- 1School of Geography and Ecotourism, Southwest Forestry University, Kunming, China
- 2Southwest Research Center of Ecological Civilization (State Forestry and Grassland Administration), Kunming, China
In order to cope with environmental changes, plants constantly adjust their morphological characteristics in order to adapt to changing environment. In the present study, populations of Bombax ceiba from Mengla area and Yuanjiang area in Yunnan Province were selected as the research objects. Six tree structure factors, such as tree height and crown width, eight leaf trait factors, such as leaf area and leaf length, and several habitat factors, such as area topography, meteorology and soil nutrients, were measured. Structural equation model and variation decomposition method were applied to analyze the effects of various habitat factors on tree structure and leaf traits of B. ceiba, and to reveal its morphological responses to habitat heterogeneity. The results showed that there was a significant negative correlation between tree structure and leaf traits in the two study habitats (Mengla area and Yuanjiang area), and the correlation coefficient was −0.47 in Mengla area and −0.22 in Yuanjiang area. Both topographic and soil factors had positive effects on tree structure of the two habitats, and the topographic factors had a greater impact on tree structure than leaf traits. The main difference was that meteorological factors had a positive effect on tree structure of Mengla, but a negative effect on leaf traits, while Yuanjiang showed the opposite patterns. The variation analysis showed that the superposition of three environmental factors in Mengla area had a greater explanation power of tree structure and leaf traits than that in Yuanjiang area, and the topographic factors had the largest explanation power of tree structure in both areas, which reflected that fact that the characteristics of Mengla habitat imposed a greater influence on B. ceiba. The soil factors in Mengla area accounted for 20.1% of the leaf traits, while the meteorological factors in Yuanjiang area accounted for 11.6%. The results showed that leaf traits were sensitive to environmental differences. In general, the responses of B. ceiba to heterogeneous habitats is based on the specific performance of its resource utilization capacity. The research results can provide references for exploring the morphological responses of plants to heterogeneous habitats.
1. Introduction
The Fifth Assessment Report (AR5) and the IPCC Special Report on Global Warming of 1.5°C published by the United Nations Intergovernmental Panel on Climate Change points out that global temperatures will continue to rise in the future, and drought levels will become more intense (IPCC, 2013), which further affect tree growth (Deslauriers et al., 2007), and alter the structure and functions of forest ecosystems (Bonan, 2008; Peng et al., 2011). Therefore, in the context of climate change, clarifying the responses of morphological characteristics and community structure to environmental factors can help us better understand the changing trends of forest ecosystems, and provide a theoretical basis for forest conservation and management.
Plants are an important part of ecosystems, and their growth is strongly influenced by environmental conditions. Therefore, small changes in the environment can directly and indirectly affect plant growth (Mc Dowell et al., 2008; Korner and Basler, 2010; Deslauriers et al., 2017), and they often exhibit varying morphological characteristics. Morphological structure and functional traits are measurable characteristics of plants that have adapted and evolved over time to the external environment (Díaz et al., 1998), and these ecological responses to environmental gradients (climatic conditions, soil nutrients, altitude) have attracted great interest (Holscher et al., 2002). Tree structure can reflect the radial growth of plants, and there are differences in the effects of different environmental gradients on tree structure. Some studies have shown that increasing temperature and changing precipitation could affect tree growth patterns (Williams, 1997). A study on the seasonal dynamics of radial growth of Qinghai spruce (Picea crassifolia) found that soil temperature was a limiting factor of tree growth (Tian et al., 2017). Similarly, Jiang et al. (2015) and Gao et al. (2019) investigated the seasonal dynamics of side cypress (Platycladus orientalis) and Helan Mountain oil pine (Pinus tabuliformis) in semi-arid areas of northern China. These plants were monitored for intra-annual radial growth, and water availability during the growing season determined the amount of radial growth of their lateral structure. Leaves are the main organs of gas exchange between plants and their external environment. They are sensitive to environmental changes, and are widely used as a measure of the trade-off between plant growth rate and resource conservation (Wright et al., 2004). Under the constraints of limited resources, in response to stress factors such as shade and soil moisture, plants tend to regulate stomatal conductance and net photosynthetic rate by selecting leaf conformation patterns to improve their adaptation to the habitat (Funk and Cornwell, 2013). Jacobs (1999) studied the relationship between plant leaf morphological characteristics and climatic factors within 30 plant communities near the equator, and showed that leaf size was most correlated with precipitation, with leaf aspect ratio positively correlated with mean annual precipitation. The present study investigates the association between tree structure and leaf traits of B. ceiba in different habitats, and reveal the response mechanisms of tree structure and leaf traits to heterogeneous habitats.
B. ceiba is a deciduous tree species in the Malvaceae family. It can reach 30 to 40 meters in height and up to 3 meters in trunk diameter, and is widely distributed in tropical and subtropical regions. Due to its large beautiful flowers, it is with high ornamental value. Various tree parts of B. ceiba have medicinal uses. In particular, it is adaptable to harsh environmental conditions with high resistance.
In the present study, the morphological structure of B. ceiba was divided into two physical traits, namely, tree structure factors (e.g., tree height, diameter at breast height, crown width, height under branches, number of branches, taperingness) and leaf traits (e.g., leaf area, leaf length, leaf width, leaf circumference, fresh weight, saturated weight, dry weight, leaf water content). By analyzing the responses of tree structure and leaf traits to heterogeneous environment, we aim to provide scientific reference to reveal the response mechanisms of plant morphological structure to habitat heterogeneity. We specifically tested the following hypotheses: 1. Habitat conditions affect the morphological and structural characteristics of B. ceibas. 2. The morphological and structural characteristics of B. ceibas are correlated, and their correlations change in different habitats. 3. In different habitat, the relative importance of meteorological, topographic and soil factors in affecting the morphological and structural characteristics of B. ceibas varies.
2. Research methods
2.1. Measurement items and methods
The morphological structure of 230 B. ceiba individuals in the Mengla area, and 200 B. ceiba individuals in the Yuanjiang dry heat valley were measured by field survey in 2019, with relevant environmental conditions measured (Figure 1).
The height (H), diameter at breast height (DBH), crown breadth (CB), and height to crown base (HCB) of each B. ceiba were measured; the number of branches (NB) was counted from four directions: east, west, north and south; the whole B. ceiba was photographed with a camera. The NB was counted from the east, west, north and south directions. The entire B. ceiba was photographed with a camera and imported into CAD software, and the taperingness (T) was calculated using the equivalence relationship.
For each B. ceiba plant, 20 fully extended and healthy leaves were taken from the middle outer ring of the canopy in the four directions of southeast and northwest. The leaf length (LL), leaf width (LW), leaf area (LA), and perimeter (P) of each leaf were measured using a portable laser leaf area meter (CID CI-202, United States). The fresh weight (LFW), saturated weight (LSW), leaf dry weight (LDW), and leaf water content (LWC) were calculated by Shimadzu analytical balance (ATY124, Japan).
The longitude, latitude, elevation and aspect of the habitat for each B. ceiba were measured using a handheld GPS instrument, and the slope was measured using a geological compass. Aspect was measured by starting from facing east and rotating in clockwise direction, and the specific quantified transformation was as follows: 1 for the north slope (247.5–292.5), 2 for the northeast slope (292.5–337.5), 3 for the northwest slope (202.5–247.5), 4 for the east slope (337.5–22.5), 5 for the west slope (167.5–202.5), 6 for the southeast slope (22.5–67.5), 7 for the southwest slope (112.5–167.5), and 8 for the south slope (67.5–112.5). In order to eliminate the dimensional relationship between the observed variables and make the data comparable, the observed indexes were standardized and normalized. Soil samples were taken from B. ceiba growing area according to the 5-point sampling method, and samples were brought back to the laboratory for the analysis of total nitrogen, total phosphorus, available phosphorus, ammonium nitrogen and nitrate-nitrogen. Meteorological data were obtained from the Chinese ground-based meteorological station Mengla (21°28′N, 101°35′E) and Yuanjiang meteorological station (23°36′N, 101°59′E) for 10 years from 2009 to 2018 for average temperature, average relative humidity, and average annual rainfall.1
2.2. Data processing
SPSS 22.0 and Canoco 5.0 software were used for statistical analysis of the data. First, principal component analysis was done separately for tree structure, leaf traits, soil factors, topographic factors and meteorological factors, and the coefficients of each observed variable were used to divide the 1st principal component axial load factor by the square root of the corresponding principal component eigenvalue. Next, Pearson coefficient was quantified to analyze the correlation among measured factors. Variance decomposition analysis was performed in Canoco 5.0 software to explore the explanatory power of each habitat factor on the structure and leaf traits of B. ceiba.
3. Analysis of results
3.1. Analysis of morphological and structural characteristics of Bombax ceibas in different study area
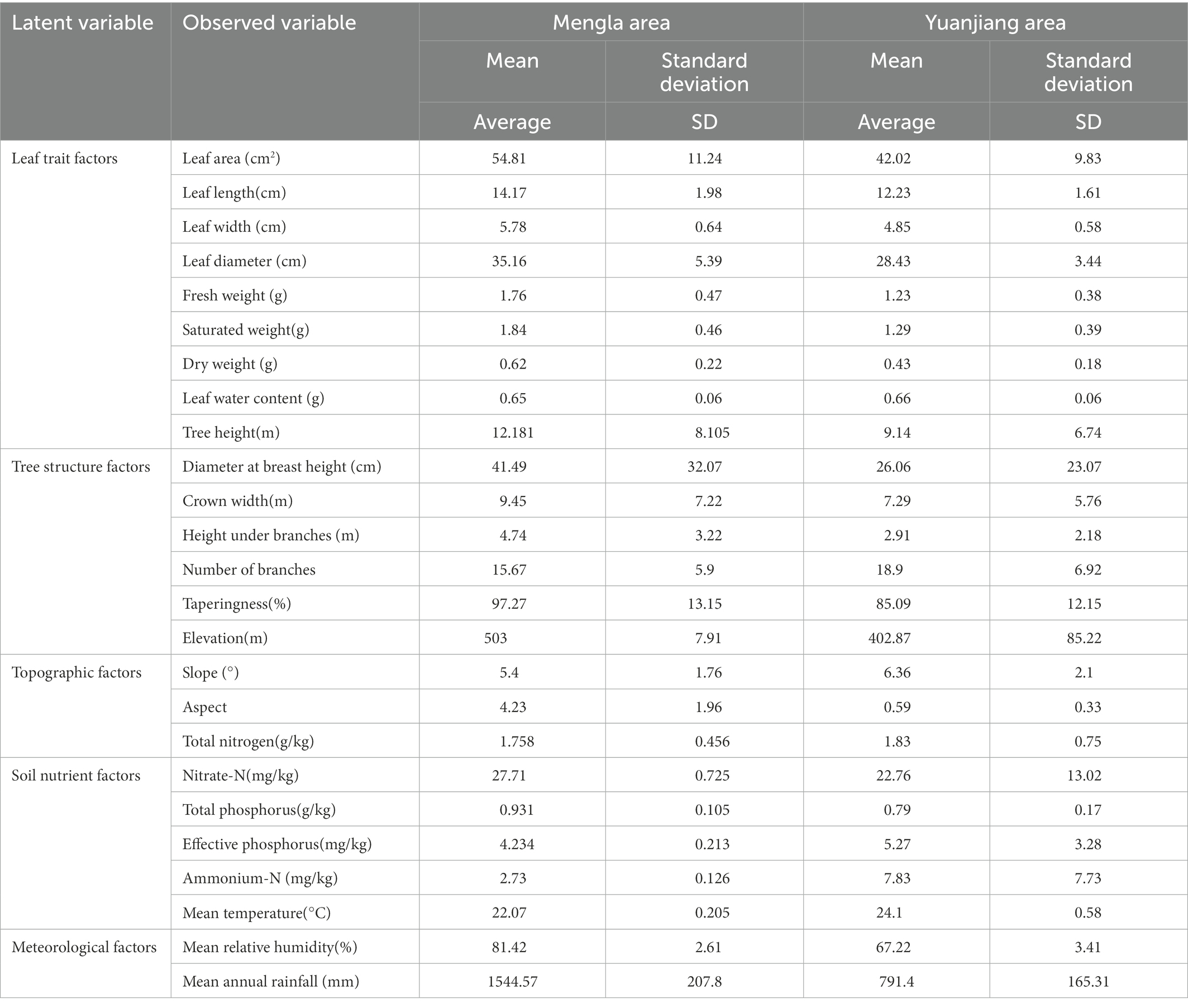
The leaf traits and structural characteristics of B. ceiba in Mengla and Yuanjiang areas were measured separately, and the overall mean values of these growth factors of B. ceiba in Mengla area were larger than those in Yuanjiang area. The average temperature in Yuanjiang is higher than that of Mengla, while the average relative humidity and average annual precipitation is lower than that of Mengla, which reflects the poor habitat conditions in Yuanjiang (Table 1).
Redundancy analysis was performed for each of the three habitat factors and each of the B. ceiba morphological structure factors. The first two axes explained 65.5 and 8.6% of the total variance, respectively, with a total explanatory power of 74.1%, which suggests that the RDA results were reliable. Slope, aspect, nitrate-N, total phosphorus, effective phosphorus, ammonia-N, nitrate-N, mean annual rainfall, and mean relative humidity were positively correlated with B. ceiba morphological structure at axis one, which explained 6.7, 0.9, 2.4, 2.6, 2.9, 4.8, 3.0, 58.3, and 1.1% of the variance, respectively, whereas elevation and mean temperature were negatively correlated with B. ceiba morphological structure, and explained 10.2 and 7.1% of the variance variation, respectively (Figure 2).
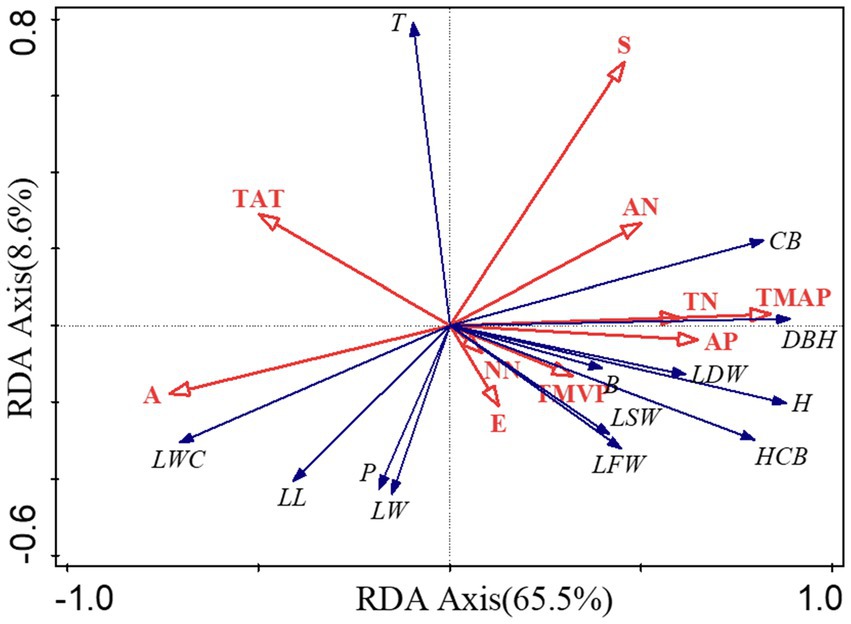
Figure 2. RDA analysis of habitat factors and growth factors of B. ceiba. Note: H: Height; DBH: Diameter at breast height; CB: Crown breadth; HCB: Height to crown base; T: Taperingness; B: Branch; LL: Leaf length; LW: Leaf width; P: Perimeter; LA: Leaf area; LFW: Leaf fresh weight; LSW: Leaf Saturated Weight; LDW: Leaf dry weight; LWC: Leaf water content; TN: Total Nitrogen; TP: Total Phosphorus; AP: Available Phosphorus; AN: Ammonium-N; NN: Nitrate-N; TAT: The Average Temperature; TMVP: The Mean Vapor pressure; TMAP: The Mean Annual Precipitation; A: Altitude; S: Slope; E: Aspect.
3.2. Correlation analysis of various factors of Bombax ceibas structure under different habitats
The correlations of the factors of tree structure of B. ceiba in different habitat conditions were different. The correlation coefficients of tree height with diameter at breast height, crown width, height under branches and dry weight in Mengla area were highly significant and positive. The correlation coefficient was greater than 0.6, and the correlation coefficient with water content was-0.685. The correlation coefficient between diameter at breast height and crown width, height under branch and dry weight was highly significant and positive, all correlation coefficients were greater than 0.6, and the correlation coefficient with water content was highly significant and negative, with the correlation coefficient as −0.721. The correlation coefficient between crown width and branch height was 0.697, and the correlation coefficient between crown width and branch height was −0.715.The correlation coefficients were above 0.6 for fresh weight, saturated weight and dry weight, and-0.66 for water content. Leaf area was highly significantly and positively correlated with leaf length, leaf width and circumference, with correlation coefficients above 0.7. Leaf length was significantly and positively correlated with leaf width and girth, with correlation coefficients of 0.73 and 0.755. Leaf width was highly significantly and positively correlated with perimeter, fresh weight and saturated weight, with correlation coefficients above 0.6. Fresh weight was highly significantly and positively correlated with saturation weight and dry weight, with correlation coefficients of 0.994 and 0.926. Saturated weight was highly significantly and positively correlated with dry weight, with a correlation coefficient of 9.26. Dry weight was highly significantly and negatively correlated with water content, with a correlation coefficient of −6.97.
The correlation coefficient between tree height and diameter at breast height, crown width, branch height and dry weight in Yuanjiang area was highly significant and positive, with correlation coefficients greater than 0.6, and highly significant and negative correlation with water content, with correlation coefficient of −0.723. The correlation coefficients between diameter at breast height, crown width and branch height were highly significant and positive, with correlation coefficients of 0.954 and 0.798, respectively, and highly significant and negative correlation with water content, with correlation coefficient of −0.805; the correlation coefficients between crown width, branch height and dry weight were highly significant and positive. The correlation coefficients were 0.85, 0.625 and −0.769; the correlation coefficients were 0.607 and 0.742; the correlation coefficients were −0.778; the correlation coefficients were 0.607 and 0.742; the correlation coefficients were −0.778. The correlation coefficients were greater than 0.6; leaf length was highly significantly positively correlated with leaf width and perimeter, with correlation coefficients of 0.709 and 0.984; leaf width was highly significantly positively correlated with perimeter, fresh weight and saturated weight, with correlation coefficients above 0.6; fresh weight was highly significantly positively correlated with saturated weight and dry weight, with correlation coefficients of 0.998 and 0.942; dry weight was highly significantly negatively correlated with water content, with correlation coefficient of −0.767 (Figure 3).
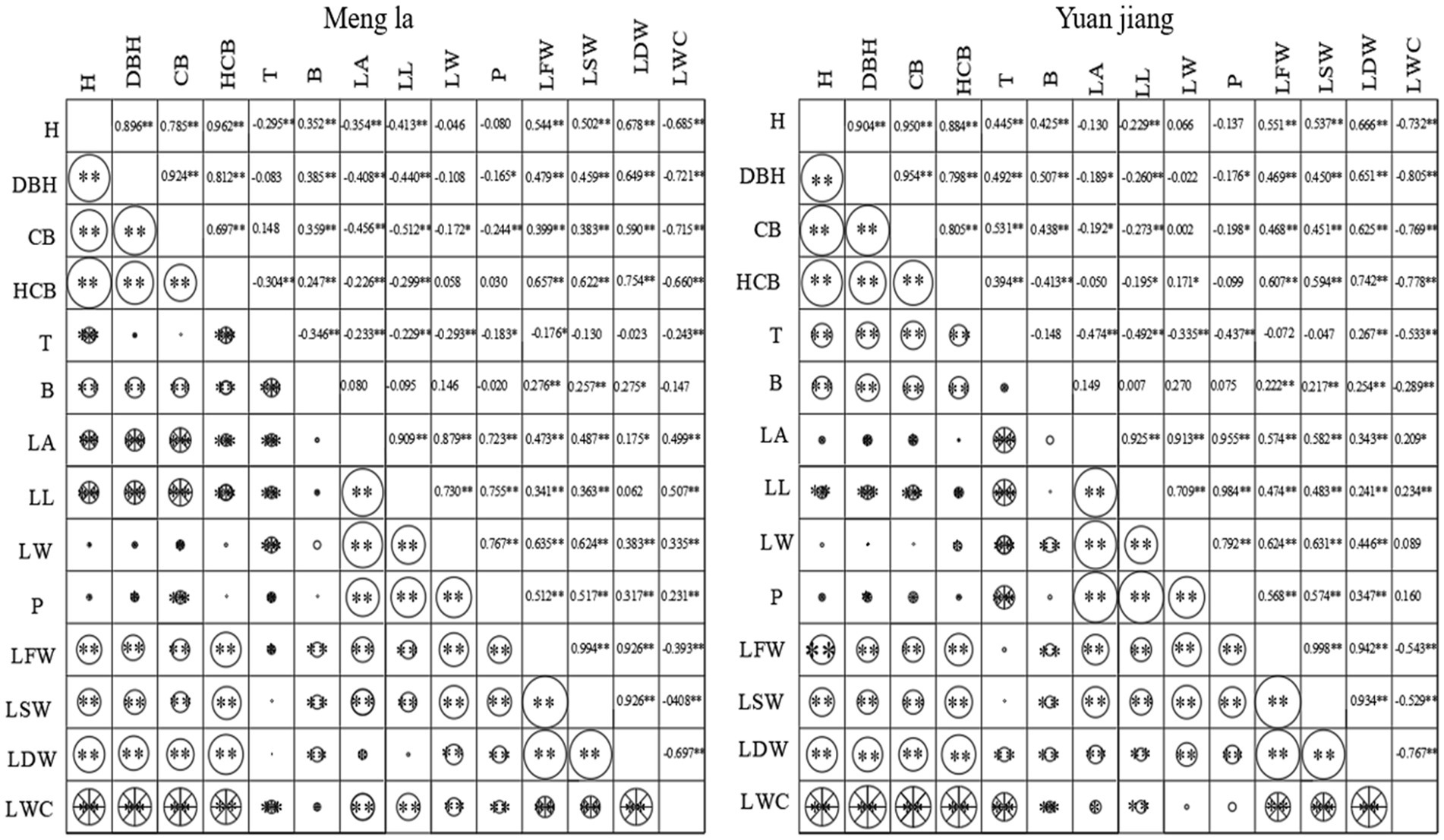
Figure 3. Correlation of different growth factors in regard to B. ceiba morphology and structure. Note: * significant level, p < 0.05, ** very significant level, p < 0.01; Lines mean negative correlation, white circles mean positive correlation, and the size of circles represent the level of correlation.
3.3. Analysis of the explanation power of habitat factors in regard to Bombax ceiba morphology
Meteorological factors, topographic factors and soil nutrients are important components of habitat factors, which play important roles in affecting plant growth. The variance decomposition showed that the total superposition of three types of habitat factors (topographic factors, meteorological factors and soil factors) in Mengla area explained 43.5% of tree structure of B. ceiba, among which topographic factors explained 18.6% of the variation, followed by meteorological factors, which explained 17.5% of the variation, and soil factors explained the smallest variation (12.4%) among the three factors. By contrast, the soil, topographic and meteorological factors had less explanatory power of leaf traits than tree structure, with a total explanation power of 12.3% of the variation. The three habitat factors of topography, soil and meteorology explained 6.0, 20.1 and 4.7% of B. ceiba tree structure, respectively. The superposition of the three habitat factors (topographic, meteorological and soil) explained 30.2% of the tree structure of B. ceibas in Yuanjiang and Mengla, with the topographic factor explaining 13.6% of B. ceiba tree structure, followed by the meteorological factor (explaining 5.6% of the tree structure), and the soil factor (explaining only 2.6% of the tree structure). The soil, topographic and meteorological factors explained less of the leaf traits than tree structure, with a total explanation power of 9.8% of the variation. The meteorological factor explained 11.6% of leaf trait variation, followed by topographic factors (10.4%), whereas the soil factor had the lowest explanatory power (only 2.1%; Figure 4).
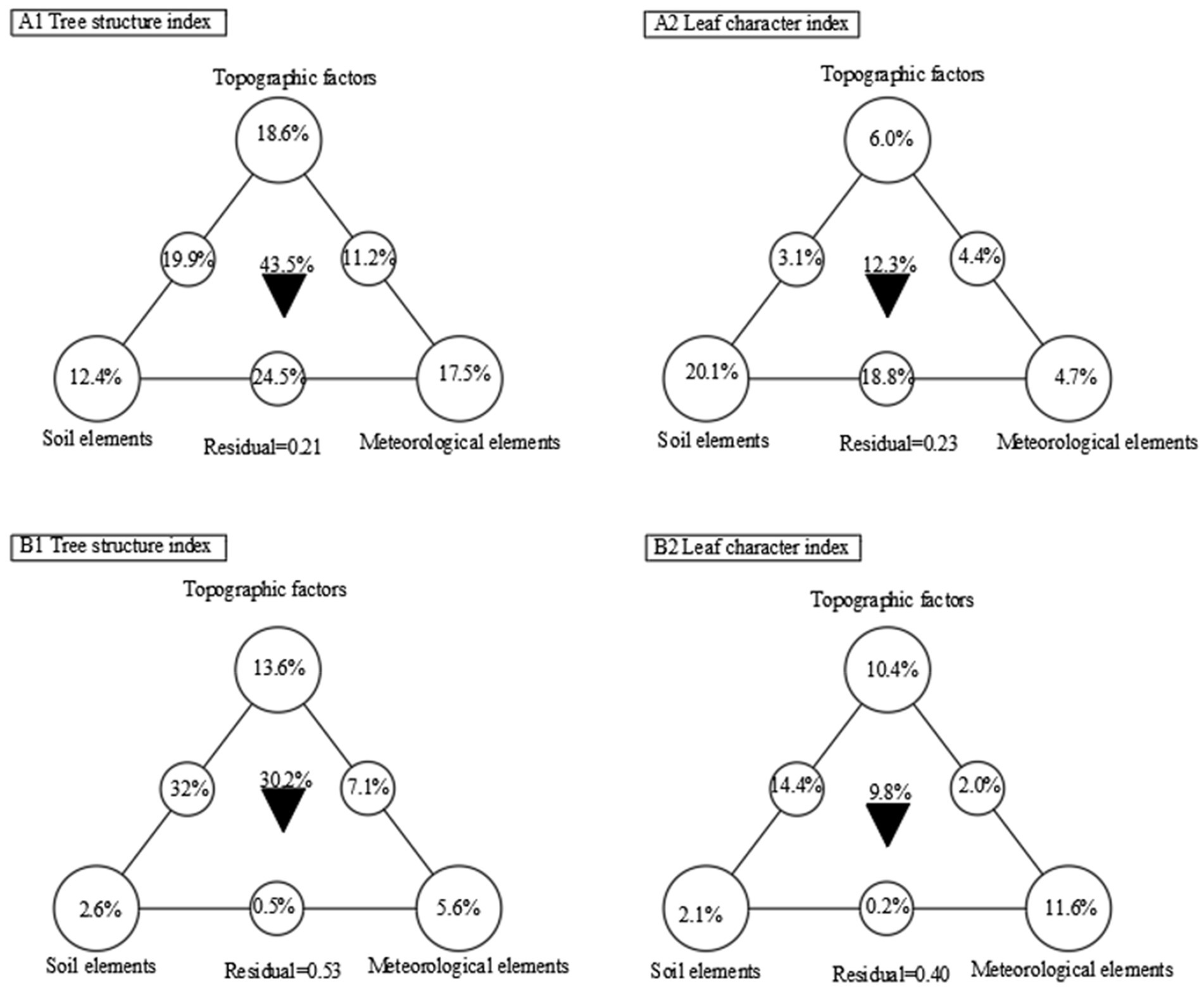
Figure 4. The single and combined effects of meteorological factors, topographic factors and soil factors on tree structure of B. ceiba using variance decomposition method. “▼” represent the combined effects of different factors, A1,A2:Mengla area; B1, B2:Yuanjiang area.
4. Discussion
4.1. Response of Bombax ceiba tree morphological structure to habitat heterogeneity
Plant morphological structure varies greatly under different habitat conditions. With changes in plant growth and resource availability, plants are able to change morphological structure, growth rate, and phenotypic plasticity to maximize the efficient use of resources and biomass production (Huang et al., 2022). It was found that the height, diameter at breast height, crown width, branch height, and number of branches of B. ceiba in Yuanjiang and Mengla areas were highly significantly and positively correlated with each other, suggesting that B. ceiba in heterogeneous habitats is able to adapt to the environment, and further adjusts its tree structure to cope with heterogeneous habitats, thus exhibiting similar growth strategies. Many studies have shown that meteorological factors (precipitation, temperature, humidity, etc.), topographic factors (elevation, aspect, slope, etc.), and soil resources (total nitrogen, total phosphorus, organic carbon content, etc.) affect tree structure (Yang et al., 2022), and the present study found that topographic, soil, and meteorological factors together explained 43.5 and 30.2% of the tree structure of B. ceiba in Mengla and Yuanjiang areas, respectively, and thus exhibited a high explanatory power of tree structure. Therefore, the topographic factors had the highest explanatory powers of tree structure in both habitats. By contrast, the soil and meteorological factors had higher explanatory powers of tree structure in Mengla than in Yuanjiang. It has been shown that C, N and P directly influence plant growth and development, and drive geochemical cycles and transformation of other nutrients (Peri et al., 2008), while climate change affects plant growth and consequently the ecological structure and function of forests (Lenoir et al., 2008). The present study shows that meteorological factors in both Mengla and Yuanjiang areas had higher explanatory power of morphological structure of B. ceiba than soil factors, suggesting that topographic factors in different habitat conditions could change soil moisture, heat, and soil nutrients in B. ceiba growing area through changes in slope relief, elevation gradient, and aspect differences, which eventually participate in the process of altering the morphological structure of B. ceiba.
4.2. Responses of Bombax ceiba leaf traits to habitat factors
As an important organ for material exchange with the external environment, leaves can be easily measured, and possess plastic functional traits due to evolutionary adaptation to spatial heterogeneity or environmental changes. B. ceiba leaves, as plant organs exposed to the atmospheric environment, are sensitive to changes in habitat conditions, mainly in the form of changes in leaf traits in order to adapt to spatial heterogeneity. In the present study, the correlations between leaf traits of B. ceiba in Mengla and Yuanjiang areas were extensive, and more than half of these correlation could reach a highly significant level (p < 0.01), indicating that B. ceiba often showed certain correlations between different leaf traits during growth, and eventually formed a series of trait combinations to adapt to changes in specific habitats through the adjustment and balance between different internal functional traits (Sack et al., 2003; Baraloto et al., 2010). This is consistent with the findings of Arredondo and Schnyder (2003) that the specific leaf area of graminaceous plants was positively correlated with leaf dry weight. Several studies have shown that plants tend to show greater leaf plasticity and thus adapt to the environment in order to adapt to changes in abiotic environments (Richardson et al., 2017). In this study, the superposition of three environmental factors explained 12.3 and 9.8% of leaf traits in Mengla and Yuanjiang areas, respectively, where soil factors explained the most leaf traits in Mengla area, while meteorological factors explained the most in Yuanjiang area, probably because Yuanjiang area was mainly affected by high temperature and drought, which would have a legacy effect on the soil, and affect the biological activity in the soil. By contrast, Mengla area was affected by high temperature, and rainfall interaction directly affects nutrient availability of the soil, thus promoting the activity of key enzymes for the morphological transformation of carbon and nitrogen nutrients, which, in turn, makes the soil more fertile and contributes to nutrient content of B. ceiba leaves (Appel, 1998).
4.3. Responses of Bombax ceiba morphological structure to different habitat conditions
The roles of plant tree structure and leaf traits are not independent. Rather, they interact to adapt to the environment through the adjustment and balance of different combinations of trait factors (Weiher et al., 1999). Studies have shown that the effects of environmental factors on the structure of B. ceiba varied in different habitats. In dry and hot environment, drought can have an inhibitory effect on plant growth, such as plant height, crown width, diameter at breast height, and other morphological indicators (Shen et al., 2019). We get similar finding that the meteorological factors in Mengla area had higher explanatory power of B. ceiba tree structure than in Yuanjiang area. This is probably because Mengla is located in a humid-heat-influenced area, while Yuanjiang is located in a dry and hot zone, and the dry and hot infertile soil plays a determining role in affecting the growth of B. ceiba trees in terms of height and crown width. Overall, the topographic factors had the highest explanatory power of B. ceiba structure, while the explanatory power of meteorological and soil factors were slightly weaker, indicating that the topographic factors influenced the water, heat and nutrient environment in which the stands were located, and then influenced the tree height as well as canopy growth of B. ceiba. Studies have shown that as a source pool of nutrients, soil directly affects plant uptake and utilization of N and P nutrients, and further influences plant N:P stoichiometry relationships and even biomass allocation and ecological strategy selection (Peri et al., 2008). In the present study, we found that soil nutrients had the highest explanatory power of leaf traits in Mengla, while meteorological factors had the highest leaf traits in Yuanjiang, showing that soil nutrients had a differential effect on the growth and development of tree structure and leaf traits in different areas. Mengla showed a resource accumulation growth strategy, whereas Yuanjiang showed a growth strategy to adapt to the environment, reflecting the ecological plasticity of B. ceiba through maximizing the utilization efficiency of environmental resources.
5. Conclusion
The morphological structure of B. ceiba in heterogeneous habitats display different performance in response to the environment, reflecting its growth strategy of adaptive adjustment to resources. This study showed that the correlations between the factors of tree morphology and leaf traits in Mengla and Yuanjiang areas were extensive, and more than half of them could reach highly significant levels, reflecting the similarity of the adjustment of B. ceiba in different habitats. In terms of the degree of explanatory power, the three environmental factors had more explanatory power of tree structure than leaf traits, with the topographic factor explaining 18.6% of the tree structure. Meanwhile, the soil factor explained 20.1% of the leaf traits in Mengla, while the topographic factor explaining 13.6% of tree structure, and the meteorological factor explaining 11.6% of the leaf traits in Yuanjiang. This reflects that fact that B. ceiba in Mengla area adopts a resource accumulation growth strategy under hot and humid conditions, whereas B. ceiba in Yuanjiang area adopts a conservation growth strategy under high temperature and drought habitats. Of course, the morphological structure of B. ceiba is influenced by both internal genes and external environment, and future studies should be expanded on the aspect of genetic traits.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YW: writing and editing. YZ and WL: review and editing. KM: investigation and visualization. XC: resource, investigation, writing, review, and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (31860206).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Appel, T. (1998). Non-biomass soil organic N-- the substrate for N mineralization flushes following soil drying-rewetting and for organic N rendered CaCl2-extractable upon soil drying. Soil Biol. Biochem. 30, 1445–1456. doi: 10.1016/S0038-0717(97)00230-7
Arredondo, J. T., and Schnyder, H. (2003). Components of leaf elongation rate and their relationship to specific leaf area in contrasting grasses. New Phytol. 158, 305–314. doi: 10.1046/j.1469-8137.2003.00745.x
Baraloto, C., Paine, C. E. T., Poorter, L., et al. (2010). Decoupled leaf and stem economics in rain forest trees. Ecol. Lett. 13, 1338–1347. doi: 10.1111/j.1461-0248.2010.01517.x
Bonan, G. B. (2008). Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449. doi: 10.1126/science.1155121
Deslauriers, A., Anfodillo, T., Rossi, S., and Carraro, V. (2007). Using simple causal modeling to understand how water and temperature affect daily stem radial variation in trees. Tree Physiol. 27, 1125–1136. doi: 10.1093/treephys/27.8.1125
Deslauriers, A., Fonti, P., Rossi, S., Rathgeber, C. B., and Gričar, J. (2017). Ecophysiology and plasticity of wood and phloem formation. Dendroecology 231, 13–33. doi: 10.1007/978-3-319-61669-8_2
Díaz, S., Cabido, M., and Casanoves, F. (1998). Plant functional traits and environmental filters at a regional scale. J. Veg. Sci. 9, 113–122. doi: 10.2307/3237229
Funk, J. L., and Cornwell, W. K. (2013). Leaf traits within communities: context may affect the mapping of traits to function. Ecology 94, 1893–1897. doi: 10.1890/12-1602.1
Gao, J. N., Yang, B., He, M. H., et al. (2019). Intra-annual stem radial increment patterns of Chinese pine, Helan Mountains, northern Central China. Trees 33, 751–763. doi: 10.1007/s00468-019-01813-w
Holscher, D., Schmitt, S., and Kupfer, K. (2002). Growth and leaf traits of four broad-leaved tree species along a hillside gradient. Eur. J. For. Res. 121, 229–239. doi: 10.1046/j.1439-0337.2002.02031.x
Huang, Z. W., Yang, L., Wang, Y. J., et al. (2022). The characteristics of tree shape structure and the influencing factors of Bombax ceiba L. in different habitats. Chinese J. Ecology 41, 1552–1559. doi: 10.13292/j.1000-4890.202208.011
IPCC (2013). Climate change: The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press.
Jacobs, B. F. (1999). Estimation of rainfall variables from leaf char-acters in tropical Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 145, 231–250. doi: 10.1016/S0031-0182(98)00102-3
Jiang, Y., Wang, B. Q., Dong, M. Y., Huang, Y. M., Wang, M. C., and Wang, B. (2015). Response of daily stem radial growth of Platycladus orientalis to environmental factors in a semi-arid area of North China. Trees 29, 87–96. doi: 10.1007/s00468-014-1089-8
Korner, C., and Basler, D. (2010). Phenology under global warming. Science 327, 1461–1462. doi: 10.1126/science.1186473
Lenoir, J., Gegout, J. C., Marquet, P. A., et al. (2008). A significant upward shift in plant species optimum elevation during the 20th century. Science 320, 1768–1771. doi: 10.1126/science.1156831
Mc Dowell, N., Pockman, W. T., Allen, C. D., et al. (2008). Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought. New Phytol. 178, 719–739. doi: 10.1111/j.1469-8137.2008.02436.x
Peng, C. H., Ma, Z. H., Lei, X. D., et al. (2011). A drought-induced pervasive increase in tree mortality across Canadas boreal forests. Nat. Clim. Chang. 1, 467–471. doi: 10.1038/nclimate1293
Peri, P. L., Gargaglione, V., and Pastur, G. M. (2008). Above-and belowground nutrients storage and biomass accumulation in marginal Nothofagus antarctica forests in southern Patagonia. For. Ecol. Manag. 255, 2502–2511. doi: 10.1016/j.foreco.2008.01.014
Richardson, B. A., Chaney, L., Shaw, N. L., and Still, S. M. (2017). Will phenotypic plasticity affecting flowering phenology keep pace with climate change. Glob. Chang. Biol. 23, 2499–2508. doi: 10.1111/gcb.13532
Sack, L., Grubb, P. J., and Marañón, T. (2003). The functional morphology of juvenile plants tolerant of strong summer drought in shaded forest understories in southern Spain. Plant Ecol. 168, 139–163. doi: 10.1023/A:1024423820136
Shen, J. Y., Li, S. F., Huang, X. B., et al. (2019). Radial growth responses to climate warming and drying in Pinus yunnanensis in Nanpan River basin. Chinese J. Plant Ecology 43, 946–958. doi: 10.17521/cjpe.2019.0169
Tian, Q. Y., He, Z. B., Xiao, S. C., et al. (2017). Response of stem radial growth of Qinghai spruce (Picea crassifolia) to environmental factors in the Qilian Mountains of China. Dendrochronologia 44, 76–83. doi: 10.1016/j.dendro.2017.04.001
Weiher, E., Van Der Werf, A., Thompson, K., et al. (1999). Challenging Theophrastus: a common core list of plant traits for functional ecology. J. Veg. Sci. 10, 609–620. doi: 10.2307/3237076
Williams, L. G. (1997). Phenology of deciduous and broadleaved-evergreen tree species in a Mexican tropical lower montane forest. Glob. Ecol. Biogeogr. 6, 115–127. doi: 10.2307/2997568
Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., et al. (2004). The world-wide leaf economics spectrum. Nature 428, 821–827. doi: 10.1038/nature02403
Keywords: Bombax ceiba, morphological structure, habitat heterogeneity, tree structure, leaf traits
Citation: Wang Y, Zhang Y, Mao K, Li W and Cheng X (2023) Morphological responses of Bombax ceiba to habitat heterogeneity in Southwest China. Front. Ecol. Evol. 10:1118045. doi: 10.3389/fevo.2022.1118045
Edited by:
Kaixiong Xing, Hainan Normal University, ChinaCopyright © 2023 Wang, Zhang, Mao, Li and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, ✉ d3cwNTkyQGdtYWlsLmNvbQ==; Xiping Cheng, ✉ eGlwaW5nY2hlbmcyMDEyQDE2My5jb20=
 Yanfang Wang1,2
Yanfang Wang1,2 Wei Li
Wei Li Xiping Cheng
Xiping Cheng