- 1Department of Zoology and Entomology, University of Pretoria, Pretoria, South Africa
- 2Department of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ, United States
Predators have profound effects on prey behavior and some adult brood parasites use predator resemblance to exploit the antipredator defenses of their hosts. Clarifying host perception of such stimuli is important for understanding the adaptive significance of adult brood parasite characteristics, and the mechanisms by which they misdirect hosts. Here I review the literature to explore the adaptive basis of predator resemblance in avian brood parasites, and natural variation in host responses to these stimuli. I also provide a framework for the information ecology of predator resemblance, which is based on the principles of signal detection theory and draws from empirical evidence from the common cuckoo, Cuculus canorus, as the most widely studied system. In this species, visual and acoustic hawk-like stimuli are effective in manipulating host defenses. Overall, contrasts across host responses suggest that different modalities of information can have independent effects on hosts, and that predator resemblance takes advantage of multiple sensory and cognitive processes. Host perception of these stimuli and the degree to which they are processed in an integrated manner, and the physiological processes underlying regulation of the responses, present new avenues for brood parasitism research.
Introduction
Predation is a powerful force of natural selection. As a consequence, systems for detecting, recognizing and responding to predators are widespread (Lima and Dill, 1990; Ruxton et al., 2018; Leavell and Bernal, 2019). In the Kalahari Desert, you might witness a fork-tailed drongo aggressively dive-bombing a tawny eagle (Dicrurus adsimilis, and Aquila rapax, respectively). At roughly sixty times lighter, why does the drongo risk harassing this gigantic predator? Typically, approaching large predators can be explained by rewards shared across the prey community via mobbing defenses (Shields, 1984; Caro, 2005). By contrast, rodents that actively approach predatory cat cues derive no benefit for themselves or conspecifics. This fatal attraction occurs under Toxoplasma gondii infection, which alters predator aversion in intermediate rat hosts. The manipulative endoparasite benefits from increased transmission to the stomach of a cat (the definitive host; Berdoy et al., 2000). Indeed, cheats regularly benefit from stimulus ambiguity in the world of predator-versus-prey, harnessing mimicry and misdirection to mislead (Ruxton et al., 2018; Leavell and Bernal, 2019). Particularly infamous amongst cheats are the brood parasites, and here, I review the role of predator resemblance in facilitating brood parasitism.
Hosts of brood parasites suffer reduced reproductive success via two main routes: the premature death of their young, and by misdirected parental effort (Rothstein, 1990; Kilner, 2005; Kilner and Langmore, 2011; Mark and Rubenstein, 2013). To avoid these costs, hosts have evolved adaptations in defense against brood parasitism. In response, counteradaptations (to avoid host detection and enhance parasitism success) are the corresponding adaptations in antagonistic coevolution between host and parasite, that explain the evolution of, for example, extreme egg mimicry (Spottiswoode and Stevens, 2010). Indeed, egg mimicry has long provided textbook examples of host-parasite coevolution (Rothstein, 1990; Davies, 2000; Feeney et al., 2014). By contrast, characteristics at the adult stage can be shaped by ecology beyond the host’s nest. In some cases, adult characteristics appear to capitalize on a fate worse than brood parasitism for the host parent: that of falling prey to a predatory hawk (Lima and Dill, 1990; Davies, 2000).
Is Hawk-Like Resemblance Adaptive for Cuckoos?
Some adult brood parasitic cuckoos (Cuculinae) share characteristics with raptors; an observation that has long enchanted natural historians (Wallace, 1889; Chance, 1940). Phenotypic overlap could have arisen through convergent evolution, or via antagonistic coevolution with hosts (Payne, 1967; Craib, 1994). Comparative analyses across cuckoos suggest brood parasitic species are more likely to resemble predatory birds than species exhibiting parental care (Payne, 1967; Krüger et al., 2007). Moreover, predator-like characteristics appear to have evolved among cuckoos after brood parasitism arose (Krüger et al., 2007), suggesting that predator resemblance is adaptive. Importantly, predator resemblance could influence host behavior in three ways. First, by provoking escape responses, which extends opportunity to access the nest for egg-laying (Welbergen and Davies, 2008, 2011; York and Davies, 2017). Second, by deterring hosts from mobbing, which could provide greater access to nests, or avoid the costs of being mobbed (Welbergen and Davies, 2008, 2011; York and Davies, 2017). Indeed, host mobbing defenses are important since highly vocal nest-defending reed warblers (Acrocephalus scirpaceus) are parasitized least (Campobello and Sealy, 2018), mobbing can result in cuckoo injury or death (Molnár, 1944; Wyllie, 1981; Davies and Brooke, 1988; Šulc et al., 2020), and mobbing can alert neighbors, thereby increasing local nest-guarding and egg-rejection defenses. That said, mobbing can also be costly for hosts, and can increase parasitism and depredation (Smith et al., 1984; Krama and Krams, 2005; Davies and Welbergen, 2009; Campobello and Sealy, 2018). Third, predator resemblance could also influence hosts by misdirecting their defenses from clutch- to self-protection, thus increasing antipredator vigilance while reducing investment in egg rejection defenses (York and Davies, 2017).
Elegant experiments by Davies and Welbergen indicated that visual sparrowhawk, Accipiter nisus, resemblance is adaptive in host interactions with common cuckoos (Cuculus canorus; hereafter “cuckoo”; Duckworth, 1991; Davies and Welbergen, 2008). Barred chest plumage deters non-host parids from approaching, and reduces mobbing defenses in reed warbler hosts—as expected if they fear and avoid hawks (Welbergen and Davies, 2008, 2011). Indeed, warblers were more aggressive in mobbing cuckoos that appeared less hawk-like (where chest-barring was concealed; Welbergen and Davies, 2011), thereby confirming that hawk resemblance is perceived and effectively enhances brood parasitism success. However, not all individuals are fooled by the imperfect visual resemblance. Hosts mobbed cuckoos three times more intensely than hawks, demonstrating a capacity for discrimination, which is important because it provides the necessary basis for antagonistic coevolution (Duckworth, 1991; Welbergen and Davies, 2011; Yu et al., 2017). Some regularly and infrequently parasitized species will aggressively mob cuckoos despite their hawk-like appearance (Trnka and Prokop, 2012; Lyon and Gilbert, 2013; Ma et al., 2018). These apparently aggressive species may have overcome cuckoo hawk resemblance by being highly discriminating, driven by intense social learning under high parasitism prevalence. Alternatively, aggressive mobbing of a hawk could represent a cost of visual hawk-like characters (Lyon and Gilbert, 2013). As illustrated by the tawny eagle and drongo, prey will occasionally mob even very large predators vigorously, but this is not the case across all prey species and is rare among reed warblers (Welbergen and Davies, 2009). Indeed, baseline responses to predators are an important consideration in cuckoo-host dynamics and in particular, with regards to local variation in predator presence.
Female cuckoo calls share characteristics with those of Accipiter hawks (Newman, 2013; Liang, 2017; York and Davies, 2017). Similarities between female cuckoo and sparrowhawk calls provoke antipredator behavior to a similar extent both among hosts and non-hosts (York and Davies, 2017). Furthermore, in a number of cuckoo host species, female cuckoo and sparrowhawk calls similarly supress host defenses against brood parasitism, supporting the view that perceiving a female cuckoo call as that of a hawk manipulates a trade-off between behaviors that promote self-protection versus those that support parental investment (York and Davies, 2017; Roncalli et al., 2019; Marton et al., 2021; Shen et al., 2021). In the case of reed warblers, rejection defenses were suppressed, by contrast, great reed warbler, A. arundinaceus, mobbing responses were dampened following exposure to female cuckoo calls, which suggests the effects of these calls can supress both major lines of defense against brood parasitism. Whether these calls also increase opportunities for brood parasitism by increasing nest access is unknown (York and Davies, 2017; Marton et al., 2021). Given that diverse hawk-like stimuli are salient and provoke varied responses among hosts, we turn our attention to their evolution in the context of wider information ecology.
How Does Hawk Resemblance Evolve in Cuckoos?
Communication involves a signal that is emitted by a sender to influence a receiver, and in turn, the response of the receiver determines signal efficacy, ultimately providing a net fitness payoff to both parties (Bradbury and Vehrencamp, 1998; Maynard-Smith and Harper, 2003). Information is also available in the form of cues; mere byproducts of an organism existing in the environment (e.g., body shape, locomotory sounds), that are not emitted to influence a receiver and, importantly, cannot evolve independently from the characteristic about which they provide information (Maynard-Smith and Harper, 2003; Stevens, 2013). Crypsis scrambles information that could be extracted from cues, thereby concealing the organism from the detection systems of a natural enemy or victim, and is not widely considered to be a form of communication (Stevens, 2013; but see: Ruxton et al., 2018). By contrast, mimicry biases the characteristics of a mimic to correspond with information that is emitted by a model. In wrongly identifying the mimic as the model, the target receiver (the dupe) releases a benefit for the mimic while paying some form of cost for being misled (Figure 1; Dalziell and Welbergen, 2016; Font, 2019). Mimetic traits persist where there is a net fitness benefit for receivers, since correct detection of a true signal from the model is nevertheless advantageous (Stevens, 2013; Font, 2019). While examples of both signal and cue mimicry have been identified, the basis for their origin and maintenance are frequently debated and revised (Stevens, 2013; Dalziell and Welbergen, 2016; Jamie, 2017; Ruxton et al., 2018; de Jager and Anderson, 2019; Font, 2019). As such, the compelling diversity of adaptive resemblance continues to provide fascinating conceptual advances.
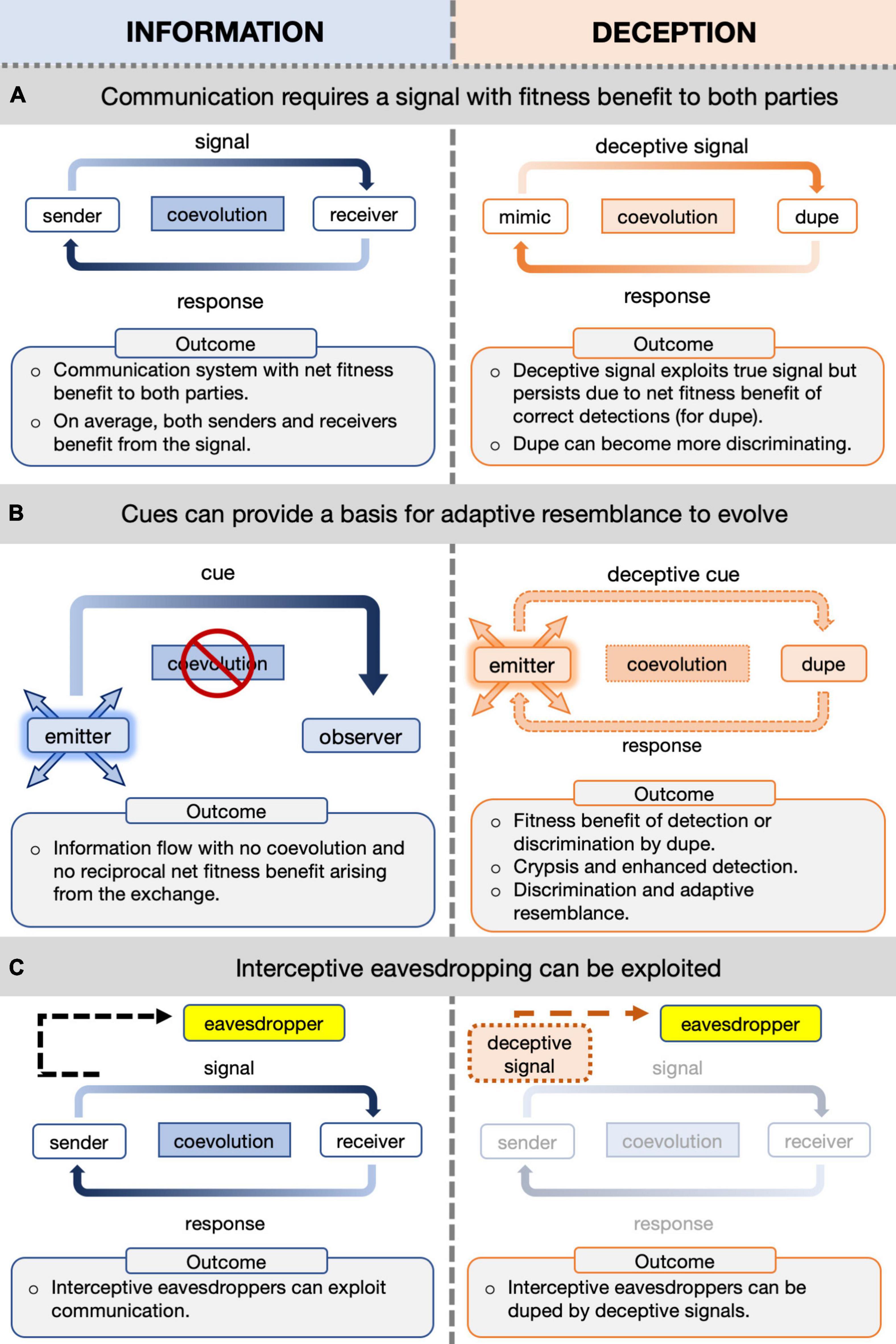
Figure 1. Schematics to illustrate potential roles and relationships in adaptive resemblance. Mutually beneficial or neutral information (left column) and deceptive information (right column) for: (A) communication (L) versus deceptive mimicry that exploits an existing relationship (R)—on average it benefits the signaller to signal and the recipient to respond due to net fitness benefits of correct detections; (B) information from cues can provide the basis for other forms of adaptive resemblance to evolve, without relying on an established signaller-receiver relationship; (C) interceptive eavesdroppers can exploit an existing communication relationship, and in turn are susceptible to deceptive signals with overlapping characteristics.
In cuckoos, visual hawk-like resemblance includes a number of features: overall shape and size, flight pattern, yellow legs and eye rings, and conspicuous barred chest plumage (Davies, 2000; Davies and Welbergen, 2008; Welbergen and Davies, 2011). Other predators that exhibit disruptive patterns (e.g., leopard spots or tiger stripes) are conspicuous in some contexts, but under dappled lighting or high speed motion, such patterns make it challenging to detect the boundaries of the predator’s form (Stevens, 2013). The barred chest plumage of hawks and cuckoos is salient for prey, and the extent of chest barring modifies prey responses to hawks (Davies and Welbergen, 2008; Welbergen and Davies, 2011). The chest barring of the cuckoo could be considered a deceptive signal (because there is a fitness benefit in resembling the hawk) and is generally classed as Batesian mimicry (Welbergen and Davies, 2011). However, the resemblance is not based on a signal because the chest barring is cryptic plumage, therefore the conventional basis of mimicry is absent (Figure 1A). Additionally, a key criteria of Batesian mimicry is the assumption that the mimic imposes costs for the model (Stevens, 2013) and such a mechanism to enforce costs on hawks is difficult to envisage, and in fact, any habituation to hawk-like characteristics due to cuckoo exposure would be beneficial to hawks. Perhaps the least troublesome approach is to place hawk-like barring cues within the broader umbrella of adaptive resemblance (Figure 1B; Starrett, 1993).
Female brood parasitic cuckoos also exhibit plumage polymorphisms. While it appears that the existence of polymorphisms in female cuckoos is not aligned with mimicking an alternative raptor model (Trnka et al., 2015), cuckoos with hawk-like features are more likely to be polymorphic (Thorogood and Davies, 2013a). Plumage polymorphism is effective because hosts use social learning to identify the gray cuckoo morph as a fake, which incurs a frequency-dependent fitness benefit for the rarer rufous morph (Thorogood and Davies, 2012). It is also possible that rufous females benefit from reduced costs of being mobbed, due to any costs associated with hawk-like appearance (Lyon and Gilbert, 2013). This additional benefit for the hepatic morph would therefore be frequency-dependent in relation to host baseline aggression toward hawks.
The ecological basis for the resemblance between female cuckoo and hawk calls differs from that underlying visual resemblance. Adult hawks produce calls in the context of communication between the pair, accompanying provisioning visits during courtship and chick rearing (Newton, 1986). Consequently, these calls are conspicuous signals and prey can benefit from interceptive eavesdropping, since hawk calls are indicative of ongoing local predation risk (Peake, 2005; Ridley et al., 2014; Billings et al., 2015). Given that small passerines are the main target of sparrowhawk hunts, female cuckoo hawk-like calls exploit an interceptive eavesdropper with dishonest information; which can be considered a distinct role in communication ecology (Figure 1C). The benefits of hawk-like female cuckoo calls may originate from signals used in intraspecific communication (York and Davies, 2017; York, 2018). Cuckoos of both sexes are responsive to playbacks of adult cuckoo calls (Moskát and Hauber, 2019). The existence of multi-function signals is widespread in animal communication, since once a beneficial signal is produced, additional benefits reinforce its advantage (Bradbury and Vehrencamp, 1998). Although adult Cuculinae vocalizations are not fully described, those with sex differences in adult calls are brood parasitic (Payne, 2005; Kim et al., 2017).
The flexible and ephemeral nature of behavioral signaling means that female cuckoo calls can be produced with the most beneficial timing to influence relevant audiences (Chance, 1940; Wyllie, 1981; York and Davies, 2017). Their brevity may explain limitations for hosts to develop counter-responses through learning (York and Davies, 2017). Opportunities for individual and social learning of calls by hosts could be more scarce than for visual characteristics, since associative learning requires a mobbing or active demonstrator to be effective (Campobello and Sealy, 2011a; and references therein). If learning does occur, temporal variation in parasitism intensity may account for between-year variation in host defenses (Campobello and Sealy, 2011a; Thorogood and Davies, 2013b). Populations with higher frequencies of parasitism where cuckoos are more abundant (e.g., Moskát et al., 2008; Campobello and Sealy, 2011b) could provide greater opportunities for habituation or learning processes.
How Do Hosts Respond to the Hawk-Like Stimuli of Cuckoos?
Processing Hawk-Like Stimuli
Signal detection theory has long provided a basis for understanding animal communication and sensory ecology (Duncan and Sheppard, 1965; Wiley, 2006). How well prey can separate a hawk from background environmental noise (detection) is analogous to how well a host can discern between cuckoo and hawk (discrimination), since both processes are influenced by sensory limits and performance (Stoddard and Stevens, 2011). We currently have a limited understanding of the perceptual processes underlying host responses to adult cuckoos.
Both the hawk-like barred chest plumage and conspicuous yellow eyes of cuckoos provoke responses from hosts (Davies and Welbergen, 2008; Trnka et al., 2012). How host responses relate to whether the characteristic of the predator model shared by the cuckoo is either contextually cryptic (barred plumage), or conspicuous (yellow skin) deserves further consideration. It is possible that these two types of information differ in perceptual processing. Detection of barring might be influenced by perceptual filtering, and its effect could be distance dependent, resulting in camouflage at distance and conspicuousness at close range, as occurs in some species (Stevens, 2013; Ruxton et al., 2018). Another interesting possibility is that the effect of barring could be conspicuous when static but cryptic in motion, since the same stimulus can vary in effect depending on observer context (Caro et al., 2013). The extent of this effect could depend on the exact plumage barring and degree of similarity to the model hawk, indeed, cuckoos do tend to be more similar to sympatric hawk species (Gluckman and Mundy, 2013). Future experimental work would fruitfully examine the integration of information to determine host perceptual processing speed and response thresholds to variation in these cryptic and conspicuous stimuli.
For hosts that appear less susceptible to the repellent effects of hawk-like appearance, vocal resemblance might provide cuckoos with another important mechanism for accessing host nests and avoiding the costs of being mobbed (Marton et al., 2021). The call may frequently follow the otherwise secretive and rapid behavioral sequence of a female gliding down to lay in the nest (Chance, 1940). Hosts observing this event may therefore be exposed to several hawk-like stimuli (body form and flight, plumage and pattern similarities, and calls) in sequence. Sequential exposure could either additively reinforce, or compensate for deficiencies in the others, depending on host discriminatory rules, iterative sampling rate, and multidimensional integration of predator stimuli (Leavell and Bernal, 2019). The effects estimated from experimental studies on singular hawk-like stimuli might therefore represent an underestimation of effects generated by an animated and multimodal live bird. Alternatively, cuckoo hosts might rely largely on discrimination rules based on single traits in isolation due to effects such as overshadowing (Kazemi et al., 2014).
Responding to Hawk-Like Stimuli
Host responses to hawk-like stimuli likely depend on species-specific baseline thresholds for predator detection and behavioral responses on detecting a predator. Prey can initiate several response types on detecting a predatory threat. Heightened vigilance combined with freezing can avoid localization by the predator, or fleeing the location can occur in the absence of, or in immediate response to, attempted attack (Ruxton et al., 2018). Alternatively, prey can aggressively mob predators, whereby easily localizable individuals approach and make violent contact with the predator (Shields, 1984; Caro, 2005). Cuckoo hosts use this range of behavioral defenses toward cuckoos, therefore it is important to examine whether host responses reflect discrimination, or costs of hawk-like resemblance (Davies and Welbergen, 2008; Lyon and Gilbert, 2013).
Detection thresholds can be modulated by individual factors (personality, state, age) and extrinsic environmental variation (Ruxton et al., 2018; Römer and Holderied, 2020). Similarly, discrimination thresholds are modulated with local parasitism or predation risk, which determines the trade-off between the costs of false alarms and correct detections for hosts in a given population (Welbergen and Davies, 2008, 2009; Davies, 2011). Behavioral responses to stimuli are regulated by neuroendocrine and endocrine mechanisms, and predator stimuli can provoke acute stress responses with sustained effects (Clinchy et al., 2013). Brood parasitism can influence stress physiology (Mark and Rubenstein, 2013), and hawk-like stimuli could contribute to modulation of this pathway. By doing so, brood parasites indirectly affect host risk-assessment physiology, which is analogous to the endoparasites that influence physiology underlying the risk-taking decisions of their host, and thereby promote parasite transmission (Poulin, 2010). Future studies could fruitfully examine the physiological mechanisms underlying responses to hawk-like stimuli, and the consequences for host life-history trade-offs. One important consideration is how such mechanisms interact with defense against other threats (e.g., egg predators; Campobello and Sealy, 2010, 2011a; Lawson et al., 2021).
Discussion
The intriguing absence of predator resemblance among other avian brood parasites remains unexplained. Parasitic cowbirds (Icteridae) exhibit a number of general adaptations to mitigate host defenses, but lack resemblance of predators that prey on adult hosts (Lawson et al., 2021). This may be a consequence of phylogenetic or body size constraints. Hawk resemblance is also relatively rare even among cuckoos (of 141 species, 17% “hawk-like,” 28% with barred plumage; Thorogood and Davies, 2013a). Another possibility for adult brood parasites is aggressive mimicry, whereby the brood parasite resembles an innocuous model (Feeney et al., 2015). This form of resemblance could be more common than is widely appreciated, and deserves further attention.
Overall, predator resemblance allows cuckoos to exploit hosts and to enhance brood parasitism by taking advantage of multiple sensory and cognitive processes. The hawk-like stimuli of brood parasitic cuckoos appear to defy satisfactory labeling using established frameworks for mimicry. I hope that considering their placement in the context of communication ecology as adaptive resemblance, as described here, will prove useful.
Author Contributions
JEY wrote the manuscript.
Funding
The author was supported by funding from Natural Environment Research Council grant NE/M00807X/1, and the European Union’s Horizon (2020) Research and Innovation Program (Marie Skłodowska-Curie IF Grant Agreement No. 837838).
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author thanks Dominic Cram, Rebecca Kilner, Cassie Stoddard, and Nick Davies for encouragement and thought-provoking discussions about cuckoos and mimicry, and Jake Dunn for the invitation to present a guest seminar on this work. The author would also like to thank editor and the two reviewers for their helpful comments.
References
Berdoy, M., Webster, J. P., and Macdonald, D. W. (2000). Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. B Biol. Sci. 267, 1591–1594. doi: 10.1098/rspb.2000.1182
Billings, A. C., Greene, E., and De La Lucia Jensen, S. M. (2015). Are chickadees good listeners? Antipredator responses to raptor vocalizations. Anim. Behav. 110, 1–8. doi: 10.1016/j.anbehav.2015.09.004
Bradbury, J. W., and Vehrencamp, S. L. (1998). Principles Of Animal Communication. Sunderland, MA: Sinauer Associates.
Campobello, D., and Sealy, S. G. (2010). Enemy recognition of reed warblers (Acrocephalus scirpaceus): threats and reproductive value act independently in nest defence modulation. Ethology 116, 498–508. doi: 10.1111/j.1439-0310.2010.01764.x
Campobello, D., and Sealy, S. G. (2011a). Nest defence against avian brood parasites is promoted by egg-removal events in a cowbird-host system. Anim. Behav. 82, 885–891. doi: 10.1016/j.anbehav.2011.07.028
Campobello, D., and Sealy, S. G. (2011b). Use of social over personal information enhances nest defense against avian brood parasitism. Behav. Ecol. 22, 422–428. doi: 10.1093/beheco/arq225
Campobello, D., and Sealy, S. G. (2018). Evolutionary significance of antiparasite, antipredator and learning phenotypes of avian nest defence. Sci. Rep. 8, 1–10. doi: 10.1038/s41598-018-28275-3
Caro, T. (2005). Antipredator Defenses in Birds and Mammals. PressChicago, IL: University of Chicago.
Caro, T., Stankowich, T., Kiffner, C., and Hunter, J. (2013). Are spotted skunks conspicuous or cryptic? Ethol. Ecol. Evol. 25, 144–160. doi: 10.1080/03949370.2012.744359
Clinchy, M., Sheriff, M. J., and Zanette, L. Y. (2013). Predator-induced stress and the ecology of fear. Funct. Ecol. 27, 56–65. doi: 10.1111/1365-2435.12007
Dalziell, A. H., and Welbergen, J. A. (2016). Mimicry for all modalities. Ecol. Lett. 19, 609–619. doi: 10.1111/ele.12602
Davies, N. B. (2011). Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. doi: 10.1111/j.1469-7998.2011.00810.x
Davies, N. B., and Welbergen, J. A. (2008). Cuckoo-hawk mimicry? An experimental test. Proc. Biol. Sci. 275, 1817–1822. doi: 10.1098/rspb.2008.0331
Davies, N. B., and Welbergen, J. A. (2009). Social transmission of a host defense against cuckoo parasitism. Science 324, 1318–1320. doi: 10.1126/science.1172227
Davies, N. B., and Brooke, M. L. (1988). Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284. doi: 10.1016/S0003-3472(88)80269-0
de Jager, M. L., and Anderson, B. (2019). When is resemblance mimicry? Funct. Ecol. 33, 1586–1596. doi: 10.1111/1365-2435.13346
Duckworth, J. W. (1991). Responses of breeding reed warblers Acrocephalus scirpaceus to mounts of sparrowhawk Accipiter nisus, cuckoo Cuculus canorus and jay Garrulus glandarius. IBIS (Lond. 1859) 133, 68–74. doi: 10.1111/j.1474-919X.1991.tb04812.x
Duncan, C. J., and Sheppard, P. M. (1965). Sensory discrimination and its role in the evolution of batesian mimicry. Behaviour 24, 269–282. doi: 10.1163/156853965X00066
Feeney, W. E., Troscianko, J., Langmore, N. E., and Spottiswoode, C. N. (2015). Evidence for aggressive mimicry in an adult brood parasitic bird, and generalized defences in its host. Proc. R. Soc. B Biol. Sci. 282, 893–896. doi: 10.1098/rspb.2015.0795
Feeney, W. E., Welbergen, J. A., and Langmore, N. E. (2014). Advances in the study of coevolution between avian brood parasites and their hosts. Annu. Rev. Ecol. Evol. Syst. 45, 227–246. doi: 10.1146/annurev-ecolsys-120213-091603
Font, E. (2019). Mimicry, camouflage and perceptual exploitation: the evolution of deception in nature. Biosemiotics 12, 7–24. doi: 10.1007/s12304-018-9339-6
Gluckman, T. L., and Mundy, N. I. (2013). Cuckoos in raptors’ clothing: barred plumage illuminates a fundamental principle of Batesian mimicry. Anim. Behav. 86, 1165–1181. doi: 10.1016/j.anbehav.2013.09.020
Jamie, G. A. (2017). Signals, cues and the nature of mimicry. Proc. R. Soc. B Biol. Sci. 284:20162080. doi: 10.1098/rspb.2016.2080
Kazemi, B., Gamberale-Stille, G., Tullberg, B. S., and Leimar, O. (2014). Stimulus salience as an explanation for imperfect mimicry. Curr. Biol. 24, 965–969. doi: 10.1016/j.cub.2014.02.061
Kilner, R. M., and Langmore, N. E. (2011). Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. 86, 836–852. doi: 10.1111/j.1469-185X.2010.00173.x
Kim, H., Lee, J. W., and Yoo, J. C. (2017). Comparing vocal structures of the parasitic and nonparasitic groups in Cuculinae. Avian Res. 8, 1–5. doi: 10.1186/s40657-017-0084-3
Krama, T., and Krams, I. (2005). Cost of mobbing call to breeding pied flycatcher, Ficedula hypoleuca. Behav. Ecol. 16, 37–40. doi: 10.1093/beheco/arh116
Krüger, O., Davies, N. B., and Sorenson, M. D. (2007). The evolution of sexual dimorphism in parasitic cuckoos: sexual selection or coevolution? Proc. Biol. Sci. 274, 1553–1560. doi: 10.1098/rspb.2007.0281
Lawson, S. L., Enos, J. K., Antonson, N. D., Gill, S. A., and Hauber, M. E. (2021). Do hosts of avian brood parasites discriminate parasitic vs. predatory threats? A meta-analysis. Adv. Study Behav. 53, 63–95. doi: 10.1016/bs.asb.2021.03.002
Leavell, B. C., and Bernal, X. E. (2019). The cognitive ecology of stimulus ambiguity: a predator–prey perspective. Trends Ecol. Evol. 34, 1048–1060. doi: 10.1016/j.tree.2019.07.004
Liang, W. (2017). Crafty cuckoo calls. Nat. Ecol. Evol. 1, 1427–1428. doi: 10.1038/s41559-017-0321-5
Lima, S. L., and Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. doi: 10.1139/z90-092
Lyon, B., and Gilbert, G. (2013). Rarely parasitized and unparasitized species mob and alarm call to cuckoos: implications for sparrowhawk mimicry by brood parasitic cuckoos. Wilson J. Ornithol. 125, 627–630. doi: 10.1097/00006205-199508000-00001
Ma, L., Yang, C., and Liang, W. (2018). Hawk mimicry does not reduce attacks of cuckoos by highly aggressive hosts. Avian Res. 9, 1–7. doi: 10.1186/s40657-018-0127-4
Mark, M. M., and Rubenstein, D. R. (2013). Physiological costs and carry-over effects of avian interspecific brood parasitism influence reproductive tradeoffs. Horm. Behav. 63, 717–722. doi: 10.1016/j.yhbeh.2013.03.008
Marton, A., Fülöp, A., Bán, M., Hauber, M. E., and Moskát, C. (2021). Female common cuckoo calls dampen the mobbing intensity of great reed warbler hosts. Ethology 127, 286–293. doi: 10.1111/eth.13126
Moskát, C., and Hauber, M. E. (2019). Sex-specific responses to simulated territorial intrusions in the common cuckoo: a dual function of female acoustic signaling. Behav. Ecol. Sociobiol. 73:60. doi: 10.1007/s00265-019-2665-0
Moskát, C., Hansson, B., Barabás, L., Bártol, I., and Karcza, Z. (2008). Common cuckoo Cuculus canorus parasitism, antiparasite defence and gene flow in closely located populations of great reed warblers Acrocephalus arundinaceus. J. Avian Biol. 39, 663–671. doi: 10.1111/j.1600-048X.2008.04359.x
Payne, R. B. (1967). Interspecific communication signals in parasitic birds. Am. Nat. 101, 363–375. doi: 10.1086/282504
Peake, T. M. (2005). “Eavesdropping in communication networks,” in Animal Communication Networks, ed. P. K. Mcgregor (New York, NY: Cambridge University Press), 13–37.
Poulin, R. (2010). Parasite manipulation of host behavior: an update and frequently asked questions. Adv. Study Behav. 41, 151–186. doi: 10.1016/S0065-3454(10)41005-0
Ridley, A. R., Wiley, E. M., and Thompson, A. M. (2014). The ecological benefits of interceptive eavesdropping. Funct. Ecol. 28, 197–205. doi: 10.1111/1365-2435.12153
Römer, H., and Holderied, M. (2020). Decision making in the face of a deadly predator: high-amplitude behavioural thresholds can be adaptive for rainforest crickets under high background noise levels: decision rule in cricket bat avoidance. Philos. Trans. R. Soc. B Biol. Sci. 375:20190471. doi: 10.1098/rstb.2019.0471
Roncalli, G., Soler, M., Ruiz-Raya, F., Serrano-Martín, A. J., and Ibáñez-Álamo, J. D. (2019). Predation risk affects egg-ejection but not recognition in blackbirds. Behav. Ecol. Sociobiol. 73:56. doi: 10.1007/s00265-019-2668-x
Rothstein, S. I. (1990). A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 21, 481–508. doi: 10.1146/annurev.es.21.110190.002405
Ruxton, G. D., Allen, W. L., Sherratt, T. N., and Speed, M. P. (2018). Avoiding Attack: The Evolutionary Ecology Of Crypsis, Aposematism, And Mimicry, 2nd Edn. Oxford: Oxford University Press.
Shen, C., Yu, J., Li, X., Yue, J., Wang, H., and Liang, W. (2021). Responses of incubating females to female cuckoo calls in 2 hole-nesting bird species. Curr. Zool. 67, 565–567. doi: 10.1093/cz/zoab004
Shields, W. M. (1984). Barn swallow mobbing: self-defence, collateral kin defence, group defence, or parental care? Anim. Behav. 32, 132–148. doi: 10.1016/S0003-3472(84)80331-0
Smith, J. N. M., Arcese, P., and McLean, I. G. (1984). Age, experience, and enemy recognition by wild song sparrows. Behav. Ecol. Sociobiol. 14, 101–106. doi: 10.1007/BF00291901
Spottiswoode, C. N., and Stevens, M. (2010). Visual modeling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc. Natl. Acad. Sci. U.S.A. 107, 8672–8676. doi: 10.1073/pnas.0910486107
Starrett, A. (1993). Adaptive resemblance: a unifying concept for mimicry and crypsis. Biol. J. Linn. Soc. 48, 299–317. doi: 10.1016/0024-4066(93)90002-6
Stoddard, M. C., and Stevens, M. (2011). Avian vision and the evolution of egg color mimicry in the common cuckoo. Evolution (N. Y) 65, 2004–2013. doi: 10.1111/j.1558-5646.2011.01262.x
Šulc, M., Štìtková, G., Procházka, P., Požgayová, M., Sosnovcová, K., Studecký, J., et al. (2020). Caught on camera: circumstantial evidence for fatal mobbing of an avian brood parasite by a host. J. Vertebr. Biol. 69:1. doi: 10.25225/jvb.20027
Thorogood, R., and Davies, N. B. (2012). Cuckoos combat socially transmitted defenses of reed warbler hosts with a plumage polymorphism. Science 337, 578–580. doi: 10.1126/science.1220759
Thorogood, R., and Davies, N. B. (2013a). Hawk mimicry and the evolution of polymorphic cuckoos. Chinese Birds 4, 39–50. doi: 10.5122/cbirds.2013.0002
Thorogood, R., and Davies, N. B. (2013b). Reed warbler hosts fine-tune their defenses to track three decades of cuckoo decline. Evolution (N. Y) 67, 3545–3555. doi: 10.1111/evo.12213
Trnka, A., and Prokop, P. (2012). The effectiveness of hawk mimicry in protecting cuckoos from aggressive hosts. Anim. Behav. 83, 263–268. doi: 10.1016/j.anbehav.2011.10.036
Trnka, A., Prokop, P., and Grim, T. (2012). Uncovering dangerous cheats: how do avian hosts recognize adult brood parasites? PLoS One 7:e37445. doi: 10.1371/journal.pone.0037445
Trnka, A., Trnka, M., and Grim, T. (2015). Do rufous common cuckoo females indeed mimic a predator? An experimental test. Biol. J. Linn. Soc. 116, 134–143. doi: 10.1111/bij.12570
Wallace, A. R. (1889). Darwinism: an Exposition of the Theory of Natural Selection With Some of Its Applications. London: Macmillan.
Welbergen, J. A., and Davies, N. B. (2008). Reed warblers discriminate cuckoos from sparrowhawks with graded alarm signals that attract mates and neighbours. Anim. Behav. 76, 811–822. doi: 10.1016/j.anbehav.2008.03.020
Welbergen, J. A., and Davies, N. B. (2009). Strategic variation in mobbing as a front line of defense against brood parasitism. Curr. Biol. 19, 235–240. doi: 10.1016/j.cub.2008.12.041
Welbergen, J. A., and Davies, N. B. (2011). A parasite in wolf’s clothing: hawk mimicry reduces mobbing of cuckoos by hosts. Behav. Ecol. 22, 574–579. doi: 10.1093/beheco/arr008
Wiley, R. H. (2006). Signal detection and animal communication. Adv. Study Behav. 36, 217–247. doi: 10.1016/S0065-3454(06)36005-6
York, J. E., and Davies, N. B. (2017). Female cuckoo calls misdirect host defences towards the wrong enemy. Nat. Ecol. Evol. 1, 1520–1525. doi: 10.1038/s41559-017-0279-3
Keywords: adaptive resemblance, imperfect mimicry, eavesdropping, perception, predator-prey, mimicry, communication, cuckoo
Citation: York JE (2021) The Evolution of Predator Resemblance in Avian Brood Parasites. Front. Ecol. Evol. 9:725842. doi: 10.3389/fevo.2021.725842
Received: 15 June 2021; Accepted: 19 October 2021;
Published: 12 November 2021.
Edited by:
Brian Peer, Western Illinois University, United StatesReviewed by:
Daniela Campobello, University of Palermo, ItalyMominul Islam Nahid, Norwegian University of Science and Technology, Norway
Copyright © 2021 York. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer E. York, ankzNjRAY2FtLmFjLnVr
†Present address: Jennifer E. York Department of Zoology, University of Cambridge, Cambridge, United Kingdom
 Jennifer E. York
Jennifer E. York