
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 16 September 2021
Sec. Conservation and Restoration Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.723026
This article is part of the Research Topic Integrating Traditional Ecological Knowledge into Ecology, Evolution, and Conservation View all 13 articles
Trophic levels can be applied to describe the ecological role of organisms in food webs and assess changes in ecosystems. Stable isotopes analysis can assist in the understanding of trophic interactions and use of food resources by aquatic organisms. The local ecological knowledge (LEK) of fishers can be an alternative to advance understanding about fish trophic interactions and to construct aquatic food webs, especially in regions lacking research capacity. The objectives of this study are: to calculate the trophic levels of six fish species important to fishing by combining data from stable isotopes analysis and fishers’ LEK in two clear water rivers (Tapajós and Tocantins) in the Brazilian Amazon; to compare the trophic levels of these fish between the two methods (stable isotopes analysis and LEK) and the two rivers; and to develop diagrams representing the trophic webs of the main fish prey and predators based on fisher’s LEK. The fish species studied were Pescada (Plagioscion squamosissimus), Tucunaré (Cichla pinima), Piranha (Serrasalmus rhombeus), Aracu (Leporinus fasciatus), Charuto (Hemiodus unimaculatus), and Jaraqui (Semaprochilodus spp.). A total of 98 interviews and 63 samples for stable isotopes analysis were carried out in both rivers. The average fish trophic levels did not differ between the stable isotopes analysis and the LEK in the Tapajós, nor in the Tocantins Rivers. The overall trophic level of the studied fish species obtained through the LEK did not differ from data obtained through the stable isotopes analysis in both rivers, except for the Aracu in the Tapajós River. The main food items consumed by the fish according to fishers’ LEK did agree with fish diets as described in the biological literature. Fishers provided useful information on fish predators and feeding habits of endangered species, such as river dolphin and river otter. Collaboration with fishers through LEK studies can be a viable approach to produce reliable data on fish trophic ecology to improve fisheries management and species conservation in tropical freshwater environments and other regions with data limitations.
Freshwater environments can be considered the most altered and threatened in the world (Geist, 2011; Reid et al., 2019; Albert et al., 2020). Due to the human interaction and dependence on riverine environments, populations of aquatic organisms are vulnerable to the effects of increasing environmental and anthropogenic changes, such as long and unusual periods of drought (climate change), dams, mining, habitat change (deforestation), and overfishing (Castilhos et al., 1998; Junk et al., 2007; Malhi et al., 2008; Latrubesse et al., 2017; Arantes et al., 2019a). The lack of available data, combined with scarce financial and human resources, are among the main current problems affecting the management of freshwater ecosystems (Castello et al., 2013; Cavole et al., 2015). Therefore, research on the ecology of freshwater environments is essential for the conservation of these aquatic ecosystems and the maintenance of their ecosystem services (Barletta et al., 2010).
One way to monitor and to evaluate environmental changes, both natural and anthropogenic, consists of studies on ecosystems’ trophic structure (Newsome et al., 2010; Andrade et al., 2019; Melo et al., 2019). The trophic level consists on the position occupied by the organism in the food web (Lindeman, 1942). In this sense, the ecological role of organisms can be described through the calculation of their trophic level (Post, 2002; Quezada-Romegialli et al., 2018). The trophic level also allows for the assessment of ecological effects of fishing, to the extent that some organisms, such as top predators (large fish), are selectively removed from the aquatic food webs (Pauly et al., 1998; Shin et al., 2005). These selective removals can alter the structure of the food webs, thus affecting the flow of matter and energy in the environments (Andersen and Pedersen, 2010; Loh et al., 2015). Some effects of these removals may be the trophic cascades (Scheffer et al., 2005; Myers et al., 2007), on which a consumer-resource interaction indirectly influences the other trophic levels (Paine, 1980; Estes et al., 2011). Another possible effect is the simplification of food webs, on which there is a decline in species with higher trophic levels (Estes et al., 2011). These effects (trophic cascades, simplification of food webs) can have consequences even for non-exploited species (Pauly et al., 1998; Estes et al., 2011).
A method that has been used to study aquatic food webs consists on the stable isotopes analysis, which can assist in the understanding of trophic interactions and use of food resources by organisms (Fry, 2006; Pereyra et al., 2016; Arantes et al., 2019b). The stable isotopes analysis allows a determination of the part of the diet that was consumed and assimilated by the organisms (De Niro and Epstein, 1978; Newsome et al., 2009; Carvalho et al., 2018). The carbon isotope allows to trace the main basal energy sources assimilated by the organisms (Fry, 2006; Correa and Winemiller, 2018; Costa et al., 2020). Conversely, nitrogen values predictably increase from prey to predator (Minagawa and Wada, 1984), being thus used to calculate the trophic position along the food chain, or the trophic level (Post, 2002; Olivar et al., 2018; Chiang et al., 2020).
However, the technique of stable isotopes analysis requires specialized machinery, detailed protocols and a considerably amount of processing time. Therefore, the local ecological knowledge (LEK) of fishers can be a reliable and alternative approach to advance understanding about fish trophic interactions and to construct aquatic food webs (Silvano and Begossi, 2002; Gerhardinger et al., 2006; Batista and Lima, 2010; Nunes et al., 2011; Ramires et al., 2015; Souza et al., 2020). Fishers’ LEK, which can be propagated over time by cultural transmission (Berkes, 1999; Diamond, 2001), can provide useful information about aquatic animals and their behaviors (Huntington, 2000; Johannes et al., 2008; Herbst and Hanazaki, 2014). Such information from fishers’ LEK, which can be mixed or incorporated into conventional research data, can be an important source for creating new ecological hypotheses (Silvano and Valbo-Jørgensen, 2008; Turvey et al., 2010). Among its many applications, fishers’ LEK can contribute to identify environmental changes and assess impacts from development projects on fisheries resources (Hallwass et al., 2013; Baird et al., 2020; Runde et al., 2020; Santos et al., 2020), to calculate fish trophic level and to indicate patterns of mercury bioaccumulation in fish (Silvano and Begossi, 2016), besides providing needed data on the abundance patterns, occurrence and distribution of threatened species (Bender et al., 2014; Zapelini et al., 2017; Lopes et al., 2018; Freitas et al., 2020; Hallwass et al., 2020b; Ribeiro et al., 2021).
In the Amazon region, studies applying stable isotopes analysis to analyze trophic relationships have been conducted mainly in white and black water rivers (Araujo-Lima et al., 1986; Oliveira et al., 2006; Mortillaro et al., 2015; Aguiar-Santos et al., 2018; Carvalho et al., 2018), whereas fewer studies have been conducted in clear water rivers (Zuluaga-Gómez et al., 2016; Andrade et al., 2019). Clear water basins covering 27.3% of the total area of the Amazon basin and are the most impacted basins of the Amazon (Goulding et al., 2003). In the Brazilian Amazon, there are two large protected areas, besides indigenous lands, in the region of the Lower Tapajós, which is a clear water river (Keppeler et al., 2017). However, dams and other projects are planned in the upstream region of the Tapajós River and its tributaries, including some projects already approved and built, which represent a challenge for the conservation of aquatic biodiversity (Fearnside, 2015; Winemiller et al., 2016; Athayde et al., 2019; Runde et al., 2020). The Tocantins River, which is another clear water river, can be considered one of the most impacted sub-basins in the Brazilian Amazon (Barthem et al., 2005), mainly due to the high rates of deforestation and the construction of highways and dams, which have caused several environmental and social impacts affecting both fish and riverine people (Fearnside, 1999, 2001; Hallwass et al., 2013). The installation of several hydroelectric projects in the Tocantins-Araguaia river basin since the 1980s, associated with high rates of deforestation for agricultural expansion, can have numerous effects on the trophic ecology of animals, such as disruption of food webs, alterations on the abundance of prey and predators, altering the functional diversity of fish (Mérona et al., 2001; Arantes et al., 2019a; Melo et al., 2019).
The main objectives of this study are to investigate the trophic structure of the ichthyofauna by calculating the trophic level of six fish species relevant for small scale fisheries, to compare fish trophic level data obtained from different methods, stable isotopes analysis and fisher’s LEK, and to compare fish trophic level values and trophic structure between two clear water rivers in the Brazilian Amazon (Tapajós and Tocantins) that differ on environmental integrity and history of environmental impacts. Another objective is to construct diagrams representing the trophic webs of the main prey and predators of fish based on the fisher’s LEK and to compare these LEK data with the literature, in the two studied rivers. We tested the following hypotheses: (1) the trophic level of the fish obtained through the LEK will be consistent with the data obtained in the stable isotopes analysis. A previous study indicates that the trophic levels of fish species, including Amazonian fish, calculated through the fishers’ LEK are consistent with the trophic levels recorded in the literature (Silvano and Begossi, 2016). In the present study, we will use an approach that considers what was assimilated by the organisms through the stable isotopes analysis (Newsome et al., 2009), thus calculating the trophic level based on nutrients assimilated to support the consumer fish (Fry, 2006), but from the same species in the same sites where we conducted the fishers’ LEK survey; (2) Due to a more accentuated history of environmental impacts in the Tocantins river basin, we expect that fishers would mention less food items and predators for the studied fish in the Tocantins than in the Tapajós River.
The clear water rivers Tapajós and Tocantins have transparent and greenish waters with low amounts of sediments and dissolved solids (Junk and Piedade, 2010). The acidity of the waters in clear water rivers can vary between pH 5 and 6 depending on the river stretch (Sioli, 1984; Junk et al., 2007). Both the basins of the Tapajós River (490.000 km2) and the Tocantins-Araguaia River (757.000 km2) are entirely located within the Brazilian territory (Latrubesse et al., 2005). Both rivers originate in the central Brazilian plateau (Cerrado biome) and have their mouth and most of their course running through the Amazon Forest (Scoles, 2014). In the areas of flooded vegetation of these rivers there is a highly specialized flora with two types of vegetation: flooded rain forest (Salomão et al., 2007) and alluvial riparian vegetation (Veloso et al., 1991).
The population that was studied in both rivers belongs to the “caboclos” cultural group, who are also called “ribeirinhos” (riverine people). These people are descendants of indigenous Brazilians and Portuguese colonizers, but more recently there has been an immigration of people from the northeast of Brazil (Begossi, 1998). The small-scale fisheries are predominant in these tropical rivers in the Brazilian Amazon (Bayley and Petrere, 1989; Hallwass et al., 2011, 2020a), where fishing is considered to be amongst the most important economic activities, both for subsistence and for commercialization, in addition to small-scale agriculture and livestock (McGrath et al., 2008; Runde et al., 2020). The level of formal education of fishers limits their reallocation to other economic activities not directly related to the use of natural resources (Lima et al., 2012).
The interviews were conducted in eight fishing communities in the Tapajós River and five in the Tocantins River (13 communities in total, Figure 1), respectively in September and October, 2018. The communities that were selected to be included in the study were located at least 5 km apart from each other and following a distance gradient from the largest cities: Itaituba (PA) in the Tapajós river and Marabá (PA) in the Tocantins river. When arriving in the communities, community leaders were initially contacted, the objectives of the work were explained to them, and agreement and permission to conduct our research in the community were requested. After agreeing with the research, the community leader indicated the first fishers to be interviewed, according to the minimum criteria for inclusion in the study: fishing as a main activity, being older than 18 years of age, and living in the region for at least 10 years. Fishers were interviewed individually, usually in their homes and before each interview the research was explained and consent was requested from the fisher to participate in the interview. After the interview, the interviewed fisher was solicited to indicate another fisher in the community who would fits the same criteria, through the snowball method, which has been successfully applied in previous studies on fisher’s LEK in the Brazilian Amazon (Hallwass et al., 2013, 2020b; Runde et al., 2020). The interviews were based on a semi-structured questionnaire (Supplementary Material 3), in which photos of the fish were shown, always in the same order, following previous methods of ethnoecological studies (Silvano and Begossi, 2002, Begossi, 2012). The questions asked addressed the fisher’s socioeconomic profile and the questions about fish analyzed in this study were: (a) What is the name of this fish? (b) What does this fish eat? (c) Who eats this fish? Six species, or groups of species that receive the same popular name, were chosen, which occur both in the Tapajós river and in the Tocantins river, because these fish belong to different trophic level (according to the literature) and because they are important for fishing (trade or consumption) (Hallwass et al., 2011, 2013, 2020a; Runde et al., 2020). The fish species chosen were Pescada (Plagioscion squamosissimus), Tucunaré (Cichla pinima), Piranha (Serrasalmus rhombeus), Aracu (Leporinus fasciatus), Charuto (Hemiodus unimaculatus), and Jaraqui (Semaprochilodus spp.). The species Jaraqui (Semaprochilodus spp.) was not collected in the Tocantins river, hence it was not included in the stable isotopes analysis comparison. This study was approved by the ethics’ committees for studies with people (CONEP/CAAE: 82355618.0.0000.5347) and animals (CEUA: 34186) at the Federal University of Rio Grande do Sul.
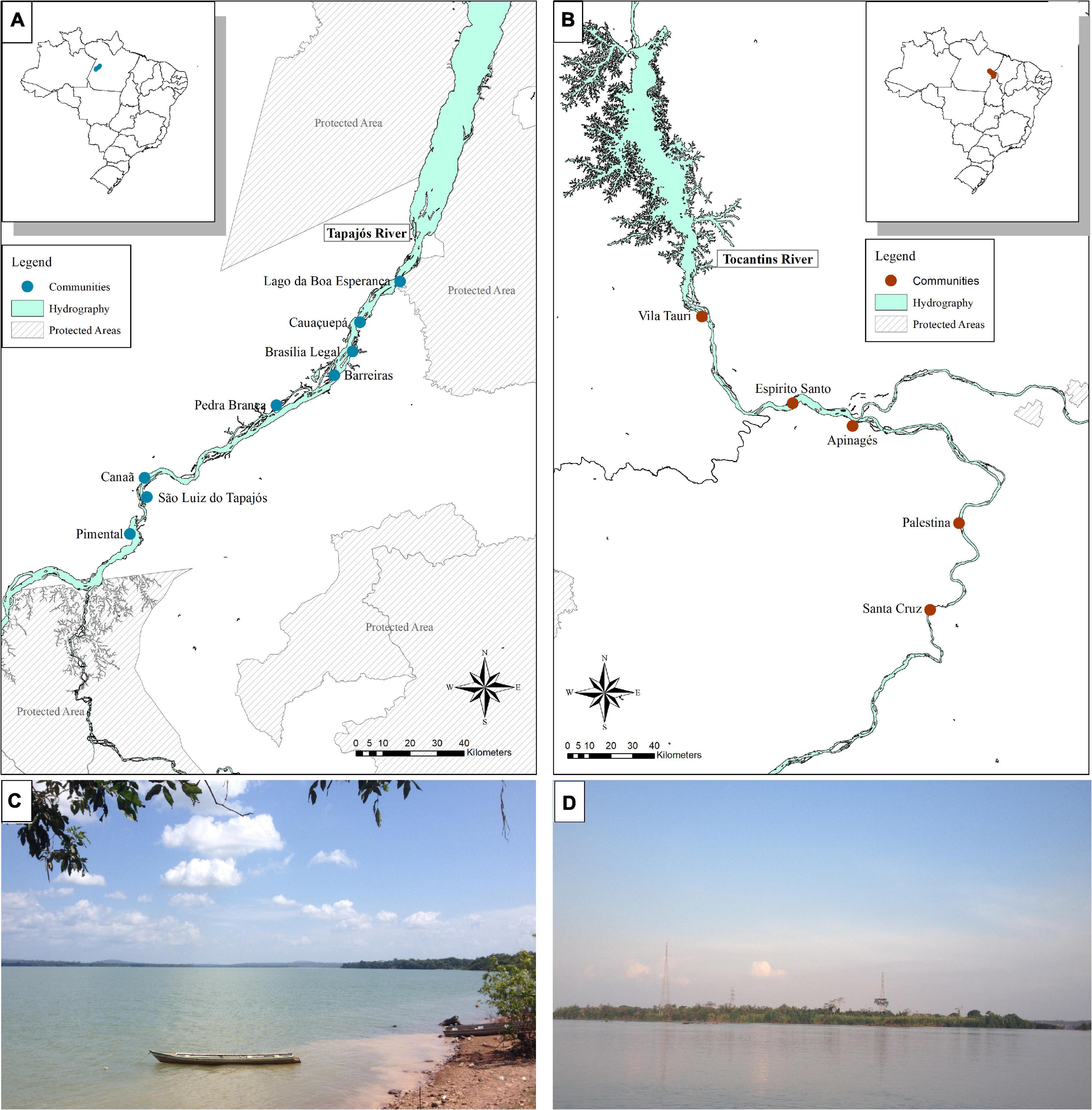
Figure 1. Location of rivers, communities and sampling sites of interviews and fish samples for stable isotopes analysis in the Tapajós (A,C) and Tocantins (B,D) rivers, in the Brazilian Amazon.
The fish were collected from lakes or river stretches close to the communities where interviews were conducted (Figure 1). The fish sampling was performed using two sets of fishing nets (420 m2 each), each set with different mesh sizes (ranging from 15 to 80 mm between adjacent nodes), over 24 h. The specimens were identified at the species level and the standard length (SL-cm) and weight (g) were measured. In addition to fish, samples from the benthic macrofauna (mollusks) were manually collected, to be used as a baseline in isotopic models. All samples were stored in plastic bags and preserved on ice until processed.
After collection, samples of antero-dorsal muscle tissue from fish and of adductor muscle from mollusks were removed for stable isotopes analysis. In the laboratory, these samples were washed with distilled water and inspected to remove only the tissue of interest. Afterward, each sample was placed in a Petri dish, which was pre-sterilized in a hydrochloric acid bath for 24 h, and then placed in the oven at 60° for 48 h. After that, the samples were transformed into fine powder with a mortar and pestle and sub-samples (approximately 1 mg) were weighed on a precision scale (∼ 1 mg) and stored in ultrapure tin capsules (Elemental-D-1008). Sample readings were performed with the Thermo iCAP6300 Duo isotope ratio mass spectrometer (Cambridge, United Kingdom) at the University of Alberta, Canada. The results were expressed in delta notation: δ13C or δ15N = [(Ramostra/Standard) -1] ∗ 1000, where R = 12C/13C/ or 14N/15N. The values obtained were compared with reference standards for carbon (PeeDee Belemnite) and nitrogen (atmospheric air) and their isotopic ratios (δ13C, δ 15N) expressed in per mil (‰) (Fry, 2006). The internal standard of known carbon and nitrogen composition was analyzed with each sequence to assess the accuracy of the instrument. The standard deviation was δ13C = 0.05 ‰ and δ15N = 0.19 ‰.
In some cases, samples may have a high lipid content, which can influence δ13C values (De Niro and Epstein, 1978; Logan et al., 2008). There are two approaches to increase the accuracy of δ13C measurements: lipids can be removed chemically (Mintenbeck et al., 2008), or mathematical corrections based on empirical equations can be used (Post et al., 2007). Lipid extraction can affect δ15N values, as other non-lipid materials can be removed (Pinnegar and Polunin, 1999; Sweeting et al., 2006; Mintenbeck et al., 2008). This method is also time-consuming and requires the use of hazardous materials, such as chloroform (Elliott et al., 2014). Due to these limitations, mathematical corrections have been quite effective and used for different types of animals (Post et al., 2007; Ehrich et al., 2011; Elliott et al., 2014; Olivar et al., 2018; Clark et al., 2019). Based on this, as all fish samples showed a C: N ratio equal to or greater than 3.5 a mathematical normalization was applied to correct the carbon values, by using the equation Δδ13C = –3.32 + 0.99 × C : N (Post et al., 2007).
The fish trophic level was calculated based on the fisher’s LEK obtained through interviews by following the methodology adopted in a previous study (Silvano and Begossi, 2016). According to this methodology, food items were grouped into main categories and a trophic level value was assigned to each category: fruits and seeds (Trophic level = 2), other plants and flowers (aquatic plants, leaves, and other plant parts, Trophic level = 2), detritus (including mud and algae, Trophic level = 2), terrestrial invertebrates (insects, spiders, earthworms, Trophic level = 3), aquatic invertebrates (crustaceans, mainly shrimp, Trophic level = 3), terrestrial vertebrates (birds, frogs, and others, Trophic level = 4) and fish that could not be identified (Trophic level = 4). First, the trophic level of each food item was multiplied by the percentage of fishers who cited that item and the trophic level of all items were summed. This sum was then divided by the sum of the percentages of fisher who cited each item. For example, fishers on the Tapajós river cited that the fish Charuto (H. unimaculatus) eats vegetables (Trophic level = 2 cited by 13.65% of fishers), detritus (Trophic level = 2, 92.43% of fishers), invertebrates (Trophic level = 3, 9.09% of fishers) and Piaba (a general name for small fish) (Trophic level = 3.8, 1.52% of fishers). Therefore, the Charuto trophic level was estimated as: (2 ∗ 13.65) + (2 ∗ 92.43) + (3 ∗ 9.09) + (3.8 ∗ 1.52) = 245.26, then 245.26/116.69 = 2.10. Considering that the basal food items (plants) will have the value of 1, the lowest value of calculated trophic level of the studied fish would be 2 (for a strictly herbivorous fish) and the highest value would be 4 for a piscivorous fish. Whenever possible to identify the species of fish that were mentioned by fishers as prey, we calculated the trophic position of these prey fish considering the food items mentioned in the literature.
Fish trophic position value were also estimated through stable isotope data using the “tRophicPosition” package in R (Quezada-Romegialli et al., 2018). This method incorporates Bayesian inference to calculate the trophic level of consumers at the population level, considering the individual variability in the data of stable isotopes. The trophic level of each species was modeled using Monte Carlo via Markov chains (MCMC) with 20,000 interactions and 20,000 adaptive samples in JAGS 4.3.0, using both isotopes of carbon and nitrogen. Two baselines were used: the scraper mollusk of the genus Doryssa spp. was chosen to represent the benthic baseline and the herbivorous fish Hemiodus unimaculatus was chosen to be the baseline referring to the pelagic pathway. The isotopic fractionation values used for carbon (δ13C) and nitrogen (δ15N) were 0.39 ± 1.3 and 3.4 ± 0.98, respectively (Post, 2002).
The paired t-test was calculated to determine if the average trophic level values for all fish species analyzed were significantly different between the fishers’ LEK data and the data generated in the models by stable isotopes analysis, in both rivers. This analysis has already been used in a previous study comparing fish trophic level between fishers’ LEK data and the biological literature (Silvano and Begossi, 2016). The t-test was performed to compare the average number of prey and fish predators cited by fishers between the two studied rivers. Before running t-tests, the Shapiro–Wilk and the Levene tests both based on residuals were performed to check normality and variance homogeneity of data, respectively. All residuals’ tests indicated normality and homogeneity of variances. All statistical analyzes were performed using the R 4.0.3 software (R Development Core Team, 2021).
Diagrams were constructed to represent the trophic webs of the main fish prey and predators based on the fisher’s LEK and these data were compared with prey and predators of fish according to the biological literature (Silvano and Begossi, 2002, Begossi, 2012). These trophic webs included all fish prey cited by fishers, whereas only those fish predators cited by more than 10% of fishers were included. This criterion was adopted to better visualize the relationships between predators and prey, given the higher variability of cited predators. The sum of cited prey or predators may exceed 100%, as fishers could cite more than one food item or predator for each fish species studied.
A total of 98 fishers were interviewed, 65 in the Tapajós river and 33 in the Tocantins river, including 61 men and four women in Tapajós and 30 men and three women in the Tocantins. The average age of the fishers interviewed in the Tapajós River was 47.2 years (±11.4 years), the average fishing experience (time since started fishing) was 25.5 years (±11.9 years) and the time residing in the region was 36.8 years (±15.3 years). The average age of the fishers interviewed in the Tocantins River was 56.5 years (±14 years), the average fishing experience was 34.8 years (±17.6 years) and the time residing in the region was 41.5 years (±16.3 years).
A total of 63 samples of fish and mollusks were analyzed through stable isotopes analysis, including 40 samples from the Tapajós river (34 fish and six mollusks) and 23 samples from the Tocantins river (20 fish and three mollusks) (Table 1). The mean trophic level considering all studied fish species did not differ (t = –0.58, df = 5, p = 0.96) between data from stable isotopes analysis (2.84 ± 0.52) and fishers’ LEK (2.83 ± 0.86) in the Tapajós river (Figure 2A). Similarly, the mean trophic level did not differ (t = 0.48, df = 4, p = 0.66) between stable isotopes analysis (2.96 ± 0.33) and LEK (3.10 ± 0.88) in the Tocantins river (Figure 2B). The trophic level values obtained through the LEK did not differ from those obtained through the stable isotopes analysis for all species in the Tocantins river and nearly all species in the Tapajós river, except for Aracu L. fasciatus, which had a lower trophic level according to LEK (Table 2).
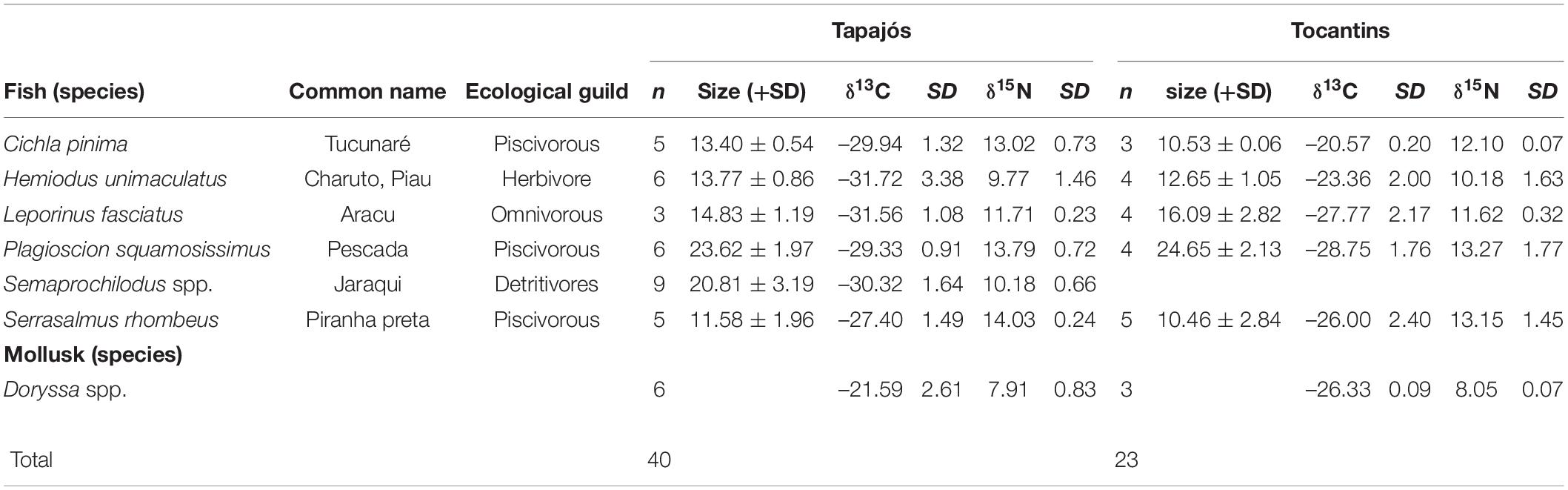
Table 1. Mean and standard deviation (SD) of size (total length), number of samples (n) and values of stable isotopes of carbon (δ13C) and nitrogen (δ15N) of fish and mollusks sampled in the Tapajós and Tocantins rivers.
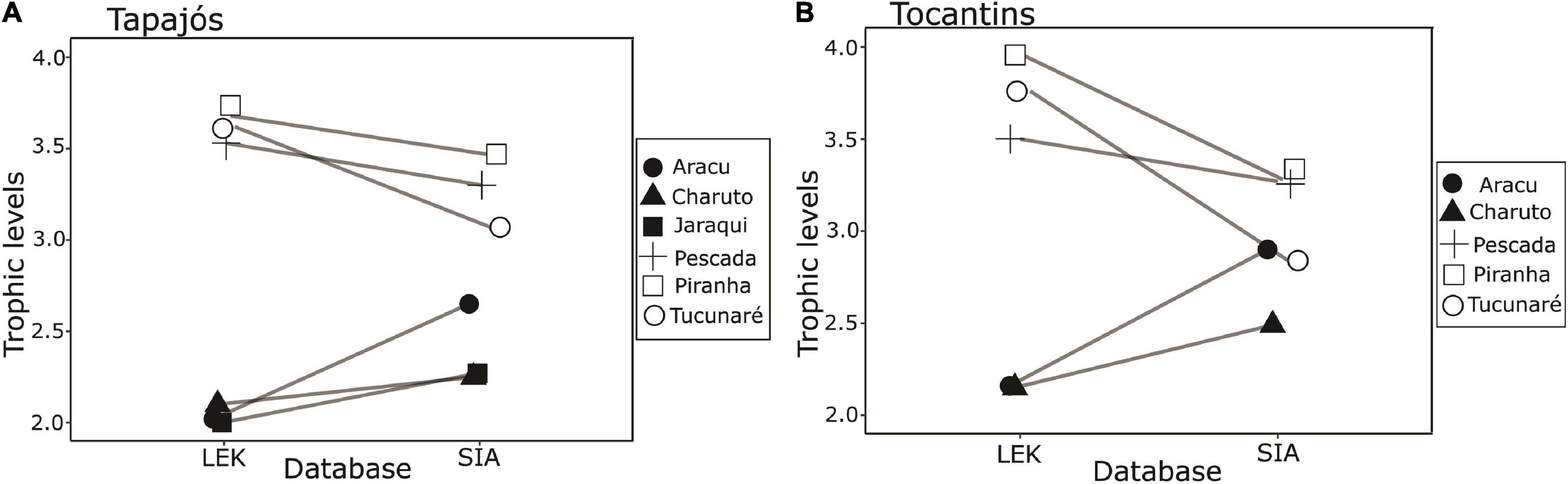
Figure 2. Paired comparison between trophic levels of fish species based on fisher’s knowledge (LEK) and stable isotope analysis (SIA) in the (A) Tapajós river (t = –0.58, df = 5, p = 0.96) and (B) Tocantins river (t = 0.48, df = 4, p = 0.66). The fish species analyzed were Aracu (Leporinus fasciatus), Charuto (Hemiodus unimaculatus), Jaraqui (Semaprochilodus spp.) except in the Tocantins river, Pescada (Plagioscion squamosissimus), Piranha (Serrasalmus rhombeus), and Tucunaré (Cichla pinima).
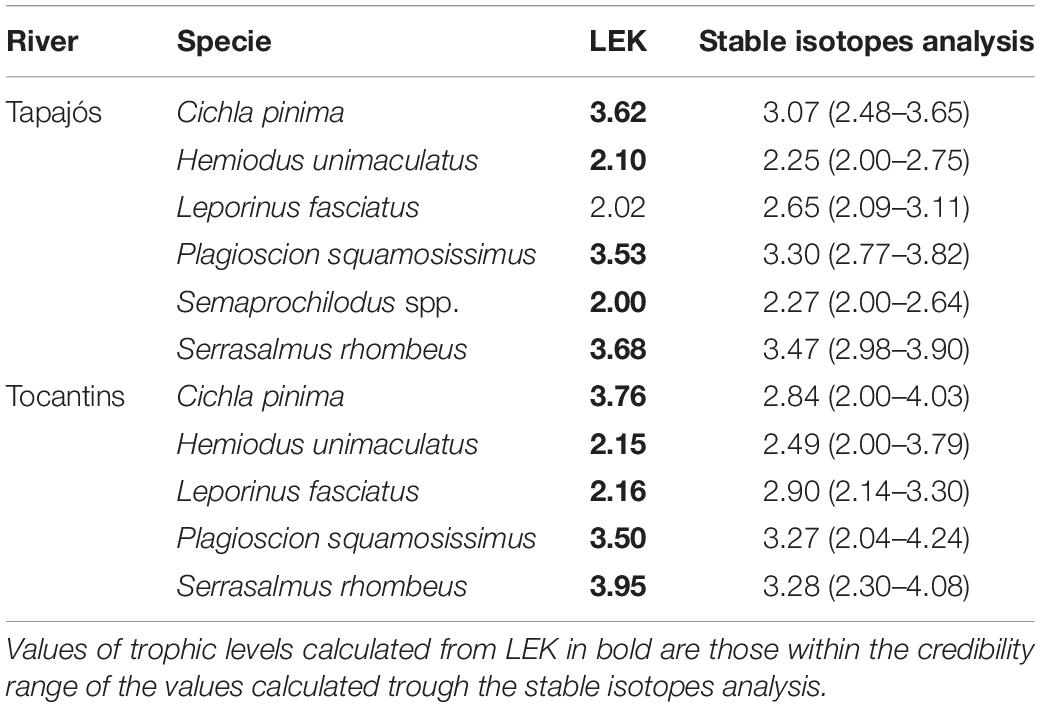
Table 2. Trophic levels calculated from the local ecological knowledge (LEK) of fishers and posterior trophic level estimates originated from Bayesian models (mean values and 95% credibility interval) based on stable isotopes analysis for the fish species studied in the Tapajós and Tocantins rivers.
The interviewed fishers cited 57 prey items and 22 fish predators in the Tapajós River and 27 prey items and 18 fish predators in the Tocantins river (Supplementary Tables 1, 2). The average number of prey cited for the studied fish species differed (t = 4.96, df = 97, p < 0.01) between fishers interviewed in the Tapajós (1.97 ± 0.46) and Tocantins (1.47 ± 0.45) rivers (Figure 3A). Conversely, the average number of predators cited did not differ (t = 0.88, df = 97, p = 0.38) between fishers interviewed in the Tapajós (1.82 ± 0.68) and Tocantins (1.69 ± 0.65) rivers (Figure 3B).
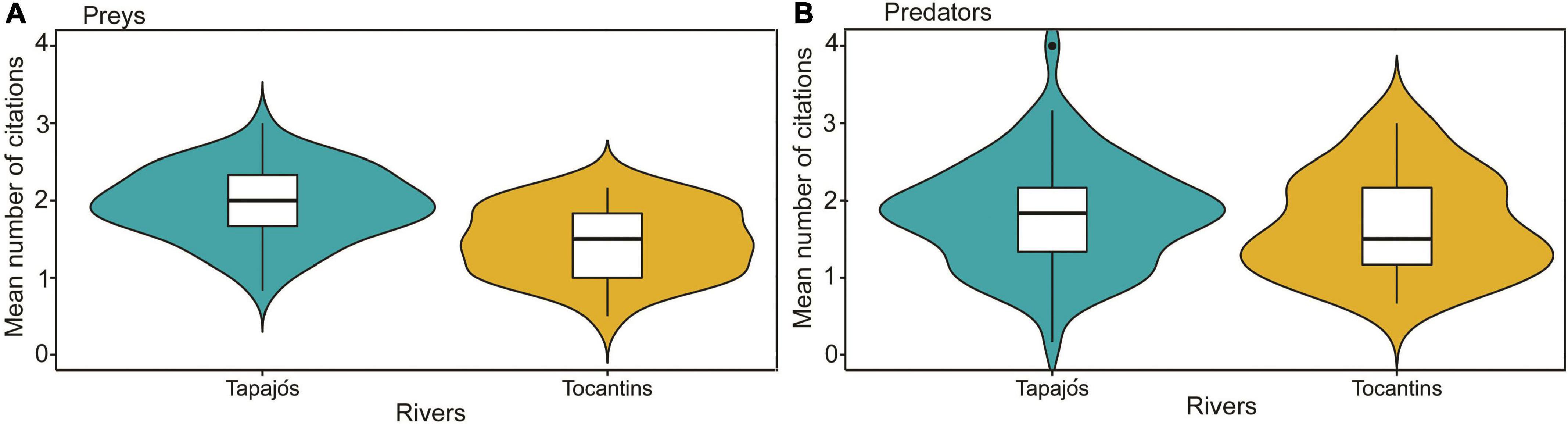
Figure 3. Comparison of the average number of fishers’ citations between the Tapajós and Tocantins rivers regarding the number of (A) fish prey (t = 4.96, df = 97, p < 0.01) and (B) fish predators (t = 0.88, df = 97, p = 0.38). Boxplot indicates the median (dark line inside box) and quartiles (25 and 75%, outer lines of the box). Data distribution is indicated by violin-colored area.
Simplified food webs were built based on fishers’ citations on fish prey (Figure 4) and predators (Figure 5) in the Tapajós (Figures 4A, 5A) and Tocantins (Figures 4B, 5B) rivers. According to the fisher’s LEK, fish species such as Pescada (P. squamosissimus), Tucunaré (C. pinima) and Piranha (S. rhombeus) can be considered mainly piscivorous in both rivers (Figure 4). According to most fishers interviewed in the Tapajós (Figure 4A) and in the Tocantins (Figure 4B) Rivers, detritus was the main food for the fishes Charuto (H. unimaculatus) and Jaraqui (Semaprochilodus spp.). The fish Aracu (L. fasciatus) can be considered as herbivorous or omnivorous species according to the fishers’ LEK in both rivers, as this fish can feed on different items, but it eats mainly fruits (Figure 4).
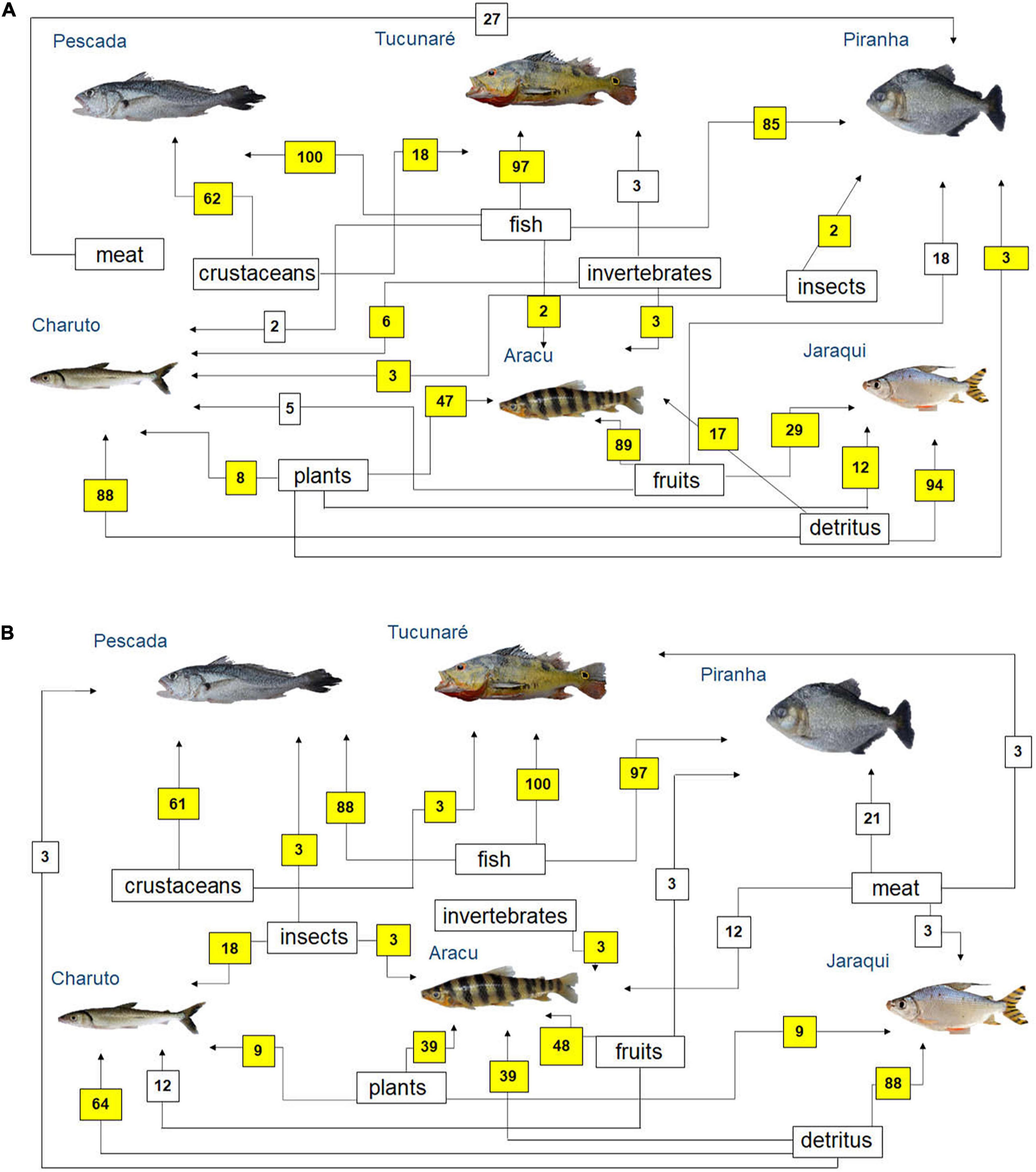
Figure 4. Diagram representing fish feeding relationships (simplified food web) of the studied fish species, based on fishers’ local ecological knowledge about fish prey (question: What this fish eats?) in the rivers (A) Tapajós (n = 65 fishers) and (B) Tocantins (n = 33 fishers), in the Brazilian Amazon. The numbers are the percentage of interviewed fishers who mentioned each feeding interaction. The sum may be greater than 100%, as fishers could cite more than one food item for each studied fish species. Those food interactions that agree with fish feeding relationships reported in the biological literature (Mérona et al., 2001; Mérona and Rankin-de-Mérona, 2004; Dary et al., 2017) are marked in yellow.
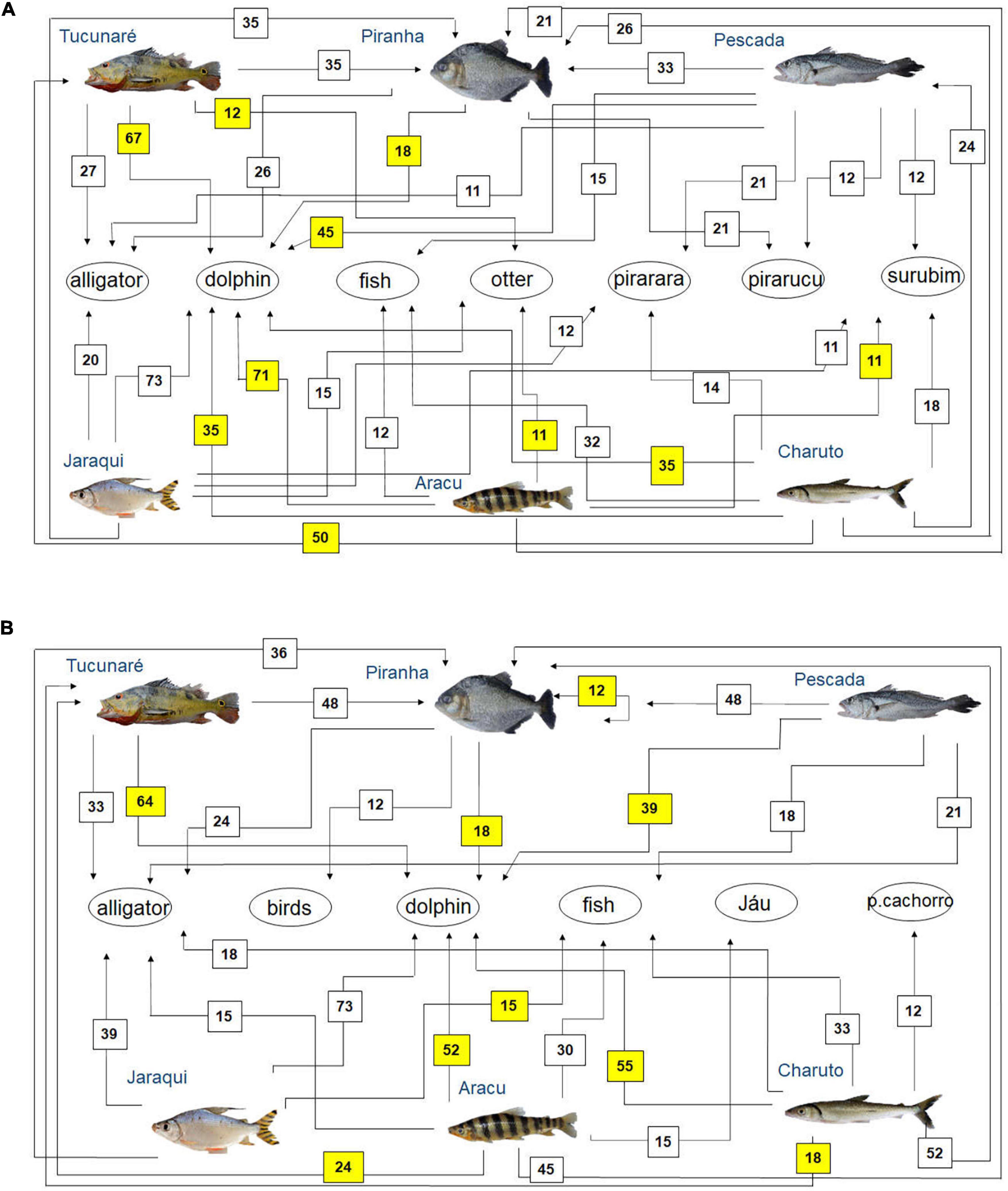
Figure 5. Diagram representing fish feeding relationships (simplified food web) of the studied fish species, based on fishers’ local ecological knowledge about fish predators (question: Who eats this fish?) in the rivers (A) Tapajós (n = 65 fishers) and (B) Tocantins (n = 33 fishers), in the Brazilian Amazon. The numbers are the percentage of interviewed fishers who mentioned each feeding interaction. The sum may be greater than 100%, as fishers could cite more than one food item for each studied fish species. Those food interactions that agree with fish feeding relationships reported in the biological literature (Best, 1984; Silva et al., 2013; Dary et al., 2017; Jacobi et al., 2020) are marked in yellow.
One of the fish predators most cited by fishers in both rivers was the red dolphin Inia spp. (Figure 5). In both rivers, the piranha (Serrasalmus spp.) was the main predatory fish mentioned by the interviewed fishers (Figure 5). Other fish identified as fish predators by the interviewed fishers were the Pirarara (Phractocephalus hemioliopterus), Pirarucu (Arapaima gigas) and Surubim (Pseudoplatystoma spp.) in the Tapajós river (Figure 5A), as well as the Jaú (Pimelodidae) and peixe-cachorro (Acestrorhynchus spp., Raphiodon vulpinus, and Hydrolicus spp.) in the Tocantins River (Figure 5B).
Fishers’ LEK was a robust estimator of trophic level in relation to stable isotopes analysis in both rivers. Ten of a total of the 11 trophic levels estimated based on LEK were within the credibility interval of estimates of trophic levels through the stable isotopes analysis. This result further demonstrates that fisher’s LEK can be a promising rapid and low cost alternative to obtain reliable data for studies on fish trophic ecology, as observed in previous studies (Ramires et al., 2015; Silvano and Begossi, 2016).
Fishers can acquire knowledge about fish diets by observing fish stomach contents while manipulating and gutting fish (Silvano and Begossi, 2002). Furthermore, fishers constantly manipulate food items to be used as baits, thus gaining knowledge about food preferences of fish, considering that those food items cited as part of fish diets are also commonly used as baits (Silvano and Begossi, 2005; Baird, 2007; Ramires et al., 2015). On the other hand, fish predators preying fish on gill nets may be commonly observed by fishers, thus fishing activity can be an important source of knowledge about the feeding behavior of fish and other animals (Silvano and Begossi, 2002; Ramires et al., 2015).
Despite an overall agreement, there were small differences between the trophic level estimates based on LEK and stable isotopes analysis: the trophic level based on the LEK of the fish Aracu fell outside the credibility interval and was thus lower than the trophic level of this fish estimated through the stable isotopes analysis model in the Tapajós river. This and other slight differences between LEK and stable isotopes analysis regarding the estimated trophic levels can be explained by the fact that fishers make their inferences about fish diets through observation of fish stomach contents, besides direct observation of fish behavior. Therefore, LEK is mostly based on what was ingested by fish, not on what was actually assimilated in fish tissues, as measured by stable isotopes analysis. Indeed, some of the food items present in fish stomachs may be refractory to digestion (e.g., vegetation, fruits, shells) and may not be digested nor assimilated by the consumer. Furthermore, the differences in trophic levels between the LEK and stable isotopes analysis observed in the present study can be at least partially attributed to the temporal differences in the methods being compared. The data from the stable isotopes analysis indicate what has been assimilated and transformed into tissues by the consumer fish, considering a time span of approximately 90 days from consumption (Mont’Alverne et al., 2016). On the other hand, data obtained through the fishers’ LEK may include a much longer time window on fish diets, as fishers can acquire this knowledge through the accumulation of observations over several years of fishing activity, along their experience in contact with the environment (Silvano and Begossi, 2002), besides the transmission of knowledge among fishers (Johnson, 2006). Therefore, these two methods or approaches can be used concurrently in studies of trophic ecology. For example, the simultaneous use of LEK and stable isotopes analysis has been successfully applied to assess habitat use by turtles in estuarine environments (Wedemeyer-Strombel, 2019).
Considering the diversity of species in the Brazilian Amazon basin (Dagosta and de Pinna, 2019), detailed information on fish feeding habits may be relatively scarce (Mérona et al., 2001; Mérona and Rankin-de-Mérona, 2004; Silvano and Begossi, 2016; Dary et al., 2017). Moreover, studies on fish trophic ecology may show some limitations, such as small sample sizes, geographically restricted sampling and not including seasonal variation. Conventional studies based on the method of stomach content analysis may have, among their main limitations, a large number of empty stomachs of piscivorous fish (Vinson and Angradi, 2011) and the difficulty to identify certain items, which may be very digested and are often only bones, not allowing identification at the species level. These limitations can complicate the identification of the diets of piscivores, such as piranhas (Prudente et al., 2016) and alligators (Magnusson et al., 1987). However, in the present study, fishers cited potential fish prey of these two species of predators, in some cases identifying even more refined taxonomic levels than those generally described in the biological literature. Another advantage of including fishers’ LEK in studies on trophic ecology is the possibility of building conceptual models of food interactions, which can be used in different environments, such as freshwater and marine (Silvano and Begossi, 2002, Begossi, 2012; Le Fur et al., 2011). Furthermore, the LEK based data can be also applied in ecosystem modeling studies, aiming to improve management of fisheries resources in environments that need management, but lack data (Ainsworth and Pitcher, 2005; Bevilacqua et al., 2016; Bentley et al., 2019).
The results of this and previous studies provide evidence that fisher’s LEK has clear potential to “fit the pieces” and fill knowledge gaps regarding ecosystem function of fish, especially in remote tropical regions where scientific knowledge is still incipient. Moreover, information about the diet of fish and large predatory species is usually scarce in the scientific literature, as well as studies addressing the interactions between trophic ecology and fishing resources. In the present study, all food items most mentioned by fishers as being important to the diet of the studied fish corroborated with data from the literature. In both rivers, the general food items mentioned for piscivorous fish, such as Pescada and Tucunaré, were fish and crustaceans, whereas fishers mentioned fish being eaten by Piranha, fruits and plants as food of the Aracu and detritus as the main food item of Jaraqui. Therefore, fishers’ LEK can help to better understand the trophic ecology of fish that may receive a higher fishing pressure and thus more urgently demand management and conservation actions, such as some large piscivorous and herbivorous fishes in the Brazilian Amazon and elsewhere (Pauly et al., 1998; Welcomme, 1999; Hallwass et al., 2020b). Furthermore, the detailed information revealed by fishers’ LEK regarding the diet and trophic interactions of freshwater fishes can be useful to identify, and hence to maintain, some of the important ecosystem services provided by fish, such as seed dispersal (Lucas, 2008; Anderson et al., 2009; Horn et al., 2011), nutrient cycling (Flecker, 1996; Winemiller et al., 2006) and the food security of riverine populations (Isaac and Almeida, 2011; Begossi et al., 2019).
The fishers’ LEK can assist in understanding ecological aspects of emblematic species, such as the endangered red dolphin or boto, Inia spp. (Silva et al., 2018; Vidal et al., 2019; Campbell et al., 2020). In this study, fishers reported that the boto consumes the fish Tucunaré, Pescada, Aracu, and Charuto, all of which corroborate with a study on the boto diet carried out in the 1980s, through stomach content analysis (Best, 1984). However, the Jaraqui fish was also mentioned as being consumed by the boto by 73% of the interviewed fishers in both rivers, but this fish has not been mentioned in the literature as being part of the boto diet. Similarly, a previous study also shows that fishers mention the river dolphin as important predators of the fish Jaraqui in the Central Amazon (Batista and Lima, 2010). This hypothesis of Jaraqui predation by botos or other freshwater dolphins can be investigated in more detail in the future, given the importance of Jaraqui for small-scale fisheries throughout the Brazilian Amazon (Hallwass and Silvano, 2015; Hallwass et al., 2020a,b; Runde et al., 2020). Therefore, the potential predation of Jaraqui by the boto, as evidenced by the interviewed fishers, indicates a possible overlap between the resources used by this river dolphin and humans (fishers), which can become a source of conservation related conflicts (Loch et al., 2009; Kelkar et al., 2010). The interviewed fishers mentioned some fish, such as Aracu, Jaraqui, and Tucunaré, as being consumed by the river otter, and these same fish have been described as being part of the river otter diet through the analysis of fecal samples in the Negro River Basin, in the Brazilian Amazon (Silva et al., 2013).
Some discrepancies between fishers’ LEK and the biological literature were recorded in relation to fish predators. Fishers indicated Pescada and Piranha as being consumed by the Pirarucu, however, these species have not yet been recorded in studies on the feeding of Pirarucu. These differences between the two knowledge bases (LEK and biological) may occur due to the natural variation in the availability and occurrence of food items throughout the aquatic ecosystems in the Amazon, since the existing studies on trophic ecology of Pirarucu have not been conducted in the same rivers addressed in this study (Tapajós and Tocantins).
Studies on trophic ecology are even more relevant in the context of the Amazon biome, which is highly dynamic and which has undergone numerous changes during the last few years (Dagosta et al., 2020; Latrubesse et al., 2020). The Tocantins-Araguaia river basin is considered as an important and priority area for conservation, due both to the high presence of endemic species and the high number of dams (Dagosta et al., 2020). Dams can alter the abundance of prey and predators in the environment (Mérona et al., 2001), besides influencing and modifying fish feeding habits due to lack of food (Melo et al., 2019). The impacts and changes to fish and fisheries already observed in the Tocantins River (Mérona et al., 2001; Hallwass et al., 2013) can be repeated in the Tapajós river in the near future, as this river is targeted for development projects directed to energy production and enhancing navigation for the export of soy and meat (Fearnside, 2015; Latrubesse et al., 2020). Even considering that the Tapajós River basin has a high diversity of species (Dagosta and de Pinna, 2019) and several protected areas, there is a rapid loss in the forest area, especially in the region of the Lower Tapajós River (Dagosta et al., 2020), that may affect all food web of the river, mainly the large-bodied fish species as the top predators (Capitani et al., 2021). Besides these potential future impacts, there have been mining activities in the middle and upper Tapajós River since the mid-1980s, thus affecting both human populations and aquatic organisms due to mercury contamination, including contamination of fish consumed by people (Harada et al., 2001; Faial et al., 2015; Vasconcellos et al., 2021). A previous study demonstrates that fish trophic levels estimated by LEK, which are equivalent to trophic levels according to literature data, are also related to mercury content on fish, thus showing the potential of fishers’ LEK as an indicator of fish trophic level in bioaccumulation studies (Silvano and Begossi, 2016). The present study corroborated and advance these previous findings on the potential value of fishers’ LEK to indicate fish trophic levels and associated ecological properties (Silvano and Begossi, 2016). The previous study compared trophic levels estimated through the LEK with those from biological literature (Silvano and Begossi, 2016), whereas this study compared trophic levels estimated by LEK with data showing what was indeed assimilated by the organisms through the use of stable isotopes analysis (Newsome et al., 2009) for the same fish species and in the same sites where the interviews with fishers were conducted. Therefore, by adopting a more refined and accurate comparison, this study showed a very close agreement between fishers’ LEK and biological data on fish trophic levels, paving the way for a collaboration between fishers and scientists to develop ecological and ecotoxicological indicators.
Contrary to the initial hypothesis, the overall fishers’ LEK about the trophic levels and feeding interactions of fish did not differ between the two studied rivers, even though they have a distinct history and intensity of environmental impacts. For example, there were no differences on the number of fish predators cited by fishers between the Tapajós (22 predators) and the Tocantins (18 predators) rivers. This may be partially due to some degree of plasticity in the feeding behavior of at least some aquatic species, which may adapt to environmental changes that are occurring over time. Alternatively, it may be that fish and aquatic predators had not changed their feeding habits yet in the more altered Tocantins River, so impacts had not lead to perceived modifications in the diet of these organisms. These suggestions or hypotheses need to be checked in future studies aimed to understand the influences of river modification on fish diets and trophic ecology. However, as expected according to the proposed hypothesis, a greater number of food items was cited by fishers in the Tapajós river (57 items) when compared to the Tocantins river (27 items). This difference may be possibly due to the lower availability of some food items, such as fruits, in the Tocantins River, as a consequence of a more intense deforestation in this river basin. The results provided from fishers’ LEK thus reinforce the need to prioritize conservation and restoration strategies for aquatic environments in the Tocantins-Araguaia river basin.
The combination of both approaches (LEK and stable isotopes analysis) can advance the knowledge base on diet and trophic interactions of fish species with greater reliability, by producing accurate data, in a fast and effective way. Although such combination is desirable whenever possible, the stable isotopes analysis technique requires financial resources, specialized machinery, and considerable processing time, which may be beyond the reach of many researchers and communities in tropical developing countries. In such a context, this study adds to previous research to show that fishers’ LEK can provide useful information on fish trophic ecology and that such information based on LEK is closely related to biological data. The fishers’ LEK can thus be reliably applied to improve fisheries management and species conservation in those regions of the world that have data limitations but need urgent management.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Federal University of Rio Grande do Sul (CONEP/CAAE: 82355618.0.0000.5347). The ethics committee waived the requirement of written informed consent for participation. The animal study was reviewed and approved by Federal University of Rio Grande do Sul, (CEUA: 34186).
PP and RS conceived and designed the experiment. PP, GH, and RS conducted the field work. PP conduced the lab work, processed the stable isotope samples, performed all the statistical analyses, and wrote the first draft of the manuscript. MP contributed to reading of isotope samples. All authors contributed to subsequent versions and revisions.
We thank to the National Council for Scientific and Technological Development (CNPq) for the Ph.D. scholarship of PP (140957/2017-0) and for the research scholarship of RS (303393/2019-0). RS thanks a scholarship from the Coordination for the Improvement of Higher Education Personnel (CAPES-PRINT, 88887.467553/2019-00). This study was funded by Social Sciences and Humanities Research Council of Canada (SSHRC) (Project Tracking Change: RES0027949).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thanks all fishers on the Tapajós and Tocantins rivers for their cooperation and contribution to the research. We would like to thank Alexandre M. Garcia, Priscila F. M. Lopes, and Sandra M. Hartz for useful comments on a previous version of this work. We would also like to thank all colleagues in the Human Ecology and Fish Laboratory at the Federal University of Rio Grande do Sul, who assisted in field trips and interviews, especially Caroline Dutra, Carolina Batista, and Camila Flores for helping with the processing of the fish samples.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.723026/full#supplementary-material
Aguiar-Santos, J., deHart, P. A. P., Pouilly, M., Freitas, C. E. C., and Siqueira-Souza, F. K. (2018). Trophic ecology of speckled peacock bass Cichla temensis Humboldt 1821 in the middle Negro River, Amazon, Brazil. Ecol. Freshw. Fish 27, 1076–1086. doi: 10.1111/eff.12416
Ainsworth, C. H., and Pitcher, T. J. (2005). “Using local ecological knowledge in ecosystem models,” in Fisheries Assessment and Management in Data-Limited, eds G. H. Kruse, V. F. Gallucci, D. E. Hay, R. I. Perry, R. M. Peterman, T. C. Shirley, et al. (Fairbanks, AK: Alaska Sea Grant College Program, University of Alaska), 289–304. doi: 10.4027/famdis.2005.17
Albert, J. S., Destouni, G., Duke-Sylvester, S. M., Magurran, A. E., Oberdorff, T., Reis, R. E., et al. (2020). Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 50, 85–94. doi: 10.1007/s13280-020-01318-8
Andersen, K. H., and Pedersen, M. (2010). Damped trophic cascades driven by fishing in model marine ecosystems. Proc. R. Soc. Lond. B Biol. Sci. 277, 795–802. doi: 10.1098/rspb.2009.1512
Anderson, J. T., Rojas, S. J., and Flecker, A. S. (2009). High-quality seed dispersal by fruit-eating fishes in Amazonian floodplain habitats. Oecologia 161, 279–290. doi: 10.1007/s00442-009-1371-4
Andrade, M. C., Fitzgerald, D. B., Winemiller, K. O., Barbosa, P. S., and Giarrizzo, T. (2019). Trophic niche segregation among herbivorous serrasalmids from rapids of the lower Xingu River, Brazilian Amazon. Hydrobiologia 829, 265–280. doi: 10.1007/s10750-018-3838-y
Arantes, C. C., Winemiller, K. O., Asher, A., Castello, L., Hess, L. L., Petrere, M., et al. (2019a). Floodplain land cover affects biomass distribution of fish functional diversity in the Amazon River. Sci. Rep. 9:16684. doi: 10.1038/s41598-019-52243-0
Arantes, C. C., Winemiller, K. O., Petrere, M., and Freitas, C. E. C. (2019b). Spatial variation in aquatic food webs in the Amazon River floodplain. Freshw. Sci. 38, 213–228. doi: 10.1086/701841
Araujo-Lima, C. A. R. M., Forsberg, B. R., Victoria, R., and Martinelli, L. (1986). Energy sources for detritivorous fishes in the Amazon. Science 234, 1256–1258. doi: 10.1126/science.234.4781.1256
Athayde, S., Duarte, C. G., Gallardo, A. L. C. F., Moretto, E. M., Sangoi, L. A., Dibo, A. P. A., et al. (2019). Improving policies and instruments to address cumulative impacts of small hydropower in the Amazon. Energy Policy 132, 265–271. doi: 10.1016/j.enpol.2019.05.003
Baird, I. G. (2007). Fishes and forests: the importance of seasonally flooded riverine habitat for Mekong River fish feeding. Nat. Hist. Bull. Siam Soc. 55, 121–148.
Baird, I. G., Manorom, K., Phenow, A., and Gaja-Svasti, S. (2020). What about the tributaries of the tributaries? Fish migrations, fisheries, dams and fishers’ knowledge in North-Eastern Thailand. Int. J. Water Resour. Dev. 36, 170–199. doi: 10.1080/07900627.2019.1611549
Barletta, M., Jaureguizar, A. J., Baigun, C., Fontoura, N. F., Agostinho, A. A., Almeida-Val, V. M. F., et al. (2010). Fish and aquatic habitat conservation in South America: a continental overview with emphasis on neotropical systems. J. Fish Biol. 76, 2118–2176. doi: 10.1111/j.1095-8649.2010.02684.x
Barthem, R., Marques, M., Charvet-Almeida, P., and Montag, L. F. A. (2005). Amazon River Basin: I – characterization and environmental impacts due to deforestation. Ecosyst. Sustain. Dev. V 81, 615–625.
Batista, V. S., and Lima, L. G. (2010). In search of traditional bio-ecological knowledge useful for fisheries co-management: the case of jaraquis Semaprochilodus spp. (Characiformes, Prochilodontidae) in Central Amazon, Brazil. J. Ethnobiol. Ethnomed. 6:15. doi: 10.1186/1746-4269-6-15
Bayley, P. B., and Petrere, M. J. (1989). “Amazon fisheries: assessment methods, current status and management points,” in Proceedings of the International Large River Symposium, ed. D. P. Dodge (Ottawa, ON: Canadian Special Publication of Fisheries and Aquatic Sciences), 385–398.
Begossi, A. (1998). “Cultural and ecological resilience among caiçaras of the Atlantic Forest and caboclos of the Amazon, Brazil,” in Linking Social and Cultural Systems for Resilience, eds C. Folke and F. Berkes (Cambridge: Cambridge U. Press), 129–157.
Begossi, A., Salivonchyk, S. V., Hallwass, G., Hanazaki, N., Lopes, P. F. M., Silvano, R. A. M., et al. (2019). Fish consumption on the Amazon: a review of biodiversity, hydropower and food security issues. Braz. J. Biol. 79, 345–357.
Bender, M. G., Machado, G. R., De Azevedo Silva, P. J., Floeter, S. R., Monteiro-Netto, C., Luiz, O. J., et al. (2014). Local ecological knowledge and scientific data reveal overexploitation by multigear artisanal fisheries in the Southwestern Atlantic. PLoS One 9:e110332. doi: 10.1371/journal.pone.0110332
Bentley, J. W., Hines, D. E., Borrett, S. R., Serpetti, N., Hernandez-Milian, G., Fox, C., et al. (2019). Combining scientific and fishers’ knowledge to co-create indicators of food web structure and function. ICES J. Mar. Sci. 76, 2218–2234. doi: 10.1093/icesjms/fsz121
Berkes, F. (1999). Sacred Ecology: Traditional Ecological Knowledge and Resource Management. Philadelphia, PA: Taylor and Francis.
Best, R. C. (1984). The Aquatic Mammals and Reptiles of the Amazon. Dordrecht: Dr. W. Junk Publishers, 371–412. doi: 10.1007/978-94-009-6542-3_15
Bevilacqua, A. H. V., Carvalho, A. R., Angelini, R., and Christensen, V. (2016). More than anecdotes: fishers’ ecological knowledge can fill gaps for ecosystem modeling. PLoS One 11:e0155655. doi: 10.1371/journal.pone.0155655
Campbell, E., Mangel, J. C., Alfaro-Shigueto, J., Mena, J. L., Thurstan, R. H., and Godley, B. J. (2020). Coexisting in the Peruvian Amazon: interactions between fisheries and river dolphins. J. Nat. Conserv. 56:125859. doi: 10.1016/j.jnc.2020.125859
Capitani, L., Angelini, R., Keppeler, F. W., Hallwass, G., Azevedo, R., and Silvano, M. (2021). Food web modeling indicates the potential impacts of increasing deforestation and fishing pressure in the Tapajós River, Brazilian Amazon. Reg. Environ. Change 21:42.
Carvalho, F., Power, M., Forsberg, B. R., Castello, L., Martins, E. G., and Freitas, C. E. C. (2018). Trophic ecology of Arapaima sp. in a ria lake—river–floodplain transition zone of the Amazon. Ecol. Freshw. Fish 27, 237–246. doi: 10.1111/eff.12341
Castello, L., Mcgrath, D. G., Hess, L. L., Coe, M. T., Lefebvre, P. A., Petry, P., et al. (2013). The vulnerability of Amazon freshwater ecosystems. Conserv. Lett. 6, 217–229. doi: 10.1111/conl.12008
Castilhos, Z. C., Bidone, E. D., and Lacerda, L. D. (1998). Increase of the background human exposure to mercury through fish consumption due to gold mining at the Tapajos river region, Para State, Amazon. Bull. Environ. Contam. Toxicol. 61, 202–209. doi: 10.1007/s001289900749
Cavole, L. M., Arantes, C. C., and Castello, L. (2015). How illegal are tropical small-scale fisheries? An estimate for arapaima in the Amazon. Fish. Res. 168, 1–5. doi: 10.1016/j.fishres.2015.03.012
Chiang, W. C., Chang, C. T., Madigan, D. J., Carlisle, A. B., Musyl, M. K., Chang, Y. C., et al. (2020). Stable isotope analysis reveals feeding ecology and trophic position of black marlin off eastern Taiwan. Deep. Res. Part II Top. Stud. Oceanogr. 175:104821. doi: 10.1016/j.dsr2.2020.104821
Clark, C. T., Horstmann, L., and Misarti, N. (2019). Lipid normalization and stable isotope discrimination in Pacific walrus tissues. Sci. Rep. 9:5843. doi: 10.1038/s41598-019-42095-z
Correa, S. B., and Winemiller, K. (2018). Terrestrial–aquatic trophic linkages support fish production in a tropical oligotrophic river. Oecologia 186, 1069–1078. doi: 10.1007/s00442-018-4093-7
Costa, A. F., Botta, S., Siciliano, S., and Giarrizzo, T. (2020). Resource partitioning among stranded aquatic mammals from Amazon and Northeastern coast of Brazil revealed through carbon and nitrogen stable isotopes. Sci. Rep. 10:12897. doi: 10.1038/s41598-020-69516-8
Dagosta, F. C. P., and de Pinna, M. (2019). The fishes of the Amazon: distribution and biogeographical patterns, with a compreehensive list of species. Bull. Am. Museum. Nat. Hist. 2019:163.
Dagosta, F. C. P., de Pinna, M., Peres, C. A., and Tagliacollo, V. A. (2020). Existing protected areas provide a poor safety-net for threatened Amazonian fish species. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 1167–1189. doi: 10.1002/aqc.3461
Dary, E. P., Ferreira, E., Zuanon, J., and Röpke, C. P. (2017). Diet and trophic structure of the fish assemblage in the mid-course of the Teles Pires river, Tapajós river basin, Brazil. Neotrop. Ichthyol. 15, 1–14. doi: 10.1590/1982-0224-20160173
De Niro, M. J., and Epstein, S. (1978). Influence of diet on the distribution of carbon isotopes in animals. Geochxm. Cosmochim. Acta 42, 495–506.
Ehrich, D., Tarroux, A., and Killengreen, S. T. (2011). Stable isotope analysis: modelling lipid normalization for muscle and eggs from arctic mammals and birds. Methods Ecol. Evol. 2, 66–76. doi: 10.1111/j.2041-210X.2010.00047.x
Elliott, K. H., Davis, M., and Elliott, J. E. (2014). Equations for lipid normalization of carbon stable isotope ratios in aquatic bird eggs. PLoS One 9:e83597. doi: 10.1371/journal.pone.0083597
Estes, J. A., Terborgh, J., Brashares, J. S., Power, M. E., Berger, J., Bond, W. J., et al. (2011). Trophic downgrading of planet earth. Science 333, 301–306. doi: 10.1126/science.1205106
Faial, K., Deus, R., Deus, S., Neves, R., Jesus, I., Santos, E., et al. (2015). Mercury levels assessment in hair of riverside inhabitants of the Tapajós River, Pará State, Amazon, Brazil: fish consumption as a possible route of exposure. J. Trace Elem. Med. Biol. 30, 66–76. doi: 10.1016/j.jtemb.2014.10.009
Fearnside, P. M. (2001). Environmental impacts of Brazil’s Tucuruí Dam: unlearned lessons for hydroelectric development in amazonia. Environ. Manage. 27, 377–396. doi: 10.1007/s002670010156
Fearnside, P. M. (2015). Amazon dams and waterways: Brazil’s Tapajós Basin plans. Ambio 44, 426–439. doi: 10.1007/s13280-015-0642-z
Flecker, A. S. (1996). Ecosystem engineering by a dominant detritivore in a diverse tropical stream. Ecology 77, 1845–1854. doi: 10.2307/2265788
Freitas, C. T., Espírito-Santo, H. M. V., Campos-Silva, J. V., Peres, C. A., and Lopes, P. F. M. (2020). Resource co-management as a step towards gender equity in fisheries. Ecol. Econ. 176:106709. doi: 10.1016/j.ecolecon.2020.106709
Geist, J. (2011). Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 11, 1507–1516. doi: 10.1016/j.ecolind.2011.04.002
Gerhardinger, L. C., Freitas, M. O., Bertoncini, ÁA., Borgonha, M., and Hostim-Silva, M. (2006). Collaborative approach in the study of the reproductive biology of the dusky grouper Epinephelus marginatus (Lowe, 1834) (Perciformes: Serranidae). Acta Sci. Biol. Sci. 28, 219–226.
Goulding, M., Barthem, R., and Ferreira, E. J. G. (2003). The Smithsonian Atlas of the Amazon. Washington, D.C: Smithsonian Books. doi: 10.4324/9780203028049
Hallwass, G., and Silvano, R. A. M. (2015). Patterns of selectiveness in the Amazonian freshwater fisheries: implications for management. J. Environ. Plan. Manag. 59, 1537–1559. doi: 10.1080/09640568.2015.1081587
Hallwass, G., da Silva, L. H., Nagl, P., Clauzet, M., and Begossi, A. (2020a). “Small-scale fisheries, livelihoods and food security of riverine people,” in Fish and Fisheries in the Brazilian Amazon: People, Ecology and Conservation in Black and Clear Water Rivers, (São Paulo: Springer International Publishing).
Hallwass, G., Lopes, P. F., Juras, A. A., and Silvano, R. A. M. (2011). Fishing effort and catch composition of Urban market and rural villages in Brazilian Amazon. Environ. Manage. 47, 188–200. doi: 10.1007/s00267-010-9584-1
Hallwass, G., Lopes, P. F., Juras, A. A., and Silvano, R. A. M. (2013). Fishers’ knowledge identifies environmental changes and fish abundance trends in impounded tropical rivers. Ecol. Appl. 23, 392–407. doi: 10.1890/12-0429.1
Hallwass, G., Schiavetti, A., and Silvano, R. A. M. (2020b). Fishers’ knowledge indicates temporal changes in composition and abundance of fishing resources in Amazon protected areas. Anim. Conserv. 23, 36–47. doi: 10.1111/acv.12504
Harada, M., Nakanishi, J., Yasoda, E., Pinheiro, M. D. C. N., Oikawa, T., De Assis Guimarães, G., et al. (2001). Mercury pollution in the Tapajos River basin, Amazon mercury level of head hair and health effects. Environ. Int. 27, 285–290. doi: 10.1016/S0160-4120(01)00059-9
Herbst, D. F., and Hanazaki, N. (2014). Local ecological knowledge of fishers about the life cycle and temporal patterns in the migration of mullet (Mugil liza) in Southern Brazil. Neotrop. Ichthyol. 12, 879–890. doi: 10.1590/1982-0224-20130156
Horn, M. H., Correa, S. B., Parolin, P., Pollux, B. J. A., Anderson, J. T., Lucas, C., et al. (2011). Seed dispersal by fishes in tropical and temperate fresh waters: the growing evidence. Acta Oecol. 37, 561–577. doi: 10.1016/j.actao.2011.06.004
Huntington, H. P. (2000). Using traditional ecological knowledge in science: methods and applications. Ecol. Appl. 10, 1270–1274.
Isaac, V. J., and Almeida, M. C. (2011). El Consumo de Pescado en la Amazonía Brasileña. COPESCAALC Documento Ocasional. No 13. Available online at: www.fao.org/icatalog/inter-e.htm (accessed July 8, 2021).
Jacobi, C. M., Villamarín, F., Campos-Silva, J. V., Jardine, T., and Magnusson, W. E. (2020). Feeding of Arapaima sp.: integrating stomach contents and local ecological knowledge. J. Fish Biol. 97, 265–272. doi: 10.1111/jfb.14372
Johannes, R. E., Freeman, M. M. R., and Hamilton, R. J. (2008). Ignore fishers’ knowledge and miss the boat. Fish Fish. 1, 257–271. doi: 10.1111/j.1467-2979.2000.00019.x
Johnson, D. S. (2006). Category, narrative, and value in the governance of small-scale fisheries. Marine Policy 30, 747–756. doi: 10.1016/j.marpol.2006.01.002
Junk, W. J., and Piedade, M. T. F. (2010). “An introduction to South American wetland forests: distribution, definitions and general characterization,” in Amazonian Floodplain Forests. Ecological Studies (Analysis and Synthesis), Vol. 210, eds W. Junk, M. Piedade, F. Wittmann, J. Schöngart, and P. Parolin (Dordrecht: Springer), 3–25. doi: 10.1007/978-90-481-8725-6_1
Junk, W. J., Soares, M. G. M., and Bayley, P. B. (2007). Freshwater fishes of the Amazon River basin: their biodiversity, fisheries, and habitats. Aquat. Ecosyst. Heal. Manag. 10, 153–173. doi: 10.1080/14634980701351023
Kelkar, N., Krishnaswamy, J., and Choudhary, S. (2010). Coexistence of fisheries with River Dolphin. Conserv. Biol. 24, 1130–1140. doi: 10.1111/j.1523-1739.2010.01467.x
Keppeler, F. W., Hallwass, G., and Silvano, R. A. M. (2017). Influence of protected areas on fish assemblages and fisheries in a large tropical river. Oryx 51, 268–279. doi: 10.1017/S0030605316000247
Latrubesse, E. M., Arima, E. Y., Dunne, T., Park, E., Baker, V. R., D’Horta, F. M., et al. (2017). Damming the rivers of the Amazon basin. Nature 546, 363–369. doi: 10.1038/nature22333
Latrubesse, E. M., d’Horta, F. M., Ribas, C. C., Wittmann, F., Zuanon, J., Park, E., et al. (2020). Vulnerability of the biota in riverine and seasonally flooded habitats to damming of Amazonian rivers. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 1136–1149. doi: 10.1002/aqc.3424
Latrubesse, E. M., Stevaux, J. C., and Sinha, R. (2005). Tropical rivers. Geomorphology 70, 187–206. doi: 10.1016/j.geomorph.2005.02.005
Le Fur, J., Guilavogui, A., and Teitelbaum, A. (2011). Contribution of local fishermen to improving knowledge of the marine ecosystem and resources in the Republic of Guinea, West Africa. Can. J. Fish. Aquat. Sci. 68, 1454–1469. doi: 10.1139/f2011-061
Lima, M. A. L., Doria, C. R. D. C., and Freitas, C. E. D. C. (2012). Pescarias artesanais em comunidades ribeirinhas na amazônia brasileira: perfil socioeconômico, conflitos e cenário da atividade. Ambient. Soc. 15, 73–90. doi: 10.1590/S1414-753X2012000200005
Loch, C., Marmontel, M., and Simões-Lopes, P. C. (2009). Conflicts with fisheries and intentional killing of freshwater dolphins (Cetacea: Odontoceti) in the Western Brazilian Amazon. Biodivers. Conserv. 18, 3979–3988. doi: 10.1007/s10531-009-9693-4
Logan, J. M., Jardine, T. D., Miller, T. J., Bunn, S. E., Cunjak, R. A., and Lutcavage, M. E. (2008). Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. J. Anim. Ecol. 77, 838–846. doi: 10.1111/j.1365-2656.2008.01394.x
Loh, T. L., McMurray, S. E., Henkel, T. P., Vicente, J., and Pawlik, J. R. (2015). Indirect effects of overfishing on Caribbean reefs: sponges overgrow reef-building corals. PeerJ 2015:e901. doi: 10.7717/peerj.901
Lopes, P. F. M., Verba, J. T., Begossi, A., and Pennino, M. G. (2018). Predicting species distribution from fishers’ local ecological knowledge: a new alternative for data-poor management. Can. J. Fish. Aquat. Sci. 76, 1423–1431. doi: 10.1139/cjfas-2018-0148
Lucas, C. M. (2008). Within flood season variation in fruit consumption and seed dispersal by two Characin fishes of the Amazon. Biotropica 40, 581–589.
Magnusson, W. E., Vieira Da Silva, E., and Lima, A. P. (1987). Diets of Amazonian crocodilians. J. Herpetol. 21, 85–95. doi: 10.2307/1564468
Malhi, Y., Roberts, J. T., Betts, R. A., Killeen, T. J., Li, W., and Nobre, C. A. (2008). Climate change, deforestation, and the fate of the Amazon. Science 319, 169–172. doi: 10.1126/science.1146961
McGrath, D. G., Cardoso, A., Almeida, O. T., and Pezzuti, J. (2008). Constructing a policy and institutional framework for an ecosystem-based approach to managing the Lower Amazon floodplain. Environ. Dev. Sustain. 10, 677–695. doi: 10.1007/s10668-008-9154-3
Melo, T., Torrente-Vilara, G., and Röpke, C. P. (2019). Flipped reducetarianism: a vegan fish subordinated to carnivory by suppression of the flooded forest in the Amazon. For. Ecol. Manage. 435, 138–143. doi: 10.1016/j.foreco.2018.12.050
Mérona, B., and Rankin-de-Mérona, J. (2004). Food resource partitioning in a fish community of the central Amazon floodplain. Neotrop. Ichthyol. 2, 75–84.
Mérona, B., Mendes dos Santos, G., and Gonçalves de Almeida, R. (2001). Short term effects of Tucuruí Dam (Amazonia, Brazil) on the trophic organization of fish communities. Environ. Biol. Fishes 60, 375–392. doi: 10.1023/A:1011033025706
Minagawa, M., and Wada, E. (1984). Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 48, 1135–1140. doi: 10.1016/0016-7037(84)90204-7
Mintenbeck, K., Brey, T., Jacob, U., Knust, R., and Struck, U. (2008). How to account for the lipid effect on carbon stable-isotope ratio (δ13C): sample treatment effects and model bias. J. Fish Biol. 72, 815–830. doi: 10.1111/j.1095-8649.2007.01754.x
Mont’Alverne, R., Jardine, T. D., Pereyra, P. E. R., Oliveira, M. C. L. M., Medeiros, R. S., Sampaio, L. A., et al. (2016). Elemental turnover rates and isotopic discrimination in a euryhaline fish reared under different salinities: implications for movement studies. J. Exp. Mar. Bio. Ecol. 480, 36–44. doi: 10.1016/j.jembe.2016.03.021
Mortillaro, J. M., Pouilly, M., Wach, M., Freitas, C. E. C., Abril, G., and Meziane, T. (2015). Trophic opportunism of central Amazon floodplain fish. Freshw. Biol. 60, 1659–1670. doi: 10.1111/fwb.12598
Myers, R. A., Baum, J. K., Shepherd, T. D., Powers, S. P., and Peterson, C. H. (2007). Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315, 1846–1850. doi: 10.1126/science.1138657
Newsome, S. D., Ralls, K., Van Horn Job, C., Fogel, M. L., and Cypher, B. L. (2010). Stable isotopes evaluate exploitation of anthropogenic foods by the endangered San Joaquin kit fox (Vulpes macrotis mutica). J. Mammal. 91, 1313–1321. doi: 10.1644/09-MAMM-A-362.1
Newsome, S. D., Tinker, M. T., Monson, D. H., Oftedal, O. T., Ralls, K., Staedler, M. M., et al. (2009). Using stable isotopes to investigate individual diet specialization in California sea otters (Enhydra lutris nereis). Ecology 90, 961–974.
Nunes, D. M., Hartz, S. M., and Silvano, R. A. M. (2011). Conhecimento ecológico local e científico sobre os peixes na pesca artesanal no sul do Brasil. Bol. Inst. Pesca 37, 209–223.
Olivar, M. P., Bode, A., Lo, C., and Hulley, P. A. (2018). Trophic position of lanternfishes (Pisces: Myctophidae) of the tropical and equatorial Atlantic estimated using stable isotopes. ICES J. Mar. Sci. 76, 649–661. doi: 10.1093/icesjms/fsx243
Oliveira, A. C. B., Soares, M. G. M., Martinelli, L. A., and Moreira, M. Z. (2006). Carbon sources of fish in an Amazonian floodplain lake. Aquat. Sci. 68, 229–238. doi: 10.1007/s00027-006-0808-7
Paine, R. T. (1980). Food webs: linkage, interaction strenght and community infrastructure. J. Anim. Ecol. 49, 666–685.
Pauly, D., Christensen, V., Dalsgaard, J., Froese, R., and Torres, F. (1998). Fishing down marine food webs. Science 279, 860–863. doi: 10.1126/science.279.5352.860
Pereyra, P. E. R., Mont’Alverne, R., and Garcia, A. M. (2016). Carbon primary sources and estuarine habitat use by two congeneric ariid catfishes in a subtropical coastal lagoon. Zoologia 33, 1–7. doi: 10.1590/S1984-4689zool-20150075
Pinnegar, J. K., and Polunin, N. V. C. (1999). Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Funct. Ecol. 13, 225–231. doi: 10.1046/j.1365-2435.1999.00301.x
Post, D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718.
Post, D. M., Layman, C. A., Arrington, D. A., Takimoto, G., Quattrochi, J., and Montaña, C. G. (2007). Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152, 179–189. doi: 10.1007/s00442-006-0630-x
Prudente, B., da, S., Carneiro-Marinho, P., Valente, R., de, M., Montag, L. F., et al. (2016). Feeding ecology of Serrasalmus gouldingi (Characiformes: Serrasalmidae) in the lower Anapu River region, Eastern Amazon, Brazil. Acta Amaz. 46, 259–270. doi: 10.1590/1809-4392201600123
Quezada-Romegialli, C., Jackson, A. L., Hayden, B., Kahilainen, K. K., Lopes, C., and Harrod, C. (2018). tRophicPosition, an r package for the Bayesian estimation of trophic position from consumer stable isotope ratios. Methods Ecol. Evol. 9, 1592–1599. doi: 10.1111/2041-210X.13009
R Development Core Team. (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ramires, M., Clauzet, M., Barrella, W., Rotundo, M. M., Silvano, R. A. M., and Begossi, A. (2015). Fishers’ knowledge about fish trophic interactions in the southeastern Brazilian coast. J. Ethnobiol. Ethnomed. 11:19. doi: 10.1186/s13002-015-0012-8
Reid, A. J., Carlson, A. K., Creed, I. F., Eliason, E. J., Gell, P. A., Johnson, P. T. J., et al. (2019). Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 94, 849–873. doi: 10.1111/brv.12480
Ribeiro, A. R., Damasio, L. M. A., and Silvano, R. A. M. (2021). Fishers’ ecological knowledge to support conservation of reef fish (groupers) in the tropical Atlantic. Ocean Coast. Manag. 204:105543. doi: 10.1016/j.ocecoaman.2021.105543
Runde, A., Hallwass, G., and Silvano, R. A. M. (2020). Fishers’ knowledge indicates extensive socioecological impacts downstream of proposed dams in a Tropical River. One Earth 2, 255–268. doi: 10.1016/j.oneear.2020.02.012
Salomão, R. D. P., Vieira, I. C. G., Suemitsu, C., Rosa, N., de, A., de Almeida, S. S., et al. (2007). As florestas de Belo Monte na grande curva do Xingu, Amazônia Oriental. Bol. Mus. Para. Emilio Goeldi Cienc. Nat. 2, 57–153.
Santos, R. E., Pinto-Coelho, R. M., Drumond, M. A., Fonseca, R., and Zanchi, F. B. (2020). Damming Amazon Rivers: environmental impacts of hydroelectric dams on Brazil’s Madeira River according to local fishers’ perception. Ambio 49, 1612–1628. doi: 10.1007/s13280-020-01316-w
Scheffer, M., Carpenter, S., and De Young, B. (2005). Cascading effects of overfishing marine systems. Trends Ecol. Evol. 20, 579–581. doi: 10.1016/j.tree.2005.08.018
Shin, Y. J., Rochet, M. J., Jennings, S., Field, J. G., and Gislason, H. (2005). Using size-based indicators to evaluate the ecosystem effects of fishing. ICES J. Mar. Sci. 62, 384–396. doi: 10.1016/j.icesjms.2005.01.004
Silva, R. E., Rosas, F. C. W., and Zuanon, J. (2013). Feeding ecology of the giant otter (Pteronura brasiliensis) and the Neotropical otter (Lontra longicaudis) in Jaú National Park, Amazon, Brazil. J. Nat. Hist. 48, 465–479. doi: 10.1080/00222933.2013.800607
Silva, V. M. F., Freitas, C. E. C., Dias, R. L., and Martin, A. R. (2018). Both cetaceans in the Brazilian Amazon show sustained, profound population declines over two decades. PLoS One 13:e0191304. doi: 10.1371/journal.pone.0191304
Silvano, R. A. M., and Begossi, A. (2002). Ethnoichthyology and fish conservation in the Piracicaba River (Brazil). J. Ethnobiol. 22, 285–306.
Silvano, R. A. M., and Begossi, A. (2005). Local knowledge on a cosmopolitan fish: Local knowledge on a cosmopolitan fish Ethnoecology of Pomatomus saltatrix (Pomatomidae) in Brazil and Australia ethnoecology of Pomatomus saltatrix (Pomatomidae) in Brazil and Australia. Fish. Res. 71, 43–59. doi: 10.1016/j.fishres.2004.07.007
Silvano, R. A. M., and Begossi, A. (2012). Fishermen’s local ecological knowledge on southeastern Brazilian coastal fishes: contributions to research, conservation, and management. Neotrop. Ichthyol. 10, 133–147. doi: 10.1590/S1679-62252012000100013
Silvano, R. A. M., and Begossi, A. (2016). From ethnobiology to ecotoxicology: fishers’ knowledge on trophic levels as indicator of bioaccumulation in tropical marine and freshwater fishes. Ecosystems 19, 1310–1324. doi: 10.1007/s10021-016-0002-2
Silvano, R. A. M., and Valbo-Jørgensen, J. (2008). Beyond fishermen’s tales: contributions of fishers’ local ecological knowledge to fish ecology and fisheries management. Environ. Dev. Sustain. 10, 657–675. doi: 10.1007/s10668-008-9149-0
Sioli, H. (1984). The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and its Basin. Dordrecht: Springer. doi: 10.1007/978-94-009-6542-3
Souza, O. G. Jr., Nunes, J. L. G., and Silvano, R. A. M. (2020). Biology, ecology and behavior of the acoupa weakfish Cynoscion acoupa (Lacepède, 1801) according to the local knowledge of fishermen in the northern coast of Brazil. Mar. Policy 115:103870. doi: 10.1016/j.marpol.2020.103870
Sweeting, C. J., Polunin, N. V. C., and Jennings, S. (2006). Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Commun. Mass Spectrom. 20, 595–601. doi: 10.1002/rcm.2347
Turvey, S. T., Barrett, L. A., Yujiang, H., Lei, Z., Xinqiao, Z., Xianyan, W., et al. (2010). Cambios rápidos en las directrices en comunidades de pescadores de yangtze y en la memoria local de especies extintas. Conserv. Biol. 24, 778–787. doi: 10.1111/j.1523-1739.2009.01395.x
Vasconcellos, A., Hallwass, G., Bezerra, J., Serrão, A., Meneses, H., Lima, M., et al. (2021). Health risk assessment from consumption of mercury-contaminated fish in Munduruku Indigenous Communities in Amazon. Int. J. Environ. Res. Public Heal. 18, 1–16.
Veloso, H. P., Rangel Filho, A. L. R., and Lima, J. C. A. L. (1991). Classificação da Vegetação Brasileira, Adaptada a um Sistema Universal. Rio de Janeiro: IBGE.
Vidal, M. D., de Moura, M. F., and Muniz, G. P. S. (2019). Conhecimentos e crenças de pescadores artesanais sobre os golfinhos fluviais do Médio Rio Tapajós, Pará. Rev. Bras. Biociências 17, 53–60.
Vinson, M. R., and Angradi, T. R. (2011). Stomach emptiness in fishes: sources of variation and study design implications. Rev. Fish. Sci. 19, 63–73. doi: 10.1080/10641262.2010.536856
Wedemeyer-Strombel, K. R. (2019). Fishers’ Ecological Knowledge and Stable Isotope Analysis: A Social-Ecological Systems Approach to Endangered Species Conservation. Available online at: https://scholarworks.utep.edu/open_etd/185 (accessed July 8, 2021).
Welcomme, R. L. (1999). A review of a model for qualitative evaluation of exploitation levels in multi-species fisheries. Fish. Manag. Ecol. 6, 1–19.
Winemiller, K. O., Montoya, J. V., Roelke, D. L., Layman, C. A., and Cotner, J. B. (2006). Seasonally varying impact of detritivorous fishes on the benthic ecology of a tropical floodplain river. J. North Am. Benthol. Soc. 25, 250–262.
Winemiller, K. O., Nam, S., Baird, I. G., Darwall, W., Lujan, N. K., Harrison, I., et al. (2016). Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 351, 128–129.
Zapelini, C., Giglio, V. J., Carvalho, R. C., Bender, M. G., and Gerhardinger, L. C. (2017). Assessing fishing experts’ knowledge to improve conservation strategies for an endangered grouper in the Southwestern Atlantic. J. Ethnobiol. 37, 478–493. doi: 10.2993/0278-0771-37.3.478
Keywords: fish ecology, food webs, local ecological knowledge, stable isotopes, Tapajós River, Tocantins River
Citation: Pereyra PER, Hallwass G, Poesch M and Silvano RAM (2021) ‘Taking Fishers’ Knowledge to the Lab’: An Interdisciplinary Approach to Understand Fish Trophic Relationships in the Brazilian Amazon. Front. Ecol. Evol. 9:723026. doi: 10.3389/fevo.2021.723026
Received: 09 June 2021; Accepted: 26 August 2021;
Published: 16 September 2021.
Edited by:
Thiago Gonçalves-Souza, Federal Rural University of Pernambuco, BrazilReviewed by:
Alejandro Casas, Universidad Nacional Autónoma de México, MexicoCopyright © 2021 Pereyra, Hallwass, Poesch and Silvano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paula Evelyn Rubira Pereyra, cGF1bGluaGFydWJpcmFAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.