
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 09 September 2021
Sec. Paleoecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.733062
Evidence is accumulating on the regular and systematic Neanderthal exploitation of birds. However, the motivations, mechanisms, and circumstances underlying this behavior remains little explored despite their potential implications on Neanderthal ecology and capabilities. Fossil remains of choughs (Pyrrhocorax, Corvidae) are among the most abundant in cave sites with Mousterian technology. We reviewed the evidence showing that Neanderthals processed choughs for food, and confirmed that it occurred frequently over a widespread spatial and temporal scale. This lead us to propose the hypothesis that the cave-like refuge is the keystone resource connecting Neanderthals and choughs captured at night in rocky shelters eventually used by both species. By adopting an actualistic approach, we documented the patterns of refuge use and population dynamics of communally roosting choughs, the strategies and technology currently used to capture them, and their behavioral response against experimental human predators at night. Actualistic experiments showed that large numbers of choughs can be captured without highly sophisticated tools at night regularly and periodically, due to their occupation year-round during long-term periods of the same nocturnal shelters, the constant turnover of individuals, and their high site tenacity at these roost-sites even after recurrent disturbance and predation. Captures even with bare hands are further facilitated because choughs tend to flee confused into the cavity in darkness when dazzled and cornered by human (experimental) predators. Given the extreme difficulty of daylight chough capturing in open country, nocturnal hunting with the help of fire in the roosting caves and consumption in situ are proposed as the most plausible explanations for the strong association of choughs and Neanderthals in fossil assemblages. Night hunting of birds has implications for the social, anatomical, technological, and cognitive capacities of Neanderthals.
Neanderthal cognitive and physical capacities remain a hotly debated topic in human evolution. Social networking, including verbal communication, planning, anticipation, and coordination of labor division for obtaining food are linked to modern cognitive abilities with broad eco-evolutionary implications (Villa and Roebroeks, 2014; Botha, 2020; Breyl, 2020; Lombard and Högberg, 2021; Wadley, 2021). Among socially coordinated activities, hunting of large terrestrial game has been argued to provide pulses of food covering most energetic and nutritional demands of Neanderthals (Richards et al., 2000; Bocherens et al., 2005). Yet dietary reconstructions are challenging from an ecological perspective, especially when faced the sources and methods used to obtain meat by distinguishing predation from scavenging (Marean and Yeun Kim, 1998; Villa et al., 2005). Smaller prey or plants providing scarcer but more regularly available food and essential nutrients for optimal or specific metabolic requirements are gaining importance in the study of Neanderthal trophic ecology, both in their strongholds in Europe (Hardy and Moncel, 2011; Nabais and Zilhao, 2019) and in the easternmost part of Neanderthal distribution in the Altai mountains of southern Siberia (Salazar-García et al., 2021). Recently, increasing evidence is accumulating on the regular and systematic Neanderthal exploitation of marine resources such as mollusks providing limiting nutrients (e.g., Zilhão et al., 2020, and references therein) and flying birds of many species (e.g., Finlayson et al., 2012; Negro et al., 2016, and references therein). Hunting elusive birds can be cognitively challenging, technologically difficult, and energetically costly. However, this type of prey can eventually represent abundant and nutritionally valuable resources for versatile omnivorous hunter-gatherers (Negro et al., 2016), especially when or where herbivore mammals were scarce or seasonally available. The frequency, circumstances, methods, and nutritional inputs associated to bird capture and exploitation have important, albeit little explored, implications in Neanderthal physical/anatomical, social, technological, and cognitive capacities.
By knowing specific features of the ecology and behavior of a food species, it is possible to infer capture and exploitation tactics by their consumers and then to conduct actualistic experimental approaches taking extant predators and scavengers as study models (Sala and Arsuaga, 2018). These approaches may also allow the making of comparative inferences about the capacities of Neanderthals for food procurement by using extant humans as smart-hunter referents (Negro et al., 2016). Generalizations about the foraging and feeding ecology of Neanderthals have proposed bird exploitation owing to a generalist and versatile foraging mode (Blasco and Fernández Peris, 2012; Negro et al., 2016). A recent non-mutually exclusive hypothesis states that birds were targeted and systematically or opportunistically predated for not only consumption, but also for feathers, claws, and bones for ornamentation and other symbolic purposes (Fiore et al., 2004; Sánchez Marco and Cacho Quesada, 2010; Peresani et al., 2011; Finlayson et al., 2012; Radovčić et al., 2015, 2020; Fiore et al., 2016; Majkić et al., 2017; Blasco et al., 2019). This appealing hypothesis has revolutionary implications by assigning unprecedented cognitive abilities to Neanderthals, thus sustaining the ongoing debate and opening new research avenues in human evolution. Even though this hypothesis is gaining momentum, the understanding of bird exploitation and its eco-evolutionary implications are constrained by partial and unbalanced databases regarding birds as compared with other prey groups in sites with Neanderthal occupation. These information sources rarely include comprehensive qualitative and quantitative data on avian prey, much less on the relative importance of this over other food types, on the potential factors governing the occurrence of particular bird species, or on the preferential target of some species over others. Therefore, information and circumstantial evidence about bird catching and the exploitation of avian populations could shed light on the ecology and cognitive abilities of Neanderthals.
Among birds exploited by hominins, raptors, pigeons, and corvids, especially the Pyrrhocorax choughs (Passeriformes, Corvidae), have been frequently recorded in the Pleistocene of the Palearctic (Tyrberg, 1998, 2008). Choughs are medium-sized (200–360 g depending on species and sex), highly social corvids widely distributed at continental scales but with currently fragmented and isolated populations throughout the Palearctic (Cramp and Perrins, 1994). Choughs are represented by two sister species, the red-billed chough (P. pyrrhocorax) (Figure 1A) and the Alpine chough (P. graculus) (Figure 1D), slightly differing in appearance and altitude-related habitat use and distribution, but sharing many aspects of their ecology and behavior. Both inhabit open, montane, and rocky inland and coastal landscapes, locally in sympatry (Cramp and Perrins, 1994). They are singular among Palearctic birds by their remarkable troglodyte habits. Large caves, cracks, potholes, and chasms are used for nesting, resting during the hottest daylight hours and inclement weather conditions, and especially for communally night roosting often congregating hundreds of individuals (Cramp and Perrins, 1994; Blanco and Tella, 1999; Blanco et al., 2009).
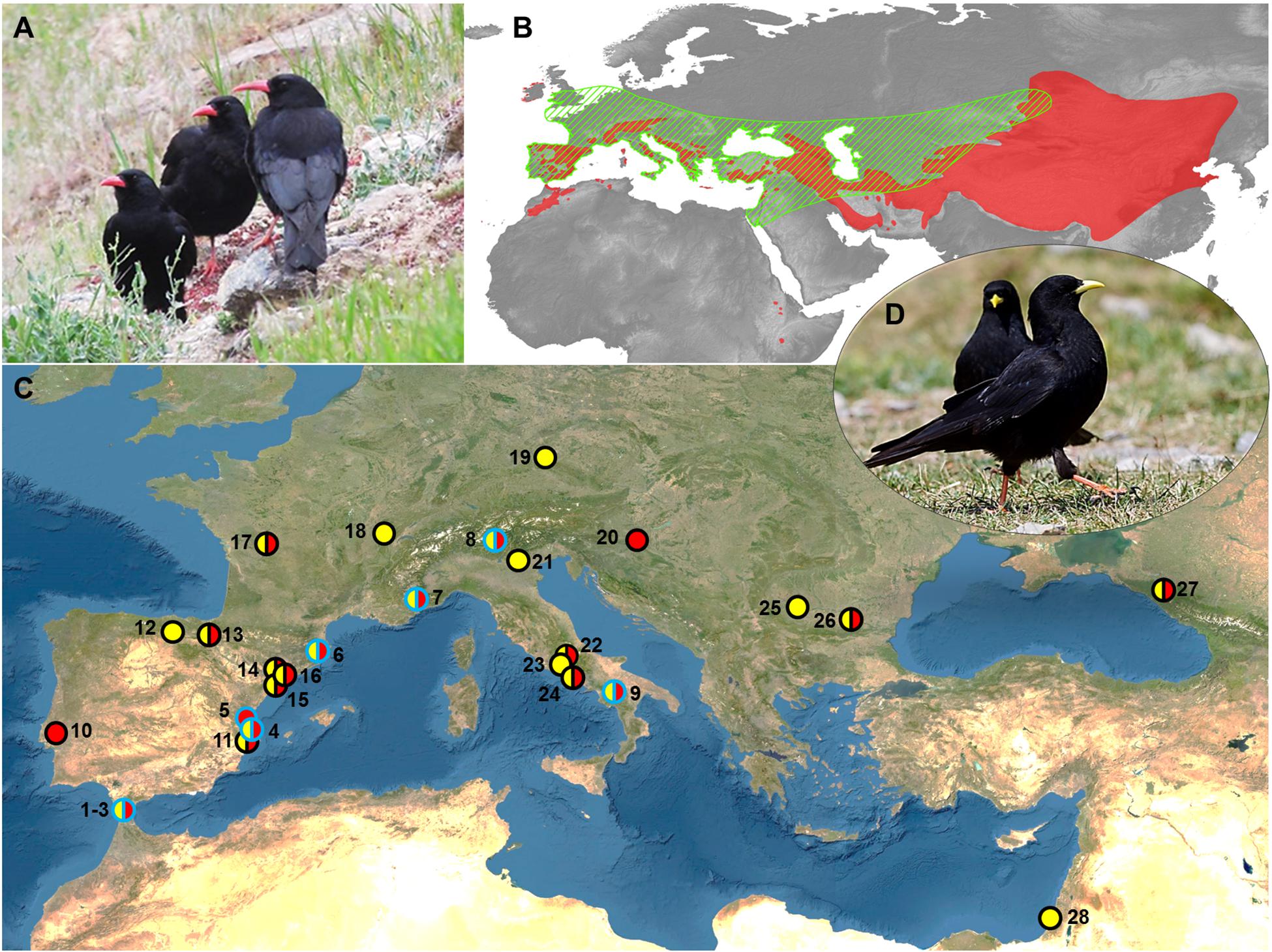
Figure 1. (A) Red-billed choughs. (B) Map showing the current pooled distribution range of red-billed and Alpine choughs (red area, according to BirdLife International and Handbook of the Birds of the World, 2019; freely available at http://datazone.birdlife.org/species/requestdis), and estimated range of Neanderthals (green barred area; modified from Ryulong, 2016; freely available at https://www.ancient.eu/image/5958/geographical-range-of-neanderthals/). (C) Sites with evidence of both Neanderthal occupation and presence of chough skeletal elements. The presences of red-billed choughs, Alpine choughs or both species in the sites are represented by red, yellow, or both-colors circular markers. Markers with blue borders represent sites with presence of processed chough fossils remains, while circles with black borders represent sites where processing marks were not searched for or not recorded. References of the sites:1–3: Gorham, Vanguard, Ibex caves; 4: Cova Negra; 5: Bolomor; 6: Arbreda; 7: Pié Lombard; 8: Fumane; 9: Grotte de Castelcivita; 10: Figueira Brava; 11: Beneito; 12: El Castillo; 13: Labeko koba; 14: Romaní; 15: Gegant; 16: Coll Verdaguer; 17: Combe Grenal; 18: La Baume de Gigny; 19: Sesselfelsgrotte; 20: Vindija; 21: Grotta del Broion; 22: Valle Radice; 23: Grotta Guattari; 24: Grotta di Sant Agostino; 25: Temnata; 26: Bacho Kiro; 27: Mezmaiskaya; 28: Kebara. Sources are shown in Table 1 and Supplementary Table 1. (D) Alpine choughs. Maps were generated with QGIS software and modified with Microsoft PowerPoint 2010 (Microsoft Corporation, Redmond, WA, United States, https://www.microsoft.com/pt-pt/microsoft-365/previous-versions/office-2010) under CSIC Organizational License. Photo credits: A (G. Blanco), D (J.J. Negro).
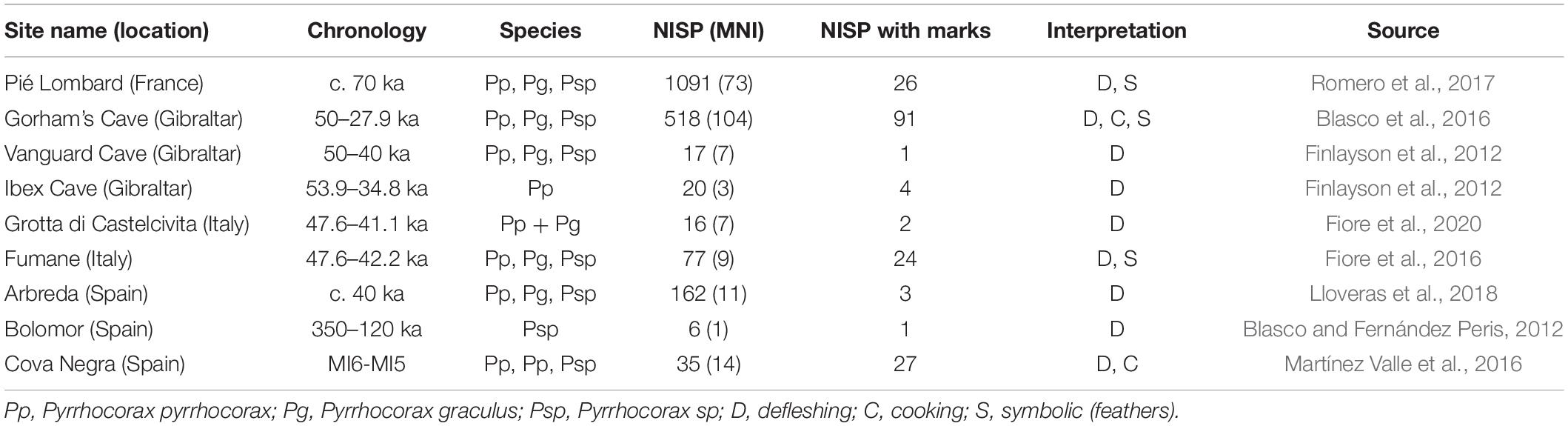
Table 1. Number of identified specimens (NISP), minimum number of individuals (MNI), and NISP with evidence of anthropogenic processing marks on chough fossil bones at Mousterian sites.
Chough fossil remains are among the most abundant elements in the paleontological avian record, as one or both chough species are present in almost all of the karstic infillings from the European Middle and Early Pleistocene (Tyrberg, 1998, 2008). Taking into account that choughs nest and rest in rocky shelters, finding their bones in fossil deposits has been treated in the literature as a consequence that did not need further explanation in the absence of anthropic cut marks. The spatiotemporal coexistence of Neanderthals and choughs across Eurasia would have favored a regular contact between these cave-dwelling species, and therefore an easy access of Neanderthals and other hominins to choughs. In fact, when searched for cut marks, an active processing of wing feather removal for meat consumption, ornamentation, or both, has been recorded in cave sites with Mousterian lithic tools (e.g., Peresani et al., 2011; Finlayson et al., 2012; Blasco et al., 2016). Therefore, the extensive occurrence and frequency of chough remains from the Mousterian onward, until the end of the Pleistocene, suggests an even stronger association than previously acknowledged. To assess the spatial and temporal extent of Neanderthals and choughs co-occurrence we performed a literature review of published information on sites where these species were present. In the case of the choughs, the evidence was fossil. Neanderthals presence was inferred through fossils or by lithic toolkits from the Middle Paleolithic. We paid particular attention to taphonomic studies determining whether chough remains were modified by human action (i.e., presenting evidence of cut or tooth marks, as well as burn signs) or accumulated on the site by other processes. This review indicated that the current distribution of choughs greatly overlaps with that estimated for Neanderthals (Figure 1B). The coincidental presence of Neanderthals and choughs at anthropological sites is common and widespread (Figure 1C and Table 1). As of today, there is evidence of consumption of both chough species in sites with Mousterian technology (Middle Paleolithic) between longitude 10° west and 40° east, and between 30° and 40° north latitude (Figure 1C). Processing marks on chough remains were confirmed in all sites (n = 9) where they were searched for Table 1, while in other sites (n = 19), evidence of processing was generally not searched for or not reported (Figure 1B and Supplementary Table 1). Cases are particularly abundant on or close to the coasts of the Iberian Peninsula. Although a majority of the levels where processed choughs were found belong to the late Middle Paleolithic, when the Neanderthals were about to become extinct, some evidence is much older, as in Bolomor Cave, Iberian Peninsula (Table 1). We cannot rule out that Neanderthals that may have used specific lithic industries such as the Micoquian in the easternmost end of their distribution (Kolobova et al., 2020), also procured choughs for themselves, as data on chough processing for those populations are currently unavailable. None-the-less, the known diet of those far-away Neanderthals was broad and therefore similar to that of other Neanderthals elsewhere (Salazar-García et al., 2021).
Choughs would have been particularly abundant in the past, but they should have been especially easy to capture or targeted preferentially by Neanderthals to explain this remarkable association. However, an easy capture conflicts with the distrustful character and great intelligence of corvids (Marzluff and Angell, 2007). Unlike scavenger species whose capture would be predictable at large animal carcasses exploited by Neanderthals (Finlayson and Finlayson, 2016), choughs perform long daily, seasonal, and altitudinal movements (Cramp and Perrins, 1994; Morinha et al., 2017b) to feed on invertebrates, seeds, and fruits, but never on carrion (Soler and Soler, 1993; Blanco et al., 1994; Sánchez-Alonso et al., 1996; Laiolo and Rolando, 1999), which makes the daylight location of foraging flocks very unpredictable (Blanco et al., 1998; Morinha et al., 2017a). Choughs are also known by their strong and acrobatic flight that makes them difficult targets for most diurnal predators (Cramp and Perrins, 1994; Cuevas and Blanco, 2009), including modern humans with relatively rudimentary hunting tools, as indicated by the chough elusive escape behavior and long flushing distance (Jiménez et al., 2013). However, choughs can be very vulnerable against human predators in their night shelters (Ibáñez, 1986; Zúñiga, 1989; Blanco et al., 2019).
The motivations, mechanisms, and circumstances underlying chough exploitation remain unresolved despite the potential implications on Neanderthal ecology and capabilities. Caves represent an essential resource tightly linked to human evolution. Suitable refuges would have been scarce and difficult to locate and defend against conspecific competitors and carnivore predators, especially for hominins venturing into unknown regions. Choughs use caves and a variety of other large indoor rocky shelters for night roosting throughout the year (Blanco et al., 1993; Cramp and Perrins, 1994), which implies that refuge and abundant food for Neanderthals would converge in these places. Therefore, cave-like refuge represents a keystone resource connecting Neanderthals and choughs captured at night in rocky shelters eventually used by both species. This hypothesis posits the nocturnal hunting activity of Neanderthals directed to choughs in their rocky shelters as the most parsimonious explanation sustaining this connection, instead of their capture during daylight in nesting and foraging sites. By adopting an actualistic approach, we document the patterns of refuge use and population dynamics of communally roosting choughs, the strategies and technology currently used to capture them, and their behavioral response against experimental human predators at night. Our main aim is to evaluate the eco-evolutionary implications of chough exploitation, by considering the generally overlooked potential nocturnal hunting activity of Neanderthals. Specifically, we aimed to deep on (1) Neanderthals’ cognitive capabilities to weave a night ambush toward elusive birds; (2) social abilities to arrange and achieve massive bird captures in darkness; (3) fire-related technology associated to enlighten themselves, and dazzle, harass, corral, and grab flying prey; (4) anatomical features related with an improved vision to locate and track roosting flocks, and cautiously approach and access the caves under low light and dark conditions, as well as climbing abilities previously overlooked; and (5) nutritional rewards associated to a chough meal.
Monitoring of population size and trends of choughs and other corvids often includes the simultaneous counts of individuals in shelters used for communally roosting over large geographic areas. With this aim, it is necessary to locate previously the active roosts each season, by monitoring foraging flocks and the flights to and from these sites at sunset and sunrise, respectively (Blanco et al., 2019). These activities were conducted during the last three decades in several regions of Spain, which allowed us to determine the size of communally roosting flocks and to locate and characterize roosting sites over a variety of habitats. Here, we focused on the more widely distributed and abundant red-billed chough, which also shows currently less restricted habitat requirements than the Alpine chough in Iberia, although both species can form mixed flocks and share roosting sites (Cramp and Perrins, 1994). The daily, seasonal, and inter-annual use of communal roosts was assessed by frequent monitoring of multiple sites across the years, which confirmed that communal roosts were generally used throughout the year with maximum numbers of individuals during winter (Blanco et al., 1993, 2019; Morinha et al., 2017b). Therefore, we considered winter counts (January) to determine roosting flock sizes in the monitored sites. The nature and characteristics of each roosting site were recorded and categorized as (i) large natural cavities in rocking terrain, and (ii) human-made cavities and structures that mimic the indoor conditions of natural ones. To evaluate roosting site tenacity, we considered the number of years that each particular site was used by choughs during the period 1988–2020. Given that the monitoring period was different for each particular site depending on the date of first occupation or localization, we considered those sites monitored for at least 5 years.
The localization of communal roosting sites allowed us to attempt capturing choughs to individually mark and study them in a sample of these sites (Blanco and Tella, 1999; Morinha et al., 2017b). The apparently best way to proceed to capture as many choughs as possible was previously planned according to the access, dimensions, structure, and escape exits in each site. We recorded the type and characteristics of each roosting site where the captures were conducted, the number of persons involved, the strategy and tools used, the behavior of choughs toward contemporary nocturnal human predators (as a proxy of prehistoric cave-dwelling humans), and the number of individuals captured in each event. Choughs were captured and handled by authorized personnel. Handling time was minimized to reduce stress and injury during capture/sampling. After manipulation choughs were released in good state where captured.
We acknowledged that a proportion of choughs was individually marked with plastic rings with unique alpha-numeric codes to assess the replacement rate of identifiable individuals in communal roosts. Thus, individual turnover in the roosts was used as a form to evaluate whether choughs could be viewed as a renewable resource for human predators. Thus, we monitored the flock size and the presence of ringed choughs identified with spotting scopes over consecutive days in communal roosts. In addition, we evaluated the impact on flock dynamics and site desertion of recurrent year-round captures in particular roosting sites across the years, and the turnover of individually marked choughs in these places by means of multiple captures. In a few cases, we were able to capture and ring all individuals present in a particular roosting site and in each nocturnal capture event, which allowed us to further evaluate the site-tenacity of flocks and the daily turnover of a proportion of individuals by monitoring the number and identity of individuals using the roosts the following days.
By tracking chough flocks, a total of 185 communal roosting sites were located. A proportion (33%) of these sites was large cavities generated by diverse geological processes in variable rocky and sedimentary substrates, including caves, crevices, and chasms, as well as smaller cavities concentrated in particular sectors of cliffs (see examples in Supplementary Figures 1A–C). These sites showed entrances of variable size (from about 1 to >20 m width and up to 30 m high or deep), shape (circular, elliptic, elongated, irregular), and accessibility for humans (from good accessibility on foot or relatively easy climbing to inaccessible without using climbing gear). The entrances (single or multiple) of these cavities invariably connect with dark and deep indoor shelters completely closed (caves) or open in its upper part, both partially (cracks in the ground or cliffs) and totally (chasms). The dimensions of a sample of accessible sites ranged from a combination of relatively narrow spaces with an elongated but variable conformation and height, to large spaces of several hundred square meters in area and up to >40 m in height and depth. The remainder communal roosts were located in anthropogenic structures, including caves, tunnels, quarries, and wells generated by mining activities, abandoned farmhouses, and wells in agricultural areas, and historical buildings, bridges, and other artifacts in urbanized areas (see examples in Figure 2A, Supplementary Figure 1D). The entrances and indoor spaces of these anthropogenic sites used as shelters by choughs are easily accessible for humans, except when located in large infrastructures such as bridges or the retaining walls of reservoir dams.

Figure 2. (A) Example of a communal roost of red-billed choughs in a well (Cuenca province, Spain); (B) Details of the position of choughs after cornered them by human (experimental) predators, and (C) their capture by hand and (D) with the help of a ladder in communal roosts located in buildings in Aragón (Spain). (E) A red-billed chough after marked with rings for individual identification. Photo credits: A (J. A. Cuevas), B (G. Blanco), C,D (J. M. García), E (Ó. Frías).
Flock size of communally roosting choughs in January ranged between 3 and 737 individuals (mean ± SD = 76 ± 91, median = 48, n = 542 counts from 93 sites from 1990 to 2020). Among winter roosting sites monitored for at least 5 years (range: 5–32), most (98.3%, n = 60) were used recurrently (mean ± SD = 8.6 ± 5.1 years, range = 1–25).
The number of choughs that used the same communal roosts in three sampled sites varied on average by 26.2% ± 15.0 between consecutive days (range = 7.7–43.4%). These variations were confirmed by the daily turnover of individuals, evaluated through the individual identification of ringed choughs. Overall, 33 (58%), 12 (21%), and 12 (21%) of the choughs identified by their rings in the three sites (n = 57) were observed in one, two, or three consecutive days in the same roosts. On the few occasions when we captured and banded all individuals in a particular site, we confirmed this partial daily turnover by determining the presence of a proportion of unmarked individuals entering the same site the following evening.
Chough captures for research purposes were mostly conducted in roosting sites whose entrances were accessible on foot. These sites were accessed through a silent night-time approach with the help of the minimum necessary artificial light 1 to 3 h after dark, or less frequently before sunrise. Chough behavior in the face of such a nocturnal threat generally varies depending on the capture sequence. At first, some choughs try and manage to escape from the human “predators,” especially the most alert individuals perching closest to the cavity exits. After this initial reaction, the most frequent chough attitude when lit with flashlights was to flee to the highest positions or into the deeper and often progressively narrower and darker parts of the cavity acting as a cul-de-sac, thus facilitating the capture of most individuals (Figure 2B). On the few occasions that captures were carried out in vertical cavities like wells (Figure 2A) and chasms (Supplementary Figure 1C), the behavior of choughs was invariably to take refuge toward the bottom. This allowed capturing most or all individuals by accessing them with climbing gear.
The number of persons involved was variable (generally a team of 4–5 persons, range: 2–10) depending on the size of the roosting flock. At relatively small and narrow roosting sites, the captures were conducted at the cavity exits by people equipped with large butterfly hand-nets and headlamps, so that the choughs could be dazzled and intercepted without damage when they tried to escape to the outside. On many (not quantified) occasions the dazzled choughs were captured by bare hand in flight (Figure 2C), on the ground, and on the indoor walls with the help of hand nets and ladders (Figure 2D). Large roost entrances were sometimes partially covered with nets to maximize the number of catches.
By using all these procedures, 5,525 full-grown choughs were captured in 296 events (mean ± SD = 19 ± 29 individuals per event, range = 1–258) at 70 different communal roosting sites, and released there after data collection. Overall, choughs were captured in large crevices in cliffs (n = 206 individuals), mining caves and quarries (n = 171), traditional agricultural wells (n = 179), chasms (n = 34), an old trench for an anti-aircraft gun (n = 101), historic buildings (n = 186), and a variety of abandoned and in-use farm houses (n = 1,969), livestock corrals (n = 2,520), and industrial warehouses (n = 159). Several capturing events were carried out at most of the sites (mean ± SD = 79 ± 114 individuals captured per particular site, n = 70, across the years; range = 3–522) without roosting flock desertion in any site. This was further supported by the large proportion of individuals recaptured in several occasions, as confirmed by the ring codes (Figure 2E). In addition, breeding choughs were captured by using the same procedures in their nesting sites, generally one or two members of the mated pair, while developing nestlings were captured during daylight in their nests (accessed by ladders and climbing gear) when feathered but before fledgling on predictable dates (population peak from mid to late May) each year.
Depending on geographic area and season, other bird species were opportunistically caught in much lower numbers during the night-time captures of choughs, including mostly kestrels (Falco tinnunculus and Falco naumanni), feral Rock dove (Columba livia), Barn owl (Tyto alba), Red start (Phoenicurus ochruros), and a variety of other birds.
Unraveling how and how often the remains of each bird species reached the fossil deposits is paramount to understand its abundance, distribution, behavior, paleoenvironmental conditions, and predator-prey relationships in the paleocenosis. With regard to archaeological sites, addressing these questions can also provide insight on human ecology, behavior, and evolution. During the Pleistocene, choughs were distributed throughout Europe, sharing caves and rocky shelters with different Homo species. Choughs and Neanderthals largely overlapped in their geographical distributions during this period, from western Europe to at least the Altai mountains in the East, and their ranges were likely further shared during the Pleistocene when choughs were more widely distributed in central and eastern Europe according to the fossil record, while in the Holocene the range of choughs became notably restricted, particularly for the Alpine chough (Tyrberg, 1998, 2008; Sánchez Marco, 2004). Our review confirms that chough fossil remains are among the most abundant in cave sites with Neandertal presence where avifaunal remains have been recorded and quantified. It may be of great interest to explore in the future the existence of this pattern of chough consumption in caves with ante- and post-Mousterian transition lithic industries (Micoquian and Szeletian) (Kolobova et al., 2020).
In this study, we aimed to attempt understanding how and why Neanderthals exploited choughs, not to review the paleontological record including these species, nor to posit hypothesis about the accumulation of chough remains due to other, generally unknown, predators. Of course, there are many sites where choughs fossil remains have been found not associated to cave occupation by Neanderthals (reviewed by Tyrberg, 1998, 2008; Finlayson et al., 2012), and others with Neanderthal lithic industries where chough consumption was suspected but anthropogenic processing marks on fossil bones were not been searched or recorded (Supplementary Table 1). Even though some chough skeletal remains may correspond to individuals dying naturally in the caves, or be the results of capture by other predators (e.g., Laroulandie, 2010), to our knowledge, there is no evidence of particular predator species accumulating chough remains in Neanderthal sites or current deposits. This may be due to the difficulty in capturing choughs during the day by daytime predators that could potentially accumulate remains in the caves. In fact, potential prey accumulators in caves could be restricted to medium-sized carnivore mammals such as canids and mustelids mostly acting at night. Diurnal raptors able to capture choughs (e.g., Aquila chrysaetos, Aquila fasciata), currently do it rarely (mostly upon juveniles) when compared to other species of birds easier to capture (Ontiveros, 2016; Arroyo, 2017). These eagles do not accumulate food remains in nests and rarely beneath perches, as demonstrated by the need of repeated snapshot samplings in these sites to collect enough of these generally scarce food remains for dietary studies (Ontiveros, 2016; Arroyo, 2017) before they disappear due to scavengers. These eagles do not access the interior of caves used by choughs as roosting sites, although their prey remains could be passively washed there or transported secondarily by scavengers (Laroulandie, 2010). In addition, nocturnal predators such as large owls and carnivore mammals cannot hunt in the complete darkness of deep caves at night, further hampered by the inaccessible positions that choughs use to sleep inside the roosting sites. Therefore, it seems unlikely that the accumulation of chough remains in sites where human-made cut marks has been found was mostly due to other predators such as owls and carnivore mammals. In fact, predators smaller than Neanderthals would probably leave more recognizable and frequent marks than the Neanderthals themselves (Laroulandie, 2010; Lloveras et al., 2020). Instead, there is extensive evidence that Neanderthal people processed choughs for food frequently over a widespread spatial and temporal range. This supports a targeted and extensive chough exploitation rather than incidental predation or scavenging, and hunting by just a few specialist Neanderthals. Evidence of chough exploitation commenced to accumulate in the last decade, even though cut marks are not necessarily associated to the consumption of this kind of relatively small prey. Therefore, we should expect analyses of fossils excavated in the past, or those from ongoing or future studies will provide a more detailed picture of the spatial and temporal extent of the Neanderthal-chough utilitarian association.
Overall, the motivations, mechanisms, and circumstances underlying the widespread and frequent Neanderthal exploitation of choughs and other birds (especially rock doves) have remained unresolved despite their broad eco-evolutionary implications. Same as contemporary humans currently do for research, Neanderthals could aurally detect and visually follow chough flocks to their shelters to establish temporary or permanent settlements, and to take advantage of an abundant and easy-to-use trophic resource consumed in situ. At present, dozens to hundreds of choughs can gather at night shelters year-round. At these sites, variable numbers of dazzled and cornered choughs (up to 250 individuals) can be captured by hand nets and bare hand, both in rocky sites as caves, crevices, and chasms, as well as in human-made cavities and buildings mimicking the conditions of caves. As an example, one of us (GB) and his collaborators in their twenties were able to catch about ten choughs each in repeated capture events conducted with bare hands and just the help of headlamps and bags by free climbing into roosting crevices and caves, although assuming the risk of dangerous falls. Choughs can be captured regularly and periodically because their occupation year-round during long-term periods of the same night shelters, the constant turnover of individuals, and their high site tenacity of roosting and nesting sites even after disturbance and recurrent predation (Zúñiga, 1989; Blanco et al., 1996; Banda and Blanco, 2009, 2014; Laiolo et al., 2009; Morinha et al., 2017b). The use of flashlights facilitates the capture providing artificial illumination and by dazzling choughs. Captures by hand are further enhanced by the behavior of choughs in the presence of experimental human predators when they enter the roosts, since they tend to flee confused into the cavity in darkness. Therefore, the relative ease of nocturnal capture of chough flocks by hominin predators without great technical means is demonstrated by comparison with modern humans used as a proxy. Unlike against other predators, choughs would be especially vulnerable to Neanderthals due to their unique use of artificial lighting by means of fire in the complete darkness of caves at night.
According to the hypothesis of a cave connection between choughs and Neanderthals, other cliff-nesting species are expected to occur also frequently in Neanderthal sites. However, the fossil remains of choughs far exceed those of other rock-dwelling species such as swallows, swifts, and raptors (Tyrberg, 1998, 2008; Sánchez Marco, 2004). In support of a targeted seeking for choughs, other similarly sized and cliff-nesting corvids roosting on trees, as well as tree-nesting species also roosting on trees (genus Corvus, Pica, Cyanopica), should be much less frequent in the Mousterian sites, which is actually true (Tyrberg, 1998, 2008; Sánchez Marco, 2004) despite their scavenging and mostly colonial habits would make them frequent prey (Finlayson and Finlayson, 2016). Choughs often form mixed foraging flocks with other corvid species (Cramp and Perrins, 1994; Cuevas and Blanco, 2009; Blanco et al., 2019) comparatively much less consumed by Neanderthals, which supports a differential capture of choughs in roosting sites (never shared with other corvids) rather than in open country. In turn, a parallel case of bird consumption by Neanderthals is offered by the rock dove. This pigeon is associated to rocky outcrops for nesting and roosting, and it has been suggested that it was regularly consumed by Neanderthals according to processing marks in fossil bones (Blasco et al., 2014). Choughs and rock doves, share not only the troglodyte nesting and communal roosting habit and sites but a similar body mass. A single individual of any of these two species would make an adequate meal for a hominin, and in fact several individuals of both species may be captured simultaneously at roost sites. In addition, pigeons are appreciated as food worldwide, while choughs are highly palatable according to personal appreciations of hunters consuming them in the past in Spain and nowadays in Morocco.
As it occurs with rock doves, choughs presenting an overlapping distribution range with the Neanderthals would have been a suitable food resource exploited regularly or as an occasional dietary backup. Perhaps an adult Neanderthal would need at least 2–3 choughs for satiation, but choughs together with rock doves could be harvested en masse at night in the roosting sites. Neanderthal group size has been estimated to be about 10–20 adults and their children (De Groote, 2018). Capturing 40–60 choughs in a single hunting bout by just a few skilled hunters in a roost site could be entirely possible according to our actualistic experiments, which suggest a valuable resource on which the whole group could feed regularly. In addition, choughs are relatively territorial at breeding, especially the red-billed, with just one couple per cave or a few in very large cavities (Blanco et al., 1997, 1998). Therefore, as currently, choughs could be only harvested regularly and in good numbers at night when they gather in large flocks in roosting caves. Breeding pairs could also be regularly captured at night in their nesting sites used for roosting year-round, while nestlings could be seasonally harvested each spring by predating accessible nests at daylight. Current nocturnal bycatches in the chough roosts of other bird species suggest a similar process to explain their relatively high frequency at Mousterian sites (Tyrberg, 1998, 2008; Sánchez Marco, 2004; Finlayson et al., 2012). Some of these species, including the colonial lesser kestrel (Negro et al., 2020), could be encountered at sites shared with choughs as nowadays (Blanco and Tella, 1997) and perhaps also hunted there by Neanderthals. In fact, kestrels of both species proved especially clumsy and easy to capture by us at night in these sites. The consumption of birds, even if occasional, may have been not necessarily driven only because of their net caloric content, but for the acquisition of micronutrients (Watts, 2020). In particular, the red-billed chough has been reported showing the highest content of circulating carotenoids recorded so far in birds (Tella et al., 2004), and these molecules are essential micronutrients playing roles in different physiological processes including vision and immunological function (Álvarez et al., 2014; Milani et al., 2017).
Translating the above knowledge to a possible regular capture of choughs by Neanderthals has multiple implications on their ecology, behavior, and capabilities. Given the extreme difficulty of daylight capturing choughs in open country without highly sophisticated tools, nocturnal hunting with the help of fire in the roosting caves is the only plausible explanation for the strong association of processed choughs and Neanderthals in fossil assemblages. The hunt resulting in chough death for consumption is doubtless easier than their current capturing alive without damage for research purposes. This could be achieved by throwing stones or by the documented use of long wooden sticks and stone-tipped spears (Aranguren et al., 2018; Hoffecker, 2018). Dazzling and bewilderment could be promoted by the light of torches or even through controlled fires with parapets of branches at the entrance to the cavities, both also producing heat, noise and smoke increasing confusion in the choughs. In fact, Neanderthals mastered the use of fire all along their existence as a species from about 400–300,000 years ago (Roebroeks and Villa, 2011; Sanz et al., 2020; MacDonald et al., 2021), and the use of constructions inside of long caves in total absence of natural light also testifies of the use of fire for illumination (Jaubert et al., 2016). This would support Neanderthal use of fire for hunting and, to our knowledge, this is the first documented suggestion of social hunting of birds at night with the help of fire technology. This necessarily implies prior organization for silent night-time approach to the caves, and episodic-like memory linked to social coordination for previously planned nocturnal hunting, which suggests cognitive capabilities for anticipation and labor division involving the use of hunting kits. Handling a torch, a spear, or launching projectiles at dark by single individuals requires great working attention (Wynn and Coolidge, 2012). In this particular case, the use of different tools seems more likely to be successful if managed by individuals coordinating tasks than by hunting pack members using the available toolkit without prior planning. In addition, the apparently repeated chough hunting and consumption events in the same caves, as assessed by stratigraphy, has been particularly well documented in Gorham’s Cave along a minimum 20,000–30,000 years (Blasco et al., 2016). This would indicate the regular or seasonal exploitation of a renewable resource, whose recurrent use is incompatible with the shared long-term occupation of the roosting caves of choughs by Neanderthals, but with an intermittent use by each species. This agrees with the regular, seasonal occupation by short periods documented in many caves associated to Neanderthal mobility (Carrion-Marco et al., 2019; Leierer et al., 2019). A temporal exclusion of choughs from their roosting sites, together with the likely long-term memory of their hominin predator, could potentially lead to a hunting tradition and sustainable exploitation strategy compatible with some degree of autonoesis (sensu Tulving, 2002), a salient feature of human condition rarely attributed to Neanderthals (Wynn and Coolidge, 2012).
Neanderthals were well-endowed technologically and also anatomically for night captures in a cave environment. First, compared with anatomical modern humans (AMH), they had features such as an improved vision in dim light (Taylor and Reimchen, 2016) that may have helped them to locate and track birds, and to hunt under low light and darkness conditions, including associated cranial morphology and eye size (Pearce et al., 2013). Second, Neanderthals had shorter lower limbs compared to AMH. A study on the locomotor abilities of hominins (Higgins and Ruff, 2011), suggests that Neanderthals were possibly better climbers in rough terrain and inside caves than AMH.
The procurement of choughs by cave people does not end with the extinction of the Neanderthals. In the Upper Paleolithic, AMH also processed choughs. For instance, Laroulandie (2010) reported the finding of 1,337 chough remains at the Upper Magdalenian site of La Vache, in France, 245 of which presented cut marks. This and similar findings in other places led Finlayson and Finlayson (2016) to suggest that the practice of capturing choughs, and particularly when the aim was to collect their feathers, may have been transmitted from Neanderthals to modern humans. The association of shelter and food in the form of choughs could have led to abstract thinking associated with this species that would reinforce the use of its feathers, and also talons (especially the posterior digit D-I claw, showing a size and shape close to a medium-sized raptor) and other parts (e.g., red tarsus and red or yellow beaks) as adornments. As an alternative hypothesis, cut marks on tibiotarsus (which lack flesh) apparently for peeling and fracturing (Fiore et al., 2016; Martínez Valle et al., 2016) could be related to the consumption of carotenoids deposited under the tarsal scales. Therefore, although Neanderthals could have used chough feathers for symbolic purposes, their capture do not necessarily imply a focused searching for feathers, but for flesh and micronutrients. This is supported by the fact that the long molting period in this species (May-November) implies that molted flight feathers in good state accumulate in large quantities in the floor of roosting sites promoted by their indoor conditions and the often large numbers of choughs using them repeatedly. In turn, this implies that molted feathers could be easily collected during daylight in the caves, while the presence and number of these and other remains such as feces represent unequivocal signs of the presence and approximate quantity of roosting individuals for future meals. Whatever the actual relevance and motivations of chough exploitation, our study sustains the ongoing debate on social, anatomical, technological and cognitive abilities associated to Neanderthal bird –nocturnal– hunting, thus contributing to open new research avenues in human ecology and evolution.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the animal study because bird captures for ringing do not requires ethical approval in Spain.
GB and JN conceived and designed the study. GB conducted the fieldwork and wrote the first draft of the manuscript. AS-M, JN, and GB reviewed and organized the data. All authors participated in improving the manuscript and gave final approval for publication.
This study was partially funded by projects from the Junta de Comunidades de Castilla-La Mancha (PPIC10-0094-3036), the Spanish Ministry of the Environment (082/2002), and the Ministry of Science and Education (BOS2003-05066 and CGL2015-66381-P). AS-M was supported by the projects CGL2016-76431-P and CGL2017-82654-P (AEI/FEDER, EU).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Conversations with Clive Finlayson and Carolina Lakunza, and comments by Ruth Blasco, helped us to focus the study and improve the manuscript. We thank all persons supporting the fieldwork and involved in the chough captures across the years. We thank Jesús A. Cuevas, José M. García, and Óscar Frías for providing pictures illustrating the study, and Pablo Salinas for help in the elaboration of maps.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.733062/full#supplementary-material
Álvarez, R., Vaz, B., Gronemeyer, H., and de Lera, A. R. (2014). Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem. Rev. 114, 1–125. doi: 10.1021/cr400126u
Aranguren, B., Revedin, A., Amico, N., Cavulli, F., Giachi, G., Grimaldi, S., et al. (2018). Wooden tools and fire technology in the early Neanderthal site of Poggetti Vecchi (Italy). PNAS 115, 2054–2059. doi: 10.1073/pnas.1716068115
Arroyo, B. (2017). “Águila real – Aquila chrysaetos,” in Enciclopedia Virtual de los Vertebrados Españoles, eds A. Salvador and M. B. Morales (Madrid: Museo Nacional de Ciencias Naturales).
Banda, E., and Blanco, G. (2009). Implications of nest-site limitation on density-dependent nest predation at variable spatial scales in a cavity-nesting bird. Oikos 118, 991–1000. doi: 10.1111/j.1600-0706.2009.17363.x
Banda, E., and Blanco, G. (2014). Strict mate fidelity and reduced breeding dispersal of widowed Red-billed Choughs. Bird Study 61, 371–377. doi: 10.1080/00063657.2014.927819
BirdLife International and Handbook of the Birds of the World. (2019). Bird species distribution maps of the world. Cambridge, MA: BirdLife International.
Blanco, G., Cuevas, J. A., and Fargallo, J. A. (1998). Breeding density and distribution of Choughs Pyrrhocorax pyrrhocorax nesting in river cliffs: the roles of nest-site availability and nest-site selection. Ardea 86, 237–244.
Blanco, G., Cuevas, J. A., Frías, Ó, and González del Barrio, J. L. (2019). A shot in the dark: Sport hunting of declining corvids promotes the inadvertent shooting of threatened red-billed choughs. J. Nat. Conserv. 52:125739. doi: 10.1016/j.jnc.2019.125739
Blanco, G., Fargallo, J. A., and Cuevas, J. A. (1993). Seasonal variations in numbers and levels of activity in a communal roost of Choughs Pyrrhocorax pyrrhocorax in Central Spain. Avocetta 17, 41–44.
Blanco, G., Fargallo, J. A., and Cuevas, J. A. (1994). Consumption rates of olives by Choughs in Central Spain: variations and importance J. Field. Orn. 65, 482–489.
Blanco, G., Fargallo, J. A., Tella, J. L., and Cuevas, J. A. (1997). Role of buildings as nest-sites in the range expansion and conservation of choughs Pyrrhocorax pyrrhocorax in Spain. Biol. Cons. 79, 117–122. doi: 10.1016/s0006-3207(96)00118-8
Blanco, G., Pais, J. L., Fargallo, J. A., Potti, J., Lemus, J. A., and Dávila, J. A. (2009). High proportion of non-breeding individuals in an isolated red-billed chough population on an oceanic island (La Palma, Canary Islands). Ardeola 56, 229–239.
Blanco, G., and Tella, J. L. (1997). Protective association and breeding advantages of choughs nesting in lesser kestrel colonies. Anim. Behav. 54, 335–342. doi: 10.1006/anbe.1996.0465
Blanco, G., and Tella, J. L. (1999). Temporal, spatial and social segregation of red-billed choughs between two types of communal roost: a role for mating and territory acquisition. Anim. Behav. 57, 1219–1227. doi: 10.1006/anbe.1999.1103
Blanco, G., Tella, J. L., and Torre, I. (1996). Age and sex determination of monomorphic non-breeding choughs: A long-term study. J. Field. Orn. 67, 428–433.
Blanco, G., Tella, J. L., and Torre, I. (1998). Traditional farming and key foraging habitats for chough Pyrrhocorax pyrrhocorax conservation in a Spanish pseudosteppe. J. Appl. Ecol. 35, 232–239. doi: 10.1046/j.1365-2664.1998.00296.x
Blasco, R., and Fernández Peris, J. (2012). A uniquely broad spectrum diet during the Middle Pleistocene at Bolomor Cave (Valencia, Spain). Quat. Int. 252, 16–31. doi: 10.1016/j.quaint.2011.03.019
Blasco, R., Finlayson, C., Rosell, J., Marco, A. S., Finlayson, S., Finlayson, G., et al. (2014). The earliest pigeon fanciers. Sci. Rep. 4:5971.
Blasco, R., Rosell, J., Rufà, A., and Finlayson, C. (2016). Pigeons and choughs, a usual resource for the Neanderthals in Gibraltar. Quat. Int. 421, 62–77. doi: 10.1016/j.quaint.2015.10.040
Blasco, R., Rosell, J., Sánchez-Marco, A., Gopher, A., and Barkai, R. (2019). Feathers and food: Human-bird interactions at Middle Pleistocene Qesem Cave, Israel. J. Hum. Evol. 136:102653. doi: 10.1016/j.jhevol.2019.102653
Bocherens, H., Drucker, D. G., Billiou, D., and Vandermeersch, B. (2005). Isotopic evidence for diet and subsistence pattern of the Saint-Cesaire I Neanderthal: review and use of a multi-source mixing model. J. Hum. Evol. 49, 71–87.
Botha, R. (2020). Neanderthal Language: Demystifying the Linguistic Powers of our Extinct Cousins. Cambridge, MA: Cambridge University Press.
Breyl, M. (2020). Triangulating Neanderthal cognition: A tale of not seeing the forest for the trees. Hoboken, NJ: Wiley Interdisciplinary Reviews, e1545.
Carrion-Marco, Y. C., Calatayud, P. G., Eixea, A., Martínez-Varea, C. M., Tormo, C., Badal, E., et al. (2019). Climate, environment and human behaviour in the Middle Palaeolithic of Abrigo de la Quebrada (Valencia, Spain): the evidence from charred plant and micromammal remains. Quat. Sci. Rev. 217, 152–168. doi: 10.1016/j.quascirev.2018.11.032
Cramp, S., and Perrins, C. M. (1994). Handbook of the Birds of Europe, the Middle East and North Africa. The birds of the western Palearctic. Vol. VIII: crows to finches. Oxford: Oxford Univ. Press.
Cuevas, J. A., and Blanco, G. (2009). “Chova piquirroja – Pyrrhocorax pyrrhocorax,” in Enciclopedia Virtual de los Vertebrados Españoles, eds A. Salvador and L. M. Bautista (Madrid: Museo Nacional de Ciencias Naturales).
De Groote, I. (2018). Neanderthals, Biosocial Models of. Internat. Encyclop. Anthrop. 2018, 1–8. doi: 10.1002/9781118924396.wbiea2177
Finlayson, C., Finlayson, C., Brown, K., Blasco, R., Rosell, J., Negro, J. J., et al. (2012). Birds of a feather: Neanderthal exploitation of raptors and corvids. PLoS One 7:e45927. doi: 10.1371/journal.pone.0045927
Finlayson, S., and Finlayson, C. (2016). The birdmen of the Pleistocene: On the relationship between Neanderthals and scavenging birds. Quat. Int. 421, 78–84. doi: 10.1016/j.quaint.2015.12.057
Fiore, I., Gala, M., Boschin, F., Crezzini, J., Tagliacozzo, A., and Moroni, A. (2020). Archeozoology and taphonomy of bird remains from Grotta di Castelcivita (Salerno, Italy) and clues for human-bird interactions. Quat. Int. 551, 224–242. doi: 10.1016/j.quaint.2019.09.004
Fiore, I., Gala, M., Romandini, M., Cocca, E., and Peresani, M. (2016). From feathers to food: Reconstructing the complete exploitation of avifaunal resources by Neanderthals at Fumane cave, unit A9. Quat. Int. 421, 134–153. doi: 10.1016/j.quaint.2015.11.142
Fiore, I., Gala, M., and Tagliacozzo, A. (2004). Ecology and subsistence strategies in the eastern Italian Alps during the Middle Palaeolithic. Int. J. Osteoarchaeol. 14, 273–286. doi: 10.1002/oa.761
Hardy, B. L., and Moncel, M. H. (2011). Neanderthal use of fish, mammals, birds, starchy plants and wood 125-250,000 years ago. PLoS One 6:e23768. doi: 10.1371/journal.pone.0023768
Higgins, R. W., and Ruff, C. B. (2011). The effects of distal limb segment shortening on locomotor efficiency in sloped terrain: implications for Neandertal locomotor behavior. Am. J. Phys. Anthropol. 146, 336–345. doi: 10.1002/ajpa.21575
Hoffecker, J. F. (2018). The complexity of Neanderthal technology. PNAS 115, 1959–1961. doi: 10.1073/pnas.1800461115
Jaubert, J., Verheyden, S., Genty, D., Soulier, M., Cheng, H., and Blamart, D. (2016). Early Neanderthal constructions deep in Bruniquel Cave in southwestern France. Nature 534, 111–114. doi: 10.1038/nature18291
Jiménez, G., Meléndez, L., Blanco, G., and Laiolo, P. (2013). Dampened behavioral responses mediate birds’ association with humans. Biol. Cons. 159, 477–483. doi: 10.1016/j.biocon.2012.10.030
Kolobova, K. A., Roberts, R. G., Chabai, V. P., Jacobs, Z., Krajcarz, M. T., Shalagina, A. V., et al. (2020). Archaeological evidence for two separate dispersals of Neanderthals into southern Siberia. PNAS 117, 2879–2885. doi: 10.1073/pnas.1918047117
Laiolo, P., Banda, E., Lemus, J. A., Aguirre, J. I., and Blanco, G. (2009). Behaviour and stress response during capture and handling of the red-billed chough Pyrrhocorax pyrrhocorax (Aves: Corvidae). Biol. J. Linnean Soc. 96, 846–855. doi: 10.1111/j.1095-8312.2008.01174.x
Laiolo, P., and Rolando, A. (1999). The diet of the chough (Pyrrhocorax pyrrhocorax) and the alpine chough (Pyrrhocorax graculus) in the Alps: seasonality, resource partitioning and population density. Revue d’écologie 54, 133–147.
Laroulandie, V. (2010). “Alpine chough Pyrrhocorax graculus from Pleistocene sites between Pyrenees and Alps: natural versus cultural assemblages,” in Proceedings of the 6th Meeting of the ICAZ Bird Working Group in Groningen: Birds in Archaeology. (23.8–7.8. 2008), Vol. 12, Barkhuis, 219.
Leierer, L., Jambrina-Enríquez, M., Herrera-Herrera, A. V., Connolly, R., Hernández, C. M., Galván, B., et al. (2019). Insights into the timing, intensity and natural setting of Neanderthal occupation from the geoarchaeological study of combustion structures: A micromorphological and biomarker investigation of El Salt, unit Xb, Alcoy, Spain. PLoS One 14:e0214955. doi: 10.1371/journal.pone.0214955
Lloveras, L., Garcia, L., Maroto, J., Soler, J., and Soler, N. (2018). The bird assemblage from the Middle Palaeolithic level I of Arbreda Cave: a taphonomic story. J. Archaeol. Sc. Reports 21, 758–770.
Lloveras, L., Garcia, L., Marqueta, M., Maroto, J., Soler, J., and Soler, N. (2020). The role of birds in Upper Palaeolithic sites: Zooarchaeological and taphonomic analysis of the avian remains from Arbreda Cave (Serinyà, northeast Iberia). Q. Internat. 2020:002. doi: 10.1016/j.quaint.2020.10.022
Lombard, M., and Högberg, A. (2021). Four-field co-evolutionary model for human cognition: variation in the Middle Stone Age/Middle Palaeolithic. J. Archaeolog. Method Theor. 28, 142–177. doi: 10.1007/s10816-020-09502-6
MacDonald, K., Scherjon, F., van Veen, E., Vaesen, K., and Roebroeks, W. (2021). Middle Pleistocene fire use: The first signal of widespread cultural diffusion in human evolution. PNAS 118:e2101108118. doi: 10.1073/pnas.2101108118
Majkić, A., Evans, S., Stepanchuk, V., Tsvelykh, A., and d’Errico, F. (2017). A decorated raven bone from the Zaskalnaya VI (Kolosovskaya) Neanderthal site, Crimea. PLoS One 12:e0173435. doi: 10.1371/journal.pone.0173435
Marean, C. W., and Yeun Kim, S. (1998). Mousterian Large-mammal remains from Kobeh Cave. Curr. Anthr. 39, S79–S114.
Martínez Valle, R., Calatayud, P. M. G., and Bonilla, V. V. (2016). Bird consumption in the final stage of Cova Negra (Xátiva, Valencia). Q. Internat. 421, 85–102. doi: 10.1016/j.quaint.2016.01.068
Marzluff, J. M., and Angell, T. (2007). In the company of crows and ravens. New Haven: Yale University Press, 2007.
Milani, A., Basirnejad, M., Shahbazi, S., and Bolhassani, A. (2017). Carotenoids: biochemistry, pharmacology and treatment. Br. J. Pharm. 174, 1290–1324. doi: 10.1111/bph.13625
Morinha, F., Bastos, R., Carvalho, D., Travassos, P., Santos, M., Blanco, G., et al. (2017a). A spatially-explicit dynamic modelling framework to assess habitat suitability for endangered species: The case of Red-billed Chough under land use change scenarios in Portugal. Biol. Cons. 210, 96–106. doi: 10.1016/j.biocon.2017.04.013
Morinha, F., Dávila, J. A., Bastos, E., Cabral, J. A., Frías, Ó, González, J. L., et al. (2017b). Extreme genetic structure in a social bird species despite high dispersal capacity. Mole. Ecol. 26, 2812–2825. doi: 10.1111/mec.14069
Nabais, M., and Zilhao, J. (2019). The consumption of tortoise among last interglacial Iberian Neanderthals. Quat. Sci. Rev. 217:225e246.
Negro, J. J., Blasco, R., Rosell, J., and Finlayson, C. (2016). Potential exploitation of avian resources by fossil hominins: An overview from ethnographic and historical data. Quat. Int. 421, 6–11. doi: 10.1016/j.quaint.2015.09.034
Negro, J. J., Prenda, J., Ferrero, J. J., Rodríguez, A., and Reig-Ferrer, A. (2020). A timeline for the urbanization of wild birds: The case of the lesser kestrel. Quat. Sci. Rev. 249:106638. doi: 10.1016/j.quascirev.2020.106638
Ontiveros, D. (2016). “Águila perdicera – Hieraaetus fasciatus,” in Enciclopedia Virtual de los Vertebrados Españoles, eds A. Salvador and M. B. Morales (Madrid: Museo Nacional de Ciencias Naturales).
Pearce, E., Stringer, C., and Dunbar, R. I. (2013). New insights into differences in brain organization between Neanderthals and anatomically modern humans. Proc. R. Soc. B: Biol. Sci. 280:20130168. doi: 10.1098/rspb.2013.0168
Peresani, M., Fiore, I., Gala, M., and Tagliacozzo, A. (2011). Late Neandertals and the intentional removal of feathers as evidenced from bird bone taphonomy at Fumane Cave 44 ky B.P. Italy. PNAS 108, 3888–3893. doi: 10.1073/pnas.1016212108
Radovčić, D., Sršen, A. O., Radovčić, J., and Frayer, D. W. (2015). Evidence for Neandertal jewelry: modified white-tailed eagle claws at Krapina. PLoS One 10:e0119802. doi: 10.1371/journal.pone.0119802
Radovčić, D., Sršen, A. O., Vaccari, L., Radovčić, J., and Frayer, D. W. (2020). Surface analysis of an eagle talon from Krapina. Sci. Rep. 10:6329.
Richards, M. P., Pettitt, P. B., Trinkaus, E., Smith, F. H., Paunović, M., and Karavanić, I. (2000). Neanderthal diet at Vindija and Neanderthal predation: the evidence from stable isotopes. PNAS 97, 7663–7666. doi: 10.1073/pnas.120178997
Roebroeks, W., and Villa, P. (2011). On the earliest evidence for habitual use of fire in Europe. PNAS 108, 5209–5214. doi: 10.1073/pnas.1018116108
Romero, A., Díez, J., and Brugal, J. (2017). Aves de caza. Estudio tafonómico y zooarqueológico de los restos avianos de los niveles musterienses de Pié Lombard (Alpes-Maritimes, Francia). Munibe 68, 73–84.
Ryulong. (2016). Geographical Range of Neanderthals. In Ancient History Encyclopedia. https://www.ancient.eu/image/5958/ (accessed date October 20, 2016).
Sala, N., and Arsuaga, J. L. (2018). Regarding beasts and humans: a review of taphonomic works with living carnivores. Quat. Int. 466, 131–140. doi: 10.1016/j.quaint.2016.03.011
Salazar-García, D. C., Power, R. C., Rudaya, N., Kolobova, K., Markin, S., Krivoshapkin, A., et al. (2021). Dietary evidence from Central Asian Neanderthals: A combined isotope and plant microremains approach at Chagyrskaya Cave (Altai, Russia). J. Hum. Evol. 156:102985. doi: 10.1016/j.jhevol.2021.102985
Sánchez Marco, A. (2004). Avian zoogeographical patterns during the Quaternary in the Mediterranean region and paleoclimatic interpretation. Ardeola 51, 91–132.
Sánchez Marco, A., and Cacho Quesada, C. (2010). Avian wings as ornaments in the Magdalenian? Archaeofauna 19, 133–139.
Sánchez-Alonso, C., Ruiz, X., Blanco, G., and Torre, I. (1996). An analysis of the diet of Red-billed Chough Pyrrhocorax pyrrhocorax nestlings in NE Spain, using neck ligatures. Ornis Fennica 73, 179–185.
Sanz, M., Daura, J., Cabanes, D., Égüez, N., Carrancho, Á, Badal, E., et al. (2020). Early evidence of fire in south-western Europe: the Acheulean site of Gruta da Aroeira (Torres Novas, Portugal). Sci. Rep. 10:12053.
Soler, J. J., and Soler, M. (1993). Diet of the red-billed chough Pyrrhocorax pyrrhocorax in the Southeast of the Iberian Peninsula. Bird Study 40, 216–222.
Taylor, J. S., and Reimchen, T. E. (2016). Opsin gene repertoires in northern archaic hominids. Genome 59, 541–549. doi: 10.1139/gen-2015-0164
Tella, J. L., Figuerola, J., Negro, J. J., Blanco, G., Rodríguez-Estrella, R., Forero, M. G., et al. (2004). Ecological, morphological and phylogenetic correlates of interspecific variation in plasma carotenoid concentration in birds. J. Evol. Biol. 17, 156–164. doi: 10.1046/j.1420-9101.2003.00634.x
Tulving, E. (2002). Episodic memory: From mind to brain. Ann. Rev. Psychol. 53, 1–25. doi: 10.1146/annurev.psych.53.100901.135114
Tyrberg, T. (1998). Pleistocene birds of the Palearctic: a catalogue. Cambridge: Nuttall Ornithological Club.
Tyrberg, T. (2008). Pleistocene birds of the Palearctic. Available: http://web.telia.com/~u11502098/pleistocene.html (accessed date February 24, 2008)
Villa, P., and Roebroeks, W. (2014). Neandertal demise: an archaeological analysis of the modern human superiority complex. PLoS One 9:e96424. doi: 10.1371/journal.pone.0096424
Villa, P., Soto, E., Santonja, M., Pérez-González, A., Mora, R., Parcerisas, J., et al. (2005). New data from Ambrona: closing the hunting versus scavenging debate. Quat. Int. 126, 223–250. doi: 10.1016/j.quaint.2004.03.001
Wadley, L. (2021). What stimulated rapid, cumulative innovation after 100,000 years ago? J. Arch. Method Theory 28, 120–141. doi: 10.1007/s10816-020-09499-y
Watts, D. P. (2020). Meat eating by nonhuman primates: A review and synthesis. J. Hum. Evol. 149:102882. doi: 10.1016/j.jhevol.2020.102882
Wynn, T., and Coolidge, F. L. (2012). How to think like a Neandertal. Oxford: Oxford University Press.
Zilhão, J., Angelucci, D. E., Igreja, M. A., Arnold, L. J., Badal, E., Callapez, P., et al. (2020). Last Interglacial Iberian Neandertals as fisher-hunter-gatherers. Science 367:6485.
Keywords: caves, choughs, fire-related technology, hunting tactics, micronutrients, Mousterian sites, Pyrrhocorax, troglodyte habits
Citation: Blanco G, Sánchez-Marco A and Negro JJ (2021) Night Capture of Roosting Cave Birds by Neanderthals: An Actualistic Approach. Front. Ecol. Evol. 9:733062. doi: 10.3389/fevo.2021.733062
Received: 29 June 2021; Accepted: 13 August 2021;
Published: 09 September 2021.
Edited by:
Pasquale Raia, University of Naples Federico II, ItalyReviewed by:
Maciej Tomasz Krajcarz, Institute of Geological Sciences (PAN), PolandCopyright © 2021 Blanco, Sánchez-Marco and Negro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guillermo Blanco, Zy5ibGFuY29AY3NpYy5lcw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.