
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 23 November 2022
Sec. Conservation and Restoration Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.1020703
This article is part of the Research TopicHuman-Wildlife Conflicts: Consequences of the Erosion of the Available Habitat, Introduction of Alien Species, and AnthropizationView all 5 articles
 Yunrui Ji1,2
Yunrui Ji1,2 Xuelei Wei1,2
Xuelei Wei1,2 Fang Liu1,2*
Fang Liu1,2* Diqiang Li1,2*
Diqiang Li1,2* Jiahua Li3
Jiahua Li3 Xiangyuan Huang4
Xiangyuan Huang4 Jiajiang Jiang4
Jiajiang Jiang4 Jianyan Tang3
Jianyan Tang3Conflicts between humans and Asiatic black bears (Ursus thibetanus) are widespread in Asia and pose challenges to human-bear coexistence. Identifying effective mitigation measures requires a thorough understanding of human-bear conflicts (HBC). We assessed spatial-temporal patterns of HBC and their impact factors around the Baoshan Section of the Gaoligongshan Nature Reserve (GNNR) between 2012 and 2020. The results suggested that crop raiding by bears occurred most commonly, followed by beehive loss, livestock depredation, and human casualties. HBC hotspots occurred near the protected area where local people frequently encountered bears. The landscapes with lower elevation and human density were at higher risk of HBC. Furthermore, villages with more fragmented forests or less fragmented croplands were more vulnerable to HBC. The differences in agricultural structures contributed to the diverse composition of HBC between the two regions. In addition, crop raiding by bears decreased significantly, probably due to the changing landscape composition and configuration derived from human behaviors, yet livestock depredation and beehive loss increased. Our findings indicated the complex interrelationship between the environment, bears, and humans, which could guide the implementation of mitigation measures. We recommend multiple approaches based on a social-ecological system to mitigate HBC.
Human-carnivore conflicts occur when the needs of wildlife or humans negatively impact each other (Xu et al., 2019; Zimmermann et al., 2020). These conflicts have existed since prehistory and persist today in areas where humans and carnivores share landscapes (Honda, 2009; Can et al., 2014; Bhatia et al., 2020). However, exacerbated human activities reduce and fragment wildlife habitats, threatening numerous endangered animals (Laurance et al., 2000; Liu et al., 2019). These species have to live near humans in fragmented natural areas, leading to frequent human-wildlife interactions (Wilson et al., 2005; Morales-González et al., 2020). Increasing negative interactions have provoked widespread and intense human-wildlife conflicts (Inskip and Zimmermann, 2009; Braczkowski et al., 2020; König et al., 2020), which impacts species conservation and jeopardizes human livelihoods and safety.
Asiatic black bear (Ursus thibetanus) is widely distributed in southern and eastern Asia (Garshelis and Steinmetz, 2020). It is adaptable to the environment by utilizing all easily accessible foods, including natural and anthropogenic foods (Herrero et al., 2005; Elfström et al., 2014; Lewis et al., 2015). Therefore, black bears regularly interact with rural people and frequently involve in conflicts with humans (Jamtsho and Wangchuk, 2016; Huang et al., 2018). Bears can cause crop damage (Kazmi et al., 2019; Letro et al., 2020), livestock depredation (Jamtsho and Wangchuk, 2016; Waseem et al., 2020), beehive loss (Liu et al., 2011), and even human injuries or deaths (Charoo et al., 2011; Ali et al., 2018). Humans kill black bears in retaliation for such losses or to prevent future losses, posing severe threats to the species’ conservation (Garshelis and Steinmetz, 2020; Letro et al., 2020; Gomez et al., 2021). Moreover, these conflicts appear to increase in many areas (Can et al., 2014; Smith and Herrero, 2018; Prajapati et al., 2021), which pose significant challenges to human-bear coexistence (Ali et al., 2018).
Identifying spatial and temporal patterns of human-bear conflicts (HBC) and their determiners is crucial for developing mitigation measures (Broekhuis et al., 2017; Klees et al., 2020; Ankit et al., 2021). HBC occurrence appeared to be associated with environmental features and human-related attractants (Rojas-VeraPinto et al., 2022). Human-bear overlap is necessary for HBC occurrence (Sharma et al., 2020), and thus features associated with bear and human distribution (i.e., elevation, forest cover, and human population) might impact HBC occurrence (Wilson et al., 2005; Gastineau et al., 2019; Rojas-VeraPinto et al., 2022). Bears are more likely to occupy areas with limited human access (high and rugged) (Morales-González et al., 2020), yet agricultural attractants near bear habitats can predispose bears to forage in human landscapes (Wilson et al., 2006; Lewis et al., 2015). The availability of anthropogenic food (i.e., crops, livestock, and honey) is significantly related to HBC occurrences (Merkle et al., 2013; Huang et al., 2018). Furthermore, landscape composition and configuration can determine the availability and access to resources, affecting bears’ behaviors and human-bear interactions (Tee et al., 2021; Khosravi et al., 2022). As found previously, HBC is more likely to occur in landscapes characterized by a complex mosaic of forest habitat patches (Boudreau et al., 2022; Khosravi et al., 2022). Therefore, conflict mitigation efforts should consider the effects of these factors on HBC. Moreover, HBC can be changeable as the changes in these features (Jampel, 2016; Heemskerk, 2020), bringing a tremendous challenge for bear conservation.
China has the most extensive geographic distribution of black bears within the species’ range (Garshelis and Steinmetz, 2020). HBC usually occur near protected areas where residents experience the most costs of coexistence with wildlife (Li et al., 2013; Huang et al., 2018; Jordan et al., 2020). Gaoligongshan Nature Reserve (GNNR) lies in the western part of Yunnan Province, China, which is considered a global hotspot for biodiversity conservation (Lan and Dunbar, 2000; Myers et al., 2000; Li et al., 2019). However, villagers around GNNR mainly depend on agriculture, leading to frequent human-bear encounters due to the attraction of agricultural products (Kirby et al., 2016). Additionally, China significantly have improved forest and rural livelihoods in recent decades (Ma et al., 2018; Yang et al., 2019), which may change the environment and human-bear interactions. A thorough understanding of HBC is the basis for identifying effective policies and measures to promote long-term human-bear coexistence. We explored the spatial-temporal patterns of HBC and their determiners around GNNR with the following objectives: (1) to identify the hotspots of HBC; (2) to examine the impact of environmental factors on HBC occurrence and composition; (3) to explore the temporal fluctuations and trends in HBC and their possible causes; and (4) provide potential measures to mitigate bear damage.
GNNR is located in the southern area of Mt. Gaoligong, Yunnan, China (Xiong and Zhu, 2006), the key area of the Southwest China biodiversity hotspot (Myers et al., 2000). It is the home to animals as diverse as takin (Budorcas taxicolor), hoolock gibbon (Hylobates hoolock), red panda (Ailurus fulgens), and Asiatic black bear (Lan and Dunbar, 2000; Chan et al., 2019; Li et al., 2019). In 1992, WWF recognized GNNR as a Grade A protected area of global importance, and in 1997 GNNR was listed as a Biosphere Reserve.1
The study was conducted in communities around the Baoshan Section of gaoligongshan nature reserve (BSGN) (Figure 1). The study area spans 3,708 km2 with 73% forests and 21% cropland covering, and the elevation ranges from 617 to 3740 m. It is located in subtropical areas and contains various vegetation types, predominantly with monsoonal broadleaf evergreen forests (Allendorf and Yang, 2013). We divided the study area into two regions due to their geography, climate, and social economic discrepancy. The western and eastern regions are parts of Tengchong city and Longyang District, respectively. The western region is dominated by the Indian Ocean monsoon climate with a mean annual temperature of 15.1°C and annual rainfall of 1531 mm (The People’s Government of Tengchong City, 2022). Influenced by the dry-hot valley, the eastern region is warmer and drier than the western region. Its annual rainfall is 972 mm, with a mean annual temperature of 16.5°C (The People’s Government of Longyang District, 2022). About 240,000 and 120,000 people live in the western and eastern regions, with most people depending on agriculture (Frayer et al., 2014; He and Sikor, 2015; Liang, 2017). Traditionally, villagers occasionally go into the reserve for herb collecting and pasturing, but these human activities decreased recently owing to strict management and law enforcement. Whereas, forests in the slopes and foothills below the reserve are managed by local communities (Xiong and Zhu, 2006; Allendorf and Yang, 2013), where humans often overlap with black bears, leading to the continuous occurrence of HBC around BSGN.
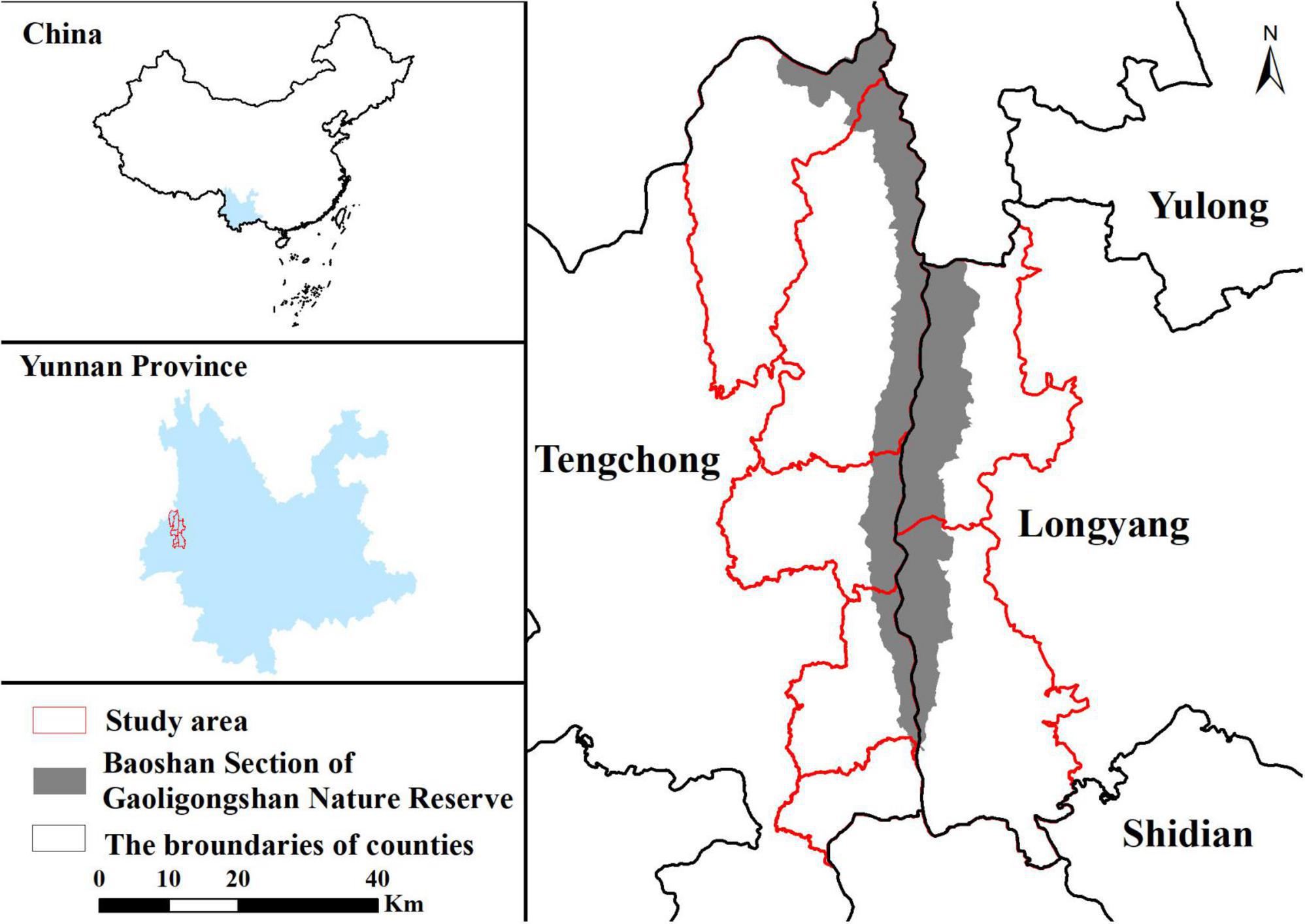
Figure 1. Map of the study area. The map shows locations of the Baoshan Section of Gaoligongshan Nature Reserve and study area.
Yunnan is one of the first provinces in China to implement a Wildlife Damage Compensation Program (WDCP) (National Forestry and Grassland Administration, 2008). Data on HBC incidents from 2012 to 2020 were sourced from the WDCP database provided by BSGN. We tabulated information (i.e., incident date, location, damage species, damage type, damage extent, and compensation) from documents such as applications, details of incidents, and final compensation. We categorized HBC occurrences as crop raiding, livestock depredation, beehive loss, and human casualties. To explore the spatial and temporal pattern of HBC, we extracted the location and time of HBC occurrence. As most incidents only included the villages where the event occurred, we could not conduct analyses at a finer spatial scale than villages.
Based on habitat characteristics, human disturbance, and bear ecology, we used (a) land use (the proportion of cropland and forest), (b) elevation, (c) human population density, and (d) fragmentation of forest and cropland [landscape shape index (LSI) and effective mesh size (MESH)] as explanatory variables for HBC occurrences. We extracted and measured these variables in each village’ administrative borders (Figure 2A). We extracted land use data from the 30 m land cover datasets in China (Yang and Huang, 2021). Then, we calculated the percentage of cropland and forest within each village (within administrative boundaries) in ArcGIS 10.6. Elevation was derived from the ASTER GDEM V2 digital elevation model at 30 m resolution.2 The population density was extracted from 1 km population count datasets in China from Worldpop.3 We calculated each village’s average elevation and population density using “Zonal” in ArcGIS 10.6.
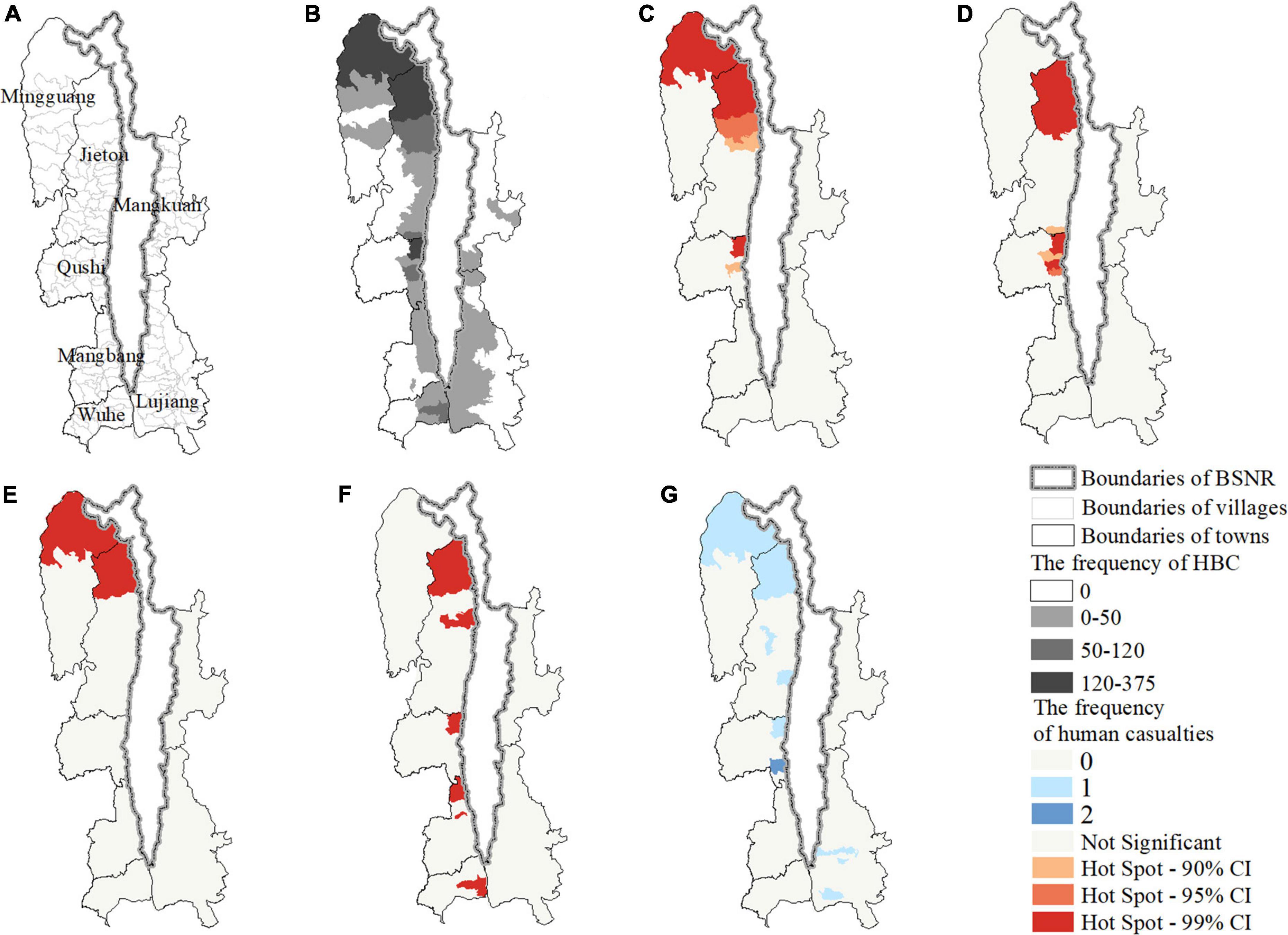
Figure 2. Maps showing the study area (A) the townships and villages, (B) the villages experienced human-bear conflicts; and the hotspot of human-bear conflicts for (C) all types combined, (D) crop raiding; (E) livestock depredation, (F) beehive loss, and (G) the frequency of human casualties, between 2012 and 2020.
We measured two fragmentation indices (LSI and MESH) for forest and cropland using landscape metric algorithms implemented in FRAGSTATS (McGarigal et al., 2002). LSI provides a standardized measure of total edge or edge density, which can characterize a landscape’s subdivision independently of its size (Jaeger, 2000). The LSI value is 1 when the landscape consists of a single square or maximally compact (i.e., almost square) patch of the corresponding type. LSI increases without limit as the patch type becomes more disaggregated. MESH characterizes the subdivision/fragmentation of a landscape independently of its size (McGarigal et al., 2002). The lower limit of MESH is constrained by the cell size ratio to landscape area and is achieved when the corresponding patch type consists of a single-pixel patch.
Firstly, spatial autocorrelation is the critical property describing a spatial pattern, which defines the dependence of a given variable’s values on the same variable recorded at neighboring locations (Valcu and Kempenaers, 2010). We investigated the local spatial autocorrelation of HBC around BSGN at the village scale using Getis-Ord analysis, which can identify the hotspots of conflict occurrences (Getis and Ord, 1992). The Getis-Ord statistic describes the degree of spatial autocorrelation. It can show whether significant clustering is apparent, with Z-scores greater than 1.96 indicating significant (a < 0.05) hot spots (Getis and Ord, 1992). This analysis was created using ArcGIS 10.6 (ESRI Inc.).
Secondly, we use a generalized linear model (GLM) to assess the relationships between environmental variables and response variable (i.e., HBC occurrences in each village). Because the response variable is a kind of count data with low arithmetic mean, a GLM with a Poisson distribution error structure and the natural log (ln) link function (i.e., Poisson regression) is appropriate (Coxe et al., 2009). Therefore, we evaluated the influence of environmental factors on HBC occurrences in each village using Poisson regression. We used Akaike’s Information Criterion (Burnham and Anderson, 1998) for model selection and evaluation. We selected the top-performing model based on their backward stepwise AIC using the “Mass” package (Venables et al., 2002) in R version 4.2.0 (R Core Team, 2022).
Finally, we compared the composition of HBC between two regions using the Chi-square test that determines if there is dependence (association) between the two classification variables (i.e., region and HBC type). We used Mann-Whitney U-test to test whether there is a difference in the occurrence of specific HBC between the two regions. Because HBC occurrence does not meet normal distribution, the Mann-Whitney U-test, the non-parametric counterpart to the t-test for independent samples, is more suitable for our response variable. All these analyses were conducted in R.
We used time series analysis to explore the temporal patterns of the total HBC and separately for crop raiding, livestock depredation, and beehive loss. Each time series comprises a trend, a cycle, and a residual. This time series decomposition isolates periodic patterns from the overall trend by fitting a Loess curve to the month-aggregated time series. The remaining seasonally decomposed time series is smoothed to identify the residual trend. Residuals were calculated as the exceptions from this combined season-trend model (Brockwell and Davis, 2002). Furthermore, we verified the significance of the overall trend in HBC using the MK test for the decomposed series (i.e., we removed the seasonality). Mann-Kendall (MK) test can statistically assess if there is a monotonic upward or downward trend of the variable of interest over time. A monotonic upward (downward) trend means that the variable consistently increases (decreases) through time, but the trend may or may not be linear. All these analyses were conducted in R, and the MK test was computed using the “Kendall” package (McLeod, 2022) in R.
From 2012 to 2020, a total of 1,966 HBC incidents were reported around BSGN (Table 1). Crop raiding was the most common HBC occurred (46.39%), followed by beehive loss (27.87%), livestock depredation (25.13%), and human casualties (0.61%). Corn was the most commonly raided crop by bears around BSGN (Table 1). Bears destroyed 2,978 beehives and killed 704 domestic animals around BSGN, with goats or sheep dominating (93%), followed by cattle (6.7%) and mules (0.3%). Twelve bear attacks on humans were recorded, resulting in 10 injuries and 2 fatalities (Table 1). Ten of those attacks occurred in forest areas, and the remaining two occurred in scrubland and farmland. A total of US$359,274 was paid as compensation for damages caused by bears around BSGN during 2012–2020 (Table 1). Most payments were provided to families as relief for livestock depredation (33.55%), followed by a relative who died or was injured in a bear attack (25.17%), crop raiding (21.51%), and beehive loss (19.77%).
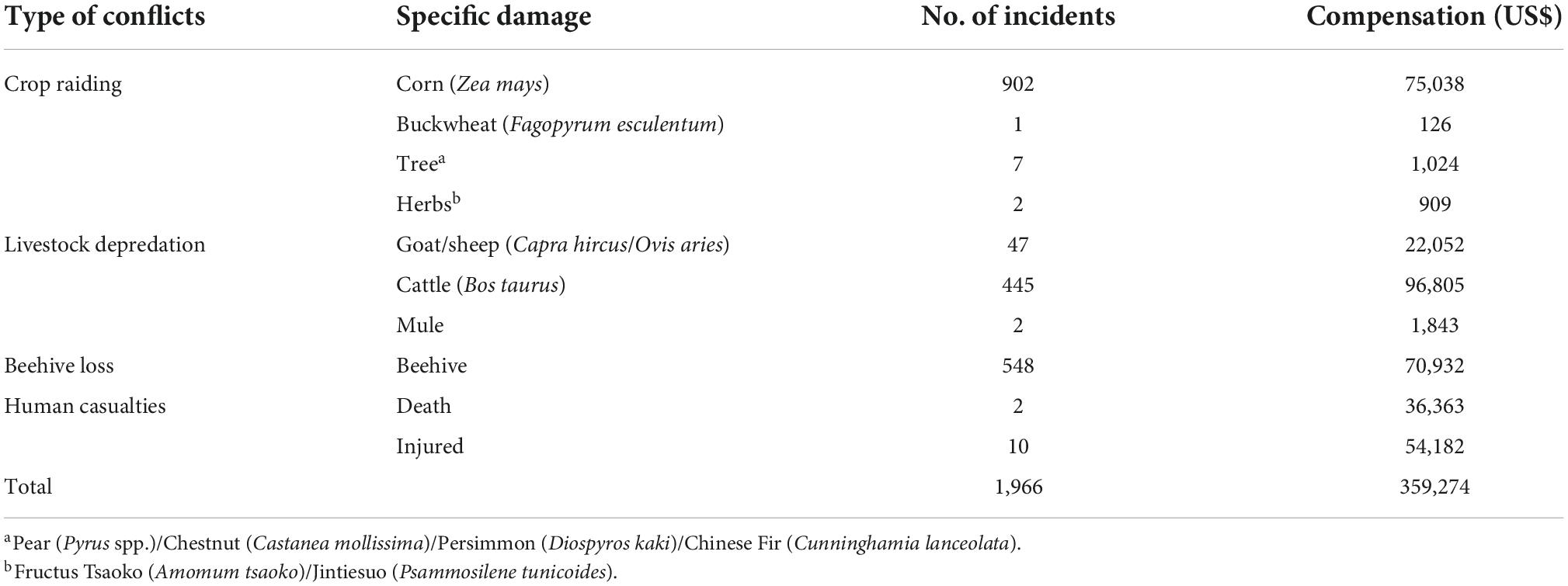
Table 1. Details of human-bear conflicts around Baoshan Section of Gaoligongshan Nature Reserve during 2012–2020.
A total of 53 villages (42.4%, n = 125; Figure 2B) experienced HBC during 2012–2020, and almost all of these villages bordered BSGN. Crop raiding occurred in the most villages (32.0%, n = 125), followed by beehive loss (24.8%), livestock depredation (24.0%), and human casualties (7.2%). The western region suffered more HBC incidents (84.51%, n = 1,969) than the eastern region (15.49%). Getis-Ord statistics indicated that HBC for all types combined (Figure 2C) and for each type (Figures 2D–F) were clustered in the western region, especially in several villages of Mingguang town, Jietou town, and Qushi town. Additionally, crop raiding (Figure 2D) and livestock depredation (Figure 2E) were more clustered spatially than beehive loss (Figure 2F). Furthermore, there are no apparent hotspots of human casualties (Figure 2G), which only occurred once in several villages. The HBC composition varied by region (χ2 = 937.9, d.f. = 1, P < 0.001). The proportion of crop raiding (W = 67, p = 0.018) and beehive loss (W = 5, p = 0.002) in the eastern region was significantly higher and lower than in the western region, respectively (Figure 3). The proportion of livestock depredation and human casualties did not vary significantly between regions (W = 39, p = 0.931; W = 44, P = 0.752; Figure 3).
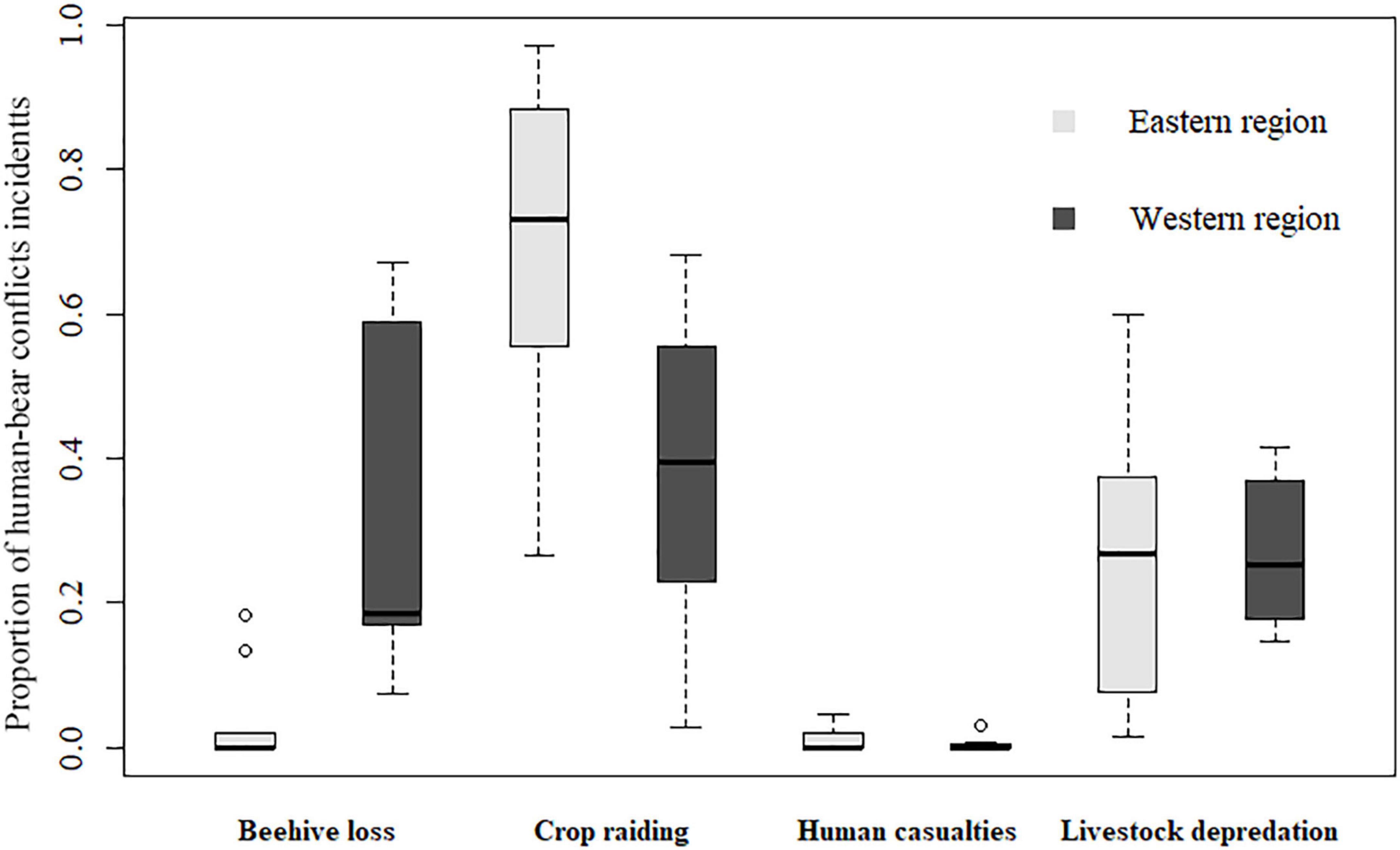
Figure 3. Proportion of different types of human-bear conflicts in the western and eastern region around Baoshan Section of Gaoligongshan Nature Reserve during 2012–2020.
Poisson regression results showed that all variables were included in the final model and significantly influenced HBC occurrences except for the LSI of cropland (Table 2). This top model indicated that HBC occurrence was negatively related to the elevation and human population density (Table 2). HBC was more likely to occur in villages with a high proportion of forest and cropland (Table 2). Moreover, the HBC occurrence increased with MESH and LSI of the forest (Table 2). However, cropland’s MESH was negatively associated with HBC occurrences (Table 2).
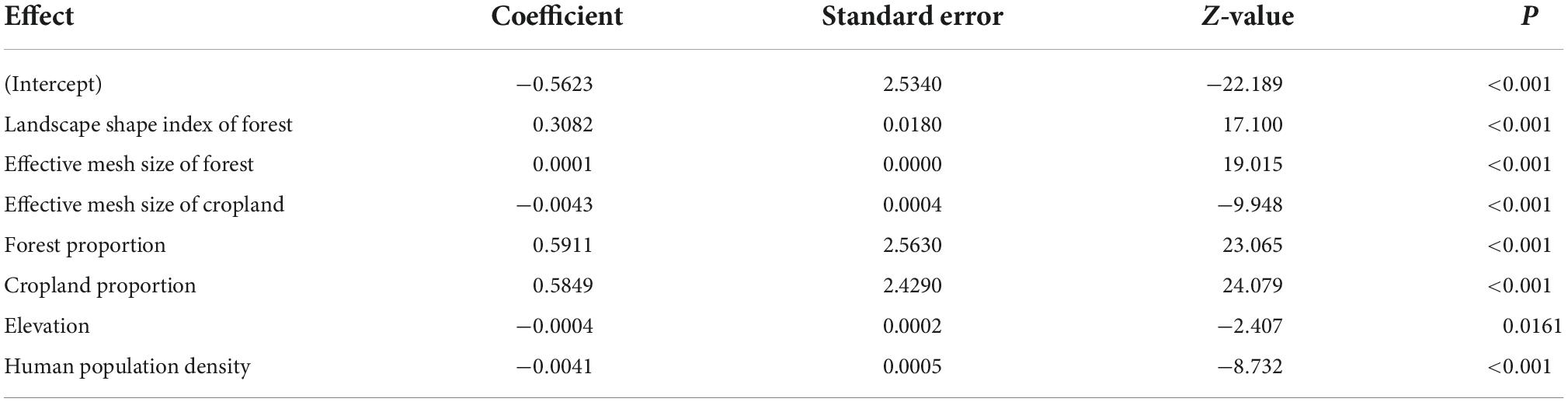
Table 2. Results of the Poisson regression analysis relating human-bear conflicts to various covariates around Baoshan Section of Gaoligongshan Nature Reserve during 2012–2020.
HBC occurred throughout the years (Figure 4A), peaking in August-October. Similar unimodal monthly patterns existed in different types of HBC, yet their peaks were staggered in time. Specifically, the peaks of beehive loss and livestock depredation occurred in August, one month earlier than crop raiding. HBC occurrence fluctuated across the years (Figure 4A), peaking in 2013 and 2018, respectively, and fell to its lowest point in 2020. Time series analysis and MK test indicated no distinct trend of HBC occurrence (tau = 0.1, p = 0.13; Figure 4A). The occurrence of livestock depredation (tau = 0.14, p = 0.03; Figure 4C) and beehive loss (tau = 0.36, p < 0.001; Figure 4D) has increased during 2017–2021, but inversely for crop raiding (tau = –0.31, p < 0.001; Figure 4B).
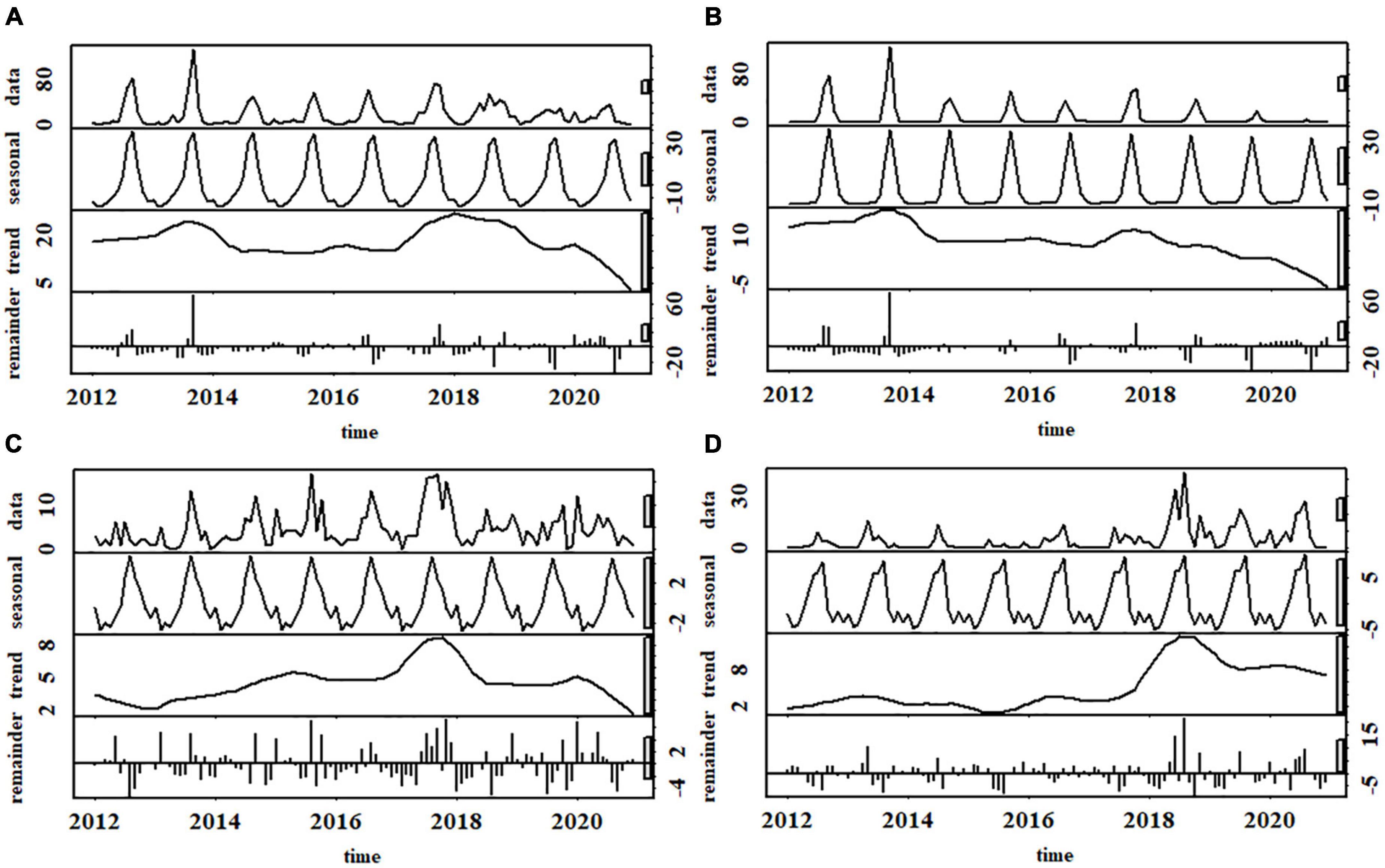
Figure 4. Temporal patterns of human-bear conflicts showing (A) all types combined, (B) crop raiding; (C) livestock depredation, (D) beehive loss around Baoshan Section of Gaoligongshan Nature Reserve during 2012–2020.
Crop raiding was the most common and severe HBC around BSGN, as reported elsewhere (Liu et al., 2011; Huang et al., 2018). Free-ranging livestock and apiaries situated in forests also were severely depredated by bears. Bears caused severe economic losses to local people and attacked people, a behavior that villagers find intolerable. Although attacks are rare, this experience can continue to elicit fear in people living near the bears’ habitat (Floyd, 1999). Bears are known to avoid humans, yet avoidance is not always possible (Bombieri et al., 2019). Most bear attacks are a defensive response to sudden encounters (Herrero et al., 2005; Parchizadeh and Belant, 2021). We had acquired from survivors that these attacks occurred when they were herding or weeding in collective forests and suddenly encountered bears, as found previously (Bombieri et al., 2019; Parchizadeh and Belant, 2021).
Either type of HBC mentioned above is the negative consequence of human-wildlife interactions. Human encroachment can negatively impact bears’ distribution and behaviors (Ordiz et al., 2014; Morrell et al., 2021). Bears are usually confined to areas with extreme conditions (e.g., higher elevations) and fewer human populations, leading to a low human-bear overlap and risk of HBC occurrences in these areas (Morales-González et al., 2020; Rojas-VeraPinto et al., 2022). Instead, HBC occurrences clustered near the BSGN and increased significantly with forest and cropland proportion. Forest is one of the essential elements to the survival of black bears (Garshelis and Steinmetz, 2020). Nevertheless, the spatially concentrated, predictable, and reliable products offered by human landscapes near forests can attract bears to forage closer to human landscapes (Baruch-Mordo et al., 2013; Lewis et al., 2015). Consequently, there is a high risk of HBC in forest-cropland interfaces where both concealment and anthropogenic could afford food to bears, especially around protected areas (Broekhuis et al., 2017; Klees et al., 2020).
Aside from the composition of landscape, the configuration, i.e., the spatial organization of the landscape elements, can also determine the bears’ behavior, and hence HBC (Linke et al., 2005; Boudreau et al., 2022). We found that HBC are more likely to occur in fragmented forests, as found elsewhere (Bombieri et al., 2018; Khosravi et al., 2022). These fragmented forests typically contain smaller, separated habitat areas with reduced natural food availability. Bears must increase their home ranges, frequently travel between patches, and even move into the surrounding human landscape for food (Khosravi et al., 2022). Additionally, the increased edge density from forest fragmentation can prompt bears to enter these unfavorable habitats (Klees et al., 2020). These frequent forest-human movements of bears increase the risk of HBC, making fragmented forest landscapes human-wildlife conflict hotspots (Bombieri et al., 2018; Boudreau et al., 2022). However, cropland fragmentation has a contrary impact on HBC. Bears are opportunistic omnivores who can make trade-off between the benefits and the risks (Baruch-Mordo et al., 2013; Lewis et al., 2015). Fragmented cropland patches probably increase bears’ cost of inter-crop-patch movement, leading to a low likelihood of crop-raiding in more fragmented cropland landscapes.
In terms of composition, HBC differed between the two regions. The eastern region suffered more crop raiding and less beehive loss than the western region, which might be related to landscape configuration and food availability. The eastern region is more suitable for cultivating tropical crops than the western region due to its hot-dry climate (Liang, 2017). In addition, these tropical crops were more profitable than traditional cereal (Liang, 2017). Therefore, villagers from the Easter region cultivate tropical fruits and vegetables in warmer areas near villages, leaving maize near forests (Jin et al., 2016; Liang, 2017). On the contrary, cereal was one of the principal crops grown in the western region. Corn was concentrated near human settlements in the western region because of the more rugged collective areas near BSGN. Therefore, cornfields in the eastern region appear nearer bear habitat, which can increase their risk of raiding, as found by previous studies (Kazmi et al., 2019; Bautista et al., 2021). Moreover, more residents in the western region are keeping bees to increase their income than in the eastern region. The higher availability of honey can bring more beehive loss in the western region, similar to the previous study (Jampel, 2016). However, there is a lack of detailed data about the availability and distribution of anthropogenic food (i.e., maize, honey, and livestock) around BSGN. Further studies are needed to verify these inferences.
Our results indicated that HBC fluctuated across months, with a peak in August-October when maize was more readily available than natural food. Furthermore, the frequency of livestock depredation and beehive loss increases as the corn matures until September, when bears mostly turn to maize, consistent with previous findings (Huang et al., 2018; Waseem et al., 2020). During this time, black bears tend to forage near villages, which increases the risk of bear encounters with livestock and humans (Merkle et al., 2013; Elfström et al., 2014). We found that HBC fluctuated throughout the year, which may be attributed to bears’ response to the annual fluctuation in food availability. Bears can increase their use in human-dominated landscapes when natural foods are scarce, and then switch to natural food sources when available (Johnson et al., 2015; Lewis et al., 2015). The most important findings were that crop raiding decreased during 2012–2020, but livestock depredation and beehive loss were in the opposite direction. These patterns might be explained by the complex effect of human behavior on the interrelations between people, the environment, and bears. With the implementation of the Grain-To-Green Program (GTGP) and special agriculture, the local government has encouraged farmers to plant economic forest or cash crops (such as nuts, Camellia spp., Ginkgo biloba, and herbs) in place of cereal crops, especially in steep mountains (Delang, 2014; Tengchong Municipal People’s Government, 2020). These changes might have reduced and fragmented cornfields near the forest, decreasing bears’ crop raiding as their relationships in spatial patterns. Jones and Pelton (2003) claimed a similar relationship that shifting from corn to cotton might reduce agricultural food resources for bears and influence the bears’ habitat use. Nonetheless, beehives and livestock are still laid out in forests near bear habitats. Hence, we inferred that bears switched from corn to honey/livestock due to the increasing cost of crop raiding, making more beehives and livestock damaged by bears. In addition, the increase in beehive loss and livestock depredation might be related to their increasing availability, which needs to be verified by field surveys.
Human-bear conflict is one of the most significant challenges for bear conservation. Our study assessed HBC around BSGN, identified hotspots, and revealed the complex association between the environmental features and HBC occurrences. We also recognized diverse trends of HBC occurrences across types owing to changing social-ecological environment. Combining these assessments is paramount to understanding the human-bear relationship better. Effective mitigations can benefit both rural livelihood and biodiversity conservation. Based on our findings, we recommend: (1) Human encroachment is a key driver of HBC. Forest restoration and adequate food resources are primary for resolving HBC. (2) Long-term investigation and scientific research are needed to evaluate how the bears respond to changing environments and identify the drivers of decreasing crop raiding and increasing beehive loss around BSGN. (3) Changing agricultural structures can reduce villagers’ dependence on maize, honey, and livestock. Furthermore, low-palatable crops should be cultivated on the edges of bears’ habitats. (4) An insurance cost-sharing mechanism involving joint payments from the government, farmers, and Chinese tourists can be attempted to enhance compensation and support the program’s long-term sustainability (Chen et al., 2013). (5) Prevention is widely recognized as by far the most effective measure to reduce human-carnivore conflicts (Goodrich, 2010). High platforms could be explored and implemented to compensate beekeepers who suffered severe damage.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YJ and XW were responsible for writing. DL and FL were responsible for coordinating the manuscript. JL, XH, JJ, and JT were co-authors and contributed to structuring of the article. All authors contributed to the article and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (Grant no. 41301054) and Chinese Academy of Forestry Research Fundamental Research Fund Project (Grant no. CAFYBB2014QB008).
We are grateful to the Animals Asia Foundation and the staff of Baoshan Section of Gaoligongshan Nature Reserve.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ali, A., Waseem, M., Teng, M., Ali, S., Ishaq, M., Haseeb, A., et al. (2018). Human–Asiatic black bear (Ursus thibetanus) interactions in the Kaghan Valley, Pakistan. Ethol. Ecol. Evol. 30, 399–415. doi: 10.1080/03949370.2017.1423113
Allendorf, T. D., and Yang, J. (2013). The role of ecosystem services in park–people relationships: The case of Gaoligongshan Nature Reserve in southwest China. Biol. Conserv. 167, 187–193. doi: 10.1016/j.biocon.2013.08.013
Ankit, K., Ghanekar, R., Morey, B., Mondal, I., Khandekar, V. R. J., Mondol, S., et al. (2021). Inhabiting terra incognita: Two-decadal patterns of negative human-leopard interactions in human-dominating landscape of Maharashtra, India. Glob. Ecol. Conserv. 29:e01740. doi: 10.1016/j.gecco.2021.e01740
Baruch-Mordo, S., Webb, C. T., Breck, S. W., and Wilson, K. R. (2013). Use of patch selection models as a decision support tool to evaluate mitigation strategies of human–wildlife conflict. Biol. Conserv. 160, 263–271. doi: 10.1016/j.biocon.2013.02.002
Bautista, C., Revilla, E., Berezowska-Cnota, T., Fernández, N., Naves, J., and Selva, N. (2021). Spatial ecology of conflicts: Unravelling patterns of wildlife damage at multiple scales. Proc. R. Soc. B Biol. Sci. 288:20211394. doi: 10.1098/rspb.2021.1394
Bhatia, S., Redpath, S. M., Suryawanshi, K., and Mishra, C. (2020). Beyond conflict: Exploring the spectrum of human–wildlife interactions and their underlying mechanisms. Oryx 54, 621–628. doi: 10.1017/S003060531800159X
Bombieri, G., Delgado, M., del, M., Russo, L. F., Garrote, P. J., López-Bao, J. V., et al. (2018). Patterns of wild carnivore attacks on humans in urban areas. Sci. Rep. 8:17728. doi: 10.1038/s41598-018-36034-7
Bombieri, G., Naves, J., Penteriani, V., Selva, N., Fernández-Gil, A., López-Bao, J. V., et al. (2019). Brown bear attacks on humans: A worldwide perspective. Sci. Rep. 9:8573. doi: 10.1038/s41598-019-44341-w
Boudreau, M. R., Gantchoff, M. G., Ramirez-Reyes, C., Conlee, L., Belant, J. L., and Iglay, R. B. (2022). Using habitat suitability and landscape connectivity in the spatial prioritization of public outreach and management during carnivore recolonization. J. Appl. Ecol. 59, 757–767. doi: 10.1111/1365-2664.14090
Braczkowski, A., Fattebert, J., Schenk, R., O’Bryan, C., Biggs, D., and Maron, M. (2020). Evidence for increasing human-wildlife conflict despite a financial compensation scheme on the edge of a Ugandan National Park. Conserv. Sci. Pract. 2:e309. doi: 10.1111/csp2.309
Brockwell, P. J., and Davis, R. A. (2002). Introduction to time series and forecasting, 2nd Edn. Berlin: Springer.
Broekhuis, F., Cushman, S. A., and Elliot, N. B. (2017). Identification of human-carnivore conflict hotspots to prioritize mitigation efforts. Ecol. Evol. 7, 10630–10639. doi: 10.1002/ece3.3565
Burnham, K. P., and Anderson, D. R. (1998). Model selection and inference. New York, NY: Springer. doi: 10.1007/978-1-4757-2917-7
Can, ÖE., D’Cruze, N., Garshelis, D. L., Beecham, J., and Macdonald, D. W. (2014). Resolving human-bear conflict: A global survey of countries, experts, and key factors. Conserv. Lett. 7, 501–513. doi: 10.1111/conl.12117
Chan, B. P. L., Bi, Z., and Duan, S.-Z. (2019). Introduction to a four-year biodiversity survey of Tengchong section of Gaoligongshan National Nature Reserve, in the footsteps of pioneering naturalists in western Yunnan, China. J. Threat. Taxa 11:11. doi: 10.11609/jott.4438.11.11.14391-14401
Charoo, S. A., Sharma, L. K., and Sathyakumar, S. (2011). Asiatic black bear–human interactions around Dachigam National Park, Kashmir, India. Ursus 22, 106–113. doi: 10.2192/URSUS-D-10-00021.1
Chen, S., Yi, Z.-F., Campos-Arceiz, A., Chen, M.-Y., and Webb, E. L. (2013). Developing a spatially-explicit, sustainable and risk-based insurance scheme to mitigate human–wildlife conflict. Biol. Conserv. 168, 31–39. doi: 10.1016/j.biocon.2013.09.017
Coxe, S., West, S. G., and Aiken, L. S. (2009). The analysis of count data: A gentle introduction to poisson regression and its alternatives. J. Pers. Assess. 91, 121–136. doi: 10.1080/00223890802634175
Delang, C. O. (2014). China’s grain for green program: A review of the largest ecological restoration and rural development program in the world. Berlin: Springer.
Elfström, M., Zedrosser, A., Støen, O.-G., and Swenson, J. E. (2014). Ultimate and proximate mechanisms underlying the occurrence of bears close to human settlements: Review and management implications: Bears near human settlements: Review and management. Mamm. Rev. 44, 5–18. doi: 10.1111/j.1365-2907.2012.00223.x
Floyd, T. (1999). Bear-inflicted human injury and fatality. Wilderness Environ. Med. 10, 75–87. doi: 10.1580/1080-60321999010
Frayer, J., Müller, D., Sun, Z., Munroe, D., and Xu, J. (2014). Processes underlying 50 years of local forest-cover change in Yunnan, China. Forests 5, 3257–3273. doi: 10.3390/f5123257
Garshelis, D. L., and Steinmetz, R. (2020). Ursus thibetanus, asiatic black bear. The IUCN red list of threatened species 2020. Available online at: https://www.iucnredlist.org/species/22824/166528664 (accessed March 10, 2022).
Gastineau, A., Robert, A., Sarrazin, F., Mihoub, J.-B., and Quenette, P.-Y. (2019). Spatiotemporal depredation hotspots of brown bears, Ursus arctos, on livestock in the Pyrenees, France. Biol. Conserv. 238:108210. doi: 10.1016/j.biocon.2019.108210
Getis, A., and Ord, J. K. (1992). The analysis of spatial association by use of distance statistics. Geogr. Anal. 24, 189–206. doi: 10.1111/j.1538-4632.1992.tb00261.x
Gomez, L., Wright, B., Shepherd, C. R., and Joseph, T. (2021). An analysis of the illegal bear trade in India. Glob. Ecol. Conserv. 27:e01552. doi: 10.1016/j.gecco.2021.e01552
Goodrich, J. M. (2010). Human-tiger conflict: A review and call for comprehensive plans. Integr. Zool. 5, 300–312. doi: 10.1111/j.1749-4877.2010.00218.x
He, J., and Sikor, T. (2015). Notions of justice in payments for ecosystem services: Insights from China’s sloping land conversion program in Yunnan province. Land Use Policy 43, 207–216. doi: 10.1016/j.landusepol.2014.11.011
Heemskerk, S. (2020). Temporal dynamics of human-polar bear conflicts in Churchill, Manitoba. Glob. Ecol. Conserv. 24:e01320.
Herrero, S., Smith, T., DeBruyn, T. D., Gunther, K., and Matt, C. A. (2005). Brown bear habituation to people–Safety, risks, and benefits. Wildl. Soc. Bull. 33, 362–373. doi: 10.2193/0091-7648200533
Honda, T. (2009). Environmental factors affecting the distribution of the Wild Boar, Sika Deer, asiatic black bear and Japanese macaque in central Japan, with implications for human-wildlife conflict. Mamm. Stud. 34, 107–116. doi: 10.3106/041.034.0206
Huang, C., Li, X. Y., Shi, L. J., and Jiang, X. L. (2018). Patterns of human-wildlife conflict and compensation practices around Daxueshan Nature Reserve, China. Zool. Res. 39, 406–412. doi: 10.24272/j.issn.2095-8137.2018.056
Inskip, C., and Zimmermann, A. (2009). Human-felid conflict: A review of patterns and priorities worldwide. Oryx 43:18. doi: 10.1017/S003060530899030X
Jaeger, J. A. G. (2000). Landscape division, splitting index, and effective mesh size: New measures of landscape fragmentation. Landsc. Ecol. 15, 115–130. doi: 10.1023/A:1008129329289
Jampel, C. (2016). Cattle-based livelihoods, changes in the taskscape, and human–bear conflict in the Ecuadorian Andes. Geoforum 69, 84–93. doi: 10.1016/j.geoforum.2016.01.001
Jamtsho, Y., and Wangchuk, S. (2016). Assessing patterns of human–asiatic black bear interaction in and around Wangchuck Centennial National Park, Bhutan. Glob. Ecol. Conserv. 8, 183–189. doi: 10.1016/j.gecco.2016.09.004
Jin, L., Duan, Z., and Yang, W. (2016). Analysis of community development adjacent to Gaoligongshan Nature Reserve based on GIS and PRA. J. West China For. Sci. 45, 121–126.
Johnson, H. E., Breck, S. W., Baruch-Mordo, S., Lewis, D. L., Lackey, C. W., Wilson, K. R., et al. (2015). Shifting perceptions of risk and reward: Dynamic selection for human development by black bears in the western United States. Biol. Conserv. 187, 164–172. doi: 10.1016/j.biocon.2015.04.014
Jones, M. D., and Pelton, M. R. (2003). Female American black bear use of managed forest and agricultural lands in coastal North Carolina. Ursus 14, 188–197.
Jordan, N. R., Smith, B. P., Appleby, R. G., Eeden, L. M., and Webster, H. S. (2020). Addressing inequality and intolerance in human–wildlife coexistence. Conserv. Biol. 34, 803–810. doi: 10.1111/cobi.13471
Kazmi, S., Minhas, R. A., Ahmad, B., Awan, M. S., Abbasi, S., Ali, U., et al. (2019). Crop raiding by Himalayan black bear: A major cause of human-bear conflict in Machiara National Park, Pakistan. J. Anim. Plant Sci. 29, 854–863.
Khosravi, R., Wan, H. Y., Sadeghi, M. R., and Cushman, S. A. (2022). Identifying human–brown bear conflict hotspots for prioritizing critical habitat and corridor conservation in southwestern Iran. Anim. Conserv. doi: 10.1111/acv.12800
Kirby, R., Alldredge, M. W., and Pauli, J. N. (2016). The diet of black bears tracks the human footprint across a rapidly developing landscape. Biol. Conserv. 200, 51–59. doi: 10.1016/j.biocon.2016.05.012
Klees, J., Badry, M., Ford, A. T., Golumbia, T., and Burton, A. C. (2020). Predicting human-carnivore conflict at the urban-wildland interface. Glob. Ecol. Conserv. 24:e01322. doi: 10.1016/j.gecco.2020.e01322
König, H. J., Kiffner, C., Kramer-Schadt, S., Fürst, C., Keuling, O., and Ford, A. T. (2020). Human–wildlife coexistence in a changing world. Conserv. Biol. 34, 786–794. doi: 10.1111/cobi.13513
Lan, D., and Dunbar, R. (2000). Bird and mammal conservation in Gaoligongshan Region and Jingdong County, Yunnan, China: Patterns of species richness and nature reserves. Oryx 34:12.
Laurance, W. F., Vasconcelos, H. L., and Lovejoy, T. E. (2000). Forest loss and fragmentation in the Amazon: Implications for wildlife conservation. Oryx 34, 39–45. doi: 10.1046/j.1365-3008.2000.00094.x
Letro, L., Wangchuk, S., and Dhendup, T. (2020). Distribution of asiatic black bear and its interaction with humans in Jigme Singye Wangchuck National Park, Bhutan. Nat. Conserv. Res. 5, 44–52. doi: 10.24189/ncr.2020.004
Lewis, D. L., Baruch-Mordo, S., Wilson, K. R., Breck, S. W., Mao, J. S., and Broderick, J. (2015). Foraging ecology of black bears in urban environments: Guidance for human-bear conflict mitigation. Ecosphere 6:art141. doi: 10.1890/ES15-00137.1
Li, F., Huang, X.-Y., Zhang, X.-C., Zhao, X.-X., Yang, J.-H., and Chan, B. P. L. (2019). Mammals of Tengchong section of Gaoligongshan National Nature Reserve in Yunnan Province, China. J. Threat. Taxa 11, 14402–14414. doi: 10.11609/jott.4439.11.11.14402-14414
Li, X., Buzzard, P., Chen, Y., and Jiang, X. (2013). Patterns of livestock predation by carnivores: Human–wildlife conflict in Northwest Yunnan, China. Environ. Manag. 52, 1334–1340. doi: 10.1007/s00267-013-0192-8
Liang, Q. Q. (2017). An empirical study on economic situation investigation Gaoligongshan ethnic villages nature reserve. China Popul. Resour. Environ. 27, 248–251.
Linke, J., Franklin, S. E., Huettmann, F., and Stenhouse, G. B. (2005). Seismic cutlines, changing landscape metrics and grizzly bear landscape use in Alberta. Landsc. Ecol. 20, 811–826. doi: 10.1007/s10980-005-0066-4
Liu, F., McShea, W. J., Garshelis, D. L., Zhu, X., Wang, D., and Shao, L. (2011). Human-wildlife conflicts influence attitudes but not necessarily behaviors: Factors driving the poaching of bears in China. Biol. Conserv. 144, 538–547. doi: 10.1016/j.biocon.2010.10.009
Liu, J., Coomes, D. A., Gibson, L., Hu, G., Liu, J., Luo, Y., et al. (2019). Forest fragmentation in China and its effect on biodiversity. Biol. Rev. 94, 1636–1657. doi: 10.1111/brv.12519
Ma, L., Liu, S., Niu, Y., and Chen, M. (2018). Village-scale livelihood change and the response of rural settlement land use: Sihe Village of Tongwei County in Mid-Gansu Loess Hilly Region as an example. Int. J. Environ. Res. Public Health 15:1801. doi: 10.3390/ijerph15091801
McGarigal, K., Cushman, S. A., Neel, M. C., and Atwill, E. (2002). FRAGSTATS: Spatial pattern analysis program for categorical maps. Amherst, MA: University of Massachusetts.
McLeod, A. I. (2022). Kendall: Kendall rank correlation and mann-kendall trend test. Available online at: https://CRAN.R-project.org/package=Kendall (accessed October 20, 2022).
Merkle, J. A., Robinson, H. S., Krausman, P. R., and Alaback, P. (2013). Food availability and foraging near human developments by black bears. J. Mammal. 94, 378–385. doi: 10.1644/12-MAMM-A-002.1
Morales-González, A., Ruiz-Villar, H., Ordiz, A., and Penteriani, V. (2020). Large carnivores living alongside humans: Brown bears in human-modified landscapes. Glob. Ecol. Conserv. 22:e00937. doi: 10.1016/j.gecco.2020.e00937
Morrell, N., Appleton, R. D., and Arcese, P. (2021). Roads, forest cover, and topography as factors affecting the occurrence of large carnivores: The case of the Andean bear (Tremarctos ornatus). Glob. Ecol. Conserv. 26:e01473. doi: 10.1016/j.gecco.2021.e01473
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
National Forestry and Grassland Administration (2008). Notice of pilot work on wildlife damage compensation. Available online at: http://www.forestry.gov.cn/ (accessed March 20, 2022).
Ordiz, A., Kindberg, J., Sæbø, S., Swenson, J. E., and Støen, O.-G. (2014). Brown bear circadian behavior reveals human environmental encroachment. Biol. Conserv. 173, 1–9. doi: 10.1016/j.biocon.2014.03.006
Parchizadeh, J., and Belant, J. L. (2021). Brown bear and Persian leopard attacks on humans in Iran. PLoS One 16:e0255042. doi: 10.1371/journal.pone.0255042
Prajapati, U., Koli, V. K., and Sundar, K. S. G. (2021). Vulnerable sloth bears are attracted to human food waste: A novel situation in Mount Abu town, India. Oryx 55, 699–707. doi: 10.1017/S0030605320000216
R Core Team (2022). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Rojas-VeraPinto, R., Bautista, C., and Selva, N. (2022). Living high and at risk: Predicting Andean bear occurrence and conflicts with humans in southeastern Peru. Glob. Ecol. Conserv. 36:e02112. doi: 10.1016/j.gecco.2022.e02112
Sharma, P., Chettri, N., Uddin, K., Wangchuk, K., Joshi, R., Tandin, T., et al. (2020). Mapping human-wildlife conflict hotspots in a transboundary landscape, Eastern Himalaya. Glob. Ecol. Conserv. 24:e01284. doi: 10.1016/j.gecco.2020.e01284
Smith, T. S., and Herrero, S. (2018). Human-bear conflict in Alaska: 1880-2015: Alaska human-bear conflict. Wildl. Soc. Bull. 42, 254–263. doi: 10.1002/wsb.870
Tee, T. L., van Manen, F. T., Kretzschmar, P., Sharp, S. P., Wong, S. T., Gadas, S., et al. (2021). Anthropogenic edge effects in habitat selection by sun bears in a protected area. Wildl. Biol. 2:wlb.00776. doi: 10.2981/wlb.00776
Tengchong Municipal People’s Government (2020). Chinese herbal medicine planting in qushi “blossom”, special agricultural open up a “new path”. Available online at: http://www.tengchong.gov.cn/info/egovinfo/1001/zfxxgkpt/zfxxgknry/01526098-0-/2020-1218001.htm (accessed October 20, 2022).
The People’s Government of Longyang District (2022). About Longyang. Available online at: http://www.longyang.gov.cn/lygk.htm (accessed January 15, 2022).
The People’s Government of Tengchong City (2022). The overview of Tengchong City. Available online at: http://www.tengchong.gov.cn/info/1074/12110.htm (accessed October 20, 2022).
Valcu, M., and Kempenaers, B. (2010). Spatial autocorrelation: An overlooked concept in behavioral ecology. Behav. Ecol. 21, 902–905. doi: 10.1093/beheco/arq107
Venables, W. N., Ripley, B. D., and Venables, W. N. (2002). Modern applied statistics with S, 4th Edn. Berlin: Springer.
Waseem, M., Mahmood, T., Hussain, A., Hamid, A., Akrim, F., Andleeb, S., et al. (2020). Ecology and human conflict of asiatic black bear Ursus thibetanus laniger in Mansehra District, Pakistan. Pak. J. Zool. 52, 1443–1451. doi: 10.17582/journal.pjz/20180209100205
Wilson, S. M., Madel, M. J., Mattson, D. J., Graham, J. M., and Merrill, T. (2006). Landscape conditions predisposing grizzly bears to conflicts on private agricultural lands in the western USA. Biol. Conserv. 130, 47–59. doi: 10.1016/j.biocon.2005.12.001
Wilson, S. M., Madel, M. J., Mattson, D. J., Graham, J. M., Burchfield, J. A., and Belsky, J. M. (2005). Natural landscape features, human-related attractants, and conflict hotspots: A spatial analysis of human–grizzly bear conflicts. Ursus 16, 117–129.
Xiong, Q., and Zhu, M. (eds). (2006). A study of the communities surrounding Mts. Gaoligong: Science Press.
Xu, J., Wei, J., and Liu, W. (2019). Escalating human–wildlife conflict in the Wolong Nature Reserve, China: A dynamic and paradoxical process. Ecol. Evol. 9, 7273–7283. doi: 10.1002/ece3.5299
Yang, J., and Huang, X. (2021). The 30 m annual land cover datasets and its dynamics in China from 1990 to 2020 (1.0.0) [Data set]. Geneva: Zenodo. doi: 10.5281/ZENODO.4417809
Yang, L., Liu, M., and Min, Q. (2019). Natural disasters, public policies, family characteristics, or livelihood assets? The driving factors of farmers’ livelihood strategy choices in a nature reserve. Sustainability 11:5423. doi: 10.3390/su11195423
Keywords: human-bear coexistence, crop raiding, beehive loss, landscape, agricultural structure
Citation: Ji Y, Wei X, Liu F, Li D, Li J, Huang X, Jiang J and Tang J (2022) Assessing the spatial-temporal patterns of conflicts between humans and Asiatic black bears (Ursus thibetanus) around the Gaoligongshan Nature Reserve, China. Front. Ecol. Evol. 10:1020703. doi: 10.3389/fevo.2022.1020703
Received: 16 August 2022; Accepted: 07 November 2022;
Published: 23 November 2022.
Edited by:
Alberto Meriggi, The University of Pavia, ItalyReviewed by:
Francesco Ferretti, University of Siena, ItalyCopyright © 2022 Ji, Wei, Liu, Li, Li, Huang, Jiang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Liu, bGl1ZmFuZy5jYWZAZm94bWFpbC5jb20=; Diqiang Li, bGlkcUBjYWYuYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.