- 1Phylogenomics Lab, Universidade de Vigo, Vigo, Spain
- 2Institute of Pathology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 3BIH Bioportal Single Cells, Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Berlin, Germany
- 4CIBIO/InBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto Vairão, Vairão, Portugal
- 5The Palaeogenomics and Bio-Archaeology Research Network, Research Laboratory for Archaeology and History of Art, University of Oxford, Oxford, United Kingdom
- 6Independent Researcher, Oxford, United Kingdom
Seven species of house geckos occur across the scattered islands of the Indian Ocean. Two of these, Hemidactylus frenatus and H. parvimaculatus are both widespread and possess distribution profiles that suggest pre-European, or perhaps natural dispersal to some islands. Of these, only H. frenatus currently has sufficient molecular data to begin exploring dispersal patterns. This species is one of the most successful reptile colonists, as demonstrated by its global, pantropical distribution. While in some areas, such as Australia and continental South America, its dispersal patterns are both recent and well-known, early historical records of Hemidactylus in the Indian Ocean islands suggest earlier and/or potentially non-human-mediated dispersals. Here, we reviewed the historical literature and combined those reports with an assessment of mitochondrial DNA diversity of a global sampling of H. frenatus samples that included modern and museum specimens. Our results corroborate previous studies and demonstrate the relatively high diversity within this species’ native range in Southeast Asia. In addition, the phylogenetic analysis suggests both a potential cryptic species complex, as well as global geographic structuring of different H. frenatus mitochondrial lineages. This has important implications for many comparative studies of this complex. Frequent and ongoing dispersals and colonizations complicate the identification of potentially older migration patterns. Further assessments including additional samples and analyses of additional genetic markers are necessary to disentangle older from more recent dispersals within this intriguing species.
Introduction
As the global human population has grown, so have the number of plant and animal species that we have collectively translocated beyond the boundaries of their natural distributions. These range expansions, combined with population and range contractions of many endemic native species have led to a generalized pattern of global biotic homogenization (Olden et al., 2004), and this phenomenon has accelerated with the commensurate increase in international shipping, human travel, and cargo movement (Mooney and Cleland, 2001). Importantly, determining whether a species’s distribution has been influenced by people is not always straightforward since long-distance, non-human-mediated dispersal has been demonstrated in a wide range of species (de Queiroz, 2005). The majority of studies that have focused on range expansions resulting from human-mediated transport have often done so with a view toward using these taxa as a proxy for understanding human migration pathways and trade networks. As a result, they have focused on those specific taxa that have been most closely interwoven into human societies, and on which people have become most reliant.
In addition to the common domestic animals (Frantz et al., 2019; Perri et al., 2021) and plants (da Fonseca et al., 2015), as well as animals introduced as game, numerous additional commensal species have also dispersed as a result of their association with people but in a less deliberate fashion. Classic animal examples include rats, mice and house shrews (Suncus murinus), all of which have been the subject of genetic (Duplantier et al., 2002; Tollenaere et al., 2010; Aplin et al., 2011; Brouat et al., 2014; Ohdachi et al., 2016) and archeological (Cucchi et al., 2020) studies investigating their dispersal beyond their native distributions, including across the Western Indian Ocean (Cheke, 2010). A comprehensive survey of taxa whose current distributions have been manipulated by people in Island Southeast Asia, for example, revealed scores of animal species that have been transported between islands over the past 10,000 years (Heinsohn, 2003). Likewise, movements of both wild, commensal and domesticated animals and plants across and around the Indian Ocean from Asia to East Africa and Madagascar have also been reviewed (Boivin et al., 2013).
Here, we explored the patterns of transport and dispersal using commensal geckos, a somewhat neglected species in animal movement studies, despite the fact that they are widespread and easily transported (Jones et al., 2013). Although there are more than 165 species within the genus Hemidactylus (Agarwal et al., 2021), the majority are restricted to small-ranging habitats in South Asia. Approximately 10 species, including the house geckos H. mabouia from Africa and the Asian H. frenatus, have achieved intercontinental distributions (Weterings and Vetter, 2018), while H. parvimaculatus reached many Indian Ocean islands. However, because the review by Weterings and Vetter (2018) regarding invasive Hemidactylus did not re-assess older identifications, the Indian Ocean distributions are not reliable for ‘H. mabouia’ or ‘H. brookii’ (= H. parvimaculatus).
Hemidactylus frenatus is the most widely introduced gecko in the world (Weterings and Vetter, 2018), and though native to tropical Asia and the Indian subcontinent, it has been identified on islands and continents spanning the globe (McKay and Milenkaya, 2020). Adaptations that permit this species to establish populations in new locations include: the females’ ability to store viable sperm for up to a year (Yamamoto and Ota, 2006); their hardshelled eggs’ resistance to immersion in seawater (Hsu et al., 2021) and the capability to reproduce year-round in tropical zones (McKay and Phillips, 2012). Furthermore, they lay eggs in crevices in objects, and are therefore often accidentally transported in cargo or baggage (Weterings and Vetter, 2018, ASC pers. obs.). These features also increase their potential to remain undetected and undamaged in transit in the past on both ancient sailing vessels and on modern shipping containers. None of these characters alone, however, explain why this species, and others such as H. mabouia and H. parvimaculatus, but not their numerous congeners, have been such successful colonists.
While the spread of H. frenatus throughout the Pacific was first described in association with troop and supply movements during World War II (Case and Bolger, 1991), this species has also been suggested as a proxy to understand older human movements, at least across the Indian Ocean (Cheke, 2010; Boivin et al., 2013). The observation that distinct haplogroups coexist across some of these islands (Rocha et al., 2010), together with their curious distribution on remote atolls suggests they may have arrived in a series of different events (Cheke, 2010). Given the modern widespread distribution of gecko species within tropical and subtropical ecotones, commensal geckos in general, and H. frenatus specifically, represent excellent models to investigate the unintentional human-mediated movement of a widespread commensal reptile. One of the problems hindering the genetic approach to understanding the spread of geckos is the use of different markers in independent studies, which precludes efforts to comprehensively and simultaneously analyze all the available data.
Here, in order to characterize the distribution of specific house gecko populations, we applied two different approaches. First, we established both the time frame and geographic distribution of house geckos in the Indian Ocean by reviewing the historic literature and early descriptions and collections of geckos in the region. We then extracted DNA and sequenced a 463 bp fragment of the mitochondrial cytochrome b gene from 111 museum and modern specimens of the most widespread and genetically diverse house gecko species, derived from over 70 locations representing 36 countries (Supplementary Table 1). Using the obtained sequence alignment, we constructed phylogenetic trees and assessed the geographic partitioning of the individuals assigned to well-supported clades. These combined approaches, including the increased resolution afforded by genetic signatures, allowed us to identify specific populations and lineages of Asian house geckos across their global and Indian Ocean distributions. These data also allowed us to discuss the results in light of human-mediated movement of taxa writ large.
Results and Discussion
Historical Accounts of the Distribution of Commensal Geckos in the Indian Ocean
In addition to H. frenatus, six other species of house geckos, mostly small gray or brown, largely facultatively anthropophilic animals, inhabit the historically uninhabited remote islands of the Indian Ocean. None of the volcanic islands of Mascarenes and Comoros, nor the continental fragments (Madagascar and the granitic Seychelles), much less the numerous atolls of the western Indian Ocean (Figure 3), have been connected to any continent for upward of 60 million years, and therefore either natural or human-mediated over water dispersal are necessary to explain their distribution.
Of the seven, two, Hemidactylus platycephalus and the H. mabouia-mercatorius complex are Afro-Malagasy species (Rocha et al., 2010), which historically were merged within the polyphyletic umbrella species ‘H. mabouia’ (Louette et al., 2004; Rocha et al., 2010; Agarwal et al., 2021). Of these, only H. mercatorius seems to have spread beyond the immediate area (Madagascar, Comoros, Aldabra group, Farquhars), to the granitic Seychelles and, unexpectedly, to Platte, but only recently via human agency (Gerlach, 2007; Rocha et al., 2009a). For example, although Boulenger (1909) reported “H. mabuia” on Mahé in 1905 (specimen confirmed by Cheke (1984) as H. mercatorius), it probably did not establish a population since it was not detected again there until 1995, and in 2002 on Frégate (Gerlach, 2007). Platte, a low coral island, is infrequently visited by zoologists, and it is possible that H. mercatorius may have been long established. H. parvimaculatus was initially misidentified as H. mercatorius (Vinson and Vinson, 1969) in the Mascarenes, but true mabouia/mercatorius was absent in the Mascarenes (Cheke and Hume, 2008; Cole, 2008) until discovered in Réunion in 2010, with molecular data suggesting their recent introduction from either the Comoros, the Seychelles or the East African coast (Sanchez et al., 2012), the most likely of these being Mayotte (Comoros), also still a French territory.
Of the other five species, one is Pacific, and four are Asian in origin, but with differing dispersal histories. Lepidodactylus lugubris is a parthenogenic Pacific species with apparently endemic Indian Ocean clones (Ineich, 1999), which suggests a long-established natural dispersal via drift. This species is also the least associated with humans, and only occurs on the most easterly of the islands including Rodrigues, the Chagos group, and Coëtivy (Gerlach, 2007; Cheke and Hume, 2008; Cole, 2009). Boulenger (1909) reported specimens of L. lugubris collected in 1905 from both Mahé and Praslin in the granitic Seychelles, but it has not been reported since (Gerlach, 2007), and no specimens exist in the United Kingdom Natural History Museum collections (Cheke, 1984), so this record may have been made in error. L. lugubris does occur sympatrically in houses in Rodrigues with three other gecko species (ASC pers. obs.), and it has also recently been found on St. Brandon/Cargados Carajos (Nik Cole, pers. comm. to ASC, 2020), directly in the path of the South Equatorial Current (New et al., 2005).
Parthenogenic Hemiphyllodactylus typus, only found in the Mascarenes, is not usually associated with buildings (Grégory et al., 2007) and was first reported in 1948 (Vinson and Vinson, 1969; Deso et al., 2020). Two studies (Grégory et al., 2007; Deso et al., 2020) however, suggested that given the difficulty in observing this species, it may have escaped notice and be native in the Mascarenes, especially since it appears to favor the native forest habitat. This narrative would require oceanographically difficult cross-equatorial drifting. Another Asian gecko species, Gehyra mutilata, is widespread in the Indian Ocean, but lacks sufficient genetic variation in this region to identify its geographical origins (Rocha et al., 2009b). G. mutilata is strictly commensal and is unlikely to have survived on islands where the human presence was only fleeting. Therefore, even if cross-ocean mariners introduced the species unintentionally, a temporary human stopover would not be sufficient for the species to maintain a presence. In fact, this species failed to establish a population on Aldabra until there was a permanent human presence at a research station (Cheke, 1984; Gerlach, 2007).
The final two species, Hemidactylus frenatus and H. parvimaculatus, are both facultative commensals, with distributions, histories and genetic signatures that warrant further investigation. Both of these species, common in southern India and Ceylon/Sri Lanka (Daniel, 2002; Das and de Silva, 2005), have long-term established populations in the Maldives where H. frenatus was likely introduced on multiple occasions (Agarwal et al., 2019). When the first faunal collections were made in the Maldives by the Stanley Gardiner expedition of 1899-1900, H. frenatus was widespread throughout, but parvimaculatus (as ‘H. gleadovii’) was only collected on Hulule, Male Atoll (Laidlaw, 1902). People from Sri Lanka are known to have settled on numerous occasions, so the presence of H. parvimaculatus is not unexpected, and H. frenatus could also have arrived from long-standing trading (Maloney, 1980; Litster, 2016) with ports to the east.
The Maldives are long-inhabited islands, possibly from 1400 BCE (Maloney, 1980; Jaufar, 2019), though Litster (2016, 2020) is more cautious about BCE dates, suggesting probable early stopover use, if not settlement. The oldest confirmed direct radiocarbon date is remarkably late, 249–393 CE, evidently after the cowry-shell (Monetaria moneta) trade for currency was well-established. While they could be the source of gecko populations elsewhere, it is the presence of geckos on further-flung islands first encountered by humans more recently, in the last 1,500 years, mostly the last 500, that concern us here.
Hemidactylus parvimaculatus
Hemidactylus parvimaculatus is part of the Indo-Asian H. brookii complex, and was only recognized as a separate species in 2010; the natural range of the species being the island of Sri Lanka and also southern India (Bauer et al., 2010). Although not reported in Mauritius until 1818 (Cheke and Hume, 2008) (as ‘H. brookii’), and still absent from the granitic Seychelles (Gerlach, 2007), it was collected on then uninhabited isolated sand cay of Coëtivy in 1803. Coëtivy, discovered in 1771 (Lionnet, 1972), was first settled in 1811 (de Froberville, 1848), though it had been visited on various occasions by Seychellois and French/Mauritians (Toussaint, 1967) seeking free coconuts and turtles. This specimen was illustrated in a remarkable watercolor of seven geckos by Baudin expedition artist Charles-Alexandre Lesueur (Collection Lesueur, Muséum du Havre MS 78-115; reproduced in Cheke (2009); Figure 1); the species was also collected in 1905 on the remote atoll of Desroches in the Amirantes (Boulenger, 1909; as H. brookii), and was still present in 1981 (Gardner, 1986).
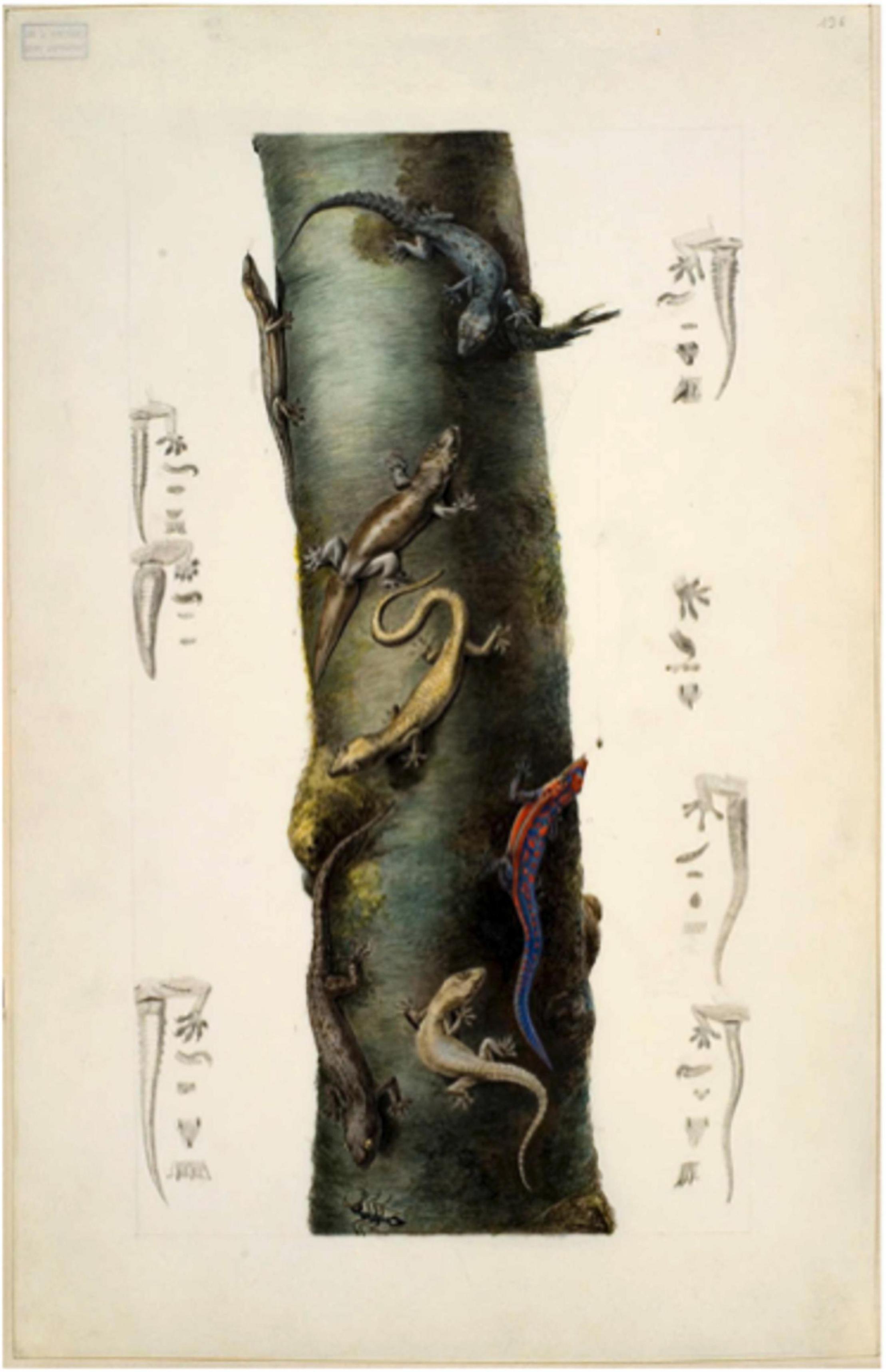
Figure 1. An image of seven geckos drawn by Charles Alexandre Lesueur in 1803. The Hemidactylus parvimaculatus from Coëtivy is bottom right (Cheke, 2009). Although it is a female without preano-femoral pores, the pattern of tubercles and the undertail scalation detailed in the side-sketch closely match photographs and details for parvimaculatus in Mahony’s (2011) review of brookii group geckos.
Its presence on these isolated low islands is anomalous, and is unlikely to result from natural drift (it would be a unique cross-equatorial case of sea drift in the Indian Ocean). It is also unlikely to have been brought from Mauritius, whence the Seychelles and their outer islands were settled, as it would have shown up on the much more populated main granitic islands, where it is still absent (Gerlach, 2007; Rocha et al., 2009a). It is therefore possible that it arrived via Austronesian cross-ocean mariners traveling via Sri Lanka or the Maldives - the species does not occur in these travelers’ source areas (Sumatra, Borneo). The species was not found on Desroches in 2005 despite targeted searches (Rocha et al., 2009a) - only frenatus, absent in 1905, (Boulenger, 1909 contra Gerlach, 2007) but present in 1981 (Cheke, 1984; Gardner, 1986) was identified, nor has it been rediscovered on Coëtivy (Cheke, 1984; Gardner, 1986). A more likely source is the Maldives: Maldivian folklore recounts tales of boats lost at sea ending up in remote islands, so, although the islands are not specifically named (apart from Chagos; Romero-Frías, 1999; Romero-Frias, 2012), this could be the source of H. parvimaculatus on Desroches and Coëtivy.
One story, Hoïïavai (= Chagos in Maldivian; Litster, 2016), was based on a 17th century BCE case of a fishing vessel wrecked in the Chagos, and its crew rescued to tell the tale (Romero-Frias, 2012). Although some Portuguese ships had been wrecked on the northern Chagos banks in the mid-late 16th century, they were traders heading for the East Indies and back (Wenban-Smith and Carter, 2016), so would have been unlikely to have carried parvimaculatus. The same applies to the first known deliberate landing on Egmont in 1605 by an English party traveling east (Wenban-Smith and Carter, 2016). There is no evidence of any European landing on Diego Garcia until much later. H. parvimaculatus also occurs on Moheli and Anjouan (Comoros) (Vences et al., 2004; Rocha et al., 2005; Hawlitschek et al., 2011), and all three Mascarenes (Vinson and Vinson, 1969; as ‘H. mercatorius’; Vences et al., 2004), but not Madagascar (Glaw and Vences, 2007).
Two clades are present in the Mascarenes (Vences et al., 2004; Rocha et al., 2005), and one extends to the Comoros, potentially indicating invasion events from different source populations. Although Austronesian and subsequent pre-European cross-ocean trading from c.800 BCE (Cheke, 2010; Boivin et al., 2013) is a possible source of the Comorian clade (with back-spread to Réunion later), a post-European origin of both clades is probably more likely since the Comoros was formerly administered colonially from Réunion. There is no reason to suppose that Hemidactylus spp. in the Mascarenes arrived other than accidental transport post-European contact since there is no evidence of pre-European landings in this group (Cheke and Hume, 2008; Cheke et al., 2017).
Hemidactylus frenatus
The most complex and interesting case is that of the common house gecko Hemidactylus frenatus. Although its almost universal presence on Indian Ocean islands suggests a generalized, probably anthropogenic distribution, there are numerous anomalies within this apparently uniform pattern. Rocha et al.’s (2010) statement that this species is ‘present throughout Indian Ocean islands without any signs of geographical structure’ overlooked the fact that some structure is evident in both this and previous papers (Vences et al., 2004; Rocha et al., 2005) as discussed below.
House geckos tend only to be reported by naturalists, and not by mariners or explorers, so it is unsurprising that the first Indian Ocean record is not until c.1770 when Philibert Commerson’s artist Paul Jossigny drew a Hemidactylus frenatus from Mauritius; the previously unpublished image is preserved in the Muséum National d’Histoire Naturelle in Paris (Cheke and Hume, 2008), reproduced by Bour (2015); Figure 2. A report (Hoffstetter, 1946) identifying some gecko subfossils as H. frenatus was made in error; the bones were those of the osteologically similar endemic gecko Cyrtodactylus (now Nactus) serpensinsula (Arnold, 2009), extinct on the Mauritian mainland, and only discovered alive on offshore islets after Hoffstetter’s study. Despite intensive fossil collection across different Indian Ocean islands (Aldabra, Reunion, Mauritius, Madagascar and Seychelles), where other gekkonid species have been identified (Cheke and Hume, 2008; Hume, 2004; Hume et al., 2018) there are no known subfossils of Hemidactylus spp. in the Indian Ocean, suggesting that a natural and old (pre-human) colonization is unlikely.
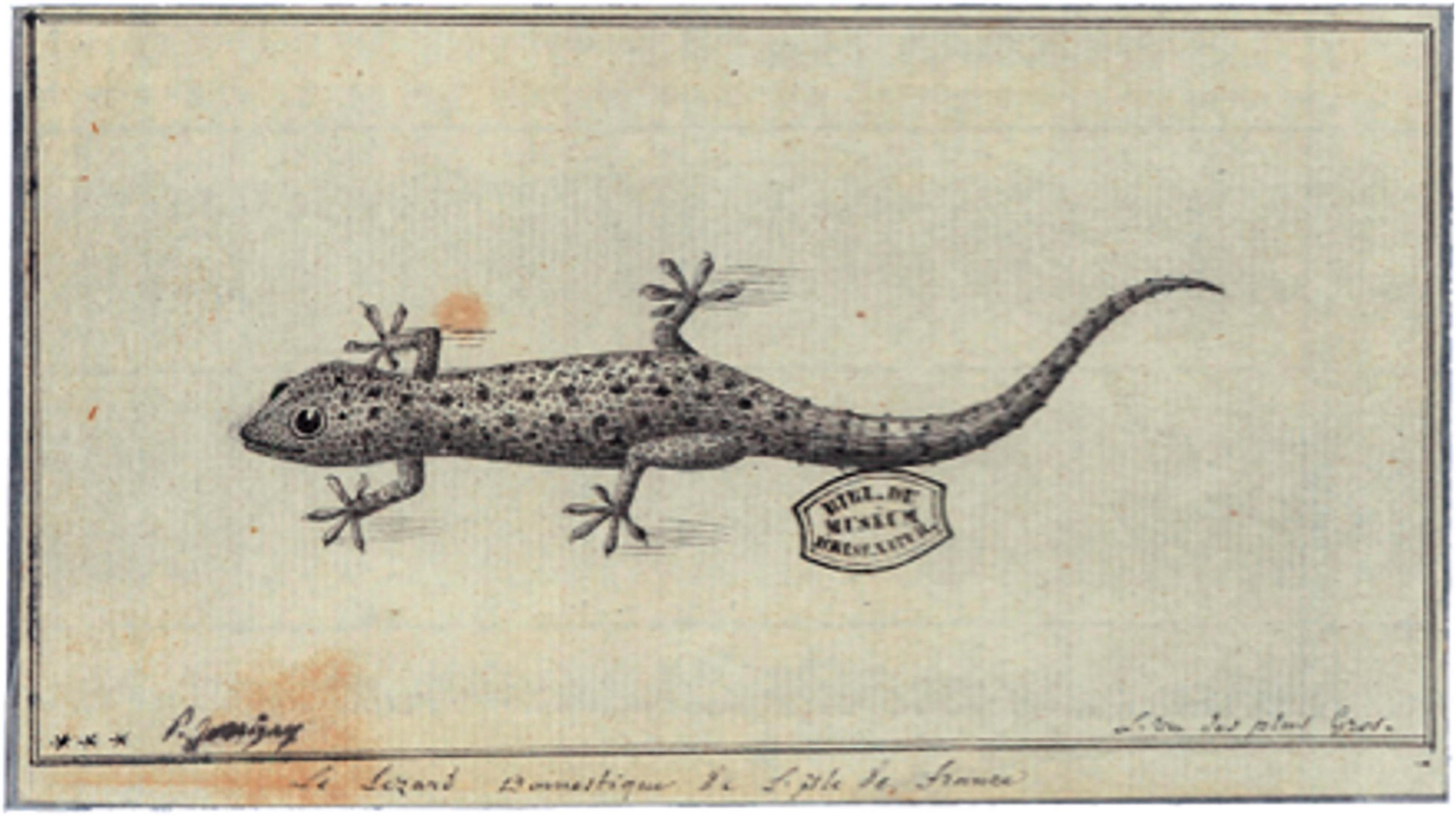
Figure 2. An image of Hemidactylus frenatus, Mauritius. Sketch by Paul Philippe Sanguin de Jossigny c.1770 (from Bour (2015)).

Figure 3. (Top) A map showing the location of modern and museum samples that yielded sufficient DNA to be included in this study. The colors on the map mirror those of the clades on the phylogenetic tree depicting the relationships of the Cytochrome b lineages. The light gray shading denotes the modern distribution of H. frenatus retrieved from GBIF records and converted into polygons (GBIF.org; 29 November 2021- GBIF Occurrence download https://doi.org/10.15468/dl.s8w3sw). (Bottom left) A schematic representation of the Bayesian consensus tree (full tree in Supplementary Figure 1). Branches leading to the outgroups are not shown and posterior probabilities are shown for the main branches only. Tips are labeled with the different localities where samples from each lineage originate, and are color-coded to match the distribution maps. (Bottom Right) An inset depicting the relevant locations across the Western Indian Ocean discussed in the main text. Location acronyms are as follows: Reunion (Re); Mauritius (Ma), Rodrigues (Ro), Peros Baños (PB), African Banks (AB), Rémire (Rm), Poivre (PV), Desroches (DR), Alphonse (Aph), Desnoeufs (Des), Farquars (Fq), Aldabra (AD), Cosmoledo (CM) and Astove (AT). St. Brandon group including Sírene and Establishment (= Île Raphael).
The interesting aspects of frenatus are its curious distribution on outlying atolls and sand cays of the Seychelles (Figure 3) – the species is widespread on these islands which have only small agricultural (coconut) or, more recently, tourist-related settlements. Despite this, the species was absent on the much more populated, visited and biologically known granitic islands until the 1990s (Gerlach, 2007), barring a doubtful record from “the Seychelles” (island unspecified) in 1863 (Peters and von der Decken, 1869).
H. frenatus occurs, or occurred, on many low islands and atolls visited by the 1905 Stanley Gardiner/Percy Sladen Trust expedition (Boulenger, 1909; Cheke, 1984). There are no earlier records since, other than Rémire (Amirantes; collection in 1882; Günther, 1884), no-one had previously collected lizards on these islands. The expedition found them on Bird (central Seychelles), Poivre, Rémire and Desroches (Amirantes), Sirène and ‘Establishment’ [ = Raphael] (St. Brandon), Diego Garcia, Salomon group, Peros Banhos group and possibly Egmont (Chagos), but none were identified on the granitic islands. This species has subsequently been found on several other low islands: Platte, Alphonse, Desnoeufs, African Banks (Cheke, 1984; Gerlach, 2007), Agalega (Cheke and Lawley, 1983; Webster and Cadinouche, 2013), and belatedly reconfirmed on St. Brandon (Nik Cole, pers. comm. 2020). The species was, and remains (Cheke, 1984; Gerlach, 2007), absent on more westerly atolls closer to Madagascar, where H. mercatorius was, and is, present (Farquhars, Aldabra, Cosmoledo, Astove) (Boulenger, 1909), and where the currents favor transport northwest from Madagascar, and at times effectively north/north-east via the SECC (Peng et al., 2015; Hawlitschek et al., 2017).
While commonly found in houses, H. frenatus is found living in rocks and trees on islands inhabited only by seabirds in the Amirantes’ (Gardner, 1986), leading him to agree with Cheke (1984) that this distribution was due to natural sea-drift from the east. However, that does not explain why the drift missed the granitic islands (but hit Bird further north), and carried the geckos further west to the Amirantes. The fullest flow of the South Equatorial Current runs south of the Seychelles Bank (New et al., 2005), which might have impacted the Amirantes more than the granitics, which would also be ‘protected’ (in a sense) by the shallow bank diverting the flow around it. Unlike many of the atolls, the granitic islands have other native lizards including geckos, but pre-existing reptiles have never been known to prevent its establishment elsewhere, and H. frenatus is known to aggressively out compete other similarly sized geckos (e.g., Cole et al., 2005; Dame and Petren, 2006; Newbery and Jones, 2007; Csurhes and Markula, 2009).
Hemidactylus frenatus is also widespread in Madagascar (Glaw and Vences, 2007) and is present on all four Comoro islands (Louette et al., 2004; Hawlitschek et al., 2011). Unlike the oceanic high islands and atolls (Maldives excepted), these have been inhabited for well over a millennium (Crowther et al., 2016; Anderson et al., 2018b), but reptile collections, apart from one Malagasy chameleon, only date from the mid-19th century BCE (Andriamialisoa and Langrand, 2003; Hawlitschek et al., 2011). Hence, there is no historical basis for insight into the means or date of arrival of the species there. Some consider three, or all four, species of Hemidactylus in the Comoros to have been introduced (Vences et al., 2004; Hawlitschek et al., 2011) but given the distribution and habitat preferences (Hawlitschek et al., 2011, 2017), the evidence for mercatorius and especially platycephalus remains ambiguous.
In summary, from the seven largely commensal house gecko species that today inhabit Indian Ocean islands, only the early records of both H. parvimaculatus and H. frenatus across some isolated islands suggest the possibility of early (pre-European) colonizations or even natural dispersals. Of these, H. frenatus is undoubtedly the widest distributed and most abundant across the Western Indian Ocean (and globally), and we therefore tried to gather a comprehensive widespread set of samples that allowed for an exploration of the geographic distribution of its genetic variation, and possibly shed some light into this species’s (early or modern) dispersal patterns.
Molecular Evidence for Global Hemidactylus frenatus Dispersal and Distribution
Several recent studies have attempted to resolve colonization patterns across parts of the range of H. frenatus using mtDNA sequences. Vences et al. (2004) analyzed partial 16S rRNA sequences from individuals from Madagascar, Grand Comoro, the Andaman islands, Mauritius, Rodrigues and Sri Lanka and considered this to show “low genetic differentiation … with no recognizable phylogeographical structure, indicating recent colonization or introductions.” Rocha et al. (2005) focused on the Comoro islands, and likewise, did not identify any notable structure with the same 16S rRNA marker.
Using partial 12S rRNA and Cytochrome b (CYTB) gene sequences to examine the origin of South American populations, Torres-Carvajal (2015) found no variation across Ecuador, Colombia, Hawaii and Papua New Guinea, although these were highly distinct from lineages in Myanmar/Burma and India. Tonione et al. (2011) also identified high diversity within the tiny island of Moorea, French Polynesia, based on Cytochrome Oxidase 1 (CO1) sequences, and Agarwal et al. (2019) determined the probable origin of H. frenatus from the Maldive Islands to be from Southeast Asia. Both these latter studies proposed multiple introductions, and the need for improved sampling of potential source populations to unravel colonization patterns.
At least five lineages have now been identified within H. frenatus, and geographic structure is present. In addition to the Indian and Sri Lankan lineages, the worldwide (Indian, Pacific and Atlantic Ocean islands) samples analyzed here fit clearly into three well-supported clades. One (red in Figure 3) is present in both Myanmar and the Western Indian Ocean (WIO) islands of Madagascar and Comoros (Moheli), another (green) is found both in southeast Asia (Vanuatu, Philippines, Malaysia, Myanmar) as well as across some WIO islands (Diego Garcia, the Seychelles -Mahé- and Madagascar) and also in the Atlantic Ocean island of St. Helena. The third (yellow) is also widespread across the Pacific and Indian Ocean islands.
Since we sequenced samples from the main 16S rRNA haplogroups in the Western Indian Ocean, we were able to match these three lineages of CYTB to the different haplogroups described in Rocha et al. (2010). Although structure was, unsurprisingly, previously less obvious using the more conserved locus than with CYTB, these three lineages are also reflected in 16S data. In this way, the yellow clade corresponds to 16S haplotypes H4-H7 and H10, the red clade to Comoros and Madagascar H8 and H9, and the green clade, to remaining haplotypes from Mahé (represented by H2 - sample 2MA21).
We did not sample additional South American localities, but extrapolating from Torres-Carvajal (2015), South American localities from Colombia to Ecuador (including Galapagos) all belong to the yellow lineage, which is further present also at least in Myanmar (Tonione et al., 2011). In addition, though not all lineages can be unambiguously associated with the ones in Agarwal et al. (2019), some correspondence is evident (e.g., lineage A from (Agarwal et al., 2019) with the blue lineage here, both solely in Sri Lanka). The remaining lineages are not comparable, highlighting the diversity harbored by this taxa, especially in Southeast Asia.
Given this, and the number of lineages identified in other studies with other mitochondrial loci (e.g., Tonione et al., 2011), it seems likely that additional lineages remain unidentified from Southeast Asia. With highly divergent lineages present in India and Sri Lanka, supporting their Indian origin (Bansal and Karanth, 2010), at least three of the lineages spread eastward and westward, giving rise to their modern global distribution. Contrary to its colonization across continental South America, which seems to have been recent and involved mostly a single lineage (Case and Bolger, 1991; Case et al., 1994; Torres-Carvajal, 2015), Tonione et al. (2011) identified three lineages within Moorea, a small island in French Polynesia, and similarly, Western Indian Ocean islands overall currently harbor at least three different lineages of this species.
As with many other widespread species of Hemidactylus assessed with genetic methods, including H. mabouia (Agarwal et al., 2021) and H. fasciatus (Wagner et al., 2014), H. frenatus appears to be a species complex. Variation between the samples from mainland India and Sri Lanka and the island populations ranges up to 14 and 11%, respectively, which is as high as the divergence between pairs of reptile (Harris, 2002) and mammalian (Allen et al., 2020) species as measured using CYTB sequences. Variation between the main lineages within the Indian Ocean islands ranges between 7.0 and 8.5%, which in some small archipelagos such as Diego Garcia (and in the Seychelles) co-occur, highlighting the intricacy of the phylogeography of H. frenatus.
These current patterns highlight both the high levels of diversity, particularly within the native range, and suggest a high number of colonization events required to explain their current distribution. All five primary lineages are currently found in India, Sri Lanka and/or Myanmar, but without a comprehensive sampling across this area and an increased number of markers with various levels of resolution, the timings of the different Western Indian Ocean island colonizations remain unknown. While there is yet no clear evidence for natural and old colonization events (e.g., differentiated lineages endemic from certain islands or groups), it does appears is possible that this species has been transported in multiple waves, perhaps initially in pre-European times, but that these few early dispersals are now predominantly obscured by a much greater number of recent and ongoing translocations associated with modern transportation networks.
The patterns revealed here related to the three primary lineages identified allow for the development of provisional and testable hypotheses. The widespread ‘yellow’ lineage (Figure 3) occurs on scattered atolls of the coralline Seychelles. H. frenatus, however, was not present on the islands from which these atolls were colonized when they were first sampled in the 19th century. In addition, the yellow lineage still does not occur in Mauritius, and H. frenatus was only confirmed in the granitic Seychelles (Mahé) in the 1990s. Since this lineage occurs widely, both on the mainland and in the islands of Wallacea and the Pacific, it is likely to have been present in Sumatra and Borneo when the Austronesian voyagers colonized Madagascar and the Comoros. No archeological remains have been found to confirm that they crossed mid-ocean (Anderson et al., 2018a). Geckos could still have been dispersed either via shipwrecks that did not result in an archeological signature, or via lost mariners who strayed from the Maldives (Agarwal et al., 2019).
The ‘red’ lineage’s presence in the Indian Ocean only on Madagascar and the Comoros (Rocha et al., 2010), could match the profile of Indian trade with East Africa and Madagascar/Comoros in the medieval and early modern period (Boivin et al., 2013). Specifically, the geographic distribution could be correlated with the distribution of the introduced Indian civet Viverricula indica found in Madagascar, Comoros, the East African islands of the Zanzibar group, and Socotra (Gaubert et al., 2017), but nowhere else in the western Indian Ocean. The third (green) lineage could correspond to more modern trade and travel (notably to formerly isolated Diego Garcia), which may also be responsible for the yellow lineage’s dispersal to Australia and the Pacific.
Higher resolution datasets, through either greater number of targeted markers such as SNPs or the retrieval of complete mitochondrial and nuclear genomes, are required to further investigate the lineage splits and chronology of these different dispersals, but the patterns presented are suggestive, and the use of these commensal species as tracers for early human movements merits further consideration. This approach would be significantly improved though denser sampling of mainland southeast Asia and the other suggested sources of all the lineages.
Furthermore, determination of dispersal patterns based on any single molecular marker, such as mtDNA, needs to be treated with caution. In Southern Europe, patterns of minimal variation in mtDNA in two unrelated gecko species conflicted with nuclear markers, suggestive of a “selective sweep” that could obscure phylogeographic history based on mtDNA (Rato et al., 2010, 2011). A higher than usual nDNA/mtDNA diversity pattern was also identified in H. frenatus (Tonione et al., 2011), which certainly warrants further investigation and highlights the need to interpret cautiously the phylogeographic patterns based on mtDNA.
Finally, it appears from the current data that some gecko species and lineages are significantly more amenable to human translocation, or are more efficient at establishing colonies thereafter. From the greater than 165 currently recognized species of genus Hemidactylus, ten have intercontinental distributions, with two that are widely present in Western Indian Ocean islands, H. mabouia-mercatorius complex and H. frenatus, among the five most widespread and invasive Hemidactylus (Rocha et al., 2010; Weterings and Vetter, 2018).
As noted earlier, geckos have specific characteristics that make them efficient colonizers, but the characteristics that make some species especially effective remain unknown. Several studies have suggested that thermal physiology may play a role. Thus, an investigation of thermal tolerances in widespread species, relative to those with more restricted ranges may be revealing. Clearly H. frenatus is able to colonize what is thought to be less optimal habitat (McKay and Milenkaya, 2020), and there is some evidence for increased cold tolerance in invasive populations (Lapwong et al., 2021). On the other hand, H. frenatus showed no evidence of increased boldness which is often (although not necessarily) associated with invasive species (Nordberg et al., 2021).
The identification of likely “cryptic species” within both H. mabouia species-complex and H. frenatus, however, complicates the situation, since it becomes unclear if the same lineages have been compared, or if some lineages have unique characteristics. Modeling approaches, to determine potential ranges, and the impact of climate change (e.g., Rödder et al., 2008), as well as analyses of diet, behavior and other ecological variables, may benefit from determining which lineages within these diverse species are being compared. The sequence dataset presented in this study may form a useful comparative framework for such future assessments.
Conclusion
Atypical distribution patterns of H. parvimaculatus and H. frenatus on outlying atolls and sand cays of the Seychelles, coupled with an historic absence on the more populated islands are compatible with a hypothesis of early, pre-European or even natural, colonization patterns. We attempted to verify this for H. frenatus using analysis of CYTB sequence data from modern and museum specimens. Our results highlight high diversity across its native range, indicating that it is a potential species complex, as well as the multiple translocation events needed to explain its current distribution. Ultimately, while it was not possible to clearly define their patterns and timings without a greatly increased sampling across its native range, it remains tempting to hypothesize that they reflect the different routes and timings of human dispersals across the region, while accepting that older patterns may be obscured by frequent and ongoing recent colonization events. Increased sampling of both specimens and genomic variation is a promising way to further investigate these questions and to better understand the reasons underlying some geckos’ extreme colonizing capabilities.
Methods
Initially, 97 tissue samples of Hemidactylus frenatus were analyzed: 51 specimens were obtained from museum collections (Field Museum, Chicago; British National History Museum, London; Smithsonian, Washington DC), and 46 modern specimens were collected in the field by Dr. J. Chris Hillman and Solomon Pomerantz (Sealinks Project). Museum samples were mainly stored in ethanol or formalin, while the modern tissue samples were dried. To enable the data collected here to be directly compared with the phylogeographic patterns obtained by Rocha et al. (2010), 14 samples representing distinct haplogroups based on 16S rRNA sequences from this earlier work were re-extracted and included in this study. An additional 15 specimens (including two outgroups) were included from GenBank (Supplementary Table 1).
All samples excluding those previously analyzed by (Rocha et al., 2010) were analyzed in the facilities at the Archeology Department of Durham University. For these, DNA extractions were performed using Qiagen Microkit following the manufacturers’ instructions. A 463 bp region of the mitochondrial cytochrome b was amplified and sequenced in one fragment, in 25 ul reactions (1U of Sigma Jump Start Taq (0.2 ul), 10 mM primers (0.625 ul each), 25 mM MgCl2 (4 ul), 10* Buffer (2.5 ul) 25 mM dNTP mix (0.25 ul) with 1 ul DNA extract and made upt to volume with H2O) and annealing temperatures of 58-59°C. Due to the fact that many specimens were from museum samples, specific primers were designed (5′-3′): CTAATGATCCTCCGCAAAGC and AATCCGCCTCAAATTCACTG, based on the sequence of the whole mtDNA of Hemidactylus frenatus (Accession number NC_012902). For the specimens previously analyzed by Rocha et al. (2010), universal Cytochrome b primers (GluDG and Cytb2H - (Palumbi, 1996) were used to amplify a fragment that was 417 bp, missing the last 46 bp relative to the remaining samples. Conditions were as above, but using 1U of MyTaq and the associated Mastermix Buffer, and with an annealing temperature of 50°C. These PCRs were carried out in the laboratories at CIBIO, University of Porto. Sequencing reactions were carried out using both primers by the DNA Sequencing Service at the School of Biological and Biomedical Sciences at Durham University, while those from CIBIO were purified and sequenced using a commercial company (Genewiz, Germany).
While all of the samples from Rocha et al. (2010) could be amplified, due to the known issues of extracting DNA from museum specimens, and especially those stored in formalin, (reviewed in Hykin et al. (2015), only one “old” museum sample yielded DNA, and in total only 64 samples were successfully sequenced (information detailed in Supplementary Table 1). Electropherograms were manually inspected and corrected, and manually aligned using both Geneious R6 version (Kearse et al., 2012) and BioEdit (Hall, 1999). All showed typical mtDNA base composition, and could be translated into expected amino acid sequences for the gene. Regarding the ingroup (H. frenatus), the alignment had 106 variable and 74 parsimony informative sites. New sequences were submitted to GenBank (accession numbers OL880471-OL880521).
Phylogenetic analyses were performed by construction of Bayesian trees using MrBayes version 3.2.2 (Ronquist et al., 2012). The best-fit nucleotide substitution model, selected in jModelTest2 (Darriba et al., 2012) under the AIC criterion was the HKY + G model. The analysis was run for 5,000,000 generations, with a sample frequency of 5,000 and with a burn-in period of 25%, with remaining trees used to infer a consensus tree and calculate Bayesian Posterior Probabilities. A Maximum likelihood (ML) approach was also employed to estimate a phylogeny, using PhyML 3.0 (Guindon et al., 2010), both for defining the most appropriate model of molecular evolution under the AIC criteria, and producing a phylogeny. The chosen model was again the HKY + G model, and support for the phylogenetic tree was inferred with 1,000 bootstrap replicates. The phylogenetic trees were imported to FigTree v.1.4.2 (Rambaut, 2014) for graphical visualization and editing. Two divergent species from the genus, Hemidactylus shihraensis and Hemidactylus dawudazraqui, were used as outgroups. The full resulting tree (BI consensus; complete tips labeled) with bootstraps and posterior probabilities is shown on Supplementary Figure 1.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Permits for fieldwork and tissue collection were obtained from SBS (Seychelles Bureau of Standards) for Seychelles specimens (granted to SR and DJH). Permission to sample museum specimens was secured from the museums after a destructive application process.
Author Contributions
ASC, and GL conceived of the study. ASC collected and analyzed historical records. SR, AT, and DJH extracted DNA and generated and analyzed mitochondrial sequences. SR, AT, GL, DJH, and ASC wrote the manuscript with contributions from all authors.
Funding
This work was supported by the European Union’s Horizon 2020 Research and Innovation Programme under the Grant Agreement Number 857251.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
ASC thanks curator Gabrielle Baglione, and also Justin Janssen, student of the 1801-4 Baudin expedition, for documents from the Lesueur archive in Muséum du Havre, France. Samples from the Seychelles were collected under fieldwork and tissue collection permits from SBS (ref A0347). SR and DJH are grateful to the Ministry of Environment and Natural Resources and Justin Gerlach (NPTS) for logistical assistance, support and information. We thank Jesse C. Hillman, Solomon Pomerantz and the SeaLinks Project for additional sample collection, and the Field Museum (Chicago), the Smithsonian Institute (Washington, DC), and the British Natural History Museum (London) for access to their herpetology collections. AT was funded by grants from Durham University and the University of Aberdeen.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.791762/full#supplementary-material
References
Agarwal, I., Ceríaco, L. M. P., Metallinou, M., Jackman, T. R., and Bauer, A. M. (2021). How the African house gecko (Hemidactylus mabouia) conquered the world. R. Soc. Open Sci. 8:210749. doi: 10.1098/rsos.210749
Agarwal, I., Jablonski, D., and Bauer, A. M. (2019). The identity and probable origin of the Hemidactylus geckos of the Maldives. Herpetol. J. 29, 230–236.
Allen, R., Ryan, H., Davis, B. W., King, C., Frantz, L., Irving-Pease, E., et al. (2020). A mitochondrial genetic divergence proxy predicts the reproductive compatibility of mammalian hybrids. Proc. R. Soc. Lond. B 287:20200690. doi: 10.1098/rspb.2020.0690
Anderson, A., Clark, G., Haberle, S., Higham, T., Nowak-Kemp, M., Prendergast, A., et al. (2018b). New evidence of megafaunal bone damage indicates late colonization of Madagascar. PLoS One 13:e0204368. doi: 10.1371/journal.pone.0204368
Anderson, A., Camens, A., Clark, G., Haberle, S., and Seetah, K. (2018a). “Investigating pre-modern colonization of the Indian Ocean: the remote islands enigma,” in Connecting Continents: Archaeology and History in the Indian Ocean, ed. K. Seetah (Athens, OH: Ohio University Press), 30–67.
Andriamialisoa, F., and Langrand, O. (2003). “The history of zoological exploration of Madagascar,” in The natural history of Madagascar, eds S. M. Goodman and J. Benstead (Chicago, IL: University of Chicago Press), 1–15. doi: 10.1093/molbev/msu329
Aplin, K. P., Suzuki, H., Chinen, A. A., Chesser, R. T., ten Have, J., Donnellan, S. C., et al. (2011). Multiple geographic origins of commensalism and complex dispersal history of Black Rats. PLoS One 6:e26357. doi: 10.1371/journal.pone.0026357
Arnold, E. N. (2009). Recently extinct reptile populations from Mauritius and Réunion, Indian Ocean. J. Zool. 191, 33–47.
Bansal, R., and Karanth, K. P. (2010). Molecular phylogeny of Hemidactylus geckos (Squamata: Gekkonidae) of the Indian subcontinent reveals a unique Indian radiation and an Indian origin of Asian house geckos. Mol. Phylogenet. Evol. 57, 459–465. doi: 10.1016/j.ympev.2010.06.008
Bauer, A. M., Jackman, T. R., Greenbaum, E., de Silva, A., Giri, V. B., and Das, I. (2010). Molecular evidence for the taxonomic status of Hemidactylus brookii group taxa (Squamata: Gekkonidae). Herpetol. J. 20, 129–138.
Boivin, N., Crowther, A., Helm, R., and Fuller, D. Q. (2013). East Africa and Madagascar in the Indian Ocean world. J. World Prehist. 26, 213–281.
Boulenger, G. A. (1909). No. XVI.- A list of the freshwater fishes, batrachians, and reptiles obtained by Mr. J. Stanley Gardiner’s Expedition to the Indian Ocean. Trans. Linn. Soc. Lond. 2nd Ser. Zool. 12, 291–300.
Bour, R. (2015). Paul Philippe Sanguin de Jossigny (1750-1827), artiste de Philibert Commerson. Les dessins de reptiles de Madagascar, de Rodrigues et des Seychelles. Zoosystema 37, 415–448.
Brouat, C., Tollenaere, C., Estoup, A., Loiseau, A., Sommer, S., Soanandrasana, R., et al. (2014). Invasion genetics of a human commensal rodent: the black rat Rattus rattus in Madagascar. Mol. Ecol. 23, 4153–4167. doi: 10.1111/mec.12848
Case, T. J., and Bolger, D. T. (1991). The role of introduced species in shaping the distribution and abundance of island reptiles. Evol. Ecol. 5, 272–290.
Case, T. J., Bolger, D. T., and Petren, K. (1994). Invasions and competitive displacement among house geckos in the tropical pacific. Ecology 75, 464–477.
Cheke, A. S. (1984). “Lizards of the seychelles,” in Biogeography and Ecology of the Seychelles Islands, ed. D. R. Stoddart (The Hague: W. Junk), 331–360.
Cheke, A. S. (2009). La faune vertebrée terrestre de l’Ile Maurice en 1803: données inédites provenant des manuscrits de Péron et Lesueur. Bull. Soc. Géol. Normandie Amis Mus. Havre 96, 61–77.
Cheke, A. S. (2010). The timing of arrival of humans and their commensal animals on Western Indian Ocean oceanic islands. Phelsuma 18, 38–69.
Cheke, A. S., and Hume, J. P. (2008). Lost Land of the Dodo: The Ecological History of Mauritius, Réunion and Rodrigues. London: Bloomsbury Publishing.
Cheke, A. S., and Lawley, J. C. (1983). Biological history of Agalega, with special reference to birds and other land vertebrates. Atoll Res. Bull. 273, 65–108.
Cheke, A. S., Pedrono, M., Bour, R., Anderson, A., Griffiths, C., Iverson, J. B., et al. (2017). Giant tortoises spread to western Indian Ocean islands by sea drift in pre-Holocene times, not by later human agency – response to Wilmé et al. (2016). J. Biogeog. 44, 1426–1440.
Cole, N. (2009). Herpetofaunal observations on Eagle Island, Middle Brother, North Brother and Diego Garcia, with an overview of previous records in the Chagos Archipelago. Phelsuma 17, 40–48.
Cole, N. C. (2008). A Field Guide to the Reptiles and Amphibians of Mauritius. Vacoas: Mauritian Wildlife Foundation.
Cole, N. C., Jones, C. G., and Harris, S. (2005). The need for enemy-free space: the impact of an invasive gecko on island endemics. Biol. Conserv. 125, 467–474.
Crowther, A., Lucas, L., Helm, R., Horton, M., Shipton, C., Wright, H. T., et al. (2016). Ancient crops provide first archaeological signature of the westward Austronesian expansion. Proc. Natl. Acad. Sci. U.S.A. 113, 6635–6640. doi: 10.1073/pnas.1522714113
Csurhes, S., and Markula, A. (2009). Pest Animal Risk Assessment: Asian House Gecko Hemidactylus frenatus. Queensland Primary Industries and Fisheries report. Queensland: Department of Employment, Economic Development and Innovation.
Cucchi, T., Papayianni, K., Cersoy, S., Aznar-Cormano, L., Zazzo, A., Debruyne, R., et al. (2020). Tracking the Near Eastern origins and European dispersal of the western house mouse. Sci. Rep. 10:8276. doi: 10.1038/s41598-020-64939-9
da Fonseca, R. R., Smith, B. D., Wales, N., Cappellini, E., Skoglund, P., Fumagalli, M., et al. (2015). The origin and evolution of maize in the Southwestern United States. Nat. Plants 1:14003. doi: 10.1038/nplants.2014.3
Dame, E. A., and Petren, K. (2006). Behavioural mechanisms of invasion and displacement in Pacific island geckos (Hemidactylus). Anim. Behav. 71, 1165–1173.
Daniel, J. C. (2002). The Book of Indian Reptiles and Amphibians. Mumbai: Bombay Natural History Society and Oxford University Press.
Darriba, D., Taboada, G. L., Doallo, R., and Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9:772. doi: 10.1038/nmeth.2109
Das, I., and de Silva, A. (2005). A Photographic Guide to Snakes and other Reptiles of Sri Lanka. London: New Holland Publishers.
de Froberville, E. (1848). “Rodrigues, Galéga, les Séchelles, les Almirantes etc,” in Iles de l’Afrique, Vol. 3, ed. M. A. P. d’Avezac (Paris: Firmin Didot), 64–114.
de Queiroz, A. (2005). The resurrection of oceanic dispersal in historical biogeography. Trends Ecol. Evol. 20, 68–73. doi: 10.1016/j.tree.2004.11.006
Deso, G., Probst, J.-M., and Dubos, N. (2020). The widespread Indo-Pacific Slender Gecko Hemiphyllodactylus typus: a single species across the Oceans? Bull. Phaethon 51, 38–41.
Duplantier, J.-M., Orth, A., Catalan, J., and Bonhomme, F. (2002). Evidence for a mitochondrial lineage originating from the Arabian peninsula in the Madagascar house mouse (Mus musculus). Heredity 89, 154–158. doi: 10.1038/sj.hdy.6800122
Frantz, L. A. F., Haile, J., Lin, A. T., Scheu, A., Geörg, C., Benecke, N., et al. (2019). Ancient pigs reveal a near-complete genomic turnover following their introduction to Europe. Proc. Natl. Acad. Sci. U.S.A. 116, 17231–17238.
Gardner, A. S. (1986). The biogeography of the lizards of the Seychelles islands. J. Biogeogr. 13:237.
Gaubert, P., Patel, R. P., Veron, G., Goodman, S. M., Willsch, M., Vasconcelos, R., et al. (2017). Phylogeography of the small indian civet and origin of introductions to western Indian Ocean islands. J. Hered. 108, 270–279. doi: 10.1093/jhered/esw085
Gerlach, J. (2007). Terrestrial and Freshwater Vertebrates of the Seychelles Islands. Leiden: Backhuys Publishers.
Glaw, F., and Vences, M. (2007). A Field Guide to the Amphibians and Reptiles of Madagascar. Köln: Vences & Glaw.
Grégory, D., Probst, J. M., and Ineich, I. (2007). Hemiphyllodactylus typus Bleeker, 1860 (Sauria: Gekkonidae) sur l’île de La Réunion: écologie et répartition. Bull. Soc. Herp. Fr. 124, 31–48.
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. doi: 10.1093/sysbio/syq010
Günther, A. (1884). “Reptilia,” in Report on the Zoological Collections Made in the Indo-Pacific Ocean During the Voyage of H.M.S. “Alert” 1881-2, ed. A. Günther (London: British Museum).
Hall, T. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Harris, D. J. (2002). Reassessment of comparative genetic distance in reptiles from the mitochondrial cytochrome b gene. Herpetol. J. 12, 85–87.
Hawlitschek, O., Brückmann, B., Berger, J., Green, K., and Glaw, F. (2011). Integrating field surveys and remote sensing data to study distribution, habitat use and conservation status of the herpetofauna of the Comoro Islands. Zookeys 144, 21–78. doi: 10.3897/zookeys.144.1648
Hawlitschek, O., Ramírez Garrido, S., and Glaw, F. (2017). How marine currents influenced the widespread natural overseas dispersal of reptiles in the Western Indian Ocean region. J. Biogeogr. 44, 1435–1440.
Heinsohn, T. (2003). Animal translocation: long-term human influences on the vertebrate zoogeography of Australasia (natural dispersal versus ethnophoresy). Aust. Zool. 32, 351–376.
Hsu, M.-H., Lin, J.-W., Liao, C.-P., Hsu, J.-Y., and Huang, W.-S. (2021). Trans-marine dispersal inferred from the saltwater tolerance of lizards from Taiwan. PLoS One 16:e0247009. doi: 10.1371/journal.pone.0247009
Hume, J. P. (2004). A preliminary vertebrate palaeontological survey of the granitic Seychelles islands. Phelsuma 12, 24–34.
Hume, J. P., Martill, D., and Hing, R. (2018). A terrestrial vertebrate palaeontological review of Aldabra Atoll, Aldabra Group, Seychelles. PLoS One 13:e0192675. doi: 10.1371/journal.pone.0192675
Hykin, S. M., Bi, K., and McGuire, J. A. (2015). Fixing formalin: a method to recover genomic-scale DNA sequence data from formalin-fixed museum specimens using high-throughput sequencing. PLoS One 10:e0141579. doi: 10.1371/journal.pone.0141579
Ineich, I. (1999). “Spatio-temporal analysis of the unisexual-bisexual Lepidodactylus lugubris complex (Reptilia, Gekkonidae),” in Tropical Island herpetofauna: Origin, Current Diversity and Conservation. Proceedings of the International Symposium Diversity of Reptiles, Amphibians, and Other Terrestrial Animals on Tropical Islands: Origin, Current Status, and Conservation, Okinawa, Japan, 6-7 June 1998, ed. H. Ota (Amsterdam: Elsevier), 199–228.
Jaufar, S. (2019). An Archaeological Study of the Maldive Islands: Investigating the Islamic Period Settlements. Doctoral thesis. Norwich: University of East Anglia.
Jones, E. P., Eager, H. M., Gabriel, S. I., Jóhannesdóttir, F., and Searle, J. B. (2013). Genetic tracking of mice and other bioproxies to infer human history. Trends Genet. 29, 298–308. doi: 10.1016/j.tig.2012.11.011
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Laidlaw, F. F. (1902). “Amphibia & reptilia,” in The Fauna and Geography of the Maldive and Laccadive Archipelagoes : Being the Account of the Work Carried on and of the Collections Made by an Expedition During the Years 1899 and 1900, ed. J. S. Gardiner (Cambridge: Cambridge university Press), 120–122.
Lapwong, Y., Dejtaradol, A., and Webb, J. K. (2021). Plasticity in thermal hardening of the invasive Asian house gecko. Evol. Ecol. 35, 631–641.
Litster, M. (2016). Cowry Shell Money and Monsoon Trade: The Maldives in Past Globalizations. Ph.D. thesis. Canberra, ACT: Australian National University.
Litster, M. (2020). “Maldivian archaeology,” in Encyclopedia of Global Archaeology, ed. C. Smith (Cham: Springer Nature). doi: 10.1007/978-3-030-30018-0_3456
Louette, M., Meirte, D., and Jocqué, R. (eds.) (2004). La faune Terrestre de l’Archipel des Comores. Studies in Afrotropical Zoology 293. Tervuren: Musée Royale de l’Afrique Centrale.
Mahony, S. (2011). Taxonomic revision of Hemidactylus brookii Gray: a re-examination of the type series and some Asian synonyms, and a discussion of the obscure species Hemidactylus subtriedrus Jerdon (Reptilia: Gekkonidae). Zootaxa 3042, 37–67.
McKay, J. L., and Milenkaya, O. (2020). The Asian House Gecko (Hemidactylus frenatus) established in natural vegetation of Oaxaca, Mexico. Reptil. Amphib. 27, 479–484.
McKay, J. L., and Phillips, B. L. (2012). Climatic determinants of the reproductive timing in the Asian house gecko, Hemidactylus frenatus Duméril and Bibron (Gekkonidae). Raffles Bull. Zool. 60:6.
Mooney, H. A., and Cleland, E. E. (2001). The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. U.S.A. 98, 5446–5451. doi: 10.1073/pnas.091093398
New, A. L., Stansfield, K., Smythe-Wright, D., Smeed, D. A., Evans, A. J., and Alderson, S. G. (2005). Physical and biochemical aspects of the flow across the Mascarene Plateau in the Indian Ocean. Phil. Trans. R. Soc. Lond. A 363, 151–168. doi: 10.1098/rsta.2004.1484
Newbery, B., and Jones, D. N. (2007). “Presence of Asian house gecko Hemidactylus frenatus across an urban gradient in Brisbane: influence of habitat and potential for impact on native gecko species,” in Pest or Guest: The Zoology of Overabundance, eds D. Lunney, P. Eby, P. Hutchings, and S. Burgin (Mosman, NSW: Royal Zoological Society of New South Wales), 59–65.
Nordberg, E., Denny, R., and Schwarzkopf, L. (2021). Testing measures of boldness and exploratory activity in native versus invasive species: geckos as a model system. Anim. Behav. 177, 215–222.
Ohdachi, S. D., Kinoshita, G., Oda, S.-I., Motokawa, M., Jogahara, T., Arai, S., et al. (2016). Intraspecific phylogeny of the house shrews, Suncus murinus – S. Montanus species complex, based on the mitochondrial Cytochrome-b Gene. Mamm. Study 41, 229–238.
Olden, J. D., Leroy Poff, N., Douglas, M. R., Douglas, M. E., and Fausch, K. D. (2004). Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 19, 18–24. doi: 10.1016/j.tree.2003.09.010
Palumbi, S. R. (1996). “Nucleic acids II: the polymerase chain reaction,” in Molecular Systematics, eds D. M. Hillis, C. Moritz, and B. K. Mable (Sunderland, MA: Sinauer Associates), 205–247.
Peng, S., Qian, Y.-K., Lumpkin, R., Li, P., Wang, D., and Du, Y. (2015). Characteristics of the near-surface currents in the Indian Ocean as deduced from satellite-tracked surface drifters. Part II: Lagrangian Statistics. J. Phys. Oceanogr. 45, 459–477.
Perri, A. R., Feuerborn, T. R., Frantz, L. A. F., Larson, G., Malhi, R. S., Meltzer, D. J., et al. (2021). Dog domestication and the dual dispersal of people and dogs into the Americas. Proc. Natl. Acad. Sci. U.S.A 118:e2010083118. doi: 10.1073/pnas.2010083118
Peters, W. C. H., and von der Decken, C. C. (1869). “Säugethiere und Amphibien: gesammelt von Baron C. C. von der Decken auf seinen Reisen im äquatorialen Ostafrika,” in Baron Carl Claus von der Decken’s Reisen in Õst-Afrika, Vol. 3(pt. 1), ed. O. Kersten (Leipzig: C.F.Winter), 18pp+plates.
Rambaut, A. (2014). FigTreev1.3.1. Available online at: http://tree.bio.ed.ac.uk/software/figtree/ (accessed February 1, 2022).
Rato, C., Carranza, S., and Harris, D. J. (2011). When selection deceives phylogeographic interpretation: the case of the Mediterranean house gecko, Hemidactylus turcicus (Linnaeus, 1758). Mol. Phylogenet. Evol. 58, 365–373. doi: 10.1016/j.ympev.2010.12.004
Rato, C., Carranza, S., Perera, A., Carretero, M. A., and Harris, D. J. (2010). Conflicting patterns of nucleotide diversity between mtDNA and nDNA in the Moorish gecko, Tarentola mauritanica. Mol. Phylogenet. Evol. 56, 962–971. doi: 10.1016/j.ympev.2010.04.033
Rocha, S., Carretero, M. A., and Harris, D. J. (2005). Diversity and phylogenetic relationships of Hemidactylus geckos from the Comoro islands. Mol. Phylogenet. Evol. 35, 292–299. doi: 10.1016/j.ympev.2004.11.023
Rocha, S., Carretero, M. A., and Harris, D. J. (2010). On the diversity, colonization patterns and status of Hemidactylus spp. (Reptilia: Gekkonidae) from the Western Indian Ocean islands. Herpetol. J. 20, 83–89.
Rocha, S., Harris, D. J., Perera, A., Silva, A., Vasconcelos, R., and Carretero, M. A. (2009a). Recent data on the distribution of lizards and snakes of the Seychelles. Herpetol. Bull. 110, 20–32.
Rocha, S., Ineich, I., and James Harris, D. (2009b). Cryptic variation and recent bipolar range expansion within the Stumped-Toed Gecko Gehyra mutilata across Indian and Pacific Ocean islands. Contrib. Zool. 78, 1–8.
Rödder, D., Solé, M., and Böhme, W. (2008). Predicting the potential distributions of two alien invasive Housegeckos (Gekkonidae: Hemidactylus frenatus, Hemidactylus mabouia). North West. J. Zool. 4, 236–246.
Romero-Frías, X. (1999). The Maldive Islanders: A Study of the Popular Culture of an Ancient Ocean Kingdom. Barcelona: Nova Ethnographica Indica.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Sanchez, M., Rocha, S., and Probst, J.-M. (2012). Un nouveau gecko nocturne naturalisé sur l’île de La Réunion : Hemidactylus mercatorius Gray, 1842 (Reptilia: Squamata: Gekkonidae). Bull. Soc. Herp. Fr. 142-143, 89–106.
Tollenaere, C., Brouat, C., Duplantier, J.-M., Rahalison, L., Rahelinirina, S., Pascal, M., et al. (2010). Phylogeography of the introduced species Rattus rattus in the western Indian Ocean, with special emphasis on the colonization history of Madagascar. J. Biogeogr. 37, 398–410.
Tonione, M. A., Reeder, N., and Moritz, C. C. (2011). High genetic diversity despite the potential for stepping-stone colonizations in an invasive species of gecko on Moorea, French Polynesia. PLoS One 6:e26874. doi: 10.1371/journal.pone.0026874
Torres-Carvajal, O. (2015). On the origin of South American populations of the common house gecko (Gekkonidae: Hemidactylus frenatus). NBER Work. Pap. Ser. 27, 69–79.
Toussaint, A. (1967). La Route des Îles: Contribution à L’histoire Maritime des Mascareignes. Paris: S.E.V.P.E.N.
Vences, M., Wanke, S., Vieites, D. R., Branch, W. R., Glaw, F., and Meyer, A. (2004). Natural colonization or introduction? Phylogeographical relationships and morphological differentiation of house geckos (Hemidactylus) from Madagascar. Biol. J. Linn. Soc. Lond. 83, 115–130.
Vinson, J., and Vinson, J. M. (1969). The saurian fauna of the Mascarene Islands. Mauritius Inst. Bull. 6, 203–320.
Wagner, P., Leaché, A. D., and Fujita, M. K. (2014). Description of four new West African forest geckos of the Hemidactylus fasciatus Gray, 1842 complex, revealed by coalescent species delimitation. Bonn Zool. Bull. 63, 1–14.
Webster, I., and Cadinouche, A. (2013). Agalega Expedition Report: Summary of Results with Recommendations for Management, Research and Monitoring. Port Louis: Report to the Outer Island Development Corporation [of Mauritius].
Wenban-Smith, N., and Carter, M. D. (2016). Chagos: A History: Exploration, Exploitation, Expulsion. London: Chagos Conservation Trust.
Weterings, R., and Vetter, K. C. (2018). Invasive house geckos (Hemidactylus spp.): their current, potential and future distribution. Curr. Zool. 64, 559–573.
Keywords: H. frenatus, house gecko, phylogenetics, evolution, human-mediated dispersal
Citation: Rocha S, Trinks A, Harris DJ, Larson G and Cheke AS (2022) The Global and Western Indian Ocean Dispersal of House Geckos From Asia Using Historical and Mitochondrial DNA Perspectives. Front. Ecol. Evol. 10:791762. doi: 10.3389/fevo.2022.791762
Received: 08 October 2021; Accepted: 07 March 2022;
Published: 17 May 2022.
Edited by:
Pasquale Raia, University of Naples Federico II, ItalyReviewed by:
Andreanna J. Welch, Durham University, United KingdomRobert William Meredith, Montclair State University, United States
Copyright © 2022 Rocha, Trinks, Harris, Larson and Cheke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Greger Larson, Z3JlZ2VyLmxhcnNvbkBhcmNoLm94LmFjLnVr
†These authors have contributed equally to this work
 Sara Rocha
Sara Rocha Alexandra Trinks2,3†
Alexandra Trinks2,3† Greger Larson
Greger Larson Anthony S. Cheke
Anthony S. Cheke