- 1Xinjiang Production and Construction Corps Key Laboratory of Protection and Utilization of Biological Resources in Tarim Basin, Tarim University, Alaer, China
- 2Key Laboratory of Tarim Animal Husbandry Science and Technology of Xinjiang Production and Construction Corps, Tarim University, Alaer, China
- 3Animal Husbandry and Veterinary Station of Zawa Town, Moyu, China
- 4Animal Health Supervision Institute, Yining, China
- 5Animal Husbandry and Veterinary Station of Jiliyuzi Town, Yining, China
The Anjian chicken is a local breed in Hotan, Xinjiang, China. Herein, we studied the morphological characteristics and genetic diversity of the Anjian chicken population. The findings of this study could inform the genetic improvement strategy of this breed. Phenotypic characteristics investigated included the diversity in the general appearance, feather color, and crowing length of the Anjian cocks. The population structure of the Anjian chicken and its relationship with other chicken breeds were also assessed based on mitochondrial DNA (mtDNA) D-loop sequence analysis. Phenotypically, the feather color of the Anjian chicken varied considerably. The sequence diversity analysis revealed the following: nucleotide diversity (Pi) was 0.00618, haplotype diversity (Hd) was 0.776, the average number of nucleotide differences (k) was 7.631, and Tajima’s (D) was −0.00407, indicating that Anjian chicken is moderately genetically diverse. Further phylogenetic analysis revealed that the Anjian chicken breed has 10 haplotypes clustered into two branches. Genetic distance and median network analysis showed that the mtDNA D-loop sequence of the Anjian chicken was distributed in many different clusters of the tree. These data demonstrate that even though the Anjian chicken mainly originated from red jungle fowl, it has multiple maternal origins. In conclusion, the Anjian chicken is highly genetically diverse.
Introduction
Biodiversity is the basis for the survival and development of human society, and livestock and poultry genetic resources are an essential part of biodiversity. China is one of the countries with the richest genetic resources of livestock and poultry in the world. Numerous livestock and poultry breeds left over from Chinese history are huge genetic treasures.
Local chickens in China are rich in genetic resources and display high phenotypic variation. Some chicken breeds have yellow, partridge, black, white, barred, and tile gray feathers, among others. The feathers could also be silky or flake. Other distinct features of certain breeds include green ears, big crests, long whiskers, five toes and claws, hairy feet, and a bald tail (Chen et al., 2010). Food and Agriculture Organization of the United Nations guidelines (FAO, 2012) provide that phenotypic characterization of chickens should include qualitative and quantitative variables. Qualitative variables include plumage color (white, black, blue, red, wheaten), skin color (non-pigmented (white), yellow, or blue-black), and comb type (single, pea, rose, walnut, cushion, strawberry, duplex, V-shaped, or double). Quantitative variables include body weight and body size.
Appropriate assessment and characterization of populations at the phenotypic and genotypic levels facilitates conservation strategies and the protection of genetic diversity (Hall and Bradley, 1995; Plante et al., 2007; Gizaw et al., 2008). Phenotypic characterization is essential for protecting and improving biological and genetic resources (Mustofa et al., 2021). The phenotypic characteristics of chickens are influenced by environmental and genetic factors (Mahfudz et al., 2011). Morphological features, including beak shape, comb, feather color, and body weight, are crucial chicken phenotypic traits and are also important factors when improving the genetic blueprint of a breed (Mustofa et al., 2021).
However, the phenotypic diversity does not reveal the genetic structure of the population, and ultimately, genetic identification based on molecular techniques is required (Mustofa et al., 2021). Genetic diversity studies can reveal the population structure for conservation purposes (Zanetti et al., 2010). Mitochondrial DNA (mtDNA) sequences have become important genetic markers for studying phylogenetic relationships due to their rapid evolutionary rate, high copy number, and maternal inheritance (Luo et al., 2019). The mitochondrial genome of the domestic chicken is about 16.7 kb and consists of a coding region containing 37 genes and a control region (CR) called the displacement loop (D-loop) (Desjardins and Morais, 1990). Due to the high mutation rate in the D-loop region, this site is used as a marker for genetic diversity, phylogenetic relationships, and maternal origin studies (Osman et al., 2016; Gao et al., 2017).
Anjian chicken is a local chicken breed in China, introduced in the country more than 200 years ago (Muhatai et al., 2012). During a resource survey in 2010, it was found that the Anjian chicken was only reared in Moyu County, Pishan County, Hotan County, Qira County, and other areas in Hotan Prefecture, Xinjiang. Conflicting accounts have been put forward to describe the origin of this chicken species. First, it is generally accepted that Pelung cocks were introduced in foreign by a merchant nicknamed Anjian. Thus, the Anjian chicken is likely a descendant of a cock first introduced in the country by the merchant. Because Anjian chicken has a unique crowing sound, it is often used in crowing competitions. The Anjian chicken can eat crude feed and has excellent foraging ability, physical agility, and high disease resistance (Muhatai et al., 2012). Data on Anjian chicken remain scanty (Muhatai et al., 2012), and there is completely no data on phenotypic characteristics and the genetic diversity of this chicken breed.
This study analyzed the phenotypic characteristics and the genetic diversity of Anjian chickens. The phenotypic features included the color of the feathers, body size and weight, and the crowing sound. The population structure of Anjian chickens was also compared to those of other chicken breeds based on the mtDNA D-loop sequences. The findings of this study could provide a theoretical basis for Anjian chicken genetic breeding strategies and future epigenetic research. This research could also provide excellent resources for Heterosis and the production of superior breeds.
Materials and methods
Animals and morphological features
Animals
Anjian chickens obtained from Moyu County (Latitude 36°36′–39°38′ N, Longitude 79°08′–80°51′ E) and Qira County (Latitude 35°18′–39°30′ N, Longitude 80°03′–82°10′ E) in Hotan Prefecture (Figure 1).
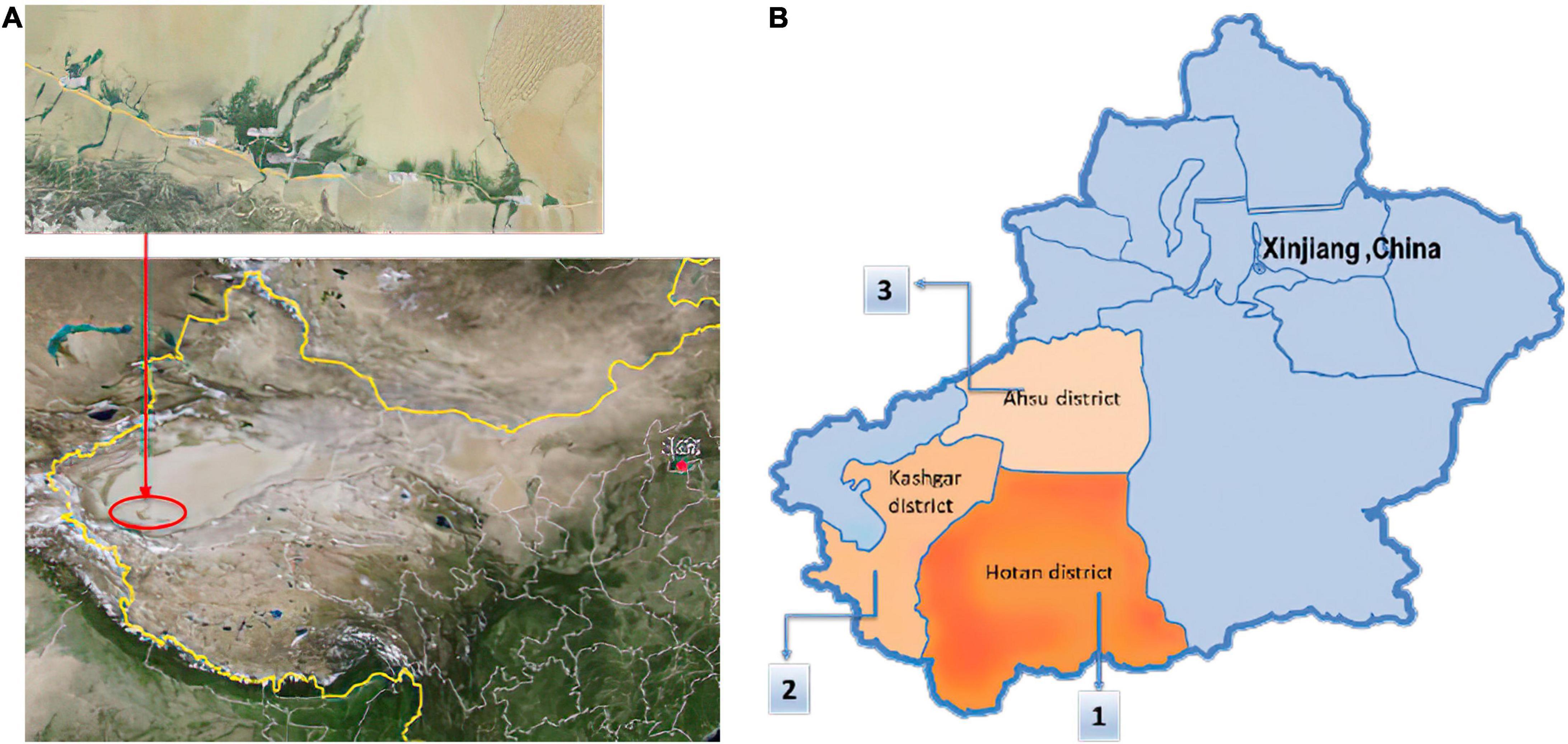
Figure 1. Anjian chickens are mainly distributed in the south of Xinjiang, China. (A) They are located in the northern foothills of the Kunlun Mountains and in Hotan Prefecture, in the southern region of the Taklimakan Desert. (B) Distribution of Anjian chickens: (1). In Hotan Prefecture (region of origin); (2). A small number of chickens are distributed in Shache County, Maigaiti, and four counties in the Kashgar area; (3). Aksu Prefecture has started breeding in recent years.
Qualitative variables
The phenotypic characteristics of 900 Anjian chickens (150 cocks and 750 hens) were observed and recorded. The phenotypic features of interest included the comb type, beak type and color, ear and ear leaf colors, eye color, droop size, presence or absence of beards, face, shank color, and skin color. For feather diversity analysis, the focus was on the color of the feather on the neck, back, wing, chest, abdomen, and tail of the Anjian chickens.
Quantitative variables
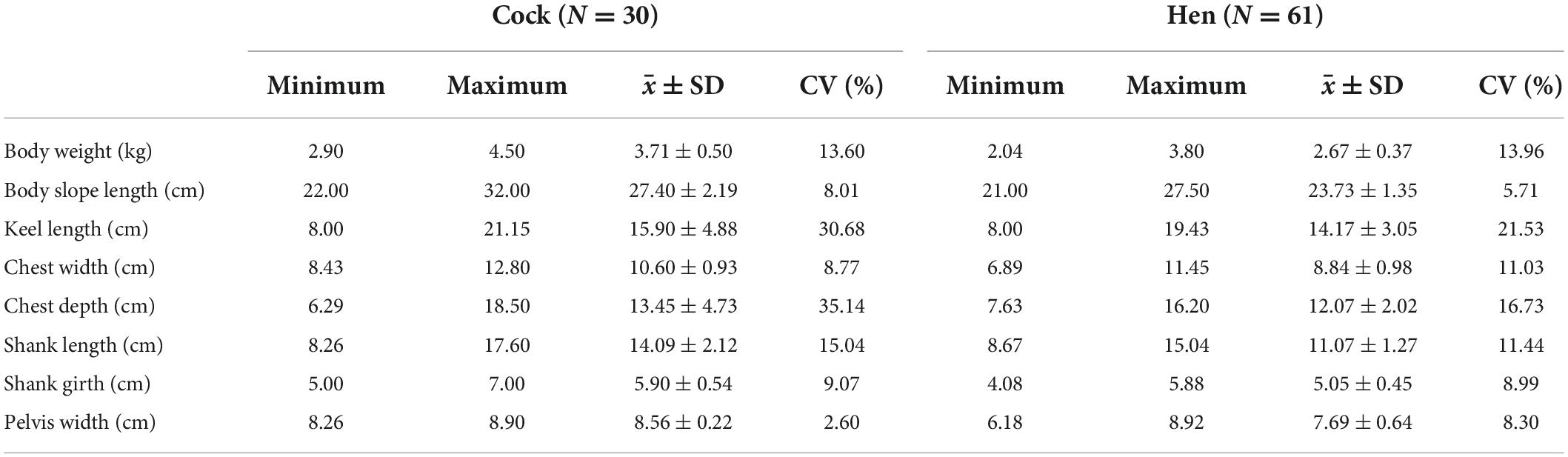
In addition to phenotypic observation, 30 mature cocks and 61 mature hens were selected for body weight (kg), body slope length (cm), keel length (cm), chest width (cm), chest depth (cm), shank length (cm), shank girth (cm), and pelvis width (cm) assessment. Finally, 96 cocks were selected for genetic diversity studies and the duration of crowing.
Genetic characterization
The 29 Anjian chickens for genetic characterization were sampled from Moyu County, Hetan County (Latitude 34°22′–38°27′N, Longitude 78°–80°30′E), Luopu County (Latitude 37°07′N, Longitude 80°18′E), Pishan County (Latitude 37°62′N, Longitude 78°28′E), and Qira County in Hotan Prefecture. The genetic diversity and matrilineality of the chickens were analyzed based on the mtDNA D-loop sequences. In addition to the sequence generated, the mtDNA D-loop sequences of 36 other chicken breeds and one turkey breed were derived from GenBank.
Briefly, 2–3 ml of blood samples were collected from the inferior pterygoid vein of each chicken and stored at −20°C until further processing. The genomic DNA was extracted using the whole blood genomic DNA rapid extraction kit purchased from Aidlab Biotechnologies Co., Ltd. (Beijing, China).
The purity and concentration of the extracted genomic DNA were assessed by UV spectrophotometry and diluted to 50 ng/μL for PCR amplification. The sequence of primers used for amplifying the mtDNA D-loop and sequencing were as follows: 5′-AGTCATATTATTCCCGCTTGGT-3′ for the forward primer and 5′-CTTTTGCCCACAGGTATAGTAGGT-3′ for the reverse primer (Liu et al., 2006). Each 25 μL PCR reaction mix comprised the following; 1 μL of DNA template (50–100 ng/μL), 10 × Buffer (2.5 μL), 2 μL of 2.5 mmol/L dNTP mix, 10 pmol forward primer (1 μL), 10 pmol reverse primer (1 μL), and 5 U Taq polymerase (EXTaqHS) (0.125 μL), and cddH2O. The PCR reaction conditions were as follows: Initial denaturation at 95°C for 5 min, subsequent denaturation through 35 cycles at 94°C for 30 s, annealing at 60°C for 30 s, extension at 72°C for 2 min and 30 s, and final extension at72°C for 12 min. PCR products were cooled to 15°C and analyzed by gel electrophoresis (1.5% agarose). The PCR products were sequenced by the Beijing Yuewei Gene Technology Co., Ltd. (Beijing, China). The mtDNA CR (D-loop) was the region of interest.
Statistical analysis
Data on quantitative morphological features were analyzed using SPSS software, version 26.0 (International Business Machines Corporation, United States of America) and Excel version 2016 (Microsoft, United States of America). To calculate the frequency distribution of traits of interest was expressed as proportions/percentages. The distribution of special traits in the cocks, the hens, and both groups was expressed as percentages. Maximum, minimum, mean measure (averages ± standard Deviation; ± SD), and coefficient of variation (CV) values for continuous variables such as body weight were also calculated. Finally, the duration of crowing was determined using a stopwatch and analyzed as described for body measurements.
Sequence alignment, cutting, and splicing were performed using the Cluster X (version 2.0.11) and DNAman (version 6.0, LynnonBiosoft, United States of America) software. The haploid typing, haploid diversity, and nucleotide diversity were analyzed by DnaSP (version 5.10) package, and genetic distances were calculated by the MEGA software (version 11.0, Mega Limited, New Zealand). In contrast, the phylogenetic tree was constructed using the neighbor-joining (NJ) and maximum likelihood (ML) methods based on the Poisson model (1,000 bootstrap iterations). A median variation network diagram was constructed using the NetWork software (version 10.2).1
Results
Morphological characterization
Qualitative variables of the Anjian chicken
The colors of feathers of the Anjian chickens were considerably diverse, whereas those of the native game chickens did not differ substantially (Figure 2). In the present study, more than 60% of the Anjian hens had light-yellow colored feathers, while most cocks were predominantly black.
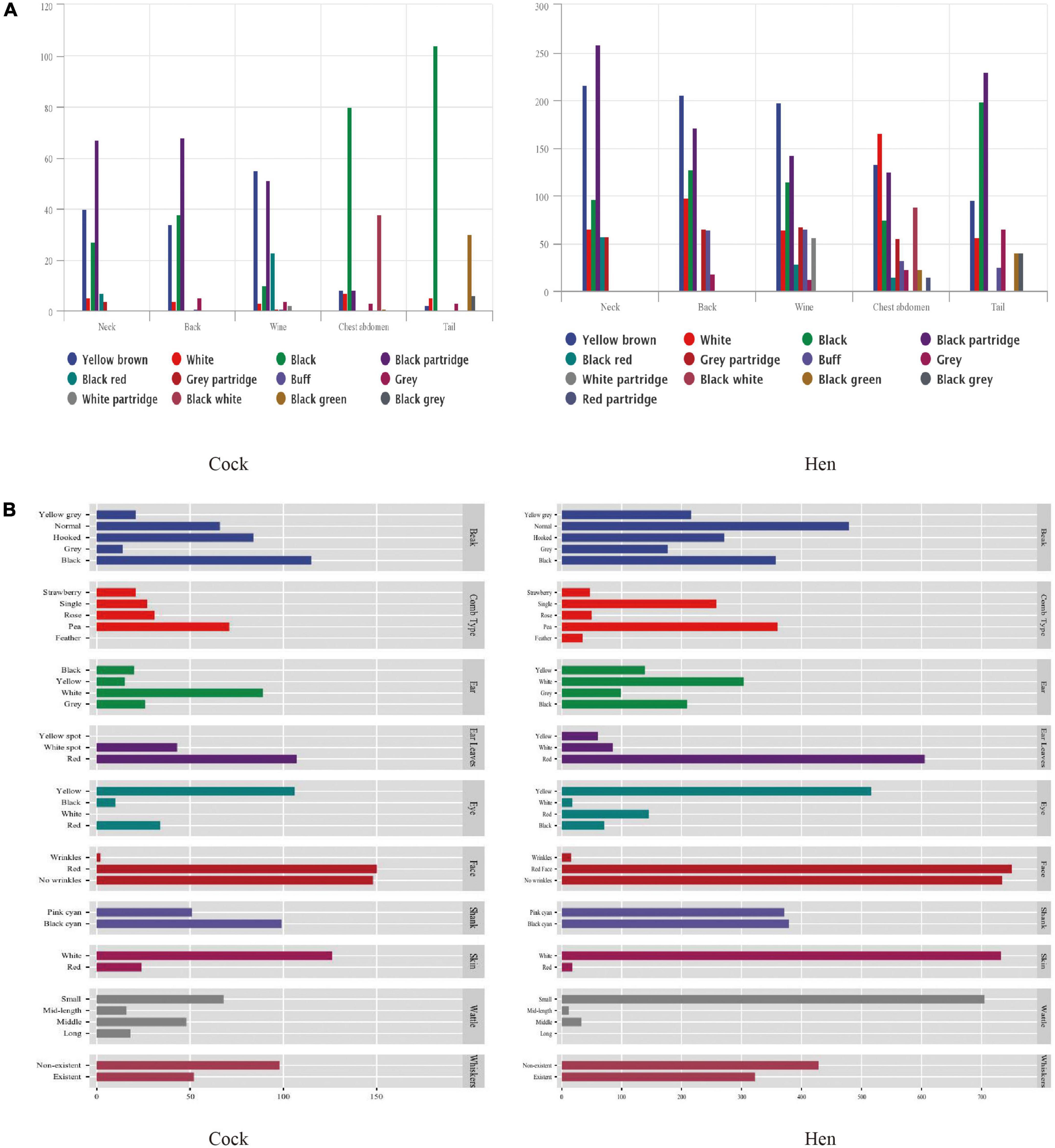
Figure 2. Plumage colors and morphological characteristics of the Anjian chickens. (A) Plumage colors; (B) morphological characteristics.
The neck and back feathers of the cocks were mainly black partridge, while the wing feathers were mainly yellow-brown and black partridge. For the cocks, the chest, abdomen, and tail feathers were mainly black. Conversely, the neck, back, and wing feathers of the hens were mainly yellow-brown, and the chest and abdomen feathers were mainly white and black partridge or buff. The tail feathers of the hens were predominantly black or black partridge.
The pea comb was predominant among the male Anjian chickens. The skin of the male chickens was predominantly white, although a few chickens had red skin on their necks and chests. The beaks were hooked and were most frequently black. The ears were predominantly white, and the ear leaves were predominantly red but occasionally with white spots. The eyes were mainly yellow, and none of the 150 cocks sampled had white eyes. The wattle was usually small. The faces of the Anjian chickens were red and mostly wrinkled. The shanks of the Anjian chickens were mainly black-cyan. The cocks and the hens were morphologically similar, although the beaks of the Anjian hens were not hooked.
Photographs of the Anjian chickens are shown in Figure 3. Given that the combs were pea-type and the skin was red, it is probable that the Anjian chicken could have been bred from the game chickens. Of note is that the Anjian cocks with a pea-shaped comb and red skin are docile.

Figure 3. Images of the morphological characteristics observed in the Anjian chickens. (A) Dominant plumage colors; (B) main comb shapes and head characteristics; (C) skin and shank colors.
Quantitative variables of the Anjian chicken
The quantitative parameters of the Anjian chicken body are shown in Table 1, while the crowing results are shown in Table 2. The variation coefficient of body weight and body size indexes (body weight, keel length, chest depth, and tibia length.) of the Anjian cock was large. Given the large body weight and size, the chicken is ideal for breeding. The average continuous crowing time of the Anjian chickens was 6.02 s but ranged from 2.51 to 11.20 s.
Genetic characterization
The genetic diversity of the 1,235 bp D-loop region of 29 Anjian chickens is shown in Table 3. Generally, 30 polymorphic loci were detected, accounting for approximately 2.43% of the total analyzed loci. There were 19 alignment gaps, eight single polymorphic loci (221, 230, 233, 260, 341, 366, 962, and 1,164 bp), 22 simple information loci (178, 223, 228, 236, 253, 254, 267, 272, 292, 317, 321, 326, 353, 374, 378, 457, 697, 722, 803, 1,225, and 1,226 bp), and 2-base variant sites. The nucleotide diversity (Pi) was 0.00618. A total of 10 haplotypes were identified, and the haploid diversity (Hd) was 0.776, demonstrating moderate genetic diversity among the haplotypes. The average number of nucleotide differences (k) between sequences in the population was 7.631. The Tajima’s D value was −0.00407, which was insignificant (P > 0.10), implying that the variations were neutral mutations. Diversity analysis and the Tajima’s D test showed that Anjian chickens have undergone a bottleneck effect in the process of evolution.
The phylogenetic tree of the Anjian chicken’s mtDNA D-loop sequences is shown in Figure 4. There was no difference in the phylogenetic trees constructed using the ML and NJ method. The phylogenetic tree showed that all 10 haplotypes (H) of the Anjian chicken were clustered into two branches, among which H5 and H9 belonged in one group, and haplotypes H1, H3, H4, and H2, H6, H7, H8, and H10 belonged in the other group.
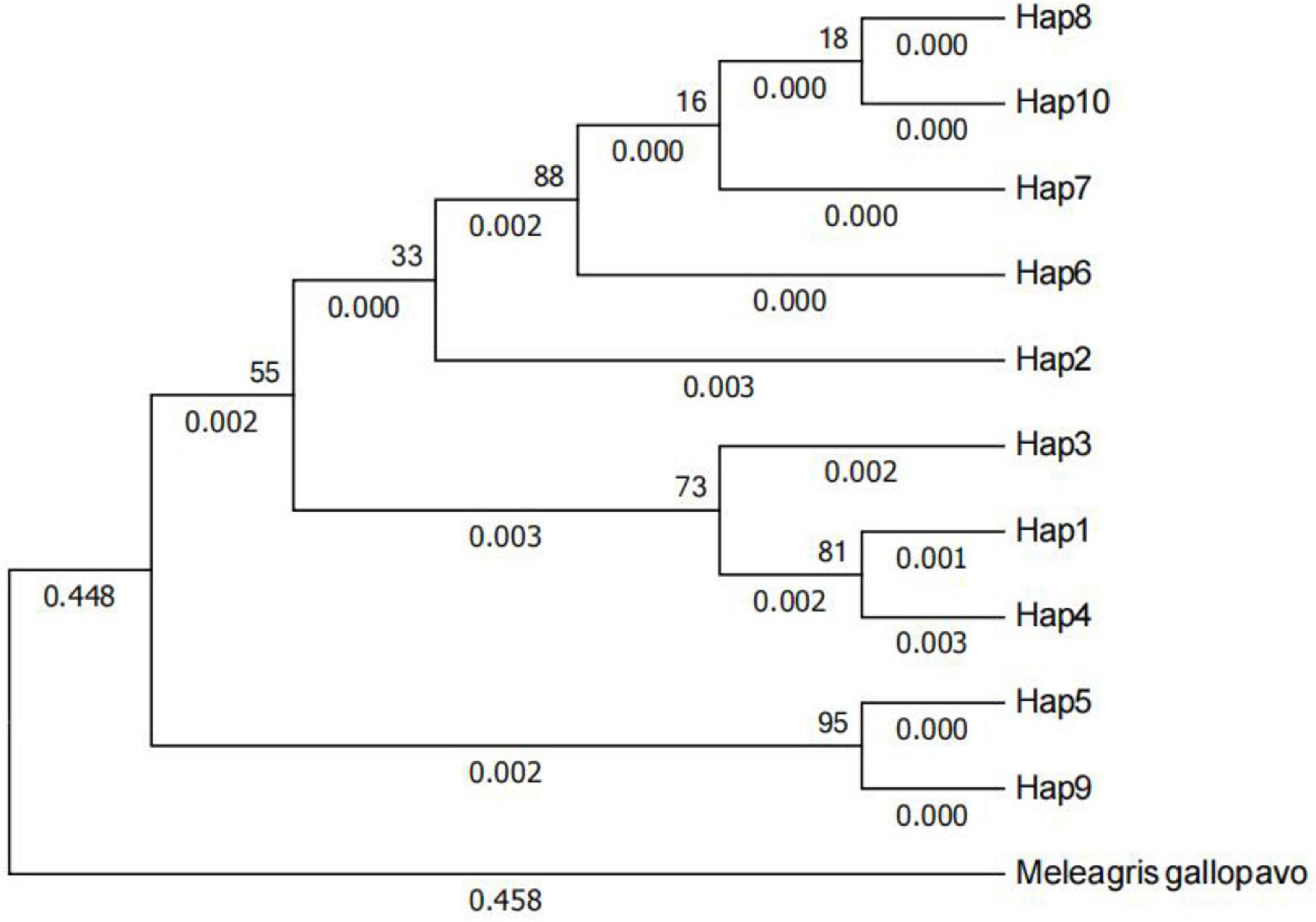
Figure 4. Phylogenetic tree of the 10 haplotypes of Anjian chicken was constructed using the adjacency method (NJ). A breed of turkey (EF153719.1) was selected as the outgroup. The bootstrap value percentage is displayed above the branch.
The NJ phylogenetic tree constructed based on the mtDNA D-loop sequences of 10 Anjian chicken haplotypes, 36 other chicken breeds, and one Turkey breed is shown in Figure 5. The topological results obtained from the phylogenetic trees constructed using the ML method and the NJ method were the same. As shown in the cluster diagram, the cluster self-development values were small, and most of them were 0. This implies that considerable differences exist between the Anjian chicken and other chicken breeds, and this may be because the Anjian chicken is reared in isolation in the remote Hotan, Xinjiang region. The Anjian chicken haplotypes were located in the same branch as multiple breeds of game chickens and red jungle fowl. The phylogenetic analysis showed that the mtDNA sequences of the Anjian chicken were widely scattered in many different clusters in the tree.
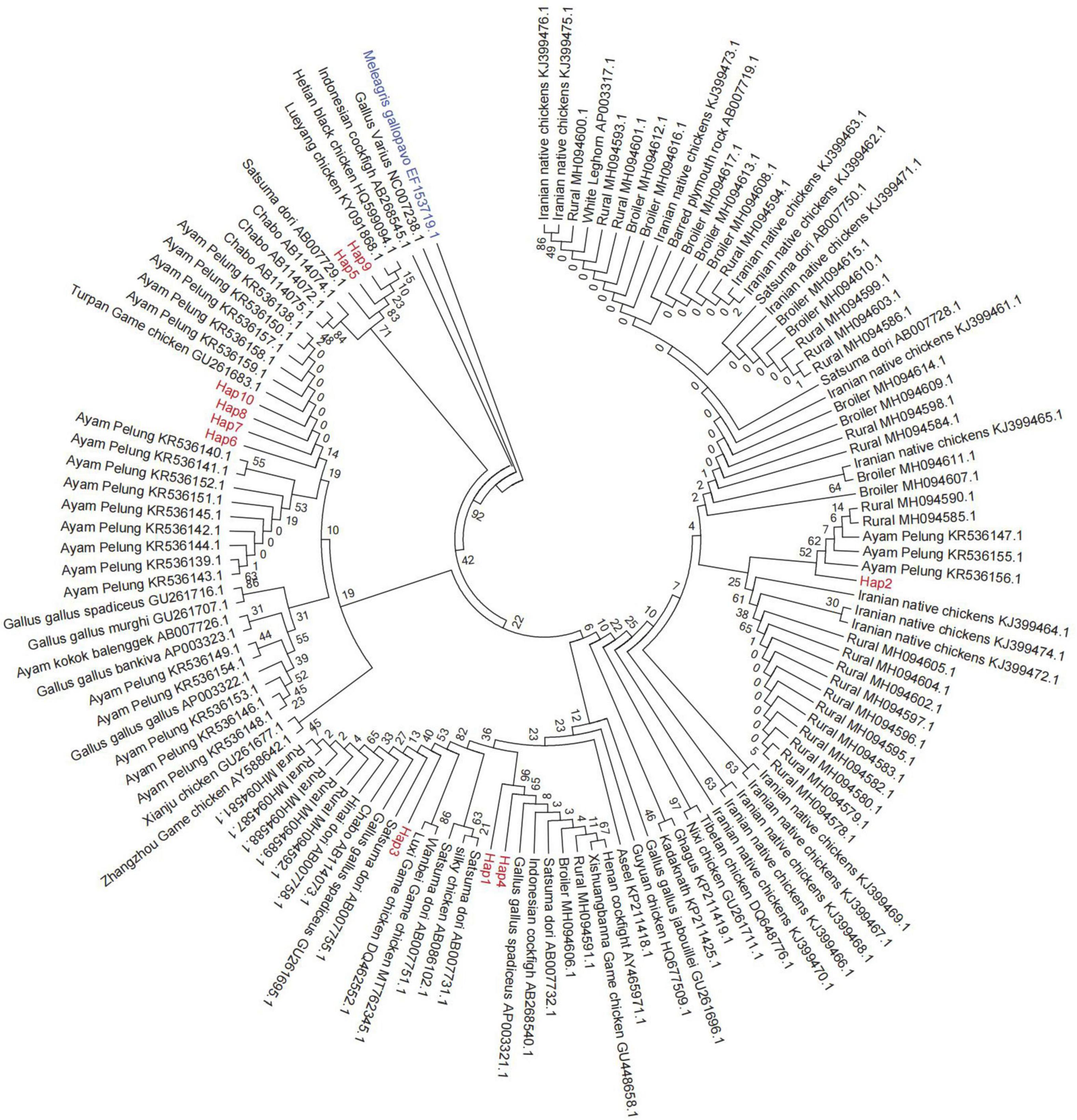
Figure 5. The phylogenetic relationships between the Anjian chicken and 36 other breeds. Anjian chicken haplotypes are highlighted in red, and a breed of turkey (EF153719.1) was selected as the outgroup. The bootstrap value percentage is displayed above the branch.
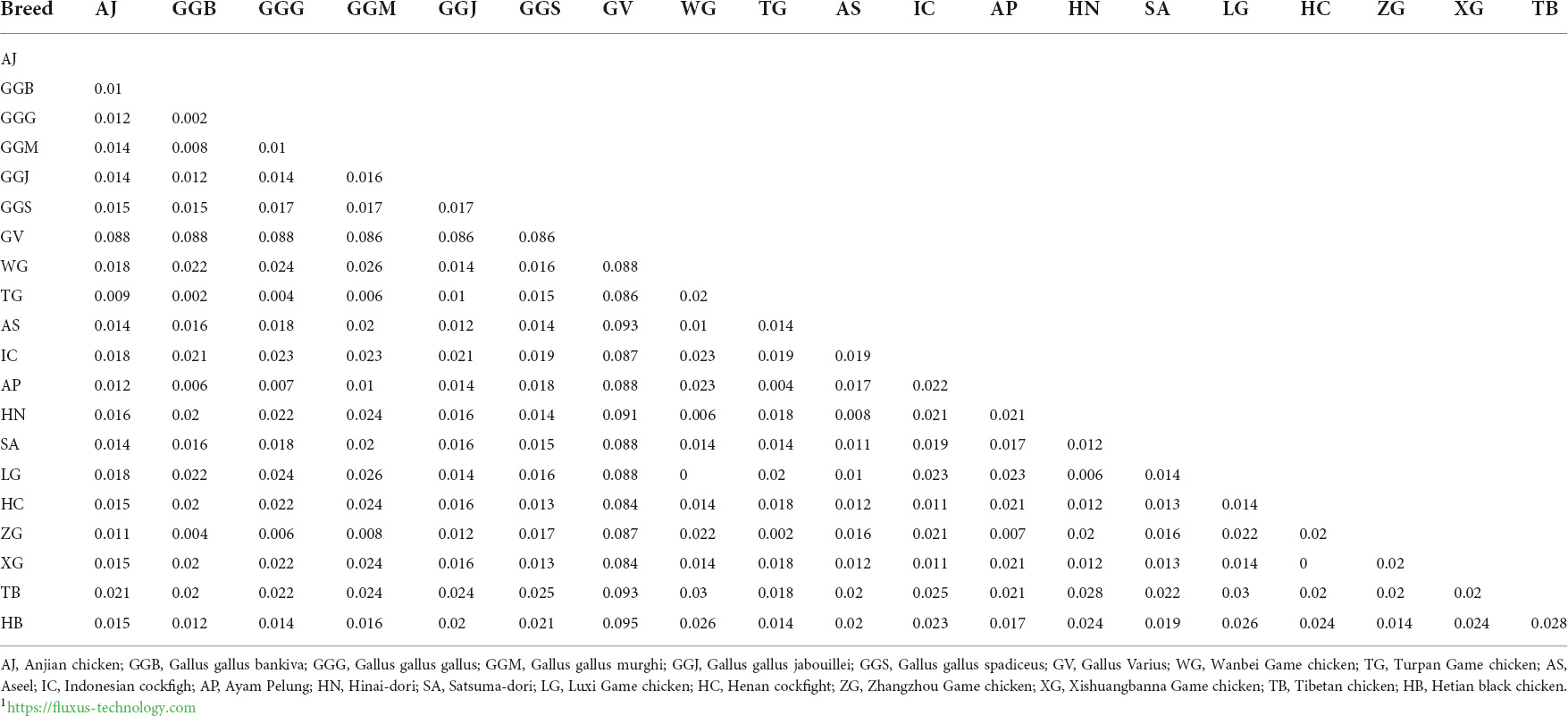
Based on the phylogenetic results, the Anjian chicken probably originated from the more primitive red jungle fowl and game chickens and was then bred with the matrilineal domestic chickens in East and Southeast Asia, implying that the Anjian chicken originated from multiple lineages. Therefore, we analyzed 59 partial sequences (510 bp) of mtDNA D-loop region of 20 Anjian chicken, Game chicken, Jungle fowl, Pelung chicken, Tibetan chicken, and Hetian black chicken, with a total of 29 haplotypes. The genetic distance between these breeds is shown in Table 4, and the median connection network in Figure 6.
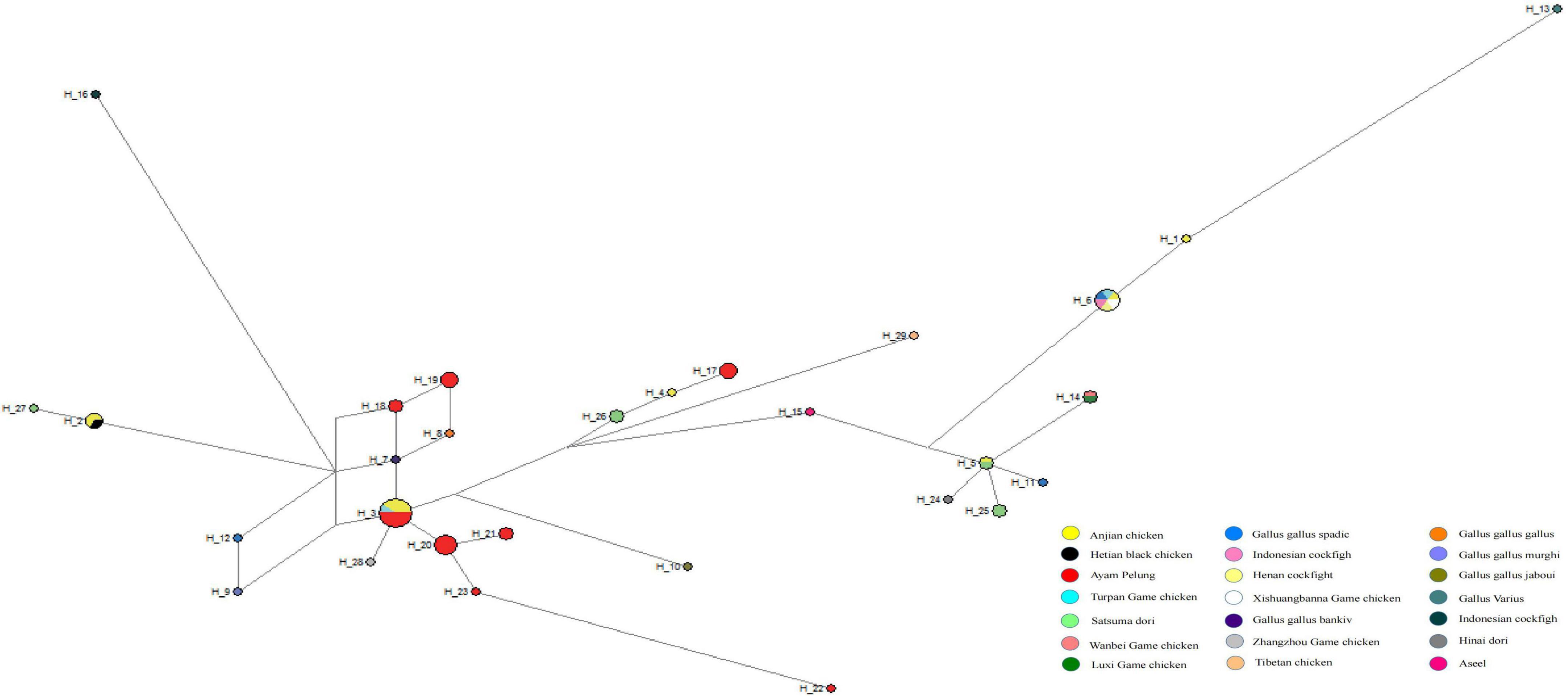
Figure 6. Median connectivity network based on the D-loop DNA sequences of 29 haplotypes from 20 chicken breeds. Each population is represented by different colors and shows the proportion of different populations in each haplotype. A breed of turkey (EF153719.1) was selected as the outgroup.
According to the genetic distance between breeds (Table 4), the closest relatives to the Anjian chicken are the Turpan game chicken, the five red jungle fowl subtypes, Aseel, Ayam pelung, Satsuma-dori, Henan cockfight, Xishuangbanna game chicken, Zhangzhou game chicken, and Hetian black chicken. The genetic distance between Gallus Varius and the Anjian chicken is relatively large, indicating that the Anjian chicken is not a descendant of Gallus Varius.
The median connectivity network (Figure 6) shows that the haplotypes of the Anjian chicken are embedded or clustered with other chicken breeds, and the shared haplotypes between the Anjian chicken and other chicken breeds and the mixing between those breeds demonstrate the gene flow and hybridization among different chicken breeds in the past. Gallus Varius (NC007238.1, haplotype H13) and the Indonesian cockfight (AB268545.1, H16) were relatively independent, consistent with the phylogenetic tree findings. Generally, the genetic analyses revealed that the Anjian chicken originated from the red prairie chicken and the cockgame chickens and has multiple matrilineal origins.
Discussion
The present study revealed that colors of feathers of Anjian chickens are considerably diverse, ranging from black, yellow, white, gray, buff, and red to brown, consistent with studies in different countries, including Pakistan, Sri Lanka, and Indonesia (Iqbal et al., 2015; Liyanage et al., 2015; Mahmood et al., 2017; Maharani et al., 2021). The MC1R gene that codes for the black color of feathers is located on the E locus and affects the synthesis of eumelanin and brown melanin by regulating tyrosinase activity (Feng et al., 2017). Eumelanin makes the feathers black and dark brown, and brown melanin makes Red and yellow on the same feather (Roulin and Ducrest, 2013). More than 60% of the Anjian hens had light-yellow colored feathers, while most cocks were predominantly black. Female (Asmara et al., 2019b) and male (Asmara et al., 2019a) Pelung chickens have predominantly black feathers. The tail feathers of the Anjian chicken were fledged and warped, similar to those of the native Hetian chicken, while the tail feathers of the game chickens were small and drooping. In some cases, the color of the feathers and skin could reveal the ancestry of birds, precisely the genetic origin of breeds and strains. Thus, it could guide the development of a hybrid breeding system.
Feather color is an important phenotypic trait that reflects breed and gender. Anjian chickens mainly have pea comb and also have single comb. Pea comb is a specifical comb type of cockfighting chickens (Iqbal et al., 2015; Liyanage et al., 2015; Mahmood et al., 2017; Asmara et al., 2019a; Maharani et al., 2021). Male Pelung chickens have a single comb (Asmara et al., 2019a), which is common in chickens in other tropical countries, such as Sri Lanka (Liyanage et al., 2015) and Nigeria (Rotimi et al., 2016). The jungle fowl only has a single comb (Desta, 2019).
The beaks of the male Anjian chickens were hooked, but those of their female counterparts were straight. Straight beaks are believed to be the best for producing pleasant crowing (Asmara et al., 2019a). Disease, environmental factors, nutrition, and genetics affect the beak shape (Bai et al., 2014).
Eye color may distinguish different types of chicken breeds. Most Anjian chickens have yellow eyes, while the eye of the cock game chickens is predominantly yellow (Mahmood et al., 2017). Yellow eyes are also the most common feature of Indonesian native chickens (Maharani et al., 2021).
The shanks of Anjian chicken were mostly black-cyan. Consumers in different regions have different preferences for chicken shank colors. For example, South China consumers prefer a yellow shank, while in Southwest China, a greenshank is most preferred. Therefore, shank color directly impacts the selling price, and this factor could impact chicken breeding, farming, and marketing in some regions (Li et al., 2020).
The skin color of the Anjian chicken was predominantly white, but few chickens had red neck and chest skin. The red skin in some Anjian chickens is caused by excessive androgens, which could have been inherited from the game chickens. Most Anjian chickens have white skin, black beaks, and black-cyan shanks. A phylogenetic analysis based on the carotene dioxygenase 2 (BCDO2) gene showed that white skin and black shank and beak are typical characteristics of the red prairie chicken, suggesting that breeds with this characteristic may have originated from this species (Gao et al., 2017).
Most Anjian chicken has pea comb, small wattles, and yellow eyes. Pea comb, small wattles, and yellow eyes are the common characteristics of all cockgame chickens (Mahmood et al., 2017), consistent with previous studies (Iqbal et al., 2015; Liyanage et al., 2015).
The average weight of the adult Anjian chickens (3.71 ± 0.50 and 2.67 ± 0.37 kg) was Significantly higher than that of the local Hetian black chicken (1.93 ± 0.20 and 1.72 ± 0.12 kg) (Li et al., 2010), Baicheng oil chicken (2.40 ± 0.33 and 1.93 ± 0.26 kg) (Yang et al., 2021), and other cockfighting species in China, such as Turpan game chickens (2.48 and 1.95 kg) (Xue et al., 2018) and Xishuangbanna game chickens (2.61 ± 0.10 and 1.93 ± 0.05 kg) (Sun et al., 2017). The average weight of Anjian chicken is comparable to that of pelung (3.55 ± 0.16 kg) (Maharani et al., 2021). The body shape of the Anjian chicken was similar but significantly larger to that of the Hetian native chicken. Thus, Anjian chicken cocks are ideal for the ternary hybridization of broilers.
The average continuous crowing length of the Anjian chickens (6.02 ± 1.67 s) was significantly shorter than that of the Pelung chicken (8.85 ± 1.38 s), popular in Indonesia (Partasasmita et al., 2020).
Genetic diversity within a species is an important characteristic that mediates adaptation to changing environments, such as harsh conditions and disease. The genetic diversity of the population demonstrates its evolutionary potential and environmental adaptability. Protecting the genetic diversity of a population is a very important consideration for any future breeding program. mtDNA is a maternal inheritance material that carries important genetic information and could be responsible for certain characteristics of breeds (Skibinski et al., 1994). Genetic studies can reveal the evolutionary origin and history of species (Lohman et al., 2008), and the degree of genetic variability is mainly reflected in the level of genetic diversity. Hd and Pi are important parameters for evaluating population genetic diversity, and their value is directly proportional to the genetic variation and adaptability of organisms to environmental changes and pressure (Zhao et al., 2017). The Anjian chickens are generally moderately genetically diverse from local chicken breeds. Its hd of 0.776 is lower than that of rural Pakistan (0.825 ± 0.051) (Nisar et al., 2019), higher than that of the Tibetan chicken (0.658 ± 0.065) (Jia et al., 2020), and the Pakistan boiler (0.455 ± 0.0170) (Nisar et al., 2019). The Pi of Anjian chicken was 0.00618, higher than that of the Tibetan chicken (0.00442 ± 0.00094) (Wang et al., 2017), rural Pakistan (0.00536 ± 0.00075), and the Pakistan broiler (0.00212 ± 0.00136) chicken (Nisar et al., 2019). Generally, the Anjian chicken has a rich genetic diversity and high selection potential.
According to the genetic distance between breeds, the red jungle fowl, Hetian black chicken, and certain species of cockgame chickens are the closest relatives to the Anjian chicken. Genetics affects phenotypic characteristics such as body length and size (Mariandayani et al., 2013), consistent with our analysis of the body weight and shape of the Anjian chicken.
Phylogenetic analysis showed that Anjian chicken originated from red prairie chicken and the cockgame chickens, demonstrating its multiple matrilineal origins. Most native chickens in China have multiple matrilineal inheritances (Bao et al., 2008). The genetic diversity analysis based on molecular markers in the mtDNA D-loop region showed that the Qingyuan partridge chicken displays a high mitochondrial genetic diversity and may have five maternal origins (Zhang et al., 2022). Phylogenetic analysis of the mtDNA CR of 73 Gushi chickens showed that this breed has multiple maternal origins (Gu et al., 2022). Based on the phenotypic characteristic and phylogenetic evolution analysis, the Tibetan chickens display multiple matrilineal inheritances (Jia et al., 2020).
Studies have shown that Anjian chickens are richly diverse in feather color and general appearance. Based on the phenotypic features and genetic analysis of the mtDNA D-loop region, the Anjian chicken is a descendant of the red jungle fowl, the game chickens, and other domestic chickens in East and Southeast Asia, which explains the rich diversity in the feather color and the size of Anjian chickens.
Conclusion
In conclusion, Anjian chickens predominantly originated from red jungle fowl and game chickens, but it is also slightly related to multiple species. The findings of this study enrich our understanding of the genetic blueprints and origin of the Anjian chicken and create the foundation for future studies and improvement of this breed.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of Tarim University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
NY and CT created the figures, performed analyses, and wrote the manuscript. WA, H’eW, XS, and CH designed and provided the guidance for the genetics methods. TT, YY, NK, HL, and AW collected the blood and extracted DNA. GM designed and supervised the project. All authors contributed to the preparation of the final manuscript and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation of China (31060294 and 31160448).
Acknowledgments
We gratefully thanks the Anjian chicken farmers in the Hotan area for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Asmara, I. Y., Garnida, D., Setiawan, I., and Partasasmita, R. (2019a). Short Communication: Phenotypic diversity of male pelung chickens in West Java Province, Indonesia. Biodiversitas 20, 2243–2248. doi: 10.13057/biodiv/d200819
Asmara, I. Y., Garnida, D., Tanwiriah, W., and Partasasmita, R. (2019b). Qualitative morphological diversity of female Pelung Chickens in West Java, Indonesia. Biodiversitas 20, 126–133. doi: 10.13057/biodiv/d200115
Bai, H., Zhu, J., Sun, Y., Liu, R., Liu, N., Li, D., et al. (2014). Identification of genes related to beak deformity of chickens using digital gene expression profiling. PLoS One 9:e107050. doi: 10.1371/journal.pone.0107050
Bao, W. B., Shu, J. T., Wang, C. B., Zhang, H. X., Weigend, S., and Chen, G. H. (2008). Investigation on genetic diversity and systematic evolution in chinese domestic fowls and red jungle fowls by analyzing the mtDNA control region. Acta Vet. Et Zootech. Sin. 39, 1449–1459.
Chen, K. W., Yang, N., Wang, G. Y., Wang, Z. Y., Wang, K. H., Wang, J. Y., et al. (2010). Animal Genetic Resources in China-Poultry. China: China Agriculture Press.
Desjardins, P., and Morais, R. (1990). Sequence and gene organization of the chicken mitochondrial genome: A novel gene order in higher vertebrates. J. Mol. Biol. 212, 599–634. doi: 10.1016/0022-2836(90)90225-b
Desta, T. T. (2019). Phenotypic characteristic of junglefowl and chicken. Worlds Poult. Sci. J. 75, 69–82. doi: 10.1017/S0043933918000752
FAO (2012). Phenotypic Characterization of Animal Genetic Resources. FAO Animal Production and Health Guidelines No. 11. Rome: FAO.
Feng, J. Q., Xu, W., Huang, L., Luo, W. X., and Cai, H. F. (2017). Research Advances on MC1R Gene. China Anim. Husb. Vet. Med. 04, 1141–1148. doi: 10.16431/j.cnki.1671-7236.2017.04.029
Gao, Y. S., Jia, X. X., Tang, X. J., Fan, Y. F., Lu, J. X., Huang, S. H., et al. (2017). The genetic diversity of chicken breeds from Jiangxi, assessed with BCDO2 and the complete mitochondrial DNA D-loop region. PLoS One 12:e0173192. doi: 10.1371/journal.pone.0173192
Gizaw, S., Komen, H., Windig, J. J., Hanotte, O., and Arendonk, J. (2008). Conservation priorities for Ethiopian sheep breeds combining threat status, breed merits and contributions to genetic diversity. Genet. Sel. Evol. 40, 433–447. doi: 10.1186/1297-9686-40-4-433
Gu, R., Tang, X. J., Fan, Y. F., Jia, X. X., Ge, Q. L., and Lu, J. X. (2022). Analyses of genetic diversity and phylogenetic evolution of Gushi chicken based on mitochondrial DNA control region sequence. J. Yangzhou Univ. 43, 56–61. doi: 10.16872/j.cnki.1671-4652.2022.01.008
Hall, S. J. G., and Bradley, D. G. (1995). Conserving livestock breed biodiversity. Trends Ecol. Evol. 10, 267–270. doi: 10.1016/0169-5347(95)90005-5
Iqbal, A., Ali, A. A., Javed, K., Akram, M., Usman, M. C., Mehmood, S., et al. (2015). Phenotypic characterization of two indigenous chicken ecotypes of Pakistan. J. Anim. Plant. Sci. 25, 346–350.
Jia, X. X., Lu, J. X., Tang, X. J., Fan, Y. F., Ge, Q. L., and Gao, Y. S. (2020). Characterization and phylogenetic evolution of mitochondrial genome in Tibetan chicken. Anim. Biotechnol. 2020, 1–7. doi: 10.1080/10495398.2020.1858846
Li, H. F., Zheng, X. C., and Deng, B. Z. (2020). Research progress on chicken shank color traits. Poult. raising Poult. Dis. Contr. 2020, 21–23.
Li, X. B., Liu, L., and Lu, H. H. (2010). Discussion on the protection and utilization of Hetian black chicken germplasm resources. Chin. Livest. Poult. Breed. 6, 124–126.
Liu, Y. P., Wu, G. S., Yao, Y. G., Miao, Y. W., Luikart, G., Baig, M., et al. (2006). Multiple maternal origins of chickens: Out of the Asian jungles. Mol. Phylogenet. Evol. 38, 12–19. doi: 10.1016/j.ympev.2005.09.014
Liyanage, R. P., Dematawewa, C. M. B., and Silva, G. L. L. P. (2015). Comparative study on morphological and morphometric features of village chicken in Sri Lanka. Trop. Agric. Res. 26, 261–273. doi: 10.4038/tar.v26i2.8090
Lohman, D. J., Peggie, D., Pierce, N. E., and Meier, R. (2008). Phylogeography and genetic diversity of a widespread Old World butterfly, Lampides boeticus (Lepidoptera: Lycaenidae). BMC Evol. Biol. 8:301. doi: 10.1186/1471-2148-8-301
Luo, H. D., Li, H. J., Huang, A., Ni, Q. Y., Yao, Y. F., Xu, H. L., et al. (2019). The complete mitochondrial genome of Platysternon megacephalum peguense and molecular phylogenetic analysis. Genes 10:487. doi: 10.3390/genes10070487
Maharani, D., Mustofa, F., Sari, A. P. Z. N., Fathoni, A., Sasongko, H., and Hariyono, D. N. H. (2021). Phenotypic characterization and principal component analyses of indigenous chicken breeds in Indonesia. Vet. World. 14, 1665–1676. doi: 10.14202/vetworld.2021.1665-1676
Mahfudz, L. D., Wulandari, A. R., and Johari, S. (2011). Genetic variation through polymorphism of blood and egg white protein in three kinds of Kedu chickens at laying period. Anim. Prod. Sci. 13, 83–88.
Mahmood, S., Rehman, A. U., Khan, M. S., Lawal, R. A., and Hanotte, O. (2017). Phenotypic diversity among indigenous cockfighting (Aseel) chickens from Pakistan. J. Anim. Plant. Sci. 27, 1126–1132.
Mariandayani, H. N., Solihin, D. D., Sulandari, S., and Sumantri, C. (2013). Phenotypic variation and estimation genetic distance between local chicken and broiler chicken using morphological analysis. Vet. J. 14, 475–484.
Muhatai, G., Azimu, W., Zhai, F., and Ahemetti, T. T. (2012). “Anjiyan chicken”—— Hotan unique precious home chicken resources. China Poult. 34, 65–66. doi: 10.16372/j.issn.1004-6364.2012.05.021
Mustofa, F., Fathoni, A., Sari, A. P. Z. N., Sasongko, H., and Maharani, D. (2021). Body weight and body size measurement of five Indonesian local chicken. Earth Environ. Sci. 788:012016. doi: 10.1088/1755-1315/788/1/012016
Nisar, A., Waheed, A., Khan, S., Feng, X., and Shah, A. H. (2019). Population structure, genetic diversity and phylogenetic analysis of different rural and commercial chickens of Pakistan using complete sequence of mtDNA D-loop. Mitochondrial DNA, A. DNA Mapp. Seq. Anal. 30, 273–280. doi: 10.1080/24701394.2018.1484118
Osman, S. A. M., Yonezawa, T., and Nishibori, M. (2016). Origin and genetic diversity of Egyptian native chickens based on complete sequence of mitochondrial DNA D-loop region1. Poult. Sci. 95, 1248–1256. doi: 10.3382/ps/pew029
Partasasmita, R., Asmara, I. Y., and Garnida, D. (2020). Crowing characteristics of Pelung chickens at different age and body weight. Biodiversita 21, 4339–4344. doi: 10.13057/biodiv/d210953
Plante, Y., Vega-Pla, J. L., Lucas, Z., Colling, D., De March, B., and Buchanan, F. (2007). Genetic diversity in a feral horse population from Sable Island, Canada. J. Hered. 98, 594–602. doi: 10.1093/jhered/esm064
Rotimi, E. A., Egahi, J. O., and Adeoye, A. A. (2016). Phenotypic characterization of indigenous chicken population in Gwer-West, Benue State, Nigeria. Word Sci. News. 53, 343–353.
Roulin, A., and Ducrest, A. L. (2013). Genetics of colouration in birds. Semin. Cell Dev. Biol. 6–7, 594–608. doi: 10.1016/j.semcdb.2013.05.005
Skibinski, D. O. F., Gallagher, C., and Beynon, C. M. (1994). Mitochondrial DNA inheritance. Nature 368, 817–818. doi: 10.1038/368817b0
Sun, Y. J., Sun, F. C., Guo, A. W., and Yang, Y. J. (2017). Investigation on breed characteristics and breeding status of fighting cocks in Xishuangbanna. Heilongjiang Anim. Sci. & Vet. Med. 11, 202–203, 299. doi: 10.13881/j.cnki.hljxmsy.2017.1928
Wang, H., Chen, Y., Yue, Q. X., Zhao, C. J., Zhou, R. Y., Li, L. H., et al. (2017). Study on sequence variation and origin based on mtDNA D-loop of Bashang Long-tail Chicken. China Poult. 20, 11–17. doi: 10.16372/j.issn.1004-6364.2017.20.003
Xue, Z. F., Ainiwal, L., Azati, Zhang, Y. H., Wang, J. X., and Yu, L. J. (2018). Research on germplasm characteristics of Turpan cockfighting. Heilongjiang Anim. Sci. & Vet. Med. 11, 217–219. doi: 10.13881/j.cnki.hljxmsy.2017.06.0132
Yang, S., Cao, Y. Q., Li, H. Y., Lu, L. Z., Li, G. Q., Zeng, T., et al. (2021). Analysis of production performance and genetic diversity of Baicheng oil chicken. J. Zhejiang Agric. Sci. 62, 1438–1442. doi: 10.16178/j.issn.0528-9017.20210751
Zanetti, E., De Marchi, M., Dalvit, C., and Cassandro, M. (2010). Genetic characterization of local Italian breeds of chickens undergoing in situ conservation. Poult. Sci. 89, 420–427. doi: 10.3382/ps.2009-00324
Zhang, J., Jia, X. X., Lu, J. X., Shen, X., Tang, X. J., Fan, Y. F., et al. (2022). Genetic diversity of Mitochondrial DNA D-loop region in Qingyuan partridge chicken. China Poult. 44, 24–28. doi: 10.16372/j.issn.1004-6364.2022.02.004
Zhao, X. B., Wang, B. J., Jian, K. L., Yang, G. Y., Lai, W. M., and Gu, X. B. (2017). Analysis of the characteristics of genetic variability within Psoroptes cuniculi isolates in some regions of China, inferred by Mitochondrial Cytochrome Oxidase b Gene. Acta Vet. Et Zootech. Sin. 48, 714–721. doi: 10.11843/j.issn.0366-6964.2017.04.015
Keywords: Anjian chicken, appearance, plumage color, crowing, mtDNA D-loop
Citation: Yang N, Tang C, Azimu W, Wang H, Tuersuntuoheti T, Yalimaimaiti Y, Kelimu N, Li HS, Wumaier A, Sun XY, Hao CS and Muhatai G (2022) Phenotypic and genetic diversity of the Anjian chicken in China. Front. Ecol. Evol. 10:1003615. doi: 10.3389/fevo.2022.1003615
Received: 26 July 2022; Accepted: 16 September 2022;
Published: 12 October 2022.
Edited by:
Guanglin He, Sichuan University, ChinaReviewed by:
Mengge Wang, Sichuan University, ChinaYi Zhou, University of Southern California, United States
Copyright © 2022 Yang, Tang, Azimu, Wang, Tuersuntuoheti, Yalimaimaiti, Kelimu, Li, Wumaier, Sun, Hao and Muhatai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gemingguli Muhatai, Z21nbC0xMTNAZm94bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Na Yang1,2†
Na Yang1,2† Chi Tang
Chi Tang Hui’e Wang
Hui’e Wang