- 1Key Laboratory of Southwest China Wildlife Resources Conservation of Ministry of Education, China West Normal University, Nanchong, China
- 2Jilin Provincial Key Laboratory of Animal Resource Conservation and Utilization, Northeast Normal University, Changchun, China
- 3College of Life Science, Jilin Agricultural University, Changchun, China
Introduction: Flight and echolocation are two crucial behaviors associated with niche expansion in bats. Previous researches have attempted to explain the interspecific divergence in flight morphology and echolocation vocalizations in some bat groups from the perspective of foraging ecology. However, the relationship between wing morphology and echolocation vocalizations of bats remains obscure, especially in a phylogenetic context.
Objectives: Here, we aimed to assess the correlated evolution of wing morphology and echolocation calls in bats within a phylogenetic comparative framework.
Methods: We integrated the information on search-phrase echolocation call duration, peak frequency, relative wing loading, aspect ratio, and foraging guilds for 152 bat species belonging to 15 families. We quantified the association among wing morphology, echolocation call parameters, and foraging guilds using phylogeny-based comparative analyses.
Results: Our analyses revealed that wing morphology and echolocation call parameters depended on families and exhibited a marked phylogenetic signal. Peak frequency of the call was negatively correlated with relative wing loading and aspect ratio. Call duration was positively correlated with relative wing loading and aspect ratio among open-space aerial foragers, edge-space aerial foragers, edge-space trawling foragers, and narrow-space gleaning foragers. Wing morphology, call duration, and peak frequency were predicted by foraging guilds.
Conclusion: These results demonstrate that adaptive response to foraging ecology has shaped the correlated evolution between flight morphology and echolocation calls in bats. Our findings expand the current knowledge regarding the link between morphology and vocalizations within the order Chiroptera.
Introduction
Flight and echolocation are two key behavioral innovations in the evolutionary history of bats (Fenton et al., 1995; Arita and Fenton, 1997; Teeling et al., 2000). Flight behavior provides benefits for long-distance dispersal over geographical barriers, avoidance of terrestrial predators, and niche expansion from the ground to the sky (Speakman, 2001; Jones and Teeling, 2006; Luo et al., 2019a). Echolocation is an active form of orientation in which echolocators emit sounds and then listen to reflected echoes from the object (Griffin, 1944; Schnitzler and Kalko, 1998). Most bats are capable of echolocation, the only exceptions being some old world fruit bats (Schnitzler et al., 2003; Jones and Teeling, 2006). Echolocating bats can perceive the environment through auditory scene analysis via echolocation, albeit their vision also functions in long-range discrimination of large objects (Simmons et al., 1979; Moss and Surlykke, 2010; Fenton et al., 2014). Echolocation and flight behaviors mediate multiple activities associated with fitness of bats, including foraging, social communication, and reproduction (Arita and Fenton, 1997; Jones and Siemers, 2010; Guo et al., 2021). Despite the sustained interest in bat flight and echolocation from ethologists, ecologists, and evolutionary biologists (Griffin, 1944; Norberg and Rayner, 1987; Arita and Fenton, 1997; Thiagavel et al., 2018), the relationship between flight morphology and echolocation vocalizations in most bat groups remains uncertain.
From a functional perspective, while bats are foraging, the operation of echolocation is mechanically linked to flight. Bats utter echolocation calls to search for prey, then catch prey through their wings, tail membrane, or mouth (Rayner, 1991). Bats control wing-flapping via contracting serratus and pectoralis muscles; this compresses the thoracic air volume and increases subglottal pressure, ultimately triggering the emission of echolocation vocalizations (Suthers et al., 1972; Lancaster et al., 1995). Echolocation calls are produced by bats at the end of the upstroke or at the start of the downstroke that coincide with expiration, resulting in an apparent connection between wingbeat frequency and repetitive rate of echolocation vocalizations (Rayner, 1991; Kalko and Schnitzler, 1993; Jones, 1994; Wong and Waters, 2001). Echolocating bats achieve prey detection and capture based on a combination of auditory feedback, motor control, and respiration (Rayner, 1991; Wong and Waters, 2001). Consequently, flight, echolocation, and respiration in bats are physiologically coupled, largely reducing the energetic cost of echolocation signal emission (Rayner, 1991; Speakman and Racey, 1991; Currie et al., 2020).
Previous researchers have shown that both wing morphology and echolocation calls are dependent on foraging ecology in some bats (Aldridge and Rautenbach, 1987; Norberg and Rayner, 1987; Neuweiler, 1989; Kingston et al., 2000; Schnitzler and Kalko, 2001; Denzinger and Schnitzler, 2013). In general, open-space aerial foragers have long pointed wings that correspond to high wing loading (WL) and aspect ratio (AR), allowing them to fly at faster speeds and greater distances (Norberg and Rayner, 1987; Rhodes, 2002; Jennings et al., 2004). In contrast, foragers in edge and narrow spaces have evolved short round wings to improve maneuverability and aerodynamic efficiency, given that environmental obstacles such as vegetation restrict their flight performance (Norberg and Rayner, 1987; Marinello and Bernard, 2014). Moreover, echolocation calls of open-space aerial foragers are characterized by comparatively long duration, low frequency, and narrow bandwidth, features that are beneficial to long-range detection of night-flying insects due to less attenuation and increased signal energy (Denzinger and Schnitzler, 2013; Luo et al., 2019b). Aerial and trawling foragers in edge spaces emit short and high-frequency calls for echolocation, and this facilitates reducing the interference of obstacle echoes with prey echoes (Kalko and Schnitzler, 1993; Schnitzler and Kalko, 1998). Narrow-space gleaning foragers locate prey via hearing the sounds generated by prey activities and prey echoes, and they utter short broadband calls with multiple harmonics (Arlettaz et al., 2001; Geipel et al., 2013). The flutter-detecting foragers in narrow spaces, which include bats in the families Rhinolophidae, Hipposideridae, and Rhinonycteridae and the mormoopid Pteronotus parnellii, emit constant-frequency calls during foraging along with a broadband sweep at the start or the end (Jones and Teeling, 2006; Fenton et al., 2012). Narrow space flutter-detecting foragers are high duty cycle bats that produce echolocation calls with long duration relative to call period (Fenton et al., 2012). Such calls maximize the performance of prey detection and localization in highly cluttered environments (Schnitzler and Kalko, 1998; Jones and Teeling, 2006; Fenton et al., 2012). These findings imply that wing morphology may be correlated with echolocation call design in bat evolution due to the selective pressure imposed by foraging ecology.
Several studies have attempted to quantify the relationship between wing morphology and characteristics of echolocation calls in some bat groups. Norberg and Rayner (1987) evaluated the relationship between wing morphology and echolocation call structure among 18 bat species. They found that bats with high wing loading and aspect ratio produce narrowband calls, while species with low wing loading and aspect ratio emit broadband frequency-modulation calls (Norberg and Rayner, 1987). Aldridge and Rautenbach (1987) revealed close links among, wing loading, aspect ratio, and habitat use for 26 bat species from South Africa. Roemer et al. (2019) demonstrated that the indices of wing morphology were significantly related to echolocation call duration and peak frequency for 21 bat species in France and Belgium. Nonetheless, these studies are subject to two major limitations: (1) the number of bat species investigated was relatively limited, making it difficult to assess the generality of the conclusions; and (2) the effects of phylogenetic history between species are ignored, yielding potential phylogenetic biases in assessing the relationship between wing morphology and echolocation call design (Martins and Garland, 1991; Ives and Zhu, 2006).
The goal of this study is to explore the relationship between wing morphology and echolocation calls in bats within a phylogenetic comparative framework. We hypothesized that foraging ecology drives the correlated evolution between wing morphology and echolocation calls in bats. To test our hypothesis, we integrated a large dataset from published literature on echolocation call duration, peak frequency, relative wing loading (RWL; wing loading after correcting for body mass), aspect ratio (AR), and foraging guilds for 152 bat species in 15 families. We determined the relationship between bat wing morphology and echolocation call parameters after controlling for phylogenetic relationships between species. We made the following predictions: (1) relative wing loading, aspect ratio, echolocation call parameters, and foraging guilds of bats would exhibit pronounced phylogenetic signals, given that biological traits depended on phylogenetic history (Collen, 2012; Luo et al., 2019b); (2) relative wing loading and aspect ratio are tightly linked to spectro-temporal parameters of echolocation calls; and (3) foraging guilds would account for interspecific variation in wing morphology and echolocation call parameters.
Materials and methods
Data collection
We obtained six variables of bats from published sources, including body mass, wing loading, aspect ratio, echolocation call duration, peak frequency, and foraging guilds. A literature search was conducted in the Web of Science, Wiley, Springer, JSTOR, and Research Gate in April 2018, October 2019, and June (IUCN SSC, 2022). The Latin name of each bat species on the Red List of the International Union for Conservation of Nature (IUCN SSC, 2022), together with the keywords “echolocation,” “wing morphology,” “foraging,” and “guild,” were used as search terms. The literature search was limited to publications from January 1972 to June (IUCN SSC, 2022). All variables were compiled according to the following criteria: (1) phylogenetic information for the species was available on the phylogenetic tree that incorporated 812 bat species (Shi and Rabosky, 2015); (2) bat echolocation calls were sampled during evening emergence from roosts, in search flight, after release in the field, or a combination of these conditions; (3) acoustic analysis was confined to the harmonic with the highest energy; (4) the average value was used if call parameters differed among geographic locations, degree of clutter, or published sources; and (5) Pipistrellus nanus, Chalinolobus variegatus, and Myotis ricketti were regarded as synonyms of Neoromicia nana, Glauconycteris variegata, and Myotis pilosus (IUCN SSC, 2022), respectively. Finally, we retained data from 152 bat species in 15 families (Supplementary Table S1), including Vespertilionidae (N = 57), Molossidae (N = 10), Emballonuridae (N = 12), Nycteridae (N = 3), Noctilionidae (N = 2), Mormoopidae (N = 5), Phyllostomidae (N = 27), Mystacinidae (N = 1), Hipposideridae (N = 9), Rhinonycteridae (N = 1), Rhinolophidae (N = 15), Craseonycteridae (N = 1), Megadermatidae (N = 3), Rhinopomatidae (N = 2), and Miniopteridae (N = 4). For detailed information on data collection, see Luo et al. (2019a,b).
Data processing
To correct for the allometric effect, we computed relative wing loading for each species according to the formula RWL = WL/body mass 1/3 (Norberg et al., 2000). The foraging guilds of bats, which were determined based on species’ habitat type and foraging mode (Schnitzler et al., 2003; Denzinger and Schnitzler, 2013), were taken from the literature (Supplementary Table S1). Bat guilds were divided into five categories, i.e., open-space aerial forager (OA, N = 26), edge-space aerial forager (EA, N = 43), edge-space trawling forager (ET, N = 8), narrow-space gleaning forager (NG, N = 49), and narrow-space flutter-detecting forager (NF, N = 26). OA forages in open spaces and captures their prey in the aerial mode. EA forages near the vegetation edges, in vegetation gaps, or above the water surfaces using an aerial mode. ET captures the prey above the water surfaces using the trawling mode. NG hunts for the prey from surfaces of vegetation or the ground using the gleaning mode. NF hunts for fluttering insects in highly cluttered spaces close to the vegetation (Schnitzler and Kalko, 2001; Denzinger and Schnitzler, 2013). We combined ET and EA into one category, given that ET had a small sample size and experienced a similar sensory challenge of auditory masking effects with EA. Previous investigations have shown that echolocating bats suffer from more serious auditory masking in cluttered environments versus in open spaces (Schnitzler et al., 2003; Siemers and Schnitzler, 2004). Therefore, we assigned species to the most complex guild based on the level of habitat clutter if they showed flexibility in the use of foraging space and modes. To fulfill the assumption of normality, we log10-transformed RWL, AR, call duration, and peak frequency for analysis.
Statistical analysis
We employed a general linear model (GLM) to test whether wing morphology and echolocation call parameters differed between families. We used Pagel’s λ and Blomberg’s K to examine phylogenetic signals in wing morphology, echolocation call parameters, and foraging guilds of bats based on a pruned phylogenetic tree via the packages APE and phytools (Paradis et al., 2004; Revell, 2012; Shi and Rabosky, 2015). Pagel’s λ and Blomberg’s K are two common measures of phylogenetic dependence of traits, with values close to zero indicating weak phylogenetic signal, and values close to one indicating strong phylogenetic signal (Kamilar and Cooper, 2013). A phylogenetic analysis of variance (PANOVA), together with post-hoc multiple comparisons, were conducted to evaluate the effects of foraging guilds on wing morphology and echolocation call parameters using phytools (Revell, 2012). The phylogenetic generalized least square (PGLS) regressions were applied to determine the association between wing morphology and echolocation call parameters using nlme (Pinheiro et al., 2014). Call duration and peak frequency were entered into PGLS regression models as the dependent variables. RWL and AR were assigned as the fixed predictor variables, and recording context was assigned as a covariate. PGLS regressions were operated under four different evolutionary scenarios: a Brownian motion (BM) model, an Ornstein-Uhlenbeck (OU) model, a lambda (λ) model, and ordinary least squares regression (OLS). The BM model assumes that phenotypic traits change gradually with evolutionary time at a constant rate. The OU model assumes that phenotypic traits change randomly around a deterministic trend. The λ model is a modified BM model obtained after correcting for the phylogenetic covariance matrix. We selected the best-fitting regression model based on the Akaike information criterion corrected for a small sample size using MuMIn (Hensley et al., 2015; Barton, 2016; Charlton and Reby, 2016). We calculated the R2 for each regression model using the function R2.pred of rr2 (Ives, 2019). Our preliminary analyses suggested that RWL and AR were not significantly related to call duration among all bats studied, since call duration in some NF (i.e., rhinolophids and the mormoopid Pteronotus parnellii) was largely deviated from that predicted by wing morphology. Therefore, we repeated our analyses excluding data from NF. All statistics were run in R 3.3.3. Means are given ± SE.
Results
Effects of phylogenetic history on wing morphology and echolocation calls
The wing morphology (GLM: RWL: df = 14, F = 6.11, p < 0.0001; AR: df = 14, F = 9.37, p < 0.0001) and echolocation call parameters (GLM: call duration: df = 14, F = 44.06, p < 0.0001; peak frequency: df = 14, F = 17.09, p < 0.0001) of bats exhibited pronounced variation among families. RWL (λ = 0.75, p < 0.0001; K = 0.49, p = 0.008) and AR (λ = 0.98, p < 0.0001; K = 1.01, p = 0.001) much depended on phylogenetic relationships between species. A strong phylogenetic signal was detected for call duration (λ = 0.91, p < 0.0001; K = 1.43, p = 0.001), peak frequency (λ = 0.91, p < 0.0001; K = 0.93, p = 0.001), and foraging guilds (λ = 0.92, p < 0.0001; K = 1.60, p = 0.001), regardless of the estimated indices.
Relationship between wing morphology and echolocation calls
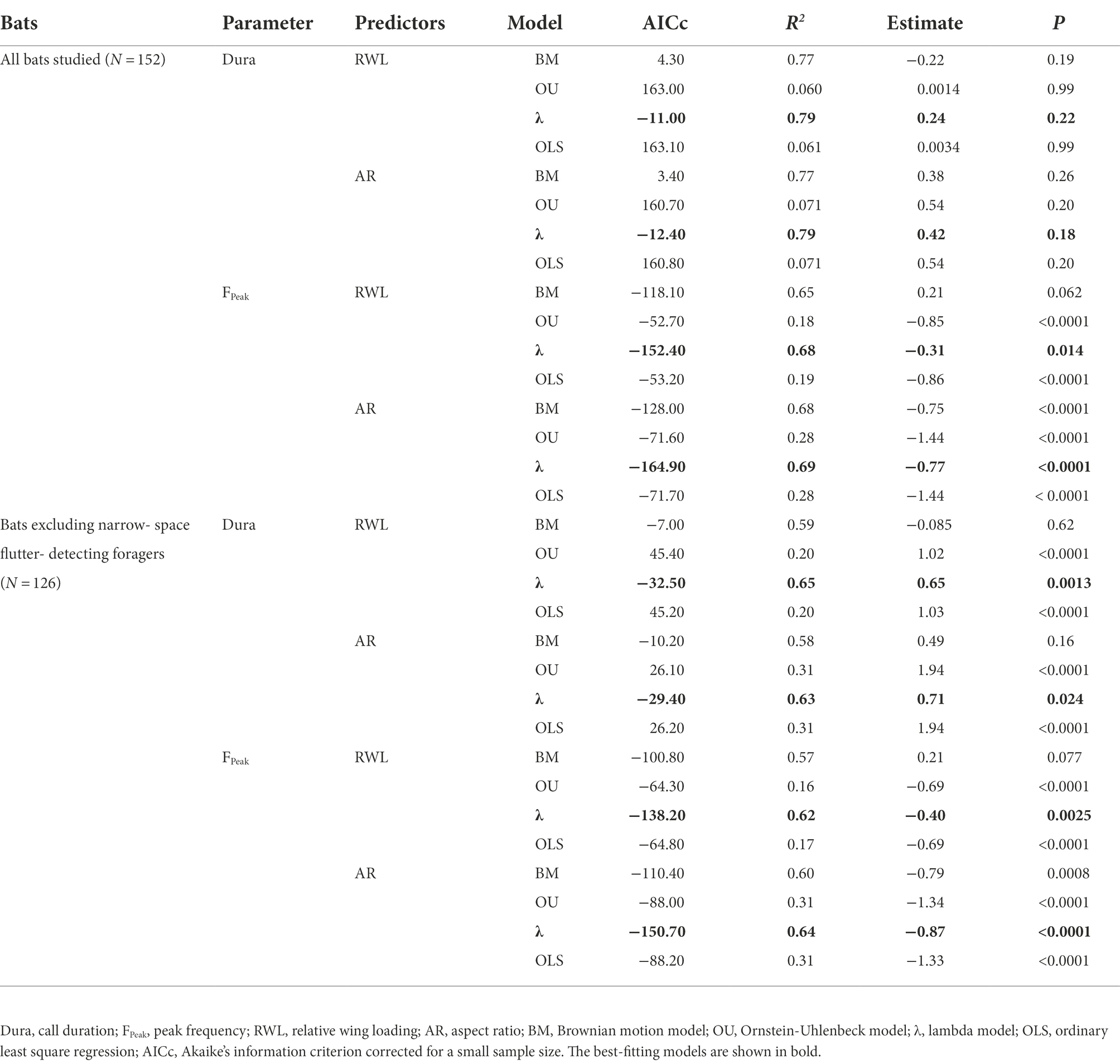
The lambda model provided the best fit for testing the relationship between wing morphology and echolocation call parameters in bats (Table 1). Despite the weak relationship between wing morphology and call duration (N = 152, all p > 0.05, Table 1; Figures 1A,B), RWL (N = 152, R2 = 0.68, estimate = −0.31, p = 0.014) and AR (N = 152, R2 = 0.69, estimate = −0.77, p < 0.0001) were significant negative predictors of peak frequency among all bats studied (Table 1; Figures 1C,D). After excluding NF, RWL (N = 126, R2 = 0.65, estimate = 0.65, p = 0.0013; Figure 2A) and AR (N = 126, R2 = 0.63, estimate = 0.71, p = 0.024; Figure 2B) were positively correlated with call duration. RWL (N = 126, R2 = 0.62, estimate = −0.40, p = 0.0025; Figure 2C) and AR (N = 126, R2 = 0.64, estimate = −0.87, p < 0.0001; Figure 2D) were negatively correlated with peak frequency among OA, EA, ET, and NG.
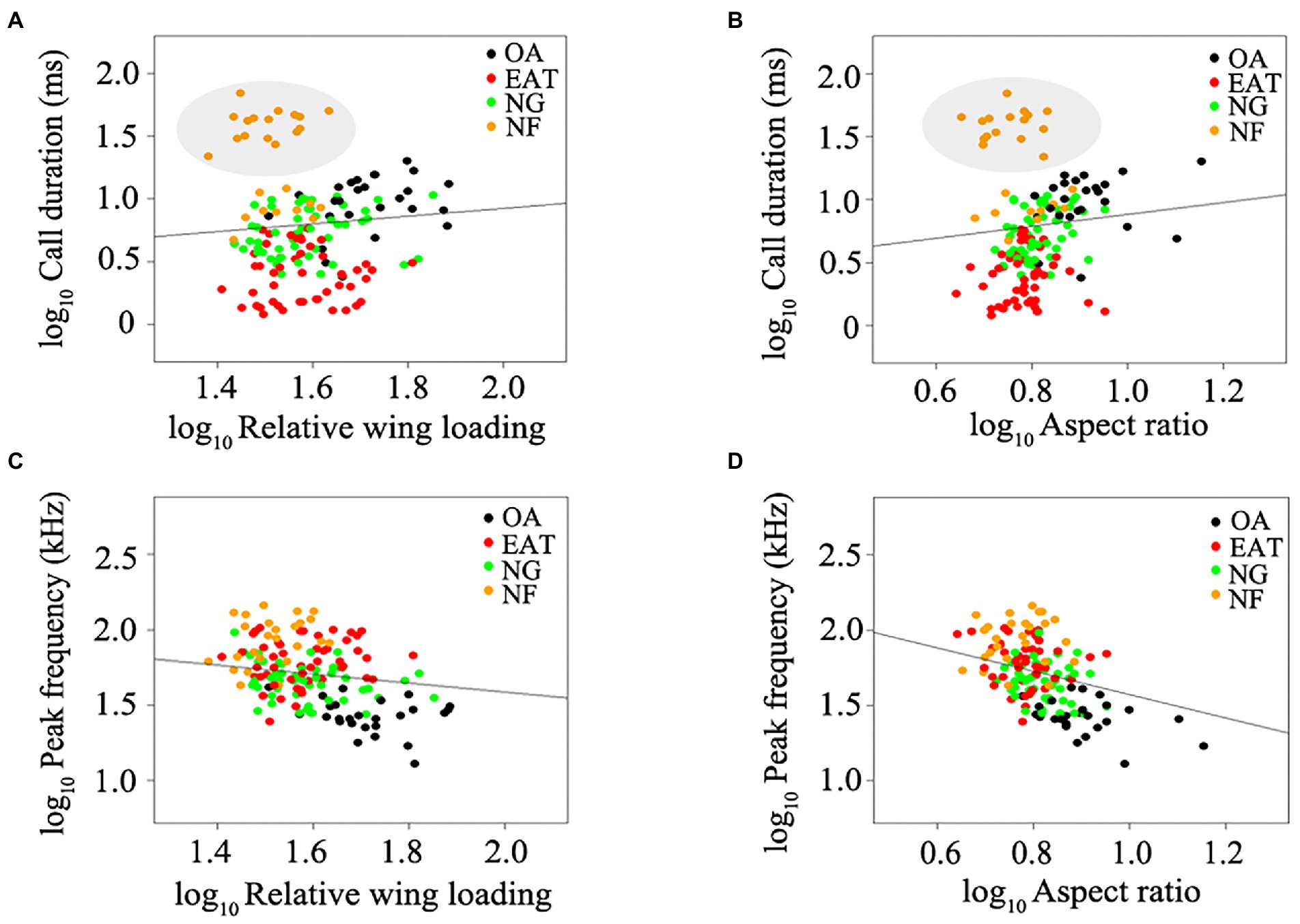
Figure 1. Relationship between wing morphology and echolocation call parameters among all bats studied. (A) Relative wing loading and call duration. (B) Aspect ratio and call duration. (C) Relative wing loading and peak frequency. (D) Aspect ratio and peak frequency. Black plots represent open-space aerial forager (OA). Red plots represent edge-space aerial and trawling forager (EAT). Green plots represent narrow-space gleaning forager (NG). Orange plots represent narrow-space flutter-detecting forager (NF). Data in ellipses represent rhinolophids and the mormoopid Pteronotus parnellii. Lines represent the best-fitting regression models after correcting for phylogeny.
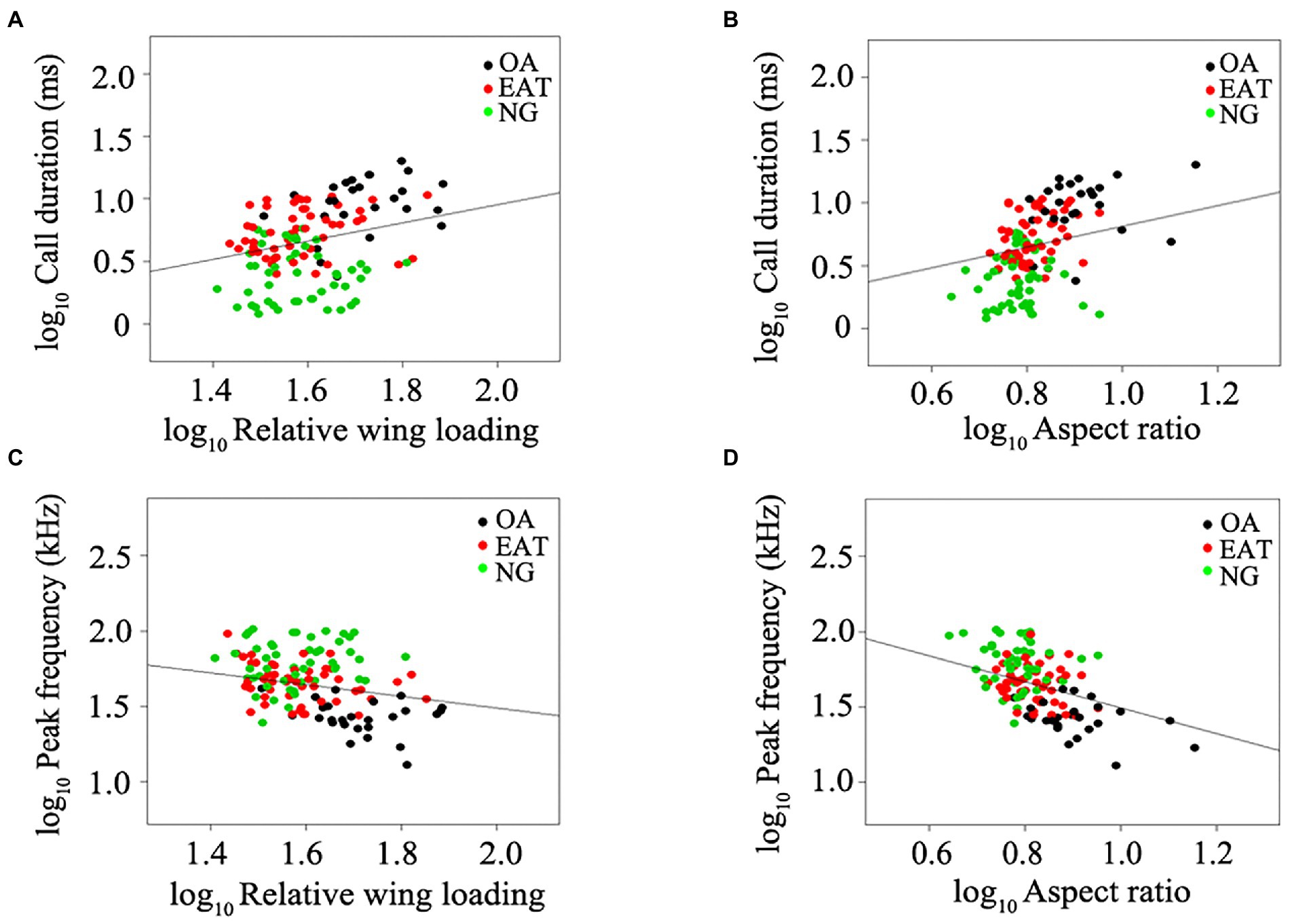
Figure 2. Relationship between wing morphology and echolocation call parameters in bats excluding narrow-space flutter-detecting foragers. (A) Relative wing loading and call duration. (B) Aspect ratio and call duration. (C) Relative wing loading and SCPF. (D) Aspect ratio and SCPF. Black plots represent open-space aerial forager (OA). Red plots represent edge-space aerial and trawling forager (EAT). Green plots represent narrow-space gleaning forager (NG). Lines represent the best-fitting regression models after correcting for phylogeny.
Ecological adaption of echolocation calls and wing morphology
The phylogenetic analysis of variance indicated that wing morphology of bats was significantly affected by foraging guilds (PANOVA: RWL: df = 3, F = 24.75, p = 0.019; AR: df = 3, F = 30.32, p = 0.006). OA had higher RWL and AR compared with those foraging in edge and narrow spaces (Figures 3A,B). Similarly, the duration (PANOVA: df = 3, F = 96.60, p = 0.001) and peak frequency (PANOVA: df = 3, F = 66.84, p = 0.001) of echolocation calls in bats were dependent on foraging guilds. NF uttered the longest echolocation calls, followed by OA, EA, and ET, and NG uttered the shortest echolocation calls (Figure 3C). OA emitted echolocation calls of lower frequency in comparison with those foraging in edge and narrow spaces (Figure 3D).
Figure 3. (A–D) Effects of foraging guilds on wing morphology and echolocation call parameters in bats. OA, open-space aerial forager; EAT, edge-space aerial and trawling forager; NG, narrow-space gleaning forager; NF, narrow-space flutter-detecting forager. Data in box plots represent the upper and lower adjacent values (highest and lowest horizontal line, respectively), 25 and 75% quartile with median value (box) and outliers (dots). Stars above and below boxplots indicate significant differences in pairwise comparisons between different foraging guilds based on phylogenetic analysis of variance. *p < 0.05. **p < 0.01.
Discussion
In this study, we employed a large dataset comprising 152 bat species from 15 families to examine the relationship between wing morphology and echolocation calls in the phylogenetic context. Our comparative analyses indicated that relative wing loading and aspect ratio were negatively related to peak frequency of echolocation calls within the order Chiroptera, even after correcting for phylogenetic relationships between species. We found that relative wing loading and aspect ratio scaled positively with echolocation call duration in bats while excluding narrow-space flutter-detecting foragers. Despite the marked phylogenetic constraint, wing morphology and spectro-temporal parameters of echolocation calls in bats were predicted by foraging guilds. All these results provide comparative evidence supporting the hypothesis that foraging ecology has shaped the correlated evolution between flight morphology and echolocation calls in extant bats.
There was a tight link among wing morphology, echolocation calls, and foraging guilds in bats within a phylogenetic comparative framework, especially among aerial foragers, trawling foragers, and narrow-space gleaning foragers. This echoes previous findings based on ordinary comparative analysis (Aldridge and Rautenbach, 1987; Jennings et al., 2004; Mancina et al., 2012; Roemer et al., 2019). As expected, open-space aerial foragers have higher wing loading and aspect ratio, and correspondingly emit echolocation calls of long duration and low peak frequency. These characteristics are conducive to rapid and long-lasting flight, allowing bats to achieve long-range detection of larger night-active insects in open spaces (Norberg and Rayner, 1987; Denzinger and Schnitzler, 2013; Jung et al., 2014). For aerial and trawling foragers in edge spaces, however, background targets such as vegetation serve as a physical barrier that restricts their flight speed (Norberg and Rayner, 1987; Norberg, 1994). The echoes from background targets and emitted signals can mask prey echoes, thereby preventing or reducing the chance of detecting prey (Schnitzler and Kalko, 1998; Schnitzler et al., 2003). Under these circumstances, edge-space aerial and trawling foragers feature intermediate wing loading and aspect ratio and produce short and high-frequency echolocation calls (Schnitzler and Kalko, 2001). The gleaning and flutter-detecting foragers in narrow spaces face multiple physical barriers imposed by habitat clutter, and they have evolved lower wing loading and aspect ratio to enhance flight maneuverability. Narrow-space gleaning foragers produce short and high-frequency calls for spatial orientation and foraging, albeit prey-generated sounds are also responsible for food acquisition (Arlettaz et al., 2001; Ratcliffe et al., 2005; Geipel et al., 2013). The flutter-detecting foragers in narrow spaces emit long constant-frequency echolocation calls followed by a downward broadband sweep, an upward broadband sweep, or both. Such a call design confers advantages in tracking the fluttering moths and beetles in extremely cluttered environments, albeit with a substantial temporal overlap between prey echoes and clutter echoes (Schnitzler and Kalko, 2001; Denzinger and Schnitzler, 2013). Nonetheless, narrow-space flutter-detecting foragers avoid acoustic masking by employing Doppler shift compensation to maintain echo frequency within the sensitive frequency range of the auditory fovea (Schnitzler, 1973; Schnitzler et al., 2003; Fenton et al., 2012). Together, these findings confirm that flight morphology and echolocation calls of bats show adaptive responses to foraging niches.
In rhinolophids and Pteronotus parnellii, the duration of echolocation calls was remarkably longer than expected based on their relative wing loading and aspect ratio (Figures 1A,B). This is not surprising, given that these bats are typically high duty cycle echolocators that emit echolocation calls of long duration (20–100 ms; Fenton et al., 2012; Luo et al., 2019b). The use of long-duration echolocation call allows Rhinolophids and P. parnellii to monitor one or several amplitude and frequency glints within an echo, and therefore can largely improve their prey detection performance (Schnitzler and Denzinger, 2011; Fenton et al., 2012). Supporting this idea, Lazure and Fenton (2011) found that echo strength of insect-like fluttering target increased with call duration and associated duty cycle through playback experiments; further monitoring confirmed that rhinolophids and P. parnellii initiated more approach toward the fluttering target in forest understory habitats than low duty cycle bats. Moreover, long-duration echolocation calls in rhinolophids and P. parnellii facilitate obtaining temporal patterning of acoustic glints that correspond to wingbeat rates of fluttering insects (von der Emde and Schnitzler, 1990; Fenton et al., 2012). This offers the potential for identifying different prey species and achieving selective foraging, since the rates of wingbeat are species-specific among flying insects (Kober and Schnitzler, 1990; von der Emde and Schnitzler, 1990; Schnitzler and Denzinger, 2011). Combined, a deviation of call duration from that predicted by wing morphology in rhinolophids and Pteronotus parnellii provides additional support for the role of foraging ecology in shaping the design of echolocation calls.
The relative wing loading, aspect ratio, and echolocation call parameters of bats contained considerable phylogenetic information, indicating that wing morphology and echolocation calls are not independent of shared phylogenetic history. A similar finding has been reported by Collen (2012), who demonstrated that echolocation call duration and frequency parameters exhibited a strong phylogenetic signal in most bat lineages. Similarly, Luo et al. (2019b) found that phylogenetic components exerted a remarkable influence on the design of echolocation calls in 207 bat species across 17 families. Social vocalizations and morphological traits also exhibit moderate to high levels of phylogenetic signals in animals ranging from anurans (Gingras et al., 2013) to birds (Arato and Fitch, 2021) and mammals (Peters and Peters, 2010; Kamilar and Cooper, 2013). Several mechanisms may account for the effects of phylogenetic history on wing morphology and echolocation calls in bats. First, the development of wing membranes and echolocation vocalizations in many bats is controlled by functional genes (Li et al., 2007; Tokita et al., 2012). This illustrates that wing morphology and echolocation calls of bats have a genetic basis and thus can be largely inherited across generations through a genetic mechanism and eventually across species through cladogenesis (Scherrer and Wilkinson, 1993; Monroy et al., 2011; Wang et al., 2014). Second, since body size and associated sound-producing organ (i.e., the larynx) in bats are not independent of phylogenetic history (Collen, 2012; Luo et al., 2019b), similar characteristics of wing morphology and echolocation calls would be found between closely related species. Therefore, the phylogenetic history of bats may affect the design of wings and echolocation calls indirectly by acting on body size. Third, we verified the presence of a pronounced phylogenetic signal for foraging guilds of bats. If these bats use niche-dependent wing morphology and echolocation vocalizations, it is likely that wing morphology and echolocation calls show similar adaptations within some lineages.
The relationship between flight and echolocation in the origin of bats remains a controversial topic. Three alternative hypotheses have been proposed, namely echolocation first (Fenton et al., 1995), flight first (Simmons and Geisler, 1998), and tandem development (Rayner, 1991; Speakman, 2001). According to the echolocation-first hypothesis, the ancestors of bats were nocturnal insectivores capable of echolocation; their enlarged hands allowed them to hunt insects through gliding, and their flight ability evolved after echolocation to enhance maneuverability and simplify returning to the hunting perch (Fenton et al., 1995; Arita and Fenton, 1997). By contrast, the flight-first hypothesis suggests that the ancestors of bats evolved powered flight from climbing and gliding, which relied on a vision for spatial orientation and obstacle detection (Norberg, 1994). Per the flight-first hypothesis, echolocation developed later than the origin of powered flight (Norberg, 1994; Simmons and Geisler, 1998). The tandem development hypothesis proposes that the first bats evolved flight and echolocation simultaneously, possibly owing to the coupling of wing beating, respiration, and echolocation call output (Speakman and Racey, 1991; Speakman, 2001). Some Eocene fossil bats such as Icaronycteris, Archaeonycteris, Palaeochiropteryx, and Hassianycteris suggest the capability of powered flight and echolocation, providing limited information in support of either hypothesis (Simmons and Geisler, 1998). Simmons et al. (2008) contend that the primitive early Eocene bats (Onychonycteris finneyi) were capable of powered flight but lacked laryngeal echolocation based on the morphological features of the forelimb, sternum, cochleae, and stylohyal bones, thus supporting the prediction of the flight-first hypothesis (Simmons et al., 2008). However, Veselka et al. (2010) documented that the articulation between the stylohyal and tympanic bones is a better predictor of laryngeal echolocation ability than the shape of the stylohyal bone among extant bats and that O. finneyi may have mastered the ability of laryngeal echolocation (Veselka et al., 2010). One recent study reconstructed the ancestral states of eye size and brain region size in bats, highlighting that the progenitors of bats had eyes too small to allow pursuit of night-flying insects but did have an auditory system sufficient to afford echolocation (Thiagavel et al., 2018). These studies have yielded mixed results. As such, discovery of more primitive fossil bats combined with the establishment of their phylogenetic relationships with extant bats would help to clarify the origin of powered flight and echolocation.
In summary, our phylogenetic comparative analyses demonstrated that foraging guilds and phylogenetic relationships between species affected interspecific divergence in wing morphology and echolocation calls among 152 bat species from 15 families. This indicated that ecological selection and phylogenetic constraint underlie the evolution of morphological and acoustic traits in bats. Upon taking phylogeny into account, we found that relative wing loading and aspect ratio were closely related to peak frequency of echolocation calls among these bats. The relationships among relative wing loading, aspect ratio, and echolocation call duration were also pronounced across open-space aerial foragers, edge-space aerial foragers, edge-space trawling foragers, and narrow-space gleaning foragers. These results provided evidence that adaptive response to foraging ecology drives the correlated evolution between flight morphology and echolocation calls in extant bats. Notably, three potential issues may affect the outcome of this study. One possible concern is that echolocation call parameters in bats are dependent on body size. Nonetheless, we still found marked associations among relative wing loading, aspect ratio, and size-corrected echolocation call parameters in bats (Supplementary Table S2). Another possible concern is the interspecific heterogeneity in the number of echolocation vocalizations. To verify the robustness of our results, we conducted comparative analyses with sample size weighting. Our additional analyses suggested that echolocation call parameters of bats were also predicted by relative wing loading and aspect ratio after weighting by sample size (Supplementary Table S3). Finally, some species may not fit into the observed foraging habitat and mode through stable isotope and DNA-based dietary analyses (Oelbaum et al., 2019; Ingala et al., 2021). Combined with fine-scale monitoring of habitat use, foraging strategy, and dietary compositions of focal species, further research is needed to assess the contribution of foraging ecology to the diversity of wing morphology and echolocation calls of bats in the phylogenetic context.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
BL and JF designed the study. WZ, HL, PW, DZ, WL, and YL collected the data. BL, JW, and LF conducted the analyses. BL and WZ wrote the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 32271561 and 32071492), Natural Science Foundation of Sichuan Province (Grant No. 2022NSFSC1761), and Undergraduate Innovation and Entrepreneurship Training Program of China (Grant No. 202110638011).
Acknowledgments
We are grateful to Huimin Gao, Weiwei Wang, and Yingchun Deng for their assistance with literature search. We acknowledge the reviewers for valuable advices and comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1031548/full#supplementary-material
References
Aldridge, H. D. J., and Rautenbach, I. L. (1987). Morphology, echolocation and resource partitioning in insectivorous bats. J. Amin. Ecol. 56, 763–778. doi: 10.2307/4947
Arato, J., and Fitch, W. T. (2021). Phylogenetic signal in the vocalizations of vocal learning and vocal non-learning birds. Philos. T. R. Soc. B 376:20200241. doi: 10.1098/rstb.2020.0241
Arita, H. T., and Fenton, M. B. (1997). Flight and echolocation in the ecology and evolution of bats. Trends Ecol. Evol. 12, 53–58. doi: 10.1016/S0169-5347(96)10058-6
Arlettaz, R., Jones, G., and Racey, P. A. (2001). Effect of acoustic clutter on prey detection by bats. Nature 414, 742–745. doi: 10.1038/414742a
Barton, K. (2016). Mumin: multi-model inference. R package version 1.15.6. Available at: https://CRANR-projectorg/package=MuMIn:18 (Accessed November 9, 2022).
Charlton, B. D., and Reby, D. (2016). The evolution of acoustic size exaggeration in terrestrial mammals. Nat. Commun. 7:12739. doi: 10.1038/ncomms12739
Collen, A. ed. (2012). The evolution of echolocation in bats: A comparative approach. Doctoral dissertation. London: University College London.
Currie, S. E., Boonman, A., Troxell, S., Yovel, Y., and Voigt, C. C. (2020). Echolocation at high intensity imposes metabolic costs on flying bats. Nat. Ecol. Evol. 4, 1174–1177. doi: 10.1038/s41559-020-1249-8
Denzinger, A., and Schnitzler, H. U. (2013). Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of micro chiropteran bats. Front. Physiol. 4:164. doi: 10.3389/fphys.2013.00164
Fenton, M., Audet, D., Orbrist, M., and Rydell, J. (1995). Signal strength, timing, and self-deafening: the evolution of echolocation in bats. Paleobiology 21, 229–242. doi: 10.1017/S0094837300013221
Fenton, M. B., Faure, P. A., and Ratcliffe, J. M. (2012). Evolution of high duty cycle echolocation in bats. J. Exp. Biol. 215, 2935–2944. doi: 10.1242/jeb.073171
Fenton, B., Jensen, F. H., Kalko, E. K. V., and Tyack, P. L. (2014). “Sonar signals of bats and toothed whales,” in Springer handbook of auditory research. Vol. 51. eds. A. Surlykke, P. Nachtigall, R. Fay, and A. Popper (New York: Springer), 11–59. doi: 10.1007/978-1-4614-9146-0_2
Geipel, I., Jung, K., and Kalko, E. K. V. (2013). Perception of silent and motionless prey on vegetation by echolocation in the gleaning bat Micronycteris microtis. P. Roy. Soc. B 280:20122830. doi: 10.1098/rspb.2012.2830
Gingras, B., Mohandesan, E., Boko, D., and Fitch, W. T. (2013). Phylogenetic signal in the acoustic parameters of the advertisement calls of four clades of anurans. BMC Evol. Biol. 13:134. doi: 10.1186/1471-2148-13-134
Griffin, D. R. (1944). Echolocation by blind men, bats and radar. Science 100, 589–590. doi: 10.1126/science.100.2609.589
Guo, D. G., Ding, J. N., Liu, H., Zhou, L., Feng, J., Luo, B., et al. (2021). Social calls influence the foraging behavior in wild big-footed myotis. Front. Zool. 18:3. doi: 10.1186/s12983-020-00384-8
Hensley, N. M., Drury, J. P., Garland, T., and Blumstein, D. T. (2015). Vivid birds do not initiate flight sooner despite their potential conspicuousness. Curr. Zool. 61, 773–780. doi: 10.1093/czoolo/61.4.773
Ingala, M. R., Simmons, N. B., Wultsch, C., Krampis, K., Provost, K. L., and Perkins, S. L. (2021). Molecular diet analysis of neotropical bats based on fecal DNA metabarcoding. Ecol. Evol. 11, 7474–7491. doi: 10.1002/ece3.7579
IUCN SSC (2022). The IUCN red list of threatened species. Available at: http://www.iucnredlist.org (January, 2022).
Ives, A. R. (2019). R2s for correlated data: phylogenetic models, lmms, and glmms. Syst. Biol. 68, 234–251. doi: 10.1093/sysbio/syy060
Ives, A. R., and Zhu, J. (2006). Statistics for correlated data: phylogenies, space, and time. Ecol. Appl. 16, 20–32. doi: 10.1890/04-0702
Jennings, N. V., Parsons, S., Barlow, K. E., and Gannon, M. R. (2004). Echolocation calls and wing morphology of bats from the West Indies. Acta. Chiropterol. 6, 75–90. doi: 10.3161/1508110042176644
Jones, G. (1994). Scaling of wingbeat and echolocation pulse emission rates in bats: why are aerial insectivorous bats so small? Funct. Ecol. 8, 450–457. doi: 10.2307/2390068
Jones, G., and Siemers, B. M. (2010). The communicative potential of bat echolocation pulses. J. Comp. Physiol. A 197, 447–457. doi: 10.1007/s00359-010-0565-x
Jones, G., and Teeling, E. C. (2006). The evolution of echolocation in bats. Trends Ecol. Evol. 21, 149–156. doi: 10.1016/j.tree.2006.01.001
Jung, K., Molinari, J., and Kalko, E. K. V. (2014). Driving factors for the evolution of species-specific echolocation call design in new world free-tailed bats (Molossidae). PLoS One 9:e85279. doi: 10.1371/journal.pone.0085279
Kalko, E. K. V., and Schnitzler, H. U. (1993). Plasticity in echolocation signals of european pipistrelle bats in search flight: implications for habitat use and prey detection. Behav. Ecol. Sociobiol. 33, 415–428. doi: 10.1007/BF00170257
Kamilar, J. M., and Cooper, N. (2013). Phylogenetic signal in primate behavior, ecology and life history. Philos. T. R. Soc. B 368:20120341. doi: 10.1098/rstb.2012.0341
Kingston, T., Jones, G., Zubaid, A., and Kunz, T. (2000). Resource partitioning in rhinolophoid bats revisited. Oecologia 124, 332–342. doi: 10.1007/PL00008866
Kober, R., and Schnitzler, H. U. (1990). Information in sonar echoes of fluttering insects available for echolocating bats. J. Acoust. Soc. Am. 87, 882–896. doi: 10.1121/1.398898
Lancaster, W. C., Henson, O., and Keating, A. (1995). Respiratory muscle activity in relation to vocalization in flying bats. J. Exp. Biol. 198, 175–191. doi: 10.1242/jeb.198.1.175
Lazure, L., and Fenton, M. B. (2011). High duty cycle echolocation and prey detection by bats. J. Exp. Biol. 214, 1131–1137. doi: 10.1242/jeb.048967
Li, G., Wang, J., Rossiter, S. J., Jones, G., and Zhang, S. Y. (2007). Accelerated foxp2 evolution in echolocating bats. PLoS One 2:e900. doi: 10.1371/journal.pone.0000900
Luo, B., Leiser-Miller, L., Santana, S. E., Zhang, L., Liu, T., Xiao, Y. H., et al. (2019b). Echolocation call divergence in bats: a comparative analysis. Behav. Ecol. Sociobiol. 73:154. doi: 10.1007/s00265-019-2766-9
Luo, B., Santana, S. E., Pang, Y. L., Wang, M., Xiao, Y. H., and Feng, J. (2019a). Wing morphology predicts geographic range size in vespertilionid bats. Sci. Rep. 9:4526. doi: 10.1038/s41598-019-41125-0
Mancina, C. A., García-Rivera, L., and Miller, B. W. (2012). Wing morphology, echolocation, and resource partitioning in syntopic cuban mormoopid bats. J. Mammal. 93, 1308–1317. doi: 10.1644/11-MAMM-A-331.1
Marinello, M. M., and Bernard, E. (2014). Wing morphology of neotropical bats: a quantitative and qualitative analysis with implications for habitat use. Can. J. Zool. 92, 141–147. doi: 10.1139/cjz-2013-0127
Martins, E. P., and Garland, T. (1991). Phylogenetic analyses of the correlated evolution of continuous characters: a simulation study. Evolution 45, 534–557. doi: 10.1111/j.1558-5646.1991.tb04328.x
Monroy, J. A., Carter, M. E., Miller, K. E., and Covey, E. (2011). Development of echolocation and communication vocalizations in the big brown bat, eptesicus fuscus. J. Comp. Physiol. A 197, 459–467. doi: 10.1007/s00359-010-0614-5
Moss, C., and Surlykke, A. (2010). Probing the natural scene by echolocation in bats. Front. Behav. Neurosci. 4:33. doi: 10.3389/fnbeh.2010.00033
Neuweiler, G. (1989). Foraging ecology and audition in echolocating bats. Trends Ecol. Evol. 4, 160–166. doi: 10.1016/0169-5347(89)90120-1
Norberg, U. M. (1994). “Wing design, flight performance, and habitat use in bats,” in Ecological morphology: Integrative organismal biology. eds. P. C. Wainwright and S. M. Reilly (Chicago: University of Chicago Press), 205–239.
Norberg, U. M., Brooke, A. P., and Trewhella, W. J. (2000). Soaring and non-soaring bats of the family pteropodidae (flying foxes, pteropus spp.): wing morphology and flight performance. J. Exp. Biol. 203, 651–664. doi: 10.1242/jeb.203.3.651
Norberg, U. M., and Rayner, J. M. V. (1987). Ecological morphology and flight in bats (mammalia; chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philos. T. R. Soc. B 316, 335–427. doi: 10.1098/rstb.1987.0030
Oelbaum, P. J., Fenton, M. B., Simmons, N. B., and Broders, H. G. (2019). Community structure of a Neotropical bat fauna as revealed by stable isotope analysis: not all species fit neatly into predicted guilds. Biotropica 51, 719–730. doi: 10.1111/btp.12700
Paradis, E., Claude, J., and Strimmer, K. (2004). Ape: analyses of phylogenetics and evolution in r language. Bioinformatics 20, 289–290. doi: 10.1093/bioinformatics/btg412
Peters, G., and Peters, M. K. (2010). Long-distance call evolution in the felidae: effects of body weight, habitat, and phylogeny. Biol. J. Linn. Soc. 101, 487–500. doi: 10.1111/j.1095-8312.2010.01520.x
Pinheiro, J. C., Bates, D. J., Debroy, S. D., and Sakar, D. (2014). Nlme: Linear and nonlinear mixed effects models. R package version 3, 1–117. Available at: http://cran.R-project.Org/package=nlme (Accessed November 9, 2022).
Ratcliffe, J. M., Raghuram, H., Marimuthu, G., Fullard, J. H., and Fenton, M. B. (2005). Hunting in unfamiliar space: echolocation in the Indian false vampire bat, megaderma lyra, when gleaning prey. Behav. Ecol. Sociobiol. 58, 157–164. doi: 10.1007/s00265-005-0912-z
Rayner, J. M. V. (1991). “Complexity and a coupled system: flight, echolocation and evolution in bats,” in Constructional morphology and evolution. eds. N. Schmidt-Kittler and K. Vogel (Berlin: Springer), 173–191.
Revell, L. J. (2012). Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. doi: 10.1111/j.2041-210X.2011.00169.x
Rhodes, M. P. (2002). Assessment of sources of variance and patterns of overlap in microchiropteran wing morphology in Southeast Queensland, Australia. Can. J. Zool. 80, 450–460. doi: 10.1139/z02-029
Roemer, C., Coulon, A., Disca, T., and Bas, Y. (2019). Bat sonar and wing morphology predict species vertical niche. J. Acoust. Soc. Am. 145, 3242–3251. doi: 10.1121/1.5102166
Scherrer, J. A., and Wilkinson, G. S. (1993). Evening bat isolation calls provide evidence for heritable signatures. Anim. Behav. 46, 847–860. doi: 10.1006/anbe.1993.1270
Schnitzler, H. U. (1973). Control of doppler shift compensation in the greater horseshoe bat, Rhinolophus ferrumequinum. J. Com. Physiol. A 82, 79–92. doi: 10.1007/BF00714171
Schnitzler, H. U., and Denzinger, A. (2011). Auditory fovea and Doppler shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals. J. Comp. Physiol. A 197, 541–559. doi: 10.1007/s00359-010-0569-6
Schnitzler, H. U., and Kalko, E. K. V. (1998). “How echolocating bats approach and acquire food,” in Bat biology and conservation. eds. T. H. Kunz and P. A. Racey (Washington: Smithsonian Institution Press), 197–204.
Schnitzler, H. U., and Kalko, E. K. V. (2001). Echolocation by insect-eating bats. Bioscience 51, 557–569.
Schnitzler, H. U., Moss, C. F., and Denzinger, A. (2003). From spatial orientation to food acquisition in echolocating bats. Trends Ecol. Evol. 18, 386–394. doi: 10.1016/S0169-5347(03)00185-X
Shi, J. J., and Rabosky, D. L. (2015). Speciation dynamics during the global radiation of extant bats. Evolution 69, 1528–1545. doi: 10.1111/evo.12681
Siemers, B. M., and Schnitzler, H. U. (2004). Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature 429, 657–661. doi: 10.1038/nature02547
Simmons, J., Fenton, M., and O'Farrell, M. (1979). Echolocation and pursuit of prey by bats. Science 203, 16–21. doi: 10.1126/science.758674
Simmons, N. B., and Geisler, J. H. (1998). Phylogenetic relationships of icaronycteris, archaeonycteris, hassianycteris, and palaeochiropteryx to extant bat lineages, with comments on the evolution of echolocation and foraging strategies in microchiroptera. Bull AMNH. 235, 1–182.
Simmons, N. B., Seymour, K. L., Habersetzer, J., and Gunnell, G. F. (2008). Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature 451, 818–821. doi: 10.1038/nature06549
Speakman, J. R. (2001). The evolution of flight and echolocation in bats: another leap in the dark. Mammal Rev. 31, 111–130. doi: 10.1046/j.1365-2907.2001.00082.x
Speakman, J. R., and Racey, P. A. (1991). No cost of echolocation for bats in flight. Nature 350, 421–423. doi: 10.1038/350421a0
Suthers, R. A., Thomas, S. P., and Suthers, B. J. (1972). Respiration, wing-beat and ultrasonic pulse emission in an echo-locating bat. J. Exp. Biol. 56, 37–48. doi: 10.1242/jeb.56.1.37
Teeling, E. C., Scally, M., Kao, D. J., Romagnoli, M. L., Springer, M. S., and Stanhope, M. J. (2000). Molecular evidence regarding the origin of echolocation and flight in bats. Nature 403, 188–192. doi: 10.1038/35003188
Thiagavel, J., Cechetto, C., Santana, S. E., Jakobsen, L., Warrant, E. J., and Ratcliffe, J. M. (2018). Auditory opportunity and visual constraint enabled the evolution of echolocation in bats. Nat. Commun. 9:98. doi: 10.1038/s41467-017-02532-x
Tokita, M., Abe, T., and Suzuki, K. (2012). The developmental basis of bat wing muscle. Nat. Commun. 3:1302. doi: 10.1038/ncomms2298
von der Emde, G., and Schnitzler, H. U. (1990). Classification of insects by echolocating greater horseshoe bats. J. Comp. Physiol. A 167, 423–430. doi: 10.1007/bf00192577
Veselka, N., McErlain, D. D., Holdsworth, D. W., Eger, J. L., Chhem, R. K., Mason, M. J., et al. (2010). A bony connection signals laryngeal echolocation in bats. Nature 463, 939–942. doi: 10.1038/nature08737
Wang, L., Lin, A. Q., Xiao, Y. H., Jiang, T. L., and Feng, J. (2014). Postnatal development in the big-footed bat, myotis macrodactylus: wing morphology, echolocation calls, and flight. Acta Theriol. 59, 435–441. doi: 10.1007/s13364-014-0182-0
Keywords: BAT, correlated evolution, echolocation calls, phylogeny, wing morphology
Citation: Zou W, Liang H, Wu P, Luo B, Zhou D, Liu W, Wu J, Fang L, Lei Y and Feng J (2022) Correlated evolution of wing morphology and echolocation calls in bats. Front. Ecol. Evol. 10:1031548. doi: 10.3389/fevo.2022.1031548
Edited by:
Gang Song, Institute of Zoology (CAS), ChinaReviewed by:
Felipe N. Moreno-Gómez, Universidad Católica del Maule, ChileYang Wang, Hebei Normal University, China
Anderson Feijo, Institute of Zoology (CAS), China
Copyright © 2022 Zou, Liang, Wu, Luo, Zhou, Liu, Wu, Fang, Lei and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Luo, bHVvYjA0MUBuZW51LmVkdS5jbg==; Jiang Feng, ZmVuZ2pAbmVudS5lZHUuY24=
†These authors have contributed equally to this work
 Wenyu Zou1†
Wenyu Zou1† Bo Luo
Bo Luo Jiang Feng
Jiang Feng