
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 06 September 2021
Sec. Coevolution
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.725222
This article is part of the Research TopicEvolution and Function of Acoustic and Visual Signals in Avian Brood ParasitismView all 17 articles
Female common cuckoo (Cuculus canorous) predator-like “bubbling” calls distract host parental attention and reduce the egg rejection rate. Such “bubbling” calls are also frequently used to attract males and deter territorial rivals in intraspecies contact, and these calls are an ancestral character in many cuckoo species. Although hosts have had sufficient time to become familiar with this call and evolve anti-parasitic strategies, why are the hosts fooled by this “bubbling” call? We propose two hypotheses. The first hypothesis proposes that call variation reduces the opportunity for host species to correctly assess cuckoo tricks. In contrast, the second hypothesis proposes that the cost of behavior may prevent the antiparasitic strategy from evolving. In the study, we tested the prerequisites of these hypotheses, by investigating whether cuckoo calls vary during the day and testing whether the predator-like calls suppress bird activities. Based on field recordings from three different areas, we found high overlap in the calls generated during different periods. Oriental great reed warblers (Acrocephalus orientalis), a host species, did not show different responses toward the playback of female common cuckoo calls generated before noon or afternoon. Based on bird count data, we found that predator-like call playback is insufficient for suppressing bird activities. Therefore, none of the prerequisites were supported by our field data. We discuss the potential reasons for our findings and hope to inspire more research examining female cuckoo vocalizations.
The arms race between the common cuckoo (Cuculus canorous) and its hosts is a classic example of coevolution (Poulin and Forbes, 2012; Moksnes et al., 2013). Theoretical models suggest that both participants are locked in an arms race, and the outcomes depend on a series of strategies shaped by coevolution (Takasu, 1998, 2003; Soler, 2014). Common cuckoos have evolved numerous strategies to increase parasitism success, such as laying mimetic eggs (Honza et al., 2014; Yang et al., 2016, 2017), adjusting the timing of egg laying (Seel, 1973; Johnsgard, 1997; Wang et al., 2020) and mimicking hawk morphology (Welbergen and Davies, 2011; Gluckman and Mundy, 2013). Host species have also evolved various strategies to reduce the risk of parasitism, including the ability to discriminate cuckoo eggs (Lang et al., 2014), mobbing behavior (Ma et al., 2018), and unique alarm calls when cuckoos are in the vicinity of host nests (Yu et al., 2017).
The “bubbling” call of female common cuckoos is also considered a parasitic strategy to mimic hawks. This predator-like call diverts the attention of reed warbler (Acrocephalus scirpaceus) parents and reduces the egg rejection rate (York and Davies, 2017). Playback of this call can effectively suppress the mobbing intensity of great reed warblers (Acrocephalus arundinaceus) (Marton et al., 2021). Even unsuitable hosts can be deceived. York and Davies (2017) found blue tits (Cyanistes caeruleus) and great tits (Parus major) increased their vigilance after hearing female “bubbling” calls, similar to how they respond to sparrowhawk (Accipiter nisus) calls; Jiang et al. (2021) found that both female common cuckoo and sparrowhawk calls elicited vigilance and escape responses from chickens (Gallus gallus domesticus). Although both female common cuckoo calls and sparrowhawk calls consist of rapidly repeated elements (York and Davies, 2017), there are clear differences between them. For example, the maximum frequency of female common cuckoo calls is always less than 2.5 kHz, and the minimum frequency of sparrowhawk calls can exceed 2.5 kHz (Deng et al., 2019b). As there are clear differences between female common cuckoo “bubbling” calls and sparrowhawk calls, why are the hosts fooled by this “bubbling” call?
One possible reason is that the hosts have not had sufficient time to evolve the ability to distinguish female common cuckoo calls from predator calls. However, this explanation is unlikely, given that predator-like female calls are a common trait in at least four cuckoo species (Kim et al., 2017; Yoo et al., 2020), indicating that they are not recently evolved strategies. Another possibility is that hosts have not had sufficient opportunities to become familiar with female common cuckoo calls and distinguish them from predator calls. This hypothesis would be supported if female common cuckoos rarely use this predator-like call (e.g., using this call after parasitizing a host’s clutch) (York and Davies, 2017). However, female common cuckoos also use “bubbling” calls to attract males and deter territorial rivals when they fly or perch on branches (Deng et al., 2019b; Moskat and Hauber, 2019; Yoo et al., 2020). More than 90% of all calls occur during the morning rather than during the egg laying time in the afternoon (Gong et al., 2020). Thus, hosts have plenty of opportunities to become familiar with this mimetic call and discriminate it from the hawk calls.
In this study, we proposed two hypotheses concerning the “bubbling” call of female common cuckoos and tested the prerequisites of these hypotheses with field data. The first concerns call variation. Non-passeriform vocalizations are widely assumed to be simple and stereotyped and show little variation; for example, there is a high degree of consistency in the number of syllables (Møller et al., 2016, 2017) and call characteristics in individual male common cuckoo calls (Jung et al., 2014; Li et al., 2017; Zsebok et al., 2017). However, recent studies have revealed that individual male common cuckoo calls are more variable than previously thought (Deng et al., 2019a), and male common cuckoos can use these versatile vocalizations to encode different messages (Tryjanowski et al., 2018b; Xia et al., 2019; Moskat et al., 2021). Inspired by these observations, our first hypothesis is that female common cuckoos use different calls for interspecific and intraspecific communication. Specifically, characteristics differ between calls generated in the afternoon (i.e., when eggs are laid) and other periods. Consequently, host species may have little opportunity to become familiar with the female cuckoos’ call used to mimic hawks and discriminate it from the hawk calls. We used field recordings from three areas to test whether there were consistent differences among calls generated at different times and conducted playback experiments to test whether a host species, oriental great reed warbler (Acrocephalus orientalis), showed different responses to female common cuckoo “bubbling” calls broadcasted at different times.
The second hypothesis concerns the benefit and cost of host antiparasitic behavior. When the cost exceeds the benefit, the behavior should be eliminated (or not evolve) (Szalai and Szamado, 2009; Higham, 2014); for example, the cost of misidentification prevents cuckoo fledglings from being identified by parents in many host species (Lotem, 1993). The benefit of distinguishing female common cuckoo calls from predator calls is clear: hosts can use female common cuckoo calls as a predictor of parasitism risk and increase antiparasitic behavior (e.g., mobbing behavior) to reduce the risk of parasitism. However, the cost of this behavior is also obvious: the hosts may be killed once they misidentify predator calls as a female common cuckoo call. If the cost exceeds the potential benefit, it is better to treat any calls similar to predator calls as a potential predatory threat (Ruxton et al., 2004). If this is the truth, we predicted other predator-like calls, besides cuckoos “bubbling” call, can also influence bird activities. So, we played calls from a neutral bird, the little grebe (Tachybaptus ruficollis), whose calls consist of rapidly repeated elements and had a similar structure as predator calls/female cuckoo calls. As prey birds escape or remain silent after hearing predator calls (Akçay et al., 2016; Santema et al., 2019), we predicted that fewer bird species should be observed after playback.
To compare call characteristics, sound recordings were collected by passive acoustic recorder Songmeters (Wildlife Acoustics Inc., United States) from Liaohe Delta Nature Reserve (41.034°N, 121.725°E), Wild Duck Lake (40.417°N, 115.850°E) and Dagangzi National Forest (43.617°N, 126.133°E), China. Reed-bed habitat is the dominant habitat type at both the Liaohe Delta Nature Reserve and Wild Duck Lake; and Dagangzi National Forest consists of natural secondary forest. The common cuckoo predominantly parasitizes the oriental great reed warbler in both Liaohe Delta Nature Reserve (Li et al., 2016) and Wild Duck Lake, and parasitizes many forest birds in Dagangzi National Forest, such as Daurian redstarts (Phoenicurus auroreus) (Zhang et al., 2021). In Liaohe Delta Nature Reserve, 10 recorders were used from June 28th to July 29th, 2018; in Wild Duck Lake, 10 recorders were used from May 7th to July 8th, 2017; in Dagangzi National Forest, 8 recorders were used from May 17th to July 10th, 2018. Recorders were attached to trees or telegraph poles at a height of 3 m above ground and were set to record continuously at a sampling rate of 44.1 kHz and a sampling accuracy of 16 bits. The adjacent recorders were separated by a minimum distance of 200 m to avoid the same call from being recorded by two recorders. Recorders were checked approximately every 10 days to replace batteries and memory cards. A total of 7,200, 14,640, and 12,720 h of recordings were collected from Liaohe Delta Nature Reserve, Wild Duck Lake and Dagangzi National Forest, respectively.
Kaleidoscope software (Wildlife Acoustics Inc., United States) was used to automatically select female common cuckoo calls from the sound recordings. First, we entered the following acoustic features of our target sound (female calls) to create a recognizer: the frequency ranged from 600 to 2900 Hz, and the duration ranged from 1.6 to 4 s. These acoustic features were slightly larger than the actual parameters of female common cuckoo calls, but this was done to increase the detectability of calls by the software. We then manually checked all calls identified by the recognizer based on listening and visual inspection of the spectrograms. In total, we obtained 1,222, 1,431, and 124 female calls from Liaohe Delta Nature Reserve, Wild Duck Lake and Dagangzi National Forest, respectively (Gong et al., 2020).
All female common cuckoo call recordings were extracted and resampled at 22.05 kHz. Avisoft software (Avisoft Bioacoustics, Germany) was used to generate spectrograms with the following settings: fast Fourier transform length 256 points; Hamming window with a frame size of 100% and an overlap of 50%; frequency resolution 86 Hz; and time resolution of 5.8 ms. Female common cuckoo calls consist of a series of rapidly repeated “kwik-kwik-kwik” notes (York and Davies, 2017). Each “kwik” note represents a continuous trace on the spectrogram. For each call, the maximum frequency, minimum frequency, duration, and the number of notes were measured (Figure 1A). For each hour, 10 randomly selected calls, or all calls (if fewer than 10 calls within this hour), were measured. A total of 118, 113, and 84 calls were measured from Liaohe Delta Nature Reserve, Wild Duck Lake and Dagangzi National Forest, respectively. The data are shown in Supplementary Appendix 1.
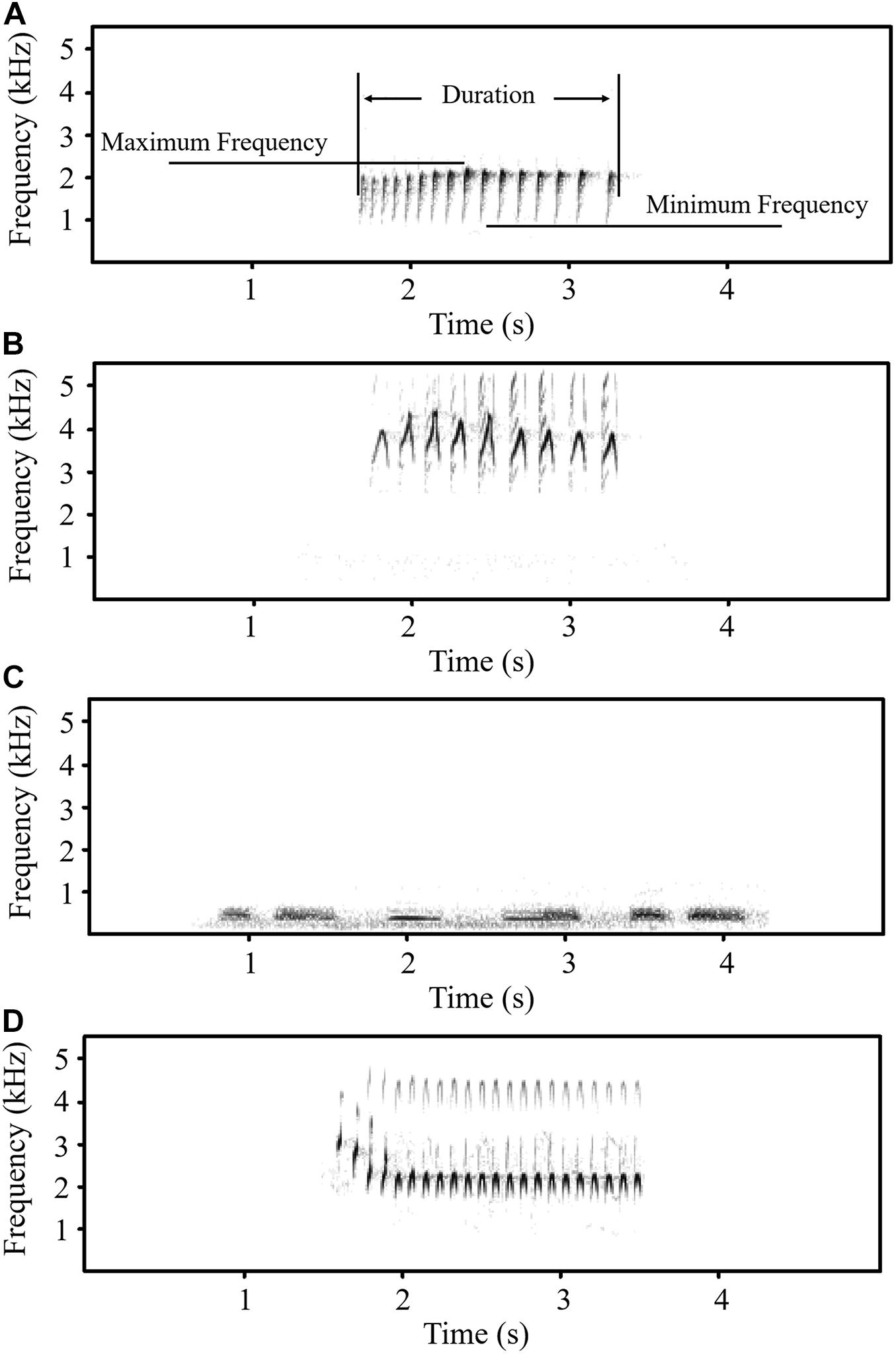
Figure 1. Spectrogram of female common cuckoo “bubbling” call (A), common kestrel call (B), oriental turtle dove call (C), and little grebe call (D).
To check whether host bird oriental great reed warblers can discriminate female common cuckoo “bubbling” calls generated in the morning or afternoon, these experiments were conducted at Wild Duck Lake on July 15th and 21st, 2021. The common cuckoo predominantly parasitizes oriental great reed warblers in this reed-bed habitat. The experiments date was near the end of breeding season, however, both common cuckoos and oriental great reed warblers were still active, frequently uttering calls or continuously singing high-pitched song.
Nine “bubbling” calls generated before noon and nine “bubbling” calls generated after noon were randomly selected from the 113 calls that were measured and recorded in the same area to create playback sounds. Calls from common kestrels (Falco tinnunculus) (Figure 1B), a common predator species in the study area, and oriental turtle doves (Streptopelia orientalis) (Figure 1C), a harmless bird, were used as a positive control and negative control, respectively. For common kestrels and oriental turtle doves, recordings from 9 individuals in each species were downloaded from Xeno-Canto1 (Supplementary Appendix 2), and one call in each individual was used to generate the playback sound. In the playback sounds, the rate was adjusted to 1 call per 10 s. A total of four 90-s playback sounds were generated, and two 15-s breaks were inserted after 30 and 60 s to generate 2-min playback sounds. The rhythm of these sounds was similar to the playback sounds used in a previous study (Marton et al., 2021).
The sounds were played by a loudspeaker (E1; SMH Company, China), with the amplitude set to approximately 85 dB measured at 1 m with a sound level meter (NL-20; Rion Company, Japan). Playback experiments were conducted in the close vicinity of a singing oriental great reed warbler. The loudspeaker was positioned within 10 m from the target oriental great reed warbler, which was singing and always perched on the middle and upper parts of reeds. As target individuals were not banded, playback experiments were conducted at least 50 m apart to avoid repeated sampling from the same individual. There were 32 oriental great reed warblers involved in the playback experiments. These 32 individuals were randomly divided into four equal-sized groups, corresponding to the above four categories of acoustics used in the playback. For each individual, only one 2-min long sound was played. The observers with binoculars and stopwatches recorded whether and when the target individual stopped singing or flew away during the 2 min of playback. Some additional behavioral variables, such as scanning the surroundings, also reflect vigilance (York and Davies, 2017), but these behaviors were not recorded because of the difficulties in observing such behaviors in the dense vegetation used by the birds.
To test whether bird activities are suppressed by predator-like calls, these experiments were conducted at Xiaolongmen National Forest Park (40.017°N, 115.467°E), China, from May 24th to 28th, 2021. This area consists of secondary temperate deciduous broad-leaf forest. We played calls from female common cuckoos (Figure 1A), common kestrels (Figure 1B), oriental turtle doves (Figure 1C), and little grebes (Figure 1D). Oriental turtle doves are resident birds in the study area, and their call was used as a control. Both common kestrels and common cuckoos are breeding birds in the study area, and little grebes do not breed in this forest park. We used common kestrel calls rather than sparrowhawk calls as in previous studies (York and Davies, 2017; Xia et al., 2019; Jiang et al., 2021) because common kestrel is a more common predator than sparrowhawk in the study area based on our observations. The calls from common kestrels, female common cuckoos, and little grebes have a similar structure and consist of rapidly repeated elements (Figure 1).
As we did not collect enough recordings in the study area, we used acoustic files from Xeno-Canto (see footnote 1, Supplementary Appendix 2) to create playback sounds. For female common cuckoo calls, we did not distinguish the calls generated before or after noon because our aim in these experiments was to test whether bird activities were suppressed by any call types similar to predator calls rather than compare differences between calls generated at different periods. In addition, the acoustic characteristics highly overlapped in the calls generated before and after noon (seen in the Results). Recordings from 9 individuals were downloaded for each species, and one call from each individual was used. Similar to a previous study (Marton et al., 2021), the rate was adjusted to 1 call per 10 s, and then two 15-s breaks were inserted after 30 and 60 s, generating four 2-min long playback sounds.
The sounds were played by a loudspeaker (E1; SMH Company, China), with the amplitude set to approximately 85 dB measured at 1 m with a sound level meter (NL-20; Rion Company, Japan). Point counts were conducted at a total of 100 sites after playback. Sites were randomly divided into four equal-sized groups, corresponding to the above four categories of acoustics used in the playback. At each site, the loudspeaker was placed at approximately 1 m of a tree branch. The observers with binoculars were positioned approximately 10 m from the loudspeaker, and all bird species heard during a 3-min period within 30 m of the loudspeaker after playback were recorded. A 30-m radius was used because birds outside that distance were barely detected by the observers in the forest. As we had no prior information regarding an appropriate timeframe to make observations, our pragmatic solution was to choose a 3-min period for observation, not too long (exceeding efficient time) or too short (few birds were observed). Although birds were not individually ringed, the probability of counting an individual twice was very low, as two successive sites were separated by at least 200 m. Moreover, all point counts were conducted over a relatively short period during the breeding season to avoid the effect of season.
Female common cuckoos generated calls from 3:00 to 20:00 in the study area, and the peak call output occurred during the morning (Gong et al., 2020). The calls were divided into two categories: calls generated before 12:00 and after 12:00. This division is based on the egg laying time by Common Cuckoos: 90% of egg laying occurred from 12:00 to 20:00 (Seel, 1973; Wang et al., 2020). As female Common Cuckoos give calls after parasitizing a host’s clutch to divert host attention away from the clutch (York and Davies, 2017), we assumed that calls generated in the afternoon were mainly for interspecific communication (i.e., misdirect host defenses), and calls generated before the afternoon were for intraspecific communication (e.g., attract males and deter territorial rivals). We admit that this division is overly simplistic and somewhat arbitrary, as intraspecific calls could also occur in the afternoon, especially when cuckoos chase each other.
Multivariate analysis of variance (MANOVA) was used to assess the overall differences in the call characteristics between different periods (i.e., before or after noon) and among areas, followed by analysis of variance (ANOVA) to assess each individual call characteristic if there was a significant difference detected by MANOVA. Principal component analysis (PCA) with varimax rotation was used to compress the original variables into independent principal components, and discriminant function analysis (DFA) was used to determine whether calls generated before or after noon could be successfully split. The results from leave-one-out cross validation are reported as percentages of recordings correctly assigned in DFA.
For playback to oriental great reed warblers, birds that stopped singing or flew away during the 2 min of playback were scored as “response,” and birds that continued to sing were scored as “no response.” In two cases, the birds flew and approached the loudspeaker and then sang, and these two instances were also scored as “no response.” Logistic regression was used to test whether “response” was affected by the time when the experiments were conducted. After confirming that there was no temporal effect, Fisher’s exact test was used to compare the frequency of “response” among the four playback sounds (female common cuckoo calls generated before noon, female common cuckoo calls generated after noon, common kestrel calls, and oriental turtle dove calls).
For bird survey data, linear regression was used to test whether the number of species or number of individuals was affected by the time when experiments were conducted. After confirming that there was no temporal effect, both the number of species and the number of individuals were compared by ANOVA among the four groups (played calls from female common cuckoos, common kestrels, oriental turtle doves, and little grebes), followed by Tukey’s test for post hoc pairwise multiple comparisons if there was a significant difference found in ANOVA.
All analyses were performed using SPSS 21.0 (IBM Corporation, United States). p values < 0.05 were considered statistically significant.
Our recordings revealed significant differences in call characteristics between periods (MANOVA: Pillai’s Trace = 0.04, F4,308 = 2.80, p = 0.026) and among areas (MANOVA: Pillai’s Trace = 0.36, F8,618 = 16.91, p < 0.001) (Figure 2). Specifically, calls generated before noon were longer in duration (ANOVA: F1,311 = 8.10, p = 0.005) than calls generated after noon, and there was no significant difference in other variables between different periods: maximum frequency (ANOVA: F1,311 = 3.68, p = 0.056), minimum frequency (ANOVA: F1,311 = 0.09, p = 0.763), and number of notes (ANOVA: F1,311 = 1.87, p = 0.173). For DFA, only 58.5, 60.2, and 61.9% of calls could be correctly classified into different periods in Liaohe Delta Nature Reserve, Wild Duck Lake and Dagangzi National Forest, respectively. There was high overlap in the calls between different periods based on the two principal components (with eigenvalues larger than 1) (Figure 3), and there was no clear timeline separating calls into different categories based on the measured acoustic variables (Figure 4).
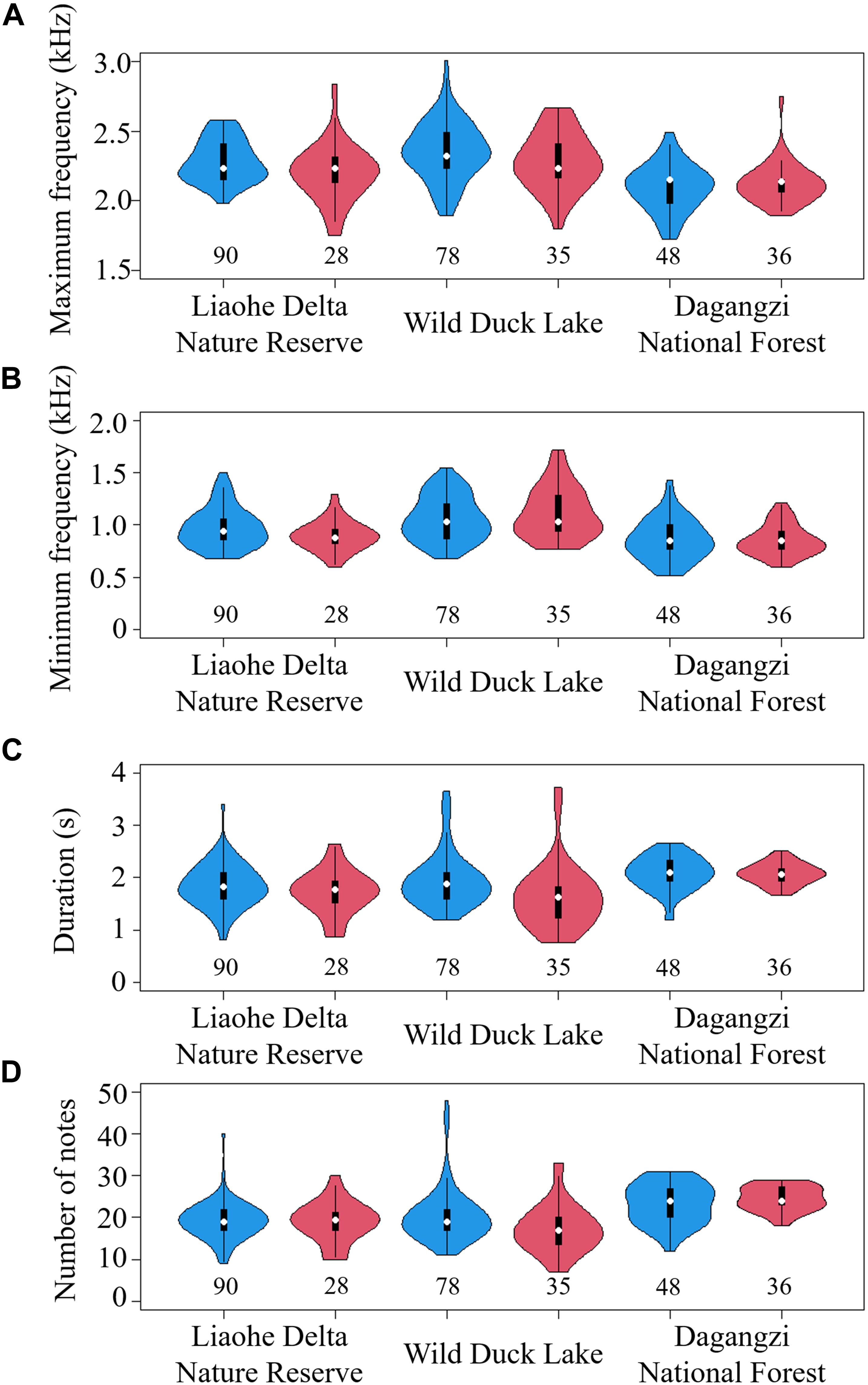
Figure 2. Call characteristics between periods and among areas: maximum frequency (A), minimum frequency (B), duration (C), and number of notes (D). The blue violins indicate calls recorded before 12:00; the red violins indicate calls recorded after 12:00. Sample sizes are shown below each violin. In the middle of each density curve is a box plot, with black rectangle showing the ends of the first and third quartiles and white dot the median.
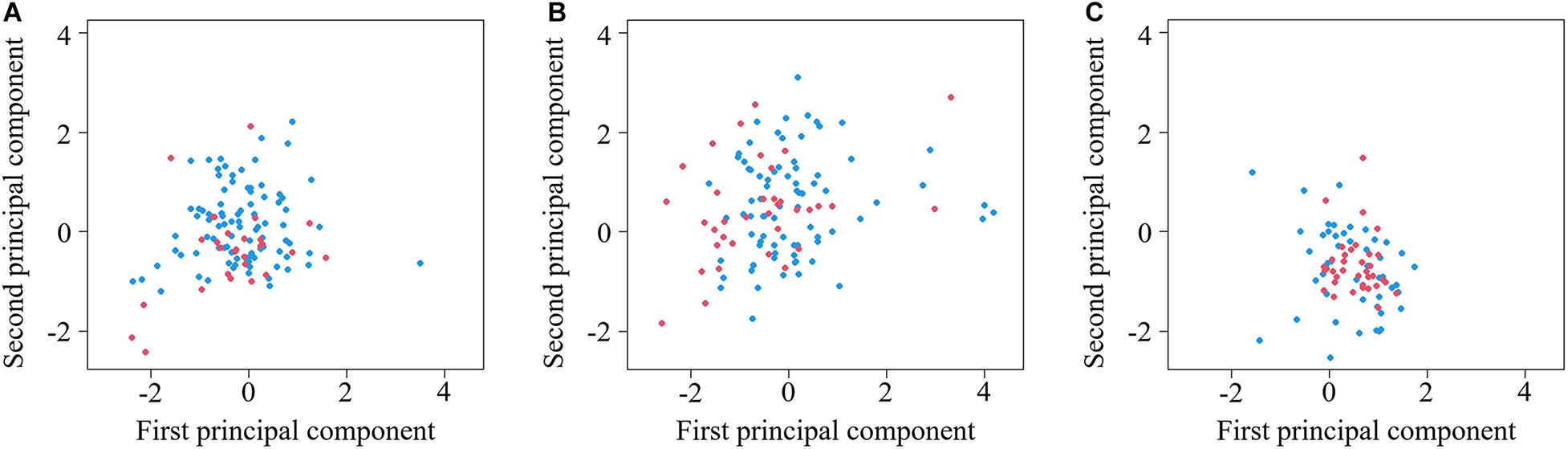
Figure 3. Female common cuckoo “bubbling” calls highly overlapped between different periods in Liaohe Delta Nature Reserve (A), Wild Duck Lake (B), and Dagangzi National Forest (C). The blue points indicate calls recorded before 12:00; the red points indicate calls recorded after 12:00.
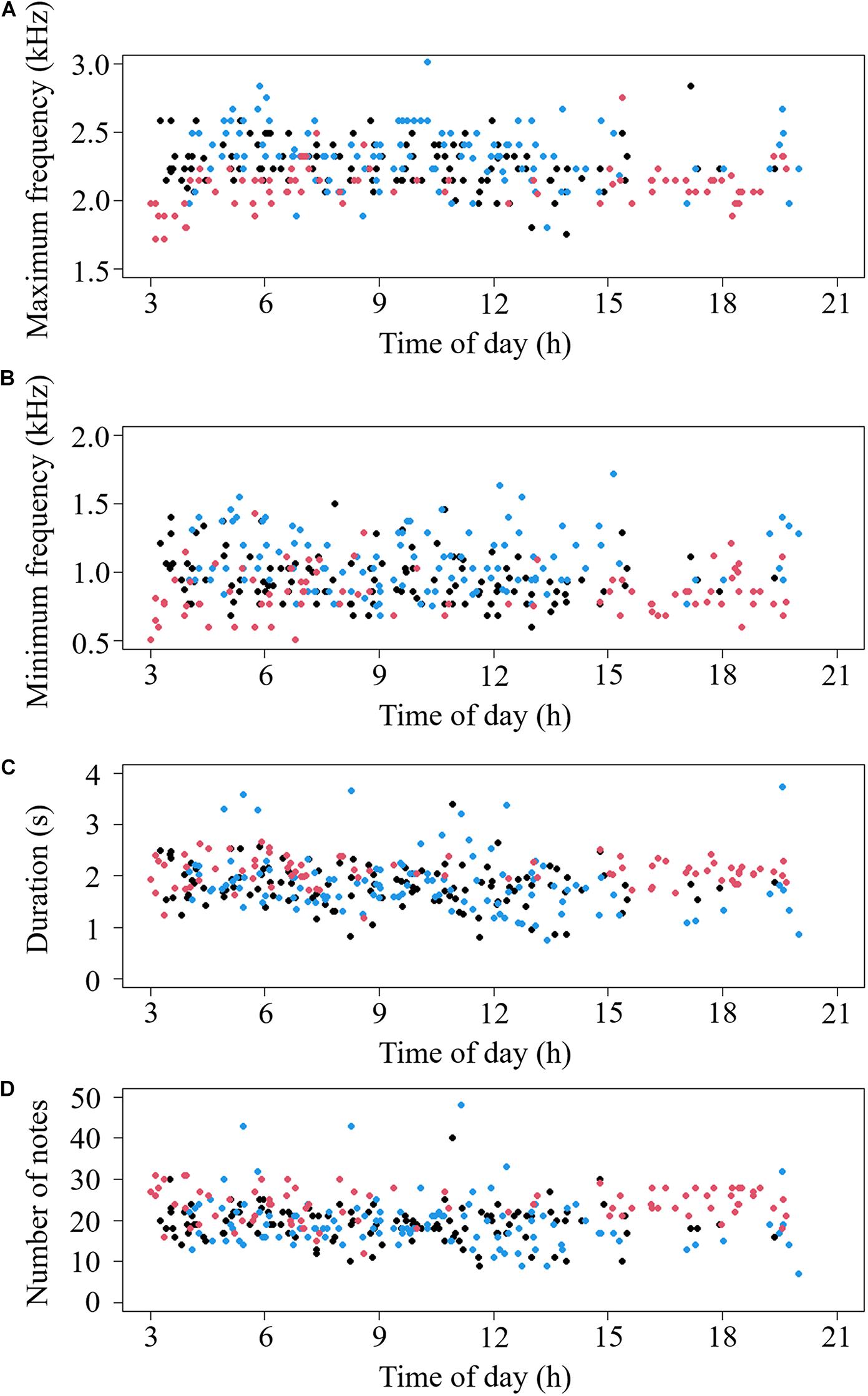
Figure 4. Characteristics of female common cuckoos “bubbling” calls plotted against time of day: maximum frequency (A), minimum frequency (B), duration (C), and number of notes (D). The black points indicate calls from Liaohe Delta Nature Reserve; the blue points indicate calls from Wild Duck Lake; and the red points indicate the calls from Dagangzi National Forest.
Nearly all oriental great reed warblers kept singing without flying during the playback of female common cuckoo calls generated before or after noon (Supplementary Appendix 3). Only two individuals flew and approached the loudspeaker at 52 and 57 s, respectively. One of these two individuals quickly began to sing at the new location, and another stayed at the new location for 15 s without singing and then flew back to the original location and sang again. During the playback of the common kestrel call, 3 individuals flew away at 12, 35, and 37 s; 1 individual stopped singing at 46 s and then flew away at 68 s; 1 individual stopped singing at 62 s, and then generated alarm calls without flying; and another 3 individuals continued to sing. During the playback of oriental turtle dove calls, all 8 individuals continued to sing and did not fly. There was no temporal effect on the response of birds to playback (logistic regression: odds ratio = 2.00, χ = 0.17, p = 0.678). Common kestrel calls increased the response probability (stop singing or fly away) compared with the other three groups (Fisher’s exact test: p = 0.026), and there were no differences observed among the other three groups (female common cuckoo calls generated before noon, female common cuckoo calls generated after noon, and oriental turtle dove calls).
A total of 33 species were heard after playback at all 100 sites (Supplementary Appendix 4). There were 3.10 ± 0.17 (mean ± standard error) species and 3.53 ± 0.20 individuals heard at each site. The number of species and the number of individuals were strongly positively correlated (Pearson correlation: r = 0.93, p < 0.001). There was no temporal effect on either the number of species (linear regression: coefficient = 0.46, F1,98 = 0.13, p = 0.716) or the number of individuals (linear regression: coefficient = −0.14, F1,98 = 0.01, p = 0.923). There was no significant difference in either the number of species (ANOVA: F3,96 = 0.44, p = 0.728) (Figure 5A) or the number of individuals (ANOVA: F3,96 = 0.38, p = 0.769) (Figure 5B) heard after the different playback sounds.
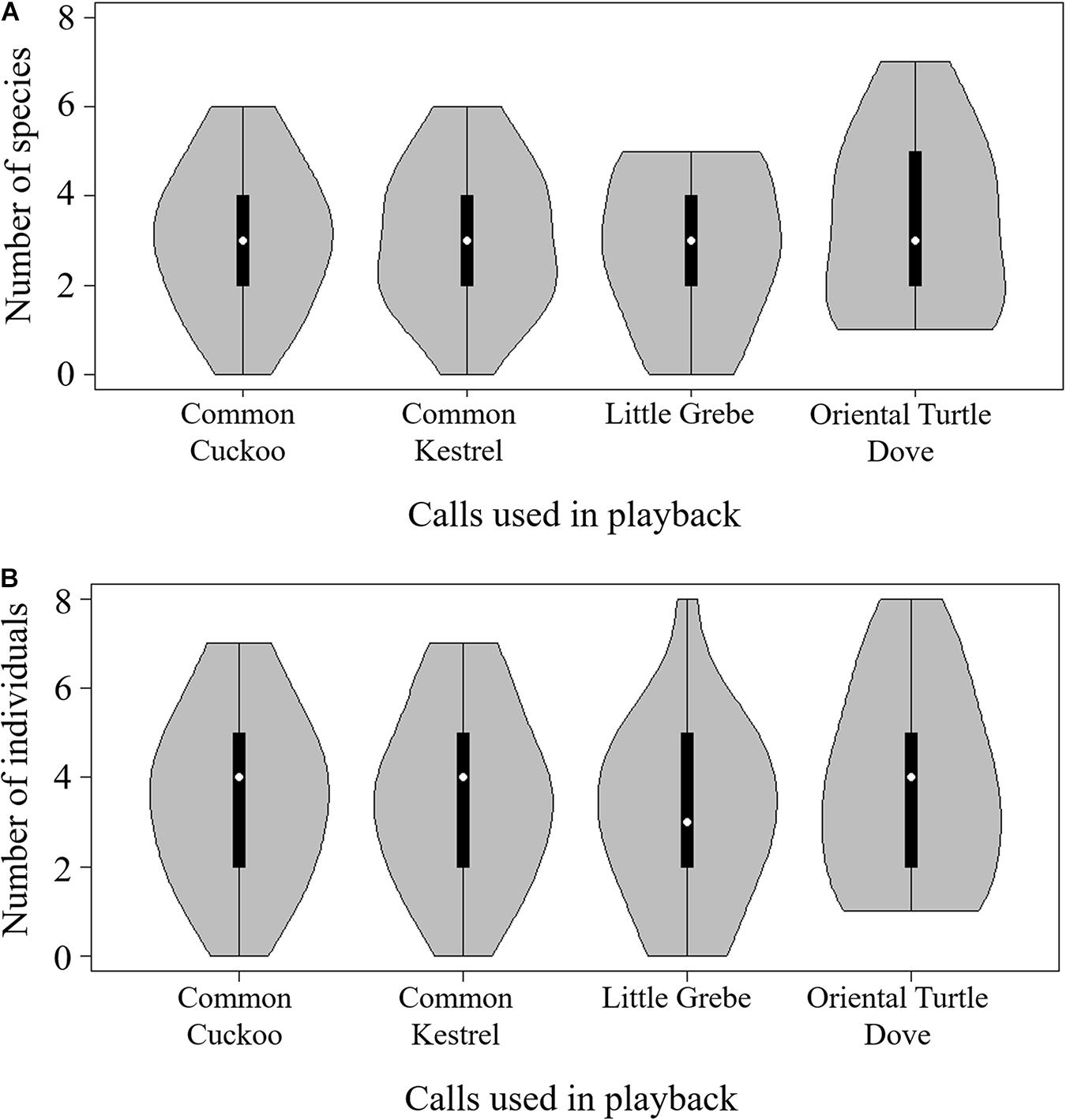
Figure 5. Number of species (A) and individuals (B) heard after playback. Sample sizes for each group is 25. In the middle of each density curve is a box plot, with black rectangle showing the ends of the first and third quartiles and white dot the median.
Common cuckoos and their hosts are an excellent system for studying coevolutionary arms races (Poulin and Forbes, 2012; Moksnes et al., 2013). Various strategies are adopted by cuckoos and their hosts. Recently, the “bubbling” call of female common cuckoos has been included in the long list of cuckoo tricks after the pioneering work by York and Davies (2017). This “bubbling” call type is frequently used when female common cuckoos fly or perch on branches (Deng et al., 2019b; Moskat and Hauber, 2019), and it is an ancestral character in many cuckoo species (Kim et al., 2017; Yoo et al., 2020). Thus, hosts have had sufficient opportunities to become familiar with this call and to evolve anti-parasitic strategies. Why are hosts still fooled by this “bubbling” call? We propose two hypotheses. The first concerns call variations in female common cuckoo and the second concerns the cost of antiparasitic behavior. In the study, we tested the prerequisites of these hypotheses, by investigating “bubbling” call variation and bird activities in predator-like calls playback. Based on the field recordings from three different areas, we found that there is a high degree of overlap in the calls generated between different periods. Oriental great reed warblers, a host species, did not vary in their responses to playback of female common cuckoo calls generated before noon or after noon. Based on the bird count data, we found that predator-like call playback is insufficient for suppressing bird activities. Therefore, none of these prerequisites are supported by our field data.
Although non-Passeriformes vocalizations are generally simple and stereotyped, many non-Passeriformes can use versatile vocalizations to encode different messages. For example, corncrakes (Crex crex) are known to express different levels of aggressive motivation through different call types (Rek and Osiejuk, 2011); African penguins (Spheniscus demersus) use four vocal categories under different circumstances (Favaro et al., 2014); and male ural owls (Strix uralensis) use different calls for territorial advertisement and for duetting with females (Zhou et al., 2020). For female common cuckoos, there are clear benefits, at least in theory, for separating call types corresponding to interspecific or intraspecific functions. The elaborate vocalizations may increase the probability that host species are fooled by cuckoos as well as the stimulation of sensory perception (Akre and Johnsen, 2014; Cui et al., 2016), which can reduce habituation in the distraction of host attention. However, our recordings do not support this idea. The acoustic characteristics largely overlapped between calls generated before or after noon, and DFA could not distinguish calls based on the measured characteristics. In this study, we split the calls based on time rather than interspecific or intraspecific functions. We admit that this division is overly simplistic, as many intraspecific calls can be mixed with interspecific calls into the afternoon group. However, we do not think the conclusions would be changed if other criteria were used to divide the calls. As the number of calls in the afternoon is quite low compared with the number of calls in the morning, we checked almost all afternoon calls by listening and visually inspecting the spectrograms when we measured the acoustic characteristics. We did not find any distinctive calls that are specially used for host species (e.g., after parasitizing a host’s clutch).
For oriental great reed warblers, a host species in the study area, the induced behaviors during playback were not affected by whether female calls were generated before or after noon. Similar behavior indicates that there was no difference between calls generated in different periods, at least for oriental great reed warblers. This result is consistent with the acoustic analysis based on the call characteristics. Another interesting finding is that an interspecific function of female common cuckoo “bubbling” calls was not supported in the study, as the induced vigilance during cuckoo call playback was similar to that of the negative control (oriental turtle dove calls) and significantly lower than that of the positive control (common kestrel calls). This finding contradicts the findings of previous research (York and Davies, 2017; Jiang et al., 2021; Marton et al., 2021). The target species in previous studies always remained in an open area in the beginning of the experiments. For example, York and Davies (2017) presented playbacks to tits at experimental feeders; Jiang et al. (2021) presented playbacks to domestic chickens inhabiting open areas; Marton et al. (2021) started to broadcast playbacks after great reed warblers showed mobbing behavior to a cuckoo decoy. In this study, target individuals were perched on dense reeds. Thus, oriental great reed warblers might reduce their vigilance because of the shelter of dense reeds. Another possibility we could not rule out is that oriental great reed warblers respond to female cuckoo calls in some subtle ways, for example, through changes in posture and heart rate during playback.
The second hypothesis we proposed concerns the cost of host antiparasitic behavior. Theoretically, if costs exceed benefits, the behavior should be eliminated or not evolve at all (Szalai and Szamado, 2009; Higham, 2014). The cost of ignoring a predator can result in death (Creel and Christianson, 2008; Lima, 2009). Therefore, natural selection may favor prey species that can detect predatory cues (Ruxton et al., 2004). We counted bird species and the number of individuals after playback predator-like calls. We predicted that fewer birds would be observed after playback because bird activity would be suppressed (e.g., escaping, remaining silent) after hearing predator calls (Akçay et al., 2016; Santema et al., 2019). However, this prediction was not supported by our data: we did not find fewer species or number of individuals after playing back calls of female common cuckoos, common kestrels, and little grebes compared with the control (oriental turtle dove calls). These negative results may not stem from a lack of statistical power. If there was a medium effect of predator-like calls with a 0.3 effect size, as suggested by Cohen (1988), the power of ANOVA with 25 data points in each of the four groups could reach 0.69 (calculated by the “pwr” package in R software). This 0.69 statistical power is greater than the power of approximately 0.44 in most animal behavior studies (Jennions and Møller, 2002). Another possible reason for the negative result is that acoustic signals alone are insufficient for stimulating the prey response (Randler and Randler, 2020). Taxidermic models could be used in future studies to create a more realistic environment (Zachau and Freeberg, 2012; Tryjanowski et al., 2018a).
Acoustic signals play a key role in modifying bird behavior (Todt and Naguib, 2000; Slater, 2003). Recent research has shown that acoustic signals are involved in the arms race between common cuckoos and their hosts (York and Davies, 2017; Jiang et al., 2021; Marton et al., 2021). Female common cuckoos frequently use “bubbling” calls for both interspecific and intraspecific functions during the breeding season (Deng et al., 2019b; Moskat and Hauber, 2019; Yoo et al., 2020). The starting point of our study is the question: Why are the hosts fooled by this “bubbling” call? Two hypotheses based on call variations and the cost of antiparasitic behavior were proposed, and the prerequisites of these hypotheses were tested. None of these prerequisites are supported by our field recordings and playback experiments. More studies should be conducted to broaden our understanding of the vocalization of female cuckoos.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The animal study was reviewed and approved by the research protocol was approved by the Animal Management Committee at the College of Life Sciences, Beijing Normal University, under license number CLS-EAW-2016-017.
CX conceived and designed the study. MT, JL, XL, and CX performed fieldwork. YW and CX analyzed the data. CX and AM wrote the manuscript with assistance from MT and YW. All authors reviewed and approved the final manuscript.
This work was supported by the 111 Project (B13008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared past co-authorships with one of the authors, AM.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.725222/full#supplementary-material
Supplementary Appendix 1 | Call characteristics in female common cuckoos.
Supplementary Appendix 2 | The following recordings were downloaded from Xeno-Canto (http://www.xeno-canto.org) and used in the playback calls: : 138120, 181351, 466764, 479827, 53403, 550539, 557084, 557087, 605602 for female common cuckoos; 142344, 264925, 300296, 366079, 377557, 388806, 431372, 509603, 590630 for common kestrels; 120409, 19795, 285728, 286084, 286091, 409198, 426927, 473070, 491550 for oriental turtle doves; and 100940, 183440, 268729, 26929, 377058, 42554, 510716, 596757, 648836 for little grebes.
Supplementary Appendix 3 | Oriental great reed warbler responses during call playback.
Supplementary Appendix 4 | Bird count data after call playback.
Akçay, Ç, Clay, A., Campbell, S. E., and Beecher, M. D. (2016). The sparrow and the hawk: aggressive signaling under risk of predation. Behav. Ecol. 27, 601–607. doi: 10.1093/beheco/arv196
Akre, K. L., and Johnsen, S. (2014). Psychophysics and the evolution of behavior. Trends Ecol. Evol. 29, 291–300. doi: 10.1016/j.tree.2014.03.007
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences, 2nd Edn. Hillsdale, NJ: Lawrence Erlbaum Associates.
Creel, S., and Christianson, D. (2008). Relationships between direct predation and risk effects. Trends Ecol. Evol. 23, 194–201. doi: 10.1016/j.tree.2007.12.004
Cui, J., Song, X., Zhu, B., Fang, G., Tang, Y., and Ryan, M. J. (2016). Receiver discriminability drives the evolution of complex sexual signals by sexual selection. Evolution 70, 922–927. doi: 10.1111/evo.12889
Deng, Z., Lloyd, H., Xia, C., Li, D., and Zhang, Y. (2019a). Within-season decline in call consistency of individual male Common Cuckoos (Cuculus canorus). J. Ornithol. 160, 317–327. doi: 10.1007/s10336-019-01631-4
Deng, Z., Lloyd, H., Xia, C., Møller, A. P., Liang, W., and Zhang, Y. (2019b). Components of variation in female Common Cuckoo calls. Behav. Process. 158, 106–112. doi: 10.1016/j.beproc.2018.10.007
Favaro, L., Ozella, L., and Pessani, D. (2014). The vocal repertoire of the african penguin (Spheniscus demersus): structure and function of calls. PLoS One 9:e103460. doi: 10.1371/journal.pone.0103460
Gluckman, T. L., and Mundy, N. I. (2013). Cuckoos in raptors’ clothing: barred plumage illuminates a fundamental principle of Batesian mimicry. Anim. Behav. 86, 1165–1181. doi: 10.1016/j.anbehav.2013.09.020
Gong, Y., Wang, M., Zhou, B., Deng, Z., He, Y., and Xia, C. (2020). Daily vocal pattern of female Common Cuckoo (Cuculus canorus) (in Chinese with an abstract in English). Chin. J. Zool. 55, 560–565.
Higham, J. P. (2014). How does honest costly signaling work? Behav. Ecol. 25, 8–11. doi: 10.1093/beheco/art097
Honza, M., Sulc, M., Jelínek, V., Pozgayová, M., and Procházka, P. (2014). Brood parasites lay eggs matching the appearance of host clutches. Proc. R. Soc. B Biol. Sci. 281, 1–7.
Jennions, M. D., and Møller, A. P. (2002). A survey of the statistical power of research in behavioral ecology and animal behavior. Behav. Ecol. 14, 438–445. doi: 10.1093/beheco/14.3.438
Jiang, X., Zhang, C., Liu, J., and Liang, W. (2021). Female cuckoo calls elicit vigilance and escape responses from wild free-range chickens. Ethol. Ecol. Evol. 33, 37–48. doi: 10.1080/03949370.2020.1792557
Johnsgard, P. A. (1997). The Avian Brood Parasites: Deception at the Nest. New York, NY: Oxford University Press.
Jung, W. J., Lee, J. W., and Yoo, J. C. (2014). “cu-coo”: can you recognize my stepparents? A study of host-specific male call divergence in the Common Cuckoo. PLoS One 9:e90468. doi: 10.1371/journal.pone.0090468
Kim, H., Lee, J. W., and Yoo, J. C. (2017). Characteristics of female calls of four Cuculus species breeding in Korea. Korean J. Ornithol. 24, 1–7.
Lang, A. K., Bollinger, E. K., and Peer, B. D. (2014). Effect of parasite-to-host egg ratio on egg rejection by a brown-headed cowbird host. Auk 131, 694–701. doi: 10.1642/auk-14-28.1
Li, D., Ruan, Y., Wang, Y., Chang, A., Wan, D., and Zhang, Z. (2016). Egg-spot matching in Common Cuckoo parasitism of the oriental reed warbler: effects of host nest availability and egg rejection. Avian Res. 7, 199–209.
Li, Y., Xia, C., Lloyd, H., Li, D., and Zhang, Y. (2017). Identification of vocal individuality in male cuckoos using different analytical techniques. Avian Res. 8, 134–140.
Lima, S. L. (2009). Predators and the breeding bird: behavioural and reproductive flexibility under the risk of predation. Biol. Rev. 84, 485–513. doi: 10.1111/j.1469-185x.2009.00085.x
Lotem, A. (1993). Learning to recognize nestlings is maladaptive for cuckoo Cuculus canorus hosts. Nature 362, 743–746.
Ma, L., Yang, C., and Liang, W. (2018). Hawk mimicry does not reduce attacks of cuckoos by highly aggressive hosts. Avian Res. 9:35. doi: 10.1186/s40657-018-0127-4
Marton, A., Fülop, A., Ban, M., Hauber, M. E., and Moskat, C. (2021). Female Common Cuckoo calls dampen the mobbing intensity of great reed warbler hosts. Ethology 127, 286–293. doi: 10.1111/eth.13126
Moksnes, A., Fossøy, F., Røskaft, E., and Stokke, B. G. (2013). Reviewing 30 years of studies on the Common Cuckoo accumulated knowledge and future perspectives. Chin. Birds 4, 3–14. doi: 10.5122/cbirds.2013.0001
Møller, A. P., Morelli, F., Mousseau, T. A., and Tryjanowski, P. (2016). The number of syllables in Chernobyl cuckoo calls reliably indicate habitat, soil and radiation levels. Ecol. Indic. 66, 592–597. doi: 10.1016/j.ecolind.2016.02.037
Møller, A. P., Morelli, F., and Tryjanowski, P. (2017). Cuckoo folklore and human well-being: cuckoo calls predict how long farmers live. Ecol. Indic. 72, 766–768. doi: 10.1016/j.ecolind.2016.09.006
Moskat, C., and Hauber, M. E. (2019). Sex-specific responses to simulated territorial intrusions in the Common Cuckoo: a dual function of female acoustic signaling. Behav. Ecol. Sociobiol. 73:60. doi: 10.1007/s00265-019-266
Moskat, C., Taylor, D. M., and Hauber, M. E. (2021). Effective conspecific communication with aberrant calls in the Common Cuckoo (Cuculus canorus). Behav. Ecol. Sociobiol. 75:7. doi: 10.1007/s00265-020-029
Poulin, R., and Forbes, M. R. (2012). Meta-analysis and research on host-parasite interactions: past and future. Evol. Ecol. 26, 1169–1185. doi: 10.1007/s10682-011-9544-0
Randler, C., and Randler, E. (2020). Territorial responses of Nuthatches Sitta europaea —evaluation of a robot model in a simulated territorial intrusion. Birds 1, 53–63. doi: 10.3390/birds1010006
Rek, P., and Osiejuk, T. S. (2011). Nonpasserine bird produces soft calls and pays retaliation cost. Behav. Ecol. 22, 657–662. doi: 10.1093/beheco/arr027
Ruxton, G. D., Sherratt, T. N., and Speed, M. P. (2004). Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford: Oxford University Press.
Santema, P., Valcu, M., Clinchy, M., Zanette, L., and Kempenaers, B. (2019). Playback of predator calls inhibits and delays dawn singing in a songbird community. Behav. Ecol. 30, 1283–1288. doi: 10.1093/beheco/arz075
Slater, P. J. B. (2003). Fifty years of bird song research: a case study in animal behaviour. Anim. Behav. 65, 633–639. doi: 10.1006/anbe.2003.2051
Soler, M. (2014). Long−term coevolution between avian brood parasites and their hosts. Biol. Rev. 89, 688–704. doi: 10.1111/brv.12075
Szalai, F., and Szamado, S. (2009). Honest and cheating strategies in a simple model of aggressive communication. Anim. Behav. 78, 949–959. doi: 10.1016/j.anbehav.2009.06.025
Takasu, F. (1998). Why do all host species not show defense against avian brood parasitism: evolutionary lag or equilibrium? Am. Nat. 151, 193–205. doi: 10.1086/286111
Takasu, F. (2003). Co-evolutionary dynamics of egg appearance in avian brood parasitism. Evol. Ecol. Res. 5, 345–362. doi: 10.1007/978-3-319-73138-4_19
Todt, D., and Naguib, M. (2000). Vocal interactions in birds: the use of song as a model in communication. Adv. Study Behav. 29, 247–296. doi: 10.1016/s0065-3454(08)60107-2
Tryjanowski, P., Morelli, F., Kwieciński, Z., Indykiewicz, P., and Møller, A. P. (2018a). Birds respond similarly to taxidermic models and live cuckoos Cuculus canorus. J. Ethol. 36, 243–249. doi: 10.1007/s10164-018-0554-z
Tryjanowski, P., Morelli, F., Osiejuk, T. S., and Møller, A. P. (2018b). Functional significance of cuckoo Cuculus canorus calls: responses of conspecifics, hosts and non-hosts. PeerJ. 6:e5302. doi: 10.7717/peerj.53022/13
Wang, L., Zhong, G., He, G., Zhang, Y., and Liang, W. (2020). Egg laying behavior of Common Cuckoos (Cuculus canorus): data based on field video-recordings. Zool. Res. 41, 458–464.
Welbergen, J. A., and Davies, N. B. (2011). A parasite in wolf’s clothing: hawk mimicry reduces mobbing of cuckoos by hosts. Behav. Ecol. 22, 574–579. doi: 10.1093/beheco/arr008
Xia, C., Deng, Z., Lloyd, H., Møller, A. P., Zhao, X., and Zhang, Y. (2019). The function of three main call types in Common Cuckoo. Ethology 125, 652–659. doi: 10.1111/eth.12918
Yang, C., Wang, L., Liang, W., and Møller, A. P. (2016). Egg recognition as antiparasitism defence in hosts does not select for laying of matching eggs in parasitic cuckoos. Anim. Behav. 122, 177–181. doi: 10.1016/j.anbehav.2016.10.018
Yang, C., Wang, L., Liang, W., and Møller, A. P. (2017). How cuckoos find and choose host nests for parasitism. Behav. Ecol. 28, 859–865. doi: 10.1093/beheco/arx049
Yoo, S., Kim, H. N., Lee, J. W., and Yoo, J. C. (2020). Seasonal and diurnal patterns of population vocal activity in avian brood parasites. Ibis 162, 1001–1011. doi: 10.1111/ibi.12741
York, J. E., and Davies, N. B. (2017). Female cuckoo calls misdirect host defences towards the wrong enemy. Nat. Ecol. Evol. 1, 1520–1525. doi: 10.1038/s41559-017-0279-3
Yu, J., Xing, X., Jiang, Y., Liang, W., Wang, H., and Møller, A. P. (2017). Alarm call-based discrimination between Common Cuckoo and Eurasian sparrowhawk in a Chinese population of great tits. Ethology 123, 542–550. doi: 10.1111/eth.12624
Zachau, C. E., and Freeberg, T. M. (2012). Chick-a-dee call variation in the context of “flying” avian predator stimuli: a field study of Carolina chickadees (Poecile carolinensis). Behav. Ecol. Sociobiol. 66, 683–690. doi: 10.1007/s00265-012-1316-5
Zhang, J., Møller, A. P., Yan, D., Li, J., and Deng, W. (2021). Egg rejection changes with seasonal variation in risk of cuckoo parasitism in Daurian redstarts, Phoenicurus auroreus. Anim. Behav. 175, 193–200. doi: 10.1016/j.anbehav.2021.03.007
Zhou, B., Xia, C., Chen, Z., and Deng, W. (2020). Individual identification of male ural owls based on territorial calls. J. Raptor Res. 54, 57–65. doi: 10.3356/0892-1016-54.1.57
Keywords: acoustic signals, call variation, common cuckoo, female vocalization, playback
Citation: Wang Y, Tian M, Liu J, Lu X, Møller AP and Xia C (2021) Testing the Interspecific Function of Female Common Cuckoo “Bubbling” Call. Front. Ecol. Evol. 9:725222. doi: 10.3389/fevo.2021.725222
Received: 15 June 2021; Accepted: 18 August 2021;
Published: 06 September 2021.
Edited by:
Jiangping Yu, Northeast Normal University, ChinaReviewed by:
Csaba Moskát, Hungarian Natural History Museum, HungaryCopyright © 2021 Wang, Tian, Liu, Lu, Møller and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Canwei Xia, eGlhY2Fud2VpQGJudS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.