
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 01 December 2022
Sec. Ecophysiology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.1028037
Habitat loss, climate change, and global agriculture have a non-negligible effect on the reduced abundance and diversity of floral resources. Malnutrition and nutritional stress are consequences of the combination of these factors with considerable impact on honey bee health and colony losses. The solution to inadequate natural sources for beekeeping is the additional feeding of honey bee colonies with food supplements. The algae Chlorella is a natural food source, with a nutrient profile similar to natural pollen, thus it has promising application in beekeeping. We evaluated Chlorella vulgaris syrup as a dietary supplement in the view of the oxidative stress that may be caused by long term administration to the colonies. Consuming Chlorella syrup did not influence the activity of digestive enzymes of summer honey bee workers, however, lipase activity insignificantly increased. After Chlorella application to colonies, we also observed insignificantly higher gene expression of antioxidant enzymes catalase and superoxid dismutase1 in adult workers; however, in larvae the expression of those genes was not affected. Surprisingly, the gene expression did not correspond with enzyme activity in adult bee abdomens. In Chlorella fed colonies, we recorded a higher concentration of vitellogenin, which plays multiple roles in honey bee physiology, i.e., antioxidant, storage protein, or immunity-related functions. Our new findings brought evidence that Chlorella did not negatively affect the digestion or oxidative balance of honey bees, thus its application as a pollen supplement can be fully recommended for maintaining the health of honey bee colonies during periods of dearth.
• Chlorella syrup was well accepted by bees and larvae
• The digestive system of bees is not influenced by consuming Chlorella syrup
• Chlorella syrup consumption increases gene expression of antioxidant enzymes without affecting their activity
• Chlorella consumption stimulated vitellogenin production
Over the past few decades, increased honey bee colony losses have been reported worldwide (Kulhanek et al., 2017; Gray et al., 2019), which brings the health and nutrition of honey bees to the forefront of general interest (Brodschneider and Crailsheim, 2010; Meixner and Le Conte, 2016). Habitat loss, climate change, and global agriculture have a non-negligible effect on the reduced abundance and diversity of floral resources. Increasing areas of non-forage habitats or monocultures have led to a strong emphasis on the quality of food sources and their impact on the vitality of bees as well (Smart et al., 2016; Alaux et al., 2017; Smart et al., 2019). Diversity and availability of nutritional resources, especially pollen, are known to be essential for the proper functioning of the honey bee colony and the immune system of individual bees (Alaux et al., 2010a; Scofield and Mattila, 2015; Di Pasquale et al., 2016; Frias et al., 2016; Danihlik et al., 2018). Therefore, malnutrition and nutritional stress can make a remarkable contribution to high colony losses (Potts et al., 2010; Goulson et al., 2015; Branchiccela et al., 2019). The solution to inadequate natural sources for beekeeping during periods of dearth is the additional feeding of honey bee colonies with commercial protein supplements.The use of commercial supplements is currently the subject of intense research due to the need to holistically determine the effectiveness of protein supplementation using diets that consider both individual bee and colony-level health metrics (Brodschneider and Crailsheim, 2010; Danihlik et al., 2018; Wright et al., 2018).
Microalgae have been tested as a potential food supplement for honey bees (Eremia et al., 2013; Jehlík et al., 2019; Ricigliano, 2020; Ricigliano and Simone-Finstrom, 2020; Jang et al., 2022; Ricigliano et al., 2022). The nutritional quality of algae is similar to that of pollen, with a high content of proteins, nutritionally relevant lipids, essential amino acids and micronutrients such as vitamins and minerals, especially within two genera—Arthrospira (also known as spirulina) and Chlorella (Ricigliano, 2020). Protein supplementation using Chlorella vulgaris was reported to increase honey bee colony productivity, as noted by increased brood production and honey stores (Eremia et al., 2013). Additionally, it has been described that organic extracts from microalgae have antimicrobial activity. Hence, the microalgae-based feed could have additional prophylactic properties for honey bee colonies (Catarina Guedes et al., 2011; Salem et al., 2014; Dostálková et al., 2021).
Little is known regarding how dietary supplements affect individual bees at the level of physiological and molecular parameters. Effectiveness of the immune system, modifications of biochemical and physiological reactions or capacity of anti-oxidative defence reactions should be studied to more holistically assess any additional impacts (positive or negative) of dietary supplements, particularly those during different developmental and behavioural states of the bee. Such fine-scale measures are often neglected but can provide a more complete picture of the value of potential nutritional products redundant (Ricigliano et al., 2022). Recent studies indicate, that supplementing bees with pollen can improve honey bee health and positively influence stimulation of the immune system on both colony and individual levels (Alaux et al., 2010b; Di Pasquale et al., 2013; Annoscia et al., 2017; Danihlik et al., 2018; Degrandi-Hoffman et al., 2020). Recent work on spirulina found that in caged bees, a diet of natural pollen or spirulina resulted in comparable proteome expression patterns indicating that microalgal supplements may be a sustainable and effective diet matching honey bee needs (Ricigliano et al., 2021). In a previous study, we documented the potential for Chlorella as a dietary supplement for honey bees (Jehlík et al., 2019). We found that colonies fed Chlorella had positive effects of supplementation during the spring on both colony level (i.e., increased brood production) and individual level metrics (i.e., larger hypopharyngeal glands in nurse bees). Pre-winter colony supplementation provided initial evidence of the modulatory influence of Chlorella feeding on basic digestive enzymes, which was supported by cage studies that also determined that the algal diet influenced transcript levels of endocrine markers involved in cell signal pathways (Jehlík et al., 2019).
As microalgae are rich in antioxidants (phenolics, carotenoids, and other photosynthetic pigments) (Li et al., 2007; Goiris et al., 2012), within this study we also aimed to investigate Chlorella’s antioxidant properties when consumed by bees. Antioxidants can scavenge and detoxify by-products of aerobic metabolism—free radicals and reactive molecules termed as reactive oxygen species (ROS). In insect tissues, ROS are constantly produced by a diverse array of metabolic processes, including mitochondrial respiration, multiple oxidases enzymes, or xenobiotic degradation. Increased ROS levels are caused by the imbalance between the ROS production rate and the antioxidant capacity of the organism. Enhanced levels of ROS are harmful as they can lead to oxidative damage of important biomolecules such as proteins, lipids, and nucleic acids, and thus negatively affect basic biochemical and physiological processes (Sies et al., 2017). Primary protection against ROS accumulation and oxidative stress is ensured by key antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) along with glutathione- and thioredoxin-dependent peroxidases (Weirich et al., 2002; Corona and Robinson, 2006). SOD primarily converts superoxide radical anions (O2−) generated by aerobic metabolism to hydrogen peroxide and oxygen. There are two SOD isoforms in all eukaryotes, cytosolic SOD1 (copper; zinc-containing SOD) and mitochondrial SOD2 [manganese-containing SOD (Weirich et al., 2002)]. CAT is an enzyme primarily located in the peroxisomes and driving the decomposition of hydrogen peroxide to water and oxygen (Ahmad et al., 1988; Ahmad and Pardini, 1990).
Vitellogenin (Vg) is known to play crucial roles in bee reproduction, nutrition and immunity (Salmela and Sundström, 2018) as well as longevity of honey bees (Fluri et al., 1982; Amdam et al., 2005). Vg synthesized in the fat body has been suggested to fulfill also an important function in the antioxidant system of honey bees. There is evidence that Vg might be preferentially oxidized, and thus represent an important component of antioxidant repertoire in bee hemolymph (Seehuus et al., 2006; Zhang et al., 2017).
This study aimed to conduct a fine-scale assessment of Chlorella vulgaris as a dietary supplement for honey bee colonies to more holistically evaluate the long-term effects of the algal supplementation on individual bees. The effects of Chlorella were measured based on the activity of digestive enzymes, antioxidant enzyme activity and gene expression, and Vg production and gene expression. The results support the assumption that Chlorella can be used as a safe protein supplement for honey bees in support of bee homeostasis.
Honey bee colonies (Apis mellifera carnica) were kept in one apiary close to Krhová (49.4842989 N, 17.9937911E) located in the Czech Republic. The colonies were at the strength of 11 occupied frames (42.5 × 17 cm) in one box (Langstroth style). Three control colonies (Control) were fed with APIVITAL® sugar syrup (Dobruška, Czech Republic). Three experimental colonies (Algae) were fed with 10% (w/v) Chlorella powder in APIVITAL® sugar syrup. Chlorella powder consisted of autotrophic alga Chlorella vulgaris provided by the Centre Algatech, Institute of Microbiology CAS, Třeboň, Czech Republic. Both groups, Control, and experimental (Algae) consumed a total of 3 liters of feed per colony over 2 weeks, equally 300 g of dry Chlorella powder for the algae treatment. After 2 weeks of feeding, approximately 300 adult bee workers and one frame with uncapped brood were collected from each hive. All samples were immediately frozen and stored at −80°C.
For Vg quantification in larval and worker hemolymph, a common direct ELISA was used. The hemolymph was taken from the bee abdomens that were gently punctured with a pin, and the leaking hemolymph was collected by a capillary; one μl (n = 20) was then used for Vg quantification. The primary anti-Vg antiserum (Koubová et al., 2021) was diluted 1:20,000 and secondary antibody – swine anti-rabbit IgG labelled with horseradish peroxidase (SwAR/HPR – LabNed) - was diluted 1:2,000. The reaction was visualized using 3,3′,5,5′-tetramethylbenzidine (Sigma Aldrich, United States), and the absorbance of the product was determined at 450 nm in a microplate reader. The results were expressed as the absorbance normalized per 1 μl of hemolymph.
The activities of basic digestive enzymes—proteases, amylases, and lipases—were determined in the midgut of the adult workers. The organs were dissected, weighed, homogenized by sonication in 0.2 M Tris pH 7.8, and centrifuged. The aliquots—0.1 midgut equivalents for proteases, 0.025 midgut equivalents for amylases, and 0.25 midgut equivalents for lipases—were tested for enzymatic activity.
For the determination of protease activity the resorufin-casein kit (Roussel et al., 2015) was used according to the manufacturer’s instructions. Sample extracts in 0.2 M Tris pH 7.8 were mixed with 0.4% resorufin-casein substrate and 0.02 M calcium chloride solution in microcentrifuge tubes. Afterward, the mixture was heated for 1 h at 37°C under gentle shaking. The reaction was terminated by 5% trichloroacetic acid, and the mixture was incubated for the next 10 min at 37°C. Non-hydrolyzed casein was then removed by centrifugation. The absorbance of the supernatant was measured at 490 nm. Protease activity was expressed in units of proteolytic activity per mg of fresh midgut weight. The activity unit (U) was defined as the amount of enzyme (mg) which caused an increase in optical density by 0.1 per min in 1 ml of the reaction mixture (Elpidina et al., 2001).
The 3,5-dinitrosalicylic acid (DNS) reagent was used for the determination of amylase activity as described previously (Kodrik et al., 2012). In brief, sample extracts were mixed with an equal volume of 2% starch (Sigma Aldrich, United States) in a phosphate buffer. The mixture was agitated for 40 min at 30°C, and the reaction was terminated by adding 200 μl of 0.005% DNS solution. Subsequently, the solution was boiled at 100°C for 5 min, cooled, centrifuged (10,000 g 10 min), and the absorbance of the supernatant was determined at 550 nm. Amylase activity was expressed in μmol maltose (maltose was served as standard) per mg of midgut fresh weight (Bernfeld, 1955).
For determination of lipase activity, 4-methylumbelliferyl butyrate (4-MUB) was used (Roberts, 1985; Kodrik et al., 2012). In brief, the 5 μl 2 mM 4-MUB substrate diluted in dimethylsulfoxide (DMSO) was mixed with the 5 μl sample extracts (diluted in 0.1 M acetic buffer, pH 5) and 195 μl 0.1 M acetate buffer, pH 5. The release of the fluorescent 4-methylumbelliferone (4-MU) was monitored at 5 min intervals for 30 min at 327 nm excitation and 449 nm emission with a Synergy 4 multi-mode microplate reader (BioTek Instr., Winooski, Vermont, United States). Lipase activity was expressed in nmol of 4-MU per min per mg of midgut fresh weight.
Sample processing and concentration of proteins - Bees were separated into body parts and the digestive system was removed from each bee. For further analysis, only abdomens were used. Samples were homogenized using TissueLyser II (Qiagen, Germany) using glass beads (3 mm in diameter, Roth, Germany). Then 200 μl of 0.1 M potassium phosphate buffer pH 7.0 was added to the homogenate. Samples were centrifuged for 10 min at 12,000 g and 4°C. The supernatant was used for further measurements. For all samples, protein concentration was determined according to Bradford (1976) by using Coomassie® brilliant blue G-250 (Sigma Aldrich, United States) and measured at 595 nm. Bovine serum albumin (Merck, Germany) was used as a calibration standard. Samples were measured in triplicates for both protein concentration and enzyme activities (i.e., CAT and SOD).
Catalase activity - for determination of CAT activity, extracts of bee abdomens were diluted 1:2 in potassium phosphate buffer pH 7.0. The enzyme reaction was measured according to Aebi (1984) on a Synergy H1 Multi-Mode Reader (BioTek Instr., Winooski, Vermont, United States). For the reaction, 220 μl of buffer and 10 μl of the sample were used; the reaction was initiated with 20 μl 150 mM H2O2 (Lach-Ner, Czech Republic). Absorbance changes at 240 nm were recorded for 5 min at 30°C. CAT activity was expressed as the amount of decomposed H2O2 (molar extinction coefficient ε240 = 39,400 M−1.cm−1) in 1 ml of bee extract.
Superoxide dismutase activity – abdomen extracts used for the measurements of SOD activity were diluted 50× in 0.02 M Tris buffer pH 8.2 containing 1 mM EDTA and then used for determination of SOD activity according to Marklund and Marklund (1974). A volume of 220 μl of Tris buffer and 20 μl of diluted sample was used for the reaction. For the initiation of the reaction 20 μl of 2 mM pyrogallol was pipetted into the well. The change in absorbance was measured for 5 min at 25°C with a wavelength of 420 nm. SOD activity was expressed in enzyme units (U) per one ml of bee extract. The unit is defined as the amount of enzyme which inhibits the auto-oxidation of pyrogallol by 50% (Marklund and Marklund, 1974).
To quantify relative gene expression, a pooled sample of 2 adult bees was used for RNA extraction. The bees were placed in plastic mesh bags (Bioreba, Switzerland), 1.2 ml of GITC lysis buffer containing 1% ß-mercaptoethanol (Sigma Aldrich, United States) were added and a sample was homogenized using a pestle. For larvae samples, one larva was homogenized in homogenizer TissueLyser II (Qiagen, Germany). After homogenization 300 μl of GITC lysis buffer was added.
Total RNA was extracted from homogenates in GITC buffer using 300 μl of homogenate for adult bees and larvae samples. The NucleoSpin RNA Plus kit was used for RNA extraction according to the manufacturer’s instructions (Marchery-Nagel, Germany). RNA was eluted in 60 μl of RNase-free water, then the concentration and quality of RNA samples were quantified by Synergy HT microplate reader (BioTek, Germany). RNA integrity was confirmed by electrophoresis in 1.1% (w/v) agarose gel stained by GelRed (Biotium, Fremont, CA, United States). Reverse transcription was performed with the Transcriptor High Fidelity kit (Roche, Switzerland) in 20 μl reaction volumes according to the manufacturer’s protocol with a final concentration of RNA 50 ng/μl per reaction. Relative gene expression was determined using SYBR Green (SYBR Select Master Mix, Applied Biosystems, United States) on CFX96 Touch Real-Time PCR Detection System (Bio-Rad, United States). Product quality and contamination were tested by measuring the melting curves of PCR products (60°C – 95°C, using increments of 0.5°C for 0.1 min). The relative expression of genes of interest (catalase [CAT], superoxide dismutase1 [SOD1], superoxide dismutase2 [SOD2], vitellogenin [Vg]) to housekeeping genes (HKG) (RPS5, EF-alpha) was calculated according to Pfaffl (2001). The stability of housekeeping genes were tested by the Excel-based tool BestKeeper™ (Pfaffl et al., 2004). For primer sequences and stability of housekeeping genes see Supplementary material S1.
Statistica version 13 software (TIBCO Inc., Palo Alto, CA, United States) was used for the data analysis; OriginPro 2019b (64-bit) was used for creation the plots. Data were tested for normality by the Shapiro–Wilk test. Based on data distribution, it was decided whether to use non-parametric Mann–Whitney U test (Figures 1, 2, 3, 4A) or parametric t-test (Figures 4B, 5, 6).
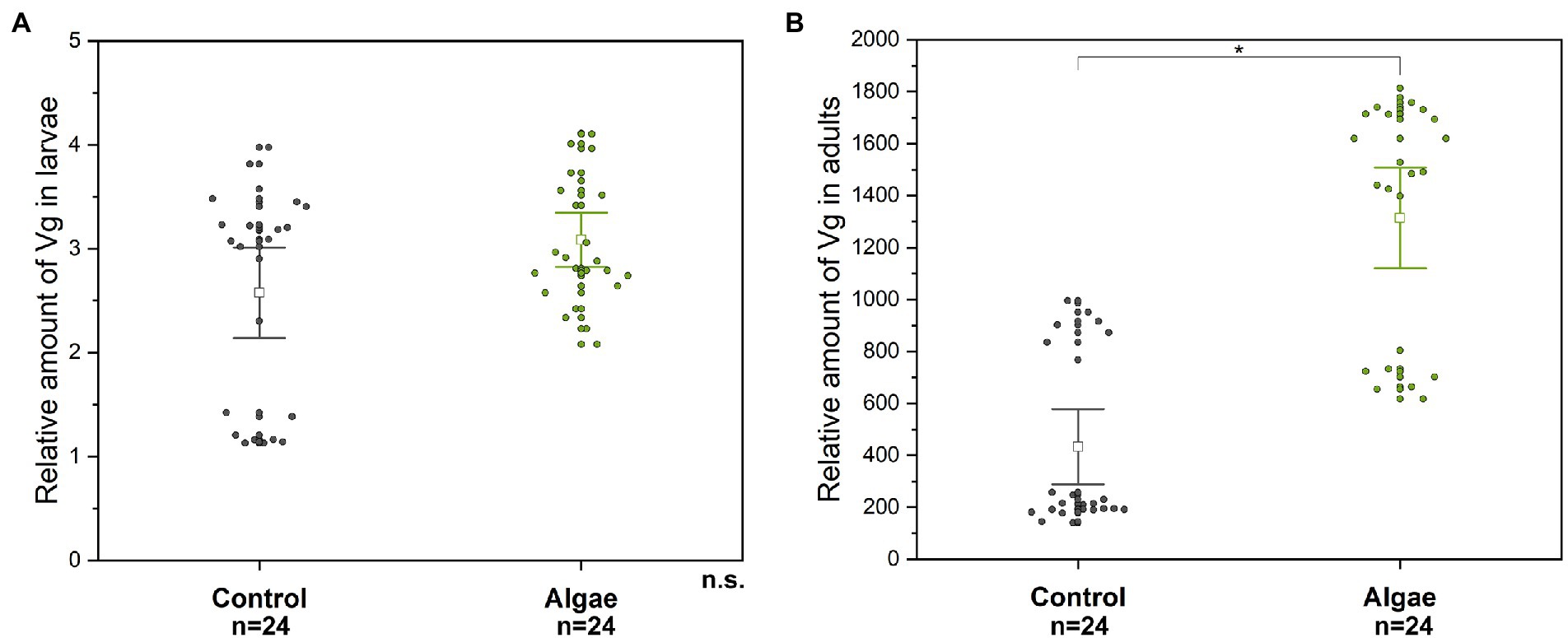
Figure 1. The relative level of Vg in larvae (A) and adult (B) hemolymph. A significant difference (p < 0.004) between the groups (Control, Algae) is indicated by an asterisk above the means. n.s. – non-significant difference. Control – bees from colonies fed with APIVITAL® sugar syrup. Algae – bees from colonies fed with 10% (w/v) Chlorella powder in APIVITAL® sugar syrup.
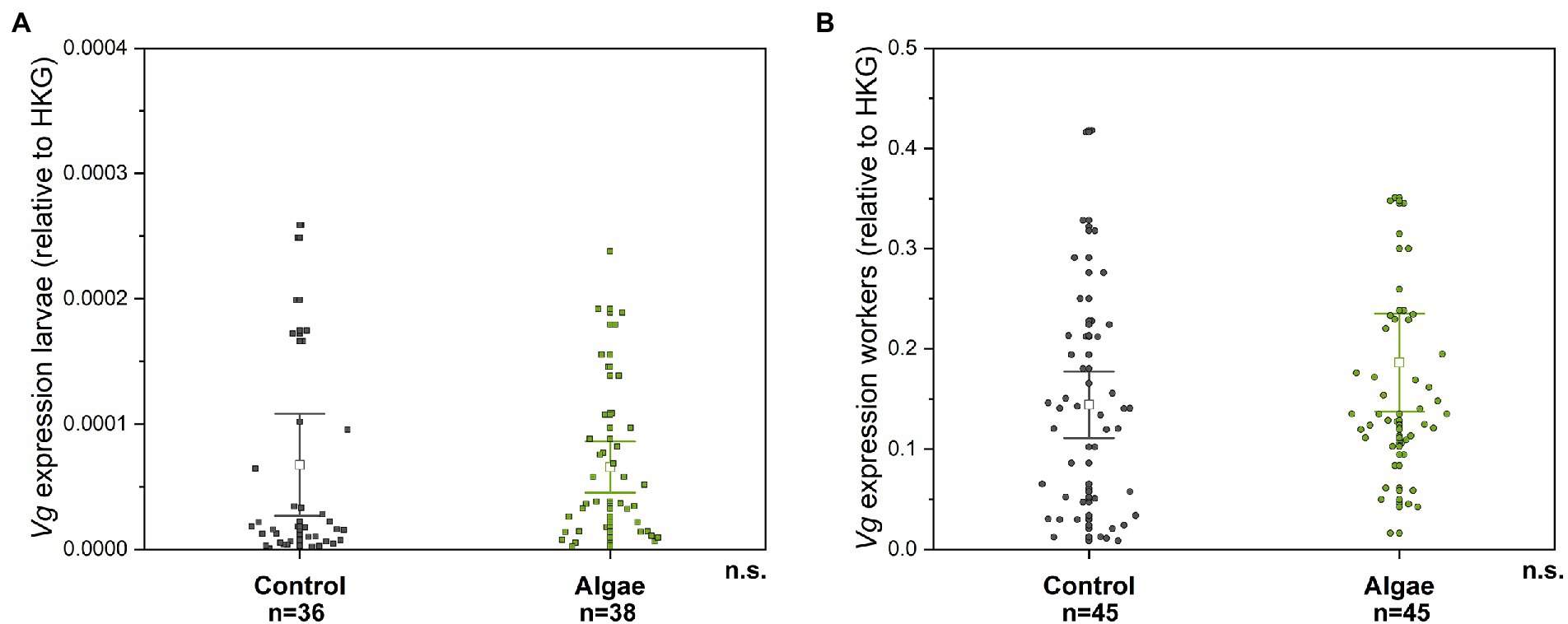
Figure 2. The relative gene expression of Vg in bee larvae (A) and adult bees (B). n.s. – non-significant difference. Control – bees from colonies fed with APIVITAL® sugar syrup. Algae – bees from colonies fed with 10% (w/v) Chlorella powder in APIVITAL® sugar syrup.
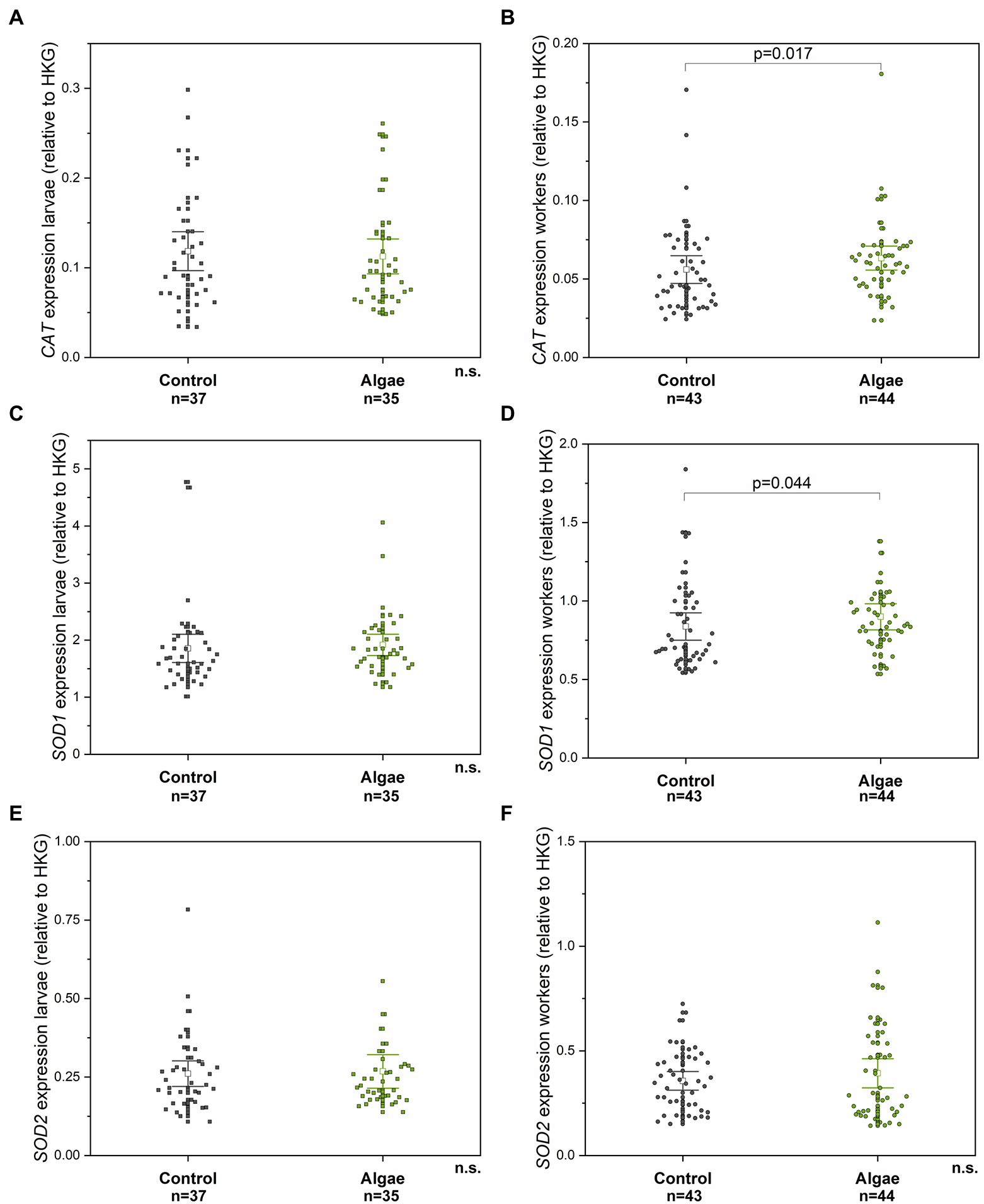
Figure 3. The relative gene expression of CAT, SOD1, and SOD2 compared to HKG in larvae (A, C, E) and adult workers (B, D, F), respectively. n. s. – non-significant difference. Control – bees from colonies fed with APIVITAL® sugar syrup. Algae – bees from colonies fed with 10% (w/v) Chlorella powder in APIVITAL® sugar syrup. HKG – housekeeping gene.
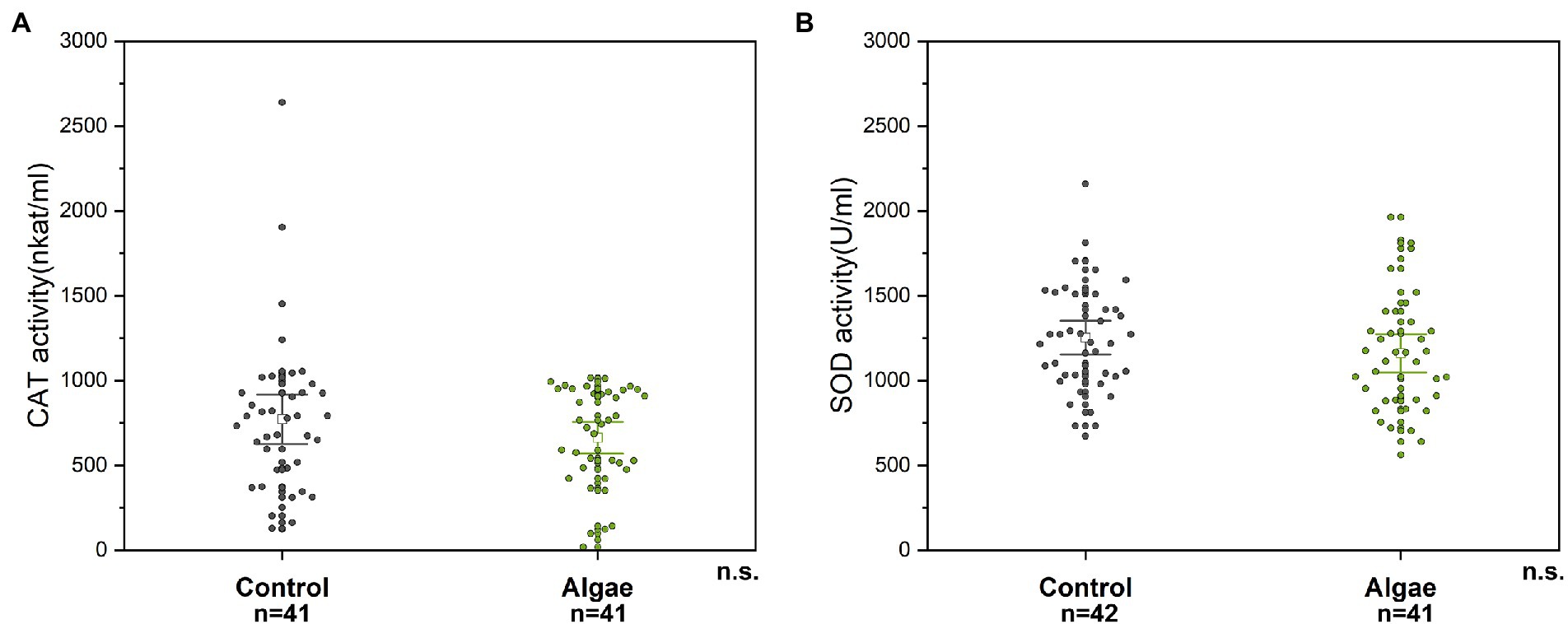
Figure 4. CAT (A) and SOD (B) enzyme activity in bee abdomen extracts; n. s. – the non-significant difference between tested groups. Control – bees from colonies fed with APIVITAL® sugar syrup. Algae – bees from colonies fed with 10% (w/v) Chlorella powder in APIVITAL® sugar syrup.
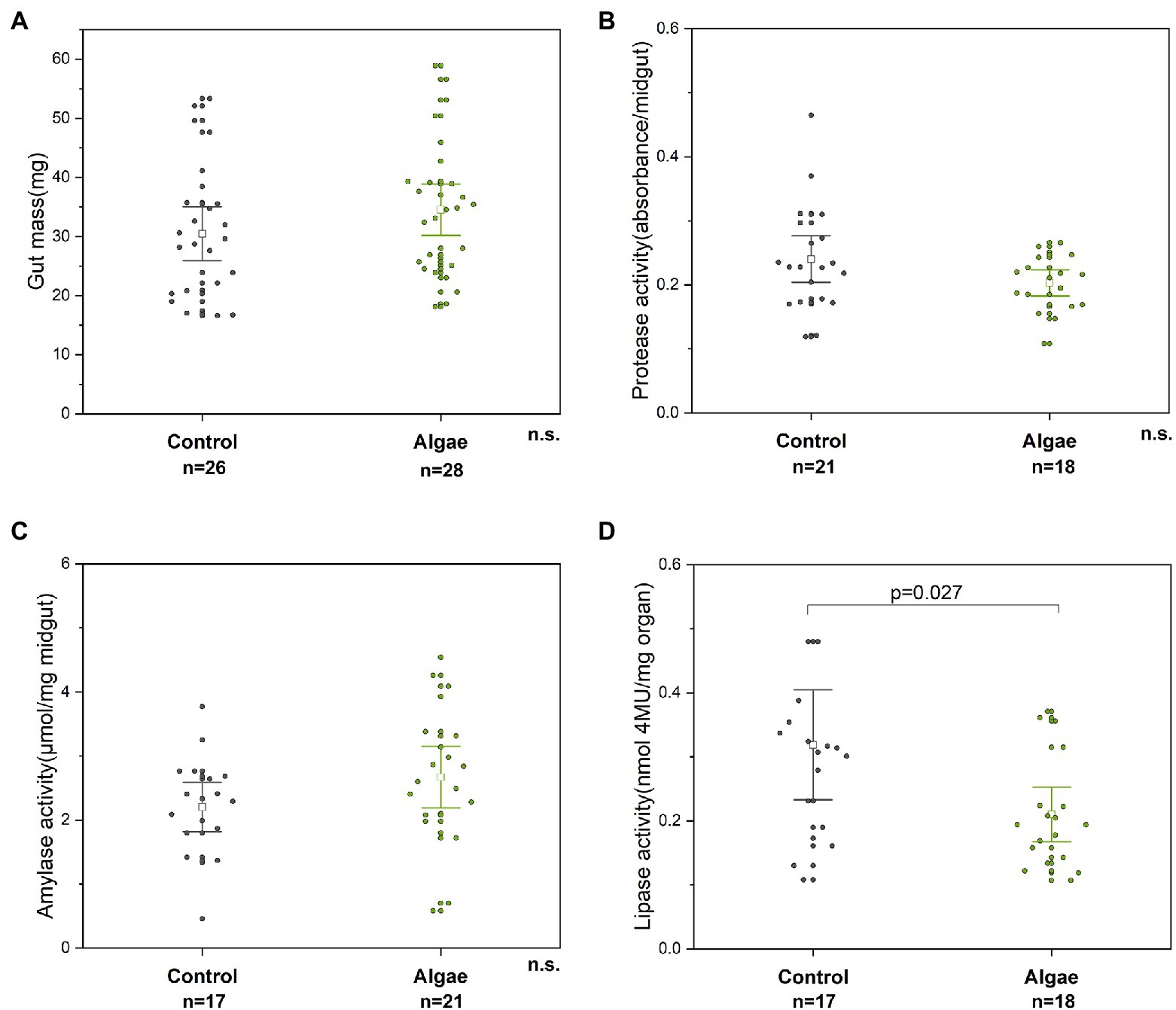
Figure 5. Parameters of the digestive system, specifically midgut mass (A), the activities of protease (B), amylase (C), and lipase (D). n. s. – non-significant difference. Control – bees from colonies fed with APIVITAL® sugar syrup. Algae – bees from colonies fed with 10% (w/v) Chlorella powder in APIVITAL® sugar syrup.
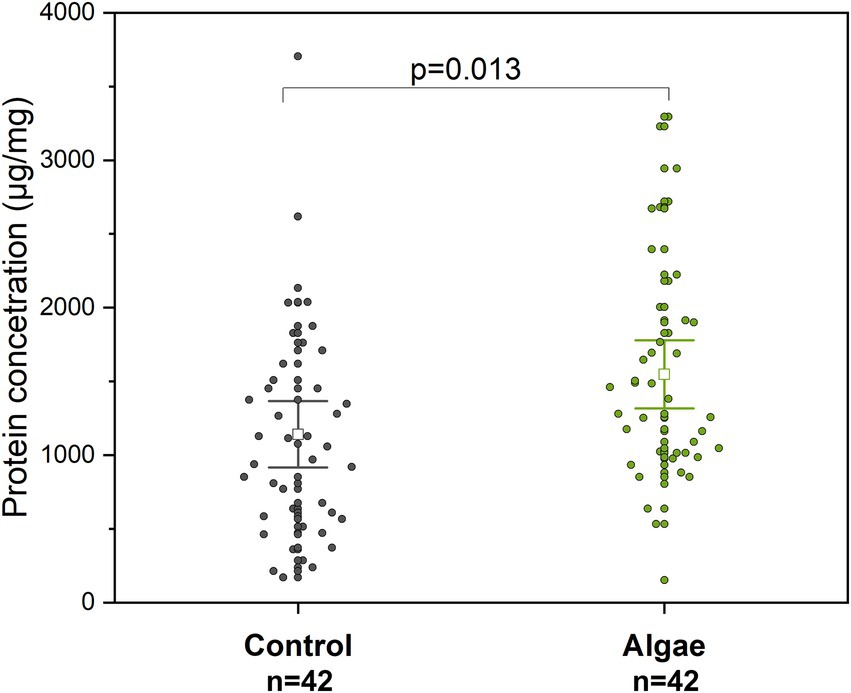
Figure 6. Protein concentration in extracts from adult bee abdomens. Control – bees from colonies fed with APIVITAL® sugar syrup. Algae – bees from colonies fed with 10% (w/v) Chlorella powder in APIVITAL® sugar syrup.
Due to multiple testing, we used Bonferroni correction, with the level of significance set to α = 0.004. The p-values (p < 0.05) were added into graphs for informative reasons. The statistical power was calculated for each statistical test, for details see Supplementary Table S2. The number of biological replicates is noted in the individual graphs; the colonies are represented equally in each group.
During the feeding experiment, it was observed that bees collected the Chlorella-enriched syrup and stored it similarly to honey (Figure 7A). Notably, adult bee workers directly transferred Chlorella to larvae as part of their diet, which could be inferred by the green colour of the larval midgut (Figure 7B). The uptake of Chlorella syrup was also clearly visible by the green colour of dissected honey crops (Figure 7C).
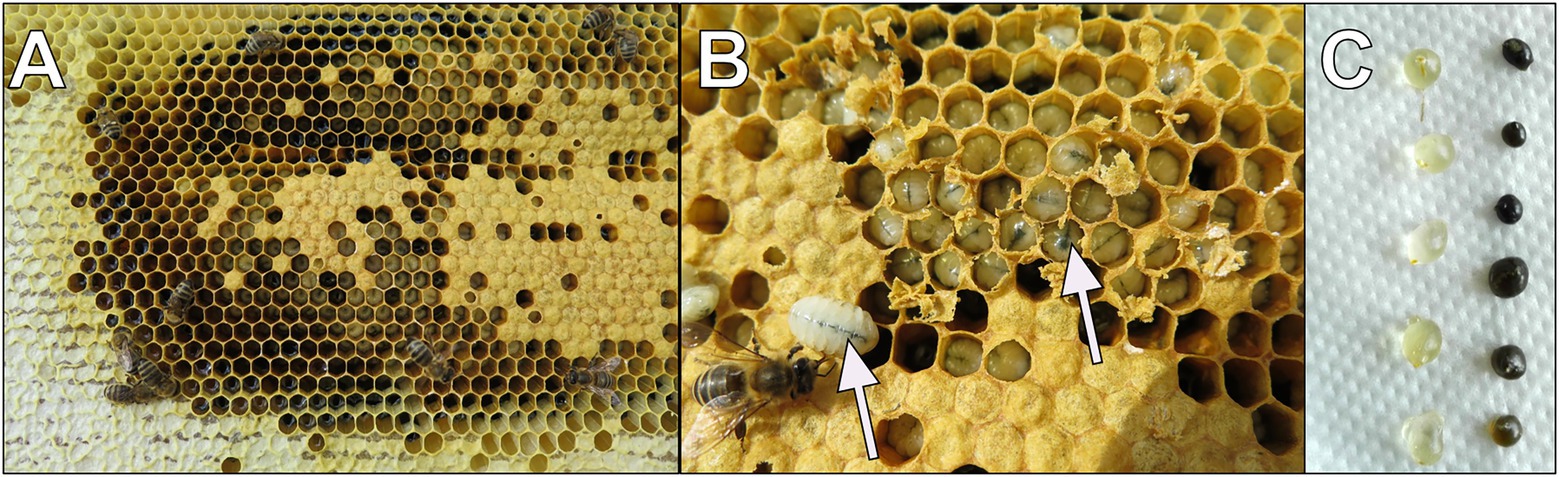
Figure 7. Documentation of the consumption of Chlorella in experimental hives. (A) Example of stored Chlorella syrup in wax combs; (B) Green color of the larval midgut due to Chlorella syrup consumption marked with arrows; (C) Honey crops dissected from adult bees with a visible green color in Chlorella fed group (right column).
To determine the effect of the algal supplement on the bee digestive system, the weight of honey bee midgut and activity of digestive enzymes were measured in bees from colonies either fed Chlorella syrup or a control diet. After the two-week feeding period, no effect was observed on the weight of the midgut mass of bees from Chlorella-fed colonies as compared to control bees (t-test, p = 0.188, df = 52) (Figure 5A). Similarly, activities of amylase and protease enzymes were not influenced by the consumption of Chlorella-enriched syrup (amylase, t-test, p = 0.221, df = 36; protease, p = 0.230, df = 37) (Figures 5B,C). Interestingly, the activity of lipase in worker midgut was higher in bees from control colonies (about 1.5-fold) than that in bees from colonies fed with Chlorella (t-test, p = 0.027, df = 33) (Figure 5D), however the difference was not statistically significant.
Extracts prepared from the abdomens of adult bees from Chlorella-fed colonies showed higher protein concentration (t-test, p = 0.013, df = 82) than the control group (Figure 6). The average concentration of proteins in extracts from abdomens was 1140.8 ± 723.4 μg/ml for bees from the Control group and 1548.1 ± 742.9 μg/ml for the Chlorella-fed group which indicates about a 1.4-fold insignificant increase.
The Chlorella supplemented diet did not influence the Vg level in the hemolymph of honey bee larvae (Mann–Whitney U-test, p = 0.317, Z = −1) (Figure 1A). On the other hand, the relative level of Vg in the adult hemolymph was significantly higher (about 3 times) in the group fed with Chlorella (Mann–Whitney U-test, p = 4 × 10−6, Z = −4.629) (Figure 1B) than in controls fed with sugar solution only. The Vg level in larvae was several 100 times lower than that in adults.
The relative gene expression of Vg gene transcripts was not significantly different between the control group and the Chlorella-fed group in adult bees (Mann–Whitney U test, p = 0.178 Z = -1.348) and in larvae (Mann–Whitney U test, p = 0.0577, Z = -1.898), Figure 2. Similarly to Vg protein abundance, the expression of the Vg gene is around three magnitudes lower compared to adults.
The relative expression of genes for antioxidant enzymes (CAT, SOD1, SOD2) was measured in larvae and adult bees. No significant differences between the control and the group of the experimental larvae from Chlorella-fed colonies were observed for all genes tested (Mann–Whitney U test, CAT p = 0.679, Z = 0.414; SOD1 p = 0.137 Z = −1.484; SOD2 p = 0.952, Z = 0.061) (Figures 3A,C,E). However, adult workers from colonies fed with Chlorella syrup showed insignificantly higher relative gene expression of CAT (Mann–Whitney U-test, p = 0.017, Z = −2.390) (Figure 3B) and SOD1 (Mann–Whitney U-test, p = 0.044, Z = −2.016) (Figure 3D) in comparison to control bees. SOD2 gene expression showed no differences between the two experimental groups (Mann–Whitney U-test, p = 0.802, Z = −0.250) (Figure 3F).
The activity of antioxidant enzymes CAT and SOD were determined in abdomens of honey bee workers with removed midgut. No differences in activity of CAT and SOD were detected within two experimental groups tested (Mann–Whitney U test, p = 0.450, Z = 0.747 for CAT in abdomens; and t-test, p = 0.219, df = 81 for SOD in abdomens) (Figure 4).
The alga Chlorella was previously described as a promising nutritional source for honey bees (Ricigliano, 2020) with a positive effect on their development and physiology (Eremia et al., 2013; Jehlík et al., 2019). As the potential for using algae as nutrient supplementation for honey bees and other livestock is of increasing interest (Madeira et al., 2017; Wells et al., 2017; Nethravathy et al., 2019), we aimed to perform a fine-scale assessment of metabolic modulations, including antioxidant enzymes, digestive enzymes, and Vg levels. Our results complement previous studies on the colony and individual levels (Al-Saif et al., 2014; Jehlík et al., 2019) to provide a more holistic evaluation of the suitability of Chlorella supplementation for bee health and vitality. The feeding trial was performed in August 2019, which is at the end of beekeeping season when pollen, nectar, or honeydew sources are very limited in this region, thus effects caused by Chlorella supplementation should not be influenced by other nutritional resources. Importantly, this is also a relevant time for supplementation from a colony management perspective, as during this period of dearth, beekeepers feed proteinaceous supplements with expectations to improve bee health prior to overwintering (Mattila and Otis, 2007).
Honey bee colonies accepted the alga syrup well with a positive effect on their development, as previously reported for Chlorella by Eremia et al. (2013) and Jehlík et al. (2019) as well as Arthrospira platensis (spirulina) (Ricigliano and Simone-Finstrom, 2020). In our experiment, the syrup was stored in honeycombs, which was clearly visible because of the typical green colour of the syrup. Consumption of algae was confirmed with the colour difference of larvae gut and honey bee crop. Ehrenberg et al. (2019) used synthetic compounds, such as Coomassie Brilliant Blue, as colourful dyes suitable for in vitro assays of food intake in honey bee larvae. We hypothesize, that Chlorella in moderate concentrations could be used as an effective, low-cost and environment-friendly dye, e.g., in feed intake and in hive storage related studies.
From our results, it is evident that there is no significant impact of Chlorella supplementation in the doses used on the digestive system of bees, with no differences found in midgut mass, protease, amylase activity, and lipase activity. We expected higher protease activity in midguts after Chlorella applications due to the higher amount of proteins in alga feed. Previous research on protein supplements detected higher protease activity in bees fed with high concentration of crude protein in a supplement compared to rape pollen (Li et al., 2012). This fact is explained by Moritz and Crailsheim (1987) who found correlation between the amounts of protein in the endo-peritrophic space and protease activity (i.e., trypsin-like and chymotrypsin-like). Pollen grains are enveloped by a polysaccharide protective wall which is probably connected to complicated digestibility and subsequent lower amount of soluble proteins (Moritz and Crailsheim, 1987). Therefore our findings showed at least similar capacity of proteolytic degradation in midguts of Chlorella supplemented bees compared to non-Chlorella control. Interestingly, lipase activity was slightly but not significantly reduced in the midgut of the Chlorella fed bees, in agreement with previous research (Jehlík et al., 2019). Corby-Harris et al. (2019) detected higher lipase activity in bees deprived of pollen. Pollen-fed bees had lower activity of lipases suggesting that Chlorella is a suitable source of nutrition. Additionally, it has been described that organic extracts from microalgae have antimicrobial activity. Hence, microalgae-based feed could have a prophylactic properties for honey bee colonies (Catarina Guedes et al., 2011; Salem et al., 2014; Dostálková et al., 2021).
Metabolic processes in aerobic organisms, including insects, are associated with the production of low physiological levels of ROS which can be efficiently regulated by the enzyme and non-enzyme components of antioxidant system (Felton and Summers, 1995; Corona and Robinson, 2006; Sies et al., 2017). Exposure of honey bees to xenobiotic compounds, such as pesticides or herbicides, often results in increased ROS production, with subsequent mobilization of antioxidant enzymes (Chakrabarti et al., 2015; Balieira et al., 2018; Paleolog et al., 2021). When focusing on parameters of oxidative stress in Chlorella fed bees, we randomly sampled workers from inside the colony. Our results did not show any significant differences in CAT and SOD enzyme activities in abdomens of control and experimental groups. Based on our results, we could hypothesize that CAT and SOD enzyme activity is sufficient for degradation of ROS in both experimental groups. Alternatively, CAT and SOD does not play significant role in ROS degradation compared to other mechanisms involved in anti-oxidative stress response (Sies et al., 2017).
No differences were also observed in Chlorella-fed bees expression patterns of SOD and CAT genes. Previously, changes in CAT and SOD1 and SOD2 gene expression levels after feeding bees with a high protein content diet were described by Li et al. (2014). The authors observed that elevated gene expression of antioxidant genes can positively affect the longevity of adult honey bees. A higher expression rate of CAT was also observed for colonies surrounded by non-agricultural forage (Ricigliano et al., 2019). No significant difference was recorded for CAT, SOD1, and SOD2 gene expression in larvae, despite showing green material in their digestive tracts. Larvae are fed with Chlorella indirectly, nurse bees mix royal jelly, a product of hypopharyngeal glands of workers, with Chlorella similarly as pollen (Crailsheim et al., 2013). Consequently the concentration of Chlorella in larval food is probably decreased. Larval food additionally contains other components such as honey, which is know for its antioxidant properties (Erejuwa et al., 2012).
Both groups of colonies were kept in one apiary with the same opportunities to forage. An insignificantly higher protein (1.4-fold) content was observed in abdomens of adult workers fed by alga diet. This finding is likely indicative of the much higher protein content in alga (about 50% in dry powder) than that in common pollen (7–35%) (data from the Centre Algatech, Institute of Microbiology CAS, Třeboň, Czech Republic). Supplementation of colonies with easy digestible protein-rich diet induces higher protein production in honey bees (Paiva et al., 2019). Higher total protein concentrations in hemolymph in September before wintering is positively correlated with the population size of colonies in the following spring (Smart et al., 2019). Thus, feeding colonies with soluble protein-based food, could increase the protein concentration in honey bee hemolymph and improve the fitness of colonies (De Jong et al., 2009; Morais et al., 2013; Watkins de Jong et al., 2019).
Our results are in agreement with the expected positive impact of Chlorella food importantly on the Vg protein level. Previous studies found that Vg production is stimulated by a protein-rich diet and increased levels of Vg are connected with extended longevity (Bitondi and Simoes, 1996; Havukainen et al., 2013; Aurori et al., 2014) and successful overwintering at the colony level (Smart et al., 2019; Ricigliano and Simone-Finstrom, 2020). We detected a 3-fold higher concentration of Vg in hemolymph of Chlorella fed bees which we attribute to the large number of nutrients that algae contain.
In previous laboratory studies, consumption of algal-based food by bees positively stimulated Vg gene expression in adult workers compared to plain sucrose food (Ricigliano and Simone-Finstrom, 2020; Jang et al., 2022). Elevated Vg expression corresponded to the pollen rich diet, therefore we conclude that in our study where bees had access to stored or naturally occurring pollen, no differences in Vg expression indicate similar effect as pollen or algal-based food consumption as in vitro. Jehlík et al. (2019) showed significantly higher Vg gene expression after in vivo application of Chlorella in winter, we explain these differences by various tissues used for the determination of gene expression. In the study of Jehlík et al. (2019) only honey bee heads were used for RNA extraction compared to whole bee body RNA extraction in the present study as we assumed Vg expression in the abdominal fat body (Corona et al., 2007). Production of larval Vg and even Vg gene expression was not influenced in the Chlorella-fed group; we have not any satisfactory explanation for this, because a real role of Vg in larvae is not fully understood.
Application of Chlorella-based pollen supplements represents an attractive measure in the current beekeeping practice. It has the potential to replace the traditional pollen supplements prepared from soya, yeast, wheat, or lentils. Although, the importance of ROS production and its regulation by antioxidant system in maintaining balanced bee homeostasis has been recognized; the actual knowledge on the effects of bee food supplements to ROS production is very limited. Chlorella-based supplements have been previously studied from the point of improving colony fitness or effects to nutritional markers such as levels of vitellogenin. Collectively, our results corroborate the expected positive effects of Chlorella-supplemented syrup to bee nutritional status assessed as the protein content in adult bees. Additionally, we brought the first evidence that feeding Chlorella to honey bee colonies does not elicit increased ROS levels. Further studies should be directed to evaluations of optimal formulations, dosages and timing of Chlorella-based supplements. More detailed studies on molecular mechanisms of the digestion and utilization of algal cellular components by different developmental stages and casts of bees are also necessary. The results should provide a solid foundation and guidelines for further implementation of Chlorella as an effective and safe pollen substitute in apiculture practice.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
JD, MS-F, DK, MP, SD: conceptualization. JD, DK, MS-F: methodology. SD, JD, and DK: performed the analysis and data collection. JD and SD: data curation and writing—original draft. SD: visualization. MP and DK: supervision. MP: project administration. DK, MSF, and MP: writing—review and editing. All authors contributed to the article and approved the submitted version.
This research was funded by the Inter-Action program of the Ministry of Education, Youth and Sport of the Czech Republic (grant no. LTAUSA17116).
We acknowledge the technical assistance of Zuzana Žvátorová and Helena Štěrbová in laboratory measurements, and Petra Urajová for providing the Chlorella powder.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1028037/full#supplementary-material
Aebi, H. (1984). Catalase in vitro. Methods Enzymol. 105, 121–126. doi: 10.1016/s0076-6879(84)05016-3
Ahmad, S., and Pardini, R. S. (1990). Mechanisms for regulating oxygen toxicity in phytophagous insects. Free Radic. Biol. Med. 8, 401–413. doi: 10.1016/0891-5849(90)90107-t
Ahmad, S., Pritsos, C. A., Bowen, S. M., Heisler, C. R., Blomquist, G. J., and Pardini, R. S. (1988). Antioxidant enzymes of larvae of the cabbage looper moth, Trichoplusia ni: subcellular distribution and activities of superoxide dismutase, catalase and glutathione reductase. Free Radic. Res. Commun. 4, 403–408. doi: 10.3109/10715768809066908
Alaux, C., Allier, F., Decourtye, A., Odoux, J. F., Tamic, T., Chabirand, M., et al. (2017). A 'Landscape physiology' approach for assessing bee health highlights the benefits of floral landscape enrichment and semi-natural habitats. Sci. Rep. 7:40568. doi: 10.1038/srep40568
Alaux, C., Ducloz, F., Crauser, D., and Le Conte, Y. (2010). Diet effects on honeybee immunocompetence. Biol. Lett. 562–565. doi: 10.1098/rsbl.2009.0986
Al-Saif, S. S., Abdel-Raouf, N., El-Wazanani, H. A., and Aref, I. A. (2014). Antibacterial substances from marine algae isolated from Jeddah coast of Red Sea Saudi Arabia. Saudi J. Biol. Sci. 21, 57–64. doi: 10.1016/j.sjbs.2013.06.001
Amdam, G. V., Aase, A. L., Seehuus, S. C., Kim Fondrk, M., Norberg, K., and Hartfelder, K. (2005). Social reversal of immunosenescence in honey bee workers. Exp. Gerontol. 40, 939–947. doi: 10.1016/j.exger.2005.08.004
Annoscia, D., Zanni, V., Galbraith, D., Quirici, A., Grozinger, C., Bortolomeazzi, R., et al. (2017). Elucidating the mechanisms underlying the beneficial health effects of dietary pollen on honey bees (Apis mellifera) infested by Varroa mite ectoparasites. Sci. Rep. 7:6258. doi: 10.1038/s41598-017-06488-2
Aurori, C. M., Buttstedt, A., Dezmirean, D. S., Marghitas, L. A., Moritz, R. F., and Erler, S. (2014). What is the main driver of ageing in long-lived winter honeybees: antioxidant enzymes, innate immunity, or vitellogenin? J. Gerontol. A Biol. Sci. Med. Sci. 69, 633–639. doi: 10.1093/gerona/glt134
Balieira, K. V. B., Mazzo, M., Bizerra, P. F. V., Guimarães, A. R. D. J. S., Nicodemo, D., and Mingatto, F. E. (2018). Imidacloprid-induced oxidative stress in honey bees and the antioxidant action of caffeine. Apidologie 49, 562–572. doi: 10.1007/s13592-018-0583-1
Bitondi, M. M. G., and Simoes, Z. L. P. (1996). The relationship between level of pollen in the diet, vitellogenin and juvenile hormone titres in Africanized Apis mellifera workers. J. Apic. Res. 35, 27–36.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1006/abio.1976.9999
Branchiccela, B., Castelli, L., Corona, M., Díaz-Cetti, S., Invernizzi, C., Martínez de la Escalera, G., et al. (2019). Impact of nutritional stress on the honeybee colony health. Sci. Rep. 9:10156. doi: 10.1038/s41598-019-46453-9
Brodschneider, R., and Crailsheim, K. (2010). Nutrition and health in honey bees. Apidologie 41, 278–294. doi: 10.1051/apido/2010012
Catarina Guedes, A., Barbosa, C. R., Amaro, H. M., Pereira, C. I., and Xavier Malcata, F. (2011). Microalgal and cyanobacterial cell extracts for use as natural antibacterial additives against food pathogens. Int. J. Food Sci. Technol. 46, 862–870. doi: 10.1111/j.1365-2621.2011.02567.x
Chakrabarti, P., Rana, S., Sarkar, S., Smith, B., and Basu, P. (2015). Pesticide-induced oxidative stress in laboratory and field populations of native honey bees along intensive agricultural landscapes in two eastern Indian states. Apidologie 46, 107–129. doi: 10.1007/s13592-014-0308-z
Corby-Harris, V., Snyder, L., and Meador, C. (2019). Fat body lipolysis connects poor nutrition to hypopharyngeal gland degradation in Apis mellifera. J. Insect Physiol. 116, 1–9. doi: 10.1016/j.jinsphys.2019.04.001
Corona, M., and Robinson, G. E. (2006). Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect Mol. Biol. 15, 687–701. doi: 10.1111/j.1365-2583.2006.00695.x
Corona, M., Velarde, R. A., Remolina, S., Moran-Lauter, A., Wang, Y., Hughes, K. A., et al. (2007). Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. U. S. A. 104, 7128–7133. doi: 10.1073/pnas.0701909104
Crailsheim, K., Brodschneider, R., Aupinel, P., Behrens, D., Genersch, E., Vollmann, J., et al. (2013). Standard methods for artificial rearing of Apis mellifera larvae. J. Apic. Res. 52, 1–16. doi: 10.3896/ibra.1.52.1.05
Danihlik, J., Skrabisova, M., Lenobel, R., Sebela, M., Omar, E., Petrivalsky, M., et al. (2018). Does the pollen diet influence the production and expression of antimicrobial peptides in individual honey bees? Insects 9. doi: 10.3390/insects9030079
De Jong, D., da Silva, E. J., Kevan, P. G., and Atkinson, J. L. (2009). Pollen substitutes increase honey bee haemolymph protein levels as much as or more than does pollen. J. Apic. Res. 48, 34–37. doi: 10.3896/IBRA.1.48.1.08
Degrandi-Hoffman, G., Corby-Harris, V., Chen, Y., Graham, H., Chambers, M., Watkins de Jong, E., et al. (2020). Can supplementary pollen feeding reduce varroa mite and virus levels and improve honey bee colony survival? Exp. Appl. Acarol. 82, 1–19. doi: 10.1007/s10493-020-00562-7
Di Pasquale, G., Alaux, C., Le Conte, Y., Odoux, J. F., Pioz, M., Vaissiere, B. E., et al. (2016). Variations in the availability of pollen resources affect honey bee health. PLoS One 11:e0162818. doi: 10.1371/journal.pone.0162818
Di Pasquale, G., Salignon, M., Le Conte, Y., Belzunces, L. P., Decourtye, A., Kretzschmar, A., et al. (2013). Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS One 8:e72016. doi: 10.1371/journal.pone.0072016
Dostálková, S., Urajová, P., Činčárová, D., Vránová, T., Hrouzek, P., Petřivalský, M., et al. (2021). Fatty acids and their derivatives from Chlorella vulgaris extracts exhibit in vitro antimicrobial activity against the honey bee pathogen Paenibacillus larvae. J. Apic. Res. 1-13. doi: 10.1080/00218839.2021.1994264
Ehrenberg, S., Lewkowski, O., and Erler, S. (2019). Dyeing but not dying: Colourful dyes as a non-lethal method of food labelling for in vitro-reared honey bee (Apis mellifera) larvae. J. Insect Physiol. 113, 1–8. doi: 10.1016/j.jinsphys.2018.12.008
Elpidina, E. N., Vinokurov, K. S., Gromenko, V. A., Rudenskaya, Y. A., Dunaevsky, Y. E., and Zhuzhikov, D. P. (2001). Compartmentalization of proteinases and amylases in Nauphoeta cinerea midgut. Arch. Insect Biochem. Physiol. 48, 206–216. doi: 10.1002/arch.10000
Erejuwa, O. O., Sulaiman, S. A., and Ab Wahab, M. S. (2012). Honey: a novel antioxidant. Molecules 17, 4400–4423. doi: 10.3390/molecules17044400
Eremia, N., Bahcivanji, M., and Zagareanu, A. (2013). Study of influence of algal "chlorella vulgaris" suspension on growth and productivity of bees families. Lucrări Științifice - Universitatea de Științe Agricole și Medicină Veterinară, Seria Zootehnie 59, 148–152.
Felton, G. W., and Summers, C. B. (1995). Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 29, 187–197. doi: 10.1002/arch.940290208
Fluri, P., Lüscher, M., Wille, H., and Gerig, L. (1982). Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. J. Insect Physiol. 28, 61–68. doi: 10.1016/0022-1910(82)90023-3
Frias, B. E. D., Barbosa, C. D., and Lourenco, A. P. (2016). Pollen nutrition in honey bees (Apis mellifera): impact on adult health. Apidologie 47, 15–25. doi: 10.1007/s13592-015-0373-y
Goiris, K., Muylaert, K., Fraeye, I., Foubert, I., De Brabanter, J., and De Cooman, L. (2012). Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 24, 1477–1486. doi: 10.1007/s10811-012-9804-6
Goulson, D., Nicholls, E., Botias, C., and Rotheray, E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957. doi: 10.1126/science.1255957
Gray, A., Brodschneider, R., Adjlane, N., Ballis, A., Brusbardis, V., Charrire, J. D., et al. (2019). Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J. Apic. Res. 58, 479–485. doi: 10.1080/00218839.2019.1615661
Havukainen, H., Munch, D., Baumann, A., Zhong, S., Halskau, O., Krogsgaard, M., et al. (2013). Vitellogenin recognizes cell damage through membrane binding and shields living cells from reactive oxygen species. J. Biol. Chem. 288, 28369–28381. doi: 10.1074/jbc.M113.465021
Jang, H., Ghosh, S., Sun, S., Cheon, K., Mohamadzade Namin, S., and Jung, C. (2022). Chlorella-supplemented diet improves the health of honey bee (Apis mellifera). Front. Ecol. Evol. 10:922741. doi: 10.3389/fevo.2022.922741
Jehlík, T., Kodrík, D., Krištůfek, V., Koubová, J., Sábová, M., Danihlík, J., et al. (2019). Effects of chlorella sp. on biological characteristics of the honey bee Apis mellifera. Apidologie 50, 564–577. doi: 10.1007/s13592-019-00670-3
Kodrik, D., Vinokurov, K., Tomcala, A., and Socha, R. (2012). The effect of adipokinetic hormone on midgut characteristics in Pyrrhocoris apterus L. (Heteroptera). J. Insect Physiol. 58, 194–204. doi: 10.1016/j.jinsphys.2011.11.010
Koubová, J., Sábová, M., Brejcha, M., Kodrík, D., and Capkova Frydrychova, R. (2021). Seasonality in telomerase activity in relation to cell size, DNA replication, and nutrients in the fat body of Apis mellifera. Sci. Rep. 11. doi: 10.1038/s41598-020-79912-9
Kulhanek, K., Steinhauer, N., Rennich, K., Caron, D. M., Sagili, R. R., Pettis, J. S., et al. (2017). A national survey of managed honey bee 2015-2016 annual colony losses in the USA. J. Apic. Res. 56, 328–340. doi: 10.1080/00218839.2017.1344496
Li, H.-B., Cheng, K.-W., Wong, C.-C., Fan, K.-W., Chen, F., and Jiang, Y. (2007). Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 102, 771–776. doi: 10.1016/j.foodchem.2006.06.022
Li, C., Xu, B., Wang, Y., Feng, Q., and Yang, W. (2012). Effects of dietary crude protein levels on development, antioxidant status, and total midgut protease activity of honey bee (Apis mellifera ligustica). Apidologie 43, 576–586. doi: 10.1007/s13592-012-0126-0
Li, C. C., Xu, B. H., Wang, Y. X., Yang, Z. B., and Yang, W. R. (2014). Protein content in larval diet affects adult longevity and antioxidant gene expression in honey bee workers. Entomol. Exp. Appl. 151, 19–26. doi: 10.1111/eea.12167
Madeira, M. S., Cardoso, C., Lopes, P. A., Coelho, D., Afonso, C., Bandarra, N. M., et al. (2017). Microalgae as feed ingredients for livestock production and meat quality: a review. Livest. Sci. 205, 111–121. doi: 10.1016/j.livsci.2017.09.020
Marklund, S., and Marklund, G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47, 469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x
Mattila, H. R., and Otis, G. W. (2007). Manipulating pollen supply in honey bee colonies during the fall does not affect the performance of winter bees. Can. Entomol. 139, 554–563. doi: 10.4039/n06-032
Meixner, M. D., and Le Conte, Y. (2016). A current perspective on honey bee health. Apidologie 47, 273–275. doi: 10.1007/s13592-016-0449-3
Morais, M. M., Turcatto, A. P., Pereira, R. A., Francoy, T. M., Guidugli-Lazzarini, K. R., Goncalves, L. S., et al. (2013). Protein levels and colony development of Africanized and European honey bees fed natural and artificial diets. Genet. Mol. Res. 12, 6915–6922. doi: 10.4238/2013.December.19.10
Moritz, B., and Crailsheim, K. (1987). Physiology of protein digestion in the midgut of the honeybee (Apis mellifera L.). J. Insect Physiol. 33, 923–931. doi: 10.1016/0022-1910(87)90004-7
Nethravathy, M. U., Mehar, J. G., Mudliar, S. N., and Shekh, A. Y. (2019). Recent advances in microalgal bioactives for food, feed, and healthcare products: commercial potential, market space, and sustainability. Compr. Rev. Food Sci. Food Saf. 18, 1882–1897. doi: 10.1111/1541-4337.12500
Paiva, J. P. L. M., Esposito, E., Honorato, D. M., De Souza, G. I., Francoy, T. M., and Morais, M. M. (2019). Effects of ensiling on the quality of protein supplements for honey bees Apis mellifera. Apidologie 50, 414–424. doi: 10.1007/s13592-019-00661-4
Paleolog, J., Wilde, J., Miszczak, A., Gancarz, M., and Strachecka, A. (2021). Antioxidation defenses of Apis mellifera Queens and workers respond to Imidacloprid in different age-dependent ways: old Queens are resistant foragers are not. Animals 11:1246.
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. doi: 10.1093/nar/29.9.e45
Pfaffl, M., Tichopad, A., Prgomet, C., and Neuvians, T. (2004). Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – excel-based tool using pair-wise correlations. Biotechnol. Lett. 26, 509–515. doi: 10.1023/b:bile.0000019559.84305.47
Potts, S. G., Biesmeijer, J. C., Kremen, C., Neumann, P., Schweiger, O., and Kunin, W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
Ricigliano, V. A. (2020). Microalgae as a promising and sustainable nutrition source for managed honey bees. Arch. Insect Biochem. Physiol. 104:e21658. doi: 10.1002/arch.21658
Ricigliano, V. A., Dong, C., Richardson, L. T., Donnarumma, F., Williams, S. T., Solouki, T., et al. (2021). Honey bee proteome responses to plant and cyanobacteria (blue-green algae) diets. ACS Food Sci. Technol. 1, 17–26. doi: 10.1021/acsfoodscitech.0c00001
Ricigliano, V. A., Mott, B. M., Maes, P. W., Floyd, A. S., Fitz, W., Copeland, D. C., et al. (2019). Honey bee colony performance and health are enhanced by apiary proximity to US conservation reserve program (CRP) lands. Sci. Rep. 9:4894. doi: 10.1038/s41598-019-41281-3
Ricigliano, V. A., and Simone-Finstrom, M. (2020). Nutritional and prebiotic efficacy of the microalga Arthrospira platensis (spirulina) in honey bees. Apidologie 51, 898–910. doi: 10.1007/s13592-020-00770-5
Ricigliano, V. A., Williams, S. T., and Oliver, R. (2022). Effects of different artificial diets on commercial honey bee colony performance, health biomarkers, and gut microbiota. BMC Vet. Res. 18:52. doi: 10.1186/s12917-022-03151-5
Roberts, I. M. (1985). Hydrolysis of 4-methylumbelliferyl butyrate: a convenient and sensitive fluorescent assay for lipase activity. Lipids 20, 243–247. doi: 10.1007/BF02534195
Roussel, M., Villay, A., Delbac, F., Michaud, P., Laroche, C., Roriz, D., et al. (2015). Antimicrosporidian activity of sulphated polysaccharides from algae and their potential to control honeybee nosemosis. Carbohydr. Polym. 133, 213–220. doi: 10.1016/j.carbpol.2015.07.022
Salem, O., Hoballah, E. M., Ghazi, M., Safia,, Hanna, N., and Suzy, (2014). Antimicrobial activity of microalgal extracts with special emphasize on Nostoc sp. Life Sci. J. 11, 752–758.
Salmela, H., and Sundström, L. (2018). Vitellogenin in inflammation and immunity in social insects. Inflamm. Cell Signal. 5:e1506. doi: 10.14800/ics.1506
Scofield, H. N., and Mattila, H. R. (2015). Honey bee workers that are pollen stressed as larvae become poor foragers and waggle dancers as adults. PLoS One 10:e0121731. doi: 10.1371/journal.pone.0121731
Seehuus, S. C., Norberg, K., Gimsa, U., Krekling, T., and Amdam, G. V. (2006). Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 103, 962–967. doi: 10.1073/pnas.0502681103
Sies, H., Berndt, C., and Jones, D. P. (2017). Oxidative stress. Annu. Rev. Biochem. 86, 715–748. doi: 10.1146/annurev-biochem-061516-045037
Smart, M. D., Otto, C. R. V., and Lundgren, J. G. (2019). Nutritional status of honey bee (Apis mellifera L.) workers across an agricultural land-use gradient. Sci. Rep. 9:16252. doi: 10.1038/s41598-019-52485-y
Smart, M., Pettis, J., Rice, N., Browning, Z., and Spivak, M. (2016). Linking measures of Colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS One 11:e0152685. doi: 10.1371/journal.pone.0152685
Watkins de Jong, E., Degrandi-Hoffman, G., Chen, Y., Graham, H., and Ziolkowski, N. (2019). Effects of diets containing different concentrations of pollen and pollen substitutes on physiology, Nosema burden, and virus titers in the honey bee (Apis mellifera L.). Apidologie 50. doi: 10.1007/s13592-019-00695-8
Weirich, G. F., Collins, A. M., and Williams, V. P. (2002). Antioxidant enzymes in the honey bee Apis mellifera. Apidologie 33, 3–14. doi: 10.1051/apido:2001001
Wells, M. L., Potin, P., Craigie, J. S., Raven, J. A., Merchant, S. S., Helliwell, K. E., et al. (2017). Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycol. 29, 949–982. doi: 10.1007/s10811-016-0974-5
Wright, G. A., Nicolson, S. W., and Shafir, S. (2018). Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 63, 327–344. doi: 10.1146/annurev-ento-020117-043423
Keywords: pollen supplement, microalgae, oxidative stress, beekeeping, malnutrition
Citation: Dostálková S, Kodrík D, Simone-Finstrom M, Petřivalský M and Danihlík J (2022) Fine-scale assessment of Chlorella syrup as a nutritional supplement for honey bee colonies. Front. Ecol. Evol. 10:1028037. doi: 10.3389/fevo.2022.1028037
Received: 25 August 2022; Accepted: 09 November 2022;
Published: 01 December 2022.
Edited by:
Carla Mucignat, University of Padua, ItalyReviewed by:
Eirik Søvik, Volda University College, NorwayCopyright © 2022 Dostálková, Kodrík, Simone-Finstrom, Petřivalský and Danihlík. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiří Danihlík, amlyaS5kYW5paGxpa0B1cG9sLmN6
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.