- 1Department of Life Sciences, University of Modena and Reggio Emilia, Modena, Italy
- 2Institute of Anthropic Impact and Sustainability in Marine Environment (IAS), National Research Council (CNR), Rome, Italy
- 3Department of Agronomy, Food, Natural Resources, Animals and Environment, University of Padua, Padua, Italy
- 4Department STEBICEF, Università degli Studi di Palermo, Palermo, Italy
Because of its parasitic habits, reproduction costs of the common cuckoo (Cuculus canorus) are mostly spent in pre-laying activities. Female costs are limited to searching host nests and laying eggs, whereas, males spend time in performing intense vocal displays, possibly with territorial purpose. This last aspect, together with a sexual plumage dimorphism, points to both intra- and inter-sexual selections operating within this species. One element triggering sexual selection is a differential fitness accrued by different phenotypes. Before analyzing possible sexual selection mechanisms operating in cuckoos, it is therefore necessary to verify whether there is a variability among male secondary characters by describing and quantifying them. Here we aimed to quantitatively characterize the main two potential candidates of sexual selection traits, i.e., calls and displays, shown by males at perches. During the 2019 breeding season, in a site within the Po Plain, we both audio and video recorded cuckoo males at five different perches. We analyzed acoustic variables as well as display sequences searching for potential correlations. We found a significant variation among calls that could be clustered into four vocal types. We also found that no visual displays were associated with vocal displays; cuckoo males were either vocal and motionless or soundless and active. We discuss our results under the perspective of the potential value of sexual selection in brood parasites and its role in its parasitic habit.
Introduction
The common cuckoo (Cuculus canorus) is an obligatory brood parasite, as it lays its eggs in nests of other species who raise its young until fledging at the expenses of their own offspring (Chance, 1940; Wyllie, 1981). While this reproductive strategy results in saving an important amount of energy devoted to parental care, both female and male cuckoos are greatly engaged in pre-laying reproductive activities (Nakamura and Miyazawa, 1997; Davies, 2015). Since the beginning of the reproductive season, females constantly search for the “right” nest to parasitize (Reboreda et al., 2017), whereas, males are often observed on obvious perches while performing long and repetitive displays.
In the context of sexual selection, acoustic signals as much as courtship rituals, can provide information regarding male quality over which potential receivers, i.e., females and males, may assess mating opportunities and confrontation prospects (Searcy and Andersson, 1986). Over evolutionary time, signals have been selected to convey honest communication serving a dual function, mate attraction and territory defense (Searcy and Andersson, 1986; Benedetti et al., 2018).
Little attention, however, has been given to the potential multimodal nature of these signals in the common cuckoo. Multimodal signals are defined as signals whose components are received by different sensory systems. They are widely diffused in the animal world and have been mainly investigated in the context of sexual selection, such as during courtship displays (Mitoyen et al., 2019). The exact function and selective role of multimodal signals is, however, not agreed upon, with several non-exclusive explanations proposed. Multimodal signals for example might have evolved to convey redundant information, with multiple components having the same meaning and retaining it also if emitted separately (Hebets and Papaj, 2005; Mitoyen et al., 2019). This is known as the back-up signal hypothesis and it is linked to environmental variability: the more channels are used in communications, the least probable it is that the signal might be missed (Johnstone et al., 1996).
On the other hand, the presence of multiple components might allow the sender to emit information about completely different topics. If that was the case, multimodal signals might evolve whenever there are different targets for the signals – for example, during simultaneous inter and intrasexual competition – or when the message itself is comprised of information about distinct aspects of the sender individual (e.g., both its position and its quality). In this case coordination between components is crucial: simultaneity or sequentiality between components might convey messages different than the sum of their parts (emergent properties, Rowe and Guilford, 1996). Part of the reason for the lack of consensus on the function of multimodal signals is that trait-based experiments tend to separate them in their single components (Groot et al., 2021). This can lead to biased or partial conclusions about the responses to these signals. Research involving common cuckoos has greatly focused on their calls, some of which have been found to be signals involved in both intra (Moskát et al., 2017, 2018) and inter-sexual selection (Moskát and Hauber, 2021). However, to our knowledge no research has been conducted on the potential multimodal nature of cuckoo signaling, i.e., if there are other components accompanying the acoustic display.
Behavioral displays have been examined in other brood parasite species, specifically in bronzed cowbirds (Molothrus aeneus) (Friedmann, 1929) and pin-tailed whydah (Vidua macroura) (Shaw, 1984), and described as a courtship ritual soliciting females at a close range. In both species, it has been described as pre-copulatory displays composed of airborne and terrestrial elements (Clotfelter, 1995). Cuckoo males perform their vocal displays from perches shared with females, who gain convenient point of view to find nests to parasite. Cuckoo males become territorial with its vocal properties (Moskát et al., 2017), but whether visual displays also serve this function has not yet been tested nor is there any evidence that they can attract a mate or transmit social information. The only clue comes from an experiment aimed to verify male cuckoo ability to discriminate different female morphs. Cuckoos attempted to mate with a decoy without any preliminary attempt to perform any courtship, indicating that male displays may not be used to attract females (Lee et al., 2019).
Unlike vocalizations, visual displays are, thus, completely unexplored in cuckoos, as there is no ethogram or evidence showing inter-individual variability or advanced hypotheses on their functions. Hypothetically, given that male cuckoo acoustic displays elicit a response from both males and females, a visual display might appear unneeded, or present only as a redundancy. However, given the extended amount of time this species devotes to the acoustic displays, it might be possible that a visual display might be a less generic, more targeted message for a specific set of other individuals – for example, a female approaching or a challenging male –. This might point to a sequentiality in the two displays, with the visual component following the acoustic one.
We studied both acoustic and visual displays in the common cuckoo, aiming: (i) to tentatively reveal how many individuals resided in the area by identifying vocal types (VTs, i.e., male individuals differing by their vocal features) and to determine VTs spatial preferences among their perches; (ii) to describe a cuckoo male ethogram and quantify consistent behavioral sequences; (iii) to verify the presence of multimodal visual/acoustic signals in male cuckoo displays and to characterize their relationship, and (iv) to verify whether there are VT-specific behavioral rituals.
Materials and Methods
Study Area
We studied cuckoo rituals from May to August 2019 within the Mirandola Plain, a 500 hectares area recently naturalized in a marsh area with large reedbed, regularly monitored by volunteers of the SOM (Modena Ornithological Station). Today the whole area is a Special Protection Area hosting a very high biodiversity (Lui and Giannella, 2003), including dense populations of reed and great reed warbler (Acrocephalus scirpaceus and A. arundinaceus, respectively), two of the most parasitized species in Italy (Campobello and Sealy, 2009) with abilities to social learn antiparasitic defenses (Campobello and Sealy, 2011). At the beginning of the season, we identified five perches, for brevity Vantage Points, VPs (VP1, VP9, VP16, VD, and T; Supplementary Figure 1), where cuckoos performed vocal and visual displays most often. Distances between perches averaged 364 m (range 160–1,189 m).
Acoustic and Video Recording
We recorded cuckoo calls and displays with three video cameras (Sony DCR-DVD650, Canon LEGRIA HF R86, Nikon D330). We recorded cuckoo calls also with a ZOOM H4 digital recorder (ZOOM Corporation; parameters: 44. 1 kc/s sampling rate, 16bit depth) connected to a directional microphone (Audio Technica AT815b). We modified a tripod so that it could hold simultaneously the microphone and one of the video cameras. Other two tripods held one video camera each. With favorable weather conditions, thus with no precipitations or strong wind, each day, we placed each of three tripods in front of a randomly chosen VP and left recording for approximately 3 h.
Call Description
Among all cuckoo calls only the most common one, the cu-coo call, has been shown to possess individual specific properties. This advertising call consists of two notes and is employed by cuckoos to recognize familiar individuals, such as close neighbors (Moskát et al., 2017). Both notes of cu-coo call are individual-specific (Moskát et al., 2018), in particular with the call frequency, duration and, especially, the maximum frequency of the first syllable being the most characterizing variables (Zsebõk et al., 2017). It is therefore possible to discriminate individuals starting from the analysis of their vocalizations by using acoustic variables as discriminatory elements (Table 1; Lei et al., 2005; Li et al., 2017; Zsebõk et al., 2017). In addition to the cu-coo call, males possess a richer vocal repertoire than the single multi-purpose call uttered by females, the bubbling call. Other male calls include a slight different variant of the advertising call, the cu-cu-coo call, and two calls used more less frequently, the gowk and gou calls (Lei et al., 2005; Moskát and Hauber, 2019). Contrarily to the cu-coo call, there is no evidence that all the other calls are characterized by an inter-individual variability.
Acoustic and Video Analysis
Acoustic Analysis
We analyzed a total of 27 h 03’ of tracks recorded by both microphone and video cameras. Recordings from video cameras were extracted with the Video Pad-Video Editor program. After a preliminary visual screening of collected recordings, we identified several cu-coo and cu-cu-coo call sequences (Supplementary Figure 2). The other calls of the cuckoo male repertoire were rarely used, making their analysis difficult if not impossible. We detected, however, a call resembling both the gowk (Moskát and Hauber, 2019) and gou (Lei et al., 2005) calls but, given the scant sample size and the lack of reference on its vocal characterization (Table 1), we preferred to assign it a new name (bark call) and provide characterization details to assist future comparisons (Supplementary Figure 3).
We used the terms call and syllable only when analyzing the sequences including cu-coo and cu-cu-coo calls. Specifically, the syllable was one single element, the calls the set of several syllables (i.e., the cu-coo together form one call, with cu the first syllable - S1 and coo the second– S2), whereas the whole sequence was composed of all call repetitions divided by a pause of less than 2 s as shown in Supplementary Figure 2. To find cuckoo sequences, we visually inspected spectrograms using Raven Pro 1.5.0 (Yang and Center for Conservation Bioacoustics, 2014; Cornell Lab of Ornithology, Ithaca, NY, United States) with the following settings: brightness 48, contrast 69, spectrogram window size at 3,268 points.
To analyze time-and-frequency parameters, we selected only the cu-coo and cu-cu-coo sequences characterized by high intensity and absence of overlap with other signals. We manually selected a maximum of three calls per sequence selecting those with a better quality on the base of the spectrogram inspection (brightness 48, contrast 85, FFT spectrogram window size at 1,329). Then we analyzed acoustic variables that were previously used in other studies (Lei et al., 2005; Wei et al., 2014; Li et al., 2017; Zsebõk et al., 2017): for each syllable (i) syllable length (ΔT, i.e., ending– starting times), (ii) minimum frequency (Fmin), (iii) maximum frequency (Fmax), (iv) bandwidth (ΔF, i.e., maximum – minimum frequencies), (v) peak frequency (Fpeak, i.e., frequency with the maximum energy); for the intra-call syllables: (viii) pause between two adjacent syllables (Tpause), and (ix) difference between maximum frequencies of two adjacent syllables (ΔFmax).
Behavioral Analysis
Out of a total of 9 h 19’ of video recordings, cuckoos were present in only 2 h 50’ distributed in 42 video clips. We identified 16 behaviors within three behavioral categories and behaviors within each category were mutually exclusive (see section “Statistical Analysis” and section “Behavioral Analysis,” Table 2). We coded all behaviors using Boris v.7.9.22 software (Friard and Gamba, 2016), while slowing down the speed of video-recording by 50%. All video clips showed one focal individual, a cuckoo male, at the time. To investigate whether the single behavioral events – i.e., any instance of focal individual activity or its absence – were replicated in consistent behavioral sequences, all behavioral data were further summarized in three categories: “Posture,” “Movement,” and “Vocalization.” Behavioral events within the same category (states) were considered mutually exclusive; all events within each category of each behavioral sequence belonged to one of the following states.
Posture category was defined as the general positions that individuals assumed when perched, with two states: either (i) Lax individual keeps their wings lower than the tail, the wingtips pointing down and the rump up; or (ii) Non-Lax, individual either moves or perches with their wings above the tail. Movements were divided in three states: (i) Still, i.e., not performing any movement, (ii) Tail swing individual moves its tail (either tail swing left to right or tail swing up): we pooled tail swing left to right together with tail swing up as the latter behavior happened almost always simultaneously with the former (92.5% of the time, see Table 2); (iii) Active, which includes several other types of movements (i.e., head movement, autogrooming, moving alongside the branch, rotating the body; see Table 2). Vocalizations were summarized in two states: (i) calling and (ii) silent (since bark call represented only the 0.05% of the total vocalizations, for the purpose of this analysis, we decided to pool all calls together). For further rationale behind these categorizations, see section “Results” and section “Behavioral analysis” and Table 2.
Statistical Analysis
Acoustic Analysis
To test whether we could discriminate different vocal types among all selected sequences, we averaged the syllable acoustic variables per song, then we quantified the intra-song coefficient of variation (CV) as CV = 100*[1+1/(4*n)]*SD/mean, where n is the sample size (following Zsebõk et al., 2017). We then selected the acoustics variables characterized by a low level of CV (less of 3%) and rescaling them to prevent biases due to data overdispersion (Zuur et al., 2007). By using SPSS software (SPSS Institute Inc., Chicago, IL, United States), we conducted a hierarchical cluster analysis (Yim and Ramdeen, 2015) with the squared Euclidean distance on the rescaled variables. All acoustics variables previously selected were tested with Kruskal-Wallis rank sum test and Dunn’s test to verify whether they differ among the vocal types.
Spatial Preferences
To test whether the vocal types were found significantly different in specific VPs, we conducted χ2 tests on contingency tables built with both the number and the percentages of each vocal type found in each VP. These analyses were run with Statistica 10 (StatSoft Inc, 2001).
Behavioral Analysis
Analyses of this section were performed by using R version 4.0.3 (R Foundation for Statistical Computing; R Core Team, 2014). Each video was decoded as a three-categories behavioral sequence, i.e., a sequence of events (42 sequences, 54.57 ± 99.58 [mean ± SD] events). Each event was characterized by three states, one for each category: for example, event 1 of sequence 1 could be lax/active/silent, followed by event 2 of sequence 1 lax/active/calling, followed by event 3 of sequence 1 lax/still/calling, and so on. From these three-categories behavioral sequences we created a contingency table with co-occurrences of Posture, Movement and Vocalization, and we tested the significance of these co-occurrence with Fisher exact test, adjusted with False Discovery Rate [fdr, packages Multcompview (Graves et al., 2015) and GmAMisc (Alberti, 2020)].
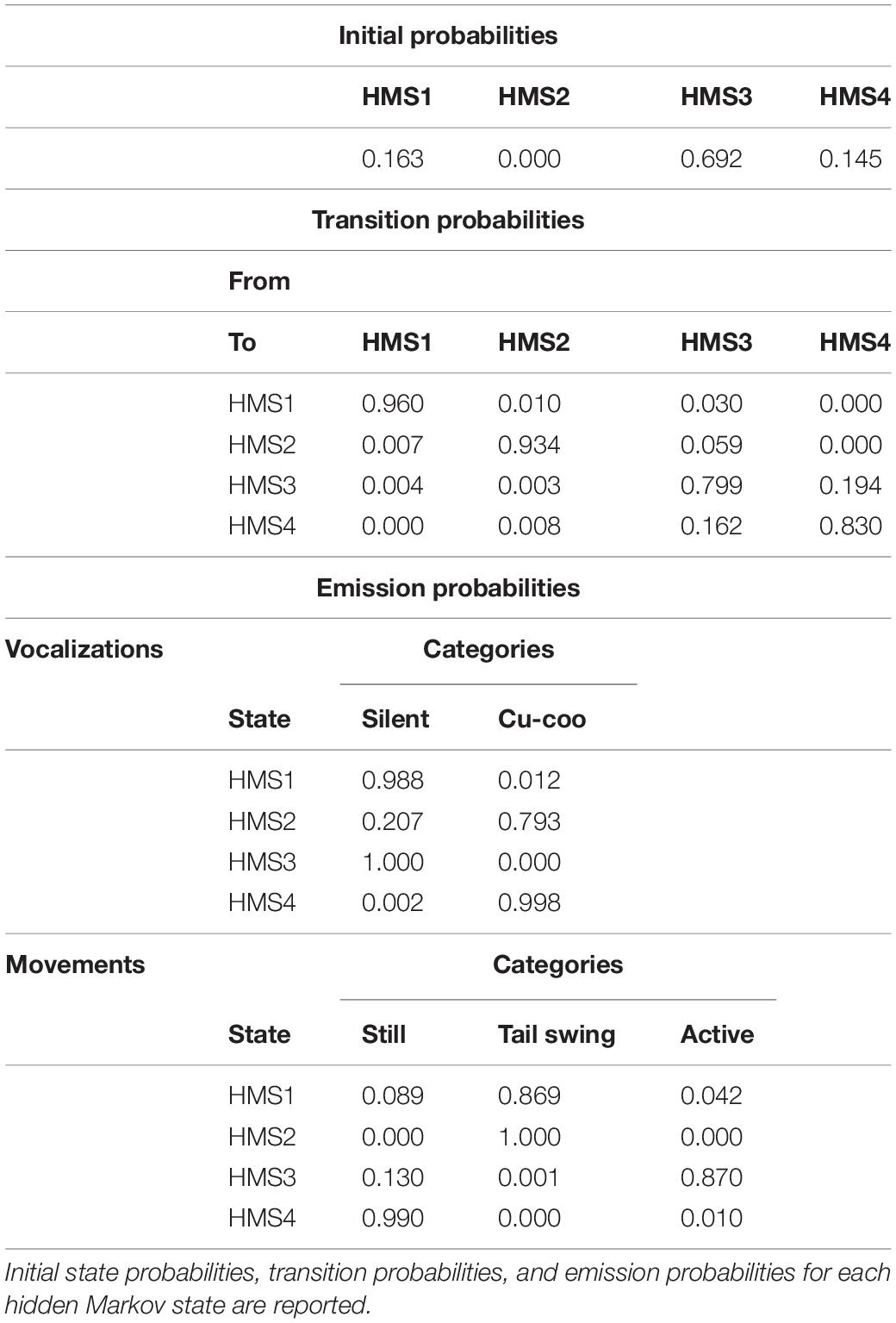
To further analyze associations between behavioral displays and vocalizations, behavioral sequences were also modeled with a hidden Markov chain model (package seq HMM, Helske and Helske, 2017). This analysis allows to search for Hidden Markov States (HMSs), i.e., partial sequences and co-occurrences of states that might be repeated within a sequence. In the models, we used Movement and Vocalization categories, because the Posture category was represented by the only state (Lax) in the vast majority of behavioral events. We created the model with the function “build_hmm” and fitted it with function “fit_model” (Helske and Helske, 2017). The model estimated several parameters through Maximum Likelihood: initial probabilities (probability to be in a specific HMS at the beginning of the sequence), transition probabilities (the probability to pass from one HMS to the others) and emission probabilities (the probability of a state in each category to be associated with an HMS). This analysis appeared particularly suited to our dataset as it can estimate more than one category (or channel) at the time, i.e., it could detect pattern of association and transition even between states not belonging to the same category, thus forming multi-category HMSs. We selected the number of HMSs via BIC-selection and fitted the model with best BIC estimates (Helske and Helske, 2017). No covariates were included.
Finally, to detect possible differences in the behavioral displays of the vocal types, we performed a finer scale Symmetry test of the two contingency tables (Alberti, 2020) considering the four most common behavioral states of those sequences: cu-coo call, tail swing left to right, tail swing up, and bark call.
Results
Vocal Types and Spatial Preferences
We counted 800 call sequences, including 368 recorded in the morning and 432 in the afternoon. For characterization, we manually selected 550 cu-coo calls, for a total of 1,100 syllables and then we characterized the syllables (S1 and S2) by their acoustic variables in terms of mean ± SD. The acoustic variables with low CVs values and therefore used to identify the potential different vocal types were the following: Fmin, Fmax, Fpmax for both the first (S1), and second syllable (S2). Entering these six variables into a cluster analysis and by using a cut-off limit of 4, we identified four groups and one isolated call which was excluded for the following analyses (Figure 1). The cluster dendrogram shows four different groups, thus four vocal types (VTs), that we labeled Red, Blue, Green and Yellow. The Kruskal-Wallis test showed significant differences between the four clusters for each variable (all tests, χ2 = 46.4–60.8 [min-max], P < 0.001, N = 79, df = 3). In more detail, the Duncan tests showed, first, that both syllables mirrored exactly the differences and similarities resulted in each of the variables (Supplementary Table 1), and, second, that although all four groups were significantly different for most of the variables, the only two vocal types that differed significantly for all of them were the Blue and the Red (Figure 2). Out of the four vocal types, only the Red one was ubiquitous, thus not showing specific VP preferences (χ2 = 7.38, P = 0.117, N = 29). The Blue was found mostly on VP9 (χ2 = 33.72, P < 0.001, N = 36), whereas the Green (χ2 = 20.36, P < 0.001, N = 11) and Yellow (χ2 = 16.00, P = 0.003, N = 4) preferred the VP1. These significant values, however, disappeared when we analyzed the percentages of the number of times vocal types spent in each VP, indicating they had no spatial preferences (all tests, χ2 = 0.25−4.00, P > 0.05, Figure 3).

Figure 1. Dendrogram of the hierarchic cluster analysis based on mean values of each acoustic variable (see text) quantified in each song. Labels at the bottom show the vantage point where the audio recording took place and the song identification number. The y-scale represents the rescaled distance for the four vocal types, Red, Blue, Green and Yellow, here identified by using a distance cut-off of 4 (Yim and Ramdeen, 2015).
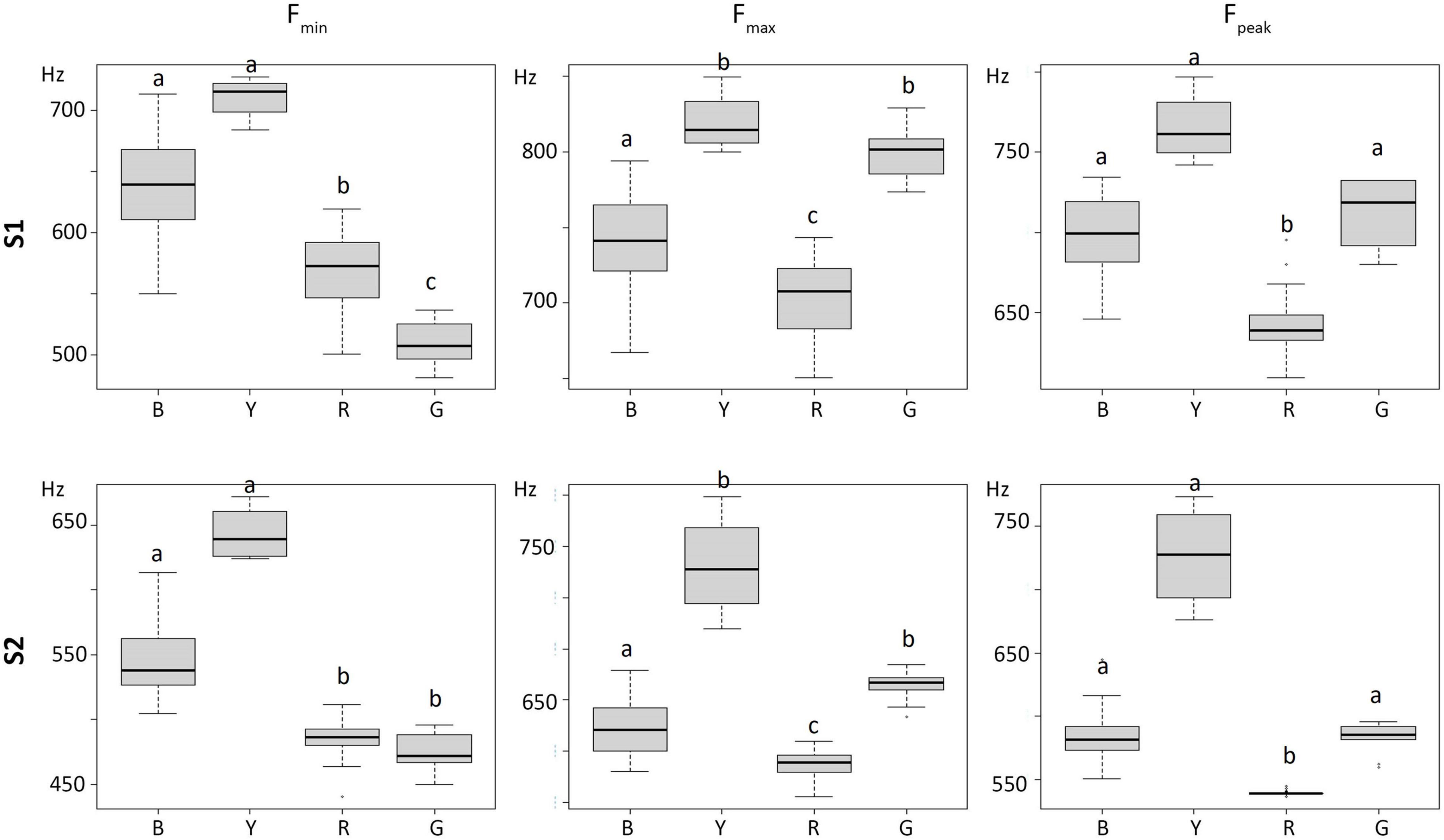
Figure 2. Boxplot of Fmin, Fmax, Fpmax variables for both the first (S1), and second syllable (S2) for the four vocal types identified by the cluster analysis (R, red; B, blue; G, green; Y, yellow). Different letters indicate significant differences between groups revealed by Dunn’s test (P < 0.05, for detailed statistics see Supplementary Table 1).
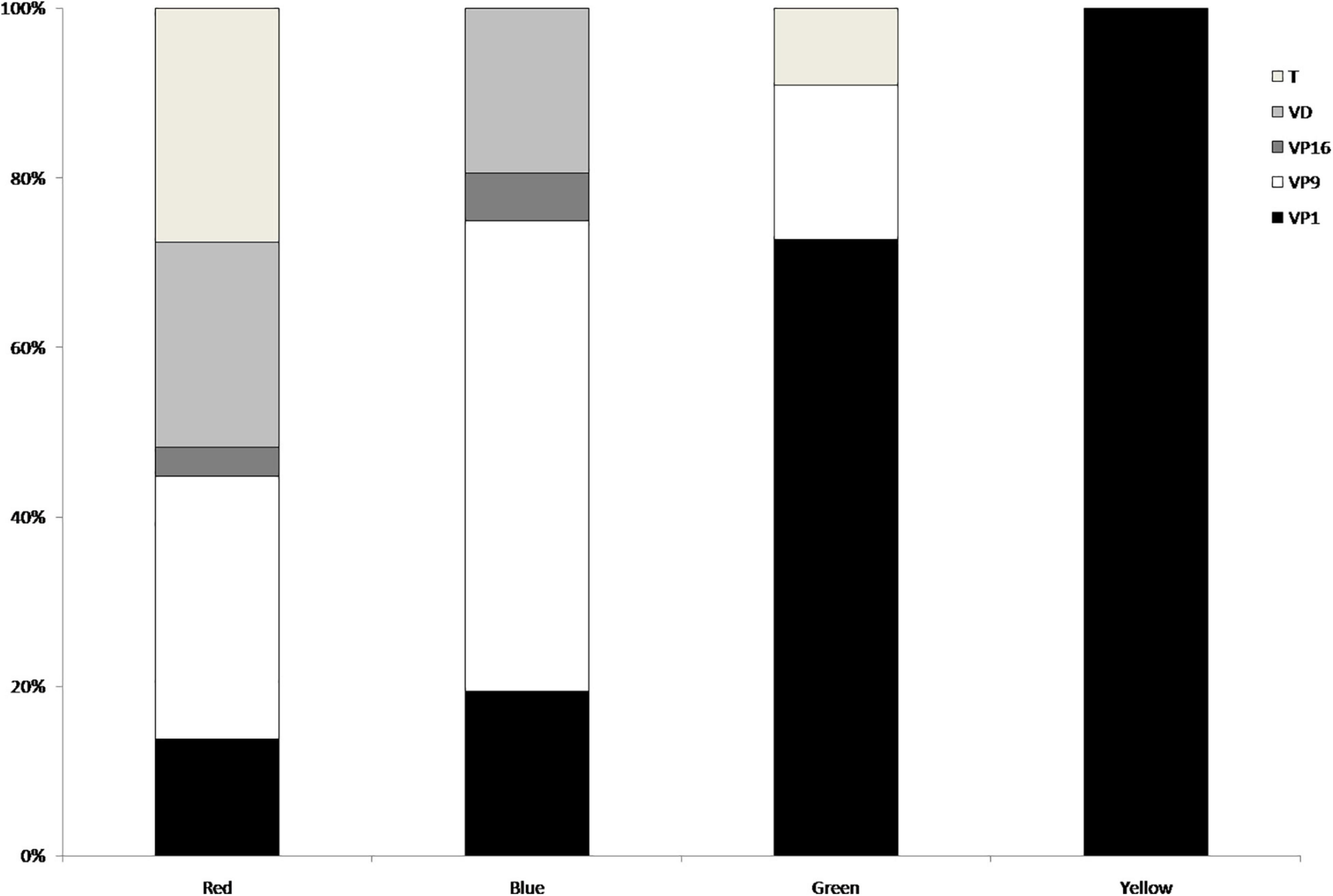
Figure 3. Percentages of the number of times each of the four vocal types (red, N = 29; blue, N = 36; green, N = 11; and yellow, N = 4) was found on each of the five Vantage Points (T, VD, VP16, VP9, and VP1).
Behavioral Analysis
We determined 2,292 behavioral events distributed in 42 sequences, each one characterized by three states according to the three categories: Posture, Movements, or Vocalizations. Within the Posture category, cuckoo males spent most of their time perching in lax posture (94.3% of all the events in the combined behavioral sequences). During our recordings, they often emitted a cu-coo call (49.3% of the events), very rarely a bark call (0.2% of all events), and otherwise they stayed silent (50.5% of the events). As for Movement, individuals either perched still (48.8% of the events), performed tail swing (16.2% of the events) or other movements (active, 34.9% of the events, see Table 2 for further details).
We found a positive association between lax posture and calling, with the non-lax posture being associated with silent (Fisher’s exact test adjusted with fdr, P < 0.001, Figure 4A). Active was associated with non-lax posture while both still and, more weakly, tail swing were associate with lax (Fisher’s exact test adjusted with fdr, P < 0.001, Figure 4B). We also found a positive association between perching still and calling and between staying silent and active (both Fisher’s exact test adjusted with fdr, P < 0.001, Figure 4C). The opposite associations – calling and being active; perching still and staying silent – were accordingly negative (both Fisher’s exact test adjusted with fdr, P < 0.001). Tail swing showed weaker associations, positive with staying silent and negative with calling (both Fisher’s exact test adjusted with fdr, p < 0.001, Figure 4C). All these results together indicate that cuckoo males adopted either a still lax posture while uttering cu-coo calls or an active perched position while being silent. Swinging tail was the only movement cuckoo males weakly associated with either a lax posture or being silent.
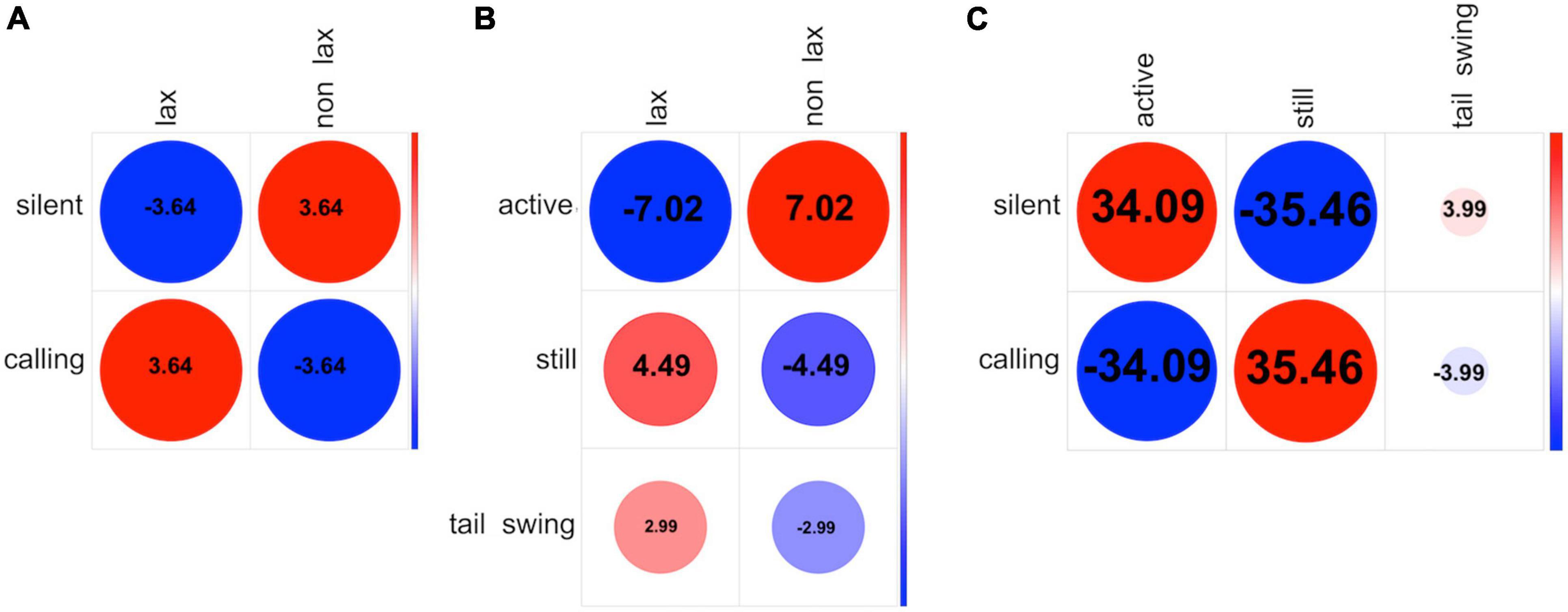
Figure 4. Strength of association between states (behavioral events) of different categories. The charts show Pearson’s Standardized Residuals (numbers within the circles), obtained through χ2 test of independence on permuted dataset. Greater positive deviation from independence (positive association) is indicated with darker hues of RED; greater negative deviation from independence (negative association) is indicated with darker hues of BLUE. Only residuals higher than threshold of 1.96 are shown. (A) Association between Vocalization and Posture categories. (B) Association between Movement and Posture categories. (C) Association between vocalization and posture categories.
The BIC of the Markov chain model was lowest with four hidden states. After fitting the model, hidden Markov state 2 (HMS2) had the lowest initial probabilities (<0.001; State 3 had highest initial probability (0.692) while HMS1 and HMS4 had initial probabilities of, respectively, 0.163 and 0.145 (Table 3 and Figure 5). In general, as expected from the results of the contingency association tables, in the model HMS3 had high emission probabilities for silent and active (respectively 1.000 and 0.870, Figure 5, Green) while HMS4 had high emission probabilities for calling (0.998) and still (0.990) (Figure 5, Blue). HMS1 described individual staying silent (0.988) and performing tail swing (0.869) (Figure 5, Light Gray), while HMS2 –the least probable – represented individuals performing a tail swing (1.000) and also calling (0.793) or stayed silent (0.207) (Table 3 and Figure 5, Red). In general, the hidden Markov models provided further confirmation that individuals either perched silent and active or were still and calling. These last results also showed that transition probabilities underscored a generally very high (0.960 < x > 0.7992, Table 3) chance that each hidden state repeated itself with a low probability to switch to another one. Thus, we did not find any evidence of an association between behavioral displays and calling, nor of other specific patterns within the sequences of behavioral events.

Figure 5. Graphical representation of behavioral sequences of events within different categories (i.e., vocalization and movement). Each horizontal bar represents one behavioral sequence (n = 42, length 1–601 events). We attributed a color to each state and its behavioral events. Behavioral sequences of (A) vocalization events. (B) Movement events. (C) Hidden Markov states (HMS) showing that cuckoo males either tend to perch motionless while repetitively calling (HMS4) or are active while being silent (HMS3).
The relatively low number of videos associated with Red and Blue vocal types did not allow an in-depth analysis, and care should be used when interpreting even significant results. With this being said, we found that the Blue VT performed more tail swing up than Red VT with respect to total events (Nominal Symmetry Test adjusted with fdr through 100000 Montecarlo simulations, P = 0.011). There was also a slight (non-significant) difference in their emission of bark call with Red VT performing some, while Blue VT never did (Nominal Symmetry Test adjusted with fdr through 100000 Montecarlo simulations, P = 0.25).
Discussion
Despite their complexity, cuckoo vocal features allowed us to discriminate different vocal types among the cuckoo males during their breeding period. While literature widely suggests that vocal types in “cu-coo” calls might be linked to inter-individual differences in male cuckoos (Lei et al., 2005; Li et al., 2017; Moskát et al., 2017, 2018; Zsebõk et al., 2017) we could not verify this, as we did not individually mark the multiple males frequenting the area. Although all but one vocal types seemed inclined to prefer specific perches, this apparent spatial preference disappeared as their sample size increased. This result suggests that no territorial behavior takes place among competing males, or at least, that vantage points are not territories to be defended. Perched on bare branches of trees beside or within reedbeds, cuckoo males showed two main behavioral states where vocal and motor activities were decoupled, as if they were almost mutually exclusive. Thus, vocal cuckoos were motionless whereas active cuckoos were soundless. Each behavioral state also tended to repeat itself with a low probability to switch into the other. Lastly, our results were not conclusive on the possible differential behavioral patterns adopted by each vocal type.
Cuckoo territoriality is suggested occurring in both sexes. In Eastern European populations, males utter cu-coo calls as a signal of territorial defense and aggression (Moskát et al., 2017, 2018; Tryjanowski et al., 2018) during the breeding season and when intrasexual aggression rate is high (Nakamura and Miyazawa, 1997). In a Japanese population, males are reported to engage in both aggressive interactions with other males and sexual contacts with females in singing areas, thus specific perches where dominant males actively sing and defend these areas from other males (Nakamura and Miyazawa, 1990, 1997). The size of singing areas of different males decreases as the density of males increases. Singing areas they might overlap with each other especially when they are within territories frequently visited by females because of a high presence of host nests. There are two causes, not necessarily mutually exclusive, explaining this territory overlapping. First, the presence of sub-dominant males sneaking some singing activity while dominant males are absent (Nakamura and Miyazawa, 1997) and, second, the polygamous nature of cuckoo males who can travel long distances to increase their mating opportunities with different females (Marchetti et al., 1998). Our results, apparently suggesting the lack of male territoriality, could instead be the outcome of either contexts driving toward singing area overlapping, thus subdominant males roaming across the entire study site and/or males searching different females to mate.
If male calls are mostly related to territoriality, our hypothesis aimed to verify a simultaneous visual component might be associated to them, indicating the presence of a multimodal visual and acoustic display. Our results did not show this being the case, as cuckoo males either call or move while were on their perches, indicating that male cuckoos do not possess a multimodal display. In fact, we argue that our results show the absence of, first, a display with simultaneous visual and acoustic components and, second, a display with a fixed and repeated alternation of the two.
There could be several explanations for a decoupling between calls and behavioral displays. First, the voice of the cuckoos is sufficiently powerful that, depending on environmental conditions, can be heard at several kilometers of distance (Meshcheryagina and Opaev, 2021). While a male sings from an obvious perch, both potential mates and competing males can hear his call from afar, well before coming into contact with the vantage point. This implies that displaying while calling could be broadly useless, a dangerous waste of energy (Cooper and Goller, 2004), and that the cu-coo call could be seen as a long-distance signal, meant to attract or repel conspecifics. Thus, there would not be the need of a visual display, neither as backup, nor to provide different information – the acoustic display of the common cuckoo is informative for both male and female conspecifics –.
Regardless from their association with calls, behavioral displays in general may play their function only if they are shown in close proximity of another individual (Bradbury and Vehrencamp, 1998). In our study, in the behavioral state in which cuckoo males moved silently, the movements involved cannot be described as potential displays for mate attraction or territoriality as they were generic movements (e.g., grooming). The only possible candidate to play a role as part of a ritual was the tail swings (Andersson and Iwasa, 1996). These resulted more evenly distributed, without a strong association with the cu-coo calls.
In social Cuculids, only anecdotic evidence is available for pre-copulatory displays (McNair, 1991; Merrett, 2014), whereas other displays have been investigated when used in communal chores (Strong et al., 2018) or toward their hosts (Davies, 2011). We cannot exclude that, since all of our video recordings showed only one male at the time, visual display might still have a role in the common cuckoo when directly confronted with a conspecific or as a response to heterospecifics. In other species acoustic and visual signals are assessed sequentially, depending on the range of the sensory system. For example, male sage grouses (Centrocercus urophasianus) attract females with their calls to their display site (Gibson and Bradbury, 1985); island flycatchers (Monarchidae) assess rival conspecifics first acoustically and then visually (Uy and Safran, 2013). Our results could be consistent with a visual component being employed and assessed only if the vocal component has managed to attract conspecific close enough, which, during our experiments, never happened at the VPs. An ideal test of tail swing function should aim to increase the sampling effort so to acquire recordings with the focal individual in close proximity to other conspecifics, both males and females.
Conclusion
Calls and visual displays are perfect candidates of traits on which sexual selection might operate as they may serve to compete for a territory (i.e., intra-sexual selection) or attract a mate (inter-sexual selection, Andersson and Iwasa, 1996; Bradbury and Vehrencamp, 1998). To our knowledge, our study is the first attempt in cuckoos to determine whether both behavioral traits show an inter-individual variability within population, the first necessary step to successively determine differential fitness of male ritual phenotypes. While we determined differential vocal types, our data did not allow to detect whether each one adopted differential rituals. In future studies, efforts should be directed to collect an adequate number of audio and video recordings of interactions between different individuals. These observations would allow the analysis of both intra- and inter-sex interactions, which may serve to better explain the apparent lack of associations between calls and visual displays we found in cuckoo males. This is a quite unusual condition in birds whose courtship rituals are often found expressed boldly together (Cooper and Goller, 2004). While the most frequent call, the cu-coo call, has been suggested to have a territorial function (Moskát et al., 2017), we found an apparent lack of territoriality of each vocal type that, on the contrary, did not appear to prefer specific perches for their vocal displays. Investigating the function of the cuckoo calls should not conducted independently from the forces selecting for call composition. The structure of the cuckoo song has been shown to depend on the probability to be mobbed by other species (Benedetti et al., 2018), suggesting that interspecific communication takes place selecting not only for individual traits but also for their extended phenotypic version (Campobello et al., 2015). Thus, potential selective factors, such as host density and host species availability, should be taken into account to examine the whole multimodal signals in cuckoos.
All the above-mentioned future directions involve activities that require a considerable field effort. The effort, however, would be proportionate to the value of the knowledge we could acquire about the mechanisms operating on the sexual selection of this species, that besides being part of one of the best coevolutionary models (Davies and Brooke, 1988; Campobello and Sealy, 2018) is also a declining bioindicator species (Tryjanowski and Morelli, 2015).
Data Availability Statement
The original contributions presented in the study are publicly available. These data can be found here: https://doi.org/10.6084/m9.figshare.16884925.
Ethics Statement
The animal study was reviewed and approved by ISPRA, via Vitaliano Brancati, 00144 Roma.
Author Contributions
DC conceived and designed the study. ME collected the data. MC and GB analyzed the acoustic recordings. BT analyzed the video recordings. ME, MC, BT, and DC wrote the manuscript. SM, LS, and MD contributed to the manuscript and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by a MIUR FFR-D15-302468 grant to DC and an A.006@ENTPUBB@02BI-SALAPROVMO 1261/06 grant to LS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very grateful to the volunteers of Modena Ornithological Station for their valuable logistic support, Mario Caffi, Bruno Massa, and Gianluca Roncalli for field assistance, and Emanuela Canale for the first important help on the bioacoustic analyses. A special thank to the late Salvo Mazzola, who created a special connection among the authors, making this work possible. We also thank two reviewers for input that greatly improved our manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.725858/full#supplementary-material
References
Alberti, G. (2020). GmAMisc: ‘Gianmarco Alberti’ Miscellaneous. R package version 1 (1), 1. Available online at: https://cran.r-project.org/package=GmAMisc (accessed June 14, 2021).
Andersson, M., and Iwasa, Y. (1996). Sexual selection. Trends Ecol. Evol. 11, 53–58. doi: 10.1016/0169-5347(96)81042-1
Benedetti, Y., Slezak, K., Møller, A. Ps, Morelli, F., and Tryjanowski, P. (2018). Number of syllables in cuckoo Cuculus canorus calls: a test using a citizen science project. Sci. Rep. 8, 1–6. doi: 10.1038/s41598-018-31329-1
Bradbury, J. W., and Vehrencamp, S. L. (1998). Principles Of Animal Communication. Sunderland, MA: Sinauer Associates.
Campobello, D., and Sealy, S. G. (2009). Avian brood parasitism in a mediterranean region: hosts and habitat preferences of common cuckoos Cuculus canorus. Bird Study 56, 389–400. doi: 10.1080/00063650903013221
Campobello, D., and Sealy, S. G. (2011). Use of social over personal information enhances nest defense against avian brood parasitism. Behav. Ecol. 22, 422–428. doi: 10.1093/beheco/arq225
Campobello, D., and Sealy, S. G. (2018). Evolutionary significance of antiparasite, antipredator and learning phenotypes of avian nest defence. Sci. Rep. 8:10569. doi: 10.1038/s41598-018-28275-3
Campobello, D., Hare, J. F., and Sarà, M. (2015). Social phenotype extended to communities: expanded multilevel social selection analysis reveals fitness consequences of interspecific interactions. Evolution 69, 916–925. doi: 10.1111/evo.12629
Clotfelter, E. D. (1995). Courtship displaying and intrasexual competition in the bronzed cowbird. Condor 97, 816–818. doi: 10.2307/1369191
Cooper, B. G., and Goller, F. (2004). Multimodal signals: enhancement and constraint of song motor patterns by visual display. Science 303, 544–546. doi: 10.1126/science.1091099
Davies, N. (2011). Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. doi: 10.1111/j.1469-7998.2011.00810.x
Davies, N. B., and Brooke, M. D. L. (1988). Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284. doi: 10.1016/S0003-3472(88)80269-0
Friard, O., and Gamba, M. (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. doi: 10.1111/2041-210X.12584
Friedmann, H. (1929). The Cowbirds: A Study in the Biology of Social Parasitism. Springfield, IL: C.C. Thomas.
Fuisz, T. I., and de Kort, S. R. (2007). Habitat-dependent call divergence in the common cuckoo: is it a potential signal for assortative mating? Proc. R. Soc. B. Biol. Sci. 274, 2093–2097. doi: 10.1098/rspb.2007.0487
Gibson, R. M., and Bradbury, J. W. (1985). Sexual selection in lekking sage grouse: phenotypic correlates of male mating success. Behav. Ecol. Sociobiol. 18, 117–123. doi: 10.1007/BF00299040
Graves, S., Piepho, H.-P., and Selzer, L. (2015). Multcompview: Visualizations of Paired Comparisons. R Package Version 0.1-8. Available online at: https://cran.r-project.org/package=multcompView (accessed June 14, 2021).
Groot, A. T., Vedenina, V., and Burfdfield-Steel, E. (2021). Editorial: multimodal mating signals: evolution, genetics and physiological background. Front. Ecol. Evol. 8:489. doi: 10.3389/fevo.2020.630957
Hebets, E. A., and Papaj, D. R. (2005). Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214. doi: 10.1007/s00265-004-0865-7
Helske, S., and Helske, J. (2017). Mixture hidden markov models for sequence data: the seqhmm package in R. arXiv [Preprint] doi: 10.18637/jss.v088.i03 arXiv: 1704.00543
Johnstone, R. A., Reynolds, J. D., and Deutsch, J. C. (1996). Mutual mate choice and sex differences in choosiness. Evolution 50, 1382–1391. doi: 10.1111/j.1558-5646.1996.tb03912.x
Jung, W. J., Lee, J. W., and Yoo, J. C. (2014). “cu-coo”: can you recognize my stepparents? – a study of host-specific male call divergence in the common cuckoo. PLoS One 9:e90468. doi: 10.1371/journal.pone.0090468
Lee, J. W., Kim, H. N., Yoo, S., and Yoo, J. C. (2019). Common cuckoo females may escape male sexual harassment by color polymorphism. Sci. Rep. 9, 1–9. doi: 10.1038/s41598-019-44024-6
Lei, F.-M., Zhao, H.-F., Wang, A.-Z., Yin, Z.-H., and Payne, R. B. (2005). Vocalizations of the common cuckoo Cuculus canorus in China. Acta Zool. Sin 51, 31–37.
Li, Y., Xia, C., Lloyd, H., Li, D., and Zhang, Y. (2017). Identification of vocal individuality in male cuckoos using different analytical techniques. Avian Res. 8, 1–7. doi: 10.1186/s40657-017-0079-0
Lui, F., and Giannella, C. (2003). Le Valli di Mirandola (Modena). Quaderni di Birdwatching, Anno V, Vol. 9. Latina: Edizioni Belvedere.
Marchetti, K., Nakamura, H., and Gibbs, H. L. (1998). Host-race formation in the common cuckoo. Science 282, 471–472. doi: 10.1126/science.282.5388.471
Merrett, S. (2014). Channel-billed Cuckoos performing a previously undescribed courtship display. Whistler 8, 61–62.
Meshcheryagina, S. G., and Opaev, A. (2021). Previously unknown behavior in parasitic cuckoo females: male-like vocalization during migratory activity. Avian Res. 12:10. doi: 10.1186/s40657-021-00246-9
Mitoyen, C., Quigley, C., and Fusani, L. (2019). Evolution and function of multimodal courtship displays. Ethology 125, 503–515. doi: 10.1111/eth.12882
Moskát, C., and Hauber, M. E. (2019). Sex-specific responses to simulated territorial intrusions in the common cuckoo: a dual function of female acoustic signaling. Behav. Ecol. Sociobiol. 73:60. doi: 10.1007/s00265-019-2665-0
Moskát, C., and Hauber, M. E. (2021). Male common cuckoos use a three-note variant of their “cu-coo” call for duetting with conspecific females. Behav Proc. 191:104472. doi: 10.1016/j.beproc.2021.104472
Moskát, C., Elek, Z., Bán, M., Geltsch, N., and Hauber, M. E. (2017). Can common cuckoos discriminate between neighbours and strangers by their calls? Anim. Behav. 126, 253–260. doi: 10.1016/j.anbehav.2017.02.013
Moskát, C., Hauber, M. E., Bán, M., Fülöp, A., Geltsch, N., Marton, A., et al. (2018). Are both notes of the common cuckoo’s call necessary for familiarity recognition? Behav. Proc. 157, 685–690. doi: 10.1016/j.beproc.2018.03.017
Moskát, C., Taylor, D. M., and Hauber, M. E. (2021). Effective conspecific communication with aberrant calls in the common cuckoo (Cuculus canorus). Behav. Ecol. Sociobiol. 75:7. doi: 10.1007/s00265-020-02946-6
Nakamura, H., and Miyazawa, Y. (1990). Social organization among cuckoo males in Kayanodaira Heights in Japan. Bull. Inst. Nat. Educ. Shiga Heights Shinshu Univ. 27, 17–27.
Nakamura, H., and Miyazawa, Y. (1997). Movements, space use and social organization of radio-tracked common cuckoos during the breeeding season in Japan. Jpn. J. Ornithol. 46, 23–54. doi: 10.3838/jjo.46.23
R Core Team (2014). R: A Language And Environment For Statistical Computing. Vienna: R Foundation for Statistical Computing.
Reboreda, J. C., Fiorini, V. D., De Mársico, M. C., Gloag, R., and Scardamaglia, R. C. (2017). “Parasitic behaviour of interspecific brood parasitic females,” in Avian Brood Parasitism. Fascinating Life Sciences, ed. M. Soler (Amsterdam: Springer), 325–342. doi: 10.1007/978-3-319-73138-4_18
Rowe, C., and Guilford, T. (1996). Hidden colour aversions in domestic chicks triggered by pyrazine odours of insect warning displays. Nature 383, 520–522. doi: 10.1038/383520a0
Searcy, W. A., and Andersson, M. (1986). Sexual selection and the evolution of song. Annu. Rev. Ecol. Syst. 17, 507–533. doi: 10.1146/annurev.es.17.110186.002451
Shaw, P. (1984). The social behaviour of the Pin-tailed Whydah Vidua macroura in northern Ghana. IBIS (Lond. 1859) 126, 463–473. doi: 10.1111/j.1474-919X.1984.tb02073.x
StatSoft Inc (2001). Statistica, Packages 10. Available online at: www.statsoft.com (accessed May 15, 2021).
Strong, M. J., Sherman, B. L., and Riehl, C. (2018). Home field advantage, not group size, predicts outcomes of intergroup conflicts in a social bird. Anim. Behav. 143, 205–213. doi: 10.1016/j.anbehav.2017.07.006
Tryjanowski, P., and Morelli, F. (2015). Presence of Cuckoo reliably indicates high bird diversity: a case study in a farmland area. Ecol. Indic. 55, 52–58. doi: 10.1016/j.ecolind.2015.03.012
Tryjanowski, P., Morelli, F., Osiejuk, T. S., and Møller, A. P. (2018). Functional significance of cuckoo Cuculus canorus calls: responses of conspecifics, hosts and non-hosts. PeerJ 6:e5302. doi: 10.7717/peerj.5302
Uy, J. A. C., and Safran, R. J. (2013). Variation in the temporal and spatial use of signals and its implications for multimodal communication. Behav. Ecol. Sociobiol. 67, 1499–1511. doi: 10.1007/s00265-013-1492-y
Wei, C., Jia, C., Dong, L., Wang, D., Xia, C., Zhang, Y., et al. (2014). Geographic variation in the calls of the common cuckoo (Cuculus canorus): isolation by distance and divergence among subspecies. J. Ornithol. 156, 533–542. doi: 10.1007/s10336-014-1153-6
Xia, C., Deng, Z., Lloyd, H., Møller, A. P., Zhao, X., and Zhang, Y. (2019). The function of three main call types in common cuckoo. Ethology 125, 652–659. doi: 10.1111/eth.12918
Yang, K. L., and Center for Conservation Bioacoustics (2014). Raven Pro: Interactive Sound Analysis Software (Version 1.5) [Computer software]. Ithaca, NY: The Cornell Lab of Ornithology.
Yim, O., and Ramdeen, K. T. (2015). Hierarchical cluster analysis: comparison of three linkage measures and application to psychological data. Quant. Methods Psychol. 11, 8–21. doi: 10.20982/tqmp.11.1.p008
York, J. E., and Davies, N. B. (2017). Female cuckoo calls misdirect host defences towards the wrong enemy. Nat. Ecol. Evol. 1, 1520–1525. doi: 10.1038/s41559-017-0279-3
Zsebõk, S., Moskát, C., and Bán, M. (2017). Individually distinctive vocalization in common cuckoos (Cuculus canorus). J. Ornithol. 158, 213–222. doi: 10.1007/s10336-016-1376-9
Keywords: cuckoo, sexual selection, courtship rituals, bioacoustics, multimodal signals
Citation: Esposito M, Ceraulo M, Tuliozi B, Buscaino G, Mazzola S, Sala L, Dal Zotto M and Campobello D (2021) Decoupled Acoustic and Visual Components in the Multimodal Signals of the Common Cuckoo (Cuculus canorus). Front. Ecol. Evol. 9:725858. doi: 10.3389/fevo.2021.725858
Received: 15 June 2021; Accepted: 19 October 2021;
Published: 16 November 2021.
Edited by:
Canchao Yang, Hainan Normal University, ChinaReviewed by:
Piotr Tryjanowski, Poznań University of Life Sciences, PolandAlexey Opaev, Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences (RAS), Russia
Copyright © 2021 Esposito, Ceraulo, Tuliozi, Buscaino, Mazzola, Sala, Dal Zotto and Campobello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beniamino Tuliozi, YmVuaWFtaW5vLnR1bGlvemlAdW5pcGQuaXQ=
 Martina Esposito
Martina Esposito Maria Ceraulo
Maria Ceraulo Beniamino Tuliozi
Beniamino Tuliozi Giuseppa Buscaino2
Giuseppa Buscaino2 Matteo Dal Zotto
Matteo Dal Zotto Daniela Campobello
Daniela Campobello