
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Ecol. Evol., 06 January 2023
Sec. Ecophysiology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.1071251
This article is part of the Research TopicInsect Pollinators in the Anthropocene: How Multiple Environmental Stressors Are Shaping Pollinator HealthView all 11 articles
Climate change, agricultural intensification, and other anthropogenic ecosystem challenges have caused declines in the diversity and abundance of insect pollinators. In response to these declines, entomologists have called for greater attention to insect pollinator conservation. Conservation primarily aims to protect groups of non-human animals—populations or species—with only secondary concern for the welfare of individual animals. While conservation and animal welfare goals are sometimes aligned, they often are not. And because animal welfare comes second, it tends to be sacrificed when in tension with conversation priorities. Consider, for example, lethal sampling to monitor many pollinator populations. Growing evidence suggests that the welfare of individual insect pollinators may be morally significant, particularly in the Hymenoptera and Diptera. Considering insect welfare in conservation practices and policies presents many challenges as, in the face of rapid, anthropogenic change, it may be impossible to avoid harming individual animals while promoting diverse populations. We suggest some practical, implementable strategies that can allow for more robust integration of animal welfare goals into insect pollinator conservation. By following these strategies, entomologists may be able to find policies and practices that promote the health of ecosystems and the individual animals within them.
Insect pollinators are in peril: anthropogenic climate and habitat changes have caused abundance, diversity, and body size declines, as well as range, phenology, and ecological relationship shifts (Cane et al., 2006; Bartomeus et al., 2011; Kuhlmann et al., 2012; Burkle et al., 2013; Barrett and Johnson, 2022; Turley et al., 2022). In the face of this rapid and unprecedented biodiversity crisis (Schachat and Labandeira, 2021), entomologists have called for greater attention to pollinators in conservation and policymaking.
Conservationists aim to maintain biological diversity, ecological health, and ecosystem integrity (Trombulak et al., 2004). These goals require focusing on populations and species—groups of non-human animals—with secondary concern for the welfare of the individual members of those groups. Although conservation goals are sometimes aligned with individual welfare—that is, how a single organism is faring from its own subjective perspective—they can conflict too. For instance, population control measures may enhance ecosystem integrity while causing harm to the individual animals being controlled. While such measures may be necessary, conservationists are increasingly concerned with minimizing such harms (Dubois et al., 2017; Sekar and Shiller, 2020). Our aim here is to consider the prospects for harm minimization in the context of conserving insect pollinators.
The welfare of sentient organisms—i.e., organisms with the capacity to feel pain (Singer, 2002)—matters morally. There is currently no scientific consensus on insect sentience (Adamo, 2016; Klein and Barron, 2016; Birch, 2020; Chittka, 2022; Gibbons et al., 2022). However, two important groups of insect pollinators– the Hymenoptera (including wasps and bees), and the Diptera (including flies) –meet many of the criteria in the Birch et al. (2021) framework for assessing animal sentience. Using this framework to review over 300 scientific studies of insect neurobiology and behavior, Gibbons et al. (2022) found “substantial evidence for sentience” in Hymenoptera and “strong evidence for sentience” in Diptera. There is also “strong evidence for sentience” in decapod crustaceans, which guaranteed their protection by the UK government in the 2022 Animal Welfare (Sentience) Act (Birch et al., 2021). Though not decisive, there is reason to take the possibility of insect sentience, and thus welfare, seriously. A precautionary approach could involve making efforts to minimize possible harm by treating insects as though they are sentient while collecting additional data (Fischer, 2016, 2019; Birch, 2017). Since most insect conservation efforts are not structured around a precautionary approach, adopting such a stance could have significant implications for the design and implementation of interventions, programs, and policies—as we will demonstrate in the following section.
Coordinated action to improve wild pollinator conservation first became highly publicized in the United States through “The Forgotten Pollinators” book and Arizona-Sonora Desert Museum pollinators’ campaign (Buchmann and Nabhan, 1996). Since the 1990s, numerous pollinator conservation actions and policies have been implemented or proposed (Williams, 2003; Byrne and Fitzpatrick, 2009; Hall and Steiner, 2019; Marselle et al., 2020; Stout and Dicks, 2022). In the next two subsections, we briefly review two examples that highlight tensions between conservation goals and individual insects’ welfare. We also suggest some ways that those tensions might be reduced via adjustments to standard practices or policies.
Most proposals for conserving insect pollinators include the need to increase community-level monitoring efforts (Dicks et al., 2016; Woodard et al., 2020; but see Tepedino and Portman, 2021) to inform interventions and assess their impact. Monitoring programs often include passive lethal sampling methods, such as pan traps, that keep field labor, expenses, and expertise relatively low while allowing for subsequent species-level identifications. Biweekly tests of pan traps, combined with sweep-netting, suggest that they do not affect long-term trends in bee abundance or diversity (Gezon et al., 2015; but see vane traps, Gibbs et al., 2017).
However, lethal monitoring programs present obvious welfare problems (Fischer and Larson, 2019). Insects drown, starve/desiccate, or die via poisoning, all of which may induce pain and stress. This is a special problem for “bycatch” insects, which comprised nearly 63% of individual captured arthropods in pan traps in Gonzalez et al. (2020). These insects are rarely used to generate data and are often discarded, offering no clear conservation benefit and constituting a negative welfare impact. Additionally, conservationists are increasingly concerned about the impact of lethal monitoring on target species (Tepedino and Portman, 2021; Montero-Castaño et al., 2022), particularly those that are vulnerable or threatened: a case study of North American bumble bees showed an increase in the number of lethal collections since the 1990s, even though data demonstrating taxonomic resilience of many recently imperiled bumble bee species are sparse (Miller et al., 2022).
There are several ways to reduce the welfare costs of monitoring initiatives. First, researchers could focus on developing protocols that minimize bycatch. For example, smaller pan traps reduce bycatch without changing bee monitoring efficacy (Gonzalez et al., 2020). Second, making bycatch (and target; Montero-Castaño et al., 2022) specimens/data more accessible could reduce the necessity for other lethal sampling studies (Spears and Ramirez, 2015; Fischer and Larson, 2019). Third, scientists could reduce suffering in lethal monitoring programs by hastening insect time-to-death via different (or increased concentrations of) lethal agents.
There is little guidance available about the appropriate level of temporal or spatial sampling effort for many monitoring initiatives (but see: Lebuhn et al., 2013). Likewise, there is little guidance about how to handle biases and deficiencies in particular methodologies (Cane et al., 2000; Baum and Wallen, 2011; Didham et al., 2020), which may lead to “more is better” or “all of the above” approaches (Rhoades et al., 2017; Portman et al., 2020). However, sampling that does not provide additional, action-relevant information to support conservation goals should be avoided for welfare, conservation, and cost/storage/effort reasons (Droege et al., 2016; Tepedino and Portman, 2021). Consider the thousands of Dialictus (Halictidae) that are collected in pan traps and often go unidentified to species due to the lack of available taxonomic expertise. Most of these individual bees offer little value to monitoring and conservation efforts (Portman et al., 2020), yet represent a significant negative welfare impact. To avoid over collection, models built from meta-analyses of capture data in different habitats with different methods could be used to estimate the actual sampling effort (temporal frequency, sites, methods) required to answer specific questions of interest before establishing a sampling protocol.
Additionally, scientists could switch wholly or partially to non-lethal sampling methods depending on research needs; when new methods are non-invasive, this may support both welfare and conservation goals (Montero-Castaño et al., 2022; Figure 1). Expert transects, where taxonomic experts go into the field to collect data on insect diversity using transects, can produce similar species accumulation curves as pan traps for hoverflies and bees in some habitats while collecting far fewer individuals (O’Connor et al., 2019; but see Rhoades et al., 2017). Conservation and insect welfare goals are thus also aligned in the need to train additional taxonomic experts (Hopkins and Freckleton, 2006) that could support less-lethal monitoring programs. Developing/validating new, non-invasive methods (like eDNA; Thomsen and Sigsgaard, 2019) or using a community- (e.g., “citizen”)-science-driven photographic BioBlitz (Bickerman-Martens et al., 2017; or iNaturalist-style databases, Gazdic and Groom, 2019) could also support conservation-relevant data collection. Barlow and O’Neill (2020) and Miller et al. (2022) review other non-lethal techniques not yet widely employed for pollinator monitoring, including: telemetry/radar, automated visual monitoring, machine-learning identification, molecular analyses, acoustic monitoring, and fecal sampling.
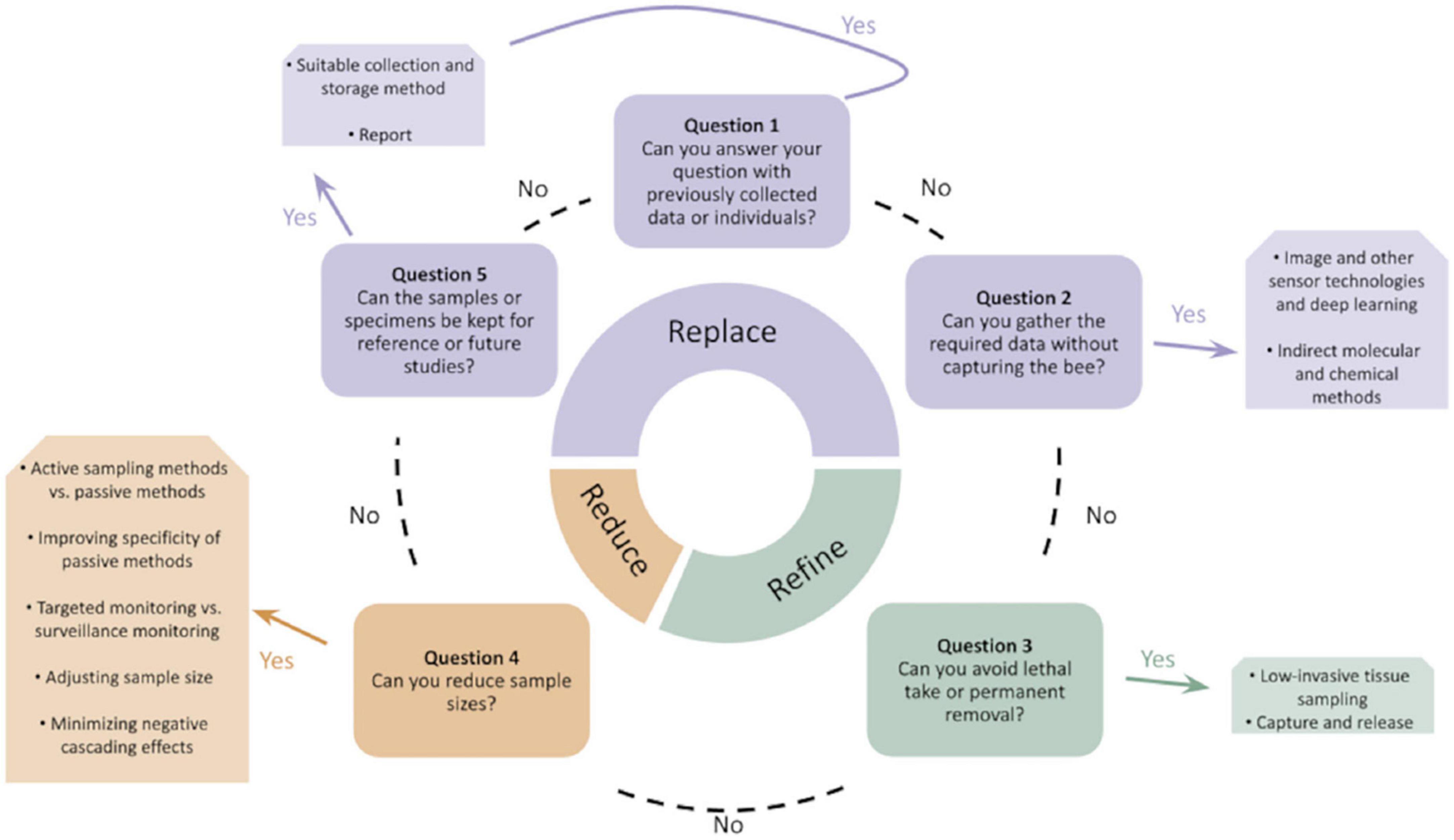
Figure 1. Decision-making framework for replacing, refining, and reducing lethal bee captures in monitoring programs, reproduced from Montero-Castaño et al. (2022) (CC BY 4.0).
Finally, some scientists have suggested that large-scale, community-level monitoring may be overemphasized for obtaining conservation-relevant data on pollinator ecology (Tepedino and Portman, 2021). Population-level studies of a few, carefully chosen pollinator species that are field- or photograph-identifiable could serve just as well for answering many action-relevant questions (Portman et al., 2020; Tepedino and Portman, 2021; Dorian and Crone, 2022). Visual monitoring is already used for some large and easily identifiable groups such as bumble bees and certain butterflies (Montgomery et al., 2021), alongside netting in areas where lethal sampling might harm endangered species (Portman et al., 2020; and see non-lethal protein mark-recapture for vulnerable species, Boyle et al., 2018).
Habitat fragmentation/simplification caused by agricultural intensification can negatively impact pollinator foraging activity and movement, thereby reducing abundance and diversity. This has led to calls for increasing “green infrastructure” for biodiversity maintenance in agricultural areas (Brown and Paxton, 2009; Dicks et al., 2016), which may include native plants alongside agricultural fields (Williams et al., 2015) or creating habitat corridors to allow for increased movement across resource-poor areas (Blüthgen et al., 2022). Green infrastructure may provide welfare benefits to wild pollinators by increasing resource availability and diversity, with positive impacts on health (St. Clair et al., 2020). However, increasing the proximity of wild pollinators to agricultural areas also harms the many animals newly inhabiting these spaces through increased exposure to agrochemicals with lethal or sublethal welfare effects (Susan et al., 2019) and other anthropogenic welfare challenges (e.g., exposure to light pollution and vehicle strikes near road verges; Phillips et al., 2019; Owens et al., 2020).
Some of these welfare effects could be mitigated by incentivizing simultaneous reductions in agrochemical usage, alongside the diversification of agricultural systems and creation of pollinator protection zones in areas where green infrastructure will be created (which may also support honey bee welfare; St. Clair et al., 2020). While this additional incentive structure may reduce the total amount of green infrastructure that can be created, each incentive is both a conservation and welfare benefit to the pollinators in that area. This holistic approach to improving pollinator conservation via multiple means demonstrates one of the ways that policy might be re-structured (and re-budgeted) if welfare and conservation were considered simultaneously. Notably, some of these same issues (and urban heat island effects) will also affect wild bee populations in urbanized areas with greenspace development (Baldrock, 2020), but different incentive structures will be needed in these spaces.
There is significant value in conserving species, populations, and ecosystems. It is also morally important to consider the welfare of non-human animals (Fischer, 2021), including many invertebrates (Koperski, 2022). We have demonstrated that conservation and welfare goals may sometimes conflict, as in the expansion of lethal pollinator monitoring programs and the creation of green infrastructure near some agricultural habitats. In many cases, it is not possible to achieve conservation goals without some harm to non-human animals. However, there appear to be ways for researchers, conservationists, farmers, and policymakers to reduce harms to non-human animals while pursuing their conservation goals. So, a precautionary approach to insect welfare is compatible with their aims (and, in some cases, may even help them achieve their aims: Capozzelli et al., 2020).
One way to promote harm reduction is to encourage welfare-oriented cost-benefit analyses in grant applications and conservation management plans—a practice that is familiar from environmental cost-benefit analysis (Atkinson and Mourato, 2008). In some cases, making the costs explicit may be sufficient to show that they are negligible relative to the potential welfare benefits.
In other cases, of course, it will be less clear what to prioritize. Eventually, then, it will be important to develop frameworks for comparing the relative importance of various costs (financial, temporal, etc.), specific conservation goals, and welfare impacts (e.g., more resource-intensive monitoring methods and the particular welfare impacts of those methods). One path forward involves developing tools that allow stakeholders to express the value they assign to avoiding negative welfare impacts in monetary terms, which could then be aggregated to determine how much stakeholders ought to be willing to pay to avoid causing those impacts (Lusk and Norwood, 2011). While economists, animal welfare scientists, and philosophers are in the early days of creating such tools—for insects and non-insects alike—entomologists can contribute to these efforts by studying insect welfare and quantifying the insect welfare impacts of different conservation practices.
In the interim, it is important simply to make welfare impacts on insects salient in discussions of conservation practices and policies. Insects warrant some consideration and we can reduce many harms to them without compromising conservation goals.
MB: conceptualization. MB, BF, and SB: writing/editing. All authors contributed to the article and approved the submitted version.
MB was currently funded as an NSF postdoctoral research fellow (2109399).
MB was an NSF postdoctoral fellow: Any opinions, findings, conclusions, or recommendations expressed in this manuscript are the authors, and do not necessarily reflect the views of the NSF.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamo, S. A. (2016). Do insects feel pain? A question at the intersection of animal behaviour, philosophy and robotics. Anim. Behav. 118, 75–79. doi: 10.1016/j.anbehav.2016.05.005
Atkinson, G., and Mourato, S. (2008). Environmental cost-benefit analysis. Annu. Rev. Environ. Resour. 33, 317–344. doi: 10.1146/annurev.environ.33.020107.112927
Baldrock, K. C. R. (2020). Opportunities and threats for pollinator conservation in global towns and cities. Curr. Opin. Insect Sci. 38, 63–71. doi: 10.1016/j.cois.2020.01.006
Barlow, S. E., and O’Neill, M. A. (2020). Technological advances in field studies of pollinator ecology and the future of e-ecology. Curr. Opin. Insect Sci. 38, 15–25. doi: 10.1016/j.cois.2020.01.008
Barrett, M., and Johnson, M. G. (2022). Centris pallida (Hymenoptera: Apidae) male body size decreases across five decades. Ecol. Entomol. 1–10. doi: 10.1111/een.13210
Bartomeus, I., Ascher, J. S., Wagner, D., Danforth, B. N., Colla, S., Kornbluth, S., et al. (2011). Climate-associated phenological advances in bee pollinators and bee-pollinated plants. PNAS 108, 20645–20649. doi: 10.1073/pnas.1115559108
Baum, K. A., and Wallen, K. E. (2011). Potential bias in pan trapping as a function of floral abundance. J. Kansas Entomol. Soc. 84, 155–159. doi: 10.2317/JKES100629.1
Bickerman-Martens, K., Swartz, B., Butler, R., and Drummond, F. A. (2017). Documenting the diversity, distribution, and status of Maine bumble bees: the Maine bumble bees atlas and citizen scientists. Mai. Policy Rev. 26, 43–49. doi: 10.53558/TMNE5631
Birch, J. (2017). Animal sentience and the precautionary principle. Anim. Sent. 2, 1–15. doi: 10.51291/2377-7478.1200
Birch, J. (2020). The search for invertebrate consciousness. Noûs 56, 133–153. doi: 10.1111/nous.12351
Birch, J., Burn, C., Schnell, A., Browning, H., and Crump, A. (2021). Review of the Evidence of Sentience in Cephalopod Molluscs and Decapod Crustaceans. London: LSE Enterprise Ltd.
Blüthgen, N., Staab, M., Achury, R., and Weisser, W. W. (2022). Unravelling insect declines: can space replace time? Proc. R. Soc. B 18:20210666. doi: 10.1098/rsbl.2021.0666
Boyle, N. K., Tripodi, A. D., Machtley, S. A., Strange, J. P., Pitts-Singer, T. L., and Hagler, J. R. (2018). A nonlethal method to examine non-Apis bees for mark-capture research. J. Insect Sci. 18:10. doi: 10.1093/jisesa/iey043
Brown, M. J. F., and Paxton, R. J. (2009). The conservation of bees: a global perspective. Apidologie 40, 410–416. doi: 10.1051/apido/2009019
Burkle, L. A., Marlin, J. C., and Knight, T. M. (2013). Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339, 1611–1615. doi: 10.1126/science.1232728
Byrne, A., and Fitzpatrick, U. (2009). Bee conservation policy at the global, regional and national levels. Apidologie 40, 194–210. doi: 10.1051/apido/2009017
Cane, J. H., Minckley, R. L., and Kervin, L. J. (2000). Sampling bees (Hymenoptera: Apiformes) for pollinator community studies: pitfalls of pan-trapping. J. Kansas Entomol. Soc. 73, 225–231.
Cane, J. H., Minckley, R. L., Kervin, L. J., Roulston, T. H., and Williams, N. M. (2006). Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol. Appl. 16, 632–644. doi: 10.1890/1051-0761(2006)016[0632:CRWADB]2.0.CO;2
Capozzelli, J. F., Hecht, L., and Halsey, S. J. (2020). What is the value of wild animal welfare for restoration ecology? Restorat. Ecol. 28, 267–270. doi: 10.1111/rec.13114
Dicks, L. V., Viana, B., Bommarco, R., Brosi, B., Arizmendi, M. D. C., Cunningham, S. A., et al. (2016). Ten policies for pollinators: what governments can do to safeguard pollination services. Science 354, 975–976. doi: 10.1126/science.aai9226
Didham, R. K., Basset, Y., Collins, C. M., Leather, S. R., Littlewood, N. A., Menz, M. H. M., et al. (2020). Interpreting insect declines: seven challenges and a way forward. Insect Conserv. Divers. 13, 103–114. doi: 10.1111/icad.12408
Dorian, N., and Crone, E. (2022). Bringing Population Ecology Back to Wild Bees. Available online at: https://d197for5662m48.cloudfront.net/documents/publicationstatus/81575/preprint_pdf/418a8e72e8cd23847c2854ddb97d0494.pdf (accessed August 22, 2022). doi: 10.22541/au.165045979.93350119/v1
Droege, S., Engler, J., Sellers, E., and O’Brien, L. (2016). “U.S. national protocol framework for the inventory and monitoring of bees, inventory and monitoring, national wildlife refuge system,” in U.S. Fish and Wildlife Service, Fort Collins, CO.
Dubois, S., Fenwick, N., Ryan, E. A., Baker, L., Baker, S. E., Beausoleil, N. J., et al. (2017). International consensus principles for ethical wildlife control. Conserv. Biol. 31, 753–760. doi: 10.1111/cobi.12896
Fischer, B. (2016). Bugging the strict vegan. J. Agric. Environ. Ethics 29, 255–263. doi: 10.1007/s10806-015-9599-y
Fischer, B. (2019). How to reply to some ethical objections to entomophagy. Ann. Entomol. Soc. Am. 112, 511–517. doi: 10.1093/aesa/saz011
Fischer, B., and Larson, B. M. H. (2019). Collecting insects to conserve them: a call for ethical caution. Insect Conserv. Divers. 12, 173–182. doi: 10.1111/icad.12344
Gazdic, M., and Groom, Q. (2019). iNaturalist is an unexpected source of plant-insect interaction data. Biodivers. Inf. Sci. Stand. 3:e37303. doi: 10.3897/biss.3.37303
Gezon, Z. J., Wyman, E. S., Ascher, J. S., Inouye, D. W., and Irwin, R. E. (2015). The effect of repeated, lethal sampling on wild bee abundance and diversity. Methods Ecol. Evol. 6, 1044–1054. doi: 10.1111/2041-210X.12375
Gibbons, M., Crump, A., Barrett, M., Sarlak, S., Birch, J., and Chittka, L. (2022). Can insects feel pain? A review of the neural and behavioural evidence. Adv. Insect Physiol. 63, 155–229. doi: 10.1016/bs.aiip.2022.10.001
Gibbs, J., Joshi, N. K., Wilson, J. K., Rothwell, N. L., Powers, K., Haas, M., et al. (2017). Does passive sampling accurately reflect the bee (Apoidea: Anthophila) communities pollinating apple and sour cherry orchards? Environ. Entomol. 46, 579–588. doi: 10.1093/ee/nvx069
Gonzalez, V. H., Osborn, A. L., Brown, E. R., Pavlick, C. R., Enríquez, E., Tscheulin, T., et al. (2020). Effect of pan trap size on the diversity of sampled bees and abundance of bycatch. J. Insect Conserv. 24, 409–420. doi: 10.1007/s10841-020-00224-4
Hall, D. M., and Steiner, R. (2019). Insect pollinator conservation policy innovations at subnational levels: lessons for lawmakers. Environ. Sci. Policy 93, 118–128. doi: 10.1016/j.envsci.2018.12.026
Hopkins, G. W., and Freckleton, R. P. (2006). Declines in the numbers of amateur and professional taxonomists: implications for conservation. Anim. Conserv. 5, 245–249. doi: 10.1017/S1367943002002299
Klein, C., and Barron, A. (2016). Insects have the capacity for subjective experience. Anim. Sent. 100, 1–19. doi: 10.51291/2377-7478.1113
Koperski, P. (2022). Freshwater invertebrates – neglected victims of biological monitoring: an ethical view. Ethics Environ. 27, 29–57.
Kuhlmann, M., Guo, D., Veldtman, R., and Doanldson, J. (2012). Consequences of warming up a hotspot: species range shifts within a centre of bee diversity. Diversi. Distrib. 18, 885–897. doi: 10.1111/j.1472-4642.2011.00877.x
Lebuhn, G., Droege, S., Connor, E. F., Gemmil-Herren, B., Potts, S. G., Minckley, R. L., et al. (2013). Detecting insect pollinator declines on regional and global scales. Conserv. Biol. 27, 113–120. doi: 10.1111/j.1523-1739.2012.01962.x
Lusk, J. L., and Norwood, F. B. (2011). Animal welfare economics. Appl. Econ. Perspect. Policy 33, 463–483. doi: 10.1093/aepp/ppr036
Marselle, M. R., Turbe, A., Shwartz, A., Bonn, A., and Colleony, A. (2020). Addressing behavior in pollinator conservation policies to combat the implementation gap. Conserv. Biol. 35, 610–622. doi: 10.1111/cobi.13581
Miller, Z. J., Lynn, A., Oster, C., Piotter, E., Wallace, M., Sullivan, L. L., et al. (2022). Unintended consequences? Lethal specimen collection accelerates with conservation concern. Am. Entomol. 68, 48–55. doi: 10.1093/ae/tmac057
Montero-Castaño, A., Koch, J. B. U., Lindsay, T. T. T., Love, B., Mola, J. M., Newman, K., et al. (2022). Pursuing best practices for minimizing wild bee captures to support biological research. Conserv. Sci. Pract. 4, 1–15. doi: 10.1111/csp2.12734
Montgomery, G. A., Belitz, M. W., Guralnick, R. P., and Tingley, M. W. (2021). Standards and best practices for monitoring and benchmarking insects. Front. Ecol. Evol. 8:579193. doi: 10.3389/fevo.2020.579193
O’Connor, R. S., Kunin, W. E., Garratt, M. P. D., Potts, S. G., Roy, H. E., Andrews, C., et al. (2019). Monitoring insect pollinators and flower visitation: the effectiveness and feasibility of different survey methods. Methods Ecol. Evol. 10, 2129–2140. doi: 10.1111/2041-210X.13292
Owens, A. C. S., Cochard, P., Durrant, J., Farnworth, B., Perkin, E. K., and Seymoure, B. (2020). Light pollution is a driver of insect declines. Biol. Conserv. 241:108259. doi: 10.1016/j.biocon.2019.108259
Phillips, B. B., Gaston, K. J., Bullock, J. M., and Osborne, J. L. (2019). Road verges support pollinators in agricultural landscapes, but are diminished by heavy traffic and summer cutting. J. Appl. Ecol. 56, 2316–2327. doi: 10.1111/1365-2664.13470
Portman, Z. M., Bruninga-Socolar, B., and Cariveau, D. P. (2020). The state of bee monitoring in the United States: a call to refocus away from bowl traps and towards more effective methods. Ann. Entomol. Soc. Am. 113, 337–342. doi: 10.1093/aesa/saaa010
Rhoades, P., Griswold, T., Waits, L., Bosque-Pérez, N. A., Kennedy, C. M., and Eigenbrode, S. D. (2017). Sampling technique affects detection of habitat factors influencing wild bee communities. J. Insect Conserv. 21, 703–714. doi: 10.1007/s10841-017-0013-0
Schachat, S. R., and Labandeira, C. C. (2021). Are insects heading toward their first mass extinction? Distinguishing turnover from crises in their fossil record. Ann. Am. Entomol. Soc. 11, 99–118. doi: 10.1093/aesa/saaa042
Sekar, N., and Shiller, D. (2020). Engage with animal welfare in conservation. Science 369, 629–630. doi: 10.1126/science.aba7271
Spears, L., and Ramirez, R. A. (2015). Learning to love leftovers: using by-catch to expand our knowledge in entomology. Am. Entomol. 61, 168–173. doi: 10.1093/ae/tmv046
St. Clair, A. L., Zhang, G., Dolezal, A. G., O’Neal, M. E., and Toth, A. L. (2020). Diversified farming in a monoculture landscape: effects on honey bee health and wild bee communities. Environ. Entomol. 49, 753–764. doi: 10.1093/ee/nvaa031
Stout, J. K., and Dicks, L. V. (2022). From science to society: implementing effective strategies to improve wild pollinator health. Philos. Trans. R. Soc. B 377:20210165. doi: 10.1098/rstb.2021.0165
Susan, D. S., Chan, W., Prosser, R. S., Rodríguez-Gil, J. L., and Raine, N. E. (2019). Assessment of risk to hoary squash bees (Peponapis pruinosa) and other ground-nesting bees from systemic insecticides in agricultural soil. Sci. Rep. 9:11870. doi: 10.1038/s41598-019-47805-1
Tepedino, V. J., and Portman, Z. M. (2021). Intensive monitoring for bees in North America: indispensable or improvident? Insect Conserv. Divers. 14, 535–542. doi: 10.1111/icad.12509
Thomsen, P. F., and Sigsgaard, E. E. (2019). Environmental DNA metabarcoding of wild flowers reveals diverse communities of terrestrial arthropods. Ecol. Evol. 9, 1665–1679. doi: 10.1002/ece3.4809
Trombulak, S. C., Omland, K. S., Robinson, J. A., Lusk, J. J., Fleischner, T. L., Brown, G., et al. (2004). Principles of conservation biology: recommended guidelines for conservation literacy from the education committee of the society for conservation biology. Conserv. Biol. 18, 1180–1190. doi: 10.1111/j.1523-1739.2004.01851.x
Turley, N. E., Biddinger, D. J., Joshi, N. K., and López-Uribe, M. M. (2022). Six years of wild bee monitoring shows changes in biodiversity within and across years and declines in abundance. Ecol. Evol. 12:e9190. doi: 10.1002/ece3.9190
Williams, I. H. (2003). The convention on biological diversity adopts the international pollinator initiative. Bee World 84, 27–31. doi: 10.1080/0005772X.2003.11099568
Williams, N. M., Ward, K. L., Pope, N., Isaacs, R., Wilson, J., May, E. A., et al. (2015). Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol. Appl. 25, 2119–2131. doi: 10.1890/14-1748.1
Keywords: ethics, pollinator conservation, insects, animal welfare, monitoring programs, green infrastructure, policy
Citation: Barrett M, Fischer B and Buchmann S (2023) Informing policy and practice on insect pollinator declines: Tensions between conservation and animal welfare. Front. Ecol. Evol. 10:1071251. doi: 10.3389/fevo.2022.1071251
Received: 16 October 2022; Accepted: 19 December 2022;
Published: 06 January 2023.
Edited by:
Pierre Lau, United States Department of Agriculture (USDA), United StatesReviewed by:
Kaitlin Deutsch, Cornell University, United StatesCopyright © 2023 Barrett, Fischer and Buchmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meghan Barrett,  bWJhcnJldHRAY3N1ZGguZWR1
bWJhcnJldHRAY3N1ZGguZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.