- 1Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville, Knoxville, TN, United States
- 2Department of Fish and Wildlife Conservation, The Global Change Center at Virginia Tech, Blacksburg, VA, United States
Editorial on the Research Topic
Sex ratios in the Anthropocene
Plants and animals began to experience a dramatic uptick in novel stressors with the onset of the current epoch, the Anthropocene (Crutzen, 2002; Ehlers and Krafft, 2006; Steffen et al., 2011; Lewis and Maslin, 2015). Introduction of non-native species, pollution, landscape modification, climate change, and over-harvesting are some of the biggest offenders (Lande, 1998; Steffen et al., 2011; Newbold et al., 2015; Maxwell et al., 2016). While human activities can cause population declines via mortality and impaired reproduction, more cryptic disturbances to population sex ratios can be just as damaging to population viability (Wedekind, 2012; Boyle et al., 2014).
For decades scientists have issued warnings about sex ratio skews and the threat of extinction in species with environmental sex determination (ESD), like sea turtles (Janzen, 1994). However, there are myriad mechanisms that can lead to a skewed sex ratio, regardless of a species' mode of sex determination, and abnormally skewed sex ratios increase the threat of extinction in most species (Wedekind, 2012). Given that sex ratios are a linchpin of population dynamics (Hartl, 1988) and significantly affect evolutionary responses to changing environments (Hendry et al., 2017; Geffroy and Wedekind, 2020), it is imperative to place sex ratios at the center of conservation research.
We conducted a literature analysis to gauge the attention paid to anthropogenic disturbances to sex ratios, including the relative focus on different taxa and anthropogenic threats (Figures 1A–C). Below, we outline these findings within three conceptual frontiers. We address major knowledge gaps and highlight the contributions in this special issue that work to fill these gaps, with the goal of impelling additional sex ratio research in the Anthropocene.
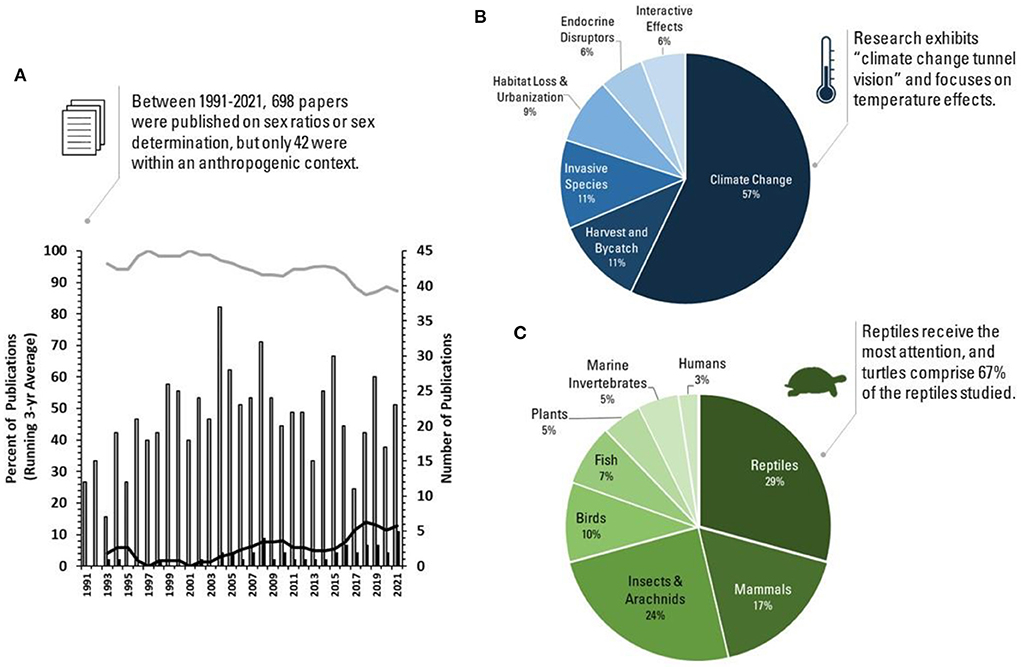
Figure 1. Publishing trends from top tier journals (Science, Nature, Proceedings of the Royal Society: B, Proceedings of the National Academy of Sciences of the USA, and Current Biology) between 1991- and 2021 on anthropogenic disturbances to sex ratios or sex determination based on the Web of Science database. (A) The number of papers published annually on sex ratios or sex determination in a fundamental context (gray bars) versus. an applied context (black bars). Trend lines are 3-year moving averages on the percentage of sex ratio or sex determination papers each year that were fundamental (gray) versus applied (black). (B) The anthropogenic threats studied within the applied sex ratio or sex determination papers. (C) The taxa represented in the applied sex ratio or sex determination research. Because we were concerned that using the above journals might bias our results toward fundamental studies, we ran a second literature analysis using eight top-tier conservation journals. Research in these journals emphasized applied sex ratio or sex determination research, yet produced strikingly similar trends and publication biases (unpublished) to the above results.
Anthropogenic effects on sex determination and juvenile sex ratios
Research on anthropogenic sex ratio skews is dominated by reptiles with temperature-dependent sex determination (Figures 1B,C). While continued work in this area is needed—particularly to determine the number of males required for population viability (Heppell et al.)—we need to broaden the scope of this research to ensure other species vulnerable to skewed sex ratios are considered. For example, there are numerous species with other forms of ESD (Korpelainen, 1990) or with labile forms of genetic sex determination that may also be at risk of skewed sex ratios as a result of anthropogenic activities (Stelkens and Wedekind, 2010; Bókony et al., 2017).
Despite the wealth of research on sex allocation theory in birds and mammals (Trivers and Willard, 1973; Rosenfeld and Roberts, 2004), these taxa are underrepresented in sex ratio conservation research (Figure 1C). Family-level sex ratio skews in response to environmental and social conditions can occur in these species, despite possessing genetic sex determination. For example, in European starlings (Sturnus vulgaris), similarity in maternal and paternal glucocorticoids, a hormone that is often responsive to anthropogenic stressors, affects a pair's likelihood of successfully fledging sons (Kilgour et al.).
Research on indirect mechanisms of sex ratio skew, like sex-specific recruitment, rarely appeared in the literature analysis. Sex-specific differences in traits that influence recruitment (e.g., growth rates and body size) can alter male and female recruitment rates, and thus, adult sex ratios. For example, wild-born female brown trout (Salmo trutta) have higher growth rates and grow larger than males, while hatchery-born individuals exhibit the opposite relationship (Palejowski et al.), which could alter recruitment and sex ratios after introduction into the wild. These indirect avenues of sex ratio skew may be cryptic, yet important drivers of population persistence.
Anthropogenic effects on adult sex ratios
Anthropogenic threats can skew sex ratios through sex-specific adult mortality. Of these threats, selective harvest and habitat loss receive the most research attention (Figure 1B).
Selective harvesting like hunting, fishing, and poaching (and the associated bycatch) can bias sex ratios by disproportionately removing one sex, especially in dimorphic species with prized ornamentation (Ginsberg and Milner-Gulland, 1994; Clutton-Brock et al., 2002; Mysterud, 2011). However, selective harvest can also affect sex ratios in more nuanced ways. For example, in many female-first sex-changing grouper, naturally female-biased sex ratios are common. Fishing practices can disrupt this sex ratio bias, which can decrease spawning and, ultimately, fishery sustainability (Chong-Montenegro and Kindsvater).
In species where males and females have different habitats, home ranges, or dispersal patterns, landscape modification can disproportionately impact the survival of one sex (Conde et al., 2010; Bonal et al., 2015). Road mortality of nesting female turtles as a function of increasing urbanization (Steen and Gibbs, 2004) is one example that has received considerable attention. Conservation efforts should include sex-specific habitat and resource use and movement patterns to better understand potential sex-specific threats that could lead to skewed sex ratios.
Engineered sex ratios
Our literature survey revealed growing exploration of intentional sex ratio manipulation for conservation, accounting for 7% of the applied papers published, mostly in the last decade. Sex ratio engineering may be used to correct a sex bias in a dwindling population, control the spread of a vector-borne diseases, or mitigate invasive species (Compton and Tu). For example, mosquito sex ratios are being engineered to curb avian malaria in threatened Hawaiian birds (Samuel et al., 2011) and sex allocation theory is being hacked to enhance population growth in New Zealand's endangered kakapo (Clout et al., 2002). Even in populations with balanced sex ratios, enhancing the production of females, which are often the limiting sex, can boost population growth (Wedekind, 2002). However, care should be taken to balance the potential benefits of sex ratio engineering with risks like increased inbreeding and reduced genetic variance (Wedekind, 2002, 2012).
Conclusions
The effects of anthropogenic disturbances on sex ratios are rippling across plant and animal taxa, yet research on the topic is broadly lacking, taxonomically biased, and dominated by a single anthropogenic threat (Figures 1A–C). We challenge researchers to think beyond the strong climate change bias apparent in publishing trends (Figure 1B), and to also consider climate change as the context through which other anthropogenic effects are interpreted. Given that wild populations are often faced with multiple human stressors (e.g., climate change and pollution), it is crucial that we account for these interactions as we gauge impacts to sex ratios in the Anthropocene.
Author contributions
AC and WH both helped conceive of the special issue topic, recruited and communicated with contributing authors, and edited the manuscript. In addition, AC conducted the literature analysis and wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
AC was funded by an AAUW American Postdoctoral Research Fellowship and WH was funded by the National Science Foundation (IOS-1755055).
Acknowledgments
We would like to express our sincere gratitude for all the authors and reviewers that contributed to this special issue.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bókony, V., Kövér, S., Nemesházi, E., Liker, A., and Székely, T. (2017). Climate-driven shifts in adult sex ratios via sex reversals: the type of sex determination matters. Philos. Transact. R. Soc. B Biol. Sci. 372, 20160325. doi: 10.1098/rstb.2016.0325
Bonal, R., Hernández, M., Espelta, J. M., Muñoz, A., and Aparicio, J. M. (2015). Unexpected consequences of a drier world: evidence that delay in late summer rains biases the population sex ratio of an insect. R. Soc. Open Sci. 2, 150198. doi: 10.1098/rsos.150198
Boyle, M., Hone, J., Schwanz, L. E., and Georges, A. (2014). Under what conditions do climate-driven sex ratios enhance versus diminish population persistence? Ecol. Evol. 4, 4522–4533. doi: 10.1002/ece3.1316
Clout, M. N., Elliott, G. P., and Robertson, B. C. (2002). Effects of supplementary feeding on the offspring sex ratio of kakapo: a dilemma for the conservation of a polygynous parrot. Biol. Conserv. 107, 13–18. doi: 10.1016/S0006-3207(01)00267-1
Clutton-Brock, T., Coulson, T., Milner-Gulland, E., Thomson, D., and Armstrong, H. (2002). Sex differences in emigration and mortality affect optimal management of deer populations. Nature 415, 633–637. doi: 10.1038/415633a
Conde, D. A., Colchero, F., Zarza, H., Christensen, N. L., Sexton, J. O., Manterola, C., et al. (2010). Sex matters: modeling male and female habitat differences for jaguar conservation. Biol. Conserv. 143, 1980–1988. doi: 10.1016/j.biocon.2010.04.049
Crutzen, P. J. (2002). Geology of mankind. Nature 415, 23–23. doi: 10.1038/415023a Ehlers, E., and Krafft, T. (eds.). (2006). Earth system science in the Anthropocene. Berlin; New York, NY: Springer.
Ehlers, E., and Krafft, T. (2006). Earth System Science in the Anthropocene. Berlin: Springer. p. 268. doi: 10.1007/b137853
Geffroy, B., and Wedekind, C. (2020). Effects of global warming on sex ratios in fishes. J. Fish Biol. 97, 596–606. doi: 10.1111/jfb.14429
Ginsberg, J. R., and Milner-Gulland, E. J. (1994). Sex-biased harvesting and population dynamics in ungulates: implications for conservation and sustainable use. Conserv. Biol. 8, 157–166. doi: 10.1046/j.1523-1739.1994.08010157.x
Hendry, A. P., Gotanda, K. M., and Svensson, E. I. (2017). Human influences on evolution, and the ecological and societal consequences. Philos. Transact. R. Soc. B Biol. Sci. 372, 20160028. doi: 10.1098/rstb.2016.0028
Janzen, F. J. (1994). Climate change and temperature-dependent sex determination in reptiles. Proc. Nat. Acad. Sci. U. S. A. 91, 7487–7490. doi: 10.1073/pnas.91.16.7487
Korpelainen, H. (1990). Sex ratios and conditions required for environmental sex determination in animals. Biol. Rev. 65, 147–184. doi: 10.1111/j.1469-185X.1990.tb01187.x
Lande, R. (1998). Anthropogenic, ecological and genetic factors in extinction and conservation. Popul. Ecol. 40, 259–269. doi: 10.1007/BF02763457
Lewis, S. L., and Maslin, M. A. (2015). Defining the Anthropocene. Nature 519, 171–180. doi: 10.1038/nature14258
Maxwell, S. L., Fuller, R. A., Brooks, T. M., and Watson, J. E. (2016). Biodiversity: the ravages of guns, nets and bulldozers. Nature 536, 143–145. doi: 10.1038/536143a
Mysterud, A. (2011). Selective harvesting of large mammals: how often does it result in directional selection? J. Appl. Ecol. 48, 827–834. doi: 10.1111/j.1365-2664.2011.02006.x
Newbold, T., Hudson, L. N., Hill, S. L., Contu, S., Lysenko, I., Senior, R. A., et al. (2015). Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50. doi: 10.1038/nature14324
Rosenfeld, C. S., and Roberts, R. M. (2004). Maternal diet and other factors affecting offspring sex ratio: a review. Biol. Reprod. 71, 1063–1070. doi: 10.1095/biolreprod.104.030890
Samuel, M. D., Hobbelen, P. H., DeCastro, F., Ahumada, J. A., LaPointe, D. A., Atkinson, C. T., et al. (2011). The dynamics, transmission, and population impacts of avian malaria in native Hawaiian birds: a modeling approach. Ecol. Appl. 21, 2960–2973. doi: 10.1890/10-1311.1
Steen, D., and Gibbs, J. (2004). Effects of roads on the structure of freshwater turtle populations. Conserv. Biol. 18, 1143–1148. doi: 10.1111/j.1523-1739.2004.00240.x
Steffen, W., Grinevald, J., Crutzen, P., and McNeill, J. (2011). The Anthropocene: conceptual and historical perspectives. Phil. Trans. R. Soc. A 369, 842–867. doi: 10.1098/rsta.2010.0327
Stelkens, R. B., and Wedekind, C. (2010). Environmental sex reversal, Trojan sex genes, and sex ratio adjustment: conditions and population consequences. Mol. Ecol. 19, 627–646. doi: 10.1111/j.1365-294X.2010.04526.x
Trivers, R. L., and Willard, D. E. (1973). Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92. doi: 10.1126/science.179.4068.90
Wedekind, C. (2002). Manipulating sex ratios for conservation: short-term risks and long-term benefits. Anim. Conserv. 5, 13–20. doi: 10.1017/S1367943002001026
Keywords: sex ratio, sex determination, Anthropocene, climate change, population dynamics
Citation: Carter AW and Hopkins WA (2022) Editorial: Sex ratios in the Anthropocene. Front. Ecol. Evol. 10:1048575. doi: 10.3389/fevo.2022.1048575
Received: 19 September 2022; Accepted: 21 October 2022;
Published: 02 November 2022.
Edited and reviewed by: Achaz von Hardenberg, University of Chester, United Kingdom
Copyright © 2022 Carter and Hopkins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda W. Carter, YWNhcnRlODJAdXRrLmVkdQ==
 Amanda W. Carter
Amanda W. Carter William A. Hopkins
William A. Hopkins