- 1Department of Molecular Biology and Biochemical Engineering, University Pablo de Olavide, Seville, Spain
- 2Swedish Collegium for Advanced Study (SCAS), Uppsala, Sweden
Sexually selected traits often depend on an individual’s physical condition, or otherwise indirectly reflect the ecological performance of individuals. When individuals disperse between populations that are locally adapted to different environments, their ecological performance may decline. This in turn may result in more poorly expressed sexual traits, and therefore in a lower reproductive success. Hence, sexual selection may reduce the effective gene flow between populations, and thereby maintain or even enhance population divergence. This hypothesis was published in a highly visible journal (van Doorn et al., 2009, Science). Here I review the subsequently published empirical tests of this hypothesis. I downloaded all metadata (incl. abstracts) of papers citing van Doorn et al. (2009) and read those papers that undertook relevant tests. To my surprise, only very few papers provided explicit tests of the hypothesis, this never involved plants, and only one study found support for it. While sexual selection may therefore not often reduce gene flow between locally adapted populations, some improvements to experimental design and choice of study system are noted. I therefore also provide a detailed list of suggestions for high quality tests of this hypothesis. This hopefully acts as a catalyst for more and better studies to test whether sexual and natural selection can work in synergy to reduce effective dispersal, and thereby protect and promote adaptive population divergence.
Introduction
The world is heterogeneous, and this often results in divergent natural selection and the local adaptation of populations (Kawecki and Ebert, 2004; Hereford, 2009). When the ecological conditions underlying this divergence are sufficiently different, they may hinder successful dispersal between locally adapted populations, effectively leading to a reduction in gene flow between them. Hence, local adaptation can be seen as a first step in a chain of events that could produce ecological speciation (Rundle and Nosil, 2005). However, many populations and species appear to be as much, if not more, diverged in sexual traits as in ecological traits. This suggests that sexual selection also plays a role in divergence and speciation, or may even be the principal driver. Many theoretical models and empirical studies have explored this possibility (Lande and Kirkpatrick, 1988; Kirkpatrick and Ravigné, 2002; van Doorn and Weissing, 2002; Arnegard and Kondrashov, 2004; Ritchie, 2007; Kraaijeveld et al., 2011).
That both natural and sexual selection are driving divergence and speciation is a bit puzzling, because they are often seen as antagonistic, in the sense that traits that increase the probability of fertilizations often decrease the probability of survival. A classical example is the long tail of the male peacock. A longer tail is appreciated by prospecting females, but makes it harder for a male to escape from predators. The average size of the tail is therefore an evolutionary trade-off between the advantages of being more attractive, and the advantages of being better at predator escape.
The development of the tail may also be a trade-off at the individual level, open to a flexible allocation depending on the individual’s situation. For example, a male that happens to have particularly good genes, or that happened to grow up under particularly good conditions, may be large and strong enough to develop a tail that is a bit longer than otherwise, because he is able to carry that burden and still fly strong for predator escape. This could be the reason why females prefer long tails in the first place, because it is an honest signal of male quality that provides the female with direct (e.g., parental care, high quality sperm, lack of parasites) and/or indirect benefits (e.g., good genes).
In this ecologically mediated individual trade-off lies the root of an idea that was also mentioned in earlier papers (Proulx, 2001; Lorch et al., 2003; Reinhold, 2004) but more fully developed and applied to divergence in van Doorn et al. (2009). We laid out there that when sexual traits are condition-dependent, i.e., somehow respond to the ecological match of an organism with its environment (Edelaar and Bolnick, 2019), then sexual selection and natural selection are not antagonistic but synergistic, and that this greatly increases the probability of speciation. Imagine for example that some peacocks live in broadleaved forests, and others in coniferous forests. If these forests are sufficiently different in resources, micro climate, parasite community etcetera, then it is possible that a peacock that disperses from one type of forest to another will do less well overall at its new location, will be in lower condition, will have to allocate less resources to the development of its sexual ornament, and ultimately will end up with a smaller tail. This is bad news for the male, since it is now less attractive. But it is good news for females, since by discarding this male as a mating partner based on his tail size, they are avoiding that their offspring inherit his locally maladaptive genes (and any maladaptive non-genetically inherited traits as well: Bonduriansky and Day, 2018). Hence, sexual selection can lead to a reduction in the effective dispersal between ecologically diverged populations, if it involves sexual traits that are somehow influenced in their expression by local adaptation (there are additional ways for this to happen, e.g., if the transmission or reception of the sexual traits depends on the local environment: Maan and Seehausen, 2011). Sexual and natural selection can thereby work hand in hand (Rowe and Rundle, 2021) to maintain or even increase population divergence.
We showed (van Doorn et al., 2009) that this synergy greatly increases the probability of speciation, making it possible to occur when divergent natural selection is weaker and/or the rate of (otherwise homogenizing) dispersal is higher. In part this is because the diverging populations do not have to evolve distinct preferences for different sexual traits, and then maintain these diverging preferences in association with the diverging ecological traits (Felsenstein, 1981; Rice and Hostert, 1993). Whenever there is dispersal between populations, the association between different traits breaks down easily due to recombination, preventing sexual selection to aid in divergence (assuming traits have a genetic component; for an alternative see Verzijden et al., 2012). Since in our hypothesis the same preference (e.g., for a longer tail) is employed in all populations, but variation in tail length reflects variation in local adaptation, sexual selection does actually (albeit indirectly) aid divergence.
While we used the example of a female preference for a male ornament, the same rationale could work for male choice on female traits, or for intrasexual competition, for example if male peacocks establish a mating hierarchy via tail length, or simply via (equally condition-dependent) fighting strength. Moreover, it will also still be relevant after gene flow actually has occurred, since the “hybrid” offspring will still be locally maladapted to a certain degree, and therefore develop poorer sexual traits and have a lower reproductive success. This mechanism therefore suggested that divergence and speciation might be easier than previously thought, and might be broadly applicable in terms of mating systems and ecological settings. We therefore expected that our hypothesis would be tested in many subsequent studies. Now 12 years later, and in connection to the Research Topic on the link between dispersal and sexual selection, I reviewed the empirical literature to see what has been done.
Materials and Methods
Even though this does not provide a full representation of all the relevant work that has been done, especially prior to the publication of van Doorn et al. (2009), I restricted myself to an analysis of the papers that cite van Doorn et al. (2009), which should still be a representative sample of the relevant recent literature. I downloaded the meta-data of all papers citing van Doorn et al. (2009) as included in Web of Science on February 2, 2021. These were 216 citations. I then read the abstract of all citing papers. Eleven entries had no summary, so I discarded those (they appeared to be comments and responses to other papers, not empirical studies). Whenever the summary gave me sufficient reason to believe that it was a paper with original empirical data that tested the hypothesis, I downloaded and read the paper. Any additional references in those papers to papers published after 2009 that might have tested our hypothesis were also downloaded and investigated. The deciding information to whether a paper counted as a valid test was based on the biology of the system and the experimental design: it should somehow involve or mimic movement of individuals between differentially adapted populations, it should test the attractiveness or mating success of local versus immigrant individuals, and it should somehow address the confounding issue of preference (or more general: the perception of the receiver) having diverged between populations. If I considered it a valid test of the hypothesis, I then established to what extent the result supported the hypothesis.
Results
Only 13 papers seemed to appear relevant upon reading the abstract. The remaining papers included surprisingly many theoretical studies, some reviews, and many papers that probably cited van Doorn et al. (2009) as context or to suggest a potential consequence or alternative explanation of their results (not quantified).
These 13 papers each had their own peculiarities, but to me only 2 were directly relevant to the hypothesis: Berdan and Fuller (2012) (killifish adapted to different salinities) and Arbuthnott and Rundle (2014) (fruit flies adapted to different temperatures). Additional references in the papers citing van Doorn et al. (2009) led me to 4 further relevant studies [which did not cite van Doorn et al. (2009) but were published after 2009]: Correia et al. (2010) (fruit flies adapted to different temperatures), Long et al. (2012) (fruit flies adapted to different toxins), Shenoi and Prasad (2016) (fruit flies adapted to different larval densities), and Tinghitella et al. (2020) (benthic and limnetic stickleback adapted to different food types).
Of these 6 studies, 1 provided support for the hypothesis (Long et al., 2012) while the other 5 did not find support for it.
Discussion
Review of the Publication Record
van Doorn et al. (2009) laid out why and how dispersal and sexual selection might interact. First, the expression of sexual ornaments is often related to ecological performance, for example when the overall condition of an individual determines the allocation of resources to that ornament. This is because investment in a sexual ornament often comes with a cost, and hence is traded-off against other costly traits: individuals with fewer resources to spend will therefore express less-developed sexual ornaments. Second, populations are often locally adapted, which means that individuals that disperse to a population with different ecological conditions will have a poorer ecological performance. As a consequence, this reduced ecological performance is then expected to affect the sexual ornament, and therefore sexual performance (in the sense of successful mating). Hence, sexual selection is expected to put a brake on the effective gene flow between locally adapted populations, thereby maintaining ecological divergence or promoting it, even up to the point of speciation.
This relatively straightforward process, published in a highly visible journal (Science) where it also received a dedicated commentary (Mank, 2009), was expected to generate a lot of empirical interest, for several reasons. It predicted that sexual selection and natural selection might work in concert instead as antagonists, as often stated. It predicted that speciation with gene flow (incl. sympatric speciation) might be more common than previously thought. It predicted that many species might be much more cryptic than we thought, because sexual ornamentation would not be different between the incipient species. It predicted that the exact type of sexual selection did not matter too much: it could be sexual selection on a condition-dependent ornament (yielding indirect genetic benefits), it could involve direct benefits (e.g., courtship feeding), it could work via intra-sexual selection (for example male-male competition) or via inter-sexual selection (e.g., female choice), it could operate via pre-zygotic assortative mating or via post-zygotic sexual selection against hybrids (van Doorn et al., 2009). And finally, it provided strong arguments in favor of the good genes hypothesis for sexual selection. This hypothesis had come under scrutiny for actually not being supported all that well both theoretically (good genes are expected to get depleted, and selection is relatively weak because it is indirect; Kokko and Heubel, 2008) and empirically (many studies did not find evidence for it; Achorn and Rosenthal, 2020), although it appears to be more relevant when environments are changing and hence more genotypes are locally maladapted (Cally et al., 2019). By pointing out that locally maladapted genes might enter populations via dispersal, the indirect (good genes) benefits of sexual selection were more likely to be operating (Proulx, 2001).
In contrast to this expectation, I encountered very few empirical studies that have addressed the key mechanism in van Doorn et al. (2009): that locally adapted mates are favored by sexual selection based on sexual ornaments whose expression depends on ecological performance. There might be several reasons for this. First, I have not exhaustively searched for all relevant papers using key words, instead I only looked for and at papers that cited van Doorn et al. (2009). There are most likely relevant papers that have been published before van Doorn et al. (2009), and there are most likely papers that have been published afterward that didn’t cite van Doorn et al. (2009), even when researchers had read the paper. As one reviewer wrote: “maybe the paper was read by many like myself with the reaction: ‘Yes, this makes a lot of sense!,’ and it was so self-evident that it was not cited when it deserved to be.” How many other relevant studies are out there is impossible to know, but since I only found 2 relevant empirical tests for the > 200 citing papers, it may not be too many. Of course there are many more studies that provide partial tests (e.g., whether there is local adaptation, whether sexual ornaments are condition-dependent, or whether local individuals are favored in sexual selection), but these are not complete tests of the hypothesis. Alternatively, it is possible that the combination of such papers on the same system does allow for a proper test, but that this final assessment has not been done by the researchers themselves and is therefore very hard to find. Second, the sexual ornament of interest needs to somehow flexibly and relatively rapidly respond to the new environment when an individual disperses. For example, if an individual first develops the ornament to its final state and then disperses (e.g., the wing patches on a butterfly), the new environment will not affect its ornament and the proposed mechanism does not operate on the dispersers (note however that it does operate via the subsequent hybrid offspring, assuming these are maladapted at some intermediate level. It could also operate with a delay on ornaments that are renewed, e.g., the plumage of birds after molt). Therefore, biological systems involving sexual ornaments that in their expression are not responsive to the environment would not be suitable to test the prediction. More general, maybe it was thought that the combination of local adaptation plus sexual selection acting on an ornament that is responsive to ecological performance was too unlikely to occur to warrant a test. However, in my view both components have a lot of empirical support, so the combination should also not be rare. A third possibility is that a test is just a lot of work or logistically more challenging, because it involves incorporating several populations. Many empirical studies focus on a single study population, so expanding this to include ecologically distinct populations might be demanding. A fourth possibility is that the study was perceived to exclusively deal with speciation, such that people working on sexual selection did not set out to test the more general prediction that local adaptation and sexual selection act in concert (and not in opposition) to reduce gene flow. And finally, for those people that did work on speciation, the prediction that ornaments and preferences do not need to diverge for the process to operate might not be in line with the common observation that ornaments and preferences actually are diverged in their system, suggesting our predictions cannot be tested meaningfully (which is not true; see below). To promote the future testing of the process and its predictions, toward the end of this paper I will give detailed suggestions for meaningful experimental designs.
Another surprise was that only 1 out of the 6 relevant studies found support for the prediction, even though the rationale behind it appears to be founded on reasonable assumptions. And even then, that study was not even a clear-cut test of the hypothesis. Long et al. (2012) based their study on Drosophila melanogaster fruit fly populations that were either adapted to cadmium or to ethanol (both toxic at high concentrations) in their larval medium. They were not specifically interested in testing our prediction, but in testing to what extent the type and amount of genetic variation in a population affects whether sexual and natural selection are aligned. This is because when populations are well-adapted, sexual selection may remove all deleterious alleles, such that the remaining sexual selection is on alleles maintained by balancing selection, including intralocus conflict. They therefore introgressed flies from the ethanol-adapted population into some but not other cadmium-adapted populations, and studied the mating success of F2 males reared on cadmium. Since the actual genotype of each male is not known, they did not study the mating success of males as a function of their degree of local adaptation. Instead, they measured the fitness of offspring of males that were previously successful or not in securing a mating under competition, and compared this between populations with versus without introgression. They found that the fitness of both daughters and sons was reduced for the males that were unsuccessful in obtaining a mating, but only in populations exposed to introgression with maladapted genotypes. It therefore appeared that in the introgressed population, the males that were sexually selected against also were locally maladapted. Hence, even though the degree of local adaptation of males was inferred from their fitness after mating success was tested, and not known beforehand, this result is in support of our prediction.
What might be the reason that the other five studies did not find support? In part this may be because the hypothesis really isn’t supported, for whatever reasons. This appears to be the case for Arbuthnott and Rundle (2014); Shenoi and Prasad (2016), and Tinghitella et al. (2020). However, there may also be issues related to the biology of the system and the design of the study that interfered with a proper test of the hypothesis, as also discussed by all studies. I synthesize these comments and my own thoughts into recommendations and ideas for future tests, which hopefully allow for a better assessment of the question to what extent sexual selection can limit the effective gene flow between locally adapted populations because of an environmentally induced reduced sexual performance.
Suggestions for Testing the Hypothesis
While the hypothesis is relatively straightforward, when applied to mate choice it involves the interaction between choosing individuals from different populations, potential mates from different populations, and environmental conditions from different populations. Therefore, a fairly large number of aspects need to be considered in order to undertake a valid test of the hypothesis. The list below can therefore serve for the design, but also for the discussion of any study.
Which Species to Use
There are of course millions of species available to test this hypothesis, and many reasons why some may be more suitable than others, or where the hypothesis has a greater fundamental or applied relevance. But I wish to highlight that plants should not be forgotten. None of the 13 potentially relevant studies reviewed in detail were about plants, and in fact van Doorn et al. (2009) was almost never cited by a plant study. Reviews on sexual selection also often exclude plants. For example, Scordato et al. (2014) excluded plants because they were interested in the role of mate choice in speciation. But sexual selection is more than mate choice or male-male conflict, it is also the amount of gametes that are produced, and the ability and competition to reach gametes of the other sex, whether this is via passive dispersal (e.g., wind, water) or dispersal mediated by other organisms (e.g., pollinators). This means that flowering plants that grow in non-local environments may be less successful in obtaining matings, because their lower ecological performance negatively affects their sexual traits. Staying close to the hypothesis as it was presented, a plant that grows in a non-local environment may be less attractive to pollinators because it is not as tall, has fewer or smaller flowers, produces less nectar or smell, etc., and therefore obtains fewer visits by pollinators and hence fewer fertilizations. Plants can be easily moved between environments as seeds or as adults, and also in the wild. Perennial plants have multiple reproductive seasons, allowing for statistically powerful within-individual comparisons. And some plants reproduce both sexually and asexually, allowing for a really interesting comparison between natural and sexual selection in terms of reproductive success. I therefore hope that plant researchers pick up this topic, or that animal researchers incorporate plants in their studies. Toward the end I also mention that species without some sort of mate choice or attractiveness can also be used to test the hypothesis, and this also applies to plants.
Which Populations to Use
The hypothesis is based on locally adapted individuals suffering lower ecological and consequently sexual performance in alternative environments. It is therefore imperative that the compared populations are sufficiently diverged and locally adapted for this to occur. Some studies suggest that in their case this may not have been so (Klappert and Reinhold, 2005; Correia et al., 2010). Local adaptation should not be assumed, but tested for [see Kawecki and Ebert (2004) for suggestions].
Which Environments to Use
It makes most sense to use as testing environments the environments actually experienced in each locally adapted population. Some studies used environments that were somewhat intermediate (Berdan and Fuller, 2012), and this may reduce the effect on the tested individuals. Depending on the shape of the function that links performance to the environment, one might consider exposing individuals to environments that are even more different than in the wild, in order to increase the probability to detect an effect, but then it will be harder to link the results back to the situation in the wild.
Which Choice Design to Use: No-Choice vs. Dyads vs. Choice in Groups
Choice is generated by preferences, but limited by actually available options. I first discuss the issue of options. To measure choice, different designs are possible: no-choice trials (is the only available potential mate accepted at all, or only with a delay, or with a lower reproductive investment?), dyadic choice trials involving one chooser and two potential mates (enabling direct comparison between the two options), or group choice trials involving several choosers and potential mates (enabling even more comparisons). Each design has its advantages and disadvantages (including in terms of logistics, sample size, and statistical analysis), so maybe the design utilized should depend on the biology of the species, reflecting the actual situation (timing and number of potential mates a chooser is exposed to) under field conditions.
Which Choosing Individuals to Use
As mentioned, choice is a combination of preferences and options, so I now discuss the issue of preferences. An individual could be more or less born with a preference, e.g., because of genetic preference alleles. Both for adaptive and non-adaptive reasons, preferences might diverge between locally adapting populations. As mentioned before, this is a problem when testing our hypothesis, because it also causes local individuals to be preferred by local choosers, similar to our prediction. A solution for this confounding effect could be to use choosing individuals from a population which is not included in the comparison across locally adapted populations. The assumption then is that any diverged preference in this “chooser population” is independent of any diverged preferences in the populations of interest, so not benefitting local males. Another solution would be to use choosers from all populations of interest, and to test if their preferences shift depending on the test environment (see below and Figure 1 for more details).
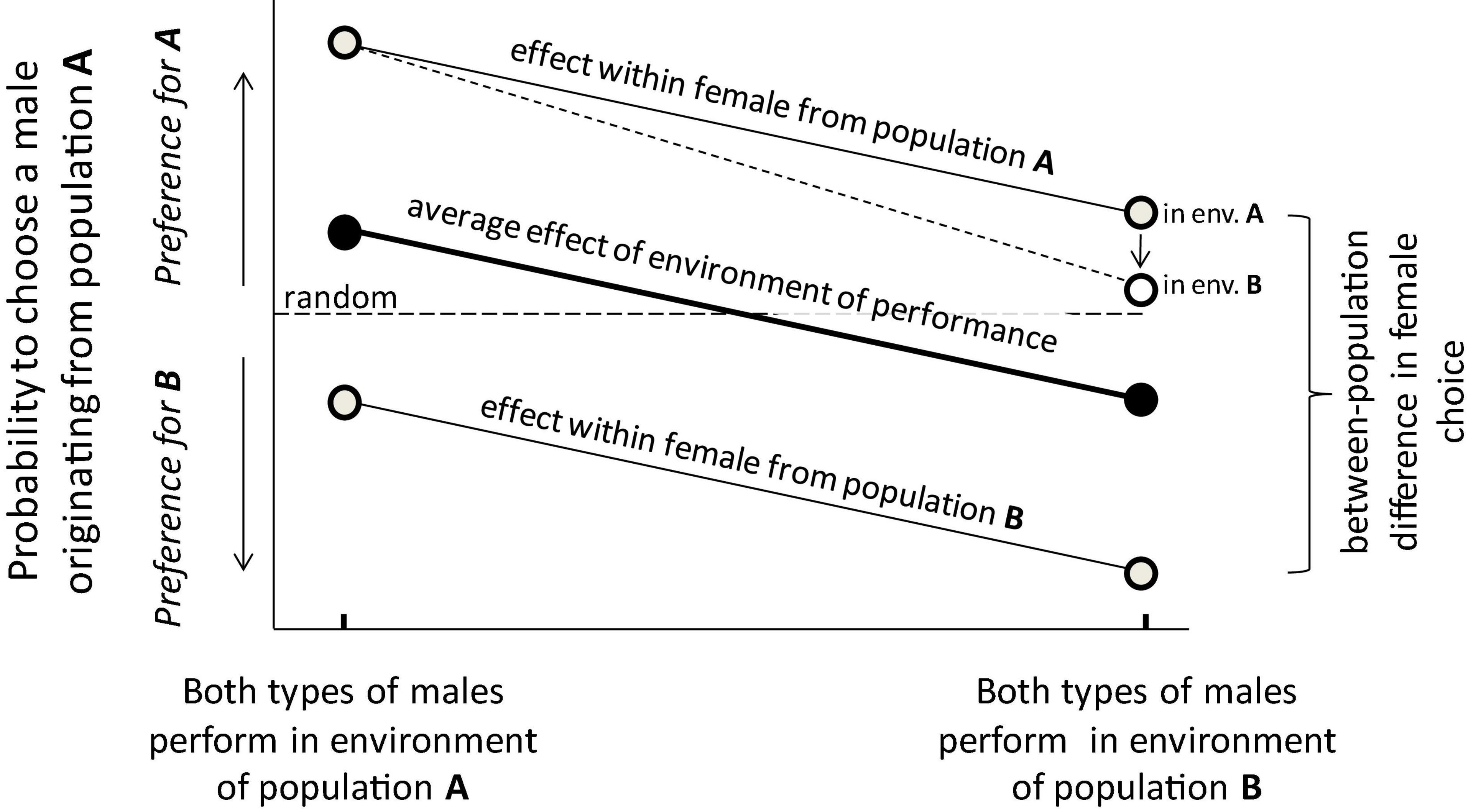
Figure 1. Disentangling the effects on mate choice (for convenience here with females as choosing individuals and males as chosen individuals, respectively). The effects are represented as the probability to choose a male originating from population A, which could also be the response variable of a statistical model to test the hypothesis. Our hypothesis predicts that males are more attractive when performing in their own environment, so males from population A should be (more) preferred when performing in the environment of population A (central thick black line; overall effect). This general effect is predicted to be true for females originating from each population, even though there might be a consistent difference in choice between them (top and bottom lines) due to diverged female preferences or male signals. In the extreme case neither type of female may even ever prefer the males from the other population (in that case their lines don’t cross the horizontal dotted line of random choice, as here), but the relevant test is whether relative preference is higher when the males perform in their native environment. Finally, if females are also exposed to the environments in which males have to perform, then their preferences might become less strong when exposed to the environment they are not adapted to (top right; here only shown for females from population A but equally relevant for females from population B). All these effects can be statistically estimated if the design involves the relevant levels and sufficient replication.
Preference could also depend on prior experience, so this needs to be taken into account. Some studies used virgin choosers, because these initiate matings more quickly. It then needs to be addressed (as in Arbuthnott and Rundle, 2014) whether the degree of choosiness is comparable between choosers that are virgin or that have mated previously, because individuals that have been maintained as virgins isolated from the other sex for an unusually long time might be very desperate to mate with the first potential mate they encounter – i.e., not be choosy. On the other hand, prior experience with potential mates and prior mating can change preferences, toward similar mates or different mates. Also here knowledge on the situation in the field might help to decide which types of choosers to use (or how to prepare them for the experiments). And if the prior experience of individuals is known and variable, this can also be statistically controlled for during data analysis (and is interesting of itself).
What if the choosing sex has (evolved) the ability to recognize locally adapted mates directly, for example because they pay attention to ecological traits or ecological performance (Snowberg and Benkman, 2009)? In that case any mating advantage for local individuals does not operate indirectly via condition-dependent traits, but directly on the locally advantageous traits. This is not the mechanism described in van Doorn et al. (2009), but produces exactly the same prediction. Note however that the consequences are exactly the same: sexual selection and natural selection operate in synergy, sexual selection removes immigrant individuals from the population, and divergence is maintained or enhanced, so depending on the question of the study this is not a problem. Detailed observations and manipulations could then maybe disentangle if mates are sexually favored directly because of their ecological traits, or indirectly via ecological performance-dependent sexual traits.
Which Environments for the Choosing Individuals to Use
If the chosen sex is affected by the experimental environments the same may be true for the choosing sex. In the extreme case, so much that it effectively doesn’t choose normally anymore (and may even suffer from increased mortality: Plath et al., 2010). To avoid this, a possibility could be to keep the chosen and the choosing individuals in separate areas (cages, tanks, etc.), each with their own relevant conditions (type of food, air temperature, water salinity, etc.) (Plath et al., 2010). Or to only restrict the environmental manipulation to the chosen sex during the development of their traits (Arbuthnott and Rundle, 2014). This way the conditions of the chosen sex can be manipulated without affecting the state of the choosing sex. A variant of this would be to present the choosing individual with recorded or computer-simulated stimuli individuals, showing their response to the environment they are exposed to Greenway et al. (2016). Whether this is a valid design depends on how well the choosing sex responds to such limited and partially unnatural interactions with the chosen sex.
But there are other solutions too. One could expose all types of chosen individuals to all types of choosing individuals in all types of environments (i.e., a fully factorial design). Even though the ability or motivation to choose might be lower in the non-local environment, what matters is whether individuals in their local environment are chosen in a greater proportion relative to when they reside in the other environment (i.e., the comparison across environments within the choosing sex of each population is what matters; Figure 1). Hence, the prediction would then be that all types of choosing individuals show a greater preference for individuals that perform in their own environment. Even when there is some population divergence in preferences and sexual traits, the effect of local performance might still operate and have an additive effect (Figure 1). Divergence in preferences is less of a problem if preference isn’t genetic but learned (Verzijden et al., 2012), but in that case experimental individuals must have been allowed to learn before being used in experimental tests. In fact, if preference is due to learning, the preference of experimental females could perhaps be beneficially homogenized by providing them the same prior experiences.
Which Chosen Individuals to Use
The larger the difference between the individuals that are chosen, the greater the expected effect. It makes sense therefore to use individuals that are “immigrants” from the other populations. However, when this is not possible or preferred, we can also compare hybrids or backcrosses with fully local individuals. In fact a range of individuals with a different degree of immigrant background can be compared with local individuals, although it helps to take this variability into account during the analysis of the data. Alternatively, the exact status of each individual is not known, but we do know the status of the different populations that are compared (their degree of immigration), or we derive the status of each individual from fitness measures, as in Long et al. (2012) discussed above.
Which Things to Measure
Sexual selection should in principle be measured in terms of fertilizations, but this is rarely done. Instead, different proxies can be measured, from the expression of sexual ornaments and armaments, to courtship behavior, to mating success. In fact all of these are worth measuring even if fertilizations are also measured, as they provide greater biological insight into what is going on (Klappert and Reinhold, 2005; Smith et al., 2013; Tinghitella et al., 2020). It is especially useful to establish that the traits of the chosen sex that supposedly respond to lower ecological performance are indeed differentially expressed across environments: if not, then the choosing sex has nothing to choose from Shenoi and Prasad (2016). Lack of responsiveness might occur if the development of the trait has completed by the time that the individual disperses to another population. For example, if a full-grown insect or bird disperses as an adult, then its structural size is not going to be affected by a new environment. If that is the case, then a focus on traits that are responsive is necessary, or the investigation should involve individuals that are immigrants before their traits have developed into their final stage – this includes hybrids and backcrosses.
What if There Is No Choice, Only Intrasexual Competition?
Above I focused on a scenario of choice between local and immigrant potential mates (a form of intersexual selection). But sexual selection might also operate via intrasexual selection, for example male-male competition. As mentioned in van Doorn et al. (2009), the hypothesis also is relevant in that case: it is quite possible that immigrant individuals are less successful in competition if their ecological performance is lower, as this may result in less time or energy available for fighting, development of smaller weaponry, etcetera. This actually simplifies our experimental design, since the choosing sex then simply becomes the object of competition and not a true participant, and some of the aspects discussed above become less or not at all relevant. It also largely removes the possibility that patterns of greater success by local individuals are driven by diverged preferences, under the assumption that individuals compete equally hard over mating opportunities, independent of the population where the potential mate comes from, i.e., local and novel mates are equally desirable; if not, the fully factorial design discussed above can again be used to deal with this. This means that species that don’t have some sort of mate choice still qualify as suitable systems to test the hypothesis.
Can We Use a Non-experimental, Observational Approach?
For whatever reason, it may not be possible to do controlled experiments in captivity. In that case, we can move individuals between populations in the wild (reciprocal transplants; Svensson et al., 2018). A disadvantage of this approach is that more variation will occur in certain aspects, e.g., the age and prior experience of individuals, or in which situations choice occurs. This will result in more noise in the data and the potential for confounding factors, making statistical tests and interpretation somewhat harder. However, one could also argue that the environments that individuals are exposed to are the relevant ones and that what happens in the wild is more relevant than what happens in the lab. So moving individuals between populations is certainly a worthwhile, valid and underutilized approach (when ethically and legally permitted).
What if experimentation is not possible - in that case, can we use natural dispersal events to test the hypothesis? To some extent, yes. We can genotype or phenotype individuals, determine their degree of “localness” (incl. for hybrids and backcrosses), assess their performance with respect to (proxies of) sexual selection, and test how this depends on the degree of localness. The prediction is that degree of localness has a positive effect on sexual performance. However, as with experiments, this could be due to a divergence in sexual preferences. This can be checked by also investigating what the choosing sex does, when it disperses into the other population (so basically doing the fully factorial comparison again): if it is more likely to mate with local mates, then apparently any preference divergence is less important than the degree of localness. Note however that as for any observational study, confounding effects that are not controlled for could influence the (causal) interpretation. For example, it is possible that ecological or sexual performance influence the dispersal decisions of individuals (Edelaar and Bolnick, 2012; Porter and Akcali, 2018; Camacho et al., 2020), in which case the comparisons are not as clean as when we perform such dispersal in a controlled (randomized) experimental manner.
Conclusion
It has yet to be more firmly established to what extent sexual selection limits the effective gene flow between locally adapted populations because of an environmentally induced reduced sexual performance. Few tests have been performed, and of those that have been performed some were suboptimal in their design. I have outlined how the study design can be improved, and how more species and more contexts of sexual selection are suitable for testing. I hope that this overview acts as a catalyst for more and better studies to test whether sexual and natural selection can work in synergy to promote and protect adaptive divergence.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author Contributions
PE had the idea for the manuscript, collected all the materials, and wrote the manuscript.
Funding
This research was supported by the Spanish Ministry of Universities (grants CGL2016-79483-P and PID2019-108971GB-I00 to PE), and by a fellowship by the Swedish Collegium for Advanced Study.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I thank the editors for their invitation and patience, and reviewers ES and MK for their constructive comments. Lock Rowe and Howard Rundle provided a preprint of their manuscript.
References
Achorn, A. M., and Rosenthal, G. G. (2020). It’s not about him: mismeasuring ‘Good Genes’ in sexual selection. Trends Ecol. Evol. 35, 206–219. doi: 10.1016/j.tree.2019.11.007
Arbuthnott, D., and Rundle, H. D. (2014). Misalignment of natural and sexual selection among divergently adapted Drosophila melanogaster populations. Anim. Behav. 87, 45–51. doi: 10.1016/j.anbehav.2013.10.005
Arnegard, M. E., and Kondrashov, A. S. (2004). Sympatric speciation by sexual selection alone is unlikely. Evolution 58, 222–237.
Berdan, E. L., and Fuller, R. C. (2012). A test for environmental effects on behavioral isolation in two species of killifish. Evolution 66, 3224–3237. doi: 10.1111/j.1558-5646.2011.01646.x
Bonduriansky, R., and Day, T. (2018). Extended Heredity. A new understanding of inheritance and evolution. Princeton and Oxford: Princeton University Press.
Cally, J. G., Stuart-Fox, D., and Holman, L. (2019). Meta-analytic evidence that sexual selection improves population fitness. Nat. Commun. 10:2017. doi: 10.1038/s41467-019-10074-7
Camacho, C., Sanabria-Fernández, A., Baños-Villalba, A., and Edelaar, P. (2020). Experimental evidence that matching habitat choice drives local adaptation in a wild population. Proc. R. Soc. B Biol. Sci. 287:20200721. doi: 10.1098/rspb.2020.0721
Correia, L., Yeaman, S., and Whitlock, M. C. (2010). Local adaptation does not always predict high mating success. J. Evol. Biol. 23, 875–878. doi: 10.1111/j.1420-9101.2010.01957.x
Edelaar, P., and Bolnick, D. I. (2012). Non-random gene flow: an underappreciated force in evolution and ecology. Trends Ecol. Evol. 27, 659–665. doi: 10.1016/j.tree.2012.07.009
Edelaar, P., and Bolnick, D. I. (2019). Appreciating the Multiple Processes Increasing Individual or Population Fitness. Trends Ecol. Evol. 34, 435–446. doi: 10.1016/j.tree.2019.02.001
Felsenstein, J. (1981). Skepticism towards Santa Rosalia, or why are there so few kinds of animals? Evolution 35, 124–138. doi: 10.1111/j.1558-5646.1981.tb04864.x
Greenway, R., Drexler, S., Arias-Rodriguez, L., and Tobler, M. (2016). Adaptive, but not condition-dependent, body shape differences contribute to assortative mating preferences during ecological speciation. Evolution 70, 2809–2822. doi: 10.1111/evo.13087
Hereford, J. (2009). A quantitative survey of local adaptation and fitness trade-offs. Am. Nat. 173, 579–588. doi: 10.1086/597611
Kawecki, T., and Ebert, D. (2004). Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. doi: 10.1111/j.1461-0248.2004.00684.x
Kirkpatrick, M., and Ravigné, V. (2002). Speciation by natural and sexual selection: models and experiments. Am. Nat. 159, S22–S35. doi: 10.1086/338370
Klappert, K., and Reinhold, K. (2005). Local adaptation and sexual selection: a reciprocal transfer experiment with the grasshopper Chorthippus biguttulus. Behav. Ecol. Sociobiol. 58, 36–43. doi: 10.1007/s00265-004-0902-6
Kokko, H., and Heubel, K. (2008). Condition-dependence, genotype-by-environment interactions and the lek paradox. Genetica 132, 209–216. doi: 10.1007/s10709-007-9166-1
Kraaijeveld, K., Kraaijeveld-Smit, F. J. L., and Maan, M. E. (2011). Sexual selection and speciation: the comparative evidence revisited. Biol. Rev. Camb. Philos. Soc. 86, 367–377. doi: 10.1111/j.1469-185X.2010.00150.x
Lande, R., and Kirkpatrick, M. (1988). Ecological speciation by sexual selection. J. Theor. Biol. 133, 85–98. doi: 10.1016/S0022-5193(88)80026-2
Long, T. A. F., Agrawal, A. F., and Rowe, L. (2012). The effect of sexual selection on offspring fitness depends on the nature of genetic gariation. Curr. Biol. 22, 204–208. doi: 10.1016/j.cub.2011.12.020
Lorch, P. D., Proulx, S., Rowe, L., and Day, T. (2003). Condition-dependent sexual selection can accelerate adaptation. Evol. Ecol. Res. 5, 867–881.
Maan, M. E., and Seehausen, O. (2011). Ecology, sexual selection and speciation. Ecol. Lett. 14, 591–602. doi: 10.1111/j.1461-0248.2011.01606.x
Mank, J. E. (2009). Sexual selection and Darwin’s Mystery of Mysteries. Science 326, 1639–1640. doi: 10.1126/science.1184680
Plath, M., Riesch, R., Oranth, A., Dzienko, J., Karau, N., Schießl, A., et al. (2010). Complementary effect of natural and sexual selection against immigrants maintains differentiation between locally adapted fish. Naturwissenschaften 97, 769–774. doi: 10.1007/s00114-010-0691-x
Porter, C. K., and Akcali, C. K. (2018). An alternative to adaptation by sexual selection: habitat choice. Trends Ecol. Evol. 33, 576–581. doi: 10.1016/j.tree.2018.05.004
Proulx, S. R. (2001). Female choice via indicator traits easily evolves in the face of recombination and migration. Evolution 55, 2401–2411. doi: 10.1111/j.0014-3820.2001.tb00755.x
Reinhold, K. (2004). Modeling a version of the good-genes hypothesis: female choice of locally adapted males. Org. Divers. Evol. 4, 157–163. doi: 10.1016/j.ode.2003.10.002
Rice, W. R., and Hostert, E. E. (1993). Laboratory experiments on speciation: what have we learned in 40 years? Evolution 47, 1637–1653. doi: 10.1111/j.1558-5646.1993.tb01257.x
Ritchie, M. G. (2007). Sexual selection and speciation. Annu. Rev. Ecol. Evol. Syst. 38, 79–102. doi: 10.1146/annurev.ecolsys.38.091206.095733
Rowe, L., and Rundle, H. D. (2021). The alignment of natural and sexual selection. Annu. Rev. Ecol. Evol. Syst. 52, 499–517. doi: 10.1146/annurev-ecolsys-012021-033324
Rundle, H. D., and Nosil, P. (2005). Ecological speciation. Ecol. Lett. 8, 336–352. doi: 10.1111/j.1461-0248.2004.00715.x
Scordato, E. S. C., Symes, L. B., Mendelson, T. C., and Safran, R. J. (2014). The role of ecology in speciation by sexual selection: a systematic empirical review. J. Hered. 105, 782–794. doi: 10.1093/jhered/esu037
Shenoi, V. N., and Prasad, N. G. (2016). Local adaptation to developmental density does not lead to higher mating success in Drosophila melanogaster. J. Evol. Biol. 29, 2036–2042. doi: 10.1111/jeb.12927
Smith, G., Fang, Y., Liu, X., Kenny, J., Cossins, A. R., de Oliveira, C. C., et al. (2013). Transcriptome-wide expression variation associated with environmental plasticity and mating success in cactophilic Drosophila mojavensis. Evolution 67, 1950–1963. doi: 10.1111/evo.12082
Snowberg, L. K., and Benkman, C. W. (2009). Mate choice based on a key ecological performance trait. J. Evol. Biol. 22, 762–769. doi: 10.1111/j.1420-9101.2009.01699.x
Svensson, E. I., Goedert, D., Gómez-Llano, M. A., Spagopoulou, F., Nava-Bolaños, A., and Booksmythe, I. (2018). Sex differences in local adaptation: what can we learn from reciprocal transplant experiments? Philos. Trans. R. Soc. B 373:20170420. doi: 10.1098/rstb.2017.0420
Tinghitella, R. M., Lackey, A. C. R., Durso, C., Koop, J. A. H., and Boughman, J. W. (2020). The ecological stage changes benefits of mate choice and drives preference divergence. Philos. Trans. R. Soc. Lond B. Biol. Sci. 357:20190546. doi: 10.1098/rstb.2019.0546
van Doorn, G. S., Edelaar, P., and Weissing, F. J. (2009). On the origin of species by natural and sexual selection. Science 326, 1704–1707. doi: 10.1126/science.1181661
van Doorn, G. S., and Weissing, F. J. (2002). Ecological versus sexual selection models of sympatric speciation: a synthesis. Selection 2, 17–40. doi: 10.1556/Select.2.2001.1-2.3
Keywords: sexual selection, condition-dependence, ecological performance, local adaptation, population divergence, speciation, dispersal, gene flow
Citation: Edelaar P (2022) Sexual Selection May Not Often Reduce Gene Flow Between Locally Adapted Populations. A Review of Some Evidence, and Suggestions for Better Tests. Front. Ecol. Evol. 10:804910. doi: 10.3389/fevo.2022.804910
Received: 29 October 2021; Accepted: 25 February 2022;
Published: 22 March 2022.
Edited by:
Jesus Martinez-Padilla, Pyrenean Institute of Ecology (IPE - CSIC) – ARAID, SpainCopyright © 2022 Edelaar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pim Edelaar, ZWRlbGFhckB1cG8uZXM=
 Pim Edelaar
Pim Edelaar