
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 02 August 2021
Sec. Ecophysiology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.722218
This article is part of the Research TopicImmunity and Disease of Aquatic Organisms Under the Combined Impact of Anthropogenic Stressors: Mechanisms and Disease OutcomesView all 7 articles
 Aleksei Krasnov1*
Aleksei Krasnov1* Erik Burgerhout1
Erik Burgerhout1 Hanne Johnsen1,2
Hanne Johnsen1,2 Helge Tveiten1,3
Helge Tveiten1,3 Anne F. Bakke4
Anne F. Bakke4 Hege Lund4
Hege Lund4 Sergey Afanasyev5
Sergey Afanasyev5 Alexander Rebl6
Alexander Rebl6 Lill-Heidi Johansen1
Lill-Heidi Johansen1Atlantic salmon is characterized with high sensitivity to low dissolved oxygen (DO) levels. Hypoxia can affect diverse biological processes with consequences that can be manifested immediately or with delay. Effects of hypoxia on the immune system and the resistance to a bacterial pathogen were investigated. Two groups were reared at, respectively, normal (NO, 80–100%) and low (LO, 60%) levels of DO over 10 months after which both groups were reared at NO. Smoltification was initiated after 13 months by a winter signal for 6 weeks, followed by constant light for 6 weeks. Samples were collected at the start and end of the constant light period. Expression of 92 immune and stress genes was analyzed in the gill, head kidney, and spleen using a Biomark HD. Most of differentially expressed genes showed higher levels in LO fish compared to NO fish; many immune genes were downregulated during smoltification and these changes were stronger in NO fish. A notable exception was pro-inflammatory genes upregulated in gill of NO fish. Further, salmon were challenged with Moritella viscosa, the causative agent of winter ulcer. Mortality was registered from 5 days post infection (dpi) to the end of trial at 36 dpi. Survival was consistently higher in NO than LO fish, reaching a maximum difference of 18% at 21–23 dpi that reduced to 10% at the end. Analyses with a genome-wide microarray at 36 dpi showed strong responses to the pathogen in gill and spleen. Notable features were the stimulation of eicosanoid metabolism, suggesting an important role of lipid mediators of inflammation, and the downregulation of chemokines. Many immune effectors were activated, including multiple lectins and acute phase proteins, enzymes producing free radicals, and matrix metalloproteinases. The transcriptomic changes induced with a bacterial challenge were similar in NO and LO. After the challenge, interferons a and g and panel of genes of innate antiviral immunity showed higher expression in LO, especially in the gill. The results from the present study suggest that chronic hypoxia in early life stimulated immune genes and attenuated their downregulation associated with smoltification. However, these changes did not improve protection against a bacterial pathogen of major concern in salmon aquaculture.
Salmonid species are evolutionarily adapted to pristine habitats with cold water and are characterized with high oxygen requirements and sensitivity to oxygen deficiency (Davis, 1975; Metcalfe et al., 1995; Remen et al., 2013). Hypoxic [low dissolved oxygen (DO) levels] conditions are often encountered in nature as well as in aquaculture environments. As DO levels play a crucial role in the maintenance of many biochemical and physiological processes, hypoxia can negatively affect development, growth, reproduction, and survival (Randall et al., 1982; Greig et al., 2006; Wang et al., 2009, 2016). The risk of hypoxia is especially high in the production units with a forced water supply including recirculation aquaculture systems (RAS) and in sea cages with large stocking densities (Remen et al., 2013; Kolarevic et al., 2016).
There is a constantly growing interest regarding the effects of hypoxia on the development, performance, and robustness of Atlantic salmon, which is associated with the expanding use of RAS in commercial aquaculture and expected climate change leading to an increase of water temperature in the sea. Many recent publications have addressed direct and remote consequences of low oxygen levels on Atlantic salmon with the observed effects ranging from complete recovery to significant changes. Daily cyclic hypoxia over 23 days with DO levels of 70% and below significantly reduced feed intake in post-smolts (Remen et al., 2012), and a 120 day exposure to low oxygen levels in large (1.5–2 kg) post-smolts showed a significant reduction in growth performance, and gene expression of liver tissue indicated clear effects of hypoxia on metabolic and protein catabolic pathways (Olsvik et al., 2013). Also, a cumulative effect of high temperature and hypoxia was found on growth and feed consumption in post-smolts (Gamperl et al., 2020). Interestingly, through metabolic measurements by swimming trials, it was suggested that especially smaller salmon (200 g) may be more vulnarable to hypoxia (50%) than their larger counterparts (3.5 kg; Oldham et al., 2019). Exposure to hypoxia from fertilization until start feeding did not appear to influence tolerance to hypoxia later in life (Wood et al., 2019, 2020).
A number of studies have focused on the immediate effects of hypoxia on disease resistance and expression of immune genes in salmonid fish. For example, hypoxia appeared not to differentially affect the severity of pancreas disease in salmon infected with salmonid alphavirus (Andersen et al., 2010). On the other hand, in Atlantic salmon post-smolts infected with amoebe Neoparamoeba perurans (the agent of amoebic gill disease), cyclic hypoxia accelerated the progression of the disease and increased amoeba counts and mortality (Oldham et al., 2020). Niklasson et al. (2011) showed that low DO levels affected the mucosal immune system of the intestine, through downregulations of nuclear factor kappa B, and differential expression of interleukins in combination with higher temperature. Furtermore, chronic hypoxia (∼50% DO) in Atlantic salmon post-smolts resulted in a distinct change of the immune response toward a viral or bacterial challenge in vitro as well as in vivo (Kvamme et al., 2013). Although the response to a bacterial challenge somewhat differed between the normoxic control and hypoxic groups, Atlantic salmon were able to mount a strong innate immune response (Zanuzzo et al., 2020). In Oncorhynchus kisutch, hypoxia generated a cortisol stress response ≤35% DO levels and several toll-like receptors and cytokines of the innate and adaptive immune response were differentially regulated (Martínez et al., 2020). Transcriptome analyses revealed combined effects of hypoxia and high water temperature (20°C) on hepatic expression of immune and stress genes in Atlantic salmon (Beemelmanns et al., 2021).
We report the immune changes induced in early development and manifested over a long period. Recently, our group showed the effects of hypoxia during early life stages on the transcriptome in start feeding larvae of Atlantic salmon (Kelly et al., 2020). This research was performed as a part of a large trial that investigated the programming effects of the environment during early life. In the present follow-up study, we investigated the prolonged effects of early life stage hypoxia. Two study groups reared at 60 and 80–100% saturation of DO were compared by the expression of 92 immune and stress genes using a test developed for assessment of the immune competence of Atlantic salmon on BioMark HD platform – ImCom (Krasnov et al., 2020). Further, challenge with Moritella viscosa, a causative agent of winter ulcer (Løvoll et al., 2009), was followed with transcriptome analyses. Despite widespread vaccination with multi-component vaccines containing the M. viscosa antigen, disease outbreaks continue to be reported in high numbers in seawater reared Atlantic salmon in Norway (Karlsen et al., 2017).
All fish handling procedures employed in the study were in accordance with national (Approval ID 11814) and EU legislation (2010/63/EU) on animal experimentation. Directly after fertilization, Atlantic salmon (AquaGen strain) eggs were kept at 60% (LO) and 80–100% NO DO levels for approximately 10 months (Figure 1). Oxygen levels above 80% are relevant to aquaculture operation and do not affect salmon growth. Eggs from each of the two groups were kept in triplicate tanks (for more details, see Kelly et al., 2020). After 10 months, the fish were PIT-tagged for individual identification and kept at 80–100% DO. At 14 months, smoltification was induced by the following light regimen: 6 weeks of 18 h darkness and 6 h light (winter), followed by 6 weeks of 24 h light (summer). Temperature during the first month after hatching was 7°C, after that the fish were kept at 10°C for the rest of the experimental period. The smoltification process was followed by seawater challenge tests (n = 10 per group) at 0, 3, and 6 weeks after the start of summer period. After 24 h, they were sacrificed using an overdose of Benzoak (0.3 mL/L) and blood was sampled for serum cortisol concentration and gill Na ATPase activity (Eurofins) analyses, to assess the development of hypo-osmoregulatory capacity. Both groups were adapted to seawater after the summer period, and no difference in plasma cortisol levels and ATPase activity was observed between the groups during smoltification. In the part of the trial described here, a total of 276 fish were used: 60 for the seawater challenge tests and 216 fish for the following bacterial challenge test. No mortalities were registered before the bacterial challenge test was started. No significant weight differences between the two groups were registered during the experimental period.
A bacterial challenge test with M. viscosa was performed after smoltification, at 17 months and at mean weight 81.2 ± 10.2 g. A total of 108 fish from each of the two groups were transferred to seawater in two 900-l circular tanks, 54 fish from each group per tank, at 10°C, and acclimated for 5 days before bath challenge (stagnant water, density 52 kg/m3, oxygenated, duration 1 h) with M. viscosa (LFI5006/2), originally isolated from the head kidney of farmed Atlantic salmon in northern Norway suffering from winter ulcer. Bacteria from frozen stock culture were grown at 12°C on blood agar (Oxoid) with 2% NaCl for 48 h and single colonies transferred to 20 ml Marine Broth (2216 Difco) and grown for 48 h at 12°C with shaking before inoculation of 600 ml MB and further growth for 48 h at 12°C. Before challenge, the OD600 nm was measured and challenge dose used was 107 cfu/ml. Challenge dose was confirmed by titration of the bacterial culture used for challenge on blood agar plates with 2% NaCl and counting of colonies. After the end of bath challenge, the fish were kept in running water at 10°C and density ca 30 kg/m3. Any background mortality during the challenge experiment was monitored in uninfected fish from the same groups and kept under the same conditions as the infected fish. Samples of gill, head kidney, and spleen were collected, at day 0 (start of continuous light of the smoltification process), day 42 of light stimulation (smolts), and 5 days before pathogen challenge, and at the end of the challenge trial. Mortality was registered for 36 days. Verification of M. viscosa as the cause of death was done by gross pathology (specifically wound development) and isolation of the pathogen from the head kidney samples of moribund fish on blood agar with 2% NaCl. At termination, all fish from the two tanks were scored for wounds, and 30 fish from each tank (five per group) were sampled for blood (plasma, erythrocytes), gill, skin, head kidney, and spleen in RNAlater.
Total RNA and DNA were isolated and purified using AllPrep DNA/RNA/miRNA Universal Kit (Qiagen) according to the manufacturer’s protocol. RNA quantity and quality were determined with Nanodrop (Thermo Scientific) and Bioanalyzer (Agilent).
To assess the immune competence (ImCom) of Atlantic salmon smolts and growers, a multigene expression assay representing the key functional groups of the immune system was designed on Biomark HD platform (Fluidigm; Krasnov et al., 2020). The first version of ImCom was used that includes 92 immune and stress genes, which were selected by the expression profiles in many challenge trials with pathogens and under inflammatory conditions, and reference genes. Analyses were performed in the gill, spleen, and head kidney of fish collected at the start and end of constant light stimulation (days 0 and 42, n = 6, totally 72 samples). The extracted RNA was adjusted at 10 ng/5 μl and reverse-transcribed using the Reverse transcription master mix (Fluidigm). Subsequently, the individual cDNA samples were added to the aforementioned 96 primer pairs (100 μM) and the PreAmp master mix (Fluidigm) and subjected to 12 pre-amplification cycles in a standard thermocycler (TAdvanced, Biometra). The pre-amplified products were treated with exonuclease I (New England BioLabs) and diluted in a SoFast EvaGreen supermix with Low ROX (Bio-Rad) and 20× DNA-binding dye sample loading reagent. The sample and primer mixes were transferred to the respective inlets of two 48.48 dynamic array IFC chips. These chips were individually primed in the BioMark IFC controller MX (Fluidigm) according to the Load mix 48.48 GE script. The loaded array chips were then placed in the BioMark HD system (Fluidigm) to proceed with the qPCR according to the GE 48 × 48 Fast PCR + Melt v2.pcl cycling program. Fluidigm RealTime PCR analysis software v. 3.0.2 was used to retrieve raw qPCR results, which were transferred in a relational database. The geometric means of two reference genes: elongation factor 1-alpha 1 and 40s ribosomal protein s20 (eef1a1b and rps20), which showed stability across samples, were used for calculation of ΔCt values. Further, the average for each gene was calculated for the entire data set and subtracted from each data point. Differential expression between the treatment groups and time-points was assessed by criteria: difference of ΔΔCt > | 0.8| and p < 0.05 (t-test).
Transcriptome analyses were carried out on gill and spleen of uninfected and challenged salmon with Nofima’s Atlantic salmon genome-wide 42.5 k DNA oligonucleotide microarray Salgeno-2 (GPL28080), totally 41 microarrays were used (n = 5). Microarrays were manufactured by Agilent Technologies, and the reagents and equipment were purchased from the same provider. Genes are annotated in Nofima’s bioinformatic pipeline STARS using GO, KEGG, and custom vocabulary (Krasnov et al., 2011a). RNA amplification and labeling were performed with a One-Color Quick Amp Labeling Kit and a Gene Expression Hybridization kit was used for fragmentation of labeled RNA. Total RNA input for each reaction was 500 ng. After overnight hybridization in an oven (17 h, 65°C, rotation speed 0.01 g), arrays were washed with Gene Expression Wash Buffers 1 and 2 and scanned with Agilent scanner. Subsequent data analyses were carried out with STARS. Global normalization was performed by equalizing the mean intensities of all microarrays. The individual values for each feature were divided by the mean value of all samples thus producing expression ratios (ER). The log2-ER were calculated and normalized with the locally weighted non-linear regression (Lowess). The differentially expressed genes (DEG) were selected by criteria: log2-ER > 0.8 (1.75-fold) and p < 0.05 (t-test). Enrichment of functional categories of gene Ontology (GO) was evaluated by comparing the numbers of genes per term in the lists of DEG, and on the microarray platform, significance was assessed with Yates’ corrected chi-square test. Data were submitted to NCBI GEO Omnibus (GSE171693).
The expression profiles of immune and stress genes in the lymphatic organs and gill were overall similar although the numbers and composition of DEG varied (Figures 2, 3). At day 0, the numbers of immune genes with lower expression in NO compared to LO ranged from 11 genes in the spleen to 19 genes in the head kidney and gill, while only one gene was upregulated in the spleen. During smoltification, many immune genes were downregulated, which is in concordance with our previous observations (Johansson et al., 2016; Robinson et al., 2017; Karlsen et al., 2018). The number of genes with decreased expression at day 42 was larger in tissues of salmon that had not been exposed to hypoxia: 42 versus 14 genes in the spleen and 28 versus 7 genes in the gill. Similar numbers of genes were downregulated in the head kidneys of LO and NO and only a few genes showed significant difference between the treatment groups at day 42, which was explained with high variance in LO. Although the decrease of expression in the end of smoltification clearly prevailed, a suite of genes showed upregulation at day 42, especially in the gill (Figure 3). A group of 15 genes included several markers of acute inflammation, such as chemokine lect2 (Mutoloki et al., 2010), a component of oxidative burst complex neutrophil cytosolic factor 1 and collagen degrading matrix metalloproteinases mmp9 and mmp13. Macrophage receptor marco (Poynter et al., 2017) and immunoglobulin receptor are involved in phagocytosis. Upregulation of these genes has been observed in Atlantic salmon under various diseases and inflammatory conditions (submitted manuscript). At day 0, the mean expression was 1.9-fold higher in LO. This group also showed a smaller reduction associated with smoltification (1.9-fold in NO and 1.5-fold in LO), the difference between the groups increased to 2.4-fold at day 42.
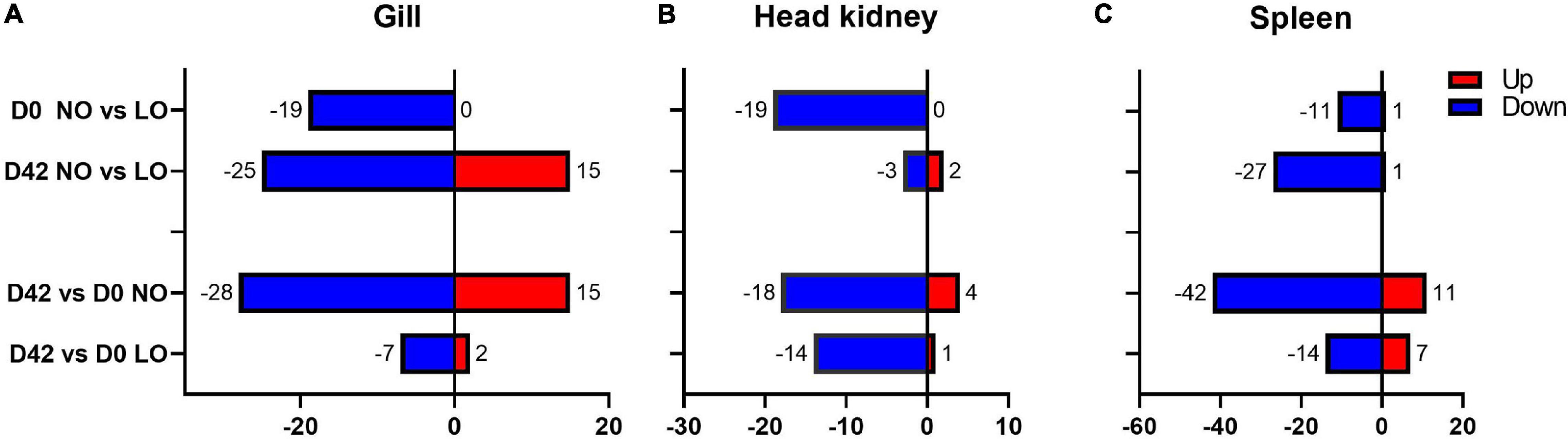
Figure 2. Gene expression in the beginning and end of light stimulation of smoltification analyzed with a multigene Biomark HD assay including 92 immune and stress genes (ImCom). Numbers of genes with expression differences between the time-points (days 42 and 0) and the treatment groups in gill (A), head kidney (B), and spleen (C). NO, LO – groups maintained at normal and low dissolved oxygen.
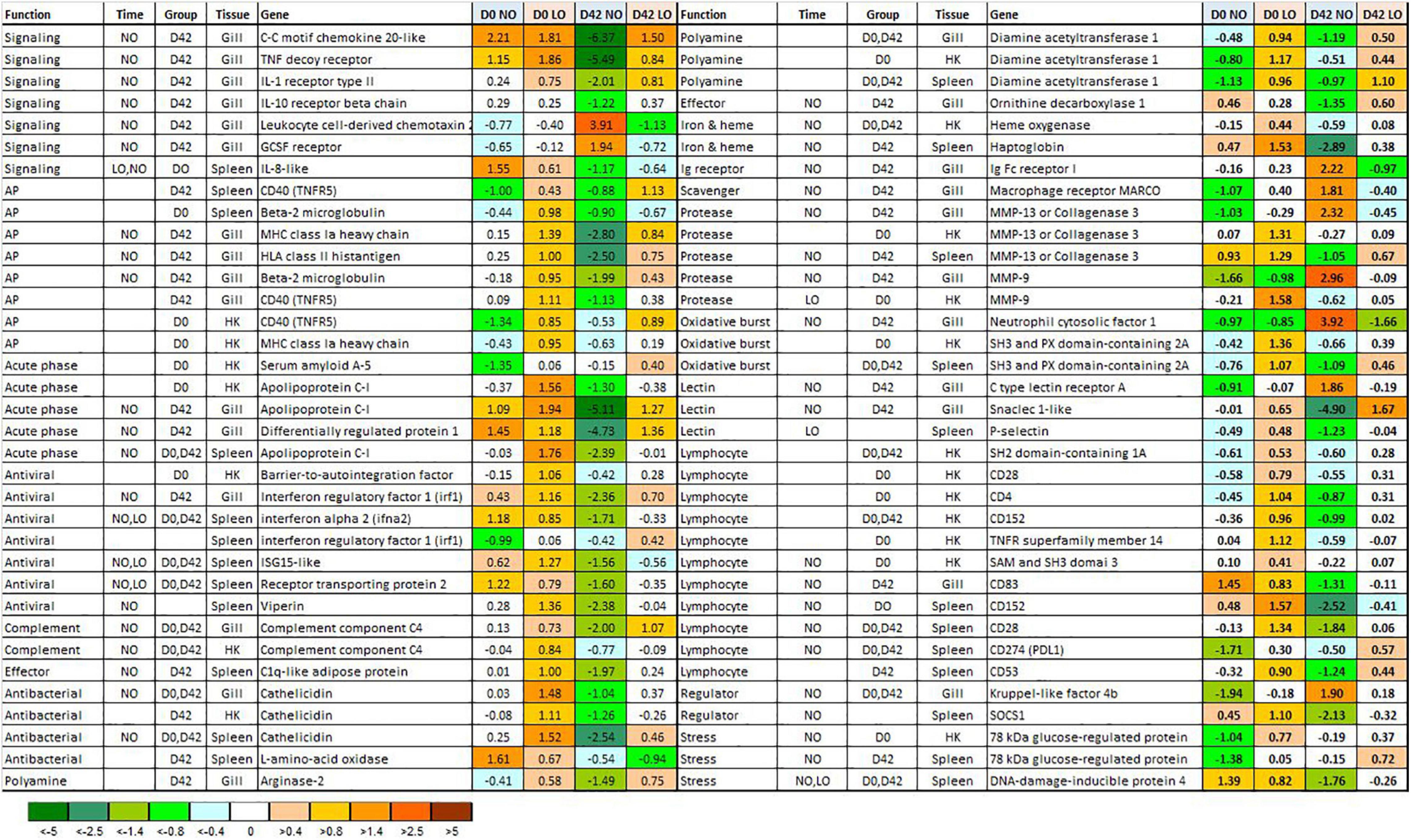
Figure 3. Differentially expressed genes BioMark HD assay. Data (ΔΔCt) are normalized so that the mean value for each gene is equal to zero. Significant differences between days 0 and 42 (beginning and end of light stimulation) and the treatment groups are denoted in columns Time and Group. NO, LO – normal and low dissolved oxygen. NO, LO – groups maintained at normal and low dissolved oxygen.
Four DEG with different immune roles showed similar expression profiles in the gill, head kidney, and spleen: an antibacterial peptide cathelicidin (Shinnar et al., 2003), diamine acetyltransferase 1, an enzyme of polyamine metabolism, apolipoprotein c-I, a cholesterol transporter involved in protection against pathogens (Fuior and Gafencu, 2019), and cd40 (tnfr5) a costimulatory protein of antigen presenting cells (Figure 3). Despite similar trends with respect to the treatment groups and time-points, the composition of DEG in the tissues was different. The number of DEG encoding cytokines, chemokines, and receptors was highest in the gill, similar to the genes involved in antigen presentation (respectively, six and four genes). Innate antiviral responses usually show the strongest suppression during smoltification. Four genes from this group [interferon a, and highly active virus responsive genes – VRG (Krasnov et al., 2011b) receptor transporting protein, viperin and isg15] were downregulated in the spleen. Several markers of T cells showed lower expression in the lymphatic organs of NO: cd4 and cd28 in the head kidney and cd28, cd152, and cd274 in the spleen. Difference between the treatment groups involved diverse functional groups and pathways of the immune system.
The first mortality in challenged salmon was recorded at 5 dpi in LO fish and at 8 dpi in NO fish (Figure 4). The difference peaked at 18% at 20–25 dpi (p < 0.05). At the end of trial at 36 dpi, mortality remained 10% lower in NO, although not significantly (Figure 3). Overall, LO showed a trend toward higher mortality throughout the trial. No significant differences in the mortality were registered in the replicate tanks during the challenge period, and data are presented as mean cumulative mortality of the two tanks.
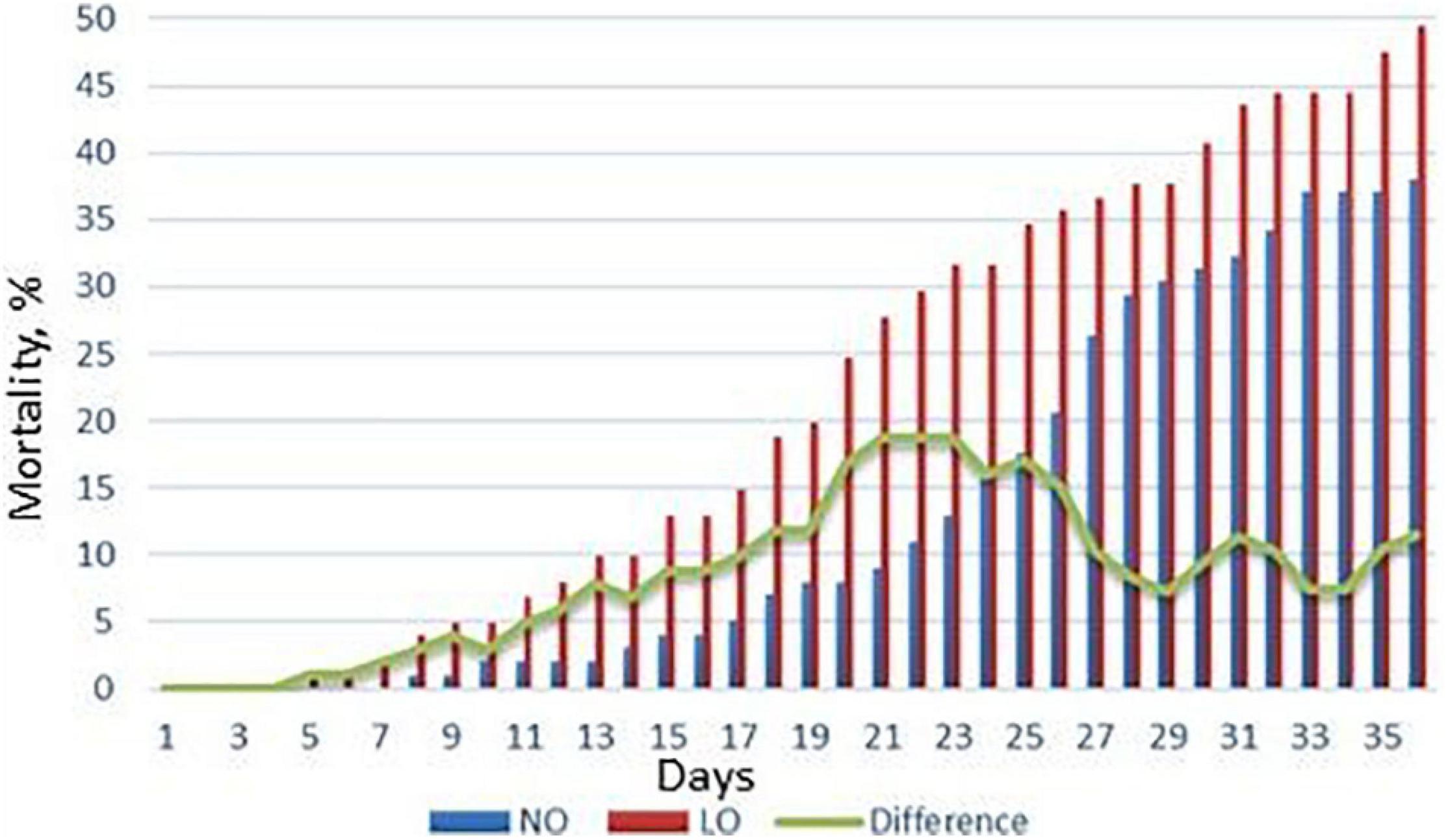
Figure 4. Mortality of Atlantic salmon challenged with M. viscosa. NO, LO – groups maintained at normal and low dissolved oxygen. Data presented are mean cumulative mortality from two replicate tanks.
The magnitude of transcriptome responses to the infection with M. viscosa reflected by the numbers of DEG was similar in the experimental groups and expression changes in the spleen were stronger in comparison with gill (Table 1). In spite of relatively small numbers of immune genes (from 7.7 to 13.1% of all DEG), the terms related to the immune system prevailed among the enriched functional categories of GO (Table 2). The enrichment analysis and inspection of DEG suggested strong and complex defensive responses to the bacterial pathogen. Putative homologs of pathogen recognition Toll-like receptors (tlr12 and tlr8) were upregulated in both tissues (Figure 5) and spleen (Figure 6B), respectively. Lipid mediators seemed to play a key part in cell signaling and communication. Six genes involved in eicosanoid metabolism were upregulated in both tissues (Figure 5). Two genes including phospholipase a2, which releases fatty acids precursors of eicosanoids from phospholipids, were activated in the gill and seven genes were upregulated in the spleen (Figure 6). Concurrent downregulation of four chemokines in the spleen and 11 chemokines in the gill suggested a trade-off between these two signaling systems. Reduced levels of chemokines transcripts in gill also indicated possible migration of immune cells to the infected sites or redistribution of cell populations in this organ. This could explain an apparent downregulation of several immune genes, such as free radicals producing inducible nitric oxide synthase (22- and 10-fold in, respectively, NO and LO fish), cytochrome b245, and neutrophil cytosolic factors – ncf2 (two other ncf2 were upregulated). Similar changes were observed in the complement factors c7 and c8 beta, rnase zf-3, an antibacterial effector with strong expression changes in gill of Atlantic salmon (Zanfardino et al., 2010; Król et al., 2020), and several other immune genes (Figure 5A). The pathogen stimulated both humoral and cellular immune responses. Diverse immune effectors were involved including antibacterial and acute phase proteins, components of the oxidative burst complex, serine and matrix metalloproteases (mmp 9 and mmp 13), and TNF-inducible metalloreductase steap 4; a hallmark of Atlantic salmon responses to M. viscosa in this trial was upregulation of a large set of lectins in both tissues and especially in the spleen. Five genes showed opposite changes in tissues including three chemokines, cathelicidin, and differentially regulated trout protein (Figure 5).
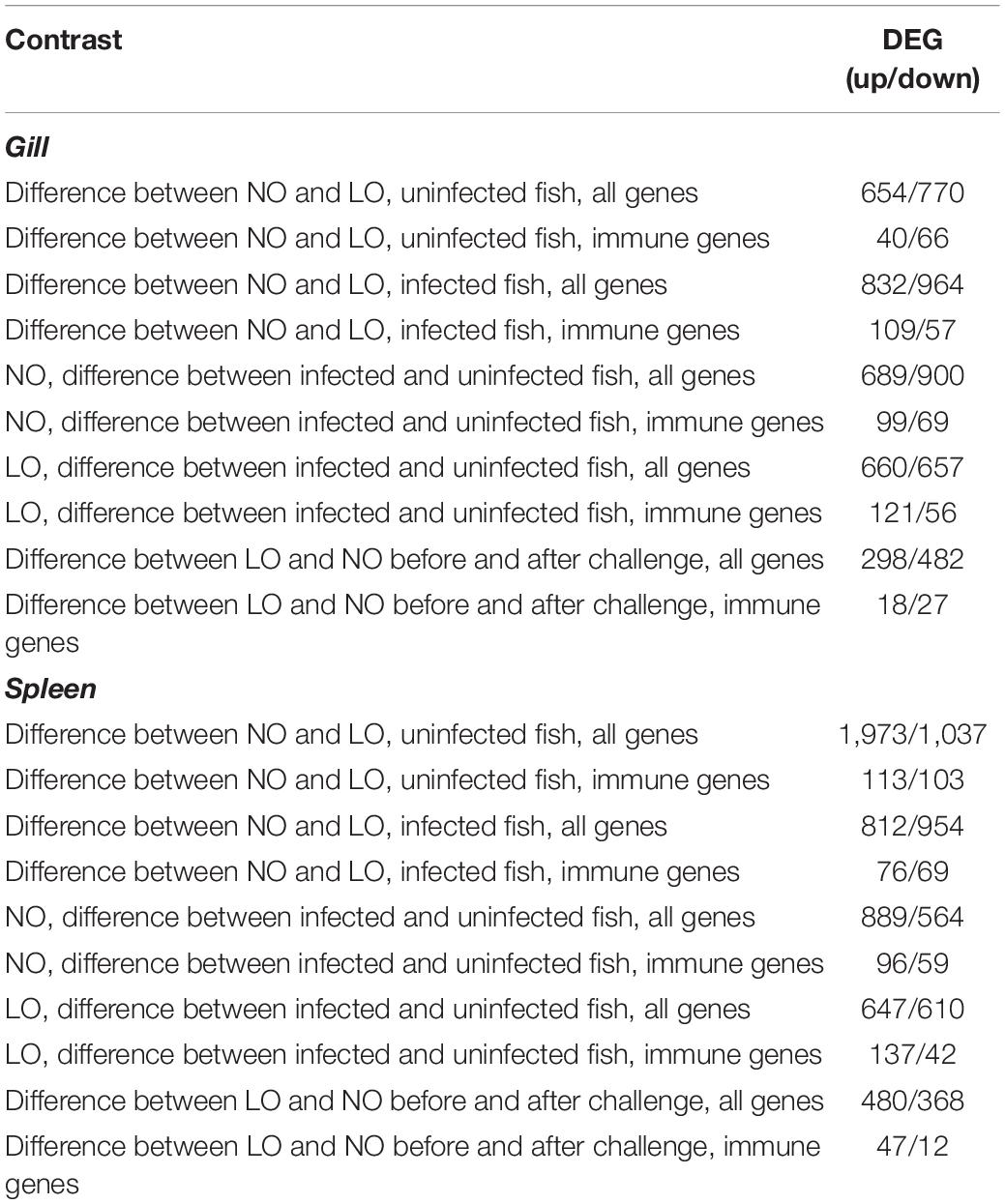
Table 1. The numbers of genes with differential expression between the treatment groups and uninfected and infected fish (microarray analyses).
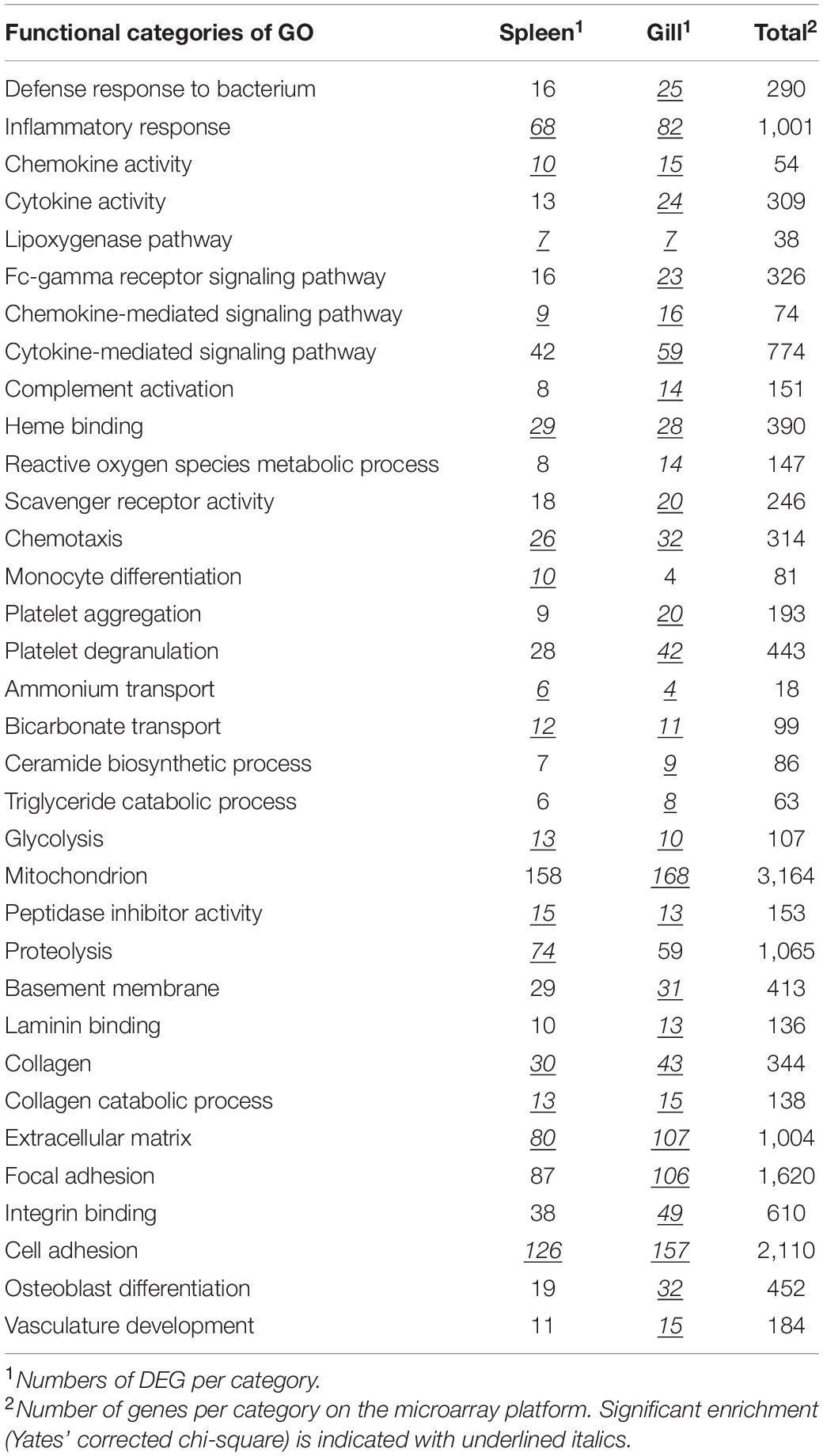
Table 2. Enrichment of functional categories of GO in the list of genes that responded to challenge with M. viscosa.
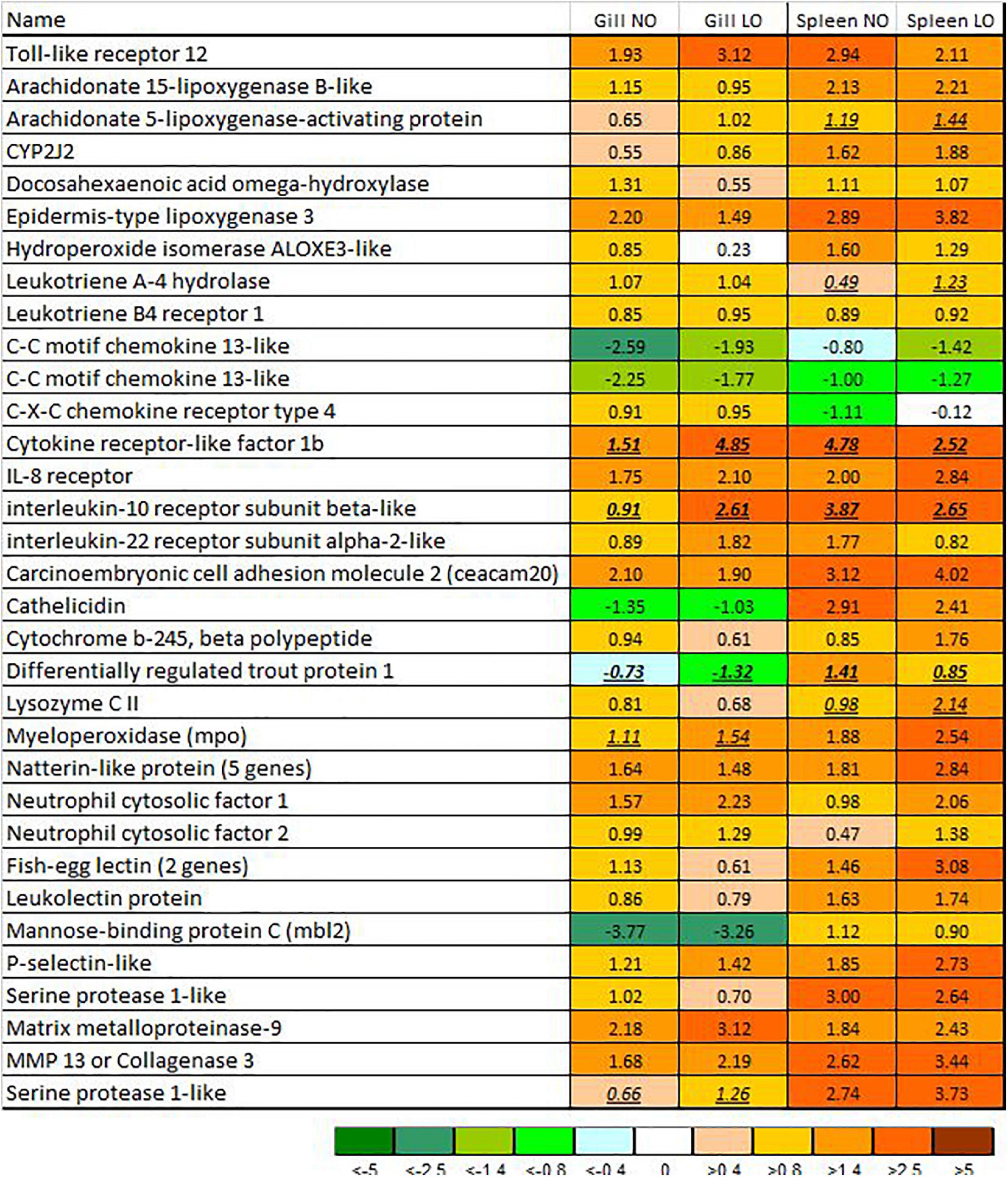
Figure 5. Immune genes with responses to M. viscosa in gill and spleen (microarrays). Data are log2-ER (expression ratios of infected to uninfected fish). Differential expression is indicated with underlined italics. NO, LO – groups maintained at normal and low dissolved oxygen. The numbers of paralogs are indicated in parentheses.
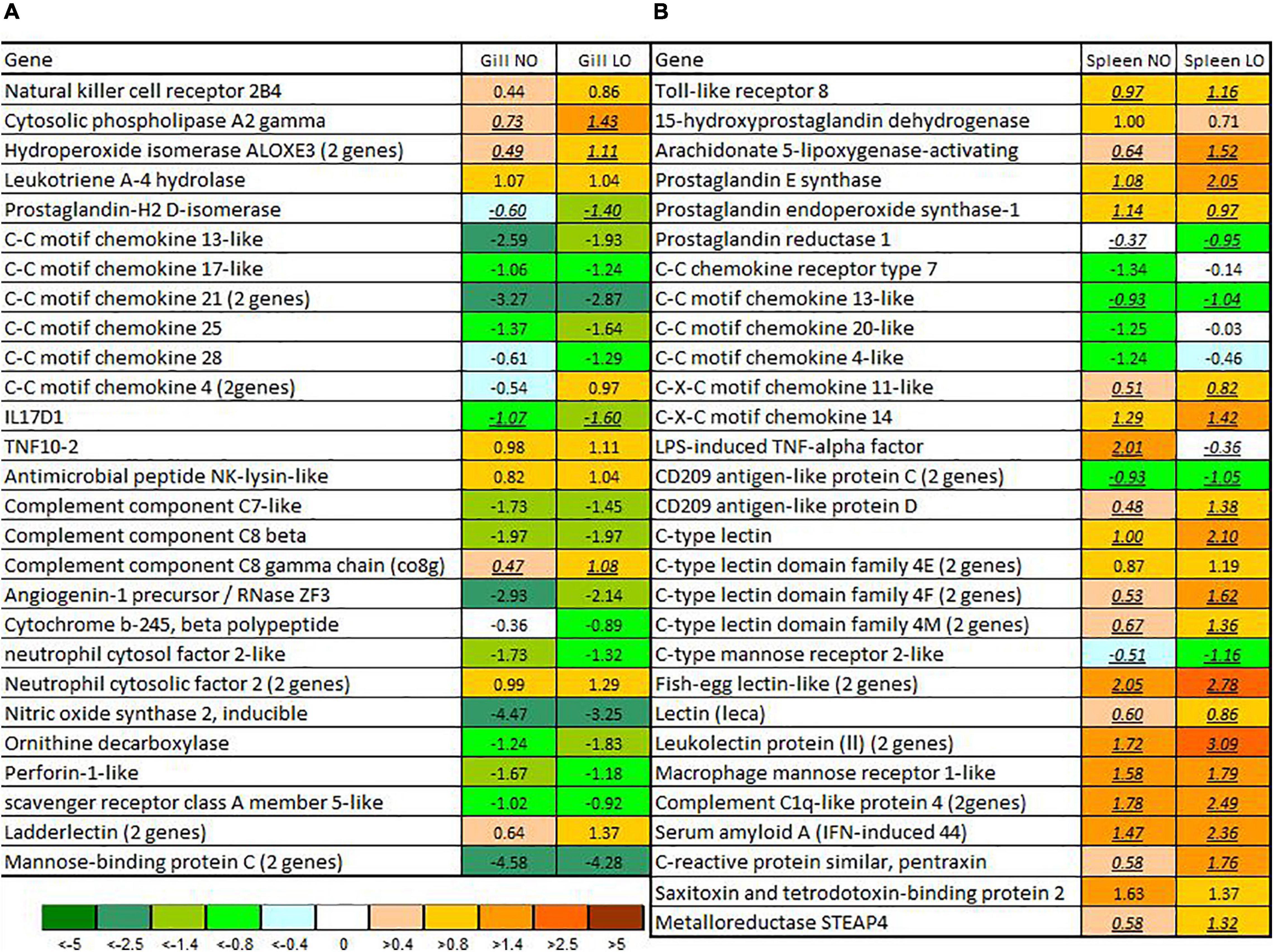
Figure 6. Immune genes with responses to M. viscosa in either gill (A) or spleen (B). Data are log2-ER (expression ratios of infected to uninfected). Differential expression is indicated with underlined italics. NO, LO – groups maintained at normal and low dissolved oxygen. The numbers of paralogs are indicated in parentheses.
Among genes that responded to M. viscosa, only a few showed differences between the treatment groups (highlighted in Figures 5, 6). The bacterial infection caused minor changes in expression of genes involved in antiviral immunity. However, differences between LO and NO fish increased after challenge, especially in the gill. A panel of genes exceeded the threshold of differential expression; higher expression in LO was shown by three interferons and a number of VRG including highly specialized antiviral effectors, such as receptor transporting protein, gig1-1, ifit5, and very large inducible gtpase (Figure 7).
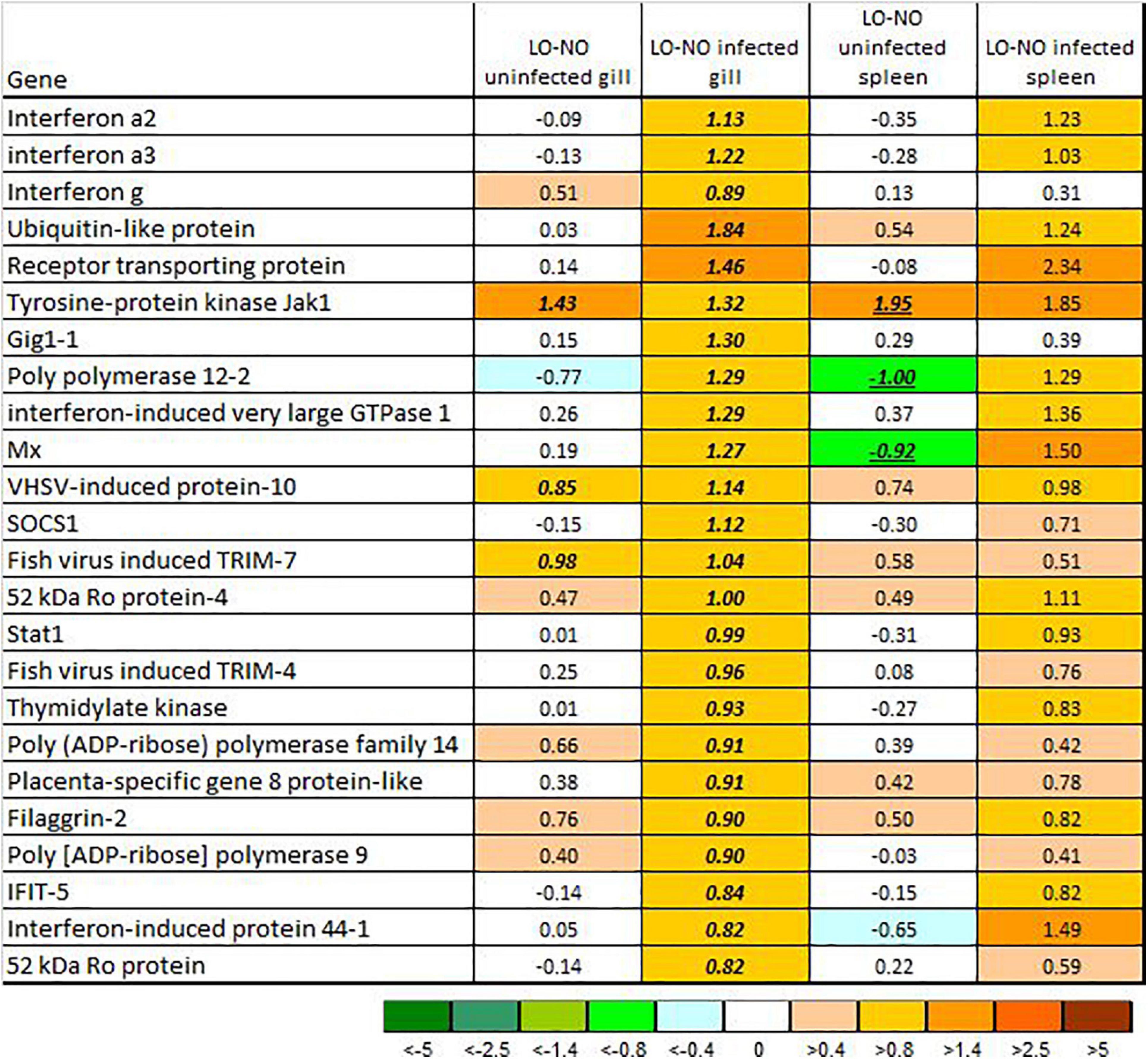
Figure 7. Expression of genes involved in innate antiviral responses (VRG). Data are log2-ER (LO to NO expression ratios). Significant differences are indicated with underlined italics. NO, LO – groups maintained at normal and low dissolved oxygen.
Oxygen deficiency, decreased energy production, shifting metabolism toward anaerobic pathways, and accumulation of by-products can affect various biological processes, and the consequences are difficult to predict. The immune system and protection against pathogens are among the main concerns. Comparisons of disease resistance in Atlantic salmon kept at normal and low levels of DO produced different results, such as absence of effect in challenge with salmon alphavirus (Andersen et al., 2010) and accelerated progression of amoebic gill disease (Oldham et al., 2020). To our knowledge, remote effects of hypoxia on the immune system and protection against pathogens in Atlantic salmon have not been reported until present. Disturbances experienced in early development may have both beneficial and detrimental consequences, which can be associated with epigenetic programming (Burgerhout et al., 2017; Liu et al., 2017; Moghadam et al., 2017; Uren Webster et al., 2018; Kelly et al., 2020). The reported research has highlighted the potential for long-term effects of hypoxia on the immunity and disease resistance of Atlantic salmon, although the results from only one study should by no means be generalized.
Smoltification was included in our study as a critical period in the life history of Atlantic salmon when massive endocrine regulation redirects the osmotic balance and induces dramatic changes in metabolism, morphology, and behavior (Barron, 1986; Björnsson et al., 2011). The effect on the immune system remained unknown until recently, though it could be anticipated considering the magnitude of the changes compared to metamorphosis and the key role of cortisol in controlling osmoregulation. Since first publication (Johansson et al., 2016), we have repeatedly observed downregulation of immune genes in Atlantic salmon smolts. Although the trend is consistent, the number and composition of DEG vary substantially, and changes would hardly be detectable without high-throughput transcriptome analyses. In developing the ImCom assay, we selected genes with a high probability of differential expression in order to ensure detection of any possible immune changes. Here, this gene set was sufficient for finding difference between the groups exposed to normoxic or hypoxic conditions in early life, although the composition of the affected genes was different between three analyzed tissues. Most of the DEG showed higher expression in LO fish and changes associated with smoltification were overall weaker in this group. A stimulatory effect of hypoxia is consistent with prevalence of a proactive coping strategy in Atlantic salmon (Damsgård et al., 2019; Rey et al., 2021), which could be established in the course of evolution and further enhanced by breeding and adaptation to the farming environment: hazards induce non-specific activation of diverse defense mechanisms including the immune system. In our experience, the transcriptome analyses find a large scale upregulation of immune genes after exposure to various stressors not related to infections. The consequences of the reduced expression of immune genes in smolts remain unknown, although in theory it may contribute to the increased occurrence of infectious diseases during first several months in the sea. This study conclusively showed that higher expression of immune genes in Atlantic salmon smolts does not necessarily improve disease resistance and may cause, or at least coincide with, higher susceptibility to pathogens.
The challenge trial with M. viscosa provided an opportunity to gain insight into the host responses to the pathogen. Transcriptome analyses with a genome-wide microarray showed immune protection from the bacterial pathogen involving many genes with well-known roles. However, with exception of the genes involved in innate antiviral responses, the results revealed only minor differences in expression of immune genes between salmon raised under normoxic and hypoxic conditions. The number of genes with expression differences between LO and NO fish was large, even greater than the number of genes affected with bacterial infection. However, it is not known if and how these differences could be related to disease resistance. Several alternative options can be discussed. The observed stimulation in LO smolts indicates a possible exhaustion of the immune system in the long term, while the activation of pro-inflammatory genes in the gills of NO smolts might enhance the protective barrier. The difference in survival, especially at the end of the trial, might be too small to be reflected in the transcriptome or depend on events, which are not detected at the gene expression levels, such as production of natural or pathogen-specific antibodies. The difference in survival can be associated with two scenarios: a better state of all or most fish in NO or higher frequency of individuals with impaired protection in LO. If the latter is true, survivors can be similar and transcriptome analyses are not expected to reveal differences. Finally, the higher activity of innate antiviral immunity might indicate an unsuccessful defense strategy in salmon exposed to hypoxia.
Hypoxia at early life stages induced sustained effects on the immune system of Atlantic salmon and defense against a pathogenic bacterium. Developmental disturbance increased expression of immune genes and attenuated their downregulation during smoltification. However, these changes did not improve survival of fish after challenge with M. viscosa.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/geo/, GSE171693.
The animal study was reviewed and approved by the Norwegian Food Safety Authority.
L-HJ, EB, AK, HJ, and HT: conceptualization. AK, AR, HL, AB, and HJ: methodology. SA: software. AK: writing—original draft preparation. EB and HJ: project administration. L-HJ, EB, HJ, and HT: funding acquisition. All authors contributed to writing and read and agreed with the submitted version of the manuscript.
This study was funded by the National Research Council of Norway (267644) and by the Nofima’s Strategic Project 11881 granted by the Research Council of Norway. SA was supported with a grant from I. M. Sechenov Institute of Evolutionary Physiology and Biochemistry (IEPHB RAS, research theme No. AAAA-A18-118012290373-7).
AK, EB, HJ, HT, and L-HJ were employed by company Nofima AS.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We wish to thank the staff at the Aquaculture Research Station in Tromsø and Mette Wesmajærvi Breiland, Gunhild S. Johansson, Marianne Hansen, and Tina Thesslund for their contributions to this project.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.722218/full#supplementary-material
Andersen, L., Hodneland, K., and Nylund, A. (2010). No influence of oxygen levels on pathogenesis and virus shedding in Salmonid alphavirus (SAV)-challenged Atlantic salmon (Salmo salar L.). Virol. J. 7:198.
Barron, M. G. (1986). Endocrine control of smoltification in anadromous salmonids. J. Endocrinol. 108, 313–319. doi: 10.1677/joe.0.1080313
Beemelmanns, A., Zanuzzo, F. S., Xue, X., Sandrelli, R. M., Rise, M. L., and Gamperl, A. K. (2021). The transcriptomic responses of Atlantic salmon (Salmo salar) to high temperature stress alone, and in combination with moderate hypoxia. BMC Genom. 22:261. doi: 10.1186/s12864-021-07464-x
Björnsson, B. T., Stefansson, S. O., and McCormick, S. D. (2011). Environmental endocrinology of salmon smoltification. Gen. Comp. Endocrinol. 170, 290–298. doi: 10.1016/j.ygcen.2010.07.003
Burgerhout, E., Mommens, M., Johnsen, H., Aunsmo, A., Santi, N., and Andersen, Ø (2017). Genetic background and embryonic temperature affect DNA methylation and expression of myogenin and muscle development in Atlantic salmon (Salmo salar). PLoS One 12:e0179918. doi: 10.1371/journal.pone.0179918
Damsgård, B., Evensen, T. H., Øverli, Ø, Gorissen, M., Ebbesson, L. O. E., Rey, S., et al. (2019). Proactive avoidance behaviour and pace-of-life syndrome in Atlantic salmon. R. Soc. Open Sci. 6:181859. doi: 10.1098/rsos.181859
Davis, J. C. (1975). Minimal dissolved oxygen requirements of aquatic life with emphasis on Canadian species: a review. J. Fish. Board Can. 32, 2295–2332. doi: 10.1139/f75-268
Fuior, E. V., and Gafencu, A. V. (2019). Apolipoprotein C1: Its pleiotropic effects in lipid metabolism and beyond. Int. J. Mol. Sci. 20:5939. doi: 10.3390/ijms20235939
Gamperl, A. K., Ajiboye, O. O., Zanuzzo, F. S., Sandrelli, R. M., Ellen de Fátima, C. P., and Beemelmanns, A. (2020). The impacts of increasing temperature and moderate hypoxia on the production characteristics, cardiac morphology and haematology of Atlantic Salmon (Salmo salar). Aquaculture 519:734874. doi: 10.1016/j.aquaculture.2019.734874
Greig, S. M., Sear, D. A., and Carling, P. A. (2006). A review of factors influencing the availability of dissolved oxygen to incubating salmonid embryos. Hydrol. Process 21, 323–334. doi: 10.1002/hyp.6188
Johansson, L. H., Timmerhaus, G., Afanasyev, S., Jorgensen, S. M., and Krasnov, A. (2016). Smoltification and seawater transfer of Atlantic salmon (Salmo salar L.) is associated with systemic repression of the immune transcriptome. Fish Shellfish Immunol. 58, 33–41. doi: 10.1016/j.fsi.2016.09.026
Karlsen, C., Thorarinsson, R., Wallace, C., Salonius, K., and Midtlyng, P. (2017). Atlantic salmon winter-ulcer disease: Combining mortality and skin ulcer development as clinical efficacy criteria against Moritella viscosa infection. Aquaculture 473, 538–544. doi: 10.1016/j.aquaculture.2017.01.035
Karlsen, C., Ytteborg, E., Timmerhaus, G., Høst, V., Handeland, S., Jørgensen, S. M., et al. (2018). Atlantic salmon skin barrier functions gradually enhance after seawater transfer. Sci. Rep. 8:9510.
Kelly, T., Johnsen, H., Burgerhout, E., Tveiten, H., Thesslund, T., Andersen, Ø, et al. (2020). Low oxygen stress during early development influences regulation of hypoxia-response genes in farmed Atlantic salmon (Salmo salar). G3 (Bethesda) 10, 3179–3188. doi: 10.1534/g3.120.401459
Kolarevic, J., Aas-Hansen, Ø, Espmark, Å, Baeverfjord, G., Terjesen, B. F., and Damsgård, B. (2016). The use of acoustic acceleration transmitter tags for monitoring of Atlantic salmon swimming activity in recirculating aquaculture systems (RAS). Aquacult. Engin. 72, 30–39. doi: 10.1016/j.aquaeng.2016.03.002
Krasnov, A., Afanasyev, S., Nylund, S., and Rebl, A. (2020). Multigene expression assay for assessment of the immune status of Atlantic salmon. Genes (Basel) 11:1236. doi: 10.3390/genes11111236
Krasnov, A., Timmerhaus, G., Afanasyev, S., and Jorgensen, S. M. (2011a). Development and assessment of oligonucleotide microarrays for Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. Part D Genom. Proteomics 6, 31–38. doi: 10.1016/j.cbd.2010.04.006
Krasnov, A., Timmerhaus, G., Schiotz, B. L., Torgersen, J., Afanasyev, S., Iliev, D., et al. (2011b). Genomic survey of early responses to viruses in Atlantic salmon, Salmo salar L. Mol. Immunol. 49, 163–174. doi: 10.1016/j.molimm.2011.08.007
Król, E., Noguera, P., Shaw, S., Costelloe, E., Gajardo, K., Valdenegro, V., et al. (2020). Integration of transcriptome, gross morphology and histopathology in the gill of sea farmed Atlantic Salmon (Salmo salar): lessons from multi-site sampling. Front. Genet. 11:610. doi: 10.3389/fgene.2020.00610
Kvamme, B. O., Gadan, K., Finne-Fridell, F., Niklasson, L., Sundh, H., Sundell, K., et al. (2013). Modulation of innate immune responses in Atlantic salmon by chronic hypoxia-induced stress. Fish Shellfish Immunol. 34, 55–65. doi: 10.1016/j.fsi.2012.10.006
Liu, J., Plagnes-Juan, E., Geurden, I., Panserat, S., and Marandel, L. (2017). Exposure to an acute hypoxic stimulus during early life affects the expression of glucose metabolism-related genes at first-feeding in trout. Sci. Rep. 7:363.
Løvoll, M., Wiik-Nielsen, C., Tunsjø, H., Colquhoun, D., Lunder, T., Sørum, H., et al. (2009). Atlantic salmon bath challenged with Moritella viscosa–pathogen invasion and host response. Fish Shellfish Immunol. 26, 877–884. doi: 10.1016/j.fsi.2009.03.019
Martínez, D., De Lázaro, O., Cortés, P., Oyarzún-Salazar, R., Paschke, K., and Vargas-Chacoff, L. (2020). Hypoxia modulates the transcriptional immunological response in Oncorhynchus kisutch. Fish Shellfish Immunol. 106, 1042–1051. doi: 10.1016/j.fsi.2020.09.025
Metcalfe, N. B., Taylor, A. C., and Thorpe, J. E. (1995). Metabolic rate, social status and life-history strategies in Atlantic salmon. Anim. Behav. 49, 431–436. doi: 10.1006/anbe.1995.0056
Moghadam, H. K., Johnsen, H., Robinson, N., Andersen, ØH., Jørgensen, E., Johnsen, H. K., et al. (2017). Impacts of early life stress on the methylome and transcriptome of Atlantic salmon. Sci. Rep. 7:5023.
Mutoloki, S., Cooper, G. A., Marjara, I. S., Koop, B. F., and Evensen, Ø (2010). High gene expression of inflammatory markers and IL-17A correlates with severity of injection site reactions of Atlantic salmon vaccinated with oil-adjuvanted vaccines. BMC Genom. 11:336. doi: 10.1186/1471-2164-11-336
Niklasson, L., Sundh, H., Fridell, F., Taranger, G. L., and Sundell, K. (2011). Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immunol. 31, 1072–1080. doi: 10.1016/j.fsi.2011.09.011
Oldham, T., Dempster, T., Crosbie, P., Adams, M., and Nowak, B. (2020). Cyclic hypoxia exposure accelerates the progression of amoebic gill disease. Pathogens 9:597. doi: 10.3390/pathogens9080597
Oldham, T., Nowak, B., Hvas, M., and Oppedal, F. (2019). Metabolic and functional impacts of hypoxia vary with size in Atlantic salmon. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 231, 30–38. doi: 10.1016/j.cbpa.2019.01.012
Olsvik, P. A., Vikeså, V., Lie, K. K., and Hevrøy, E. M. (2013). Transcriptional responses to temperature and low oxygen stress in Atlantic salmon studied with next-generation sequencing technology. BMC Genom. 14:817. doi: 10.1186/1471-2164-14-817
Poynter, S. J., Monjo, A. L., Micheli, G., and DeWitte-Orr, S. J. (2017). Scavengers for bacteria: Rainbow trout have two functional variants of MARCO that bind to gram-negative and -positive bacteria. Dev. Comp. Immunol. 77, 95–105. doi: 10.1016/j.dci.2017.07.021
Randall, D. J., Perry, S. F., and Heming, T. A. (1982). Gas transfer and acid/base regulation in salmonids. Compar. Biochem. Physiol. Part B 73, 93–103. doi: 10.1016/0305-0491(82)90203-6
Remen, M., Oppedal, F., Imsland, A. K., Olsen, R. E., and Torgersen, T. (2013). Hypoxia tolerance thresholds for post-smolt Atlantic salmon: dependency of temperature and hypoxia acclimation. Aquaculture 416, 41–47. doi: 10.1016/j.aquaculture.2013.08.024
Remen, M., Oppedal, F., Torgersen, T., Imsland, A. K., and Olsen, R. E. (2012). Effects of cyclic environmental hypoxia on physiology and feed intake of post-smolt Atlantic salmon: initial responses and acclimation. Aquaculture 326, 148–155. doi: 10.1016/j.aquaculture.2011.11.036
Rey, S., Jin, X., Damsgård, B., Bégout, M. L., and Mackenzie, S. (2021). Analysis across diverse fish species highlights no conserved transcriptome signature for proactive behaviour. BMC Genom. 22:33. doi: 10.1186/s12864-020-07317-z
Robinson, N. A., Timmerhaus, G., Baranski, M., Andersen, O., Takle, H., and Krasnov, A. (2017). Training the salmon’s genes: influence of aerobic exercise, swimming performance and selection on gene expression in Atlantic salmon. BMC Genom. 18:971. doi: 10.1186/s12864-017-4361-7
Shinnar, A. E., Butler, K. L., and Park, H. J. (2003). Cathelicidin family of antimicrobial peptides: proteolytic processing and protease resistance. Bioorg. Chem. 31, 425–436. doi: 10.1016/s0045-2068(03)00080-4
Uren Webster, T. M., Rodriguez-Barreto, D., Martin, S. A. M., Van Oosterhout, C., Orozco-terWengel, P., Cable, J., et al. (2018). Contrasting effects of acute and chronic stress on the transcriptome, epigenome, and immune response of Atlantic salmon. Epigenetics 13, 1191–1207. doi: 10.1080/15592294.2018.1554520
Wang, S. Y., Lau, K., Lai, K. P., Zhang, J. W., Tse, A. C. K., Li, J. W., et al. (2016). Hypoxia causes transgenerational impairments in reproduction of fish. Nat. Commun. 7, 1–9.
Wang, T., Lefevre, S., Huong, D. T. T., Cong, N. V., and Bayley, M. (2009). The effects of hypoxia on growth and digestion. Fish Physiol. 27, 361–396. doi: 10.1016/s1546-5098(08)00008-3
Wood, A. T., Andrewartha, S. J., Elliott, N. G., Frappell, P. B., and Clark, T. D. (2019). Hypoxia during incubation does not affect aerobic performance or haematology of Atlantic salmon (Salmo salar) when re-exposed in later life. Conserv. Physiol. 7:coz088.
Wood, A. T., Clark, T. D., Elliott, N. G., Frappell, P. B., and Andrewartha, S. J. (2020). The effects of constant and cyclical hypoxia on the survival, growth and metabolic physiology of incubating Atlantic salmon (Salmo salar). Aquaculture 527:735449. doi: 10.1016/j.aquaculture.2020.735449
Zanfardino, A., Pizzo, E., Di Maro, A., Varcamonti, M., and D’Alessio, G. (2010). The bactericidal action on Escherichia coli of ZF-RNase-3 is triggered by the suicidal action of the bacterium OmpT protease. FEBS J. 277, 1921–1928. doi: 10.1111/j.1742-4658.2010.07614.x
Keywords: Atlantic salmon, hypoxia, development, immune response, transcriptomics, Moritella viscosa
Citation: Krasnov A, Burgerhout E, Johnsen H, Tveiten H, Bakke AF, Lund H, Afanasyev S, Rebl A and Johansen L-H (2021) Development of Atlantic Salmon (Salmo salar L.) Under Hypoxic Conditions Induced Sustained Changes in Expression of Immune Genes and Reduced Resistance to Moritella viscosa. Front. Ecol. Evol. 9:722218. doi: 10.3389/fevo.2021.722218
Received: 08 June 2021; Accepted: 12 July 2021;
Published: 02 August 2021.
Edited by:
Elisabeth Holen, Norwegian Institute of Marine Research (IMR), NorwayReviewed by:
Lindsey Moore, University of Bergen, NorwayCopyright © 2021 Krasnov, Burgerhout, Johnsen, Tveiten, Bakke, Lund, Afanasyev, Rebl and Johansen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksei Krasnov, QWxla3NlaS5LcmFzbm92QG5vZmltYS5ubw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.