
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 29 March 2022
Sec. Coevolution
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.830441
This article is part of the Research TopicWomen in Coevolution 2022View all 7 articles
Cuckoo nestlings thrive as avian brood parasites. To acquire sufficient food from the host parents, cuckoo nestlings generally make louder begging calls than host nestlings, but this may cause them to be more likely to attract the attention of predators. Studies have shown that nestlings would respond to the alarm calls of their parents by begging less, or crouching and remaining silent as an adaptation to reduce the risk of being heard by predators. Nevertheless, research is lacking on how parasite nestlings respond to alarm calls of their host parents. We studied the common cuckoo (Cuculus canorus) and one of the most common cuckoo host species, the oriental reed warbler (Acrocephalus orientalis), using a playback experiment in Yongnianwa National Wetland Park during the breeding seasons from June to July, 2020–2021. The begging behaviors of either cuckoo or host nestlings were quantified by playing back the alarm calls of host adults toward common cuckoo, sparrowhawk (Accipiter nisus), or oriental turtle doves (Streptopelia orientalis). Meanwhile, normal begging without playback, playback of the natural singing (NS) of host adults, and background noise (BN) were included as behavioral reference, non-threatening comparison, and playback control, respectively. The results showed that the cuckoo and host nestlings produced similar levels of begging with or without playback of NS and BN; however, both types of nestlings inhibited their begging intensity after hearing the playback of alarm calls, although they did not respond differently to the various alarm call playbacks. This study therefore elucidated that coevolution has selected the common cuckoo nestlings that adapt their begging behavior to the parent–offspring communication of alarm signaling in their host, oriental reed warblers.
Birds such as cuckoos, which are obligate brood parasites, generally transfer all parental care to hosts, which causes a significant loss of reproduction in the latter; as a response, hosts have evolved a series of anti-parasitic strategies (Davies, 2011; Ma and Liang, 2021). In turn, parasitic birds have tuned their parasitic strategies to better counter the adaptations of hosts (e.g., Marton et al., 2021). Once a female parasitic bird lays her eggs in a host nest, which are subsequently incubated, and hatched by the host, the parasitic nestlings need to intrude into the acoustic communication between hosts and their offspring in order to obtain enough food (Rothstein, 1990; Davies, 2011). In altricial hosts, begging calls of nestlings serve as an important part of communication between nestlings and their parents; these calls carry some important information to convey the needs of nestlings (Kilner et al., 1999). Generally speaking, the more frequently and strongly a nestling begs, the more food it will receive from the parents (Kacelnik et al., 1995; Budden and Wright, 2001); therefore, effective mechanisms are needed to ensure the fidelity of begging calls. Studies have shown that begging increases energy expenditure (Chappell and Bachman, 2002), and that excessive begging will increase the risk of predation (Haskell, 1994; Briskie et al., 1999; Haff and Magrath, 2011). Because nestlings lack an effective means of escape from a nest, nest predation poses a significant danger to the nestlings, and also acts as one of the main causes of failure during reproduction (Martin, 1993). Under these circumstances, birds need to find a balance in the trade-off between the intensity of begging and the risk of nest predation.
The feeding calls parent birds make before their arrival for feeding can be used as a strategy to reduce the risk of discovery by predators (Haskell, 1994; Leech and Leonard, 1997; Briskie et al., 1999; Dearborn, 1999). These calls are effective clues to “open” begging, reminding the nestlings that the parent is approaching, thereby resulting in a begging response (Madden et al., 2005a). In contrast, when faced with danger, the parent birds send alarm calls to the nestlings as an “off” begging signal, so that the nestlings will stop begging (Davies et al., 2004; Platzen and Magrath, 2004; Madden et al., 2005a). In addition, some studies have shown that offspring can also assess predation risk according to predator clues by themselves (Magrath et al., 2007; Yasukawa et al., 2020). At present, many studies have shown using playback that parental alarm calls can induce nestlings to engage in less begging, so they begin crouching or silent behavior (Platzen and Magrath, 2004; Madden et al., 2005a; Haff and Magrath, 2012). At the same time, the alarm calls of parent birds can also encode information about a sense of urgency and relay the type of predators to nestlings, which enables nestlings to respond in the most appropriate way (Platzen and Magrath, 2005). For example, in great tits (Parus major) different alarm signals from the parents caused the nestlings to respond specifically to predators; nestlings will leave the nests when they hear the alarm calls indicating the presence of snakes while they will squat in the nest when they hear the alarm calls of corvids (Suzuki, 2011). However, individual studies have found that eastern phoebe (Sayornis phoebe) nestlings do not react to the alarm calls of their parents (Madden et al., 2005a). In addition, sometimes nestlings also engage in begging behavior even though their parents are not present (Dor et al., 2007), which increases the risk of predation. However, because nestlings have a strong ability to learn (Kedar et al., 2000; Grodzinski et al., 2008; Raihani and Ridley, 2008); they may also learn from the calls of other vulnerable species and gain the ability to evaluate the external predation risk independently. For example, research related to the nestlings of white-browed scrubwrens (Sericornis frontalis) found that they can recognize heterospecific alarm calls through learning calls (Haff and Magrath, 2012).
Previous studies have found that nestlings that are louder and more visible during begging are confronted with greater risk of predation (Haskell, 1994; Leech and Leonard, 1997; Briskie et al., 1999; Dearborn, 1999). Because parasitic nestlings are generally larger and produce begging calls that are louder and more frequent than those of the host nestlings (Davies et al., 1998; Soler, 2017), they are believed to face a higher risk of predation (Martin et al., 2000). Nevertheless, Soler et al. (2019) showed cuckoo nestlings have a lower misjudgment rate in begging behavior than host nestlings, implying that cuckoo nestlings may be more sensitive to the risk perception in the surrounding environment than host nestlings, or this may be a self-protection strategy for cuckoo nestlings to compensate for their larger body size and stronger begging calls. There is another possibility: cuckoo nestlings have lower sibling competition since they are raised without the host nestlings. In addition, some studies have found that cuckoo nestlings show an extraordinary ability to defend themselves when they are about a week old (Davies, 2000), so they may be more efficient in resisting external dangers than host nestlings.
For the hosts, they usually send out alarm calls when they spot a cuckoo, not only because the cuckoo is a parasite (Davies, 2000; Welbergen and Davies, 2008), but also because cuckoos can play the role of nest predator and because cuckoos may also kill host nestlings (Šulc et al., 2020). Therefore, the signal transmission and communication of alarm calls between adults and nestlings in a parasite-host system is more diverse and complex than that in a predator-prey system. However, research in this direction are still relatively weak; only a few studies have verified the response of parasites to the alarm calls of host parents (Khayutin, 1985; Madden et al., 2005b; Davies et al., 2006). Research on alarm call communication between parasite/host nestlings and host parents therefore needs to be further expanded.
In this study, we performed a playback experiment to investigate nestling begging behaviors of common cuckoo (Cuculus canorus) and its host, oriental reed warbler (Acrocephalus orientalis), under different risk conditions. We played back the alarm calls of a host adult directed to alert others of the presence of common cuckoo, sparrowhawk (Accipiter nisus), or oriental turtle dove (Streptopelia orientalis) which represented a nest parasite, predator, or harmless intruder, respectively. We also included normal begging (NB) calls without playback as a behavioral reference, and a playback of natural singing (NS) of a host adult or background noise (BN) as non-threatening comparisons or playback controls, respectively. We hypothesized that cuckoo nestlings should adapt to the parent–offspring alarm signaling system of the host. Therefore, we predicted that cuckoo nestlings would produce a similar level of begging behavior as that of host nestlings so as to obtain sufficient food from host parents, but both types of nestlings should reduce the intensity of begging under playback of alarm calls. In addition, we also predicted that both the cuckoo and host nestlings would maintain a higher level of begging behavior under the alarm calls indicating the presence of doves than which under the alarm calls for cuckoo or sparrowhawk because individuals of the former species do not present a risk of harm nestlings.
Our research site was located in Yongnianwa National Wetland Park (46°48′–47°31′N, 123°51′–124°37′E), Hebei Province, People’s Republic of China (China). Yongnianwa has a temperate semi-humid continental monsoon climate with an elevation of 40.3 m. The annual average precipitation totals 527.8 mm, mainly falling in summer, and the annual average temperature is 12.9°C (Ma et al., 2018). Reed (Phragmites australis), cattails (Typha latifolia), and other herbaceous plants form the backbone of the wetland (Ma et al., 2018). This study was performed during the breeding season from June to July in 2020 and 2021. The common cuckoo (Cuculidae, Cuculiformes) is the most common obligate brood parasitic bird in Europe and Asia (Moksnes et al., 2013; Zheng, 2017). The oriental reed warbler (Acrocephalidae, Passeriformes) is one of the main hosts of common cuckoo. The oriental reed warbler, which breeds among reeds, has a very rich population, with 172.50 ± 45.96 nests found in the past 5 years. The interaction between these two species is believed to involved a high level co-evolution (Yang et al., 2014, 2016, 2017; Li et al., 2016); the probability of an oriental reed warbler nest being parasitized by common cuckoo in the study area is about 14.8% (Ma et al., 2018). The animal study was reviewed and approved by the Animal Research Ethics Committee of Hainan Provincial Education Centre for Ecology and Environment, Hainan Normal University.
Alarm calls of oriental reed warbler had been recorded during previous experiments. The alarm calls were uttered by oriental reed warbler against individuals of common cuckoo, sparrowhawk (predator), and dove (harmless control) [hereinafter referred to as cuckoo alarm (CA), sparrowhawk alarm (SA), and dove alarm (DA), respectively; Yu et al., 2019; Wang and Yang, 2020]. It is feasible to use a specimen instead of a live cuckoo to obtain alarm calls in the avian brood parasitism system (Tryjanowski et al., 2018). To avoid pseudo-replication, three replicates of each type of alarm with high quality were selected from three different nest sites. Each alarm call was selected using Raven Pro 1.4 (Cornell Laboratory of Ornithology, Ithaca, NY, United States) software, with low-frequency noise removed to produce a 30-s soundbite (Bernath-Plaisted and Yasukawa, 2011). BN was randomly selected and recorded at the alarm call sites to be used as the control, and each treatment of sounds was played at the same volume, about 75 dB at 1 m away from the player. The NS of oriental reed warbler was recorded for 30 s as a control, and three replicates of each sample were also obtained. Therefore, a total of five different types of sounds were produced for the playback experiment.
Nestlings of oriental reed warbler aged 5–7 days were randomly selected for use in playback experiments. We chose nestlings 5–7 days old because nestlings at this age are not timid and would present obvious begging behavior as a response to artificial stimuli (Bernath-Plaisted and Yasukawa, 2011). In order to avoid the influence of parent birds or other birds on the responses of nestlings, the playback experiment was performed in our indoor residence near the study site (<5 min journey by bicycle). The host nests that were randomly chosen for experiment contained at least three nestlings, and one nestling of each nest was randomly chosen and brought to the residence (Madden et al., 2005a; Bernath-Plaisted and Yasukawa, 2011). The samples of cuckoo nestlings were collected from parasitized nests. The selected host/cuckoo nestlings were carefully placed in an empty nest which was previously collected using deserted nests while traveling to the study site. An adequate number of cuckoo nestlings was difficult to locate because they were fewer in number than host nestlings and because of nest predation; thus, we did not limit the age of cuckoo nestlings (8 ± 1.86 days) for playback. Because only one cuckoo nestling was in each of the parasitized nests, host nestlings from other nests were moved to these nests to avoid nest abandonment when the cuckoo nestlings were transported for playback experiment. We played back the alarm calls to 16 cuckoo nestlings and 21 oriental reed warbler nestlings, respectively. The samples of host nestlings were collected during the 2020 breeding season while those of the cuckoo nestlings were collected during the 2020 and 2021 breeding seasons. The begging behavior of cuckoo nestlings did not differ between these 2 years (see section “Statistical Analyses”), and thus we merged the data for further analyses.
The sampled nestlings were left alone to allow them to adapt to the indoor environment and were deprived of food for 40 min prior to each playback experiment. A Bluetooth player (BV370, SEE ME HERE Electronic Corporation, Shenzhen, China) was placed at 0.5 m away from the nestlings and used to play back the alarm calls. A recorder (Lotoo L300E, Infomedia Electronic Technology Corporation, Beijing, China) and a digital video recorder were placed at 0.5 and 1 m away, respectively, from the nestlings to record their begging behaviors during the entire experiment process. Before hearing the experimental sounds, nestlings were induced to produce begging by an experimenter gently touching the nest every 3 s with fingers, so as to record 30 s of NB as behavioral reference for comparison with which to compare during playback. After the nestlings stopped begging, they were allowed to calm down for 1 min before sound playback. Subsequently, the five types of sound were played back in a random order with 30 s of playback time for each sound and 5 min of intervals between each two sounds (Madden et al., 2005a,b). Nest touching as described above was also performed during each round of playback to control for the effect of such manipulation while the begging behavior of the nestlings was recorded at the same time. In each playback experiment, we touched the nest immediately after the beginning of the playback of the sound. We touched the nest 10 times in 30 s. All manipulated nestlings were returned to their original nests immediately after each experiment. We visited these nests on the day after the experiment and confirmed that the parents were attending to the nestlings and that all nestlings were in good condition.
We quantified begging behavior in response to the following manipulations: (1) NB, (2) CA, (3) SA, (4) DA, (5) NS, and (6) BN. The playback sequences from (2) to (6) were done randomly for each manipulation of different individuals while the NB was always used first in order to provide a baseline reference of begging behavior. DA refers to the presence of a harmless intruder, NS provides a non-threatening comparison, and BN provides a control for playback manipulation. We quantified nine parameters from 30 s of begging behavior for the host nestlings in each manipulation by analyzing the audio and video: (1) crouched behavior (yes/no); (2) begging time, as total begging time during 30 s of observation; (3) begging score (the max begging intensity which was divided into five levels, 0 = no begging; 1 = beak open and tarsi curved; 2 = level 1 plus neck extension; 3 = level 2 plus tarsi extension; 4 = level 3 plus wing flapping or body rocking); (4) number of bouts of begging (total number of begging bouts in 30 s); (5) begging frequency (number of begging bouts/begging time); (6) begging index (sum up of the products between begging score and its corresponding begging time in 30 s), (7) number of calls (the total number of syllables uttered), (8) call frequency (number of calls/begging time), (9) begging latency (the time from the start of playback to the appearance of begging; Lichtenstein, 2001). The quantification of begging behavior in cuckoo nestlings was the same as which in host nestlings except that the scoring of begging was different. The begging score of cuckoo nestlings was quantified as follows: 0 = no begging; 1 = mouth open without sound; 2 = mouth open with begging calls and head swing; 3 = level 2 plus wing flapping.
We used principal component (PC) analysis to extract the principal factors that affect begging behavior from the nine parameters listed above for the host nestlings by analyzing the criteria of eigenvalues larger than one. Three and two principal factors were extracted from cuckoo and host nestlings, respectively, and we used the first two factors to represent nestling begging behavior (Table 1). We merged the data of cuckoo nestlings between the 2 years of experiments because neither the year nor the interaction between experimental treatment and year had an effect on cuckoo nestling begging behavior (year for PC 1: F = 1.417, df = 1, P = 0.237; treatment × year for PC1: F = 1.679, df = 1, P = 0.099; year for PC2: F = 0.565, df = 1, P = 0.454; treatment × year for PC2: F = 0.828, df = 10, P = 0.603, multivariate analysis of variance). Generalized linear mixed models (glmm or GLMM) using Markov chain Monte Carlo (MCMC) techniques (Hadfield, 2010) were used to estimate the effects of treatment (the six experimental treatments) on PC1 or PC2 of begging behavior in either cuckoo or host nestlings while the individual identity was included as the random effect. Playback order (a value of either 2, 3, 4, 5, or 6) was assigned to temporal position in which a signal was played. For example, if the SA was played fifth, it would receive an ordinal value of 5. Note that NB was always played first, and thus its “order” value was always one. The playback order and the interactions between treatments and order were also tested in GLMM. A post hoc test with Tukey’s adjustment was used for pairwise comparison. All statistical tests were two-tailed and P = 0.05 was the level of significance. Statistical analyses were performed by using MCMCglmm and emmeans packages in R (Version 4.1.0) for Windows.1 Figures were generated by JASP (Version 0.15) for Windows (University of Amsterdam, The Netherlands).
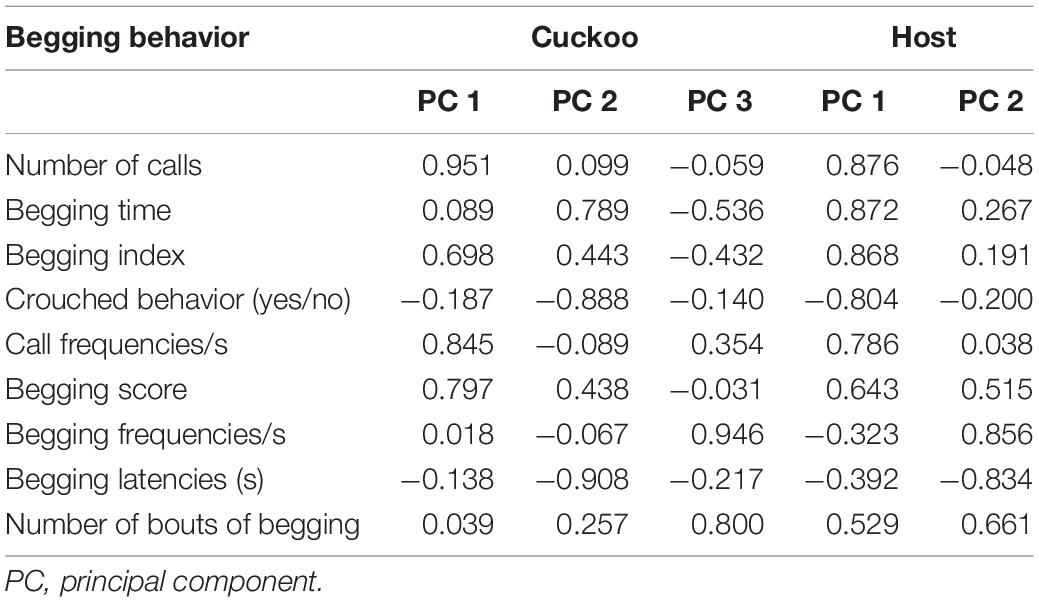
Table 1. Component matrix of begging behavior in common cuckoo (cuckoo, n = 16) and oriental reed warbler (Host, n = 21) nestlings by PC analysis.
All the nestlings responded to human touch when the sound was not played back (NB). Only one oriental reed warbler nestling did not respond to BN, whereas the other oriental reed warbler and common cuckoo nestlings showed different intensities of begging behavior in response to BN and NS. Among the cuckoo nestlings, one nestling did not respond to the CA, two did not respond to the SA, and one did not respond to the DA; with regard to the oriental reed warbler nestlings, five nestlings had no response to the CA, four did not respond to the SA, and five did not respond to the DA. The result of PC analysis indicated that the first three principal factors accounted for 85.79% of the total variation of response data for cuckoo nestlings (31.17% for PC1, 30.12% for PC2, and 24.50% for PC3). Here, PC 1 mainly represent the number of calls, call frequency, begging score, and begging index; PC 2 mainly represents begging latency, crouched behavior (yes/no), and begging time; PC 3 mainly represents begging frequency and number of bouts of begging. Two principal factors were extracted from the begging behavior of host nestlings (Table 1), which explained 75.30% of total variation of the response data. The first two principal factors explained 49.94 and 25.35% of the total variation, respectively. In addition, PC 1 mainly represents the number of calls, begging time, begging index, crouched behavior (yes/no), call frequency, and begging score; PC 2 mainly represents the begging frequency, begging latency, and number of bouts of begging. Because the first two principal factors accounted for the largest percentage of the original variable, we used PCs 1 and 2 to represent the begging behavior of nestlings during further analyses.
The MCMCglmm method showed that the treatment of experiment and playback order had a significant effect on cuckoo PC1 of begging behavior. Either different treatments or playback order of the experiment significantly predicted the begging behavior of cuckoo nestlings (P for MCMC < 0.001, GLMM, n = 96; Table 2). No significant differences were found in cuckoo PC2 (Table 2). Similarly, for host PC1, both the treatment and order significantly predicted the begging behavior of host nestlings (treatment: P for MCMC < 0.001, GLMM; order: P for MCMC = 0.004, GLMM, n = 126). Nevertheless, for host PC2, the treatments, order, and their interaction all significantly predicted the begging behavior of host nestlings (Table 2). A post hoc test indicated that the host nestlings had similar begging intensities between treatments without alarm calls (i.e., BN, NB, and NS), and this was significantly higher than with those in DA, SA, and CA when alarm calls were played back (P < 0.001 for all pairs of comparisons, post hoc test; Figure 1). For the cuckoo nestlings, the begging intensity was the highest in BN and NB while it was lowest in DA with significant differences (P < 0.001 for both, post hoc test). The begging intensity in NS did not differ to BN and NB, and was insignificantly different from SA and CA (NS vs. SA: P = 0.056, NS vs. CA: P = 0.052, post hoc test), but significantly higher than DA (P = 0.037, post hoc test; Figure 1). Finally, no significant differences were detected between treatments with alarm calls (i.e., DA, SA, and CA; Figure 1).
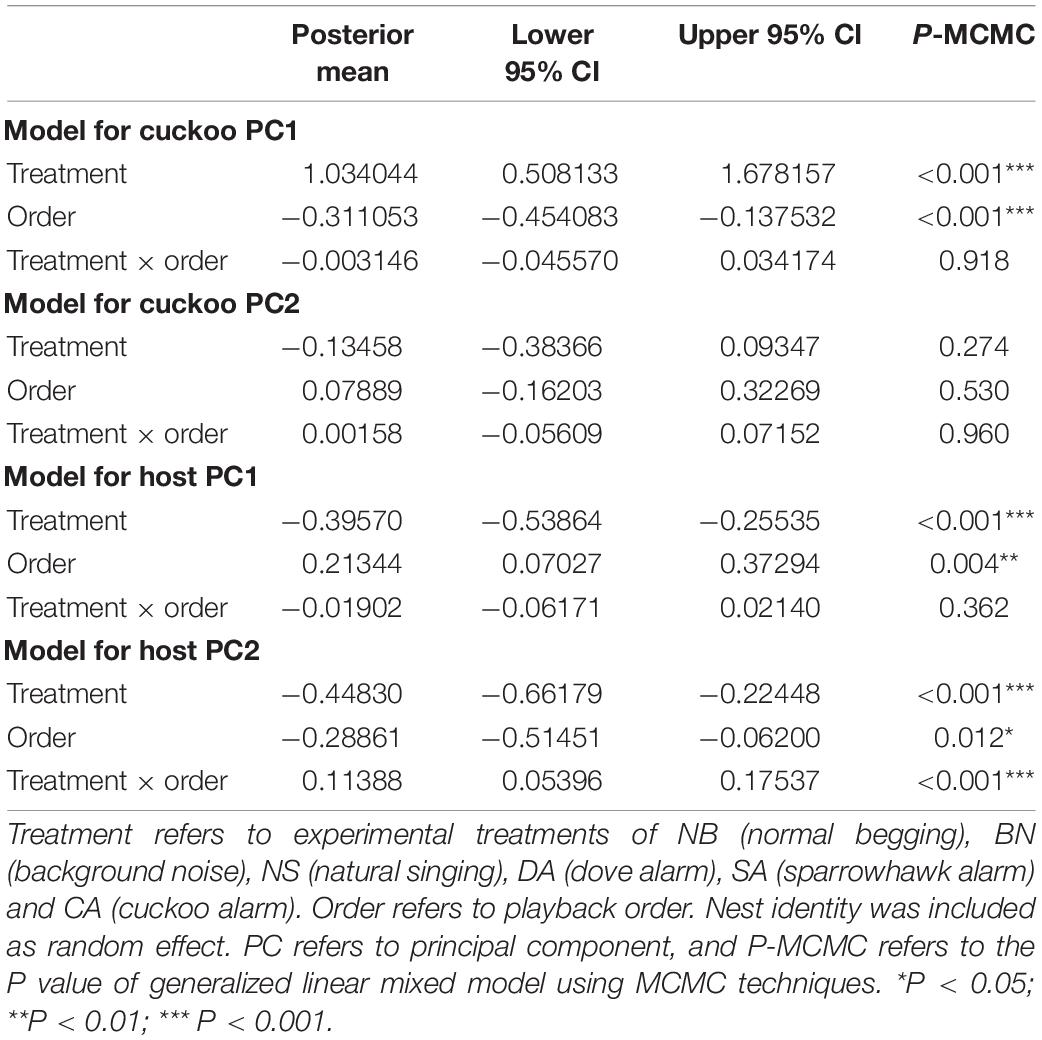
Table 2. The responses of common cuckoo (Cuckoo) and oriental reed warbler (host) nestlings in different experimental treatments by generalized linear mixed model.
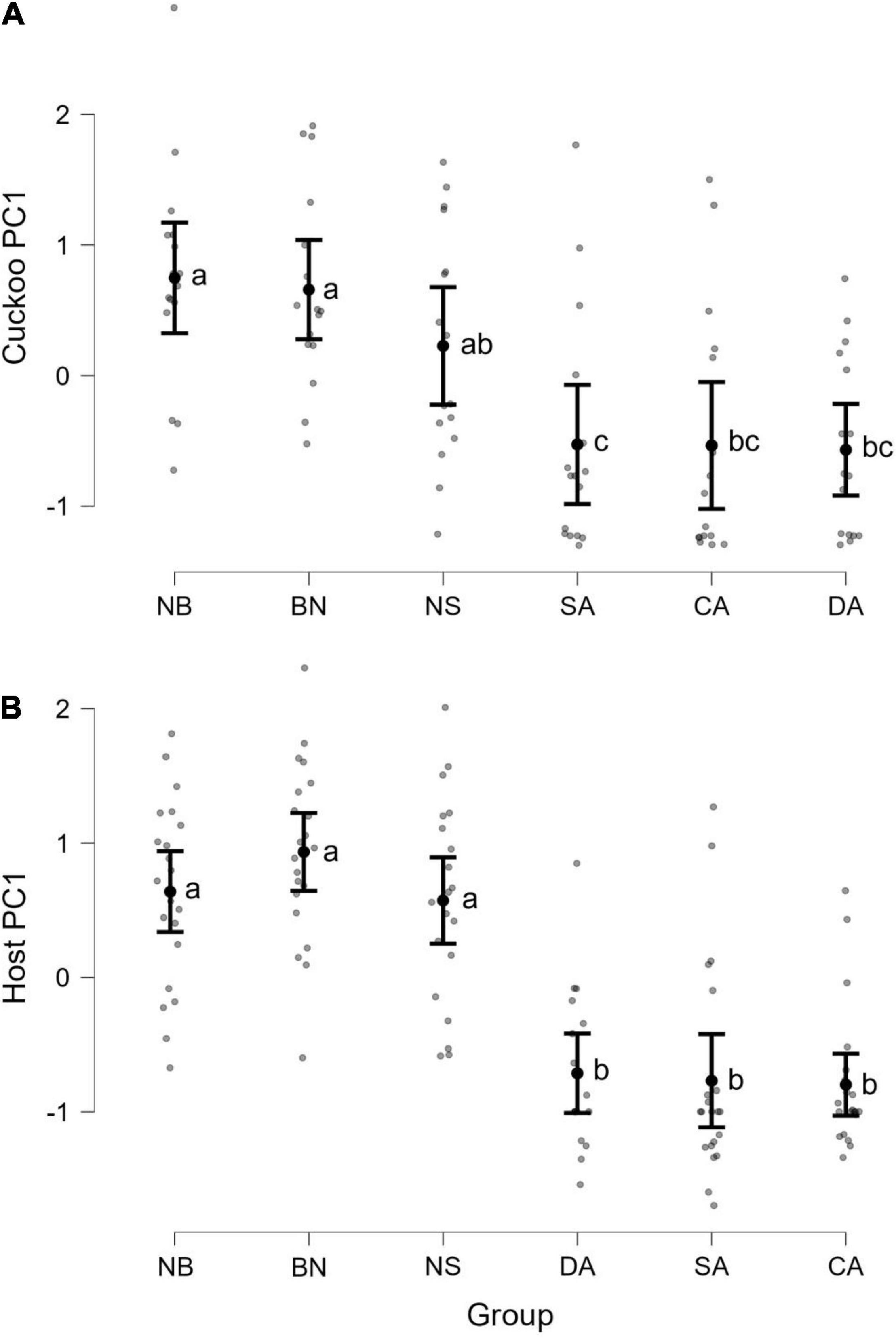
Figure 1. Pairwise comparisons of principal component 1 (PC1) between different treatments of experiments used to analyze the begging behaviors of common cuckoo (A: Cuckoo, n = 16) and oriental reed warbler (B: Host, n = 21) nestlings. NB, normal begging; BN, background noise; NS, natural singing; DA, dove alarm; SA, sparrowhawk alarm; CA, cuckoo alarm. Values are presented as mean ± SE with different letters indicating statistically significant differences between treatments in cuckoo or host nestlings (P < 0.05). No statistical significance of PC1 was detected between cuckoo and host nestlings within each treatment.
As we predicted, this study elucidates that common cuckoo nestlings behaved similarly to nestlings of their hosts, oriental reed warblers, in that they produced similar begging intensity without alarm calls but inhibited their begging intensity when alarm calls were played back. This indicates that the cuckoo nestlings can perceive the information in the alarm calls of hosts, implying that in order to reduce predation risk during parasitism they have adapted to the parent–offspring communication of alarm signaling in their hosts. Nevertheless, although the response between cuckoo and host nestlings was similar in the PC1 model, it became inconsistent in the PC2 model. This implied that the cuckoo and host nestlings presented different patterns of begging behavior toward the same information they perceived. The playback order had a certain effect on the begging behavior of nestlings; however, this can be explained by the fixed order of a NB treatment (the NB was fixed as the first treatment for a baseline reference of begging behavior). This can be proved by the post hoc test, which indicated that significant differences of order were only found between NB and other treatments but did not occur between the orders of other treatments (Supplementary Figure 1). There were three possible and non-mutually exclusive explanations for such order effects. First, this implied that the nestlings were most sensitive to the first detection of information, which was related to the first visit by parents in a certain time of food provision. Second, the reaction intensity to stimuli by nestlings decreased from the second stimulus, which implied that begging behavior was energy intensive, and the following stimuli such as NS treatment were not strong enough to trigger their response in the same manner. Third, NB treatment without sound stimuli was stronger than that with non-threatening sound stimuli such as BN, which implied that such sound stimuli influence the begging response from nestlings even though they do not encode information of predation risk.
An important part of understanding the evolution of begging behavior is to determine the selective forces that limit it (Kilner and Johnstone, 1997). Nestlings, especially altricial nestlings, lack effective escape means in the face of danger, and excessive begging will reveal the nest location and thus increase the risk of predation (Haskell, 1994; Briskie et al., 1999; Haff and Magrath, 2011). Therefore, the trade-off between food acquisition and predation cost may be an important selection pressure related to begging signals. Nestlings will initiate begging calls based on cues that their parents are coming (Madden et al., 2005a); at the same time, nestlings can also understand the risk of predation in the environment through the alarm calls of parents, and reduce the probability of being detected by predators by inhibiting begging behavior (Davies et al., 2004; Platzen and Magrath, 2004; Madden et al., 2005a). Many studies have shown that adult alarm calls can inhibit the begging behavior of nestlings (Ryden, 1978; Greig-Smith, 1980; Knight and Temple, 1986; Kleindorfer et al., 1996; Davies et al., 2004; Platzen and Magrath, 2004; Madden et al., 2005a). As we predicted, the present study found that the begging behaviors of cuckoo and its host nestlings were also inhibited by the parental bird alarm calls. This indicates that both nestlings of both species can respond appropriately to the danger information contained in the alarm calls sent by the host and respond by reducing their begging sound and activity, which is consistent with relevant previous research results (Platzen and Magrath, 2004; Madden et al., 2005a; Haff and Magrath, 2012). In addition, the oriental reed warbler builds its nest in reeds, and the shaking of the nest affected by the wind may cause the nestlings to make begging calls at an inappropriate time. Therefore, it is beneficial for the adults to use alarm calls to transmit danger signals to the chicks. However, previous studies have found that cuckoo or other birds nestlings often ignore alarm calls issued by adult birds and maintained their begging behavior (Khayutin, 1985; Maurer et al., 2003; Anderson et al., 2010). The alarm calls of the adult birds may not always be used to warn the young birds; the purpose of these calls may be to distract the attention of predators or send danger signals to their spouses (Madden et al., 2005a).
The present study found that both the nestlings of common cuckoo and the host oriental reed warbler responded similarly to different types of alarm calls sent by host adults. This indicates that they could not further identify the types of alarm calls, which was inconsistent with our prediction and with the findings of Platzen and Magrath (2005) who found that nestlings could recognize different types of alarm calls of their parents. This difference may be due to the fact that the types of alarm calls used in this study did not contain specific information that allowed the nestlings to discriminate between the types of calls (Wang and Yang, 2020; Wang et al., 2021). In two previous studies, we found that adults of the oriental reed warbler had different behavioral responses to different invaders, but that there was no difference in alarm call characteristics and their responses to different types of alarm calls (Wang and Yang, 2020; Wang et al., 2021). Furthermore, the common cuckoo is also a potential predator (Šulc et al., 2020), so their alarm calls may also contain information about predation risk. Although doves do not present a risk of harm to adults and nestlings, adult oriental reed warblers are very territorial, and their alarm calls may also contain messages designed to expel any avian or other intruders out of their territories. Haff and Magrath (2012) found that the nestlings of white-browed scrubwrens could learn to address external risks by eavesdropping on the alarm calls of different species, and thereby reduce the risk of predation by inhibiting begging behavior. In other words, nestlings may hear and respond to calls not only of their caregivers, but also of several bird species. It is beneficial for nestlings to get information about environmental risks from other species that are also sounding the alarm about the risk of predation. Few studies have addressed the issue of how parasitic nestlings respond to and adapt to the alarm calls of adoptive parents. For example, a study on the common cuckoo nestlings in nests of redstart (Phoenicurus phoenicurus) found that the response of cuckoo nestlings to the host alarm calls will inhibit begging and nestlings will exhibit immobility (Khayutin, 1985). Madden et al. (2005b) also found that nestlings of the brown headed cowbird (Molothrus ater) also responded to the alarm calls of the host red-winged blackbird (Agelaius phoeniceus) by inhibiting begging; these studies are consistent with our findings. However, Davies et al. (2006) found that common cuckoo nestlings would respond to the “churr” alarm calls of their major host, reed warbler (Acrocephalus scirpaceus), against predators by reducing their begging; nevertheless, the nestlings did not respond to the alarm calls of two new hosts, the robin (Erithacus rubecula) or dunnock (Prunella modularis). It was supposed that conspecific parasitic nestlings associated with different host races would react specifically to the alarm calls of different host races, but this hypothesis is based on only a few studies and needs to be further verified. In addition, whether the nestlings would eavesdrop on the alarm calls of other birds that are sympatric but not being utilized remains to be further studied. In addition, we found that the playback order of nestlings’ begging behavior has a significant impact due to the fact that the NB was always in first place; however, from the second order onward, we chose the sound playback randomly, including three types of alarm calls, so the cause of each order of reaction was an average effect, meaning that the effect would be lower than the first order. However, we also conducted a post hoc test and found no significant difference between the other orders except for the first. In general, based on the experimental results, our experimental method appears to be feasible.
In summary, our study found that common cuckoo nestlings, like host nestlings, quickly adjust their begging strategies when they receive information about potential danger from host parents. This suggests that the cuckoo nestlings have successfully adapted to the communication system of alarm signals between parents and offspring in hosts. These studies will facilitate our understanding of the co-evolution of the brood parasite-host system at the nestling stage.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Animal Research Ethics Committee of Hainan Provincial Education Centre for Ecology and Environment, Hainan Normal University.
CY designed the study and carried out statistical analyses. JW performed the field experiments. LM and XC reviewed and analyzed the video data. CY and JW wrote the draft of the manuscript. All authors approved the final submission.
This work was funded by the Hainan Provincial Natural Science Foundation of China (320CXTD437 and 2019RC189 to CY), the Natural Science Foundation of Hebei Province of China (C2020101002 to LM), and the National Natural Science Foundation of China (Nos. 31672303 to CY and 32101242 to LM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the Yongnianwa National Wetland Park for its support and permission to carry out this study. We also thank the three reviewers whose feedback helped to improve the quality of our manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.830441/full#supplementary-material
Anderson, M. G., Brunton, D. H., and Hauber, M. E. (2010). Species specificity of grey warbler begging solicitation and alarm calls revealed by nestling responses to playbacks. Anim. Behav. 79, 401–409. doi: 10.1016/j.anbehav.2009.11.017
Bernath-Plaisted, J., and Yasukawa, K. (2011). Effect of alarm calling by male Red-winged Blackbirds on nestling begging and female provisioning behavior. J. Field Ornithol. 82, 395–405. doi: 10.1111/j.1557-9263.2011.00342.x
Briskie, J. V., Martin, P. R., and Martin, T. E. (1999). Nest predation and the evolution of nestling begging calls. P. Roy. Soc. B: Biol. Sci. 266, 2153–2159. doi: 10.1098/rspb.1999.0902
Budden, A. E., and Wright, J. (2001). Falling on deaf ears: the adaptive significance of begging in the absence of a parent. Behav. Ecol. Sociobiol. 49, 474–481. doi: 10.1007/s002650100323
Chappell, M. A., and Bachman, G. C. (2002). “Energetic costs of begging behaviour,” in The Evolution of Begging: Competition, Cooperation and Communication, eds J. Wright and M. L. Leonard (Dordrecht: Kluwer Academic), 143–162. doi: 10.1007/0-306-47660-6_8
Davies, N. B. (2011). Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. doi: 10.1111/j.1469-7998.2011.00810.x
Davies, N. B., Kilner, R. M., and Noble, D. G. (1998). Nestling cuckoos, Cuculus canorus, exploit hosts with begging calls that mimic a brood. P. Roy. Soc. B: Biol. Sci. 273, 693–699. doi: 10.1098/rspb.1998.0346
Davies, N. B., Madden, J. R., and Butchart, S. H. (2004). Learning fine-tunes a specific response of nestlings to the parental alarm calls of their own species. P. Roy. Soc. B: Biol. Sci. 271, 2297–2304. doi: 10.1098/rspb.2004.2835
Davies, N. B., Madden, J. R., Butchart, S. H. M., and Rutila, J. (2006). A host-race of the cuckoo Cuculus canorus with nestlings attuned to the parental alarm calls of the host species. P. Roy. Soc. B: Biol. Sci. 273, 693–699. doi: 10.1098/rspb.2005.3324
Dearborn, D. C. (1999). Brown-headed cowbird nestling vocalizations and risk of nest predation. Auk. 116, 448–457. doi: 10.2307/4089378
Dor, R., Kedar, H., Winkler, D. W., and Lotem, A. (2007). Begging in the absence of parents: a “quick on the trigger” strategy to minimize costly misses. Behav. Ecol. 18, 97–102. doi: 10.1093/beheco/arl056
Greig-Smith, P. W. (1980). Parental investment in nest defence by stonechats (Saxicola torquata). Anim. Behav. 28, 604–619. doi: 10.1016/S0003-3472(80)80069-8
Grodzinski, U., Erev, I., and Lotem, A. (2008). Can hungry nestlings be trained to reduce their begging? Behav. Ecol. 19, 116–125. doi: 10.1093/beheco/arm107
Hadfield, J. (2010). MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. doi: 10.18637/jss.v033.i02
Haff, T. M., and Magrath, R. D. (2011). Calling at a cost: elevated nestling calling attracts predators to active nests. Biol. Lett. 7, 493–495. doi: 10.1098/rsbl.2010.1125
Haff, T. M., and Magrath, R. D. (2012). Learning to listen? Nestling response to heterospecific alarm calls. Anim. Behav. 84, 1401–1410. doi: 10.1016/j.anbehav.2012.09.005
Haskell, D. (1994). Experimental evidence that nestling begging behaviour incurs a cost due to nest predation. P. Roy. Soc. B: Biol. Sci. 257, 161–164. doi: 10.1098/rspb.1994.0110
Kacelnik, A., Cotton, P. A., Stirling, L., and Wright, J. (1995). Food allocation among nestling starlings: sibling competition and the scope of parental choice. P. Roy. Soc. B: Biol. Sci. 259, 259–263. doi: 10.1098/rspb.1995.0038
Kedar, H., Rodriguez-Girones, M. A., Yedvab, S., Winkler, D. W., and Lotem, A. (2000). Experimental evidence for offspring learning in parent-offspring communication. P. Roy. Soc. B: Biol. Sci. 267, 1723–1727. doi: 10.1098/rspb.2000.1201
Khayutin, S. N. (1985). Sensory factors in the behavioral ontogeny of altricial birds. Adv. Study Behav. 15, 105–152. doi: 10.1016/s0065-3454(08)60488-x
Kilner, R., and Johnstone, R. A. (1997). Begging the question: are offspring solicitation behaviours signals of need? Trends. Ecol. Evol. 12, 11–15.
Kilner, R. M., Noble, D. G., and Davies, N. B. (1999). Signals of need in parent-offspring communication and their exploitation by the common cuckoo. Nature 397, 667–672. doi: 10.1038/17746
Kleindorfer, S., Hoi, H., and Fessl, B. (1996). Alarm calls and chick reactions in the moustached warbler, Acrocephalus melanopogon. Anim. Behav. 51, 1199–1206. doi: 10.1006/anbe.1996.0125
Knight, R. L., and Temple, S. A. (1986). Nest defence in the American goldfinch. Anim. Behav. 34, 887–897. doi: 10.1016/S0003-3472(86)80075-6
Leech, S. M., and Leonard, M. L. (1997). Begging and the risk of predation in nestling birds. Behav. Ecol. 8, 644–646. doi: 10.1093/beheco/8.6.644
Li, D., Zhang, Z., Grim, T., Liang, W., and Stokke, B. G. (2016). Explaining variation in brood parasitism rates between potential host species with similar habitat requirements. Evol. Ecol. 30, 905–923. doi: 10.1007/s10682-016-9850-7
Lichtenstein, G. (2001). Selfish begging by screaming cowbirds, a mimetic brood parasite of the bay-winged cowbird. Anim. Behav. 61, 1151–1158. doi: 10.1006/anbe.2000.1688
Ma, L., and Liang, W. (2021). Egg rejection and egg recognition mechanisms in Oriental Reed Warblers. Avian. Res. 12:47. doi: 10.1186/s40657-021-00283-4
Ma, L., Yang, C., Liu, J., Zhang, J., Liang, W., and Moller, A. P. (2018). Costs of breeding far away from neighbors: isolated host nests are more vulnerable to cuckoo parasitism. Behav. Proc. 157, 327–332. doi: 10.1016/j.beproc.2018.07.017
Madden, J. R., Kilner, R. M., and Davies, N. B. (2005a). Nestling responses to adult food and alarm calls: 1. Species-specific responses in two cowbird hosts. Anim. Behav. 70, 619–627. doi: 10.1016/j.anbehav.2004.11.019
Madden, J. R., Kilner, R. M., and Davies, N. B. (2005b). Nestling responses to adult food and alarm calls: 2. Cowbirds and red-winged blackbirds reared by eastern phoebe hosts. Anim. Behav. 70, 629–637. doi: 10.1016/j.anbehav.2004.11.020
Magrath, R. D., Pitcher, B. J., and Dalziell, A. H. (2007). How to be fed but not eaten: nestling responses to parental food calls and the sound of a predator’s footsteps. Anim. Behav. 74, 1117–1129. doi: 10.1016/j.anbehav.2007.01.025
Martin, T. E., Scott, J., and Menge, C. (2000). Nest predation increases with parental activity: separating nest site and parental activity effects. P. Roy. Soc. B: Biol. Sci. 267, 2287–2293. doi: 10.1098/rspb.2000.1281
Marton, A., Fülöp, A., Bán, M., Hauber, M. E., Moskát, C., and Goymann, W. (2021). Female common cuckoo calls dampen the mobbing intensity of great reed warbler hosts. Ethology 2021:13126. doi: 10.1111/eth.13126
Maurer, G., Magrath, R. D., Leonard, M. L., Horn, A. G., and Donnelly, C. (2003). Begging to differ: scrubwren nestlings beg to alarm calls and vocalize when parents are absent. Anim. Behav. 65, 1045–1055. doi: 10.1006/anbe.2003.2148
Moksnes, A., FossØY, F., RØSkaft, E., and Stokke, B. G. (2013). Reviewing 30 years of studies on the Common Cuckoo: accumulated knowledge and future perspectives. Chin. Birds 4, 3–14. doi: 10.5122/cbirds.2013.0001
Platzen, D., and Magrath, R. D. (2004). Parental alarm calls suppress nestling vocalization. P. Roy. Soc. B: Biol. Sci. 271, 1271–1276. doi: 10.1098/rspb.2004.2716
Platzen, D., and Magrath, R. D. (2005). Adaptive differences in response to two types of parental alarm call in altricial nestlings. P. Roy. Soc. B: Biol. Sci. 272, 1101–1106. doi: 10.1098/rspb.2005.3055
Raihani, N. J., and Ridley, A. R. (2008). Experimental evidence for teaching in wild pied babblers. Anim. Behav. 75, 3–11. doi: 10.1016/j.anbehav.2007.07.024
Rothstein, S. I. (1990). A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 21, 481–508. doi: 10.2307/2097034
Ryden, O. (1978). Differential responsiveness of great tit nestlings, Parus major, to natural auditory stimuli-response strength as related to stimulus significance and previous individual exposure. Ethology 47, 236–253. doi: 10.1111/j.1439-0310.1978.tb01834.x
Soler, M. (2017). “Begging Behaviour, Food Delivery and Food Acquisition in Nests with Brood Parasitic Nestlings,” in Avian Brood Parasitism, ed. M. Soler (Cham: Springer), 493–515. doi: 10.1007/978-3-319-73138-4_27
Soler, M., de Neve, L., Macias-Sanchez, E., and Pérez-Contreras, T. (2019). Great spotted cuckoos respond earlier to the arrival of feeding foster parents and perform less erroneous begging when hungry than their magpie host nest-mates. J. Avian Biol. 50:e01952. doi: 10.1111/jav.01952
Šulc, M., Štětková, G., Jelínek, V., Czyż, B., Dyrcz, A., Karpińska, O., et al. (2020). Killing behaviour of adult brood parasites. Behaviour 157, 1099–1111. doi: 10.1163/1568539X-bja10033
Suzuki, T. N. (2011). Parental alarm calls warn nestlings about different predatory threats. Curr. Biol. 21, R15–R16. doi: 10.1016/j.cub.2010.11.027
Tryjanowski, P., Morelli, F., Kwieciński, Z., Indykiewicz, P., and Møller, A. P. (2018). Birds respond similarly to taxidermic models and live cuckoos Cuculus canorus. J. Ethol. 36, 243–249. doi: 10.1007/s10164-018-0554-z
Wang, J., Ma, L., Chen, X., and Yang, C. (2021). Behavioral and acoustic responses of the oriental reed warbler (Acrocephalus orientalis), at egg and nestling stages, to the common cuckoo (Cuculus canorus). Front. Ecol. Evol. 9:705748. doi: 10.3389/fevo.2021.705748
Wang, J., and Yang, C. (2020). Specific responses of cuckoo hosts to different alarm signals according to breeding stage: a test of the offspring value hypothesis. Curr. Zool. 66, 649–655. doi: 10.1093/cz/zoaa021/5838188
Welbergen, J. A., and Davies, N. B. (2008). Reed warblers discriminate cuckoos from sparrowhawks with graded alarm signals that attract mates and neighbours. Anim. Behav. 76, 811–822. doi: 10.1016/j.anbehav.2008.03.020
Yang, C., Li, D., Wang, L., Liang, G., Zhang, Z., and Liang, W. (2014). Geographic variation in parasitism rates of two sympatric cuckoo hosts in China. Zool. Res. 35, 67–71. doi: 10.11813/j.issn.0254-5853.2014.1.067
Yang, C., Wang, L., Liang, W., and Møller, A. P. (2016). Egg recognition as antiparasitism defence in hosts does not select for laying of matching eggs in parasitic cuckoos. Anim. Behav. 122, 177–181. doi: 10.1016/j.anbehav.2016.10.018
Yang, C., Wang, L., Liang, W., and Møller, A. P. (2017). How cuckoos find and choose host nests for parasitism. Behav. Ecol. 28, 859–865. doi: 10.1093/beheco/arx049
Yasukawa, K., Sollenberger, J., Lindsey-Robbins, J., and DeBruyn, E. (2020). Calling in the face of danger: Do nestling Red-winged Blackbirds (Agelaius phoeniceus) suppress begging in response to predator playbacks? Auk. 137:ukz071. doi: 10.1093/auk/ukz071/5666151
Yu, J., Lu, H., Sun, W., Liang, W., Wang, H., and Møller, A. P. (2019). Heterospecific alarm-call recognition in two warbler hosts of common cuckoos. Anim. Cogn. 22, 1149–1157. doi: 10.1007/s10071-019-01307-9
Keywords: alarm signals, avian brood parasitism, begging behavior, coevolution, parent-offspring communication
Citation: Wang J, Ma L, Chen X and Yang C (2022) Common Cuckoo Nestling Adapts Its Begging Behavior to the Alarm Signaling System of a Host. Front. Ecol. Evol. 10:830441. doi: 10.3389/fevo.2022.830441
Received: 07 December 2021; Accepted: 04 March 2022;
Published: 29 March 2022.
Edited by:
Benjamin Jerry Ridenhour, University of Idaho, United StatesReviewed by:
Roi Dor, Open University of Israel, IsraelCopyright © 2022 Wang, Ma, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Canchao Yang, Y2N5YW5nQGhhaW5udS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.