- 1Institute of Organismic and Molecular Evolution, Johannes Gutenberg University, Mainz, Germany
- 2Senckenberg Biodiversity and Climate Research Centre (SBiK-F), Molecular Ecology, Frankfurt, Germany
Aging is associated with diverse molecular processes such as oxidative damage, decrease in immunocompetence, or increase in epigenetic abnormalities, mutations, and inflammations. Many of these processes are linked to nutrient-sensing signalling pathways, suggesting that diet plays a critical role in the aging process. In fact, the protein content in the diet can affect both longevity and fecundity, but often in opposite directions. In many solitary organisms, protein-rich diets dramatically shorten lifespan, but increase egg production. We used the ant Temnothorax rugatulus to investigate the effect of the protein to carbohydrate ratio in the diet on the survival and fecundity of fertile workers. We fed colonies either a moderately high-carbohydrate or high-protein diet (1:2 and 2:1 respectively) and monitored worker survival and egg production over 9 weeks. The protein-rich diet did not alter the ability of workers to lay eggs, but reduced worker survival, suggesting that consuming large amounts of protein may shorten lifespan in fertile ant workers without promoting reproduction. Our study shows for the first time that a protein-rich diet reduces the overall fitness of fertile workers.
1. Introduction
Nutrient uptake is a process by which organisms obtain energy for growth, metabolism, and repair. Food usually consists of macronutrients, i.e., proteins, carbohydrates and fats, and micronutrients that are essential vitamins and minerals (Dato et al., 2016). Carbohydrates are the main source of energy, while fats serve to store energy that is available when the organism runs low on carbohydrates. Proteins can be broken down into amino acids, which are involved in important biological functions such as cell signalling, gene expression regulation, and protein phosphorylation cascades.
Many biological pathways can sense nutrients and respond to caloric availability. The nutrient-sensing pathways target of rapamycin (TOR) and insulin/IGF-I signalling (IIS) are major regulators of metabolism, growth, and development (Kenyon, 2005, 2010). These pathways respond to nutritional changes by regulating downstream genes with antioxidant, antimicrobial, or metabolic functions, and can also regulate lifespan as a consequence (Kenyon, 2005, 2010). For example, studies on various organisms such as yeast, Drosophila, or mice, have shown that dietary interventions like caloric restriction increase resistance to oxidative stress and reduce macromolecular damage, extending lifespan sometimes up to 50% (Fontana et al., 2010; Fontana and Partridge, 2015). More specifically, large amounts of amino acids proved to be harmful to a wide range of organisms (Grandison et al., 2009; Wu, 2009; Dato et al., 2016; Arganda et al., 2017). An increase in proteins might therefore fuel nutrient-sensing pathways like TOR or IIS, leading to accelerated senescence. Indeed, experimental work confirmed that protein-rich diets were detrimental to the lifespan of mice (Solon-Biet et al., 2014) and the solitary insect Drosophila (Mair et al., 2005), although it increased female reproduction in the latter (Lee et al., 2008; Lee, 2015).
In solitary organisms, lifespan and reproductive output are mostly negatively correlated. Social insects, however, exhibit a reproductive division of labor wherein longevity and fecundity are positively associated (Monroy Kuhn and Korb, 2016; Negroni et al., 2016). The queens, which can live up to several decades in ants and termites, have the monopoly on reproduction, while the short-lived workers take over all the other tasks, such as brood care and foraging (Hölldobler and Wilson, 1990). Given their social lifestyle, we can ask whether the detrimental effect of a high-protein diet on longevity also exists in social insects, and whether such a diet affects their fecundity. In a study using the Argentine ant Linepithema humile, colonies fed with a protein-rich diet produced more sexuals than workers (Aron et al., 2001). Another ant study using Camponotus colonies showed that a high-protein diet led to the production of more eggs by the queen (Nonacs, 1991). Nevertheless, it remains difficult to directly manipulate the diet of queens in mature insect societies, as they are fed by workers, and to investigate diet effects on lifespan, as they are so long-lived. Hence, more recent studies focused on sterile workers of various species, including the ants Lasius niger (Dussutour and Simpson, 2012; Poissonnier et al., 2014) and L. humile (Arganda et al., 2017) as well as honeybees (Pirk et al., 2010; Paoli et al., 2014) and revealed a detrimental effect of proteins on survival, resembling findings in solitary insects. However, the influence of diet on both longevity and fecundity has not yet been studied in fertile workers of social insects.
Here we investigate the effect of the dietary protein to carbohydrate ratio on the survival and fecundity of fertile ant workers in queenless Temnothorax rugatulus colonies. In many social Hymenoptera, workers can develop ovaries after queen loss and lay haploid, male-destined eggs, sometimes even in queenright colonies (Monnin and Peeters, 1999; Beekman and Oldroyd, 2008; Heinze, 2008; Giehr et al., 2020a, 2020b). In our species, worker reproduction is associated with lifespan extension as well as shifts in molecular pathways involved in lifespan regulation, such as IIS and TOR (Negroni et al., 2020, 2021b; Majoe et al., 2021; Choppin et al., 2021a). We conducted an experiment during which we fed ant colonies with a 1:2 or 2:1 protein:carbohydrate diet. We removed queens from their colony to induce worker fecundity and monitored worker survival and egg production over 9 weeks. In Drosophila, proteins benefit reproduction but are detrimental to lifespan (Mair et al., 2005; Lee et al., 2008; Lee, 2015). However, since fecundity and longevity are often positively associated in social insects, i.e., fertile individuals live longer (Monroy Kuhn and Korb, 2016; Blacher et al., 2017), we did not expect opposite effects of proteins on these two life-history traits.
2. Materials and methods
2.1. Ant collection and maintenance
For our study, we used the small Myrmicine ant Temnothorax rugatulus, which is widely distributed in the western part of North America. Colonies of this species live in small crevices or under rocks in high-elevation forests and contain one to several queens and between a few dozen to a few hundred workers (Rüppell et al., 1998, 2001; Choppin et al., 2021b). We collected these ants in the Chiricahua Mountains (Arizona, USA) in August 2018 and thereafter maintained them in our laboratory in a climate chamber at 21°C and 70% humidity with a 12:12 light:dark cycle. Colonies were kept in nests consisting of plastic inserts forming a cavity between two glass slides, placed in three-chambered boxes with plastered floors. Nests were covered with red foil to darken the cavity.
2.2. Worker survival and egg production
We selected seven polygynous source colonies with two macrogyne queens and at least 120 workers, which we each divided into two queenless sub-colonies containing 60 workers and 30 larvae. The sub-colonies were fed either a carbohydrate-rich (1:2) or protein-rich (2:1) diet (Dussutour and Simpson, 2008; Table 1). Sub-colonies were fed fresh cubes (ca. 1 cm3) of the jelly-like artificial diet four times a week for 9 weeks, so that they were provided with food ad libitum. We counted the number of workers each week. Every 2 weeks, newly developed pupae were removed to prevent the emergence of new workers. Every 4 weeks, each colony was anesthetized with CO2, and eggs were removed to be counted. To analyze the effect of diet on worker number over time, we conducted a Cox survival analysis using the packages “survival” (Therneau, 2021) and “coxme” (Therneau, 2020) and we verified that the model conformed to the assumption of proportional hazards using the package “survminer” (Kassambara et al., 2021). To analyze the effect of diet on the number of eggs produced after 8 weeks, we constructed a generalized mixed-effects model (GLMM; Poisson family) using the package “lme4” (Bates et al., 2015) and confirmed the model fit using the package “DHARMa” (Hartig, 2020). Source colony identification (ID) and sub-colony ID were used as random factors in both models. Statistical analyses were conducted in R v3.5.1 (R Core Team, 2020).

Table 1. Composition of the artificial diets with a 1:2 and 2:1 protein to carbohydrate ratio based on Dussutour and Simpson (2008).
3. Results
Worker survival was lower in sub-colonies fed with a protein-rich diet (X2 = 7.202, df = 1, p = 0.007; Figure 1). However, the cumulative number of worker-laid eggs at the end of the experiment was not affected by the protein content in the diet (X2 = 0.114, df = 1, p = 0.736; Figure 2).
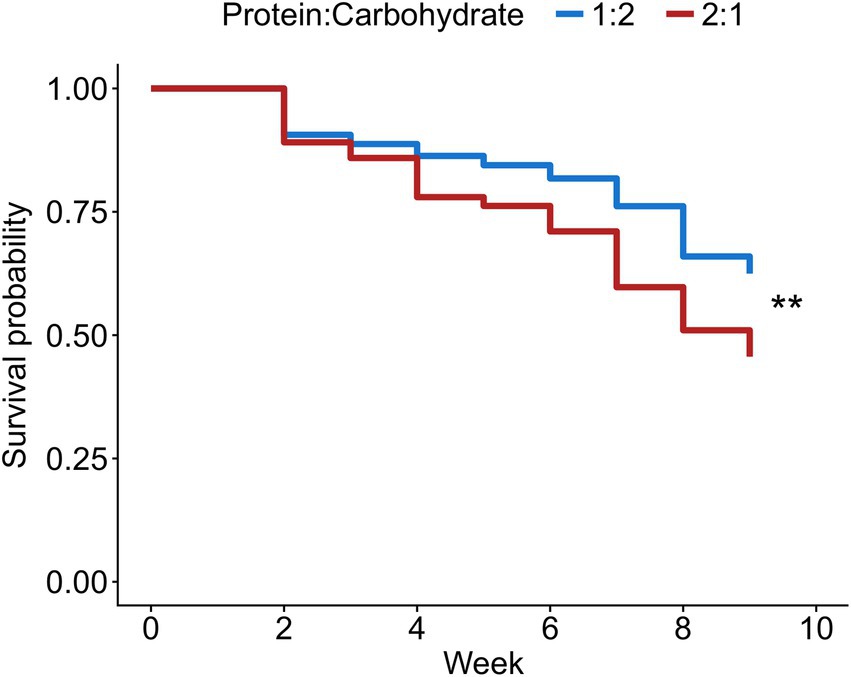
Figure 1. Probability of worker survival over time depending on the protein to carbohydrate ratio in the diet. The level of significance is **p < 0.01.
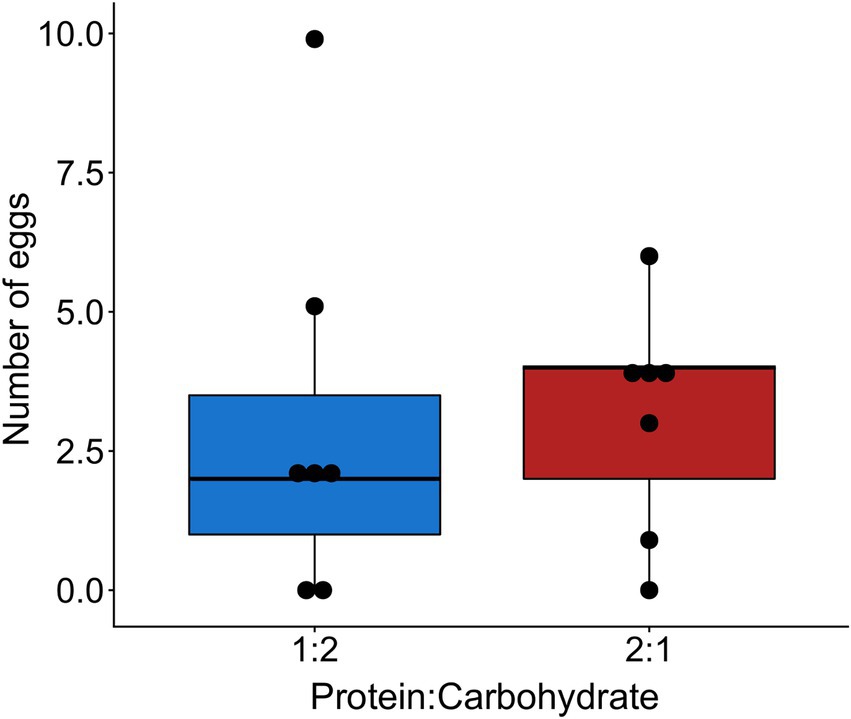
Figure 2. Cumulative number of worker-laid eggs after 8 weeks depending on the protein to carbohydrate ratio in the diet (p = 0.736).
4. Discussion
Diet composition affects life-history traits in diverse organisms (Aron et al., 2001; Naya et al., 2007; Anagnostou et al., 2010; Harvey et al., 2012; Fontana and Partridge, 2015; Bryndová et al., 2020). In this experiment, we investigated whether the survival and egg production of fertile workers in queenless colonies were affected by different protein to carbohydrate ratios in their diet. Our results demonstrate a detrimental effect of proteins on worker survival but not on egg production. The negative consequences of large amounts of protein in the diet are especially interesting since Temnothorax rugatulus workers show an extraordinary resistance to starvation and can survive without food for several months (Rüppell and Kirkman, 2005). This indicates that the lack of carbohydrates, or other nutrients, in the high-protein diet is not responsible for the higher mortality observed in that group. Supporting our findings, it has been experimentally demonstrated that protein-rich diets shorten the lifespan of both solitary insects like the German cockroach (Cooper and Schal, 1992) and social insects like Argentine ants Linepithema humile (Arganda et al., 2017), black garden ants Lasius niger (Dussutour and Simpson, 2012; Poissonnier et al., 2014), and honey-bees (Pirk et al., 2010; Paoli et al., 2014). Authors from these studies have suggested that the digestion of large amounts of proteins could be detrimental because of its cost, the production of toxic nitrogen waste, the overstimulation of nutrient-sensing pathways like TOR, and imbalances in amino acids, which can alter longevity (Grandison et al., 2009; Dato et al., 2016; Arganda et al., 2017). Besides, social insect colonies can be seen as “superorganisms” wherein the germline and soma are represented by queens and workers, respectively (Wheeler, 1911; Hölldobler and Wilson, 2009). We can speculate that, on one hand, queens that constitute the germline benefit from food intake (especially proteins) for egg-laying, while workers primarily rely on carbohydrates to conduct their task because they represent the non-reproductive soma and may thus not require high protein intake. However, when workers are forced to ingest large amounts of proteins – like in our experiment – their survival may be negatively impacted, similarly to what is found in solitary insects. Here we discuss differential diet effects between ant queens and workers, but such within-species nutritional asymmetries can be found beyond the scope of social insects. Indeed, nutrients affect reproduction and lifespan in a sex-specific manner in both fruit flies (Jensen et al., 2015) and field crickets (Maklakov et al., 2008). To summarize, our findings indicate that protein intake should be regulated to optimize survival, as shown in a study using workers of the trap-jaw ant Odontomachus hastatusi, where complementary diets minimized mortality in the context of nutritional challenges (Bazazi et al., 2016).
Conversely, we did not find diet effects on worker egg production. We know that ants rely on proteins for egg-laying (Hölldobler and Wilson, 2009). For instance, queens of the ant Camponotus floridanus produced more eggs when their colony received increased amounts of proteins (Nonacs, 1991) and colonies fed with a protein-rich diet produced more sexuals in Linepithema humile (Aron et al., 2001). Thus, we can speculate that the required amount of protein for egg laying was sufficient in both diets in our experiment. Interestingly, dietary restriction decreased fecundity in queens of the same ant species (Negroni et al., 2021a). Hence, the absence of diet effect on worker fecundity in our study could also be due to differential effects of proteins on the two female castes. Indeed, castes are not genetically determined but develop through phenotypic plasticity (Corona et al., 2016) and gene expression differences are stronger between castes of the same species than within the same caste of two closely related species (Hunt et al., 2011). Also, fertile worker gene expression is more similar to sterile worker gene expression than queen gene expression (Feldmeyer et al., 2014). And the quantity and quality of food during larval development often play a major role in caste determination (Weaver, 1966; Smith et al., 2008; Slater et al., 2020), potentially explaining the different responses of queens and workers to the food they are provided in the adult stage. In short, the diet-induced changes observed in ant queens that we mentioned above may not apply to ant workers. Finally, we can speculate that the ratios of proteins to carbohydrates 1:2 and 2:1 may have been too similar to detect diet effects on egg production. Follow-up experiments using a broader range of protein to carbohydrate ratios, as per Dussutour and Simpson (2012), which investigated diet effects on survival in the ant L. niger, would allow to better characterize the effects of protein on worker egg production.
In summary, we did not find a trade-off between longevity and fecundity, as the survival of fertile ant workers was impaired by the high-protein diet, while the ability of ant workers to lay eggs was unaffected by diet composition. We hypothesize that proteins adversely affect longevity, but do not alter fecundity, in ant workers because they may not have the same active molecular pathways that allow queens to benefit from a high-protein diet during sexual reproduction. Further molecular studies are now needed to investigate how the nutrient-sensing pathways shift between workers, which normally constitute the soma of ant colonies, and queens.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://datadryad.org/stash, doi: 10.5061/dryad.v15dv41zv.
Author contributions
MS conducted the experiment. MC analyzed the data. All authors designed the study, contributed to the article, and approved the submitted version.
Funding
This study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG) to SF (FO 298/19-2) and BF (FE 1333/6-2).
Acknowledgments
We are grateful to Romain Libbrecht for his guidance regarding the survival analysis. We thank the Southwestern Research Station (Portal, Arizona) for the support in the obtention of an ant collection permit from the Coronado National Forest.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anagnostou, C., Dorsch, M., and Rohlfs, M. (2010). Influence of dietary yeasts on Drosophila melanogaster life-history traits. Entomol. Exp. Appl. 136, 1–11. doi: 10.1111/j.1570-7458.2010.00997.x
Arganda, S., Bouchebti, S., Bazazi, S., le Hesran, S., Simpson, S. J., Dussutour, A., et al. (2017). Parsing the life-shortening effects of dietary protein: effects of individual amino acids. Proc. R. Soc. B Biol. Sci. 284:20162052. doi: 10.6084/m9.fig-share.c.3649901
Aron, S., Keller, L., and Passera, L. (2001). Role of resource availability on sex, caste and reproductive allocation ratios in the argentine ant Linepithema humile. J. Anim. Ecol. 70, 831–839. doi: 10.1046/j.0021-8790.2001.00545.x
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bazazi, S., Arganda, S., Moreau, M., Jeanson, R., and Dussutour, A. (2016). Responses to nutritional challenges in ant colonies. Anim. Behav. 111, 235–249. doi: 10.1016/j.anbehav.2015.10.021
Beekman, M., and Oldroyd, B. P. (2008). When workers disunite: intraspecific parasitism by eusocial bees. Annu. Rev. Entomol. 53, 19–37. doi: 10.1146/annurev.ento.53.103106.093515
Blacher, P., Huggins, T. J., and Bourke, A. F. G. (2017). Evolution of ageing, costs of reproduction and the fecundity–longevity trade-off in eusocial insects. Proc. R. Soc. B Biol. Sci. 284:20170380. doi: 10.1098/rspb.2017.0380
Bryndová, M., Stec, D., Schill, R. O., Michalczyk, Ł., and Devetter, M. (2020). Dietary preferences and diet effects on life-history traits of tardigrades. Zool. J. Linnean Soc. 188, 865–877. doi: 10.1093/zoolinnean/zlz146
Choppin, M., Feldmeyer, B., and Foitzik, S. (2021a). Histone acetylation regulates the expression of genes involved in worker reproduction in Temnothorax rugatulus. BMC Genomics 22:871. doi: 10.1186/s12864-021-08196-8
Choppin, M., Graf, S., Feldmeyer, B., Libbrecht, R., Menzel, F., and Foitzik, S. (2021b). Queen and worker phenotypic traits are associated with colony composition and environment in Temnothorax rugatulus (Hymenoptera: Formicidae), an ant with alternative reproductive strategies. Myrmecol News 31, 61–69. doi: 10.25849/myrmecol.news_031:061
Cooper, R. A., and Schal, C. (1992). Effects of protein type and concentration on development and reproduction of the German cockroach, Blattella germanica. Entomol. Exp. Appl. 63, 123–134. doi: 10.1111/j.1570-7458.1992.tb01567.x
Corona, M., Libbrecht, R., and Wheeler, D. E. (2016). Molecular mechanisms of phenotypic plasticity in social insects. Curr. Opin. Insect. Sci. 13, 55–60. doi: 10.1016/j.cois.2015.12.003
Dato, S., Bellizzi, D., Rose, G., and Passarino, G. (2016). The impact of nutrients on the aging rate: a complex interaction of demographic, environmental and genetic factors. Mech. Ageing Dev. 154, 49–61. doi: 10.1016/j.mad.2016.02.005
Dussutour, A., and Simpson, S. J. (2008). Description of a simple synthetic diet for studying nutritional responses in ants. Insect. Soc. 55, 329–333. doi: 10.1007/s00040-008-1008-3
Dussutour, A., and Simpson, S. J. (2012). Ant workers die young and colonies collapse when fed a high-protein diet. Proc. R. Soc. B Biol. Sci. 279, 2402–2408. doi: 10.1098/rspb.2012.0051
Feldmeyer, B., Elsner, D., and Foitzik, S. (2014). Gene expression patterns associated with caste and reproductive status in ants: worker-specific genes are more derived than queen-specific ones. Mol. Ecol. 23, 151–161. doi: 10.1111/mec.12490
Fontana, L., and Partridge, L. (2015). Promoting health and longevity through diet: from model organisms to humans. Cells 161, 106–118. doi: 10.1016/j.cell.2015.02.020
Fontana, L., Partridge, L., and Longo, V. D. (2010). Dietary restriction, growth factors and aging: from yeast to humans. Science 1979:328. doi: 10.1126/science.1172539.Dietary
Giehr, J., Senninger, L., Ruhland, K., and Heinze, J. (2020a). Ant workers produce males in queenless parts of multi-nest colonies. Sci. Rep. 10:2152. doi: 10.1038/s41598-020-58830-w
Giehr, J., Wallner, J., Senninger, L., Ruhland, K., Krüger, T., and Heinze, J. (2020b). Substantial direct fitness gains of workers in a highly eusocial ant. Mol. Ecol. 29, 3720–3730. doi: 10.1111/mec.15586
Grandison, R. C., Piper, M. D. W., and Partridge, L. (2009). Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064. doi: 10.1038/nature08619
Hartig, F. (2020). DHARMa: Residual Diagnostics For Hierarchical (Multi-level/Mixed) Regression Models. R package version 0.3.1.
Harvey, J. A., Cloutier, J., Visser, B., Ellers, J., Wäckers, F. L., and Gols, R. (2012). The effect of different dietary sugars and honey on longevity and fecundity in two hyperparasitoid wasps. J. Insect Physiol. 58, 816–823. doi: 10.1016/j.jinsphys.2012.03.002
Heinze, J. (2008). Hierarchy length in orphaned colonies of the ant Temnothorax nylanderi. Naturwissenschaften 95, 757–760. doi: 10.1007/s00114-008-0375-y
Hölldobler, B., and Wilson, E. O. (2009). The Superorganism: The Beauty, Elegance, And Strangeness of Insect Societies. W. W. Norton New York, USA.
Hunt, B. G., Ometto, L., Wurm, Y., Shoemaker, D., Yi, S. V., and Keller, L. (2011). Relaxed selection is a precursor to the evolution of phenotypic plasticity. PNAS 108, 15936–15941. doi: 10.1073/pnas.1104825108
Jensen, K., McClure, C., Priest, N. K., and Hunt, J. (2015). Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell 14, 605–615. doi: 10.1111/acel.12333
Kassambara, A., Kosinski, M., and Biecek, P. (2021). Survminer: Drawing Survival Curves Using “ggplot2”. R package version 0.4.9.
Kenyon, C. (2005). The plasticity of aging: insights from long-lived mutants. Cells 120, 449–460. doi: 10.1016/j.cell.2005.02.002
Lee, K. P. (2015). Dietary protein:carbohydrate balance is a critical modulator of lifespan and reproduction in Drosophila melanogaster: a test using a chemically defined diet. J. Insect Physiol. 75, 12–19. doi: 10.1016/j.jinsphys.2015.02.007
Lee, K. P., Simpson, S. J., Clissold, F. J., Brooks, R., Ballard, J. W. O., Taylor, P. W., et al. (2008). Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl. Acad. Sci. U S A 105, 2488–2503. doi: 10.1007/978-3-319-16778-7_5
Mair, W., Piper, M. D. W., and Partridge, L. (2005). Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 3:e223. doi: 10.1371/journal.pbio.0030223
Majoe, M., Libbrecht, R., Foitzik, S., and Nehring, V. (2021). Queen loss increases worker survival in leaf-cutting ants under paraquat-induced oxidative stress. Philos. Trans. R. Soc. Lond. B Biol. Sci. 376:20190735. doi: 10.1098/rstb.2019.0735
Maklakov, A. A., Simpson, S. J., Zajitschek, F., Hall, M. D., Dessmann, J., Clissold, F., et al. (2008). Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 18, 1062–1066. doi: 10.1016/j.cub.2008.06.059
Monnin, T., and Peeters, C. (1999). Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behav. Ecol. 10, 323–332. doi: 10.1093/beheco/10.3.323
Monroy Kuhn, J. M., and Korb, J. (2016). Editorial overview: social insects: aging and the re-shaping of the fecundity/longevity trade-off with sociality. Curr. Opin. Insect. Sci. 16, vii–vix. doi: 10.1016/j.cois.2016.06.002
Naya, D. E., Lardies, M. A., and Bozinovic, F. (2007). The effect of diet quality on physiological and life-history traits in the harvestman Pachylus paessleri. J. Insect Physiol. 53, 132–138. doi: 10.1016/j.jinsphys.2006.11.004
Negroni, M. A., Feldmeyer, B., and Foitzik, S. (2021a). Experimental increase in fecundity causes upregulation of fecundity and body maintenance genes in the fat body of ant queens. Biol. Lett. 17:20200909. doi: 10.1098/rsbl.2020.0909
Negroni, M. A., Jongepier, E., Feldmeyer, B., Kramer, B. H., and Foitzik, S. (2016). Life history evolution in social insects: a female perspective. Curr. Opin. Insect. Sci. 16, 51–57. doi: 10.1016/j.cois.2016.05.008
Negroni, M. A., Macit, M. N., Stoldt, M., Feldmeyer, B., and Foitzik, S. (2021b). Molecular regulation of lifespan extension in fertile ant workers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 376:20190736. doi: 10.1098/rstb.2019.0736
Negroni, M. A., Segers, F. H. I. D., Vogelweith, F., and Foitzik, S. (2020). Immune challenge reduces gut microbial diversity and triggers fertility-dependent gene expression changes in a social insect. BMC Genomics 21:816. doi: 10.1186/s12864-020-07191-9
Nonacs, P. (1991). Less growth with more food: how insect-prey availability changes colony demographics in the ant, Camponotus floridanus. J. Insect Physiol. 37, 891–898. doi: 10.1016/0022-1910(91)90004-J
Paoli, P. P., Donley, D., Stabler, D., Saseendranath, A., Nicolson, S. W., Simpson, S. J., et al. (2014). Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids 46, 1449–1458. doi: 10.1007/s00726-014-1706-2
Pirk, C. W. W., Boodhoo, C., Human, H., and Nicolson, S. W. (2010). The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutellata). Apidologie 41, 62–72. doi: 10.1051/apido/2009055
Poissonnier, L. A., Simpson, S. J., and Dussutour, A. (2014). Observations of the “egg white injury” in ants. PLoS One 9:e112801. doi: 10.1371/journal.pone.0112801
R Core Team (2020). R: A Language and Environment For Statistical Computing. R Foundation For Statistical Computing (Vienna, Austria).
Rüppell, O., Heinze, J., and Hölldobler, B. (1998). Size-dimorphism in the queens of the north American ant Leptothorax rugatulus (Emery). Insect. Soc. 45, 67–77. doi: 10.1007/s000400050069
Rüppell, O., Heinze, J., and Hölldobler, B. (2001). Alternative reproductive tactics in the queen-size-dimorphic ant Leptothorax rugatulus (Emery) and their consequences for genetic population structure. Behav. Ecol. Sociobiol. 50, 189–197. doi: 10.1007/s002650100359
Rüppell, O., and Kirkman, R. W. (2005). Extraordinary starvation resistance in Temnothorax rugatulus (Hymenoptera, Formicidae) colonies: demography and adaptive behavior. Insect. Soc. 52, 282–290. doi: 10.1007/s00040-005-0804-2
Slater, G. P., Yocum, G. D., and Bowsher, J. H. (2020). Diet quantity influences caste determination in honeybees (Apis mellifera): caste determination in honey bees. Proc. R. Soc. B Biol. Sci. 287:20200614. doi: 10.1098/rspb.2020.0614
Smith, C. R., Anderson, K. E., Tillberg, C. V., Gadau, J., and Suarez, A. V. (2008). Caste determination in a polymorphic social insect: nutritional, social, and genetic factors. Am. Nat. 172, 497–507. doi: 10.1086/590961
Solon-Biet, S. M., McMahon, A. C., Ballard, J. W. O., Ruohonen, K., Wu, L. E., Cogger, V. C., et al. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19, 418–430. doi: 10.1016/j.cmet.2014.02.009
Weaver, N. (1966). Physiology of caste determination. Annu. Rev. Entomol. 11, 79–102. doi: 10.1146/annurev.en.11.010166.000455
Wheeler, W. M. (1911). The ant-colony as an organism. J. Morphol. 22, 307–325. doi: 10.1002/jmor.1050220206
Keywords: life history evolution, trade-offs, senescence, reproduction, social insects, mortality
Citation: Choppin M, Schall M, Feldmeyer B and Foitzik S (2023) Protein-rich diet decreases survival, but does not alter reproduction, in fertile ant workers. Front. Ecol. Evol. 10:1098245. doi: 10.3389/fevo.2022.1098245
Edited by:
Silvio Erler, Julius Kühn-Institut – Braunschweig, GermanyReviewed by:
Ruth Archer, University of Ulm, GermanyGiuseppe Passarino, University of Calabria, Italy
Copyright © 2023 Choppin, Schall, Feldmeyer and Foitzik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina Choppin,  bWNob3BwaW5AdW5pLW1haW56LmRl
bWNob3BwaW5AdW5pLW1haW56LmRl
 Marina Choppin
Marina Choppin Miriam Schall1
Miriam Schall1 Barbara Feldmeyer
Barbara Feldmeyer Susanne Foitzik
Susanne Foitzik