- 1Ministry of Education Key Laboratory for Ecology of Tropical Islands, College of Life Sciences, Hainan Normal University, Haikou, China
- 2Department of Biology and Food Science, Hebei Normal University for Nationalities, Chengde, China
Cuckoo nest parasites lay eggs in host nests and thereby transfer all reproduction costs to the hosts. This greatly reduces host fitness. Parasitism has selected for the evolution of anti-parasitic strategies in hosts, including nest defense. The dynamic risk assessment hypothesis holds that nest parasitism only threatens the nests during the egg stage, so hosts should reduce the level of defense against nest parasites after the egg stage. We studied the behavioral and acoustic responses of oriental reed warblers (Acrocephalus orientalis), during both the egg and nestling stages, toward the common cuckoo (Cuculus canorus), sparrowhawks (Accipiter nisus) and oriental turtle doves (Streptopelia orientalis). A. orientalis can visually distinguish cuckoos from sparrowhawks and doves, indicating that hawk mimicry did not work for the cuckoos. The behavioral response of hosts in the nestling stage was stronger than in the egg stage, which supports the offspring value hypothesis and suggests that cuckoos may also act as nest predators. However, there was no difference in the alarm calls A. orientalis produce in response to different invaders, indicating that different types of alarm calls may not contain specific information.
Introduction
Nest parasitism is a special reproductive behavior in which parasitic birds such as common cuckoos (Cuculus canorus) do not build nests themselves but lay their eggs in the nests of other birds (hosts). This transfers all of the reproductive costs to their hosts, who incubate the parasite eggs and raise parasite young (Davies, 2011; Soler, 2014). Successful nest parasitism severely reduces host fitness and compels them to invest time and energy in caring for alien eggs or nestlings, while reducing their chances of re-nesting and reproducing (Rothstein, 1990; Yang et al., 2019). In addition to being nest parasites, these birds are also potential nest predators. Many cuckoos remove or eat at least one of the host eggs before laying their own eggs during parasitism (Davies, 2000; Soler, 2014). Moreover, many adult parasites (including some cuckoos, Cuculus spp., and cowbirds, Molothrus spp.) destroy entire eggs or nestlings in host nests at advanced breeding stages that are unsuitable for parasitism. This forces the hosts to rebuild nests and increases their chance of parasitism (Arcese et al., 1996; Swan et al., 2015; Soler et al., 2017). In addition, parasites may revisit host nests after parasitism and destroy the eggs or nestlings of the hosts who have rejected parasite eggs (Tate, 1967; Soler et al., 1995, 2017; Ponton et al., 2006; Hoover and Robinson, 2007). Finally, there are also nest predation cases involving brood parasites without parasitism intent (Su et al., 2017; Šulc et al., 2020).
Hosts have evolved responses to parasitism with a series of countering strategies. Nest defense is the first line of defense, and successful nest defense can greatly improve host fitness (Moore, 2002; Welbergen and Davies, 2009; Feeney et al., 2012). Some hosts recognize the parasites as a particular threat, and exhibit aggressive behavior that successfully prevents the parasites from approaching their nests (Duckworth, 1991; Welbergen and Davies, 2008; Trnka and Prokop, 2012; Yang et al., 2014b; Li et al., 2015; Ma et al., 2018a). Some hosts adjust their nest defense strategies according to the species of intruder and at different stages of reproduction (Patterson and James, 1980; Montgomerie and Weatherhead, 1988; Redondo and Carranza, 1989; Caro, 2005; Welbergen and Davies, 2009; Campobello and Sealy, 2010, 2018). The hypothesis of dynamic risk assessment (Kleindorfer et al., 2005) assumes that nest parasitism only poses a threat to the hosts during the egg stage, so the level of defense against parasites should be reduced after the egg stage. However, the response to predators should be the opposite. For example, Duckworth (1991) found that the reed warbler (Acrocephalus scirpaceus) showed a strong aggressive response to the common cuckoo during the egg stage, but the cuckoo was ignored by the host after the chicks had hatched. In addition, many species make specific alarm calls in response to different threats (Robertson and Norman, 1977; Briskie and Sealy, 1989; Gill and Sealy, 1996; Lawson et al., 2020). For example, the yellow warbler (Setophaga petechia) makes specific “seet” calls toward the brown-headed cowbird (Molothrus ater) in order to warn intraspecific or interspecific individuals of the danger (Gill and Sealy, 1996; Lawson et al., 2020) so that they can take corresponding defensive measures.
Most studies have focused on the behavioral response of hosts to the presence of brood parasite individuals (Smith et al., 1984; Honza et al., 2004; Welbergen and Davies, 2009; Neudorf and Sealy, 2012; Feeney et al., 2015). Fewer studies have documented quantitative analyses of the alarm calls (Feeney et al., 2013; Yu et al., 2017b) due to their complexity (Marler, 2004). Alarm calls are an important part of the defense of nest owners against intruders (Marler, 2004) because they may contain information about the type of intruder. For example, the barn swallow (Hirundo rustica) or great tit (Parus major) showed no behavioral response differences to cuckoo and sparrowhawk models (Liang and Møller, 2015; Yu et al., 2017b), but acoustic playback revealed that the alarm calls carried information about the types of threat (Yu et al., 2016, 2017b). Therefore, it is helpful to understand the coevolution of acoustic communication between hosts and parasites if they reveal the specific meaning of the alarm calls emitted by hosts. We studied both the behavioral and acoustic responses of oriental reed warblers (Acrocephalus orientalis) to nest intruders (including common cuckoos) across egg and nestling stages by investigating a variety of host traits.
Materials and Methods
Study Site and Species
The research was performed in Yongnianwa National Wetland Park (36°40′–36°41′N, 114°41″–114°45′E) in Handan city, Hebei Province of China from May to August 2019. Yongnianwa has a temperate sub-humid continental monsoon climate and is 40.3 m above sea level. The annual average rainfall and annual average temperature are 527.8 mm and 12.9°C, respectively. The low-lying land is dominated by a large area of reed, calamus and lotus (Ma et al., 2018b). The Oriental reed warbler (Acrocephalus orientalis) belongs to the Acrocephalidae, Passeriformes and breeds in the reeds (Zheng, 2017). A. orientalis is a host of the common cuckoo in Asia, and the interaction between them has reached a high level of intensity during their coevolution (Yang et al., 2014a, 2016, 2017; Li et al., 2016). In the population studied in Yongnianwa, 14.8% of the nests were parasitized by the common cuckoo (Ma et al., 2018b).
Measure of Behavioral Response
Mounted specimens of nest intruders were presented in the incubation stage (3rd day of incubation, n = 22) and nestling stage (ca. 4-day-old nestlings, n = 14) to investigate the behavioral response of A. orientalis. Due to the high predation rate, only three nests were tested at both egg and chick stages. Each observed nest was exposed to three species (common cuckoo: native parasitic bird, sparrowhawks Accipiter nisus: unusual predator and oriental turtle doves Streptopelia orientalis: native harmless bird species and often encounter hosts) during experiment, with an interval of at least 60 min between them. To avoid pseudo-replication, two specimen replicates of each intruder were randomly selected for the experiment. Each specimen was presented at a distance of 0.5 m from the host nests, with the bill of the specimen toward the nest. A digital video recorder (HDR-PJ510E, Sony Corporation, Tokyo, Japan) was placed at a distance of 5 m from the nest to record A. orientalis behavior. An observer (JW), dressed in camouflage and wearing a camouflage hat, squatted or stood 5 m away from the host’s nest, so that reed bushes could shade the observer, and host responses were recorded for 5 min after the hosts returned to the nests while alarm calls from the hosts were recorded using a tape recorder (Lotoo L300E, Infomedia Inc., Beijing, China) connected to a gun microphone (MKH418, Sennheiser Inc., Wiedmark, Germany) with a sampling frequency of 44.1 kHz and a sampling resolution of 24 bits (Yu et al., 2016). Neighbor nests were not tested on the same day (Yu et al., 2019a). The following parameters of host response were recorded: (1) response intensity, which was classified to watching (the host was only observed around the specimen without any other apparent response; score = 1), alert (birds produced alarm calls when they saw a specimen, but they had no physical contact with the specimen and did not appear to be in an aggressive posture; score = 2), mobbing (birds made alarm calls and flew past the specimen in a feint of aggression; score = 3) or attack (birds produced alarm calls when they attacked the specimen and had physical contact with the specimen; score = 4); (2) number of attracted individuals (the largest number of conspecific individuals attracted during the experiment); (3) number of responsive individuals (the number of attracted individuals showing alarm and above-mentioned response intensity); (4) response time (the time from hosts arrival to the strongest reaction they produced), (5) attack frequency (recorded within the first 1 min from the attack initiated to avoid host fatigue); (6) the alarm duration of 5 min.
Measure of Acoustic Response
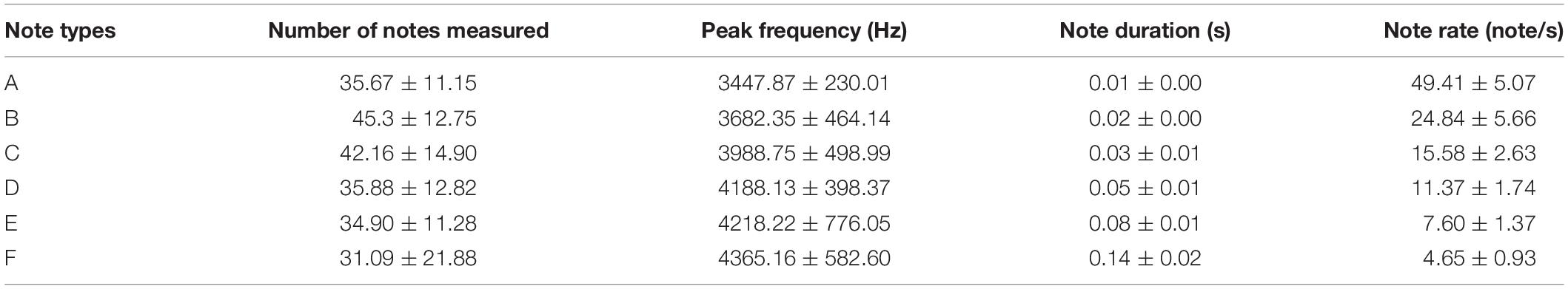
The alarm calls recorded (egg stage: n = 17; nestling stage: n = 10) in the specimen experiment were imported into the Raven Pro (version 1.4; Cornell Lab of Ornithology, Ithaca, NY, United States) sound analysis software, and were divided into six types according to the different note types presented in the spectrogram (Figure 1 and Table 1). Only the non-overlapping alarm calls with low noise were analyzed (Courter and Ritchison, 2010; Suzuki, 2014). Referring to the relevant literature (Butchart et al., 2003; Madden and Davies, 2006; Samaš et al., 2020), we selected several parameters commonly used in song measurement. Because it was difficult to define the low frequency and the high frequency in the alarm calls of A. orientalis, these two parameters were excluded from the measurement, along with the bandwidth. Moreover, to cover the characteristics of different note types, the sound parameters measured included (1) the number of note types, (2) the longest duration of a note, (3) the average duration of a note, (4) the fastest note rate, (5) the average note rate, (6) the highest peak frequency, and (7) the average peak frequency (Suzuki, 2014).
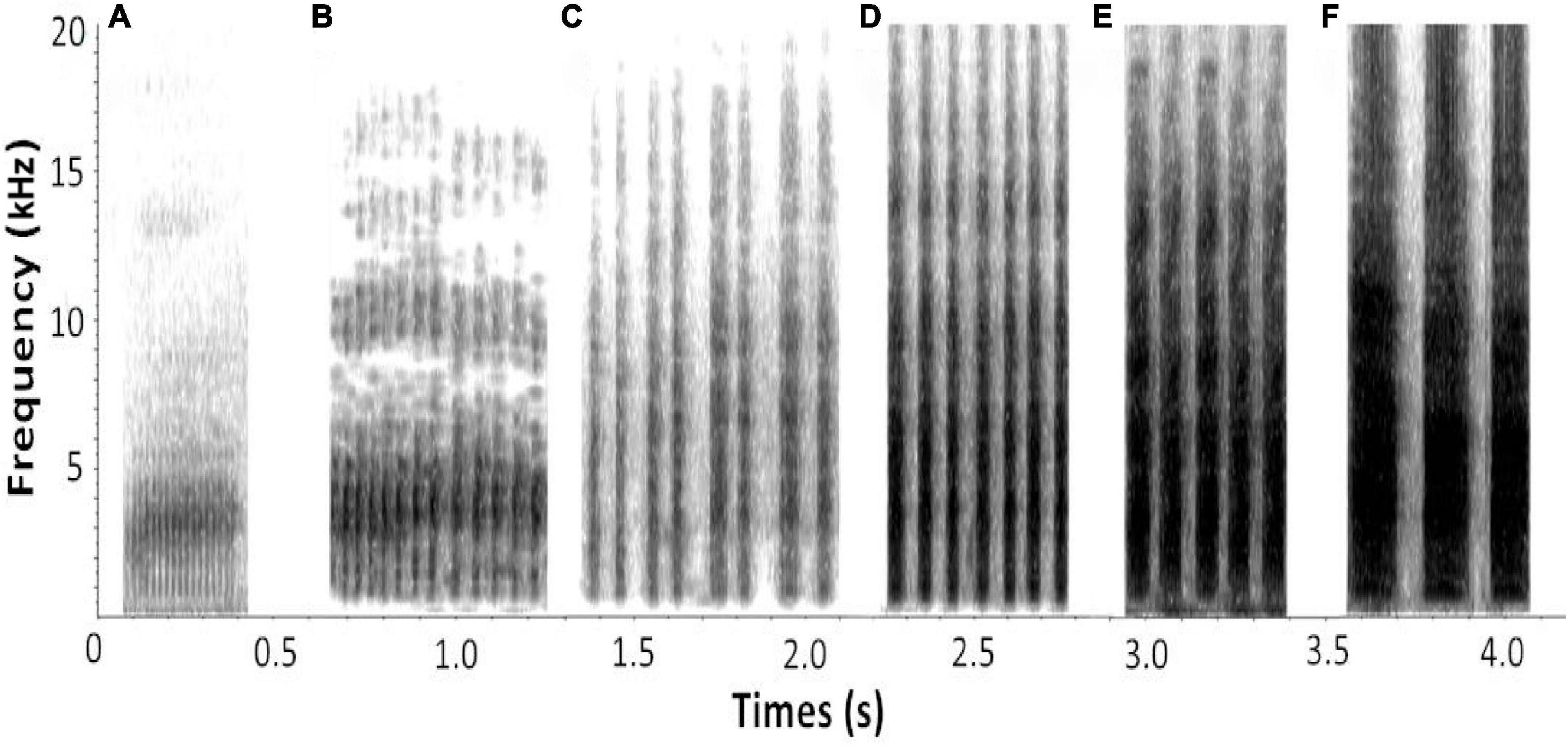
Figure 1. Alarm calls spectrogram of six note types produced by A. orientalis (aggressive behavior is escalated from A to F, where F is related to the attack behavior).
Statistical Analyses
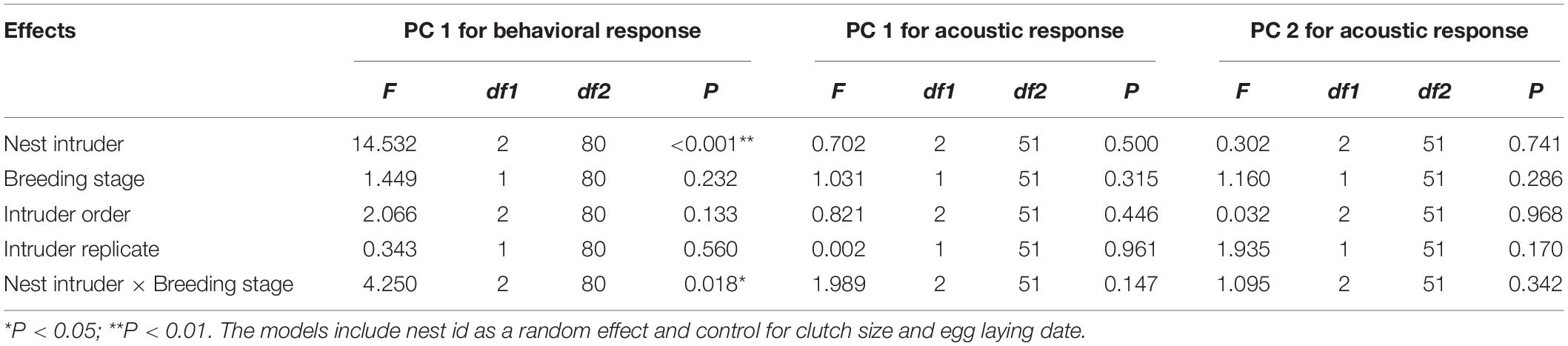
Principal component analysis (PCA) was used to extract the important principal components (PC) from the behavioral or acoustic variables, and generalized linear mixture models (GLMMs) were used to analyze the components. In GLMMs for either behavioral or acoustic analyses, the PC were the response variables while the fixed effects included nest intruder (cuckoo, sparrowhawk, or dove), breeding stage (egg or nestling stage), intruder order (presented order of specimens to each nest during experiment), and intruder replicate (identity of two replicates for each specimen type). The interaction between nest intruder and breeding stage was also tested and the nest ID was included as random effect while controlling for clutch size and egg laying date. Pairwise comparisons were conducted by the least significant differences (LSD) test. Statistical analyses used IBM SPSS 25.0 for Windows (International Business Machines Inc., Armonk, NY, United States). All the tests were two-tailed, and data are presented as mean ± SD, and the P-value significance level was 0.05.
Results
One principal component (PC1) with a characteristic value > 1.0 was extracted that explained 70.14% of the total variation of the behavioral response data, while two principal components (PC1 and PC2), both with characteristic values > 1.0, which explained 80.06% of the total variance, were extracted for the acoustic response (Table 2). The results of GLMMs showed that the responses of A. orientalis to different nest intruders were significantly different (F2,80 = 14.532, P < 0.001, GLMMs), and the interaction between the nest intruder and breeding stage also had a significant effect on the behavioral response (F2,80 = 4.250, P = 0.018, GLMMs; Table 3). The results of LSD showed that the behavioral response in the nestling stage contributed to the significant difference of total response toward intruders in the breeding stage (Figure 2). For the egg stage, the response intensity to cuckoo was slightly higher than that to the sparrowhawk and dove, but it was not significant (P > 0.05, LSD; Figure 2). The response to the cuckoo in the nestling stage was more aggressive than that to the sparrowhawk and dove (P < 0.001 for both, LSD), and there was no significant difference between the sparrowhawk and dove (P > 0.05, LSD). The maximum numbers of conspecific individuals recruited by A. orientalis to specimens of cuckoo, sparrowhawk, and dove were 2.56 ± 1.42, 1.82 ± 0.73, and 1.88 ± 1.02 at the egg stage, and 2.79 ± 1.19, 2.08 ± 0.79, and 1.83 ± 0.72 at the nestling stage, respectively, with no significant differences between dummies. All types of alarm calls appeared as an acoustic response to different nest intruders in A. orientalis, except for type A that was not present in the response to the cuckoo. The note type F was related to attacking behavior and was most frequently used by hosts toward the cuckoo (Figure 3). However, there was no significant difference in acoustic response toward different nest intruders, neither for PC1 (F2,51 = 0.702, P = 0.500, GLMMs) nor for PC2 (F2,51 = 0.302, P = 0.741, GLMMs). The breeding stage also had no significant effect on the acoustic response PC1 (F1,51 = 1.031, P = 0.315, GLMMs) and PC2 (F1,51 = 1.160, P = 0.286, GLMMs; Table 3).
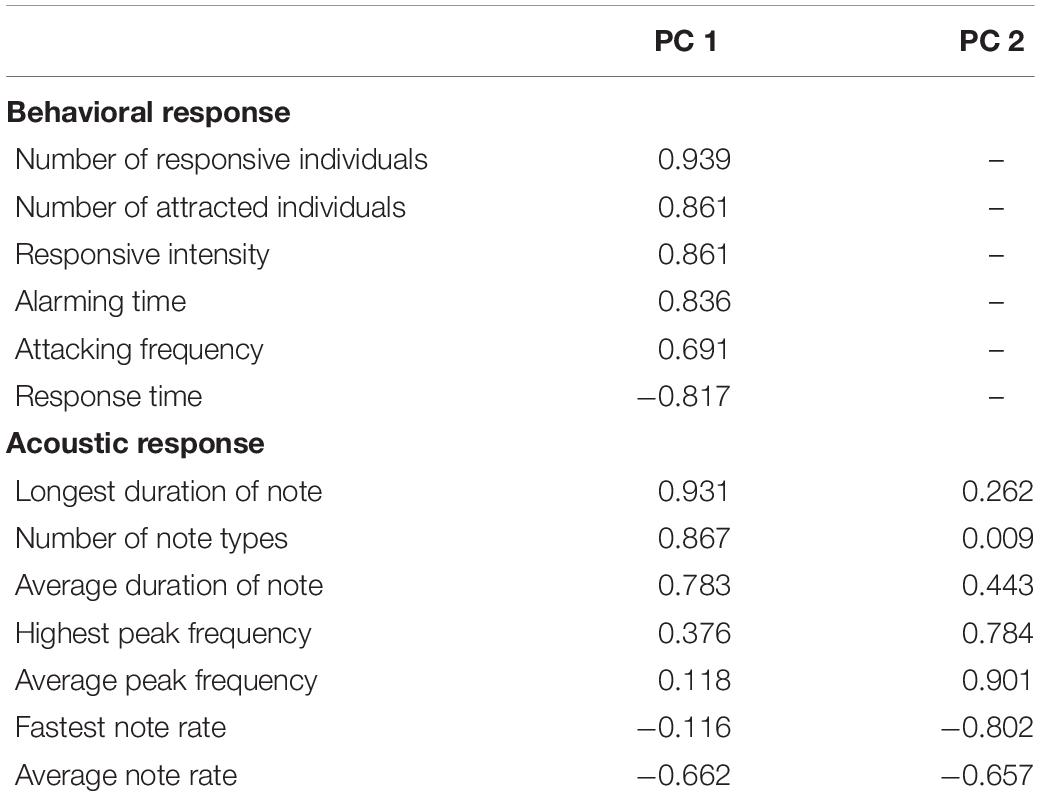
Table 2. Components, extracted by principal component analysis, for behavioral and acoustic responses in A. orientalis.
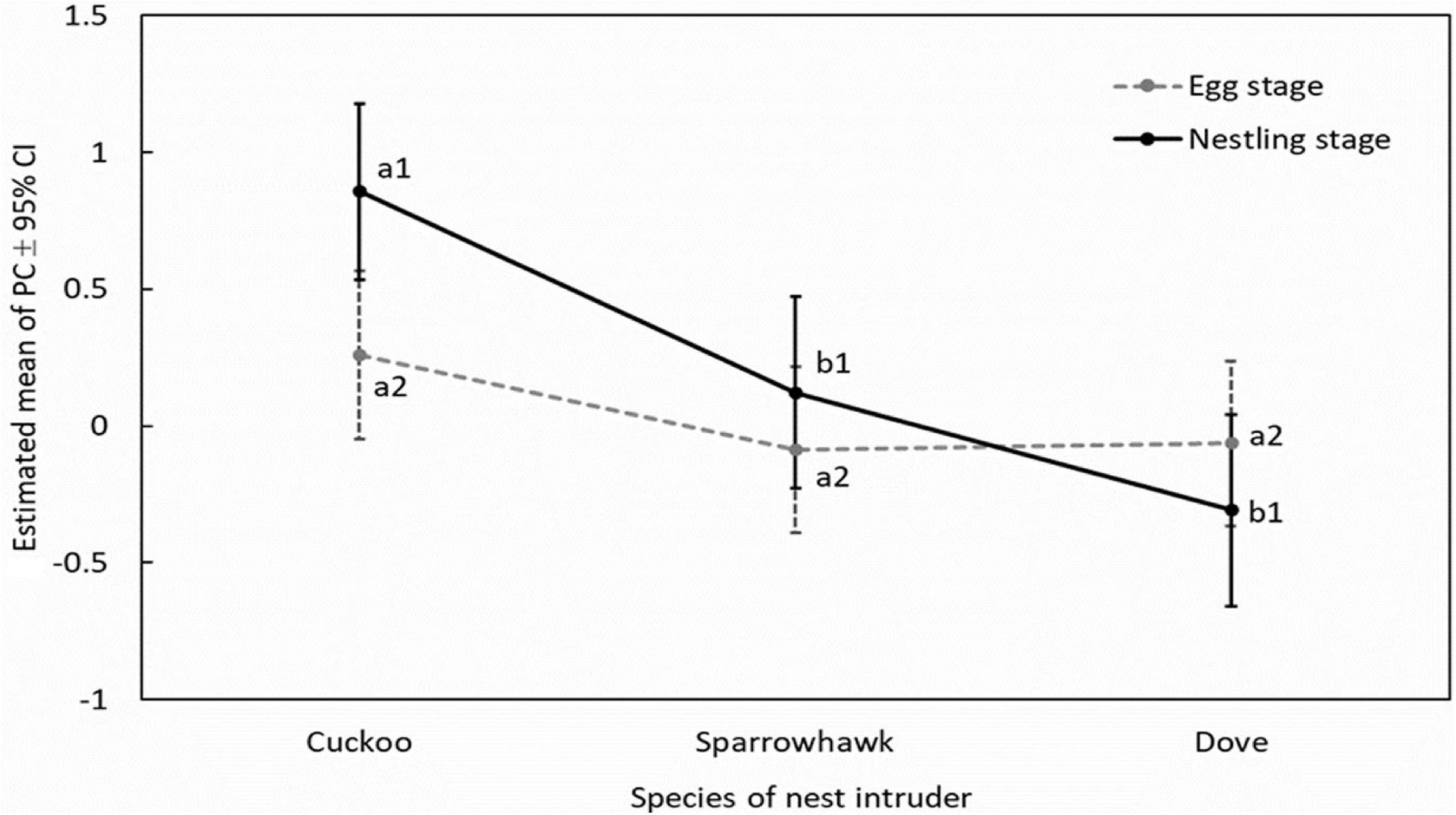
Figure 2. Pairwise comparisons for behavioral responses between nest intruders by least significant difference in A. orientalis. Significant differences are indicated by different letters; a1 and b1 refer to nestling stage, and a2 refers to egg stage.
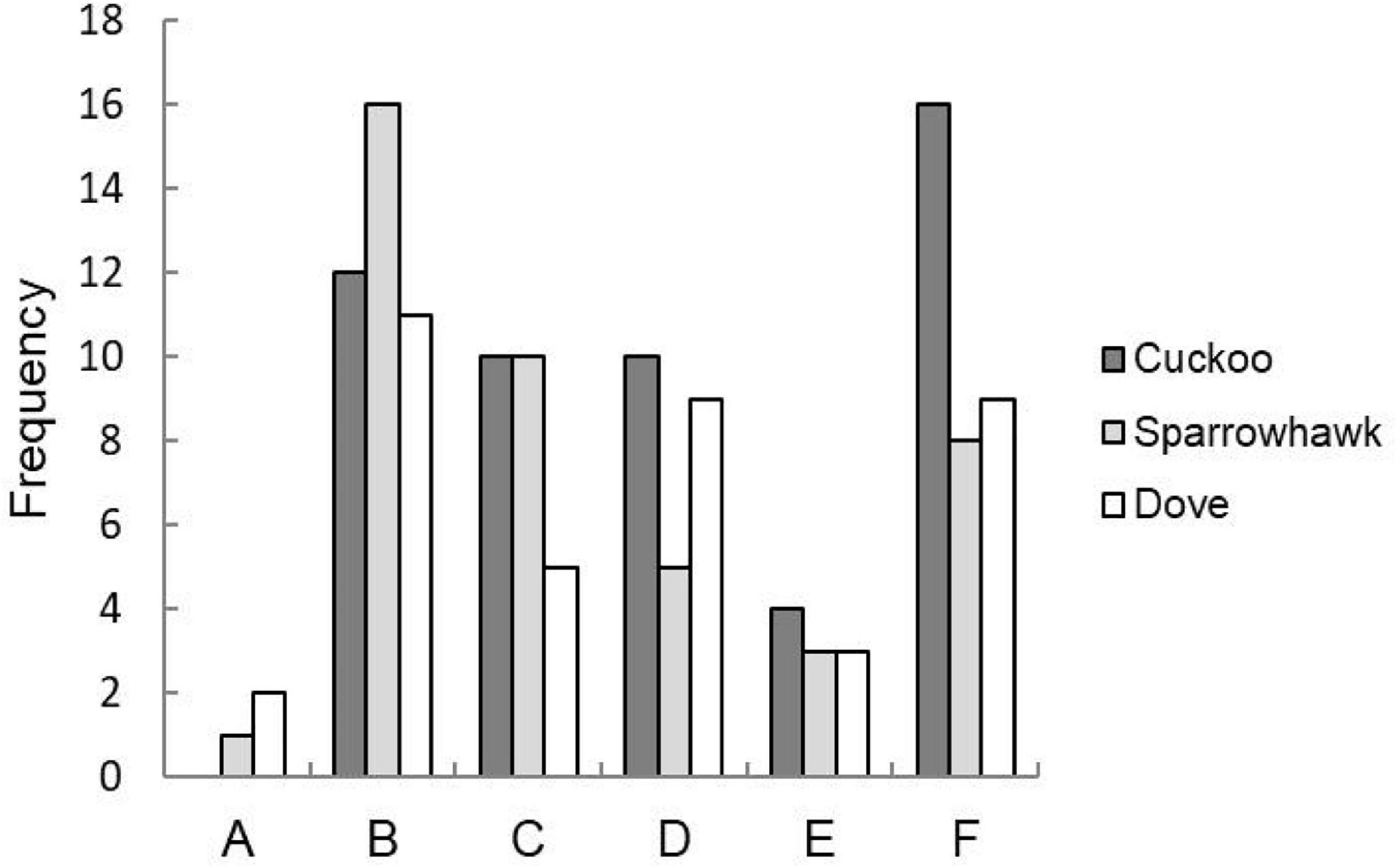
Figure 3. Frequency of note types in alarm calls produced by A. orientalis toward cuckoo, sparrowhawk, and dove.
Discussion
Our results revealed that A. orientalis responded to nest intruders in a similar way at the egg stage; however, they were more aggressive to the cuckoo than to the sparrowhawk and dove at the nestling stage, suggesting that they can visually distinguish the parasite from the sparrowhawks and doves, suggesting that hawk mimicry did not work for the cuckoos. In addition, they were able to adjust their nest defense strategies at different stages of breeding. However, there was no difference in the alarm calls they produced to different specimens, indicating that A. orientalis makes general alarm calls in response to different nest intruders without specific information of each one.
The large breeding cost of nest parasitism provides strong selection on the host to evolve anti-parasitism strategies. Among these, nest defense is the first response. Many other hosts have evolved aggressive nest protection behaviors to prevent cuckoos from approaching their nest (Welbergen and Davies, 2009), and they can also use social information to better tune their responses to various threats (Davies and Welbergen, 2009; Campobello and Sealy, 2011). This study found that the response intensity of A. orientalis to different nest intruders varied with the breeding stage. There was no difference in their responses to the three types of intruders at the egg stage, which may be because the A. orientalis is a highly territorial species, with an extremely high response to any intruders that come close to the nest during the egg stage, whereas the responses of A. orientalis to different intruders differed at the nestling stage, and the birds reacted more strongly to the cuckoo than to the sparrowhawk and the dove, which may be because cuckoos themselves are harmless to adult birds, while sparrowhawks, though adult predators, are uncommon in the study site. Therefore, our study suggested that A. orientalis could visually distinguish the cuckoo from the sparrowhawk and the dove. This was consistent with the results of other studies (Duckworth, 1991; Trnka and Prokop, 2012; Li et al., 2015; Ma et al., 2018a). In addition, our study also suggested that the visual simulation of cuckoo to sparrowhawk may not be successful for A. orientalis, which was different from the conclusions of some studies (Davies and Welbergen, 2008; Welbergen and Davies, 2011). The response intensity of A. orientalis to cuckoo and sparrowhawk was stronger in the nestling stage than in the egg stage. This supports the offspring value hypothesis that adult birds invest more in offspring during the nestling stage than the egg stage (Smith, 1977). However, previous studies on the closely related great reed warbler (A. arundinaceus) did not find any difference between the breeding stages (Briskie and Sealy, 1989; Moskát, 2005; Avilés and Parejo, 2006). In addition, Trnka and Prokop (2012) found that the aggressive behavior of great reed warbler to cuckoos decreased as the breeding stage progressed.
Contrary to the hypothesis of dynamic risk assessment, this study found that the response of A. orientalis to the cuckoo was stronger in the nestling stage than in the egg stage. Two mutually non-exclusive explanations may contribute to this result. First, this behavior may reflect the possibility that the cuckoo is also an important nest predator, and this explanation is supported by recent research by Lawson et al. (2021). Many studies have found that cuckoos may kill host nestlings, and the amount of killing varies from a single chick to the entire brood (Kinoshita and Kato, 1995; Briskie, 2007; Kawaji, 2009; Soler et al., 2017; Šulc et al., 2020). There are two hypotheses to explain the behavior of destroying host nests by brood parasites including the mafia hypothesis and the farming hypothesis (Soler et al., 2017). The mafia hypothesis suggests that the parasites will return to the host nests after laying eggs. If their eggs are rejected by the hosts, they will destroy the host nests as a punishment so that the host will be more willing to accept their eggs in the future. According to the farming hypothesis, when the parasite finds a host nest that is not suitable for parasitism (i.e., nest in late incubation or nestling stage), they will destroy it, forcing the host to build a new nest, and thus increase the chance of parasitism in the future (Soler et al., 2017). The mafia hypothesis seems only applicable to non-evicting parasitic birds because the hosts can benefit from raising their own offspring without rejecting the parasitic eggs or nestlings (Zahavi, 1979; Soler et al., 2017). The farming hypothesis, however, is suitable for any parasitic bird (Soler et al., 2017). Therefore, the cuckoos in our studied population may play an important role as nest predators, predating host nests so as to manipulate their breeding progress for suitable parasitism. Second, A. orientalis may be a general defender that shows similar aggression to different intruders. They exhibited higher aggression to cuckoos in the nestling stage than in the egg stage because they have invested more time and energy in this stage.
When many species encounter intruders, they produce alarm calls, which carry information about the size, type and speed of intruders (Suzuki, 2012, 2014; Book and Freeberg, 2015; Yu et al., 2016, 2017a,2017b, 2019a,2019b; Cunningham and Magrath, 2017; Dawson Pell et al., 2018; Kalb and Randler, 2019; Kalb et al., 2019; Walton and Kershenbaum, 2019). Given that species may differ in their behavioral and vocal responses to intruders (Liang and Møller, 2015; Yu et al., 2017b), it is necessary to conduct quantitative analysis of alarm calls. For example, yellow warbler studies found that the host can send out specific alarm calls responding to the parasitic cowbird (Gill and Sealy, 1996; Grim, 2008; Lawson et al., 2020). However, in this study we found that A. orientalis did not produce specific alarm calls in response to different nest intruders. This result was consistent with our previous study, which played back the alarm calls against different nest intruders to A. orientalis but these did not trigger specific responses (Wang and Yang, 2020). Here the main intention of alarm calls may be to attract intraspecific neighbors (Wang et al., 2020) so that they can join to expel intruders from their territories more effectively (Goodale and Ruxton, 2010). This was supported by a previous study on A. orientalis which found that neighboring conspecifics would assist the nest owner to defend against nest intruders. Nests located far from neighbors were more likely to be parasitized by cuckoos (Ma et al., 2018b).
In conclusion, we found that A. orientalis can visually identify the common cuckoo, indicating that the hawk mimicry of the cuckoo was not working in this parasite–host system. In the nestling stage, the host increased its response intensity to the cuckoo, which may be related to the possibility that the cuckoo is also a nest predator.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Animal Research Ethics Committee of Hainan Provincial Education Centre for Ecology and Environment, Hainan Normal University.
Author Contributions
CY designed and carried out laboratory and statistical analyses. JW, LM, and XC performed the field experiments. CY and JW wrote the first draft of the manuscript. All authors approved the final submission.
Funding
This work was supported by the Hainan Provincial Natural Science Foundation of China (320CXTD437 and 2019RC189) and the National Natural Science Foundation of China (31672303) to CY, and the Open Foundation of Hebei Key Laboratory of Wetland Ecology and Conservation (hklk201903) and the Natural Science Foundation of Hebei Province of China (C2020101002) to LM.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the reviewers for helpful suggestions that improved the manuscript. We would also like to thank the Yongnianwa National Wetland Park for support and permission to carry out this study.
References
Arcese, P., Smith, J. N. M., and Hatch, M. I. (1996). Nest predation by cowbirds and its consequences for passerine demography. Proc. Natl. Acad. Sci. U S A. 93, 4608–4611. doi: 10.1073/pnas.93.10.4608
Avilés, J. M., and Parejo, D. (2006). Nest defense by Iberian azure-winged magpies (Cyanopica cyanus): do they recognize the threat of brood parasitism? Ethol. Ecol. Evol. 18, 321–333. doi: 10.1080/08927014.2006.9522699
Book, D. L., and Freeberg, T. M. (2015). Titmouse calling and foraging are affected by head and body orientation of cat predator models and possible experience with real cats. Anim. Cogn. 18, 1155–1164. doi: 10.1007/s10071-015-0888-7
Briskie, J. V. (2007). Direct observations of shining cuckoos (chrysococcyx lucidus) parasitising and depredating grey warbler (gerygone igata) nests. Notornis 54, 15–19.
Briskie, J. V., and Sealy, S. G. (1989). Changes in nest defense against a brood parasite over the breeding cycle. Ethology 82, 61–67. doi: 10.1111/j.1439-0310.1989.tb00487.x
Butchart, S. H. M., Kilner, R. M., Fuisz, T., and Davies, N. B. (2003). Differences in the nestling begging calls of hosts and host-races of the common cuckoo, Cuculus canorus. Anim. Behav. 65, 345–354. doi: 10.1006/anbe.2003.2066
Campobello, D., and Sealy, S. G. (2010). Enemy recognition of reed warblers (Acrocephalus scirpaceus): threats and reproductive value act independently in nest defence modulation. Ethology 116, 498–508. doi: 10.1111/j.1439-0310.2010.01764.x
Campobello, D., and Sealy, S. G. (2011). Use of social over personal information enhances nest defense against avian brood parasitism. Behav. Ecol. 22, 422–428. doi: 10.1093/beheco/arq225
Campobello, D., and Sealy, S. G. (2018). Evolutionary significance of antiparasite, antipredator and learning phenotypes of avian nest defence. Sci. Rep. U K. 8:10569. doi: 10.1038/s41598-018-28275-3
Caro, T. M. (2005). Antipredator Defenses in Birds and Mammals. Chicago, IL: University of Chicago Press.
Courter, J. R., and Ritchison, G. (2010). Alarm calls of tufted titmice convey information about predator size and threat. Behav. Ecol. 21, 936–942. doi: 10.1093/beheco/arq086
Cunningham, S., and Magrath, R. D. (2017). Functionally referential alarm calls in noisy miners communicate about predator behaviour. Anim. Behav. 129, 171–179. doi: 10.1016/j.anbehav.2017.05.021
Davies, N. B. (2011). Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. doi: 10.1111/j.1469-7998.2011.00810.x
Davies, N. B., and Welbergen, J. A. (2008). Cuckoo-hawk mimicry? an experimental test. P. Roy. Soc. B: Biol. Sci. 275, 1817–1822. doi: 10.1098/rspb.2008.0331
Davies, N. B., and Welbergen, J. A. (2009). Social transmission of a host defense against cuckoo parasitism. Science 324, 1318–1320. doi: 10.1126/science.1172227
Dawson Pell, F. S. E., Potvin, D. A., Ratnayake, C. P., Fernández-Juricic, E., Magrath, R. D., and Radford, A. N. (2018). Birds orient their heads appropriately in response to functionally referential alarm calls of heterospecifics. Anim. Behav. 140, 109–118. doi: 10.1016/j.anbehav.2018.04.010
Duckworth, J. W. (1991). Responses of breeding reed warblers Acrocephalus scirpaceus to mounts of sparrowhawk Accipiter nisus, cuckoo Cuculus canorus and jay Garrulus glandarius. IBIS 133, 68–74. doi: 10.1111/j.1474-919X.1991.tb04812.x
Feeney, W. E., Medina, I., Somveille, M., Heinsohn, R., Hall, M. L., Mulder, R. A., et al. (2013). Brood parasitism and the evolution of cooperative breeding in birds. Science 342, 1506–1508. doi: 10.1126/science.1240039
Feeney, W. E., Troscianko, J., Langmore, N. E., and Spottiswoode, C. N. (2015). Evidence for aggressive mimicry in an adult brood parasitic bird, and generalized defences in its host. Proc. R. Soc. B Biol. Sci. 282, 893–896. doi: 10.1098/rspb.2015.0795
Feeney, W. E., Welbergen, J. A., and Langmore, N. E. (2012). The frontline of avian brood parasite-host coevolution. Anim. Behav. 84, 3–12. doi: 10.1016/j.anbehav.2012.04.011
Gill, S. A., and Sealy, S. G. (1996). Nest defence by yellow warblers: recognition of a brood parasite and an avian nest predator. Behaviour 133, 263–282. doi: 10.1163/156853996X00143
Goodale, E., and Ruxton, G. D. (2010). “Antipredator benefits from heterospecifics,” in Encyclopedia of Animal Behavior, eds M. D. Breed and J. Moore (Oxford: Academic Press), 94–99.
Grim, T. (2008). Are blackcaps (Sylvia atricapilla) defending their nests also calling for help from their neighbours? J. Ornithol. 149, 169–180. doi: 10.1007/s10336-007-0257-7
Honza, M., Grim, T., Capek, M., Moksnes, A., and Røskaft, E. (2004). Nest defence, enemy recognition and nest inspection behaviour of experimentally parasitized Reed Warblers Acrocephalus scirpaceus. Bird Study 51, 256–263. doi: 10.1080/00063650409461361
Hoover, J. P., and Robinson, S. K. (2007). Retaliatory mafia behavior by a parasitic cowbird favors host acceptance of parasitic eggs. Proc. Natl. Acad. Sci. U. S. A. 104, 4479–4483. doi: 10.1073/pnas.0609710104
Kalb, N., Anger, F., and Randler, C. (2019). Subtle variations in mobbing calls are predator-specific in great tits (Parus major). Sci. Rep. U K. 9:6572. doi: 10.1038/s41598-019-43087-9
Kalb, N., and Randler, C. (2019). Behavioral responses to conspecific mobbing calls are predator-specific in great tits (Parus major). Ecol. Evol. 9, 9207–9213. doi: 10.1002/ece3.5467
Kawaji, N. (2009). Removal of short-tailed bush warbler’s Uroshena squameiceps nestlings by the oriental cuckoo Cuculus saturatus. Jpn. J. Ornithol. 58, 118–120. doi: 10.3838/jjo.58.118
Kinoshita, M., and Kato, C. (1995). Killing nestlings stonechats by the Common Cuckoo. Jpn. J. Ornithol. 44, 99–100. doi: 10.3838/jjo.44.99
Kleindorfer, S., Fessl, B., and Hoi, H. (2005). Avian nest defence behaviour: assessment in relation to predator distance and type, and nest height. Anim. Behav. 69, 307–313. doi: 10.1016/j.anbehav.2004.06.003
Lawson, S. L., Enos, J. K., Antonson, N. D., Gill, S. A., and Hauber, M. E. (2021). Do hosts of avian brood parasites discriminate parasitic vs. predatory threats? A meta-analysis. Adv. Study Behav. 53, 63–95. doi: 10.1016/bs.asb.2021.03.002
Lawson, S. L., Enos, J. K., Mendes, N. C., Gill, S. A., and Hauber, M. E. (2020). Heterospecific eavesdropping on an anti-parasitic referential alarm call. Commun. Biol. 3, 143–150. doi: 10.1038/s42003-020-0875-7
Li, D., Wei, H., Zhang, Z., Liang, W., and Stokke, B. G. (2015). Oriental reed warbler (Acrocephalus orientalis) nest defence behaviour towards brood parasites and nest predators. Behaviour 152, 1601–1621. doi: 10.1163/1568539x-00003295
Li, D., Zhang, Z., Grim, T., Liang, W., and Stokke, B. G. (2016). Explaining variation in brood parasitism rates between potential host species with similar habitat requirements. Evol. Ecol. 30, 905–923. doi: 10.1007/s10682-016-9850-7
Liang, W., and Møller, A. P. (2015). Hawk mimicry in cuckoos and anti-parasitic aggressive behavior of barn swallows in Denmark and China. J. Avian Biol. 46, 216–223. doi: 10.1111/jav.00515
Ma, L., Yang, C., and Liang, W. (2018a). Hawk mimicry does not reduce attacks of cuckoos by highly aggressive hosts. Avian Res. 9:35. doi: 10.1186/s40657-018-0127-4
Ma, L., Yang, C., Liu, J., Zhang, J., Liang, W., and Moller, A. P. (2018b). Costs of breeding far away from neighbors: Isolated host nests are more vulnerable to cuckoo parasitism. Behav. Process. 157, 327–332. doi: 10.1016/j.beproc.2018.07.017
Madden, J. R., and Davies, N. B. (2006). A host-race difference in begging calls of nestling cuckoos Cuculus canorus develops through experience and increases host provisioning. Proc. R. Soc. B Biol. Sci. 273, 2343–2351. doi: 10.1098/rspb.2006.3585
Marler, P. (2004). “Bird calls: a cornucopia for communication,” in Nature’s music, eds P. Marler and H. Slabbekoorn (San Diego: Academic Press), 132–177.
Montgomerie, R. D., and Weatherhead, P. J. (1988). Risk and rewards of nest defence by parent birds. Q. Rev. Biol. 63, 167–187. doi: 10.2307/2830999
Moskát, C. (2005). Nest defence and egg rejection in great reed warblers over the breeding cycle: are they synchronised with the risk of brood parasitism? Ann. Zool. Fenn. 42, 579–586.
Neudorf, D. L., and Sealy, S. G. (2012). Reactions of four passerine species to threats of predation and cowbird parasitism: enemy recognition or generalized responses? Behaviour 123, 84–105. doi: 10.1163/156853992X00138
Patterson, T. L., and James, L. P. K. (1980). Reproductive value and appropriateness of response to predators by white-crowned sparrows. Behav. Ecol. Sociobiol. 7, 227–231. doi: 10.1007/BF00299368
Ponton, F., Biron, D. G., Moore, J., Moller, A. P., and Thomas, F. (2006). Facultative virulence: a strategy to manipulate host behaviour? Behav. Process. 72, 1–5. doi: 10.1016/j.beproc.2005.10.005
Redondo, T., and Carranza, J. (1989). Offspring reproductive value and nest defense in the magpie (Pica pica). Behav. Ecol. Sociobiol. 25, 369–378. doi: 10.1007/BF00302995
Robertson, R. J., and Norman, R. F. (1977). The function and evolution of aggressive host behavior towards the Brown-headed Cowbird (Molothrus ater). Can. J. Zool. 55, 508–518. doi: 10.1139/z77-066
Rothstein, S. I. (1990). A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 21, 481–508. doi: 10.2307/2097034
Samaš, P., Žabková, K., Petrusková, T., Procházka, P., Požgayová, M., and Honza, M. (2020). Nestlings of the common cuckoo do not mimic begging calls of two closely related Acrocephalus hosts. Anim. Behav. 161, 89–94. doi: 10.1016/j.anbehav.2020.01.005
Smith, J. M. (1977). Parental investment: A prospective analysis. Anim. Behav. 25, 1–9. doi: 10.1016/0003-3472(77)90062-8
Smith, J. N., Arcese, P., and McLean, I. G. (1984). Age, experience, and enemy recognition by wild song sparrows. Behav. Ecol. Sociobiol. 14, 101–106. doi: 10.1007/BF00291901
Soler, M. (2014). Long-term coevolution between avian brood parasites and their hosts. Biol. Rev. 89, 688–704. doi: 10.1111/brv.12075
Soler, M., Pérez-Contreras, T., and Soler, J. J. (2017). “Brood parasites as predators: farming and mafia strategies,” in Avian Brood Parasitism, ed. M. Soler (Cham: Springer), 271–286.
Soler, M., Soler, J. J., Martinez, J. G., and Ap, M. L. (1995). Magpie host manipulation by great spotted cuckoos: evidence for an avian mafia? Evolution 49, 770–775. doi: 10.1111/j.1558-5646.1995.tb02312.x
Su, T. P., Shao, L., Huo, J., Yang, C. C., and Liang, W. (2017). Himalayan Cuckoo fed on eggs of Bianchi’s Warbler. Chinese J. Ecol. 36, 89–93.
Šulc, M., Štětková, G., Jelínek, V., Czyż, B., Dyrcz, A., Karpińska, O., et al. (2020). Killing behaviour of adult brood parasites. Behaviour 157, 1099–1111. doi: 10.1163/1568539X-bja10033
Suzuki, T. N. (2012). Referential mobbing calls elicit different predator-searching behaviours in Japanese great tits. Anim. Behav. 84, 53–57. doi: 10.1016/j.anbehav.2012.03.030
Suzuki, T. N. (2014). Communication about predator type by a bird using discrete, graded and combinatorial variation in alarm calls. Anim. Behav. 87, 59–65. doi: 10.1016/j.anbehav.2013.10.009
Swan, D. C., Zanette, L. Y., and Clinchy, M. (2015). Brood parasites manipulate their hosts: experimental evidence for the farming hypothesis. Anim. Behav. 105, 29–35. doi: 10.1016/j.anbehav.2015.03.012
Trnka, A., and Prokop, P. (2012). The effectiveness of hawk mimicry in protecting cuckoos from aggressive hosts. Anim. Behav. 83, 263–268. doi: 10.1016/j.anbehav.2011.10.036
Walton, B., and Kershenbaum, A. (2019). Heterospecific recognition of referential alarm calls in two species of lemur. Bioacoustics 28, 592–603. doi: 10.1080/09524622.2018.1509375
Wang, J., Ma, L., Liang, W., and Yang, C. (2020). Responses of cuckoo hosts to alarm signals of different nest intruders in non-nesting areas. Zool. Res. 41, 345–350. doi: 10.24272/j.issn.2095-8137.2020.030
Wang, J., and Yang, C. (2020). Specific responses of cuckoo hosts to different alarm signals according to breeding stage: a test of the offspring value hypothesis. Curr. Zool. 66, 649–655. doi: 10.1093/cz/zoaa021/5838188
Welbergen, J. A., and Davies, N. B. (2008). Reed warblers discriminate cuckoos from sparrowhawks with graded alarm signals that attract mates and neighbours. Anim. Behav. 76, 811–822. doi: 10.1016/j.anbehav.2008.03.020
Welbergen, J. A., and Davies, N. B. (2009). Strategic variation in mobbing as a front line of defense against brood parasitism. Curr. Biol. 19, 235–240. doi: 10.1016/j.cub.2008.12.041
Welbergen, J. A., and Davies, N. B. (2011). A parasite in wolf’s clothing: hawk mimicry reduces mobbing of cuckoos by hosts. Behav. Ecol. 22, 574–579. doi: 10.1093/beheco/arr008
Yang, C., Li, D., Wang, L., Liang, G., Zhang, Z., and Liang, W. (2014a). Geographic variation in parasitism rates of two sympatric cuckoo hosts in China. Zool. Res. 35, 67–71. doi: 10.11813/j.issn.0254-5853.2014.1.067
Yang, C., Wang, L., Cheng, S. J., Hsu, Y. C., Liang, W., and Moller, A. P. (2014b). Nest defenses and egg recognition of yellow-bellied prinia against cuckoo parasitism. Naturwissenschaften 101, 727–734.
Yang, C., Liang, W., and Moller, A. P. (2019). Similar immediate costs of raising cuckoo and host chicks can hardly explain low levels of antiparasite defence in hosts. A Comment on: Samas et al. (2018). Proc. R. Soc B Biol. Sci. 286:20182430. doi: 10.1098/rspb.2018.2430
Yang, C., Wang, L., Liang, W., and Møller, A. P. (2016). Egg recognition as antiparasitism defence in hosts does not select for laying of matching eggs in parasitic cuckoos. Anim. Behav. 122, 177–181. doi: 10.1016/j.anbehav.2016.10.018
Yang, C., Wang, L., Liang, W., and Møller, A. P. (2017). How cuckoos find and choose host nests for parasitism. Behav. Ecol. 28, 859–865. doi: 10.1093/beheco/arx049
Yu, J., Lu, H., Sun, W., Liang, W., Wang, H., and Møller, A. P. (2019a). Heterospecific alarm-call recognition in two warbler hosts of common cuckoos. Anim. Cogn. 22, 1149–1157. doi: 10.1007/s10071-019-01307-9
Yu, J., Sun, W., Liang, W., Wang, H., and Møller, A. P. (2019b). Differently sized cuckoos pose different threats to hosts. Curr. Zool. 22, 1149–1157. doi: 10.1093/cz/zoz049/5583759
Yu, J., Lv, W., Xu, H., Bibi, N., Yu, Y., Jiang, Y., et al. (2017a). Function of note strings in Japanese tit alarm calls to the common cuckoo: a playback experiment. Avian Res. 8:22. doi: 10.1186/s40657-017-0080-7
Yu, J., Xing, X., Jiang, Y., Liang, W., Wang, H., Møller, A. P., et al. (2017b). Alarm call-based discrimination between common cuckoo and Eurasian sparrowhawk in a Chinese population of great tits. Ethology 123, 542–550. doi: 10.1111/eth.12624
Yu, J., Wang, L., Xing, X., Yang, C., Ma, J., Møller, A. P., et al. (2016). Barn swallows (Hirundo rustica) differentiate between common cuckoo and sparrowhawk in China: alarm calls convey information on threat. Behav. Ecol. Sociobiol. 70, 171–178. doi: 10.1007/s00265-015-2036-4
Keywords: alarm calls, brood parasite, hawk mimicry, nest parasitism, nest predator
Citation: Wang J, Ma L, Chen X and Yang C (2021) Behavioral and Acoustic Responses of the Oriental Reed Warbler (Acrocephalus orientalis), at Egg and Nestling Stages, to the Common Cuckoo (Cuculus canorus). Front. Ecol. Evol. 9:705748. doi: 10.3389/fevo.2021.705748
Received: 06 May 2021; Accepted: 28 July 2021;
Published: 19 August 2021.
Edited by:
Danielle June Whittaker, Michigan State University, United StatesReviewed by:
Daniela Campobello, University of Palermo, ItalyShelby Lawson, University of Illinois at Urbana-Champaign, United States
Copyright © 2021 Wang, Ma, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Canchao Yang, Y2N5YW5nQGhhaW5udS5lZHUuY24=
 Jiaojiao Wang
Jiaojiao Wang Laikun Ma2
Laikun Ma2 Canchao Yang
Canchao Yang