
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 16 December 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.1067923
Food resources, including food types, quantity, and quality, are the key factors that determine the survival and reproduction of wild animals. However, the most basic requirement is access to food. The choice of sleeping sites plays a crucial role in efficiently acquiring food and provides a useful starting point for studying foraging strategies. We collected data on sleeping site and foraging patch uses of wild Tibetan macaques (Macaca thibetana) in Huangshan, Anhui, China, from September 2020 to August 2021. We found that Tibetan macaques used 50 different sleeping sites, mostly located on cliffs, some of which they reused. Sleeping site altitude differed significantly according to season, with higher altitudes recorded in summer and winter. Tibetan macaques did not sleep as much as expected in the peripheral regions of their home range. The sleeping sites were often distributed in proximity to foraging patches, and there was a positive correlation between the use of sleeping sites and surrounding foraging patches. The utilization of foraging patches by Tibetan macaques is inclined towards the multiple central place foraging strategy. Our results provide supportive evidence for the proximity to food resource hypothesis and indicate the important role of sleeping sites in food resource utilization in Tibetan macaques.
Suitable sleeping sites play an essential role in animal survival, as they provide a place of rest, shelter, and access to necessary diurnal resources (Li et al., 2011; Liu et al., 2022). These diurnal resources, including food types, quantity, and quality, are key determinants of the survival and reproduction of wild animals (Paniw et al., 2019; DiGiorgio et al., 2020). Wild primates must cope with complex environments and unpredictable spatial and temporal distributions of resources (Shaffer, 2014; Allritz et al., 2022). One of the critical elements determining foraging success and efficiency is the way an animal obtains food. The choice of sleeping sites plays a crucial role in this process and its analysis is useful for studying foraging strategies.
In the context of the patchy distribution of food resources, the energy expenditure of animals transferred between foraging patches greatly influences foraging patches (Teichroeb and Smeltzer, 2018; Trapanese et al., 2019). The choice of sleeping sites has a direct effect on the reduction of foraging costs (Nakagawa, 1990). To maximize feeding benefits, animals often exhibit one of two different foraging strategies: the central place foraging (CPF) strategy and the multiple central place foraging (MCPF) strategy (Andersson, 1981; Chapman et al., 1989; McAleer and Giraldeau, 2006; Pirotta et al., 2018). The CPF model assumes that animals return daily to a single central site to sleep, which could be their nest or a commonly used resting place (Chapman et al., 1989). Species with fixed sleeping sites tend to choose CPF strategies, foraging heavily in proximity to the sleeping site. For example, CPF strategies have been observed in Whinchat (Saxicola rubetra) and eastern chipmunks (Tamias striatus; Andersson, 1981; McAleer and Giraldeau, 2006). Foragers will first depart from the sleeping site for available foraging patches and, after a period of travel and foraging, return to the sleeping place (Michelot et al., 2017). Animals adopting the CPF strategy must spend considerable time and effort commuting between the food patches and the central site if they forage at patches far from the center. Consequently, the area of their home range they can utilize is relatively small. The MCPF model assumes that animals use multiple, scattered central places for sleeping, usually close to the current foraging area (McLaughlin and Montgomerie, 1989; Smith et al., 2007), as reported in white-headed langur (Trachypithecus leucocephalus) and northern pig-tailed macaques (Macaca leonine; Li et al., 2011; José-Domínguez et al., 2015). MCPF differs from CPF in that animal travel costs include travel between central places and foraging patches and between different central places. Thus, animals that engage in MCPF can use larger foraging areas for the same foraging benefits. MCPF strategies pay more attention to the spatial distribution of food. When food is scarce or unevenly distributed, the MCPF strategy may be more beneficial to the forager than the CPF strategy because animals can reduce foraging costs by resting overnight in or near foraging patches (Chapman et al., 1989; Shaffer, 2014). If the rewards of foraging are equal, the shortest paths to foraging patches should be selected.
Animals move between multiple food patches throughout the day and generally try to maximize their foraging efficiency (Teichroeb and Smeltzer, 2018). The choice of the sleeping site plays a crucial role in improving foraging efficiency (Gazagne et al., 2020), which can explain the adaptive process of foraging strategies from another perspective. Sleeping sites have many functions, and current studies have proposed functions including predation avoidance (Smith et al., 2007; Fei et al., 2022), territory or resource defense (Li et al., 2011; Fei et al., 2022), and proximity to food resources (José-Domínguez et al., 2015; Gazagne et al., 2020). However, one of the most important functions is the proximity to food resources. Geoffroy’s spider monkeys (Ateles geoffroyi) and black crested gibbons (Nomascus concolor jingdongensis) have both been observed sleeping at sites in proximity to food patches to improve foraging efficiency (Chapman et al., 1989; Fan and Jiang, 2008). Additionally, one study on Assamese macaques (Macaca assamensis) found that the foraging patches they used on a particular day influenced their choice of sleeping site that night, which in turn affected their foraging efficiency the following day (Liu et al., 2022). Sleeping sites are a potentially limiting resource for many primates, and their use pattern may reflect the adaptive evolution of foraging strategies.
The Tibetan macaque is endemic to China, where they inhabit temperate and subtropical mountainous forest environments and are widely distributed across 13 provinces (Li J.H. et al., 2020). They mainly feed on fruits, leaves, and flowers and can flexibly adapt to spatial and temporal environmental changes (Li B. et al., 2022). Tibetan macaques are highly terrestrial primates (Li P.H. et al., 2022). During the summer, they sleep in trees near cliffs for improved ventilation and to avoid dangerous encounters with the hundred-pace viper (Deinagkistrodon acutus) and other poisonous snakes (Li and Wang, 1994). Tibetan macaques have extensive home ranges (18.54 km2) in which food is unevenly distribution (Li W.B. et al., 2022). To our knowledge, no previous research has examined the effects of sleeping sites choice on the foraging strategy of Tibetan macaques. It is also unclear what foraging strategy this species adopts for the patchy distribution of food resources. Exploring primate sleeping site selection and food patch utilization strategies is essential for effective conservation planning.
We conducted this study under natural conditions and bridged the association between sleeping sites and food patch utilization strategies. We present detailed information on the sleeping site distribution, sleeping site use preference, and the relationship between sleeping sites and foraging patch utilization in the Tianhu Mountain group of wild Tibetan macaques in Huangshan. We analyzed the distance between the sleeping sites and the food patches to explore Tibetan macaques’ food patch utilization strategies. We aimed to identify the use and distribution of Tibetan macaque sleeping sites and the relationship between the use of sleeping sites and utilization of food patches. We hypothesized that (1) if Tibetan macaques adopt the CPF strategy, the number of sleeping sites may be minimal, and that the location of the foraging patches will not affect the choice of sleeping sites. (2) If Tibetan macaques adopt the MCPF strategy, we predicted that their sleeping sites may be spread throughout their home range, and that the selection of sleeping sites will be closely related to the location of the foraging patches.
The study was conducted at the Niejiashan Research Base (30°12’N, 118°27′E) near the east gate of Mt. Huangshan in Anhui Province, China (Figure 1). The altitude ranges from 200 to 650 m above sea level and the area has a subtropical monsoon climate. Total annual rainfall is 2639.4 mm, and the average yearly temperature is 15.5°C, with the highest temperature in July (38.1°C) and the lowest in February (−13.1°C). Following Li et al. (2021), four seasons were defined according to the monthly average temperature: an average temperature >22°C was classified as summer (June–September), <10°C as winter (December–February), and 10–22°C as spring (March–May) and autumn (October–November). The main vegetation types include evergreen broad-leaved, evergreen deciduous mixed, coniferous, and coniferous and broad-leaved mixed forests (Li et al., 2021). There are also many cliffs, exposed rocks, and streams in this area. Rich and diverse vegetation and complex terrain provide an ideal habitat for Tibetan macaques (Li et al., 2021). Tibetan macaques mostly avoid human beings and inhabit dense forests with mountains and cliffs (Li J.H. et al., 2020).
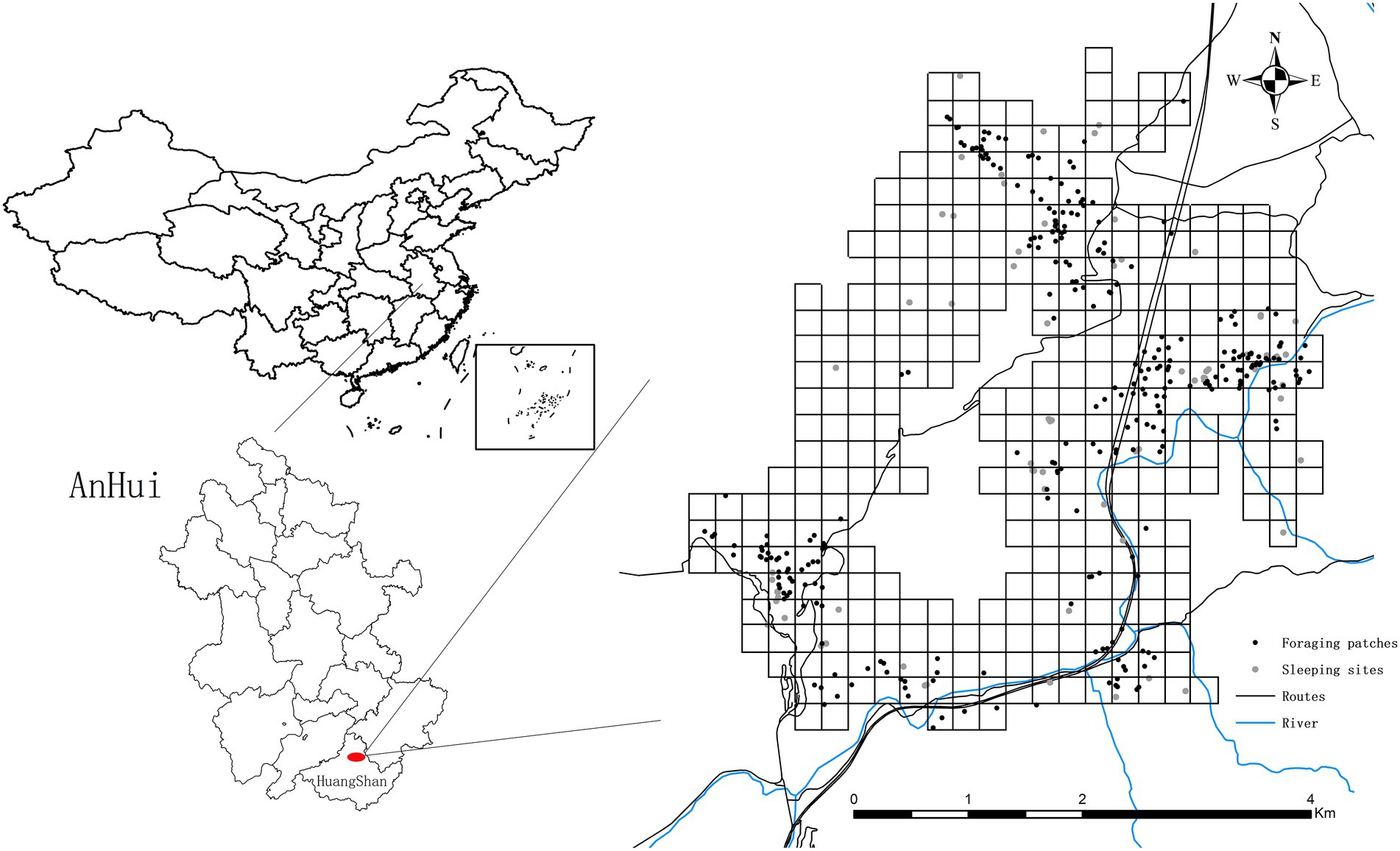
Figure 1. Locations of the study site and all Tibetan macaque feeding points and sleeping sites from September 2020 to August 2021.
We studied a group of wild Tibetan macaques for 12 consecutive months (September 2020 to August 2021). In 2018, we discovered this group of wild Tibetan macaques and began tracking them in 2019. After a year of tracking and habituation, we continuously collected data on the macaques from September 2020 to August 2021. The group consisted of 29 individuals, including 5 adult males, 8 adult females, 10 juveniles, and 6 infants. At least two groups of Tibetan macaques and two groups of rhesus macaques were also observed in the home range of the studied Tibetan macaque group during the study period. The home ranges of these groups overlapped spatially.
We set up 30 automatic return infrared cameras (UML4, UOVISION) in the Tibetan macaques’ home range to facilitate the location of the group. We combined the infrared camera feedback with the previous day’s tracking results to determine the sleeping site used by the Tibetan macaques on that day. We arrived at the sleeping site of the Tibetan macaques every morning before 06:00. After searching for the group, we followed them until they were either out of sight for more than 30 min or entered the sleeping site for the day. We used the instantaneous scan sampling method to collect data (Altmann, 1974), each sampling lasted for 5 min, and the interval between scans was 5 min. During each scan, we observed every individual sequentially from left to right for 5 s and recorded their main behaviors (classified as resting, moving, eating, socializing, and other behaviors). During tracking, we recorded the geographical location of the group every 10 min using Liangbulu GPS software (Shenzhen Liangbulu Information Technology Co., Ltd.). We obtained 93 days of observation data during the study period, including 76 full-day observations (3–12 days per month) and 17 half-day observations. Our data analysis was based on full-day data.
We defined the sleeping site as sites where the Tibetan macaque group stopped moving at dusk and rested until the following morning. Sleeping sites included cliff platforms or a group of several trees. Significant fecal deposits were present in commonly-used sleeping sites. We recorded the location of sleeping sites and habitat characteristics, including altitude, topography, and vegetation characteristics. We obtained 76 recordings of sleeping site use in Tibetan macaques involving 50 sleeping sites. To assess the spatial distribution of sleeping sites in Tibetan macaques, we recorded the locations of sleeping sites on topographic maps (1, 50,000) using the grid cell method (Huang et al., 2017). Because the group spread is usually <200 m, we divided their habitat into 200 × 200-m grid cells. This work was completed in ArcMap 10.4.1. Accordingly, the grid cells in which the Tibetan macaques were active were designated as sleeping and foraging sites, based on their behavior. When the group entered a grid cell and more than half of the individuals of the group were feeding simultaneously for >20 min, we defined the occupied cell as a foraging patch (Li et al., 2011; Liu et al., 2022). We recorded 117 foraging patches used 295 times during the study. The foraging intensity was calculated by dividing the number of records in a particular foraging patch by the total number of records.
To test the preference for sleeping sites in different parts of Tibetan macaques’ home range, we divided the annual home range of Tibetan macaques into core and peripheral areas. We defined the peripheral area as the area <200 m from the edge of the home range and considered the remaining area as the core area (Liu et al., 2022). We calculated Tibetan macaque home range as the number of 200 × 200-m grid cells that were occupied by the macaques during the study (Boyle et al., 2009). We calculated the expected value for the sleeping sites distributed separately in the core and periphery areas. We used the Chi-square test to examine the difference in sleeping site use between expected and observed values within the peripheral and core areas.
To investigate the effect of season on the altitudinal distribution of Tibetan macaques’ sleeping sites we used Kruskal–Wallis tests. We used Spearman rank correlations to investigate the relationship between the number of sleeping sites used and the number of surrounding food patches used. We calculated the number of food patches in the grid cell where the sleeping site was and in the adjacent grid cells to determine the number of surrounding food patches used.
To examine whether Tibetan macaques tended to use CPF or MCPF strategies, we estimated the linear distances between the last foraging patch used each day and the sleeping site used the same day (Used), between the last foraging patch and the nearest sleeping site used (MCPF), and between the last foraging patch and the central place (CPF). We used a paired Wilcoxon signed-rank test to compare the differences between Used and MCPF and between Used and CPF (Li et al., 2011). If there was no significant difference between the distance Used and MCPF, we assumed that Tibetan macaques adopted the MCPF strategy. If there was no significant difference between the distance Used and CPF, we assumed that Tibetan macaques adopted the CPF strategy. If both of the above hypotheses were accepted, we calculated the deviation between the distances Used and MCPF (the number of Used-MCPF), the deviation between the distance Used and CPF (the number of Used-CPF), respectively. Accordingly, we used a paired Wilcoxon signed-rank test to test the difference between two values of deviations to indicate Tibetan macaques tend to adopt the MCPF strategies or CPF strategies. If the number of Used-MCPF was smaller than the number of Used-CPF, we assumed Tibetan macaques adopted MCPF strategy. If the number of Used-CPF was smaller than the number of Used-MCPF, we assumed Tibetan macaques adopted CPF strategy. The central place in a CPF strategy was generated based on the arithmetic mean of all the sleeping site coordinates used (Brotcorne et al., 2014).
We examined the normality of all variables using one-sample Kolmogorov–Smirnov tests. We performed all statistical tests in SPSS 26.0. Statistical analyses were two-tailed, and the default significance level was set at p < 0.05.
The sleeping sites of Tibetan macaques could be divided into multiple (n = 11) and single-use (n = 39) sleeping sites; there were obvious fecal deposits in the multiple-use sleeping sites. The sleeping sites of Tibetan macaques were mainly distributed on the cliffs (n = 66, 86.8% of recorded nights), and the cliffs were dominated by shrubs, including Loropetalum chinense and Syzygium buxifolium. Neyraudia montana bushes were present on the cliff on which the macaques often slept. Only 13.2% of the sleeping sites were on hillside or trees in the forest (n = 10). And Tibetan macaques sleep in trees only in spring and summer (Table 1). The average altitude of sleeping sites was 407.71 ± 118.76 m, and differed significantly among seasons (Kruskal–Wallis tests: H = 14.617, df = 3, p = 0.002). The altitudes of sleeping sites were highest in summer and winter (Figure 2).
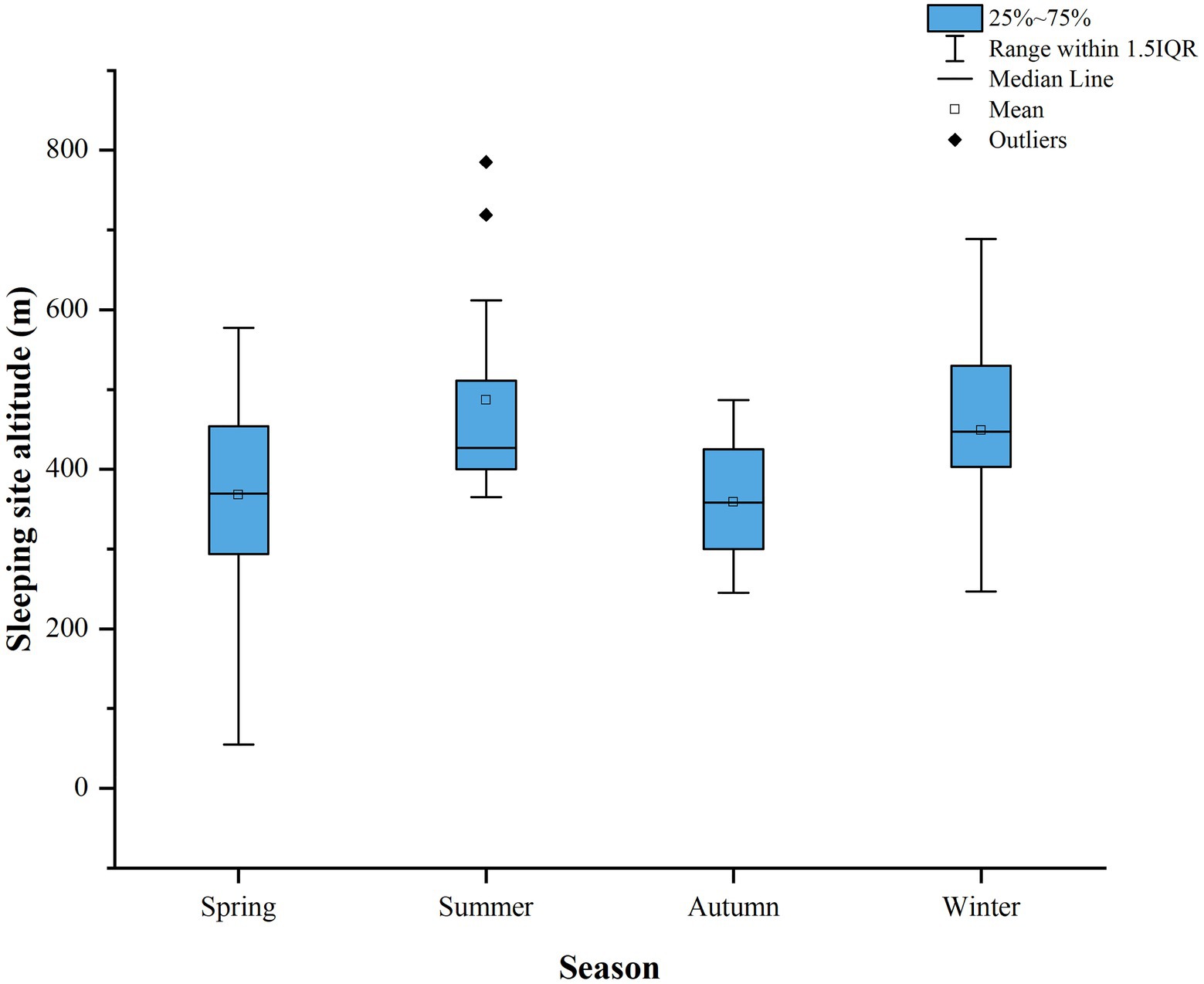
Figure 2. Sleeping site altitude of Tibetan macaques in different seasons (spring: n = 26, summer: n = 15, autumn: n = 19, winter: n = 19).
During the study, 50 sleeping sites were used a total of 76 times by Tibetan macaques; 12 of these were reused up to 5 times. The remaining 38 sleeping sites were used only once during the study. There was a preference for 8 specific sleeping sites, which accounted for 40.79% of all usage records (Table 1).
The 50 sleeping sites were distributed throughout the home range of Tibetan macaques, including the peripheral and core areas (Figure 3). Based on the annual active sites of Tibetan macaques, the total home range size was 13.56 km2. The peripheral area accounted for 56.6% of the entire home range, including 23 sleeping sites and accounted for 46% of all sleeping sites. The proportion of Tibetan macaques sleeping in the peripheral area was 38.2% (n = 29). The core area accounted for 43.4% of the home range, including 27 sleeping sites and accounting for 54% of all sleeping sites. The proportion of Tibetan macaques sleeping in the core area was 61.8% (n = 47). The chi-square goodness of fit test showed that the number of sleeping sites in the peripheral region was less than the expected value; whereas, the number of sleeping sites in the core area was more than the expected value (χ2 = 4.574, df = 1, p = 0.032). In addition, the use of sleeping sites in the peripheral area was less than expected, whereas it was higher than expected in the core area (χ2 = 14.084, df = 1, p < 0.001).
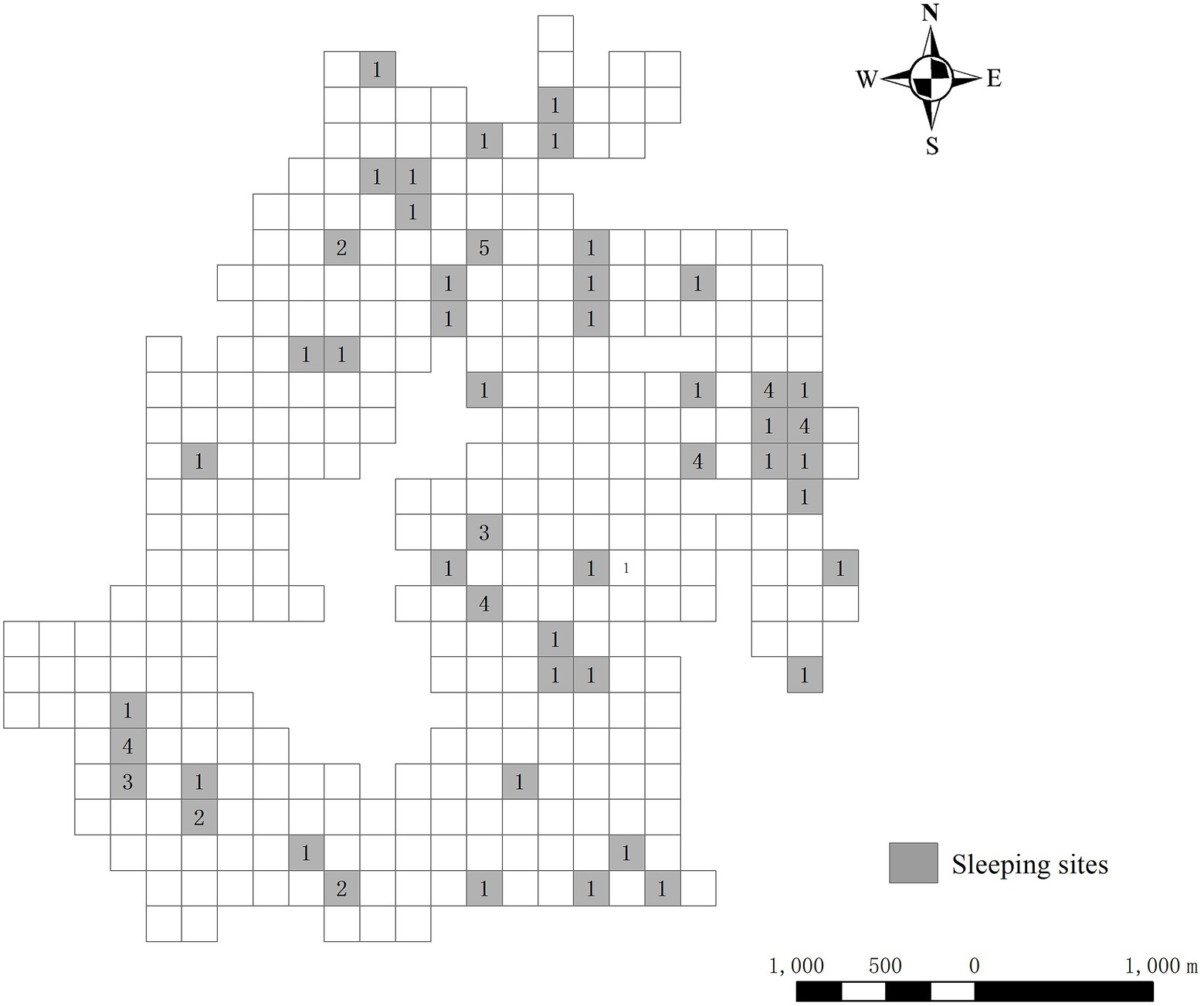
Figure 3. Distribution of sleeping sites used by Tibetan macaques in their home range during the study. Numbers indicate the frequency of sleeping site use.
We recorded 117 foraging patches used 292 times during the study. The location of sleeping sites was closely related to the location of foraging patches. Frequently used sleeping sites were often within or adjacent to the grid of commonly used foraging patches (Figure 4). The number of sleeping sites used was positively correlated with the number of surrounding foraging patches used (Spearman rank correlation: rs = 0.291, n = 50, p = 0.04).
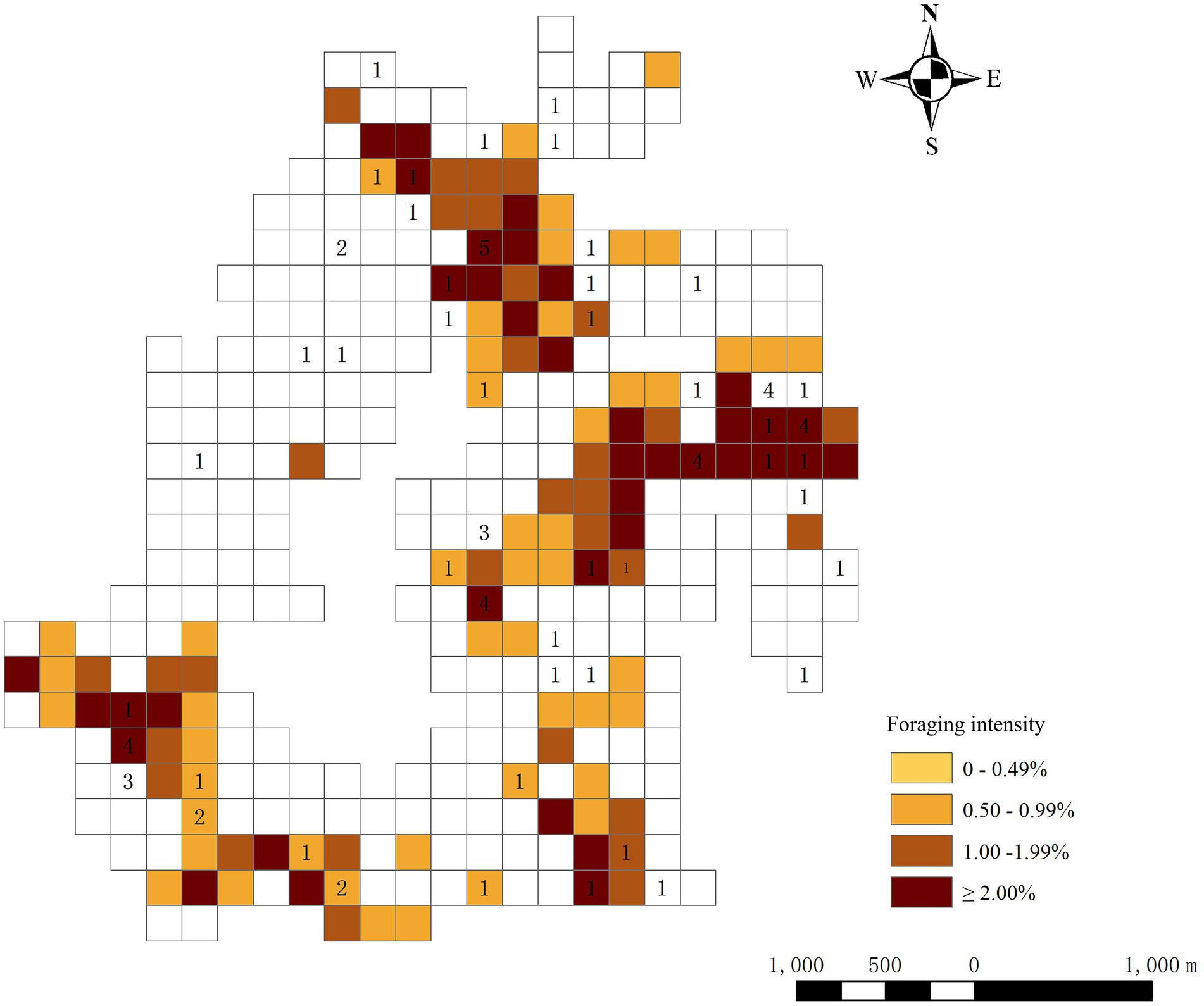
Figure 4. Distribution of foraging patches and sleeping sites of Tibetan macaques in different 200 × 200-m quadrats in a whole-year home range. Numbers indicate the locations of sleeping sites and the frequency of use, and percentages indicate the classification level of foraging intensity.
We compared the distances between the last foraging patch of the day and the sleeping site used on the day (Used) to that between the last foraging patch and the nearest sleeping site (MCPF), as well as to that between the last foraging patch and the central place (CPF). All differences were significant (Wilcoxon signed-rank test: Used vs. MCPF: Z = −5.855, n = 76, p < 0.001; Used vs. CPF: Z = −7.486, n = 76, p < 0.001). However, the difference between the distance from the last foraging patch to the sleeping site used on the day and the distance from the nearest sleeping site was significantly smaller than that between the distance from the last foraging patch to the sleeping site used and the central place (Wilcoxon signed-rank test: Z = −7.554, n = 76, p < 0.001; Figure 5).
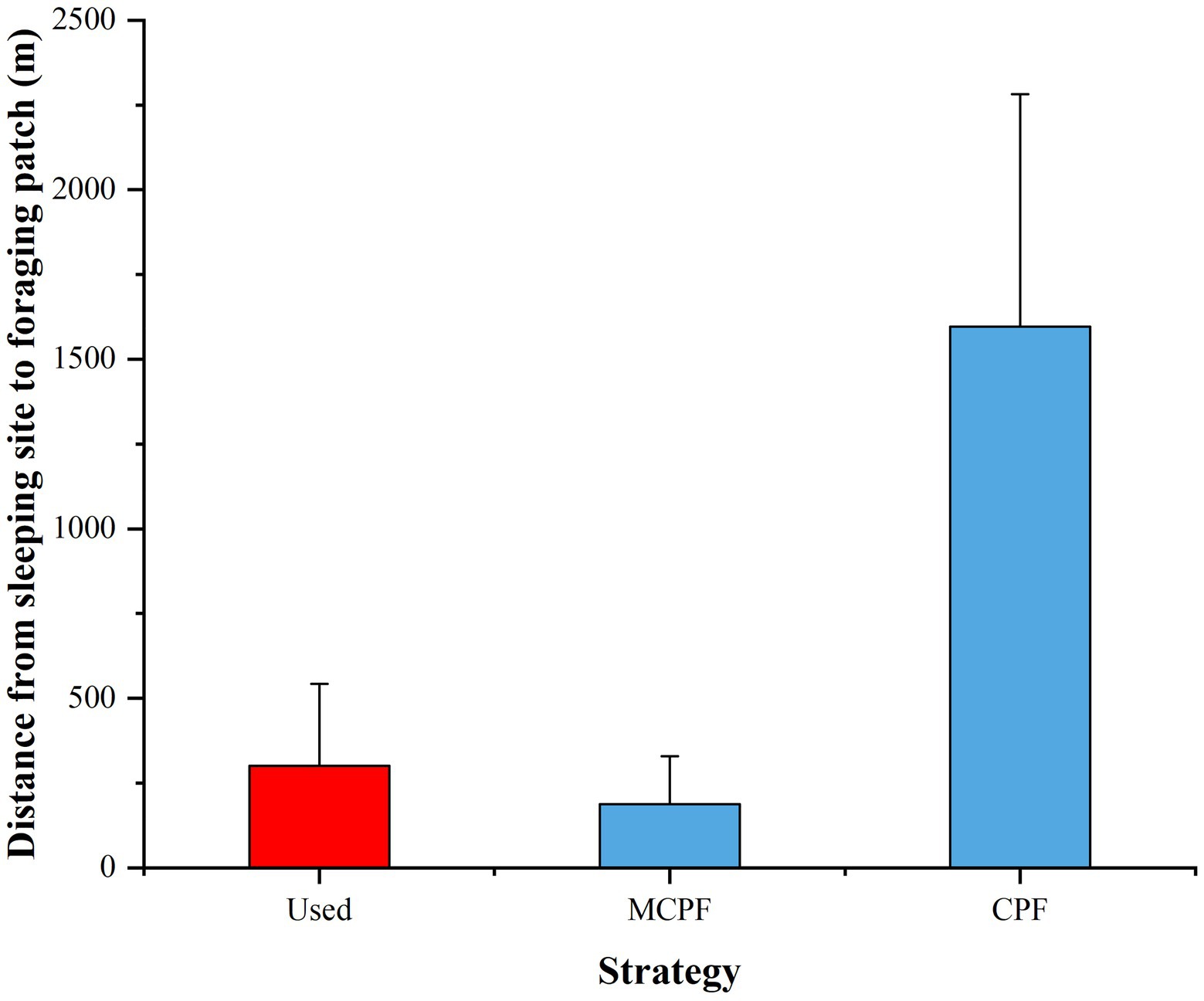
Figure 5. Mean distances between the last foraging patch of the day and the used sleeping site, the nearest sleeping site (multiple central place foraging [MCPF]), and the central sleeping site (central place foraging [CPF]) of Tibetan macaques.
Exploring primate sleeping site selection and foraging patch utilization is critical to understanding their ecological adaptation and guiding conservation. In this study, we analyzed the distribution of sleeping sites and their relationship with foraging patch utilization in wild Tibetan macaques by observing them under natural conditions. The results showed that Tibetan macaques used 50 sleeping sites throughout the year in their home range, that they preferred to sleep on cliffs, and that the altitude of sleeping sites differed significantly between seasons. Tibetan macaques reused some sleeping sites. They had more sleeping sites in the core area of their home range than in the peripheral areas, and used them more often than expected. In contrast, the peripheral areas were utilized less than expected. Sleeping site use was positively correlated with the number of foraging patches around the sleeping site, and the Tibetan macaques tended to adopt the MCPF strategy. These results indicate that food resources were one of the factors influencing sleeping site selection in Tibetan macaques, and that the choice of sleeping sites also played an important role in their foraging strategy.
Tibetan macaques used a total of 50 sleeping sites which were spread throughout their home range, consistent with our hypothesis (2). Multiple sleeping sites are conducive to full utilization of resources in the home range (Chapman et al., 1989). Primate sleeping site use may vary with the availability of food resources (Brotcorne et al., 2014). In the home range of the studied Tibetan macaques, food availability showed marked seasonal fluctuations (Li B. et al., 2022) and food resources were unevenly distributed (Li et al., 2021). This switching of sleeping sites may help the macaques utilize an unevenly distributed food resource in their active home range of up to 13.56 km2. Previous studies on Assamese macaques, white-headed langurs, and François’ langurs (T. francoisi) also found that they used multiple sleeping sites, and that the use of numerous sleeping sites reduced the cost of traveling to and from a single sleeping site (Qihai et al., 2009; Li et al., 2011; Liu et al., 2022).
We also found that Tibetan macaques tended to sleep on cliffs, and in slept in trees in summer. Earlier studies of Tibetan macaques also found that they slept in trees during summer for ventilation and protection from poisonous snakes (Li and Wang, 1994). Although we did not observe Tibetan macaques encountering predators during our study, and large predators such as the black bear (Ursus thibetanus) and clouded leopard (Neofelis nebulosa) have not been reported in the study area for a long time, some Viverridae species may still pose a potential threat to infant macaques (Li W.B. et al., 2022). The cliffs provide a natural barrier for Tibetan macaques from potential predators. Similar behaviors have been observed in Assamese macaques (Liu et al., 2022). Their sleeping sites are mainly located in the middle-upper areas of the cliff, which protects them from predators. Studies of white-headed langur monkeys have also found that they use caves in cliffs to reduce the chance of being spotted by predators (Li et al., 2011). The selection of sleeping sites on cliffs may therefore play a role in predator avoidance.
Our results showed that the altitude of Tibetan macaques’ sleeping sites is higher in summer and winter than in spring and autumn. In summer and winter, Tibetan macaques choose more sleeping sites at high altitudes, which may improve foraging efficiency and be associated with factors such as comfort and temperature regulation. Species richness and food availability were higher at lower altitudes than at higher altitudes, with spring and autumn being the most productive seasons for food (Korner, 2007). Tibetan macaques may spend more time at lower altitudes to satisfy their food needs (Zhou et al., 2022). However, in winter, Tibetan macaques rely on the fruits of Pinus massoniana and Fagaceae when other food resources are relatively scarce (Zhao, 1996). P. massoniana in the home range of Tibetan macaques is mostly distributed in areas >600 m above sea level (Li et al., 2021). To obtain food more conveniently, Tibetan macaques may choose to sleep in proximity to areas with a higher abundance of P. massoniana. Of course, comfort and temperature regulation may also be considered when selecting a sleeping site. Primates generally enjoy sunbathing during winter (Xiang et al., 2009). Summers are hot and the alpine region is cooler; whereas, the winters are colder and the bare rocks at higher altitudes receive more sun exposure. Therefore, Tibetan macaques may choose to sleep at relatively higher altitudes in the summer for cooler temperatures and better ventilation. In winter, sleeping at relatively high altitudes gives macaques early exposure to the morning sun. The choice of sleeping sites at different altitudes reflects the adaptability of Tibetan macaques to changes in resources and environment.
Although Tibetan macaques had sleeping sites throughout their home range, both the number of sleeping sites and the frequency used of sleeping sites in the peripheral region were less than expected. The territory or resource defense hypothesis suggests that sleeping at the edge of their territory helps primates detect and prevent encroachment by neighboring groups in advance (Day and Elwood, 1999). For example, white-headed langurs sleep more in the peripheral areas of their home range to prevent other males from invading the group (Li et al., 2011). Some primates do not tend to sleep at the edge of their home range, such as pileated gibbons (Hylobates pileatus), Assamese macaques, and Lar gibbons (H. lar; (Reichard, 1998; Phoonjampa et al., 2010; Liu et al., 2022). These studies found food resources to be more important than territorial defense in sleeping site selection. The same was true for Tibetan macaques, which had more than the expected number of sleeping sites and a higher-than-expected frequency of use in the core area. The number of sleeping sites used was positively correlated with the distribution of important foraging patches. Sleeping in the core area makes it easier to monopolize and protect food resources. Although other populations of Tibetan macaques and rhesus macaques were observed during the study period, no inter-group conflicts were recorded. Our results suggest that the selection of sleeping sites is mainly dependent on food resource distribution.
Our results suggest that the choice of sleeping sites plays an important role in the foraging strategy of Tibetan macaques. We found that Tibetan macaques usually slept in or near the last foraging patch of the day. As such Tibetan macaques were more likely to adopt the MCPF strategy than the CPF strategy for foraging patches. Although Tibetan macaques would repeatedly use a sleeping site, they rarely used the same sleeping site for several days consecutively. Changing sleeping sites appeared to facilitate the use of different food patches. Similar findings have been found in studies of black and gold howler monkeys (Alouatta caraya), saddleback (Saguinus fuscicollis), mustached tamarins (Saguinus mystax), François’ langurs, and white-headed langurs, which choose to sleep near food patches to improve foraging efficiency (Smith et al., 2007; Zhou et al., 2007; Li et al., 2011; Brividoro et al., 2019). In a previous study, food resources in the home range of Tibetan macaques had an uneven distribution with periodic temporal and spatial fluctuations (Li W.B. et al., 2022). In response to periodic resource shortages, primates minimize energy expenditure in their daily activities (Li Y.H. et al., 2020). In Tibetan macaques, the time for foraging and resting accounts for >70% of their total time allocation (Zhou et al., 2022). This pattern of sleeping site selection near foraging patches in Tibetan macaques can reduce foraging costs and increase the time spent foraging and resting, which may reflect their adaptation to uneven resource distribution.
In summary, there is a close relationship between the choice of sleeping site and the foraging strategy of Tibetan macaques. Tibetan macaques were more likely to use the MCPF strategy than the CPF strategy to exploit foraging patches. They selected their sleeping sites based on the foraging patch distribution. They slept more frequently near foraging patches than in the peripheral areas of their home range, highlighting that they may have selected their sleeping sites with greater foraging efficiency in mind. At the same time, the choice of sleeping sites by Tibetan macaques may also consider factors such as predators and comfort. Although this study provides detailed information on foraging patch utilization and sleeping site selection in Tibetan macaques, our results only involved one population; therefore, our next step will be to compare our results with other populations of Tibetan macaques. In future studies, environmental factors such as temperature and rainfall should be investigated more comprehensively. Studies on the selection and utilization of foraging patches and sleeping sites in Tibetan macaques will help to further understand how they adapt to their habitat and, in turn, provide positive implications for the conservation management of this species.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee, Anhui University.
B-WL and J-HL conceived and designed the study. B-WL and W-BL collected data and samples in the field. B-WL, D-PX, TZ, and P-PY analyzed the data. B-WL wrote the manuscript. J-HL provided funding support. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (no. 31971404) and the Central Government of Anhui Province guided the development of local science and technology (no. 2019b11030018).
We thank the management of Huangshan National Park and all the villagers of Niejiashan for facilitating and supporting our research. We thank Zhong-Bao Yang’s family for their help with the logistical arrangements. Many thanks go to the members of the International Collaborative Research Center for Huangshan Biodiversity and Tibetan Macaque Behavioral Ecology, who provided more help with data analysis and paper writing, especially Xi Wang, Bing-Hua Sun, Ya-Dong Li, and other members.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Allritz, M., Call, J., Schweller, K., McEwen, E. S., de Guinea, M., Janmaat, K. R. L., et al. (2022). Chimpanzees (pan troglodytes) navigate to find hidden fruit in a virtual environment. Sci. Adv. 8:eabm4754. doi: 10.1126/sciadv.abm4754
Altmann, J. (1974). Observational study of behavior: sampling methods. Behaviour 49, 227–266. doi: 10.1163/156853974x000534
Andersson, M. (1981). Central place foraging in the whinchat, Saxicola Rubetra. Ecology 62, 538–544. doi: 10.2307/1937718
Boyle, S. A., Lourenco, W. C., da Silva, L. R., and Smith, A. T. (2009). Home range estimates vary with sample size and methods. Folia Primatol (Basel) 80, 33–42. doi: 10.1159/000201092
Brividoro, M. V., Kowalewski, M. M., Scarry, C. J., and Oklander, L. I. (2019). Patterns of sleeping site and sleeping tree selection by black-and-gold howler monkeys (Alouatta caraya) in northern Argentina. Int. J. Primatol. 40, 374–392. doi: 10.1007/s10764-019-00094-x
Brotcorne, F., Maslarov, C., Wandia, I. N., Fuentes, A., Beudels-Jamar, R. C., and Huynen, M. C. (2014). The role of anthropic, ecological, and social factors in sleeping site choice by long-tailed macaques (Macaca fascicularis). Am. J. Primatol. 76, 1140–1150. doi: 10.1002/ajp.22299
Chapman, C. A., Chapman, L. J., and McLaughlin, R. L. (1989). Multiple central place foraging by spider monkeys: travel consequences of using many sleeping sites. Oecologia 79, 506–511. doi: 10.1007/BF00378668
Day, R. T., and Elwood, R. W. (1999). Sleeping site selection by the golden-handed tamarin Saguinus midas midas: the role of predation risk, proximity to feeding sites, and territorial defence. Ethology 105, 1035–1051. doi: 10.1046/j.1439-0310.1999.10512492.x
DiGiorgio, A. L., Upton, E. M., Susanto, T. W., and Knott, C. D. (2020). Wild Bornean orangutan (Pongo pygmaeus wurmbii) feeding rates and the marginal value theorem. Am. J. Primatol. 82:e23183. doi: 10.1002/ajp.23183
Fan, P. F., and Jiang, X. L. (2008). Sleeping sites, sleeping trees, and sleep-related behaviors of black crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, Central Yunnan, China. Am. J. Primatol. 70, 153–160. doi: 10.1002/ajp.20470
Fei, H. L., de Guinea, M., Yang, L., Chapman, C. A., and Fan, P. F. (2022). Where to sleep next? Evidence for spatial memory associated with sleeping sites in Skywalker gibbons (Hoolock tianxing). Anim. Cogn. 25, 891–903. doi: 10.1007/s10071-022-01600-0
Gazagne, E., Savini, T., Ngoprasert, D., Poncin, P., Huynen, M.-C., and Brotcorne, F. (2020). When northern pigtailed macaques (Macaca leonina) cannot select for ideal sleeping sites in a degraded habitat. Int. J. Primatol. 41, 614–633. doi: 10.1007/s10764-020-00173-4
Huang, Z., Yuan, P., Huang, H., Tang, X., Xu, W., Huang, C., et al. (2017). Effect of habitat fragmentation on ranging behavior of white-headed langurs in limestone forests in Southwest China. Primates 58, 423–434. doi: 10.1007/s10329-017-0600-4
José-Domínguez, J. M., Asensio, N., García, C. J. G., Huynen, M.-C., and Savini, T. (2015). Exploring the multiple functions of sleeping sites in northern pigtailed macaques (Macaca leonina). Int. J. Primatol. 36, 948–966. doi: 10.1007/s10764-015-9865-x
Korner, C. (2007). The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 22, 569–574. doi: 10.1016/j.tree.2007.09.006
Li, P. H., Li, W. B., Li, B. W., Li, Y. D., Wang, X., and Li, J. H. (2022). Positional behavior and substrate use in wild Tibetan macaques. Animals (Basel) 12:767. doi: 10.3390/ani12060767
Li, B., Li, W., Liu, C., Yang, P., and Li, J. (2022). Diverse diets and low-fiber, low-tannin foraging preferences: foraging criteria of Tibetan macaques (Macaca thibetana) at low altitude in Huangshan. Ecol. Evol. 12:e9338. doi: 10.1002/ece3.9338
Li, Y. H., Ma, G. Z., Zhou, Q. H., and Huang, Z. H. (2020). Seasonal variation in activity budget of Assamese macaques in limestone Forest of Southwest Guangxi, China. Folia Primatol. 91, 495–511. doi: 10.1159/000506593
Li, J.-H., Sun, L., and Kappeler, P. M. (2020). Behavioral Ecology of Tibetan Macaque. Cham: Springer Nature.
Li, J.-H., and Wang, Q.-S. (1994). Summer sleep site selection in Tibetan macaques. Chin. J. Zool. 29:58.
Li, W., Yang, P., Li, B., Liu, C., Sun, L., and Li, J. (2021). Habitat characteristics or protected area size: what is more important for the composition and diversity of mammals in nonprotected areas? Ecol. Evol. 11, 7250–7263. doi: 10.1002/ece3.7540
Li, W.-B., Yang, P.-P., Xia, D.-P., Huffman, M. A., Li, M., and Li, J.-H. (2022). Ecotourism disturbance on an endemic endangered primate in the Huangshan man and the biosphere Reserve of China: a way to move forward. Biology 11:1042. doi: 10.3390/biology11071042
Li, D. Y., Zhou, Q. H., Tang, X. P., Huang, H. L., and Huang, C. M. (2011). Sleeping site use of the white-headed langur Trachypithecus leucocephalus: the role of predation risk, territorial defense, and proximity to feeding sites. Curr. Zool. 57, 260–268. doi: 10.1093/czoolo/57.3.260
Liu, G., Liu, S., Li, Y., and Huang, Z. (2022). Predator avoidance and food patch proximity influence sleeping site use of Assamese macaques in a limestone forest of Southwest Guangxi, China. Am. J. Biol. Anthropol. 178, 244–256. doi: 10.1002/ajpa.24503
McAleer, K., and Giraldeau, L.-A. (2006). Testing central place foraging in eastern chipmunks, Tamias striatus, by altering loading functions. Anim. Behav. 71, 1447–1453. doi: 10.1016/j.anbehav.2006.01.005
McLaughlin, R. L., and Montgomerie, R. D. (1989). Brood dispersal and multiple central place foraging by Lapland longspur parents. Behav. Ecol. Sociobiol. 25, 207–215. doi: 10.1007/bf00302920
Michelot, T., Langrock, R., Bestley, S., Jonsen, I. D., Photopoulou, T., and Patterson, T. A. (2017). Estimation and simulation of foraging trips in land-based marine predators. Ecology 98, 1932–1944. doi: 10.1002/ecy.1880
Nakagawa, N. (1990). Choice of food patches by Japanese monkeys (Macaca fuscata). Am. J. Primatol. 21, 17–29. doi: 10.1002/ajp.1350210103
Paniw, M., Maag, N., Cozzi, G., Clutton-Brock, T., and Ozgul, A. (2019). Life history responses of meerkats to seasonal changes in extreme environments. Science 363:631. doi: 10.1126/science.aau5905
Phoonjampa, R., Koenig, A., Borries, C., Gale, G. A., and Savini, T. (2010). Selection of sleeping trees in pileated gibbons (Hylobates pileatus). Am. J. Primatol. 72, 617–625. doi: 10.1002/ajp.20818
Pirotta, E., Edwards, E. W. J., New, L., and Thompson, P. M. (2018). Central place foragers and moving stimuli: a hidden-state model to discriminate the processes affecting movement. J. Anim. Ecol. 87, 1116–1125. doi: 10.1111/1365-2656.12830
Qihai, Z., Chengming, H., Ming, L., and Fuwen, W. (2009). Sleeping site use by Trachypithecus francoisi at Nonggang nature reserve, China. Int. J. Primatol. 30, 353–365. doi: 10.1007/s10764-009-9348-z
Reichard, U. (1998). Sleeping sites, sleeping places, and presleep behavior of gibbons (Hylobates lar). Am. J. Primatol. 46, 35–62. doi: 10.1002/(sici)1098-2345(1998)46:1<35::Aid-ajp4>3.0.Co;2-w
Shaffer, C. A. (2014). Spatial foraging in free ranging bearded sakis: traveling salesmen or levy walkers? Am. J. Primatol. 76, 472–484. doi: 10.1002/ajp.22227
Smith, A. C., Knogge, C., Huck, M., Lottker, P., Buchanan-Smith, H. M., and Heymann, E. W. (2007). Long-term patterns of sleeping site use in wild saddleback (Saguinus fuscicollis) and mustached tamarins (S. mystax): effects of foraging, thermoregulation, predation, and resource defense constraints. Am. J. Phys. Anthropol. 134, 340–353. doi: 10.1002/ajpa.20676
Teichroeb, J. A., and Smeltzer, E. A. (2018). Vervet monkey (Chlorocebus pygerythrus) behavior in a multi-destination route: evidence for planning ahead when heuristics fail. PLoS One 13:e0198076. doi: 10.1371/journal.pone.0198076
Trapanese, C., Meunier, H., and Masi, S. (2019). What, where and when: spatial foraging decisions in primates. Biol. Rev. 94, 483–502. doi: 10.1111/brv.12462
Xiang, Z.-F., Nie, S.-G., Chang, Z.-F., Wei, F.-W., and Li, M. (2009). Sleeping sites of Rhinopithecus brelichi at Yangaoping, Guizhou. Int. J. Primatol. 31, 59–71. doi: 10.1007/s10764-009-9378-6
Zhao, Q. K. (1996). “Etho-ecology of Tibetan macaques at mount Emei, China,” in Evolution and Ecology of Macaque Societies. eds. J. E. Fa and D. G. Lindburg (Cambridge: Cambridge University Press), 263–289.
Zhou, Q. H., Huang, C. M., Li, Y. B., and Cai, X. W. (2007). Ranging behavior of the francois’ langur (Trachypithecus francoisi) in the fusui nature reserve, China. Primates 48, 320–323. doi: 10.1007/s10329-006-0027-9
Keywords: foraging patch, foraging strategy, food proximity, sleep, primate
Citation: Li B-W, Li W-B, Xia D-P, Zhang T, Yang P-P and Li J-H (2022) Sleeping sites provide new insight into multiple central place foraging strategies of Tibetan macaques (Macaca thibetana). Front. Ecol. Evol. 10:1067923. doi: 10.3389/fevo.2022.1067923
Received: 12 October 2022; Accepted: 02 December 2022;
Published: 16 December 2022.
Edited by:
Pedro Dias, Universidad Veracruzana, MexicoReviewed by:
Denise Spaan, Universidad Veracruzana, MexicoCopyright © 2022 Li, Li, Xia, Zhang, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Hua Li, amhsaUBhaHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.