- 1Department of Environmental Sciences, University of Toledo, Toledo, OH, United States
- 2Department of Biological Sciences, California State University at Long Beach, Long Beach, CA, United States
Differing selection pressures on stationary nest contents compared to mobile offspring mean that the nest-site characteristics resulting in the highest nest success may not be the same characteristics that result in the highest survival of juveniles from those nests. In such cases, maternal nest-site choice may optimize productivity overall by selecting nest sites that balance opposing pressures on nest success and juvenile survival, rather than maximizing survival of either the egg or the juvenile stage. Determining which macro- and microhabitat characteristics best predict overall productivity is critical for ensuring that land management activities increase overall recruitment into a population of interest, rather than benefiting one life stage at the inadvertent expense of another. We characterized nest-site choice at the macro- and microhabitat scale, and then quantified nest success and juvenile survival to overwintering in two declining turtle species, eastern box turtles and spotted turtles, that co-occur in oak savanna landscapes of northwestern Ohio and southern Michigan. Nest success in box turtles was higher in nests farther from macrohabitat edges, constructed later in the year, and at greater total depths. In contrast, survival of juvenile box turtles to overwintering was greater from nests under less shade cover and at shallower total depths. Spotted turtle nest success and juvenile survival were so high that we were unable to detect relationships between nest-site characteristics and the small amount of variation in survival. Our results demonstrate, at least for eastern box turtles, a tradeoff in nest depth between favoring nest success vs. juvenile survival to overwintering. We suggest that heterogeneity in microhabitat structure within nesting areas is important for allowing female turtles to both exercise flexibility in nest-site choice to match nest-site characteristics to prevailing weather conditions, and to place nests in close proximity to habitat that will subsequently be used by hatchlings for overwintering.
Introduction
In egg-laying animals, a female’s choice of nest site must balance a variety of sometimes conflicting selection pressures (Refsnider and Janzen, 2010). The location and microhabitat characteristics of a nest site can impact the survival of multiple life-stages, including the ovipositing or incubating adult female (e.g., Ghalambor and Martin, 2001; Spencer, 2002), the eggs during embryonic development (e.g., Resetarits and Wilbur, 1989; Martin, 1993; Madsen and Shine, 1999), and the juveniles emerging from the nest (e.g., Anders et al., 1998; Kolbe and Janzen, 2001). In addition to directly affecting the survival of different life stages, physical characteristics of nest sites can also impact a variety of phenotypic traits in the offspring produced from those nests, which may influence survival or quality of those offspring later in life, and thereby indirectly impacts the reproductive fitness of the female who chose the nest site in the first place (reviewed in Noble et al., 2018; Refsnider et al., 2019).
Importantly, nest sites that are optimal for one reason, such as minimizing risk to an ovipositing or incubating female, may be sub-optimal for a different reason, such as maximizing likelihood of the eggs hatching (e.g., Madsen and Shine, 1999; Spencer, 2002; Amat and Masero, 2004). In such situations, a female’s choice of nest site may have to take into account conflicting selection pressures, which may result in a maternal nest-site choice that optimizes the overall benefits to a female’s lifetime reproductive success, while individual components of nest-site choice may appear to be maladaptive if examined in isolation (Martin, 1992; Chalfoun and Schmidt, 2012). For example, in golden-winged warblers, nest success was highest in nests in shrublands farthest from the shrub-forest edge, whereas fledgling survival was highest from nests farthest into the forest from those same edges (Streby et al., 2014a). The opposing selection pressures on nest location for nest success vs. fledgling survival resulted in a population mean nest-site choice of nests located in close proximity to the shrub-forest edge, where neither nest success nor fledgling survival were maximized, but where the number of young raised to independence from adult care was maximized (Streby et al., 2014a).
The potential ramifications to management of nest-site choice having to balance between opposing selection pressures on different life stages are profound. Traditionally, management to impact bird population productivity was, and in most cases still is, based solely on increasing nest success: that is, habitat types that resulted in the highest nest success (defined as the probability of a nest producing at least one fledgling) were assumed to be “best,” and management actions were designed to preferentially maintain such habitats over other habitat types with lower nest success (e.g., Hartway and Mills, 2012). The problem with this approach is that it assumes nest success is a complete or representative measure of productivity, and it ignores other life stages that may be impacted differentially by the same habitat type (reviewed in Streby et al., 2014b). In the golden-winged warbler example above, when management decisions are based solely on nest success, management plans assume that shrubland is the “best” habitat for increasing golden-winged warbler productivity, and therefore endeavor to create more shrubland to increase nest success. In reality, the habitat type with the highest overall productivity is actually forest-shrubland edge, which requires a habitat mosaic consisting of forest patches in various stages of succession (Streby et al., 2014b). Thus, management that takes into account only a single life stage could be creating an ecological trap if opposing selection pressures acting on a different life stage are actually driving nest-site choice (e.g., Flaspohler et al., 2001). Therefore, conservation and management decisions made on the basis of nest-site choice need to take into account the consequences of nest-site choice for multiple life stages, as well as the potential indirect effects of nest-site choice on survival or reproduction, to ensure that they are not favoring one life stage at the expense of another and inadvertently lowering overall productivity in a population of interest.
Freshwater turtles are among the world’s most imperiled taxa, and threatened turtle species are therefore a common target for conservation and management (Rhodin et al., 2017; Stanford et al., 2020). A major threat to many threatened turtle species, particularly in areas with high anthropogenic disturbance, is nest predation by mammals [in North America, primarily raccoons (Procyon lotor) and skunks (Mephitis sp.); Kolbe and Janzen, 2002]. Indeed, predation rates sometimes exceed 90% in turtle populations in areas with high human activity (e.g., Strickland et al., 2010; Refsnider et al., 2015). However, turtle nests may also fail to hatch for a variety of other reasons, including infestation by ants, plant roots suffocating eggs, nest substrate that becomes too wet or too dry, or incubation temperatures that are too cool to support embryonic development or become lethally warm (e.g., Schwarzkopf and Brooks, 1987; Packard and Packard, 1988; Buhlmann and Coffman, 2001; Socci et al., 2005). In contrast to the nest stage, generally far less is known about rates of juvenile survival in turtles due to the difficulty in studying this small, cryptic, and mobile life stage (Pike, 2006; Paterson et al., 2012). Upon emerging from the nest, hatchling turtles of many species must travel from the nest site to habitat that is suitable for finding food, shelter, and potentially overwintering (e.g., Salmon et al., 1995; Putman et al., 2010). For many aquatic turtle species, the journey from a nest site in open, sunny habitat to aquatic habitat suitable for the juvenile stage exposes hatchlings to predators, desiccation, and potentially lethal temperature extremes (Janzen, 1993; Wilbur and Morin, 1988; Janzen et al., 2000, 2007). Therefore, as in other species (e.g., Kamel and Mrosovsky, 2004; Streby et al., 2014a), nest-site choice by female turtles likely requires balancing opposing selection pressures on different life stages. For example, nest-site characteristics that produce the highest hatching success may differ from those of nests that experience the highest hatchling survival rates. Determining the characteristics of nest sites that maximize overall recruitment into the population is important, particularly for threatened species, so that management actions such as covering nests with predator-proof cages can be targeted at nests with the greatest likelihood of producing surviving hatchlings that will contribute to the population.
Here, we characterized nest-site choice at the macro- and microhabitat scale in two declining turtle species, eastern box turtles and spotted turtles, that co-occur in oak savanna landscapes in northwestern Ohio and southern Michigan. We quantified nest success and survival of juveniles to first overwintering in both species to determine which aspects of maternal nest-site choice best predicted overall productivity, so that conservation efforts can prioritize nest sites with the highest chance of contributing offspring to each turtle population.
Materials and Methods
Study Sites and Species
Historically, the glacial and lake sand plains of the Great Lakes region in North America contained abundant oak savanna, prairie, and wet prairie habitat (Nuzzo, 1986). More recently, this same landscape and its associated habitats have undergone some of the highest rates of conversion to agricultural land of any native habitats in the United States (Leach and Givnish, 1999). Much of the remaining oak savanna and wet prairie habitat in the Great Lakes region is extensively managed to preserve structure and natural communities. Indeed, these habitat types support a substantial number of rare and declining taxa (Grigore, 2009). In particular, eastern box turtles (Terrapene carolina carolina) and spotted turtles (Clemmys guttata) co-occur in the oak savanna-wet prairie landscape in the Great Lakes region.
Eastern box turtles and spotted turtles are declining throughout their geographic range due to habitat destruction and, to a lesser extent, over-collection for the pet trade (International Union of Concerned Scientists, 2011a,b). Both species are long-lived, produce relatively small clutches, and often experience high rates of nest predation, meaning that their population sizes are acutely sensitive to losses of even a few adults (Williams and Parker, 1987; Stickel and Bunck, 1989; Hall et al., 1999; Litzgus, 2006; Enneson and Litzgus, 2008; Feng et al., 2019). We studied eastern box turtles in oak savanna and mixed hardwood forest in Lucas County, Ohio, and Calhoun and Kalamazoo Counties, Michigan, United States. We studied spotted turtles in seasonally wet prairie, swamp forest, and fen habitat in Lucas County, Ohio, and Barry County, Michigan, United States. Exact study site locations are being withheld due to the susceptibility of these species to poaching and collection for the pet trade. Management activities at our study sites include prescribed burns, invasive plant species removal, brush-cutting, herbicide application, mowing, and wetland restoration.
Characterizing Nest-Site Choice
We located nests of both turtle species in 2018 and 2019 by intensively radio-tracking gravid females to their nest sites (as in Refsnider and Linck, 2012; Figure 1). Turtles of both species were hand-captured during visual encounter surveys of our study sites in April and May, prior to the nesting season, and adult females were fitted with radio-transmitters (R1-2B, Holohil; 14.5 g for box turtles; 9.5 g for spotted turtles). We quantified plastron length of all females as a measure of body size. Radio-marked females were tracked at least once weekly until the nesting season began in approximately mid-May, at which time they were located daily. Females with shelled eggs detected during palpation, traveling toward known nesting areas, or actively moving in late afternoon or early evening were monitored continuously from 1600 and 2200 h until they were either observed nesting, or became inactive for the night. Females actively nesting were monitored from a distance to avoid disturbance. Once a nest had been completed, we recorded GPS coordinates with a handheld GPS unit, covered some nests with a wire cage to prevent mammalian predation (see below), and returned the following day to characterize the microhabitat within 1 m of the nest site.
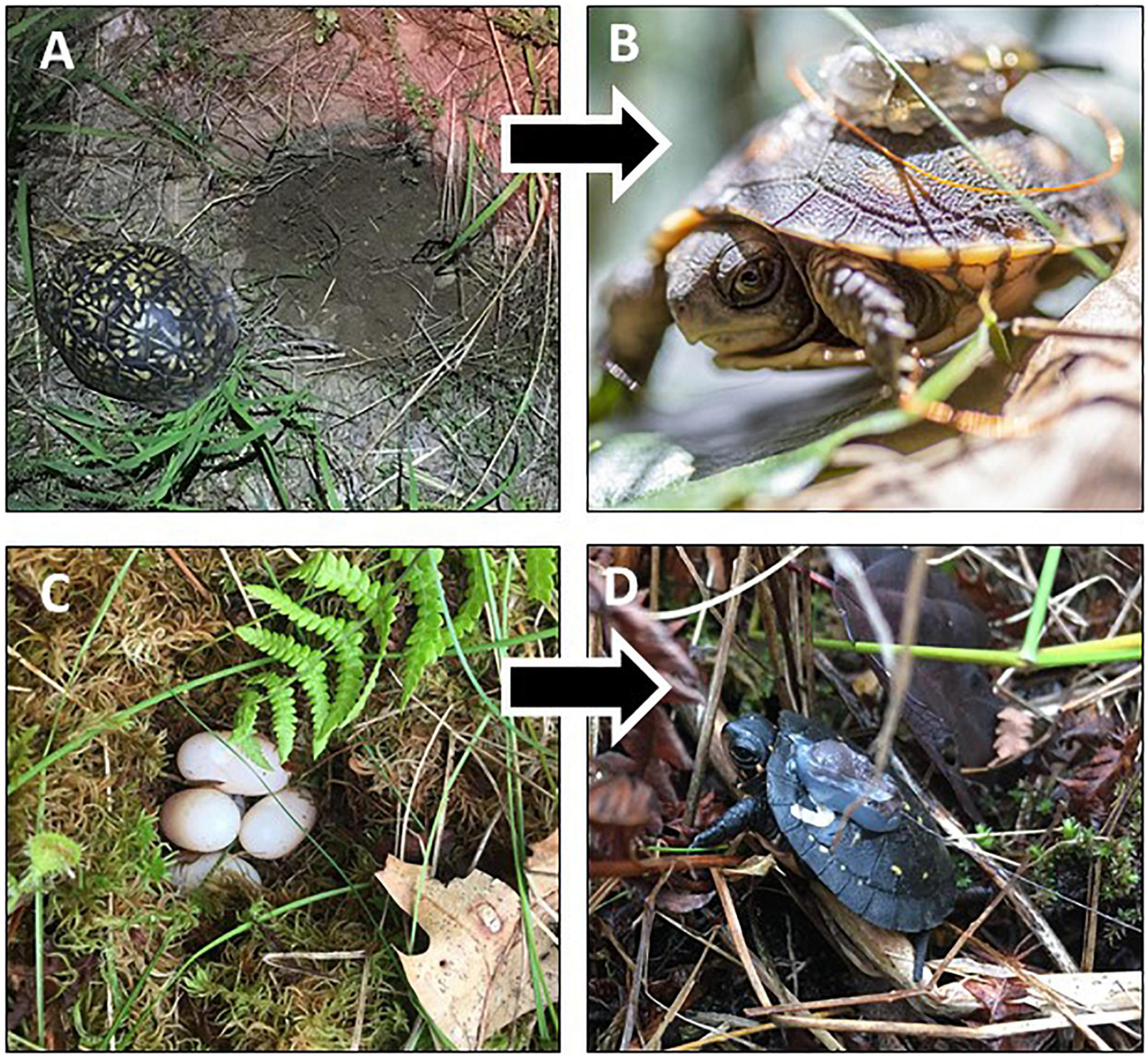
Figure 1. Nest sites were located by radio-tracking gravid female turtles to nest sites in northwestern Ohio and southern Michigan in 2018–2019. We monitored eastern box (A) and spotted (C) turtle nests until hatchling emergence, at which point miniature radio-transmitters were attached to juvenile eastern box (B) and spotted (D) turtles. Hatchlings were then monitored via radio-telemetry to determine whether they survived to enter overwintering. Photo credits: A. Hulbert.
Within 24 h of nest completion, we briefly excavated each nest to determine clutch size and to measure the total nest depth. We then replaced eggs in the nest cavity and refilled the nest. We used a 180° fisheye lens to take a hemispherical photograph directly over each nest, and we used Gap Light Analysis software (Frazer et al., 1999) to quantify shade cover from the hemispherical photographs over each nest. We classified the macrohabitat (i.e., land cover type within 10 m of the nest site) of each nest as deciduous forest, developed/residential, flooded grassland, grassland, mixed coniferous/deciduous forest, open fen, savanna, shrub swamp, or vernal wetland. We classified the microhabitat of each nest as grass; rocky soil (high organic content, < 1 mm grains interspersed with pebbles > 2 mm); rotten log; sand (well-drained, little organic matter, 1–2 mm grains); sedge; soil (high organic content, < 1 mm grains); or sphagnum. Finally, we re-covered some nests with a wire mesh cage which served both to exclude mammalian predators, and to contain recently emerged hatchlings (as in Refsnider, 2009). In 2019 we left a subset of nests unprotected to estimate mammalian predation rates in each species. For each species, we determined whether a nest would be covered with a predator-proof cage or left unprotected by caging every second nest that was constructed.
Using a combination of aerial photographs and ground-truthing, we created a georeferenced map of land cover types in ArcGIS (ESRI) for study sites in Ohio and Michigan at 0.5 m resolution (1:800). We plotted all nests in this GIS, and used the digitized land-cover map to measure the distance from each nest to the nearest edge of a different macrohabitat type, and the distance from each nest to the nearest road as an indicator of degree of habitat fragmentation in the vicinity of the nest site.
Quantifying Nest Success and Juvenile Survival
From August 1 to late October, we checked nests every 24–72 h for signs of hatchling emergence. If hatchlings did not emerge within 100 days of nest construction, we carefully excavated the nest to determine why the nest had failed (which was most often predation by a burrowing snake or rodent, or desiccation of eggs). For nests where some hatchlings successfully emerged, we excavated the nest cavity, searched the area within the cage to locate remaining hatchlings, and determined the fate of any unhatched eggs or dead hatchlings (e.g., desiccated eggs, eggs entangled by plant roots, egg eaten by ants, dead hatchlings still within the eggshell, or apparently unviable eggs). For each nest, “nest success” was assigned as 1 if any live hatchlings were recovered from the nest. Nest success was 0 if no live hatchlings were recovered and there was evidence of predation or egg mortality as described above.
We weighed hatchlings with a digital scale to the nearest 0.1 g. From each nest, we randomly selected two hatchlings to be radio-tracked, with the caveat that radio-transmitters had to weigh < 10% of a hatchling’s mass. We first painted radio-transmitters (Blackburn Transmitters) brown for camouflage, placed a bead of silicone rubber aquarium sealant (Marineland) on the center midline of a hatchling’s carapace, and placed the transmitter directly on the sealant bead. We also attached a 5–8 cm piece of blaze orange thread to the sealant bead to aid in locating hatchlings that were not visible on the ground surface. We allowed the glue to dry overnight, and then released radio-marked hatchlings at their nest site the following day. Hatchlings not radio-tracked were released at their nest site after they were measured and weighed.
To quantify juvenile survival to overwintering, we radio-tracked hatchlings 1–3 times per week until the signal was lost, mortality was confirmed, or hatchlings began overwintering. Due to the small size of the transmitters attached to hatchling turtles, batteries lasted for 25–30 days; therefore, we collected active hatchlings after approximately 25 days to replace transmitters, and then continued to radio-track the hatchlings until mortality or onset of overwintering. Each time we tracked a hatchling turtle, we recorded its behavior (e.g., actively moving, burrowed under leaf litter, etc.), microhabitat, and macrohabitat. We also recorded hatchlings’ location using a handheld GPS unit with an accuracy of 5 m, and its distance from its previous location using a tape measure. Once a hatchling was observed buried in the same location for > 2 weeks in October or November, we assumed the hatchling had begun overwintering. Juvenile survival to overwintering was assigned as either 0 or 1. Hatchlings that were either found dead or whose transmitters were recovered with damage consistent with a predator attack were assigned a survival value of 0. Hatchlings that were known to have entered hibernation were assigned a survival value of 1. Hatchlings for which we lost the radio signal more than 3 days from predicted transmitter battery expiration were assumed to have been depredated (i.e., the transmitter was either broken or carried out of signal range by a predator) and were assigned a survival value of 0. The final group of hatchlings were those whose radio signals were lost within 3 days of predicted transmitter battery expiration. For these hatchlings, if transmitter expiration occurred after 1 October (the date at which most surviving hatchlings had reached the location at which they subsequently overwintered), we presumed the hatchling had survived to enter hibernation and assigned a survival value of 1. If transmitter expiration occurred before 1 October for hatchlings in this group, we assigned the hatchling an “unknown fate” and excluded it from analysis. All animals were handled in accordance with all required state and local scientific research permits, and with the University of Toledo’s Institutional Animal Care and Use Committee (protocol #108797).
Statistical Analysis/Modeling
In all analyses, box turtles and spotted turtles were modeled separately. We first tested for correlations among the variables (i.e., Pearson’s correlation coefficient), and subsequently retained all variables because none were strongly correlated (all r ≤ 0.44). For each species, we modeled the relationship between nest-site macrohabitat variables (distance to nearest road and distance to nearest macrohabitat edge) and nest-site microhabitat variables (day of year, shade cover over the nest, and nest depth) on nest success and juvenile survival to overwintering separately. We used binomial logistic regression to construct models of all possible combinations of macrohabitat and microhabitat variables and their effects on both nest success and juvenile survival. Models for box turtles included year as a random effect because preliminary analysis indicated a difference in nest success between years in this species only. Models for spotted turtles included individual female as a random effect because some female spotted turtles nested twice in the same year (Carter, 2021). We considered the best-supported macrohabitat and microhabitat models predicting nest success and juvenile survival to be those with the lowest AICc. We further evaluated the effect sizes of the parameters in the best-supported models of nest success and juvenile survival. Finally, we used analysis of variance to determine whether either nest success or juvenile survival differed with macrohabitat type or microhabitat type in either species.
Results
We monitored a total of 83 box turtle nests and 36 spotted turtle nests in 2018 and 2019 combined. Mean values for clutch size, hatching success, and nest-site macrohabitat and microhabitat parameters in each species are shown in Table 1. The number of nests of each species constructed in each macrohabitat and microhabitat type are shown in Table 2. From these nests, we radio-tracked 68 hatchling box turtles and 34 hatchling spotted turtles.
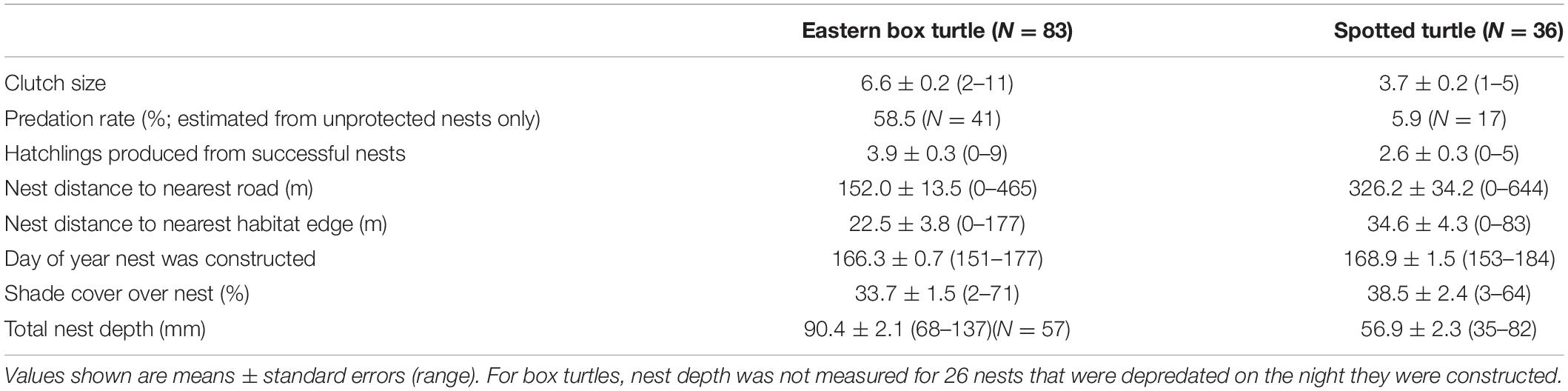
Table 1. Summary of clutch, microhabitat, and macrohabitat characteristics of eastern box turtle (Terrapene carolina carolina) and spotted turtle (Clemmys guttata) nests in northwestern Ohio and southern Michigan in 2018–2019.
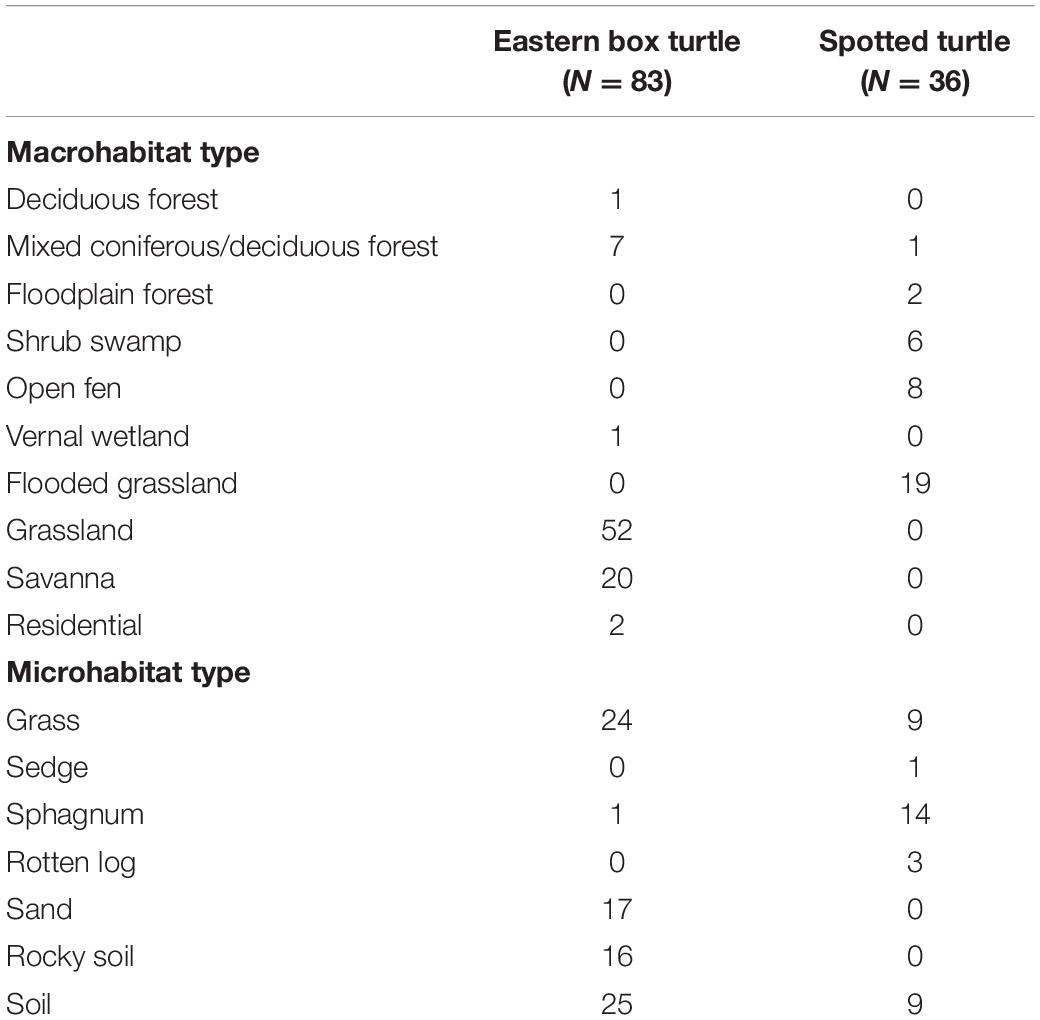
Table 2. Number of nests of eastern box turtle (Terrapene carolina carolina) and spotted turtle (Clemmys guttata) in northwestern Ohio and southern Michigan in 2018–2019 constructed in each macrohabitat and microhabitat class.
Nest Predation Rates
We caged 42 box turtle nests and 19 spotted turtle nests, and we left 41 box turtle nests and 17 spotted turtle nests unprotected to estimate nest predation rates. Two of the 42 protected box turtle nests were depredated, and 24 of the 41 unprotected nests were depredated, for an estimated natural predation rate of 58.5% in box turtle nests at our study sites. None of the 19 protected spotted turtle nests was depredated, and only 1 of the 17 unprotected nests was depredated, for an estimated natural predation rate of 5.9% in spotted turtle nests (Table 1).
Predictors of Nest Success
Only unprotected nests were included in the models predicting nest success. In box turtles, there was no difference in nest success among macrohabitat classes [F(4,36) = 1.48, P = 0.23]. The best-supported macrohabitat model predicting nest success included only distance to nearest macrohabitat edge (mean = 23 m; range 0–177 m; Supplementary Table 1), with approximately a 25% increase in the probability of nest success with every 50-m farther from macrohabitat edge (Figure 2). There was also no difference in nest success among microhabitat classes in box turtles [F(3, 37) = 0.46, P = 0.71]. The best-supported microhabitat model predicting nest success in box turtles included nest date (mean = 166; range 151–177) and total nest depth (mean = 90 mm; range 68–137 mm; Supplementary Table 2). Probability of nest success increased from ∼15% for the earliest nesting attempts to nearly 100% for those constructed 3 weeks later (Figure 3A), and increased from ∼60% in the shallowest nests to nearly 100% for nests 60 mm deeper (Figure 3B). That is, nest success in box turtles was greater in deeper nests constructed later in the season. In box turtles, female body size (measured as plastron length) was positively correlated with total nest depth [F(1, 52 = 5.47, P = 0.023], although there was substantial among-individual variation in this relationship (R2 = 0.08).
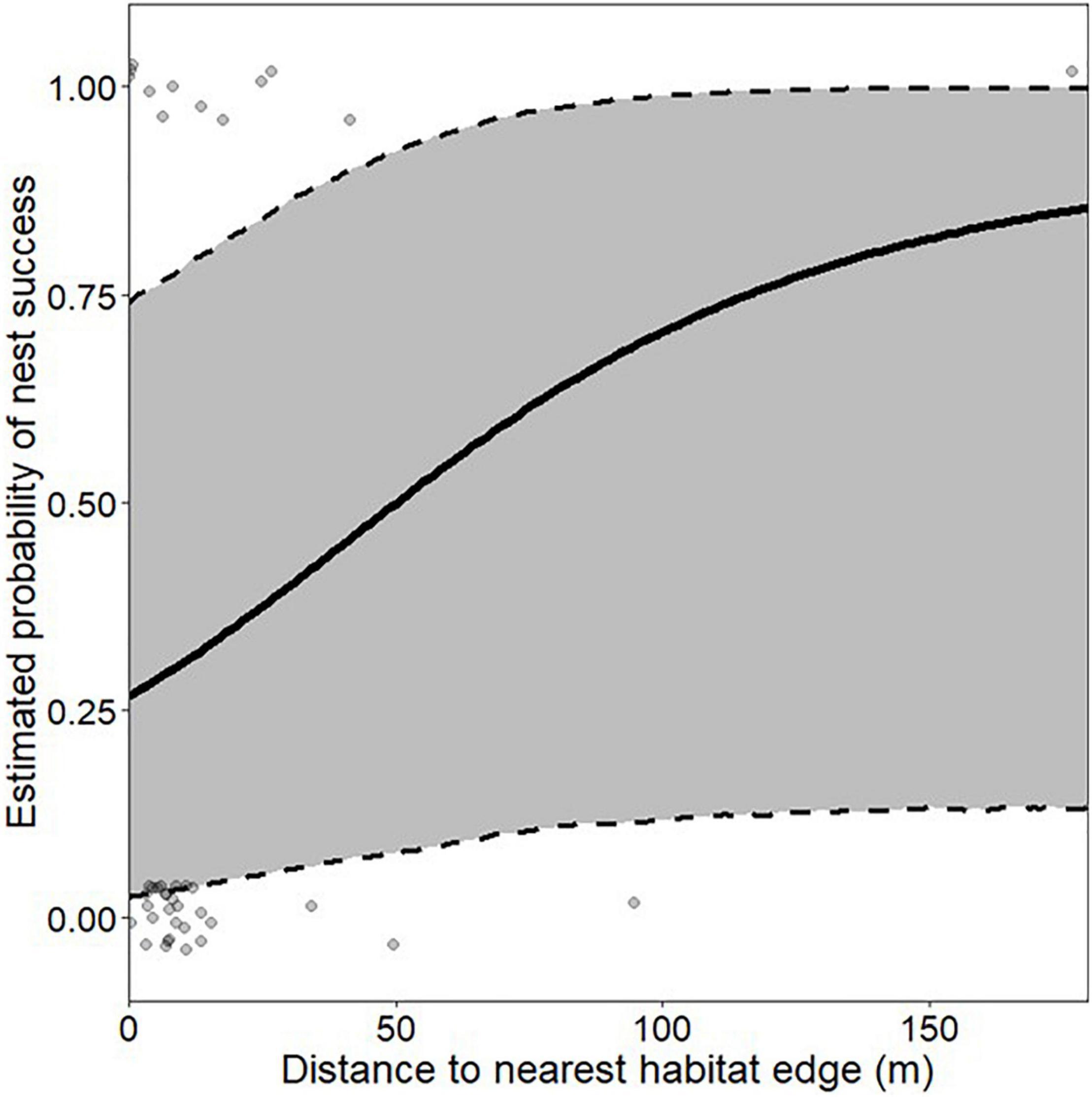
Figure 2. Effect size of nest distance to nearest habitat edge on probability of nest success in eastern box turtles (Terrapene carolina carolina) in northwestern Ohio and southern Michigan in 2018–2019. Effect size was estimated from the best-supported macrohabitat model predicting box turtle nest success, which included only distance to nearest macrohabitat edge. All non-depicted variables were held at their mean values.
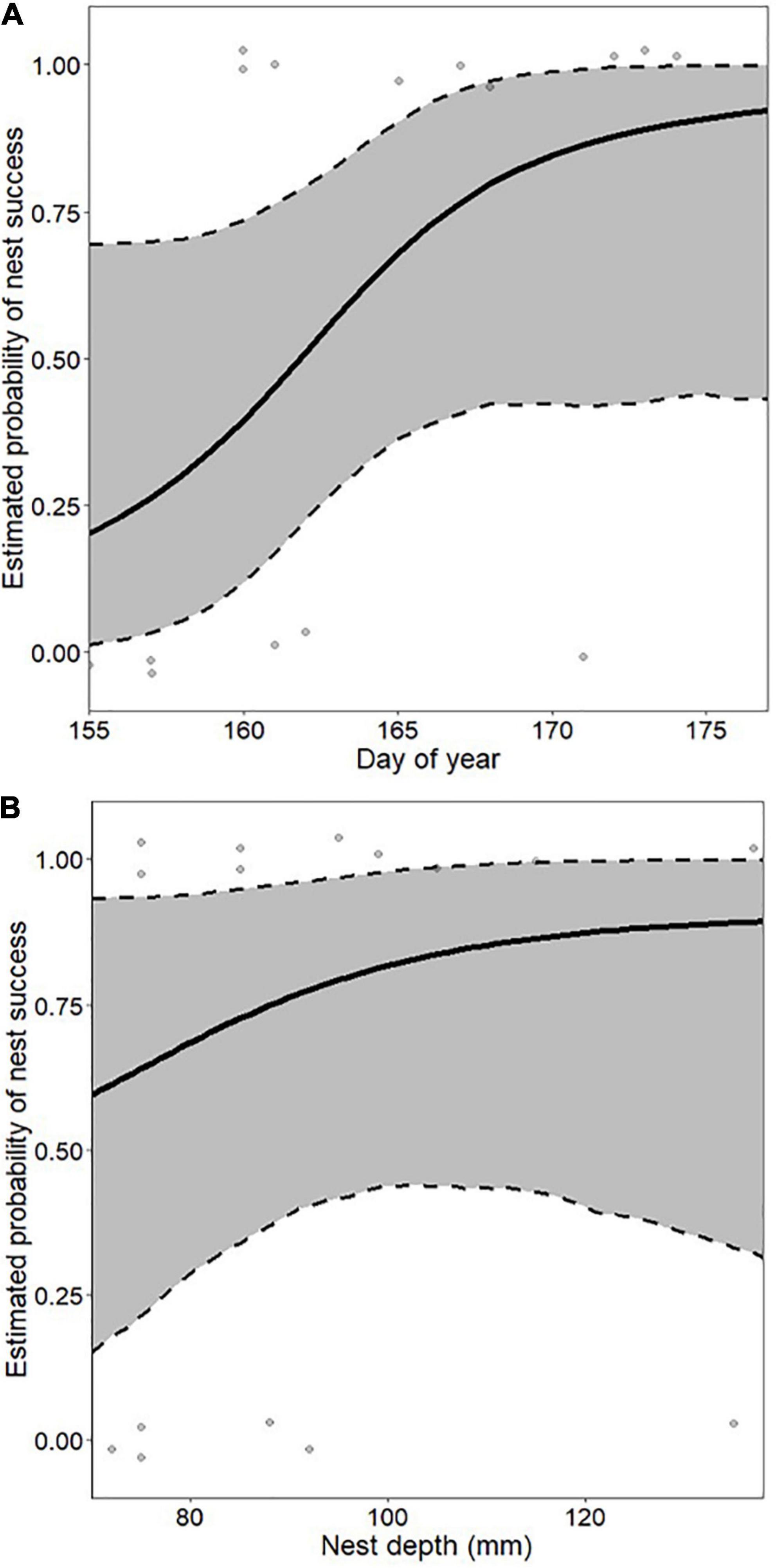
Figure 3. Effect size of nest construction date (A) and total nest depth (B) on probability of nest success in eastern box turtles (Terrapene carolina carolina) in northwestern Ohio and southern Michigan in 2018–2019. Effect sizes were estimated from the best-supported microhabitat model predicting box turtle nest success, which included nest date and total nest depth. In each panel, non-depicted variables were held at their mean values.
In spotted turtles, only one of the 17 unprotected nests was depredated, and the other 16 unprotected nests successfully produced at least one live hatchling. Due to this lack of variation in spotted turtle nest success, our models of macrohabitat and microhabitat variables predicting nest success failed to converge for this species.
Predictors of Juvenile Survival to Overwintering
We radio-tracked 68 box turtle hatchlings following nest emergence. Of those, 12 were known mortalities, 27 were observed to have entered or survived overwintering, 16 were assumed to have been depredated when their signals were lost before expected battery expiration, and 13 were assumed to have survived to overwintering because they were observed alive until their transmitter batteries expired after 1 October. Juvenile survival to overwintering did not differ with macrohabitat class in box turtles [F(3, 64 = 1.79, P = 0.16], and the null model for predicting juvenile survival to overwintering was the best-supported model for nest-site macrohabitat analysis in this species (Supplementary Table 1).
The best-supported nest-site microhabitat model predicting juvenile survival to overwintering in box turtles included shade cover and total nest depth (Supplementary Table 2). Overall, probability of juvenile survival to overwintering decreased with both shade cover (mean = 34%; range 2–71%) and nest depth (mean = 90 mm; range 68–137 mm). Probability of hatchling survival to overwintering decreased from ∼80% from a nest with 10% shade cover to ∼40% from a nest with 70% shade cover (Figure 4A), and decreased from ∼80% in the shallowest nests to ∼20% in the deepest nests (Figure 4B). That is, juvenile survival to overwintering in box turtles was greater for hatchlings from shallower nests with less shade cover.
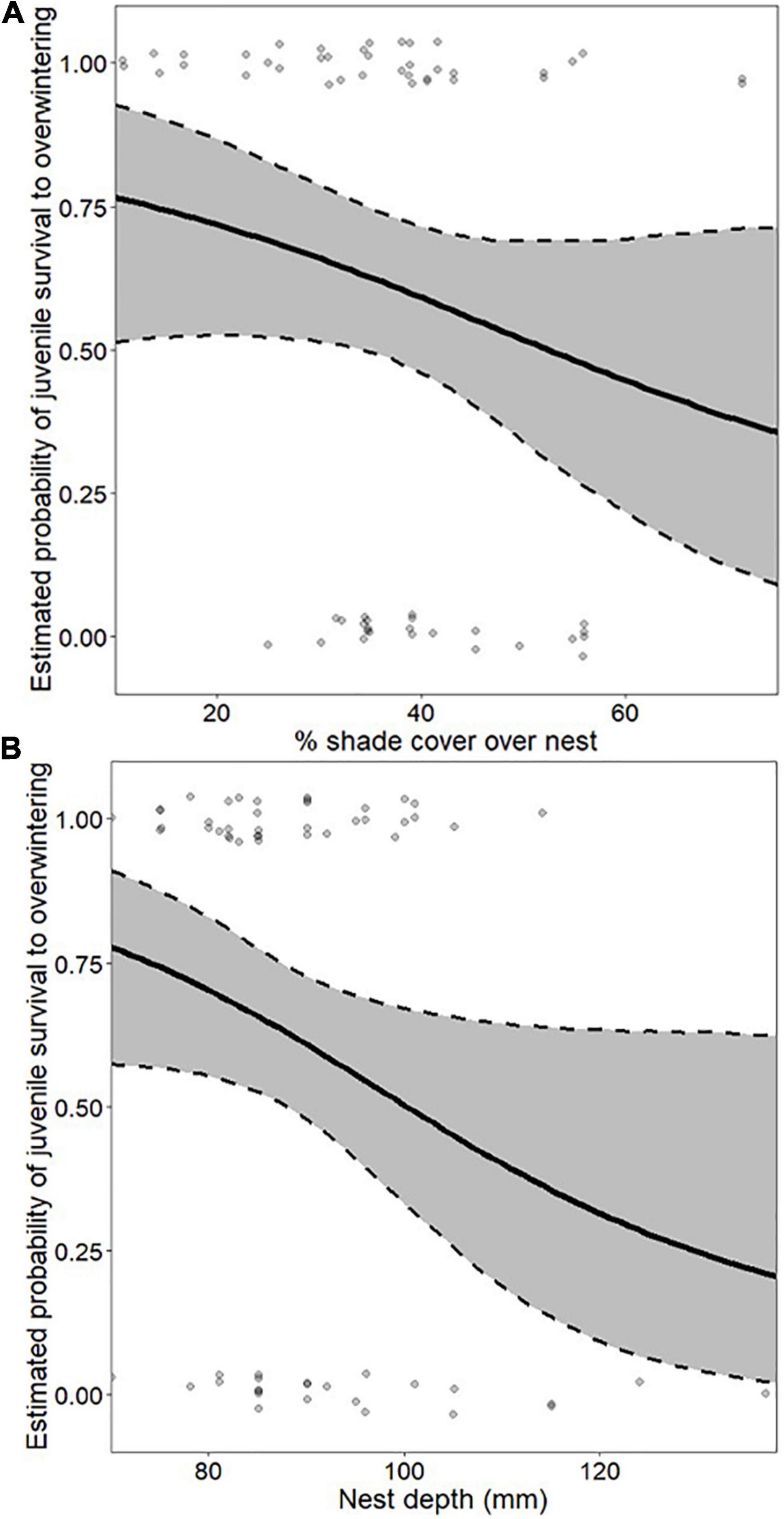
Figure 4. Effect size of shade cover over the nest (A) and total nest depth (B) on probability of juvenile survival to overwintering in eastern box turtles (Terrapene carolina carolina) in northwestern Ohio and southern Michigan in 2018–2019. Effect sizes were estimated from the best-supported microhabitat model predicting juvenile survival to overwintering in box turtles, which included shade cover over the nest and total nest depth. In each panel, non-depicted variables were held at their mean values.
We radio-tracked 34 spotted turtle hatchlings. One hatchling was found depredated, and 19 were observed to have entered or survived overwintering. Transmitter batteries expired for nine hatchlings after 1 October, and these hatchlings were assumed to have survived to overwintering. The signals for five hatchlings were lost before expected battery expiration, and these hatchlings were assumed to have been depredated.
Juvenile survival to overwintering did not differ with macrohabitat class in spotted turtles [F(4, 29) = 2.24, P = 0.09]. However, juvenile survival to overwintering differed with microhabitat class in spotted turtles [F(4, 29 = 4.4; P = 0.007], such that juveniles from nests constructed in soil had lower survival to overwintering than juveniles from nests in other microhabitat types (Figure 5). Finally, the null model for predicting juvenile survival to overwintering was the best-supported model for both nest-site macrohabitat (Supplementary Table 3) and microhabitat (Supplementary Table 4) in spotted turtles; no other models were competitive.
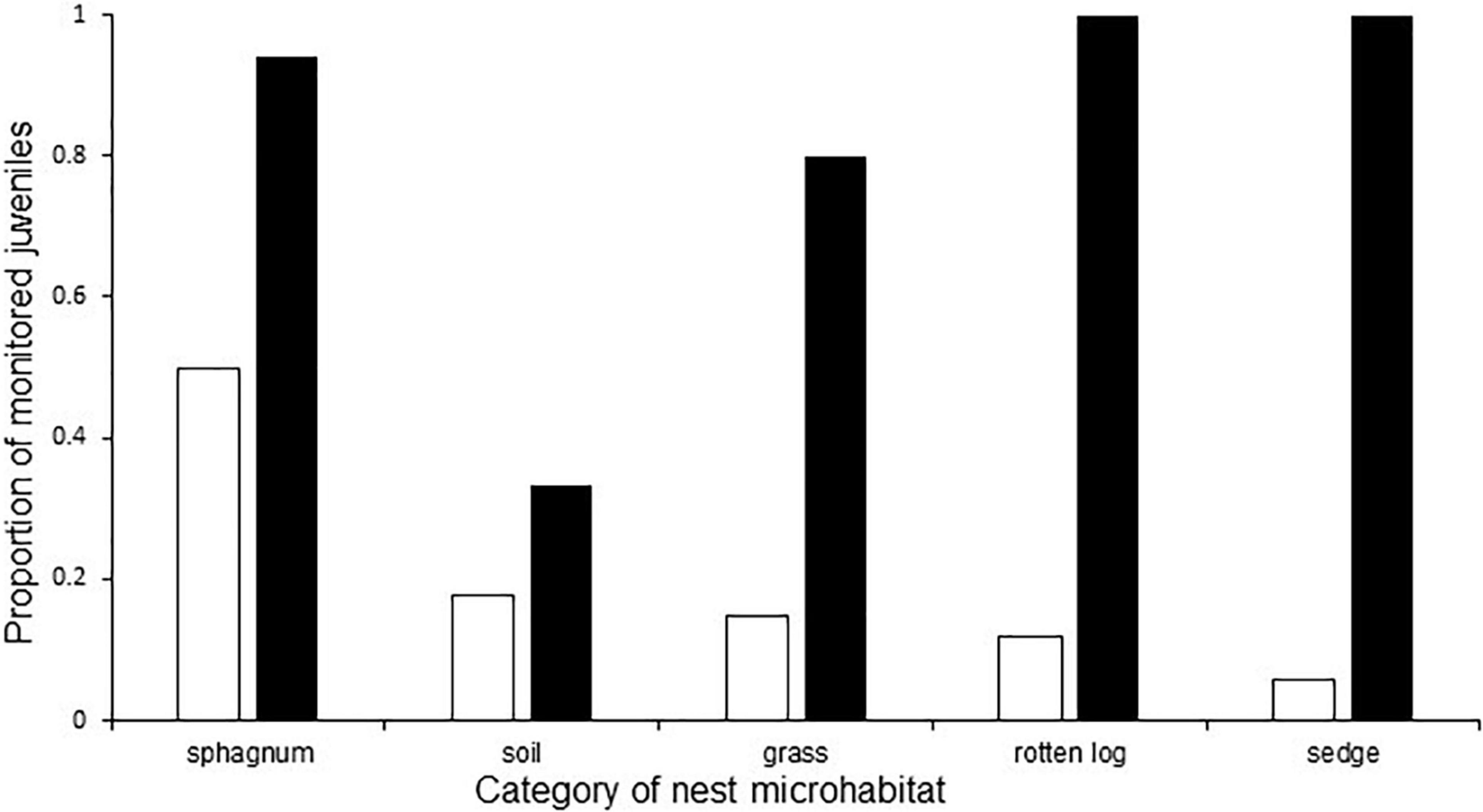
Figure 5. Proportion of spotted turtle (Clemmys guttata) juveniles monitored via radio-telemetry from nests constructed in each of five microhabitat types (white bars; sample size indicates total number of nests constructed in each microhabitat type), and rate of survival to overwintering of those juveniles (black bars), in northwestern Ohio and southern Michigan in 2018–2019. Juvenile survival to overwintering differed with nest microhabitat class [F(4,29) = 4.4, P = 0.0067].
Discussion
Maternal choice of nest site often entails balancing a variety of potential risks that may differentially affect the nesting female, the successful hatching of eggs, or the survival of juveniles (Refsnider and Janzen, 2010). In balancing these risks, females may select nest sites that minimize risk to one life stage, but at a potential cost to a different life stage. Therefore, if researchers focus only on the effect of maternal nest-site choice on the survival outcome of a single life stage, nest-site choice may appear to be maladaptive, when in fact females are selecting nest sites that optimize overall survival and reproductive output over sites that maximize survival of a single life stage (Mitchell et al., 2013; Streby et al., 2014b).
We modeled effects of nest-site characteristics at the macrohabitat and microhabitat scale on both nest success and juvenile survival to overwintering in two declining turtle species occurring in oak savanna landscapes. We found that spotted turtle nest success overall was very high (∼94%), and did not vary meaningfully with the nest-site variables we measured at either the macrohabitat or microhabitat scale. Survival of juvenile spotted turtles was lower from nests constructed in soil substrate than in other substrates, which predominantly included sphagnum mounds and rotten logs, with a few nests also constructed in sedge or grass mounds. We did not quantify nest hydric conditions in this study, but our results suggest that moisture levels within the nest may play an important role in the survival of emerging spotted turtle hatchlings. It is likely that the spotted turtle nests constructed in soil substrate were drier than nests constructed in sphagnum mounds or rotten logs, and hatchlings of several reptile species are known to have higher survival from nests with greater moisture content than from drier nests (e.g., Miller, 1993; Brown and Shine, 2004; Socci et al., 2005). Future research should investigate the influence of hydric conditions on hatchling survival in spotted turtle nests.
Nest success at our study sites was also relatively high for eastern box turtles, with approximately 40% of unprotected box turtle nests producing at least one live hatchling. Nest predation rates of > 95% have been reported in closely related turtle species (Strickland et al., 2010; Refsnider et al., 2015), so it is encouraging from a conservation standpoint that nearly 40% of nests in our study populations succeeded even without any management intervention such as predator-proof cages. We found a considerable effect of macrohabitat on nest success, such that nests constructed farther from habitat edges had higher probability of producing hatchlings than nests closer to habitat edges, despite most nests being constructed relatively close to edges. Nesting relatively close to edges, despite lower nest success near edges, is suggestive of a trade-off between selection pressure on a different life stage: perhaps hatchlings’ access to other macrohabitat types that may confer higher juvenile survival is an important driver of nesting relatively close to edges. We found no evidence of increased juvenile survival from nests near macrohabitat edges, but nest distance to forest edge was the strongest predictor of a juvenile’s eventual overwintering site in a more northern box turtle population (Laarman et al., 2018), and in a separate study on these same populations we found that most hatchling box turtles overwintered in forest or forest edge habitat (Hulbert, 2020). A useful avenue for future research would be to explore other potential life-history tradeoffs that might explain the propensity to nest near edges that confer relatively low nest success or determine if this is simply a negative edge effect, and to identify the mechanism that might underlie such an edge effect. For example, lower nest success closer to habitat edges could be due to increased predator abundance near edges. Alternatively, edges are often warmer and drier compared to core habitat, and both thermal and hydric conditions strongly influence hatchling survival and phenotype in turtles (e.g., Miller et al., 1987; Brooks et al., 1991). It is also possible that nests near edges, while apparently suboptimal for nest success, are safer for females during nest construction. We did not consider risks to nesting females here, but research on a related, aquatic species found that predation risk to nesting females was not related to nest distance from wetland edges (Refsnider et al., 2015).
At the microhabitat scale, box turtle nest success was greater in nests constructed on later dates. The benefits of nesting later are likely highest within a certain date range, with nests constructed after that range resulting in suboptimal phenotypes, lower nest success, or lower hatchling survival (Telemeco et al., 2013). In particular, following completion of embryonic development within the egg, hatchlings of species that do not overwinter within the nest cavity must have sufficient time after nest emergence to reach suitable overwintering habitat before the onset of lethally cold weather (e.g., Laarman et al., 2018). Nests constructed too late in the year may hatch with too little time remaining before the onset of winter for hatchlings to successfully reach overwintering habitat.
Box turtle nest success was also higher in nests constructed at greater total depths compared to shallower nests. Although nest temperature decreases with increasing nest depth in large turtle species that construct deep nest cavities (e.g., Roosenburg, 1996), temperature variation in relatively shallow nests such as those constructed by box turtles and closely related painted turtles is minimal and likely insufficient to affect incubation regime or offspring sex (Refsnider et al., 2013a; also see Telemeco et al., 2009). Instead, deeper nests may be more difficult for predators to detect, thereby increasing the probability that deeper nests will successfully produce hatchlings. Importantly, however, turtles construct nests using their rear limbs, meaning that females’ maximum nest depths are physically constrained by the length of their rear limbs (Refsnider, 2012). Therefore, older and larger females may be at a reproductive advantage if they can excavate deeper nests with a lower probability of being depredated, compared to younger and smaller females that are constrained to excavating more superficial nests. Indeed, in our study, nest depth increased with female plastron length, suggesting that larger females construct deeper nests than smaller females. Interestingly, there was substantial variation in nest depth even among similarly sized individuals, which could be due to individuals adjusting nest depth rather than constructing the deepest possible nests for their body size. Moreover, painted turtles constructed deeper nests in years where May temperatures were higher (Refsnider et al., 2013a), suggesting that individuals have some capacity to adjust nest depth relative to prevailing environmental conditions. Regardless of the mechanism(s) underlying variation in nest depth, the potential advantages of constructing deeper nests would only hold if increasing nest success confers a reproductive advantage, which would require no opposing selection pressure acting on the same trait (i.e., nest depth) but in a different life stage.
Box turtle hatchlings had a higher probability of surviving to overwintering if they hatched from nests that were shallower and constructed under less shade cover, compared to hatchlings from deeper, more shaded nests. Shade cover is a critical driver of incubation temperature in turtle nests, and is known to influence a variety of hatchling phenotypes, including sex in species with temperature-dependent sex determination (Janzen, 1994a). Furthermore, choice of shade cover over a nest site is a behaviorally plastic trait that females can adjust in order to match nest incubation conditions with prevailing environmental conditions (e.g., Refsnider and Janzen, 2012). Our results suggest that female choice of shade cover can also influence hatchling survival, further emphasizing the importance of this aspect of maternal nest-site choice. Importantly, in order for behavioral plasticity in maternal choice of shade cover over nest sites to be expressed, a range of shade cover options must be available within nesting areas (Refsnider et al., 2013b). For example, in an unusually warm year female turtles may nest at sites with greater shade cover than they would choose in average or cool years, in order to compensate for warmer air temperatures. Indeed, at our study sites mean May air temperatures were 3.4°C (Ohio) and 4.5°C (Michigan) cooler in 2019 than in 2018 (NOAA), and box turtles nested under 40.5% shade cover in 2018, but 30.1% shade cover in 2019 (t = 3.17; P = 0.0027). Thus, box turtles in our study appear to show similar behavioral plasticity in maternal nest-site choice to the painted turtles in Refsnider and Janzen (2012), wherein female turtles compensate for prevailing climatic conditions on nest incubation conditions by adjusting the amount of shade cover under which they choose to nest. However, if nesting areas lack variability in shade cover (which could include low ground cover as well as tree canopy), females would be unable to express their inherent behavioral plasticity and may be forced to nest at sites that could be lethally warm (Refsnider et al., 2013b). We recommend that managers endeavor to maintain heterogeneity in habitat structure at the microhabitat scale (i.e., < 1 m) within known turtle nesting areas such that open, bare patches as well as more densely vegetated patches are interspersed throughout the nesting area.
The effect of nest depth was in opposite directions for nest success compared to juvenile survival to overwintering in box turtles. The probability of nest success was higher from deeper nests, whereas the probability of juvenile survival to overwintering was higher from shallower nests. Predators may have a more difficult time detecting eggs in deeper nests, which likely explains our finding of higher nest success from deeper nests. However, hatchlings likely expend more energy digging their way out of deeper nests compared to shallower nests, which may explain why hatchlings from shallower nests were more likely to survive to overwintering. In painted turtles, deeper nests produced smaller and faster hatchlings than shallower nests, which demonstrates that even if nest depth does not affect incubation temperature or hatchling sex, there may still be effects on other phenotypes in the hatchling stage (Refsnider et al., 2013a), which would further support a potential tradeoff in the benefits of nest depth to the egg stage vs. the hatchling stage. Future research is needed to determine whether energetic costs to emerge from a nest cavity differ with depth, and whether the additional energetic costs of traveling to suitable overwintering sites could be offset in some way, perhaps by providing patches of refuge habitat in areas through which hatchlings are likely to travel from nests to overwintering sites.
Our results identify an important tradeoff between nest success and juvenile survival in box turtles: deeper nests were more likely to successfully hatch, but hatchlings were more likely to survive if they came from shallower nests. This tradeoff means that, when constructing their nests, females must balance opposing risks on two different life stages. Due to physical constraints of body size (i.e., rear limb length) on the maximum depth to which a female is capable of digging, it is likely that small females are unable to adjust their nest depth and therefore may be inadvertently favoring the survival of juveniles over nest success. However, older and larger females may have greater capacity to adjust their total nest depth, in which case they could “choose” to dig a deeper nest that would favor nest success over juvenile survival, perhaps under conditions where nest predators were abundant, or when the nest site is in close proximity to suitable overwintering habitat for juveniles. One way to test for evidence of this ability to adjust nest depth would be to compare nest depth to rear limb length in female box turtles and determine if there is more variation in nest depth in longer-limbed females.
We did not investigate the effects of nest-site characteristics on offspring sex in this study, but this is a critical knowledge gap that needs to be filled in order to better predict the impacts of climate change on these two declining turtle species. Determining the precise incubation temperature ranges that produce each sex in box and spotted turtles, and comparing incubation temperatures in wild nests with those reaction norms, will provide crucial data regarding potential sex ratio skews that could result from a warming climate (Janzen, 1994b). In particular, if specific turtle populations are at risk of producing primarily the warmer sex (females, in the case of box and spotted turtles) as the climate continues to warm, managers may be able to reverse such a trend through strategic placement of shade-providing vegetation within nesting areas such that nesting females could choose shadier nest sites in warmer years, and thereby reduce potential skews in sex ratios (Refsnider and Janzen, 2012).
Maternal nest-site choice is an important mechanism by which females can influence both the survival and the phenotypes of their offspring across multiple life stages. Knowledge of the specific nest-site characteristics chosen by females, as well as the fitness outcomes across multiple life stages resulting from those nest-site characteristics, will inform managers as to the specific habitat characteristics that result in the highest overall productivity. We recommend that nesting areas for eastern box turtles and spotted turtles be maintained with structural variation at the microhabitat scale (i.e., <1 m) to allow nesting females to express plasticity in nest-site choice by matching nest incubation conditions with the prevailing local climate through choice of shade cover over the nest, while continuing to use historical nesting habitat at the macrohabitat scale (i.e., >10 m). Furthermore, managers should avoid fragmenting nesting areas by roads or trails in order to minimize potential edge effects. Finally, a useful avenue for future research would be to investigate whether small refuge microhabitats, such as small patches of dense vegetation or small brush piles, within nesting areas would improve survival rates of box turtle hatchling turtles traveling from nests to overwintering sites in adjacent forest or forest edge habitat, particularly in large nesting areas in which nests are located far from suitable overwintering habitat.
Data Availability Statement
Data are deposited in Mendeley Data, V1, 10.17632/jd64t5prvn.1.
Ethics Statement
The animal study was reviewed and approved by the University of Toledo’s Institutional Animal Care and Use Committee (protocol #108797).
Author Contributions
JR: conceptualization, formal analysis, investigation, and writing. SC and AH: formal analysis and investigation. AD and PM: investigation. GK: formal analysis. HS: conceptualization, formal analysis, and investigation. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by a United States Fish and Wildlife Service Competitive State Wildlife Grant to the Ohio and Michigan Departments of Natural Resources and to HS and JR. Additional support was provided by the University of Toledo’s Graduate Student Association (to SC and AH) and a National Science Foundation Research Experience for Undergraduates Site Grant to JR (DBI-1852245).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank many volunteers and technicians for field data collection, including K. Altabet, J. Behrmann, Z. Bragg, O. Brooks, I. Clifton, L. Cruse, P. Delisle, J. DeVries, L. Denby, B. Furlong, N. Garwood, K. Grab, M. Griffin, T. Guo, A. Jamison, C. Kiel, J. Lee, M. Mills, K. Novak, J. Otten, R. Pagel, M. Palese, C. Piper, W. Robinson, C. Roemer, H. Thill, K. Watkins, T. Wantman, and M. Wilcox. Logistical support was provided by D. Bomia, J. Bossenbroek, D. Burkett, T. Crail, A. Derosier, R. Gardner, C. Hanaburgh, A. Ihnken, K. Menard, K. Parsons, A. Sakas, T. Schetter, R. Schroeder, W. Ulrey, S. Woods, and B. Yahn. I. Clifton provided expertise on data analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.788025/full#supplementary-material
References
Amat, J. A., and Masero, J. A. (2004). Predation risk on incubating adults constrains the choice of thermally favourable nest sites in a plover. Anim. Behav. 67, 293–300. doi: 10.1016/j.anbehav.2003.06.014
Anders, A. D., Faaborg, J., and Thompson, F. R. I. I. I. (1998). Postfledging dispersal, habitat use, and home-range size of juvenile wood thrushes. Auk 115, 349–358. doi: 10.2307/4089193
Brooks, R. J., Bobyn, M. L., Galbraith, D. A., Layfield, J. A., and Nancekivell, E. G. (1991). Maternal and environmental influences on growth and survival of embryonic and hatchling snapping turtles (Chelydra serpentina). Can. J. Zool. 69, 2667–2676. doi: 10.1139/z91-375
Brown, G. P., and Shine, R. (2004). Maternal nest-site choice and offspring fitness in a tropical snake (Tropidonophis mairii, Colubridae). Ecology 85, 1627–1634. doi: 10.1890/03-0107
Buhlmann, K. A., and Coffman, G. (2001). Fire ant predation of turtle nests and implications for the strategy of delayed emergence. J. Elisha Mitchell Sci. Soc. 117, 94–100.
Carter, S. A. (2021). Habitat Use and Nest-Site Characteristics of Ohio and Michigan Populations of Two Imperiled Freshwater Turtle Species. M.S. thesis. Toledo, OH: University of Toledo.
Chalfoun, A. D., and Schmidt, K. A. (2012). Adaptive breeding-habitat selection: is it for the birds? Auk 129, 589–599. doi: 10.1525/auk.2012.129.4.589
Enneson, J. J., and Litzgus, J. D. (2008). Using long-term data and a stage-classified matrix to assess conservation strategies for an endangered turtle (Clemmys guttata). Biol. Conserv. 141, 1560–1568. doi: 10.1016/j.biocon.2008.04.001
Feng, C. Y., Ross, J. P., Mauger, D., and Dreslik, M. J. (2019). A long-term demographic analysis of spotted turtles (Clemmys guttata) in Illinois using matrix models. Diversity 11, 1–23. doi: 10.3390/d11120226
Flaspohler, D. J., Temple, S. A., and Rosenfield, R. N. (2001). Species-specific edge effects on nest success and breeding bird density in a forested landscape. Ecol. Appl. 11, 32–46. doi: 10.1890/1051-0761(2001)011[0032:sseeon]2.0.co;2
Frazer, G. W., Canham, C. D., and Lertzman, K. P. (1999). Gap Light Analyzer (GLA), Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs, Users Manual and Program Documentation. Millbrook, NY: Simon Fraser University and the Institute of Ecosystem Studies.
Ghalambor, C. K., and Martin, T. E. (2001). Fecundity-survival trade-offs and parental risk-taking in birds. Science 292, 494–497. doi: 10.1126/science.1059379
Grigore, M. T. (2009). Living in the Oak Openings: A Guide to One of the World’s Last Great Places. Toledo, OH: Homewood Press.
Hall, R. J., Henry, P. F. P., and Bunck, C. M. (1999). Fifty-year trends in a box turtle population in Maryland. Biol. Conserv. 88, 165–172. doi: 10.1016/S0006-3207(98)00107-4
Hartway, C., and Mills, L. S. (2012). A meta-analysis of the effects of common management actions on the nest success of North American birds. Conserv. Biol. 26, 657–666. doi: 10.1111/j.1523-1739.2012.01883.x
Hulbert, A. C. (2020). Threatened Turtle Species in Ohio and Michigan: The Ecology of Hatchlings and Analysis of GPS Devices. M.S. thesis. Toledo, OH: University of Toledo.
International Union of Concerned Scientists (2011a). Clemmys guttata. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T4968A11103766.en (accessed September 07, 2021).
International Union of Concerned Scientists (2011b). Terrapene carolina. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T21641A9303747.en (accessed September 07, 2021).
Janzen, F. J. (1993). An experimental analysis of natural selection on body size of hatchling turtles. Ecology 74, 332–341. doi: 10.2307/1939296
Janzen, F. J. (1994a). Vegetational cover predicts the sex ratio of hatchling turtles in natural nests. Ecology 75, 1593–1599. doi: 10.2307/1939620
Janzen, F. J. (1994b). Climate change and temperature-dependent sex determination in reptiles. Proc. Natl. Acad. Sci. U.S.A. 91, 7487–7490. doi: 10.1073/pnas.91.16.7487
Janzen, F. J., Tucker, J. K., and Paukstis, G. L. (2000). Experimental analysis of an early life-history stage: selection on size of hatchling turtles. Ecology 81, 2290–2304.
Janzen, F. J., Tucker, J. K., and Paukstis, G. L. (2007). Experimental analysis of an early life-history stage: direct or indirect selection on body size of hatchling turtles? Funct. Ecol. 21, 162–170. doi: 10.1111/j.1365-2435.2006.01220.x
Kamel, S. J., and Mrosovsky, N. (2004). Nest site selection in leatherbacks, Dermochelys coriacea: individual patterns and their consequences. Anim. Behav. 68, 357–366. doi: 10.1016/j.anbehav.2003.07.021
Kolbe, J. J., and Janzen, F. J. (2001). The influence of propagule size and maternal nest-site selection on survival and behaviour of neonate turtles. Funct. Ecol. 15, 772–781. doi: 10.1046/j.0269-8463.2001.00587.x
Kolbe, J. J., and Janzen, F. J. (2002). Spatial and temporal dynamics of turtle nest predation: edge effects. Oikos 99, 538–544. doi: 10.1034/j.1600-0706.2002.11853.x
Laarman, P. B., Keenlance, P. W., Altobelli, J. T., Schumacher, C. M., Huber, P., Jacquot, J. J., et al. (2018). Ecology of neonate eastern box turtles with prescribed fire implications. J. Wildl. Manag. 82, 1385–1395. doi: 10.1002/jwmg.21503
Leach, M. K., and Givnish, T. J. (1999). Gradients in the composition, structure, and diversity of remnant oak savannas in southern Wisconsin. Ecol. Monogr. 69, 353–374. doi: 10.1890/0012-9615(1999)069[0353:gitcsa]2.0.co;2
Litzgus, J. D. (2006). Sex differences in longevity in the spotted turtle (Clemmys guttata). Copeia 2006, 281–288. doi: 10.1643/0045-8511(2006)6[281:sdilit]2.0.co;2
Madsen, T., and Shine, R. (1999). Life history consequences of nest-site variation in tropical pythons (Liasis fuscus). Ecology 80, 989–997.
Martin, T. E. (1992). “Breeding productivity considerations: what are the appropriate habitat features for management?,” in Ecology and Conservation of Neotropical Migrant Landbirds, eds J. M. Hagan III and D. W. Johnston (Washington, DC: Smithsonian Institution Press), 455–473. doi: 10.1065/espr2007.05.426
Martin, T. E. (1993). Nest predation and nest sites: new perspectives on old patterns. Bioscience 43, 523–532. doi: 10.2307/1311947
Miller, K. (1993). The improved performance of snapping turtles (Chelydra serpentina) hatched from eggs incubated on a wet substrate persists through the neonatal period. J. Herpetol. 27, 228–233. doi: 10.2307/1564943
Miller, K., Packard, G. C., and Packard, M. J. (1987). Hydric conditions during incubation influence locomotor performance of hatchling snapping turtles. J. Exp. Biol. 127, 401–412. doi: 10.1242/jeb.127.1.401
Mitchell, T. S., Warner, D. A., and Janzen, F. J. (2013). Phenotypic and fitness consequences of maternal nest-site choice across multiple early life stages. Ecology 94, 336–345. doi: 10.1890/12-0343.1
Noble, D. W. A., Stenhouse, V., and Schwanz, L. E. (2018). Developmental temperatures and phenotypic plasticity in reptiles: a systematic review and meta-analysis. Biol. Rev. 93, 72–97. doi: 10.1111/brv.12333
Nuzzo, V. A. (1986). Extent and status of Midwest Oak Savanna: presettlement and 1985. Nat. Areas J. 6, 6–36.
Packard, G. C., and Packard, M. J. (1988). “The physiological ecology of reptilian eggs and embryos,” in Biology of the Reptilia, eds C. Gans and R. B. Huey (New York, NY: Liss), 523–605. doi: 10.1111/j.1469-185x.1977.tb01346.x
Paterson, J. E., Steinberg, B. D., and Litzgus, J. D. (2012). Revealing a cryptic life-history stage: differences in habitat selection and survivorship between hatchlings of two turtle species at risk (Glyptemys insculpta and Emydoidea blandingii). Wildl. Res. 39, 408–418. doi: 10.1071/WR12039
Pike, D. A. (2006). Movement patterns, habitat use, and growth of hatchling tortoises, Gopherus polyphemus. Copeia 2006, 68–76. doi: 10.1643/0045-8511(2006)006[0068:mphuag]2.0.co;2
Putman, N. F., Shay, T. J., and Lohmann, K. J. (2010). Is the geographic distribution of nesting in the Kemp’s Ridley turtle shaped by the migratory needs of offspring? Integr. Comp. Biol. 50, 305–314. doi: 10.1093/icb/icq041
Refsnider, J., and Linck, M. (2012). Habitat use and movement patterns of Blanding’s turtles (Emydoidea blandingii) in Minnesota, USA: a landscape approach to species conservation. Herpetol. Conserv. Biol. 7, 185–195.
Refsnider, J. M. (2009). High frequency of multiple paternity in Blanding’s Turtle (Emys blandingii). J. Herpetol. 43, 74–81.
Refsnider, J. M. (2012). Effects of Climate Change on Reptiles with Temperature-Dependent Sex Determination and Potential Adaptation via Maternal Nest-Site Choice. Ph.D. dissertation. Ames, IA: Iowa State University.
Refsnider, J. M., Bodensteiner, B., Reneker, J., and Janzen, F. J. (2013a). Nest depth does not compensate for sex ratio skews caused by climate change in turtles. Anim. Conserv. 16, 481–490. doi: 10.1111/acv.12034
Refsnider, J. M., Warner, D. A., and Janzen, F. J. (2013b). Does shade cover availability limit nest-site choice in two populations of turtles with temperature-dependent sex determination? J. Therm. Biol. 38, 152–158. doi: 10.1016/j.jtherbio.2013.01.003
Refsnider, J. M., Clifton, I. T., and Vazquez, T. K. (2019). Developmental plasticity of thermal ecology traits in reptiles: trends, potential benefits, and research needs. J. Therm. Biol. 84, 74–82. doi: 10.1016/j.jtherbio.2019.06.005
Refsnider, J. M., and Janzen, F. J. (2010). Putting eggs in one basket: ecological and evolutionary hypotheses for variation in oviposition-site choice. Ann. Rev. Ecol. Evol. Systemat. 41, 39–57. doi: 10.1146/annurev-ecolsys-102209-144712
Refsnider, J. M., and Janzen, F. J. (2012). Behavioural plasticity may compensate for climate change in a long-lived reptile with temperature-dependent sex determination. Biol. Conserv. 152, 90–95. doi: 10.1016/j.biocon.2012.03.019
Refsnider, J. M., Reedy, A. M., Warner, D. A., and Janzen, F. J. (2015). Do tradeoffs between predation pressures on females vs. nests drive nest-site choice in painted turtles? Biol. J. Linn. Soc. 116, 847–855. doi: 10.1111/bij.12671
Resetarits, W. J. Jr., and Wilbur, H. M. (1989). Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors. Ecology 70, 220–228. doi: 10.2307/1938428
Rhodin, A. G. J., Iverson, J. B., Bour, R., Fritz, U., Georges, A., Shaffer, H. B., et al. (2017). Turtles of the World: Annotated Checklist and Atlas of Taxonomy, Synonymy, Distribution, and Conservation Status. Chelonian Research Monographs, Number 7, 8th Edn. Lunenburg, MA: Chelonian Research Foundation, 1–292.
Roosenburg, W. M. (1996). Maternal condition and nest site choice: an alternative for the maintenance of environmental sex determination? Am. Zool. 36, 157–168. doi: 10.1093/icb/36.2.157
Salmon, M., Tolbert, M. G., Painter, D. P., Goff, M., and Reiners, R. (1995). Behavior of loggerhead sea turtles on an urban beach. II. Hatchling orientation. J. Herpetol. 29, 568–576. doi: 10.2307/1564740
Schwarzkopf, L., and Brooks, R. J. (1987). Nest-site selection and offspring sex ratio in painted turtles, Chrysemys picta. Copeia 1987, 53–61. doi: 10.2307/1446037
Socci, A. M., Schlaepfer, M. A., and Gavin, T. A. (2005). The importance of soil moisture and leaf cover in a female lizard’s (Norops polylepis) evaluation of potential oviposition sites. Herpetologica 61, 233–240. doi: 10.1655/04-67.1
Spencer, R. (2002). Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles. Ecology 83, 2136–2144. doi: 10.1890/0012-9658(2002)083[2136:etnssf]2.0.co;2
Stanford, C. B., Iverson, J. B., Rhodin, A. G. J., van Dijk, P. P., Mittermeier, R. A., Kuchling, G., et al. (2020). Turtles and tortoises are in trouble. Curr. Biol. 30, R721–R735. doi: 10.1016/j.cub.2020.04.088
Stickel, L. F., and Bunck, C. M. (1989). Growth and morphometrics of the box turtle, Terrapene c. carolina. J. Herpetol. 23, 216–223. doi: 10.2307/1564442
Streby, H. M., Refsnider, J. M., Peterson, S. M., and Andersen, D. E. (2014a). Retirement investment theory explains patterns in songbird nest-site choice. Proc. Roy. Soc. Lond. 281:20131834. doi: 10.1098/rspb.2013.1834
Streby, H. M., Refsnider, J. M., and Andersen, D. E. (2014b). Redefining reproductive success in songbirds: a challenge to the nest success paradigm. Auk 131, 718–726. doi: 10.1642/AUK-14-69.1
Strickland, J., Cobert, P., and Janzen, F. J. (2010). Experimental analysis of effects of markers and habitat structure on predation of turtle nests. J. Herpetol. 44, 467–470. doi: 10.1670/08-323.1
Telemeco, R. S., Abbott, K. C., and Janzen, F. J. (2013). Modeling the effects of climate change-induced shifts in reproductive phenology on temperature-dependent traits. Am. Nat. 181, 637–648. doi: 10.1086/670051
Telemeco, R. S., Elphick, M. J., and Shine, R. (2009). Nesting lizards (Bassiana duperreyi) compensate partly, but not completely, for climate change. Ecology 90, 17–22. doi: 10.1890/08-1452.1
Wilbur, H. M., and Morin, P. J. (1988). “Life history evolution in turtles,” in Biology of the Reptilia, Vol. 16, eds C. Gans and R. B. Huey (New York, NY: Alan R. Liss), 387–439.
Keywords: juvenile, nest depth, nest-site choice, predation, shade cover
Citation: Refsnider JM, Carter SE, Diaz A, Hulbert AC, Kramer GR, Madden P and Streby HM (2022) Macro- and Microhabitat Predictors of Nest Success and Hatchling Survival in Eastern Box Turtles (Terrapene carolina carolina) and Spotted Turtles (Clemmys guttata) in Oak Savanna Landscapes. Front. Ecol. Evol. 9:788025. doi: 10.3389/fevo.2021.788025
Received: 01 October 2021; Accepted: 07 December 2021;
Published: 24 January 2022.
Edited by:
Hope Klug, University of Tennessee at Chattanooga, United StatesReviewed by:
James Paterson, Institute for Wetland and Waterfowl Research, Ducks Unlimited Canada, CanadaTom Langen, Clarkson University, United States
Copyright © 2022 Refsnider, Carter, Diaz, Hulbert, Kramer, Madden and Streby. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeanine M. Refsnider, SmVhbmluZS5yZWZzbmlkZXJAdXRvbGVkby5lZHU=
 Jeanine M. Refsnider
Jeanine M. Refsnider Sarah E. Carter
Sarah E. Carter Alexis Diaz2
Alexis Diaz2 Austin C. Hulbert
Austin C. Hulbert Paige Madden
Paige Madden Henry M. Streby
Henry M. Streby