
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 30 June 2021
Sec. Chemical Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.703134
This article is part of the Research Topic Chemical Ecology and Conservation Biological Control View all 8 articles
Within the Brassicaceae, wild as well as crop species are challenged by specialist herbivores including cabbage white butterflies (Pieris spp.). The wild crucifer Brassica nigra responds to oviposition by Pieris butterflies by the synergistic expression of two egg-killing traits. Genotypes that express a hypersensitive response (HR)-like necrosis (direct egg-killing) also emit oviposition-induced plant volatiles (OIPVs) attracting Trichogramma egg parasitoids (indirect egg-killing). This so-called double defense line can result in high butterfly egg mortalities. It remains unknown whether this strategy is unique to B. nigra or more common in Brassica species. To test this, we examined the response of different Trichogramma evanescens lines to OIPVs emitted by B. nigra and three close relatives (Brassica napus, Brassica rapa, and Brassica oleracea). Furthermore, we evaluated whether HR-like necrosis played a role in the attraction toward plant volatiles. Our results show a specificity in wasp attraction to different plant species. Three out of four plant species attracted a specific T. evanescens strain, including the crops B. rapa and B. napus. Parasitoid attraction was positively affected by presence of HR-like necrosis in one plant species. Our findings imply that, despite being a true generalist in terms of host range, T. evanescens shows intraspecific variation during host searching, which should be taken into account when selecting parasitoid lines for biocontrol of certain crops. Finally, we conclude that also crop plants within the Brassicaceae family possess egg-killing traits and can exert the double-defense line which may enable effective selection of egg-killing defense traits by cabbage breeders.
Eggs of herbivorous insects deposited on leaves, stems and branches are often the start of herbivory. Plants have evolved to recognize egg deposition as a warning signal of herbivory and developed an array of defenses to reduce egg survival. Such direct egg-killing traits include wound tissue growth, formation of neoplasm or necrotic tissue, and production of ovicidal substances, and have been reported in several plant families after oviposition of diverse herbivorous insect species (Hilker and Fatouros, 2015, 2016; Fatouros et al., 2016). Moreover, egg deposition can induce plant cues that recruit natural enemies, mainly egg parasitoids, either by the emission of oviposition-induced plant volatiles (OIPVs) or by changes in the chemical profile of leaf surfaces (Colazza et al., 2010).
Recent studies on the Brassicaceae family have shown that several species can develop a hypersensitive response (HR)-like leaf necrosis against the eggs of specialist Pieridae butterflies, mainly species of lineage II that includes many of the common cabbage crops (Griese et al., 2021). The black mustard Brassica nigra forms necrotic lesions specifically in response to eggs of Pieris spp. So far, attraction of Trichogramma wasps to OIPVs induced after Pieris oviposition has been only shown for B. nigra (Fatouros et al., 2016). Interestingly, B. nigra plants were shown to use a so-called double defense line: plants that express HR-like necrosis also emit Trichogramma-attracting OIPVs, together leading to total egg mortalities up to 80% (Fatouros et al., 2015). In addition to the presence of HR-like necrosis, attraction of Trichogramma parasitoids to egg-infested B. nigra plants depends on the time elapsed since oviposition (either 24 h and/or 72 h), the identity of the herbivore, the place of origin of Trichogramma parasitoids, and the presence of other herbivores (Fatouros et al., 2012, 2014; Cusumano et al., 2015; Ponzio et al., 2016).
Induction of plant responses by eggs has been shown to involve compounds present in the egg glue produced by female accessory reproductive glands (ARGs) (Hilker and Fatouros, 2015; Paniagua Voirol et al., 2020). In B. nigra, the ARG secretion of Pieris brassicae induced OIPVs when female ARGs were used that were dissected 48 h after mating (Fatouros et al., 2015). The male-derived anti-sex pheromone benzyl cyanide (BC), present in ARGs and previously shown to induced short-range plant cues arresting Trichogramma wasps to Brassica oleracea leaves (Fatouros et al., 2008), does not play a role in elicitation of OIPVs in B. nigra (Fatouros et al., 2015).
Egg-killing traits are highly desirable in crop protection because they kill an immobile stage of the pest before feeding damage occurs (Fatouros et al., 2016). In general, plant breeding programs have prioritized yield-related traits and thus over-looked, or inadvertently selected against, plant defenses. This is especially true for indirect plant defenses (HIPVs and OIPVs) where the defense against the pest is not directly visible, but it is mediated by a third organism (Chen et al., 2015). Many studies have reported beneficial effects of egg-killing for plants, but little is known about the presence of egg-killing traits in crop plants and their wild ancestors, nor about the underlying genetics and molecular mechanisms (Tamiru et al., 2011). It is hypothesized that wild species could serve as source of identifying and incorporating egg-related defensive traits to crop plants through the currently available breeding tools (Tamiru et al., 2015).
The utilization of indirect defenses for effective pest-killing requires successful host location and parasitism/predation from third trophic level organisms. Different populations of natural enemies present high genetic variability in locating and parasitizing their host and this issue has been addressed by recent research programs (Leung et al., 2020). Recently, it has been proposed that this intra-specific variation can be exploited for the creation of next generation biological control agents (Lommen et al., 2017).
In this paper, we tested the attractiveness of OIPVs emitted by different Brassica species on different lines of Trichogramma evanescens. First, we investigated the expression of the direct egg-killing trait, HR-like necrosis induced by P. brassicae in different plant species of the genus Brassica. We then tested different Brassica species on different lines of T. evanescens bred from single wild caught-females, also known as isofemale lines. Up to now, there is no evidence whether other wild or cultivated brassicaceous species besides B. nigra produce OIPVs attracting Trichogramma wasps to prevent herbivory by Pieris caterpillars. It is also important to gain more information regarding the role of HR-like necrosis in other Brassica species and whether synergistic expression of OIPVs and HR-like necrosis is common. We selected representatives of the most predominant cabbage crop species: cultivars of Brassica napus and Brassica rapa, and a wild B. oleracea. In addition, as a positive control, we used an accession of B. nigra known to strongly express both egg-killing defenses. To gain further insight into the compounds that elicit Trichogramma-attracting OIPVs, we applied a wash made from the outer surface of the P. brassicae eggs that includes secretions from the ARG. We recently showed that such an egg wash induces HR-like in B. nigra in the same manner as deposited eggs (Griese et al., 2021).
Specifically, we asked the following questions: (i) Which of the three Brassica species express HR-like necrosis to eggs and what is the severity of the response compared to B. nigra? (ii) Which of the three Brassica species emit Trichogramma-attracting volatiles 24 h and/or 72 h after egg deposition? (iii) Does an egg wash treatment on B. nigra and B. rapa plants induce Trichogramma-attracting volatiles? (iv) Do T. evanescens isofemale lines display variability in attraction to OIPVs? and (v) Apart from B. nigra, do other Brassica species display a synergistic effect between the two egg-killing traits (OIPVs and HR-like necrosis)?
We used an accession of the wild crucifer B. nigra in the experiments, SF48-O1, that serves a positive control because of the high severity and frequency of HR-like necrosis and proven emission of OIPVs (Fatouros et al., 2012, 2014, 2015; Griese et al., 2017). The accession is derived from open pollinated-field multiplication of the SF48 accession previously reported by Griese et al. (2017). This genotype derives from seeds collected in 2009 from a black mustard population near the Rhine river in Wageningen, the Netherlands (E. Poelman, personal communication). B. oleracea var. oleracea plants were grown from seeds that were collected from wild cabbage plants in Kimmeridge, Dorset, United Kingdom in 2005. These plants are wild relatives of B. oleracea cultivars and occur in coastal and cliffy areas (Gols et al., 2008, 2011; Wichmann et al., 2008). This wild cabbage genotype “Kimmeridge” has been shown to express HR-like necrosis in 7% of tested plants (Pashalidou et al., 2015). B. rapa var. parachinensis (L58) is a leaf vegetable line (Cai Xin) with origin in China. Seeds were provided by the Laboratory of Genetics of Wageningen University. B. napus var. annua (CR3195) plants were grown from seeds that were provided by the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Germany and originate from Marnoo, Australia. It is a cultivar grown in spring for oil production. Other accessions of B. napus and B. rapa were previously shown to respond with an HR-like necrosis of a medium strength after egg deposition (Griese et al., 2020). B. nigra, B. rapa, and B. napus were 4 weeks old and B. oleracea 6 weeks old at the time of butterfly oviposition. All the plants were grown from seed stage and were kept at greenhouse conditions (18–20°C, 50–70% RH, photoperiod L16: D8) at Experimental Farm of Wageningen University (Unifarm).
The herbivore P. brassicae (Lepidoptera: Pieridae) was reared on Brussels sprouts plants (B. oleracea var. gemmifera cv. Cyrus) in a climatized room at the Laboratory of Entomology, Wageningen University (18–20°C, 50–70% RH, photoperiod L16: D8). Adult butterflies were kept in a cage without plants for oviposition prior to plant induction and were fed with a 10% honey/water solution that was delivered in cotton balls.
Three T. evanescens isofemale lines were made from wasps emerging from lepidopteran eggs in the field and subsequently reared on Ephestia kuehniella Zeller eggs (Lepidoptera: Pyralidae) provided by Koppert Biological Systems, the Netherlands. The wasps were reared in a climate chamber of the Laboratory of Genetics of Wageningen University (25 ± 1°C, 50–70% RH, photoperiod L18:D6). The first isofemale line (DG018) was made from a female wasp that emerged from a Pieris rapae egg found on B. nigra plants in 2018, at the river Rhine in Wageningen. The second isofemale line (Bawc) was started from a female that emerged in 2016 from Mamestra brassicae eggs deposited on white cabbage plant (B. oleracea var. capitata) in a cultivated field in Ballenhausen, Göttingen, Germany. The third isofemale line (BB) was started from a female collected from a Pieris egg found on Brussels sprouts (B. oleracea var. gemmifera) in a private garden in Wageningen, the Netherlands in 2018.
Plants for experiments were covered with a zippered mesh bag apart from the first fully developed leaf from the bottom (usually the fourth leaf of a B. nigra, B. napus, and B. rapa plant and the third leaf of a B. oleracea). The plants were subsequently inserted into a cage with about 20–30 mated females of P. brassicae to allow butterfly oviposition on the exposed leaf. After obtaining one egg clutch (20–30 eggs), plants were removed from the cage and were kept under greenhouse conditions (18–20°C, 50–70% RH, photoperiod L16: D6) for 24 or 72 h. The time points were selected because of previous findings and the development of HR-like necrosis (Fatouros et al., 2012). AN HR-like necrosis develops within 72 h after egg deposition (see below). If more than 30 eggs were deposited, the surplus of eggs was carefully removed with a brush after the end of every oviposition event. Control plants received no eggs but were kept in the same conditions in the greenhouse for 24 or 72 h. Just before testing, plants were transferred to the Laboratory of Entomology of Wageningen University and were tested in Y-tube bioassays.
To obtain a wash from freshly laid P. brassicae eggs, we used the same protocol as Caarls et al. (2021). In short, eggs laid on filter paper were washed in 1 mL MES buffer per 400 eggs. A wash of filter paper without eggs was used as control. A fully developed leaf was treated by applying ten droplets of 10 μL of egg wash each on the bottom side of the leaf; 24 or 72 h prior to testing. Just before testing, plants were transferred to a climatized room to conduct Y-tube bioassays. To test the attraction of parasitoids to egg-wash treated B. nigra and B. napus, at least 20 individuals of every plant species were treated.
Dual choice experiments were conducted by using a dynamic Y-tube olfactometer previously described in detail (Fatouros et al., 2012). The same conditions in terms of air filtering, air flow, temperature, and lighting were applied. The setup was specifically modified for group release of parasitic wasps and it has been previously shown that group release does not influence the choice of an individual. When the wasps make a choice, they are trapped inside a collection tube which is attached to each branch of the olfactometer. For each pair of odor sources, a group of 10 females was released. All wasps used were between 2 and 5 days old, mated, and naïve to plant cues. After 45 min, the number of wasps that were found in either side of the collection tubes were recorded. Parasitoids that did not select between the odor sources were recorded as “non-responding.” Every time that a new group of wasps was about to be released, the position of the treatments was alternated to avoid biased effects. Each group of parasitoids was tested only once. To test the attraction to OIPVs, we tested an infested plant (induced by egg clutch or egg wash) was tested against a control plant of the same plant species in each two-choice bioassay. Volatiles of uninfested control plants were previously shown to not be attractive to Trichogramma wasps when tested against clean air (Fatouros et al., 2012). Therefore, a preference toward volatiles of egg-infested plants can give an indication of an attraction toward OIPVs. No tests between plant species were conducted. At least eight plant pairs per plant species and treatment (24 or 72 h after egg deposition) were tested and therefore a minimum of 80 wasps/plant species/treatment were tested in total. A maximum of two replicates per experimental day were conducted and plant-parasitoid combinations were randomized within each block.
For parasitoid species identification, an individual wasp from each line was used for sequencing of its DNA barcode, the “Folmer region” of the mitochondrial cytochrome c oxidase sub-unit 1 (CO1) gene (see Supplementary Data: parasitoid DNA barcoding of the CO1 gene). These sequences were also used to asses intra-specific variation and relative similarity in this mtDNA marker between lines (see Supplementary Data: phylogenetic analysis).
Hypersensitive response-like necrosis usually develops within 72 h depending on the plant species. Plants were scored for HR-like necrosis after five days based on the size of the necrotic tissue following the severity scorings of Griese et al. (2017): no response (HR: 0), weak response (HR: 1) when necrotic spots were visible only on the underside of the leaf, medium response (HR: 2) when a small necrotic tissue was visible on both sides of the leaf, or strong response (HR: 3) when necrosis was visible on both sides of the leaf and covered or expanded the borders treated surface of the droplet or the egg clutch.
To test whether the effect of HR was affected by plant species, two different analyses were performed: one considering the occurrence of HR as a binomial response variable (i.e., no HR compared with all other categories combined), and another one considering the severity of HR as multinomial response variable (i.e., none, weak, medium, or strong). The plant species was included in the models as categorical fixed factor in both analyses. HR occurrence was tested with a logistic regression model using the function glm in R1, whereas the HR severity was tested with an ordinal response model using the function polr of the package MASS in R. If the multinomial regression model detected significant differences amongst factor levels, we proceeded with pairwise comparisons to determine which differed using the glht function found in the multcomp package in R. To analyze the attraction of the different parasitoid lines to volatiles from different plant species, a logistic regression model with a binomial distribution was used, testing the H0: logit = 0. As a response variable, the proportion of responding wasps that selected a treated plant out of the total responsive wasps was used. Non-responding parasitoids were excluded from the analysis. In cases of overdispersion, a quasibinomial distribution was fitted in the data. All the analyses were performed with R statistical software (R Core Team, 2020).
All species tested were capable of expressing HR-like necrosis in response to P. brassicae eggs, although the occurrence and severity of HR-like necrosis differed between species: all B. nigra plants tested responded with HR-like necrosis to egg deposition, while almost none of the B. oleracea plants did. For B. rapa and B. napus, slightly more than half of the plants developed HR-like necrosis in response to eggs. A significant effect of the plant species on the occurrence of HR-like necrosis induced by P. brassicae egg deposition was found (χ2 = 30.256, df = 3, p < 0.001). Severity of HR-like necrosis was also significantly affected by the plant species tested (χ2 = 45.739, df = 3, p < 0.001; Figure 1). The strongest HR severity was found in B. nigra compared with all the other species tested. Statistically significant differences were also found between B. napus and B. oleracea (z = 2.457, p = 0.014) and between B. rapa and B. oleracea (z = 2.474, p = 0.013). Both cultivated plants displayed overall intermediate-strong HR levels whereas most of the wild B. oleracea plants showed no or weak HR responses (Figure 1).
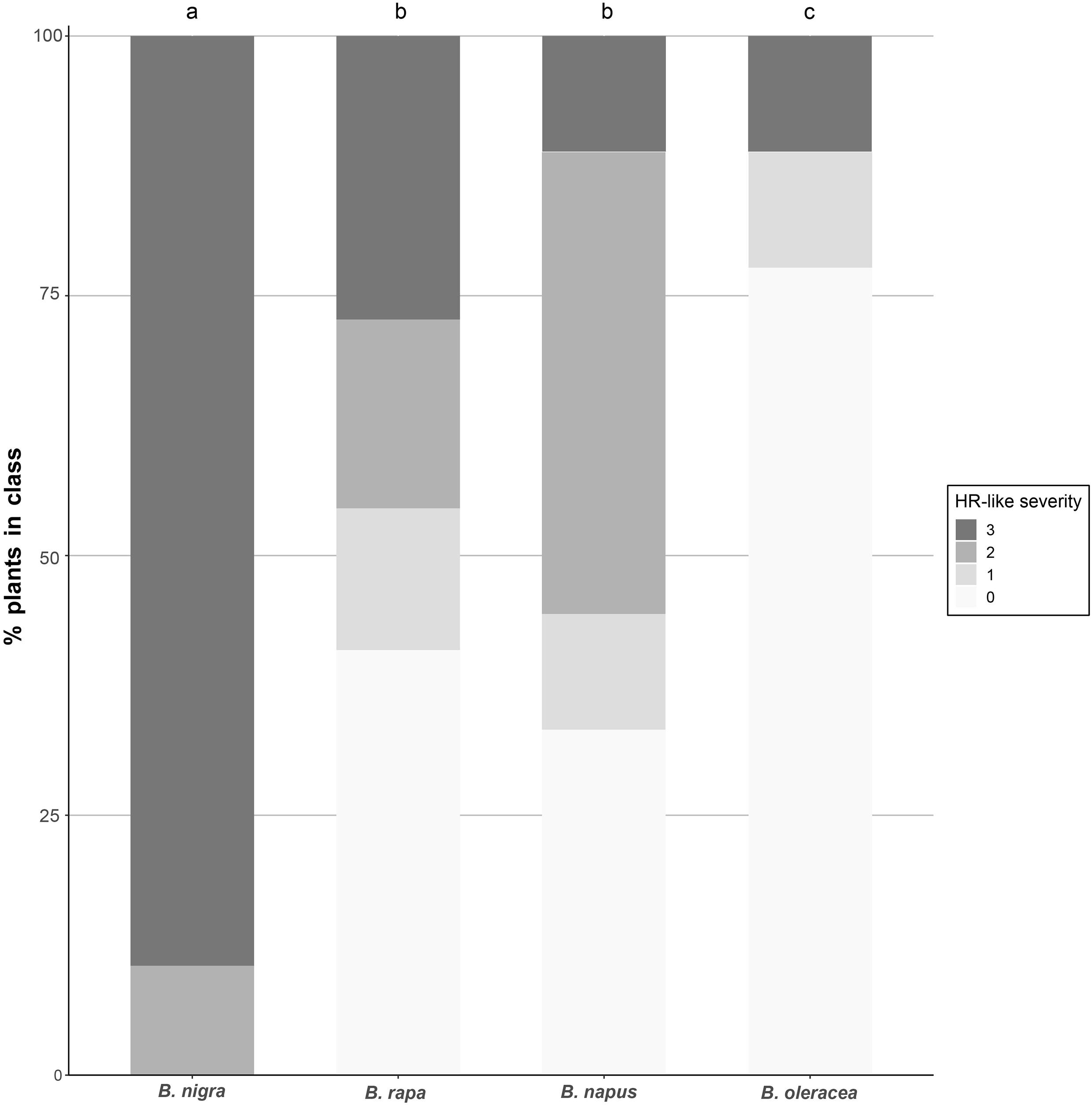
Figure 1. Severity of HR-like necrosis induced by P. brassicae eggs in different Brassica species. White color indicates no HR (0), light gray indicates a weak response (1), medium gray indicates a medium response (2), and dark gray indicates a strong response (3). Different letters indicate significant differences in the HR severity between plant species (p < 0.05, logistic regression). Sample sizes: B. nigra (n = 19 plants), B. oleracea (n = 18 plants), B. rapa (n = 22 plants), B. napus (n = 18 plants).
DNA-barcoding of mt-CO1 genes of an individual female wasp from each line showed that they are all T. evanescens, and that each line has a unique haplotype (Supplementary Table 1). Isofemale lines Bawc and DG018 differ at a single site, and line BB differs from Bawc and DG018 at two and three sites, respectively (Supplementary Figure 1).
For B. nigra, from the three lines tested, DG018 preferred volatiles of egg-infested plants, compared to control plants. This choice was most obvious and significant three days after oviposition (24 h: z = 1.946, p = 0.052, 72 h: z = 1.971, p = 0.048, GLM; Figure 2). Volatiles from egg-infested B. napus were attractive to the Bawc line 24 h after egg-laying (GLM, z = 2.098, p = 0.035). However, this attraction was not as long-lasting as Bawc wasps did not make a choice between volatiles from control and egg-infested plants 72 h after treatment. Overall, females of the BB line were not attracted to OIPVs from any of the tested Brassica species.
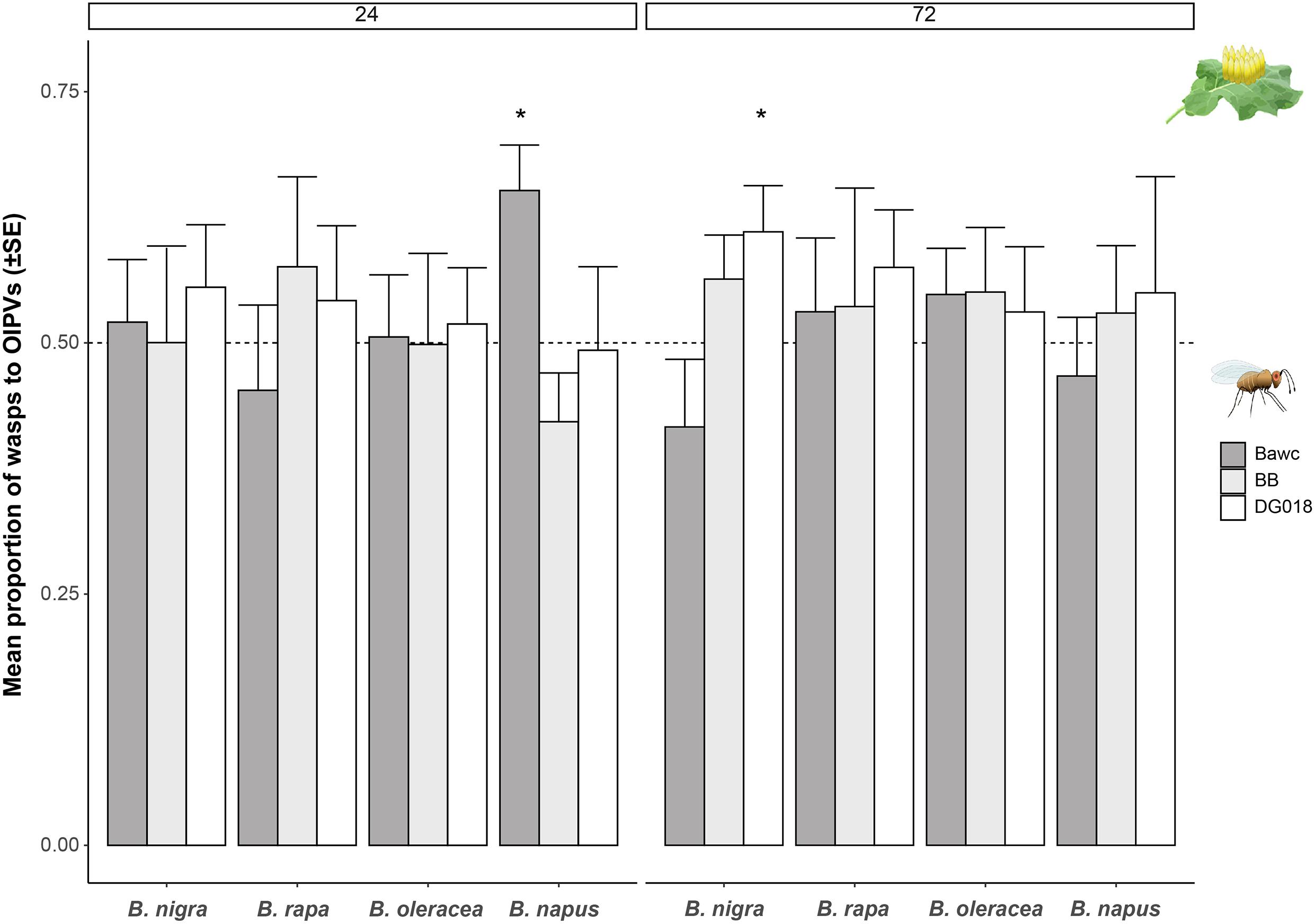
Figure 2. Proportion of wasps (mean ± SE) attracted to volatiles of P. brassicae egg-infested Brassica plants. Three different T. evanescens isofemale lines were tested with volatiles emitted by four different Brassica species, 24 h (left) and 72 h (right) after egg deposition. Different lines of T. evanescens were: Bawc (dark gray), BB (light gray), and DG018 (white). Statistically significant results (p < 0.05) are indicated with an asterisk (logistic regression). Bars without asterisk are not significant. Every plant-parasitoid combination was tested eight times and 10 female wasps were released per plant pair (n = 80 wasps per treatment/strain).
On average, when considering the combined responses of the three parasitoid lines toward volatiles of uninfested and egg-infested B. oleracea, more than 50% were attracted to volatiles of egg-induced plants for both time points. Although there is a slight increase of the percentages after 72 h, none of the B. oleracea – parasitoid interactions were statistically significant (Figure 2). Similarly, volatiles of egg-infested B. rapa plants tended to be more attractive to Trichogramma than of control plants, but none of these preferences were significant.
Further bioassays were conducted to address the question of whether HR presence/absence has an effect on the attraction of Trichogramma wasps to volatiles of egg-infested plants especially for plant species where we observed a trend for attraction to HR positive plants after the first eight bioassays. We found that 65% of DG018 wasps significantly preferred volatiles of egg-infested B. rapa plants expressing HR-like above volatiles of control plants (GLM, t = 3.485, p = 0.039; Supplementary Figure 1). This was not the case for volatiles of plants not expressing HR-like necrosis.
Regardless of the parasitoid line or the time after oviposition, we found that presence of HR-like necrosis has a significant effect on T. evanescens preferences to volatiles of egg-infested B. rapa plants (χ2 = 5.658, df = 1, p = 0.017; Figure 3A). The synergistic effect of HR-like and parasitoid-attraction was not found for B. napus or B. oleracea plants and other isofemale lines. As all tested B. nigra plants expressed an HR-like we could not assess whether or not there was a synergistic effect for this accession (SF48-O1).
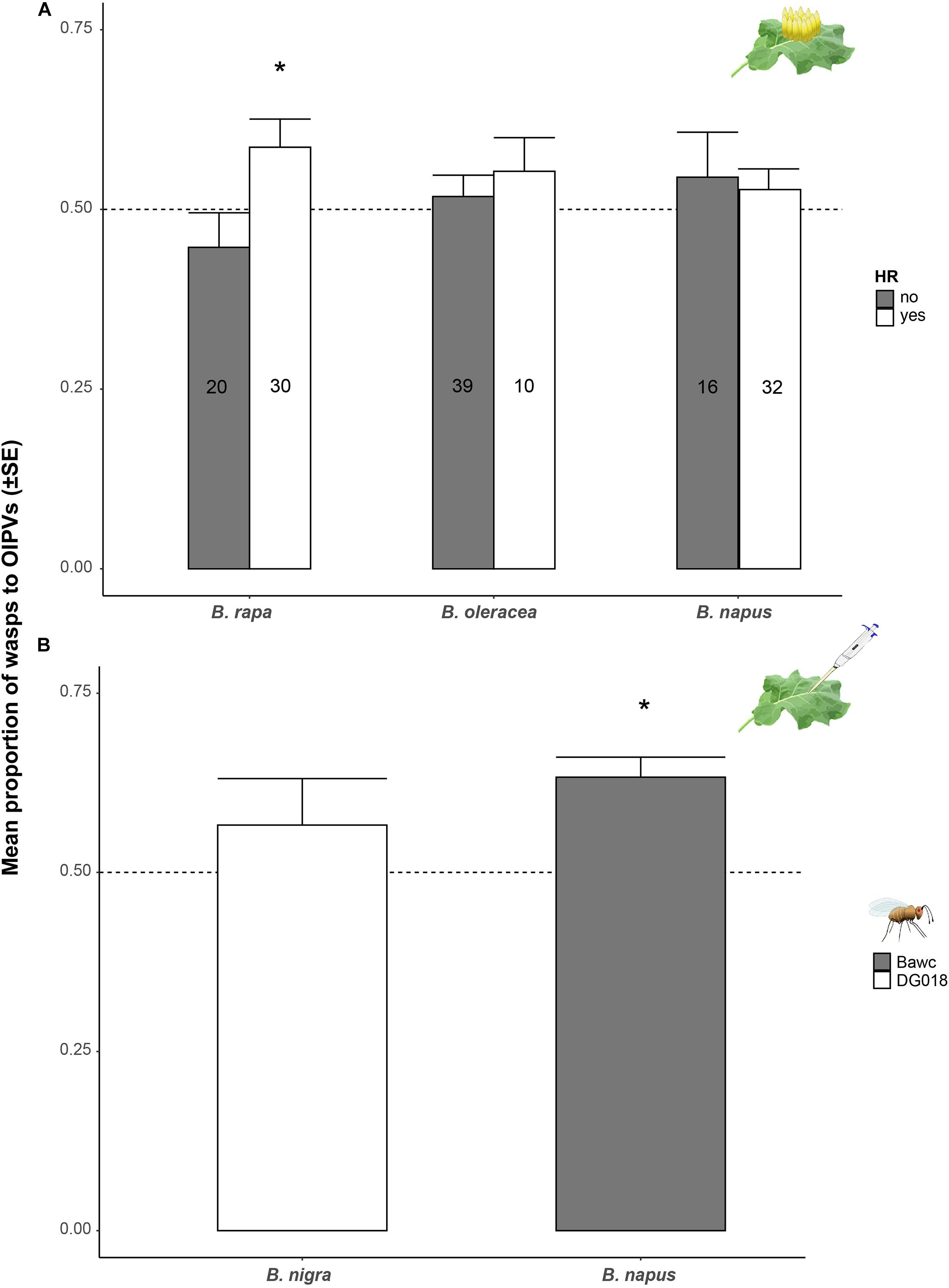
Figure 3. Proportion of wasps (mean ± SE) attracted to volatiles of plants induced by eggs or egg wash. (A) Proportion of T. evanescens females attracted to volatiles of P. brassicae egg-infested Brassica plants depending on expression of HR-like necrosis. Proportion of preference to plants expressing an HR-like (white) or not (dark gray) regardless of the parasitoid strain or time after induction is shown. Every plant-parasitoid combination was replicated eight times and 10 female wasps were released per plant pair (n = 80 wasps per treatment/strain). (B) Proportion of T. evanescens isofemale lines Bawc (dark gray) and DG018 (white) attracted to volatiles of B. rapa or B. nigra plants treated with P. brassicae egg wash 24 h (B. napus) and 72 h (B. nigra) after induction. Every plant-parasitoid combination was replicated 8–10 times with 10–12 female wasps. Asterisks indicate a preference which is significantly different from a 50:50 distribution within a choice test (GLM).
The significant plant-parasitoid interactions were used to test whether egg-derived compounds induce parasitoid-attracting volatiles. We used the isofemale line DG018, previously shown to be attracted to volatiles of egg-infested B. nigra plants, 72 h after oviposition and the Bawc line attracted to OIPVs of B. napus 24 h after oviposition. To test egg-derived compounds, we used a wash of eggs, which was previously shown to induced a similar HR-like necrosis as eggs in B. nigra (Caarls et al., 2021). Here, egg wash induced HR-like necrosis in several of the tested plants (Supplementary Figure 3).
Wasps from DG018 did not discriminate between volatiles of B. nigra plants treated with egg wash or control plants (Figure 3B). Interestingly, wasps from the Bawc line were significantly attracted to volatiles of egg wash-treated B. napus plants compared to plants treated with the solvent 24 h after treatment (GLM, z = 2.295, p = 0.021; Figure 3B).
In this study, we show that two isofemale lines of T. evanescens were attracted to volatiles of either B. nigra 72 h after egg deposition or B. napus 24 h after egg deposition. HR-like necrosis was expressed in all Brassica species tested but at different frequency and severity. Volatiles of B. rapa expressing HR were more attractive to the parasitoids than non-HR plants indicating a synergistic effect between the two egg-killing traits. Notably, wash made from P. brassicae eggs contains elicitors that can trigger parasitoid-attracting volatiles in one plant species (B. napus). We thus confirm that emission of OIPVs and synergistic effects with HR-like necrosis are found in three of the Brassica species tested, but attraction depends on the Trichogramma line used, and the expression of HR-like necrosis.
Overall, it is shown that HR-like necrosis underneath eggs is expressed in all Brassica species tested, confirming results from previous studies that used wild and cultivated plants (Pashalidou et al., 2015; Griese et al., 2021). Emission of egg parasitoid-attracting volatiles was shown for three of the four tested Brassica species. Interestingly, egg-induced volatiles emitted by the two crops, B. rapa and B. napus, attracted different isofemale wasp lines. According to the “plant domestication-reduced defence” hypothesis, domestication has reduced chemical resistance against herbivorous insects (Chen et al., 2015). For example, maize landraces released eightfold higher (E)-caryophyllene attractive to Cotesia parasitoids than a commercial maize line (Tamiru et al., 2017). Interestingly, a meta-analysis on eco-evolutionary factors that drive the induction of plant volatiles actually show that domestication reduces complexity of volatile blend while the emission of specific volatiles like green leaf volatiles and sesquiterpenes increased in some domesticated species compared to wild species (Rowen and Kaplan, 2016). Our data suggest that, in addition to the wild crucifer B. nigra, crop plants in the Brassicaceae family possess indirect plant defenses and thus, domestication seems to not have caused the loss of this trait. Yet, in B. oleracea, we have not found attraction to OIPVs in either the wild “Kimmeridge,” nor the crop B. oleracea var. gemmifera (Fatouros et al., 2005). To understand whether the trait is variable between genotypes or absent, a larger germplasm screening of OIPVs in B. oleracea is needed. In fact, we cannot exclude that other plant accessions or field populations could respond differently in terms of OIPV emission or expression of HR-like necrosis than the accessions tested here.
In our study, we found specificity in terms of attraction of T. evanescens to OIPVs emitted by specific plant species. Each of the responding plant species attracted only one parasitoid strain. Black mustard and B. rapa plants attracted the DG018 strain while B. napus attracted the Bawc strain. The two responding species were collected from eggs on different plants and herbivore hosts. T. evanescens strain DG018 was collected from the Rhine river in Wageningen, The Netherlands, which is also the place of origin of the used B. nigra seeds. Hence, it seems that black mustard – P. brassicae – T. evanescens (DG018) is a well-established tri-trophic interaction in this local ecosystem. Similarly, the RU263 strain, which was collected in the same area, was shown previously to be attracted to HR-expressing B. nigra 72 h post egg deposition (Fatouros et al., 2014). The habitat specificity of Trichogramma has been examined in the past by many authors and Trichogramma parasitoids are known to learn to associate oviposition experience with plant volatiles (Romeis et al., 2005). Moreover, rearing history of parasitoids was previously shown to affect odor discrimination in the aphid parasitoid Diaeretiella rapae (Cascone et al., 2019). In this context, it can be hypothesized that the observed pattern in the attraction of parasitoids is the outcome of experience from the interaction of the parasitoid with the original host and host plant. However, it still remains under investigation whether this can be maintained after several generations in a laboratory host like E. kuehniella.
Another explanation about the observed specificity in the attraction to OIPVs is that different parasitoid isofemale lines have adapted to parasitize eggs of a certain “quality.” Attraction of DG018 strain occurred 72 h after egg deposition in plants that had developed an HR-like necrosis. For DG018 parasitoid wasps, HR positive plants may provide chemical information about eggs that are in the stage of desiccation; weaker to activate innate defenses; but still suitable for Trichogramma development. Furthermore, herbivore eggs are known to activate innate responses after parasitism by T. evanescens which can lead to a much lower parasitoid emergence (Abdel-Latief and Hilker, 2008). On the other hand, parasitoids belonging to Bawc strain were attractive to B. napus only 24 h after oviposition. Bawc parasitoid wasps may have learned to recognize only volatiles that reveal that eggs are still young and therefore suitable for parasitism. Pieris egg quality deteriorates with time: when eggs are about 96 h old, they become unsuitable for parasitism (Fatouros et al., 2005). Further tests are needed to link preference with performance for these parasitoids to get insight on whether females (from different populations of the same species) choose the best host for their offspring.
We did not find a Trichogramma line that was attracted to all host plants. T. evanescens is known to have a broad host range (Polaszek, 2010). Here, we show intra-specific variation in the use of host-finding cues from different plant species. It is not yet clear what underlies the demonstrated intra-specific variation in T. evanescens’ response to OIPVs. The CO1 barcodes show that there is genetic variability between these three lines. CO1 barcodes have been used for species identification, as well as for analysis of intra-specific variation between populations (Bergsten et al., 2012). The two most similar haplotypes, DG018 and Bawc, were collected from distant sites in the Netherlands and Germany, respectively. Conversely, the two lines from Wageningen, the Netherlands (BB and DG018) are less similar. While lacking in geographically driven patterns of similarity, interestingly, the haplotypes of both responding lines, DG018 and Bawc, are more similar to each other than either are to BB, which was not attracted to the OIPVs of any plant. These differences in CO1 genes are not indicative of a causative relationship with OIPV attraction but rather reflect genetic distance between populations that could have affected the genome elsewhere in a way that is responsible for the differences we observe. A larger sampling of different CO1 haplotypes and expanded behavioral testing could reveal a correlative relationship between specific haplotypes, or barcodes, and OIPV responses. Thus, the specific barcodes could be used to identify wild-type specimens with a known desirable trait, or useful for a particular crop-type, as well as to track the status of a particular strain in a given field before and/or after parasitoid release (Lommen et al., 2017). With the ease and speed with which DNA barcodes can be obtained, this could serve as a promising strategy for harnessing intra-specific variation in Trichogramma for increased biocontrol efficacy.
The synergy between HR-like necrosis and attraction of egg parasitoids could be a desirable trait to be used in crop protection (Fatouros et al., 2016). Previously, we showed that mortalities of singly laid P. rapae/Pieris napi eggs went up to 80% in a natural population of B. nigra due to the combination of necrosis and egg parasitism (Fatouros et al., 2016). Subsequently conducted Y-tube experiments showed that OIPVs emitted by plants expressing HR-like necrosis were more attractive to the native T. evanescens isofemale line (RU263) than OIPVs of non-HR plants (Fatouros et al., 2014). In this study we show that both cultivars expressed HR-like necrosis with medium strength compared to the tested B. nigra accession that almost exclusively expressed strong necrosis. In B. napus, parasitoid attraction was independent of presence of HR-like necrosis. On the contrary, egg parasitoids were attracted to OIPVs from B. rapa plants only when HR-like necrosis was expressed, highlighting a potential synergistic association between the two types of defenses in this species. Whether the presence of the necrosis and attraction of parasitoids in B. rapa leads to higher egg mortalities compared to non-expressing plants remains to be investigated. Moreover, as expression and strength of HR-like highly varies between accessions within Brassica species (Fatouros et al., 2014; Griese et al., 2017, 2021; Bassetti et al., to be submitted), we expect that the synergistic expression with OIPVs shows natural variation too and therefore is more common in Brassicaceae.
It was initially hypothesized that elicitors in the glue-like secretions produced by the ARGs of Pieris butterflies are responsible for activating emission of OIPVs. B. nigra plants treated with ARGs from P. brassicae females 48 h after mating emitted volatiles attractive to Trichogramma wasps (Fatouros et al., 2015). Here, this hypothesis was studied by treating plants with wash made of P. brassicae eggs. It is likely that egg wash contains mostly compounds from the outside of eggs and egg glue, as eggs are not harmed in this method of compound extraction (Caarls et al., 2021). We found attraction of T. evanescens Bawc line toward volatiles emitted by egg wash-treated B. napus plants while volatiles of egg wash-treated B. nigra plants tended to attract DG018 parasitoids. Egg wash that was prepared in the same manner was previously shown to induce HR-like necrosis and related defense responses in B. nigra (Caarls et al., 2021). This suggests that similar compounds are present in the egg wash that induce indirect defense responses as well. Whether these compounds are indeed located in the egg glue, and what the identity of the elicitors is, needs further investigation. Moreover, the use of egg wash over eggs has the advantage that it can be easily treated to study characteristics of the eliciting molecules (Caarls et al., 2021) or used as a reproducible egg-mimicking treatment in germplasm screenings to find genes underlying the trait (Griese et al., 2017; Bassetti et al., to be submitted).
The confirmation that egg wash can be successfully used to trigger defending responses also opens up possibilities for manipulating herbivore and natural enemy behavior and enhance conservational biocontrol in the open field. In a previous study, mated P. brassicae females were found to significantly avoid B. nigra plants that had already received egg deposition by P. brassicae (Fatouros et al., 2012). This property can be combined with the attraction of egg parasitoids as we showed in this study to design a “push and pull” system (Cook et al., 2007). Plants that are induced by egg secretion could be used as push factors due to their oviposition deterrent properties while at the same time eliciting attraction of natural enemies. Other stimuli such as the sex pheromones of the pest can be used as pull factors. In this scenario, P. brassicae butterflies in the environment will be led to areas outside of the target crop causing minimal damage.
In summary, we have shown that besides the wild crucifer B. nigra also close relatives and domesticated Brassica species, i.e., B. rapa and B. napus emit Trichogramma-attracting volatiles. We have found that parasitoid responses show intraspecific variation. The tested wasps seem genetically different enough to explain the observed variation. There is an emerging need of selecting parasitoids adapted to the different agroecosystems to achieve a more successful augmentative biocontrol. However, recognition of OIPVs is just the first step for host location by egg parasitoids. Parasitoid wasps should be accordingly assessed for parasitism rates before selected as suitable biocontrol agent. Future efforts should also analyze the chemical composition of OIPVs in the different Brassicas and screen more accessions between and within species to eventually understand how common they are and unravel the genetic basis. On the molecular level, we need work on the recognition and chemical characterization of egg-related elicitors that trigger direct and indirect plant defenses. The use of such elicitors could boost up conservational biocontrol in the field.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. The following accession numbers can be found at: NCBI (accession: MW722247–MW722250).
DA, AC, and NF planned and designed the research. DA, LC, AC, and LG performed the experiments and/or analyzed the data. All authors executed data interpretation and wrote the manuscript.
This research was made possible by support of the Dutch Technology Foundation TTW, which was part of the Netherlands Organisation for Scientific Research (NWO) (NWO/TTW VIDI grant 14854 to NF).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Sean Geurts from Unifarm (Wageningen University and Research) for cultivating and caring of the plants used in the experiment. We thank Pieter Rouweler, André Gidding, and Frans van Aggelen for rearing of Pieris brassicae. We are thankful to Gabriella Bukovinskine Kiss for assisting the parasitoid rearing. Furthermore, we thank Rieta Gols, Niccolo Bassetti, and Eddie Griese for providing seeds.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.703134/full#supplementary-material
Abdel-Latief, M., and Hilker, M. (2008). Innate immunity: eggs of Manduca sexta are able to respond to parasitism by Trichogramma evanescens. Insect Biochem. Mol. Biol. 38, 136–145. doi: 10.1016/j.ibmb.2007.10.001
Bassetti, N., Caarls, L., Bouwmeester, K., Bukovinszkine’kiss, G., Van Veen, J., El-Soda, M., et al. (to be submitted). Genetic dissection of a butterfly egg-induced plant defence trait in Brassica rapa by QTL analysis.
Bergsten, J., Bilton, D. T., Fujisawa, T., Elliott, M., Monaghan, M. T., Balke, M., et al. (2012). The effect of geographical scale of sampling on DNA barcoding. Syst. Biol. 61, 851–869.
Caarls, L., Bassetti, N., Van Doesburg, F., Verbaarschot, P., Van Loon, J. J. A., Schranz, M. E., et al. (2021). Deciphering Brassica plant defence responses to cabbage white butterfly egg-associated molecular patterns. bioRxiv [Preprint]. doi: 10.1101/2021.03.29.437462
Cascone, P., Gols, R., Fatouros, N. E., Ponzio, C., Dicke, M., and Guerrieri, E. (2019). The effect of rearing history and aphid density on volatile-mediated foraging behaviour of Diaeretiella rapae. Ecol. Entomol. 44, 255–264. doi: 10.1111/een.12704
Chen, Y. H., Gols, R., and Benrey, B. (2015). Crop domestication and its impact on naturally selected trophic interactions. Annu. Rev. Entomol. 60, 35–58. doi: 10.1146/annurev-ento-010814-020601
Colazza, S., Peri, E., Salerno, G., and Conti, E. (2010). “Host searching by egg parasitoids: exploitation of host chemical cues,” in Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma, eds F. L. Consoli, J. R. P. Parra, and R. Zucchi (Dordrecht: Springer), 97–147. doi: 10.1007/978-1-4020-9110-0_4
Cook, S. M., Khan, Z. R., and Pickett, J. A. (2007). The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 52, 375–400. doi: 10.1146/annurev.ento.52.110405.091407
Cusumano, A., Weldegergis, B. T., Colazza, S., Dicke, M., and Fatouros, N. E. (2015). Attraction of egg-killing parasitoids toward induced plant volatiles in a multi-herbivore context. Oecologia 179, 163–174. doi: 10.1007/s00442-015-3325-3
Fatouros, N. E., Broekgaarden, C., Bukovinszkine’kiss, G., Van Loon, J. J. A., Mumm, R., Huigens, M. E., et al. (2008). Male-derived butterfly anti-aphrodisiac mediates induced indirect plant defense. Proc. Natl. Acad. Sci. U.S.A. 105, 10033–10038. doi: 10.1073/pnas.0707809105
Fatouros, N. E., Bukovinszkine’kiss, G., Kalkers, L. A., Soler Gamborena, R., Dicke, M., and Hilker, M. (2005). Oviposition-induced plant cues: Do they arrest Trichogramma wasps during host location? Entomol. Exp. Appl. 115, 207–215. doi: 10.1111/j.1570-7458.2005.00245.x
Fatouros, N. E., Cusumano, A., Danchin, E. G. J., and Colazza, S. (2016). Prospects of pest-killing defenses for sustainable crop protection. Ecol. Evol. 6, 6906–6918. doi: 10.1002/ece3.2365
Fatouros, N. E., Lucas-Barbosa, D., Weldegergis, B. T., Pashalidou, F. G., Van Loon, J. J. A., Dicke, M., et al. (2012). Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS One 7:e43607. doi: 10.1371/journal.pone.0043607
Fatouros, N. E., Paniagua Voirol, L. R., Drizou, F., Doan, Q. T., Pineda, A., Frago, E., et al. (2015). Role of Large Cabbage White butterfly male-derived compounds in elicitation of direct and indirect egg-killing defenses in the black mustard. Front. Plant Sci. 6:794. doi: 10.3389/fpls.2015.00794
Fatouros, N. E., Pineda, A., Huigens, M. E., Broekgaarden, C., Shimwela, M. M., Figueroa, I. A., et al. (2014). Synergistic effects of direct and indirect defences on herbivore egg survival in a wild crucifer. Proc. Biol. Sci. 281:20141254. doi: 10.1098/rspb.2014.1254
Gols, R., Bullock, J. M., Dicke, M., Bukovinszky, T., and Harvey, J. A. (2011). Smelling the wood from the trees: non-linear parasitoid responses to volatile attractants produced by wild and cultivated cabbage. J. Chem. Ecol. 37, 795–807. doi: 10.1007/s10886-011-9993-5
Gols, R., Wagenaar, R., Bukovinszky, T., Dam, N. M. V., Dicke, M., Bullock, J. M., et al. (2008). Genetic variation in defense chemistry in wild cabbages affects herbivores and their endoparasitoids. Ecology 89, 1616–1626. doi: 10.1890/07-0873.1
Griese, E., Caarls, L., Bassetti, N., Mohammadin, S., Verbaarschot, P., Bukovinszkine’kiss, G., et al. (2021). Insect egg-killing: a new front on the evolutionary arms-race between brassicaceous plants and pierid butterflies. New Phytol. 230, 341–353. doi: 10.1111/nph.17145
Griese, E., Dicke, M., Hilker, M., and Fatouros, N. E. (2017). Plant response to butterfly eggs: inducibility, severity and success of egg-killing leaf necrosis depends on plant genotype and egg clustering. Sci. Rep. 7:7316.
Griese, E., Pineda, A., Pashalidou, F. G., Iradi, E. P., Hilker, M., Dicke, M., et al. (2020). Plant responses to butterfly oviposition partly explain preference-performance relationships on different brassicaceous species. Oecologia 192, 463–475. doi: 10.1007/s00442-019-04590-y
Hilker, M., and Fatouros, N. E. (2015). Plant responses to insect egg deposition. Annu. Rev. Entomol. 60, 493–515. doi: 10.1146/annurev-ento-010814-020620
Hilker, M., and Fatouros, N. E. (2016). Resisting the onset of herbivore attack: plants perceive and respond to insect eggs. Curr. Opin. Plant Biol. 32, 9–16. doi: 10.1016/j.pbi.2016.05.003
Leung, K., Ras, E., Ferguson, K., Ariëns, S., Babendreier, D. B., Bijma, P., et al. (2020). Next generation biological control: the need for integrating genetics and evolution. Biol. Rev. 95, 1838–1854. doi: 10.1111/brv.12641
Lommen, S. T. E., De Jong, P. W., and Pannebakker, B. A. (2017). It is time to bridge the gap between exploring and exploiting: prospects for utilizing intraspecific genetic variation to optimize arthropods for augmentative pest control - a review. Entomol. Exp. Appl. 162, 108–123.
Paniagua Voirol, L. R., Valsamakis, G., Lortzing, V., Weinhold, A., Johnston, P. R., Fatouros, N., et al. (2020). Plant responses to insect eggs are not induced by egg-associated microbes, but by a secretion attached to the eggs. Plant Cell Environ. 43, 1815–1826.
Pashalidou, F. G., Fatouros, N. E., Van Loon, J. J. A., Dicke, M., and Gols, R. (2015). Plant-mediated effects of butterfly egg deposition on subsequent caterpillar and pupal development, across different species of wild Brassicaceae. Ecol. Entomol. 40, 444–450.
Polaszek, A. (2010). “Species diversity and host associations of Trichogramma in Eurasia,” in Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma, eds F. L. Consoli, J. R. P. Parra, and R. Zucchi (Dordrecht: Springer), 237–266.
Ponzio, C., Cascone, P., Cusumano, A., Weldegergis, B. T., Fatouros, N. E., Guerrieri, E., et al. (2016). Volatile-mediated foraging behaviour of three parasitoid species under conditions of dual insect herbivore attack. Anim. Behav. 111, 197–206.
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Romeis, J., Babendreier, D., Wäckers, F. L., and Shanower, T. G. (2005). Habitat and plant specificity of Trichogramma egg parasitoids—underlying mechanisms and implications. Basic Appl. Ecol. 6, 215–236.
Rowen, E., and Kaplan, I. (2016). Eco-evolutionary factors drive induced plant volatiles: a meta-analysis. New Phytol. 210, 284–294.
Tamiru, A., Bruce, T. J., Woodcock, C. M., Caulfield, J. C., Midega, C. A., Ogol, C. K., et al. (2011). Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol. Lett. 14, 1075–1083.
Tamiru, A., Bruce, T. J. A., Richter, A., Woodcock, C. M., Midega, C. A. O., Degenhardt, J., et al. (2017). A maize landrace that emits defense volatiles in response to herbivore eggs possesses a strongly inducible terpene synthase gene. Ecol. Evol. 7, 2835–2845.
Tamiru, A., Khan, Z. R., and Bruce, T. J. A. (2015). New directions for improving crop resistance to insects by breeding for egg induced defence. Curr. Opin. Insect Sci. 9, 51–55.
Keywords: tritrophic interactions, host location, hypersensitive response, indirect defense, egg parasitoids, Pieris
Citation: Afentoulis DG, Cusumano A, Greenberg LO, Caarls L and Fatouros NE (2021) Attraction of Trichogramma Wasps to Butterfly Oviposition-Induced Plant Volatiles Depends on Brassica Species, Wasp Strain and Leaf Necrosis. Front. Ecol. Evol. 9:703134. doi: 10.3389/fevo.2021.703134
Received: 30 April 2021; Accepted: 07 June 2021;
Published: 30 June 2021.
Edited by:
Cesar Rodriguez-Saona, The State University of New Jersey, United StatesReviewed by:
Maria Fernanda Gomes Villalba Peñaflor, Universidade Federal de Lavras, BrazilCopyright © 2021 Afentoulis, Cusumano, Greenberg, Caarls and Fatouros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nina E. Fatouros, bmluYS5mYXRvdXJvc0B3dXIubmw=
†Present address: Lotte Caarls, Plant Breeding, Wageningen University and Research, Wageningen, Netherlands
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.