- 1Nara National Research Institute for Cultural Properties, Nara, Japan
- 2Institute of Archaeology named after A.Kh. Margulan, Almaty, Kazakhstan
- 3National Museum of the Republic of Kazakhstan, Astana, Kazakhstan
- 4BioArCh - University of York, York, United Kingdom
- 5National Research Institute of Cultural Heritage, Daejeon, South Korea
The Bronze Age—Iron Age transition in Central Asia (ca. 800 BCE) was a period of significant cultural change that was heavily influenced by greater population interaction and mobility. Indeed, scholars have increasingly emphasized the role that “food globalization in prehistory” has played in defining this period. In the mountain foothills of Kazakhstan, culinary traditions from across Eurasia were combined through the use of Southwest Asian wheat, barley, and livestock (cattle, sheep, and goats) with East Asian foxtail and broomcorn millets. The development of food cultures during this period has been investigated by archaeobotanical and isotopic analysis, yet lipid residues from pottery, which directly represent culinary practices, have not been adequately examined. In this study, lipid residue analysis was conducted on 72 pottery sherds, excavated from three burial mounds and one non-burial, ritual site located in Kazakhstan, dating to ca. 700–200 BCE. A particularly informative observation was the frequency of miliacin, a biomarker of broomcorn millet, detected in residues that corresponded well with previously published regional differences observed in carbon isotope ratios of human remains that indicate the consumption of C4 plants. This study also demonstrates continuity of Bronze Age dairying traditions into the Iron Age. Finally, this study sheds new light on the diversity of food cultures and mortuary practices in this region, which were not uniform across either space or time.
Introduction
Pottery production first developed among East Asian populations almost 20,000 years ago and among Southwest Asian populations around 9,000 years ago. These developments represented significant expansions in the range of foods available to humans and enabled the development of novel cooking techniques (Barnett et al., 1995; Tsuneki et al., 2017; Jordan and Gibbs, 2019). Adding to the typical toolkit of ceramic specialists (Skibo, 2015), researchers are increasingly applying biomolecular techniques to better understand the ways that archaeological pottery was used. While ancient proteins have been identified in Neolithic Anatolian pottery, providing exciting new avenues of investigation (Hendy et al., 2018), the primary biomolecular approach to understanding past pottery use remains lipid residue analysis, as these compounds are more resistant to diagenesis during heating and deposition (Eglinton et al., 1991).
Lipid residue analysis has been extensively applied to historic and prehistoric pottery, from many parts of the world, successfully identifying the use of diverse ingredients, including dairy products (Evershed et al., 2008a) and broomcorn millet (Heron et al., 2016; Standall et al., 2022), and has shed new light on a variety of different culinary practices (Cramp et al., 2011; Craig et al., 2013; Dunne et al., 2016; Shoda et al., 2020). However, this technique has only been applied to a limited number of sites from Kazakhstan and, while these studies have demonstrated the possible domestication of horses and use of horse milk around 3,500 BCE and the use of ruminant dairy products from ca. 2,100–900 BCE, no study has previously explored the diverse mixtures of foodstuffs and traditions that epitomize the beginning of food globalization in prehistory. This period of extensive East–West interaction in Central Asia incorporated the translocation of foxtail and broomcorn millet, wheat, barley, and other plant species, domesticated livestock, including cattle, sheep, and goats, in addition to material culture, people, and ideas (Jones et al., 2011; Hermes et al., 2019; Liu et al., 2019; Matuzevičiūtė and Liu, 2021). Therefore, great potential exists to investigate and elucidate how these cultural interactions are represented in the selection and combination of foodstuffs and the methods and materials used to process them, i.e., culinary practices.
In the past decade, archaeologists working in Central Asia have made major discoveries regarding: (1) the earliest dispersals of Southwest Asian crops into northern Central Asia (Zhou et al., 2020); (2) the earliest spread of domesticated sheep and goat into the Central Asian foothills (Hermes et al., 2022); and (3) the earliest westward dispersals of East Asian crops, such as broomcorn millet (Spengler et al., 2018; Yatoo et al., 2020). While archaeologists, such as Akishev, working at village sites in Southern Kazakhstan have recognized the prominence of agriculture in the economy of certain populations during the first millennium BCE (see Spengler et al., 2021), this period is often characterized by a perceived focus on specialized mobile pastoralism. Increasingly, scholars are recognizing the regionally diverse economies of this period and part of the world, with people adapting to localized environmental constraints. Among these adaptations, extensive archaeological excavation has illustrated that farming villages existed in ecologically rich mountains foothills during this time (Chang, 2022). While scholars increasingly accept that these people expressed marked regional differences in their economies, comparative data on their diets remains scarce and verifications of these interpretations with modern molecular methods, exploring culinary practices, are still lacking. Here, we apply this method to pottery excavated from three mound burials “kurgans” and one non-burial ritual “sanctuary” site, in different parts of Kazakhstan, that demonstrate clear evidence of pottery belonging to the first millennium BCE. Our aim is to better understand the diversity of cooking practices performed in the first millennium BCE, such as the frequency of millet and dairy product use and the contribution from other kinds of natural resources to cuisine.
Materials and methods
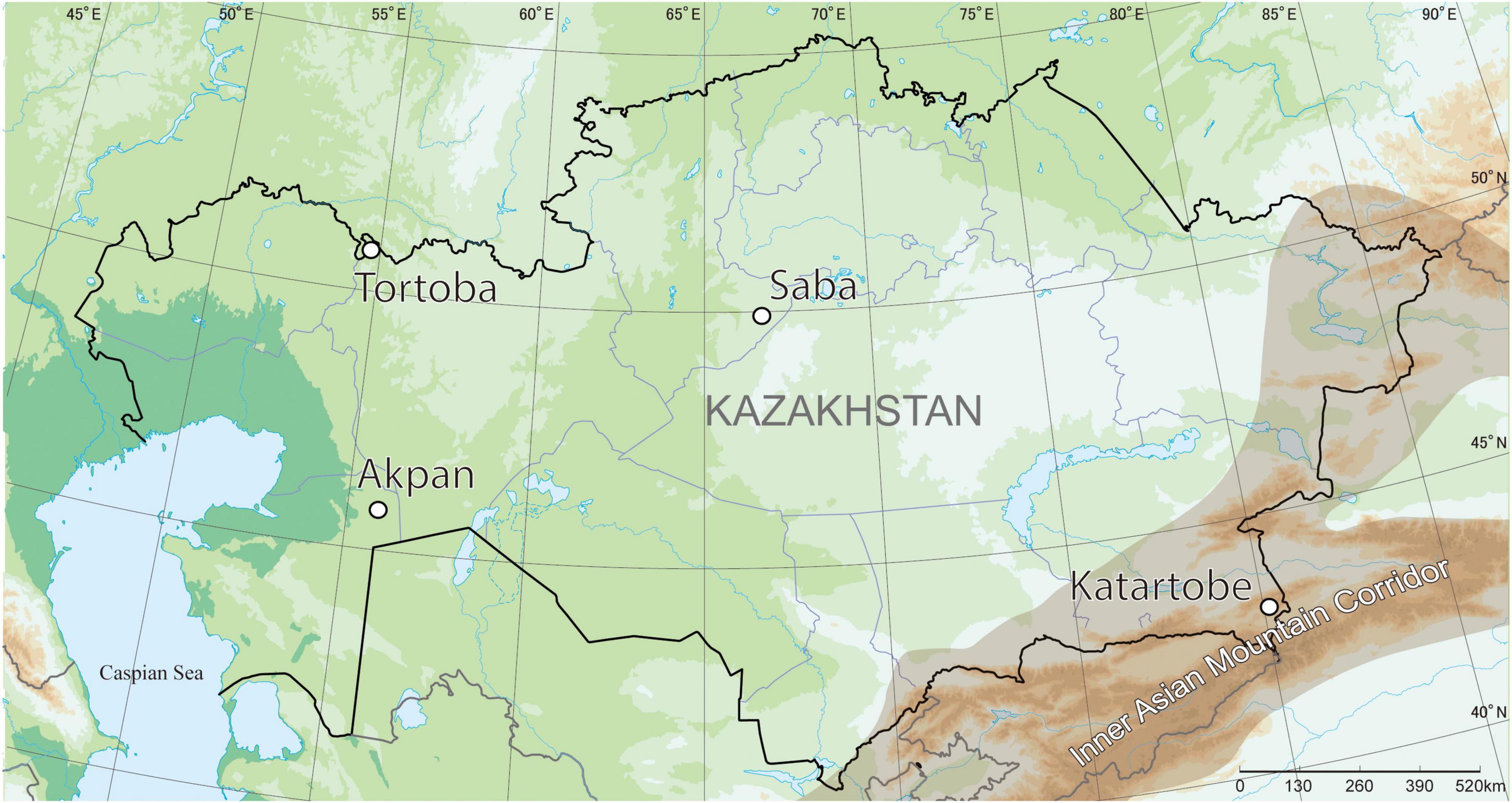
Ceramic sherds were sampled from four Early Iron Age archaeological sites across Kazakhstan, including the Tortoba kurgan site in western Kazakhstan (50°44′33″N, 54°44′59″E, n = 17, 6th to 4th century BCE), the Saba kurgan site in central Kazakhstan (49°49′41″N, 66°09′42″E, n = 11, 7th to 5th century BCE), the Katartobe kurgan site in Southeastern Kazakhstan (43°11′24″N, 80°08′27″E, n = 20, 5th century BCE), and the Akpan sanctuary site in Southwestern Kazakhstan (45°44′12″N, 55°48′26″E, n = 24, 4th to 2nd century BCE). The location of these sites is presented in Figure 1.
Lipid extraction and gas chromatography mass spectrometry (GC-MS) analysis was undertaken at the Nara National Research Institute for Cultural Properties and gas chromatography combustion isotope ratio mass spectrometry (GC-c-IRMS) analysis was conducted at the University of York. Samples were obtained from the internal surface of ceramic sherds using an electric model (Dremel) drill equipped with a tungsten carbide bit. Firstly, the sampling area was cleaned by removing the ceramic surface, to a depth of <1 mm, using the drill. A clean drill bit was then used to obtain approximately 2 g of ceramic powder from the sampling area by drilling to a depth of between 2 and 5 mm. Clean drill bits were used for each sherd sampled. Around 1 g of ceramic powder was accurately weighed for each extraction.
Acid extraction
We followed the protocol detailed in Craig et al. (2013) for acidified methanol extraction. Clean glass vials containing around 1 g (±5%) of ceramic powder and 10 μl of a n-tetratriacontane internal standard (1 μg μl–1) were prepared. Approximately 4 ml of methanol (CH3OH) was added to each vial before they were placed in an ultrasonic bath, set to 25°C, for 15 min. Approximately 800 μl of sulfuric acid (H2SO4) was then added to each vial before they were placed in a heating block, set to 70°C, for 4 h. The vials were cooled to room temperature then centrifuged at 4,000 rpm for 5 min. The supernatant was transferred to a new vial. Approximately 2 ml of hexane (C6H14) was added to the new vials, mixed using a vortex mixer, and allowed to stand for several minutes for static separation. The supernatant (hexane) layer containing extracted lipids was then transferred to a third vial. The process of adding, mixing, and transferring hexane was repeated two additional times. The extract was then dried under a gentle nitrogen gas flow. Hexane was added to the dried extract, mixed, and transferred to a GC vial containing 10 μl of the internal standard n-hexatriacontane (1 μg μl–1), in two stages (first time: 90 μl and second time: 50 μl), to produce a final extract suspended in 150 μl of hexane. One blank and standard was processed with each batch to identify any potential contamination that may have occurred during the extraction process.
Solvent extraction
We followed the protocol detailed in Evershed et al. (1990) for solvent extraction. Vials containing around 1 g (±5%) of ceramic powder and 10 μl of a n-tetratriacontane internal standard (1 μg μl–1) were prepared. Approximately 5 ml of dichloromethane-methanol (DCM:MeOH, 2:1 V/V) was added to each vial before they were placed in an ultrasonic bath, set to 25°C, for 15 min. The vials were then centrifuged at 4,000 rpm for 15 min followed by transfer of the supernatant to a new vial. The process of adding, mixing, and transferring DCM:MeOH was repeated two additional times. The extract was then dried under a gentle nitrogen gas flow. Hexane was added to the dried extract, mixed, and transferred to a GC vial containing 10 μl of the internal standard n-hexatriacontane (1 μg μl–1), in two stages (first time: 90 μl and second time: 50 μl), to produce a final extract suspended in 150 μl of hexane. One blank and standard was processed with each batch to identify any potential contamination that may have occurred during the extraction process. Solvent extracts were derivatized using BSTFA + TMCS, 99:1 [N, O-bis (trimethylsilyl) trifluoroacetamide with 1% trimethyl-chlorosilane] prior to analysis by GC-MS.
Gas chromatography mass spectrometry
Extracts were analyzed by gas chromatography–mass spectrometry using a Shimadzu GCMS-QP2010Ultra. The inlet temperature was set to 300°C and 1 μl sample was introduced to the GC-MS using the splitless injection method. An Ultra ALLOY-5 (Frontier Laboratories Ltd., Japan: 30 m × 0.25 mm, film thickness 0.25 μm) column was used. The oven temperature was set to 50°C for 2 min, then raised to 325°C, at 10°C/min, and held for 12 min. The m/z scan range was between 50 and 800, with a total acquisition time of 41.5 min. The ion source temperature was set to 230°C and ionization voltage to 70 eV. Helium was the carrier gas, with a flow of 3 ml/min.
Samples were also analyzed in SIM mode for the detection of miliacin. The oven temperature was set to 50°C for 1 min, raised to 280°C at 20°C/min, then raised to 325°C at 5°C/min, and held for 8.5 min. Selected ions were m/z 189, 294, 231, 425, and 440, with a total acquisition time of 30 min. The ion source temperature was set to 230°C and ionization voltage to 70 eV. Helium was the carrier gas, with a flow of 3 ml/min.
Gas chromatography combutsion isotope ratio mass spectrometry
Acidified methanol extracts that contained sufficient quantities of C16:0 and C18:0 n-alkanoic acids were subjected to GC-c-IRMS according to the method detailed in Craig et al. (2012). The GC-c-IRMS used consisted of an Agilent 7890B GC-MS connected to an Isoprime GC5 interface and an Isoprime 100 IRMS. The inlet temperature was set to 300°C and 1 μl sample was introduced to the GC-MS using the splitless injection method. A DB-5MS UI (J&W Scientific technologies, USA: 60 m × 0.25 mm, film thickness 0.25 μm) column was used. The GC oven temperature was set to 50°C for 0.5 min, then raised to 175°C at 25°C/min, and maintained for 20 min. The eluate was introduced directly from the column to the interface and carbonized in a GC5 tube (CuO) held at 850°C. Clear separation of the analyzed peaks was achieved with high resolution. The eluate from the interface was ionized by electroshock in the IRMS, with intensities at m/z 44, 45, and 46 measured and the 13C/12C ratios calculated on a computer using Ion Vantage and IonOS software (Isorime, Cheadle, UK). Results of the measurements were expressed as per mill (‰) relative to the international standard V-PDB. For accuracy and precision of the measurements, standards with known isotopic ratios (Indiana standard F8-3) were used.
Results
Gas chromatography mass spectrometry
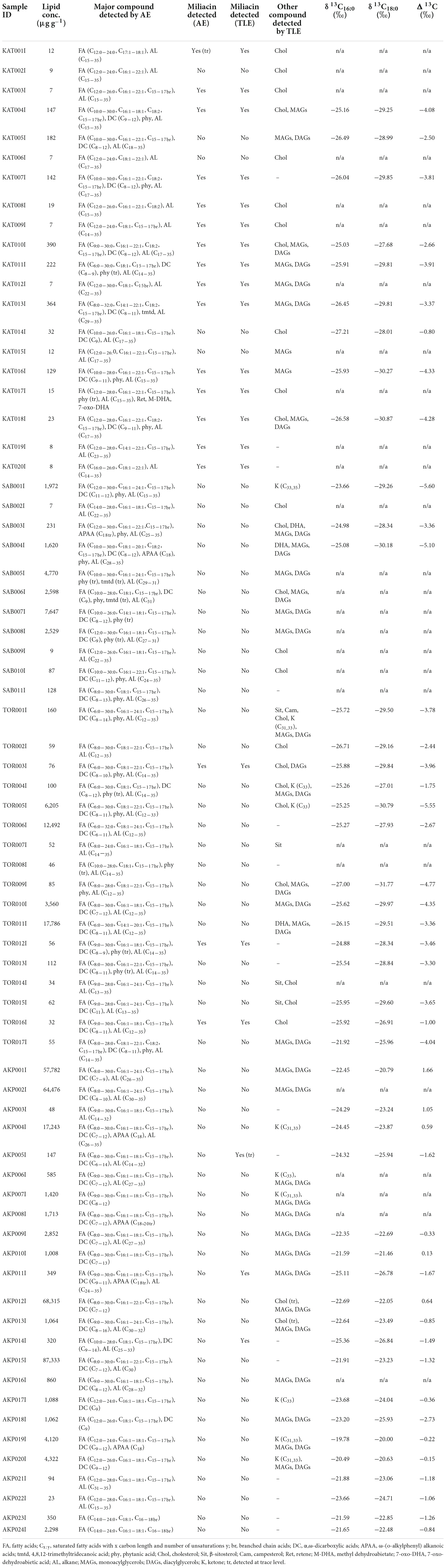
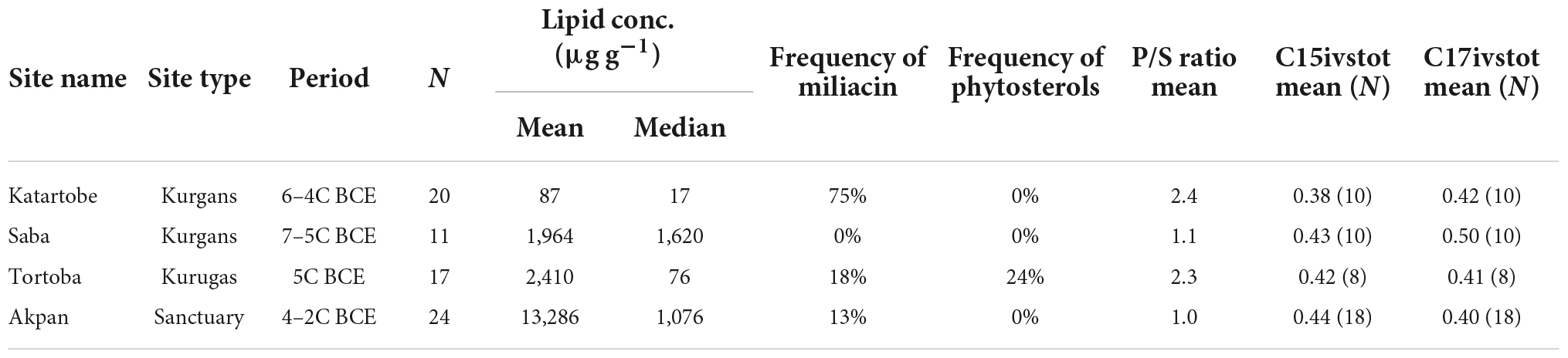
Quantified lipid residue concentrations and a list of major compounds detected in each sample are presented in Table 1, with a summary of results from each site presented in Table 2. All of the samples analyzed in this study contained interpretable quantities of lipids (>5 μg g–1 for pottery sherds, Craig et al., 2013). Differences in lipid concentration between sites (Table 2) may derive from either variation in environmental factors or the original context of their use.
Acidified methanol extracts primarily consisted of palmitic (C16:0) and stearic (C18:0) acids, with a range of other saturated (C6:0–C30:0), mono-unsaturated (C16:1–C22:1), poly unsaturated (C18:2), and branched chain (C15 and C17) fatty acids, dicarboxylic acids (C6–C14), and alkanes (C12–C35), present in some of the samples (Figure 2). Phytanic acid was frequently identified in samples from Katartobe (6 of 20), Saba (9 of 11), and Tortoba (9 of 17), and 4,8,12-trimethyltridecanoic acid (TMTD) was observed in samples from Katartobe (n = 1) and Saba (n = 2). In addition, a low abundance of C18 APAAs were identified in samples from Saba (n = 2) and Akpan (n = 4). However, these compounds were often present in distinct samples. Therefore, while aquatic product processing may be identified by the observation of C18–22 ω-(o-alkylphenyl)alkanoic acids (APAAs) and isoprenoid fatty acids, i.e., TMTD, phytanic, and pristanic acids (Hansel et al., 2004; Evershed et al., 2008b), it is difficult to assess the extent to which aquatic resources contributed to these residues with confidence. A series of compounds characteristic of heated Pinaceae resin, including retene, methyl dehydroabietate, and 7-oxo-dehydroabietic acid (Modugno and Ribechini, 2009), were identified in one sample (KAT017I) from Katartobe. These compounds may derive from wood smoke, cooking, and firing while using Pinaceae species as fuel.
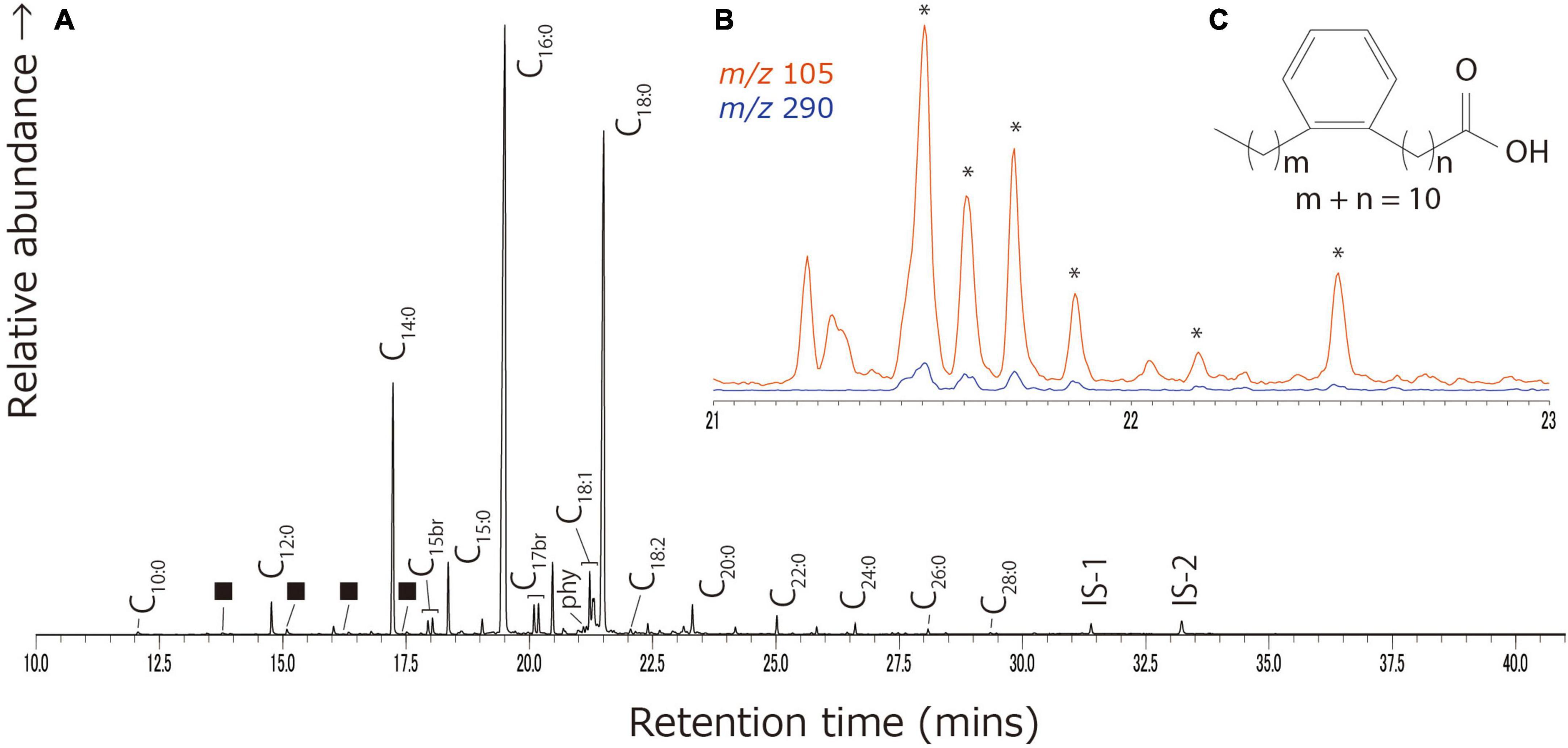
Figure 2. Typical partial gas chromatogram of the Saba potsherd (SAB004IAE) lipid extract. (A) Partial total-ion chromatogram of the acidified methanol extracts. Peak identities–Cx:y: saturated fatty acids with x carbon length and number of unsaturations y, ■: α,ω-dicarboxylic acids with carbon chain C8-C11. phy, phytanic acid; IS-1, n-tetratriacontane; IS-2, n-hexatriacontane. (B) The summed m/z 105 and 290 ion chromatogram shows the presence of ω-(o-alkylphenyl) alkanoic acids with 18 carbon atoms (*). (C) Chemical structure of APAAs.
A series of phytosterols, including campesterol and β-sitosterol were identified in several solvent extracts from Tortoba (n = 4) (Figure 3). Cholesterol was frequently observed together with monoacylglycerols (MAGs), diacylglycerols (DAGs), and long chain ketones. Dehydroabietic acid, a compound present in species from the family Pinaceae (Costa et al., 2016), was detected in samples from Saba (n = 2) and Tortoba (n = 1).
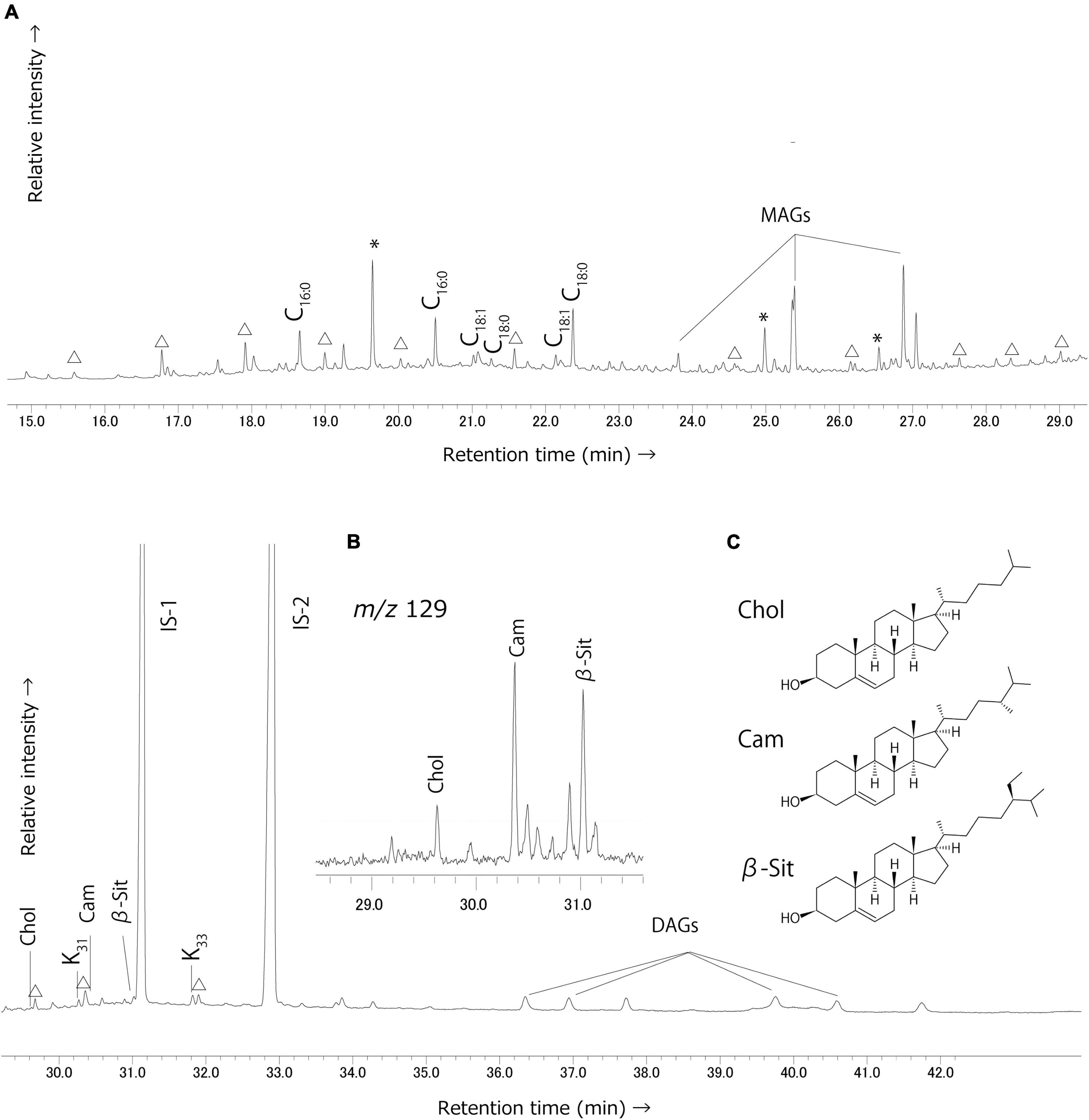
Figure 3. Typical partial gas chromatogram of the Tortoba potsherd (TOR001ITLE) lipid extract. (A) Partial total-ion chromatogram of the solvent extracts. Peak identities–Cx:y: saturated fatty acids with x carbon length and number of unsaturations y,△: n-Alkanes, *plasticizer contamination. MAGs, monoglycerides; DAGs, diglycerides; IS-1, n-tetratriacontane; IS-2, n-hexatriacontane. (B) The summed m/z 129 ion chromatogram shows the presence of sterols. (C) Chemical structure of the Cholesterol, Campesterol, and β-sitosterol.
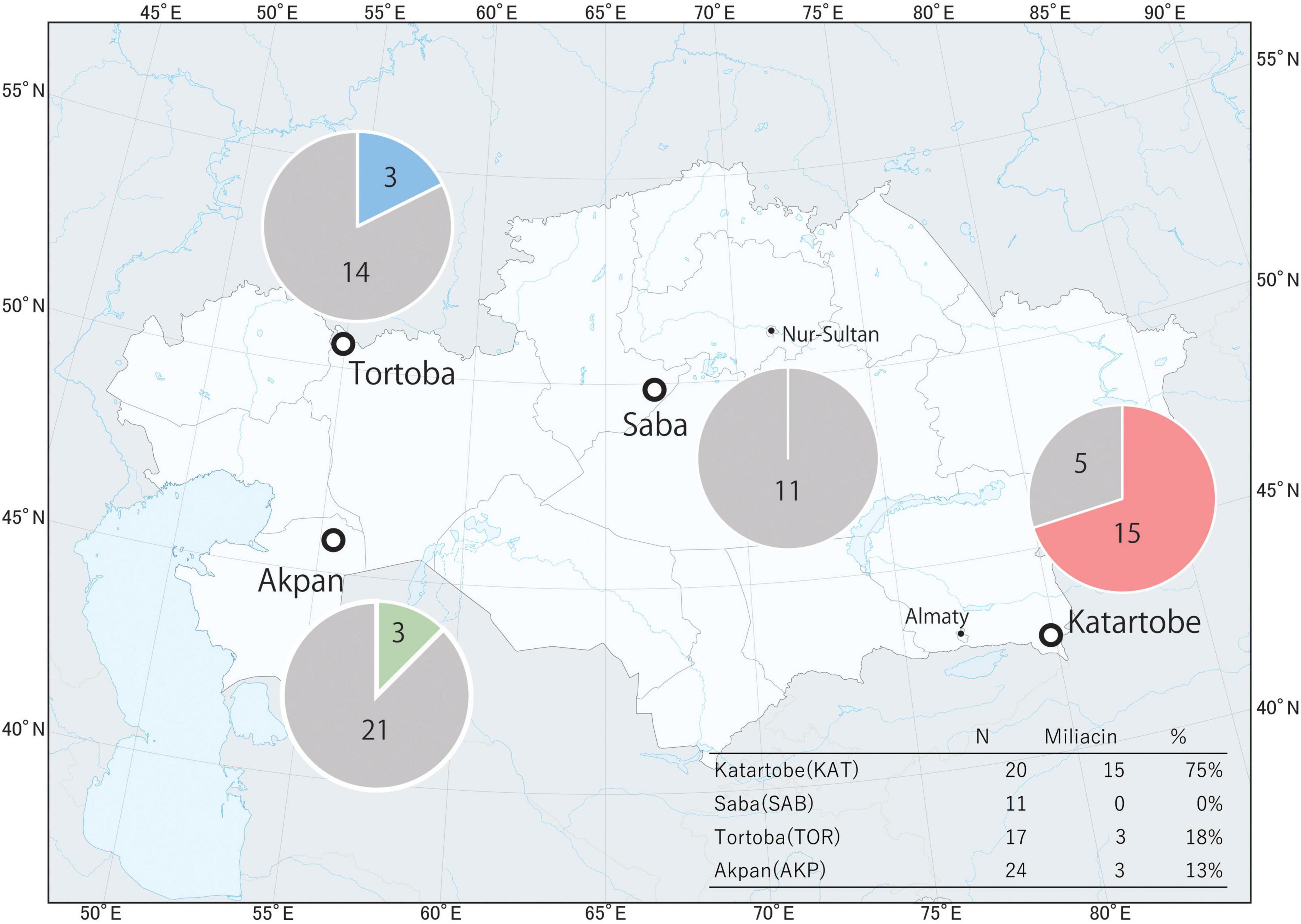
Miliacin (olean-18-en-3β-ol methyl ether) was identified in acidified methanol and solvent extracts from Katartobe (n = 15), Akpan (n = 3), and Tortoba (n = 3), although it was absent from Saba (Figure 4). Interestingly, there was a notable difference in the relative abundance of miliacin between Katartobe, Tortobe, and Akpan, with much lower abundances observed at the latter two sites. This data may indicate a difference in either the frequency or quantity of broomcorn millet processed in these vessels, although other factors, such as preferential preservation conditions, may have also contributed to the observed difference.
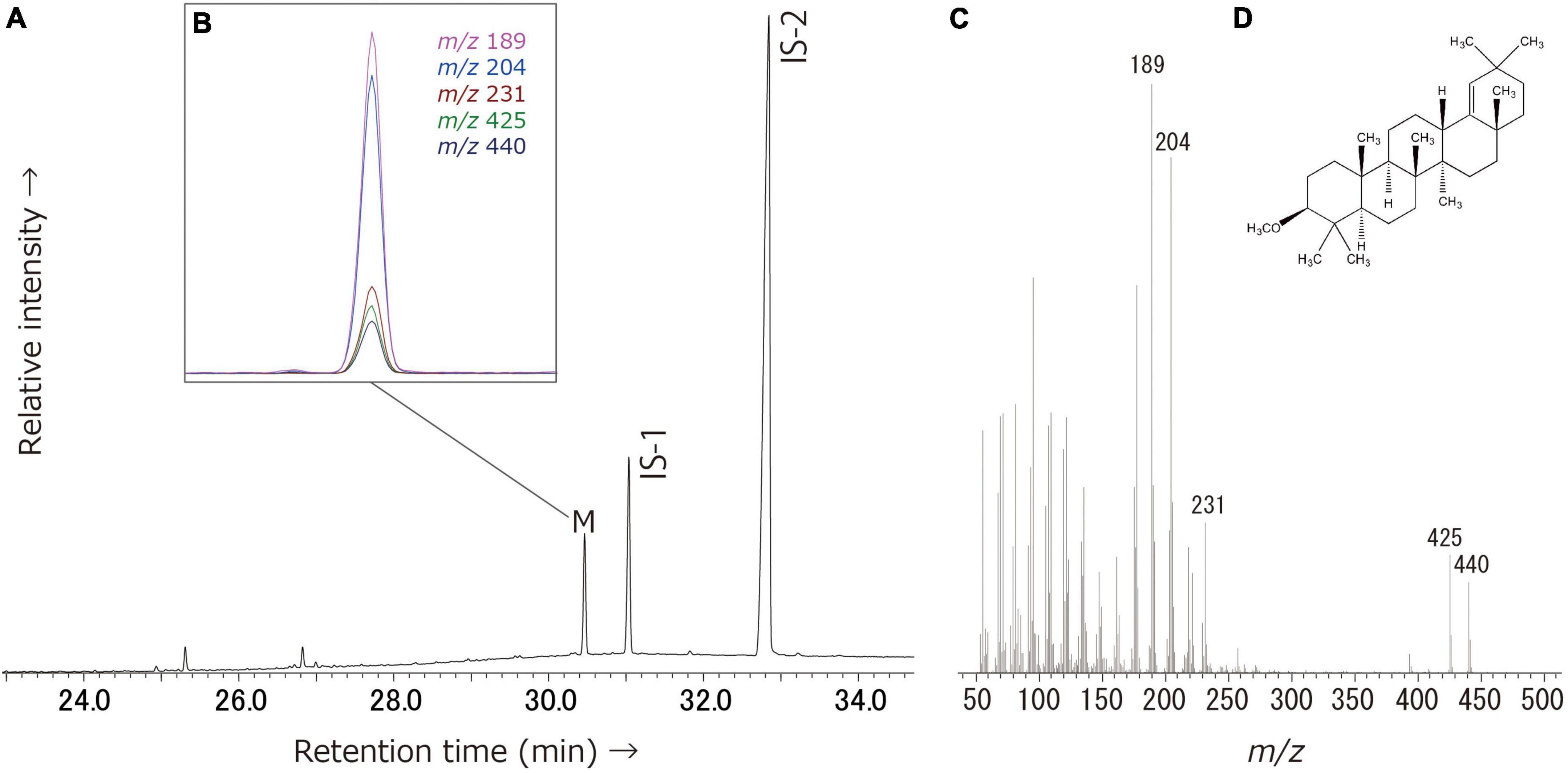
Figure 4. Typical partial gas chromatogram of the Katartobe potsherd (KAT003ITLE) lipid extract. (A) Partial total-ion chromatogram of the solvent extracts. Peak identities–M, miliacin; IS-1, n-tetratriacontane; IS-2, n-hexatriacontane. (B) The summed m/z ion 189, 204, 231, 425, and 440 chromatograms show the presence of miliacin. (C) Mass spectrum of miliacin detected from the extracts. (D) Chemical structure of miliacin.
The relative abundance of iso- and anteiso- branched chain C15 and C17 fatty acids present in acidified methanol extracts was calculated to assess the contribution of aquatic resources to residues (C15ivstot = i15:0/i15:0 + a15:0, C17ivstot = i17:0/i17:0 + a17:0, Demirci et al., 2021). There was no significant difference between C15ivstot/C17ivstot values from the four sites (Katartobe: 0.38/0.42, Saba: 0.43/0.50, Tortoba: 0.42/0.41, and Akpan: 0.44/0.40), all of which demonstrated lower ratios than predominantly aquatic residues from Europe (C15ivstot range = 0.39–0.82 and mean = 0.61; C17ivstot range = 0.22–0.66 and mean = 0.53, Demirci et al., 2021) and corresponding values from modern fish (C15ivstot 0.59 ± 0.1; C17ivstot 0.59 ± 0.5, Hauff and Vetter, 2010).
Finally, the ratio of palmitic and stearic acids (P/S) was calculated to assess the contribution of plant lipids to residues (Dunne et al., 2016). The mean P/S values were greater at Katartobe (2.4) and Tortoba (2.3) than at Saba (1.1) and Akpan (1.0), corresponding to the frequency of miliacin and phytosterols at the former two sites, respectively. However, it is difficult to accurately evaluate the extent of plant lipid contribution to residues from this data alone.
Gas chromatography combustion isotope ratio mass spectrometry
Stable carbon isotope values (δ13C) of two major n-alkanoic acids (C16:0 and C18:0) were measured to obtain further information on the origin of the residues. The results demonstrate a broad range of δ13C16:0 (−27.21 to −19.78‰) and δ13C16:0 (−31.77 to −20.00‰) values (Table 1). In Figure 5, the δ13C16:0 and Δ13C (δ13C18:0–δ13C16:0) values of samples from this study are plotted against reference ranges of modern and authentic archaeological plant and animal lipids (Lucquin et al., 2018; Cubas Morera et al., 2020; Taché et al., 2021), enabling the discrimination of ruminant adipose and dairy oils (Evershed et al., 1999; Copley et al., 2003). Residues from Tortoba, Saba, and Katartobe predominantly comprise ruminant adipose and dairy fats, whereas most samples from Akpan plot within the range of non-ruminant adipose and aquatic oils. However, these results must be carefully interpreted given the potential effects that mixing products, such as millet, may have on the isotopic composition of residues (Taché et al., 2021) as is discussed below.
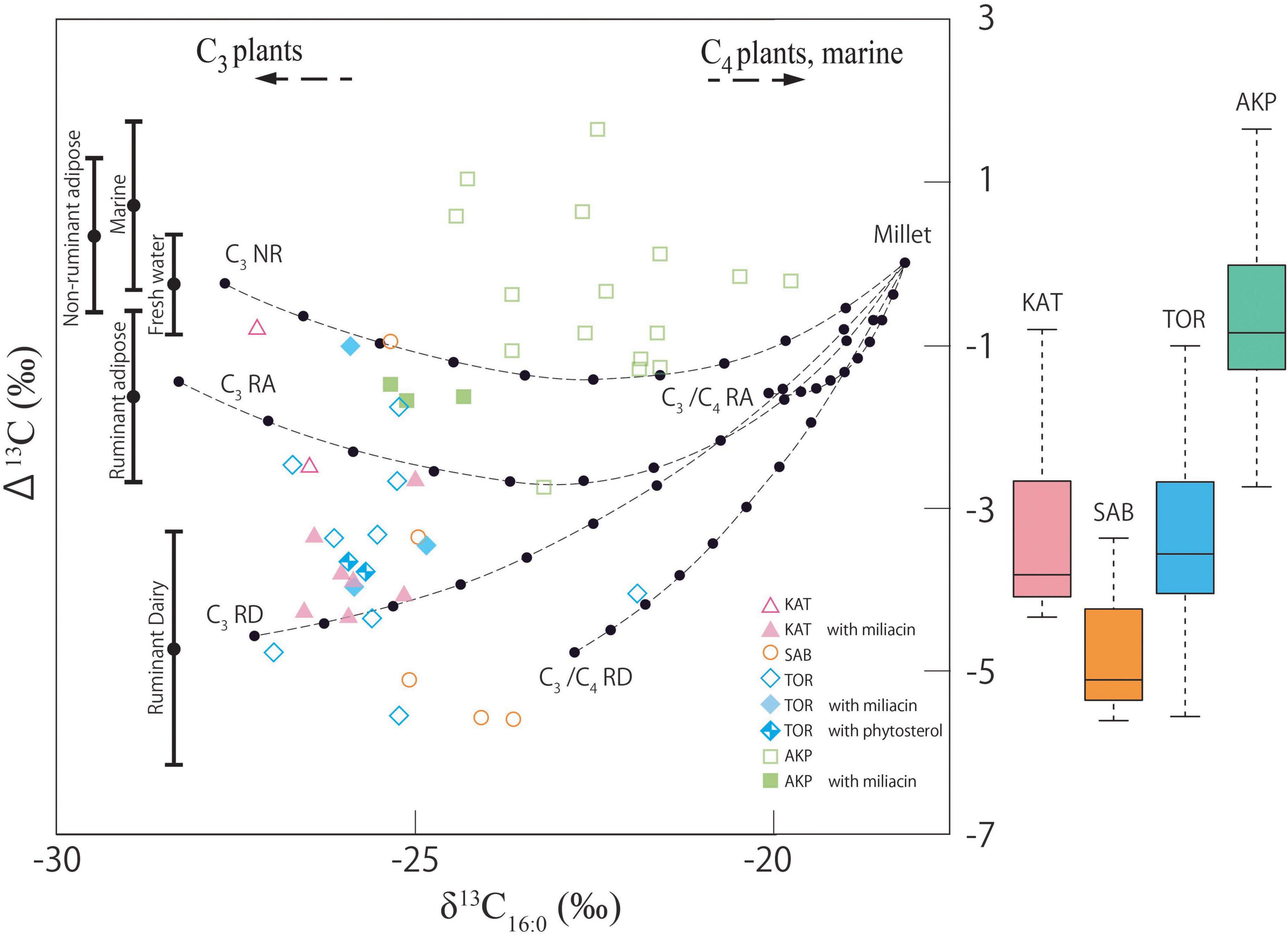
Figure 5. Plot of the offset between the δ13C values of stearic and palmitic acids (Δ13C value) against the δ13C value of the palmitic acid. Reference ranges show quartile ranges based on data from existing studies (Dudd and Evershed, 1998; Spangenberg et al., 2006, 2010; Gregg et al., 2009; Outram et al., 2009; Dunne et al., 2012; Spiteri, 2012; Craig et al., 2013; Horiuchi et al., 2015; Miyata et al., 2015; Taché and Craig, 2015; Choy et al., 2016; Heron et al., 2016; Lucquin et al., 2016; Courel et al., 2020; Pääkkönen et al., 2020; Taché et al., 2021). The hypothetical mixing curves (Taché et al., 2021) are compared with the plots to verify when the offset values are large due to the mixing of millets.
Discussion
The frequency and abundance of miliacin, a biomarker for broomcorn millet
Miliacin is a pentacyclic triterpene methyl ether (PTME) concentrated in the seeds of P. miliaceum (broomcorn millet). Species in closely related genera of the panicoid and chloridoid subfamilies—Panicum, Pennisetum, Paspalum, Digitaria, Chionochloa, Eragrostis, Glyceria, and Microstegium—are also reported to produce miliacin, in varying abundance, in addition to other PTMEs (Jacob et al., 2005; Bossard et al., 2013). However, it is unlikely that any of these species, other than P. miliaceum, was actively used as an important food source in East and Central Asia. Therefore, the detection of miliacin in cooking pots may be directly attributed to the processing of P. miliaceum (Shoda et al., 2021).
While it is not possible to reliably compare the frequency of miliacin observations at each site, due to varied and small sample sizes, this dataset potentially highlights regional differences in the extent to which P. miliaceum was processed in cooking pots (Figure 6). It is evident that P. miliaceum was processed in a proportionally greater number of vessels at Katartobe than other sites examined in this study. This may reflect either greater or more varied use of the cereal in cooking pots, perhaps representing regional culinary culture. However, it is important to note that an absence of miliacin does not necessarily demonstrate that P. miliaceum was not processed, as questions remain as to the development of broomcorn millet residues and the survivability of miliacin (Standall et al., 2022). Complicating this matter further are the high δ13C16:0 values observed at Akpan, relative to other sites examined, that may reflect a contribution of lipids from either P. miliaceum, aquatic resources, or combinations of the two. Therefore, while a substantial contribution of lipids from P. miliaceum is perhaps unlikely, our findings remain tentative and warrant further investigation. In addition, it is difficult to assess the economic significance of P. miliaceum from these data alone, as only culturally significant sites were examined. Further research is necessary to assess the use and uses of this cereal beyond culturally significant sites in order to understand the significance of P. miliaceum in subsistence strategies.
Other plant biomarkers
Phytosterols, including β-sitosterol, campesterol and stigmasterol, were identified in four samples from Tortoba indicating the presence of plant products in pottery, although it is not possible to identify their source.
The identification of retene in KAT017I demonstrates the exploitation of Pinaceae species. However, there is no indication as to how this resource was exploited, as the transfer of resin markers may have been either deliberate or indirect. These compounds may be transferred during either the firing process, cooking, pitch production, or the application of pitch as a sealant (Colombini et al., 2005; Drieu et al., 2020). The presence of dehydroabietic acid in other samples cannot be confidently attributed to the exploitation of Pinaceae species as this compound is a potential environmental contaminant (Horiuchi et al., 2013).
Mixing of foodstuffs
Ceramic-absorbed organic residues represent an accumulation of lipids throughout the use-life of a vessel (Miller et al., 2020). Therefore, it is important to consider the effects that both concurrent and sequential mixing of different foodstuffs may have on the isotopic composition of a residue. For instance, recent studies have demonstrated that isotopic values indicative of ruminant adipose fats (Dudd and Evershed, 1998; Dunne et al., 2012) may be formed by the mixing of millet with either C3-fed ruminant carcass or dairy fats (Taché et al., 2021). In addition, it is important to consider that the isotopic composition of a product may be influenced by environmental conditions (Fernandes et al., 2018) and that any such differences between archaeological residues and modern reference material must be accounted for during interpretation.
Samples that contain miliacin and phytosterols plot within the range of C3-fed ruminant adipose, dairy, and non-ruminant products (Figure 5), likely indicating that plant products were processed with a variety of different ingredients, either concurrently or sequentially. Understanding the composition of residues containing miliacin becomes difficult when isotopic data is viewed with theoretical mixing models. Samples that occupy the space between C3-fed non-ruminant—millet and C3-fed ruminant—millet mixing curves, such as TOR016I, AKP003I, and AKP011I, cannot be confidently attributed to either, as there is substantial natural variation in the isotopic values of different products resulting in overlapping values. In the same manner, while KAT013I and TOR012I produced Δ13C values that correspond to the ruminant dairy products, they plot between C3-fed ruminant—millet, and C3-fed ruminant dairy—millet mixtures, making their interpretation more difficult.
Differences in pottery use between sites
There is a clear isotopic distinction between the dominant source of lipids at Akpan and the other three sites investigated. While ruminant (e.g., sheep, goat, and cattle) products were the primary ingredient at Saba, Katartobe, and Tortoba, residues from Akpan primarily comprise non-ruminant lipids. Higher δ13C16:0 and Δ13C values at Akpan may derive from the processing of products including aquatic resources, horse meat and dairy, C4-fed non-ruminant animals, and C4 plants. However, given the low frequency of miliacin observations at Akpan (13%) and the low abundance in which it was observed, it is unlikely that P. miliaceum was the dominant source of 13C enrichment in these samples. Indeed, it is questionable whether P. miliaceum directly contributed to residue formation in any of the samples that do not contain miliacin. The proximity of Akpan to the Caspian Sea raises the possibility of aquatic resource processing, yet the absence of aquatic biomarkers, which are often poorly preserved, prevents confirmation of this hypothesis.
Previous analysis conducted on ceramics from Bronze Age settlement and cemetery sites in northern Kazakhstan demonstrated dominant contributions of ruminant adipose and dairy fats to residues (Outram et al., 2011, 2012). The data from this study, specifically from Saba, Katartobe, and Tortoba, indicate that dairying traditions may have continued into the Iron Age.
The isotopic distinction between residues from Akpan and the three kurgan sites raises the possibility that different foodstuffs were processed during different activities, such as burials and ritual sacrifices, practiced at kurgan and sanctuary sites. While this data indicates a distinction in the use of 13C enriched products at Akpan and dairy products at the kurgan sites, further research is necessary to fully explore these observations and determine their reliability and significance to local cultures.
Comparing lipid residue and human and animal bone collagen stable isotope datasets
Bronze Age human remains from the Gansu Corridor and Xinjiang Uyghur Autonomous Region in China demonstrate a significant East–West distinction in carbon isotope values that is attributable to the degree of C4 plants incorporated into diets (Wang et al., 2017). Late Bronze Age and Iron Age human isotope data from Kazakhstan are presented in Figure 7. These data demonstrate the greatest degree of 13C enrichment in South–Eastern Kazakhstan that corresponds with the most frequent and abundant observation of miliacin, observed during this study, at Katartobe. These datasets suggest that broomcorn millet was more important to subsistence in this region, during the LBA and IA, than the other regions of Kazakhstan studied. Indeed, South–Eastern Kazakhstan occupies part of the “Inner Asian Mountain Corridor” that is currently understood to have been integral in the translocation and adoption of this crop throughout Eurasia (Frachetti, 2012; Spengler et al., 2014).
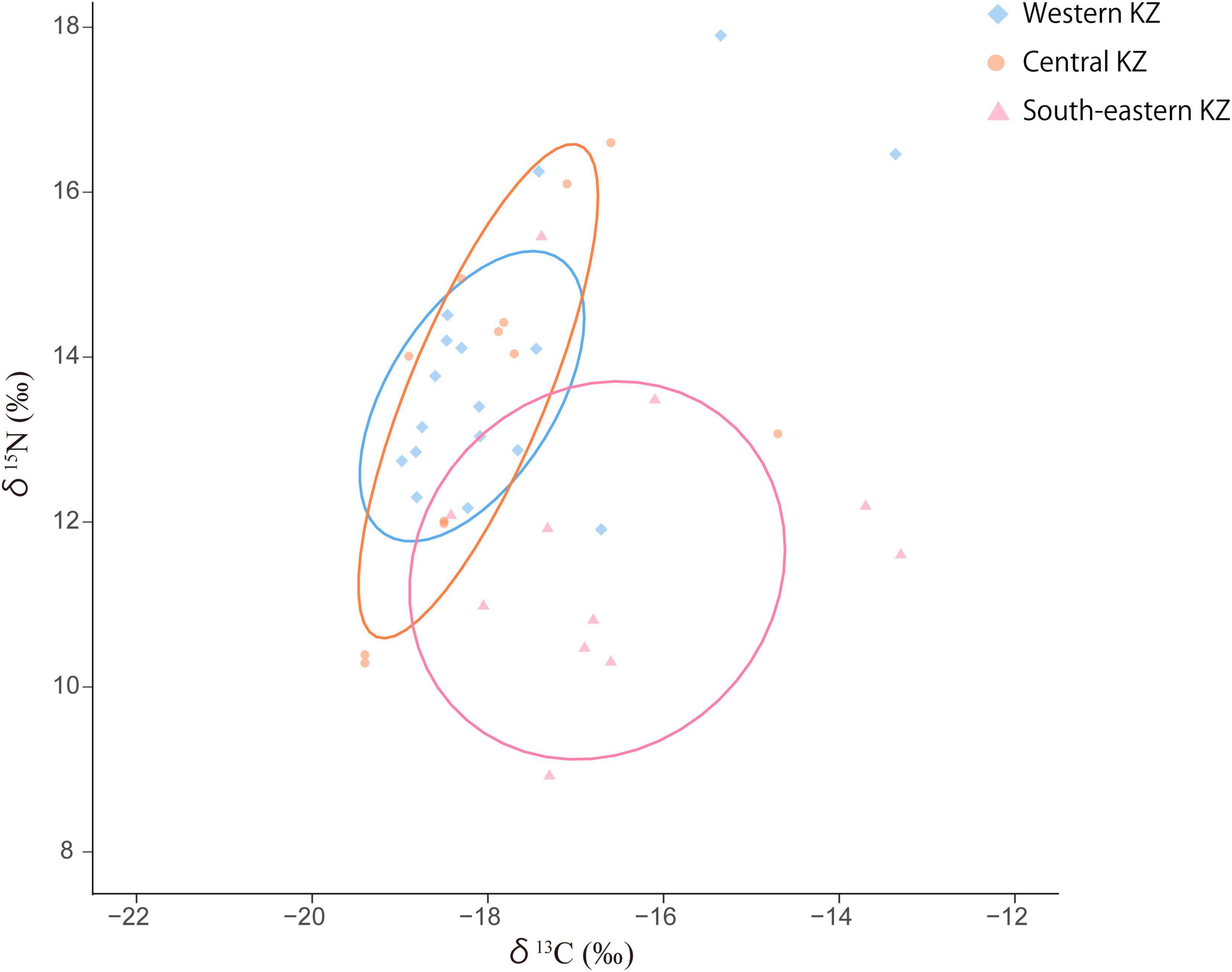
Figure 7. Scatter plots with 68.3% (1σ) confidence ellipses of the stable carbon and nitrogen isotope values of human bone collagen from the Late Bronze Age to the Iron Age Kazakhstan (after Ananyevskaya et al., 2020; Figure 5).
As previously mentioned, the population of Akpan may have extensively exploited aquatic resources from the Caspian Sea. Human isotope data potentially supports this hypothesis, as higher δ15N values of individuals from western Kazakhstan (Figure 7) correspond to higher δ15N values of fish from the Caspian and Aral Sea regions, relative to their freshwater counterparts (Haruda et al., 2020; Itahashi et al., 2020).
Although there are no animal remains reported from the Akpan site, this supports the assumption that the enriched δ13C values could originated from aquatic resources.
Conclusion
This study illustrates different pottery cooking patterns at four cemetery and ritual sites across Kazakhstan, dating between 700 and 200 BCE. The results demonstrate some correspondence to trends observed in human and animal bone collagen isotope analyses in the studied regions. Specifically, the data seem to indicate that broomcorn millet was more intensively processed in the foothill zones of eastern Kazakhstan, during the first millennium BCE, than in Central and Western Kazakhstan, as previously discussed by Spengler et al. (2021) although the sample size is limited compared to the size of the study area. The observation of miliacin in ceramic-absorbed residues suggests that broomcorn millet was a readily prepared ingredient in these vessels and connects the grain to the East Asian boiling and steaming culinary traditions from which it originated (Fuller and Rowlands, 2011). In the light of this observation, there is an interesting comparison with wheat and barley that traveled into eastern and central China from Central Asia, during the second and first millennium BCE. Contrary to broomcorn millet, wheat and barley grain morphotypes and western grinding and baking cuisine traditions did not spread together (Ritchey et al., 2021).
In addition to climatic and environmental conditions, dietary patterns can be closely related to political and economic factors, such as human mobility and trade. Therefore, further investigation of transitions in dietary and cooking traditions at these sites will shed new light on dynamic East–West interactions in the later part of Central Asian prehistory. Comparison of lipid residue and isotopic datasets will enable consideration of cooking methods and the extent of consumption, in addition to elucidating the type (age, sex, and social status) of people that consumed broomcorn millet. Certainly, such combined analysis will demonstrate the diversity of food cultures in different regions and lead to a better understanding of the history of East–West exchanges in the Eurasian continent.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
NM, MS, HT, AL, and SS performed the analyses in the laboratory. NM, MS, ES, and SS processed the analytical data. NM, ES, and SS wrote the initial manuscript. NM and SS created the figures. OC and SS conceptualized the study and supervised the work, as well as added to the draft. AO, SR, AK, AN, and S-WN carried out excavations, sample collection, and curation of the material. All authors discussed the results and commented on the manuscript.
Funding
This work was supported by the JSPS KAKENHI Grant Numbers 20H05820, 22F21304 and the Networking Core Centres for International Cooperation on Conservation of Cultural Heritage Project “KOPIR: Knowledge Transfer on the Methodology and Practice of Investigation, Recording and Conservation of Archaeological Remains” by Japanese Agency for Cultural Affairs.
Acknowledgments
We would like to thank Elina Ananyevskaya, Robert Spengler, and Paula Doumani Dupuy for useful comments to this manuscript. We also thank the technical staffs at the Nara National Research Institute for Cultural Properties for the support and the two reviewers for their thoughtful comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ananyevskaya, E., Akhatov, G., Loman, V., Dmitriev, E., Ermolayeva, A., Evdokimov, V., et al. (2020). The effect of animal herding practices on the diversity of human stable isotope values in North Central Asia. Reports 34:102615.
Barnett, W. K., Barnett, W. K., and Hoopes, J. W. (1995). The emergence of pottery: technology and innovation in ancient societies. New York: Smithsonian Institution Press.
Bossard, N., Jacob, J., Le Milbeau, C., Sauze, J., Terwilliger, V., Poissonnier, B., et al. (2013). Distribution of miliacin (olean-18-en-3β-ol methyl ether) and related compounds in broomcorn millet (Panicum miliaceum) and other reputed sources: Implications for the use of sedimentary miliacin as a tracer of millet. Organ. Geochem. 63, 48–55. doi: 10.1016/j.orggeochem.2013.07.012
Chang, C. (2022). Models for Iron Age agriculture and pastoralism in Kazakhstan. J. Historic. Archaeol. Anthropol. Sci. 7, 47?49.
Choy, K., Potter, B. A., McKinney, H. J., Reuther, J. D., Wang, S. W., and Wooller, M. J. (2016). Chemical profiling of ancient hearths reveals recurrent salmon use in Ice Age Beringia. Proc. Natl. Acad. Sci. U.S.A. 113, 9757–9762. doi: 10.1073/pnas.1606219113
Colombini, M. P., Modugno, F., and Ribechini, E. (2005). Direct exposure electron ionization mass spectrometry and gas chromatography/mass spectrometry techniques to study organic coatings on archaeological amphorae. J. Mass Spectr. 40, 675–687. doi: 10.1002/jms.841
Copley, M. S., Berstan, R., Dudd, S. N., Docherty, G., Mukherjee, A. J., Straker, V., et al. (2003). Direct chemical evidence for widespread dairying in prehistoric Britain. Proc. Natl. Acad. Sci. U.S.A. 100, 1524–1529. doi: 10.1073/pnas.0335955100
Costa, M. S., Rego, A., Ramos, V., Afonso, T. B., Freitas, S., Preto, M., et al. (2016). The conifer biomarkers dehydroabietic and abietic acids are widespread in Cyanobacteria. Scientif. Rep. 6:23436. doi: 10.1038/srep23436
Courel, B., Robson, H. K., Lucquin, A., Dolbunova, E., Oras, E., Adamczak, K., et al. (2020). Organic residue analysis shows sub-regional patterns in the use of pottery by Northern European hunter-gatherers. Roy. Soc. Open Sci. 7:192016. doi: 10.1098/rsos.192016
Craig, Oliver, E., Richard, B., Allen, Anu Thompson, Rhiannon, E., et al. (2012). Distinguishing Wild Ruminant Lipids by Gas Chromatography/combustion/isotope Ratio Mass Spectrometry. RCM 26, 2359–2364. doi: 10.1002/rcm.6349
Craig, O. E., Saul, H., Lucquin, A., Nishida, Y., Taché, K., Clarke, L., et al. (2013). Earliest evidence for the use of pottery. Nature 496, 351–354. doi: 10.1038/nature12109
Cramp, L. J. E., Evershed, R. P., and Eckardt, H. (2011). What was a mortarium used for? Organic residues and cultural change in Iron Age and Roman Britain. Antiquity 85, 1339–1352. doi: 10.1017/S0003598X00062098
Cubas Morera, M., Lucquin, A. J. A., Robson, H. K., Colonese, A. C., and Arias, P. (2020). Latitudinal gradient in dairy production with the introduction of farming in Atlantic Europe. Nat. Commun. 11, 1–9.
Demirci, Ö, Lucquin, A., Çakırlar, C., Craig, O. E., and Raemaekers, D. C. M. (2021). Lipid residue analysis on Swifterbant pottery (c. 5000–3800 cal BC) in the Lower Rhine-Meuse area (the Netherlands) and its implications for human-animal interactions in relation to the Neolithisation process. J. Archaeol. Sci. 36:102812.
Drieu, L., Lepère, C., and Regert, M. (2020). The Missing Step of Pottery chaîne opératoire: Considering Post-firing Treatments on Ceramic Vessels Using Macro- and Microscopic Observation and Molecular Analysis. J. Archaeol. Method Theory 27, 302–326. doi: 10.1007/s10816-019-09428-8
Dudd, S. N., and Evershed, R. P. (1998). Direct demonstration of milk as an element of archaeological economies. Science 282, 1478–1481. doi: 10.1126/science.282.5393.1478
Dunne, J., Evershed, R. P., Salque, M., Cramp, L., Bruni, S., Ryan, K., et al. (2012). First dairying in green Saharan Africa in the fifth millennium BC. Nature 486, 390–394. doi: 10.1038/nature11186
Dunne, J., Mercuri, A. M., Evershed, R. P., Bruni, S., and di Lernia, S. (2016). Earliest direct evidence of plant processing in prehistoric Saharan pottery. Nat. Plants 3:16194. doi: 10.1038/nplants.2016.194
Eglinton, G., Logan, G. A., Ambler, R. P., Boon, J. J., and Perizonius, W. R. K. (1991). Molecular Preservation [and Discussion]. Philosoph. Transact. Roy. Soc. Lon. Ser. B 333, 315–328. doi: 10.1098/rstb.1991.0081
Evershed, R. P., Heron, C., and Goad, L. J. (1990). Analysis of Organic Residues of Archaeological Origin by High-Temperature Gas Chromatography and Gas Chromatography-Mass Spectrometry. Analyst 115, 1339–1342. doi: 10.1039/an9901501339
Evershed, R. P., Dudd, S. N., Charters, S., Mottram, H., Stott, A. W., Raven, A., et al. (1999). Lipids as carriers of anthropogenic signals from prehistory. Philosoph. Transact. Roy. Soc. Lon. Ser. B 354, 19–31.
Evershed, R. P., Payne, S., Sherratt, A. G., Copley, M. S., Coolidge, J., and Urem-Kotsu, D. (2008a). Earliest date for milk use in the Near East and southeastern Europe linked to cattle herding. Nature 455, 528–531. doi: 10.1038/nature07180
Evershed, R. P., Copley, M. S., Dickson, L., and Hansel, F. A. (2008b). Experimental evidence for the processing of marine animal products and other commodities containing polyunsaturated fatty acids in pottery vessels. Archaeometry 50, 101–113. doi: 10.1111/j.1475-4754.2007.00368.x
Fernandes, R., Eley, Y., Brabec, M., Lucquin, A., Millard, A., and Craig, O. E. (2018). Reconstruction of prehistoric pottery use from fatty acid carbon isotope signatures using Bayesian inference. Organ. Geochem. 117, 31–42. doi: 10.1016/j.orggeochem.2017.11.014
Frachetti, M. D. (2012). Multiregional Emergence of Mobile Pastoralism and Nonuniform Institutional Complexity across Eurasia. Curr. Anthropol. 53, 2–38. doi: 10.1086/663692
Fuller, D., and Rowlands, M. (2011). “Ingestion and Food Technologies: Maintaining differences over the long-term in West, South and East Asia,” in Interweaving Worlds – systematic interactions in Eurasia, 7th to 1st millennia BC. Essays from a conference in memory of Professor Andrew Sherratt, eds J. Bennet, S. Sherratt, and T. C. Wilkinson (Oxford: Oxbow Books Ltd), 37–60. doi: 10.2307/j.ctvh1dr2k.9
Gregg, M. W., Banning, E. B., Gibbs, K., and Slater, G. F. (2009). Subsistence practices and pottery use in Neolithic Jordan: molecular and isotopic evidence. J. Archaeol. Sci. 36, 937–946. doi: 10.1016/j.jas.2008.09.009
Hansel, F. A., Copley, M. S., Madureira, L. A. S., and Evershed, R. P. (2004). Thermally produced ω-(o-alkylphenyl)alkanoic acids provide evidence for the processing of marine products in archaeological pottery vessels. Tetrahed. Lett. 45, 2999–3002. doi: 10.1016/j.tetlet.2004.01.111
Haruda, A. F., Ventresca Miller, A. R., Paijmans, J. L. A., Barlow, A., Tazhekeyev, A., and Bilalov, S. (2020). The earliest domestic cat on the Silk Road. Scientif. Rep. 10:11241. doi: 10.1038/s41598-020-67798-6
Hauff, S., and Vetter, W. (2010). Quantification of branched chain fatty acids in polar and neutral lipids of cheese and fish samples. J. Agricult. Food Chem. 58, 707–712. doi: 10.1021/jf9034805
Hendy, J., Colonese, A. C., Franz, I., Fernandes, R., Fischer, R., Orton, D., et al. (2018). Ancient proteins from ceramic vessels at Çatalhöyük West reveal the hidden cuisine of early farmers. Nat. Commun. 9:4064. doi: 10.1038/s41467-018-06335-6
Hermes, T. R., Frachetti, M. D., Doumani Dupuy, P. N., Mar’yashev, A., Nebel, A., and Makarewicz, C. A. (2019). Early integration of pastoralism and millet cultivation in Bronze Age Eurasia. Proc. Roy. Soc. B 286:20191273. doi: 10.1098/rspb.2019.1273
Hermes, T. R., Schmid, C., Tabaldiev, K., and Motuzaite Matuzeviciute, G. (2022). Carbon and oxygen stable isotopic evidence for diverse sheep and goat husbandry strategies amid a Final Bronze Age farming milieu in the Kyrgyz Tian Shan. Int. J. Osteoarchaeol. 32, 792–803. doi: 10.1002/oa.3103
Heron, C., Shoda, S., Breu Barcons, A., Czebreszuk, J., Eley, Y., and Gorton, M. (2016). First molecular and isotopic evidence of millet processing in prehistoric pottery vessels. Scientif. Rep. 6:38767. doi: 10.1038/srep38767
Horiuchi, A., Miyata, Y., and Evershed, R. P. (2013). Unexpected Compounds in Archaeological Pottery: Issues of Organic Residue Analysis. Archaeol. Nat. Sci. 65, 27–34.
Horiuchi, A., Miyata, Y., Kamijo, N., Cramp, L., and Evershed, R. P. (2015). A dietary study of the Kamegaoka culture population during the final Jomon period, Japan, using stable isotope and lipid analyses of ceramic residues. Radiocarbon 57, 721–736. doi: 10.2458/azu_rc.57.18455
Itahashi, Y., Ananyevskaya, E., Yoneda, M., Ventresca Miller, A. R., Nishiaki, Y., and Motuzaite Matuzeviciute, G. (2020). Dietary diversity of Bronze-Iron Age populations of Kazakhstan quantitatively estimated through the compound-specific nitrogen analysis of amino acids. J. Archaeol. Sci. 33:102565. doi: 10.1016/j.jasrep.2020.102565
Jacob, J., Disnar, J.-R., Boussafir, M., Spadano Albuquerque, A. L., Sifeddine, A., and Turcq, B. (2005). Pentacyclic triterpene methyl ethers in recent lacustrine sediments (Lagoa do Caçó, Brazil). Organ. Geochem. 36, 449–461. doi: 10.1016/j.orggeochem.2004.09.005
Jones, M., Hunt, H., Lightfoot, E., Lister, D., Liu, X., and Motuzaite-Matuzeviciute, G. (2011). Food globalization in prehistory. World Archaeol. 43, 665–675. doi: 10.1080/00438243.2011.624764
Jordan, P., and Gibbs, K. (2019). Ceramics in Circumpolar Prehistory: Technology, Lifeways and Cuisine. Cambridge: Cambridge University Press. doi: 10.1017/9781316339374
Liu, X., Jones, P. J., Motuzaite Matuzeviciute, G., Hunt, H. V., Lister, D. L., and An, T. (2019). From ecological opportunism to multi-cropping: Mapping food globalisation in prehistory. Quatern. Sci. Rev. 206, 21–28. doi: 10.1016/j.quascirev.2018.12.017
Lucquin, A., Gibbs, K., Uchiyama, J., Saul, H., Ajimoto, M., Eley, Y., et al. (2016). Ancient lipids document continuity in the use of early hunter-gatherer pottery through 9,000 years of Japanese prehistory. Proc. Natl. Acad. Sci. U.S.A. 113, 3991–3996. doi: 10.1073/pnas.1522908113
Lucquin, A., Robson, H. K., Eley, Y., Shoda, S., Veltcheva, D., Gibbs, K., et al. (2018). The impact of environmental change on the use of early pottery by East Asian hunter-gatherers. Proc. Natl. Acad. Sci. U.S.A. 115, 7931–7936. doi: 10.1073/pnas.1803782115
Matuzevičiūtė, G. M., and Liu, X. (2021). “Prehistoric Agriculture in China: Food Globalization in Prehistory,” in Oxford Research Encyclopedia of Environmental Science, (Oxford: University Press: New York), 1–23. doi: 10.1093/acrefore/9780199389414.013.168
Miller, M. J., Whelton, H. L., Swift, J. A., Maline, S., Hammann, S., and Cramp, L. J. E. (2020). Interpreting ancient food practices: stable isotope and molecular analyses of visible and absorbed residues from a year-long cooking experiment. Scientif. Rep. 10:13704. doi: 10.1038/s41598-020-70109-8
Miyata, Y., Horiuchi, A., Takada, H., and Nakamura, T. (2015). Evaluation of sea mammals as marine resource by lipid analysis in pottery excavated from Mawaki Archaeological Site, Ishikawa, Japan. JSSSCP 2015, 40–41.
Modugno, F., and Ribechini, E. (2009). “GC/MS in the Characterisation of Resinous Materials,” in Organic Mass Spectrometry in Art and Archaeology, eds M. P. Colombini and F. Modugno (New York: John Wiley & Sons), 215–235. doi: 10.1002/9780470741917.ch8
Outram, A. K., Stear, N. A., Bendrey, R., Olsen, S., Kasparov, A., Zaibert, V., et al. (2009). The earliest horse harnessing and milking. Science 323, 1332–1335. doi: 10.1126/science.1168594
Outram, A. K., Stear, N. A., Kasparov, A., Usmanova, E., Varfolomeev, V., and Evershed, R. P. (2011). Horses for the dead: funerary foodways in Bronze Age Kazakhstan. Antiquity 85, 116–128. doi: 10.1017/S0003598X00067478
Outram, A. K., Kasparov, A., Stear, N. A., Varfolomeev, V., Usmanova, E., and Evershed, R. P. (2012). Patterns of pastoralism in later Bronze Age Kazakhstan: new evidence from faunal and lipid residue analyses. J. Archaeol. Sci. 39, 2424–2435. doi: 10.1016/j.jas.2012.02.009
Pääkkönen, M., Holmqvist, E., Bläuer, A., Evershed, R. P., and Asplund, H. (2020). Diverse Economic Patterns in the North Baltic Sea Region in the Late Neolithic and Early Metal Periods. Europ. J. Archaeol. 23, 4–21.
Ritchey, M. M., Sun, Y., Motuzaite Matuzeviciute, G., Shoda, S., Pokharia, A. K., and Spate, M. (2021). The wind that shakes the barley: the role of East Asian cuisines on barley grain size. World Archaeol. 53, 287–304. doi: 10.1080/00438243.2022.2030792
Shoda, S., Lucquin, A., Yanshina, O., Kuzmin, Y., Shevkomud, I., and Medvedev, V. (2020). Late Glacial hunter-gatherer pottery in the Russian Far East: Indications of diversity in origins and use. Quatern. Sci. Rev. 229:106124. doi: 10.1016/j.quascirev.2019.106124
Shoda, S., Standall, E. A., and Murakami, N. (2021). Biomolecular Archaeology of Broomcorn Millet: A Review. Millet Res. 36, 1–8.
Skibo, J. M. (2015). “Pottery Use-Alteration Analysis,” in Use-Wear and Residue Analysis in Archaeology, eds J. M. Marreiros, J. F. G. Bao, and N. F. Bicho (Netherlands: Springer International Publishing), 189–198. doi: 10.1007/978-3-319-08257-8_10
Spangenberg, J. E., Jacomet, S., and Schibler, J. (2006). Chemical analyses of organic residues in archaeological pottery from Arbon Bleiche 3, Switzerland – evidence for dairying in the late Neolithic. J. Archaeol. Sci. 33, 1–13. doi: 10.1016/j.jas.2005.05.013
Spangenberg, J. E., Ferrer, M., Tschudin, P., Volken, M., and Hafner, A. (2010). Microstructural, chemical and isotopic evidence for the origin of late neolithic leather recovered from an ice field in the Swiss Alps. J. Archaeol. Sci. 37, 1851–1865. doi: 10.1016/j.jas.2010.02.003
Spengler, R., Frachetti, M., Doumani, P., Rouse, L., Cerasetti, B., Bullion, E., et al. (2014). Early agriculture and crop transmission among Bronze Age mobile pastoralists of Central Eurasia. Proc. Biol. Sci. 281:20133382. doi: 10.1098/rspb.2013.3382
Spengler, R. N., de Nigris, I., Cerasetti, B., Carra, M., and Rouse, L. M. (2018). The breadth of dietary economy in Bronze Age Central Asia: Case study from Adji Kui 1 in the Murghab region of Turkmenistan. J. Archaeol. Sci. 22, 372–381. doi: 10.1016/j.jasrep.2016.03.029
Spengler, R. N., Miller, A. V., Schmaus, T., Matuzevičiūtė, G. M., Miller, B. K., and Wilkin, S. (2021). An Imagined Past? Nomadic Narratives in Central Asian Archaeology. Curr. Anthropol. 62, 251–286. doi: 10.1086/714245
Spiteri, C. D. (2012). Pottery Use at the Transition to Agriculture in the Western Mediterranean. Evidence from Biomolecular and Isotopic Characterisation of Organic Residues in Impressed/Cardial Ware Vessels. Ph.D thesis, Heslington: University of York.
Standall, E. A., Craig, O. E., and Heron, C. (2022). “Putting millet into a culinary context: Organic residue analysis and the identification of Panicum miliaceum in pottery vessels,” in Millet And What Else? The wider context of the adoption of millet cultivation in Europe, eds W. Kirleis, M. Dal Corso, and D. Filipovic (Leiden: Sidestone Press), 219–230.
Taché, K., and Craig, O. E. (2015). Cooperative harvesting of aquatic resources and the beginning of pottery production in north-eastern North America. Antiquity 89, 177–190. doi: 10.15184/aqy.2014.36
Taché, K., Jaffe, Y., Craig, O. E., Lucquin, A., Zhou, J., Wang, H., et al. (2021). What do “barbarians” eat? Integrating ceramic use-wear and residue analysis in the study of food and society at the margins of Bronze Age China. PLoS One 16:e0250819. doi: 10.1371/journal.pone.0250819
Tsuneki, A., Nieuwenhuyse, O., and Campbell, S. (2017). The emergence of pottery in West Asia. Oxford: Oxbow Books.
Wang, T., Wei, D., Chang, X., Yu, Z., Zhang, X., Wang, C., et al. (2017). Tianshanbeilu and the Isotopic Millet Road: reviewing the late Neolithic/Bronze Age radiation of human millet consumption from north China to Europe. Natl. Sci. Rev. 6, 1024–1039. doi: 10.1093/nsr/nwx015
Yatoo, M. A., Spate, M., Betts, A., Pokharia, A. K., and Shah, M. A. (2020). New evidence from the Kashmir Valley indicates the adoption of East and West Asian crops in the western Himalayas by 4400 years ago. Quatern. Sci. Adv. 2:100011. doi: 10.1016/j.qsa.2020.100011
Keywords: Central Asia, Early Iron Age, millet, cuisine, organic residue analysis, compound-specific isotope analysis
Citation: Murakami N, Onggaruly A, Rakhimzhanova S, Standall EA, Talbot HM, Lucquin A, Suzuki M, Karimagambetov A, Nuskabay A, Nam S-W, Craig OE and Shoda S (2022) Lipid residues in ancient pastoralist pottery from Kazakhstan reveal regional differences in cooking practices. Front. Ecol. Evol. 10:1032637. doi: 10.3389/fevo.2022.1032637
Received: 31 August 2022; Accepted: 27 September 2022;
Published: 10 November 2022.
Edited by:
Jean Nicolas Haas, University of Innsbruck, AustriaReviewed by:
Francesco Carrer, Newcastle University, United KingdomJohn Dodson, Institute of Earth Environment (CAS), China
Copyright © 2022 Murakami, Onggaruly, Rakhimzhanova, Standall, Talbot, Lucquin, Suzuki, Karimagambetov, Nuskabay, Nam, Craig and Shoda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natsuki Murakami, bXVyYWthbWktbjMzQG5pY2guZ28uanA=; Shinya Shoda, c2hpbnlhLnNob2RhQHlvcmsuYWMudWs=
 Natsuki Murakami
Natsuki Murakami Akhan Onggaruly2
Akhan Onggaruly2 Edward A. Standall
Edward A. Standall Alexandre Lucquin
Alexandre Lucquin Miho Suzuki
Miho Suzuki Sang-Won Nam
Sang-Won Nam Oliver E. Craig
Oliver E. Craig Shinya Shoda
Shinya Shoda