
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 16 February 2022
Sec. Ecophysiology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.817864
This article is part of the Research Topic Individual Variation and Responses of Animals to Changing Environments View all 10 articles
 Christopher G. Goodchild1,2*
Christopher G. Goodchild1,2* Isaac VanDiest1
Isaac VanDiest1 Samuel J. Lane1
Samuel J. Lane1 Michelle Beck1,3
Michelle Beck1,3 Hallum Ewbank2
Hallum Ewbank2 Kendra B. Sewall1
Kendra B. Sewall1A central theme in the field of ecology is understanding how environmental variables influence a species’ distribution. In the last 20 years, there has been particular attention given to understanding adaptive physiological traits that allow some species to persist in urban environments. However, there is no clear consensus on how urbanization influences physiology, and it is unclear whether physiological differences in urban birds are directly linked to adverse outcomes or are representative of urban birds adaptively responding to novel environmental variables. Moreover, though low-density suburban development is the fastest advancing form of urbanization, most studies have focused on animals inhabiting high intensity urban habitats. In this study, we measured a suite of physiological variables that reflect condition and immune function in male song sparrows (Melospiza melodia) from rural and suburban habitats. Specifically, we measured hematological indices [packed cell volume (PCV), hemoglobin concentration, mean corpuscular hemoglobin concentration (MCHC)], circulating glutathione (total, reduced, and oxidized), oxidative damage (d-ROM concentration), antioxidant capacity, and components of the innate immune system [bacteria killing ability (BKA), white blood cell counts]. We also measured whole-animal indices of health, including body condition (scaled mass index length) and furcular fat. Song sparrows inhabiting suburban environments exhibited lower hemoglobin and MCHC, but higher body condition and furcular fat scores. Additionally, suburban birds had higher heterophil counts and lower lymphocyte counts, but there were no differences in heterophil:lymphocyte ratio or BKA between suburban and rural birds. PCV, glutathione concentrations, and oxidative damage did not differ between suburban and rural sparrows. Overall, suburban birds did not exhibit physiological responses suggestive of adverse outcomes. Rather, there is some evidence that sparrows from rural and suburban habitats exhibit phenotypic differences in energy storage and metabolic demand, which may be related to behavioral differences previously observed in sparrows from these populations. Furthermore, this study highlights the need for measuring multiple markers of physiology across different types of urban development to accurately assess the effects of urbanization on wildlife.
Urbanization dramatically restructures ecosystems by introducing novel environmental variables (Marzluff, 2001; Hobbs et al., 2006) and poses one of the largest threats to wildlife (Aronson et al., 2014). Remarkably, in some species, individuals are able to persist in both urban and rural habitats (i.e., “urban adapters”; Blair, 1996; McKinney, 2006), yet the underlying physiological traits that allow individuals of the same species to live in disparate habitats remain unclear. Animals inhabiting urban environments must cope with anthropogenic light and noise, increased exposure to toxicants, altered predator and prey communities, and shifts in disease exposure (Marzluff, 2001; Isaksson, 2018). While some animals are able to thrive under these conditions (i.e., “urban exploiters”; Blair, 1996; McKinney, 2006), there is an underlying assumption that inhabiting urban habitats is costly for most individuals (Birnie-Gauvin et al., 2016; Murray et al., 2019). Several studies have examined the physiological and fitness consequences of urbanization by comparing urban and rural dwelling birds within species and found urban birds exhibit lower body condition (Capilla-Lasheras et al., 2017; Murray et al., 2019) and reduced reproductive success (Chatelain et al., 2021). However, there are also cases where birds inhabiting urban environments exhibit higher body condition (Auman et al., 2008; Minias, 2016), reproductive success (Lane et al., unpublished data) and survival (Møller, 2009b; Phillips et al., 2018) compared to rural birds of the same species. These contradictory results among previous studies highlight the need to understand the physiological processes underlying broader condition and fitness outcomes for individuals living in urban environments (Isaksson, 2015; Ouyang et al., 2018).
A range of physiological mechanisms allow birds to cope with varying environmental conditions, but such mechanisms may carry fitness costs or result in trade-offs with other physiological processes. Consequently, the strongest studies measure several “biomarkers” of physiological condition to more accurately assess costs or benefits of dwelling in urban habitats (e.g., Bókony et al., 2012; Ibáñez-Álamo et al., 2020). Additionally, while it is often assumed that physiological differences among rural and urban birds are directly or indirectly linked to adverse health and fitness outcomes, it is also possible that physiological differences are indicative of urban birds adaptively responding to novel environmental variables (Isaksson, 2015). Therefore, to accurately characterize physiological responses to urbanization and determine if they reflect costs or adaptive responses requires examining multiple physiological variables that reflect a range of processes, including cellular damage, metabolic performance, and immune function.
Previous studies indicate birds inhabiting urban environments often exhibit higher oxidative stress, shifts in hematological variables, and altered immune function (Isaksson, 2015, 2018). Oxidative stress generally describes an imbalance between pro-oxidants (e.g., reactive oxygen and nitrogen species) and antioxidants [e.g., glutathione (GSH)] (Costantini, 2008), and is known to disrupt a myriad of physiological processes, including damage to red blood cell membranes and denaturation of hemoglobin molecules (Mohanty et al., 2014). In some cases, birds inhabiting urban environments exhibit lower packed cell volume (PCV; i.e., hematocrit) and hemoglobin concentrations (Llacuna et al., 1996; Cid et al., 2018), and shifts in these hematological indices may indicate adverse health outcomes in urban dwelling birds associated with increased oxidative stress (Minias, 2015; Johnstone et al., 2017). Alternatively, lower PCV and hemoglobin concentration in urban birds may reflect different metabolic demands related to habitat-specific differences in behaviors (Lowry et al., 2013; Charmantier et al., 2017). That is, in some cases, urban dwelling birds have smaller territory size, are less neophobic, and have shorter flight initiation distances (Senar et al., 2017; Juárez et al., 2020; Fossett and Hyman, 2021). These shifts in behavior may reduce aerobic requirements and thus hematological indices. The fact that urban and rural birds often differ in body condition, which is a rough of estimate of a bird’s total energy reserve, further suggests urban and rural dwelling birds experience different energetic demands (Capilla-Lasheras et al., 2017; Phillips et al., 2018).
Shifts in energy budgets and higher oxidative stress in urban birds also may be associated with altered immune function (Watson et al., 2017; Cummings et al., 2020a,b). Indeed, urban dwelling birds tend to exhibit infection more often than rural birds, which could cause or be the result of altered immune function (Hamer et al., 2012; Bichet et al., 2013; Giraudeau et al., 2014; Rouffaer et al., 2017; Jiménez-Peñuela et al., 2019; Sykes et al., 2021). Interspecific comparisons among urban and rural dwelling avifauna indicate urban birds cope with increased disease exposure by investing in a more robust immune system (Møller, 2009b). Although investment in immune function may improve disease defense, chronic immune activation in urban habitats has been shown to occur at a cost to body condition (Capilla-Lasheras et al., 2017; but see Merrill et al., 2019). Taken as a whole, there is strong evidence that physiological responses to urbanization can lead to trade-offs that generate distinct urban and rural phenotypes (Isaksson, 2018). Nonetheless, determining if physiological responses to urbanization reflect fitness costs or adaptive coping requires further investigation (Isaksson, 2015).
In particular, it is critical to understand the effects of low-density urban environments on avian physiology because the main driver of urbanization globally is the expansion of small and medium sized cities (McKinney, 2002; Fragkias et al., 2013). While most existing studies on the effects urbanization on avian physiology focus on comparisons among birds from rural and highly urbanized environments, prior work has shown that the impact of urbanization on avian richness and abundance can vary within and among urban areas depending on the intensity and recency of urbanization (MacGregor-Fors and Schondube, 2011; Ferenc et al., 2014; Evans et al., 2015, 2018). Given the rate of low density suburban expansion is predicted to increase, it is critical to examine physiological differences between rural birds and birds inhabiting moderately urbanized environments in order to predict how suburban expansion will impact avian populations (Marzluff, 2001; Isaksson, 2015).
In this study, we examined antioxidant capacity and oxidative damage, hematological indices, immune endpoints, and body condition in male song sparrows (Melospiza melodia) living across replicate suburban and rural study sites (see section “Site Selection”; Supplementary Table 1). Song sparrows are a common songbird native to North America and are an excellent model to study physiological differences across urban-rural gradients because they are considered urban adapters and are present in both rural and urban habitats. While our previous studies have detected consistent behavioral differences between rural and suburban song sparrows (Davies and Sewall, 2016; Davies et al., 2018), hormone concentrations to not reliably differ across habitats (Beck et al., 2018; Lane et al., 2021), suggesting other physiological variables may be associated with behavioral differences between rural and suburban dwelling birds. Additionally, there is no evidence of genetic differences between rural and suburban populations in our song sparrow system (Brewer et al., 2020). We predicted suburban birds would exhibit higher oxidative stress, lower PCV and hemoglobin, greater immune activation, and lower body condition.
We sampled male song sparrows from three rural and three suburban field sites in and around the low-density cities of Blacksburg (human population: 44,074; human density: 1,390 per square km) and Radford (human population: 18,255; human density: 1,169 per square km) in Montgomery County, VA, United States (U.S. Census Bureau, 2021). We previously characterized the urbanization level of field sites using a technique described by Seress et al. (2014), whereby we divided an aerial image of the 1 km2 area around each study site into 100 m × 100 m cells and scored the abundance of vegetation, buildings, and paved surfaces, such as roads and parking lots, in each cell. From these cell scores, we calculated the following summary land-cover measures for each study site: mean building density score, number of cells with high building density (>50% cover), number of cells with paved surfaces, mean vegetation density score, and number of cells with high vegetation density (>50% cover). We then used the PC1 score from a principal components analysis (PCA) of these landscape variable to calculate an “urbanization index” [see Seress et al. (2014) and Davies et al. (2018) for method validation and further details on site selection and characteristics]. In this study, we described sites with an urbanization index >3.0 as “suburban” and sites with an urbanization index <−1.70 as “rural” (Supplementary Table 1), though it is worth emphasizing that urbanization index is a continuous variable.
We sampled a total of 136 male song sparrows from suburban and rural sites over 3 years (Supplementary Table 1). In a separate study, we were examining nest attendance behavior and reproductive physiology of female song sparrows in our study population; therefore, we only sampled male sparrows in an effort to avoid interfering with concurrent studies by repeatedly sampling female song sparrows. We captured male song sparrows during the breeding season (April–June) by playing conspecific calls at the center of previously mapped territories to lure males into mist nets [see Hyman et al. (2004) for how male territories were defined). All captures occurred between 500 and 1,200 h. We collected 150–175 μl of blood from each male sparrow (mass: 21.16 ± 1.09 mean ± SD) via venipunction of the basilar vein using a 26-gauge needles and heparinized capillary tubes. After blood sampling, we collected morphological measurements to determine body condition (i.e., scaled mass index; see section “Scaled Mass Index”) We measured hemoglobin concentration and prepared blood smears in the field immediately after blood collection (see section “Hematological Indices”). We stored remaining blood on ice until we returned to the laboratory to further prepare blood for later analysis. An aliquot of whole blood was frozen at −80°C for later analysis of glutathione (see section “Oxidative Stress”). We centrifuged hematocrit tubes to separate plasma and measured PCV (see section “Hematological Indices”), then stored plasma at −80°C until analysis of oxidative damage and antioxidant capacity (see section “Oxidative Stress”) and bacteria killing ability (BKA) (see section “Bacteria Killing Ability”). Data were collected opportunistically while conducting a separate concurrent study of female song sparrows, thus sample sizes varied across years. We collected plasma for BKA from 20 rural and 41 suburban males in year 2016. We collected plasma for d-ROMs and antioxidant capacity measurements from 15 rural and 20 suburban males in year 2017. We collected hematological indices, blood smears, glutathione concentrations, and scaled mass index from 20 rural and 20 suburban males in year 2020. We also measured furcular fat (scored 0–3) for birds collected during each field season. Researchers adhered to social distancing and safety precautions while collecting data for the 2020 field season during the COVID-19 global health crisis. Study design and methods were approved by the Virginia Tech Institutional Animal Care and Use Committee; all animals were immediately released after blood collection and were in good health.
To assess body condition, we calculated scaled mass index according to Peig and Green (2009). The scaled mass index has been proposed as a more robust estimation of body condition because it accounts for a changing relationship between mass and body length as body size changes during growth (Peig and Green, 2010). Following Peig and Green (2009), we conducted Pearson correlations between mass and length measurements (head length, bill width, bill length, tarsus length, wing chord, and tail length) to determine which length measurement was the best predictor of body mass. For song sparrows in this study, wing chord was the best predictor of body mass (R = 0.50), therefore we used this variable in the calculation of scaled mass index.
We measured total hemoglobin in whole blood (∼5 μl) in duplicate using the HemoCue system (Ängelholm, Sweden) and used the mean of replicate measurements (intraassay variation: 2.19%) in the statistical analysis. We measured PCV as the percent red blood cells v/v whole blood. Additionally, we calculated mean corpuscular hemoglobin concentration (MCHC; i.e., amount of hemoglobin per red blood cell volume) as the quotient of hemoglobin concentration divided by PCV.
To count white blood cells, we stained blood smears with the JorVet Dip Quick Stain Kit (Jorgensen Labs, Loveland, CO, United States), and a single observer determined white blood cell differentials for each bird by examining at least 100 leukocytes at 100× magnification and counting the number of lymphocytes, heterophils, monocytes, and eosinophils (sensu Ots et al., 1998; Ewenson et al., 2001; Davis et al., 2004). We calculated heterophil:lymphocyte (H:L) ratio for each bird from the white blood cell differential.
The concentrations of total GSH (tGSH) and free GSH in whole blood were measured using the DetectX Glutathione fluorescent detection kit (Arbor Assays, Inc., Ann Arbor, MI, United States) and following the manufacturer’s instructions. Briefly, whole blood was diluted 1:2 with equal parts 5% sulfosalicylic acid, then kept on ice until assay buffer and sample diluent were added to increase the dilution to 1:300, which we validated as the optimal dilution for our study species. Diluted samples were transferred to a flat-bottom 96-well plate. Each plate contained duplicate 50 μl standards, controls, and 1:300 diluted samples. ThioStar (25 μl) reagent was added to each well, after which the plate was lightly tapped and incubated for 15 min. Free GSH concentration was measured fluorometrically at excitation/emission of 405/510 nm using a Tecan Infinite M200 microplate reader. After measuring free GSH, we measured tGSH by adding 25 μl of reaction mixture provided by the manufacturer to all wells. We then tapped the plate gently to mix constituents, incubated for 15 min, and measured fluorescence a second time at excitation/emission of 405/510 nm. We calculated the mean for duplicate sample results for both tGSH and free GSH. The intra- and interassay variations were 2.52–4.5 and 4.39%, respectively. We calculated oxidized GSH (GSSG) by subtracting free GSH from tGSH, then divided the result by two, per the manufacturer’s instructions. Additionally, we calculated the ratio of GSH:GSSG.
We quantified two aspects of oxidative status using d-ROMs and OXY-Adsorbent kits (Diacron; Grosseto, Italy). The d-ROMs kit measures concentrations of reactive oxygen metabolites in plasma and is a measure of the amount of oxidative damage an individual has sustained. For this assay, we added 10 μl of plasma to each well followed by 200 μl of reagent mix. The plate was incubated for 1 min in the plate reader before being read. Second, we quantified the ability of the plasma antioxidant barrier to neutralize the oxidative action of hypochlorous acid (HOCl) using the Oxy-Adsorbent kit. We diluted plasma 1:100 with distilled water and then added 5 μl of diluted plasma to each sample well followed by 200 μl of HOCl solution. The plate was gently rocked while incubating at 37°C for 10 min after which we added 5 μl of chromagen solution to each well and mixed the wells with additional pipetting. We included blanks and calibration standards in triplicate on the plates for both types of assays. For both assays, we used a microplate reader (BioTek Synergy HTX) set to 37°C to measure sample absorbance at 505 nm and the plates were read kinetically once per minute for a total of 10 min. For both assays, we ran 20 samples in duplicate per plate and used the average of these in the analysis. The intraassay variation for the d-ROMs and Oxy adsorbent assays were 2.42 and 12.86%, respectively.
We first optimized the assay using a pooled aliquot of six plasma samples collected in the field from adult males in the previous 48 h. Plasma was diluted 1:5, 1:10, and 1:20 in sterile PBS and mixed with Escherichia coli (ATCC 8739, Epower microorganisms; Microbiologics® St. Cloud, MN, United States) at concentrations of 104, 105, and 106 colony forming units. Samples were incubated for 30 min at 37°C after which we added 250 μl of Tryptic soy broth (TSB, Sigma-Aldrich, St. Louis, MO, United States) to each sample. Samples were incubated at 37°C and the absorbance recorded after 8, 12, and 24 h of incubation. Samples were vigorously vortexed and absorbance quantified using a NanoDrop Spectrophotometer (ND-2000, Thermo Scientific, Pittsburgh, PA, United States) at OD300. The absorbance of each sample and the positive controls were each averaged and used to calculate the proportion of bacteria killed as 1-(sample absorbance/positive control absorbance). We found that the 1:20 plasma dilution with 105 E. coli concentration and 8 h of incubation resulted in approximately 50% bacteria killing. Following optimization, we evaluated bactericidal capacity of samples from individual males in triplicate (intraassay variation: 12.90%), and calculated the killing capacity using the average absorbance from triplicate measurements.
We used R (Version 4.0.2; R Core Team, 2021) to statistically analyze whether physiological variables differed between rural and suburban birds. Many of the physiological traits we measured can fluctuate throughout the day and over the breeding season, thus Julian date and time of day birds were sampled are included as covariates in all models. We analyzed the effect of habitat on scaled mass index using a linear model (LM), we then included scaled mass index as a covariate in all models of physiological variables. To assess differences in fat score across habitats, we used a generalized linear model (GLM), with a Gamma distribution and included sample year as a covariate. We used LMs to separately analyze the effect of habitat on PCV, hemoglobin, tGSH, free GSH, GSSG, and GSSG:tGSH. To analyze heterophil, lymphocyte, and monocyte counts, we used GLMs with a Poisson distribution for count data. For BKA, we arcsin square-root transformed E. coli counts to meet the assumption of normality, then compared habitat differences using a GLM with a Gamma distribution. We analyzed HOCl neutralization and d-ROMs data using separate GLMs, with a Gamma distribution. For both HOCl neutralization and d-ROMs data, we log-transformed data to meet assumptions of normality. All models were additive. Finally, we tested for correlations among physiological variables for data collected from the same individuals during the 2020 field season. BKA, d-ROMs, and HOCl neutralization were not included in correlation analyses because these data were collected during separate field seasons.
Scaled mass index was higher in birds collected from suburban sites compared to birds from rural sites during the 2020 season (Figure 1A; | t| = 2.05, p = 0.048) but did not vary within the season or by time of day (Table 1). Additionally, across all field seasons, suburban birds had a higher fat score compared to rural birds (Figure 1B; | t| = 2.03, p = 0.04). Regardless of habitat, fat score increased as the breeding season progressed (| t| = 2.00, p = 0.047), but fat score did not vary by body condition or time of day (Table 1).
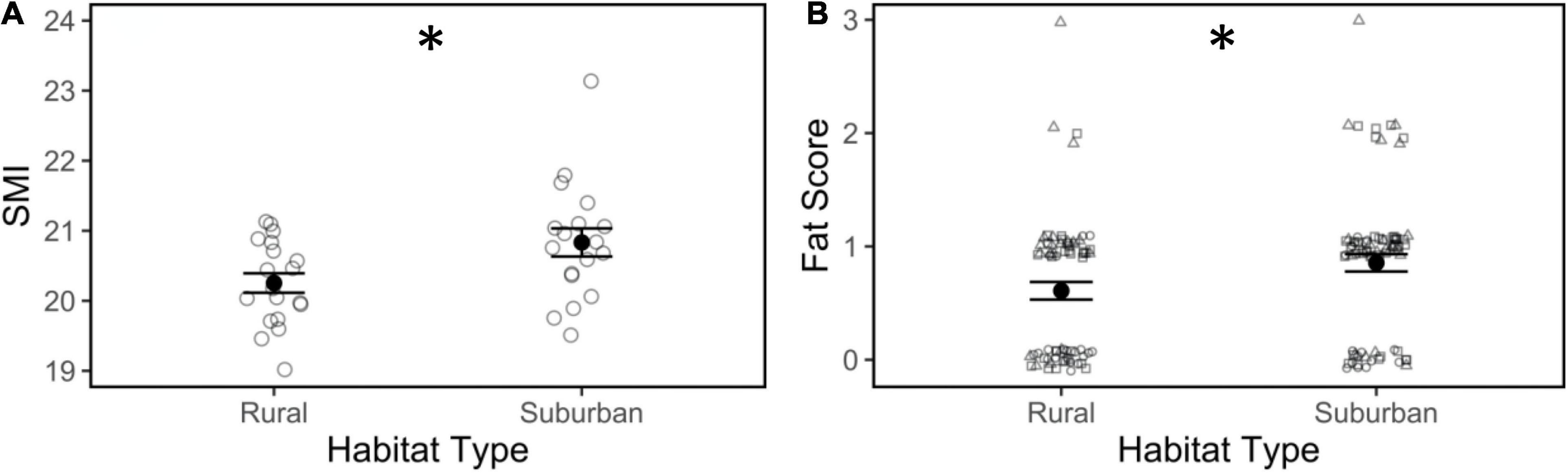
Figure 1. Scaled mass index (SMI; A) and fat score (B) of male song sparrows from rural and suburban habitats. Closed circles and whiskers are mean ± SE; open shapes are measurements of individuals birds; (B) open squares: 2016; open circles: 2017; open triangles: 2020; asterisk denotes difference at p < 0.05.

Table 1. Physiological measurements of male song sparrows collected from rural or suburban habitats.
Packed cell volume did not differ between suburban and rural birds (Figure 2A; | t| = 1.12, p = 0.27), but total hemoglobin was lower in suburban birds compared to rural birds (Figure 2B; | t| = 2.96, p < 0.01). Additionally, MCHC was lower in suburban birds compared to rural birds (Figure 2C; | t| = 2.96, p < 0.01). PCV, hemoglobin, and MCHC did not vary within the season, by time of day, or by body condition (Table 1).
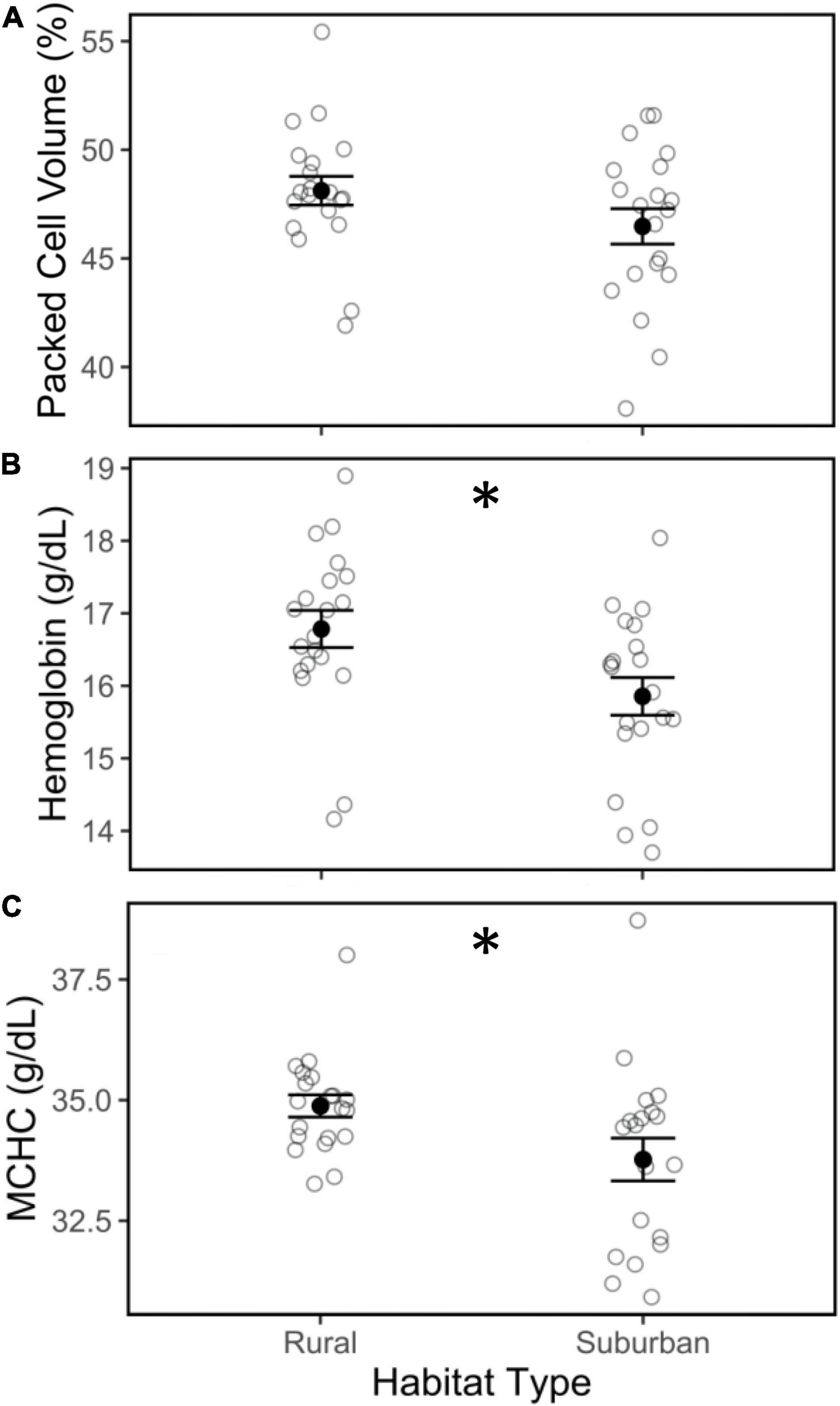
Figure 2. Packed cell volume (PCV; A), total hemoglobin concentration (B), and mean corpuscular hemoglobin concentration (MCHC; C) in blood collected from male song sparrows inhabiting rural and suburban sites. Closed circles and whiskers are mean ± SE; open circles are measurements of individuals birds; asterisk denotes difference at p < 0.05.
There was no effect of habitat on tGSH (Figure 3A; | t| = 0.84, p = 0.41), free GSH (Figure 3B; | t| = 0.57, p = 0.58), GSSG (Figure 3C; | t| = 0.82, p = 0.42), or GSSG:tGSH (Figure 3D; | t| = 0.02, p = 0.98), nor were these glutathione concentrations influenced by Julian date, time of day, or body condition (Table 1). Our measure of reactive oxygen metabolites, d-ROMs, did not differ between suburban and rural birds (Figure 4A; | t| = 1.24, p = 0.23), and did not vary within the season, by time of day, or by body condition (Table 1). Likewise, suburban and rural birds did not differ in antioxidant capacity (i.e., HOCl neutralization; Figure 4B; | t| = 0.54, p = 0.59). Antioxidant capacity was negatively related to body condition (| t| = 2.27, p = 0.03) but did not vary within the season or by time of day (Table 1).
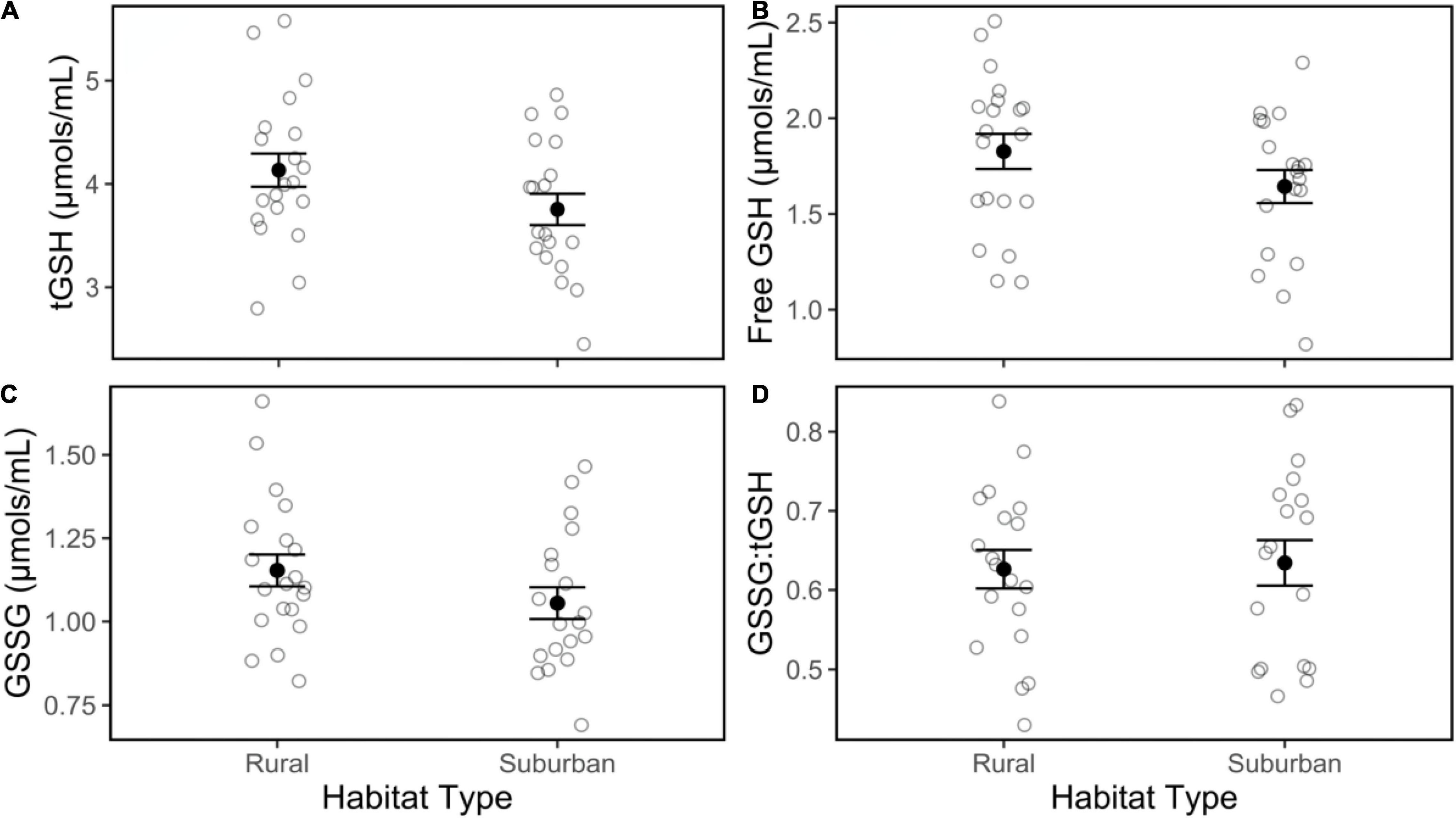
Figure 3. Total glutathione (tGSH; A), free glutathione (GSH; B), oxidized glutathione (GSSG; C) concentrations and the ratio of GSSG:tGSH (D) in blood collected from male song sparrows inhabiting rural and suburban sites. Closed circles and whiskers are mean ± SE; open circles are measurements of individuals birds. No significant habitat differences were detected.
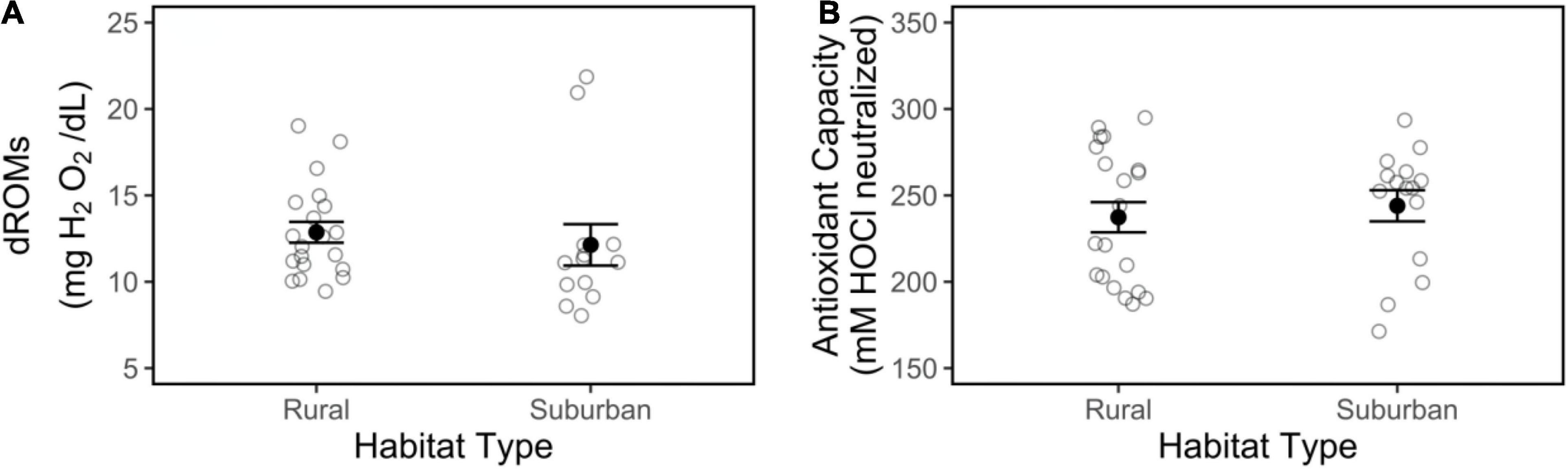
Figure 4. Reactive oxygen metabolites (dROMs; A) and antioxidant capacity (B) in plasma collected from male song sparrows from rural and suburban sites. Closed circles and whiskers are mean ± SE; open circles are measurements of individuals birds. No significant habitat differences were detected.
Heterophil counts were higher in suburban birds compared to rural birds (Figure 5A; | t| = 4.35, p < 0.01). Conversely, suburban birds had lower lymphocyte counts compared to rural birds (Figure 5B; | t| = 2.40, p = 0.02). Monocyte counts did not differ between suburban and rural birds (Figure 5C; | t| = 1.37, p = 0.17). The abundance of heterophils (| t| = 2.45, p = 0.02) and monocytes (| t| = 4.67, p < 0.01) decreased as the breeding season progressed, whereas the abundance of lymphocytes (| t| = 2.72, p < 0.01) increased as the breeding season progressed. Additionally, there as a positive relationship between monocyte counts and body condition (| t| = 2.98, p < 0.01), but neither lymphocyte nor heterophil abundance varied by body condition (Table 1). White blood cell counts did not vary by time of day (Table 1). Although suburban birds exhibited a higher mean heterophil count and a lower mean lymphocyte count, there was no difference in H:L ratio between suburban and rural birds (Figure 5D; | t| = 0.55, p = 0.59), nor did H:L ratio vary within the season, by time of day, or by body condition (Table 1). We observed eosinophils in some cases, but overall abundance of this cell type was too low to compare across habitats.
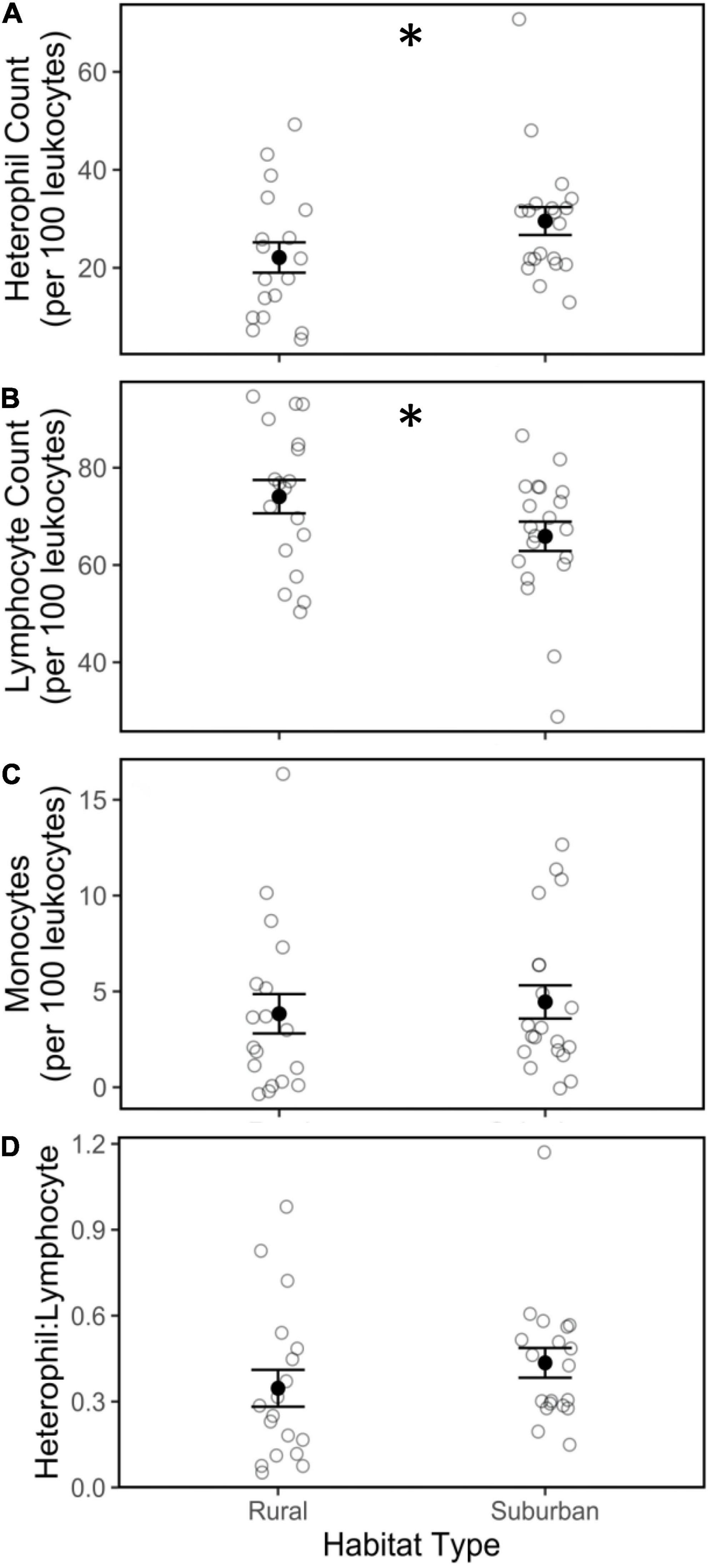
Figure 5. Heterophil (A), lymphocyte (B), and monocyte (C) cell counts and heterophil: lymphocyte ratio (D) from male song sparrows from rural and suburban sites. Closed circles and whiskers are mean ± SE; open circles are measurements of individuals birds; asterisk denotes difference at p < 0.05.
Bacteria killing ability did not differ between rural and suburban birds (Figure 6; | t| = 0.71, p = 0.48) and did not vary by time of day or body condition (Table 1), but BKA did increase as the breeding season progressed (| t| = 2.67, p = 0.01).
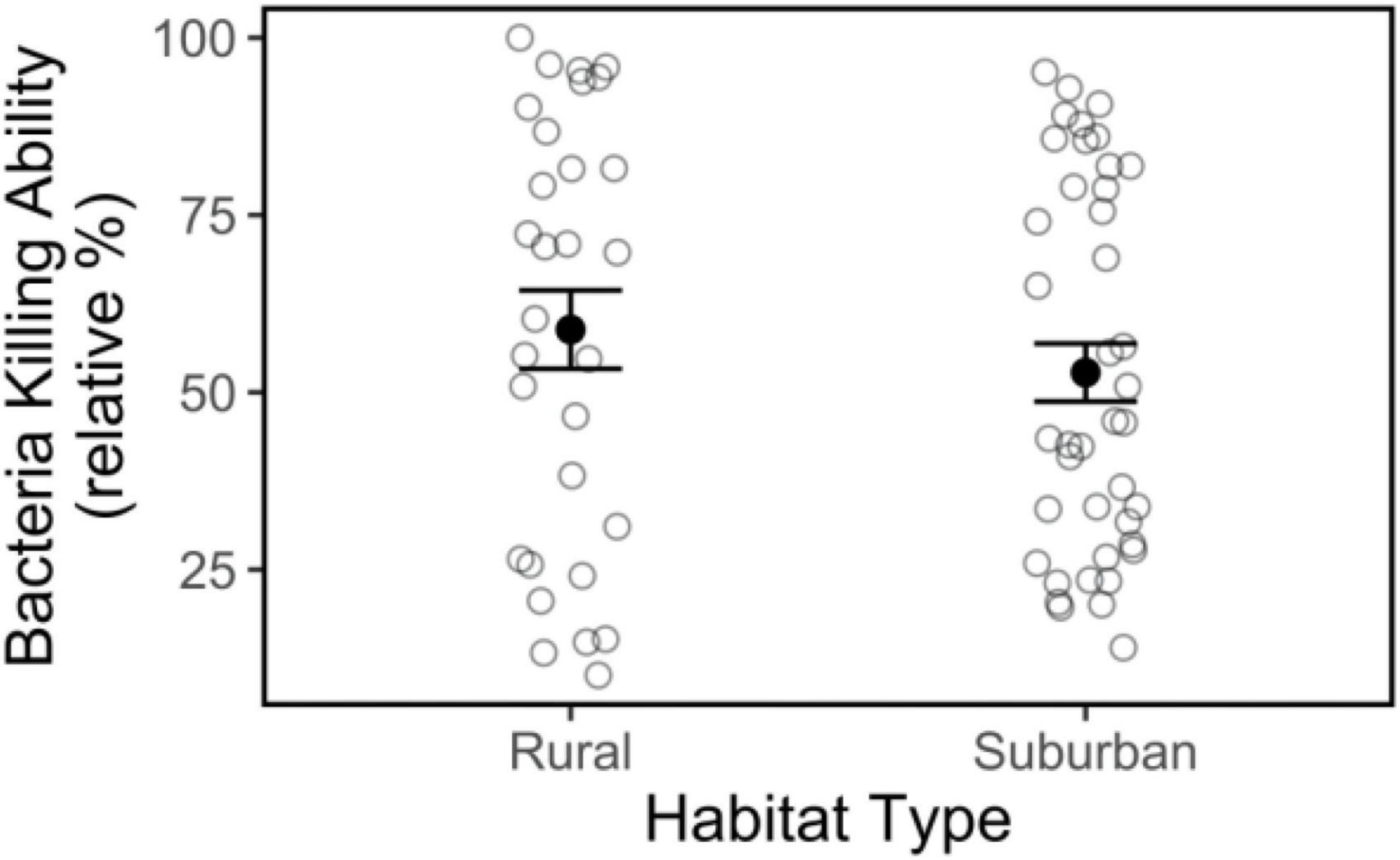
Figure 6. Bacteria killing ability of plasma from male song sparrows from rural and suburban sites. Closed circles and whiskers are mean ± SE; open circles are measurements of individuals birds. No significant habitat differences were detected.
We examined relationships among physiological traits for measurements collected in year 2020. Although we detected some significant correlations between variables that were experimentally derived from each other (e.g., mass and scaled mass index; tGSH and free GSH; MCHC and hemoglobin), there were no notable relationships among physiological variables (Supplementary Table 2).
Over the last 20 years, the field of urban ecology has burgeoned as the rate of urban expansion has accelerated around the globe (Hedblom and Murgui, 2017; Barot et al., 2019). However, even with increasing attention paid to interactions among wildlife and urban landscapes, it remains difficult to predict the costs and benefits of urbanization for wildlife due to a lack of consensus on how animals respond physiologically to urbanization. Recently, there has been growing recognition that site-specific differences in biotic and abiotic variables are major factors determining how animals respond to urbanization (Isaksson, 2020). For example, the anthropogenic landscape of high-density metropolitan habitats differs dramatically from low-density suburban habitats (Chace and Walsh, 2006; Aronson et al., 2014). Though several studies have examined physiological responses in animals inhabiting high-density urban environments, comparatively few studies have examined physiological responses in wildlife inhabiting suburban environments.
In this study, we found that male song sparrows from rural and suburban habitats exhibited differences in physiological traits indicative of shifts in energy budgets. Specifically, song sparrows from the suburban habitat exhibited higher body condition and fat score but lower hemoglobin concentrations. We previously observed that song sparrows from these rural and suburban habitats exhibit behavioral differences (Beck et al., 2018; Davies et al., 2018) that may contribute to rural song sparrows experiencing a greater aerobic demand, thereby causing a depletion of fat reserves and an adaptive increase in hemoglobin concentration to facilitate oxygen delivery to tissues. Furthermore, given that we did not observe higher oxidative stress in suburban song sparrows, the lower hemoglobin concentration in suburban birds does not appear to be caused by oxidative damage to erythrocytes (discussed below). We also found some evidence that suburban and rural song sparrows differed in their white blood cell counts, but overall, low-density urbanization did not appear to substantially impact immune function (discussed below). Taken as a whole, the results from the present study (1) suggest suburban environments do not cause severe adverse physiological responses in song sparrows but may result in shifts in energy budgets, and (2) underscore the importance of measuring multiple physiological variables across sites that differ in the intensity of urbanization in order to avoid misinterpreting the impact of urbanization on avian health.
The finding that suburban song sparrows exhibited lower hemoglobin concentrations compared to rural counterparts is consistent with previous studies that examined hemoglobin concentration in noisy miners (Manorina melanocephala) and house sparrows (Passer domesticus) from rural and urban habitats (Herrera-Duenas et al., 2014; Powell et al., 2014; but see Minias, 2016). Exposure to urban contaminants and anthropogenic noise can increase oxidative stress, resulting in urban birds exhibiting oxidative damage to red blood cells and lower PCV and hemoglobin concentrations in urban birds (Herrera-Duenas et al., 2014; Bauerová et al., 2017). However, unlike these previous findings, we did not detect differences in GSH concentrations, d-ROMs, or antioxidant capacity (HOCl neutralization) in song sparrows at our suburban sites. Therefore, the cause for this difference in hemoglobin concentrations may not be a direct effect of urbanization as it is in prior studies.
There are two possible explanations for the lack of habitat-specific differences in oxidative stress. It may be that song sparrows are excellent urban adapters, whereby urbanization does not substantially alter oxidative status. Alternatively, it is possible that song sparrows can cope with low-density urbanization but exposure to higher intensity urbanization of large metropolitan habitats would increase oxidative status. Collecting song sparrows from high density urban habitats would aid in the ability to interpret the effect of low-density suburban habitats on song sparrows; however, due to the absence of high density urbanization on the landscape of our study system, we were unable to measure physiological variables in song sparrows from highly urbanized environments.
Similar to our results, Herrera-Dueñas et al. (2017) found minimal evidence of oxidative stress in house sparrows inhabiting suburban habitats, though these authors did observe higher oxidative stress in house sparrows from highly urbanized habitats compared to birds from rural habitats. Given birds inhabiting suburban environments are less likely to be exposed to contaminants (e.g., nitrogen oxides, heavy metals, and pesticides) found at higher concentrations in high-density urban environments (e.g., Espín et al., 2014; Salmón et al., 2018), it is unsurprising that we did not detect higher oxidative stress in suburban song sparrows in our study. Yet, the absence of oxidative stress in song sparrows from our study system also rules out the possibility that damaging effects of oxidative stress are causing a reduction in hemoglobin concentrations in male song sparrows from the suburban habitat.
Though it is difficult to identify the specific environmental or ecological factors driving differences in hemoglobin concentrations among rural and suburban song sparrows, the overall pattern of physiological differences suggests an indirect effect of urbanization on energy budgets. Specifically, in addition to higher hemoglobin concentrations, rural song sparrows also exhibited lower body condition (i.e., scaled mass index) and fat scores. These findings suggest that rural birds may experience a greater metabolic demand than their suburban counterparts and that these differences reflect adaptive physiological responses to environmental variation.
Our previous work in this song sparrow system suggests habitat-specific differences in behavior may contribute to rural birds allocating more energy toward activity. For instance, suburban song sparrows are more aggressive than their rural counterparts when exposed to simulated conspecific territorial intrusions conducted with taxidermic mounts (Davies and Sewall, 2016; Beck et al., 2018; Davies et al., 2018). Interestingly, although suburban song sparrows exhibit a higher aggression score in any single interaction, conspecific density is nearly ninefold greater in the rural song sparrow population (unpublished data), and higher neighbor density can increase the frequency of aggressive interactions in territorial songbirds (Yoon et al., 2012), even while decreasing the intensity of those conflicts (the so-called “Dear Enemy” effect, Fisher, 1954; Temeles, 1994). Therefore, even though rural song sparrows are less aggressive in a given interaction, rural song sparrows likely allocate more energy toward frequently defending their territories from conspecific intrusions compared to suburban song sparrows. Furthermore, based on personal observation (KBS) over 8 years of working with this song sparrow population, rural birds typically take longer to respond to conspecific playbacks than suburban males, and we often attract multiple rural males during a single playback, whereas we rarely encounter multiple males on territories of suburban sparrows. These anecdotal observations suggest rural song sparrows are more likely to travel further from their territory. If rural song sparrows must defend their territories more frequently and tend to travel further from their territory than suburban song sparrows, it is likely that rural birds experience a higher aerobic demand for greater activity.
Other behavioral differences between urban and rural birds may also be associated with rural birds experiencing a greater aerobic demand. Several studies, including a recent study in our lab (unpublished data), have found that rural song sparrows have a longer flight initiation distance (i.e., birds tended to fly away when observers were further way) compared to suburban birds (Evans et al., 2010; Fossett and Hyman, 2021), a response that has been observed in other avian species as well (Lin et al., 2012; Møller et al., 2013; Carlen et al., 2021). Flight initiation distance, in turn, is positively correlated with basal metabolic rate (BMR), suggesting BMR may be higher in rural birds as a consequence of longer flight initiation distance and the frequency of such flights (Møller, 2009a). Considering the behavioral differences in our song sparrow population as well as potential associations between habitat-specific differences in flight initiation distance and BMR, it is possible that rural birds are adaptively increasing hemoglobin concentrations to facilitate greater aerobic scope. Such an increase is consistent with previous studies that report birds adaptively adjust hemoglobin concentrations to meet energetically demanding situations (e.g., Jaeger and McGrath, 1974; Prats et al., 1996; Bury et al., 2019). Although several studies have examined hematological indices and oxidative stress in urban and rural populations, fewer studies have quantified whether urban and rural birds have distinct metabolic phenotypes. Future research should examine whether habitat-specific differences in hemoglobin concentrations translate to differences in metabolic rates to determine if the differences we observed in hemoglobin concentrations indeed reflect shifts in aerobic demand.
Song sparrows from suburban habitats exhibited slightly elevated heterophil counts compared to their rural counterparts, though the biological significance of this finding is somewhat unclear. Several previous studies have found birds inhabiting urban environments experience increased risk of infection from avian diseases (e.g., avian malaria, poxvirus, and coccidia) compared to rural dwelling birds (Bichet et al., 2013; Jiménez-Peñuela et al., 2019; Sykes et al., 2021). Consequently, birds inhabiting high-density urban environments tend to have greater investment in innate immunity (Møller, 2009b; Audet et al., 2016). Despite this evidence, the incidence and severity of infection in birds tends to be positively correlated with the intensity of urbanization, suggesting both infection and immune activation will be highest in large cities (Giraudeau et al., 2014). Because the suburban sites in our study were characterized by low-intensity urbanization, it is unlikely that suburban song sparrows experience greater disease exposure, which may explain why we did not observe a substantial difference in heterophil counts between rural and suburban song sparrows.
Although male song sparrows in our study exhibited higher heterophil counts, there was no difference in BKA or H:L ratio, which is a measure of innate immune response that is closely associated with the long-term activation of the corticosterone-mediated stress response in birds (Davis et al., 2008; Minias, 2019). Given that we did not observe differences in BKA or H:L ratios, the suburban sites do not appear to induce a readiness to cope with infection or a state of chronic stress in male song sparrows. This finding is generally consistent with results of our previous studies, where we did not detect differences in baseline or stress-induced plasma corticosterone levels in song sparrows from rural and suburban habitats (Beck et al., 2018; Davies et al., 2018). Additionally, there was no difference between suburban and rural males in their ability to terminate the stress response following dexamethasone injection (Lane et al., 2021). While some other studies have detected differences in corticosterone levels between rural and urban dwelling birds (e.g., Bonier et al., 2007; Ibáñez-Álamo et al., 2020), overall there does not appear to be a consistent relationship between urbanization and the corticosterone-mediated stress response in birds (Bonier, 2012; Injaian et al., 2020).
We observed that suburban birds exhibited higher body condition and fat scores but lower hemoglobin concentrations, indicating song sparrows adaptively respond to habitat-specific aerobic demands by altering hemoglobin concentration. To test the hypothesis that habitat differences shift energy budgets in song sparrows, future research should focus on quantifying differences in aerobic performance among rural and suburban song sparrows. For example, future work should examine whether variation in hemoglobin concentrations among rural and suburban sparrows is associated with resting and maximum metabolic rates. Additionally, hemoglobin synthesis can be suppressed by poor nutrition (Minias, 2015), so future studies should also examine whether availability of micronutrients on the landscape influences the metabolic phenotypes of rural and suburban song sparrows (Coogan et al., 2018; Cummings et al., 2020a). Finally, to better understand the ecological relevance of varying hemoglobin levels, it is critical to understand whether hemoglobin concentration is related to breeding ecology and fitness outcomes (e.g., survival and reproductive success). We note that characterization of urban environments and the use of “urban” vs. “suburban” vary among studies, which limits the ability to perform comparative analyses. Therefore, greater emphasis should be placed on employing similar methodologies for characterizing urban environments among studies in order to facilitate comparisons of phenotypic responses to urbanized habitats. In the context of the present study, low-intensity urbanization does not appear to cause severe adverse physiological responses in song sparrows, rather physiological differences among rural and suburban sparrows appear to be adaptive to habitat specific biotic and abiotic factors.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Virginia Tech IACUC.
CG and KS designed the study. SL, IV, MB, and HE contributed to data collection. CG and SL contributed statistical analysis. CG, KS, and IV wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by National Science Foundation grant IOS 2114288 to KS.
MB is employed by Industrial Economics Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.817864/full#supplementary-material
Aronson, M. F. J., La Sorte, F. A., Nilon, C. H., Katti, M., Goddard, M. A., Lepczyk, C. A., et al. (2014). A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 281:20133330. doi: 10.1098/rspb.2013.3330
Audet, J. N., Ducatez, S., and Lefebvre, L. (2016). The town bird and the country bird: problem solving and immunocompetence vary with urbanization. Behav. Ecol. 27, 637–644. doi: 10.1093/beheco/arv201
Auman, H. J., Meathrel, C. E., and Richardson, A. (2008). Supersize me: does anthropogenic food change the body condition of silver gulls? A comparison between urbanized and remote, non-urbanized areas. Waterbirds 31, 122–126.
Barot, S., Abbadie, L., Auclerc, A., Barthélémy, C., Bérille, E., Billet, P., et al. (2019). Urban ecology, stakeholders and the future of ecology. Sci. Total Environ. 667, 475–484. doi: 10.1016/j.scitotenv.2019.02.410
Bauerová, P., Vinklerová, J., Hraníček, J., Čorba, V., Vojtek, L., Svobodová, J., et al. (2017). Associations of urban environmental pollution with health-related physiological traits in a free-living bird species. Sci. Total Environ. 60, 1556–1565. doi: 10.1016/j.scitotenv.2017.05.276
Beck, M. L., Davies, S., and Sewall, K. B. (2018). Urbanization alters the relationship between coloration and territorial aggression, but not hormones, in song sparrows. Anim. Behav. 142, 119–128. doi: 10.1016/j.anbehav.2018.06.012
Bichet, C., Scheifler, R., Cœurdassier, M., Julliard, R., Sorci, G., and Loiseau, C. (2013). Urbanization, trace metal pollution, and malaria prevalence in the house sparrow. PLoS One 8:53866. doi: 10.1371/journal.pone.0053866
Birnie-Gauvin, K., Peiman, K. S., Gallagher, A. J., De Bruijn, R., and Cooke, S. J. (2016). Sublethal consequences of urban life for wild vertebrates. Environ. Rev. 24, 416–425. doi: 10.1139/er-2016-0029
Blair, R. B. (1996). Land use and avian species diversity along an urban gradient. Ecol. Appl. 6, 506–519. doi: 10.2307/2269387
Bókony, V., Seress, G., Nagy, S., Lendvai, A. Z., and Liker, A. (2012). Multiple indices of body condition reveal no negative effect of urbanization in adult house sparrows. Landsc. Urban Plan. 104, 75–84. doi: 10.1016/j.landurbplan.2011.10.006
Bonier, F. (2012). Hormones in the city: endocrine ecology of urban birds. Horm. Behav. 61, 763–772. doi: 10.1016/J.YHBEH.2012.03.016
Bonier, F., Martin, P. R., Sheldon, K. S., Jensen, J. P., Foltz, S. L., and Wingfield, J. C. (2007). Sex-specific consequences of life in the city. Behav. Ecol. 18, 121–129. doi: 10.1093/beheco/arl050
Brewer, V. N., Lane, S. J., Sewall, K. B., and Mabry, K. E. (2020). Effects of low-density urbanization on genetic structure in the Song Sparrow. PLoS One 15:e0234008. doi: 10.1371/journal.pone.0234008
Bury, A., Niedojadlo, J., Sadowska, E. T., Bauchinger, U., and Cichon, M. (2019). Contrasting response of haematological variables between long-term training and short exercise bouts in zebra finches (Taeniopygia guttata). J. Exp. Biol. 222:jeb193227. doi: 10.1242/jeb.193227
Capilla-Lasheras, P., Dominoni, D. M., Babayan, S. A., O’Shaughnessy, P. J., Mladenova, M., Woodford, L., et al. (2017). Elevated immune gene expression is associated with poor reproductive success of urban blue tits. Front. Ecol. Evol. 5:64. doi: 10.3389/fevo.2017.00064
Carlen, E. J., Li, R., and Winchell, K. M. (2021). Urbanization predicts flight initiation distance in feral pigeons (Columba livia) across New York City. Anim. Behav. 178, 229–245. doi: 10.1016/j.anbehav.2021.06.021
Chace, J. F., and Walsh, J. J. (2006). Urban effects on native avifauna: a review. Landsc. Urban Plan. 74, 46–69. doi: 10.1016/j.landurbplan.2004.08.007
Charmantier, A., Demeyrier, V., Lambrechts, M., Perret, S., and Grégoire, A. (2017). Urbanization is associated with divergence in pace-of-life in great tits. Front. Ecol. Evol. 5:53. doi: 10.3389/fevo.2017.00053
Chatelain, M., Massemin, S., Zahn, S., Kurek, E., Bulska, E., and Szulkin, M. (2021). Urban metal pollution explains variation in reproductive outputs in great tits and blue tits. Sci. Total Environ. 776:145966. doi: 10.1016/j.scitotenv.2021.145966
Cid, F. D., Fernández, N. C., Pérez-Chaca, M. V., Pardo, R., Caviedes-Vidal, E., and Chediack, J. G. (2018). House sparrow biomarkers as lead pollution bioindicators. Evaluation of dose and exposition length on hematological and oxidative stress parameters. Ecotoxicol. Environ. Saf. 154, 154–161. doi: 10.1016/j.ecoenv.2018.02.040
Coogan, S. C. P., Raubenheimer, D., Zantis, S. P., and Machovsky-Capuska, G. E. (2018). Multidimensional nutritional ecology and urban birds. Ecosphere 9:e02177. doi: 10.1002/ECS2.2177
Costantini, D. (2008). Oxidative stress in ecology and evolution: lessons from avian studies. Ecol. Lett. 11, 1238–1251. doi: 10.1111/j.1461-0248.2008.01246.x
Cummings, C. R., Hernandez, S. M., Murray, M., Ellison, T., Adams, H. C., Cooper, R. E., et al. (2020a). Effects of an anthropogenic diet on indicators of physiological challenge and immunity of white ibis nestlings raised in captivity. Ecol. Evol. 10, 8416–8428. doi: 10.1002/ece3.6548
Cummings, C. R., Khan, N. Y., Murray, M. M., Ellison, T., Welch, C. N., Hernandez, S. M., et al. (2020b). Foraging in urban environments increases bactericidal capacity in plasma and decreases corticosterone concentrations in white ibises. Front. Ecol. Evol. 8:575980. doi: 10.3389/fevo.2020.575980
Davis, A. K., Cook, K. C., and Altizer, S. (2004). Leukocyte profiles in wild house finches with and without mycoplasmal conjunctivitis, a recently emerged bacterial disease. Ecohealth 1, 362–373. doi: 10.1007/s10393-004-0134-2
Davies, S., and Sewall, K. B. (2016). Agonistic urban birds: elevated territorial aggression of urban song sparrows is individually consistent within a breeding period. Biol. Lett. 12:20160315. doi: 10.1098/rsbl.2016.0315
Davies, S., Beck, M. L., and Sewall, K. B. (2018). Territorial aggression in urban and rural song sparrows is correlated with corticosterone, but not testosterone. Horm. Behav. 98, 8–15. doi: 10.1016/j.yhbeh.2017.11.010
Davis, A. K., Maney, D. L., and Maerz, J. C. (2008). The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct. Ecol. 22, 760–772. doi: 10.1111/j.1365-2435.2008.01467.x
Espín, S., Martínez-López, E., León-Ortega, M., Martínez, J. E., and García-Fernández, A. J. (2014). Oxidative stress biomarkers in Eurasian eagle owls (Bubo bubo) in three different scenarios of heavy metal exposure. Environ. Res. 131, 134–144. doi: 10.1016/j.envres.2014.03.015
Evans, B. S., Reitsma, R., Hurlbert, A. H., and Marra, P. P. (2018). Environmental filtering of avian communities along a rural-to-urban gradient in greater Washington, D.C., USA. Ecosphere 9:e02402. doi: 10.1002/ecs2.2402
Evans, B. S., Ryder, T. B., Reitsma, R., Hurlbert, A. H., and Marra, P. P. (2015). Characterizing avian survival along a rural-to-urban land use gradient. Ecology 96, 1631–1640. doi: 10.1890/14-0171.1
Evans, J., Boudreau, K., and Hyman, J. (2010). Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116, 588–595. doi: 10.1111/J.1439-0310.2010.01771.X
Ewenson, E. L., Zann, R. A., and Flannery, G. R. (2001). Body condition and immune response in wild zebra finches: effects of capture, confinement and captive-rearing. Naturwissenschaften 88, 391–394. doi: 10.1007/s001140100250
Ferenc, M., Sedláček, O., and Fuchs, R. (2014). How to improve urban greenspace for woodland birds: site and local-scale determinants of bird species richness. Urban Ecosyst. 17, 625–640. doi: 10.1007/s11252-013-0328-x
Fisher, J. (1954). “Evolution and bird sociality,” in Evolution as a Process, eds J. Huxley, A. Hardy, and E. Ford (London: Allen & Unwin), 71–83.
Fossett, T. E., and Hyman, J. (2021). The effects of habituation on boldness of urban and rural song sparrows (Melospiza melodia). Behaviour 1, 1–15. doi: 10.1163/1568539X-BJA10113
Fragkias, M., Güneralp, B., Seto, K. C., and Goodness, J. (2013). “A synthesis of global urbanization projections,” in Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities: A Global Assessment, eds T. Elmqvist, M. Fragkias, J. Goodness, B. Güneralp, P. J. Marcotullio, R. I. McDonald, et al. (New York, NY: Springer), 409–436. doi: 10.1007/978-94-007-7088-1_21
Giraudeau, M., Mousel, M., Earl, S., and McGraw, K. (2014). Parasites in the city: degree of urbanization predicts poxvirus and coccidian infections in house finches (Haemorhous mexicanus). PLoS One 9:e86747. doi: 10.1371/journal.pone.0086747
Hamer, S. A., Lehrer, E., and Magle, S. B. (2012). Wild birds as sentinels for multiple zoonotic pathogens along an urban to rural gradient in greater Chicago, Illinois. Zoonoses Public Health 59, 355–364. doi: 10.1111/j.1863-2378.2012.01462.x
Hedblom, M., and Murgui, E. (2017). “Urban bird research in a global perspective,” in Ecology and Conservation of Birds in Urban Environments, eds E. Murgui and M. Hedblom (Cham: Springer), 3–10. doi: 10.1007/978-3-319-43314-1_1
Herrera-Duenas, A., Pineda, J., Antonio, M. T., and Aguirre, J. I. (2014). Oxidative stress of house sparrow as bioindicator of urban pollution. Ecol. Indic. 42, 6–9. doi: 10.1016/j.ecolind.2013.08.014
Herrera-Dueñas, A., Pineda-Pampliega, J., Antonio-García, M. T., and Aguirre, J. I. (2017). The influence of urban environments on oxidative stress balance: a case study on the house sparrow in the Iberian Peninsula. Front. Ecol. Evol. 5:106. doi: 10.3389/fevo.2017.00106
Hobbs, R. J., Arico, S., Aronson, J., Baron, J. S., Bridgewater, P., Cramer, V. A., et al. (2006). Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 15, 1–7. doi: 10.1111/j.1466-822X.2006.00212.x
Hyman, J., Hughes, M., Nowicki, S., and Searcy, W. (2004). Individual variation in the strength of territory defense in male song sparrows: correlates of age, territory tenure, and neighbor aggressiveness. Behaviour 141, 15–27. doi: 10.1163/156853904772746574
Ibáñez-Álamo, J. D., Jimeno, B., Gil, D., Thomson, R. L., Aguirre, J. I., Díez-Fernández, A., et al. (2020). Physiological stress does not increase with urbanization in European blackbirds: evidence from hormonal, immunological and cellular indicators. Sci. Total Environ. 721:137332. doi: 10.1016/j.scitotenv.2020.137332
Injaian, A. S., Francis, C. D., Ouyang, J. Q., Dominoni, D. M., Donald, J. W., Fuxjager, M. J., et al. (2020). Baseline and stress-induced corticosterone levels across birds and reptiles do not reflect urbanization levels. Conserv. Physiol. 8:coz110. doi: 10.1093/conphys/coz110
Isaksson, C. (2015). Urbanization, oxidative stress and inflammation: a question of evolving, acclimatizing or coping with urban environmental stress. Funct. Ecol. 29, 913–923. doi: 10.1111/1365-2435.12477
Isaksson, C. (2018). Impact of Urbanization on Birds. Cham: Springer, 235–257. doi: 10.1007/978-3-319-91689-7_13
Isaksson, C. (2020). Urban ecophysiology: beyond costs, stress and biomarkers. J. Exp. Biol. 223:jeb203794. doi: 10.1242/JEB.203794/226425
Jaeger, J. J., and McGrath, J. J. (1974). Hematologic and biochemical effects of simulated high altitude on the Japanese quail. J. Appl. Physiol. 37, 357–361. doi: 10.1152/jappl.1974.37.3.357
Jiménez-Peñuela, J., Ferraguti, M., Martínez-de la Puente, J., Soriguer, R., and Figuerola, J. (2019). Urbanization and blood parasite infections affect the body condition of wild birds. Sci. Total Environ. 651, 3015–3022. doi: 10.1016/j.scitotenv.2018.10.203
Johnstone, C. P., Lill, A., and Reina, R. D. (2017). Use of erythrocyte indicators of health and condition in vertebrate ecophysiology: a review and appraisal. Biol. Rev. 92, 150–168. doi: 10.1111/brv.12219
Juárez, R., Chacón-Madrigal, E., and Sandoval, L. (2020). Urbanization has opposite effects on the territory size of two passerine birds. Avian Res. 11:11. doi: 10.1186/s40657-020-00198-6
Lane, S. J., Emmerson, M. G., VanDiest, I. J., Hucul, C., Beck, M. L., Davies, S., et al. (2021). Hypothalamic-pituitary-adrenal axis regulation and organization in urban and rural song sparrows. Gen. Comp. Endocrinol. 310:113809. doi: 10.1016/j.ygcen.2021.113809
Lin, T., Coppack, T., Lin, Q. X., Kulemeyer, C., Schmidt, A., Behm, H., et al. (2012). Does avian flight initiation distance indicate tolerance towards urban disturbance? Ecol. Indic. 15, 30–35. doi: 10.1016/j.ecolind.2011.09.018
Llacuna, S., Gorriz, A., Riera, M., and Nadal, J. (1996). Effects of air pollution on hematological parameters in passerine birds. Arch. Environ. Contam. Toxicol. 31, 148–152. doi: 10.1007/BF00203919
Lowry, H., Lill, A., and Wong, B. B. M. (2013). Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537–549. doi: 10.1111/brv.12012
MacGregor-Fors, I., and Schondube, J. E. (2011). Gray vs. green urbanization: relative importance of urban features for urban bird communities. Basic Appl. Ecol. 12, 372–381. doi: 10.1016/j.baae.2011.04.003
Marzluff, J. M. (2001). “Worldwide urbanization and its effects on birds,” in Avian Ecology and Conservation in an Urbanizing World, eds J. M. Marzluff, R. Bowman, and R. Donnelly (Boston, MA: Springer), 19–47. doi: 10.1007/978-1-4615-1531-9_2
McKinney, M. L. (2002). Urbanization, biodiversity, and conservation. Bioscience 52, 883–890. doi: 10.1641/0006-3568(2002)052[0883:ubac]2.0.co;2
McKinney, M. L. (2006). Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247–260. doi: 10.1016/j.biocon.2005.09.005
Merrill, L., Stewart Merrill, T. E., Barger, A. M., and Benson, T. J. (2019). Avian health across the landscape: nestling immunity covaries with changing landcover. Integr. Comp. Biol. 59, 1150–1164. doi: 10.1093/icb/icz037
Minias, P. (2015). The use of haemoglobin concentrations to assess physiological condition in birds: a review. Conserv. Physiol. 3:cov007. doi: 10.1093/conphys/cov007
Minias, P. (2016). Reproduction and survival in the city: which fitness components drive urban colonization in a reed-nesting waterbird? Curr. Zool. 62, 79–87. doi: 10.1093/cz/zow034
Minias, P. (2019). Evolution of heterophil/lymphocyte ratios in response to ecological and life-history traits: a comparative analysis across the avian tree of life. J. Anim. Ecol. 88, 554–565. doi: 10.1111/1365-2656.12941
Mohanty, J. G., Nagababu, E., and Rifkind, J. M. (2014). Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 5:84. doi: 10.3389/fphys.2014.00084
Møller, A. P. (2009a). Basal metabolic rate and risk-taking behaviour in birds. J. Evol. Biol. 22, 2420–2429. doi: 10.1111/j.1420-9101.2009.01850.x
Møller, A. P. (2009b). Successful city dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159, 849–858. doi: 10.1007/s00442-008-1259-8
Møller, A. P., Grim, T., Ibáñez-Álamo, J. D., Markó, G., and Tryjanowski, P. (2013). Change in flight initiation distance between urban and rural habitats following a cold winter. Behav. Ecol. 24, 1211–1217. doi: 10.1093/beheco/art054
Murray, M. H., Sánchez, C. A., Becker, D. J., Byers, K. A., Worsley-Tonks, K. E. L., and Craft, M. E. (2019). City sicker? A meta-analysis of wildlife health and urbanization. Front. Ecol. Environ. 17:575–583. doi: 10.1002/fee.2126
Ots, I., Murumägi, A., and Hõrak, P. (1998). Haematological health state indices of reproducing Great Tits: methodology and sources of natural variation. Funct. Ecol. 12, 700–707. doi: 10.1046/j.1365-2435.1998.00219.x
Ouyang, J. Q., Isaksson, C., Schmidt, C., Hutton, P., Bonier, F., and Dominoni, D. (2018). A new framework for urban ecology: an integration of proximate and ultimate responses to anthropogenic change. Integr. Comp. Biol. 58, 915–928. doi: 10.1093/icb/icy110
Peig, J., and Green, A. J. (2009). New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. doi: 10.1111/J.1600-0706.2009.17643.X
Peig, J., and Green, A. J. (2010). The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct. Ecol. 24, 1323–1332. doi: 10.1111/J.1365-2435.2010.01751.X
Phillips, J. N., Gentry, K. E., Luther, D. A., and Derryberry, E. P. (2018). Surviving in the city: higher apparent survival for urban birds but worse condition on noisy territories. Ecosphere 9:e02440. doi: 10.1002/ecs2.2440
Powell, C., Lill, A., and Johnstone, C. P. (2014). Body condition and chronic stress in urban and rural noisy miners. Open Ornithol. J. 6, 25–31. doi: 10.2174/1874453201306010025
Prats, M. T., Palacios, L., Gallego, S., and Riera, M. (1996). Blood oxygen transport properties during migration to higher altitude of wild quail, Coturnix coturnix coturnix. Physiol. Zool. 69, 912–929. doi: 10.1086/physzool.69.4.30164235
R Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rouffaer, L. O., Strubbe, D., Teyssier, A., Hudin, N. S., Van den Abeele, A. M., Cox, I., et al. (2017). Effects of urbanization on host-pathogen interactions, using Yersinia in house sparrows as a model. PLoS One 12:e0189509. doi: 10.1371/journal.pone.0189509
Salmón, P., Stroh, E., Herrera-Dueñas, A., von Post, M., and Isaksson, C. (2018). Oxidative stress in birds along a NOx and urbanisation gradient: an interspecific approach. Sci. Total Environ. 622–623, 635–643. doi: 10.1016/j.scitotenv.2017.11.354
Senar, J. C., Garamszegi, L. Z., Tilgar, V., Biard, C., Moreno-Rueda, G., Salmón, P., et al. (2017). Urban great tits (Parus major) show higher distress calling and pecking rates than rural birds across Europe. Front. Ecol. Evol. 5:163. doi: 10.3389/fevo.2017.00163
Seress, G., Lipovits, Á, Bókony, V., and Czúni, L. (2014). Quantifying the urban gradient: a practical method for broad measurements. Landsc. Urban Plan. 131, 42–50. doi: 10.1016/j.landurbplan.2014.07.010
Sykes, B. E., Hutton, P., and McGraw, K. J. (2021). Sex-specific relationships between urbanization, parasitism, and plumage coloration in house finches. Curr. Zool. 67, 237–244. doi: 10.1093/cz/zoaa060
Temeles, E. J. (1994). The role of neighbours in territorial systems: when are they “dear enemies”? Anim. Behav. 47, 339–350. doi: 10.1006/ANBE.1994.1047
U.S. Census Bureau (2021). Subcounty Resident Population Estimates: April 1, 2010 to July 1, 2019; April 1, 2020; and July 1, 2020 (SUB-EST2020). Available online at: https://www.census.gov/programs-surveys/popest/technical-documentation/research/evaluation-estimates/2020-evaluation-estimates/2010s-cities-and-towns-total.html (accessed January 15, 2022).
Watson, H., Videvall, E., Andersson, M. N., and Isaksson, C. (2017). Transcriptome analysis of a wild bird reveals physiological responses to the urban environment. Sci. Rep. 7:44180. doi: 10.1038/srep44180
Keywords: urbanization, avian physiology, oxidative stress, immune function, hemoglobin
Citation: Goodchild CG, VanDiest I, Lane SJ, Beck M, Ewbank H and Sewall KB (2022) Variation in Hematological Indices, Oxidative Stress, and Immune Function Among Male Song Sparrows From Rural and Low-Density Urban Habitats. Front. Ecol. Evol. 10:817864. doi: 10.3389/fevo.2022.817864
Received: 18 November 2021; Accepted: 25 January 2022;
Published: 16 February 2022.
Edited by:
Edward Narayan, The University of Queensland, AustraliaReviewed by:
Pierre J. Deviche, Arizona State University, United StatesCopyright © 2022 Goodchild, VanDiest, Lane, Beck, Ewbank and Sewall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher G. Goodchild, Y2dvb2RjaGlsZEB1Y28uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.