- Department of Biology, Institute of Evolutionary Ecology and Conservation Genomics, Ulm University, Ulm, Germany
Insect species richness and abundance has declined rapidly over the last few decades. Various stressors, such as the conversion of natural habitats, climate change, land-use intensification, agrochemicals and pathogens, are thought to be major factors in this decline. We treated female bees of two common pollinator species in Europe, Osmia bicornis and Bombus terrestris, with a field-realistic dose of the neonicotinoid clothianidin. We tested its effects on the foraging behavior of O. bicornis under semi-natural conditions and on the antennal sensitivity of both bee species to common floral volatiles by using electroantennography. Clothianidin negatively affected the foraging behavior in O. bicornis by decreasing the number of flowers visited per foraging flight and by increasing the time per flower visit and the searching time between two flowers. It also decreased the antennal sensitivity to 2-phenylethanol in the two bee species. Thus, clothianidin is clearly a threat for bees via its effects on their foraging behavior and antennal sensitivity and is hence probably detrimental for pollination and the reproductive success of bees.
Introduction
Biodiversity, especially species richness, abundance and the distribution of pollinators is globally declining (Potts et al., 2010; van der Sluijs et al., 2013; Godfray et al., 2014; Goulson et al., 2015; Hallmann et al., 2017). The limited availability of food and nesting resources and the occurrence of parasites and pathogens, climate change, and pesticides are considered to be the main drivers of this decline (Goulson et al., 2015). With regard to pesticides, the use of neonicotinoids is a major threat for the most important agents in pollination, namely honeybees, bumblebees, and solitary bees (Elbert et al., 2008; Godfray et al., 2014).
The intensification of agriculture has transformed the agrochemical landscape and resulted in a massive overuse of pesticides in recent years (Casida and Durkin, 2013; Gross, 2013; van der Sluijs et al., 2013). Among the neonicotinoids, imidacloprid, thiamethoxam, and clothianidin (a breakdown product of thiamethoxam) are the most toxic (Scott-Dupree et al., 2009; Stokstad, 2013; Botías et al., 2015). They have been used as a seed coating or have been applied via foliar or soil treatment until their ban in Germany by the end of 2020 (Elbert et al., 2008; Fent, 2013). However, the EU Pesticides Database has revealed that some of these banned neonicotinoids are still authorized for use at national level in a few European countries. Because of their long persistence in soil, neonicotinoids can be detected even in untreated plants and soil over years (Hopwood et al., 2012; Botías et al., 2015). Neonicotinoids target the central nervous system of insects in which they act as an agonist of insect nicotinic acetylcholine receptors (nAChR) at the postsynaptic membrane in the nervous system; however, they are not degraded by acetylcholine esterase as is the natural transmitter acetylcholine (Tomizawa and Casida, 2005; Elbert et al., 2008; Fent, 2013; Fischer et al., 2014). Acetylcholine is a highly important transmitter and is suggested to play an important role during transmission from olfactory receptor neurons via the antennal lobe to the mushroom bodies (Fischer et al., 2014). Because of their higher affinity and higher selectivity for insect nAChR over vertebrate nAChR, neonicotinoids are more toxic to insects, as has been clearly shown by the much higher lethal doses (LD50) recorded for vertebrates (Jeschke and Nauen, 2008; Matsuda et al., 2011; Uneme, 2011). In addition to their direct lethal effects, they also exhibit sublethal effects that do not directly cause death in animals (Artz and Pitts-Singer, 2015).
Many of the adverse effects of neonicotinoids have been demonstrated in honeybees. Treatment with clothianidin significantly reduces the life span of Apis mellifera workers (Sgolastra et al., 2015; Tsvetkov et al., 2017). Furthermore, Tomé et al. (2012) and Williamson et al. (2014) have found a sublethal effect and confirmed that low doses of clothianidin affect the motor function and the walking behavior in adult neotropical stingless bees and honeybees. Interestingly, two other studies in honeybees and in Osmia cornuta have revealed no effects of neonicotinoids on locomotion or even increased locomotive activity (El Hassani et al., 2008; Jin et al., 2015). Neonicotinoids also seem to affect memory and learning by damage to the central nervous system in bees (Tomé et al., 2012; van der Sluijs et al., 2013). In a cognition experiment with O. cornuta, Jin et al. (2015) have found a blockage of memory retrieval for learned cues guiding to a food source after neonicotinoid treatment. Further, neonicotinoids affect foraging success, with the treatment of various bee taxa resulting in less directed flights and a lower pollen and nectar foraging efficiency (Desneux et al., 2007; Hopwood et al., 2012; van der Sluijs et al., 2013; Feltham et al., 2014; Fischer et al., 2014; Tan et al., 2014; Tison et al., 2016). In contrast, in the bumblebee Bombus terrestris, thiamethoxam does not appear to affect the total length of foraging flights or searching time between two flowers (Stanley and Raine, 2016). However, field experiments and even experiments carried out under semi-field conditions to determine the effects of neonicotinoids on wild bees under natural conditions are scarce. Rundlöf et al. (2015) have performed a huge field study and found a reduced density of wild bees, reduced nesting activity near treated fields and negatively affected colony growth in B. terrestris. Since all of the bee species have a function as pollinators, we need to understand the effects of neonicotinoids on foraging behavior and pollination. Moreover, the amount of pollen that females collect affects larval fitness and reproductive success (Radmacher and Strohm, 2010; Seidelmann, 2014; Stanley et al., 2015).
In social insects, semiochemicals are crucial for maintaining the colony (Ayasse and Jarau, 2014). Insect pheromones such as cuticular hydrocarbons (CHCs) or cuticular lipids play a key function and are vital for sustaining the intra-colonial network; pheromones regulate and control worker reproduction and underpin behavioral patterns such as mating or worker reproduction in social insects (Ayasse and Jarau, 2014). In social insects, scent not only has an intraspecific function in communication, but also plays an important role in foragers finding their host plants. In solitary and social insects, floral scent is thought to be a major cue for discriminating and identifying different flowers (Schiestl, 2015). It also serves as a cue enabling bees to distinguish between rewarding and non-rewarding flowers and even the amount of reward that is present within a flower (Dötterl and Vereecken, 2010; Schiestl, 2015). Bees perceive a multitude of semiochemicals via chemical receptors that are located on their antennae (Kaib, 2003). The semiochemical signal is then transmitted via the antennal lobe to the mushroom bodies (Heisenberg, 1998). Here, acetylcholine is intimately involved in transmission (Fischer et al., 2014). However, the effects of neonicotinoids on antennal sensitivity, and especially on receptor level, are poorly investigated and only a few studies are available (i.e., Tappert et al., 2017).
Although wild bees and bumblebees are clearly as important as honeybees in terms of pollination, most studies on the effects of neonicotinoids have focused on various honeybee species and have been performed under laboratory conditions (Michener, 2000; van der Sluijs et al., 2013; Godfray et al., 2014). Only a few studies have focused on solitary bee species but most have shown negative effects after insecticide treatment (Artz and Pitts-Singer, 2015; Jin et al., 2015; Sgolastra et al., 2015). In order to increase our knowledge concerning the effects of neonicotinoids on wild bees, we have studied the effects of clothianidin on the foraging behavior and antennal sensitivity in the red mason bee Osmia bicornis and the buff-tailed bumblebee B. terrestris. Both O. bicornis, the most abundant solitary bee of the genus Osmia in Central Europe, and B. terrestris are important pollinators in orchards and plantations (Westrich and Dathe, 1997; Gruber et al., 2011). We have treated female bees of both species with field-realistic and sub-lethal doses of clothianidin and looked for differences in their foraging behavior and antennal sensitivity to various floral volatiles. We have also tested floral volatiles in both species and a pheromone component in B. terrestris, because both play important roles in colony maintenance and the finding of host plants.
We hypothesized that clothianidin would negatively affect the foraging behavior of female O. bicornis. We expected that the number of flowers per foraging flight would decrease and the time per flower and the time between two flowers would increase after clothianidin treatment. Because flower morphology might influence flower handling time, we chose two plant species that differed in their floral morphology, namely one Asteraceae (Crepis biennis) and a Ranunculaceae (Ranunculus spp.). We further hypothesized a negative effect of clothianidin on the sensitivity of antennal scent receptors in both O. bicornis and B. terrestris and expected that clothianidin would decrease antennal sensitivity in both species.
Materials and Methods
Study Species
The female O. bicornis LINNAEUS 1778 that were used in both experiments were reared in trap nests in the Botanical Garden at Ulm University. Cocoons that had overwintered in a cardboard box at 6°C in a refrigerator were placed into small rearing cages or flight cages (24.5 × 24.5 × 24.5 cm). After hatching and mating, female bees were able to start their own brood in wooden nesting blocks (49.5 × 20 × 17.5 cm) that had holes (diameter 7 mm) drilled into them. The bees were allowed to feed ad libitum on a 50% sugar solution, namely a dilution of two-thirds of a 73% sugar solution of API-Invert® (Südzucker AG, Mannheim, Germany) and one-third water. We added 3 g potassium sorbate and 1 g citric acid per 1 l of sugar solution as a preservative. We offered the sugar solution on small pieces of foam in a Petri dish, which was replaced every second or third day. During recordings of the foraging flights of the bees in the flight cages, we removed the Petri dish with the sugar solution. For the antennal sensitivity experiments, bees that had hatched in small flight cages in the laboratory were allowed to mate before they were used for electrophysiological recordings.
For the antennal response experiment, we also used female B. terrestris LINNAEUS 1758 reared in the laboratory at Ulm University. After mating and hibernation at 6°C for 10–12 weeks, queens were allowed to found new colonies (for details, see Rottler-Hoermann et al., 2016). As for O. bicornis, the bumble bees were fed ad libitum on a 50% sugar solution and additionally on fresh pollen (Koppert Biological Systems, Germany), which was replaced every 2 days. The new colonies were kept in wooden boxes (39 × 16.5 × 16 cm) with two separated compartments at a temperature of 27 ± 2°C and a relative humidity of 60–70% under constant darkness. The founding queens of all colonies were originally derived from commercial colonies (Koppert Biological Systems, Germany).
Clothianidin Treatment
To ensure that all bees were treated with a field-realistic amount of clothianidin [(E)-1-(2-chloro-1,3-thiazol-5-ylmethyl)-3-methyl-2-nitroguanidine], we chose a concentration of 0.75 ng (1 Osmia Equivalent OE) clothianidin per bee for O. bicornis (Jin et al., 2015) and 2.55 ng (1 Bombus Equivalent BE) for B. terrestris. We diluted clothianidin (>98.0%, Sigma-Aldrich, Hamburg, Germany) in the respective amount in acetone (>99.8%, Sigma-Aldrich, Hamburg, Germany). For the experiment we used the pure compound instead of a formulation to avoid any potential effects of additives. Test solutions were stored in brown screw-cap micro-vials (CZT, Kriftel, Germany) at 6°C in a refrigerator to prevent photolysis ([EPA] U.S. Environmental Protection Agency., 2003).
Because the controlled uptake of clothianidin was important to ensure that the same conditions were experienced by all bees, we did not feed them with clothianidin via the sugar solution (see Tan et al., 2014; Jin et al., 2015). Instead, we applied 1 OE/1 BE clothianidin solution or acetone to the soft skin between the last sternite and tergite of the abdomen of each individual (Tappert et al., 2017). This topical application was used as it was comparable with foliar spray treatment in the field, spray experiments with clothianidin having been shown to be the most toxic for O. lignaria (Scott-Dupree et al., 2009). We conducted the experiments 1 day after treatment.
Measuring the Effects of Clothianidin
Foraging Behavior of Osmia bicornis
Foraging flights of O. bicornis (N = 22) were recorded simultaneously in two flight cages (3.00 × 2.00 × 2.20 m) in the Botanical Garden of Ulm University with one flight cage for each treatment. We conducted the experiments between May and June 2017 at comparable ambient air temperatures in the morning since the activity of bees rapidly decreased at high air temperatures. Ranunculus spp. and C. biennis, which were derived from a wild meadow in the Botanical Garden, served as a pollen and nectar source and were randomly distributed within the flight cage. Because flower morphology influences handling time, we tested two different plant species, namely Ranunculus spp. (Ranuculaceae) and C. biennis (Asteraceae). The two plant species differ clearly in their flower morphology and are within the broad food spectra of O. bicornis and B. terrestris. Plants remained in the same position within the flight cages until they wilted (maximum 2 days). For each bee, we recorded the time that they interacted with a flower. In addition, we registered the number of visited flowers, their species identity, and the time between visits from one flower to the next (searching time) per 3-min period. We chose this constant time, because completed foraging flights representing the period that the bees were absent from the nest, with no resting phase but under constant foraging, were rare. To facilitate the recordings, bees were labeled with an individual color code (Revell, Bünde, Germany) on the mesonotum. Foraging behavior after neonicotinoid treatment was only performed with O. bicornis because similar studies with B. terrestris have previously been performed (see Stanley and Raine, 2016) and have not revealed any effects of neonicotinoid treatment on their foraging behavior.
Antennal Sensitivity of Osmia bicornis and Bombus terrestris
We performed Electroantennographic analysis (EAG) at Ulm University. EAG is a good method to show the summed receptor potential and thus the response to an odorant at the periphery of the olfactory system (Schiestl and Poll, 2002). We diluted two floral semiochemicals, namely 2-phenylethanol (99%, Sigma-Aldrich, St. Louis, MO, United States) and linalool (racemic mixture, 97%, Sigma-Aldrich, St. Louis, MO, United States), which are common occurring volatile organic compounds (VOCs) of flowers (Knudsen et al., 1993, 2006), to various concentrations in hexane. One μl of the respective compound was diluted in 999 μl hexane (98%, Merck, Darmstadt, Germany) to produce the first test solution (dilution of 10–3). For the following dilution stages, the first test solution (dilution of 10–3) served as a stock solution and was diluted, respectively, to obtain dilutions of 10–4, 10–5, 10–6, and 10–7. This series of five different dilution stages was only applied to O. bicornis. For B. terrestris, we only used three dilutions (10–3, 10–5, and 10–7) because preliminary studies had shown that differences in the antennal response were only found at a dilution of 10–3. Furthermore, we also tested ethyl palmitate (ethyl hexadecanoate, 99%; Sigma-Aldrich, St. Louis, MO, United States), which is a pheromone component in bumblebees (Rottler-Hoermann et al., 2016), at three concentrations (dilutions of 10–3, 10–5, and 10–7) as a third volatile. In social insects, in particular, pheromones play an important role and are indispensable in maintaining intra-colonial communication and the regulation and control of reproduction (Ayasse and Jarau, 2014). Hexane served as a control in all the experiments. All test solutions were stored in screw-cap micro-tubes (CZT, Kriftel, Germany) at –20°C.
For EAG analysis, we cut off the right antenna of a female bee (N = 40 for O. bicornis and B. terrestris, respectively) at the scapus with spring-scissors. Detached antennas were cut at the first and last segment of the flagellum with a razorblade. Using two micromanipulators (Märzhäuser Wetzlar GmbH & Co. KG, Wetzlar, Germany), we mounted each antenna between two borosilicate glass capillaries (GC150TF-10, Harvard Apparatus Ltd., Edenbridge, United Kingdom) filled with insect Ringer’s solution (5 g NaCl, 0.42 g KCl, and 0.19 g CaCl2 ⋅ 2H2O dissolved in 1 l demineralized water) and connected to gold electrodes. The electrode at the base of the antenna was grounded, while the electrode at the tip was connected to a signal acquisition controller (Intelligent Data Acquisition Controller IDAC 2, Ockenfels SYNTECH GmbH, Kirchzarten, Germany) to record differences in receptor potential. The antenna was placed in front of a glass tube that directed a humidified air stream (volume 30 ml/min) toward the antenna and prevented it from rapidly drying out. Scents were applied to the antenna in a constant order (2-phenylethanol, linalool, ethyl palmitate for B. terrestris) with increasing concentration, which means decreasing dilution, and starting with 2-phenylethanol. At the beginning, between the first and second scent compound being presented to O. bicornis, between the second and third scent compound being presented to B. terrestris and at the end of each test series, we applied hexane and air individually to the antenna in order to normalize data and to correct for possible losses in sensitivity over time. For the stimulus, 10 μl of the respective solution was added to a filter paper strip (VWR International, Leuven, Belgium) and, after evaporation of the solvent for 1 min, the filter paper was inserted into a Pasteur pipette (150 mm, Soda Lime Glass, VWR International, Darmstadt, Germany). For each stimulus measurement, the Pasteur pipette was connected via a silicone tube to a stimulus controller (Syntech Stimulus Controller CS-05, Ockenfels SYNTECH GmbH, Kirchzarten, Germany) that delivered air puffs (30 ms, 25 ml/s) onto the antenna. Antennal responses were analyzed by Syntech EAG software EAGPro (v 2.0, Syntech, Hilversum, Netherlands).
Statistical Analysis
All statistical analyses were conducted with R (version 3.5.2, R Core Team, 2018). We compared recorded data of the neonicotinoid group and the control group of foraging flights (number of flowers per foraging flight, time between two flowers, time per flower visit and time per C. biennis flower and Ranunculus spp. flower) using a Mann–Whitney U test, since the data were not normally distributed. For a comparison of antennal responses, we first normalized the responses by using EAGPro software to correct for possible changes in the sensitivity of an antenna. Response to hexane was set as a response of 100%, whereas all other responses were calculated as values relative to hexane and were log-transformed. We calculated linear mixed-effect models (LME) for each compound by using the lme function from the nlme package (version 3.1-137, Pinheiro et al., 2014). Treatment and concentration were set as fixed factors, individual as a random factor. We ran a post hoc test by using the function glht (General Linear Hypotheses) from the multcomp package (version 1.4-16, Hothorn et al., 2008). All model assumptions were validated and were sufficient. A t-test followed by a Benjamini–Hochberg correction was used to analyse the response to a certain compound at a certain concentration compared with hexane (100%). If one of the tested scent compounds at a certain concentration showed a significantly higher response than hexane, we assumed that the bees were able to detect that substance at that concentration (Brandt et al., 2017).
Results
Foraging Behavior
Clothianidin altered the foraging behavior in O. bicornis females (Figure 1 and Table 1). During a time period of 3 min, females treated with clothianidin visited fewer flowers than untreated bees (Figure 1A, Mann–Whitney U test: W1,21 = 97.5, p = 0.016) and the searching time was significantly longer (Figure 1B, Mann–Whitney U test: W1,21 = 9, p < 0.001). Clothianidin had no effect on the average handling time per flower (Mann–Whitney U test: W1,16 = 18, p = 0.093). Bees treated with clothianidin exhibited a significantly longer flower visiting time for Ranunculus spp. (Figure 1C, Mann–Whitney U test: W1,16 = 7, p = 0.006) as compared with C. biennis. For C. biennis flowers, flower handling time was the same for untreated and treated bees (Figure 1C, Mann–Whitney U test: W1,16 = 36, p = 1).
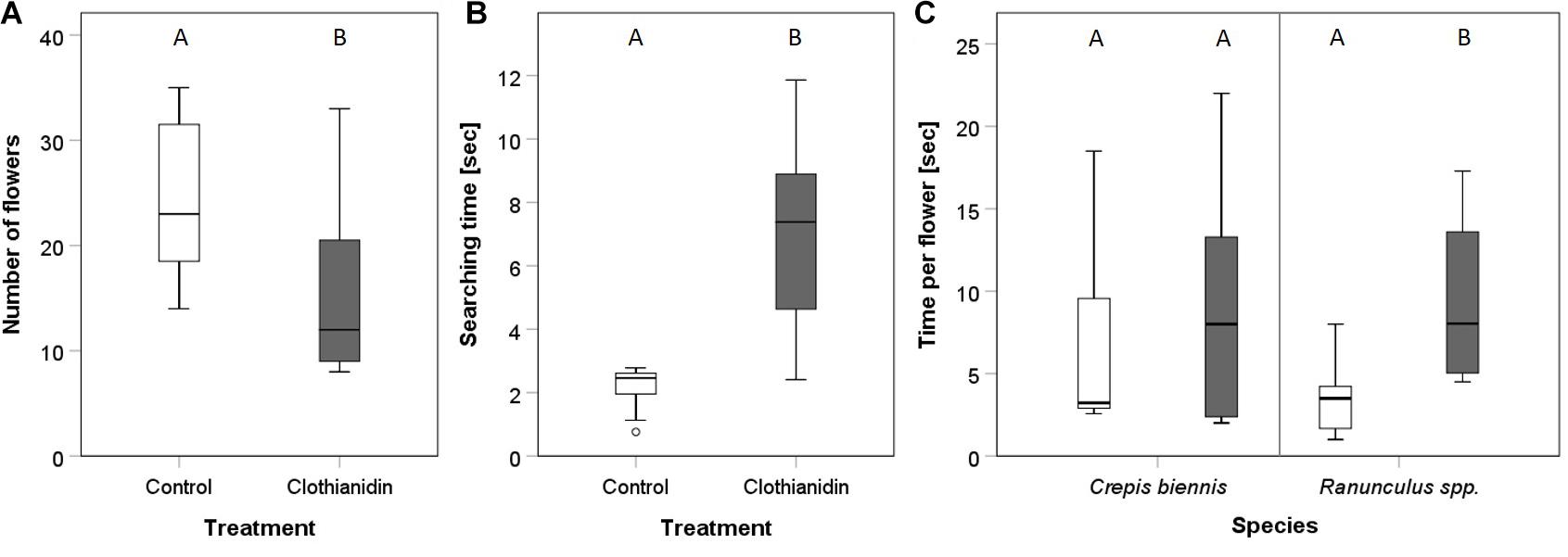
Figure 1. Comparison of the effect of clothianidin treatment on various parts of the foraging flights of Osmia bicornis. (A) Average number of visited flowers per bee. (B) Average time between two flowers (searching time) per bee. (C) Average time per flower separated for both plant species Crepis biennis and Ranunculus spp. Boxes represent the 1st and 3rd quartiles, the median is shown as a solid line. Whiskers show the confidence interval of 95%. Outliers are plotted as individual dots. Different capital letters indicate significant differences between the control (n = 11) and the clothianidin (n = 11) treatment groups.
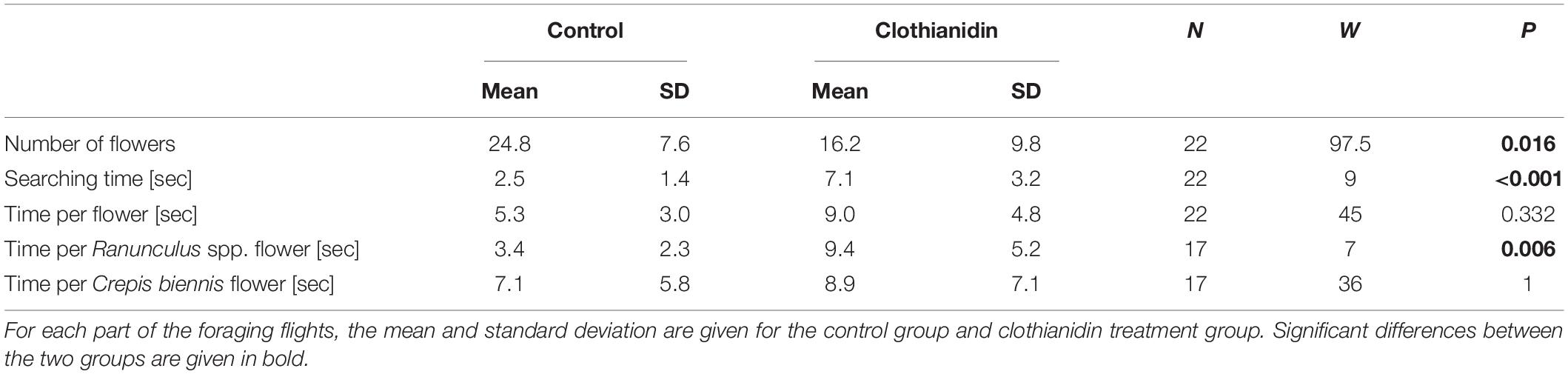
Table 1. Comparison of various parts of the foraging flights of clothianidin-treated and untreated Osmia bicornis females by means of t-tests.
Antennal Sensitivity
A comparison of antennal responses of O. bicornis to 2-phenylethanol and linalool revealed no treatment-dependent differences (Table 2). Antennal responses to 2-phenylethanol (LME: F1,4 = 434.448, p < 0.001) and linalool (LME: F1,4 = 467.743, p < 0.001) were significantly different for concentration (Table 2). Further, a combined effect of treatment and concentration was detected for 2-phenylethanol (LME: F1,4 = 3.861, p < 0.01). To validate whether a bee can detect a given compound at its respective concentration, we compared the antennal responses with the response to hexane, which was set 100% (Supplementary Table 2). For 2-phenylethanol, the responses to the dilutions 10–4 and 10–3 were significantly higher in both treatment groups compared with hexane (Figure 2). For the concentration 10–5, only the antennal response of the bees in the control group was higher than that for hexane (t-test: t19 = 3.3057, p < 0.01). Thus, bees without clothianidin treatment were able to detect these higher concentrations, whereas clothianidin-treated bees could not. Responses to the two lowest concentrations did not differ from hexane, either in the control group or in the treatment group. For linalool, the responses to the concentration 10–4 and 10–3 were also significantly higher in both treatment groups compared with hexane and thus were detectable by the bees, whereas the remaining concentrations were not (Figure 2).
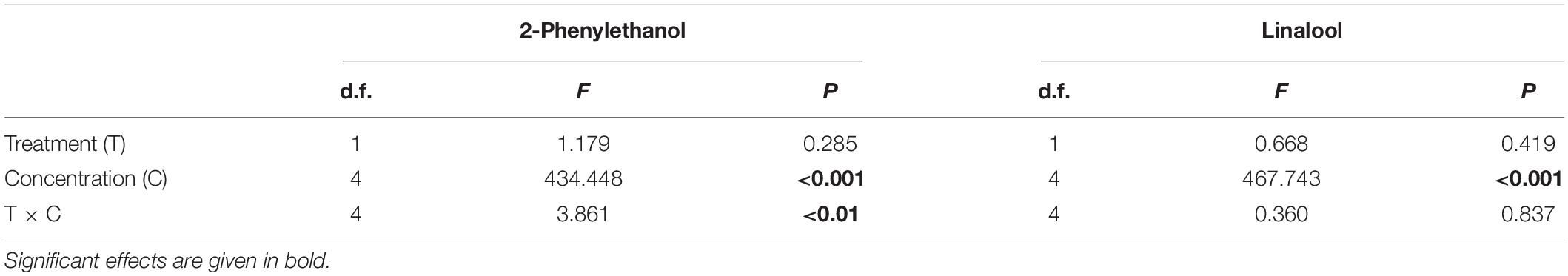
Table 2. Results of linear mixed-effect models (LME) for the antennal responses of clothianidin-treated O. bicornis females to two different scent compounds, namely 2-phenylethanol and linalool, in comparison with an untreated control.
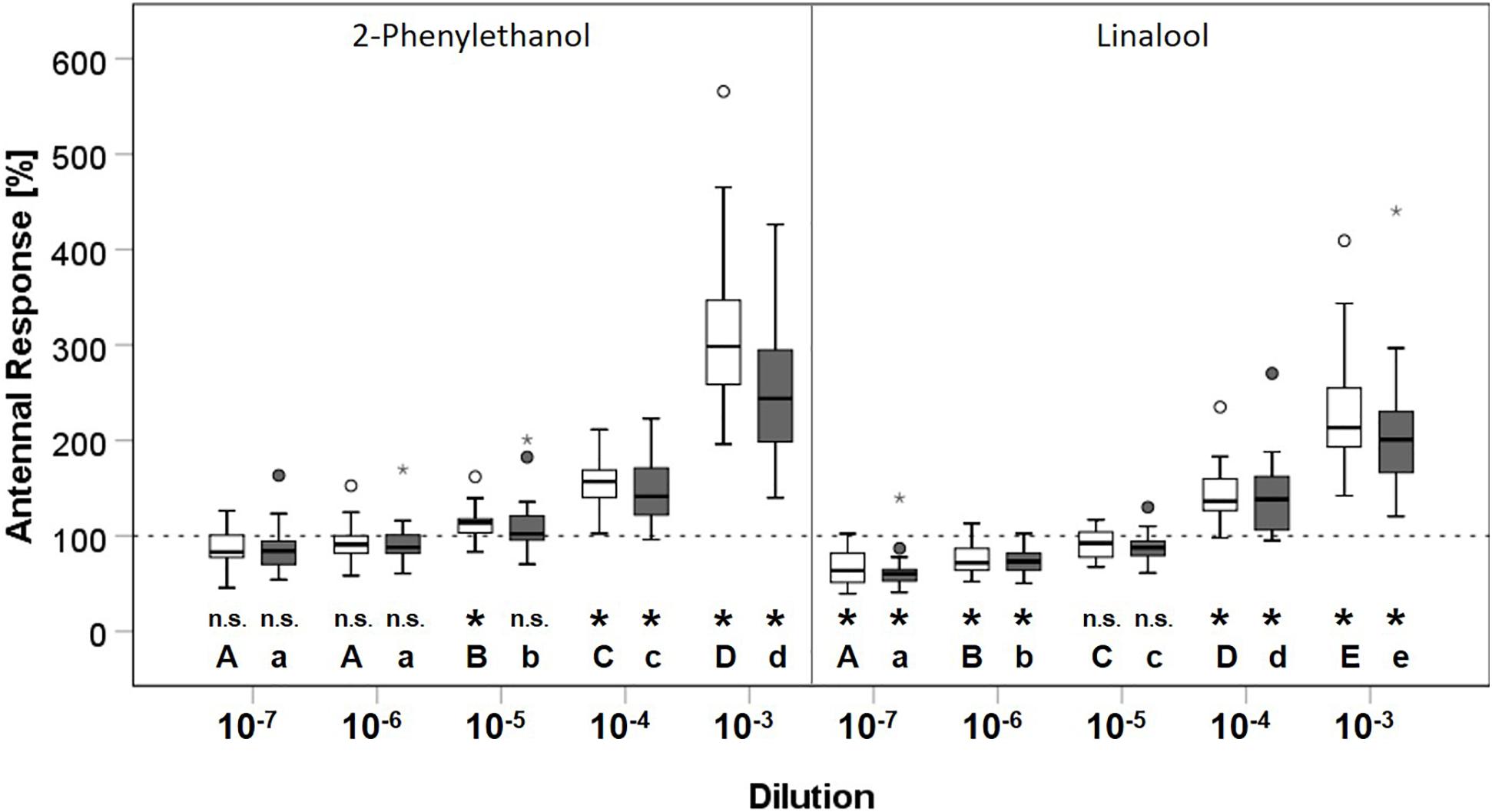
Figure 2. Comparison of the effect of clothianidin treatment on antennal responses of O. bicornis (N = 40) to 2-phenylethanol and linalool. White boxes represent the control group treated with acetone; the clothianidin treatment group is shown by gray boxes. Boxes represent the 1st and 3rd quartiles, the median is shown as a solid line. Whiskers show the minimum and maximum range of values no further than the 1.5-fold inter-quartile range from the respective hinge. Outliers are plotted as individual dots, extreme outliers as stars. Hexane (response 100%) is shown as a broken line. Different letters indicate significant differences between concentrations for each odor compound within the control (capital letters) or clothianidin (small letters) group. Asterisks (p < 0.05) indicate significant differences compared with hexane (n.s.: p > 0.05).
We found similar results for B. terrestris. Antennal responses revealed no treatment-dependent differences (Table 3). Antennal response differed significantly by concentration for 2-phenylethanol (LME: F1,2 = 71.895, p < 0.001), linalool (LME: F1,2 = 85.003, p < 0.001) and ethyl palmitate (LME: F1,2 = 11.851, p < 0.001). As for O. bicornis, an interactive effect of treatment and concentration on the antennal response was present for 2-phenylethanol (LME: F1,2 = 3.578, p < 0.05) and ethyl palmitate (LME: F1,2 = 4.32, p < 0.05). With regard to 2-phenylethanol and ethyl palmitate, the antennal response in the control group was significantly higher than that for hexane at all concentrations, whereas it was only significantly higher for the concentration 10–3 in bees of the treatment group (Figure 3 and Supplementary Table 4). Thus, B. terrestris without clothianidin treatment were able to detect 2-phenylethanol and ethyl palmitate at lower concentrations than clothianidin-treated bees. Linalool was only detectable in the highest concentration 10–3 in both groups, with and without clothianidin treatment.
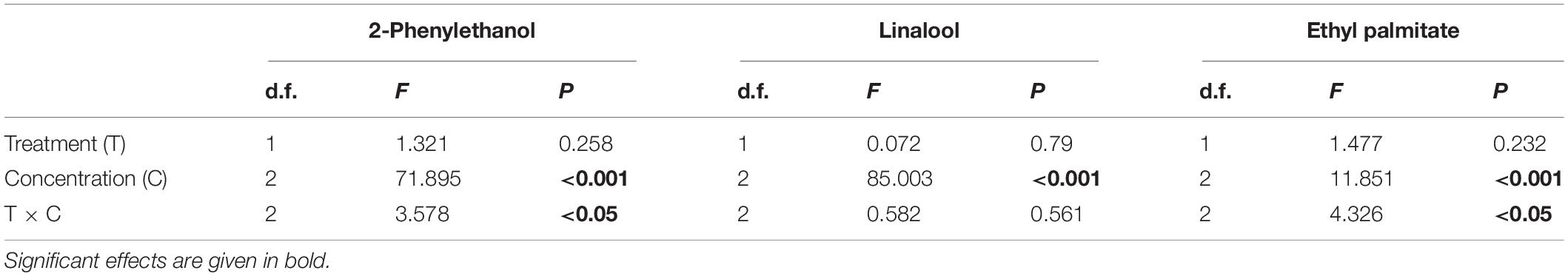
Table 3. Results of LME for the antennal responses of clothianidin-treated Bombus terrestris females to three different scent compounds, namely 2-phenylethanol, linalool, and ethyl palmitate, in comparison with an untreated control.
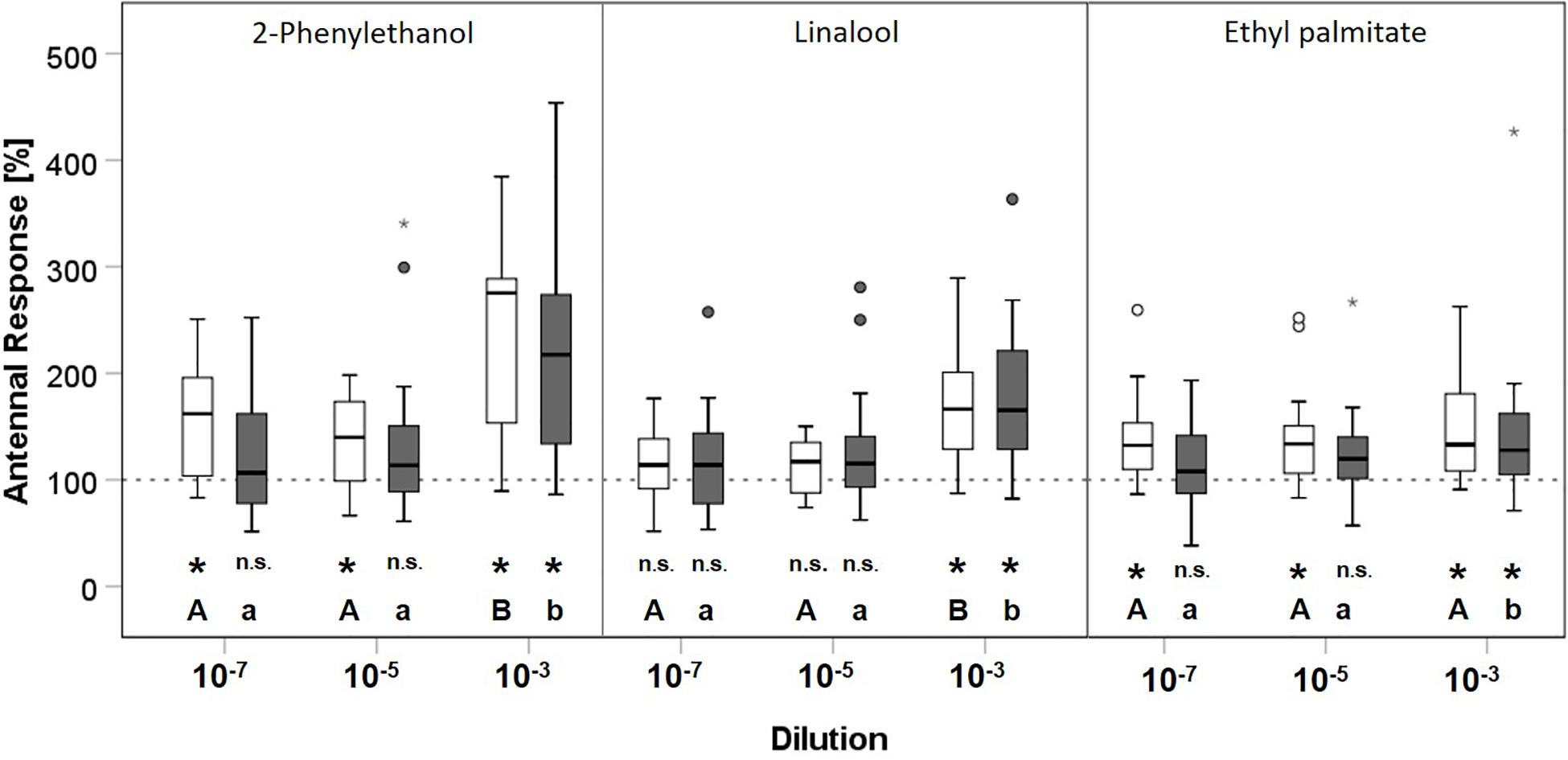
Figure 3. Comparison of the effect of clothianidin treatment on antennal responses of Bombus terrestris (N = 40) to 2-phenylethanol, linalool and ethyl palmitate. White boxes represent the control group treated with acetone; the clothianidin treatment group is shown by gray boxes. Boxes represent the 1st and 3rd quartiles, the median is shown as a solid line. Whiskers show the minimum and maximum range of values no further than the 1.5-fold inter-quartile range from the respective hinge. Outliers are plotted as individual dots, extreme outliers as stars. Hexane (response 100%) is shown as a broken line. Different letters indicate significant differences between concentrations for each odor compound within the control (capital letters) or clothianidin (small letters) group. Asterisks (p < 0.05) indicate significant differences compared to hexane (n.s.: p > 0.05).
Discussion
The results of our behavioral experiments showed that treatment with a field-realistic dose of clothianidin negatively affected the foraging behavior of O. bicornis. Treated bees visited significantly fewer flowers than the control group and exhibited a significantly longer searching time between two flowers and a longer visiting time on Ranunculus spp. flowers. The EAG analyses showed a decreased sensitivity of antennal scent receptors for 2-phenylethanol in O. bicornis and B. terrestris and in the sensitivity for ethyl palmitate in B. terrestris. Thus, bees treated with clothianidin on average were not able to detect 2-phenylethanol and ethyl palmitate at small concentrations, unlike bees that were not exposed to clothianidin.
Foraging Behavior
In our study, treated females spent a longer time on a flower and needed more time to reach the next flower resulting in lower visitation rates. Bees may need more time per flower, if they have problems with handling flowers, particularly while collecting pollen and nectar. A possible explanation is that bees have problems with learning how to manipulate flowers (Stanley and Raine, 2016). They face a blockage of memory retrieval (Jin et al., 2015) for learned handling strategies, thereby increasing their handling time for flowers. The lower flower visitation rates that we found in clothianidin-treated O. bicornis females in our study were also observed in a former investigation after the treatment of bees with thiamethoxam (Stanley et al., 2015; Stanley and Raine, 2016). Furthermore, if females try to gain the same amount of pollen per flower from a certain plant species, they may need more time to exploit a flower if they experience problems manipulating it. To test this, future studies should focus on pollen foraging efficiency after neonicotinoid treatment by weighing bees before and after pollen-collecting flights.
In our experiments, we used two different plant families, Asteraceae (C. biennis) and Ranuculaceae (Ranunculus spp.) in order to test whether the effect of clothianidin treatment is different depending on flower morphology. Bees treated with clothianidin spent more time handling Ranunculus flowers than bees in the control group. For C. biennis, which has composite flowers, handling time did not differ in our experiments. In Ranunculus spp. flowers, bees have to find the pollen in the center of each flower, whereas in the composite flowers of C. biennis, they can pick pollen from the whole flower head. This shows that bees have problems in handling flowers, depending on the complexity of a flower. A more than two-fold increase in the flower visiting time of B. terrestris after neonicotinoid treatment was observed in a study using the complex flowers of Lotus corniculatus (Stanley and Raine, 2016). Further, the authors mentioned that bumble bees experimentally exposed to a chronic dose of thiamethoxam learnt how to manipulate these complex flowers much more slowly than untreated bees.
In addition to the longer handling time of flowers by treated O. bicornis females, our study clearly showed that the time for searching for a new flower was also increased. To visit a further food source, bees have to fly from one flower to another, if these are not arranged in inflorescences. One reason for the increasing searching time is a disruption of the physical ability to fly. Tosi et al. (2017) have described an alteration in flight ability in honeybees after thiamethoxam treatment, which leads to decreased flight duration, distance and velocity. In further studies, honeybees treated with neonicotinoids also showed less well directed flights; this is a possible explanation for an increase in searching time and thus a decrease in the number of visited flowers (van der Sluijs et al., 2013; Fischer et al., 2014; Tison et al., 2016). In particular, the homing flights of honeybees and thus their navigation were affected in these studies. The longer searching times shown by our treated bees also suggest that their navigation skills are reduced within their three-dimensional surroundings. Disorientation after treatment with neonicotinoids might be the result of disturbed memory and learning behavior as shown in former studies (Desneux et al., 2007; Fent, 2013; van der Sluijs et al., 2013). Because flowers with nectar and pollen are an unreliable food source, bees rely on their memory to find good resources (Gross, 2013). Bees also clearly use floral traits such as color, shape and scent plus landmarks to find valuable food sources such as flowers (Gross, 2013; Knauer and Schiestl, 2015). Thus, a blockage of memory retrieval for learned cues that guide the bee to a food source might increase searching time (Jin et al., 2015). The effects of the increasing handling time and increasing searching time result in an increase of the total time of a foraging flight and, thus, the risk of pollinators being confronted with potential predators also increases. The reason for disturbed memory and olfactory learning might be a disturbance of the mushroom bodies, which are important in olfactory learning and which have been shown to be reduced in volume after neonicotinoid treatment (Heisenberg, 1998; Rybak and Menzel, 2010; Tomé et al., 2012; Fent, 2013). However, we have not investigated this aspect, because we have focused on the antennal receptors and not on higher brain structures in our study. Bees use floral scents to find flowers as a nectar and pollen source. Problems in finding new flowers, leading to increased searching times between two flowers, probably arise because of the effect of decreased antennal sensitivity, as we have found for certain of the tested chemical volatile compounds.
Antennal Sensitivity
Our result showed an effect of clothianidin on the antennal sensitivity in O. bicornis and B. terrestris for certain of the tested compounds that included not only typical floral volatiles (Knudsen et al., 1993, 2006) but also pheromone components (Rottler-Hoermann et al., 2016). Bees treated with clothianidin were unable to detect 2-phenylethanol and ethyl palmitate in small concentrations, unlike bees that had not been exposed to clothianidin. Scent plays an important role in the finding of host plants and also serves as a cue in long-range attraction (Dötterl and Schäffler, 2007; Burger et al., 2010). Thus, it is crucial for bees to be able to detect low concentrations of floral scent compounds. A decreased sensitivity towards floral volatiles might lead to disturbances in the finding of host plants in both our studied species. If bees cannot find flowers or at least have problems locating them, they will not find appropriate amounts of pollen, a resource that plays and important role affecting the fitness of bees (Radmacher and Strohm, 2010). With regard to bumble bees, we should also mention disturbances in pheromone perception, since pheromones are crucial for intracolonial communication (Ayasse and Jarau, 2014), the disruption of which can lead to severe changes in colony maintenance and the stability of the colony. To the best of our knowledge, this is the first study that has shown an effect of neonicotinoids on antennal sensitivity in bees. In contrast to our findings, Artz and Pitts-Singer (2015) detected no changes in antennal sensitivity after fungicide treatment in O. lignaria. However, they used a different treatment mode involving nocturnal spray applications of their plants. In their approach, bees do not come into direct contact with the pesticide but take it up via nectar or pollen. This shows clearly that treatment modes can differ from each other in their effects on pollinators.
However, a study by Hesselbach and Scheiner (2018) has revealed a loss in taste sensitivity in honeybees after treatment with flupyradifurone, which binds nAChR similarly to neonicotinoids. Because neonicotinoids target receptors in the insect nervous system, receptors at the antennal level might also be affected and, thus, antennal sensitivity might be reduced. Comparing the substance classes of 2-phenylethanol (a benzenoid) and linalool (a monoterpene), clothianidin only reduced antennal sensitivity for 2-phenylethanol indicating that it might affect different scent receptor classes differently. To test this possibility, a broader range of scent compounds from various common substance classes should be tested in further approaches. As our EAG investigations compare the summed receptor potential per antenna they offer good evidence for the strength of total neurological activity within the antenna. Although we cannot identify single neuron activity, it is clearly seen that there is a difference in comparison to the control. In order to investigate the effects of clothianidin on the olfactory-receptor-neuron processing system at higher brain levels (e.g., antennal lobe and mushroom bodies), as suggested by Artz and Pitts-Singer (2015), it would be necessary to perform single cell recordings of peripheral olfactory neurons or calcium imaging.
Conclusion
Our study has clearly shown that clothianidin impairs the foraging behavior of O. bicornis and the antennal sensitivity of O. bicornis and B. terrestris. Since we have used field-realistic doses of clothianidin, we can expect similar effects in field populations of pollinating wild bees. The effect of neonicotinoids and other insecticides is probably twofold. On one hand, the ecosystem service of pollination provided by bees is negatively affected; in particular, the chances of flowers being pollinated decrease and, consequently, the number of fruits or seeds produced also decreases. On the other hand, in the longer term, a decrease in the biodiversity and abundance of wild bee populations can be expected, since disturbed foraging behavior will also affect the number of progeny and therefore the reproductive success of bees. However, our results in two pollinator species cannot necessarily be extrapolated to other pollinator groups, which might show different responses to insecticides. Thus, we need urgently to study of a variety of pollinators and pollinator groups (such as solitary bees, bumblebees, or even hoverflies), and not only honeybees.
In nature, bees collect pollen and nectar from several plants and fields, all possibly treated with a variety of pesticides over several days or weeks. Bees are therefore exposed to mixtures of agrochemicals over long periods of time. Thus, the effects of pesticides under real life conditions might be much more drastic than those determined in our study.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
FS, IJO, JK, and MA designed the experiments. FS, IJO, and JK performed the experiments. FS and MA wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the staff of the Botanical Garden Ulm for their help during the flight cage experiments and to Elisabeth Arnold and Elke Stamm for their support in the laboratory. Further, we thank Theresa Jones for linguistic advice and Jonas Kuppler for statistical advice and helpful comments that improved this manuscript. We also thank two independent reviewers for their helpful comments that greatly improved this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.697355/full#supplementary-material
References
Artz, D. R., and Pitts-Singer, T. L. (2015). Effects of fungicide and adjuvant sprays on nesting behavior in two managed solitary bees, osmia lignaria and megachile rotundata. PloS One 10:1371. doi: 10.1371/journal.pone.0135688
Ayasse, M., and Jarau, S. (2014). Chemical ecology of bumble bees. Annu. Rev. Entomol. 59, 299–319. doi: 10.1146/annurev-ento-011613-161949
Botías, C., David, A., Horwood, J., Abdul-Sada, A., Nicholls, E., Hill, E., et al. (2015). Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 49, 12731–12740. doi: 10.1021/acs.est.5b03459
Brandt, K., Dötterl, S., Francke, W., Ayasse, M., and Milet-Pinheiro, P. (2017). Flower visitors of campanula: are oligoleges more sensitive to host-specific floral scents than polyleges? J. Chem. Ecol. 43, 4–12. doi: 10.1007/s10886-016-0802-z
Burger, H., Dötterl, S., and Ayasse, M. (2010). Host-plant finding and recognition by visual and olfactory floral cues in an oligolectic bee. Funct. Ecol. 24, 1234–1240. doi: 10.1111/j.1365-2435.2010.01744.x
Casida, J. E., and Durkin, K. A. (2013). Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 58, 99–117. doi: 10.1146/annurev-ento-120811-153645
Desneux, N., Decourtye, A., and Delpuech, J. M. (2007). The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106. doi: 10.1146/annurev.ento.52.110405.091440
Dötterl, S., and Schäffler, I. (2007). Flower scent of floral oil-producing lysimachia punctata as attractant for the oil-bee macropis fulvipes. J. Chem. Ecol. 33, 441–445. doi: 10.1007/s10886-006-9237-2
Dötterl, S., and Vereecken, N. J. (2010). The chemical ecology and evolution of bee-flower interactions: a review and perspectives. Can. J. Zool. 88, 668–697. doi: 10.1139/Z10-031
El Hassani, A. K., Dacher, M., Gary, V., Lambin, M., Gauthier, M., and Armengaud, C. (2008). Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera). Arch. Environ. Contam. Toxicol. 54, 653–661. doi: 10.1007/s00244-007-9071-8
Elbert, A., Haas, M., Springer, B., Thielert, W., and Nauen, R. (2008). Applied aspects of neonicotinoid uses in crop protection. Pest. Manage. Sci. 64, 1099–1105. doi: 10.1002/ps.1616
Feltham, H., Park, K., and Goulson, D. (2014). Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology. 23, 317–323. doi: 10.1007/s10646-014-1189-7
Fischer, J., Müller, T., Spatz, A.-K., Greggers, U., Grünewald, B., and Menzel, R. (2014). Neonicotinoids interfere with specific components of navigation in honeybees. PloS One 9:91364. doi: 10.1371/journal.pone.0091364
Godfray, H. C. J., Blacquière, T., Field, L. M., Hails, R. S., Petrokofsky, G., Potts, S. G., et al. (2014). A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. Biol. Sci. 281:558. doi: 10.1098/rspb.2014.0558
Goulson, D., Nicholls, E., Botías, C., and Rotheray, E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957. doi: 10.1126/science.1255957
Gross, M. (2013). EU ban puts spotlight on complex effects of neonicotinoids. Curr. Biol. 23, 462–464. doi: 10.1016/j.cub.2013.05.030
Gruber, B., Eckel, K., Everaars, J., and Dormann, C. F. (2011). On managing the red mason bee (Osmia bicornis) in apple orchards. Apidologie. 42, 564–576. doi: 10.1007/s13592-011-0059-z
Hallmann, C. A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N., Schwan, H., et al. (2017). More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PloS One 12:1371. doi: 10.1371/journal.pone.0185809
Heisenberg, M. (1998). What do the mushroom bodies do for the insect brain? An introduction. Learn Mem. 5, 1–10. doi: 10.1101/lm.5.1.1
Hesselbach, H., and Scheiner, R. (2018). Effects of the novel pesticide flupyradifurone (Sivanto) on honeybee taste and cognition. Sci. Rep. 28, 354–366. doi: 10.1038/s41598-018-23200-0
Hopwood, J., Vaughan, M., Shepherd, M., Biddinger, D., Mader, E., Black, S. H., et al. (2012). Are neonicotinoids killing bees: a review of research into the effects of neonicotinoid insecticides on bees, with recommendations for action. Portland: The Xerces Society for Invertebrate Conservation.
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical J. 50, 346–363. doi: 10.1002/bimj.200810425
Jeschke, P., and Nauen, R. (2008). Neonicotinoids-from zero to hero in insecticide chemistry. Pest. Manage. Sci. 64, 1084–1098. doi: 10.1002/ps.1631
Jin, N., Klein, S., Leimig, F., Bischoff, G., and Menzel, R. (2015). The neonicotinoid clothianidin interferes with navigation of the solitary bee Osmia cornuta in a laboratory test. J. Exp. Biol. 218, 2821–2825. doi: 10.1242/jeb.123612
Kaib, M. (2003). “Chemorezeption,” in Lehrbuch der Entomologie: Teil 1, eds K. Dettner and W. Peters (Berlin: Spektrum Akademischer Verlag), 302–320.
Knauer, A. C., and Schiestl, F. P. (2015). Bees use honest floral signals as indicators of reward when visiting flowers. Ecol. Lett. 18, 135–143. doi: 10.1111/ele.12386
Knudsen, J. T., Eriksson, R., Gershenzon, J., and Ståhl, B. (2006). Diversity and distribution of floral scent. Bot. Rev. 72, 1–120. doi: 10.1663/0006-8101200672
Knudsen, J. T., Tollsten, L., and Bergström, L. (1993). Floral scents—a checklist of volatile compounds isolated by head-space techniques. Phytochemistry. 33, 253–280. doi: 10.1016/0031-9422(93)85502-I
Matsuda, K., Buckingham, S. D., Kleier, D., Rauh, J. J., Grauso, M., and Sattelle, D. B. (2011). Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 22, 573–580. doi: 10.1016/S0165-6147(00)01820-4
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., and Team, R. C. (2014). nlme: Linear and nonlinear mixed effects models. R package version 3.1-117. http://CRAN.R-project.org/package=nlme.
Potts, S. G., Biesmeijer, J. C., Kremen, C., Neumann, P., Schweiger, O., and Kunin, W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
R Core Team. (2018). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Radmacher, S., and Strohm, E. (2010). Factors affecting offspring body size in the solitary bee Osmia bicornis (Hymenoptera, Megachilidae). Apidologie 41, 169–177. doi: 10.1051/apido/2009064
Rottler-Hoermann, A.-M., Schulz, S., and Ayasse, M. (2016). Nest wax triggers worker reproduction in the bumblebee Bombus terrestris. R. Soc. Open Sci. 3:599. doi: 10.1098/rsos.150599
Rundlöf, M., Andersson, G. K. S., Bommarco, R., Fries, I., Hederström, V., Herbertsson, L., et al. (2015). Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80. doi: 10.1038/nature14420
Rybak, J., and Menzel, R. (2010). “Mushroom Body of the Honeybee,” in Handbook of Brain Microcircuits, eds G. M. Shepherd and S. Grillner (Oxford: Oxford University Press), 433–438. doi: 10.1093/med/9780195389883.003.0044
Schiestl, F. P. (2015). Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytol. 206, 571–577. doi: 10.1111/nph.13243
Schiestl, F. P., and Poll, F. M. (2002). “Detection of physiologically active flower volatiles using gas chromatography coupled with electroantennography,” in Molecular methods of plant analysis, volume 21: analysis of taste and aroma, eds J. F. Jackso, H. F. Linskens, and R. Inman (Berlin: Springer), 173–198. doi: 10.1007/978-3-662-04857-3_9
Scott-Dupree, C. D., Conroy, L., and Harris, C. R. (2009). Impact of Currently Used or Potentially Useful Insecticides for Canola Agroecosystems on Bombus impatiens (Hymenoptera: Apidae), Megachile rotundata (Hymentoptera: Megachilidae), and Osmia lignaria (Hymenoptera: Megachilidae. J. Econ. Entomol. 102, 177–182. doi: 10.1603/029.102.0125
Seidelmann, K. (2014). Optimal progeny body size in a solitary bee, Osmia bicornis (Apoidea: Megachilidae. Ecol. Entomol. 39, 656–663. doi: 10.1111/een.12145
Sgolastra, F., Tosi, S., Medrzycki, P., Porrini, C., and Burgio, G. (2015). Toxicity of Spirotetramat on Solitary Bee Larvae, Osmia cornuta (Hymenoptera: Megachilidae), in Laboratory Conditions. J. Apic. Sci. 59:24. doi: 10.1515/jas-2015-0024
Stanley, D. A., Garratt, M. P. D., Wickens, J. B., Wickens, V. J., Potts, S. G., and Raine, N. E. (2015). Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528, 548–550. doi: 10.1038/nature16167
Stanley, D. A., and Raine, N. E. (2016). Chronic exposure to a neonicotinoid pesticide alters the interactions between bumblebees and wild plants. Funct. Ecol. 30, 1132–1139. doi: 10.1111/1365-2435.12644
Stokstad, E. (2013). Pesticides under fire for risks to pollinators. Science 340, 674–676. doi: 10.1126/science.340.6133.674
Tan, K., Chen, W., Dong, S., Liu, X., Wang, Y., and Nieh, J. C. (2014). Imidacloprid alters foraging and decreases bee avoidance of predators. PloS One 9:2725. doi: 10.1371/journal.pone.0102725
Tappert, L., Pokorny, T., Hofferberth, J., and Ruther, J. (2017). Sublethal doses of imidacloprid disrupt sexual communication and host finding in a parasitoid wasp. Sci. Rep. 7:42756. doi: 10.1038/srep42756
Tison, L., Hahn, M.-L., Holtz, S., Rößner, A., Greggers, U., Bischoff, G., et al. (2016). Honey Bees’ behavior is impaired by chronic exposure to the neonicotinoid thiacloprid in the field. Environ. Sci. Technol. 50, 7218–7227. doi: 10.1021/acs.est.6b02658
Tomé, H. V. V., Martins, G. F., Lima, M. A. P., Campos, L. A. O., and Guedes, R. N. C. (2012). Imidacloprid-induced impairment of mushroom bodies and behavior of the native stingless bee Melipona quadrifasciata anthidioides. PloS One 7:6. doi: 10.1371/journal.pone.0038406
Tomizawa, M., and Casida, J. E. (2005). Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 45, 247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930
Tosi, S., Burgio, G., and Nieh, J. C. (2017). A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 7:1361-8. doi: 10.1038/s41598-017-01361-8
Tsvetkov, N., Samson-Robert, O., Sood, K., Patel, H. S., Malena, D. A., Gajiwala, P. H., et al. (2017). Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 356, 1395–1397. doi: 10.1126/science.aam7470
Uneme, H. (2011). Chemistry of clothianidin and related compounds. J. Agric. Food Chem. 59, 2932–2937. doi: 10.1021/jf1024938
van der Sluijs, J. P., Simon-Delso, N., Goulson, D., Maxim, L., Bonmatin, J.-M., and Belzunces, L. P. (2013). Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 5, 293–305. doi: 10.1016/j.cosust.2013.05.007
Westrich, P., and Dathe, H. H. (1997). Die bienenarten deutschlands (Hymenoptera, Apidae): ein aktualisiertes verzeichnis mit kritischen anmerkungen. Mitt. Entomol. Verein Stuttg. 32, 3–34.
Williamson, S. M., Willis, S. J., and Wright, G. A. (2014). Exposure to neonicotinoids influences the motor function of adult worker honeybees. Ecotoxicology 23, 1409–1418. doi: 10.1007/s10646-014-1283-x
Keywords: Osmia bicornis, Bombus terrestris, neonicotinoid, clothianidin, foraging flight, antennal sensitivity
Citation: Straub F, Orih IJ, Kimmich J and Ayasse M (2021) Negative Effects of the Neonicotinoid Clothianidin on Foraging Behavior and Antennal Sensitivity in Two Common Pollinator Species, Osmia bicornis and Bombus terrestris. Front. Ecol. Evol. 9:697355. doi: 10.3389/fevo.2021.697355
Received: 19 April 2021; Accepted: 16 August 2021;
Published: 03 September 2021.
Edited by:
Sara Diana Leonhardt, Technical University of Munich, GermanyReviewed by:
Denis Thiery, Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement (INRAE), FranceJeremy N. McNeil, Western University, Canada
Copyright © 2021 Straub, Orih, Kimmich and Ayasse. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manfred Ayasse, bWFuZnJlZC5heWFzc2VAdW5pLXVsbS5kZQ==
 Florian Straub
Florian Straub Ihotu Joy Orih
Ihotu Joy Orih Manfred Ayasse
Manfred Ayasse