
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 13 December 2021
Sec. Conservation and Restoration Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.741069
This article is part of the Research Topic Human Impacts on Bats in Tropical Ecosystems: Sustainable Actions and Alternatives View all 10 articles
Some animal species exhibit sex-specific patterns as an adaptation to their habitats, however, adaptability to a human-dominated landscape is commonly explored without considering intraspecific sexual differences. Differences between males and females lead to a sexual segregation in habitat use. In southern Mexico, we explored sex-specific responses to landscape modification of six common species of phyllostomid bats: Artibeus jamaicensis, A. lituratus, Sturnira lilium, Carollia perspicillata, Glossophaga soricina, and Platyrrhinus helleri using riparian corridors within continuous forest and cattle pastures. Furthermore, we explored sex related responses to vegetation attributes (i.e., tree height and basal area) and seasonality (i.e., wet and dry seasons). Overall, capture rates were significantly skewed toward females and riparian corridors in pastures. Females of G. soricina exhibited a strong positive relationship with greater tree height and basal area. Seasonality was important for A. lituratus and S. lilium females, only. The results indicate a sexual driven response of bats to habitat modification. The high energetic demands of females associated to reproduction could lead to foraging into riparian corridors in pastures. The presence of large trees along riparian corridors in pastures may help maintaining a diverse and dynamic bat community in modified tropical landscapes.
The configuration of tropical landscapes is highly dynamic as a consequence of changes in land-use and cover (Mayaux et al., 2005; Fagan et al., 2013). A common feature in tropical agricultural landscapes is the presence of relict natural vegetation along streams which persists even when being exposed to long-term agricultural practices and small-scale land use changes (Lundy and Montgomery, 2010). Habitat disturbance can alter the spatial arrangement of critical resources for animals within a given landscape, potentially resulting in disrupted demographic patterns (e.g., sex-ratio, abundance, age classes, etc.) among habitat patches. The degree to which habitat disturbance alters the demography of animal populations, however, should vary as a function of the behavioral plasticity of individual species (Bender et al., 1998; Nupp and Swihart, 2000).
Males and females of the same species may differ in several aspects of their biology (e.g., sexual dimorphism, different thermoregulatory strategies), which may result in sexual segregations of habitat use (Lintott et al., 2014) and/or between sex competition (Lemaître et al., 2014; Benitez-Malvido et al., 2016). Sexual segregation can be broadly categorized into the following types: habitat segregation and social segregation. Habitat segregation occurs where the sexes differ in the use of the physical environment, whilst social segregation when a species tends to form single-sex groups (Wearmouth and Sims, 2008). Habitat segregation hypothesis suggests that inherent sexual differences in reproductive strategies i.e., reproductive energy demands, breeding period and predation risk (Dietz et al., 2006; Nardone et al., 2015; Beerman et al., 2016; Benitez-Malvido et al., 2016) result in females trading off habitat quality in favor of offspring safety (Wearmouth and Sims, 2008).
Most studies on the effects of human-modified landscapes on bat communities have focused at the species level responses, while the potential importance of intra-specific differences are often ignored (Lintott et al., 2014). Bats are an ideal taxon for studying sexual segregation, since sexual dimorphism in bats is rare but sexual segregation is widespread (Senior et al., 2005). Seasonal and maternal sexual segregation have been documented for many bat species (Sgroi and Wilkins, 2010; Encarnação, 2012; Diamond and Diamond, 2014). In many tropical bat species, females in resource-rich habitats roost in groups with few or no males present (harem groups) (Ortega and Arita, 1999; McCracken and Wilkinson, 2000; Altringham, 2011). Particularly due to reproductive and parental costs, females have higher energy requirements, so they are less abundant in habitats with limited food resources (Racey et al., 1987; Ramos Pereira et al., 2010). Sex should be considered separately whenever possible in the study of bats because males and females of the same bat species may have different seasonal distribution and roosts with different characteristics (Broders et al., 2006; Safi et al., 2007; Weller et al., 2009). For instance, the response of two Neotropical frugivorous bats to local and landscape scale attributes were sex and seasonally specific; females were more abundant than males in edge and matrix habitats, and females seem to increase their foraging movements during pregnancy and low fruit availability (Rocha et al., 2017). In another study, bats showed sexual differences in the habitat use within urban landscapes, with males being more widely distributed and females more abundant in highly connected areas. Moreover, access to water was a limiting factor in determining female distribution (Lintott et al., 2014; Patriquin et al., 2019). The importance of fine-scale spatiotemporal and demographically precise data is essential for effective conservation strategies (Hutson et al., 2001; Russo et al., 2010; van Toor et al., 2011).
Habitat loss and fragmentation are important threats to bat populations as they eliminate or reduce suitable foraging habitats and forest structures for roost (Kingston, 2010). Information on the abundance and sex ratios of bat populations throughout the year is important for understanding their ecology in periods of resource scarcity (Perry et al., 2010). Therefore, obtaining sex-specific information on the behavior and habitat requirements of bats should be one of the primary goals in conservation efforts (Weller et al., 2009; Perry et al., 2010). In order to understand the mechanisms by which some bat species are affected by habitat loss, it is necessary to determine not only if habitat disturbance affects life-history parameters, but also if habitat loss generates changes in their social structure. In this study we assessed six common species of phyllostomid bats to identify sex-related patterns within a human-dominated landscape. Our study provides insights into the importance of habitat type on sex ratio and on sex distribution throughout the year. The objective of this study was to determine if habitat affects bat sex ratio in a human dominated landscape in Southern Mexico. For this, we sampled individuals from six abundant bat species in conserved continuous forests and cattle pastures along and away from riparian corridors. We expected that because riparian corridors provide food, water and roosts, capture rates of females would be greater along them (Naiman et al., 2000). We hypothesized that sex specific differences in habitat use will be caused by the reproductive energetic demands in females (i.e., pregnancy and lactation). At the local scale, vegetation attributes such as tree height may affect female abundance, because females are frequently restricted to high-quality habitats for foraging (Lintott et al., 2014).
The study was carried out in the tropical region of Lacandona, south of the state of Chiapas, Mexico. The original vegetation consists mainly on lowland tropical rain forests. Deforestation of the region began in the 1970’s, resulting in the reduction of old-growth continuous forest from 95% in 1976 to 56% in 1996 (De Jong et al., 2000); only 36% of the original old-growth continuous forest remains today (Carabias et al., 2012). Currently, the main land-use practices in the region consist of cattle pastures, the cultivation of maize and other crops (De Jong et al., 2000; Zermeño-Hernández et al., 2015). The resulting landscape comprise a mosaic of human-modified habitats that include semi-urban settlements, agricultural land, open pastures for cattle, riparian zones, patches of secondary and old-growth forests of various sizes. The region has a mean annual temperature of 24°C; average annual rainfall is 3,000 mm with June to October as the wettest months (551 mm month–1) and February to April as the driest months (<100 mm month–1) (Comisión Federal de Electricidad, 2006; van Breugel et al., 2006).
Four different habitat types were selected for this study including the following: (i) riparian habitat within old-growth continuous forest (RM); (ii) riparian habitat in active cattle pastures (RP); (iii) old-growth continuous forest 1,000 m away from riparian vegetation (MF); and (iv) active cattle pastures (P) 1,000 m away from riparian vegetation. For a total of 12 sampling sites. Each habitat type was replicated three times and study sites were at least 1.5 km away from each other (Figure 1). Streams were all permanent (although with variable amounts of running water throughout the year) while stream width varied from 2 to 8 m. Study sites in pastures were active cattle pastures, although these sites were predominantly devoid of a tree cover, all sites had isolated trees and a few trees that serve as live fences. It is common that isolated trees are left standing to provide shade for cattle, firewood for cooking, or for aesthetic reasons (Galindo-González et al., 2000). The cattle pastures were located in the Marqués de Comillas municipality, on the south side of the Lacantún River. Old-growth continuous forest sites were located in the 330,000 ha Montes Azules Biosphere Reserve (MABR) on the north side of the river (16°04′ N to 90°45′ W; INE, 2000, Figure 1).
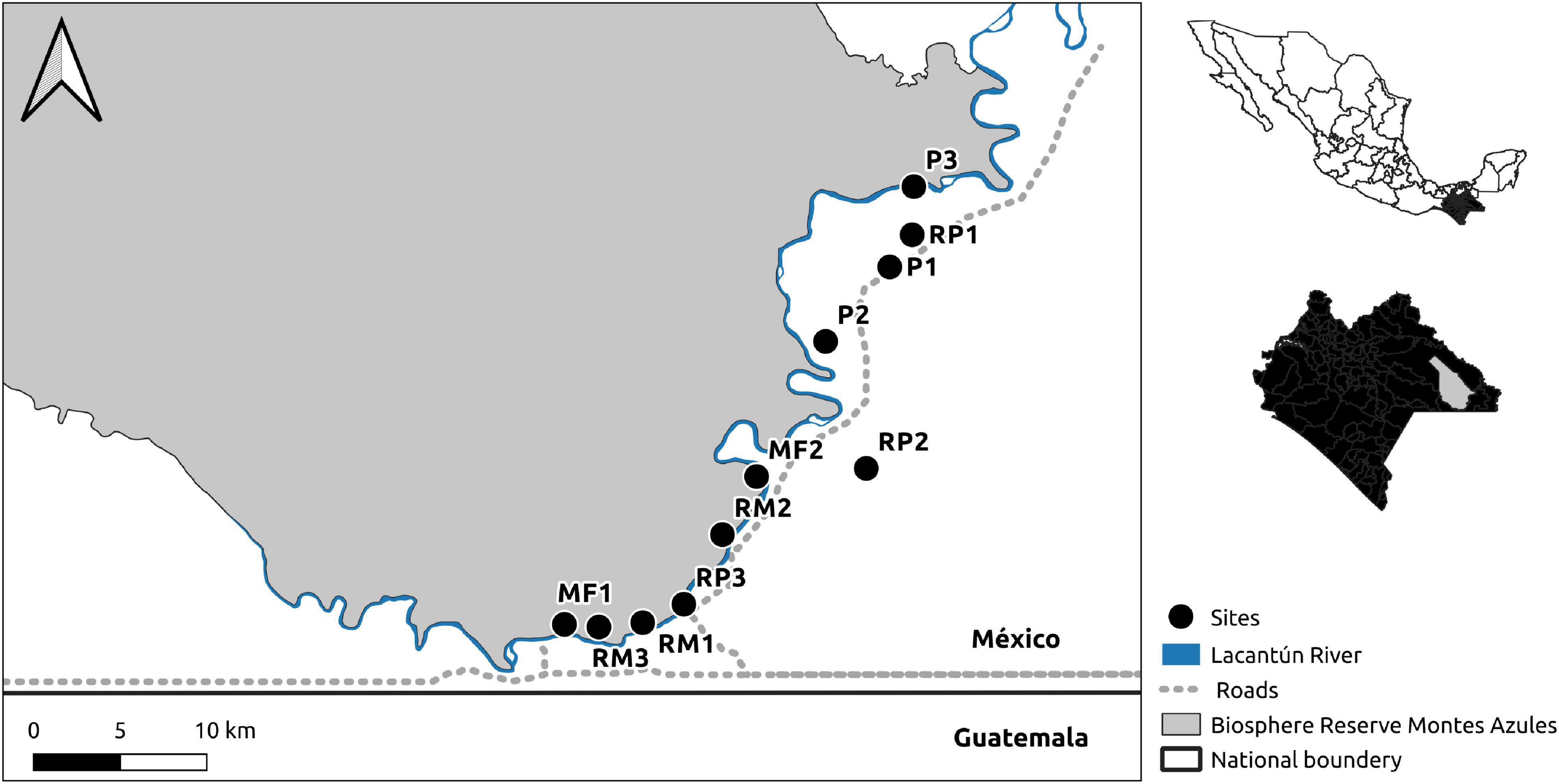
Figure 1. Study area and bat sampling sites at the Lacandona rain forest, Chiapas, Mexico. The map shows the distribution of the habitat types used to sample bat species: riparian vegetation in mature forest (RM), riparian vegetation in pasture (RP), mature forest (MF), Pasture (P).
Sampling of bats was conducted twice during the dry season (December to May) and twice during the wet season (June to November) for 3 consecutive years (2011, 2012, and 2013), using a standardized method of four nights per site. A previous study in the same locations showed that over 70 sampling nights, 34 during the dry season and 36 during the rainy season, a total of 1,752 individuals belonging to 28 species of Phyllostomidae were captured (de la Peña-Cuéllar et al., 2015). For this study however, we considered the six most abundant species of phyllostomid bats in the region, including the following: Artibeus jamaicensis, A. lituratus, Sturnira lilium, Carollia perspicillata, Glossophaga soricina, and Platyrrhinus helleri, the minimum number of captures needed to be considered in the study were one capture per habitat per season, species abundance cut off of n = 60.
Five nets (12 m long × 2.6 m high) were set at ground level and were opened at dusk (1800–1830) for four consecutive hours, which corresponds to the peak foraging time for most phyllostomid species (La Val, 1970). The bat sampling nets were arranged according to habitat type: (1) in the riparian habitats (including continuous forest and pastures), nets were located parallel and/or diagonally across the stream, depending on site characteristics; (2) in continuous forest, nets were positioned across natural flying corridors; (3) in active pastures, nets were located under the canopy of isolated trees. In all sites we searched for similar physical characteristics that allow the same mist net arrangement, this is one individual net and two pairs of nets in an “L” shape (two nets connected perpendicularly). Nets were located ca. 50 m apart. Nights with a full moon or heavy rain were avoided during bat sampling in order to prevent variation in capture success associated with these conditions (Morrison, 1978). Captured individuals were temporarily stored in cloth bags and identified to species following Medellín et al. (2011). For all captured bats, we determined sex by inspecting genitalia (Racey and Speakman, 1987). In females, we detected pregnancy by palpation (Racey and Speakman, 1987), and lactation by the occurrence of enlarged nipples surrounded by a hairless skin area and by extruding milk with a gentle finger pressure on the nipple base. Sex ratio was calculated as the ratio of males to females in each site (Russo et al., 2010).
There is a risk of exposure to some significant zoonotic agents for any person handling bats in the field, following biosecurity recommendations (Newman et al., 2011). For this, all participants that sampled bats were appropriately trained to handle bats; bites and scratches were avoided by using leather gloves, previous anti-rabies vaccination and hand washing before and after bat manipulation.
To determine the influence of vegetation structure on the abundance of male and female bats, for each site we recorded all trees ≥10 cm diameter at breast height (dbh) within a 0.1 ha (20 × 50 m) plot (Gentry, 1982). Transects were located along streams in riparian continuous forest and riparian pasture habitats and randomly located in continuous forest and pasture habitats. We considered the following vegetation attributes: density of individuals, species richness, forest basal area, and tree height.
First, we compared capture rates for each sex among habitat types by using a standardized capture rate (captures/1,000 mist net hour) that compensated for differences in number of nets, size of nets and length of time nets were open (Perry et al., 2010). We compared capture rates using analysis of variance on ranks (ANOVA). Data were checked to meet assumption of homoscedasticity and normality. We performed non-metric multidimensional scaling analyses (NMDS) based on the identity and abundance of tree species occurring in the sampling sites, to obtain a continuous synthetic variable summarizing dissimilarity patterns among vegetation species composition. The matrix used in the analysis was built using the Bray-Curtis index (Magurran, 2004). This iterative method of ordination has the advantage of properly handling non-linear species response of any shape (Oksanen, 2013) and has a good performance even when beta diversity is high (McCune and Grace, 2002). It is one of the preferred ordination methods for analyzing community data (McCune and Grace, 2002). The scores of axis 1 were used as an explanatory variable for evaluating differential sex response to tree species composition. Second, we fitted a general linear mixed model (GLMM) for each species separately with binomial error distribution and logit link function to determine the influence of vegetation traits on male and female abundance. In order to assess the relative effects of the explanatory variables on males in comparison to females, the model was run with the proportion of females to males per night (n = 70) as the response variable, with “site” as a random factor (Lintott et al., 2014). We considered the following explanatory variables: habitat type; season (dry and rainy), Vba, forest basal area per site; Vab, total number of trees; Vrich, trees species richness; Vh, average tree height; Vspcomp, scores of NMDS axis 1 (see Supplementary Tables 1, 2). For each model, we calculated Akaike’s information criterion (AICc) corrected for small sample size following (Burnham and Anderson, 2004). This approach allowed us to select the most plausible models from a set of models. The set of models considered for every response variable, at each scale, included the null model (without explanatory power) and other models that considered each explanatory variable independently. We compared the model using Δi, which is the difference of AICc between a given model and the best (lowest AICc) model. We also calculate the AIC weights (wi) for each model. The wi represents the weight of the evidence that a certain model is the best model given the data and the set of candidate models. The 95% confidence set of the best models was defined by summing the wi, from the largest to the smallest, until the sum is = 0.95. Only models with an AICc lower than the null model were considered to define the 95% confidence set of plausible models.
All analyses were performed with R v. 1.0.136 (R Core Team, 2019).
We completed 70 nights of capture effort, 34 during the dry season, and 36 during the rainy season, resulting in a total capture effort of 180 net hours in RM, RP, and P, and 140 net hours in MF habitats. The total number of captures of the six most common bat species was 1,365 individuals (78% of all captures) including the following: Artibeus jamaicensis (n = 199, 11%), Artibeus lituratus (n = 396, 23%), Sturnira lilium (n = 521, 30%), Carollia perspicillata (n = 60, 3%), Glossophaga soricina (n = 109, 6%) and Platyrrhinus helleri (n = 81, 5%). These species were also present at all study sites. Overall, 43% of the sampled individuals from the species considered were males, while 57% were females, 1.131 proportion of females to males. The capture rates were significantly skewed toward females (F = 5.282, P < 0.001) (Figure 2). Riparian pasture, was the habitat with the highest female capture rates (433 individuals, 2.14 capture rate), followed by riparian continuous forest (152, 0.75) and active pasture (141, 0.69). Continuous forest was the habitat with the lowest female capture rates (50, 0.28) (Figure 2).
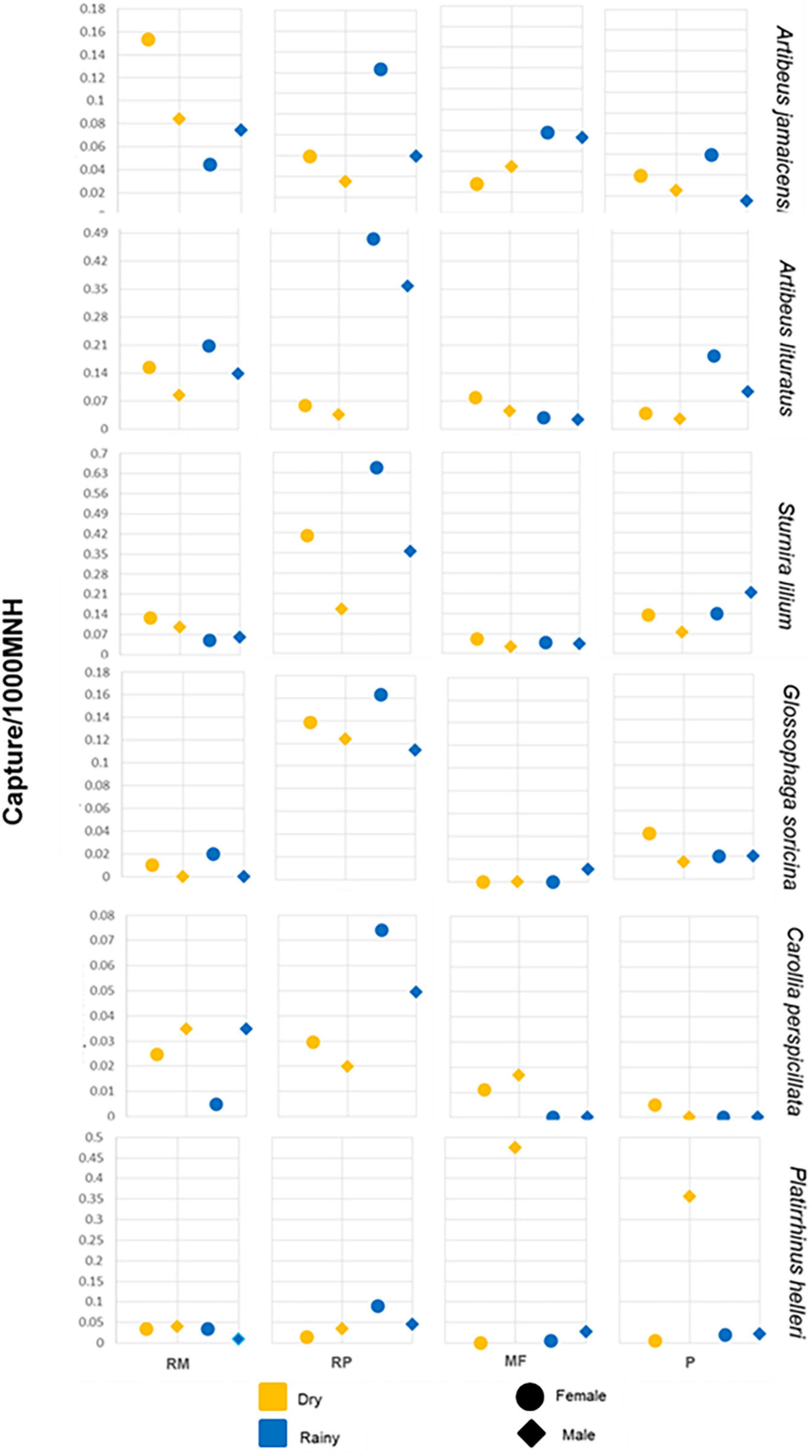
Figure 2. Capture rate (bats/mist net hour) of males and females of six bat species across different habitat types at the Lacandona rain forest during the dry and rainy seasons.
Riparian habitats in mature forests (RM) and pastures (RP) showed higher vegetation structural complexity than non-riparian habitats. Despite of the fact that RM contained more trees than RP, these habitats showed similar average canopy height (Table 1), whereas the lowest canopy height was recorded in pastures. On the other hand, basal area was greater in riparian habitats that in non-riparian habitats, in both old-growth forests and pastures (Table 1). The bioplot resulting of NMDS ordination is in Supplementary Figure 1.
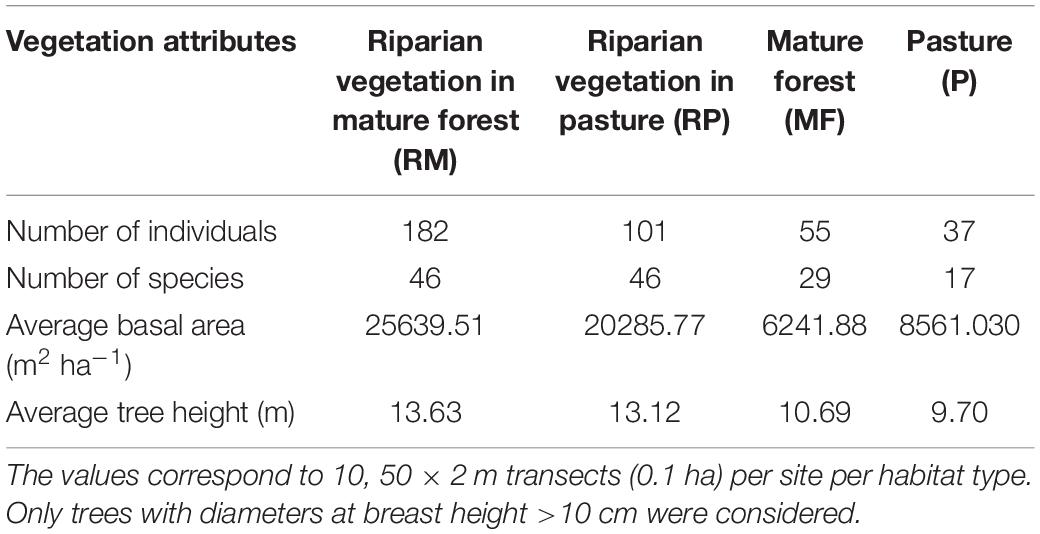
Table 1. Tree community attributes in four different tropical habitat types replicated three times, Chiapas, Mexico.
Seasonality and habitat type were the best explanatory variables describing the presence of A. lituratus females; while for S. lilium, tree species composition and seasonality appeared as the most important variables explaining the incidence of females. In the case of G. soricina we found that the presence of females was positively related to vegetation structure including forest basal area and average tree height (Table 2). The results for all species are in Supplementary Table 3.
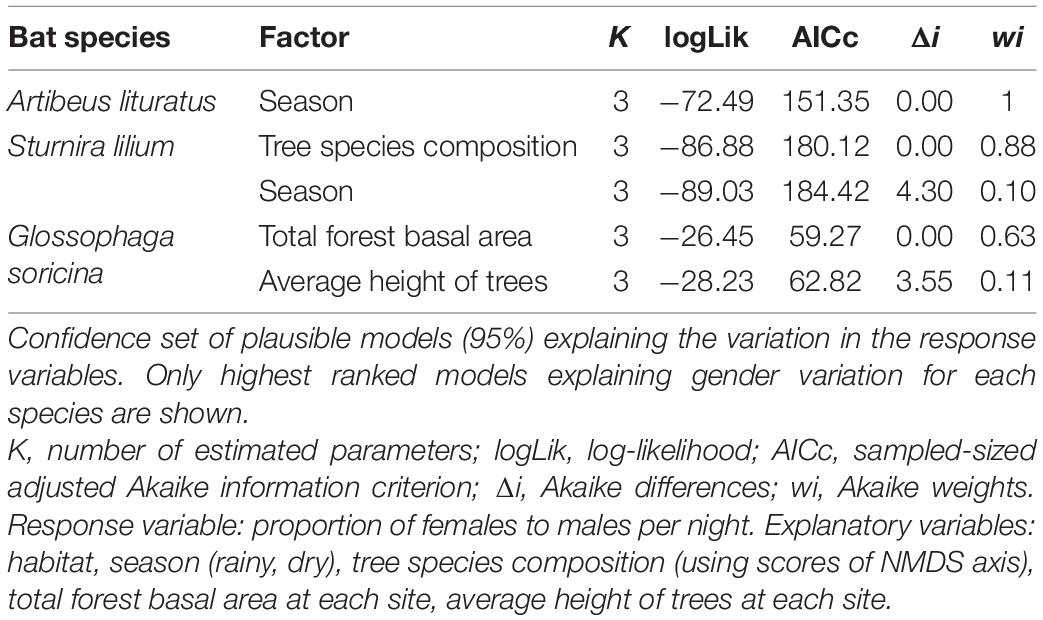
Table 2. Results of Akaike information criterion (AIC)-based model selection, assessing the association between the proportion of females to males of three bat species with seasonality and vegetation attributes.
Overall, the results showed that males and females of the selected bat species cope differently with habitat disturbance. Sex ratio was skewed toward females, which is an expected pattern for harem species (A. jamaicensis and A. lituratus) (de Mello and Fernandez, 2000). Furthermore, the results provide insights into the relative importance of habitat type for a specific sex. Except for mature forest, overall capture rates of females were greater than those of males, for the six studied species implying that during reproductive period, females of some bat species, may increase their activity in these habitats (e.g., foraging and/or drinking). Female bats can show changes in foraging activity probably due to lactation when energy and water requirements increase (Adams and Hayes, 2008; Barclay, 2012).
Unlike males, females need to return at night to the maternity roosts to nurse their young which probably limits female foraging areas and restricts foraging females to the rewarding areas located in the proximity of their roosts (van Toor et al., 2011). Moreover, males may have greater survival than females (Keen and Hitchcock, 1980; Kurta and Matson, 1980), because males are not subject to the additional energetic pressures associated with pregnancy and lactation. For instance, in arid environments the drinking passes of lactating female bats were significantly higher than those of non-reproductive adult females, thus, survival of reproductive female bats seems to be conditioned to the availability or frequent and uninterrupted access to free-standing water sources (Adams and Hayes, 2008). We found that capture rates in active pastures were predominately toward females. The prevalence of females may be related to the high energy demanding of flying in cluttered habitat than flying in more open areas (Grodzinski et al., 2009), due to the elevated energetic costs associated with higher vegetation complexity might represent a particularly high burden for females during pregnancy and while nursing, males otherwise prefer sites with greater vegetation cover possibly related to roost defense (Henry and Kalko, 2007; Rocha et al., 2017).
Contrary to our hypothesis, we found that females of A. lituratus did not exhibit relationship with habitat quality. The largest species of Stenodermatinae is A. lituratus, and as in other mammals, larger species could be more sensitive to human habitat disturbance (Lande, 1987). Nevertheless, the high number of females in this bat species, reflects selective foraging in a resource rich environment and higher roost availability in forested habitats. Even though Artibeus species may cross inhospitable matrix areas in fragmented landscapes, covering different vegetation types and flying distances ranging from 5 to 10 km (Galindo-González, 1998), females of A. lituratus might locally depend on temporal foliar roosts, and prefer larger trees within the dense and shaded mature forest that can provide energetic and thermal requirements to leave the young while foraging (Evelyn and Stiles, 2003; Bianconi et al., 2006; Arnone et al., 2016). For the same study area, A. lituratus has shown to select roosts with high humidity located in trees with the greatest basal areas (Ortiz-Ramírez et al., 2006).
Furthermore, some studies have argued that Glossophaginae are resilient to land use change (Willig et al., 2007). Our data indicate that the presence of G. soricina females is significantly and positively correlated to vegetation attributes such as tree height and forest basal area, supporting the idea that the species is an habitat specialist (Aguiar et al., 2014). The association between females of G. soricina with large trees could be due to the fact that large trees provide greater availability of roosts and foraging opportunities (Evelyn and Stiles, 2003; Ortiz-Ramírez et al., 2006). There is evidence showing food differentiation between specimens of G. soricina in the same areas where females preferred a plant food item different from males (Alvarez and Sánchez-Casas, 1999). During pregnancy and breeding seasons females might feed on the nearest available resource, whereas males fly larger distances in search of other feeding areas (Sosa et al., 1996). This foraging behavior might reduce the activity of G. soricina to habitats with high resource availability limiting its activity to small home ranges increasing its susceptibility to local extinction (Arita and Santos-del-Prado, 1999). This increases the importance of vegetation traits associated to the reproductive energetic demand (pregnancy, lactation and roost defense) (Charles-Dominique, 1991; Klingbeil and Willig, 2010).
Tree species composition was the strongest predictor variable for S. lilium, this frugivorous bat is known for its preference for understory shrubs and pioneer tree species, females of S. lilium are able to forage among forest elements as a result of non-random distribution of resources across the landscape (Loayza and Loiselle, 2008). Moreover, frugivorous bats like S. lilium which can visit different vegetation types can be considered as indicator taxa of habitat change in riparian vegetation, rather than highly specialized taxa in which population decline rapidly under environmental changes (de la Peña-Cuéllar et al., 2015).
The importance of seasonality for A. lituratus and S. lilium females might reside on differences in abundance and diversity of food resources between the wet and dry seasons. Seasonal fluctuations in rainfall influence phenology of fruiting plants and affects productivity in tropical forests (Ramos Pereira et al., 2010). In tropical regions, usually the rainy season corresponds to greatest fruit abundance than the dry season (Smythe, 1986). Even, many tropical bats timing their reproductive phenology to match periods of peak food availability, female bats may be constrained by the energetic requirements associates with reproduction, which might force them to alter their foraging behavior (Lintott et al., 2014). Resource availability due to seasonality can result in shortened flights during the exploration for food and shelter, whereas during the dry season when food resources are often scarce, females respond to local-scale vegetation structure increasing foraging movements into resource-rich pioneer fruiting plant species areas like secondary forests, while males tend to select areas close to old-growth forests (Rocha et al., 2017).
Our results show that responses of bat to human disturbance are sex-specific. Taking into account sex ratios of bat populations may help to a better understanding of the pervasive consequences of habitat loss and fragmentation. Sex-specific studies are important for bat conservation practices in order to promote habitat conditions favorable for both, females and males (Perry et al., 2007). Vegetation attributes like three height and basal area reflect the age and vertical complexity of forest, and more varied niche opportunities for bats, enhancing greater taxonomic and phylogenetic biodiversity (Martins et al., 2017). Our results suggest that the structural complexity of the vegetation and large trees influence the presence of females of G. soricina. In this sense, management efforts should promote riparian vegetation cover with large forest basal areas, important for the conservation of the entire bat community. Even though our analysis was restricted to six common bat species, we assumed that the maintenance of habitats that favor habitat generalist species should also benefit bat species that are habitat specialists (Istvanko et al., 2016; Rocha et al., 2018). Therefore, we encourage the inclusion of species of sensitive trophic guilds (gleaning insectivores and carnivores) and particularly roosting habits emphasizing adequate protection of females in conservation plans. Conservation actions toward female protection are particularly important due to their high level of parental investment associated with rearing pups (Istvanko et al., 2016). Management decisions that do not guarantee the protection of the habitat frequently used by female bats would likely have detrimental long term consequences on their reproduction, jeopardizing the dynamics and long-term persistence (van Toor et al., 2011; Frank et al., 2016).
In human dominated landscapes, the presence of isolated trees in pasture promote bat flights across pasture and increase bat detectability (Galindo-González et al., 2000), based on foraging behavior frugivorous bats travel across pastures and visit isolated trees while foraging, and use canopies for roosts or to decrease predation risks (Galindo-González, 1998), also isolated trees in pastures act as stepping stones for traveling across fragmented landscapes to different forest remnants (Guevara et al., 1989). However, pastures cannot sustain the same species richness of bats as old-growth forest and riparian vegetation (de la Peña-Cuéllar et al., 2015). Moreover, land use change in tropical landscapes seems to have considerable effects on bat population dynamics, for instance evidence suggests that forest-adapted insectivorous species are particularly sensitive to habitat conversion (Medellín et al., 2000; Williams-Guillén and Perfecto, 2010). Furthermore, frugivorous bats respond to matrix quality in different manners, whereas studies have found that frugivorous abundance was positively associated with the proportion of high quality habitats (Pinto and Keitt, 2008; Avila-Cabadilla et al., 2012; de la Peña-Cuéllar et al., 2015), some other studies have shown that frugivorous bat richness and abundance are higher in moderately fragmented landscapes than in old-growth forest (Willig et al., 2007; Klingbeil and Willig, 2009). Overall agricultural intensification may cause detrimental effects on bats and thus presumably on the ecosystem services they provide (Williams-Guillén et al., 2015). Additional research is needed to directly examine the effects of pregnancy and lactation on habitat selection by bats. We encourage radio-tracking studies that can show specific habitat use of males and females (roost and foraging areas) and if there is temporal segregation between sexes; this could provide information about how different habitats have an impact in the demography of bat populations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the animal study because the handling was very brief and we did not perform animal sacrifices.
EP-C and JB-M conceived and designed the experiments, contributed reagents, materials, and analysis tools, wrote the manuscript, and revised the manuscript. EP-C performed the experiments and analyzed the data. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the Comisión Nacional de Áreas Naturales Protegidas (CONANP) and the National Autonomous University of Mexico (UNAM). EP-C acknowledges the scholarship and financial support provided by the Posgrado en Ciencias Biológicas (UNAM) and the National Council of Science and Technology (CONACYT), as well as additional support from the Bat Conservation International (BCI Student Research Scholarship Program). We are grateful to R. Lombera-Estrada, G. and I. Lombera, G. Rodríguez-Barrera, and J. L. Peña-Mondragón for their assistance in the field. We are also grateful for logistical support provided by J. M. Lobato-García.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.741069/full#supplementary-material
Adams, R. A., and Hayes, M. A. (2008). Water availability and successful lactation by bats as related to climate change in arid regions of western North America. J. Anim. Ecol. 77, 1115–1121. doi: 10.1111/j.1365-2656.2008.01447.x
Aguiar, L. M. S., Bernard, E., and Machado, R. B. (2014). Habitat use and movements of Glossophaga soricina and Lonchophylla dekeyseri (Chiroptera: Phyllostomidae) in a Neotropical savannah. Zoologia 31, 223–229. doi: 10.1590/S1984-46702014000300003
Altringham, J. D. (2011). Bats: From Evolution To Conservation, 2nd Edn. Oxford: Oxford University Press.
Alvarez, T., and Sánchez-Casas, N. (1999). Diferenciación alimentaria entre los sexos de Glossophaga soricina (Chiroptera: Phyllostomidae) en México. Rev. Biol. Trop. 47, 1129–1136. doi: 10.3161/150811012X654871
Arita, H. T., and Santos-del-Prado, K. (1999). Conservation Biology of Nectar-Feeding Bats in Mexico. J. Mammal. 80, 31–41. doi: 10.2307/1383205
Arnone, I. S., Trajano, E., Pulchério-Leite, A., and Passos, F. C. (2016). Long-distance movement by a great fruit-eating bat, Artibeus lituratus (Olfers, 1818), in southeastern Brazil (Chiroptera, Phyllostomidae): evidence for migration in Neotropical bats? Biota Neotrop. 16:e0026. doi: 10.1590/1676-0611-BN-2015-0026
Avila-Cabadilla, L. D., Sanchez-Azofeifa, G. A., Stoner, K. E., Alvarez-Añorve, M. Y., Quesada, M., and Portillo-Quintero, C. A. (2012). Local and landscape factors determining occurrence of phyllostomid bats in tropical secondary forests. PLoS One 7:e35228. doi: 10.1371/journal.pone.0035228
Barclay, R. M. R. (2012). Variable variation: annual and seasonal changes in offspring sex ratio in a bat. PLoS One 7:e36344. doi: 10.1371/journal.pone.0036344
Beerman, A., Ashe, E., Preedy, K., and Williams, R. (2016). Sexual segregation when foraging in an extremely social killer whale population. Behav. Ecol. Sociobiol. 70, 189–198. doi: 10.1007/s00265-015-2038-2
Bender, D. J., Contreras, T. A., and Fahrig, L. (1998). Habitat Loss and Population Decline: a Meta-Analysis of the Patch Size Effect. Ecology 79, 517–533.
Benitez-Malvido, J., Martínez-Falcón, A. P., Dattilo, W., González-DiPierro, A. M., Lombera Estrada, R., and Traveset, A. (2016). The role of sex and age in the architecture of intrapopulation howler monkey-plant networks in continuous and fragmented rain forests. PeerJ 4:e1809. doi: 10.7717/peerj.1809
Bianconi, G. V., Mikich, S. B., and Pedro, W. A. (2006). Movements of bats (Mammalia, Chiroptera) in Movements Chiropteroptera) Atlantic For est remnants in southern Brazil Forest remnants southern Brazil. Rev. Bras. Zool. 23, 1199–1206.
Broders, H. G., Forbes, G. J., Woodley, S., and Thompson, I. D. (2006). Range extent and stand selection for roosting and foraging in forest-dwelling Northern Long-eared Bats and Little Brown Bats in the Greater Fundy ecosystem, New Brunswick. J. Wildl. Manage. 70, 1174–1184.
Burnham, K. P., and Anderson, D. R. (2004). Multimodel inference: understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304. doi: 10.1177/0049124104268644
Carabias, J., Meli, P., and Hernández, G. (2012). Evaluación de los impactos de proyectos de desarrollo sustentable sobre la reducción del cambio de uso de suelo en ejidos de Marqués de Comillas, Chiapas. México: INECC.
Charles-Dominique, P. (1991). Feeding strategy and activity budget of the frugivorous bat Carollia perspicillata (Chiroptera: Phyllostomidae) in French Guiana. J. Trop. Ecol. 7:243. doi: 10.1017/S026646740000540X
Comisión Federal de Electricidad (2006). División Hidrométrica Sureste. México: Marqués de Comillas.
De Jong, B. H. J., Ochoa-Goana, S., Castillo-Santiago, M. A., Ramirez-Marcial, N., and Cairns, M. A. (2000). Carbon flux and patterns of land-use/land-cover change in het Selva Lacandona, Mexico. J. Hum. Environ. 29, 504–511. doi: 10.1579/0044-7447-29.8.504
de la Peña-Cuéllar, E., Benítez-Malvido, J., Avila-Cabadilla, L. D., Martínez-Ramos, M., and Estrada, A. (2015). Structure and diversity of phyllostomid bat assemblages on riparian corridors in a human-dominated tropical landscape. Ecol. Evol. 5, 903–913. doi: 10.1002/ece3.1375
de Mello, M. A. R., and Fernandez, F. A. S. (2000). Reproductive ecology of the bat Carollia perspicillata (Chiroptera: Phyllostomidae) in a fragment of the Brazilian Atlantic coastal forest. Z. Saeugetierkd. 65, 340–349.
Diamond, G. F., and Diamond, J. M. (2014). Bats and Mines: evaluating Townsend’s Big-Eared Bat (Corynorhinus townsendii) Maternity Colony Behavioral Response to Gating. West. N. Am. Nat. 74, 416. doi: 10.3398/064.074.0407
Dietz, M., Kalko, E. K. V., and Encarnação, J. A. (2006). Small scale distribution patterns of female and male Daubenton’s bats (Myotis daubentonii). Acta Chiropterol. 8, 403–415.
Encarnação, J. A. (2012). Spatiotemporal pattern of local sexual segregation in a tree-dwelling temperate bat Myotis daubentonii. J. Ethol. 30, 271–278. doi: 10.1007/s10164-011-0323-8
Evelyn, M. J., and Stiles, D. A. (2003). Roosting requirements of two frugivorous bats (Sturnira lilium and Arbiteus intermedius) in fragmented Neotropical forest. Biotropica 35, 405–418. doi: 10.1646/02063
Fagan, M. E., DeFries, R. S., Sesnie, S. E., Arroyo, J. P., Walker, W., Soto, C., et al. (2013). Land cover dynamics following a deforestation ban in northern Costa Rica. Environ. Res. Lett. 8:034017. doi: 10.1088/1748-9326/8/3/034017
Frank, H. K., Mendenhall, C. D., Judson, S. D., Daily, G. C., and Hadly, E. A. (2016). Anthropogenic impacts on Costa Rican bat parasitism are sex specific. Ecol. Evol. 6, 4898–4909. doi: 10.1002/ece3.2245
Galindo-González, J. (1998). Dispersión de semillas por murciélagos: su importancia en la conservación y regeneración del bosque tropical. Acta Zool. Mex. 73, 57–74.
Galindo-González, J., Guevara, S., and Sosa, V. J. (2000). Bat- and bird-generated seed rains at isolated trees in pastures in a tropical rainforest. Conserv. Biol. 14, 1693–1703. doi: 10.1046/j.1523-1739.2000.99072.x
Gentry, A. H. (1982). “Patterns of neotropical plant species diversity,” in Evolutionary Biology, eds M. K. Hecht, B. Wallace, and G. T. Prance (Boston: Springer).
Grodzinski, U., Spiegel, O., Korine, C., and Holderied, M. W. (2009). Context-dependent flight speed: evidence for energetically optimal flight speed in the bat Pipistrellus kuhlii? J. Anim. Ecol. 78, 540–548. doi: 10.1111/j.1365-2656.2009.01526.x
Guevara, S., Laborde, J., and Sánchez, G. (1989). Are Isolated Remnant Fragmented Trees in Pastures a Canopy? Selbyana 19, 34–43.
Henry, M., and Kalko, E. K. V. (2007). Froraging Strategy and Breeding Constraints of Rhynophyla Pumilio (PHYLLOSTOMIDAE) in the Amazon Lowlands. J. Mammal. 88, 81–93. doi: 10.1644/06-mamm-a-001r1.1
Hutson, A. M., Mickleburgh, S. P., and Racey, P. A. (2001). Global Status Survey and Conservation Action Plan: Microchiropteran Bats. Cambridge: IUCN, doi: 10.4103/0250-474X.84603
Istvanko, D. R., Risch, T. S., and Rolland, V. (2016). Sex-specific foraging habits and roost characteristics of Nycticeius humeralis in north-central Arkansas. J. Mammal. 97, 1336–1344. doi: 10.1093/jmammal/gyw102
Keen, R., and Hitchcock, H. H. B. (1980). Survival and longevity of the little brown bat (Myotis lucifugus) in southeastern Ontario. J. Mammal. 61, 1–7. doi: 10.2307/1379951
Kingston, T. (2010). Research priorities for bat conservation in Southeast Asia: a consensus approach. Biodivers. Conserv. 19, 471–484. doi: 10.1007/s10531-008-9458-5
Klingbeil, B. T., and Willig, M. R. (2009). Guild-specific responses of bats to landscape composition and configuration in fragmented Amazonian rainforest Brian. J. Appl. Ecol. 46, 203–213. doi: 10.1111/j.1365-2664.2007.0
Klingbeil, B. T., and Willig, M. R. (2010). Seasonal differences in population-, ensemble- and community-level responses of bats to landscape structure in Amazonia. Oikos 119, 1654–1664. doi: 10.1111/j.1600-0706.2010.18328.x
Kurta, A., and Matson, J. O. (1980). Disproportionate Sex Ratio in the Big Brown Bat (Eptesicus fuscus). Am. Midl. Nat. 104, 367–369. doi: 10.2307/2424878
La Val, R. (1970). Banding Returns and Activity Periods of Some Costa Rican Bats. Southwest. Nat. 15, 1–10. doi: 10.2307/3670196
Lande, R. (1987). Extinction Thresholds in Demographic Models of Territorial Populations. Am. Nat. 130, 624–635. doi: 10.1086/284734
Lemaître, J.-F., Gaillard, J.-M., Pemberton, J. M., Clutton-Brock, T. H., and Nussey, D. H. (2014). Early life expenditure in sexual competition is associated with increased reproductive senescence in male red deer. Proc. Biol. Sci. 281:20140792. doi: 10.1098/rspb.2014.0792
Lintott, P. R., Bunnefeld, N., Fuentes-Montemayor, E., Minderman, J., Mayhew, R. J., Olley, L., et al. (2014). City life makes females fussy: sex differences in habitat use of temperate bats in urban areas. R. Soc. Open Sci. 1:140200. doi: 10.1098/rsos.140200
Loayza, A. P., and Loiselle, B. A. (2008). Preliminary Information on the Home Range and Movement Patterns of Sturnira lilium (Phyllostomidae) in a Naturally Fragmented Landscape in Bolivia. Biotropica 40, 630–635. doi: 10.1111/j.1744-7429.2008.00422.x
Lundy, M., and Montgomery, I. (2010). Summer habitat associations of bats between riparian landscapes and within riparian areas. Eur. J. Wildl. Res. 56, 385–394. doi: 10.1007/s10344-009-0330-z
Martins, A. C. M., Willig, M. R., Presley, S. J., and Marinho-Filho, J. (2017). Effects of forest height and vertical complexity on abundance and biodiversity of bats in Amazonia. For. Ecol. Manage. 391, 427–435. doi: 10.1016/j.foreco.2017.02.039
Mayaux, P., Holmgren, P., Achard, F., Eva, H. D., Stibig, H.-J. J., and Branthomme, A. (2005). Tropical forest cover change in the 1990s and options for future monitoring. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 360, 373–384. doi: 10.1098/rstb.2004.1590
McCracken, G. F., and Wilkinson, G. S. (2000). “Bat Mating Systems,” in Reproductive Biology of Bats, eds E. G. Crichton and P. H. Krutzsch (San Diego: Academic Press), 321–362. doi: 10.1016/B978-012195670-7/50009-6
McCune, B., and Grace, J. B. (2002). PCORD Analysis of Ecological Communities. Gleneden Beach: MjM Software Design.
Medellín, R. A., Equihua, M., and Amin, M. A. (2000). Bat Diversity and Abundance as Indicators of Disturbance in Neotropical Rainforests\rDiversidad y Abundancia de Murciélagos como Indicadores de Perturbaciones en Selvas Húmedas Neotropicales. Conserv. Biol 14, 1666–1675. doi: 10.1111/j.1523-1739.2000.99068.x
Medellín, R. A., Equihua, M., and Amin, M. A. (2011). Identificación de los murciélagos de México: clave de campo. México: Universidad Nacional Autónoma de México.
Morrison, D. W. (1978). Lunar phobia in a neotropical fruit bat, Artibevs jamaicensis (Chiroptera: Phyllostomidae). Anim. Behav. 26, 852–855. doi: 10.1016/0003-3472(78)90151-3
Naiman, R. J., Bilby, R. E., and Bisson, P. A. (2000). Riparian Ecology and Management in the Pacific Coastal Rain Forest. Bioscience 50:996. doi: 10.1641/0006-3568(2000)050[0996:reamit]2.0.co;2
Nardone, V., Cistrone, L., Di Salvo, I., Ariano, A., Migliozzi, A., Allegrini, C., et al. (2015). How to be a male at different elevations: ecology of intra-sexual segregation in the trawling bat Myotis daubentonii. PLoS One 10:e0134573. doi: 10.1371/journal.pone.0134573
Newman, S. H., Field, H., Epstein, J., and de Jong, C. (2011). Investigating The Role Of Bats In Emerging Zoonoses. Rome: FAO.
Nupp, T., and Swihart, R. (2000). Landscape-level correlates of small-mammal assemblages in forest fragments of farmland. J. Mammal. 81, 512–526. doi: 10.1093/jmammal/81.2.512
Oksanen, J. (2013). Multivariate Analysis of Ecological Communities in R: vegan tutorial. R Packag. Version. 1–43.
Ortega, J., and Arita, H. T. (1999). Structure and Social dynamics of harem groups in Artibeus jamaicensis (Chiroptera: Phyllostomidae). J. Mammal. 80, 1173–1185. doi: 10.2307/1383168
Ortiz-Ramírez, D., Lorenzo, C., Naranjo, E., and León-Paniagua, L. (2006). Selección de refugios por tres especies de murciélagos frugívoros (Chiroptera: Phyllostomidae) en la Selva Lacandona, Chiapas, México. Rev. Mex. Biodivers. 77, 261–270.
Patriquin, K. J., Guy, C., Hinds, J., and Ratcliffe, J. M. (2019). Male and female bats differ in their use of a large urban park. J. Urban Ecol. 5:juz015. doi: 10.1093/jue/juz015
Perry, R. W., Carter, S. A., and Thill, R. E. (2010). Temporal Patterns in Capture Rate and Sex Ratio of Forest Bats in Arkansas. Am. Midl. Nat. 164, 270–282. doi: 10.1674/0003-0031-164.2.270
Perry, R. W., Thill, R. E., and Carter, S. A. (2007). Sex-specific roost selection by adult red bats in a diverse forested landscape. For. Ecol. Manage. 253, 48–55. doi: 10.1016/j.foreco.2007.07.007
Pinto, N., and Keitt, T. H. (2008). Scale-dependent responses to forest cover displayed by frugivore bats. Oikos 117, 1725–1731. doi: 10.1111/j.1600-0706.2008.16495.x
R Core Team (2019). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Racey, P. A., and Speakman, J. R. (1987). The energy costs of pregnancy and lactation in heterothermic bats. Symp. Zool. Soc. Lond. 57, 107–125.
Racey, P. A., Speakman, J. R., and Swift, S. M. (1987). Reproductive adaptations of heterothermic bats at the northern borders of their distribution. S. Afr. J. Sci. 83, 635–638.
Ramos Pereira, M. J., Marques, J. T., and Palmeirim, J. M. (2010). Ecological responses of frugivorous bats to seasonal fluctuation in fruit availability in amazonian forests. Biotropica 42, 680–687. doi: 10.1111/j.1744-7429.2010.00635.x
Rocha, R., Ferreira, D. F., López-Baucells, A., Farneda, F. Z., Carreiras, J. M. B., Palmeirim, J. M., et al. (2017). Does sex matter? Gender-specific responses to forest fragmentation in Neotropical bats. Biotropica 49, 881–890. doi: 10.1111/btp.12474
Rocha, R., Ovaskainen, O., López-Baucells, A., Farneda, F. Z., Sampaio, E. M., Bobrowiec, P. E. D., et al. (2018). Secondary forest regeneration benefits old-growth specialist bats in a fragmented tropical landscape. Sci. Rep. 8:3819. doi: 10.1038/s41598-018-21999-2
Russo, D., Cistrone, L., Garonna, A. P., and Jones, G. (2010). Reconsidering the importance of harvested forests for the conservation of tree-dwelling bats. Biodivers. Conserv. 19, 2501–2515. doi: 10.1007/s10531-010-9856-3
Safi, K., König, B., and Kerth, G. (2007). Sex differences in population genetics, home range size and habitat use of the parti-colored bat (Vespertilio murinus, Linnaeus 1758) in Switzerland and their consequences for conservation. Biol. Conserv. 137, 28–36. doi: 10.1016/j.biocon.2007.01.011
Senior, P., Butlin, R. K., and Altringham, J. D. (2005). Sex and segregation in temperate bats. Proc. R. Soc. B Biol. Sci. 272, 2467–2473. doi: 10.1098/rspb.2005.3237
Sgroi, M. P., and Wilkins, K. T. (2010). Roosting Behavior of the Mexican Free-Tailed Bat (Tadarida Brasiliensis) in a Highway Overpass. West. N. Am. Nat. 63, 366–373.
Smythe, N. (1986). Competition and resource partitioning in the guild of neotropical terrestrial frugivorous mammals. Annu. Rev. Ecol. Syst. 17, 169–188. doi: 10.1146/annurev.es.17.110186.001125
Sosa, M., De Ascencao, A., and Soriano, P. (1996). Dieta y patrón reproductivo de Rhogessa minutilla (Chiroptera: Vespertilionidae) en una zona árida de Los Andes de Venezuela. Rev. Biol. Trop. 44, 867–875.
van Breugel, M., Martínez-Ramos, M., Bongers, F., Martinez-Ramos, M., and Bongers, F. (2006). Community dynamics during early secondary succession in Mexican tropical rain forests. J. Trop. Ecol. 22, 663–674. doi: 10.1017/s0266467406003452
van Toor, M. L., Jaberg, C., and Safi, K. (2011). Integrating sex-specific habitat use for conservation using habitat suitability models. Anim. Conserv. 14, 512–520. doi: 10.1111/j.1469-1795.2011.00454.x
Wearmouth, V. J., and Sims, D. W. (2008). Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Adv. Mar. Biol. 54, 108–170. doi: 10.1016/S0065-2881(08)00002-3
Weller, T. J., Cryan, P. M., and O’Shea, T. J. (2009). Broadening the focus of bat conservation and research in the USA for the 21st century. Endanger. Species Res. 8, 129–145. doi: 10.3354/esr00149
Williams-Guillén, K., Olimpi, E., Maas, B., Taylor, P. J., and Arlettaz, R. (2015). “Bats in the Anthropogenic Matrix: Challenges and Opportunities for the Conservation of Chiroptera and Their Ecosystem Services in Agricultural Landscapes Kimberly,” in Bats in the Anthropocene: Conservation of Bats in a Changing World, eds C. C. Voigt and T. Kingston (Berlin: Springer), 151–186. doi: 10.1007/978-3-319-25220-9
Williams-Guillén, K., and Perfecto, I. (2010). Effects of agricultural intensification on the bat assemblage in a coffee landscape in Chiapas, Mexico. Biotropica 42, 605–613. doi: 10.2472/jsms.23.470
Willig, M., Presley, S. J., Bloch, C. P., Yanoviak, S. P., Díaz, M. M., Arias Chauca, L., et al. (2007). Phyllostomid Bats of Lowland Amazonia: effects of Habitat Alteration on Abundance. Biotropica 39, 737–746. doi: 10.1111/j.1744-7429.2007.00322.x
Keywords: forest disturbance, bats, riparian corridors, tropical forests, sex ratio
Citation: de la Peña-Cuéllar E and Benítez-Malvido J (2021) Sex-Biased Habitat Use by Phyllostomid Bats on Riparian Corridors in a Human Dominated Tropical Landscape. Front. Ecol. Evol. 9:741069. doi: 10.3389/fevo.2021.741069
Received: 14 July 2021; Accepted: 22 November 2021;
Published: 13 December 2021.
Edited by:
Paulo Estefano Bobrowiec, National Institute of Amazonian Research (INPA), BrazilReviewed by:
Diogo F. Ferreira, Centro de Investigacao em Biodiversidade e Recursos Geneticos (CIBIO-InBIO), PortugalCopyright © 2021 de la Peña-Cuéllar and Benítez-Malvido. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erika de la Peña-Cuéllar, ZXJpa2FwY0BjaWVjby51bmFtLm14
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.