
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Ecol. Evol., 30 July 2021
Sec. Behavioral and Evolutionary Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.682504
This article is part of the Research TopicFrom Ecology to Cancer Biology and Back AgainView all 18 articles
 Joy Peplinski1*
Joy Peplinski1* Margaret A. Malone1
Margaret A. Malone1 Katherine J. Fowler1
Katherine J. Fowler1 Emily J. Potratz1
Emily J. Potratz1 Alexander G. Pergams1
Alexander G. Pergams1 Kristie L. Charmoy1
Kristie L. Charmoy1 Kiran Rasheed2
Kiran Rasheed2 Stanislav S. Avdieiev3
Stanislav S. Avdieiev3 Christopher J. Whelan1,2
Christopher J. Whelan1,2 Joel S. Brown1,3
Joel S. Brown1,3In nature, many multicellular and unicellular organisms use constitutive defenses such as armor, spines, and noxious chemicals to keep predators at bay. These defenses render the prey difficult and/or dangerous to subdue and handle, which confers a strong deterrent for predators. The distinct benefit of this mode of defense is that prey can defend in place and continue activities such as foraging even under imminent threat of predation. The same qualitative types of armor-like, spine-like, and noxious defenses have evolved independently and repeatedly in nature, and we present evidence that cancer is no exception. Cancer cells exist in environments inundated with predator-like immune cells, so the ability of cancer cells to defend in place while foraging and proliferating would clearly be advantageous. We argue that these defenses repeatedly evolve in cancers and may be among the most advanced and important adaptations of cancers. By drawing parallels between several taxa exhibiting armor-like, spine-like, and noxious defenses, we present an overview of different ways these defenses can appear and emphasize how phenotypes that appear vastly different can nevertheless have the same essential functions. This cross-taxa comparison reveals how cancer phenotypes can be interpreted as anti-predator defenses, which can facilitate therapy approaches which aim to give the predators (the immune system) the upper hand. This cross-taxa comparison is also informative for evolutionary ecology. Cancer provides an opportunity to observe how prey evolve in the context of a unique predatory threat (the immune system) and varied environments.
Most organisms experience the risk of mortality from predators. In response to this risk, natural selection has imbued prey with a strikingly broad range of anti-predator behaviors, physiologies, and morphologies. One ubiquitous anti-predator defense is fleeing, whether by the legs of a gazelle, the wings of a grasshopper, the flick of a lobster’s tail, the hydro-jet propulsion of an octopus, or the flagellum of a single-celled ciliate. Escape into burrows or to refugia inaccessible to predators also provides safety for many would-be prey, including marine species that escape into rocky interstices to escape predatory fish or into sand to escape shorebirds. Camouflage is another defense pervasive across taxa, which allows prey as diverse as stick-insects, octopuses, and nightjars to blend imperceptibly into the background. In almost all cases, the aforementioned adaptations entail a tradeoff between safety and foraging (McNamara and Houston, 1987; Lima and Dill, 1990; Brown and Kotler, 2004). That is, while fleeing or remaining hidden, an organism must cease feeding; and while actively feeding, it becomes vulnerable.
An intriguing subset of anti-predator adaptations minimizes this tradeoff between foraging and safety, allowing prey to carry on with fitness-enhancing activities even after they are detected by predators. Such defenses include armor, spines, and noxious chemicals. Consider a pangolin digging and foraging at a termite mound. When approached by a lion, it does not have to flee the scene or take refuge. Instead, it can continue foraging, waiting until virtually the last moment of the lion’s approach before curling into a ball and defending itself. This provides the pangolin with valuable foraging time. It can remain next to the termite mound and resume foraging as soon as the immediate threat subsides. Critically, many predators will not even attempt to attack the pangolin because of the excessive amount of handling time and effort that would be required to circumvent the armor. This deterrent attribute of armor, spines, and noxiousness may be equally or more important to the prey than the capability of reducing predator lethality in the event of attack.
Here, we focus on the deterrent functions of armor, spines, and noxiousness, which differ somewhat from some other classifications of prey defenses which focus on when in the “predation cycle” a defense is effective (e.g., Jeschke, 2006). We agree that armor affects the prey search step because it increases predator handling time, as well as the final meal step because it decreases predator lethality. Further, we expect that armor will act as a predation deterrent, which should reduce the likelihood of attack. This also applies to spiny defenses. While noxiousness, specifically in the form of toxins, should affect the search step because it increases predator digestion time (Jeschke, 2006), we emphasize that toxins and other forms of noxiousness will serve as deterrents to attack. Warning signals have been identified as affecting the likelihood of attack (Jeschke, 2006), but warning signals are only as useful as the dangerous defenses with which they are associated. As long as predators are foraging optimally, they should be somewhat or completely deterred from attacking armored, spiny, and noxious prey.
Just about all major taxa of living things, from bacteria to single-celled eukaryotes to invertebrates to vertebrates, have members exhibiting armor, spines, noxious chemicals, or combinations of these adaptations (Stankowich and Campbell, 2016; Pančić and Kiørboe, 2018; Klompmaker et al., 2019; Sugiura, 2020). In some cases, possession of one of these adaptations might mean that the other two are unnecessary or can be less pronounced. Slugs without shells or spines may be quite noxious and even poisonous, while snails with shells or sea urchins with their spines are generally not (Lindquist, 2002). In other cases, possessing one of these adaptations, such as armor, may actually amplify the advantages of possessing spines or noxious chemicals (Rice, 1985). This might explain why so many species possess two or all three of these adaptations. Across life forms, armor, spines, and noxiousness display wonderful examples of parallel and convergent evolution.
Though variations of these adaptations are seen across taxa, species exhibiting armor, spines, or noxiousness are usually the exception rather than the rule. This is probably because these adaptations tend to be permanent and costly, more-so than strategies such as camouflage, fleeing, and fixed activity schedules (e.g., nocturnality), which are more common. The production and maintenance of armor, spines, and noxious chemicals all incur an extra energetic and nutritional cost. Exaggerated armor also renders an organism heavier, clumsy, and inflexible. Spines encumber an organism’s movements by dragging and snagging on obstacles in the environment. Noxious defenses require the maintenance of specialized physiologies, organs, or diets even if the chemicals are not constantly deployed. Since these defenses are partially to entirely constitutive, if predators are never or rarely encountered, possessing these defenses would be excessively taxing and maladaptive. But, if predators are ever-present, even a costly constitutive defense would be more of an asset than a burden. By allowing would-be prey to continue fitness-enhancing activities in the proximity of aware predators, it essentially gives the prey more enemy-safe space.
Now, consider cancer cells inhabiting their tumor ecosystem. Cancer cells also suffer a form of predation: from the host’s immune system. In fact, cancer’s ability to evade the immune system may be among its most necessary and ubiquitous features. Immune evasion ranks as a hallmark of cancer (Hanahan and Weinberg, 2011; Fouad and Aanei, 2017). How does this come about?
It might seem that upon initiation the cancer cell would already be immune-evasive, possessing near-identical properties to its progenitor normal cells. But, in transitioning from being part of the whole organism to becoming its own unit of selection, the cancer cell must modify or dispense with several of the properties of normal cells. In becoming its own organism, it must resist programmed cell death, ignore anti-growth signaling and tissue control, and achieve proliferative immortality. Once the cell has become a cancer, natural selection favors adaptations that modify or upregulate intra-cellular metabolic pathways, cell-cell signaling processes, nutrient transporters and membrane pumps, and self-sufficiency in growth factors (Brown, 2016). Heritable variation available to natural selection occurs in cancers via mutations, fixed epigenetic changes, chromosomal rearrangements, copy number variation, and aneuploidy brought on by actual cell fusion or by incomplete cell division (Nam et al., 2020; Pienta et al., 2020b). To be more successful at acquiring nutrients, occupying space, and outcompeting other cancer cells they present antigens to the immune system (Houghton, 1994). In particular, some adaptations of cancer cells result in neo-antigens, novel proteins, and molecules absent from normal cells (Lee et al., 2018). Any of these cancer cell adaptations may invite attack from the immune system. To survive, cancer cells must evolve effective immune evasion (Vinay et al., 2015).
Studies of anti-predator adaptations in nature and cancer have developed along somewhat separate lines. Much of this difference results from different interests and goals. However, approaches play a large part. Much research has been dedicated to understanding how cancer cells become resistant to the immune system, with the aim of developing immunotherapies to bolster immune system attacks on cancer (Oiseth and Aziz, 2017). A large portion of this work has focused on genes, proteins, and metabolic and signaling pathways that permit cancer cells to avoid detection, attack, or even any response by immune cells. Such genes, proteins, and pathways provide targets for developing chemo- and immunotherapies (Esfahani et al., 2020; Tan et al., 2020). Less has been studied regarding the categories of “anti-predator” behaviors, physiologies, and morphologies of cancer cells. Genes or molecular pathways in cancer may be identified as immunosuppressive without us having knowledge of why the individual cancer cells are in less danger because of these adaptations. Our limited understanding of cancer cell-immune system predator-prey interactions hinders our capacity to anticipate cancer cell responses to altered tissue environments, cell communities, and treatment regimes.
Here we take an ecological perspective on the evolution and utility of different anti-predator adaptations in nature and in cancer (cancer is also a part of nature, but for purposes of terminology we will use “nature” as a shorthand for all other organisms other than cancer). Our reference to the “ecology of fear” is based on the premise that fear is an adaptation for assigning a cost to activities that incur a risk of injury or death. In response to threatening immune cells, cancer cells may be able to evolve some degree of fleeing, hiding, or camouflaging like prey seen in nature, but probably not to the same degree. Furthermore, we shall argue that cancer cells must be able to maintain foraging and proliferation activities in the presence of potentially lethal immune cells. Cancer cells likely enjoy very little enemy-free space. We argue that cancer cells should and do evolve the equivalence of armor, spines, and noxiousness. In fact, these may be some of the most advanced and important adaptations of cancers, evolving again and again in parallel and as convergent evolution from patient to patient, from tumor to tumor within a patient, and perhaps even multiple times among the cancer cells of a tumor.
In what follows, we begin with a discussion of mammals because these species are likely familiar to readers across disciplines; for them, spines, armor and noxiousness are literal both in terms of form and function. We then proceed to describe anti-predator defenses in fishes, insects, and microorganisms; the goal being to transition to taxa that become gradually more similar to single-celled cancer organisms. In this overview, we categorize defenses as being armor-like, spine-like, or noxious. We use these terms because they are easily recognizable in well-known species (e.g., mammals), and therefore serve as convenient references–shorthand, if you will–when we describe systems and traits that are perhaps less familiar to many readers. We categorize defenses into the categories of armor, spines, and noxiousness primarily based on their functions, starting with mammalian examples as a model. We take this approach to emphasize the convergent functionality (more-so than appearance) of defenses across taxa.
Next, we briefly detail how the immune system poses threats to cancer cells in terms of types of immune cells, their activation and proliferation, and how they actually kill cancer cells. While the immune system and its cells do not operate under the same ecological and evolutionary principles as predators in nature, they do represent a mortality threat to cancer and exert a selection pressure on cancer cells that is remarkably like that of predators on their prey. We then seek parallel and similar categories of adaptations in cancer that can best be described as armor, spines, and noxiousness. Finally, we note how drawing such parallels between nature and cancer can enrich studies of anti-predator adaptations in nature and suggest ways for how drugs and the immune system can be better deployed to improve patient care.
Armored defenses take two main forms in mammals, keratinous scales and osteoderms. Scales are derivatives of hair and are found only in pangolins (Pholidota) (Figure 1A). Osteoderms are dermal bone deposits and are (or were) found in plate form in armadillos, glyptodonts, and pampatheres (Cingulata), and as small ossicles in ground sloths (Pilosa) (Hill, 2006). Of these groups, only pangolins and armadillos are extant. Scales and osteoderms often do not provide impenetrable protection against predators’ teeth and claws, but they make prey more difficult and time-consuming to kill and ingest. They function as effort deterrents.
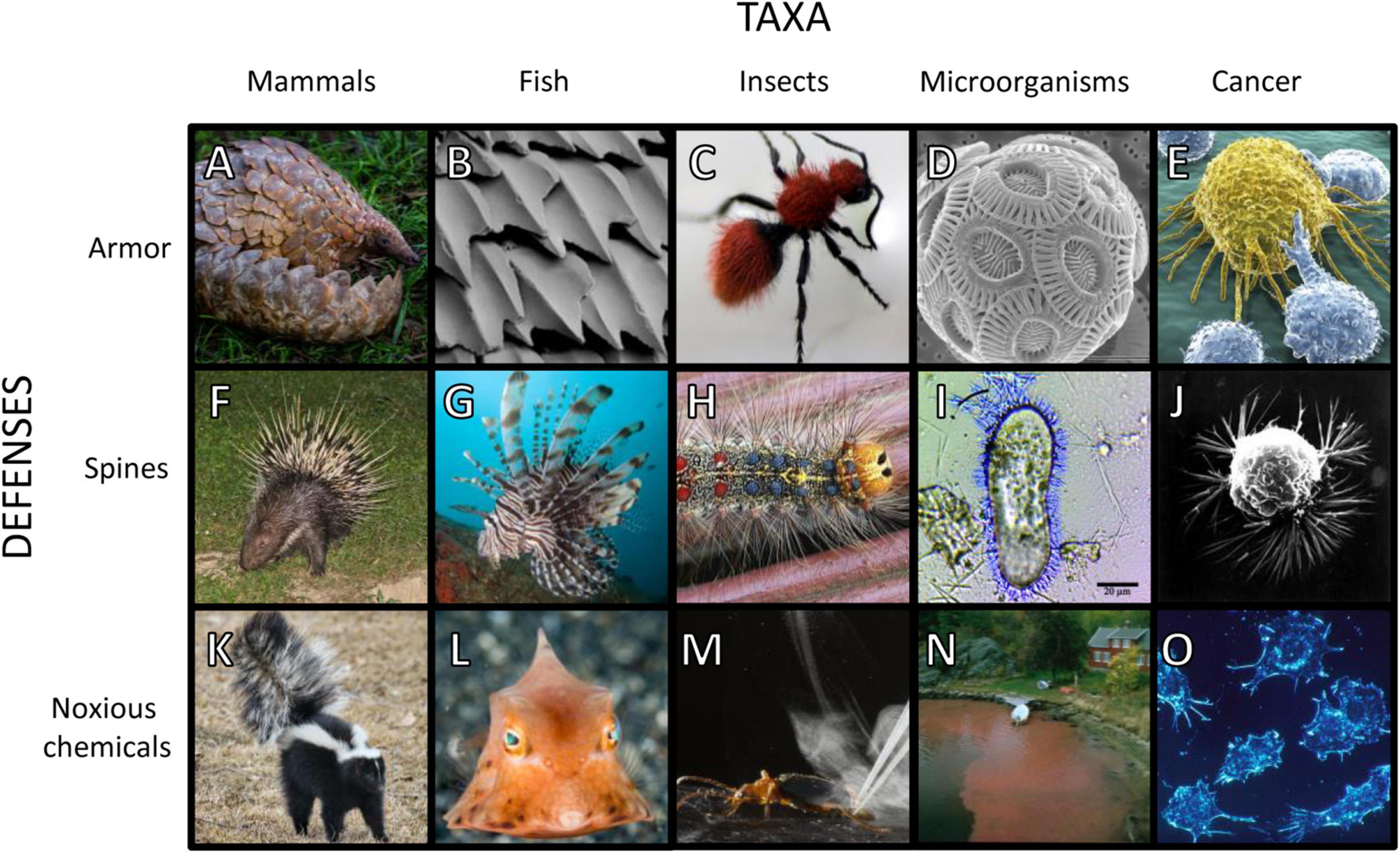
Figure 1. Images of species with armor, spines, and noxious chemical defenses. Examples of armor: (A) Temminck’s ground pangolin (Smutsia temminckii) (Wursten, 2017); (B) scanning electron micrograph of placoid scales on a white shark (Carcharodon carcharias) (Lindsay, 2019); (C) velvet ant (Mutillidae) (Pemberton, 2009); (D) scanning electron micrograph of coccolithophore (Emiliania huxleyi) (Taylor, 2011); (E) cancer cell being attacked by T-cells (Gartner, 2021). Examples of spines: (F) Malayan porcupine (Hystrix brachyura) (Villemagne, 2008); (G) lionfish (Pterois sp.) (Mistry, 2019); (H) Lymantria dispar (Materialscientist, 2009); (I) Paramecium tetraurelia with trichocysts (DavidpBowman, 2014); (J) scanning electron micrograph of breast cancer cell (Wetzel and Schaefer, 1980). Examples of noxious chemical defenses: (K) striped skunk (Mephitis mephitis) (Keck, 2011); (L) thornback cowfish (Lactoria fornasini) (Zerpe, 2018); (M) bombardier beetle (Brachinus elongatulus) (Hedgcock, 2013); (N) red tide Dinophysis bloom (Dahl, 2019); (O) cancer cells impeding immune cells by creating an acidic environment (Fox, 2001). Images were cropped but not otherwise altered.
In mammals, spiny defenses take the form of modified hairs that are exaggeratedly thick, stiff, and sharp. Taxa with spines (or foam-cored quills) include echidnas (Tachyglossidae), tenrecs (Tenrecinae), hedgehogs (Erinaceinae), Afro-Eurasian porcupines (Hystricidae), and American porcupines (Erethizontidae), with spines evolving independently in each lineage. The extremely long quills of Afro-Eurasian porcupines (Figure 1F) and the barbed quills of American porcupines can seriously injure and even kill attacking predators (Afro-Eurasian: Mori et al., 2014; Kerbis Peterhans et al., 2019; Lazzeri et al., 2020; American: Katzner et al., 2015; Elbroch et al., 2016; Forti et al., 2018). These structures will therefore deter predators for risk of physical harm and incapacitation. The relatively short spines of other taxa may not incapacitate predators, and instead, may function like armor by making the prey difficult to handle. Supporting this hypothesis, tenrecs, hedgehogs, and echidnas, which have relatively short spines, will roll up into a ball when threatened, a behavior similar to (armored) pangolins and three-banded armadillos (Tolypeutes tricinctus). Spines serve jointly as effort and injury deterrents.
Noxious chemical defenses in mammals include venom, anointed toxins, and foul odors. Slow lorises (Nycticebus sp.) produce venom by mixing their saliva with an oily exudate from their brachial glands. They retain the substance in their mouths for a venomous bite or spread it over their fur (Alterman, 1995; Nekaris et al., 2013). Other species “self-anoint” their bodies with toxins or odiferous compounds produced by other organisms. African crested rats (Lophiomys imhausi) chew the bark of African poison arrow trees (Acokanthera schimperi), then apply the toxic material to specialized hairs on their flanks (Kingdon et al., 2012; Weinstein, 2020; Weinstein et al., 2020). Interestingly, African crested rats also have armor-like traits. Their reinforced skulls and remarkably tough skin are resilient to “all but the sharpest of teeth, claws or beaks” (Kingdon et al., 2012).
Odiferous anal-gland secretions and urine are widespread across mammal species and are often used for scent marking and communication (Mengak, 2005; Stankowich et al., 2011; McLean, 2014; Jansen et al., 2020). Skunks (Figure 1K) and stink badgers (Musteloidea: Mephitidae) and striped polecats (Musteloidea: Mustelidae) have co-opted anal secretions for defense (Stankowich et al., 2011). When sprayed on potential predators, a skunk’s thiol-containing musk causes a burning sensation in the eyes (Cuyler, 1924; Wood et al., 2002). Skunks, stink badgers, striped polecats, slow lorises, and African crested rats all have black and white aposematic coloring which warns predators of their noxiousness (Stankowich et al., 2011; Nekaris et al., 2019). Noxiousness serves as an injury deterrent.
Several characteristics unite mammals with armored, spiny, and noxious defenses. Relative to the mammalian norm, mammals with spines, armor, and noxious chemicals exhibit locomotion associated with stability rather than speed (Lovegrove, 2001). Furthermore, they often have low metabolic rates (McNab, 1984, 2008; Haim et al., 1990; Stephenson and Racey, 1994; Lovegrove, 2000). While attributing causation can be tricky, armor, spines, and noxiousness may both be associated with and permit slower speeds and lower metabolic rates. Most intriguing and compelling is that these defenses (unlike fleeing, camouflage, and escape to refugia) allow the mammal to maintain feeding activities in the presence of predators that have detected them, even at close proximity. Approach distance (or flight initiation distance) describes how close a predator can get to a prey before it flees. We posit that armor, spines, and noxiousness significantly reduce approach distances while discouraging predators from approaching at all. This theme will be repeated in the following sections.
Armor and spines occur early in fish evolution. Extinct jawless fishes in the clade Osteostraci exhibited a conspicuous armored endoskeleton headshield, with plates and spines (Klompmaker et al., 2019). With the evolution of jaws, gnathostome fish emerged as predators of other fish. Their prey often evolved armor in defense. Extinct placoderms possessed articulated armored plates that covered their head and body (Klompmaker et al., 2019). Cartilaginous fishes (Chondrichthyes; sharks, skates, and rays) have armored placoid scales (Figure 1B; Raschi and Tabit, 1992). Extinct lobe-finned fishes (Sarcopterygii) were covered in tough bone and keratin and extant coelacanths (Coelacanthidae) have specialized scales that are resistant to damage from predators (Quan et al., 2018). Ganoid scales in Polypteriformes (bichirs) and Lepisosteiformes (gars), and bony plates in Acipenseriformes (sturgeons) also provide armored deterrence (Song et al., 2011; Yang et al., 2013; Wishingrad et al., 2015). Armature is persistent throughout spiny ray-fined fishes (Percomorpha) such as boxfishes (Ostraciidae), which have dermal scutes that provide protection from both penetrating and crushing forces (Yang et al., 2015). Even the seemingly inconspicuous scales of small mouth bass (Morone saxatilis) provide protection from predators by resisting punctures (Zhu et al., 2012). The effort deterrence of armor gives way to injury deterrence when these features are also spiny or associated with spines.
Being spiny allow fish to evade predation by threatening harm, preventing capture, increasing handling time, or otherwise reducing predator efficiency (Forbes, 1989; Nilsson et al., 1995; Nilsson and Brönmark, 2000). Head and fin spines and deep bodies are common defensive traits. Many piscivores are gape-limited predators, therefore a deep body effectively frees the prey from these predators. In fact, spines may have evolved with deepening or widening body shape for this very reason (Price et al., 2015). Spines may be erected as needed to increase body depth in groups like triggerfish and filefish (Balistidae and Monacanthidae). Other Tetraodontiformes (including porcupinefish, Diodontidae, and pufferfish, Tetraodontidae) possess a behavioral startle response to predation threats that includes increasing body size and erecting body spines (Greenwood et al., 2010; Pleizier et al., 2015). The evolution of fin spine length in butterflyfish (Chaetodontidae) correlates with foraging in risky habitats and situations (Hodge et al., 2018). Spines serve jointly as effort and injury deterrents.
Noxious slimes, venoms and toxins occur frequently throughout the evolutionary history of fishes. Pre-vertebrates and jawless fishes (hagfishes, Myxinidae) deploy a noxious slime that smothers the gills and suffocates would-be predators (Zintzen et al., 2011). Venom is observed in at least 58 fish families and serves both predatory and anti-predatory functions (Smith et al., 2016; Harris and Jenner, 2019). In some species, modified fin and body spines form hypodermic needles capable of injecting venom into predators (Harris and Jenner, 2019). This defense is hypothesized to contribute to lionfishes’ (Pterois miles and Pterois volitans) (Figure 1G) wide niche breadth and P. volitan’s expansive range and success as an invasive species (Harris and Jenner, 2019). Noxious chemical defenses can take the form of ichthyotoxins which are secreted from the skin and aid in escape. To reduce parasitic load or when threatened, pufferfish (Tetraodontidae) release tetrodotoxin through their skin (Saito, 1985; Munday et al., 2003). Boxfish and cowfish (Ostraciidae) (Figure 1L) release ostracitoxin as a poisonous secretion (Thomson, 1964). Notably, both these families are comprised of relatively slow swimming omnivores found on coral reefs.
Conspicuous defensive traits such as armor, spines, or noxious chemicals play a large role in fish behavior and foraging. Fish without defensive armor mitigate predation risk with behavioral responses such as fleeing (McLean and Godin, 1989). Consequently, unarmored fish may reduce the amount of time foraging and flee sooner than those that are defended. Armored stickleback (Culaea inconstans) preferred to associate with non-defended fathead minnows (Pimephales promelas) in high-risk environments, perhaps because they were the predator’s less preferred prey (Mathis and Chivers, 2003). Such tradeoffs between foraging and predation risk have evolutionary consequences. For example, butterflyfish species (Chaetodontidae) with longer spines have riskier foraging strategies such as being solitary or venturing farther from the safety of the reef (Hodge et al., 2018). As with mammals, armor, spines, and noxious chemicals in fish may permit tenacious foraging under high risk, permit closer approach distances, and discourage predators from attacking at all.
Insects have evolved a spectacular array of defenses. Sugiura (2020) classified insect defenses into chemical, morphological, physical, and behavioral categories. In a manner similar to the other taxa we discuss, Sugiura’s morphological and physical categories include armor and spines, and chemical and behavioral categories include toxic or noxious exudates, venoms, and regurgitated gut fluids.
An insect’s exoskeleton acts as armor among other functions (Davies, 1988). As body armor, the exoskeleton is made of stiff sheets or lamellates of chitinous and proteinaceous material connected by a flexible membrane which allows the entire exoskeleton to move (Waldbauer, 2012). In some insect species, the chitinous exoskeleton can be so hard that it is all but impervious to crushing and digestion. A striking example first observed by Alfred Russel Wallace (Wallace, 1867, 1895, as seen in Wang et al., 2018b) are the Pachyrhynchus weevils (Coleoptera: Curculionidae: Entiminae: Pachyrhynchini). The exceptional strength of mature weevil exoskeletons results from a thickly sclerotized cuticle, combined with fibrous ridges in the endocuticle layer of the exoskeleton, apparently unique to these weevils (Wang et al., 2018a). Such defenses act primarily as an effort deterrent.
Many insects have evolved defensive hairs (setae) and spines. The lubber grasshopper (Romalea guttata) has sharp spines on its hindlegs which deter predators (Eisner et al., 2005). Many lepidopteran taxa possess hairs and spines. Some of these are “urticating” or stinging structures, while others, e.g., those of the mulberry tiger moth (Lemyra imparilis) and the moth, Lymantria dispar (Figure 1H), provide only a physical deterrence to predators (Whelan et al., 1989; Sugiura and Yamazaki, 2014) or parasites (Kageyama and Sugiura, 2016). On at least some species, the hairs increase in length and/or density in later larval instars, and these developmental changes appear to increase the deterrence effect of the hairs (Whelan et al., 1989; Sugiura and Yamazaki, 2014).
Insects manifest an extraordinary diversity of chemical defenses (Eisner, 1970; Blum, 1981; Eisner et al., 2005). These defenses are found on the surface, in blood, the gut, or systemically (Eisner et al., 2005). Glandular chemical defenses may be injected into the enemy or secreted in other ways (Eisner, 1970). In some species, venoms may be used both for defense and to acquire prey. Finally, chemical defenses may be produced endogenously or acquired exogenously (Eisner et al., 2005). Many herbivorous insects sequester plant secondary compounds intended for defense against herbivores. In these cases, specialized herbivores are often better defended against their own predators than are generalist herbivores (Zvereva and Kozlov, 2016).
Given their soft bodies, larvae of Coleoptera and Lepidoptera are particularly vulnerable to physical attack, and, unsurprisingly, many are well defended by spines (brushy setae) or noxious chemicals. The unicorn caterpillar moth (Schizura unicornis) sprays its defensive chemical cocktail from a saclike gland located behind the head (Eisner et al., 2005). The bombardier beetles (Coleoptera) (Figure 1M) produce their defensive chemicals, benzoquinones, which combine explosively when ejected. The reactants, hydrogen peroxide and hydroquinones, are forced through a “reaction chamber,” where catalases and peroxidases drive the chemical reaction, ejecting “their spray at the temperature of boiling water” (Eisner et al., 2005, p. 159–160). Their spray repels spiders, ants, frogs, and birds.
Insects commonly have two or even all three forms of effort/injury deterrents. Velvet ants (Hymenoptera: Mutillidae) (Figure 1C) possess a suite of formidable defenses, including aposematically colored, coarse, dense hair (setae), a chemical alarm signal, stridulatory warning sounds, and potent stings (Hertz, 2007; Schmidt, 2016). In addition, they are protected by a round, slippery, and extremely hard exoskeleton (Schmidt and Blum, 1977; Gall et al., 2018). They can even survive over 20 min in the stomach of a toad prior to rejection by regurgitation (Mergler and Gall, 2021).
Many insect species flee at the approach of a potential predator (e.g., cockroaches, houseflies, and grasshoppers), exhibit amazing camouflage (e.g., peppered moth, stick insects, planthoppers), or overwhelm their predators numerically with occasional emergences (e.g., periodical cicada, mayflies) or outbreaks (swarming locusts). We hypothesize that these species are less likely to exhibit armor, spines, and noxious chemicals. Such species should be more likely to cease feeding activities at the approach of predators. Insects that use effort/injury deterrents pay a price for their defenses (Flenner et al., 2009) but can maintain activities in the presence of predators (Witz and Mushinsky, 1989; Ge et al., 2019). In fact, most insects that seem “easy” to catch are likely defended with some combination of armor, spines, and noxiousness (or sheer numbers).
We see cancer as a speciation event in which a protist evolves from the cells of its host (Gatenby and Brown, 2017; Gatenby et al., 2020; Pienta et al., 2020a). From this perspective, microorganisms provide the closest examples for cancer of armored, spiny, and noxious defenses, both in form and function. The term “microorganism” includes archaea, bacteria, protozoa, fungi, and algae. For this diverse polyphyletic grouping, our goal is to highlight how and when armored, spiny, and noxious defenses are seen in unicellular organisms.
Armor-like defenses in unicellular species include reinforced cell walls (armored plates and sheaths) and robust extracellular matrices. In phytoplankton (single-celled algae), these provide effort deterrence from zooplankton grazing (Hamm et al., 2003). Most species of dinoflagellates have cell walls surrounded by armor-like cellulosic sheaths known as thecae. Diatoms have frustules, silicified cell walls (Hamm et al., 2003), which allow them to survive passage through a predator’s gut (Fowler and Fisher, 1983). Diatoms thicken their frustule walls in response to copepod grazing (Pondaven et al., 2007; Grønning and Kiørboe, 2020). Coccolithophore phytoplankton are named for their calcium carbonate plates, or coccoliths, which surround their cell walls (Figure 1D). Heterotrophic protist predators exhibit significantly reduced population growth rates when fed calcified rather than non-calcified (less armored) strains of Emiliania huxleyi coccolithophores (Harvey et al., 2015; Pančić and Kiørboe, 2018). E. huxleyi have a haplo-diplontic life cycle and only the diploid cells, which are non-motile, are calcified (Kolb and Strom, 2013). This is consistent with our hypothesis of a relationship between armored defenses and less mobile (or sessile) lifestyles.
Cell and colony size and shape also influence host-grazer interactions. Prey morphologies that are large or unwieldy increase handling time for predators and thereby function as armor despite the lack of a thick outer covering. The presence of some species of Daphnia (zooplankton grazers) will induce populations of Scenedesmus phytoplankton to transition into linked-up colonies with spiny morphologies (van Donk and Hessen, 1993; Pančić and Kiørboe, 2018). Lürling et al. (1997) confirmed that Daphnia species grazed Scenedesmus at lower rates when Scenedesmus formed large, linked colonies. Similarly, protozoan grazers promote planktonic bacteria with elongated filamentous morphologies (Jürgens et al., 1994; Pernthaler et al., 1996; Hahn et al., 1999), which can manifest as individual cells with strongly elongated shapes or thin chains of multiple cells (Hahn et al., 1999). Various bacterial species are permanently or facultatively filamentous, but the increased prevalence of filamentous bacteria in the presence of protozoan grazers reflects differential survival, not an induction of filamentous morphology (Hahn et al., 1999).
Another type of armor-like defense in microorganisms is observed in biofilms, which adhere together by extracellular matrices of polymeric substances (polysaccharides, nucleic acids, and proteins). The exopolymeric matrix constitutes a “biofilm shield” that is difficult for predators to penetrate, and the unwieldy masses of cells resist phagocytosis by unicellular predators (Matz and Kjelleberg, 2005). Phagocyte predators are less hindered when this shield is broken. For example, neutrophils have an elevated response (oxidative burst) to Pseudomonas aeruginosa surface biofilms when they have been mechanically disturbed (Kharazmi, 1991; Jensen et al., 1992). Biofilms are effective at defending against suspension-feeding protozoans in aquatic/marine habitats (Matz et al., 2002; Seiler et al., 2017), and immune cells such as neutrophils and macrophages in host systems (Jesaitis et al., 2003; Chandra et al., 2007; Thurlow et al., 2011; Roilides et al., 2015).
Spines and distinctive adaptations that function like spines provide microorganisms with safety from predation through both effort and injury deterrence. Some ciliates and dinoflagellates produce filamentous trichocysts, silicified needle-like extrusive organelles (Figure 1I). When triggered, these protists discharge a barrage of trichocysts outside their cells, creating a spikey obstruction between the protist and its predator (Knoll et al., 1991). To avoid injury, a persistent predator must detour around the trichocyst mass, resulting in increased subduing time. The dinoflagellate Prorocentrum micans possesses both trichocysts and armor-like theca (Rhiel et al., 2018). The diatom Chaetoceros peruvianus produces spine-like extensions of its already armor-like siliceous frustule. These extensions (setae) are believed to deter predators by increasing handling effort (Pickett-Heaps et al., 1994; Smodlaka Tanković et al., 2018).
Toxins and other noxious chemical defenses are employed by diverse species of archaea, bacteria, phytoplankton, and yeasts. Perhaps the most infamous are the neurotoxins of dinoflagellates, diatoms, and cyanobacteria, which can lead to harmful algal blooms or red tides. These neurotoxins include chemicals of the saxitoxin family, commonly known as paralytic shellfish toxins, as well as spiroimines, goniodomin A, and lytic compounds (Xu et al., 2017). Most of the noxious chemicals of phytoplankton are retained in their cells, delivered to predators only upon ingestion. Some Alexandrium species also produce extracellular allelopathic compounds (Ma et al., 2009). Harmful algal blooms can originate from several genera of dinoflagellates, including Dinophysis, shown in Figure 1N.
Noxious chemical defenses are commonly deployed by yeast (and other fungi) and bacteria, especially in biofilms. Because fungi, including yeasts, are generally immobile, high-nutrient patches are desirable and competition for them can be fierce. Fungi have thus evolved to produce an array of noxious chemicals which they employ against bacterial and other fungal competitors (Künzler, 2018). They also provide injury deterrence against predators. In yeasts, these substances, known as killer toxins, are secreted from the cell. Some species of marine bacteria produce the alkaloid compound violacein, which induces a cell death program in protozoan predators (Matz et al., 2008). These bacteria do not excrete the violacein, but retain it in their cells, making them toxic upon consumption. Matz et al. (2008) found that violacein production per bacterium was 3–59 times higher for cells in biofilms than planktonic cells.
As they do for multicellular species, armored, spiny and noxious defenses allow microorganisms to defend in place, a behavior that is especially useful when foraging from resource-rich patches, or when a microorganism is sessile. The advantage of using effort or injury deterrents to remain active, in place, is especially evident when considering biofilms. Bacteria can reach much higher densities in biofilms than in the water column (Matz et al., 2008). Some researchers have hypothesized that these high cell densities can be reached because biofilms provide refuge from predators (Matz and Kjelleberg, 2005), and others have noted that surfaces tend to concentrate nutrients (Baty et al., 2000; Hall-Stoodley et al., 2004). We expect that these two conditions are not coincidental, but closely related. Planktonic bacteria can escape predators by fleeing, but this is an undesirable strategy when swimming away from a nutrient-rich patch.
In response to the immune system, do cancer cells evolve some subset of armor, spines and noxious chemicals as seen among microorganisms?
The National Cancer Institute describes the immune system as “a complex network of cells, tissues, organs, and the substances they make that helps the body fight infections and other diseases” (NCI, 2021). The human immune system is a marvel with layers of complexity for the simple purpose of killing pathogens and pathogen-infected normal cells, and for removing debris and malfunctioning cells. It involves a large number of cell types, diverse signaling molecules, and a variety of spatial scales near and far from the actual location of infection or attack. It provides both surveillance, memory, adaptability, and attack. Specialized cells of the immune system, particularly killer T-cells, do threaten and kill cancer cells. To the cancer cells, they exert selection pressures just like predators on their prey in nature.
In this section we describe some of the features of the immune system and highlight the striking differences between cytotoxic immune cells and most predators in natural systems. Compared to traditional predators, they should not and do not respond in the same way to effort and injury deterrents from their pathogen prey. However, the immune system does drive the evolution of immune evasion and suppression by the cancer cells within the patient, and the suite of adaptations deployed by cancer cells can, in many cases, resemble the effort and injury deterrents described for the other taxa in function if not always in form.
The cancer cells face threats from the innate and the adaptive immune system, though the latter likely exerts stronger selection on the cancer cells’ anti-immune adaptations. But, the innate immune response of natural killer (NK) cells, macrophages, and dendritic cells may be crucial for priming the adaptive response that includes antigen presenting cells (APC) and cytotoxic T-cells. NK cells can kill from a distance by releasing proteases and other substances in close proximity to the cancer cell. These substances puncture holes in the cancer cell’s membrane and permit additional lethal proteases to enter and kill the cancer cell. T-cells operate similarly, but they must be in contact with the cancer cell. Macrophages kill cancer cells by engulfing them through phagocytosis. In the tumor, macrophages and NK-cells, through their killing of cancer cells, and APC and dendritic cells, through their transport of bound antigens to lymph nodes, can recruit, prime, and activate cytotoxic T-cells. The T-cells flow through the blood to the tumor from the lymph nodes, where they can continue to proliferate as well as attach to cancer cells (Figure 1 in Demaria et al., 2019 provides an excellent illustration).
There are three noteworthy features of the interaction of the immune system with cancer cells. First, while it is not a predator-prey system in the usual sense, the action of cytotoxic T-cells can be conceptualized and modeled as a predator (de Pillis et al., 2005; Kareva et al., 2010; Robertson-Tessi et al., 2012; Kaur and Ahmad, 2014). As a “predator,” T-cells are subsidized in the sense that new ones can be recruited from the lymph nodes, independent of killing rate and success (Blank et al., 2016). This could be compared to house cats (T-cells) preying on song birds (cancer cells) where the fate of the cat population is uncoupled from the fate of the song bird population because the cats are supported by pet food (Lepczyk et al., 2004). Second, as modeled and noted by Kareva et al. (in review), cytotoxic T-cells do not work on commission like natural predators. Their proliferation can be stimulated by the overall presence of the antigen and the number of cancer cells. But, a given cytotoxic T-cell’s proliferation rate does not increase with the rate at which it kills cancer cells. In fact, in a phenomenon known as T-cell exhaustion (Zarour, 2016; Wang et al., 2018; Philip and Schietinger, 2019), a T-cell may become injured and incapacitated in the process of attaching to and killing a cancer cell. Hence, the direct effect of T-cells on cancer cells, and vice-versa, bears a greater resemblance to extreme interference competition than predation (Kareva et al., in review). Third, other immune cells such as regulatory T-cells (T-regs) act as a break on the proliferation of cytotoxic T-cells. Their proliferation is stimulated by cytotoxic T-cells even as T-regs suppress the proliferation of cytotoxic T-cells. In its simplest guise, T-regs, cytotoxic T-cells and cancer cells form a kind of tri-trophic level system: T-regs “prey” upon and benefit from T-cells, and T-cells prey upon and benefit from cancer cells (Dullens et al., 1986; Eftimie et al., 2016; Walker and Enderling, 2016).
In the previous sections we discussed examples of species that defend themselves from predators for whom successful foraging leads to increased individual fitness. Like other organisms, these predators make foraging decisions that balance nutritional and energetic rewards against the risk of bodily harm and missed opportunity costs. We shall refer to these types of predators as traditional predators. Immune cells fall into a different category of predator. While they are predators insomuch as they exert selection on prey (pathogens, cancer cells) as traditional predators would, cytotoxic immune cells are not traditional predators. They do not individually benefit from successful foraging (killing cancer cells). This is because the unit of natural selection is not the individual immune cell but the entire host organism. Immune cells’ behaviors serve to benefit the entire organism, even at the expense of the individual immune cell. In this way, they may be likened to the soldier caste of a eusocial species, such as soldier ants, which will walk into the face of danger for the benefit of the colony.
For systems with traditional predators, armor-like defenses are effective primarily as an effort deterrent. All else equal, traditional predators will opt for the easier (unarmored) prey. This strategy maximizes time and energy efficiency. Soldier-caste-type predators, meanwhile, may not be deterred from pursuing difficult prey. Their preference should reflect the needs of the whole organisms or colony. For example, neutrophils and macrophages attempt to phagocytize biofilms even when they are too large to engulf, which frustrates phagocytosis (Leid, 2009; Thurlow et al., 2011). Prey defenses that incapacitate predators function as injury deterrents. When alternatives are available, traditional predators will opt for less dangerous prey. When the fitness reward from a prey does not counterbalance the risk of lethal or incapacitating injury to the predator, then the predator should forgo that prey entirely. Soldier-caste-type predators, meanwhile, will not be deterred from pursuing dangerous prey, even if there is a high probability that the soldier will be killed. Attempts by neutrophils and macrophages to engulf bacterial biofilms will trigger the bacteria to release cytotoxic chemicals that are effective in killing the cells (Thurlow et al., 2011; Hirschfeld, 2014; Scherr et al., 2015). These phagocytes lose twice, first by the prey’s armored defense and second by the prey’s noxious defense, yet they still pursue the prey until their deaths. Figure 2 summarizes how defenses that either increase subduing-handling time or threaten incapacitation will affect traditional and soldier-caste-type predators. Though soldier-caste-type predators will not necessarily be deterred for their own self-preservation, they can be deterred for the sake of the whole organism’s or eusocial colony’s wellbeing.
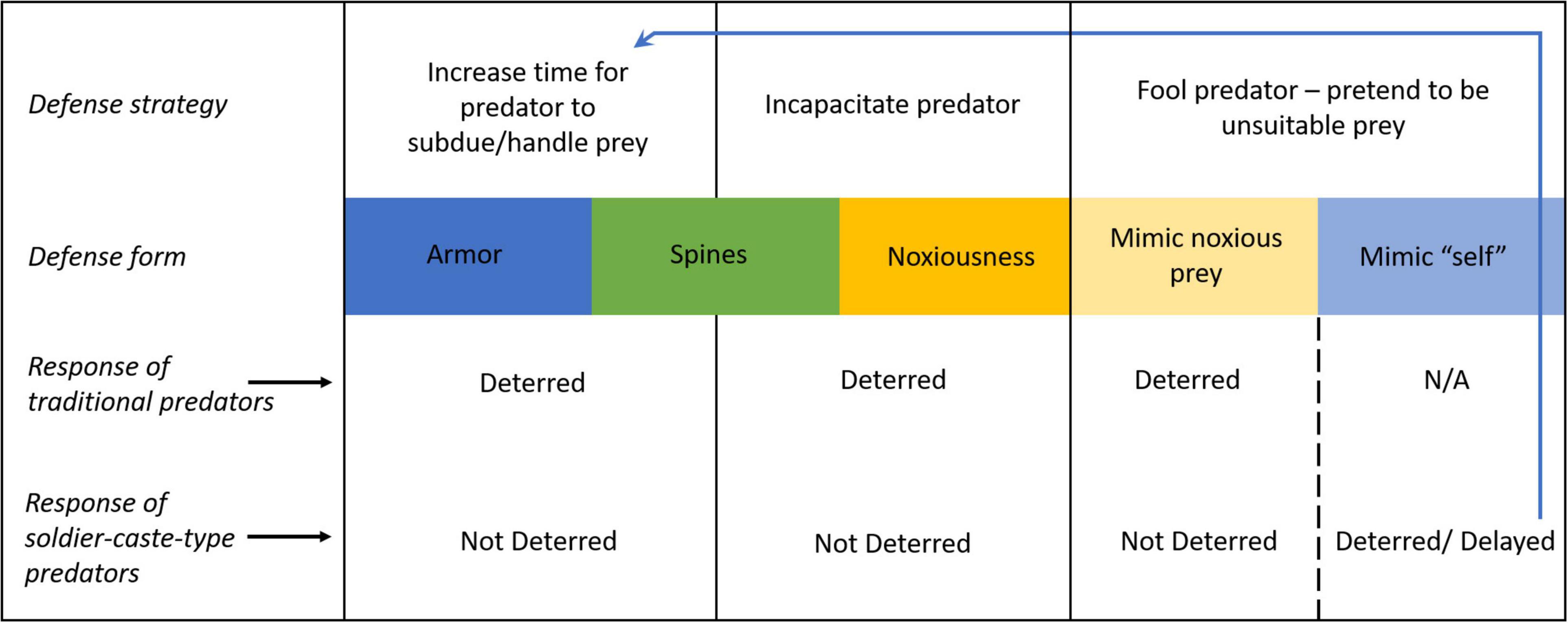
Figure 2. Defenses which function to increase predator handling time and/or subduing time (armor and, in some instances, spines) will deter traditional predators but not soldier-caste-type predators. Defenses which incapacitate predators (noxious chemicals and, in some instances, spines) will also deter traditional predators but not soldier-caste-type predators. Prey can also fool predators with false signals, pretending to be unsuitable prey (Batesian mimics). For traditional predators, noxious prey are unsuitable prey. Mimicking noxious prey will deter traditional predators but not soldier-caste-type predators. For soldier-caste-type prey, “self” cells/individuals from the same organism/superorganism are unsuitable prey. Mimicking “self” cells/individuals could deter soldier-caste-type predators, or simply increase the time required for proper identification. By delaying proper identification, these mimics are effectually increasing predator subduing time, making this an armor-like defense. For traditional predators not part of a superorganism, the distinction between self and non-self is obvious so self-mimicry is N/A.
Self-attack, where the attacker misidentifies benevolent cells or individuals of the (super)organism as threats, is a particularly relevant concern for soldier-caste-type predators. In eusocial animal colonies, discernment of colony members decreases the chance of both self-attack (Michener, 1974; Crosland, 1990; Fishwild and Gamboa, 1992), which is analogous to autoimmunity, and parasitism, which is analogous to infection or cancer. Naked mole rats, which are eusocial, use odors to recognize colony members, with individuals’ odors mixing to create a unique and dynamic colony scent (O’Riain and Jarvis, 1997). Almost universal among eusocial insects (Breed and Bennett, 1987; Smith and Breed, 1995), nest mate recognition is accomplished in a similar way, by picking up the chemical profile unique to the colony with their antenna. This strategy is useful for detecting colony threats such as parasites, unless the parasites can convincingly mimic colony members or the nest itself. Eusocial stingless bees (Melipona subnitida) will swiftly attack and kill full adult (post-eclosion) parasitic mantisflies (Plega hagenella) that enter their colony but will not kill younger adults (pharates) still in pupa (Maia-Silva et al., 2013). Perhaps because the pharates convincingly mimic the scent profile of the nest, the bee workers simply gently remove the pharates with the nest’s waste, at which point the mantisflies continue their life cycle (Maia-Silva et al., 2013). Maia-Silva et al. (2013) conclude that delayed adult eclosion in these mantisflies is an important adaptation to avoid attack by host bees.
Immune cells depend on antigen recognition to discern self and non-self. To evade immune cell predation, cancer cells should disguise themselves as host cells, similar to the strategy of the parasitic mantisfy pharates. However, this presents a tradeoff to the cancer cells. Novel adaptations that would make them more successful at acquiring nutrients, occupying space, and outcompeting other cancer cells–e.g., modification or upregulation of intra-cellular metabolic pathways, cell-cell signaling processes, nutrient transporters, membrane pumps, and self-sufficiency in growth factors (Brown, 2016)–will also result in conspicuous antigen presentation (Houghton, 1994; Lee et al., 2018). Having supplementary anti-predator defenses such as armor, spines, or noxious defenses could allow cancer cells to incorporate novel adaptations with impunity, even if the accompanying antigens ultimately increase immune cell attack.
The adaptive immune system creates a coevolutionary arms race between cancer cells and the host immune system which bears some similarities to that between traditional prey and predators (Kareva et al., in review). This arms race reoccurs de novo within each cancer patient. In traditional predator-prey models, predators directly convert consumed prey into more predators (predator biomass), but this is typically not the case for immune cells (Merlo et al., 2006, Kareva et al., in review). However, the adaptive immune system will produce cytotoxic T-cell variants that successfully target invader cells (Merlo et al., 2006). The direct conversion of prey into predator biomass has evolutionary consequences because successful predators will have more offspring, selecting for superior predatory traits. Within the lifespan of an individual host, cancer cells have the opportunity to evolve immune evasion over many generations, but the host does not. The adaptability of the immune system enables the host to modulate how it attacks the changing cancer cell community.
Cancer cells subjected to NK cells, macrophages, cytotoxic T-cells and other associated regulator cells find themselves being the prey, so their immune evasion responses are akin to standard anti-predator adaptations that emerge from traditional predator-prey systems. Tumors can be classified as hot versus cold depending upon the amount of immune infiltration (Maley et al., 2017; Vareki, 2018), and hot tumors are thought to be more responsive to immunotherapies that challenge or target the cancer’s anti-predator adaptations (de Guillebon et al., 2020). While often novel in form, cancer cells’ anti-predator adaptations against the immune system function much like armor, spines, and noxiousness in other species.
As noted by Fridman (2018), the observation that the immune system might suppress cancers dates back to 1891 (Coley, 1891). However, the immune system’s therapeutic value did not become fully appreciated until this century. Not until 2011, did Hanahan and Weinberg add immune evasion to their original 2000 “hallmarks of cancer” (Hanahan and Weinberg, 2000, 2011). The field of cancer biology has progressed from noting how cancer cells may have adaptations to avoid the immune system to accepting that all successful cancer cells possess one or more evasion strategies. NK cells, macrophages, cytotoxic T-cells, and more are an ever-present feature of tumors, even in cold tumors or regions of a tumor where immune infiltration is weak. Cancer cells must and do maintain feeding, normal activity, and proliferation while surrounded by threats from immune cells. Cancer cells cannot truly flee, hide, or remain camouflaged (entirely unnoticed) from immune attack. For all these reasons, they need armor, spines, and noxiousness for defense. Though these adaptations can take on forms quite different from those in other taxa, they still function to deter “predators,” in this case predatory immune cells.
As the number of cancer cells grows, natural selection promotes increasingly effective anti-predator adaptations, thus tipping the scales in favor of the cancer cells and against the immune system (Solinas et al., 2009). This temporal progression toward cancer cells winning the arms race is termed cancer immunoediting (Shankaran et al., 2001). It has three recognized phases (Dunn et al., 2004; Pandya et al., 2016). In the first (elimination phase), the cancer cells are so few and so vulnerable that the immune system can eliminate them completely (Burnet, 1957; Corthay, 2014). In the second (equilibrium phase), the number of cancer cells and the sophistication of their immune evasion adaptations result in an equilibrium where the tumor is neither growing nor shrinking or being eliminated by the immune system (Koebel et al., 2007; Teng et al., 2008). In the third (escape phase), the number of cancer cells and their adaptations allow them to thrive and expand their range (tumor growth and metastases) even in the face of a fully functioning immune system (Khong and Restifo, 2002; Grivennikov et al., 2010).
There are three general ways by which cancer cells evade the immune system (Wildes et al., 2020). First, cancer cells alter surface membrane molecules that fool cytotoxic T-cells into perceiving them as unsuitable prey. Second, cancer cells modify their extracellular environment in a manner that repels immune cells or renders them less effective. Third, cancer cells release or present molecules that render immune cells inoperable or that alter the immune cell composition from one that is tumor-suppressive to one that is pro-tumor (Mohme et al., 2017).
Whereas natural predators try not to waste time on unprofitable prey, cytotoxic immune cells go out of their way to avoid killing healthy, normal cells. Armor–in the functional sense of increasing handling time–for cancer cells takes the form of increasing the time required for cytotoxic immune cells to recognize the cancer cell as prey (non-self), even to the point of ceasing to see the cancer cells as anything but a normal cell to be avoided (Figure 2). Cancer cells acquire this armor by changing surface proteins, altering the expression of MHC molecules, down-regulating NK cell activating ligands, or simply forgoing the advantages of antigen presenting membrane properties (Beatty and Gladney, 2015; Steven and Seliger, 2018; Anichini et al., 2020). “Armor” by this interpretation is no longer a barrier that frustrates physical processing by the predator, but now a barrier that frustrates diagnosis processing by the predator. This is because only the latter type of barrier will be effective at deterring predation by immune cells. This defense by the cancer cells can also be recognized as a form of mimicry.
A fascinating example of an armor-like immune escape comes from a mouse model of adoptive cell transfer therapy (ACT) against melanoma. In this model, an infusion of T-cells specifically recognizes a melanoma differentiation antigen, gp100, leading the melanoma cells to adapt by decreasing expression of gp100 and switching to a less differentiated neural crest phenotype (Landsberg et al., 2012). This response is mediated by TNFα, released by tumor-infiltrating cells as a part of a normal immune predation program. A downregulation of gp100 is accompanied by the expression of the nerve growth factor receptor (NGFR) and the loss of the expression of several melanosomal antigens. Thus, the cancer cells mimic embryological tissue. Even as the immune cells constantly encounter these cancer cells, they perceive them as unsuitable prey.
Cytotoxic immune cells are not completely devoid of behaviors of self-preservation. They will avoid prey perceived as “self” and they will avoid toxic circumstances. Spines to a cancer cell can be literal protrusions that prevent cytotoxic T-cells from contacting the cell membrane, or, more frequently, defenses that function like “spines.” Cancer cells do this via changes to the microenvironment that cause cytotoxic immune cells to avoid approaching the cancer cells. Literal spines or protuberances appear in single cell microscopy of cancer cells (Figure 1J). They may be invadopodia facilitating collagen degradation within the extracellular matrix (Weaver, 2006; Augoff et al., 2020), pseudopodia for movement (Guirguis et al., 1987), or extracellular extensions of intermediate filament proteins (usually associated with the cell’s cytoskeleton) that may serve for immune evasion (Sharma et al., 2019).
At present, the role of such true spines and filaments is poorly studied. How cancer cells generate microenvironments that repulse immune cells or render them inactive is better understood. Cancer cells produce hypoxic and acidic environments that are immunosuppressive (Huber et al., 2017; Multhoff and Vaupel, 2020; Vito et al., 2020; Figure 1O). In particular, cancer cells upregulating carbonic anhydrase IX (CAIX) have been shown to produce both hypoxic and acidic conditions. Such cancer cells are more aggressive, metastatic, and immunosuppressive (Pastorekova and Gillies, 2019). In breast cancer, Lloyd et al. (2016) showed that CAIX-expressing cancer cells predominated at the edges of tumors where immune infiltration was highest.
The anti-predator adaptations of cancer cells that most closely align with those seen in natural predator-prey systems are noxious defenses. Though, here again, cancer cells exploit some of the unique regulatory properties of the immune system designed to minimize injury to self. Antigen expression by a cancer cell stimulates several signaling cascades within tumor-specific activated T-cells including regulatory receptors such as the programmed cell death protein 1 (PD-1) (Pardoll, 2012). As an extra precaution against T-cells killing the wrong cells, PD-1 on the T-cell interacts with its ligand PD-L1 on the surface of normal cells. Frequently, across multiple cancer types and across patients, cancer cells independently evolve to upregulate PD-L1, covering their cell surfaces with these transmembrane ligand binding proteins PD-L1 (Dong et al., 1999; Atefi et al., 2014). When a T-cell encounters the cancer and the PD-L1 binds to the T-cell’s PD-1 protein a “no killing” command ensues. In response, the T-cell may leave the cancer alone, or, in terms of inducing injury, the T-cell may cease to divide, deactivate, or even undergo apoptosis (Butte et al., 2007; Francisco et al., 2009). This form of immune checkpoint adaptation by the cancer cells also manifests in other death inducing FAS-FAS ligand binding, CD47, and HLA-G (Pettersen, 2000; Horton et al., 2018; Zhu et al., 2019). When the cancer cells present receptors that induce death or deactivation of the T-cells, they possess a noxious defense.
The noxious anti-predator repertoire of cancer cells includes releasing chemicals that deactivate or induce apoptosis in cytotoxic cells (e.g., release of NK-cell ligands). The secretion of interferons and TNFα by infiltrating lymphocytes amplifies the immune response by attracting cytotoxic T-cells, NK cells, and macrophages. However, experimental data from both mice (Spranger et al., 2013) and humans (Rooney et al., 2015) show that interferons also induce the expression of indolamine 2,3 dioxygenase (IDO). Increased level of IDO in the tumor microenvironment leads to metabolic suppression of the lymphocytes and a reduction of cytotoxic immune cells. Cancer cells also evolve to release extracellular vesicles (EV) that can contain immune checkpoints, signaling molecules that attract pro-tumor immune cells such as T-regs and M2 macrophages. These pro-tumor cells suppress the proliferation of cytotoxic immune cells and even co-feed cancer cells. In effect, the cancer cells can evolve adaptions to co-opt and hijack the immune system (Heusinkveld and van Der Burg, 2011; Kareva, 2011). In this ultimate form of noxiousness, the cancer cells call in the “enemy” of their “enemy.” Cancer cells have developed indirect defenses very much akin to plants releasing volatile chemicals to attract predatory wasps of the plant’s arthropod pests (Halitschke et al., 2008).
We have presented examples of diverse species employing armor, spines, and noxious chemicals as anti-predator adaptations. It is evident from this overview that defenses with the same essential function do not always look similar, and traits that look similar do not always function similarly. One essential defense strategy we have described is specialized morphology that increases predator subduing and/or handling time. In mammals and other animals, this defense typically looks like armor, that is, exceptionally durable integument. In other species, this defense strategy often takes the form of a robust exterior, but sometimes it does not. For example, filamentous morphology in bacteria and cancer increases predator handling time even without the thickening of cell walls (Jürgens et al., 1994; Pernthaler et al., 1996; Hahn et al., 1999).
Spine-like morphologies are widespread across taxa, but the function of these morphologies varies. Some species’ spines are clearly dangerous to predators, such as the exaggerated spines of porcupines (Figure 1F). In other cases, it is less evident that spine-like morphologies are capable of hurting predators and may instead function primarily to increase predator subduing or handling time. For example, the presence of Daphnia (zooplankton grazers) triggers Scenedesmus phytoplankton form linked-up colonies and develop spines (van Donk and Hessen, 1993; Pančić and Kiørboe, 2018). This morphology reduces predation by small Daphnia, suggesting that the function of the spines is to make the Scenedesmus colonies larger and thereby more difficult to handle. This echoes the hypothesis of Price et al. (2015) that fish spines evolved to thwart gape-limited piscivores. Spine-like morphologies in cancer may serve multiple purposes including movement, degradation of extra-cellular matrix, and to keep cytotoxic T-cells at bay. These functions need not be mutually exclusive. The protuberances of PD-L1 transmembrane proteins in cancer cells may be more akin functionally to the rays of lionfish (Pterois spp.) (Figure 1G). While cancer cells and lionfish face very different predators, each can result in the predator’s injury or death. The lesson from this overview is that defenses functioning like armor and spines (terms based on mammalian examples) are widespread across nature, but the function of morphological defenses cannot be assumed from their appearance alone.
The benefits of armor, spines, and noxious defenses will vary according to the frequency of defended prey in the system as well as the type of predator. If all prey of an ecosystem had 100% effective defenses, then their predators would starve and there would be none. Similarly, if cancer cells within their tumor exerted 100% effective immunosuppression, there would be no activated immune response. But, in the absence of any predators or immune response, natural selection would favor prey and cancer cells without spines, armor, or noxiousness. Conversely, if prey were poorly defended or defenses were rare, then the population size of predators would be abundant, thus favoring more highly defended prey. Predator-prey systems in nature should equilibrate on a mix of vulnerable and defended prey; or prey with some intermediate level of defense. For example, though North American porcupines (Erethizon dorsatum) are robustly defended by barbed spines, their defense is not so robust to be able to thwart specialist predators such as fishers (Pekania pennanti) (DeWitt et al., 2019).
Because vulnerable prey support the predators that select for armored, spiny, and noxious prey, and defended prey support few predators, thus favoring undefended prey, we imagine a mix of vulnerable and defended prey species in most natural ecosystems. In particular, defenses such as armor, spines, and noxiousness seem to be favored when the prey experience high encounter rates with predators and when they need to maintain conspicuous feeding activities in the face of these threats. Cancer cells almost always live in microenvironments with cytotoxic threats from the innate and adaptive immune system. They cannot really flee nor hide (entirely escape detection), so armor-like, spine-like, and noxious defenses should be the norm. While camouflage, as such, is not an option because immune cells will encounter just about all cells in the tumor, there can be a form of serendipitous or perhaps adaptive protection by having a ring of cancer-associated fibroblasts form a physical enclosure of cells and extracellular matrix that blocks off immune cells (Hilmi et al., 2020).
For prey with armor, spines, and noxious defenses that are targeted by immune systems, it is less straightforward to predict how the frequency of defended prey in the system will affect the effectiveness of each defense. Soldier-caste-type predators will not avoid attacking defended prey just because easier prey are available. A lot will depend on the feedback between the frequency of defended cells and the degree of anti-tumor immune infiltration. If, for instance, defense comes in the form of not presenting antigens, then cytotoxic T-cells will largely ignore them and be more available to attack antigen presenting cancer cells. If all other cancer cells are defended, then it behooves a cancer cell to conform. In this case, all “armored” cancer cells become an evolutionarily stable strategy (ESS). If the cancer’s defense comes in the form of directly or indirectly causing the inactivation or death of cytotoxic immune cells, then these cancer cells provide a “public good” that may promote freeloading at the ESS. A cancer cell surrounded by noxiously defended cancer cells may need no defense; while a cancer cell surrounded by undefended ones may do best by being noxious. With noxious defenses, the ESS community may be a mix of defended and less defended cancer cells. The coexistence of immune-evasive and immune-susceptible cancer cell types is an interesting and important avenue for research.
Aposematism, or warning signaling, is widespread among animals with noxious defenses (Berenbaum, 1995; Stankowich et al., 2011; Wang et al., 2018b) and dangerous spines (Inbar and Lev-Yadun, 2005). When the prey can induce unacceptable acute or chronic injury, the interests of the prey and predator become aligned: the prey does not want to be attacked and the predator does not want to risk injury. Aposematic signals are not inherently deterring but are so because they are paired with dangerous (noxious and/or spiny) defenses. Likely for this reason, aposematic signals are associated with slow mobility, such as the slow and non-evasive flight styles of noxious butterflies (Srygley, 1994). Since aposematic prey have dangerous defenses, they do not need to flee as a primary defense, so speed is unimportant. Slow movement may also increase the visibility of the warning signals (Srygley, 1994).
Recognition of aposematic signals will increase with instances of Müllerian mimicry, when similar-looking noxious species with shared predators evolve to look even more similar. However, aposematism also opens the door for Batesian mimicry, where a non-dangerous prey dishonestly displays the aposematic signal, taking advantage of predators’ reluctance to pursue dangerous prey. The Batesian mimic essentially freeloads off the dangerous prey, reaping the benefits of the defense without incurring the associated costs.
A variety of anti-immune adaptations by cancer cells amount to Batesian mimicry, where the normal cells are the model and the cancer cells are the mimic. For example, cultured melanoma cells that decrease expression of the melanoma differentiation antigen gp100 evade detection by introduced T-cells (Landsberg et al., 2012) by mimicking normal cells. They do this while remaining cancer cells and not by becoming normal cells. At first glance it seems odd to classify this type of mimicry as Batesian. Unlike traditional predators that are working on commission, immune cells are not deterred from pursuing dangerous prey. However, a danger which immune cells do avoid is auto-immunity or self-attack. Cancer cells take advantage of immune cells’ reluctance to attack host cells by pretending to be host cells. This might seem like a form of camouflage. It is not. In nature camouflage prevents predator detection, it does not involve the predator finding the prey and deciding to pass it by. Cancer cells pretending to be normal cells do not evade immune cell detection, after all the immune cells are contacting normal cells and cancer cells alike to detect the antigens on their surfaces. Rather, what the mimic cancer cells really evade is accurate identification by immune cells. In this sense, it would be useful to interpret disguised cancer cells as Batesian mimicry. They do not imitate dangerous prey, but they imitate the thing that would be dangerous for immune cells to attack. Figure 2 outlines these dynamics. One will note that for cancer cells mimicking normal cells, this strategy conforms to Batesian mimicry (mimicking the prey that is dangerous to attack) and armor (increasing predator handling time). Both perspectives may prove useful for future research.
Anti-predator defenses in the form of armor, spines, and noxious chemicals are found widely across taxa in nature. They are also displayed by cancer cells. Prey using these modes of defense can continue activities such as foraging and proliferating even when predators are numerous and nearby, which can offer a distinct advantage over strategies such as fleeing, camouflage, or seeking refuge. Armor-like defenses are those which make prey difficult and time-consuming for predators to subdue and/or handle. Noxious defenses make prey dangerous; they can temporarily or permanently incapacitate predators. Spiny defenses may fall into either or both functional categories. Traditional predators will be effort-deterred and injury-deterred from pursuing prey defended in these ways.
Cancer cells experience incessant predation pressure from the immune system. By the nature of their morphology and environment, cancer cells cannot flee, hide, or remain camouflaged. Armor, spines, and noxious chemicals are thus their primary recourse for defense. These defense modes take considerably different forms in cancer cells than in other taxa, but their essential functions are the same. However, the motives of the predators are quite different in cancer versus natural systems. The cytotoxic cells of the immune system are non-traditional, soldier-caste-type predators which do not work on commission. They are not deterred from pursuing difficult or dangerous prey. Because immune cells carefully avoid attacking normal cells of the whole organism, cancer cells, like Batesian mimics, frequently capitalize on this reticence by mimicking normal cells. Furthermore, the regulatory agents of the immune system can control the deployment, proliferation, and death of cytotoxic immune cells. Cancer cells “hijack” these communications to not only suppress cytotoxic immune cells but to amplify pro-tumor immune cells.
For ecologists, cancer provides replicated worlds for studying the parallel and convergent evolution of anti-predator adaptations within different microenvironments of a tumor (habitat scale in ecology) and separate tumors of the host (biome scale with the same taxa). The same evolutionary ecology can be studied in the same cancer across patients (replaying the tape of life for roughly the same taxa) or different cancers across patients (replaying the tape of life with different taxonomic origins; for instance, colon cancer versus liver cancer patients). Furthermore, cancer research centers often possess technologies and equipment related to cell culturing, cell sorting, molecular analyses, mouse model experiments, and histologies that might be unavailable to ecologists studying natural systems.
For oncologists, empirical and conceptual work on anti-predator adaptations draws attention to the functional role that traits play in deterring predation, not just the form. Most immunotherapies, or cancer therapies in general, take advantage of a molecular target, or in the case of recent advances like CAR T-cell therapy, the actual cancer cells themselves as targetable. In our context, this therapy makes cancer cells distinguishable from normal cells, thus acting to strip away the cancer cells’ armor or Batesian mimicry. This can create a bit of tunnel vision where two things are overlooked. First, the entire context of the cancer cells’ adaptations, both to their microenvironment and for immune evasion, might be overlooked. Second, less attention is paid to how the cancer cells might or will evolve therapy resistance and effective countermeasures to the therapy.
CAR T-cell therapy can be highly effective in promoting a complete or partial response in some patients while barely hindering progressive disease in others (Wagner et al., 2020). Majzner and Mackall (2018) note that CAR T-cell therapy failure in solid tumors often results from the cancer cells downregulating or eliminating the T-cell presenting antigen. In response to issues of toxicity, T-cell infiltration into the tumor, downregulation of immunogenicity, and tumor heterogeneity, much research is going into manufacturing safer, more effective, and more applicable CAR T-cell products (Rafiq et al., 2020), which is all well and good. But, little of this work considers the ecological context of the cancer cells, the cost and benefits of their current immune evasion strategies, and the ease or difficulty that they will have in evolving an effective anti-predator response. The same applies to research on the efficacy of other current immunotherapies such as Pembrolizumab (anti PD-1 inhibitor, which works against “spines” and “noxiousness”), Nivolumab (anti PD-1 inhibitor), and Ipilimumab (CTLA-4 blocker, which works against “noxiousness”). Often Nivolumab and Ipilimumab are given together, and any of these immunotherapies may be at times combined with chemotherapy, radiation therapy, and surgery. Such therapies act against the current adaptations of the cancer cells but do not anticipate how the cancer cells will evolve new forms of immune evasion using armor, spines, or noxiousness. How easily can they evolve and at what cost to the cancer cells’ performance?
We feel that incorporating the perspectives of this overview can provide insights and direct research into the successes and failures of diverse immunotherapies and, perhaps, suggest novel therapeutic strategies. These concepts may help with anticipating rather than reacting to the cancer’s evolution of immunosuppression and resistance to immunotherapies. Just as the cancer cells exploit weaknesses in the immune system, so should the physicians find weaknesses in the current strategies of the cancer cells and anticipate how they will respond to various immunotherapies. If the physician is going to use the immune system for biological control, the therapy regime must be dynamic and change as the cancer cells’ anti-predator strategies change. Knowledge from ecology may assist in framing the anti-predator options available to the cancer cells and suggest how to anticipate the kinds of armor, spines, and noxiousness that might occur in response the therapeutic regimens. Most therapies are given until disease progression, at which point the cancer cells have long since evolved countermeasures. With this in mind, physicians can use immunotherapies in a more dynamic fashion to anticipate and steer the cancers’ evolution while driving down the population of cancer cells (Cunningham et al., 2012).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
JP and JB developed the concept and guided the collaboration. JP, MM, CW, and JB made major writing contributions. KF, EP, AP, KC, and SA contributed to writing. JP and KF designed the figures. MM, EP, KC, and JB contributed to the figures. JP formatted the manuscript. All authors conducted the literature research and contributed comments and edits to the manuscript.
JB acknowledges funding from NIH/NCI 1U54CA193489-01A1, “Cancer as a Complex Adaptive System,” and NIH/NCI U54 Supplement, “The tumor-host evolutionary arms race.”
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alterman, L. (1995). “Toxins and toothcombs: potential allospecific chemical defenses in Nycticebus and Perodicticus,” in Creatures of the Dark: The Nocturnal Prosimians, eds L. Alterman, G. A. Doyle, and M. K. Izard (Boston, MA: Springer), 413–424. doi: 10.1007/978-1-4757-2405-9_24
Anichini, A., Perotti, V. E., Sgambelluri, F., and Mortarini, R. (2020). Immune escape mechanisms in non small cell lung cancer. Cancers (Basel). 12:3605. doi: 10.3390/cancers12123605
Atefi, M., Avramis, E., Lassen, A., Wong, D. J. L., Robert, L., Foulad, D., et al. (2014). Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin. Cancer Res. 20, 3446–3457. doi: 10.1158/1078-0432.ccr-13-2797
Augoff, K., Hryniewicz-Jankowska, A., and Tabola, R. (2020). Invadopodia: clearing the way for cancer cell invasion. Ann. Transl. Med. 8:902. doi: 10.21037/atm.2020.02.157
Baty, A. M., Eastburn, C. C., Techkarnjanaruk, S., Goodman, A. E., and Geesey, G. G. (2000). Spatial and temporal variations in chitinolytic gene expression and bacterial biomass production during chitin degradation. Appl. Environ. Microbiol. 66, 3574–3585. doi: 10.1128/aem.66.8.3574-3585.2000
Beatty, G. L., and Gladney, W. L. (2015). Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 21, 687–692. doi: 10.1158/1078-0432.ccr-14-1860
Blank, C. U., Haanen, J. B., Ribas, A., and Schumacher, T. N. (2016). The “cancer immunogram”. Science 352, 658–660. doi: 10.1126/science.aaf2834
Breed, M. D., and Bennett, B. (1987). “Kin recognition in highly eusocial insects,” in Kin Recognition in Animals, eds D. J. C. Fletcher and C. D. Michener (New York, NY: John Wiley & Sons), 243–285.
Brown, J. S. (2016). Why Darwin would have loved evolutionary game theory. Proc. R. Soc. Lond. B Biol. Sci. 283:20160847. doi: 10.1098/rspb.2016.0847
Brown, J. S., and Kotler, B. P. (2004). Hazardous duty pay and the foraging cost of predation. Ecol. Lett. 7, 999–1014. doi: 10.1111/j.1461-0248.2004.00661.x
Burnet, M. (1957). Cancer: a biological approach: I. The processes of control. Br. Med. J. 1, 779–786. doi: 10.1136/bmj.1.5022.779
Butte, M. J., Keir, M. E., Phamduy, T. B., Sharpe, A. H., and Freeman, G. J. (2007). Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27, 111–122. doi: 10.1016/j.immuni.2007.05.016
Chandra, J., McCormick, T. S., Imamura, Y., Mukherjee, P. K., and Ghannoum, M. A. (2007). Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro-and anti-inflammatory cytokines. Infect. Immun. 75, 2612–2620. doi: 10.1128/iai.01841-06
Coley, W. B. (1891). II. Contribution to the knowledge of sarcoma. Ann. Surg. 14, 199–220. doi: 10.1097/00000658-189112000-00015
Corthay, A. (2014). Does the immune system naturally protect against cancer? Front. Immunol. 5:197. doi: 10.3389/fimmu.2014.00197
Crosland, M. W. J. (1990). Variation in ant aggression and kin discrimination ability within and between colonies. J. Insect Behav. 3, 359–379. doi: 10.1007/bf01052114
Cunningham, J. J., Gatenby, R. A., and Brown, J. S. (2012). Evolutionary dynamics in cancer therapy. Mol. Phylogenet. 8, 2094–2100. doi: 10.1021/mp2002279.Evolutionary
Cuyler, W. K. (1924). Observations on the habits of the striped skunk (Mephitis mesomelas varians). J. Mammal. 5, 180–189. doi: 10.2307/1373285
Dahl, E. (2019). Dinophysis Bloom in Norway. US National Office Harmful Algal Bloom. Woods Hole Oceanographic Institution. Available online at: https://hab.whoi.edu/regions-resources/gallery/photos-blooms/ (accessed March 13, 2021).
DavidpBowman (2014). Trichocyst. Wikimedia Commons, CC BY-SA 4.0. Available online at: https://commons.wikimedia.org/wiki/File:Trichocyst.jpg (accessed March 13, 2021).
Davies, R. G. (1988). “Insect structure and function,” in Outlines of Entomology, ed. R. G. Davies (Dordrecht: Springer), 7–96. doi: 10.1007/978-94-009-1189-5_2
de Guillebon, E., Dardenne, A., Saldmann, A., Séguier, S., Tran, T., Paolini, L., et al. (2020). Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int. J. Cancer 147, 1509–1518. doi: 10.1002/ijc.32889
de Pillis, L. G., Radunskaya, A. E., and Wiseman, C. L. (2005). A validated mathematical model of cell-mediated immune response to tumor growth. Cancer Res. 65, 7950–7958. doi: 10.1158/0008-5472.can-05-0564
Demaria, O., Cornen, S., Daëron, M., Morel, Y., Medzhitov, R., and Vivier, E. (2019). Harnessing innate immunity in cancer therapy. Nature 574, 45–56. doi: 10.1038/s41586-019-1593-5
DeWitt, P. D., Visscher, D. R., Schuler, M. S., and Thiel, R. P. (2019). Predation risks suppress lifetime fitness in a wild mammal. Oikos 128, 790–797. doi: 10.1111/oik.05935
Dong, H., Zhu, G., Tamada, K., and Chen, L. (1999). B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5, 1365–1369. doi: 10.1038/70932
Dullens, H. F. J., Van Der Tol, M. W. M., De Weger, R. A., and Den Otter, W. (1986). A survey of some formal models in tumor immunology. Cancer Immunol. Immunother. 23, 159–164. doi: 10.1007/bf00205644
Dunn, G. P., Old, L. J., and Schreiber, R. D. (2004). The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360. doi: 10.1146/annurev.immunol.22.012703.104803
Eftimie, R., Gillard, J. J., and Cantrell, D. A. (2016). Mathematical models for immunology: current state of the art and future research directions. Bull. Math. Biol. 78, 2091–2134. doi: 10.1007/s11538-016-0214-9
Eisner, T. (1970). “Chemical defense against predation in arthropods,” in Chemical Ecology, eds J. B. Simeone and E. Sondheimer (Cambridge, MA: Academic Press), 157–217. doi: 10.1016/b978-0-12-654750-4.50014-1
Eisner, T., Eisner, M., and Siegler, M. (2005). Secret Weapons: Defenses of Insects, Spiders, Scorpions, and Other Many-Legged Creatures. Cambridge, MA: Harvard University Press.
Elbroch, L. M., Hoogesteijn, R., and Quigley, H. (2016). Cougars (Puma concolor) killed by North American porcupines (Erethizon dorsatum). Can. Field Nat. 130, 53–55. doi: 10.22621/cfn.v130i1.1793
Esfahani, K., Roudaia, L., Buhlaiga, N., Del Rincon, S. V., Papneja, N., and Miller, W. H. Jr. (2020). A review of cancer immunotherapy: from the past, to the present, to the future. Curr. Oncol. 27:S87.
Fishwild, T. G., and Gamboa, G. J. (1992). Colony defence against conspecifics: caste-specific differences in kin recognition by paper wasps, Polistes fuscatus. Anim. Behav. 43, 95–102. doi: 10.1016/s0003-3472(05)80075-2
Flenner, I. D. A., Olne, K., Suhling, F., and Sahlén, G. (2009). Predator-induced spine length and exocuticle thickness in Leucorrhinia dubia (Insecta: Odonata): a simple physiological trade-off? Ecol. Entomol. 34, 735–740. doi: 10.1111/j.1365-2311.2009.01129.x
Forbes, L. S. (1989). Prey defences and predator handling behaviour: the dangerous prey hypothesis. Oikos 55, 155–158. doi: 10.2307/3565418
Forti, L. R., da Silva, R. M., Manzani, P. R., and Toledo, L. F. (2018). Snakes vs. porcupines: when preys leave mortal injuries. Herpetol. Notes 11, 715–718.
Fouad, Y. A., and Aanei, C. (2017). Revisiting the hallmarks of cancer. Am. J. Cancer Res. 7, 1016–1036.
Fowler, S. W., and Fisher, N. S. (1983). Viability of marine phytoplankton in zooplankton fecal pellets. Deep Sea Res. Part A. Oceanogr. Res. Pap. 30, 963–969. doi: 10.1016/0198-0149(83)90051-1
Fox, C. (2001). Cancer Cells, AV-8711-3170. National Cancer Institute. Available online at: https://visualsonline.cancer.gov/details.cfm?imageid=2306 (accessed March 13, 2021).
Francisco, L. M., Salinas, V. H., Brown, K. E., Vanguri, V. K., Freeman, G. J., Kuchroo, V. K., et al. (2009). PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029. doi: 10.1084/jem.20090847
Fridman, W. H. (2018). From cancer immune surveillance to cancer immunoediting: birth of modern immuno-oncology. J. Immunol. 201, 825–826. doi: 10.4049/jimmunol.1800827
Gall, B. G., Spivey, K. L., Chapman, T. L., Delph, R. J., Brodie, E. D. Jr., and Wilson, J. S. (2018). The indestructible insect: velvet ants from across the United States avoid predation by representatives from all major tetrapod clades. Ecol. Evol. 8, 5852–5862. doi: 10.1002/ece3.4123
Gartner, J. (2021). Lymphocytes and Cancer Cell, Image 488635633. New York, NY: Getty Images Science Photo Library.
Gatenby, R. A., and Brown, J. (2017). Mutations, evolution and the central role of a self-defined fitness function in the initiation and progression of cancer. Biochim. Biophys. Acta Rev. Cancer 1867, 162–166. doi: 10.1016/j.bbcan.2017.03.005
Gatenby, R. A., Avdieiev, S., Tsai, K. Y., and Brown, J. S. (2020). Integrating genetic and nongenetic drivers of somatic evolution during carcinogenesis: the biplane model. Evol. Appl. 13, 1651–1659. doi: 10.1111/eva.12973
Ge, Y., Zhang, L., Qin, Z., Wang, Y., Liu, P., Tan, S., et al. (2019). Different predation capacities and mechanisms of Harmonia axyridis (Coleoptera: Coccinellidae) on two morphotypes of pear psylla Cacopsylla chinensis (Hemiptera: Psyllidae). PLoS One 14:e0215834. doi: 10.1371/journal.pone.0215834
Greenwood, A. K., Peichel, C. L., and Zottoli, S. J. (2010). Distinct startle responses are associated with neuroanatomical differences in pufferfishes. J. Exp. Biol. 213, 613–620. doi: 10.1242/jeb.037085
Grivennikov, S. I., Greten, F. R., and Karin, M. (2010). Immunity, inflammation, and cancer. Cell 140, 883–899.
Grønning, J., and Kiørboe, T. (2020). Diatom defence: grazer induction and cost of shell-thickening. Funct. Ecol. 34, 1790–1801. doi: 10.1111/1365-2435.13635
Guirguis, R., Margulies, I., Taraboletti, G., Schiffmann, E., and Liotta, L. (1987). Cytokine-induced pseudopodial protrusion is coupled to tumour cell migration. Nature 329, 261–263. doi: 10.1038/329261a0
Hahn, M. W., Moore, E. R. B., and Höfle, M. G. (1999). Bacterial filament formation, a defense mechanism against flagellate grazing, is growth rate controlled in bacteria of different phyla. Appl. Environ. Microbiol. 65, 25–35. doi: 10.1128/aem.65.1.25-35.1999
Haim, A., Van Aarde, R. J., and Skinner, J. D. (1990). Metabolism and thermoregulation in the Cape porcupine, Hystrix africaeaustralis. Physiol. Zool. 63, 795–802. doi: 10.1086/physzool.63.4.30158177
Halitschke, R., Stenberg, J. A., Kessler, D., Kessler, A., and Baldwin, I. T. (2008). Shared signals–‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol. Lett. 11, 24–34.
Hall-Stoodley, L., Costerton, J. W., and Stoodley, P. (2004). Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108. doi: 10.1038/nrmicro821
Hamm, C. E., Merkel, R., Springer, O., Jurkojc, P., Maier, C., Prechtel, K., et al. (2003). Architecture and material properties of diatom shells provide effective mechanical protection. Nature 421, 841–843. doi: 10.1038/nature01416
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. doi: 10.1016/j.cell.2011.02.013
Harris, R. J., and Jenner, R. A. (2019). Evolutionary ecology of fish venom: adaptations and consequences of evolving a venom system. Toxins (Basel). 11, 60. doi: 10.3390/toxins11020060
Harvey, E. L., Bidle, K. D., and Johnson, M. D. (2015). Consequences of strain variability and calcification in Emiliania huxleyi on microzooplankton grazing. J. Plankton Res. 37, 1137–1148.
Hedgcock, C. (2013). Bombardier beetle, Brachinus elongatulus (Chaudoir). Available online at: https://charleshedgcock.photoshelter.com/gallery-image/Charismatic-Microfauna/G0000rqy5jilPT5w/I00008wHp8dL3NhY (accessed June 7, 2021).
Hertz, J. C. (2007). Velvet Ants, Mutillidae (Insecta: Hymenoptera): EENY-378. Gainesville, FL: Florida Cooperative Extension Service Institute of Food and Agricultural Sciences University of Florida.
Heusinkveld, M., and van Der Burg, S. H. (2011). Identification and manipulation of tumor associated macrophages in human cancers. J. Transl. Med. 9, 1–14.
Hill, R. V. (2006). Comparative anatomy and histology of xenarthran osteoderms. J. Morphol. 267, 1441–1460. doi: 10.1002/jmor.10490
Hilmi, M., Nicolle, R., Bousquet, C., and Neuzillet, C. (2020). Cancer-associated fibroblasts: accomplices in the tumor immune evasion. Cancers (Basel). 12:2969. doi: 10.3390/cancers12102969
Hirschfeld, J. (2014). Dynamic interactions of neutrophils and biofilms. J. Oral Microbiol. 6:26102. doi: 10.3402/jom.v6.26102
Hodge, J. R., Alim, C., Bertrand, N. G., Lee, W., Price, S. A., Tran, B., et al. (2018). Ecology shapes the evolutionary trade-off between predator avoidance and defence in coral reef butterflyfishes. Ecol. Lett. 21, 1033–1042. doi: 10.1111/ele.12969
Horton, B. L., Williams, J. B., Cabanov, A., Spranger, S., and Gajewski, T. F. (2018). Intratumoral CD8+ T-cell apoptosis is a major component of T-cell dysfunction and impedes antitumor immunity. Cancer Immunol. Res. 6, 14–24. doi: 10.1158/2326-6066.cir-17-0249
Houghton, A. N. (1994). Cancer antigens: immune recognition of self and altered self. J. Exp. Med. 180, 1–4. doi: 10.1084/jem.180.1.1
Huber, V., Camisaschi, C., Berzi, A., Ferro, S., Lugini, L., Triulzi, T., et al. (2017). “Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation,” in Seminars in Cancer Biology, eds S. Harguindey and S. J. Reshkin (Amsterdam: Elsevier), 74–89. doi: 10.1016/j.semcancer.2017.03.001
Inbar, M., and Lev-Yadun, S. (2005). Conspicuous and aposematic spines in the animal kingdom. Naturwissenschaften 92, 170–172. doi: 10.1007/s00114-005-0608-2
Jansen, R., Sodeinde, O., Soewu, D., Pietersen, D. W., Alempijevic, D., and Ingram, D. J. (2020). “White-bellied pangolin Phataginus tricuspis (Rafinesque, 1820),” in Pangolins, eds D. W. S. Challender, H. C. Nash, and C. Waterman (Amsterdam: Elsevier), 139–156. doi: 10.1016/b978-0-12-815507-3.00009-5
Jensen, E. T., Kharazmi, A., Høiby, N., and Costeron, J. W. (1992). Some bacterial parameters influencing the neutrophil oxidative burst response to Pseudomonas aeruginosa biofilms. APMIS 100, 727–733. doi: 10.1111/j.1699-0463.1992.tb03991.x
Jesaitis, A. J., Franklin, M. J., Berglund, D., Sasaki, M., Lord, C. I., Bleazard, J. B., et al. (2003). Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171, 4329–4339. doi: 10.4049/jimmunol.171.8.4329
Jeschke, J. M. (2006). Density-dependent effects of prey defenses and predator offenses. J. Theor. Biol. 242, 900–907. doi: 10.1016/j.jtbi.2006.05.017
Jürgens, K., Arndt, H., and Rothhaupt, K. O. (1994). Zooplankton-mediated changes of bacterial community structure. Microb. Ecol. 27, 27–42.
Kageyama, A., and Sugiura, S. (2016). Caterpillar hairs as an anti-parasitoid defence. Sci. Nat. 103, 86.
Kareva, I. (2011). What can ecology teach us about cancer? Transl. Oncol. 4, 266–270. doi: 10.1593/tlo.11154
Kareva, I., Berezovskaya, F., and Castillo-Chavez, C. (2010). Myeloid cells in tumour–immune interactions. J. Biol. Dyn. 4, 315–327. doi: 10.1080/17513750903261281
Katzner, T., Miller, T. A., Rodrigue, J., and Shaffer, S. (2015). A most dangerous game: death and injury to birds from porcupine quills. Wilson J. Ornithol. 127, 102–108. doi: 10.1676/14-066.1
Kaur, G., and Ahmad, N. (2014). On study of immune response to tumor cells in prey-predator system. Int. Sch. Res. Notices 2014:346597.
Keck, W. (2011). Skunk about to Spray. National Park Service. Available online at: https://commons.wikimedia.org/wiki/File:Skunk_about_to_spray.jpg (accessed March 13, 2021).
Kerbis Peterhans, J. C., Celesia, G. G., and Gnoske, T. P. (2019). Lion-porcupine interactions in Africa, including impacts on lion predatory behavior. J. East African Nat. Hist. 108, 1–15. doi: 10.2982/028.108.0101
Kharazmi, A. (1991). Mechanisms involved in the evasion of the host defence by Pseudomonas aeruginosa. Immunol. Lett. 30, 201–205. doi: 10.1016/0165-2478(91)90026-7
Khong, H. T., and Restifo, N. P. (2002). Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat. Immunol. 3, 999–1005. doi: 10.1038/ni1102-999
Kingdon, J., Agwanda, B., Kinnaird, M., O’Brien, T., Holland, C., Gheysens, T., et al. (2012). A poisonous surprise under the coat of the African crested rat. Proc. R. Soc. Lond. B Biol. Sci. 279, 675–680. doi: 10.1098/rspb.2011.1169
Klompmaker, A. A., Kelley, P. H., Chattopadhyay, D., Clements, J. C., Huntley, J. W., and Kowalewski, M. (2019). Predation in the marine fossil record: studies, data, recognition, environmental factors, and behavior. Earth Sci. Rev. 194, 472–520. doi: 10.1016/j.earscirev.2019.02.020
Knoll, G., Haacke-Bell, B., and Plattner, H. (1991). Local trichocyst exocytosis provides an efficient escape mechanism for Paramecium cells. Eur. J. Protistol. 27, 381–385. doi: 10.1016/S0932-4739(11)80256-7
Koebel, C. M., Vermi, W., Swann, J. B., Zerafa, N., Rodig, S. J., Old, L. J., et al. (2007). Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907. doi: 10.1038/nature06309
Kolb, A., and Strom, S. (2013). An inducible antipredatory defense in haploid cells of the marine microalga Emiliania huxleyi (Prymnesiophyceae). Limnol. Oceanogr. 58, 932–944. doi: 10.4319/lo.2013.58.3.0932
Künzler, M. (2018). How fungi defend themselves against microbial competitors and animal predators. PLoS Pathog. 14:e1007184. doi: 10.1371/journal.ppat.1007184
Landsberg, J., Kohlmeyer, J., Renn, M., Bald, T., Rogava, M., Cron, M., et al. (2012). Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 490, 412–416. doi: 10.1038/nature11538
Lazzeri, L., Senini, C., and Mori, E. (2020). Interspecific aggressions between crested porcupines and roe deer. Animals 10:623. doi: 10.3390/ani10040623
Lee, C.-H., Yelensky, R., Jooss, K., and Chan, T. A. (2018). Update on tumor neoantigens and their utility: why it is good to be different. Trends Immunol. 39, 536–548. doi: 10.1016/j.it.2018.04.005
Lepczyk, C. A., Mertig, A. G., and Liu, J. (2004). Landowners and cat predation across rural-to-urban landscapes. Biol. Conserv. 115, 191–201. doi: 10.1016/s0006-3207(03)00107-1
Lima, S. L., and Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. doi: 10.1139/z90-092
Lindquist, N. (2002). Chemical defense of early life stages of benthic marine invertebrates. J. Chem. Ecol. 28, 1987–2000. doi: 10.1023/A:1020745810968
Lindsay, S. (2019). Placoid Scales. Austrailian Museum. Available online at: https://australian.museum/learn/animals/fishes/placoid-scales/#main (accessed March 13, 2021).
Lloyd, M. C., Cunningham, J. J., Bui, M. M., Gillies, R. J., Brown, J. S., and Gatenby, R. A. (2016). Darwinian dynamics of intratumoral heterogeneity: not solely random mutations but also variable environmental selection forces. Cancer Res. 76, 3136–3144. doi: 10.1158/0008-5472.can-15-2962
Lovegrove, B. (2000). The zoogeography of mammalian basal metabolic rate. Am. Nat. 156, 201–219. doi: 10.1086/303383
Lovegrove, B. G. (2001). The evolution of body armor in mammals: plantigrade constraints of large body size. Evolution (N. Y.) 55, 1464–1473. doi: 10.1111/j.0014-3820.2001.tb00666.x
Lürling, M., De Lange, H. J., and Van Donk, E. (1997). Changes in food quality of the green alga Scenedesmus induced by Daphnia infochemicals: biochemical composition and morphology. Freshw. Biol. 38, 619–628. doi: 10.1046/j.1365-2427.1997.00225.x
Ma, H., Krock, B., Tillmann, U., and Cembella, A. (2009). Preliminary characterization of extracellular allelochemicals of the toxic marine dinoflagellate Alexandrium tamarense using a Rhodomonas salina bioassay. Mar. Drugs 7, 497–522. doi: 10.3390/md7040497
Maia-Silva, C., Hrncir, M., Koedam, D., Machado, R. J. P., and Imperatriz-Fonseca, V. L. (2013). Out with the garbage: the parasitic strategy of the mantisfly Plega hagenella mass-infesting colonies of the eusocial bee Melipona subnitida in northeastern Brazil. Naturwissenschaften 100, 101–105. doi: 10.1007/s00114-012-0994-1
Majzner, R. G., and Mackall, C. L. (2018). Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8, 1219–1226. doi: 10.1158/2159-8290.cd-18-0442
Maley, C. C., Aktipis, A., Graham, T. A., Sottoriva, A., Boddy, A. M., Janiszewska, M., et al. (2017). Classifying the evolutionary and ecological features of neoplasms. Nat. Rev. Cancer 17:605. doi: 10.1038/nrc.2017.69
Materialscientist (2009). Gypsy Moth Caterpillar. Wikimedia Commons, CC BY-SA 3.0. Available online at: https://commons.wikimedia.org/wiki/File:Gypsy_moth_caterpillar.JPG (accessed March 13, 2021).
Mathis, A., and Chivers, D. P. (2003). Overriding the oddity effect in mixed-species aggregations: group choice by armored and nonarmored prey. Behav. Ecol. 14, 334–339. doi: 10.1093/beheco/14.3.334
Matz, C., and Kjelleberg, S. (2005). Off the hook–how bacteria survive protozoan grazing. Trends Microbiol. 13, 302–307. doi: 10.1016/j.tim.2005.05.009
Matz, C., Deines, P., and Jürgens, K. (2002). Phenotypic variation in Pseudomonas sp. CM10 determines microcolony formation and survival under protozoan grazing. FEMS Microbiol. Ecol. 39, 57–65. doi: 10.1111/j.1574-6941.2002.tb00906.x
Matz, C., Webb, J. S., Schupp, P. J., Phang, S. Y., Penesyan, A., Egan, S., et al. (2008). Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. PLoS One 3:e2744. doi: 10.1371/journal.pone.0002744
McLean, E. B., and Godin, J.-G. J. (1989). Distance to cover and fleeing from predators in fish with different. Oikos 55, 281–290. doi: 10.2307/3565586
McLean, S. (2014). Scent glands of the common brushtail possum (Trichosurus vulpecula). New Zeal. J. Zool. 41, 193–202. doi: 10.1080/03014223.2014.899506
McNab, B. K. (1984). Physiological convergence amongst ant-eating and termite-eating mammals. J. Zool. Lond. 203, 485–510. doi: 10.1111/j.1469-7998.1984.tb02345.x
McNab, B. K. (2008). An analysis of the factors that influence the level and scaling of mammalian BMR. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 151, 5–28. doi: 10.1016/j.cbpa.2008.05.008
McNamara, J. M., and Houston, A. I. (1987). Starvation and predation as factors limiting population size. Ecology 68, 1515–1519. doi: 10.2307/1939235
Mengak, M. T. (2005). Nine-Banded Armadillo (Dasypus novemcinctus): Natural History Publication Series 4. Athens, GA: Universit of Georgia Warnell School of Forestry & Natural Resources.
Mergler, C. J., and Gall, B. G. (2021). Has the indestructible insect met its match? Velvet ants as prey to bufonid toads. Ethol. Ecol. Evol. 33, 15–24. doi: 10.1080/03949370.2020.1789747
Merlo, L. M. F., Pepper, J. W., Reid, B. J., and Maley, C. C. (2006). Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 6, 924–935. doi: 10.1038/nrc2013
Mistry, U. (2019). Lionfish in India’s Andaman Islands. Coral Reef Image Bank. Available online at: https://www.coralreefimagebank.org/fish (accessed March 13, 2021).
Mohme, M., Riethdorf, S., and Pantel, K. (2017). Circulating and disseminated tumour cells—mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 14:155. doi: 10.1038/nrclinonc.2016.144
Mori, E., Maggini, I., and Menchetti, M. (2014). When quills kill: the defense strategy of the crested porcupine Hystrix cristata L., 1758. Mammalia 78, 229–234.
Multhoff, G., and Vaupel, P. (2020). “Hypoxia compromises anti-cancer immune responses,” in Oxygen Transport to Tissue XLI. Advances in Experimental Medicine and Biology, Vol. 1232, eds P. D. Ryu, J. LaManna, D. Harrison, and S. S. Lee (Cham: Springer), 131–143. doi: 10.1007/978-3-030-34461-0_18
Munday, P. L., Schubert, M., Baggio, J. A., Jones, G. P., Caley, M. J., and Grutter, A. S. (2003). Skin toxins and external parasitism of coral-dwelling gobies. J. Fish Biol. 62, 976–981. doi: 10.1046/j.1095-8649.2003.00078.x
Nam, A. S., Chaligne, R., and Landau, D. A. (2020). Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat. Rev. Genet. 22, 3–18. doi: 10.1038/s41576-020-0265-5
NCI (2021). NCI Dictionaries. National Cancer Institute. Available online at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cigarette (accessed May 17, 2021).
Nekaris, K. A.-I., Moore, R. S., Rode, E. J., and Fry, B. G. (2013). Mad, bad and dangerous to know: the biochemistry, ecology and evolution of slow loris venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 19:21. doi: 10.1186/1678-9199-19-21
Nekaris, K., Weldon, A., Imron, M. A., Maynard, K. Q., Nijman, V., Poindexter, S. A., et al. (2019). Venom in furs: facial masks as aposematic signals in a venomous mammal. Toxins (Basel). 11:93. doi: 10.3390/toxins11020093
Nilsson, P. A., and Brönmark, C. (2000). Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos 88, 539–546. doi: 10.1034/j.1600-0706.2000.880310.x
Nilsson, P. A., Brönmark, C., and Pettersson, L. B. (1995). Benefits of a predtor-induced morphology in crucian carp. Oecologia 104, 291–296. doi: 10.1007/bf00328363
O’Riain, M. J., and Jarvis, J. U. M. (1997). Colony member recognition and xenophobia in the naked mole-rat. Anim. Behav. 53, 487–498. doi: 10.1006/anbe.1996.0299
Oiseth, S. J., and Aziz, M. S. (2017). Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat. 3, 250–261. doi: 10.20517/2394-4722.2017.41
Pančić, M., and Kiørboe, T. (2018). Phytoplankton defence mechanisms: traits and trade-offs. Biol. Rev. 93, 1269–1303. doi: 10.1111/brv.12395
Pandya, P. H., Murray, M. E., Pollok, K. E., and Renbarger, J. L. (2016). The immune system in cancer pathogenesis: potential therapeutic approaches. J. Immunol. Res. 2016:4273943.
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264. doi: 10.1038/nrc3239
Pastorekova, S., and Gillies, R. J. (2019). The role of carbonic anhydrase IX in cancer development: Links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 38, 65–77. doi: 10.1007/s10555-019-09799-0
Pemberton, C. (2009). Velvet Ant. Wikimedia Commons, CC BY-SA 3.0. Available online at: https://commons.wikimedia.org/wiki/File:Velvet_Ant.jpg (accessed March 13, 2021).
Pernthaler, J., Sattler, B., Simek, K., Schwarzenbacher, A., and Psenner, R. (1996). Top-down effects on the size-biomass distribution of a freshwater bacterioplankton community. Aquat. Microb. Ecol. 10, 255–263. doi: 10.3354/ame010255
Philip, M., and Schietinger, A. (2019). Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr. Opin. Immunol. 58, 98–103. doi: 10.1016/j.coi.2019.04.014
Pickett-Heaps, J. D., Carpente, J., and Koutoulis, A. (1994). “Valve and seta (spine) morphogenesis in the centric diatom Chaetoceros peruvianus Brightwell,” in The Protistan Cell Surface, eds R. Wetherbee, J. D. Pickett-Heaps, and R. A. Andersen (Vienna: Springer), 269–282. doi: 10.1007/978-3-7091-9378-5_16
Pienta, K. J., Hammarlund, E. U., Axelrod, R., Amend, S. R., and Brown, J. S. (2020a). Convergent evolution, evolving evolvability, and the origins of lethal cancer. Mol. Cancer Res. 18, 801–810. doi: 10.1158/1541-7786.mcr-19-1158
Pienta, K. J., Hammarlund, E. U., Axelrod, R., Brown, J. S., and Amend, S. R. (2020b). Poly-aneuploid cancer cells promote evolvability, generating lethal cancer. Evol. Appl. 13, 1626–1634. doi: 10.1111/eva.12929
Pleizier, N., Wilson, A. D. M., Shultz, A. D., and Cooke, S. J. (2015). Puffed and bothered: personality, performance, and the effects of stress on checkered pufferfish. Physiol. Behav. 152, 68–78. doi: 10.1016/j.physbeh.2015.09.011
Pondaven, P., Gallinari, M., Chollet, S., Bucciarelli, E., Sarthou, G., Schultes, S., et al. (2007). Grazing-induced changes in cell wall silicification in a marine diatom. Protist 158, 21–28. doi: 10.1016/j.protis.2006.09.002
Price, S. A., Friedman, S. T., and Wainwright, P. C. (2015). How predation shaped fish: the impact of fin spines on body form evolution across teleosts. Proc. R. Soc. Lond. B Biol. Sci. 282, 20151428. doi: 10.1098/rspb.2015.1428
Quan, H., Yang, W., Schaible, E., Ritchie, R. O., and Meyers, M. A. (2018). Novel defense mechanisms in the armor of the scales of the “living fossil” coelacanth fish. Adv. Funct. Mater. 28:1804237. doi: 10.1002/adfm.201804237
Rafiq, S., Hackett, C. S., and Brentjens, R. J. (2020). Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17, 147–167. doi: 10.1038/s41571-019-0297-y
Raschi, W., and Tabit, C. (1992). Functional aspects of placoid scales: a review and update. Mar. Freshw. Res. 43, 123–147. doi: 10.1071/mf9920123
Rhiel, E., Wöhlbrand, L., Rabus, R., and Voget, S. (2018). Candidates of trichocyst matrix proteins of the dinoflagellate Oxyrrhis marina. Protoplasma 255, 217–230. doi: 10.1007/s00709-017-1148-2
Rice, S. H. (1985). An anti-predator chemical defense of the marine pulmonate gastropod Trimusculus reticulatus (Sowerby). J. Exp. Mar. Bio. Ecol. 93, 83–89. doi: 10.1016/0022-0981(85)90150-9
Robertson-Tessi, M., El-Kareh, A., and Goriely, A. (2012). A mathematical model of tumor–immune interactions. J. Theor. Biol. 294, 56–73.
Roilides, E., Simitsopoulou, M., Katragkou, A., and Walsh, T. J. (2015). How biofilms evade host defenses. Microb. Biofilms 3, 287–300. doi: 10.1128/9781555817466.ch14
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G., and Hacohen, N. (2015). Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61. doi: 10.1016/j.cell.2014.12.033
Saito, T. (1985). Resistibility of toxic and nontoxic pufferfish against tetrodotoxin. Nippon Suisan Gakkaishi 51:1371. doi: 10.2331/suisan.51.1371
Scherr, T. D., Hanke, M. L., Huang, O., James, D. B. A., Horswill, A. R., Bayles, K. W., et al. (2015). Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. MBio 6:e01021-15.
Schmidt, J. O., and Blum, M. S. (1977). Adaptations and responses of Dasymutilla occidentalis (Hymenoptera: Mutillidae) to predators. Entomol. Exp. Appl. 21, 99–111. doi: 10.1111/j.1570-7458.1977.tb02663.x
Seiler, C., van Velzen, E., Neu, T. R., Gaedke, U., Berendonk, T. U., and Weitere, M. (2017). Grazing resistance of bacterial biofilms: a matter of predators’ feeding trait. FEMS Microbiol. Ecol. 93:fix112. doi: 10.1093/femsec/fix112
Shankaran, V., Ikeda, H., Bruce, A. T., White, J. M., Swanson, P. E., Old, L. J., et al. (2001). IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111. doi: 10.1038/35074122
Sharma, P., Alsharif, S., Fallatah, A., and Chung, B. M. (2019). Intermediate filaments as effectors of cancer development and metastasis: a focus on keratins, vimentin, and nestin. Cells 8:497. doi: 10.3390/cells8050497
Smith, B. H., and Breed, M. D. (1995). “The chemical basis for nestmate recognition and mate discrimination in social insects,” in Chemical Ecology of Insects II, eds R. T. Carde and W. J. Bell (New York, NY: Chapman and Hall), 287–317. doi: 10.1007/978-1-4615-1765-8_8
Smith, W. L., Stern, J. H., Girard, M. G., and Davis, M. P. (2016). Evolution of venomous cartilaginous and ray-finned fishes. Integr. Comp. Biol. 56, 950–961. doi: 10.1093/icb/icw070
Smodlaka Tanković, M., Baričević, A., Ivančić, I., Kužat, N., Medić, N., Pustijanac, E., et al. (2018). Insights into the life strategy of the common marine diatom Chaetoceros peruvianus Brightwell. PLoS One 13:e0203634. doi: 10.1371/journal.pone.0203634
Solinas, G., Germano, G., Mantovani, A., and Allavena, P. (2009). Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 86, 1065–1073. doi: 10.1189/jlb.0609385
Song, J., Ortiz, C., and Boyce, M. C. (2011). Threat-protection mechanics of an armored fish. J. Mech. Behav. Biomed. Mater. 4, 699–712. doi: 10.1016/j.jmbbm.2010.11.011
Spranger, S., Spaapen, R. M., Zha, Y., Williams, J., Meng, Y., Ha, T. T., et al. (2013). Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 5:200ra116. doi: 10.1126/scitranslmed.3006504
Srygley, R. B. (1994). Locomotor mimicry in butterflies? The associations of positions of centres of mass among groups of mimetic, unprofitable prey. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 343, 145–155. doi: 10.1098/rstb.1994.0017
Stankowich, T., and Campbell, L. A. (2016). Living in the danger zone: exposure to predators and the evolution of spines and body armor in mammals. Evolution (N. Y.) 70, 1501–1511. doi: 10.1111/evo.12961
Stankowich, T., Caro, T., and Cox, M. (2011). Bold coloration and the evolution of aposematism in terrestrial carnivores. Evol. Int. J. Org. Evol. 65, 3090–3099. doi: 10.1111/j.1558-5646.2011.01334.x
Stephenson, P. J., and Racey, P. A. (1994). Seasonal variation in resting metabolic rate and body temperature of streaked tenrecs, Hemicentetes nigriceps and H. semispinosus (Insectivora: Tenrecidae). J. Zool. 232, 285–294. doi: 10.1111/j.1469-7998.1994.tb01573.x
Steven, A., and Seliger, B. (2018). The role of immune escape and immune cell infiltration in breast cancer. Breast Care 13, 16–21. doi: 10.1159/000486585
Sugiura, S. (2020). Predators as drivers of insect defenses. Entomol. Sci. 23, 316–337. doi: 10.1111/ens.12423
Sugiura, S., and Yamazaki, K. (2014). Caterpillar hair as a physical barrier against invertebrate predators. Behav. Ecol. 25, 975–983. doi: 10.1093/beheco/aru080
Tan, S., Li, D., and Zhu, X. (2020). Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 124:109821. doi: 10.1016/j.biopha.2020.109821
Taylor, A. R. (2011). Emiliania Huxleyi Coccolithophore (PLoS). PLoS Biol., CC BY 2.5. Available online at: https://commons.wikimedia.org/wiki/File:Emiliania_huxleyi_coccolithophore_(PLoS).png (accessed March 13, 2021).
Teng, M. W. L., Swann, J. B., Koebel, C. M., Schreiber, R. D., and Smyth, M. J. (2008). Immune-mediated dormancy: an equilibrium with cancer. J. Leukoc. Biol. 84, 988–993. doi: 10.1189/jlb.1107774
Thomson, D. A. (1964). Ostracitoxin: an ichthyotoxic stress secretion of the boxfish, Ostracion lentiginosus. Science 146, 244–245. doi: 10.1126/science.146.3641.244
Thurlow, L. R., Hanke, M. L., Fritz, T., Angle, A., Aldrich, A., Williams, S. H., et al. (2011). Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 186, 6585–6596. doi: 10.4049/jimmunol.1002794
van Donk, E., and Hessen, D. O. (1993). Grazing resistance in nutrient-stressed phytoplankton. Oecologia 93, 508–511. doi: 10.1007/bf00328958
Vareki, S. M. (2018). High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 6:157.
Villemagne, M. (2008). Photo 56639549. iNaturalist, CC BY-NC 4.0. Available online at: https://www.inaturalist.org/photos/56639549 (accessed March 13, 2021).
Vinay, D. S., Ryan, E. P., Pawelec, G., Talib, W. H., Stagg, J., Elkord, E., et al. (2015). Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35, S185–S198.
Vito, A., El-Sayes, N., and Mossman, K. (2020). Hypoxia-driven immune escape in the tumor microenvironment. Cells 9:992. doi: 10.3390/cells9040992
Wagner, J., Wickman, E., DeRenzo, C., and Gottschalk, S. (2020). CAR T cell therapy for solid tumors: bright future or dark reality? Mol. Ther. 28, 2320–2339. doi: 10.1016/j.ymthe.2020.09.015
Waldbauer, G. (2012). How Not to Be Eaten: The Insects Fight Back. Berkeley, CA: University of California Press.
Walker, R., and Enderling, H. (2016). From concept to clinic: mathematically informed immunotherapy. Curr. Probl. Cancer 40, 68–83. doi: 10.1016/j.currproblcancer.2015.10.004
Wallace, A. R. (1867). Mimicry, and other protective resemblances among animals (1867). Westminster Rev. 32, 1–43.
Wallace, A. R. (1895). Natural Selection and Tropical Nature: Essays on Descriptive and Theoretical Biology. London: Macmillan and Company.
Wang, J., Xu, Y., Huang, Z., and Lu, X. (2018). T cell exhaustion in cancer: mechanisms and clinical implications. J. Cell. Biochem. 119, 4279–4286. doi: 10.1002/jcb.26645
Wang, L.-Y., Huang, W.-S., Tang, H.-C., Huang, L.-C., and Lin, C.-P. (2018a). Too hard to swallow: a secret secondary defence of an aposematic insect. J. Exp. Biol. 221:jeb172486.
Wang, L.-Y., Rajabi, H., Ghoroubi, N., Lin, C.-P., and Gorb, S. N. (2018b). Biomechanical strategies underlying the robust body armour of an aposematic weevil. Front. Physiol. 9:1410. doi: 10.3389/fphys.2018.01410
Weaver, A. M. (2006). Invadopodia: specialized cell structures for cancer invasion. Clin. Exp. Metastasis 23, 97–105. doi: 10.1007/s10585-006-9014-1
Weinstein, S. B. (2020). Displaying. Available online at: https://smithsonian.figshare.com/articles/media/Displaying/12185397?backTo=/collections/Lophiomys_imhausi_crested_rat_behavior_in_captive_and_natural_environments/4948773 (accessed March 14, 2021).
Weinstein, S. B., Malanga, K. N., Agwanda, B., Maldonado, J. E., and Dearing, M. D. (2020). The secret social lives of African crested rats, Lophiomys imhausi. J. Mammal. 101, 1680–1691. doi: 10.1093/jmammal/gyaa127
Wetzel, B., and Schaefer, H. (1980). Breast Cancer Cell, AV-8000-0302-B. National Cancer Institute. Available online at: https://visualsonline.cancer.gov/details.cfm?imageid=1989 (accessed March 13, 2021).
Whelan, C. J., Holmes, R. T., and Smith, H. R. (1989). Bird predation on gypsy moth (Lepidoptera: Lymantriidae) larvae: an aviary study. Environ. Entomol. 18, 43–45. doi: 10.1093/ee/18.1.43
Wildes, T. J., Dyson, K. A., Francis, C., Wummer, B., Yang, C., Yegorov, O., et al. (2020). Immune escape after adoptive T-cell therapy for malignant gliomas. Clin. Cancer Res. 26, 5689–5700. doi: 10.1158/1078-0432.ccr-20-1065
Wishingrad, V., Musgrove, A. B., Chivers, D. P., and Ferrari, M. C. O. (2015). Risk in a changing world: environmental cues drive anti-predator behaviour in lake sturgeon (Acipenser fulvescens) in the absence of predators. Behaviour 152, 635–652. doi: 10.1163/1568539x-00003246
Witz, B. W., and Mushinsky, H. R. (1989). Pygidial secretions of Pasimachus subsulcatus (Coleoptera: Carabidae) deter predation by Eumeces inexpectatus (Squamata: Scincidae). J. Chem. Ecol. 15, 1033–1044. doi: 10.1007/bf01015197
Wood, W. F., Sollers, B. G., Dragoo, G. A., and Dragoo, J. W. (2002). Volatile components in defensive spray of the hooded skunk, Mephitis macroura. J. Chem. Ecol. 28, 1865–1870.
Wursten, B. (2017). Photo 80983346. iNaturalist. Available online at: https://www.inaturalist.org/photos/80983346 (accessed March 13, 2021).
Xu, J., Hansen, P. J., Nielsen, L. T., Krock, B., Tillmann, U., and Kiørboe, T. (2017). Distinctly different behavioral responses of a copepod, Temora longicornis, to different strains of toxic dinoflagellates, Alexandrium spp. Harmful Algae 62, 1–9. doi: 10.1016/j.hal.2016.11.020
Yang, W., Gludovatz, B., Zimmermann, E. A., Bale, H. A., Ritchie, R. O., and Meyers, M. A. (2013). Structure and fracture resistance of alligator gar (Atractosteus spatula) armored fish scales. Acta Biomater. 9, 5876–5889. doi: 10.1016/j.actbio.2012.12.026
Yang, W., Naleway, S. E., Porter, M. M., Meyers, M. A., and McKittrick, J. (2015). The armored carapace of the boxfish. Acta Biomater. 23, 1–10. doi: 10.1016/j.actbio.2015.05.024
Zarour, H. M. (2016). Reversing T-cell dysfunction and exhaustion in cancer. Clin. Cancer Res. 22, 1856–1864. doi: 10.1158/1078-0432.ccr-15-1849
Zerpe, R. (2018). Thornback cowfish (Lactoria fornasini). Wikimedia Commons. Available online at: https://commons.wikimedia.org/wiki/File:Thornback_cowfish_(Lactoria_fornasini)_(43629194360).jpg (accessed March 13, 2021).
Zhu, D., Ortega, C. F., Motamedi, R., Szewciw, L., Vernerey, F., and Barthelat, F. (2012). Structure and mechanical performance of a “modern” fish scale. Adv. Eng. Mater. 14, B185–B194.
Zhu, J., Petit, P.-F., and Van den Eynde, B. J. (2019). Apoptosis of tumor-infiltrating T lymphocytes: a new immune checkpoint mechanism. Cancer Immunol. Immunother. 68, 835–847. doi: 10.1007/s00262-018-2269-y
Zintzen, V., Roberts, C. D., Anderson, M. J., Stewart, A. L., Struthers, C. D., and Harvey, E. S. (2011). Hagfish predatory behaviour and slime defence mechanism. Sci. Rep. 1:131.
Keywords: predator deterrence, immune evasion, cancer, predator-prey, convergent evolution, armor, spines, noxiousness
Citation: Peplinski J, Malone MA, Fowler KJ, Potratz EJ, Pergams AG, Charmoy KL, Rasheed K, Avdieiev SS, Whelan CJ and Brown JS (2021) Ecology of Fear: Spines, Armor and Noxious Chemicals Deter Predators in Cancer and in Nature. Front. Ecol. Evol. 9:682504. doi: 10.3389/fevo.2021.682504
Received: 18 March 2021; Accepted: 08 July 2021;
Published: 30 July 2021.
Edited by:
Elise Huchard, UMR 5554 Institut des Sciences de l’Evolution de Montpellier (ISEM), FranceReviewed by:
Thomas John Hossie, Trent University, CanadaCopyright © 2021 Peplinski, Malone, Fowler, Potratz, Pergams, Charmoy, Rasheed, Avdieiev, Whelan and Brown. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joy Peplinski, anBlcGxpMkB1aWMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.