- 1Department of Entomology and Plant Pathology, North Carolina State University, Raleigh, NC, United States
- 2Department of Entomology and Nematology, University of Florida, Lake Alfred, FL, United States
- 3Department of Horticultural Science, North Carolina State University, Raleigh, NC, United States
The feeding habits of adult Brachycera are understudied and may provide important context for understanding invasive pest biology, as with the polyphagous small fruit pest Drosophila suzukii. We developed molecular methods to study adult D. suzukii gut content in order to understand its feeding habits. We designed and verified two primer pairs specific for either blueberries or blackberries and used a qPCR melt curve analysis to determine whether we can detect the presence or absence of berry feeding by adult flies. In a laboratory assay, the blueberry fly meal DNA can be detected for longer periods than the blackberry meal DNA. Generally, female gut contents are less variable than male gut contents. We also tested recently emerged flies that were not fed as adults but developed as larvae in either blueberries or blackberries. Some adult flies from each fruit had detectable fruit DNA in their gut, which could be due to pupal meconium feeding after emergence. Next, we aimed to test the primers in the field to develop techniques to track fruit feeding by D. suzukii in its natural field environment. First, to identify the most appropriate collection method, we determined how long we could detect fruit DNA, using previously developed primers within D. suzukii gut preserved in four types of trap fluid in the laboratory. The likelihood of detecting blackberry DNA differed by day, trap fluid, and between sexes. For the blueberry primer, the possibility of detecting blueberry DNA differed by trap fluid only. Based on those results, we used RV antifreeze with a Scentry SWD lure in field trials at two research station locations, one containing blackberries and one with blueberries. We established transects away from each fruit planting and collected up to 120 total flies at each point along transects. There were no significant differences in the number of flies containing berry DNA among collection points along the transect in both locations. These results suggest that adult flies move between crop and non-crop habitats and may not be highly dependent on fruit food resources.
Introduction
Food resource use by adult, non-animal feeding higher flies (Brachycera) has been understudied because it is often considered secondary to adult oviposition in and larval damage of crops (Clarke et al., 2011; Walsh et al., 2011). In contrast, larval resource use, specifically that of invasive polyphagous pest species such as Drosophila suzukii (Matsumura) has been well studied (Parrella, 1983; Headrick and Goeden, 1990; Burrack et al., 2009; Ekesi et al., 2009; Emiljanowicz et al., 2014; Jaramillo et al., 2014; Nash and Chapman, 2014; Hardin et al., 2015; Hamby et al., 2016; Silva-Soares et al., 2017; Lewis and Hamby, 2019). However, chemical and cultural control tactics primarily target adult flies (Mahmoud and Shoeib, 2008; Ur Rehman et al., 2009; Van Timmeren and Isaacs, 2013; Erland et al., 2015; Ghabbari et al., 2018; Siddique et al., 2018; Mawtham et al., 2019).
Higher fly morphology determines and restricts feeding (Yeates and Wiegmann, 1999). While blood-feeding flies in the family Muscidae have prestomal teeth that cause the characteristic “bite” felt when they puncture skin, most Brachycera have “lapping” mouthparts (Elzinga and Broce, 1986; Yeates and Wiegmann, 1999). The “lapping” mouthparts evolved 100 million years before flowering plants and limited flies to liquid resources, such as extrafloral nectaries, hemipteran honeydew, flower nectar, decaying materials, fecal matter, yeast, fungi, and other similar substrates (Elzinga and Broce, 1986; Yeates and Wiegmann, 1999). Drosophilidae in particular may be capable of making minute scratches to soft materials to release fluids for feeding but “particulates released would have to be very narrow to pass through the very narrow pseudotracheae found in this family” (Elzinga and Broce, 1986).
In female Brachycera flies, protein appears important to egg production. At the same time, carbohydrate intake promotes adult fly longevity, while diet may have a more species-dependent impact on male reproductive success and survivorship. Anastrepha (Diptera: Tephritidae) males of some species had more copulatory success when they fed on higher protein diets while the copulatory success of males in other, closely related species were unaffected by diet (Aluja et al., 2001). Female diet resource has also been linked to reproductive success and longevity. Protein appears to be an important resource for females in developing eggs. In one study, the number of eggs produced was three times higher in Hermetia illucens (L.) adult females given a milk-powder-based protein source (Bertinetti et al., 2019). Female Anastrepha flies given sucrose and protein diet had a higher egg load than those given sucrose alone (Jacome et al., 1995; Aluja et al., 2001). In developing a fly diet for colony care, researchers found that removing key diet components (amino acid cocktail, cholesterol, etc.) reduced the egg load of Ceratitis capitata (Wiedemann) (Chang et al., 2001). Similarly, Drosophila melanogaster (Meigen) egg load was suppressed when fed on a diet lacking protein (Lee et al., 2011). Additionally, D. melanogaster egg cells may perceive environmental, nutritional status and determine whether they develop or undergo cell death (Terashima and Bownes, 2004). Perhaps unsurprisingly then, the closely related D. suzukii have also been shown to increase yeast feeding following mating (Mori et al., 2017), and egg maturation in D. suzukii has shown to be low when fed fruit juice alone (Plantamp et al., 2017). While low protein to carbohydrate levels improves D. suzukii fecundity, a high ratio of protein to carbohydrates can slow fecundity within the species (Rendon et al., 2019).
Sucrose concentration has been shown to affect C. capitata survival but not egg load (Chang et al., 2001). An intermediate amount of carbohydrates, increases lipid-storage within D. melanogaster and increases their longevity (Lee et al., 2011). Similarly, female D. suzukii given floral resources had increased carbohydrate reserves, which increased their survival compared to those treatments only given water (Tochen et al., 2016).
Many of the studies discussed so far looked at laboratory-raised colonies of flies in order to understand their biology better. In the research described here, we created a tool to study higher fly molecular gut content at the field-level. For the most part, molecular gut content research has been dominated by studies of predator feeding behavior (Juen and Traugott, 2005; Greenstone et al., 2007; Harwood et al., 2009; Szendrei et al., 2010; Günther et al., 2014; Rondoni et al., 2015; Li et al., 2017; Albertini et al., 2018; Macías-Hernández et al., 2018). We use molecular gut content to understand how a polyphagous, phytophagous fly and major small fruit crop pest, D. suzukii, may use cultivated small fruit resources within a field setting. Briem et al. (2018) in Germany recently conducted an assay that evaluated total adult food resource. They captured 40 adult male and female flies within a raspberry field, and sequenced them using high-throughput sequencing on an Illumina MiSeq platform (Briem et al., 2018). The vast majority of their flies had detectable raspberry DNA within their guts, in addition to two other plants (Urtica dioica and Polygonum humifusum). Half their samples included environmental DNA, or eDNA, from the outside of flies where they did not surface sterilize those samples (Briem et al., 2018). This research provided insight into the total feeding behavior of a small sample of flies. In our experiments, we sought to develop a tool to test large numbers of flies trapped within a field to understand what proportion of D. suzukii are using specific fruit resources in a cultivated monoculture resource. This larger scale of testing would likely be more economical for future field-based research.
We built from previous work by our group which tested a putative strawberry primer (see note in section “Materials and Methods”) (Diepenbrock et al., 2018). That primer was used to detect fruit feeding using a qPCR melt curve reaction and detected a fruit meal within flies up to 7 days after feeding in a mixed-sex population (Diepenbrock et al., 2018). We further expanded these methods by designing and testing blueberry- and blackberry-specific primers. We then conducted a series of laboratory experiments to inform the interpretation of results from field-collected flies.
Materials and Methods
Source Material
All flies from this experiment originated from a colony of D. suzukii raised for over 100 generations. These flies stem from wild flies collected in Laurel Springs, NC in 2010 and have had periodic wild-trapped flies added to maintain genetic diversity. The colony is kept at 20°C 16 L h: 8 D h and fed a cornmeal-based diet as described in Hardin et al. (2015). Adult flies between 3- and 7-days old were used for these experiments.
Primer Design and Selection
We used the D. suzukii primer (fwd: AATTGTTACCGCA CATGC; rev: GGAATGCTATATCTGGGTCC) which amplifies a 117-bp region of the COI gene (Dhami and Kumarasinghe, 2014) as a positive control for successful DNA extraction in our samples. All fruit primers were tested on adult flies starved and then fed on fruit. Early assays found that the melt curve temperature profile shifts depending on whether the DNA was extracted straight from the plant or from the D. suzukii gut. Throughout this research project, we tested 130 primer pairs. Over time, we built a library of D. suzukii fed known varieties of fruit and tested our final primers on multiple varieties of the same fruit type to determine specificity (Please, see the Supplementary Material for more information, including a list of primers tested).
We successfully found a blackberry-specific primer by searching the literature for published blackberry genes (fwd: CT TCCCCCTATAAATCCCGA/rev: CGTCTCTCTGCAATTCCT CC). The blackberry primer comes from an SSR region (RH_MEa0007aG06, Zurn et al., 2018 from Bassil et al., 2016) that we confirmed was selective for cvs. Von, Ouachita, and wild blackberries fed to adult D. suzukii without picking up most of the raspberry accessions grown at Salisbury Research Station (Supplementary Table 1).
The blueberry-specific primer comes from a maturase K gene found within the chloroplast (Accession Number: MH551878) and picks up a large number of blueberry varieties from both highbush (Vaccinium corymbosum) and rabbiteye (Vaccinium virgatum formerly V. ashei) (fwd: GGGCAT TCTATGGGTTTTCA/rev: TGGATCCTTCTTGGTTGAGC). The primer was designed using Primer 3 software (Untergasser et al., 2012).
Unfortunately, the strawberry primer published in Diepenbrock et al. (2018) was not specific to strawberries and picked up caneberries and potentially other members in the family Rosaceae. A fully annotated blackberry genome was not yet published at the time of these experiments.
Larval Feeding-Adult Gut Content Assay
To determine whether adults contain detectable remains of larval diet, we infested fruit following methods described in Kraft et al. (2020). We held infested blackberries and blueberries in separate containers with mesh bottoms at 20°C for 8 days until pupation. At that time, all pupae were removed from fruit containers and placed on a damp paper towel inside a 60 mm Petri dish until adults emerged. Replicates for each fruit type were processed on the same day. Adult flies were frozen within 12 h of adult emergence and then placed in 96% ethanol in microcentrifuge tubes and held at –20°C until DNA extraction.
Pupal Meconium Extraction Assay
To test whether berry DNA was detectible from pupal meconium, we infested blueberries and blackberries using the same methods described above. When pupae were freshly sclerotized 7 days later, we removed pupae of larvae that had wandered away from the fruit (to reduce potential external contamination). The pupae were rinsed under tap water three times on fresh dish towels to remove external fruit material as in Schnetzer and Tyler (1996). Then, pupae were placed on a damp paper towel inside a 60 mm Petri dish until adults emerged. We extracted DNA from empty pupal cases within 3 h of adult emergence over the course of 2 days. In a follow-up study, after the same rinse protocol, we additionally subjected pupae to UV light within the fume hood for 15 min to further decrease the chances of external fruit DNA presence.
Detectability Over Time Assay
In this assay, we wanted to determine for how long berry DNA would be detected after a single feeding event. Adult flies aged 3–7 days were held for ∼60 h on a dilute sugar water (5 g sugar in 400 mL of tap water) soaked cotton ball. Flies were then fed a fruit puree made from fresh fruit purchased from the grocery store (blueberries, unknown variety) or from blackberries cv. Von picked at the Piedmont Research Station, Salisbury, NC during summer 2020 with ∼4 g sugar added to increase feeding. Seeds remained present in the puree, but D. suzukii mouthparts are a magnitude of size too small to feed on seeds or skin and only fed on juice from the puree. Flies were allowed to feed on the fruit puree for 4–6 h and were checked visually for a purple or lavender stripe in their gut to confirm feeding.
After fruit feeding, flies were moved to vials of a cornmeal-based diet and placed into two separate growth chambers. For each cohort of the flies that fed on blackberry and the flies that fed on blueberry, one growth chamber held the flies at 20°C with a 16:8 light/dark cycle while the other growth chamber was set to resemble field-typical temperature and light schedule during fruit ripening periods. These field-typical temperatures were selected by taking the mean minimum and maximum temperatures from each Piedmont Research Station, Salisbury, NC (for blackberries) and Castle Hayne Research Station, Castle Hayne, NC (for blueberries) from research station weather data for the last 5 years. For each day by temperature by fruit type treatment, there were a total of 15 male and 15 female flies tested. All treatments by fruit type were set up on the same day with the same cohort of flies.
A group of control flies was placed in empty vials immediately after feeding, frozen overnight, put into 96% ethanol, and held at –20°C until DNA extraction. Five vials totaling at least 15 live flies of each sex were removed from growth chambers each day for up to 7 days, frozen, and then moved to microcentrifuge tubes with 96% ethanol and held at –20°C until DNA extraction. Therefore, there were a total of 30 fruit by temperature by time treatments, 15 for blackberry, and 15 for blueberry.
Trap Fluid Experiment
Our objective for this assay was to determine the appropriate trap fluid to use in the field that would best maintain intact gut berry DNA. We starved adult D. suzukii for 72 h on a 5 g sucrose/400 mL water solution. Adult flies were then given a puree of either fresh, store-bought blackberries or blueberries, each with ∼4 g of sucrose, on which to feed for 6 h. To best mimic conditions similar to those in the field, the live adult D. suzukii were then immediately placed into small 5 mL plastic condiment containers with 4 mL of trap liquid. One container per trap fluid was set up per day for a total of seven containers; each container had at least 5 male and 5 female flies. The lid was immediately fitted, and the trap fluid was swirled to drown all flies.
We compared four trap fluids, including: (1) molecular grade ethylene glycol, (2) apple cider vinegar, (3) tap water with a drop of liquid detergent added, and (4) propylene glycol (Peak RV & Marine Antifreeze, Northbrooke, IL). Apple cider vinegar and water are commonly used in D. suzukii traps along with commercial lures (Lee et al., 2012; Frewin et al., 2017), and ethylene and propylene glycol are used as DNA preservatives in both field and laboratory settings.
Flies in trap fluid containers were then placed in an incubator with a temperature ramping program typical of field temperatures during ripening times for each blueberry and blackberry, since trap fluids would be used in the field exposed to fluctuating, high temperatures. The blueberry program started at 21.1°C for 8 h in dark, then gradual increased for 6 h, stayed at 30.9°C for 4 h, and then gradually decreased to 21.1°C for 6 h. The blackberry program followed the same cycle with a minimum of 19.2°C and maximum of 31.2°C. In a preliminary assay, we put raspberry-fed flies outside in July to subject them to typical summer UV and temperatures; we found no difference in feeding based on UV within flies held in the incubator or outside when using a pan-Rosaceae primer (see Supplementary Material). Individual containers were removed daily for up to 7 days. Flies were removed from the liquid, then carefully placed in 1.5 mL microcentrifuge tubes containing 96% ethanol and held at –20°C until DNA extraction and qPCR.
Field Experiment
We sought to test the molecular gut content of wild-trapped D. suzukii adults from two different field locations. Field trapping experiments were conducted when fruit load in fields was at a peak from two research stations: Piedmont Research Station, Salisbury, NC for blackberries, and Sandhills Research Station, Jackson Springs, NC for blueberries. At Piedmont Research Station in a blackberry plot containing cvs. Von and Ouachita, adult flies were collected every 3 days from June 1, 2020 to July 27, 2020. At Sandhills Research Station in a blueberry germplasm field (with mostly rabbiteye blueberries ripe during the experiment), samples were collected from July 10, 2020 to July 28, 2020. Traps were created from liter-sized plastic food containers (Lee et al., 2012) filled with ∼250 mL of RV antifreeze with a Scentry D. suzukii lure hanging over the trap fluid (Great Lakes IPM, Vestaburg, MI, United States). Traps were hung at the height of 1 m on plastic stakes. To maximize the number of flies trapped, from 3 to 7 traps were placed at each location. Traps were checked every 3 days, and trapped D. suzukii were filtered from the fluid. Captured flies were then collected in microcentrifuge tubes with 96% ethanol and stored at –20°C until DNA extraction and qPCR.
We sought to collect flies under field conditions during peak fruit ripening along a transect, including two edges of cultivated fruit plantings, the center of the cultivated fruit plot, and a distant wooded field margin. In the case of Sandhills Research Station, a second wooded margin was also included (Figures 1a,b). Between 30 and 120 adult flies were trapped at each trap location.
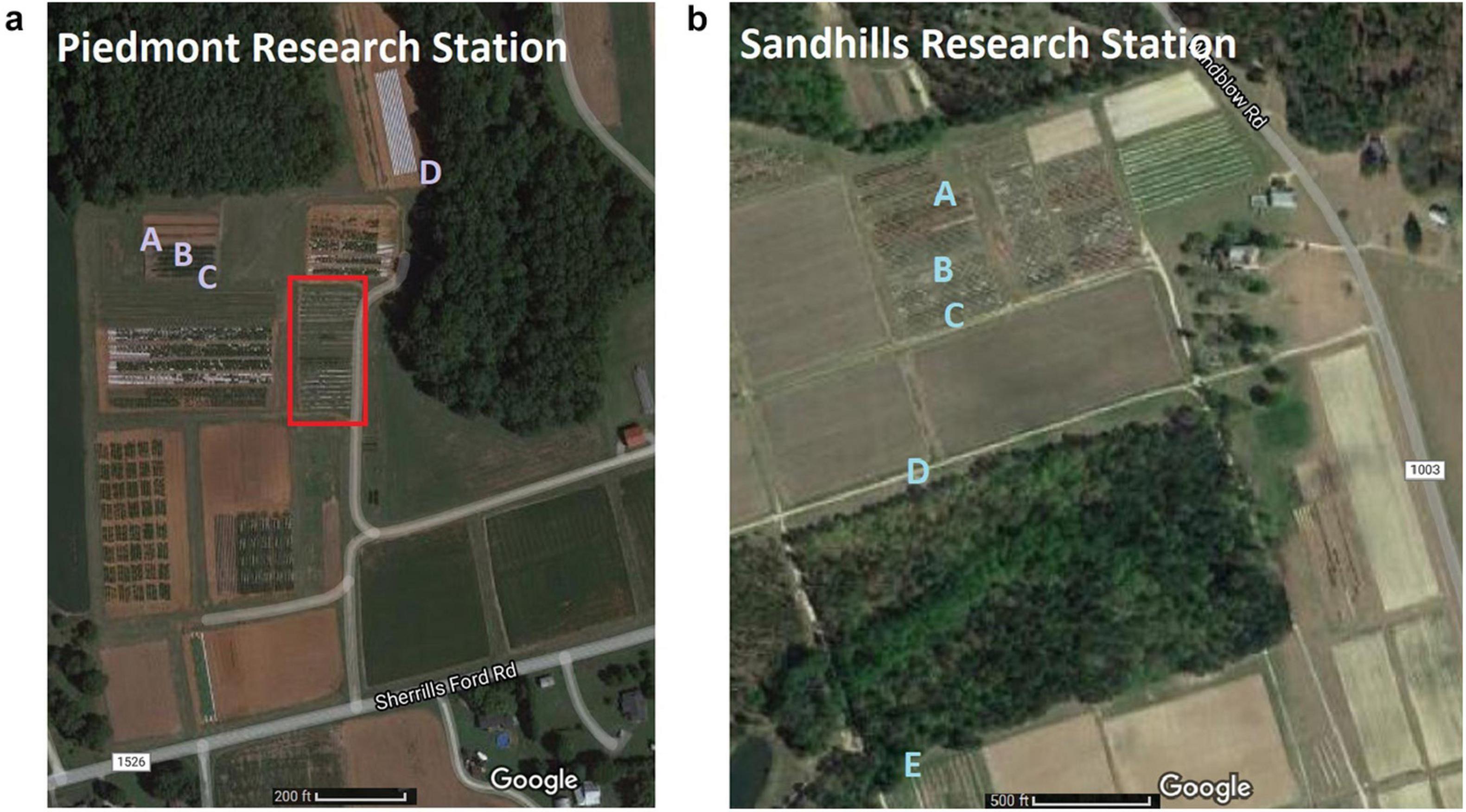
Figure 1. (a) Piedmont Research Station blackberry plots. Traps A, B, and C, are at the edge, center, and edge, respectively, of a Von and Ouachita blackberry plot. Trap D represents the wooded edge. Note that in the outlined square are caneberry breeding plots with blackberry, raspberry, black raspberry, golden raspberry, and other caneberries present at the same time. Coordinates are 35°41′49″N 80°37′45″W (Google, 2021a). (b) Sandhills Research Station blueberry plots. Traps A, B, and C were placed in a rabbiteye blueberry germplasm plot. The early-season blueberry plots in the two fields to the right were no longer fruiting during this experiment. Trap D is on a near wooded edge, and E represents a distant wooded edge. Coordinates are 35°11′39″N 79°41′06″W (Google, 2021b).
DNA Extraction
Flies were removed from the –20°C freezer in their solution of 96% ethanol, gently held by the wing and rinsed in a 5% bleach solution, and then twice rinsed in distilled water as described in Diepenbrock et al. (2018). Flies were decapitated before DNA extraction to reduce interference from DNases in the head during DNA extraction. We used a DNeasy blood and tissue kit (catalog no. 69506, Qiagen Inc., Germantown, MD, United States1) for all DNA extractions.
DNA Amplification and Comparative CT
Quantitative polymerase chain reaction (qPCR) reactions were performed in triplicate in 96-well plates (United States Scientific catalog no. 1402-9100) with blackberry, blueberry, or D. suzukii primers. Each well contained a total of 5 μl of iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, United States), 1 μl each of 5 μM forward and reverse primer, 2 μl of dd-H2O, and 1 μl of DNA template. Amplification was performed using an Applied Biosystems QuantStudio 6 Flex System in Fast mode preset for SYBR reagents in a comparative CT experiment. The subsequent PCR products were then subjected to a melt curve analysis to confirm identity. Each plate contained 30 samples, followed by a positive control of a D. suzukii starved female fed known fruit and a negative control of dd-H2O instead of DNA template.
Data Analyses
All data was considered binomial based on whether an individual fly was positive or negative for berry DNA for the given primer. Treatments tested were temperature regime, sex, day, and whether the individual fly was positive or negative. No variables were considered random. Data were analyzed using SAS version 9.4 University Edition run on Oracle VM VirtualBox, and all data conformed to the necessary assumptions. The Proc Logistic command was used to run a logistic regression of all treatments except field-trapped flies. “Day” was considered a continuous variable for these analyses.
For the field transects, treatments included the trap location, sex, and whether an individual fly was positive or negative. No variables were considered random. Data were analyzed using a Proc Glimmix command to run a binomial regression of all treatments, adding the “dist = binomial” and “link = logit” to our model statement.
Results
Data herein are presented in log-odds. Log-odds is the logarithm of the odds of success. It is a way of looking at the probability of success over the probability of failure of a binomial dataset.
Larvae Raised in Fruit and Pupal Meconium
No blackberry DNA was present in a total of 30 newly emerged adults who fed on blackberry as larvae. However, out of 30 adults that fed on blueberry as larvae, 11 flies tested positive with no difference by sex (7 females and 4 males, logistic regression χ2 = 1.2665, DF = 1, P = 0.2604).
After this observation, we set out to determine if pupal meconium was a potential source of the berry DNA within newly emerged fly guts. We found that 25 out of 30 pupal cases from larvae which fed on blackberries were positive for blackberry DNA and 30 out of 30 pupal cases were positive from larvae that fed on blueberries. In the follow-up study where rinsed pupae were subjected to 15 min of UV light to degrade any remaining DNA on the outside of the pupal case, 16 out of 30 pupal cases from larvae fed on blackberries tested positive, while 30 out of 30 pupal cases from larvae that fed on blueberries tested positive.
Blackberry Detectability Over Time
No blackberry DNA was detected after Day 4 in 15 total flies tested for each treatment. In fact, blackberry was detectible in most D. suzukii only on Day 1 and not afterward (Figure 2). Only females held at 20°C contained detectible blackberry for up to 4 days. We also found there were more males who tested positive for feeding on Day 1 than Day 0 (10 females, 2 males were positive).
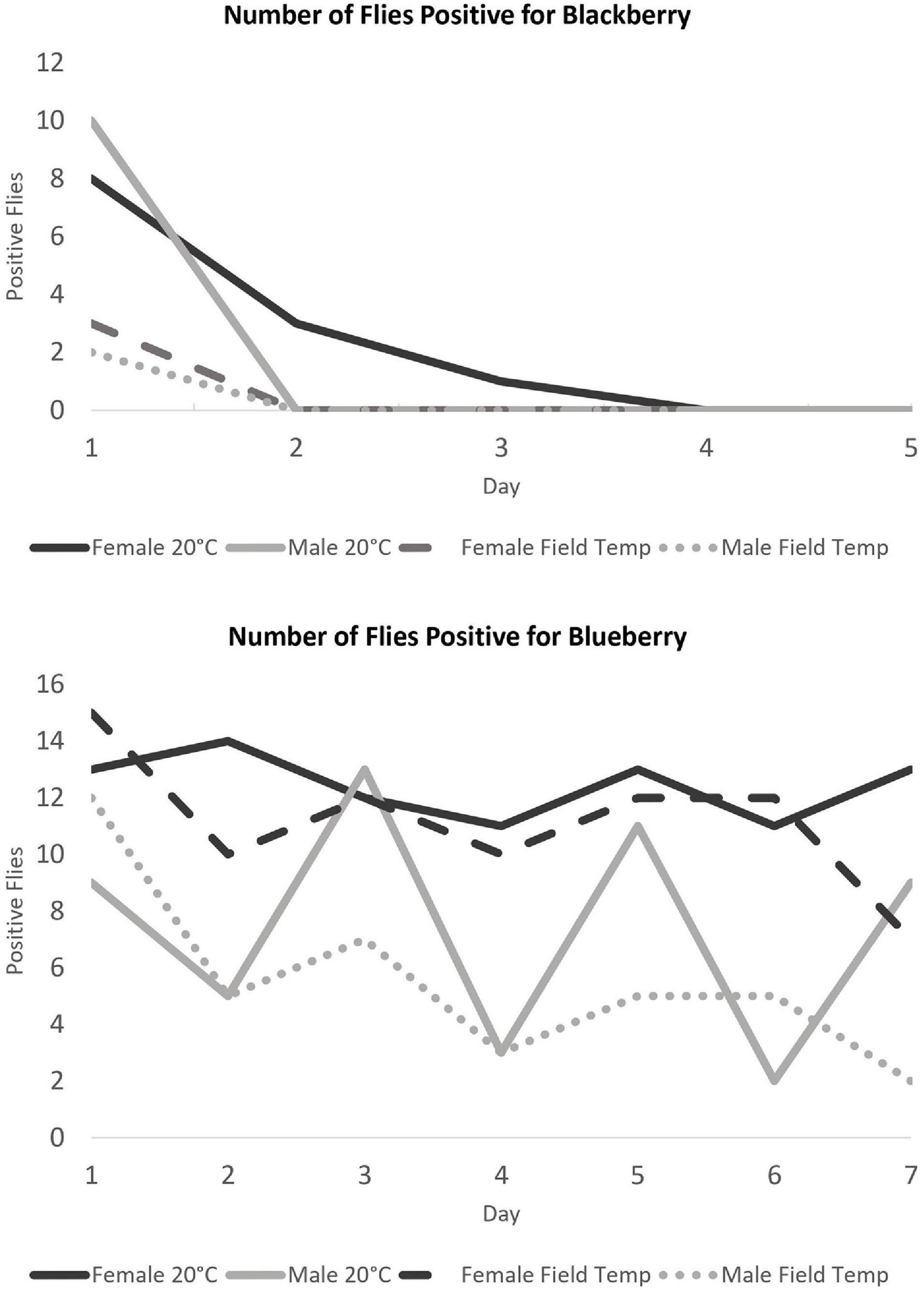
Figure 2. Number of flies positive for each type of berry DNA out of 15 total flies tested per treatment. The Y-axis represents the total positive flies and the X-axis represents days after the initial berry meal.
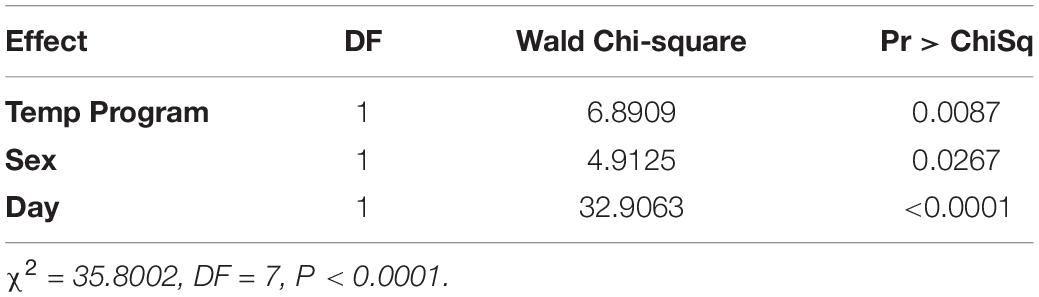
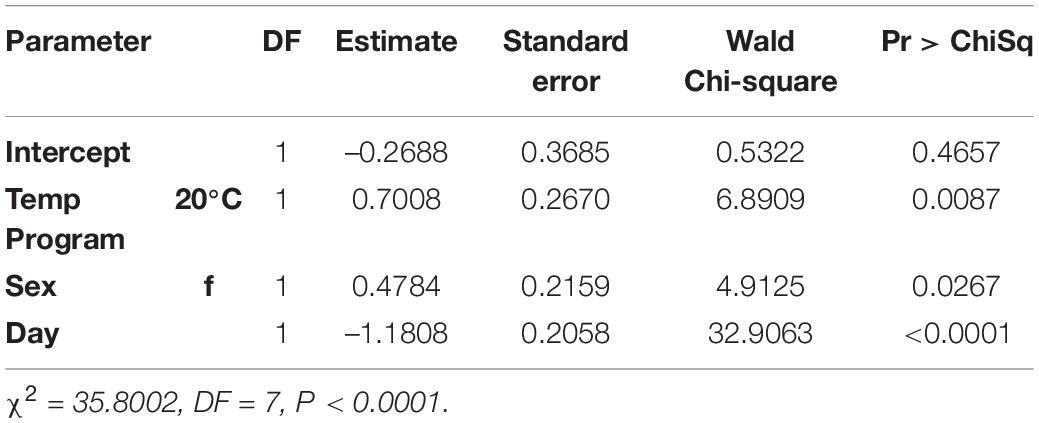
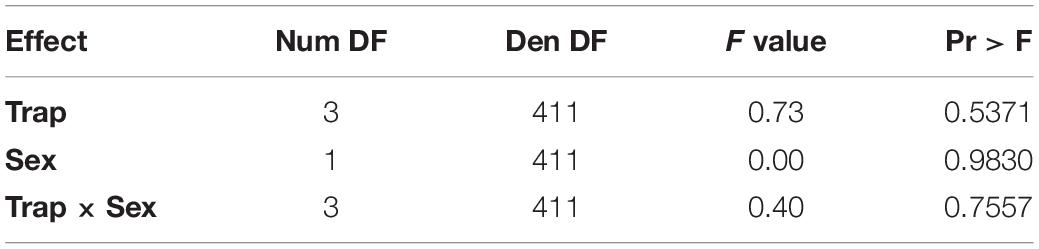
All variables (temperature program, sex, and day) were statistically significant via logistic regression. Day (Wald Chi-Square or WCS = 32.9) had a much stronger effect on whether a fly had detectible blackberry in its gut than temperature program (WCS = 6.9) and sex (WCS = 4.9) (Table 1).
For a one unit increase in day, the log-odds of having a fly positive for blackberry DNA decreased by a factor of 1.2. The log-odds of a female fly being positive for blackberry DNA increased by a factor of 0.48, and flies held at the cooler 20°C temperature program had increased log-odds of being positive for blackberry DNA by a factor of 0.7 (Table 2).
Due to the low number of positive flies in this assay (out of 15 total flies tested), we were unable to make predictions over time at field temperatures, which was our original objective for this assay (χ2 = 0.2631, DF = 4, P = 0.9921). Despite that, our raw data showed that no flies had detectible DNA after the first day held at field temperature.
Blueberry Detectability Over Time
In stark contrast to the blackberry primer results, blueberry DNA was detectible for a proportion of flies regardless of temperature program or sex for up to 7 days. Detection in males was more variable than in females (Figure 2).
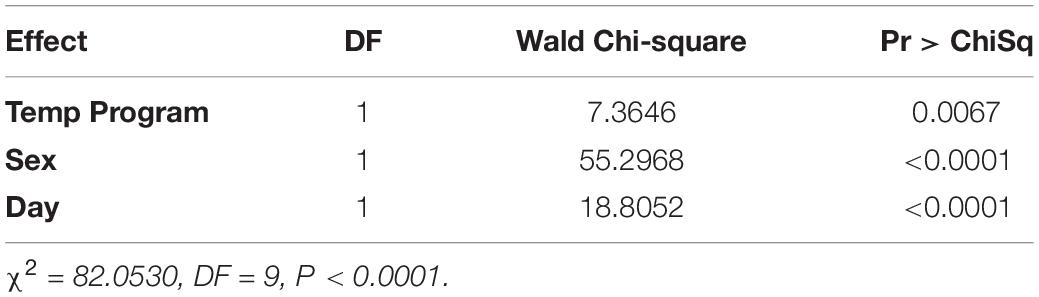
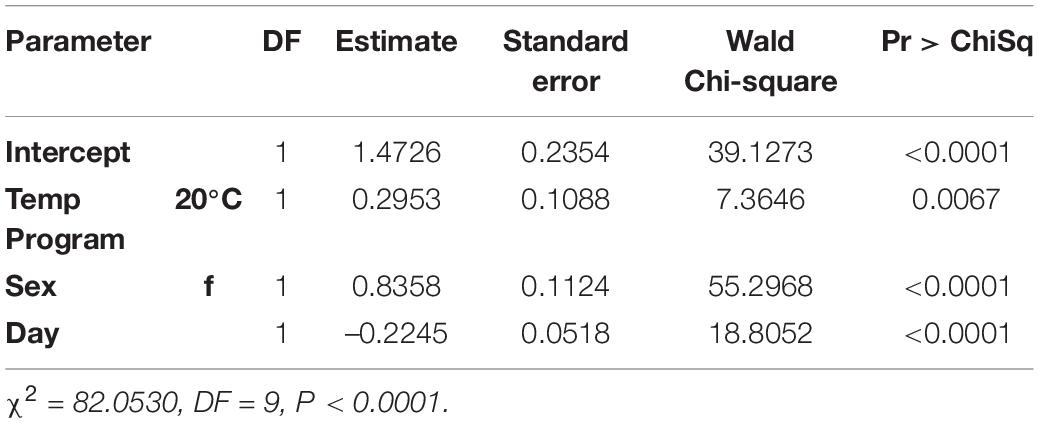
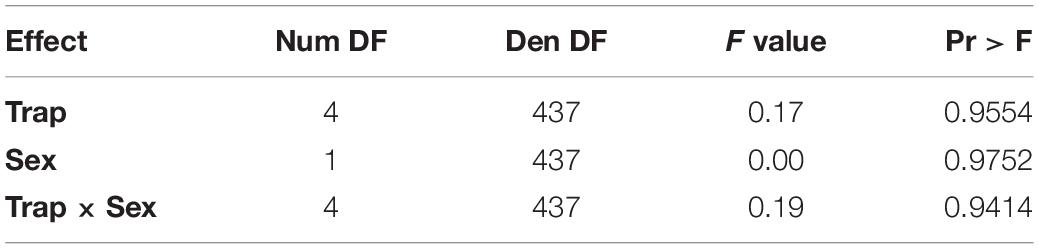
In the logistic regression, all variables were statistically significant (temperature program p = 0.0067, sex p < 0.0001, day p < 0.0001). With the blueberry primer, unlike the blackberry primer, the variable resulting in the largest effect size was sex (WCS = 55.3) followed by day (WCS = 18.8) with a smaller effect size due to the temperature program (WCS = 7.4) (Table 3).
For each additional day after feeding, a fly decreased its log-odds of being positive by a factor of 0.22. Flies held at the cooler 20°C temperature increased their log-odds of having a detectible blueberry meal by a factor of 0.3. In the case of female flies, the log-odds of having a positive blueberry DNA test increased by a factor of 0.84 (Table 4).
For flies held at field temperatures only, the log-odds of a fly being positive on day 1 versus day 7 were increased by a factor of 34.762 (95% CI 7.53-160.407) (χ2 = 43.2291, DF = 7, P < 0.0001). No other day-to-day comparisons were statistically significant.
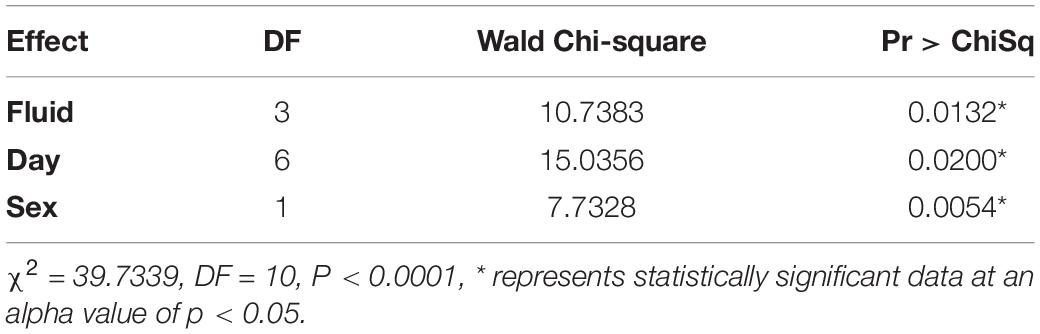
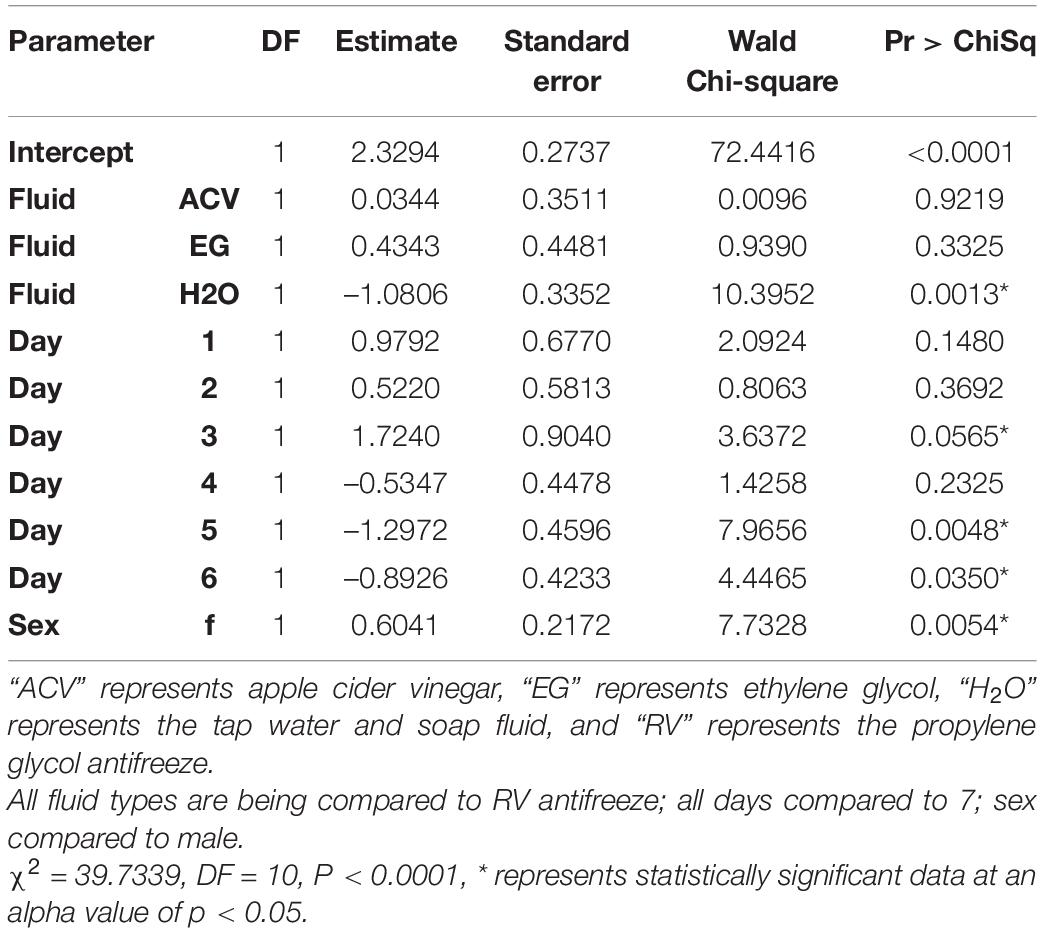
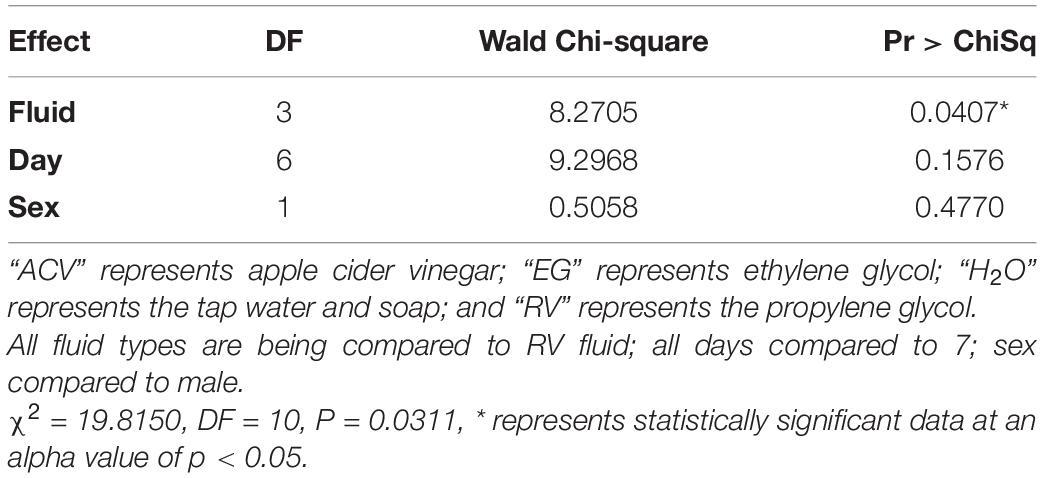
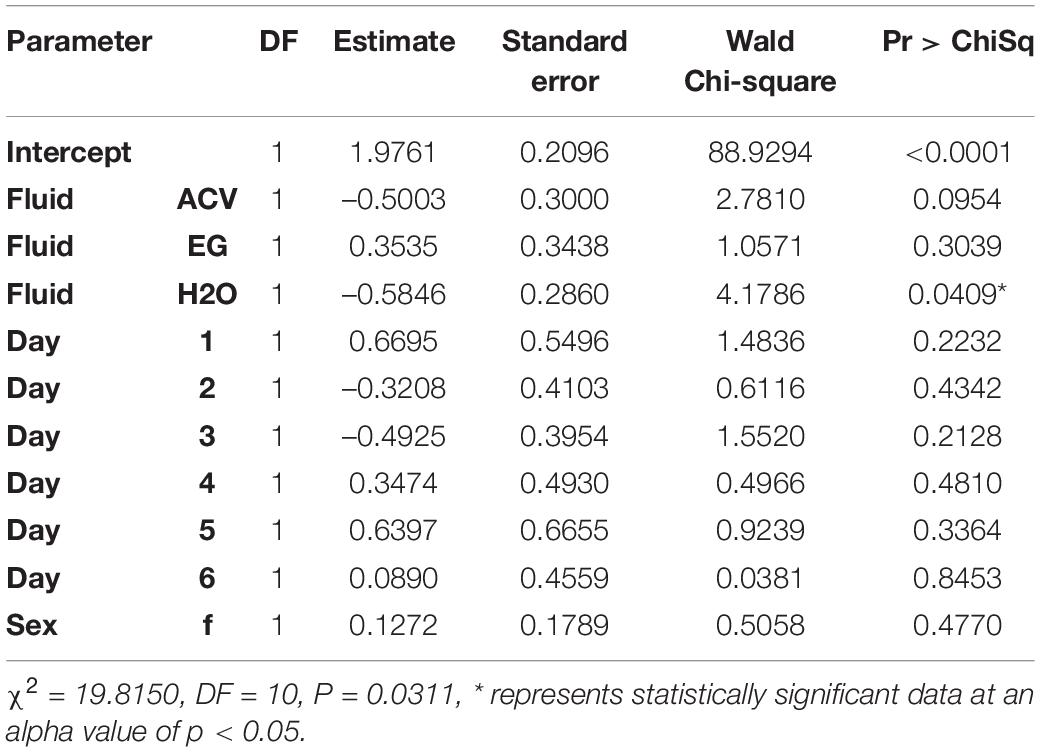
Trap Fluid
Blackberry Primers
All variables tested (trap fluid, day, and sex) were statistically significant via logistic regression. Day (Wald Chi-Square or WCS = 15.0) had a more significant effect size on whether a fly had a detectible blackberry meal in its gut than trap fluid (WCS = 10.7) followed by sex (WCS = 7.7; Table 5).
A fly fed on blackberry and held in tap water with soap had a log-odds decrease of detectible DNA of 1.1 units compared to a fly held in RV antifreeze. Other comparisons were not statistically significant. Flies tested on day 3 had increased log-odds of being positive by a factor of 1.7. Despite not being consistently significant, there is a small trend toward having increased log-odds of detecting blackberry DNA within a fly within the first 3 days with a slight decrease in the estimate for all later days. The log-odds of a female fly being positive for blackberry DNA increased by a factor of 0.6 (Table 6).
Blueberry Primers
Of the three variables tested, only trap fluid was statistically significant via logistic regression for the blueberry primer. The effect size for trap fluid was moderate (WCS = 8.3; Table 7). A blueberry-fed fly stored in tap water with soap had a decrease in its DNA detectability based on log-odds by a factor of 0.6 compared to RV antifreeze which may be due to bloating. When running the experiment, some of the flies held only in the water and soap solution became so bloated with water that they would pop at the slightest touch. None of the comparisons for day or sex were statistically significant (Table 8).
Based on these data, we used RV antifreeze as the trap fluid for the field-experiment and trapped flies every 2–3 days.
Field Tests
There was no statistically significant difference among trap locations nor sex trapped at the Piedmont Research station (Table 9). In our testing, between 0 and 11% of flies, by sex, tested positive for blackberry DNA (Table 10).
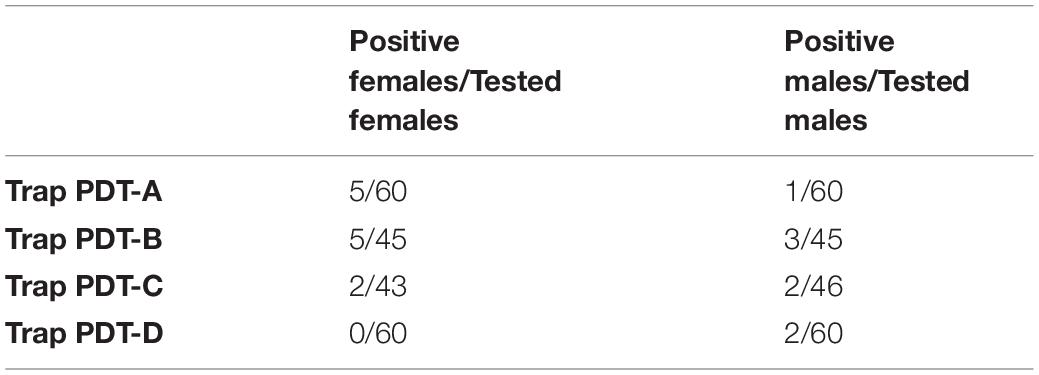
Table 10. Total positive flies out of flies tested at Piedmont Research Station blackberry plot by trap location.
There was no difference between the different trap locations tested nor between fly sex at Sandhills Research Station (Table 11). Between 0 and 15% of flies tested by sex were positive for blueberry DNA (Table 12).

Table 12. Total positive flies out of total flies tested at Sandhills Research Station blueberry plot by trap location.
Discussion
Laboratory-Raised Flies
The 20°C treatments for both blackberry and blueberry detectability over time assays had different results. Time had the most substantial effect on whether a fly would test positive for blackberry DNA after a known feeding event. Both sex and temperature also had an effect on the log-odds of testing positive for blackberry DNA but were smaller in effect size. Additionally, there was no detectible blackberry DNA in most flies after Day 1 for both temperature treatments. This could be because the adult flies starved and given blackberry DNA ate a smaller food meal than those for the blueberry experiment. The blackberry puree with ∼4 g of added sugar may have been less sweet than the blueberry puree with ∼4 g of sugar, since blackberries have fewer sugars than blueberries (Mikulic-Petkovsek et al., 2012; de Souza et al., 2014). It is also possible that a blackberry fruit meal was more easily digestible and was broken down faster than the blueberry fruit meal. In this study, there was no way to test the berry meal volume taken by each fly. Further research feeding individual D. suzukii different volumes of fruit could help elucidate answers to this question.
Alternatively, decreased detection of blackberry fruit meal over time compared to blueberry could be due to the nature of the primer region selected to test for blackberry DNA. Because both of the designed primers come from different regions within the whole plant genome, and because they are different lengths with various sequence complexity they could have different melt curve profiles; therefore, they cannot be directly compared. Additionally, since the blueberry primer comes from chloroplast DNA versus the blackberry primer from nuclear DNA, there are more copies of chloroplast DNA in a sample (Daniell et al., 2016). Therefore, there is an increased amount of blueberry DNA present within each sample which is likely adding to its increased detection time, even in non-green tissues like fruit which have low chloroplast protein synthesis but still contain plastids in the chromoplast (Daniell et al., 2016). It is possible that larval nutrition within blackberry was significantly better than within blueberry, resulting in less hungry newly emerged adults who did not need to immediately feed on pupal meconium (Hardin et al., 2015; Young et al., 2018; Olazcuaga et al., 2019). It has been shown that preferred hosts (blackberry) may result in heavier pupal weight, and that adult flies may preferentially choose a preferred host, like blackberry, if their natal host was an inferior host (i.e., pokeweed, Phytolacca americana L.) (Diepenbrock et al., 2016); thus, there is evidence that larval host influences adult behavior.
Blueberry DNA was detected for longer durations than blackberry, but detection was more variable within males than females. The dependence of male mating metrics on adult nutrition is species-specific (Aluja et al., 2001). It is possible that males within D. suzukii are less dependent on regular adult nutrition than females, perhaps because sperm is less energetically costly than developing eggs. An alternate hypothesis may be that males require less nutrition because they have less locomotor function than females and require less energy for movement (Ferguson et al., 2015). One study found that adult females of D. suzukii are four times more active within a semi-natural field environment than males, which may be specific to females’ mated status based on follow-up laboratory experiments (Ferguson et al., 2015). Starved adults of D. melanogaster typically ingest a larger than typical food meal than flies fed ad libitum (Bowdan and Dethier, 1986; Edgecomb et al., 1994). It is possible that the relatively short feeding period (6 h) may have served to better capture female feeding behavior and that males may take smaller meals more frequently. Together, reduced male activity and differential feeding behavior may impact the predictions made when testing these methods in a field setting.
Because newly emerged adults that fed on blueberry as larvae have detectible fruit DNA within their guts, it will be important to incorporate that understanding of fly biology into the interpretation of field data. Because pupal meconium may be a source of this berry DNA, this study provides evidence that adult flies are feeding on pupal meconium following eclosion, which has long been suspected as a means of acquiring symbiotic bacteria (Leach, 1934; Moll et al., 2001; Broderick and Lemaitre, 2012).
Our study of D. suzukii molecular gut content detectability over time provides important evidence that adult resource use within D. suzukii is species-specific and potentially substrate-specific even within fruit types. Additionally, it provides the first evidence that adult D. suzukii use pupal meconium as a nutritional resource after eclosion.
Field-Trapped Flies
The difference in detectible DNA by sex seen in the blackberry primers may be an artifact of variability in fly feeding behavior, particularly because it is only seen in the blackberry primers and not the blueberry primers, as discussed in the laboratory assay above. Because they were held at different temperatures, the trap fluid samples from each fruit type cannot be directly compared to each other statistically. Based on previous research by our group, the blackberry primers detect a fruit meal for flies that fed on blackberry from 1 to 4 days after feeding, with detection dropping precipitously after the first 24 h. The blueberry primers were able to detect a blueberry meal for 7 or more days. Our hypothesis that there would be a higher proportion of flies that had recently fed on either berry type within the crop was rejected. Similar proportions of flies having fed on fruit were found at in-crop, edge-of-crop, and woodline locations. This adds to the body of evidence that within a crop field and its surrounding suitable habitat, adult D. suzukii are likely moving short distances and are evenly dispersed (Klick et al., 2016; Leach et al., 2019).
Our trapping method may have selected for flies that had not been fruit fed. Previous studies have showed that traps such as those we used attract more female flies who have not yet found ovipositional substrate, more unmated flies than mated, and—importantly for our study—more starved flies than fed (Swoboda-Bhattarai et al., 2017; Wong et al., 2018).
We found a low proportion of adult feeding in individuals assessed with either set of primers. For the blackberry primers, the range of proportion of feeding was 0 to 11% of all flies. Briem et al. (2018) found that 38% of their trapped flies had fed on raspberry from their raspberry crop, though half of their samples were not surface-sterilized. Alternatively, the NGS method used by their group may be more sensitive and detect lower DNA levels than the qPCR methods used here (Briem et al., 2018, Chapter 2).
For flies feeding on blueberries, the proportions may have been low because blueberries are a less preferred ovipositional substrate compared to caneberries (Lee et al., 2011; Burrack et al., 2013; Kinjo et al., 2013). Blueberries may also be a less attractive adult food source for related reasons. The thick skin and firmer fruit of the blueberry require greater penetration force, which is why it is less appealing for oviposition (Lee et al., 2011; Burrack et al., 2013; Kinjo et al., 2013). This may result in fewer opportunities for adults to feed due to fewer ovipositional wounds. That said, the blueberry bushes in the breeding plots at Sandhills Research Station that were evaluated in this study had many fruit clusters with large feeding wounds created by paper wasps (Polistes sp.), honeybees (Apis mellifera), and/or Japanese beetles (Popilia japonica). There were also opportunities for D. suzukii to feed on punctured blueberries on the ground.
It is impossible to truly compare the blackberry-feeding flies to the blueberry-feeding flies because they were separated geographically, living under different weather conditions, and tested using different primers. It stands to reason that the blueberry-feeding flies, if all things were the same, would have detectible blueberry in their guts at least twice as often as blackberry-feeding flies simply because the blueberry primers can be detected for more than 7 days compared to less than 4 days in blackberry. Furthermore, since female flies are up to four times more active than male flies (Ferguson et al., 2015), we would have expected to see a difference in fruit feeding by sex, yet we did not. Additionally, D. suzukii that fed on blossoms, fruit, or laboratory-based diet fly longer and farther than starved flies (Wong et al., 2018). Altogether, the low proportion of fruit feeding, inconsistent with past data on feeding behavior, may instead point to a different narrative. Perhaps adult D. suzukii are more dependent on a different nutritional resource than berry juice in the field or are less dependent on nutritional resources as adults than we hypothesized. Judging by the high energetic cost of mating and finding ovipositional substrate, as well as the clues from this study and others that flies are moving up to 120 m within a few days (Klick et al., 2016; Leach et al., 2019), fruit may be a less-important nutritional resource for adults than some other substances. Mushrooms and bird manure have both been suggested as alternative host resources for oviposition (Stockton et al., 2019). Clearly, fruit feeding provides a small but meaningful proportion of D. suzukii with nutritional resources, but they may depend more heavily on other nutritional resources than fruit juice alone.
The results of this research support past findings that D. suzukii are regularly moving between crop and edge of crop locations at regular time intervals. Adult D. suzukii may depend more heavily on other nutritional resources than berry juice. This research contributes to a growing body of evidence that elucidating adult resource use in higher flies is crucial to understanding the biology of these and other polyphagous invasive pest species. Studying Brachycera feeding behavior in the wild has been difficult due to the high motility of adult flies and a lack of funding, so using pest species to further develop our knowledge of Dipteran biology can fill in gaps in our understanding of this diverse group of insects.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI (accession: FF683693.1 and MH551878).
Author Contributions
LK conceived the research, conducted the experiments and statistical analyses, analyzed the data, and wrote the manuscript. LD, TS, and HB assisted with experimental design and research. LK, RA, HA, and GF contributed material. HB secured funding. All authors reviewed, read, and approved the manuscript.
Funding
This research was funded by a grant from the USDA NIFA Specialty Crop Research Initiative 2015-51181-24252.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.719645/full#supplementary-material
Footnotes
References
Albertini, A., Marchi, S., Ratti, C., Burgio, G., Petacchi, R., and Magagnoli, S. (2018). Bactrocera oleae pupae predation by Ocypus olens detected by molecular gut content analysis. BioControl 63, 227–239. doi: 10.1007/s10526-017-9860-6
Aluja, M., Dıaz-Fleischer, F., Papaj, D. R., Lagunes, G., and Sivinski, J. (2001). Effects of age, diet, female density, and the host resource on egg load in Anastrepha ludens and Anastrepha obliqua (Diptera: Tephritidae). J. Insect Physiol. 47, 975–988. doi: 10.1016/s0022-1910(01)00072-5
Aluja, M., Jácome, I., and Macías-Ordóñez, R. (2001). Effect of adult nutrition on male sexual performance in four neotropical fruit fly species of the genus Anastrepha (Diptera: Tephritidae). J. Insect Behav. 14, 759–775.
Bassil, N. V., Nyberg, A. M., Finn, C. E., Clark, J. R., Peace, C. P., and Iezzoni, A. (2016). Development of a multiplexed fingerprinting set in blackberry. Acta Horticult. 1133, 89–96. doi: 10.17660/actahortic.2016.1133.14
Bertinetti, C., Samayoa, A. C., and Hwang, S. Y. (2019). Effects of feeding adults of Hermetia illucens (Diptera: Stratiomyidae) on longevity, oviposition, and egg hatchability: Insights into optimizing egg production. J. Insect Sci., 19:19.
Bowdan, E., and Dethier, V. G. (1986). Coordination of a dual inhibitory system regulating feeding behaviour in the blowfly. J. Comp. Physiol. A 158, 713–722.
Briem, F., Zeisler, C., Guenay, Y., Staudacher, K., Vogt, H., and Traugott, M. (2018). Identifying plant DNA in the sponging–feeding insect pest Drosophila suzukii. J. Pest Sci. 91, 985–994. doi: 10.1007/s10340-018-0963-3
Broderick, N. A., and Lemaitre, B. (2012). Gut-associated microbes of Drosophila melanogaster. Gut. Microb. 3, 307–321. doi: 10.4161/gmic.19896
Burrack, H. J., Fernandez, G. E., Spivey, T., and Kraus, D. A. (2013). Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumura (Diptera: Drosophilidae), an invasive frugivore. Pest Manage. Sci. 69, 1173–1180. doi: 10.1002/ps.3489
Burrack, H. J., Fornell, A. M., Connell, J. H., O’Connell, N. V., Phillips, P. A., Vossen, P. M., et al. (2009). Intraspecific larval competition in the olive fruit fly (Diptera: Tephritidae). Environ. Entomol. 38, 1400–1410. doi: 10.1603/022.038.0508
Chang, C. L., Albrecht, C., El-Shall, S. S., and Kurashima, R. (2001). Adult reproductive capacity of Ceratitis capitata (Diptera: Tephritidae) on a chemically defined diet. Annals of the Entomological Society of America, 94, 702–706.
Clarke, A. R., Powell, K. S., Weldon, C. W., and Taylor, P. W. (2011). The ecology of Bactrocera tryoni (Diptera: Tephritidae): what do we know to assist pest management? Ann. Appl. Biol. 158, 26–54. doi: 10.1111/j.1744-7348.2010.00448.x
Daniell, H., Lin, C. S., Yu, M., and Chang, W. J. (2016). Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genom. Biol. 17, 1–29. doi: 10.1007/978-3-540-68696-5_1
de Souza, V. R., Pereira, P. A. P., da Silva, T. L. T., de Oliveira Lima, L. C., Pio, R., and Queiroz, F. (2014). Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 156, 362–368. doi: 10.1016/j.foodchem.2014.01.125
Dhami, M. K., and Kumarasinghe, L. (2014). A HRM real-time PCR assay for rapid and specific identification of the emerging pest Spotted-Wing Drosophila (Drosophila suzukii). PLoS One 9:e98934. doi: 10.1371/journal.pone.0098934
Diepenbrock, L. M., Lundgren, J. G., Sit, T. L., and Burrack, H. J. (2018). Detecting specific resource use by Drosophila suzukii (Diptera: Drosophilidae) using gut content analysis. J. Econ. Entomol. 111, 1496–1500. doi: 10.1093/jee/toy077
Diepenbrock, L. M., Swoboda-Bhattarai, K. A., and Burrack, H. J. (2016). Ovipositional preference, fidelity, and fitness of Drosophila suzukii in a co-occurring crop and non-crop host system. J. Pest Sci. 89, 761–769. doi: 10.1007/s10340-016-0764-5
Edgecomb, R. S., Harth, C. E., and Schneiderman, A. M. (1994). Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J. Exper. Biol. 197, 215–235. doi: 10.1242/jeb.197.1.215
Ekesi, S., Billah, M. K., Nderitu, P. W., Lux, S. A., and Rwomushana, I. V. I (2009). Evidence for competitive displacement of Ceratitis cosyra by the invasive fruit fly Bactrocera invadens (Diptera: Tephritidae) on mango and mechanisms contributing to the displacement. J. Econ. Entomol. 102, 981–991. doi: 10.1603/029.102.0317
Elzinga, R. J., and Broce, A. B. (1986). Labellar modifications of muscomorpha flies (Diptera). Ann. Entomol. Soc. Am. 79, 150–209. doi: 10.1093/aesa/79.1.150
Emiljanowicz, L. M., Ryan, G. D., Langille, A., and Newman, J. (2014). Development, reproductive output and population growth of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae) on artificial diet. J. Econ. Entomol. 107, 1392–1398. doi: 10.1603/ec13504
Erland, L. A., Rheault, M. R., and Mahmoud, S. S. (2015). Insecticidal and oviposition deterrent effects of essential oils and their constituents against the invasive pest Drosophila suzukii (Matsumura)(Diptera: Drosophilidae). Crop. Protect. 78, 20–26. doi: 10.1016/j.cropro.2015.08.013
Ferguson, C. T., O’Neill, T. L., Audsley, N., and Isaac, R. E. (2015). The sexually dimorphic behaviour of adult Drosophila suzukii: elevated female locomotor activity and loss of siesta is a post-mating response. J. Exper. Biol. 218, 3855–3861.
Frewin, A. J., Renkema, J., Fraser, H., and Hallett, R. H. (2017). Evaluation of attractants for monitoring Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 110, 1156–1163. doi: 10.1093/jee/tox081
Ghabbari, M., Guarino, S., Caleca, V., Saiano, F., Sinacori, M., Baser, N., et al. (2018). Behavior-modifying and insecticidal effects of plant extracts on adults of Ceratitis capitata (Wiedemann) (Diptera Tephritidae). J. Pest Sci. 91, 907–917. doi: 10.1007/s10340-018-0952-6
Google. (2021). [Google Earth View of Sandhills Research Station Above Research Plot]. Available online at: https://www.google.com/maps/place/Sandhills+Research+Station/@35.1931806,-79.6856195,789m/data=!3m1!1e3!4m5!3m4!1s0x8854bee9388e39c7:0xfac4fad170286c65!8m2!3d35.1829902!4d-79.6774542 (accessed January 4, 2021).
Google. (2021). [Google Earth View of Piedmont Research Station Above Research Plot]. Available online at: https://www.google.com/maps/place/NCDA%26CS+Piedmont+Research+Station/@35.6965801,-80.627101,566m/data=!3m1!1e3!4m5!3m4!1s0x8853f072199aacdf:0x27078a49ed8b5a86!8m2!3d35.6966673!4d-80.6226754 (accessed January 4, 2021).
Greenstone, M. H., Rowley, D. L., Weber, D. C., Payton, M. E., and Hawthorne, D. J. (2007). Feeding mode and prey detectability half-lives in molecular gut-content analysis: an example with two predators of the Colorado potato beetle. Bull. Entomol. Res. 97, 201–209. doi: 10.1017/s000748530700497x
Günther, B., Rall, B. C., Ferlian, O., Scheu, S., and Eitzinger, B. (2014). Variations in prey consumption of centipede predators in forest soils as indicated by molecular gut content analysis. Oikos 123, 1192–1198. doi: 10.1111/j.1600-0706.2013.00868.x
Hamby, K. A., and Becher, P. G. (2016). Current knowledge of interactions between Drosophila suzukii and microbes, and their potential utility for pest management. J. Pest. Sci. 89, 621–630. doi: 10.1007/s10340-016-0768-1
Hamby, K. A., Bellamy, D. E., Chiu, J. C., Lee, J. C., Walton, V. M., Wiman, N. G., et al. (2016). Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J. Pest. Sci. 89, 605–619. doi: 10.1007/s10340-016-0756-5
Hardin, J. A., Kraus, D. A., and Burrack, H. J. (2015). Diet quality mitigates intraspecific larval competition in Drosophila suzukii. Entomol. Exper. Appl. 156, 59–65. doi: 10.1111/eea.12311
Harwood, J. D., Yoo, H. J. S., Greenstone, M. H., Rowley, D. L., and O’Neil, R. J. (2009). Differential impact of adults and nymphs of a generalist predator on an exotic invasive pest demonstrated by molecular gut-content analysis. Biol. Invasions 11, 895–903. doi: 10.1007/s10530-008-9302-6
Headrick, D. H., and Goeden, R. D. (1990). Resource utilization by larvae of Paracantha gentilis (Diptera: Tephritidae) in capitula of Cirsium californicum and C. proteanum (Asteraceae) in southern California. Proc. Entomol. Soc. Washington 92, 512–520.
Jacome, I., Aluja, M., Liedo, P., and Nestel, D. (1995). The influence of adult diet and age on lipid reserves in the tropical fruit fly Anastrepha serpentina (Diptera: Tephritidae). J. Insect. Physiol. 41, 1079–1086. doi: 10.1016/0022-1910(95)00067-5
Jaramillo, S. L., Mehlferber, E., and Moore, P. J. (2014). Life-history trade-offs under different larval diets in Drosophila suzukii (Diptera: Drosophilidae). Physiol. Entomol. 40, 2–9. doi: 10.1111/phen.12082
Juen, A., and Traugott, M. (2005). Detecting predation and scavenging by DNA gut-content analysis: a case study using a soil insect predator-prey system. Oecologia 142, 344–352. doi: 10.1007/s00442-004-1736-7
Kinjo, H., Kunimi, Y., Ban, T., and Nakai, M. (2013). Oviposition efficacy of Drosophila suzukii (Diptera: Drosophilidae) on different cultivars of blueberry. J. Econ. Entomol. 106, 1767–1771. doi: 10.1603/ec12505
Klick, J., Yang, W. Q., Walton, V. M., Dalton, D. T., Hagler, J. R., Dreves, A. J., et al. (2016). Distribution and activity of Drosophila suzukii in cultivated raspberry and surrounding vegetation. J. Appl. Entomol. 140, 37–46. doi: 10.1111/jen.12234
Koressaar, T., and Remm, M. (2007). Enhancements and modifications of primer design program Primer3. Bioinformatics 23, 1289–1291. doi: 10.1093/bioinformatics/btm091
Kraft, L. J., Yeh, D. A., Gómez, M. I., and Burrack, H. J. (2020). Determining the effect of postharvest cold storage treatment on the survival of immature Drosophila suzukii (Diptera: Drosophilidae) in small fruits. J. Econ. Entomol. 113, 2427–2435. doi: 10.1093/jee/toaa185
Leach, H., Hagler, J. R., Machtley, S. A., and Isaacs, R. (2019). Spotted wing drosophila (Drosophila suzukii) utilization and dispersal from the wild host Asian bush honeysuckle (Lonicera spp.). Agric. Forest Entomol. 21, 149–158. doi: 10.1111/afe.12315
Leach, J. G. (1934). The method of survival of bacteria in the puparia of the seed-corn maggot (Hylemyia cilicrura Rond.) 1. Zeitschrift für Angewandte Entomol. 20, 150–161. doi: 10.1111/j.1439-0418.1934.tb00358.x
Lee, J. C., Bruck, D. J., Curry, H., Edwards, D., Haviland, D. R., Van Steenwyk, R. A., et al. (2011). The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest. Manage. Sci. 67, 1358–1367. doi: 10.1002/ps.2225
Lee, J. C., Burrack, H. J., Barrantes, L. D., Beers, E. H., Dreves, A. J., Hamby, K. A., et al. (2012). Evaluation of monitoring traps for Drosophila suzukii (Diptera: Drosophilidae) in North America. J. Econ. Entomol. 105, 1350–1357.
Lewis, M. T., and Hamby, K. A. (2019). Differential impacts of yeasts on feeding behavior and development in larval Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 9, 1–12.
Li, J., Yang, F., Wang, Q., Pan, H., Yuan, H., and Lu, Y. (2017). Predation by generalist arthropod predators on Apolygus lucorum (Hemiptera: Miridae): molecular gut-content analysis and field-cage assessment. Pest. Manage. Sci. 73, 628–635. doi: 10.1002/ps.4346
Macías-Hernández, N., Athey, K., Tonzo, V., Wangensteen, O. S., Arnedo, M., and Harwood, J. D. (2018). Molecular gut content analysis of different spider body parts. PLoS One, 13:e0196589.
Mahmoud, M. F., and Shoeib, M. A. (2008). Sterilant and oviposition deterrent activity of neem formulation on Peach fruit fly Bactrocera zonata (Saunders) (Diptera: Tephritidae). J. Biopest. 1, 177–181.
Mawtham, M. M., Gailce Leo Justin, C., Sheeba Joyce Roseleen, S., and Kumanan, K. (2019). Oviposition deterrent and repellent activity of bio-inputs and insecticides against melon fruit fly, Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) in bitter gourd. Pharma Innov. J. 8, 412–416.
Mikulic-Petkovsek, M., Schmitzer, V., Slatnar, A., Stampar, F., and Veberic, R. (2012). Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 77, C1064–C1070.
Moll, R. M., Romoser, W. S., Modrakowski, M. C., Moncayo, A. C., and Lerdthusnee, K. (2001). Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J. Med. Entomol. 38, 29–32. doi: 10.1603/0022-2585-38.1.29
Mori, B. A., Whitener, A. B., Leinweber, Y., Revadi, S., Beers, E. H., Witzgall, P., et al. (2017). Enhanced yeast feeding following mating facilitates control of the invasive fruit pest Drosophila suzukii. J. Appl. Ecol. 54, 170–177. doi: 10.1111/1365-2664.12688
Nash, W. J., and Chapman, T. (2014). Effect of dietary components on larval life history characteristics in the Medfly (Ceratitis capitata: Diptera, Tephritidae). PloS One 9:e86029. doi: 10.1371/journal.pone.0086029
Olazcuaga, L., Rode, N. O., Foucaud, J., Facon, B., Ravigné, V., Ausset, A., et al. (2019). Oviposition preference and larval performance of Drosophila suzukii (Diptera: Drosophilidae), spotted-wing Drosophila: effects of fruit identity and composition. Environ. Entomol. 48, 867–881. doi: 10.1093/ee/nvz062
Parrella, M. P. (1983). Intraspecific competition among larvae of Liriomyza trifolii (Diptera: Agromyzidae): effects on colony production. Environ. Entomol. 12, 1412–1414. doi: 10.1093/ee/12.5.1412
Plantamp, C., Estragnat, V., Fellous, S., Desouhant, E., and Gibert, P. (2017). Where and what to feed? Differential effects on fecundity and longevity in the invasive Drosophila suzukii. Basic Appl. Ecol. 19, 56–66. doi: 10.1016/j.baae.2016.10.005
Rendon, D., Walton, V., Tait, G., Buser, J., Lemos Souza, I., Wallingford, A., et al. (2019). Interactions among morphotype, nutrition, and temperature impact fitness of an invasive fly. Ecol. Evol. 9, 2615–2628. doi: 10.1002/ece3.4928
Renkema, J. M., Buitenhuis, R., and Hallett, R. H. (2017). Reduced Drosophila suzukii infestation in berries using deterrent compounds and laminate polymer flakes. Insects 8:117. doi: 10.3390/insects8040117
Revadi, S., Vitagliano, S., Rossi Stacconi, M. V., Ramasamy, S., Mansourian, S., Carlin, S., et al. (2015). Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol. Entomol. 40, 54–64.
Rondoni, G., Athey, K. J., Harwood, J. D., Conti, E., Ricci, C., and Obrycki, J. J. (2015). Development and application of molecular gut-content analysis to detect aphid and coccinellid predation by Harmonia axyridis (Coleoptera: Coccinellidae) in Italy. Insect Sci. 22, 719–730. doi: 10.1111/1744-7917.12165
Schnetzer, J. W., and Tyler, M. S. (1996). Endogenous β-galactosidase activity in the larval, pupal, and adult stages of the fruit fly, Drosophila melanogaster, indicates need for caution in lacZ fusion-gene studies. Biol. Bull. 190, 173–187. doi: 10.2307/1542537
Siddique, A. B., Bachchu, M. A. A., Uddin, M. N., Rahman, M. H., Bhuyain, M. M. H., and Rana, M. S. (2018). Ovipositional deterrent and repulsive effect of six botanicals against Bactrocera cucurbitae (Coquillett)(Diptera: Tephritidae). J. Entomol. Zool. Stud. 6, 2092–2097.
Silva-Soares, N. F., Nogueira-Alves, A., Beldade, P., and Mirth, C. K. (2017). Adaptation to new nutritional environments: larval performance, foraging decisions, and adult oviposition choices in Drosophila suzukii. BMC Ecol. 17:21. doi: 10.1186/s12898-017-0131-2
Stockton, D. G., Brown, R., and Loeb, G. M. (2019). Not berry hungry? Discovering the hidden food sources of a small fruit specialist, Drosophila suzukii. Ecol. Entomol. 44, 810–822. doi: 10.1111/een.12766
Swoboda-Bhattarai, K. A., McPhie, D. R., and Burrack, H. J. (2017). Reproductive status of Drosophila suzukii (Diptera: Drosophilidae) females influences attraction to fermentation-based baits and ripe fruits. J. Econ. Entomol. 110, 1648–1652. doi: 10.1093/jee/tox150
Szendrei, Z., Greenstone, M. H., Payton, M. E., and Weber, D. C. (2010). Molecular gut-content analysis of a predator assemblage reveals the effect of habitat manipulation on biological control in the field. Basic Appl. Ecol. 11, 153–161. doi: 10.1016/j.baae.2009.10.006
Terashima, J., and Bownes, M. (2004). Translating available food into the number of eggs laid by Drosophila melanogaster. Genetics 167, 1711–1719. doi: 10.1534/genetics.103.024323
Tochen, S., Walton, V. M., and Lee, J. C. (2016). Impact of floral feeding on adult Drosophila suzukii survival and nutrient status. J. Pest Sci. 89, 793–802. doi: 10.1007/s10340-016-0762-7
Untergasser, A., Cutcutache, I., Koressaar, T., Ye, J., Faircloth, B. C., Remm, M., et al. (2012). Primer3 - new capabilities and interfaces. Nucleic Acids Res. 40:e115. doi: 10.1093/nar/gks596
Ur Rehman, J., Jilani, G., Khan, M. A., Masih, R., and Kanvil, S. (2009). Repellent and oviposition deterrent effects of indigenous plant extracts to Peach Fruit Fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae). Pakistan J. Zool. 41, 108–110.
Van Timmeren, S., and Isaacs, R. (2013). Control of spotted wing drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop Protect. 54, 126–133. doi: 10.1016/j.cropro.2013.08.003
Walsh, D. B., Bolda, M. P., Goodhue, R. E., Dreves, A. J., Lee, J., Bruck, D. J., et al. (2011). Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manage. 2, G1–G7.
Wong, J. S., Cave, A. C., Lightle, D. M., Mahaffee, W. F., Naranjo, S. E., Wiman, N. G., et al. (2018). Drosophila suzukii flight performance reduced by starvation but not affected by humidity. J. Pest Sci. 91, 1269–1278. doi: 10.1007/s10340-018-1013-x
Wong, J. S., Wallingford, A. K., Loeb, G. M., and Lee, J. C. (2018). Physiological status of Drosophila suzukii (Diptera: Drosophilidae) affects their response to attractive odours. J. Appl. Entomol. 142, 473–482. doi: 10.1111/jen.12497
Yeates, D. K., and Wiegmann, B. M. (1999). Congruence and controversy: toward a higher-level phylogeny of Diptera. Annu. Rev. Entomol/ 44, 397–428. doi: 10.1146/annurev.ento.44.1.397
Young, Y., Buckiewicz, N., and Long, T. A. (2018). Nutritional geometry and fitness consequences in Drosophila suzukii, the Spotted-Wing Drosophila. Ecol. Evol. 8, 2842–2851. doi: 10.1002/ece3.3849
Keywords: spotted wing Drosophila, integrated pest management, invasive species, gut content, small fruit IPM
Citation: Kraft LJ, Sit TL, Diepenbrock LM, Ashrafi H, Aryal R, Fernandez GE and Burrack HJ (2021) Detection of Fruit Meals Within Laboratory-Raised and Field-Trapped Adult Drosophila suzukii (Diptera: Drosophilidae) Guts. Front. Ecol. Evol. 9:719645. doi: 10.3389/fevo.2021.719645
Received: 02 June 2021; Accepted: 09 July 2021;
Published: 23 August 2021.
Edited by:
Cesar Rodriguez-Saona, Rutgers, The State University of New Jersey, United StatesReviewed by:
Jana Lee, Agricultural Research Service, United States Department of Agriculture, United StatesNicholas Aflito, Cornell University, United States
Copyright © 2021 Kraft, Sit, Diepenbrock, Ashrafi, Aryal, Fernandez and Burrack. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura J. Kraft, bGF1cmFqa3JhZnRAZ21haWwuY29t
 Laura J. Kraft
Laura J. Kraft Tim L. Sit1
Tim L. Sit1 Lauren M. Diepenbrock
Lauren M. Diepenbrock Gina E. Fernandez
Gina E. Fernandez Hannah J. Burrack
Hannah J. Burrack