- 1Key Laboratory of Molecular Cardiovascular Science, Ministry of Education, Department of Physiology and Pathophysiology, School of Basic Medical Sciences, Peking University, Beijing, China
- 2Key Laboratory Experimental Teratology of the Ministry of Education and Department of Physiology, School of Basic Medical Sciences, Shandong University, Jinan, China
- 3Key Laboratory Experimental Teratology of the Ministry of Education and Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Shandong University, Jinan, China
The sex pheromone receptors (SPRs) of Lepidopteran insects play important roles in chemical communication. In the sex pheromone detection processes, sex pheromone molecule (SPM), SPR, co-receptor (Orco), pheromone binding protein (PBP), sensory neuron membrane protein (SNMP), and pheromone degradation enzyme (PDE) play individual and cooperative roles. Commonly known as butterfly and moth, the Lepidopteran insects are widely distributed throughout the world, most of which are pests. Comprehensive knowledge of the SPRs of Lepidopteran insects would help the development of sex lure technology and the sex communication pathway research. In this review, we summarized SPR/Orco information from 10 families of Lepidopteran insects from corresponding studies. According to the research progress in the literature, we speculated the evolution of SPRs/Orcos and phylogenetically analyzed the Lepidopteran SPRs and Orcos with the neighbor-joining tree and further concluded the relationship between the cluster of SPRs and their ligands; we analyzed the predicted structural features of SPRs and gave our prediction results of SPRs and Orcos with Consensus Constrained TOPology Prediction (CCTOP) and SwissModel; we summarized the functional characterization of Lepidopteran SPRs and SPR-ligand interaction and then described the progress in the sex pheromone signaling pathways and metabotropic ion channel. Further studies are needed to work out the cryo-electron microscopy (EM) structure of SPR and the SPR-ligand docking pattern in a biophysical perspective, which will directly facilitate the understanding of sex pheromone signal transduction pathways and provide guidance in the sex lure technology in field pest control.
Introduction
Belonging to general odorant receptors, insect sex pheromone receptors (SPRs) are expressed by olfactory sensory neurons (OSNs) and can detect volatile sex pheromones or other chemical signals to coordinate their social behaviors such as mating, reproduction, and alarming (Fleischer and Krieger, 2018). In the sex pheromone detection processes, sex pheromone molecule (SPM), SPR, co-receptor (Orco), pheromone binding protein (PBP), sensory neuron membrane protein (SNMP), and pheromone degradation enzyme (PDE) play individual and cooperative roles (Leal, 2013). Usually, SPM was released from female insects and then sensed by specific SPR of conspecific male insects; meanwhile, the specific PBP surrounding the SPR transports the SPM to SPR and increases the magnitude of SPR response to SPM (Grosse-Wilde et al., 2006; Krieger et al., 2009); Orco was expressed in the same OSN of SPR and had been reported to increase the response strength of SPR to SPM through an ionotropic channel or a metabotropic channel (Nakagawa et al., 2005). After a series of elusive signal transduction pathways, the PDE contributes to the signal inactivation through degrading the SPM (Leal, 2013). Above all, the native mechanism of sex pheromone reception and signal transduction has not been fully elucidated until present.
Commonly known as butterfly and moth, the Lepidopteran insects are widely distributed throughout the world. In recent years, many odorant receptors had been characterized following the public annotation of Drosophila melanogaster odorant receptor family based on the whole-genome data (Wang and Anderson, 2010). Importantly, several SPRs have been deorphanized in Bombyx mori and other insect species according to the analysis of antennal transcriptome data (Nakagawa et al., 2005). As a consequence, sex attractants have been used as sex lures to wipe out pests without using chemical insecticides (Witzgall et al., 2010), and this environment-friendly pest control policy had been growing up gradually.
In this review, we summarized SPR and Orco information from 10 families (i.e., Bombycidae, Plutellidae, Sphingidae, Saturniidae, Geometridae, Nymphalidae, Noctuidae, Tortricidae, Pyralidae, and Crambidae) of Lepidopteran insects (Krieger et al., 2004, 2005; Sakurai et al., 2004; Miura et al., 2005; Nakagawa et al., 2005; Grosse-Wilde et al., 2007, 2010, 2011; Mitsuno et al., 2008; Jordan et al., 2009; Patch et al., 2009; Zhang et al., 2009, 2010, 2013, 2014, 2015; Wanner et al., 2010; Legeai et al., 2011; Wang et al., 2011; Yasukochi et al., 2011; Zhan et al., 2011; Bengtsson et al., 2012; Carraher et al., 2012; Leary et al., 2012; Liu et al., 2012, 2014; Montagne et al., 2012; Xu et al., 2012, 2015; Liu C. et al., 2013; Liu Y. et al., 2013; Sun et al., 2013; Wu et al., 2013; Zhang and Lofstedt, 2013; Jiang et al., 2014; Corcoran et al., 2015; De Fouchier et al., 2015; Feng et al., 2015; Garczynski and Leal, 2015; Lin et al., 2015; Steinwender et al., 2015; Chang et al., 2016; Ge et al., 2016; Jia et al., 2016; Walker et al., 2016; Zhang D. D. et al., 2016; Zhang Y. N. et al., 2016; Gonzalez et al., 2017; Li et al., 2017; Wicher et al., 2017; Yang S. et al., 2017; Du et al., 2018; Grapputo et al., 2018; Rojas et al., 2018; Table 1). Among all the Lepidopteran SPRs, several of them have been characterized to be sex pheromone sensing receptors. First of all, we reviewed the phylogenetic analyses of Lepidopteran SPRs, and the evolution of the summarized Lepidopteran SPRs was analyzed through MEGA X (Whelan and Goldman, 2001; Kumar et al., 2018). Second, we reviewed the transmembrane predictions of Lepidopteran SPRs, and the protein structure of Lepidopteran SPRs was predicted by online software Consensus Constrained TOPology Prediction (CCTOP) (Dobson et al., 2015) and SwissModel (Bertoni et al., 2017; Bienert et al., 2017; Waterhouse et al., 2018; Guex et al., 2019; Studer et al., 2020). Third, the interaction of Lepidopteran SPM and SPR was reviewed. Finally, the research status of downstream signaling responses and ligand-gated ion channels by the coupling of SPR and Orco was depicted.
Evolution of Lepidopteran Sex Pheromone Receptors
The olfactory receptor (OR) repertoire of several Lepidopteran species was usually phylogenetically analyzed, and SPRs always belong to the same clade. The neighbor-joining tree of sequences of all identified ORs of Heliothis virescens revealed a very high degree of diversity, i.e., a group that comprises 6 SPRs has at least 40% of their amino acids in common (Krieger et al., 2004). The neighbor-joining tree of H. virescens OR repertoires and BmOR1/3/4/5/6 receptors showed apparent relatedness of SPRs in B. mori and H. virescens (Krieger et al., 2005). The phylogenetic tree of B. mori and H. virescens ORs and also PxylOR1/3/4 and DindOR1/3 showed that pheromone receptors were clustered and were different from that of the Or83b family (Mitsuno et al., 2008; Patch et al., 2009). The phylogenetic analysis of B. mori, Manduca sexta, Helicoverpa armigera, and H. virescens ORs and PxylOR1/3/4/5/6/7 shows that the 6 candidate SPRs cluster together in the group of SPRs (Sun et al., 2013). SPRs formed a single subgroup in a phylogenetic tree by the ORs of B. mori, H. armigera, H. virescens, and Plutella xylostella, and the Orcos of the four species form a clade (Yang S. et al., 2017). The phylogenetic analysis of B. mori, H. armigera, and H. virescens ORs and PxylOR1/3/4/5/6/7/8/41/45 shows that the 8 candidate SPRs cluster together in the group of SPRs, and they are phylogenetically distinct from general odorant receptors (Liu et al., 2018). Neighbor-joining tree based on MUSCLE multiple sequence alignment of MsexOR1-5 and B. mori and H. virescens ORs shows that MsexOR1-4 belongs to the subgroup of SPRs, and a highly conserved Or83b group was indicated (Grosse-Wilde et al., 2010, 2011). The neighbor-joining tree including the ORs of B. mori, H. virescens, and MsexOR1 and ApolOR1/AperOR1 revealed that ApolOR1/AperOR1 are categorized in the subfamily of the candidate and functionally verified SPRs (Forstner et al., 2009). A phylogenetic analysis that was performed using candidate SexiOR and OR repertoires from H. armigera, Helicoverpa assulta, Spodoptera littoralis, and B. mori revealed a highly conserved Orco that was clustered with orthologs from all four of these species, and another group of relatively conserved SexiOR6/11/13/16 belongs to the same clade as SPRs (Du et al., 2018).
The phylogenetic tree that contained 21 OR sequences from Sesamia inferens, 43 from M. sexta, 21 from H. virescens, and 60 from B. mori, SinfOR2 was clustered with other Lepidopteran Orco sequences, and three SinfOR21/27/29 were clustered in the Lepidopteran SPR clade (Zhang Y.N. et al., 2016). A phylogenetic tree using a dataset containing all AlepOR sequences and all HassOR, HarmOR, HvirOR, and BmOR sequences revealed that AlepOrco was clustered with other Lepidopteran Orco sequences, and four AlepOR3/4/5/6 with full-length open reading frame (ORFs) were clustered in the Lepidopteran SPR clade (Zhang Y.N. et al., 2016). Multiple alignments of H. armigera SPRs and their homologs in H. virescens showed that the orthologous SPRs in these two insects had a high similarity. The phylogenetic analyses showed that these SPRs were clustered together with other B. mori and H. virescens SPRs separated from general odorant receptors (Liu Y. et al., 2013). All identified chemosensory receptors of H. armigera and H. assulta were used to construct a phylogenetic tree with the known ORs of H. virescens, HvOrco, HarmOrco, and HassOrco which were grouped together in a single lineage named the Orco subfamily. OR6/11/13/14/15/16 from the three species were clustered in the SPR subfamily, which included the functionally identified SPRs in H. virescens (Jiang et al., 2014). Notably, 10 OR sequences were used in the phylogenetic analysis with another 41 H. assulta ORs identified, 68 B. mori ORs, and 60 H. armigera ORs. One group of ORs which was formed by 7 B. mori SPRs, 7 H. armigera SPRs, and 7 HassORs was identified as the SPR group (Xu et al., 2015).
In a neighbor-joining tree including OR repertoires of Ctenopseustis obliquana, Ctenopseustis herana, and Epiphyas postvittana, of which the clade predicted to contain the SPRs of many moth species is well supported by the bootstrap analysis (Steinwender et al., 2015). The phylogenetic analysis of the EposORs was performed against comprehensive OR datasets from B. mori, H. virescens, and C. pomonella, in which 8 receptors (EposOR1/6/7/21/22/41/43/45) fall into a well-supported clade that contains SPRs from other moth species, including BmOR1/3 and HvirOR6/13/14/16 (Corcoran et al., 2015). In the sequence similarity analysis of the C. pomonella ORs, the OR repertoires of B. mori, H. virescens, M. sexta, S. littoralis, and several ORs, Cpom1/3/4/5/6 are grouped in a conserved clade containing Lepidopteran SPRs, and Orco forms a clade (Bengtsson et al., 2012).
The C. pomonella ORs are presented phylogenetically within the context of other tortricid moth species (C. obliquana, C. herana, and E. postvittana) from which large OR repertoires have been published, along with B. mori ORs serving as a Lepidopteran out-group, Orco clade, and SPR clade (Walker et al., 2016). The phylogenetic analyses of odorant receptors from Planotortrix octo, Planotortrix excessana, C. obliquana, C. herana, and E. postvittana showed that the tree is rooted with Orco, and the SPR clade is formed (Steinwender et al., 2016). The phylogenetic relationships of odorant receptors from L. botrana and other insects, such as C. pomonella, E. postvittana, B. mori, Ostrinia nubilalis, Spodoptera exigua, S. littoralis, P. xylostella, H. armigera, and H. assulta, indicated that 10 LbotOR sequences were predicted to be closely related to the SPR clade proposed for C. pomonella, B. mori, M. sexta, S. littoralis, and O. nubilalis (Rojas et al., 2018). In the phylogenetic tree of odorant receptors, orthologous OR1s of the genus Ostrinia formed a clade, and the OR1 group was included in a single lineage of the SPR subfamily (Miura et al., 2009). In the phylogenetic tree of ORs constructed using the sequences of 162 ORs from B. mori, Ostrinia furnacalis, and Conogethes punctiferalis, the OR sequences were clustered into SPRs, Orco, and other divergent ORs (Ge et al., 2016). In a neighbor-joining tree of 130 OR sequences built from three different Lepidoptera species, including C. punctiferlis, B. mori, and O. furnacalis, the Orco was clustered with other Lepidoptera Orco sequences (Jia et al., 2016).
The SPRs of Lepidopteran insects had been phylogenetically analyzed in several studies. The phylogeny of Lepidopteran SPRs shows four orthologous clades, in which a cluster only contains candidate SPRs of noctuid species, and paralogous SPRs from Cluster I differ dramatically in ligand selectivity and sensitivity (Zhang Y.N. et al., 2016). The neighbor-joining analysis of highly conserved Noctuidae SPRs and their ligands had been summarized (Jiang et al., 2019, 2020). In a maximum-likelihood phylogeny of Lepidopteran candidate SPRs from Yponomeutoidea, Pyraloidea, Tortricoidea, Papilionoidea, Bombycoidea, and Noctuoidea, SPRs were grouped into 5 different paralogous lineages, i.e., each containing SPRs from different Lepidopteran superfamilies (De Fouchier et al., 2015). The phylogenetic tree showed that the SPRs of different moths were clustered into four branches; while the moth Orcos were clustered into one branch that was separated from the SPRs (Zhang et al., 2014, Zhang Y.N. et al., 2016). Phylogenetic analysis constructed with 10 SPRs from Cydia fagiglandana, 7 SPRs from Hedya nubiferana, 8 SPRs from B. mori, 6 SPRs from E. postvittana, and 14 SPRs from C. pomonella revealed that the four genes (i.e., CpomOR1, CpomOR2a, CpomOR5, and CpomOR7) were clustered with Lepidopteran SPRs (Tian et al., 2020). In the phylogenetic tree of SPR subfamily proteins, OR1/3/4/5/6/7/8 of different Ostrinia species, respectively, formed a clade (Miura et al., 2010). The phylogenetic relationship of OnOR1-6 with SPRs from the superfamily of Bombycoidea, Noctuoidea, Pyraloidea, Yponomeutoidea, and Tortricoidea suggests that there is no clear relationship between the phylogeny of SPRs and their ligand (Wanner et al., 2010). In a phylogenetic tree, the OR4,5,8 genes of several Ostrinia moths and the OnOr6 gene formed a definite clade, which did not include any known SPRs of other Lepidoptera (Yasukochi et al., 2011). The neighbor-joining phylogenetic tree of SPRs of 8 Ostrinia species forms 5 orthologous lineages, i.e., each with 100% bootstrap support, belonging to the Lepidopteran SPR lineage (Leary et al., 2012).
In our summary, the phylogenetic tree of 256 SPRs and Orcos showed some similar information as former reports (Figure 1). The Orcos of 40 species from 10 families of Lepidoptera form a clade with a bootstrap value of 100 distinct from SPRs, indicating the high conservation of Orcos, implied the fixed function of Orcos (Zhang et al., 2019). In the Orco clade, only the 10 Orcos from Noctuidae are in a single branch; the Orcos from Tortricidae are distributed in two branches, with one branch clustered with Orcos from Crambidae and Plutellidae. As to SPRs, SPRs from Noctuidae, Tortricidae, and Crambidae are with the major number, and there is no strict affiliation between SPRs and families.
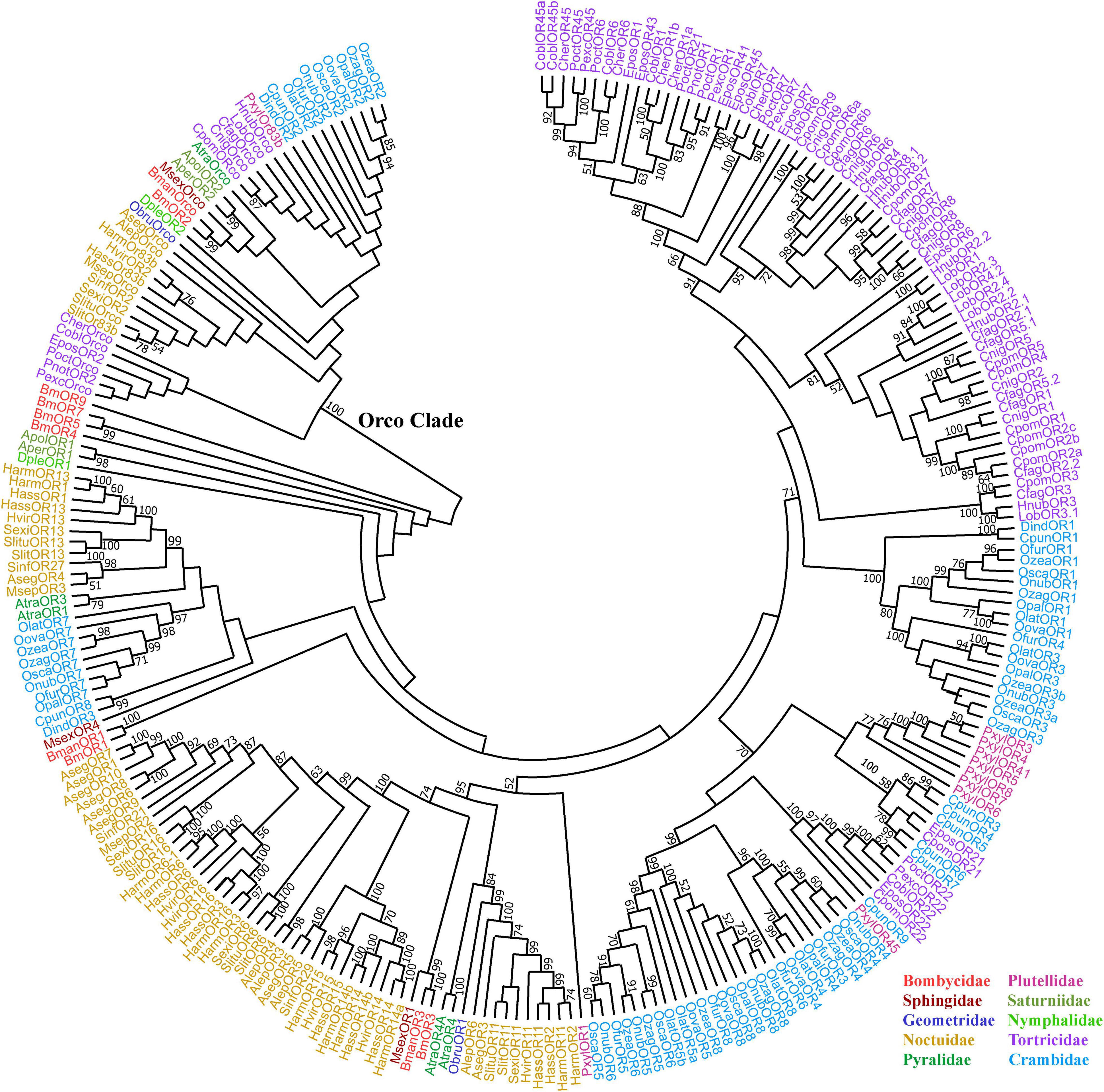
Figure 1. Phylogenetic tree of candidate sex pheromone receptors (SPRs) and Orcos of Lepidopteran insects. Lepidopteran SPR labels and their corresponding family name (at the bottom right corner) are in the same color. Node support was assessed with 1,000 bootstrap replicates, and values greater than 50% are shown. The model used was JTT according to the model prediction by MEGA X, and the neighbor-joining method was used. All the GenBank IDs of receptors are shown in Table 1.
We then summarized some SPRs and their corresponding ligands (Figure 2), and the branch position of each SPRs are the same as in Figure 1. The 10 SPRs share the same ligand Z11-16:Ald; AtraOR3, MsepOR3, HvirOR13, HassOR1/13, and HarmOR1/13 are in a branch with a bootstrap support value of 99; and PxylOR1, HvirOR16, HassOR14b, and DindOR1 are in different clusters. Z11-16:Ald is the pheromone component of H. armigera, H. assulta, H. virescens, and P. xylostella. SexiOR13/SlitOR13/SlituOR13/SinfOR27 and SlitOR6/SlituOR6 are tuned to the ligand Z9,E12-14:OAC, which is the pheromone component of the 3 corresponding species. E10,Z12-16:Ald is the ligand of BmOR3 and MsexOR1 on the same branch with a bootstrap value of 99; AlepOR3 and AlepOR4 are both tuned to Z7-12:Ac, which is their corresponding pheromone component. HarmOR16, HvirOR16, and SinfOR21 are dispersed on different branches but share one ligand Z11-16:OH. PxylOR4/41/AlepOR4, OnubOR1/3/OfurOR3, and OnubOR5/OscaOR3, respectively, tune to their corresponding major ligands Z9-14:Ac, E12-14:OAc, and Z12-14:OAc. OscaOR1/OlatOR1/OovaOR1/OscaOR3 are tuned to E11-14:OH. As the SPRs from Tortricidae, CpomOR2a, and CpomOR5 are tuned to E8,E10-12:Ac, CoblOR7 and CherOR7 both tuned to Z8-14:OAc. Similar to former research (Wanner et al., 2010), there is no clear relationship between the phylogeny of SPRs and their ligands.
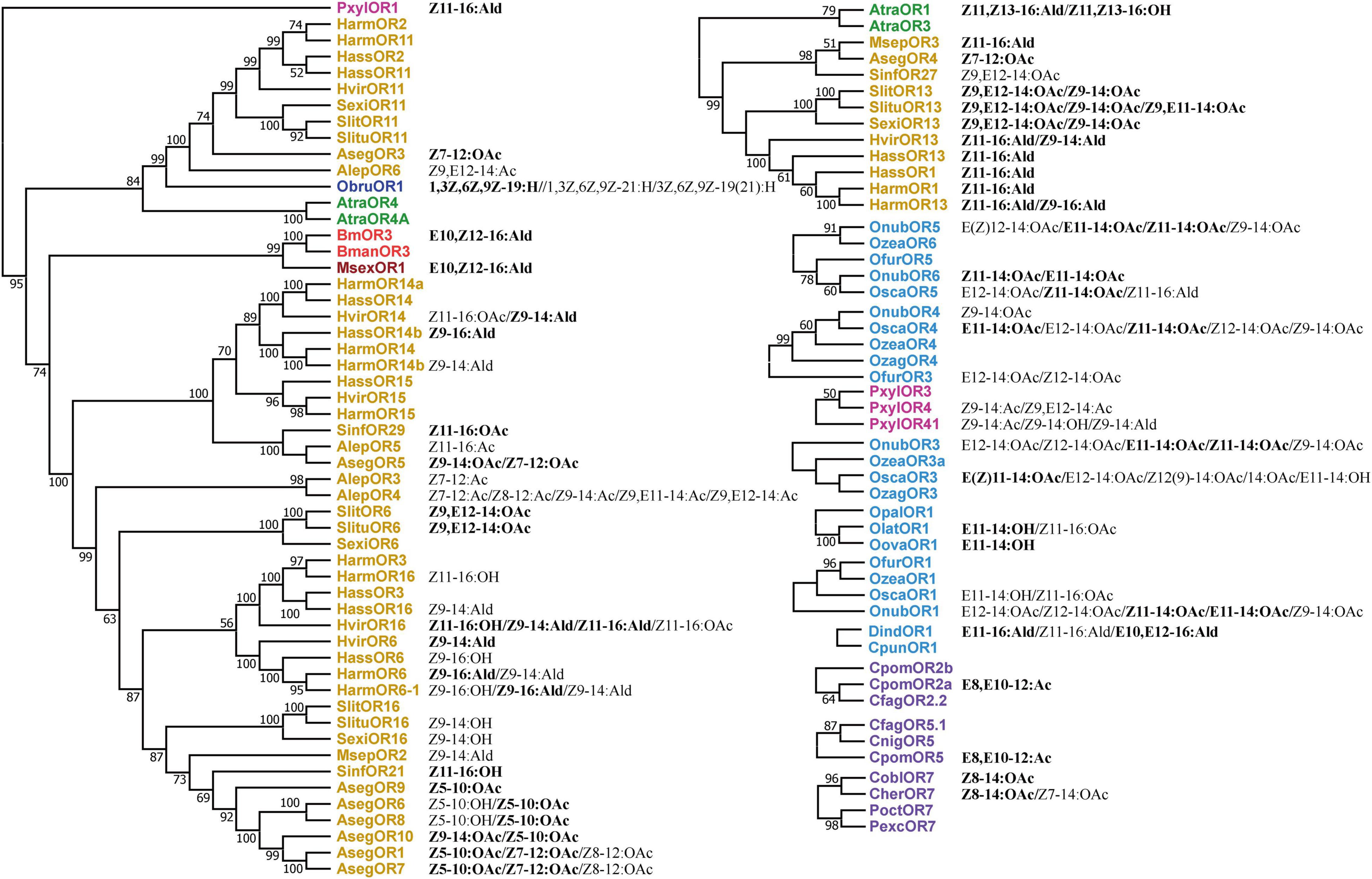
Figure 2. Lepidopteran SPRs and their ligands in the form of phylogenetic branches. Lepidopteran SPR labels and their corresponding family name are in the same color as shown in Figure 1. The ligands of SPRs are on the right side of each SPR, among which the chemical compound in bold is the sex pheromone molecule (SPM) of the corresponding species.
Structure of Lepidopteran Sex Pheromone Receptors
Several studies have predicted the transmembrane and topology of Lepidopteran SPRs and Orcos. The sequence analysis of PxylOR1/3/4 and DindOR1/3 by transmembrane domain prediction tools TMpred and TMHMM indicated that proteins coded by the genes possess the seven transmembrane domains that are the characteristics of the G protein-coupled receptors (GPCR) superfamily (Mitsuno et al., 2008). TMHMM2.0 was used for the prediction of transmembrane domains of PxylOR8/41/45, and these SPRs contain seven putative transmembrane domains (Liu et al., 2018). As with ORs from other insects, SexiOR3 hypothetically contains seven transmembrane domains with a predicted intracellular N-terminus and an extracellular C-terminus by TMHMM Server version 2.0 (Liu C. et al., 2013). SinfOR21/27/29 predicted to have the typical characteristics of an OR, including seven putative transmembrane domains, an intracellular N-terminus, and an extracellular C-terminus by TMHMM2.0 (Zhang et al., 2014). Predicted by the Phobius and MANSAT3, the SlituOR3 has seven transmembrane domains (Lin et al., 2015). According to TMBase and the SFINX package, SlituOR6/11/13/16 were predicted to possess 7 transmembrane domains (Zhang et al., 2015). The predicted transmembrane topology of CoblOR7 and CherOR7 was using SPLIT 4.0 at the transmembrane prediction server (Steinwender et al., 2015). Through TMHMM2.0, TMAP, and TMpred, EposOR1 was predicted to contain 7 transmembrane domains and an intracellular N-terminus with the exception of TMpred (Jordan et al., 2009). SlitOrco and other Noctuidae of the Orcos of Lepidoptera insects were predicted with seven transmembrane domains by TMHMM2.0 (Wu et al., 2013).
The transmembrane topology of the SPRs and Orcos of Lepidopteran insects was predicted by online software CCTOP (Dobson et al., 2015), and the structural features of these receptors according to these predictions were speculated (Table 1). All the SPRs showed no significant common features (such as transmembrane numbers) according to the CCTOP prediction results, but the intracellular N-terminus and extracellular C-terminus locations of these SPRs were similar to the previous reports of Lepidopteran insects (Mitsuno et al., 2008). The single-particle cryo-electron microscopy (EM) structure of an Orco homomer from the parasitic fig wasp at 3.5 Å resolution had been reported, which confirmed the predicted topology of the Orco in Lepidopteran insects (Butterwick et al., 2018). Through SwissModel online predictions, the cryo-EM structures of the Orco mentioned earlier (SMTL ID: 6c70.1) were the best templates of all the SPRs according to sequence identities, and thus, all the receptors have similar structural characteristics with 7 transmembrane helixes, of which BmOR3 is shown in Figure 3 as a sample.
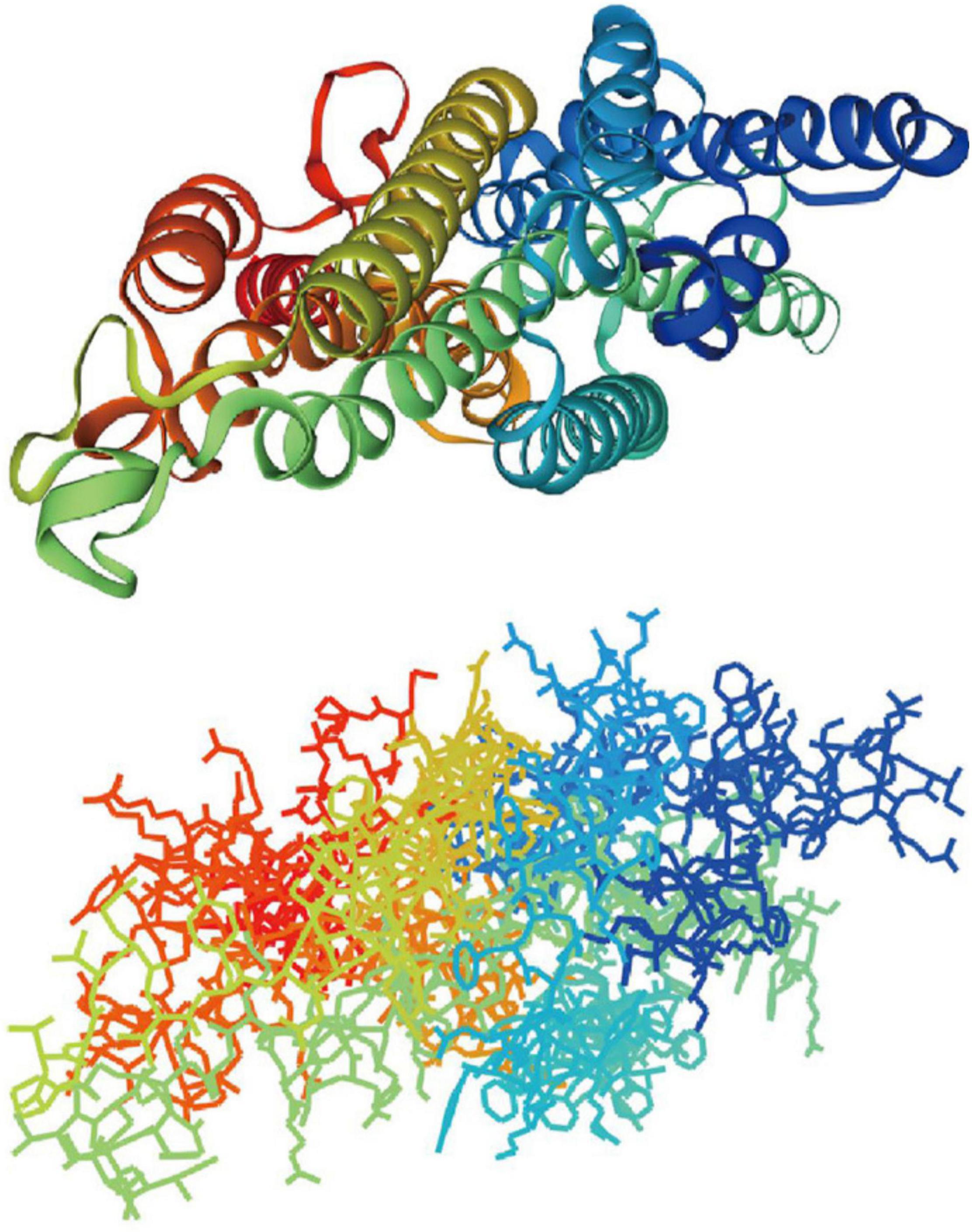
Figure 3. Predicted structure of Lepidopteran SPRs BmOR3 and Orcos through SwissModel. In total, 7 transmembrane helixes were colored rainbow in the form of silk ribbon (Cartoon) and lines.
Characterization and Interaction of Sex Pheromone Receptors and Specific Sex Pheromone Molecules
The functional characterization of SPRs in Lepidopteran insects can be divided into two types, namely, in vivo and in vitro. Almost all the Lepidopteran SPRs were characterized through classical in vitro electrophysiological recording (i.e., two-electrode voltage-clamp) of heterologous expression system on SPM activation. The two-electrode voltage-clamp recording was conducted in Xenopus oocytes coinjected with complementary RNAs encoding BmOR1 and BmOR2 to test the bombykol-inducing current response (Nakagawa et al., 2005). The similar method was used in a specific pheromone detection of PxylOR1/4/8/41/45 (Sun et al., 2013; Liu et al., 2018), MsexOR1 (Wicher et al., 2017), EgirOR1 (Li et al., 2017), MsepOR2/3 (Jiang et al., 2019, 2020), HvirOR6/13/14/16 (Wang et al., 2011), SexiOR13/16 (Liu C. et al., 2013), SinfOR21/29 (Zhang et al., 2014), AlepOR3/4/6 (Zhang et al., 2019), HarmORs/HassORs (Liu Y. et al., 2013; Liu et al., 2014; Jiang et al., 2014; Xu et al., 2015), AsegOR1/4/5/6/9 (Zhang and Lofstedt, 2013), SlituOR6/13/16 (Zhang et al., 2015), CpomOR2a/5 (Tian et al., 2020), AtraOR1/3 (Xu et al., 2012), and OlatOR1/OovaOR1/OscaOR1/3/4/5 (Miura et al., 2009, 2010). HEK293/sf9 cell calcium assay verified the sex pheromone component of ApolOR1, HassOR13, CoblOR7, and CherOR7 (Forstner et al., 2009; Jordan et al., 2009; Liu et al., 2014; Steinwender et al., 2015; Xu et al., 2015). In calcium imaging, calcium indicator Fluo-AM was used to detect the ion flow response induced by a specific ligand. In sf9 cells, endogenous Orco was provided to SPRs. Recent research adopted the Bioluminescence Resonance Energy Transfer (BRET)-based calcium sensor CalfluxVTN to detect ligand (Gu et al., 2009). In vivo, it means that the heterologous expression system is Drosophila antennae. In the characterization of S. littoralis SPRs, SlitOR6/SlitOR13 was expressed in a majority of Drosophila OSN in addition to endogenous receptors, and the responses to SPM stimuli were monitored by electroantennography, or Drosophila OR67d was replaced with SlitOR6 and the response was monitored by single sensillum recordings (Montagne et al., 2012; De Fouchier et al., 2015).
The interaction of SPR and specific SPM can be classified into receptor-ligand interaction in the perspective of biophysics, and the affinity between receptor and ligand is usually quantified by receptor-ligand complex dissociation constant Kd. According to Figure 2, most SPRs are tuned not only to the SPMs of their corresponding species but also to the SPMs of sibling species/analogs and antagonists, with different response amplitudes or different SPR-ligand affinities. In some researches, amino acid mutations of SPRs are responsible for the alteration of ligands. HarmOR14b and HassOR14b share 90% identities, and F232I + T355I are the key mutations that alter HassOR14b tuning to Z9-16:Ald to HarmOR14b tuning to Z9-14:Ald (Yang K. et al., 2017), which indicates that 232 and 355 are key residues in SPR and ligand docking. In Ostrinia species, OR3 amino acid mutation A148T in TM3 domain alters the pheromone recognition pattern by selectively reducing the E11-14:OAc response (EC50 of the dose-response curve) approximately 14-fold (Leary et al., 2012). Until present, no researches about SPR mutation and SPM recognition in a species have been reported. From the abovementioned studies, we can speculate that SPR and ligand docking pattern and the structure of SPR-ligand will be the future direction in SPR-ligand interaction studies.
Downstream Signaling Pathways of Sex Pheromone Receptors
Early research revealed the presence of G-protein, belonging to the Gαq family, in antennal preparations (especially the pheromone-sensitive sensilla trichodea) of B.mori and Antheraea pernyi, implied a participation of G-protein of the Gαq family in the signal transduction of OR cells in moths (Laue et al., 1997; Nakagawa et al., 2005). The bombykol stimulation of Xenopus laevis oocytes expressing BmOR-1 and BmGαq elicited robust dose-dependent inward Ca2 +-dependent Cl– currents on two-electrode voltage-clamp recordings, demonstrating that the binding of bombykol to BmOR-1 leads to the activation of a BmGαq-mediated signaling cascade (Sakurai et al., 2004). MsexOR1 and MsexOrco coexpressed in HEK293 and CHO cells caused bombykal-dependent increases in the intracellular free Ca2 + concentration, and inhibitor evidence showed that phospholipase C (PLC) and protein kinase C (PKC) activities are involved in the bombykal-receptor-mediated Ca2 + signals of hawk moths. It could be hypothesized that MsexOrs couple to Gαq proteins, requiring the activation of PLC for pheromone transduction (Wicher et al., 2017). Immunocytochemistry research showed that anti-Gαq and anti-Gαs antisera stained the inner and outer dendritic segments of the putative OR neuron in male and female antennae, which suggested that each subunit mediates a subset of the odorant response (Miura et al., 2005). In addition, a computational model of the insect pheromone transduction cascade had been used to calculate the presence of the G-protein pathway in pheromone detection (Gu et al., 2009). Furthermore, recent research showed that in HEK293A cells expressing BmOR3 and human Gαi, the dose-dependent coupling of BmOR3 and Gαi on bombykal stimulation was detected through BRET (Lin et al., 2021). From the biophysical perspective, a conservation residue W103 in transmembrane 2 of BmOR3 is the key that determines receptor-Gi coupling (Lin et al., 2021). Pretreatment with specific Gi inhibitor PTX had no significant effects on bombykal-induced BmOR3-BmOrco complex formation or complex-regulated calcium influx, suggesting that Gi coupling and BmOrco coupling are the two independent processes in the case of BmOR3 (Lin et al., 2021).
The GPCRs usually direct the recruitment, activation, and scaffolding of the cytoplasmic signaling complexes via two multifunctional adaptor and transducer molecules, β-arrestins 1 and 2, and arrestins also function to activate signaling cascades independently of G-protein activation or mediate receptor desensitization (Lefkowitz and Shenoy, 2005; DeWire et al., 2007). Individual arrestins had been reported to function in both olfactory and visual pathways in Dipteran insects (Merrill et al., 2001) but not in Lepidopteran insects. Recent research reported that bombykal robustly stimulated the recruitment of human α-arrestin-1/2 and B. mori intrinsic arrestin to BmOR3 in HEK293A cells in a concentration-dependent manner, and the arrestin, in turn, regulated BmOR3 internalization (Lin et al., 2021). Bombykal also induced downstream kinase (i.e., ERK, SRC, AKT, and JNK) activation (phosphorylation) through arrestin (Lin et al., 2021). These results confirmed the arrestin-mediated signaling downstream of BmOR3. The knockdown of β-arrestins significantly reduced bombykal-induced calcium influx through BmOR3-BmOR2, which was accompanied by the collapse of the receptor complex, suggesting that the α-arrestins mediate Ca2 + response mainly by regulating the structural and functional integrity of the BmOR3-BmOR2 complex (Lin et al., 2021). The summarized researches show that insect pheromone receptors may both have G-protein and arrestin downstream pathways (Figure 4).
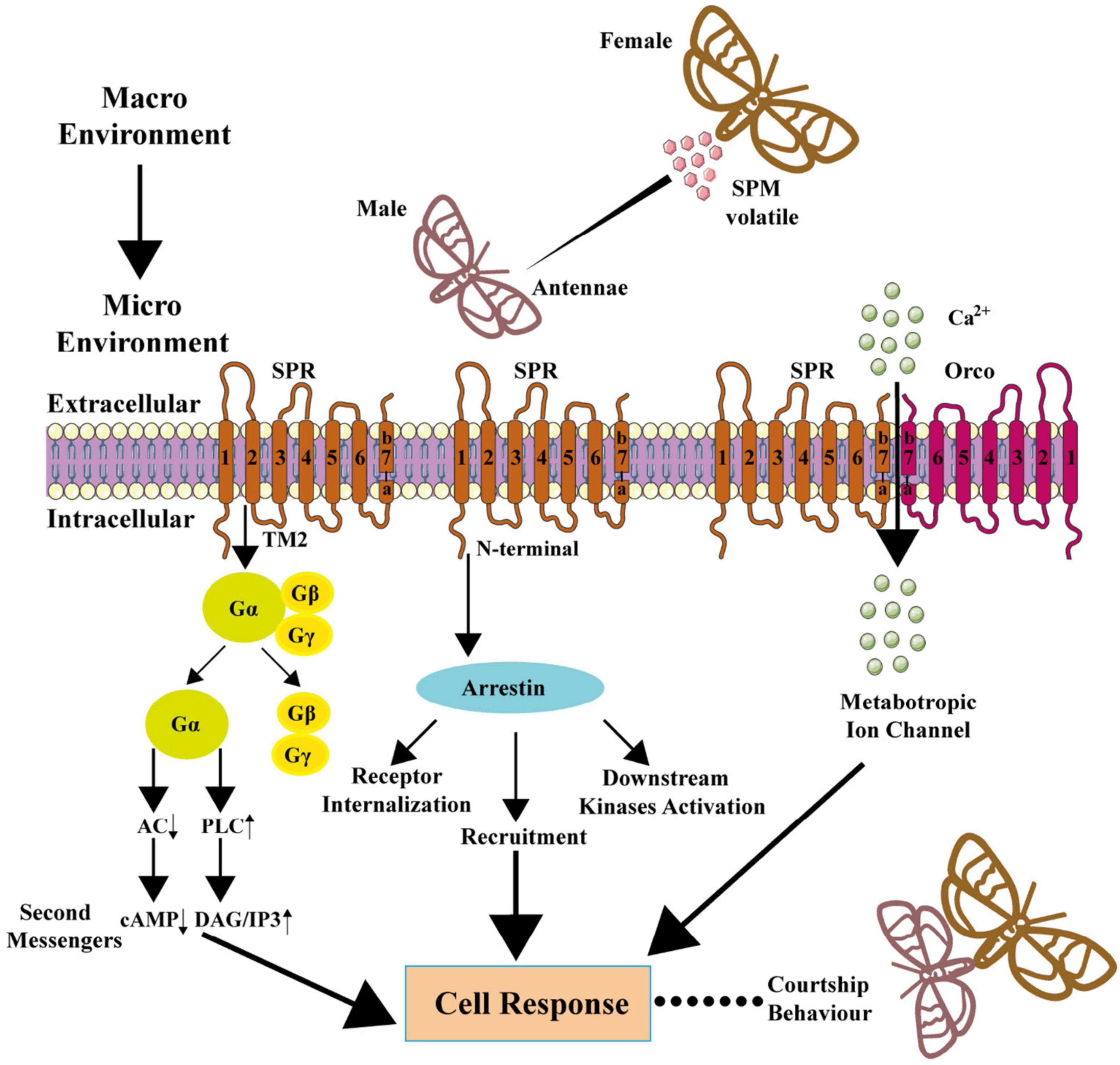
Figure 4. Downstream signaling pathway of SPR and metabotropic channel of SPR-Orco. In macro environment, male insects sense the SPM released by female insects through an olfactory sensory neuron in antennae, and SPM works as an agonist to SPR, and signal transduction leads to ultimate mating behavior.
Metabotropic Ion Channel by the Coupling of Lepidopteran Sex Pheromone Receptors and Orcos
BmOR1 and BmOR3 of B. mori are mutually exclusively expressed in a pair of adjacent pheromone-sensitive neurons of male antennae, and both of which are coexpressed with the highly conserved insect Orco. Heterologous cells coexpressing BmOR2 can greatly enhance the sensitivity of BmOR1 to bombykol, and the current-voltage analysis showed that bombykol activated a non-selective cation channel in oocytes expressing BmOR1 and BmOR2, which is different from Ca2 +-activated Cl– channel through BmGαq, and the non-selective cation channel activity in response to bombykol was also observed when BmOR1 was coexpressed with HvirOR2 or Or83b (Whelan and Goldman, 2001; Nakagawa et al., 2005; Figure 4). As reported in MsexOR1, the PLC/PKC activity is a prerequisite to bombykal-receptor-mediated Ca2 + signals in HEK293 and CHO cells, and it could be hypothesized that MsexOR1 and MsexOrco need to be phosphorylated before they can be gated by bombykal as an ionotropic odor receptor-ion channel complex (Wicher et al., 2017).
In a recent study, the BmOR3-BmOR2 combination elicited a response to bombykal and showed similar channel properties, and the coupling of BmOR3 and BmOR2 forms a cation channel with the detection of calcium influx (Lin et al., 2021). From the view of biophysics, there was also physical interaction between BmOR3 and its Orco BmOR2. On bombykal stimulation, the cytoplasmic parts intracellular loop 1 (ICL1), ICL2, and ICL3 moved away from the N-terminus, while the C-terminal helical kink moved close to the N-terminus of BmOR3. On the contrary, the lower part of loop7a-7b moved away from the N-terminus in BmOR2. ICL1, ICL2, and ICL3 also moved away from the N-terminus of BmOR2 (Lin et al., 2021). The replacement of transmembrane 7 in both receptors confirmed its indispensable role in BmOR3-BmOrco coupling for ionotropic functions (Lin et al., 2021). Several key motifs determine the BmOR3-BmOR2 coupling, the charged residue pair of BmOR3-E403 and BmOrco-K437 represents an important “ionic lock” in regard to mediating BmOR3-BmOrco coupling, and the hydrophobic patches F428/F433 of BmOR3 and zipper Y464/V467/L468/L471 of BmOrco are spatially close to each other, suggesting that they might form hydrophobic interactions (Lin et al., 2021). These reports suggest that the coupling of both SPR and Orco plays a vital role in sex pheromone signal detection and transduction.
There is some evidence that the SPR-Orco channel is metabotropic but not ionotropic. In M. sexta, the agonist induced SPR to activate the Gq-signaling pathway (Nakagawa et al., 2005; Nolte et al., 2013, 2016; Wicher et al., 2017). In B. mori, the SPM elicited G-protein and arrestin pathway, and arrestin knockdown had an effect on ion influx (Lin et al., 2021). Thus, we presume that the SPR-Orco coupling forms a metabotropic channel as before (Fleischer and Krieger, 2018). Both the metabotropic channel and the downstream signaling of SPR may be teamwork in sex pheromone signal transduction (Figure 4).
Future Directions
By using the mature technology in transcriptome sequencing and bioinformatic analyses, more sex pheromone components and SPRs of Lepidopteran insects are needed to be identified and characterized, which will help the development of sex lure technology and its usage in pest control.
Further researches are needed to work out the cryo-EM structure of SPR and the SPR-ligand docking pattern in a biophysical perspective, which will directly facilitate the understanding of sex pheromone signal transduction pathways and provide guidance in the sex lure technology in field pest control.
Author Contributions
JS and XY proposed the title of the review and provided funding required. CY wrote the original draft, performed the data processing, and prepared figures. JC, JL, and YZ contributed to review editing. All authors contributed to the article and approved the submitted version.
Funding
This work was primarily supported by the National Science Fund for Excellent Young Scholars Grant (81822008 to XY), the National Science Fund for Distinguished Young Scholars Grant (81825022 to JS), and the National Natural Science Foundation of China (31671197 to XY and 81773704 to JS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bengtsson, J. M., Trona, F., Montagne, N., Anfora, G., Ignell, R., Witzgall, P., et al. (2012). Putative chemosensory receptors of the codling moth, Cydia pomonella, identified by antennal transcriptome analysis. PLoS One 7:e31620. doi: 10.1371/journal.pone.0031620
Bertoni, M., Kiefer, F., Biasini, M., Bordoli, L., and Schwede, T. (2017). Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci. Rep. 7:10480. doi: 10.1038/s41598-017-09654-8
Bienert, S., Waterhouse, A., de Beer, T. A. P., Tauriello, G., Studer, G., Bordoli, L., et al. (2017). The SWISS-MODEL Repository - new features and functionality. Nucleic Acids Res. 45, D313–D319. doi: 10.1093/nar/gkw1132
Butterwick, J. A., Del Marmol, J., Kim, K. H., Kahlson, M. A., Rogow, J. A., Walz, T., et al. (2018). Cryo-EM structure of the insect olfactory receptor Orco. Nature 560, 447–452. doi: 10.1038/s41586-018-0420-8
Carraher, C., Authier, A., Steinwender, B., and Newcomb, R. D. (2012). Sequence comparisons of odorant receptors among tortricid moths reveal different rates of molecular evolution among family members. PLoS One 7:e38391. doi: 10.1371/journal.pone.0038391
Chang, H., Guo, M., Wang, B., Liu, Y., Dong, S., and Wang, G. (2016). Sensillar expression and responses of olfactory receptors reveal different peripheral coding in two Helicoverpa species using the same pheromone components. Sci. Rep. 6:18742. doi: 10.1038/srep18742
Corcoran, J. A., Jordan, M. D., Thrimawithana, A. H., Crowhurst, R. N., and Newcomb, R. D. (2015). The peripheral olfactory repertoire of the lightbrown apple moth, Epiphyas postvittana. PLoS One 10:e0128596. doi: 10.1371/journal.pone.0128596
De Fouchier, A., Sun, X., Monsempes, C., Mirabeau, O., Jacquin-Joly, E., and Montagné, N. (2015). Evolution of two receptors detecting the same pheromone compound in crop pest moths of the genus Spodoptera. Front. Ecol. Evol. 3:95. doi: 10.3389/fevo.2015.00095
DeWire, S. M., Ahn, S., Lefkowitz, R. J., and Shenoy, S. K. (2007). Beta-arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510.
Dobson, L., Reményi, I., and Tusnády, G. E. (2015). CCTOP: a consensus constrained TOPology prediction web server. Nucleic Acids Res. 43, W408–W412. doi: 10.1093/nar/gkv451
Du, L. X., Liu, Y., Zhang, J., Gao, X. W., Wang, B., and Wang, G. R. (2018). Identification and characterization of chemosensory genes in the antennal transcriptome of Spodoptera exigua. Comp. Biochem. Physiol. Part D Genom. Proteom. 27, 54–65. doi: 10.1016/j.cbd.2018.05.001
Feng, B., Lin, X., Zheng, K., Qian, K., Chang, Y., and Du, Y. (2015). Transcriptome and expression profiling analysis link patterns of gene expression to antennal responses in Spodoptera litura. BMC Genomics 16:269. doi: 10.1186/s12864-015-1375-x
Fleischer, J., and Krieger, J. (2018). Insect pheromone receptors - key elements in sensing intraspecific chemical signals. Front. Cell Neurosci. 12:425. doi: 10.3389/fncel.2018.00425
Forstner, M., Breer, H., and Krieger, J. (2009). A receptor and binding protein interplay in the detection of a distinct pheromone component in the silkmoth Antheraea polyphemus. Int. J. Biol. Sci. 5, 745–757. doi: 10.7150/ijbs.5.745
Garczynski, S. F., and Leal, W. S. (2015). Alternative splicing produces two transcripts encoding female-biased pheromone subfamily receptors in the navel orangeworm, Amyelois transitella. Front. Ecol. Evol. 3:115. doi: 10.3389/fevo.2015.00115
Ge, X., Zhang, T. T., Wang, Z. Y., He, K. L., and Bai, S. X. (2016). Identification of putative chemosensory receptor genes from yellow peach moth Conogethes punctiferalis (Guenée) antennae transcriptome. Sci. Rep. 6, 32636–32648. doi: 10.1038/srep32636
Gonzalez, F., Witzgall, P., and Walker, W. B. (2017). Antennal transcriptomes of three tortricid moths reveal putative conserved chemosensory receptors for social and habitat olfactory cues. Sci. Rep. 7, 41829–41840. doi: 10.1038/srep41829
Grapputo, A., Thrimawithana, A. H., Steinwender, B., and Newcomb, R. D. (2018). Differential gene expression in the evolution of sex pheromone communication in New Zealand’s endemic leafroller moths of the genera Ctenopseustis and Planotortrix. BMC Genomics 19:94. doi: 10.1186/s12864-018-4451-1
Grosse-Wilde, E., Gohl, T., Bouche, E., Breer, H., and Krieger, J. (2007). Candidate pheromone receptors provide the basis for the response of distinct antennal neurons to pheromonal compounds. Eur. J. Neurosci. 25, 2364–2373. doi: 10.1111/j.1460-9568.2007.05512.x
Grosse-Wilde, E., Kuebler, L. S., Bucks, S., Vogel, H., Wicher, D., and Hansson, B. S. (2011). Antennal transcriptome of Manduca sexta. PNAS 108, 7449–7454.
Grosse-Wilde, E., Stieber, R., Forstner, M., Krieger, J., Wicher, D., and Hansson, B. S. (2010). Sex-specific odorant receptors of the tobacco hornworm manduca sexta. Front. Cell Neurosci. 4:22. doi: 10.3389/fncel.2010.00022
Grosse-Wilde, E., Svatos, A., and Krieger, J. (2006). A pheromone-binding protein mediates the bombykol-induced activation of a pheromone receptor in vitro. Chem. Senses 31, 547–555. doi: 10.1093/chemse/bjj059
Gu, Y., Lucas, P., and Rospars, J. (2009). Computational model of the insect pheromone transduction cascade. PLoS Comput. Biol. 5:e1000321. doi: 10.1371/journal.pcbi.1000321
Guex, N., Peitsch, M. C., and Schwede, T. (2019). Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30, S162–S173. doi: 10.1002/elps.200900140
Jia, X. J., Wang, H. X., Yan, Z. G., Zhang, M. Z., Wei, C. H., Qin, X. C., et al. (2016). Antennal transcriptome and differential expression of olfactory genes in the yellow peach moth, Conogethes punctiferalis (Lepidoptera: Crambidae). Sci. Rep. 6, 29067–29082. doi: 10.1038/srep29067
Jiang, N. J., Tang, R., Guo, H., Ning, C., Li, J. C., Wu, H., et al. (2020). Olfactory coding of intra- and interspecific pheromonal messages by the male Mythimna separata in North China. Insect Biochem. Mol. Biol. 125:103439. doi: 10.1016/j.ibmb.2020.103439
Jiang, N. J., Tang, R., Wu, H., Xu, M., Ning, C., Huang, L. Q., et al. (2019). Dissecting sex pheromone communication of Mythimna separata (Walker) in North China from receptor molecules and antennal lobes to behavior. Insect Biochem. Mol. Biol. 111:103176. doi: 10.1016/j.ibmb.2019.103176
Jiang, X. J., Guo, H., Di, C., Yu, S., Zhu, L., Huang, L. Q., et al. (2014). Sequence similarity and functional comparisons of pheromone receptor orthologs in two closely related Helicoverpa species. Insect Biochem. Mol. Biol. 48, 63–74. doi: 10.1016/j.ibmb.2014.02.010
Jordan, M. D., Anderson, A., Begum, D., Carraher, C., Authier, A., Marshall, S. D., et al. (2009). Odorant receptors from the light brown apple moth (Epiphyas postvittana) recognize important volatile compounds produced by plants. Chem. Senses 34, 383–394. doi: 10.1093/chemse/bjp010
Krieger, J., Gondesen, I., Forstner, M., Gohl, T., Dewer, Y., and Breer, H. (2009). HR11 and HR13 receptor-expressing neurons are housed together in pheromone-responsive sensilla trichodea of male Heliothis virescens. Chem. Senses 34, 469–477. doi: 10.1093/chemse/bjp012
Krieger, J., Grosse-Wilde, E., Gohl, T., and Breer, H. (2005). Candidate pheromone receptors of the silkmoth Bombyx mori. Eur. J. Neurosci. 21, 2167–2176.
Krieger, J., Grosse-Wilde, E., Gohl, T., Dewer, Y. M. E., Raming, K., and Breer, H. (2004). Genes encoding candidate pheromone receptors in a moth (Heliothis virescens). PNAS 101, 11845–11850. doi: 10.1073/pnas.0403052101
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Laue, M., Maida, R., and Redkozubov, A. (1997). G-protein activation, identification and immunolocalization in pheromone-sensitive sensilla trichodea of moths. Cell Tissue Res. 288, 149–158. doi: 10.1007/s004410050802
Leal, W. S. (2013). Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58, 373–391. doi: 10.1146/annurev-ento-120811-153635
Leary, G. P., Allen, J. E., Bunger, P. L., Luginbill, J. B., Linn, C. E., Macallister, I. E., et al. (2012). Single mutation to a sex pheromone receptor provides adaptive specificity between closely related moth species. PNAS 109, 14081–14086. doi: 10.1073/pnas.1204661109
Lefkowitz, R. J., and Shenoy, S. K. (2005). Transduction of receptor signals by β-Arrestins. Science 308, 512–517. doi: 10.1126/science.1109237
Legeai, F., Malpel, S., Montagné, N., Monsempes, C., Cousserans, F., Merlin, C., et al. (2011). An expressed Sequence Tag collection from the male antennae of the Noctuid moth Spodoptera littoralis: a resource for olfactory and pheromone detection research. BMC Genomics 12:86. doi: 10.1186/1471-2164-12-86
Li, J., Wang, X., and Zhang, L. (2020). Sex pheromones and olfactory proteins in Antheraea moths: A. pernyi and A. polyphemus (Lepidoptera: Saturniidae). Arch. Insect Biochem. Physiol. 105, 1–10. doi: 10.1002/arch.21729
Li, Z. Q., Cai, X. M., Luo, Z. X., Bian, L., Xin, Z. J., Chu, B., et al. (2018). Comparison of olfactory genes in two ectropis species: emphasis on candidates involved in the detection of Type-II Sex pheromones. Front. Physiol. 9:1602. doi: 10.3389/fphys.2018.01602
Li, Z. Q., Luo, Z. X., Cai, X. M., Bian, L., Xin, Z. J., Liu, Y., et al. (2017). Chemosensory gene families in ectropis grisescens and candidates for detection of Type-II sex pheromones. Front. Physiol. 8:953. doi: 10.3389/fphys.2017.00953
Lin, J. Y., Yang, Z., Yang, C., Du, J. X., Yang, F., Cheng, J., et al. (2021). An ionic lock and a hydrophobic zipper mediate the coupling between an insect pheromone receptor BmOR3 and downstream effectors. J. Biol. Chem. 297:101160. doi: 10.1016/j.jbc.2021.101160
Lin, X., Zhang, Q., Wu, Z., and Du, Y. (2015). Identification and differential expression of a candidate sex pheromone receptor in natural populations of Spodoptera litura. PLoS One 10:e0131407. doi: 10.1371/journal.pone.0131407
Liu, C., Liu, Y., Walker, W. B., Dong, S., and Wang, G. (2013). Identification and functional characterization of sex pheromone receptors in beet armyworm Spodoptera exigua (Hubner). Insect Biochem. Mol. Biol. 43, 747–754. doi: 10.1016/j.ibmb.2013.05.009
Liu, N. Y., Xu, W., Papanicolaou, A., Dong, S. L., and Anderson, A. (2014). Identification and characterization of three chemosensory receptor families in the cotton bollworm Helicoverpa armigera. BMC Genomics 15:597. doi: 10.1186/1471-2164-15-597
Liu, Y., Gu, S., Zhang, Y., Guo, Y., and Wang, G. (2012). Candidate olfaction genes identified within the Helicoverpa armigera antennal transcriptome. PLoS One 7:e48260. doi: 10.1371/journal.pone.0048260
Liu, Y., Liu, C., Lin, K., and Wang, G. (2013). Functional specificity of sex pheromone receptors in the cotton bollworm Helicoverpa armigera. PLoS One 8:e62094. doi: 10.1371/journal.pone.0062094
Liu, Y., Liu, Y., Jiang, X., and Wang, G. (2018). Cloning and functional characterization of three new pheromone receptors from the diamondback moth, Plutella xylostella. J. Insect Physiol. 107, 14–22. doi: 10.1016/j.jinsphys.2018.02.005
Merrill, C. E., Riesgo-Escovar, J., Pitts, R. J., Kafatos, F. C., Carlson, J. R., and Zwiebel, L. J. (2001). Visual arrestins in olfactory pathways of Drosophila and the malaria vector mosquito Anopheles gambiae. PNAS 99, 1633–1638. doi: 10.1073/pnas.022505499
Mitsuno, H., Sakurai, T., Murai, M., Yasuda, T., Kugimiya, S., Ozawa, R., et al. (2008). Identification of receptors of main sex-pheromone components of three Lepidopteran species. Eur. J. Neurosci. 28, 893–902. doi: 10.1111/j.1460-9568.2008.06429.x
Miura, N., Atsumi, S., Tabunoki, H., and Sato, R. (2005). expression and localization of three G protein alpha subunits, Go, Gq, and Gs, in adult antennae of the silkmoth (Bombyx mori). J. Comp. Neurol. 485, 143–152. doi: 10.1002/cne.20488
Miura, N., Nakagawa, T., Tatsuki, S., Touhara, K., and Ishikawa, Y. (2009). A male-specific odorant receptor conserved through the evolution of sex pheromones in Ostrinia moth species. Int. J. Biol. Sci. 5, 319–330. doi: 10.7150/ijbs.5.319
Miura, N., Nakagawa, T., Touhara, K., and Ishikawa, Y. (2010). Broadly and narrowly tuned odorant receptors are involved in female sex pheromone reception in Ostrinia moths. Insect Biochem. Mol. Biol. 40, 64–73. doi: 10.1016/j.ibmb.2009.12.011
Montagne, N., Chertemps, T., Brigaud, I., Francois, A., Francois, M. C., Fouchier, A., et al. (2012). Functional characterization of a sex pheromone receptor in the pest moth Spodoptera littoralis by heterologous expression in Drosophila. Eur. J. Neurosci. 36, 2588–2596. doi: 10.1111/j.1460-9568.2012.08183.x
Nakagawa, T., Sakurai, T., Nishioka, T., and Touhara, K. (2005). Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 307, 1638–1641. doi: 10.1126/science.1106267
Nolte, A., Funk, N. W., Mukunda, L., Gawalek, P., Werckenthin, A., Hansson, B. S., et al. (2013). In situ tip-recordings found no evidence for an Orco-based ionotropic mechanism of pheromone-transduction in Manduca sexta. PLoS One 8:e62648. doi: 10.1371/journal.pone.0062648
Nolte, A., Gawalek, P., Koerte, S., Wei, H., Schumann, R., Werckenthin, A., et al. (2016). No evidence for ionotropic pheromone transduction in the hawkmoth Manduca sexta. PLoS One 11:e0166060. doi: 10.1371/journal.pone.0166060
Patch, H. M., Velarde, R. A., Walden, K. K., and Robertson, H. M. (2009). A candidate pheromone receptor and two odorant receptors of the hawkmoth Manduca sexta. Chem. Senses 34, 305–316. doi: 10.1093/chemse/bjp002
Rojas, V., Jimenez, H., Palma-Millanao, R., Gonzalez-Gonzalez, A., Machuca, J., Godoy, R., et al. (2018). Analysis of the grapevine moth Lobesia botrana antennal transcriptome and expression of odorant-binding and chemosensory proteins. Comp. Biochem. Physiol. Part D 27, 1–12. doi: 10.1016/j.cbd.2018.04.003
Sakurai, T., Nakagawa, T., Mitsuno, H., Mori, H., Endo, Y., Tanoue, S., et al. (2004). Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. PNAS 101, 16653–16658. doi: 10.1073/pnas.0407596101
Steinwender, B., Crowhurst, R. N., Thrimawithana, A. H., and Newcomb, R. D. (2015). Pheromone receptor evolution in the cryptic leafroller species, Ctenopseustis obliquana and C. herana. J. Mol. Evol. 80, 42–56. doi: 10.1007/s00239-014-9650-z
Steinwender, B., Thrimawithana, A. H., Crowhurst, R., and Newcomb, R. D. (2016). Odorant receptors of the New Zealand Endemic leafroller moth species Planotortrix octo and P. excessana. PLoS One 11:e0152147. doi: 10.1371/journal.pone.0152147
Studer, G., Rempfer, C., Waterhouse, A. M., Gumienny, G., Haas, J., and Schwede, T. (2020). QMEANDisCo - distance constraints applied on model quality estimation. Bioinformatics 36, 1765–1771.
Sun, M., Liu, Y., Walker, W. B., Liu, C., Lin, K., Gu, S., et al. (2013). Identification and characterization of pheromone receptors and interplay between receptors and pheromone binding proteins in the diamondback moth, Plutella xyllostella. PLoS One 8:e62098. doi: 10.1371/journal.pone.0062098
Tang, R., Jiang, N. J., Ning, C., Li, G. C., Huang, L. Q., and Wang, C. Z. (2020). The olfactory reception of acetic acid and ionotropic receptors in the Oriental armyworm, Mythimna separata Walker. Insect Biochem. Mol. Biol. 118:103312. doi: 10.1016/j.ibmb.2019.103312
Tian, K., Liu, W., Feng, L. K., Huang, T. Y., Wang, G. R., and Lin, K. J. (2020). Functional characterization of pheromone receptor candidates in codling moth Cydia pomonella (Lepidoptera: Tortricidae). Insect Sci. 28, 445–456. doi: 10.1111/1744-7917.12775
Walker, W. B., Gonzalez, F., Garczynski, S. F., and Witzgall, P. (2016). The chemosensory receptors of codling moth Cydia pomonella-expression in larvae and adults. Sci. Rep. 6, 23518–23534. doi: 10.1038/srep23518
Wang, G., Vasquez, G. M., Schal, C., Zwiebel, L. J., and Gould, F. (2011). Functional characterization of pheromone receptors in the tobacco budworm Heliothis virescens. Insect Mol. Biol. 20, 125–133. doi: 10.1111/j.1365-2583.2010.01045.x
Wang, L., and Anderson, D. J. (2010). Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463, 227–231. doi: 10.1038/nature08678
Wanner, K. W., Nichols, A. S., Allen, J. E., Bunger, P. L., Garczynski, S. F., Linn, C. E., et al. (2010). Sex pheromone receptor specificity in the European corn borer moth, Ostrinia nubilalis. PLoS One 5:e8685. doi: 10.1371/journal.pone.0008685
Waterhouse, A., Bertoni, M., Bienert, S., Studer, G., Tauriello, G., Gumienny, R., et al. (2018). SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303. doi: 10.1093/nar/gky427
Whelan, S., and Goldman, N. (2001). A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 18, 691–699. doi: 10.1093/oxfordjournals.molbev.a003851
Wicher, D., Morinaga, S., Halty-deLeon, L., Funk, N., Hansson, B., Touhara, K., et al. (2017). Identification and characterization of the bombykal receptor in the hawkmoth Manduca sexta. J. Exp. Biol. 220, 1781–1786. doi: 10.1242/jeb.154260
Witzgall, P., Kirsch, P., and Cork, A. (2010). Sex pheromones and their impact on pest management. J. Chem. Ecol. 36, 80–100. doi: 10.1007/s10886-009-9737-y
Wu, Z. N., Chen, X., Du, Y. J., Zhou, J. J., and ZhuGe, Q. C. (2013). Molecular identification and characterization of the Orco orthologue of Spodoptera litura. Insect Sci. 20, 175–182. doi: 10.1111/j.1744-7917.2011.01483.x
Xu, P., Garczynski, S. F., Atungulu, E., Syed, Z., Choo, Y. M., Vidal, D. M., et al. (2012). Moth sex pheromone receptors and deceitful parapheromones. PLoS One 7:e41653. doi: 10.1371/journal.pone.0041653
Xu, W., Papanicolaou, A., Liu, N. Y., Dong, S. L., and Anderson, A. (2015). Chemosensory receptor genes in the Oriental tobacco budworm Helicoverpa assulta. Insect Mol. Biol. 24, 253–263. doi: 10.1111/imb.12153
Yang, K., Ning, C., and Wang, C. Z. (2017). Two single-point mutations shift the ligand selectivity of a pheromone receptor between two closely related moth species. eLife 6:e29100.
Yang, S., Cao, D., Wang, G., and Liu, Y. (2017). Identification of genes involved in chemoreception in Plutella xyllostella by antennal transcriptome analysis. Sci. Rep. 7:11941. doi: 10.1038/s41598-017-11646-7
Yasukochi, Y., Miura, N., Nakano, R., Sahara, K., and Ishikawa, Y. (2011). Sex-linked pheromone receptor genes of the European corn borer, Ostrinia nubilalis, are in tandem arrays. PLoS One 6:e18843. doi: 10.1371/journal.pone.0018843
Zhan, S., Merlin, C., Boore, J. L., and Reppert, S. M. (2011). The monarch butterfly genome yields insights into long-distance migration. Cell 147, 1171–1185. doi: 10.1016/j.cell.2011.09.052
Zhang, D. D., and Lofstedt, C. (2013). Functional evolution of a multigene family: orthologous and paralogous pheromone receptor genes in the turnip moth, Agrotis segetum. PLoS One 8:e77345. doi: 10.1371/journal.pone.0077345
Zhang, D. D., Wang, H. L., Schultze, A., Fross, H., Francke, W., Krieger, J., et al. (2016). Receptor for detection of a Type II sex pheromone in the winter moth Operophtera brumata. Sci. Rep. 6:18576. doi: 10.1038/srep18576
Zhang, D. D., Zhu, K. Y., and Wang, C. Z. (2010). Sequencing and characterization of six cDNAs putatively encoding three pairs of pheromone receptors in two sibling species, Helicoverpa armigera and Helicoverpa assulta. J. Insect Physiol. 56, 586–593. doi: 10.1016/j.jinsphys.2009.12.002
Zhang, J., Yan, S., Liu, Y., Jacquin-Joly, E., Dong, S., and Wang, G. (2015). Identification and functional characterization of sex pheromone receptors in the common cutworm (Spodoptera litura). Chem. Senses 40, 7–16. doi: 10.1093/chemse/bju052
Zhang, S. X., Xu, S. Q., Kong, L. F., Sima, Y. H., and Cui, W. Z. (2009). Cloning of pbp1, or1 and or3 from wild silkworm Bombyx mandarina and evolutional analysis with the orthologous genes of domesticated silkworm Bombyx mori. Acta Entomol. Sinica 52, 917–922.
Zhang, Y. N., Du, L. X., Xu, J. W., Wang, B., Zhang, X. Q., Yan, Q., et al. (2019). Functional characterization of four sex pheromone receptors in the newly discovered maize pest Athetis lepigone. J. Insect Physiol. 113, 59–66. doi: 10.1016/j.jinsphys.2018.08.009
Zhang, Y. N., Jin, J. Y., Jin, R., Xia, Y. H., Zhou, J. J., Deng, J. Y., et al. (2013). Differential expression patterns in chemosensory and non-chemosensory tissues of putative chemosensory genes identified by transcriptome analysis of insect pest the purple stem borer Sesamia inferens (Walker). PLoS One 8:e69715. doi: 10.1371/journal.pone.0069715
Zhang, Y.N., Ma, J. F., Sun, L., Dong, Z. P., Li, Z. Q., Zhu, X. Y., et al. (2016). Molecular identification and sex distribution of two chemosensory receptor families in Athetis lepigone by antennal transcriptome analysis. J. Asia-Pac Entomol. 19, 571–580. doi: 10.1016/j.aspen.2016.05.009
Zhang, Y. N., Qian, J. L., Xu, J. W., Zhu, X. Y., Li, M. Y., Xu, X. X., et al. (2018). Identification of chemosensory genes based on the transcriptomic analysis of six different chemosensory organs in Spodoptera exigua. Front. Physiol. 9:432. doi: 10.3389/fphys.2018.00432
Keywords: sex pheromone receptor, structure, signal transduction, Lepidopteran insects, evolution, function
Citation: Yang C, Cheng J, Lin J, Zheng Y, Yu X and Sun J (2022) Sex Pheromone Receptors of Lepidopteran Insects. Front. Ecol. Evol. 10:797287. doi: 10.3389/fevo.2022.797287
Received: 18 October 2021; Accepted: 04 January 2022;
Published: 15 February 2022.
Edited by:
Rui Tang, Institute of Zoology, Guangdong Academy of Sciences, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Cong Huang, Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences (CAAS), ChinaZhongzhen Wu, Zhongkai University of Agriculture and Engineering, China
Copyright © 2022 Yang, Cheng, Lin, Zheng, Yu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Yu, eXV4aWFvQHNkdS5lZHUuY24=; Jinpeng Sun, c3VuamlucGVuZ0BzZHUuZWR1LmNu
 Chan Yang
Chan Yang Jie Cheng2
Jie Cheng2 Xiao Yu
Xiao Yu Jinpeng Sun
Jinpeng Sun