instance, the one proposed by Burkhard et al. (2012). It claims that the ecosystem potential is the ability to provide services due to natural factors (climate, relief, habitat, and vegetation) and human activities (land and forest management, industrial activities, etc.). The following definition better describes pollination services. The ecosystem potential is a service supply calculated for environmental conditions best for providing resources to the beekeeping industry while simultaneously meeting this type of ecosystem (Yumashev et al., 2016; Bosma et al., 2017; Häussler et al., 2017; Roberts et al., 2017; Affek, 2018; Samsonova et al., 2019).
A significant part of the potential natural honey-bearing resources in the Russia belongs to different categories of forest lands with numerous woody honey plants. The total forest area of the primary honey-bearing woody plants in Russia, including Tilia cordata Mill., Acer platanoides L., Salix caprea is 4,886 thousand ha, of which 67% are occupied by T. cordata Mill. stands. The Bashkirs is a leader in forest stocks with the dominant composition of T. cordata Mill., occupying 1.14 million ha of 5.7 million ha of forest lands. Mature and overmature linden stands cover 637.1 thousand hectares (55.9%), young plants – 72.2 thousand hectares (6.3%), with an average age of lime tree forests of 61.1 years. Russia fully meets domestic requirements for honey. Since 2014 it has become a net honey exporter, ranking 39th among other countries between Taiwan and Serbia. Nevertheless, the country’s forest is often considered a source of timber and not a natural resource for bee-keeping products. In addition, there are available resources for the effective development of beekeeping in the republic, but there is still a problem in listing nectar lime tree forests on the inventory. Electronic card filing could improve the efficiency of nomadic beekeeping (Sokolov, 1983; Sultanova et al., 2017; Gabdelkhakov et al., 2021; Martynova et al., 2021).
There has been little discussion of forest use in beekeeping. The research in this field is at an early stage of development and, as a rule, presented by monographic studies. Studies are mainly devoted to higher timber and ecological efficiency of the forest, determined by its biomass or protective properties, the ability to compensate for man-made, recreational and other loads (Bürgi, 1999; Matula et al., 2012; Pukkala et al., 2014; Jacobsen et al., 2018; Konashova et al., 2018; Smith et al., 2019; Sultanova et al., 2019; Samburova et al., 2022). The lack of research in contemporary beekeeping has a significant impact on the distribution of hives over an area for better honey production. There is an urgent need to assess the honey-bearing potential of lime tree forests as a food supply for beekeeping, which will contribute to targeted farming in linden stands, higher yield of commercial honey and more bee colonies on the territory of the forest fund.
The given study aimed to investigate the honey-bearing potential of T. cordata Mill. stands in the Southern Urals using forest management and taxation methods.
The research objectives were to distribute T. cordata Mill. forests on the republic’s territory by zones by forest districts; to analyze honey productivity of plantings including T. cordata Mill. taking into account the linden age, its share in the forest composition, the average number of flowers on the tree; to develop an information file of the honey-bearing potential of nectar linden trees of the Southern Urals.
Materials and Methods
The research was conducted in pure and mixed even- and uneven-aged forests with T. cordata Mill. in different natural zones of the Southern Urals on the territory of the Bashkirs. These included forests of the South Ural forest-steppe region, the forest-steppe region of the European part of Russia, the area of coniferous-deciduous (mixed) forests of the European part of Russia. The study relied on the following initial data: the natural and climatic conditions of the Bashkirs, specially protected forest areas (honey-bearing forest areas). The T. cordata Mill. forests in the republic were divided into zones based on forest districts (Figure 1) as follows: zone I – linden trees occupy less than 100 ha; zone II – linden trees cover 100–15,000 ha; zone III – 15,000–55,000 ha; and zone IV – 55,000–115,000 ha. The linden area and stocks were classified as “nectar lime tree” by the rotation age of mature forest of 81–100 years old.
The research employed laying temporary and permanent trial sites under forestry standard OST 56-69-83 “Trial sites in forest management. Trial establishment.” There were long-term observations and experimental work at the trial sites. The flowering of lime tree forests was studied in a permanent control trial area. All the trees there were numbered, their diameter, age class and average height for every diameter class were determined. The number of flowers was analyzed on sample trees in the north-south- and west-east-oriented crown direction. The crown was divided into 2-m segments. The flowering rate of T. cordata Mill. trees was determined by a 5-point scale of visual flowering evaluation: 0 – a total absence of flowers; 1 – flowers are available in the upper quarter of the crown; 2 – flowers are available in the upper half of the crown; 3 – flowers are available in three quarters of the crown; and 4 – flowers are available in the entire crown.
The undergrowth layer in the stands under examination: Cerasus fruticosa (Pall), Padus racemosa (Lam.) Gilib, Viburnum opulus L., Rosa acicularis Lindl, Rubus idaeus L., Lonicera xyloxteum, Coryllus avellana, Sorbus aucuparia L. Forest live cover: Aegopodium podagraria, Chamerion angustifolium, Heracleum sibiricum, Archangelica officinalis, Melilotus officinalis L., Polygonum alpinum, Achillea millefolium, Echinops sphaerocephalus L., Lavatera thuhngiaca L., Origanum vulgare L., Carduus crispus.
The honey productivity of plantings including small-leaved linden was determined based on the stand density, age, the share of linden in the forest composition, the average number of flowers on a linden tree, the average amount of nectar per 1 ha of linden, the average linden flowering period according to the formula:
where HP is the honey productivity of the linden on the plot; L is the linden coefficient in the forest composition (Table 1); N is the amount of nectar per 1 ha based on the stand density; F is the linden flowering time; S is the area of the plot, ha.
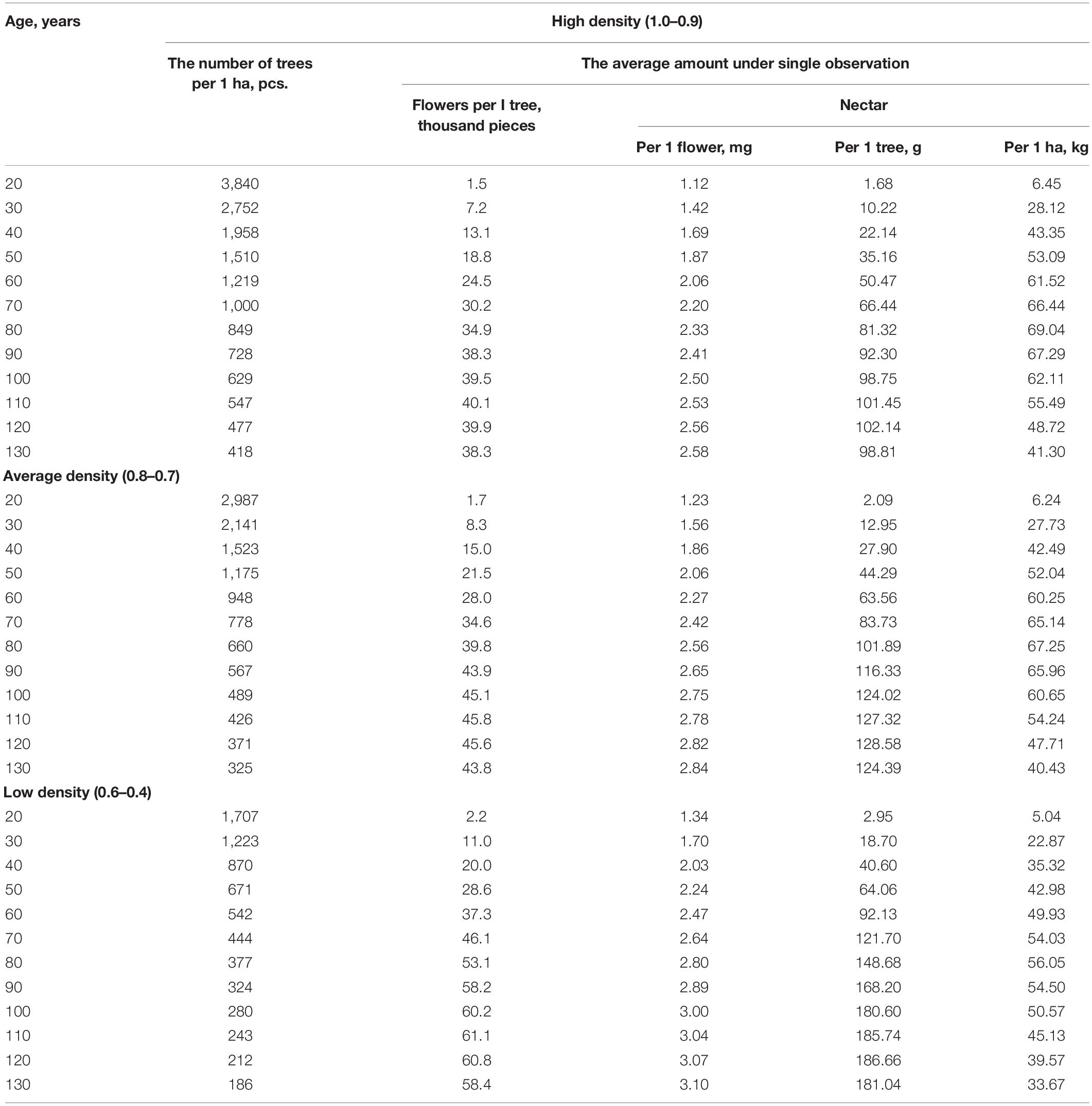
Table 1. Honey productivity of Tilia cordata Mill. depending on the stand density (Murakhtanov, 1977).
This can be illustrated by a forest stand of a high density (0.8) with a composition of 8NTC2B + A. growing on an area of 10 ha. Honey productivity for an average linden flowering period of 12 days is calculated as follows based on the Murakhtanov (1977) dynamics of average nectar-bearing capacity indicators and field studies of the present research:
Nectar from linden flowers was extracted by rinsing. Fifty flowers had been collected from the southern and northern sides of the crown and placed in a glass flask. Then the flask was poured with an accurately measured amount of distilled water. The flask with flowers and a distiller was clogged. shaken for 20–30 min. Next. the flask contents were to be filtered and 20 cm3 filtrate drained into a vessel for examination. This method of studying the amount of nectar in flowers requires strict isolation of flowers from bees and other insects. Four samples during the flowering of the lime tree are sufficient to conduct an analysis (Murakhtanov, 1977). The data were processed using variational statistics. correlation and regression analysis. Microsoft Excel and Statistica 6.0 programs.
Results
Considering the high value of linden trees as honey plants, their felling was limited in the Bashkirs since late 1950s. Lime trees were protected for the sake of beekeeping legislatively. including resolutions of the Bashkir Republic’s Council of Ministers No. 693 “On measures for farming in lime stands located in the area of developed beekeeping” dated October 01, 1959 and No. 347 “On measures to develop beekeeping and increase honey production” dated July 30, 1968. The cutting of T. cordata Mill. for harvesting wood in 3-km zones around beekeeping apiaries and in areas of developed beekeeping was banned in more than 80% of the forest area. These regulations along with the “Logging rules for lime tree forests in the zone of stationary apiaries” caused a sharp drop in the volume of harvested wood from 1994 to 2004. The estimated cutting area reduced from 42.1 in 1991 to 11.7% in 2002. Large amounts of merchantable wood underused until 2004 resulted in accumulation of ripe and overmature T. cordata Mill. forests. Ripe and overgrown forests still prevail though cutting of T. cordata Mill. increased from 168.0 to 262.0 thousand m3 in 2017–2019. This fact has both positive and negative impacts. Ripening and mature stands have more inflorescences and nectar per one tree. The nectar yield from one flower of a 50-year-old T. cordata Mill. sample tree amounted 0.944 ± 0.351 mg while it was 1.402 ± 0.112 mg from a flower of 100–year-old trees. The nectar production is reducing in the years following. The crown extension (south-north) in the conditions of the research area affects the flowering intensity: 60–70-year-old trees have 36,960 inflorescences on the south side and 30,310 flowers on the north side. The flowering rate is more dependent on the crown height. The top of the crown produces from 30 to 59% inflorescences of the total while the middle part from 31 to 49% flowers. At the base of the crown there are from 4 to 21% of inflorescences. With age, the flowering production ratio in different parts of the crown does not change significantly. The observed significant differences in the inflorescence number depending on age (r = 0.96; Sr = 0.04) are not confirmed by the reliable differences in honey-producing characteristics: the sugar-in-solution concentration (max 16.4 ± 1.1; min 11.8 ± 1.3%). nectar-forming capacity (max 0.656 ± 0.21; min 0.472 ± 0.08 mg). nectar bearing capacity (max 1.312 ± 0.092; min 0.944 ± 0.351 mg) and honey productivity (max 0.82 ± 0.05; min 0.59 ± 0.07 mg). The maximum and minimum values are common both for middle-aged and overripe stands.
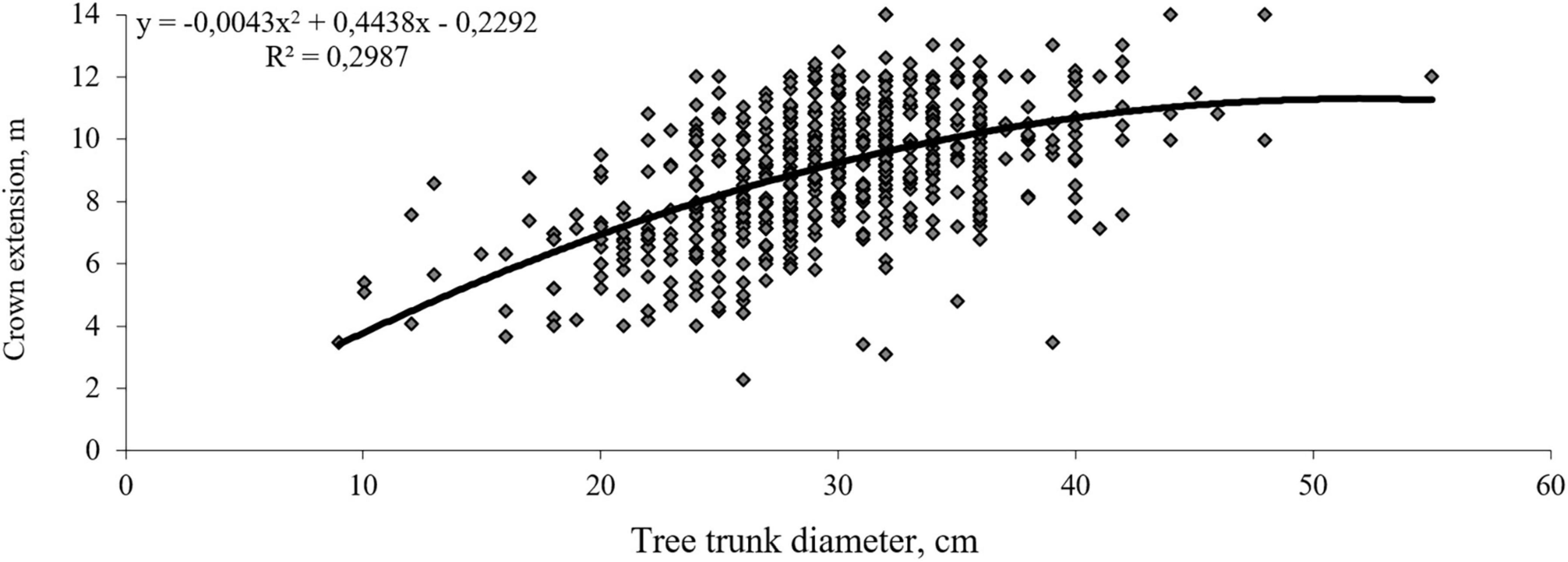
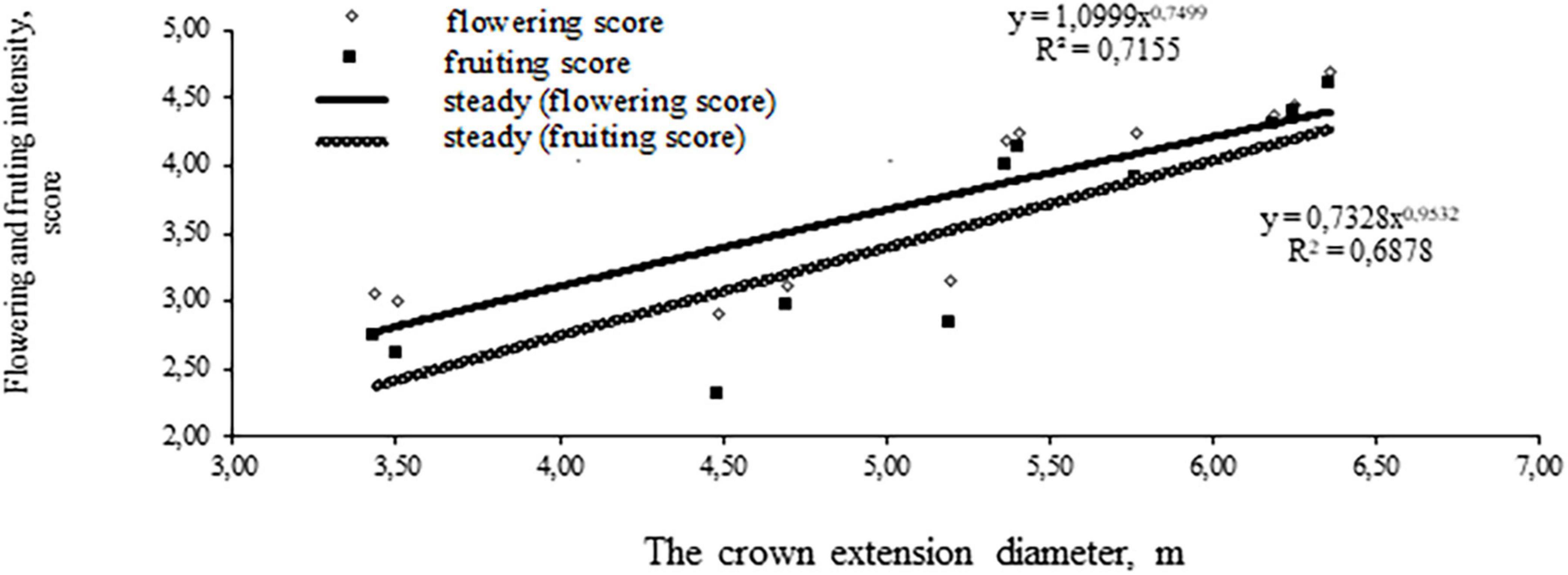
Small-leaved linden has a powerful crown with the diameter variation coefficient up to 42.2%. There is a close relationship between the crown extension and the trunk diameter expressed by the correlation coefficient r = 0.547 (Figure 1). The intensity of flowering and fruiting is closely related to the crown diameter (Figure 2).
Several factors make nectar-bearing forests in the Southern Urals attractive: sustainable growth of external and internal demand for beekeeping products; sufficient amount of available nectar-bearing plants; free access to forest resources under the provisions of the state forest policy; comprehensive state support and constant attention of the government to beekeeping as one of the essential branches of agriculture; implemented investment projects and provided business benefits. Meeting the growing demand of the population for organic beekeeping products requires the expanded nectar-bearing base and identifying forests as a primary component in the production system of the beekeeping industry.
The most common subspecies of bees for the South Ural region on the territory of the Bashkortostan Republic is the Burzyan honey bee Apis mellifera mellifera. It is resistant to invasive diseases and aggressiveness, being an important factor for preventing infections and preserving the population. In addition, it has high resistance to cold, it can collect honey actively and in the short run. Bees are locally preserved in the mountain-forest zone in the state nature reserve “Shulgan-Tash,” the Altyn Solok Nature Reserve and the Bashkiria National Park. Though wild-honey farming has been a traditional occupation of Bashkirs, 99% of the Burzian A. mellifera mellifera are kept in apiaries with frame hives. About 1% bees live in natural and artificial hollows in tree trunks – boards and decks.
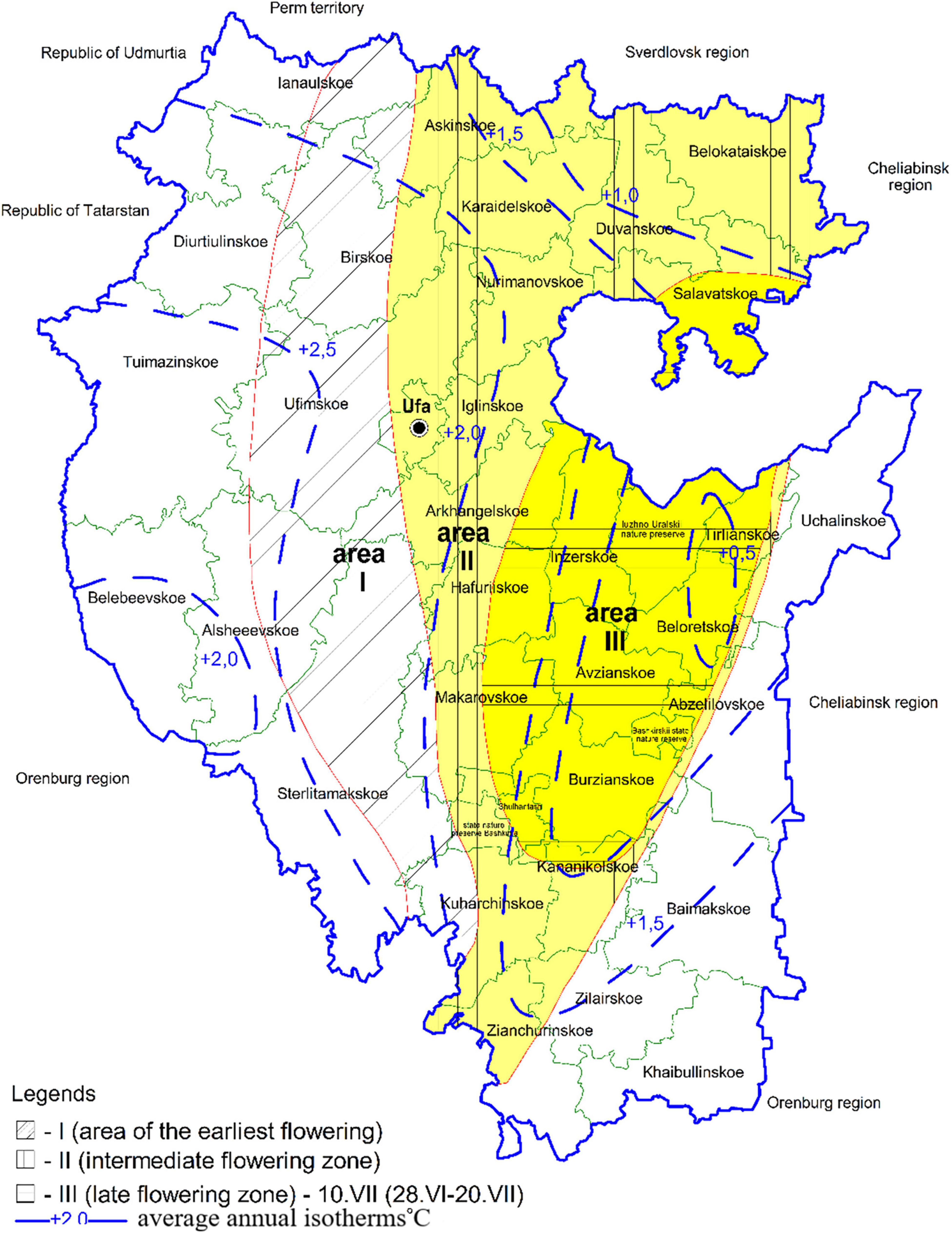
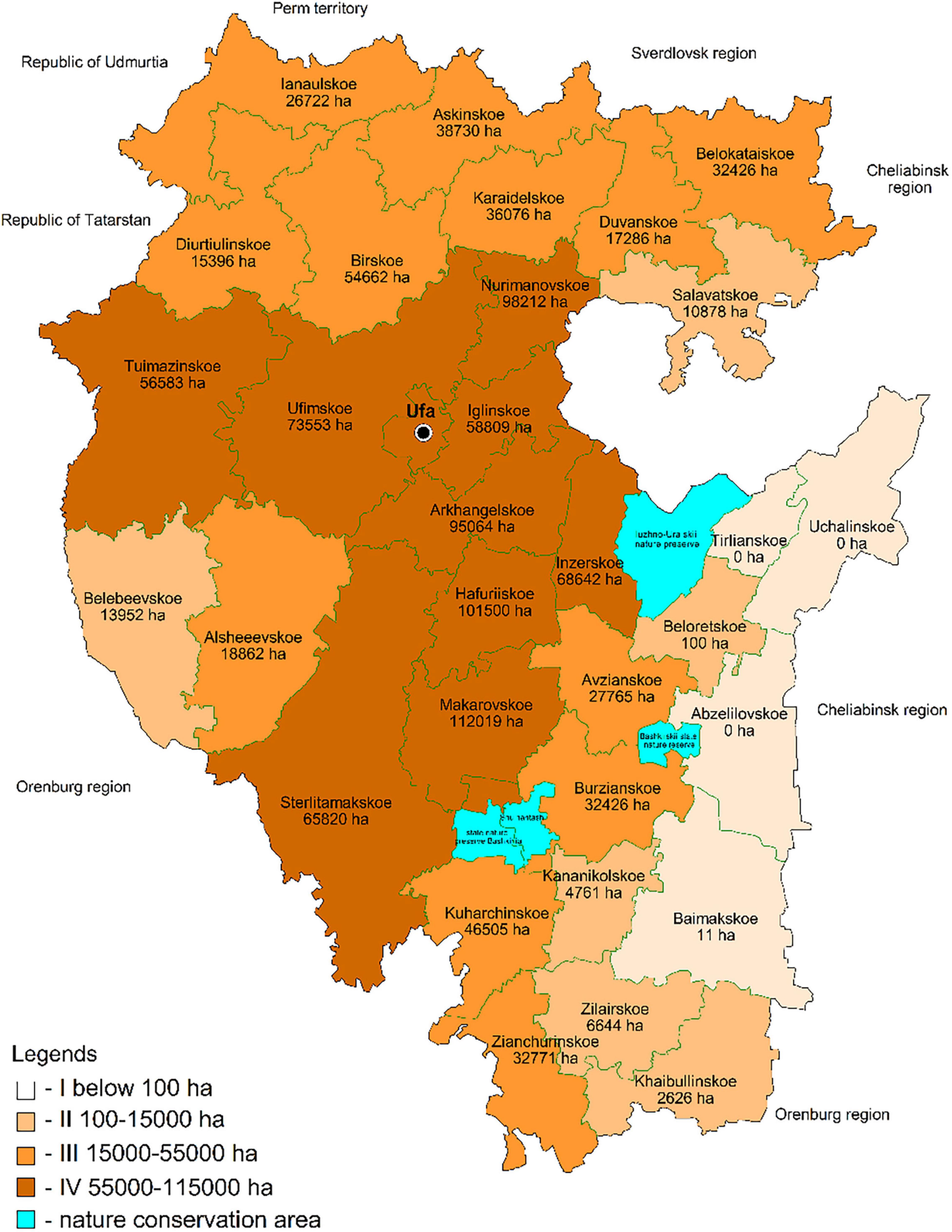
The potential honey-bearing resources. including the unique linden stands of T. cordata Mill. differing in structure. distribution scale. reproduction and honey productivity. are distributed unevenly across the natural zones of the region. Depending on natural zones. climatic conditions and landscape. the size of honey-bearing resources range from complete absence to 107.7 thousand ha. Linden in several forest districts located in the south-eastern part of the republic does not grow or occupies an area of less than 100 ha with a total reserve of about 1 thousand m3. It grows to a greater extent in the central. southern and western regions of Bashkortostan: Arkhangelskoe (18,377.4 thousand m3). Gafuriiskoye (20,910.9 thousand m3). Iglinskoie. Makarovskoie. Nurimanovskoie. Ufa forestry districts. where it occupies up to 50% of the total forested area. The flowering time and duration. honey harvest conditions are also different. Some climatic factors “shift” the beginning of T. cordata Mill. flowering and its duration. Based on the timing of linden flowering. three zones are identified that are comparable to the average annual isotherms of the republic’s territories and the spread of linden trees throughout the region (Figure 3).
With an increase in the average annual temperature. the T. cordata Mill. flowering starts earlier (r = −0.5 at Sr = 0.12. tr = 4.08. t0.05 = 2.01). The sum of temperatures for April–September also affects the annual volume of honey from one bee family (r = 0.51. F = 0.69).
Zone (I) is the area of the earliest flowering of T. cordata Mill. Linden trees make up the largest and the average share in the forest compositions (from 15,000 to 115,000 ha. Figure 4). It has the highest average annual air temperature of 2.0–2.5°C. The flowering begins on June 25. The intermediate flowering zone (II) is on the territory with the average annual isotherm of 1.5–2.0°C. The average date of flowering is July 1. The late flowering zone (III) is characterized by the lowest average air temperature up to 0.5°C. Flowering starts on July 10. There is the smallest number of linden trees.
In total. T. cordata Mill. stands referred to as the economic section “nectar linden.” and being the melliferous sources of the region. occupy an area of 157.7 thousand ha with a total wood stock of 29 thousand m3 (Figure 5).
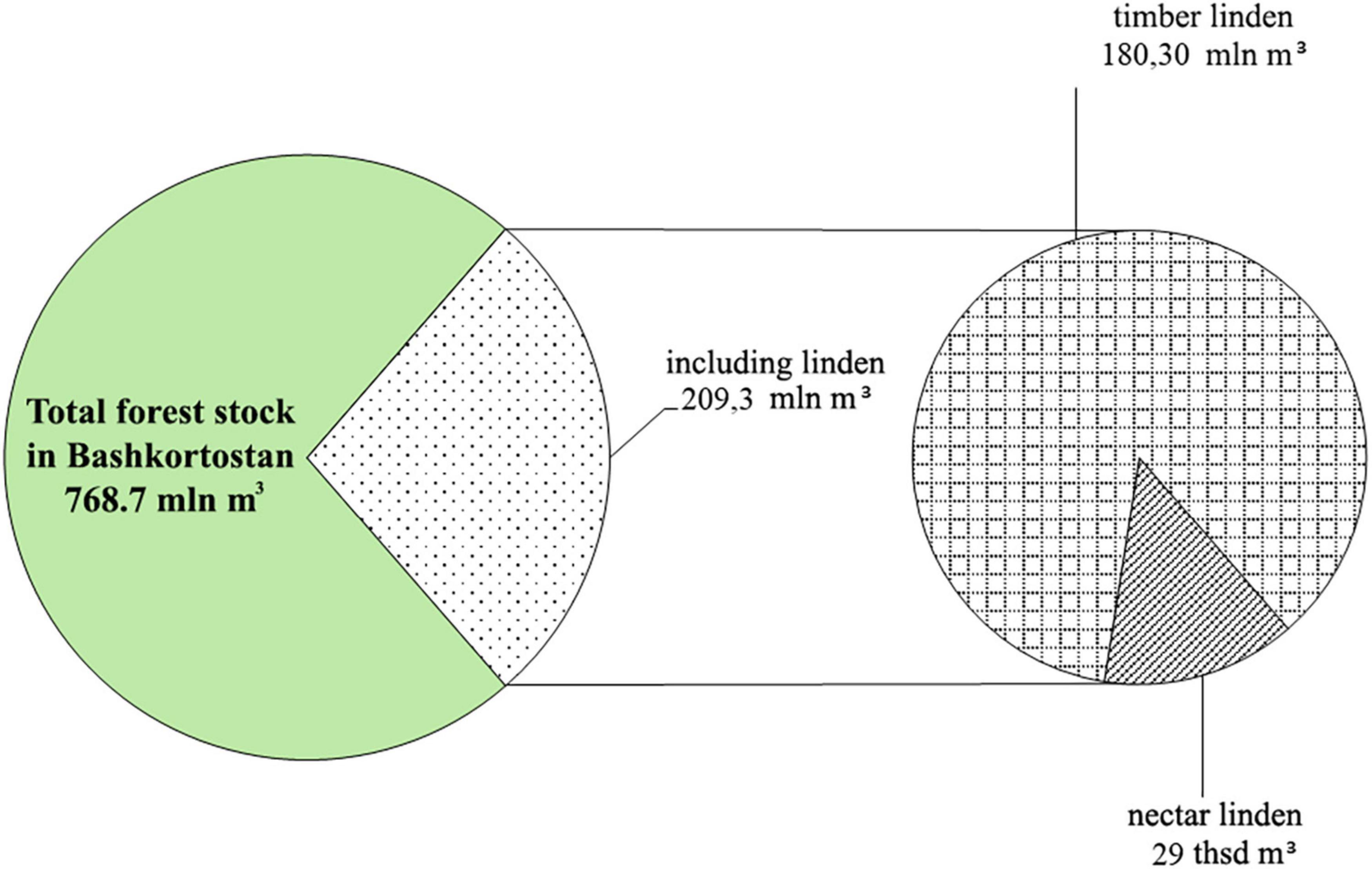
Figure 5. Distribution of the total Tilia cordata Mill. wood stock by economic sections – commercial linden and nectar linden.
Honey-bearing areas of forests are distinguished as specially protected areas (SPA) in stands with a predominance of maturing. mature and overmature T. cordata Mill. trees. They adjoin the 3-km zone around apiaries with a special farming mode. SPAs can be allocated in protective. operational and reserve forests. Linden trees in honey-bearing areas of forests around stationary apiaries are mostly cut because of not being included in the SPA category. The SPA legal status protects the linden trees from logging. which contributes to their preservation.
The development of beekeeping depends on the knowledge of the forage base. The specific. qualitative. quantitative and territorial representation of forest honey-bearing plants is a decisive factor when choosing places for the apiary. The available data characterizing forest melliferous resources make it possible to solve the following tasks: to determine the potential honey productivity of forests; to choose the most suitable place for apiaries; to determine the optimal number of bee colonies; to set the timing of honey flows and their duration; to develop a system of forestry management measures for nectar-bearing plants.
To address uneven distribution of melliferous sources and the resulting competition between bee colonies for food, it is necessary to compile an inventory of honey-bearing lands and ensuring twofold migration of bees from forests to fields planted with honey crops during the entire honey harvest season. It can increase the number of bee colonies in small apiaries up to 150, providing one bee family (50–60 thousand bees) with at least 130 kg of nectar. Meanwhile, it should be taken into account that 1 ha of forest, depending on forestry and taxation indicators, can produce from 500–700 to 150–200 kg of nectar.
Based on the above mentioned. an information file of the honey-bearing potential of nectar lime tree forests in the Southern Urals was developed. Table 2 presents a fragment of the card file for mixed deciduous plantings of 34 allotments in six quarters of the Iglinskii forestry district on an area of 278.5 ha. The T. cordata Mill. composition here ranges from five to eight units. In addition. the stands include Betula pendula Roth. Quercus robur L. and P. racemosa Lam. Gilib. S. aucuparia L. Depending on the age. the number of flowers on one T. cordata Mill. tree varies from 0 to 60.2 thousand pieces. The maximum amount of nectar is 69.04 kg/ha at 12-day linden flowering.
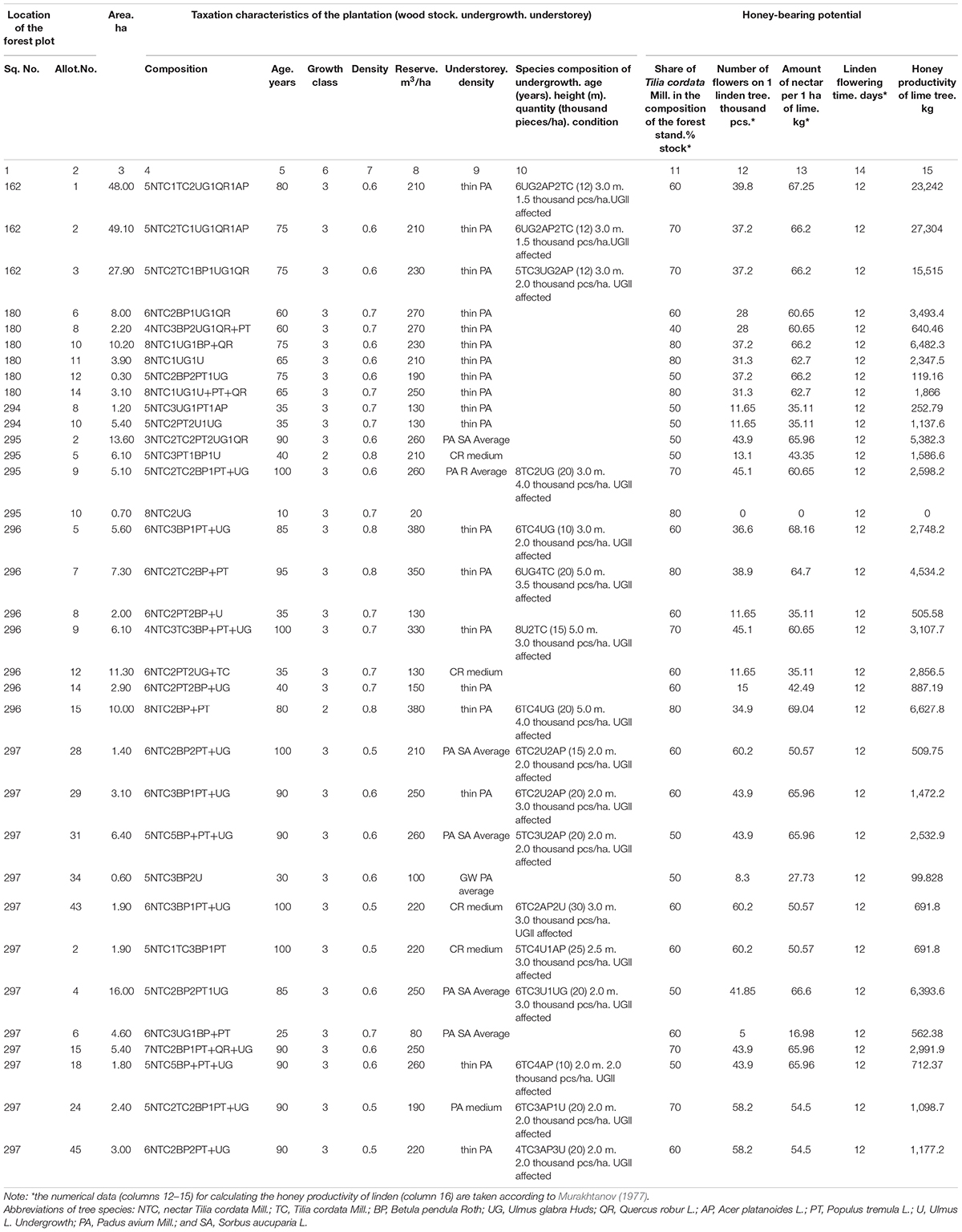
Table 2. Honey-bearing potential of specially protected areas (SPA) – “honey-bearing forest areas” (fragment). Object: Iglinskoe. Ulu-Teliakskoe forest districts.
The honey productivity of plants including T. cordata Mill. calculated concerning the linden age. its share in the forest composition. the average number of flowers on the tree. nectar per 1 ha of linden and the flowering period ranged from 252.8 to 662.8 kg/ha.
Discussion
Polish scientists found that there are 32 species of honey-bearing plants out of 85 plant species growing in 51 phytosociological regions. They are the food base for bees. The researchers distinguished forest plants with the highest honey-bearing capacity: Scrophularia nodosa L. (up to 700 kg/ha) and Solidago gigantea Aiton (700 kg/ha). The latter is an introduced species in these forests. The group of plants with a high honey-bearing potential (more than 100 kg/ha) also includes Angelica archangelica. Stachys sylvatica L. Aronia melanocarpa. Ajuga reptans L. R. idaeus L. T. cordata Mill. and A. platanoides L. They have a wide ecological growth amplitude and can grow in moist habitats (Affek et al., 2021). The honey production potential of some species of honey-bearing plants. having no quantitative data in the literature. was estimated based on the calculation of the nectar bearing capacity of similar species (Szklanowska, 1979).
Numerous botanical studies of honey have revealed the dominance of forest tree species as the main food supply for beekeeping (Elzaki and Tian, 2020). The authors showed that the botanical origin of honey influences its marketing in Sudan and most Arab countries. Honey from the Ziziphus spina-christi L. Desf. and Acacia nilotica L. Delile nectar is especially appealing to many consumers.
For example. in Sudan. the maximum density of hives is 10. 8. 7 and 8 per hectare for Acacia seyal. A. nilotica. Z. spina-Christi and Eucalyptus spp., respectively. This result shows a very low number of hives per hectare compared to Ethiopia. where about 0.03 hectares are required for every three hives (Jenkins and Miklyaev, 2014). Meanwhile it is higher than in Saudi Arabia with one hive per two hectares (Al-Ghamdi et al., 2016). The authors indicate two reasons for the low yield of honey in Sudan and most Arab countries: the lack of experience in managing modern beekeeping for optimal honey production and the lower bee feed quality.
Tilia cordata Mill. is essential in adapting forests to climate change due to the wide environmental sustainability and more ecosystem opportunities (De Jaegere et al., 2016). including food supply for beekeeping. This study found irregular distribution of melliferous sources. resulting in competition between the bee colonies for food resources that is consistent with findings of other researchers. Honey-bearing plants are distributed unevenly in the natural environment. It has an impact on the density of hives (Torné-Noguera et al., 2014); the area of honey plants is changing dramatically throughout the season and from year to year (Flo et al., 2018).
Comparing the calculated honey productivity potential with the corresponding apiary’s demand for nectar during the flowering period of the linden tree draws to the conclusion on the possible number of bee colonies on the territory. T. cordata Mill. honey-bearing lands on the area of 157.716 ha classified as the economic category “nectar linden” make it possible to place up to 1,261.7 thousand bee colonies based on the density of 8 bee colonies per 1 ha.
Conclusion
In forest stands with the prevailing linden composition. it is advisable to farm linden. create target conditions for its growth and use its nectar qualities for beekeeping.
1. The republic’s high potential in the beekeeping industry development is determined by the available honey-bearing resources of small-leaved linden. The difference in its volume across the region is enormous. There are no linden trees in the southeastern part (Abzelilovskoe. Uchalinskoe. Tirlianskoe forestry districts) while lime trees are abundant in the central. southern and western parts (Gafuriiskoe. Iglinskoe. Nurimanovskoe. Ufimskoe. Arkhangelskoe forest districts. where linden occupies up to 50% of the area covered by forest). For example. the lime tree takes up more than 112 thousand ha in the Makarovskoe forestry district.
2. The small-leaved linden’s flowering time and duration. the conditions of the honey collection are different and depend on natural zones. climatic conditions. differ. In this regard. it was entirely justified to assess the development rate of forest territories for beekeeping activities. It is not well developed. There are 991 forest plots on 1,059.5 ha.
3. There is a discrepancy between the territorial localization of “nectar” (honey-bearing) linden and specially protected honey-bearing forest areas on the republic’s territory. There is no developed methodology for assigning linden to these categories. The Russian national forest register does not distinguish between “commercial linden” and “nectar linden” (honey-bearing). It complicates the procedure for allocating plots for apiaries on forest fund lands.
4. The increased rotation age of linden trees to 81–90 years for several decades. a ban on final cutting. reduced annual cut of soft-leaved forests by 15% enlarged the stocks of overmature forest to 48%. Taking into account the age limit of T. cordata Mill., a source of high-quality lime honey, the set of measures should include logging of ripe T. cordata Mill. stands and regeneration cut of overripe trees losing their target functions to promote natural renewal and favorable conditions to grow for the younger generation of T. cordata Mill.
The linden honey production card file will contribute to the development of industrial beekeeping in the Bashkirs. Both nomadic and stationary beekeeping can increase the forest food supply potential to 60–70% and enlarge honey production by 20–25%.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
RiS: conceptualization, validation, investigation, data curation, writing – original draft, review, and editing, and visualization. MM: methodology, formal analysis, investigation, data curation, visualization, and writing – review and editing. ReS: conceptualization, methodology, resources, and writing – original draft, review, and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
References
Affek, A. N. (2018). Indicators of ecosystem potential for pollination and honey production. Ecol. Indic. 94, 33–45. doi: 10.1016/j.ecolind.2017.04.001
Affek, A. N., Regulska, E., Kołaczkowska, E., Kowalska, A., and Affek, K. (2021). Pollination potential of riparian hardwood forests—a multifaceted field-based assessment in the Vistula valley. Poland. Forests 12:907. doi: 10.3390/f12070907
Agbelemoge, A., and Adekola, P. J. (2020). Assessment of traditional harvesting techniques of wild honey in forest reserves of Oyo state. Nigeria. Futo J. Series (FUTOJNLS) 6, 14–22.
Al-Ghamdi, A., Adgaba, N., Getachew, A., and Tadesse, Y. (2016). New approach for determination of an optimum honeybee colony’s carrying capacity based on productivity and nectar secretion potential of bee forage species. Saudi J. Biol. Sci. 23, 92–100. doi: 10.1016/j.sjbs.2014.09.020
Bosma, W., Suti, S., and Deeks, P. (2017). “Beekeeping as pro-forest income diversification in Solomon Islands,” in Climate Change Adaptation in Pacific Countries, ed. W. Leal Filho (Cham: Springer), 371–387.
Bürgi, M. (1999). A case study of forest change in the Swiss lowlands. Landsc. Ecol. 14, 567–576. doi: 10.1023/A:1008168209725
Burkhard, B., Kroll, F., Nedkov, S., and Müller, F. (2012). Mapping ecosystem service supply. demand and budgets. Ecol. Indic. 21, 17–29. doi: 10.1016/j.ecolind.2011.06.019
Bystriakova, N., Griswold, T., Ascher, J. S., and Kuhlmann, M. (2018). Key environmental determinants of global and regional richness and endemism patterns for a wild bee subfamily. Biodivers. Conserv. 27, 287–309. doi: 10.1007/s10531-017-1432-7
Cunningham-Minnick, M. J., and Crist, T. O. (2020). Floral resources of an invasive shrub Alter native bee communities at different vertical strata in forest-edge habitat. Biol. Invasions 22, 2283–2298. doi: 10.1007/s10530-020-02248-y
De Jaegere, T., Hein, S., and Claessens, H. (2016). A review of the characteristics of small–leaved lime (Tilia cordata Mill.) and their implications for silviculture in a changing climate. Forests 7:56. doi: 10.3390/f7030056
Elzaki, E., and Tian, G. (2020). Economic evaluation of the honey yield from four forest tree species and the future prospect of the forest beekeeping in Sudan. Agrofor. Syst. 94, 1037–1045. doi: 10.1007/s10457-019-00478-1
Flo, V., Bosch, J., Arnan, X., Primante, C., Martín González, A. M., Barril-Graells, H., et al. (2018). Yearly fluctuations of flower landscape in a Mediterranean scrubland: consequences for floral resource availability. PLoS One 13:e0191268. doi: 10.1371/journal.pone.0191268
Gabdelkhakov, A., Rakhmatullin, Z., Martynova, M., Fazlutdinov, I., and Mullagaleev, I. (2021). Evaluating diameter distribution series of small-leaved lime (Tilia cordata Mill.) in forest stands. Plant Methods 17:45. doi: 10.1186/s13007-021-00741-6
Häussler, J., Sahlin, U., Baey, C., Smith, H. G., and Clough, Y. (2017). Pollinator population size and pollination ecosystem service responses to enhancing floral and nesting resources. Ecol. Evol. 7, 1898–1908. doi: 10.1002/ece3.2765
Jacobsen, J. B., Jensen, F., and Thorsen, B. J. (2018). Forest value and optimal rotations in continuous cover forestry. Environ. Resour. Econ. 69, 713–732. doi: 10.1007/s10640-016-0098-z
Jenkins, G. P., and Miklyaev, M. (2014). Honey production in Ethiopia: A Cost-benefit Analysis of Modern Versus Traditional Beekeeping Technologies (No. 2013-17). Cambridge: Cambridge Resources International Inc.
Konashova, S. I., Sultanova, R. R., Khairetdinov, A. F., Gabdrakhimov, K. M., Konovalov, V. F., Rakhmatullin, Z. Z., et al. (2018). Forestry and ecological aspects of the broad-leaved forest formation. J. Eng. Appl. Sci. 13, 8789–8795.
Krishnan, S., Wiederkehr Guerra, G., Bertrand, D., Wertz-Kanounnikoff, S., and Kettle, C. J. (2020). The Pollination Services of Forests: A Review of Forest and Landscape Interventions to Enhance Their Cross-Sectoral Benefits. Rome: FAO and Biodiversity International. Forestry Working Paper No. 15.
Martynova, M., Sultanova, R., Blonskaya, L., Gabdelkhakov, A., Volkova, E. Z., and Odintsov, G. (2021). Effectiveness of tending activities in broadleaved forests. J. Environ. Account. Manag. 9, 319–330. doi: 10.5890/JEAM.2021.12.001
Matula, R., Svátek, M., Kůrová, J., Úradníče, L., Kadavý, J., and Kneifl, M. (2012). The sprouting ability of the main tree species in Central European coppices: implications for coppice restoration. Eur. J. For. Res. 131, 1501–1511. doi: 10.1007/s10342-012-0618-5
Perennes, M., Diekötter, T., Groß, J., and Burkhard, B. (2021). A hierarchical framework for mapping pollination ecosystem service potential at the local scale. Ecol. Model. 444:109484. doi: 10.1016/j.ecolmodel.2021.109484
Pukkala, T., Lähde, E., and Laiho, O. (2014). Stand management optimization–the role of simplifications. For. Ecosyst. 1:3. doi: 10.1186/2197-5620-1-3
Roberts, H. P., King, D. I., and Milam, J. (2017). Factors affecting bee communities in forest openings and adjacent mature forest. For. Ecol. Manag. 394, 111–122. doi: 10.1016/j.foreco.2017.03.027
Samburova, M., Safonov, V., and Avdushko, S. (2022). Ecological and biological features of the primrose distribution in transbaikalia as the model territory of Eastern Siberia. Bot. Rev. 88, 50–62. doi: 10.1007/s12229-021-09264-0
Samsonova, I., Gryazkin, A., Smirnov, A., Mannapov, A., and Beljaev, V. (2019). “Bioresource potential of forest lands as the source of honey yield in steppe area of the river Don,” in Proceedings of the IOP Conference Series: Earth and Environmental Science (Bristol: IOP Publishing).
Smith, C., Weinman, L., Gibbs, J., and Winfree, R. (2019). Specialist foragers in forest bee communities are small. social or emerge early. J. Anim. Ecol. 88, 1158–1167. doi: 10.1111/1365-2656.13003
Sokolov, P. A. (1983). “Specifics in composition of seed and coppice linden stands,” in Forest Taxation and Management, ed. P. A. Sokolov (Krasnoyarsk: Krasnoyarsk Polytechnic Institute Publ.), 75–78.
Sultanova, R. R., Martynova, M. V., Khanov, D. A., and Bunkova, N. P. (2017). Evaluation of the use of forest for the implementation of beekeeping and other agricultural activities. Agrar. Bull. Urals. 2, 59–65.
Sultanova, R., Gabitov, I. I., Yanbaev, Y. A., Yumaguzhin, F. G., Martynova, M. V., Chudov, I. V., et al. (2019). Forest melliferous resources as a sustainable development factor of beekeeping. Isr. J. Ecol. Evol. 65, 77–84.
Szklanowska, K. (1979). Nektarowanie i wydajno’s’c miodowa wazniejszych ro’slin runa lasu li’sciastego [Nectar secretion and honey potential of some more important undergrowth plants in deciduous forest]. Pszczelnicze Zeszyty Naukowe 23, 123–130.
Torné-Noguera, A., Rodrigo, A., Arnan, X., Osorio, S., Barril-Graells, H., da Rocha-Filho, L. C., et al. (2014). Determinants of spatial distribution in a bee community: nesting resources. flower resources. and body size. PLoS One 9:e97255. doi: 10.1371/journal.pone.0097255
Keywords: Tilia cordata Mill., beekeeping, honey productivity, bee colonies, nectar productivity, tree stand, composition of plantings
Citation: Sultanova R, Martynova M and Sazgutdinova R (2022) Honey-Bearing Potential of Tilia cordata Mill. Forests in the Southern Urals. Front. Ecol. Evol. 10:832442. doi: 10.3389/fevo.2022.832442
Received: 09 December 2021; Accepted: 11 May 2022;
Published: 21 June 2022.
Edited by:
Carmen-Mihaela Popescu, Institute of Macromolecular Chemistry “Petru Poni”, RomaniaReviewed by:
Bartolomeo Schirone, University of Tuscia, ItalyRakesh Bhutiani, Gurukul Kangri Vishwavidyalaya, India
Copyright © 2022 Sultanova, Martynova and Sazgutdinova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rida Sultanova, c3VsdGFub3ZhX3JpQHJhbWJsZXIucnU=
 Rida Sultanova
Rida Sultanova Maria Martynova
Maria Martynova