
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 16 May 2022
Sec. Urban Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.790340
A key challenge in urban biodiversity conservation is to understand the drivers that govern the population establishment of different groups of species in different urban ecosystems. Here, we ask whether and to what extent vascular plant species establishment (i.e., the ability to establish self-sustaining populations within a certain time span) is driven by interactions of species functional traits, native status, and the type of ecosystem species occur in, with types of ecosystems distinguished by their degree of ecosystem novelty. To answer this question, we use a dataset of 1,178 vascular plant species occurring in Berlin, Germany that originally had been compiled to substantiate the Berlin Red List of endangered plant species. This dataset classifies native and non-native species into casual and established species based on a minimum of 25 years of expert observation. Whether a species is established or casual is distinguished among four broad types of ecosystems: natural remnant, hybrid, novel immature, and novel mature ecosystems. Moreover, we classify species into those native to Berlin and non-native species (split into archaeophytes and neophytes), and link species to selected functional traits and indicator values. By applying ordinal regression within a Bayesian framework, we show that traits are key drivers of these establishment processes and that the traits that drive species establishment differ across types of ecosystems. While across traits, more established species are present in natural remnants, low canopy height, annual life span, and late end of flowering specifically promote establishment in novel immature ecosystems. In hybrid ecosystems, low canopy height and reproduction by seeds are beneficial traits, with the latter promoting establishment in novel mature ecosystems, too. Traits were less important in predicting species establishment in native as compared to non-native species. All types of ecosystems add to urban biodiversity, and trait analyses refine our knowledge on how they can be supported in doing so on the long term. This can help in sharpening conservation measures.
Urban and urbanizing areas support both high biodiversity (e.g., Kühn et al., 2004a; Ives et al., 2016) and biodiversity loss (IPBES, 2019). This seeming discrepancy might in part be due to the lack of long-term studies of species population development in urban environments (but see Planchuelo et al., 2020a). Cities “potentially carry a large extinction debt” (Hahs et al., 2009, p. 1165). Therefore, by solely investigating today’s species occurrences, we cannot reliably foresee population development. Rather, today’s species richness might overestimate the real capacity of urban areas to host stable populations in the long-term. Key research questions for urban biodiversity conservation have thus been raised recently, such as whether urban ecosystems act as sources or sinks—or neither—of biological diversity (Shwartz et al., 2014; Lepczyk et al., 2017) or how urban environmental filters (Williams et al., 2009; Aronson et al., 2016; Piana et al., 2019) affect the establishment of viable populations within urban areas (Kowarik and von der Lippe, 2018).
Urban environments are characterized by a mosaic of different ecosystems (Niemelä, 1999), many of which have been highly transformed by humans. More specifically, a range of so-called hybrid and novel ecosystems (Hobbs et al., 2006, 2009) are present in urban areas (Kowarik, 2011). Hobbs et al. (2009) defined these as ecosystems that differ from their historic state to an extent that they—in the case of hybrid ecosystems—combine mixtures of “old” and “new” ecosystem features or—in the case of novel ecosystems—are so deeply transformed that no return to the historic state is possible. The questions arise what the contribution of hybrid and novel ecosystems is to (urban) biodiversity and if these ecosystems offer similar opportunities for establishing sustainable populations as natural ecosystems do.
Similar to containing a range of novel ecosystems, urban environments harbor a high share of non-native species (Kowarik, 1990; van Ham et al., 2013). While the establishment of native species (especially rare or threatened ones) within ecosystems might be a process valued by society, the establishment of non-native (especially invasive) species is often judged as an unwanted process, specifically within natural ecosystems. Identifying drivers of species establishment of different groups of species in different kinds of urban ecosystems can thus help us to identify conservation and management priorities (Kowarik and von der Lippe, 2018; Planchuelo et al., 2020a). A previous study by Kowarik and von der Lippe (2018) revealed different habitat functions of remnant, hybrid, and novel ecosystems, and a considerable gap between the occurrence and population establishment of native, non-native, and endangered species in these ecosystems. Yet, the role of functional traits in governing whether species manage to establish or not remains unclear. Research on the functional traits of native vs. non-native species (van Kleunen et al., 2010) and of endangered species in novel vs. hybrid and natural ecosystems (Planchuelo et al., 2020a,b) suggests that traits influence both species’ ability to establish and local extinction.
Traits shown to govern the occurrence of species differ between urban and non-urban areas (see Williams et al., 2015 for a review of vascular plants). A range of functional traits and other species attributes has been identified that promote plant life in urban areas such as large seed mass, large canopy height, short lifespan, dispersal by humans, or adaptation to warm, dry, alkaline, and nutrient rich conditions (Lososová et al., 2006; Knapp et al., 2008; Williams et al., 2015). Moreover, functional flexibility in the sense of intraspecific trait variation might prove beneficial in urban environments (Borowy and Swan, 2020). Consequently, traits also play a role in determining extinction risk. In urban areas across the world, plant species’ extinction debt relates to selected functional traits, with low canopy height, light seeds, and affinity to forests or riparian ecosystems promoting extinction (Duncan et al., 2011). Similarly, in Berlin, Germany, Planchuelo et al. (2020b) showed that traits predict local extinction in target species of conservation concern. Still, as many urban biodiversity studies rely on spatial analysis, only representing snapshots in time (Knapp et al., 2021), extinction risk might well be masked by species’ life span (Williams et al., 2015). Also, by looking at heterogeneous urban systems as if they were homogeneous entities, it is hard to gain a deeper understanding of urban biodiversity (Lososová et al., 2011; Kalusová et al., 2017), such as identifying mechanisms that drive extinction risk. Similarly, establishment failures will be masked by simply looking at the presence and absence of species at a certain point in time without considering urban heterogeneity (Kowarik and von der Lippe, 2018).
To overcome these shortages, Kowarik and von der Lippe (2018) introduced a framework for studying plant population establishment across different types of urban ecosystems. They categorized ecosystems within urban areas following a previous approach (Kowarik, 1991) into (i) natural remnant, (ii) hybrid, (iii) novel immature, and (iv) novel mature ecosystems. These categories can be paralleled with the novel ecosystem concept (Hobbs et al., 2006, 2009) and relate to the “novelty of landscapes, ecosystems, and communities” according to Heger et al. (2019), which is a site-specific perspective, defining novelty based on changes to a site (e.g., increasing the share of sealed surface in a site thus increasing temperature), not on whether environmental conditions in a site are new to a species (e.g., species occurring on urban sites characterized by high temperatures might be adapted to high temperatures anyway). The four different types of urban ecosystems comprise (i) relicts of ecosystems resembling historical benchmarks of natural landscapes such as deciduous forests or wetlands; (ii) ecosystems altered by humans that might still be able to return to historical conditions by natural succession, such as grazed grassland or forest plantings on natural soils; (iii, iv) novel ecosystems deeply altered by humans that are unlikely to return to historical conditions due to irreversible changes to soils or other environmental features as on urban vacant land. These novel ecosystems had been split in two subtypes to account for different levels of self-regulation: (iii) the first subtype is still subjected to recent or ongoing anthropogenic disturbance and thus being dominated by early successional stages with a low level of self-regulation; the second subtype (iv) has not been subjected to severe anthropogenic disturbance for more than about 20 years, leading to novel ecosystems mainly being governed by natural processes again such as in urban woodlands on vacant lots (Kowarik and von der Lippe, 2018). To establish populations within these ecosystems, species need to overcome a number of environmental filters: They need to disperse to patches of an ecosystem, survive in prevailing environmental conditions, and reproduce. Species that occur in an ecosystem but do not manage to reproduce, are so-called casual species, whose long-term occurrence is dependent on ongoing migration from other locations (such as plants cultivated in gardens as source of many spontaneously occurring non-native casual species). The framework allows testing hypotheses about potential drivers of species establishment such as species native status (Kowarik and von der Lippe, 2018) or species functional traits (Planchuelo et al., 2020a).
Here, we made use of a dataset comprising information about the population establishment of 1,178 vascular plant species across ecosystems of different novelty in Berlin, Germany. By applying the framework suggested by Kowarik and von der Lippe (2018), we asked whether and to what extent species establishment is driven by interactions of species’ functional traits, native status, and the ecosystem species occur in—and thus ecosystem novelty. While Kowarik and von der Lippe (2018) showed that established species in natural remnant and novel mature ecosystems are mostly native but non-native species have a higher share among established species in hybrid and novel immature ecosystems, we hypothesized that—on top of that—both species characterized by functional flexibility and species characterized by traits promoting occurrence in urban areas will establish more often than other species, but the relevance of traits for species establishment will vary depending on ecosystem novelty and native status (see Table 1 for more detailed hypotheses).
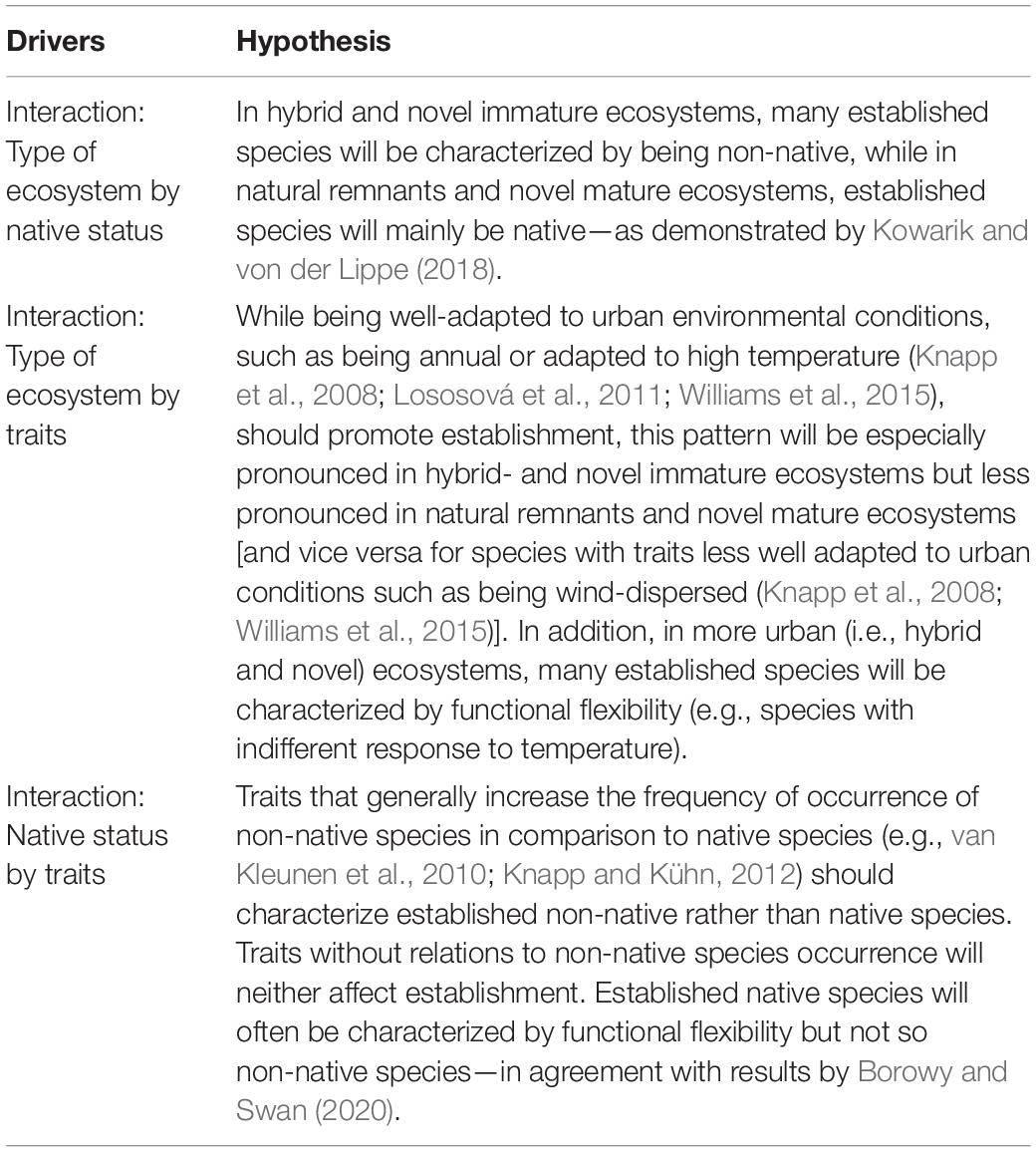
Table 1. Hypotheses about how interactions of species traits with different types of ecosystems (characterized by ecological novelty) and with species native status will influence the establishment of vascular plant species within an urban area.
We used an existing dataset on the vascular flora of Berlin, Germany (Kowarik, 1991) that originally had been compiled to substantiate the regional Red List of Berlin (Böcker et al., 1991). This Red List includes all wild growing native and non-native species that are known to occur or once have occurred in the area of today’s Berlin. The endangerment assessment distinguishes between species at different categories of endangerment, following the IUCN Red List approach. For our analyses, we chose all species listed in the dataset in Kowarik (1991), independent of their category of endangerment.
As an innovation, the dataset in Kowarik (1991) included information about the presence of species in four broad types of ecosystems that represent the total of Berlin’s ecosystems, and about species’ population status (i.e., established vs. casual) in each of the four ecosystem types, based on expert assessments. Berlin’s ecosystems were distinguished into four broad types that reflect how human agency modified ecosystems in a metropolitan region. The four ecosystem types being differentiated in the original study from 1991 can be roughly paralleled to the later established novel ecosystem concept by Hobbs et al. (2009)—as done in previous analyses (Kowarik and von der Lippe, 2018, see Supplementary Table 1). This allows us to discuss the results in relation to the novel ecosystem concept. The four ecosystem types are: Natural remnant (e.g., patches of alluvial forest), hybrid (e.g., dry grassland on natural soils), novel immature (e.g., herbaceous ruderal communities on vacant land), and novel mature (e.g., emerging urban forests sensu Kowarik et al., 2019). This distinction was based on the origin of ecosystems (natural vs. anthropogenic) and on the prevalence of natural ecosystem processes (low, mean, high) as illustrated in Supplementary Table 1 (adapted from Kowarik and von der Lippe, 2018). Natural processes (such as natural growth and mortality cycles) dominate in natural remnants and novel mature ecosystems, being medium for hybrid and low for novel immature ecosystems. Natural remnants are of natural origin, both types of novel ecosystems are of anthropogenic origin, while hybrid ecosystems can be of both, natural and anthropogenic origin.
Following a well-established approach in invasion ecology, native status differed between native species and non-native species. While the former are defined to have colonized a region by natural means, the latter are defined as being introduced accidentally or deliberately by humans. Non-native species are being divided into archaeophytes (introduced before 1500 AD) and neophytes (introduced after 1500 AD). Due to a long tradition in floristic research in Berlin (Sukopp, 1987), information on native status, specified for the Berlin region, was available and included in the 1991 Red List (Böcker et al., 1991) and in the original dataset (Kowarik, 1991).
For every species, information on whether it is casual or established is available, per type of ecosystem. The concept of casual vs. established species refers to the degree of naturalization in invasion science (Blackburn et al., 2011) but was also applied here to native species because they must overcome dispersal and establishment filters when colonizing urban habitat patches, just as introduced species have to (Kowarik and von der Lippe, 2018). Based on expert judgment, species were classified as established in a given type of ecosystem if a minimum of two generations of spontaneous offspring had been observed within a period of ≥ 25 years (Kowarik, 1991). All species that do not meet this criterion were included as casual species. Accordingly, a species can be casual in one, but established in another type of ecosystem. We used the distinction between “casual” and “established” as response variable (cf. section “Analysis”).
It has to be noted that all data from the original 1991 dataset refer to occurrence and establishment in the four ecosystem types, without geographic data on where in Berlin patches of the four types of ecosystems are located. Rather than on spatial patterns, we here focus on the long-term occurrence of species and its drivers.
In summary, the dataset we used here contains those 1,178 vascular plant species with information on established vs. casual occurrence within one or several of four types of ecosystems, together with species native status.
Traits (Supplementary Table 2) were chosen in relation to four environmental filters that species need to pass in order to establish in urban ecosystems, which we call the dispersal, environmental, reproduction, and survival filter (Kowarik and von der Lippe, 2018). Trait-by-species data was taken from databases, namely BiolFlor (Klotz et al., 2002; Kühn et al., 2004b) and LEDA (Knevel et al., 2003; Kleyer et al., 2008). In addition to functional traits, Ellenberg values were chosen (Ellenberg, 1974), i.e., species attributes that describe the association of plant species to given environmental conditions. For simplicity, we call both functional traits and attributes “traits” from now on. Species that are able to express several states of a trait (e.g., reproducing both clonally and by seed or with indifferent response to environmental conditions) were treated as flexible state of a trait.
Not every trait was available for every species. Still, we did not impute data as the amount of missing values seemed acceptable (traits chosen all cover > 75% of species; Supplementary Table 2) and phylogenetic imputation was not an option as we included the phylogenetic relationships of plant species into our models.
We applied Daphne (Durka and Michalski, 2012, 2016), a dated phylogeny that covers the vascular flora of the British Isles, Germany, The Netherlands, and Switzerland, including 4,685 species in total. We pruned this phylogeny to our dataset of plant species (and to subsets of the dataset if single traits were analyzed; see below), by using packages “ape” (Paradis and Schliep, 2019) and “picante” (Kembel et al., 2010) in R 3.6.1 (R Core Team, 2019). Consequently, only species present in all, the list of species, trait databases, and Daphne were included into analyses.
Our main aim was to explain species establishment (whether a species is casual or established) by interactions of functional traits with ecosystem novelty and species native status. Also, we aimed to account for the relatedness of species, because such autocorrelation can invert statistical pattern, as shown for spatial autocorrelation by Kühn (2007). Our models thus comprise an ordinal response variable (casual/established, with casual = 0 and established = 1), a set of categorical, numerical, and/or integer fixed predictors (type of ecosystem, native status, traits), species identity as a random predictor, and a covariance matrix of species based on phylogenetic relationships (Hadfield and Nakagawa, 2010). Ordinal regression within a Bayesian framework allows to model such data. We applied function “brm” within R-package “brms” (Bürkner, 2017, 2018), setting the family-argument to “Bernoulli”, choosing species identity as a grouping factor, and including a covariance matrix of our study species. We did so for several models:
First, we ran models that included one trait each, embedded in three two-way interactions as predictors of species establishment: type of ecosystem by native status, type of ecosystem by trait, native status by trait. This was done separately for each trait (“single trait models”), in order to identify traits that have an effect on species establishment, and to reduce the number of predictors in the full model (see below). Within single trait models, for categorical traits, we compared the most flexible trait value or trait combination to all other values. For example, species able to reproduce both clonally and by seeds seem more flexible than species reproducing by seeds only or clonally only, so the two latter were compared to the former. We ran all single trait models by applying one Markov chain and a minimum of 4,000 iterations (depending on the number of iterations needed for a model to converge). For continuous and integer traits, we applied both, models as described above and models containing t2-smooths, which internally choose the best fit, so that potential non-linearity in the combined effects of two interacting variables can be modeled (Bürkner, 2018). We compared the outcome and LOO-adjusted R2 for the model with and the model without t2-smooths. Categorical traits that contained ≤ 5 species in a trait-ecosystem or trait-status combination (e.g., archaeophytes dispersing their seeds by wind) were excluded because models did not converge reliably. Also, if two closely related traits (such as beginning, duration, and end of flowering) showed similar effects on species establishment, only one of them was chosen for inclusion into the full model.
Second, in order to further reduce the number of predictors in the full model and to make sure that predictors are not too similar to each other, we tested for correlation among those traits that showed significant effects on species establishment in single trait models. Numeric traits were tested for correlation using Kendall’s rank correlation (after testing trait distribution for normality). Categorical and integer traits (the latter—Ellenberg values—sorting species into groups of 1–9 or 1–12, thus being pseudo-categorical) were tested for correlation using Principal Component Analysis.
Lastly, we included all traits chosen in the two steps above into one model with the interactions of type of ecosystem by native status, type of ecosystem by traits, and native status by traits as fixed predictors, and casual vs. established again as response variable (“full model”; see the list of species with all other variables included into the full model in Supplementary Table 3). Applying stepwise backward selection, we deleted non-significant predictors from the model, i.e., those where the two-sided 95%-credible interval contained zero. Backward selection was performed until no non-significant predictors were left.
This procedure enabled us to test whether different groups of species vary in being established or casual across the four types of ecosystems, and which traits are most relevant in determining these differences.
All calculations were performed in R 3.6.1 (R Core Team, 2019).
The original list of species contained 1,178 vascular plant species. Of these, 49 were excluded because they did not appear in functional trait databases, the Daphne phylogeny, or both. Among excluded species, there were many aggregates (e.g., Alchemilla vulgaris agg., Ranunculus auricomus agg., Rubus fruticosus agg.). Of the remaining 1,129 species, 715 occurred within natural remnant ecosystems, 741 within hybrid ecosystems, 527 within novel immature, and 576 within novel mature ecosystems. Note that a species can occur in up to four types of ecosystems, so numbers sum to > 1,129 (cf. Supplementary Figure 1 for intersections among the four types of ecosystems). 673 of the 1,129 species were native (59.61%), 148 archaeophytes (13.11%), and 308 neophytes (27.28%; cf. Table 2 for numbers of casual and established species per status group and type of ecosystem, and Supplementary Table 2 for numbers of species per level of a categorical trait). After linking the list of 1,129 species to trait data, missing trait-by-species values ranged from 0 for type of reproduction and life span to 24.18% for dispersal vector and Ellenberg values (Supplementary Table 2).
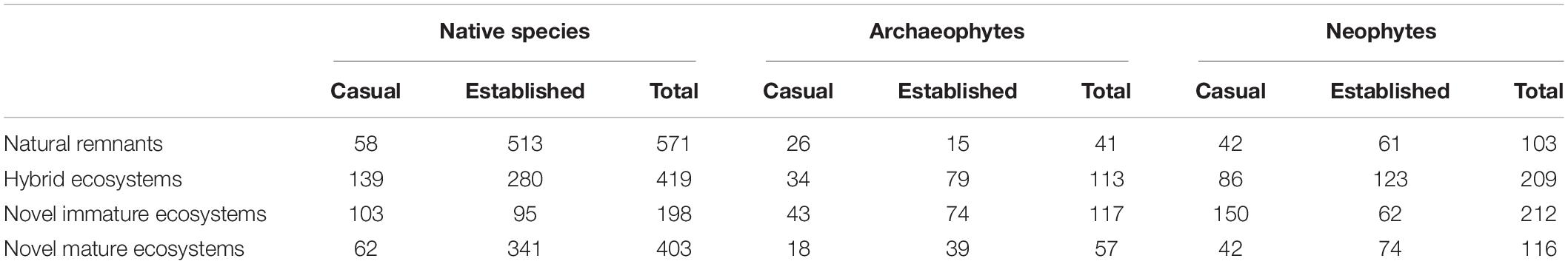
Table 2. Casual, established, and total number of vascular plant species per status group (native, archaeophyte, neophyte) and type of ecosystem (natural remnant, hybrid, novel immature, novel mature) in the flora of Berlin, Germany.
Phylogenetic relationships always had a weak positive effect on the response variable, indicating that if a species is established, its close relatives tend to be established, too. The interaction of type of ecosystem and native status was always significant. Across all types of ecosystems, there were more established native than established non-native species but less established native species were present in hybrid and novel ecosystems than in natural remnants.
The traits maximum and minimum canopy height, end of flowering, life span, and type of reproduction did not interact with native status but with type of ecosystem. Overall, there were more low-growing than tall growing species among established species and this difference was more pronounced in hybrid and novel immature ecosystems as compared to natural remnants and novel mature ecosystems. As this applied to both minimum and maximum height, only maximum height was included into the full model. Further, there were more established species with an early than late end of flowering in natural remnants and novel mature ecosystems, while hybrid and novel immature ecosystems showed an opposite pattern. For life span, there were less perennial established species in hybrid and novel immature than in other ecosystems. Compared to species with multiple life span types, there were less biennial established species in all ecosystems but in novel immature ecosystems. For type of reproduction, there were more established species reproducing exclusively by seeds in hybrid and both types of novel ecosystems than in natural remnants when compared to flexible species. There was no difference between flexible species and species that exclusively reproduce clonally.
Ellenberg’s continentality did not interact with type of ecosystem but with native status: For archaeophytes, there were more continental species and species with medium response to continentality among established species, while for neophytes, more medium and oceanic species were present among established species. For native species, there were slightly more indifferent and continental species among established species.
Ellenberg values of light, nitrogen, reaction, and temperature interacted with both type of ecosystem and native status. Compared to indifferent species, there were more established species adapted to sunny or medium light, medium temperatures, and medium or high nutrient loads within novel immature ecosystems. In hybrid and novel mature ecosystems, species adapted to acid soils were less common among established species than in the other types of ecosystems when compared to pH-indifferent species. An indifferent response to soil pH characterized established species in most cases. For archaeo- and neophytes, species adapted to shady sites, to either high or low nutrient loads, and to medium temperatures were more common among established species than was the case for indifferent species. Patterns were opposite for native species. Among native established species, there were less species adapted to alkaline soils than pH-indifferent species but this pattern was less pronounced for the two groups of non-native species. Archaeophytes were the only group characterized by more established species with adaptation to neutral pH than pH-indifferent species.
Both the beginning and duration of flowering interacted with type of ecosystem and native status, but only in the models including t2-smooths. More species with a late start of flowering were present among established species in hybrid ecosystems, while the opposite was true for novel mature ecosystems. Less species with long flowering duration were present among established species in natural remnants and novel mature ecosystems but not in the two other types of ecosystems. Differences among native species, archaeo- and neophytes were weak and only present in the smoother-model with slightly less species with a late start of flowering present among established archaeophytes but an opposite pattern for established native species. Moreover, there were less species with long vs. short flowering duration among established neophytes. As patterns of end and duration of flowering were similar, only the former was included into the full model.
Seed mass interacted with both type of ecosystem and native status but only in the model including smooths. However, estimated errors in this model were very high compared to estimates. No smooth-model results are mentioned for any other continuous or integer trait as model results with and without smoothers were identical. Pollination vector interacted neither with type of ecosystem, nor with native status. Some combinations of dispersal type and Ellenberg value for moisture with type of ecosystem and native status contained ≤ 5 species. Consequently, traits principally suitable for inclusion into the full model were maximum canopy height, beginning and end of flowering, life span, and type of reproduction, Ellenberg values for continentality, light, nitrogen, reaction, and temperature.
Of the traits principally suitable for inclusion into the full model, all numerical traits were correlated with < 0.7, not providing any reason for exclusion from the full model (Dormann et al., 2013). For categorical and integer traits, principal component analysis showed that Ellenberg continentality, light and temperature, i.e., all climate-related attributes—were correlated, and Ellenberg nitrogen and reaction, i.e., both soil-related attributes—were correlated. Consequently, besides the numerical traits maximum canopy height, beginning and end of flowering, life span, and type of reproduction, Ellenberg temperature and Ellenberg nitrogen were included into the full model.
Results of the starting full model (before applying stepwise backward selection) with and without smoothers were identical. The model without smoothers was chosen for backward selection. In the course of seven backward selection steps, the following traits were excluded (in the order given) because they had no significant effect: Ellenberg temperature with all its interactions, interaction of type of ecosystem by Ellenberg nitrogen, interaction of native status by maximum canopy height, interaction of native status by beginning of flowering, interaction of native status by type of reproduction, interaction of native status by end of flowering, and interaction of type of ecosystem by beginning of flowering. Thus, predictors with significant effects included in the final full model (Table 3) were the interactions of type of ecosystem by native status, life span (Figure 1A), type of reproduction (Figure 1B), flowering end (Figure 1C), and maximum canopy height (Figure 1D), the interactions of native status by life span (Figure 2A) and by Ellenberg nitrogen (Figure 2B), as well as species identity and phylogenetic relationships among species.
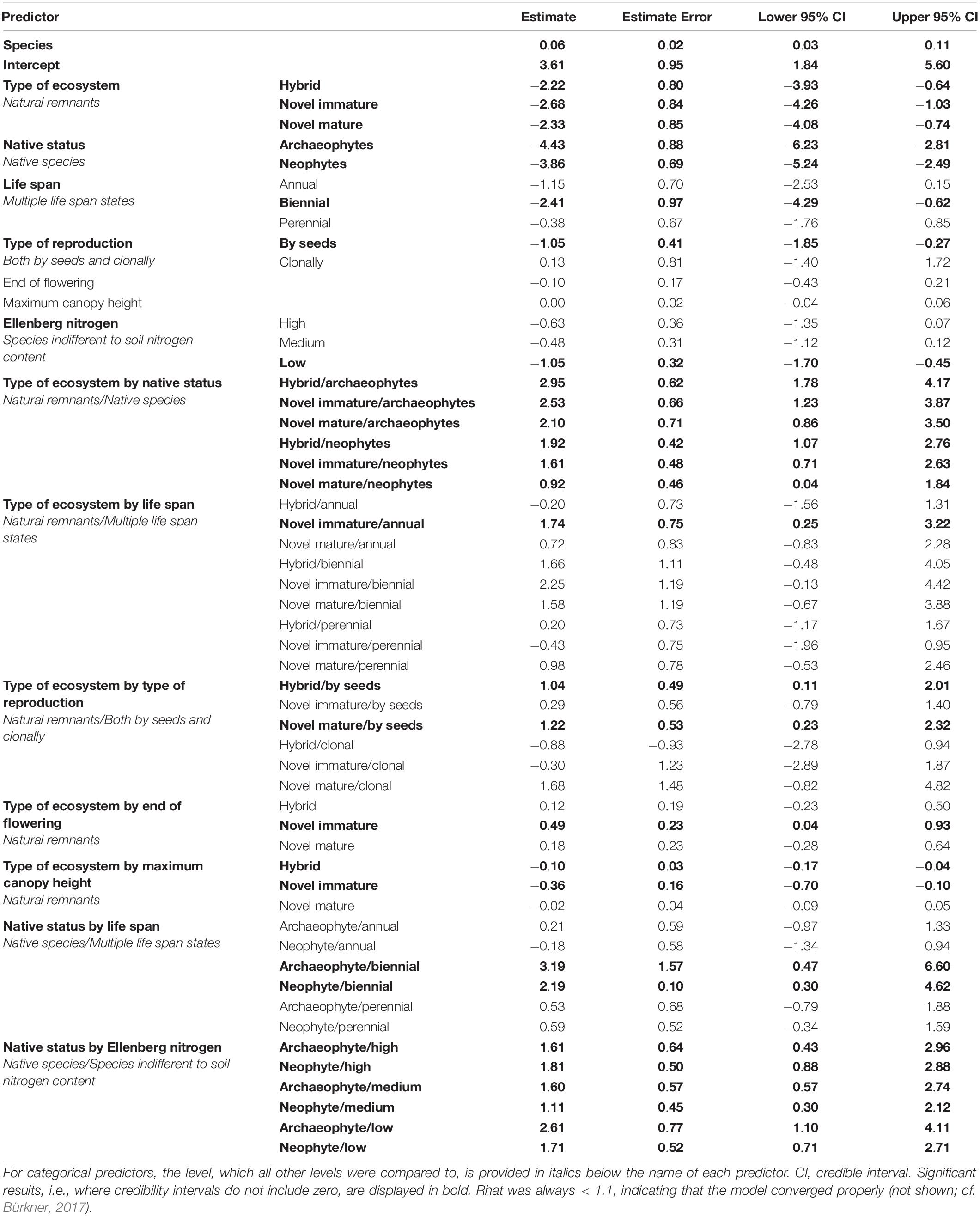
Table 3. Result of the final full model (as result of stepwise backward selection) explaining the establishment of vascular plant species across the city of Berlin, Germany with interactions of type of ecosystem by native status, type of ecosystem by traits, and native status by traits as fixed predictors, together with species identity as a random predictor and a covariance matrix of species based on phylogenetic relationships (summed as predictor “species”).
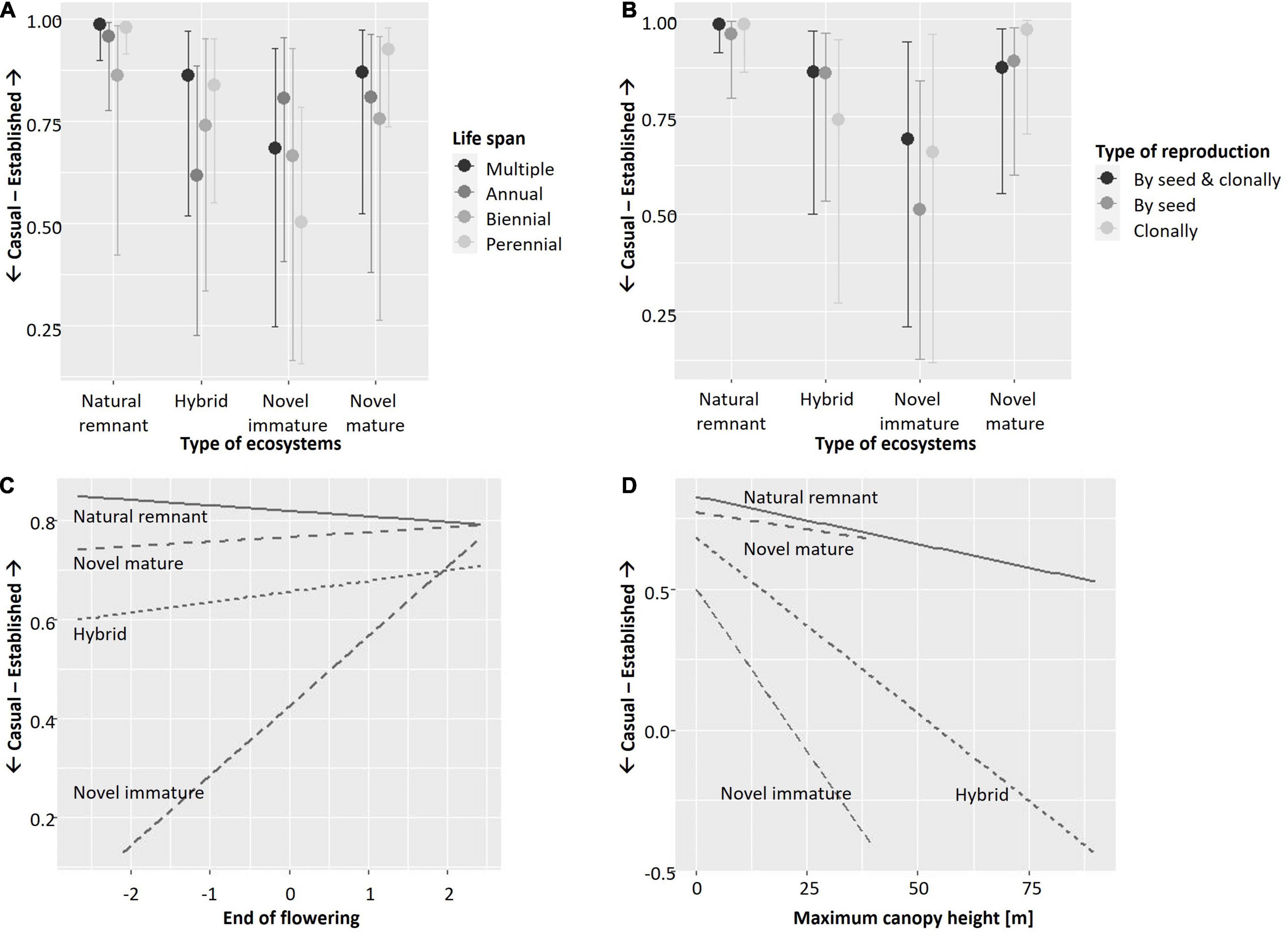
Figure 1. Plots illustrating significant interactions of (A) life span, (B) type of reproduction, (C) end of flowering, and (D) maximum canopy height with type of ecosystem in explaining the establishment of vascular plant species in Berlin, Germany. Results shown are based on the full model (cf. Table 3). Y-axes show casual vs. established species as an ordinal response variable; for (A,B) x-axes show the four types of ecosystems, while for (C,D), x-axes show the respective trait. (A,B) Were created using function “conditional_effects.brmsfit” in R-package brms (Bürkner, 2017). (C,D) Were created using function “ggplot” in R-package ggplot2 with geometric smoother (Wickham, 2016).
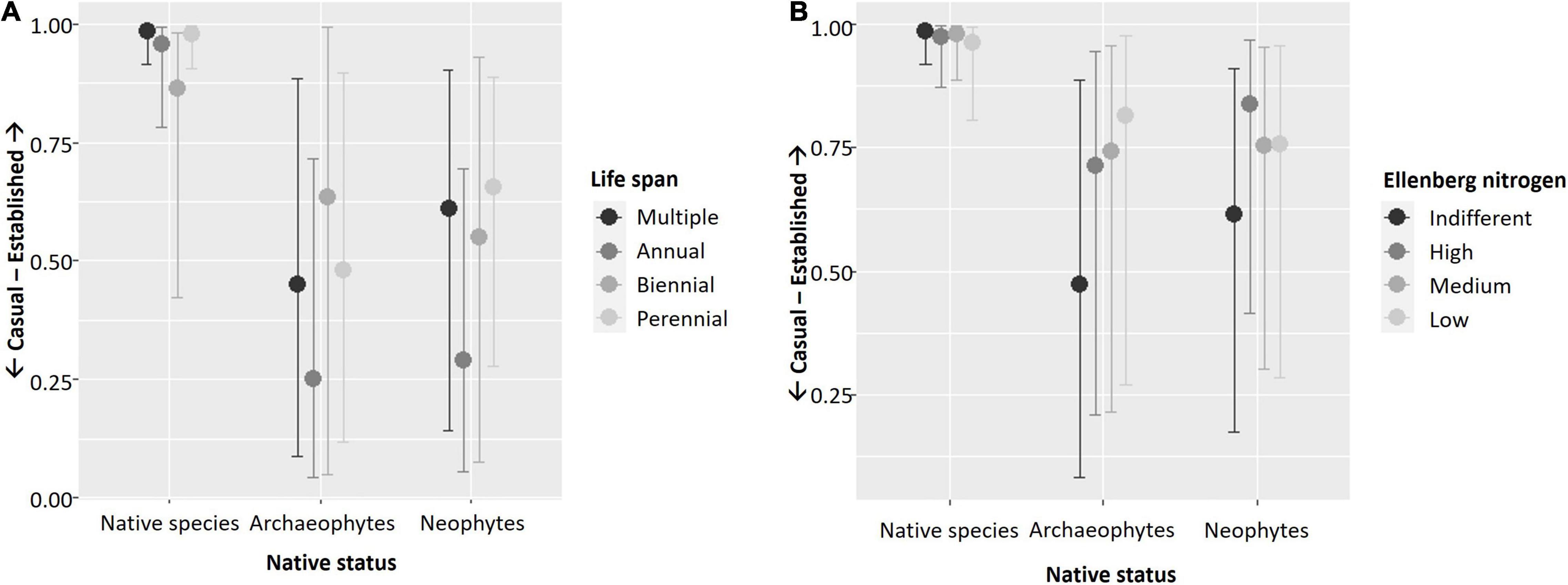
Figure 2. Plots illustrating significant interactions of (A) life span and (B) Ellenberg nitrogen with native status in explaining the establishment of vascular plant species in Berlin, Germany. Results shown are based on the full model (cf. Table 3). Y-axes show casual vs. established species as an ordinal response variable and x-axes show the four types of ecosystems. Plots were created using function “conditional_effects.brmsfit” in R-package brms (Bürkner, 2017).
Similar to single trait models, the interaction of type of ecosystem and native status was significant, with less archaeo- and neophytes among established species in natural remnants than in all other ecosystems compared to native species. With respect to traits, patterns for maximum canopy height resembled those of the single model. Again, end of flowering did only interact with type of ecosystem, but in contrast to the single model, only in novel immature ecosystems were there more established species with late vs. early end of flowering. As in the single trait model, type of reproduction interacted with type of ecosystem only. However, in the full model, there were less species exclusively reproducing by seeds than species reproducing both clonally and by seeds among established species within natural remnants and novel immature ecosystems. Life span interacted with both type of ecosystem and native status in the full model but in contrast to the single model, there were no differences any longer for biennial and perennial species. Rather, in novel immature ecosystems, there were more established annual species than established species with multiple life span types, opposite to all other types of ecosystems. Also in contrast to the single model, Ellenberg nitrogen interacted with native status only, and for both archaeo- and neophytes, there were less established species characterized by indifferent response to soil nitrogen concentration, compared to all other groups, while this was not the case for native species.
A key challenge in urban biodiversity conservation is to understand which and how drivers govern plant population establishment in different urban ecosystems (Shwartz et al., 2014; Lepczyk et al., 2017; Kowarik and von der Lippe, 2018; Piana et al., 2019). Still, the majority of urban biodiversity studies so far relied on purely spatial analysis, not incorporating temporal trends in biodiversity (Knapp et al., 2021). Also, comparative analyses of urban biodiversity often focus on rural-to-urban gradients, urban vs. rural patterns, or on selected urban ecosystems, such as forests or residential locations (Rega-Brodsky et al., 2022). Fewer analyses focus on comparing different ecosystems within a city (but see Lososová et al., 2011, 2012; Kalusová et al., 2019; Planchuelo et al., 2019). Here, we analyzed the role of functional traits in governing the establishment of vascular plant species across urban ecosystems of different ecological novelty, and across native and non-native species. While Kowarik and von der Lippe (2018) elucidated differences in the establishment of native vs. non-native species across ecosystems of varying novelty, we show that traits are key drivers of these establishment processes. Our results are in line with Kowarik and von der Lippe (2018) in that native species establish specifically well in natural remnants, while this is less obvious for non-native species. Many non-native species are pre-adapted to disturbed urban ecosystems (e.g., Borowy and Swan, 2020) and so their proportion decreases with age of succession and increases with frequency of disturbance across European cities (Lososová et al., 2012).
In addition, our results show that the traits that drive plant species establishment differ across types of ecosystems. Some traits are especially beneficial in novel immature ecosystems, in line with our second hypothesis. Here, the increased establishment of annual in comparison to other species corresponds to frequent disturbance and mirrors the high frequency of annual species that has been found in urban areas globally (Williams et al., 2015) and in early successional urban ecosystems specifically (Lososová et al., 2011). Annual herbs tend to be smaller in size than perennial woody species, and so it fits with the aforementioned pattern that established species in novel immature ecosystems tend to be small. The same was true for hybrid ecosystems. Both novel immature and hybrid ecosystems include relatively young ecosystems, such as initial and intermediate stages of succession after abandonment, as well as open ecosystems, such as grasslands in agricultural context (Planchuelo et al., 2019), where herbaceous species dominate.
Novel immature ecosystems tend to be overrepresented in sites strongly subjected to the urban heat island (such as vacant lots or industrial brownfields in dense urban areas). Moreover, due to their openness compared to novel mature ecosystems, they might experience both higher temperatures and higher fluctuation in temperatures. As a result, species with late end of flowering are promoted. Additionally, the high share of late-flowering species among non-native species in general introduces a bias (Kowarik, 2005; Küster et al., 2008; Knapp and Kühn, 2012; Pyšek et al., 2015), with late flowering non-natives benefiting of reduced competition by native species (Celesti-Grapow et al., 2003; Godoy et al., 2009).
Interestingly, exclusive reproduction by seeds was not the main trait characterizing species established in novel immature ecosystems, but flexibility (reproducing both by seeds and clonally) ranked higher. This partly fits patterns shown by Lososová et al. (2006). They compared sites in settlements and agricultural fields, showing that plant species typical of settlements reproduce both by seeds and clonally, while species exclusively reproducing by seeds are more typical of agricultural sites. Novel immature ecosystems in Berlin comprise both intensively managed agricultural fields, and highly urban sites (ecosystems along roads or tracks, initial stages of wasteland succession, etc.; Kowarik and von der Lippe, 2018) but the latter prevail. It seems that these highly urban sites, which are often characterized by disturbance, soil pollution, and other stressors are so demanding that functional flexibility becomes an important feature in plant reproduction. Similarly, Westermann et al. (2011) showed that on abandoned railway sites of Berlin, seed production correlated negatively with the presence of ruderal ecosystems. Williams et al. (2015) showed that heavier seeded plants are promoted in urban environments, with no clear patterns of clonality. Still, non-native species in general tend to benefit from clonality (Pyšek et al., 2015)—and this might explain the pattern we found for novel mature ecosystems, where species that exclusively reproduce clonally were best in establishing.
However, we did not find much support for our third hypothesis that traits, which have been found to generally increase the occurrence frequency of non-native species as compared to native species (van Kleunen et al., 2010; Knapp and Kühn, 2012) also characterize established non-native vs. established native species. Rather, type of ecosystem was involved in most interactions of our full model. This highlights the importance of comparing different types of urban ecosystems in the identification of drivers of species success and failure. Comparing trait-patterns across broad urban vs. rural floras masks such detailed insights (Kalusová et al., 2017). However, we did not test for three-way interactions (type of ecosystem by native status by traits), because models failed to converge. Identifying those more complicated interactions, as well as interactions among traits (Küster et al., 2008) will be another step forward in our understanding of ways to promote urban biodiversity.
Our hypothesis that functional flexibility more strongly supports the establishment of native as compared to non-native plant species, as had been suggested by experimental work of Borowy and Swan (2020), was partly supported. In Ellenberg nitrogen values, this was clearly the case, with established non-native species being mainly not indifferent toward soil nitrogen loads, but not so in native species. In life span, which was the only other trait whose interaction with native status affected species establishment, reduced benefits of flexibility in non-native species were not that obvious. Overall, our approach of classifying those species as flexible that are able to express different states of a trait might have been too coarse. It acknowledges that species express intraspecific trait variability (Bolnick et al., 2011), but does not measure the full range of this variability. Borowy and Swan (2020) showed that in response to urban environmental conditions, native species’ traits more often diverge (meaning that their mean trait values shift but not trait variance) than is the case for non-native species. Also, trait evolution affects the ability of species to cope and persist in urban environments (Cheptou et al., 2008; Donihue and Lambert, 2014; Alberti, 2015; Johnson and Munshi-South, 2017). Intraspecific trait variation and trait evolution add more levels of complexity to the identification of drivers and mechanisms of species success and failure, which need more research attention (Rivkin et al., 2019).
Our results confirm findings by Kowarik and von der Lippe (2018) that vascular plant species establishing within natural remnants are rather native than non-native species. These findings highlight the chance to remove (potentially) invasive non-native species from natural remnants, before they manage to establish. On top of that, we show that traits help to identify the sources and mechanisms by which vascular plant species migrate and establish within different types of ecosystems. The traits we identified as less beneficial in natural remnants—being short-lived, reproducing by seeds, flowering until late in the year, growing not that tall—seem to be some of these decisive traits (as supported by literature, e.g., van Kleunen et al., 2010; Pyšek et al., 2015). All types of ecosystems add to urban biodiversity, and trait analyses refine our knowledge on how they can be supported in doing so on the long term. This can help in sharpening conservation measures.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
IK and ML provided data on species per type of ecosystem together with background knowledge. SK analyzed the data and drafted the manuscript. All authors together interpreted data, conceived the study, and contributed to the writing and revisions of the manuscript.
SK was funded by the Helmholtz-Centre for Environmental Research—UFZ in the frame of Program-oriented Funding IV (2021–2027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Marina Golivets and Ingolf Kühn at the Helmholtz-Centre for Environmental Research—UFZ for methodological advice. We thank all those who created the dataset of 1,178 vascular plant species used here.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.790340/full#supplementary-material
Supplementary Table 3 | List of vascular plant species occuring in Berlin, Germany with selected traits, the type of ecosystem species occur in within Berlin (natural remnant, hybrid, novel immature, and novel mature ecosystems), species native status (whether species are native or non-native to Berlin — the latter distinguishing among archaeophytes and neophytes), and whether species have established or casual occurrence in the four types of ecosystems (0 = casual occurrence, 1 = established occurrence). This list is underlying the full model described in this article.
Alberti, M. (2015). Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 30, 114–126. doi: 10.1016/j.tree.2014.11.007
Aronson, M. F. J., Nilon, C. H., Lepczyk, C. A., Parker, T. S., Warren, P. S., Cilliers, S. S., et al. (2016). Hierarchical filters determine community assembly of urban species pools. Ecology 97, 2952–2963. doi: 10.1002/ecy.1535
Blackburn, T. M., Pyšek, P., Bacher, S., Carlton, J. T., Duncan, R. P., Jarošík, V., et al. (2011). A proposed unified framework for biological invasions. Trends Ecol. Evol. 26, 333–339.
Böcker, R., Auhagen, A., Brockmann, H., Kowarik, I., Scholz, H., Sukopp, H., et al. (1991). “Liste der wildwachsenden Farn- und Blütenpflanzen von Berlin (West) mit Angaben zur Gefährdung der Sippen, zum Zeitpunkt ihres ersten spontanen Auftretens und zu ihrer Etablierung im Gebiet sowie zur Bewertung der Gefährdung,” in Rote Listen der gefährdeten Pflanzen und Tiere in Berlin 1990, eds A. Auhagen, R. Platen, and H. Sukopp (und Umweltforschung: Landschaftsentwicklung), S6,57–88.
Bolnick, D. I., Amarasekare, P., Araujo, M. S., Burger, R., Levine, J. M., Novak, M., et al. (2011). Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192. doi: 10.1016/j.tree.2011.01.009
Borowy, D., and Swan, C. M. (2020). A Multi-Trait Comparison of an Urban Plant Species Pool Reveals the Importance of Intraspecific Trait Variation and Its Influence on Distinct Functional Responses to Soil Quality. Front. Ecol. Evol. 8:68. doi: 10.3389/fevo.2020.00068
Bürkner, P. C. (2017). Brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28. doi: 10.18637/jss.v080.i01
Bürkner, P. C. (2018). Advanced Bayesian multilevel modeling with the R package brms. R J. 10, 395–411. doi: 10.32614/rj-2018-017
Celesti-Grapow, L., Di Marzio, P., and Blasi, C. (2003). “Temporal niche separation of the alien flora of Rome (Italy),” in Plant Invasions: Ecological threats and Management Solutions, eds L. E. Child, J. H. Brock, G. Brundu, K. Prach, P. Pysek, and P. M. Wade (Netherlands: Backhuys Publishers), 101–111.
Cheptou, P. O., Carrue, O., Rouifed, S., and Cantarel, A. (2008). Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. PNAS 105, 3796–3799. doi: 10.1073/pnas.0708446105
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Donihue, C. M., and Lambert, M. R. (2014). Adaptive evolution in urban ecosystems. Ambio 44, 194–203.
Dormann, C. F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Carré, G., et al. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. doi: 10.1111/j.1600-0587.2012.07348.x
Duncan, R. P., Clemants, S. E., Corlett, R. T., Hahs, A. K., McCarthy, M. A., McDonnell, M. J., et al. (2011). Plant traits and extinction in urban areas: a meta-analysis of 11 cities. Glob. Ecol. Biogeogr. 20, 509–519. doi: 10.1111/j.1466-8238.2010.00633.x
Durka, W., and Michalski, S. (2012). Daphne: a dated phylogeny of a large European flora for phylogenetically informed ecological analyses. Ecology 93:2297.
Durka, W., and Michalski, S. (2016). Daphne: a Dated Phylogeny of a Large European Flora for Phylogenetically Informed Ecological Analyses. Figshare. Available online at: https://figshare.com/collections/Daphne_a_dated_phylogeny_of_a_large_European_flora_for_phylogenetically_informed_ecological_analyses/3305040
Godoy, O., Castro-Díez, P., Valladares, F., and Costa-Tenorio, M. (2009). Different flowering phenology of alien invasive species in Spain: evidence for the use of an empty temporal niche? Plant. Biol. 11, 803–811. doi: 10.1111/j.1438-8677.2008.00185.x
Hadfield, J. D., and Nakagawa, S. (2010). General quantitative genetic methods for comparative biology: Phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508. doi: 10.1111/j.1420-9101.2009.01915.x
Hahs, A. K., McDonnell, M. J., McCarthy, M. A., Vesk, P. A., Corlett, R. T., Norton, B. A., et al. (2009). A global synthesis of plant extinction rates in urban areas. Ecol. Lett. 12, 1165–1173. doi: 10.1111/j.1461-0248.2009.01372.x
Heger, T., Bernard-Verdier, M., Gessler, A., Greenwood, A. D., Grossart, H. P., Hilker, M., et al. (2019). Towards an Integrative, Eco-Evolutionary Understanding of Ecological Novelty: Studying and Communicating Interlinked Effects of Global Change. Bioscience 69, 888–899. doi: 10.1093/biosci/biz095
Hobbs, R. J., Arico, S., Aronson, J., Baron, J. S., Bridgewater, P., Cramer, V. A., et al. (2006). Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 15, 1–7. doi: 10.1111/j.1466-822x.2006.00212.x
Hobbs, R. J., Higgs, E., and Harris, J. A. (2009). Novel ecosystems: implications for conservation and restoration. Trends Ecol. Evol. 24, 599–605.
IPBES (2019). Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, eds E. S. Brondizio, J. Settele, S. Díaz, and H. T. Ngo (Bonn: IPBES secretariat), 1148. doi: 10.5281/zenodo.3831673
Ives, C. D., Lentini, P. E., Threlfall, C. G., Ikin, K., Shanahan, D. F., Garrard, G. E., et al. (2016). Cities are hotspots for threatened species. Glob. Ecol. Biogeogr. 25, 117–126. doi: 10.1111/geb.12404
Johnson, M. T. J., and Munshi-South, J. (2017). Evolution of life in urban environments. Science 358:eaam8327. doi: 10.1126/science.aam8327
Kalusová, V., Čeplová, N., Chytrý, M., Danihelka, J., Dřevojan, P., Fajmon, K., et al. (2019). Similar responses of native and alien floras in European cities to climate. J. Biogeogr. 46, 1406–1418. doi: 10.1111/jbi.13591
Kalusová, V., Čeplová, N., and Lososová, Z. (2017). Which traits influence the frequency of plant species occurrence in urban habitat types? Urban. Ecosyst. 20, 65–75. doi: 10.1007/s11252-016-0588-3
Kembel, S. W., Cowan, P. D., Helmus, M. R., Cornwell, W. K., Morlon, H., Ackerly, D. D., et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. doi: 10.1093/bioinformatics/btq166
Kleyer, M., Bekker, R. M., Knevel, I. C., Bakker, J. P., Thompson, K., Sonnenschein, M., et al. (2008). The LEDA Traitbase: A database of life-history traits of Northwest European flora. J. Ecol. 96, 1266–1274. doi: 10.1111/j.1365-2745.2008.01430.x
Klotz, S., Kühn, I., and Durka, W. (2002). BiolFlor - Eine Datenbank mit biologisch-ökologischen Merkmalen zur Flora von Deutschland. Schriftenr. Für. Veg. 38, 1–333.
Knapp, S., Aronson, M. F. J., Carpenter, E., Herrera-Montes, A., Jung, K., Kotze, D. J., et al. (2021). A Research Agenda for Urban Biodiversity in the Global Extinction Crisis. Bioscience 71, 268–279. doi: 10.1093/biosci/biaa141
Knapp, S., and Kühn, I. (2012). Origin matters: widely distributed native and non-native species benefit from different functional traits. Ecol. Lett. 15, 696–703. doi: 10.1111/j.1461-0248.2012.01787.x
Knapp, S., Kühn, I., Wittig, R., Ozinga, W. A., Poschlod, P., and Klotz, S. (2008). Urbanization causes shifts in species’ trait state frequencies. Preslia 80, 375–388.
Knevel, I. C., Bekker, R. M., Bakker, J. P., and Kleyer, M. (2003). Life-history traits of the northwest European flora: The LEDA database. J. Veg. Sci. 14, 611–614.
Kowarik, I. (1990). “Some responses of flora and vegetation to urbanization in Central Europe,” in Urban Ecology, eds H. Sukopp, S. Hejni, and I. Kowarik (The Hague: SPB Academic publishing), 5–74.
Kowarik, I. (1991). “Berücksichtigung anthropogener Standort- und Florenveränderungen bei der Aufstellung Roter Listen,” in Rote Listen der gefährdeten Pflanzen und Tiere in Berlin 1990, eds A. Auhagen, R. Platen, and H. Sukopp (Umweltforschung: Landschaftsentwicklung), 25–56.
Kowarik, I. (2005). “Urban ornamentals escaped from cultivation,” in Crop Ferality and Volunteerism: A Threat to Food Security in the Transgenic Era?, ed. G. Jonathan (Boca: Taylor & Francis), 97–121.
Kowarik, I. (2011). Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 159, 1974–1983. doi: 10.1016/j.envpol.2011.02.022
Kowarik, I., Hiller, A., Planchuelo, G., Seitz, B., von der Lippe, M., and Buchholz, S. (2019). Emerging urban forests: Opportunities for promoting the wild side of the urban green infrastructure. Sustain 11:6318. doi: 10.3390/su11226318
Kowarik, I., and von der Lippe, M. (2018). Plant population success across urban ecosystems: A framework to inform biodiversity conservation in cities. J. Appl. Ecol. 55, 2354–2361. doi: 10.1111/1365-2664.13144
Kühn, I. (2007). Incorporating spatial autocorrelation may invert observed patterns. Divers. Distrib. 13, 66–69.
Kühn, I., Brandl, R., and Klotz, S. (2004a). The flora of German cities is naturally species rich. Evol. Ecol. Res. 6, 749–764.
Kühn, I., Durka, W., and Klotz, S. (2004b). BiolFlor - a new plant-trait database as a tool for plant invasion ecology. Divers. Distrib. 10, 363–365.
Küster, E. C., Kühn, I., Bruelheide, H., and Klotz, S. (2008). Trait interactions help explain plant invasion success in the German flora. J. Ecol. 96, 860–868. doi: 10.1111/j.1365-2745.2008.01406.x
Lepczyk, C. A., Aronson, M. F. J., Evans, K. L., Goddard, M. A., Lerman, S. B., and Macivor, J. S. (2017). Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. Bioscience 67, 799–807. doi: 10.1093/biosci/bix079
Lososová, Z., Chytrý, M., Kühn, I., Hájek, O., Horáková, V., Pyšek, P., et al. (2006). Patterns of plant traits in annual vegetation of man-made habitats in central Europe. Perspect. Plant Ecol. Evol. Syst. 8, 69–81.
Lososová, Z., Chytrý, M., Tichý, L., Danihelka, J., Fajmon, K., Hájek, O., et al. (2012). Native and alien floras in urban habitats: a comparison across 32 cities of central Europe. Glob. Ecol. Biogeogr. 21, 545–555. doi: 10.1111/j.1466-8238.2011.00704.x
Lososová, Z., Horsák, M., Chytrý, M., Čejka, T., Danihelka, J., Fajmon, K., et al. (2011). Diversity of Central European urban biota: effects of human-made habitat types on plants and land snails. J. Biogeogr. 38, 1152–1163. doi: 10.1111/j.1365-2699.2011.02475.x
Paradis, E., and Schliep, K. (2019). Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528. doi: 10.1093/bioinformatics/bty633
Piana, M. R., Aronson, M. F. J., Pickett, S. T. A., and Handel, S. N. (2019). Plants in the city: understanding recruitment dynamics in urban landscapes. Front. Ecol. Environ. 17:455–463. doi: 10.1002/fee.2098
Planchuelo, G., Kowarik, I., and von der Lippe, M. (2020a). Plant traits, biotopes and urbanization dynamics explain the survival of endangered urban plant populations. J. Appl. Ecol. 57, 1581–1592. doi: 10.1111/1365-2664.13661
Planchuelo, G., Kowarik, I., and von der Lippe, M. (2020b). Endangered Plants in Novel Urban Ecosystems Are Filtered by Strategy Type and Dispersal Syndrome. Front. Ecol. Evol. 8:18. doi: 10.3389/fevo.2020.00018
Planchuelo, G., von der Lippe, M., and Kowarik, I. (2019). Untangling the role of urban ecosystems as habitats for endangered plant species. Landsc. Urban. Plan. 189, 320–334. doi: 10.1016/j.landurbplan.2019.05.007
Pyšek, P., Manceur, A. M., Alba, C., McGregor, K. F., Pergl, J., Stajerova, K., et al. (2015). Naturalization of central European plants in North America: species traits, habitats, propagule pressure, residence time. Ecology 96, 762–774.
Rega-Brodsky, C., Aronson, M., Piana, M., Carpenter, E.-S., Hahs, A., Herrera-Montes, A., et al. (2022). Urban biodiversity: state of the science and future directions. Urban Ecosyst. doi: 10.1007/s11252-022-01207-w
Rivkin, L. R., Santangelo, J. S., Alberti, M., Aronson, M. F. J., de Keyzer, C. W., Diamond, S. E., et al. (2019). A roadmap for urban evolutionary ecology. Evol. Appl. 12, 384–398. doi: 10.1111/eva.12734
Shwartz, A., Turbé, A., Julliard, R., Simon, L., and Prévot, A. C. (2014). Outstanding challenges for urban conservation research and action. Glob. Environ. Chang. 28, 39–49. doi: 10.1016/j.gloenvcha.2014.06.002
Sukopp, H. (1987). On the history of plant geography and plant ecology in Berlin. Englera 7, 85–103.
van Ham, C., Genovesi, P., and Scalera, R. (2013). Invasive Alien Species: The Urban Dimension, Case Studies on Strengthening Local Action in Europe. Belgium: IUCN European Union Representative Office, 103.
van Kleunen, M., Weber, E., and Fischer, M. (2010). A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 13, 235–245. doi: 10.1111/j.1461-0248.2009.01418.x
Westermann, J. R., von der Lippe, M., and Kowarik, I. (2011). Seed traits, landscape and environmental parameters as predictors of species occurrence in fragmented urban railway habitats. Basic. Appl. Ecol. 12, 29–37. doi: 10.1016/j.baae.2010.11.006
Williams, N. S. G., Hahs, A. K., and Vesk, P. A. (2015). Urbanisation, plant traits and the composition of urban floras. Perspect. Plant Ecol. Evol. Syst. 17, 78–86. doi: 10.1016/j.ppees.2014.10.002
Keywords: alien plant species, Bayesian framework, biodiversity conservation, functional traits, novel ecosystems, population establishment, urban land use, urbanization
Citation: Knapp S, von der Lippe M and Kowarik I (2022) Interactions of Functional Traits With Native Status and Ecosystem Novelty Explain the Establishment of Plant Species Within Urban Ecosystems: Evidence From Berlin, Germany. Front. Ecol. Evol. 10:790340. doi: 10.3389/fevo.2022.790340
Received: 06 October 2021; Accepted: 13 April 2022;
Published: 16 May 2022.
Edited by:
Federico Morelli, Czech University of Life Sciences Prague, CzechiaReviewed by:
Roderick Fensham, University of Queensland, AustraliaCopyright © 2022 Knapp, von der Lippe and Kowarik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sonja Knapp, c29uamEua25hcHBAdWZ6LmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.