
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 09 December 2021
Sec. Urban Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.681959
An increasing number of studies have focused on the response and adaptation of plants to urbanization by comparing differences in leaf functional traits between urban and rural sites. However, considerable uncertainties remain because differences in land-use type have not frequently been taken into account when assessing the effect of urbanization on leaf traits. In this study, we sampled the needles of Chinese pine (Pinus tabuliformis Carr.) in areas with three land-use types (roadsides, parks, and neighborhoods) along an urban–rural gradient in Beijing, China to determine the effect of urbanization on leaf functional traits. There were significant differences in the values of leaf functional traits between the needles of the current and previous year and across land-use types. Pines growing on roadsides had leaves with smaller length, width, and area, as well as lower stomatal density, compared with those growing in parks and neighborhoods. This implies that on roadsides, plant capacity to acquire resources (e.g., light and carbon dioxide) was degraded. Stomatal density, leaf width, and leaf P concentration increased with increasing distance from the city center, while leaf K concentration decreased with increasing distance from the city center. Importantly, there were significant differences in the urban–rural gradient of leaf functional traits between leaves of different ages, and across land-use types. Leaf age was the most important factor influencing leaf nutrient traits, while land-use type was the most important factor influencing leaf morphological traits in urban environments. Thus, considering the effects of the plant characteristic and land-use type on traits is important for assessing the urban–rural gradients of plant functional traits.
With the rapid acceleration of urbanization (Brenner and Keil, 2014), more than half of the world’s population today lives and works in urban areas (Kabisch and Haase, 2011). Urban areas are human-dominated spaces, consisting primarily of such anthropogenic infrastructure as buildings, roads, and leisure parks. Little land is left for plants, despite them providing many ecosystem services for residents. Urban environments are not conducive to plant development because of air and stormwater pollution, alteration of soil conditions (e.g., drought and heat), and light regimes (Brackx et al., 2017; Chen et al., 2017; Goyal et al., 2018); however, they benefit plants through increased CO2 concentration and N availability and effective management practices (e.g., irrigation, fertilization, and pest and insect control (Lovett et al., 2000; Gregg et al., 2003; Ziska et al., 2003; Zhao et al., 2016).
Factors that are favorable or unfavorable to plant development in urban areas are highly spatially heterogeneous (Pickett et al., 2017). This spatial heterogeneity of urban environments is characterized by both patterns of urban–rural gradient (Hope et al., 2003; Searle et al., 2012) and land-use types (Pouyat et al., 2007; Balasooriya et al., 2009). Along the urban–rural gradient, decreasing pollution, air temperature, CO2, and soil fertility influence plants both positively and negatively (Gregg et al., 2003; Ziska et al., 2004; Fortuniak et al., 2006; Pouyat et al., 2007). For example, higher soil nutrients in urban areas positively affect leaf nutrient traits, while higher CO2 concentration negatively affects leaf nutrient traits (Yin, 2002; Song et al., 2019). In addition, cities comprise a variety of land-use types (Balasooriya et al., 2009), each with unique physical environments (e.g., pollution level, soil origination and nutrients, etc.) and management practices (e.g., irrigation, fertilization, etc.), which also affect plant development (Pouyat et al., 2007; Balasooriya et al., 2009).
Plant functional traits are morphological, physiological, and phenological features that determine a plant’s ability to acquire, use, and preserve resources (Cornelissen et al., 2003; Reich et al., 2003). In recent years, plant functional traits have been investigated widely in cities to explore plant responses and adaptation to heterogeneous urban environments. Plant functional traits may reflect urban environmental conditions because some are sensitive to climate, soil, and human effects (Wright et al., 2004). In addition, changes in plant functional traits alter the ecosystem services that vegetation provides because ecosystem services and plant functional traits have a close relationship (de Bello et al., 2010).
Significant differences in plant leaf functional traits have been found in comparisons between urban and rural sites (Table 1). However, changes in leaf functional traits along an urban–rural continuum have rarely been explored in the field, and these need further validation by sampling at more field sites along the urban–rural transect. The characteristics of plants themselves influence leaf functional traits (Ghimire et al., 2018; Díaz-Barradas et al., 2020). For plants with evergreen leaves, leaf functional traits change with leaf age (Adebooye et al., 2012; Bucher et al., 2019). Land-use change as a representation of the impact of human activities on the environment is also one of the major factors affecting leaf functional traits (Knapp et al., 2009; Kalusova et al., 2017). Besides the urban–rural gradient, leaf age and land-use type also affect leaf functional traits. The effects of interactions between these factors on leaf functional traits in urban environments have been poorly investigated. It is therefore imperative to consider the effects of leaf age and land-use type on leaf traits when assessing changes along an urban–rural gradient and to determine the dominant factor controlling leaf functional traits.
We selected Chinese pine (Pinus tabuliformis Carr.) for this study because: (1) it is a native species of China and has been planted for a 100 years in Beijing, China; (2) it is one of the top five evergreen tree species in Beijing in terms of number of individuals and ecological importance, such as forest regeneration, carbon storage, and aesthetic value (Wang et al., 2014); (3) it is distributed widely across various land-use types along the urban–rural gradient in Beijing, China (Zhao, 2010; Guo et al., 2018); and (4) its leaf traits have been widely studied and show significant variations (Wang et al., 2014; Liu et al., 2016). We selected eight leaf functional traits—five morphological traits: stomatal density, leaf length, leaf width, leaf area, and specific leaf area (SLA); and three physiological traits: leaf nitrogen (N), phosphorus (P), and potassium (K) concentrations—based on three considerations: (1) They play key roles in plant adaptation to environmental changes that accompany urban–rural gradients (Woodward and Kelly, 1995; Reich and Oleksyn, 2004; Wright et al., 2004; Barwise and Kumar, 2020); (2) they are related closely to plant resource utilization strategies, growth, and production (Wright et al., 2004; Garnier and Navas, 2012); and (3) they are simple to measure (Cornelissen et al., 2003). We measured the traits of both current- and previous-year needles of Chinese pine growing in areas with three land-use types (roadsides, parks, and neighborhoods) along the urban–rural gradient. We focused on revealing the effects of the urban–rural gradient on leaf functional traits under certain conditions of leaf age and land-use type and exploring the most important factors influencing these traits, to better understand the effects of urbanization on plants and promote the design, management, growth, and production of urban plants. We sought to answer the following questions: (1) Do leaf functional traits differ significantly in current- and previous-year needles? (2) Are there significant changes in leaf functional traits across land-use types (roadsides, parks, and neighborhoods)? (3) How do leaf functional traits change along the urban–rural gradient and do these changes differ with leaf age and across land-use types? (4) How do interactions between leaf age, land-use type, and urban–rural gradient affect the spatial pattern of leaf functional traits in urban environments?
Beijing is an international metropolis located in the North China Plain, with a history of over 1,000 years. In the past four decades, it has spread out rapidly with concentric ring roads, during which time its population has reached over 21 million. It has a temperate, semi-humid, continental monsoon climate with a mean annual temperature of 11–12°C and mean annual precipitation of 500 mm.
The study sites were located along a south–north transect that runs through the urban center (Figure 1) and covers both the fastest-growing urban regions and rural areas of Beijing (Peng et al., 2016). Along this transect, 64 Chinese pine stands located on 24 roadsides, in 25 parks, and in 15 neighborhoods were selected (Figure 1 and Table 2). A roadside is a strip of plants, mainly located between a roadway (carriageway) and a sidewalk (pavement). A park is an area of open space provided for recreational use, usually owned and maintained by a local government. A neighborhood is a district, especially one forming a community within a town or city and sharing the same services and management. The distance of the chosen sites from the city center ranged from 1.00 to 24.89 km. Study sites were sampled at 1-km intervals to make the distribution of sites spatially balanced.
Chinese pine (Pinus tabuliformis Carr.) is a monoecious, wind-pollinated, and predominantly outcrossing species (Wang et al., 2010). It is a light-loving tree species with low water consumption and slow growth but strong drought tolerance (Zheng and Fu, 1978). Because of its high adaptivity to poor environments, Chinese pine has been widely planted in urban areas of Beijing for a long time.
Between July and August 2016, three well-developed pines at each site (a total of 64 sites and 192 trees) were selected to examine their traits. The age of each tree was estimated by counting the number of whorls of branches along the main trunk because pines typically grow in annual spurts, putting on one whorl of branches each year (Hartman et al., 2009; Rollinson, 2012). The average age of the trees chosen was 22 years, their average height was 5.85 m, and average diameter at breast height was 16.62 cm. To determine leaf functional traits, 80–100 current- and previous-year needles were collected randomly from each selected tree; these were fully expanded, sun-exposed, in good health, and at a height of approximately 1.8 m (Cornelissen et al., 2003).
Stomatal density was determined using scanning electron microscopy in accordance with the methods of Yang et al. (2017). Three fresh needles wiped with a wet towel were placed immediately in a plastic bottle with a fixative solution (70%, v/v, ethanol:glacial acetic acid:formalin; 90:5:5) to maintain the integrity of cellular structure. Before examination with a scanning electron microscope (SEM; FE-SEM-EDS, SU-8020, Hitachi, Tokyo, Japan), the middle segment of each needle, approximately 1 cm long, was dehydrated with ethanol, incubated overnight in isoamyl acetate, and then dried to the critical point. The number of stomata within the images was counted manually and then converted to stomatal density (number of stomata per square-millimeter of adaxial leaf surface). Images of needles captured by the scanning electron microscope were also used to measure leaf width using ImageJ software (US National Institutes of Health, Bethesda, MD, United States). Leaf length of three fresh needles was measured using a digital caliper. Twelve fresh needles were scanned using a digital scanner (HP Scanjet G3110, Hewlett-Packard Development Company, Beijing, China) to measure leaf area using ImageJ software. After leaf area measurement, needles were dried at 80°C to a constant weight. SLA was calculated as the leaf area divided by its oven-dried mass (Cornelissen et al., 2003). The remaining needles were dried and ground to measure leaf nutrient concentrations. Leaf N concentration was measured using an automatic elemental analyzer (Vario EL III, Elementar, Germany), and leaf P and K concentrations were measured using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES; Prodigy, Leeman, Hudson, NH, United States) after digestion with freshly distilled concentrated HNO3/H2O2 in a microwave oven (Oliva et al., 2003).
All indicators were tested for normality using the Shapiro–Wilk test and were log-transformed before analysis if they were distributed non-normally. Levene’s test and Q-Q plots of residuals were used to test the homogeneity of variance. The Mantel test was performed to test the spatial autocorrelation using the “mantel.rtest” function in the “ade4” package. Among the traits studied, only stomatal density showed significant spatial autocorrelation (Supplementary Table 1). Analysis of variance was used to evaluate leaf age, land-use type, and the effects of their interactions on traits. Analysis of variance was performed using the “Anova” function with type II tests in the “car” package to control unequal sample size (Lewsey et al., 1997; Langsrud, 2003). The “emmeans” function in the “emmeans” package, which provides the t-value and corrected p-values automatically for unequal sample size, was executed for multiple comparisons of trait indicators (Russell, 2018).
For traits without significant spatial autocorrelation, to test changes in traits along the urban–rural gradient, a generalized linear mixed model (GLMM) was fitted with traits as the response variable, distance of the site from the city center as the fixed effect, and land-use type as the random intercept (the gamma distribution with LOG LINK function) in R software. Likelihood ratio tests of a full model against a null model were used to measure the significance of the fixed effect (Fajardo and Siefert, 2018). The explanatory power of the model was evaluated by calculating the conditional R2 (Nakagawa and Schielzeth, 2013). For stomatal density with significant spatial autocorrelation, we performed generalized linear mixed models via Penalized Quasi-Likelihood estimation (GLMMPQL) using “glmmPQL” function in “MASS” package to test changes in stomatal density along the urban–rural gradient. The GLMMPQL enables the building of spatial models with dependent data not normally distributed (Dormann et al., 2007). The response variable, fixed effect, random intercept, the error distribution and link function in the GLMMPQL were set to be the same as in the GLMM. Taking into account the unequal sample size, these generalized linear mixed models were also performed in SAS software (PROC GLIMMIX procedure, METHOD = RMPL, DDFM = KENWARDROGER) followed by a type II sum of squares ANOVA (Langsrud, 2003; Spilke et al., 2005). To test whether changes in traits along the urban–rural gradient varied with leaf age and land-use type, general linear models with traits as the response variable and distance as the predictor variable were fitted for each leaf age class and land-use type.
Variation partitioning analysis was performed using the ‘‘varpart’’ function in the ‘‘vegan’’ package to compare the percentage of trait variations explained by leaf age, land-use type, urban–rural gradient, and their intersections. Statistical analyses were performed using the statistical software R v. 3.6.21 and SAS v. 9.4 (SAS Institute, Cary, NC, United States).
The mean trait values of current- and previous-year needles were 65.37 and 59.61 n/mm2 for stomatal density, 13.73 and 14.25 cm for leaf length, 0.25 and 0.27 cm for leaf width, 2.09 and 2.15 cm2 for leaf area, 57.45 and 48.34 cm2/g for SLA, 15.69 and 14.48 mg/g for leaf N concentration, 1.24 and 0.81 mg/g for leaf P concentration, and 7.38 and 4.48 mg/g for leaf K concentration, respectively.
Stomatal density, leaf width, SLA, and leaf N, P, and K concentrations differed significantly between leaves of different ages (Figure 2). Compared with previous-year needles, current-year needles had 10% greater stomatal density, 8% smaller leaf width, 20% greater SLA, 8% higher leaf N concentration, 53% higher leaf P concentration, and 51% higher leaf K concentration (p < 0.05, Figure 2).
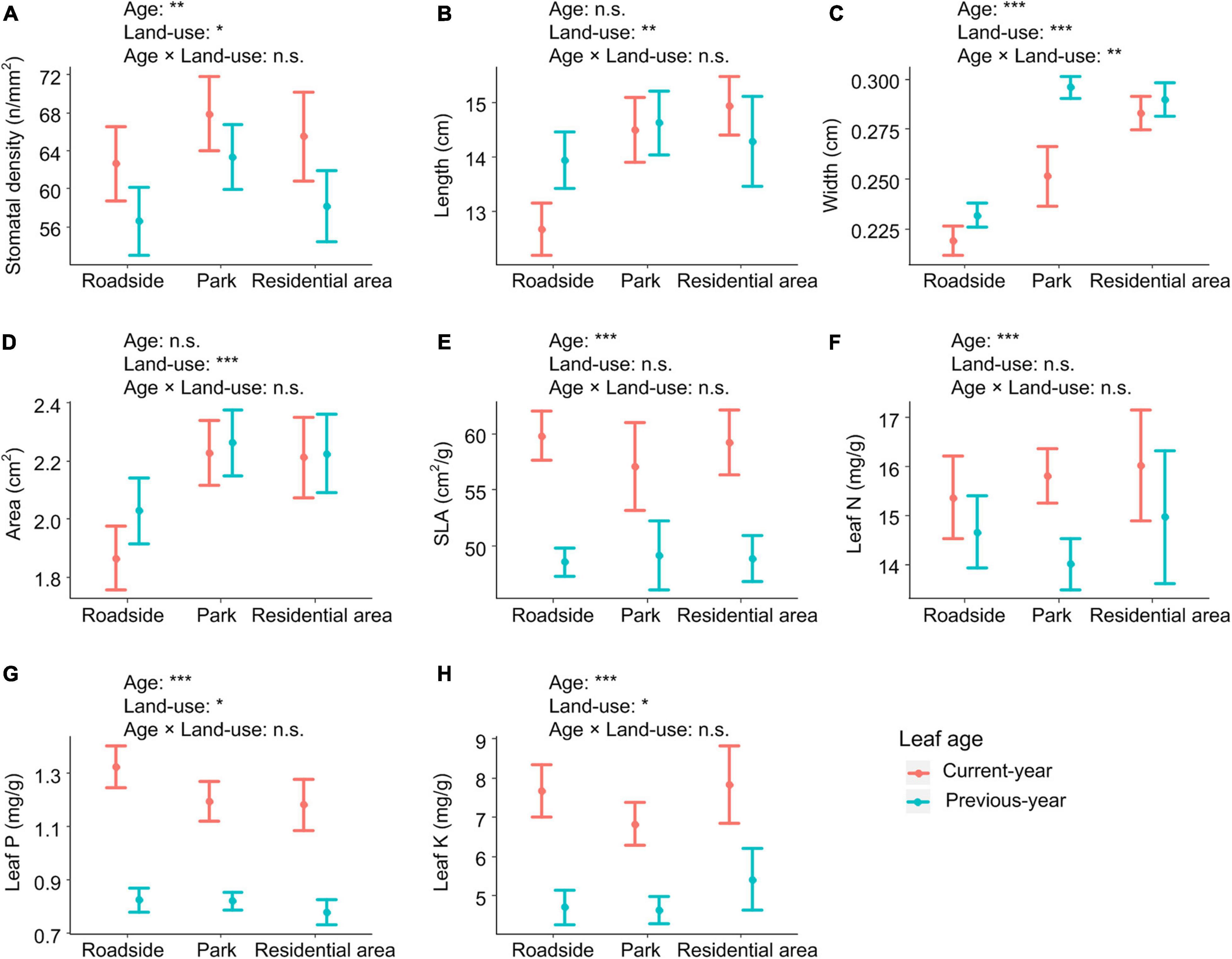
Figure 2. Leaf functional traits (mean values and 95% confidence intervals) of Chinese pine (Pinus tabuliformis Carr.) differ with leaf age and among land-use types. (A) Stomatal density, (B) Leaf length, (C) Leaf width, (D) Leaf area, (E) Specific leaf area, (F) Leaf N concentration, (G) Leaf P concentration, (H) Leaf K concentration. Significance level: ***p < 0.001; **p < 0.01; *p < 0.05; n.s., not significant.
The mean trait values of plants on roadsides and in parks and neighborhoods were 62.65, 67.90, and 65.50 n/mm2 for stomatal density, 12.55, 14.31, and 14.65 cm for leaf length, 0.22, 0.25, and 0.28 cm for leaf width, 1.87, 2.22, and 2.21 cm2 for leaf area, 59.81, 57.09, and 54.28 cm2/g for SLA, 15.36, 15.80, and 16.02 mg/g for leaf N concentration, 1.33, 1.19, and 1.18 mg/g for leaf P concentration, and 7.66, 6.83, and 7.83 mg/g for leaf K concentration, respectively.
Stomatal density, leaf length, width, and area, and leaf P and K concentrations varied significantly with land-use types (Figure 2). Compared with plants on roadsides, those in parks and neighborhoods had 9 and 9% shorter leaf length, 18 and 21% smaller leaf width, and 13 and 12% smaller leaf area, but 7 and 9% higher leaf P concentration, respectively (p < 0.05, Figure 2). In addition, compared with plants in parks, those in neighborhoods had 11% smaller leaf width and 13% lower leaf K concentration, while the values of other traits showed no significant differences between plants in parks and those in neighborhoods (Figure 2).
Stomatal density, leaf width, and leaf P concentration increased significantly along the urban–rural gradient, while leaf K concentration decreased significantly (p < 0.05, Table 3 and Figure 3).
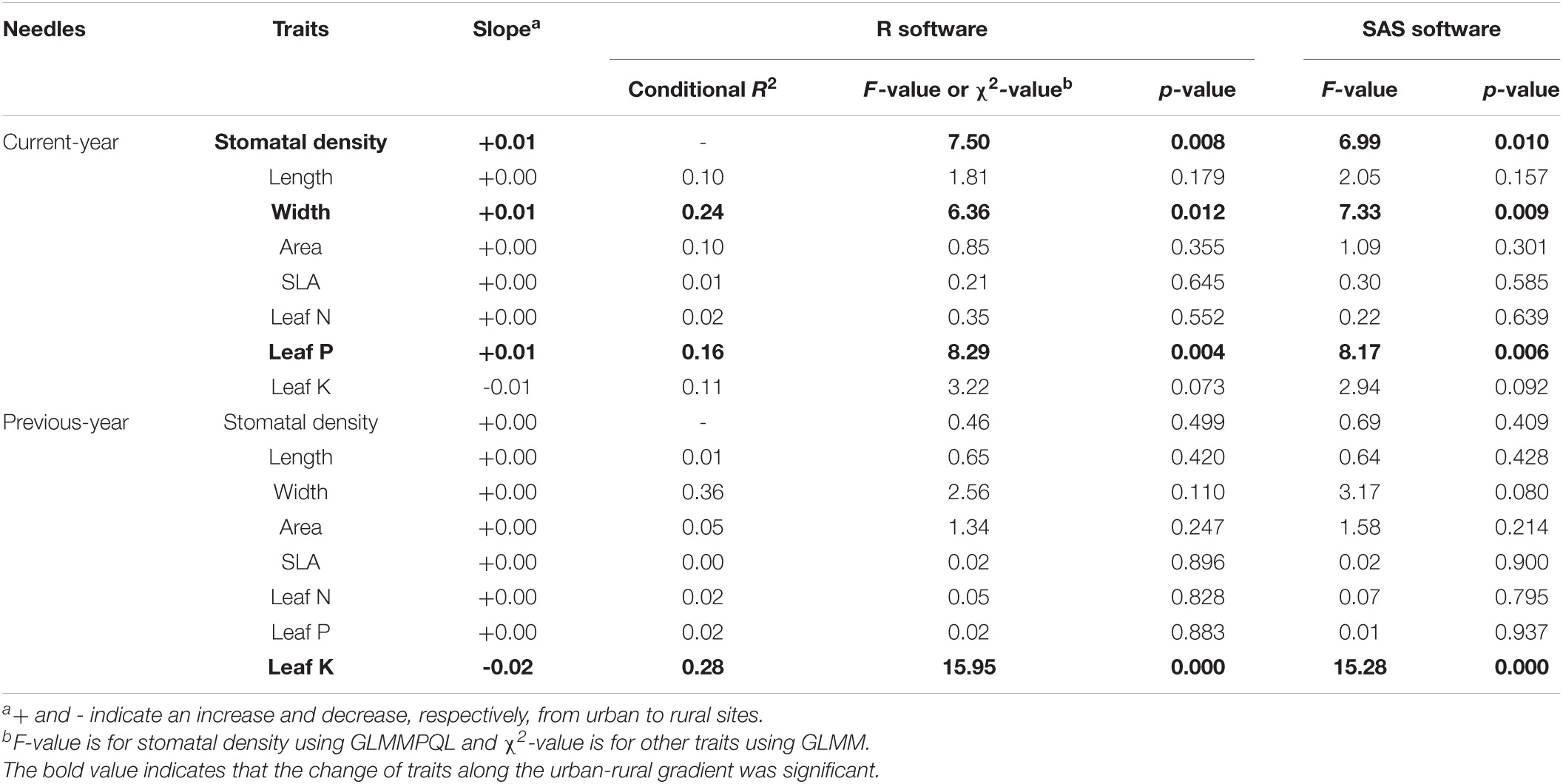
Table 3. Changes in leaf functional traits of Chinese pine (Pinus tabuliformis Carr.) along the urban–rural gradient modeled using generalized linear mixed models (GLMM) and generalized linear mixed models via Penalized Quasi-Likelihood estimation (GLMMPQL) with traits as the response variable, distance of sites from the city center as the fixed effect, and land-use type as the random intercept.

Figure 3. The relationship between leaf functional traits of Chinese pine (Pinus tabuliformis Carr.) and the distance of sites from the city center. (A) Stomatal density of current-year needles, (B) Leaf width of current-year needles, (C) Leaf P concentration of current-year needles, (D) Leaf K concentration of previous-year needles. Regression lines are shown when changes are significant. Significance level: ***p < 0.001; **p < 0.01; *p < 0.05.
Importantly, urban–rural gradients of leaf functional traits varied with leaf age and land-use type (Figure 3). Urban–rural gradients of stomatal density, leaf width, and leaf P concentration were identified only for current-year needles, and gradients in leaf K concentration were only observed for previous-year needles. Urban–rural gradients in stomatal density and leaf K concentration were found on roadsides, gradients in leaf width and leaf P and K concentrations were found in parks, while only gradients in leaf P concentration were found in neighborhoods.
The results of variation partitioning analysis revealed that among leaf age, land-use type, and urban-rural gradient, leaf age explained the greatest variations in leaf nutrient traits (leaf N, P, and K concentrations), while land-use type explained the greatest variations in leaf morphological traits (stomatal density, and leaf length, width and area) (Figure 4). This suggests that leaf age and land-use type were the most important factors influencing leaf nutrient and morphological traits in urban environments, respectively.
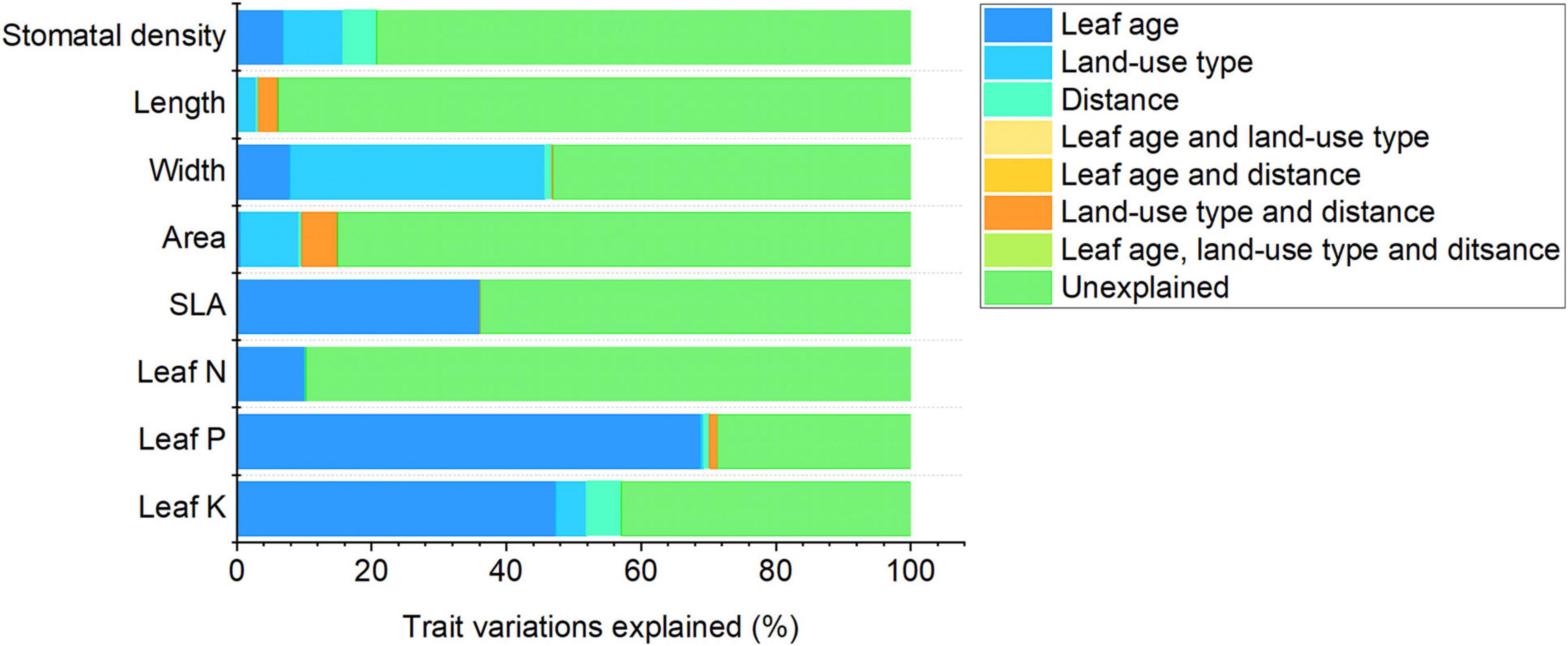
Figure 4. Variations in leaf functional traits explained by leaf age alone, land-use type alone, distance from the city center alone, and the intersection of these three factors, based on variation partitioning analysis.
Leaf functional traits are associated with plant resource acquisition, damage resistance, and mechanical strength (Wang et al., 2016; Blonder et al., 2020). Our results implied that previous-year needles tended to have greater mechanical strength (smaller SLA), but less production capacity (lower stomatal density, and lower N, P, and K concentrations) than current-year needles (Zheng et al., 2017; Bucher et al., 2019; Liu Z. et al., 2020).
Leaf functional traits changed with age, which is consistent with results reported previously. For example, as leaf age increases, SLA decreases in another Pinus species (Pinus sylvestris var. mongolica; Zheng et al., 2017; Liu X. et al., 2020), non-pine woody species (Römermann et al., 2016), and herbaceous species (Bucher et al., 2019). Stomatal density decreases with age in Tsuga heterophylla (Kouwenberg et al., 2004) and Trichosanthes cucumerina (Adebooye et al., 2012). Leaf nutrient concentrations also decrease with age in Pinus species (Pinus koraiensis; Liu X. et al., 2020; Liu Z. et al., 2020) and other tree species (Fraxinus mandshurica and Acer mono; Liu et al., 2019).
More organic matter might accumulate in leaves as they age, explaining the smaller SLA for previous-year needles than current-year needles (Liu Z. et al., 2020). A potential explanation for the decrease in stomatal density as leaf age increases may be that leaf growth and expansion widen the spaces between stomata, thereby leading to fewer stomata per given leaf area (Adebooye et al., 2012). Decreases in leaf nutrient concentrations as leaves grow may result from the transport of nutrients from old leaves to young leaves (Zheng et al., 2017; Liu et al., 2019) to maintain favorable nutrient status in young leaves, thus maintaining optimal metabolic activities and maximizing plant growth (Schreeg et al., 2014).
Leaf functional traits varied with land-use types (Figure 2). Plants growing on roadsides had traits (lower levels of stomatal density, and smaller leaf length, width and area) representative of a reduced capacity to acquire resources (e.g., light and carbon dioxide) compared with plants growing in parks and neighborhoods (Figure 2). This decrease in resource acquisition capacity is detrimental to plant growth and production potential (Fanourakis et al., 2015; Wright et al., 2017). Our results therefore imply that roadside environments do not favor the performance of plant functions (de Bello et al., 2010; Bussotti and Pollastrini, 2015; Fanourakis et al., 2015). Similarly, compared with trees of the same species growing on roadsides, the deciduous, broad-leaved tree species Zelkova serrata (Thumb.) Makino growing in parks in Tokyo, Japan exhibits lower levels of stomatal density (Osone et al., 2014), and the evergreen, broad-leaved tree species Quercus ilex growing in parks in Naples, Italy exhibits smaller leaves (Maisto et al., 2013).
Different levels of leaf functional traits among land-use types are related to each type’s unique environment. Stomata are important channels for plant water and gas exchange. The potential explanation can be proposed for the lower levels of stomatal density in plants on roadsides than in parks and neighborhoods. Drier and hotter environmental conditions on roadsides than in parks and neighborhoods may cause lower levels of stomatal density in plants (Fraser et al., 2009; Lau et al., 2018; Lu et al., 2019). Bao et al. (2001) reported that the relative humidity of air on roadsides was 12 and 7% lower than that in parks and neighborhoods, respectively, while the air temperature on roadsides was 1.49 and 0.95°C higher than that in parks and neighborhoods, respectively. Lau et al. (2018) and Lu et al. (2019) even revealed the molecular mechanism of reduced stomatal density caused by high temperature, during which expression of SPEECHLESS, a basic-helix-loop-helix transcription factor, is repressed. Deformation and curling of leaves in a dry and hot environment may cause the surface of epidermal cells to twist and fold, and the stoma to become trapped in mesophyll cells, leading to a further decrease in stomatal density. In addition, damage to leaves may inhibit stomata formation, reducing the number of stomata and finally resulting in a decrease in stomatal density.
Leaf size determines plants’ ability to capture light resources and carbon directly, and is affected by a variety of environmental factors, such as temperature, precipitation, and CO2 concentration (Mcdonald et al., 2003; Wright et al., 2017; Wang et al., 2018). Plants tend to have smaller leaves when environmental conditions are stressful, and those growing on roadsides had smaller leaves than those growing in parks and neighborhoods (Figure 2), which may be attributable to the relatively drier and hotter environmental conditions on roadsides (Bao et al., 2001; Maisto et al., 2013; Wright et al., 2017). High levels of pollutants may be another reason for reduced leaf size on roadsides; because of high traffic activity, roads are often more polluted than parks and neighborhoods. Mukherjee and Agrawal (2018) reported that total suspended particulate matter, inhalable particulate matter, and SO2 concentration levels on roadsides were 64, 96, and 202% higher than those in neighborhoods. Pollutants cannot only hinder gas exchange in photosynthesis, but also enter the plant directly and destroy photosynthetic tissue, leading to a decline in photosynthesis and subsequent reduction in leaf size (Bhatti and Iqbai, 1988; Leghari and Zaidi, 2013). Leghari and Zaidi (2013) found that the leaf length, width, and area of plants in polluted areas decreased by 20–73, 18–51, and 23–58%, respectively, compared with those of plants in unpolluted areas. Finally, lower soil nutrient levels may also contribute to reduced leaf size on roadsides (Mcdonald et al., 2003). The level of soil organic carbon, calcium, and magnesium contents in parks and neighborhoods was 36 and 62, 70 and 113, and 81 and 168% higher, respectively, than that on roadsides in Beijing (unpublished data).
Mean values of functional traits in plant communities have been used widely to determine the way the urban environment filters species into urban plant communities (Williams et al., 2015; Kalusova et al., 2017). In this study, we explored plant responses and adaptations to urban environments based on trait values measured in situ at the species level. We found that stomatal density, leaf width, and leaf P concentration increased gradually along the urban–rural gradient, while leaf K concentration decreased (p < 0.05); the other traits studied did not change significantly (p > 0.05, Figure 3 and Table 3).
Similarly, other studies have found that plants tend to have lower stomatal density and smaller leaves in urban areas than in rural areas (Pourkhabbaz et al., 2010; Leghari and Zaidi, 2013; Alotaibi et al., 2020). However, opposite results have been reported (Table 1). In addition, significant differences in SLA and leaf N concentration between urban and rural sites have been reported (Table 1).
The reasons for the inconsistences in urban-rural gradients of plant traits between different this study and other studies (Figure 3 and Table 1) may be as follows. First, the urban–rural gradients of traits depend upon the species studied. In this study, we found no significant urban–rural gradients for leaf length or area, SLA, or leaf N concentration in Chinese pine, an evergreen coniferous species; however, significant urban–rural gradients for these traits have been found in deciduous species, such as Populus tremula and Quercus robur (Nikula et al., 2010; Searle et al., 2011, 2012). This is consistent with a study by Jiahao et al. (2018), who found more moderate variations in stoichiometric and morphological traits of leaves of evergreen conifers than those of evergreen and deciduous broad-leaved species with changing environmental conditions. Differences in leaf N concentration may also be attributable to the fact that urban–rural gradients of leaf N concentration in broad-leaved forests are related closely to urban–rural gradients in aerial N deposition (Nikula et al., 2010; Searle et al., 2012); however, Chinese pine, an evergreen coniferous-leaved tree species, captures less deposition-derived N and acquires this N earlier than broad-leaved species (Rothe et al., 2002; Kristensen et al., 2004; Gundersen et al., 2009). Second, urban–rural gradients in traits depend upon the local climate. In New York, with a temperate climate, leaf area and leaf N concentration levels in Quercus robur are 730 and 20% higher, respectively, in urban areas compared with rural areas (Searle et al., 2012); meanwhile, in Potchefstroom, South Africa, with a savanna climate, leaf area and leaf N concentration are 35 and 33% lower, respectively, in urban areas (vanRensburg et al., 1997). Third, urban–rural gradients in traits vary inter-annually. Nikula et al. (2010) found that the mean leaf N concentration level of Populus tremula leaves was similar in 2007 and 2006, approximately 2.19 and 2.22%, respectively; however, the urban–rural difference in leaf N concentration in 2007 was approximately 33 times the urban–rural difference in 2006. Fourth, land-use type can enhance or weaken the degree of urban–rural gradient or even change its direction. The difference in leaf area of Ficus altissima between urban and rural sites when roadsides were used as urban sites was approximately twice that when neighborhoods were the urban sites (Alotaibi et al., 2020). Moreover, when industrial areas or roadsides were used as urban sites, the SLA of Ficus nitida in urban areas was approximately 5% lower or 74% higher, respectively, than that in rural areas (El-Khatib et al., 2020).
The occurrence of urban–rural gradients in leaf functional traits varies with leaf age (Figure 3). Functional traits of current-year needles were more sensitive to the urban–rural gradient than were those of previous-year needles (Figure 3). This may be attributable to the following: (1) the trait plasticity of evergreen woody species decreases with increasing leaf age (Niinemets, 2016); (2) old needles function primarily in mechanical protection, while young needles are the primary location of rapid photosynthesis, transpiration, and growth, which are influenced strongly by environmental changes (Tissue et al., 2001; Sariyildiz and Anderson, 2006); and (3) redistribution of nutrients to young needles may cause greater sensitivity of young needles to environmental changes (Turner and Olson, 1976; Yuan et al., 2018). Therefore, environmental changes along an urban–rural gradient, such as temperature fluctuations (Xu et al., 2001) and aerial ammonia pollution (Judžentienė et al., 2006), are more likely to alter traits in younger needles than in older needles.
The occurrence of urban–rural gradients in leaf functional traits also varies with land-use type (Figure 3), probably because the range of environmental factors along the urban–rural gradient varies with land-use type. There are more traits that show significant variation along an urban–rural gradient in parks and on roadsides than there are in neighborhoods, perhaps because neighborhoods have a more homogeneous environment attributable to similar landscape structures and management practices (Polsky et al., 2014; Wang et al., 2015). Urban–rural gradients in stomatal traits appear only on roadsides, which may be related to the urban–rural gradient in pollution levels (Bhatti and Iqbai, 1988; Leghari and Zaidi, 2013; Osone et al., 2014). There is more traffic pollution on roadsides than in parks and neighborhoods, and vehicle traffic is heavier on urban than on rural roads. Leaf width showed urban–rural gradient changes only in parks, which may be attributable to their greater temperature gradients. During the planning and development stages, a certain proportion of green spaces and impervious surfaces for roadsides and neighborhoods needs to be met. However, the proportion of land cover is more diverse in parks; for example, parks located in the city center often have more historic buildings (impervious surfaces) than those located in rural areas, which would widen urban–rural temperature gradients in parks.
High temperature, CO2 and pollution, and low moisture content in cities may decrease stomatal density, leaf width, and leaf P concentration, and increase leaf K concentration in urban plants (Woodward and Kelly, 1995; Luomala et al., 2005; Fraser et al., 2009; Pourkhabbaz et al., 2010; Wright et al., 2017). Alterations in these traits may represent adaptive adjustment of plants to environmental changes. Reduction in stomatal density cannot only restrict pollutants and the entry of CO2 into leaves (Woodward and Kelly, 1995; Verma et al., 2006; Pourkhabbaz et al., 2010), but also improve water use efficiency to prevent excessive water loss (Woodward and Kelly, 1995; Luomala et al., 2005; Wright et al., 2017). Narrowed leaves can evacuate heat quickly (Smith, 1978; Shin et al., 2000). Lower P concentration in leaves of urban plants can constrain high temperature-induced increases in metabolic reaction rates and growth rates to avoid rapid aging (Reich and Oleksyn, 2004; He et al., 2008; Pretzsch et al., 2017). In future studies, leaf functional traits and environmental factors (e.g., soil and air conditions) should be monitored simultaneously to reveal the mechanism of urban environmental influence on leaf functional traits.
Leaf age and land-use type were the most important factors influencing leaf nutrient and morphological traits, respectively, in urban environments (Figure 4). Leaf nutrients are transferred from old leaves to new leaves to prevent nutrient loss as old leaves wither (Zheng et al., 2017; Liu et al., 2019). Therefore, leaf age has a greater impact on leaf nutrients than land-use type or urban–rural gradient. Leaf morphological traits were more sensitive to land-use type than leaf age or urban–rural gradient. This might be because (1) the contribution of ontogeny to variations in leaf size and stomatal density are relatively small once leaves mature, and (2) changes in environmental conditions across land-use types are greater than those along the urban–rural gradient. For example, soil organic C ranged from 8.52 to 15.04 g/kg across land-use types in Beijing, China, but only from 10.19 to 11.68 g/kg along the urban–rural gradient (Mao et al., 2014).
Spatial heterogeneity is one of the main characteristics of urban ecosystems (Pickett et al., 2017). This characteristic is not only manifested in non-biological factors such as social economic activities and land use, but also reflected in biological components (Pouyat et al., 2007; Pickett et al., 2017). Within the city, there are great spatial variations in plants species, as well as in plant traits (Gregg et al., 2003; Guo et al., 2018). The result of this study shows that stomatal density, leaf width and leaf P concentration increased along the urban–rural gradient, while leaf K concentration decreased (Figure 3). These changes in plant traits would affect the ability of plants to use resources and strategies to respond to environmental changes, and subsequently ecosystem services beneficial to urban residents (Wright et al., 2004; de Bello et al., 2010).
The mechanism underlying changes in plant traits in cities are complex. In this study, the spatial variations in plant traits are affected by both human activities, such as land-use changes and urban-rural gradients caused by urbanization, and biological factors, such as leaf age (Figure 4). Human activities alter temperature, water and nutrients that are essential biophysical factors controlling plant development and growth, and then incur the changes in plant traits. Environmental changes alter plant physiological and ecological processes, then influencing plant production and allocation, and at last, plant traits would be altered. Therefore, measurements of the essential biophysical factors and related physiological parameters are necessary for understanding how human activities affect plant traits in urban settings.
Our study pinpoints that plant traits have changed with urban-rural gradient and across land use types and plant developmental stages. However, the genetic basis and fitness consequences of these trait changes are less explored. Manipulative experiments, such as common garden experiments or reciprocal translocation, would be useful to assess evolutionary and ecological consequences of plant trait changes.
The alteration of leaf functional traits by land-use type and urban–rural gradient indicates that plants are stressed in urban environments. It is therefore necessary to select tolerant plant species and conduct intensive management in urban planting. Pines we studied, even as a tolerant species, had smaller leaf length, width and area on roadsides than in parks and neighborhoods, implying that the production capacity of plants on roadsides may be reduced and that roadsides are poor to plant growth. The stresses faced by plants on roadsides might be mitigated by irrigation. The influence of urbanization on leaf traits is primarily dependent on the land-use type, management measures specific to the type of land use are necessary to guarantee plant health and growth.
Differences in plant traits between urban and rural sites have been reported widely in efforts to assess the responses and adaptations of plants to urban environments. Here, we examined an urban–rural continuum in leaf functional traits while also measuring the effects of leaf age and land-use type on traits. Stomatal density, leaf width, and leaf P and K concentrations changed significantly along the urban–rural gradient in response to urban environments with high temperatures, CO2 concentrations, pollution levels, and drought stress. Not only the trait values but also the urban–rural gradients in leaf functional traits varied with leaf age and land-use type. Traits in previous-year needles were less sensitive to the urban–rural gradient than those in current-year needles. The values for traits in plants on roadsides differed from those for plants in parks and neighborhoods, which might be attributable to the drier, hotter, and more polluted environment along roads. Traits exhibited more urban–rural gradients on roadsides and parks than in neighborhoods, implying that the range of environmental factors along the urban–rural gradient varies with land-use type. Leaf age and land-use type were the most important factors influencing leaf nutrient and morphological traits, respectively, in urban environments. These findings highlight the importance of considering the effects of leaf age and land-use type on traits when assessing changes in leaf functional traits along urban–rural gradients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
YS, ZO, and XIW designed the study. YS, BC, and JW performed the experiment and analyzed the data. YS and XIW wrote the first draft. YS, YL, XUW, and XIW revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Key Research and Development Program of China (2017YFE0127700), the China Postdoctoral Science Foundation (2017T100112), and the National Natural Science Foundation of China (71533005 and 41571053).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the editors and reviewers for their valuable comments, which improved the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.681959/full#supplementary-material
Adebooye, O. C., Hunsche, M., Noga, G., and Lankes, C. (2012). Morphology and density of trichomes and stomata of Trichosanthes cucumerina (Cucurbitaceae) as affected by leaf age and salinity. Turk. J. Bot. 36, 328–335.
Alotaibi, M. D., Alharbi, B. H., Al-Shamsi, M. A., Alshahrani, T. S., Al-Namazi, A. A., Alharbi, S. F., et al. (2020). Assessing the response of five tree species to air pollution in Riyadh City, Saudi Arabia, for potential green belt application. Environ. Sci. Pollut. Res. 27, 29156–29170. doi: 10.1007/s11356-020-09226-w
Balasooriya, B., Samson, R., Mbikwa, F., Vitharana, U. W. A., Boeckx, P., and Van Meirvenne, M. (2009). Biomonitoring of urban habitat quality by anatomical and chemical leaf characteristics. Environ. Exp. Bot. 65, 386–394. doi: 10.1016/j.envexpbot.2008.11.009
Bao, C., Lou, J., Zeng, X., and Xiang, Z. (2001). Effect of landscaping and greening on microclimate in Hangzhou city. J. Zhejiang Univ. 27, 415–418.
Barwise, Y., and Kumar, P. (2020). Designing vegetation barriers for urban air pollution abatement: a practical review for appropriate plant species selection. NPJ Clim. Atmos. Sci. 3, 1–19.
Bhatti, G., and Iqbai, M. Z. (1988). Investigations into the effect of automobile exhausts on the phenology, periodicity and productivity of some roadside trees. Acta Soc. Bot. Pol. 57, 395–399. doi: 10.5586/asbp.1988.038
Blonder, B., Both, S., Jodra, M., Xu, H., Fricker, M., Matos, I. S., et al. (2020). Linking functional traits to multiscale statistics of leaf venation networks. New Phytol. 29, 631–648. doi: 10.1111/nph.16830
Brackx, M., Wittenberghe, S., Verhelst, J., Scheunders, P., and Samson, R. (2017). Hyperspectral leaf reflectance of Carpinus betulus L. saplings for urban air quality estimation. Environ. Pollut. 220, 159–167. doi: 10.1016/j.envpol.2016.09.035
Brenner, N., and Keil, R. (2014). From global cities to globalized urbanization. Glocalism J. Cult. Polit. Innov. 3, 1–17. doi: 10.18356/93e0a206-en
Bucher, S. F., Feiler, R., Buchner, O., Neuner, G., Rosbakh, S., Leiterer, M., et al. (2019). Temporal and spatial trade-offs between resistance and performance traits in herbaceous plant species. Environ. Exp. Bot. 157, 187–196. doi: 10.1016/j.envexpbot.2018.10.015
Bussotti, F., and Pollastrini, M. (2015). Evaluation of leaf features in forest trees: methods, techniques, obtainable information and limits. Ecol. Indic. 52, 219–230. doi: 10.1016/j.ecolind.2014.12.010
Chen, Y., Wang, X., Jiang, B., Wen, Z., Yang, N., and Li, L. (2017). Tree survival and growth are impacted by increased surface temperature on paved land. Landsc. Urban Plan. 162, 68–79.
Cornelissen, J., Lavorel, S., Garnier, E., Díaz, S., Buchmann, N., Gurvich, D., et al. (2003). A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 51, 335–380. doi: 10.1071/bt02124
de Bello, F., Lavorel, S., Díaz, S., Harrington, R., Cornelissen, J. H., Bardgett, R. D., et al. (2010). Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 19, 2873–2893.
Díaz-Barradas, M., Gallego-Fernández, J., and Zunzunegui, M. (2020). Plant response to water stress of native and non-native Oenothera drummondii populations. Plant Physiol. Biochem. 154, 219–228. doi: 10.1016/j.plaphy.2020.06.001
Dormann, C. F., Mcpherson, J. M., Araújo, M. B., Bivand, R., Bolliger, J., Carl, G., et al. (2007). Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628. doi: 10.1111/j.2007.0906-7590.05171.x
El-Khatib, A. A., Youssef, N. A., Barakat, N. A., and Samir, N. A. (2020). Responses of Eucalyptus globulus and Ficus nitida to different potential of heavy metal air pollution. Int. J. Phytoremediation 22, 986–999. doi: 10.1080/15226514.2020.1719031
Fajardo, A., and Siefert, A. (2018). Intraspecific trait variation and the leaf economics spectrum across resource gradients and levels of organization. Ecology 99, 1024–1030. doi: 10.1002/ecy.2194
Fanourakis, D., Giday, H., Milla, R., Pieruschka, R., Kjaer, K. H., Bolger, M., et al. (2015). Pore size regulates operating stomatal conductance, while stomatal densities drive the partitioning of conductance between leaf sides. Ann. Bot. 115, 555–565. doi: 10.1093/aob/mcu247
Fortuniak, K., Kłysik, K., and Wibig, J. (2006). Urban–rural contrasts of meteorological parameters in Łódź. Theor. Appl. Climatol. 84, 91–101. doi: 10.1016/j.chemosphere.2016.06.025
Fraser, L. H., Greenall, A., Carlyle, C., Turkington, R., and Friedman, C. R. (2009). Adaptive phenotypic plasticity of Pseudoroegneria spicata: response of stomatal density, leaf area and biomass to changes in water supply and increased temperature. Ann. Bot. 103, 769–775. doi: 10.1093/aob/mcn252
Garnier, E., and Navas, M.-L. (2012). A trait-based approach to comparative functional plant ecology: concepts, methods and applications for agroecology. a review. Agron. Sustain. Dev. 32, 365–399. doi: 10.1007/s13593-011-0036-y
Ghimire, C. P., Bruijnzeel, L. A., Lubczynski, M. W., Zwartendijk, B. W., Odongo, V. O., Ravelona, M., et al. (2018). Transpiration and stomatal conductance in a young secondary tropical montane forest: contrasts between native trees and invasive understorey shrubs. Tree Physiol. 38, 1053–1070. doi: 10.1093/treephys/tpy004
Goyal, N., Shah, K., and Sharma, G. P. (2018). Does intrinsic light heterogeneity in Ricinus communis L. monospecific thickets drive species’ population dynamics? Environ. Monit. Assess. 410:190. doi: 10.1007/s10661-018-6791-3
Gregg, J. W., Jones, C. G., and Dawson, T. E. (2003). Urbanization effects on tree growth in the vicinity of New York City. Nature 424, 183–187. doi: 10.1038/nature01728
Gundersen, P., Sevel, L., Christiansen, J. R., Vesterdal, L., Hansen, K., and Bastrup-Birk, A. (2009). Do indicators of nitrogen retention and leaching differ between coniferous and broadleaved forests in Denmark? For. Ecol. Manage. 258, 1137–1146. doi: 10.1016/j.foreco.2009.06.007
Guo, P., Su, Y., Wan, W., Liu, W., Zhang, H., Sun, X., et al. (2018). Urban plant diversity in relation to land use types in built-up areas of Beijing. Chin. Geogr. Sci. 28, 100–110. doi: 10.3390/ijerph15122832
Hartman, J. R., Vaillancourt, L. J., Flowers, J. L., and Bateman, A. M. (2009). Managing Diplodia tip blight of landscape Austrian pines. J. Arboric. 35:27.
He, J.-S., Wang, L., Flynn, D. F., Wang, X., Ma, W., and Fang, J. (2008). Leaf nitrogen: phosphorus stoichiometry across Chinese grassland biomes. Oecologia 155, 301–310. doi: 10.1007/s00442-007-0912-y
Hope, D., Gries, C., Zhu, W. X., Fagan, W. F., Redman, C. L., Grimm, N. B., et al. (2003). Socioeconomics drive urban plant diversity. Proc. Natl. Acad. Sci. U.S.A. 100, 8788–8792. doi: 10.1073/pnas.1537557100
Jiahao, W., Huawei, J., Ningxiao, S., Huimin, T., Baoming, D., Dafeng, H., et al. (2018). Imbalanced plant stoichiometry at contrasting geologic-derived phosphorus sites in subtropics: the role of microelements and plant functional group. Plant Soil 430, 113–125. doi: 10.1007/s11104-018-3728-0
Judžentienė, A., Šližytė, J., Stiklienė, A., and Kupčinskienė, E. (2006). Characteristics of essential oil composition in the needles of young stand of Scots pine (Pinus sylvestris L.) growing along aerial ammonia gradient. Chemija 17, 67–73.
Kabisch, N., and Haase, D. (2011). Diversifying european agglomerations: evidence of urban population trends for the 21st century. Popul. Space Place 17, 236–253.
Kalusova, V., Ceplova, N., and Lososova, Z. (2017). Which traits influence the frequency of plant species occurrence in urban habitat types? Urban Ecosyst. 20, 65–75.
Knapp, S., Kuhn, I., Bakker, J. P., Kleyer, M., Klotz, S., Ozinga, W. A., et al. (2009). How species traits and affinity to urban land use control large-scale species frequency. Divers. Distrib. 15, 533–546. doi: 10.1111/j.1472-4642.2009.00561.x
Kouwenberg, L. L., Kürschner, W. M., and Visscher, H. (2004). Changes in stomatal frequency and size during elongation of Tsuga heterophylla needles. Ann. Bot. 94, 561–569. doi: 10.1093/aob/mch175
Kristensen, H. L., Gundersen, P., Callesen, I., and Reinds, G. J. (2004). Throughfall nitrogen deposition has different impacts on soil solution nitrate concentration in European coniferous and deciduous forests. Ecosystems 7, 180–192.
Lambrecht, S. C., Mahieu, S., and Cheptou, P. O. (2016). Natural selection on plant physiological traits in an urban environment. Acta Oecol. Int. J. Ecol. 77, 67–74. doi: 10.1016/j.actao.2016.09.002
Langsrud, Ø (2003). ANOVA for unbalanced data: use type II instead of type III sums of squares. Stat. Comput. 13, 163–167.
Lau, O. S., Song, Z., Zhou, Z., Davies, K. A., and Bergmann, D. C. (2018). Direct control of SPEECHLESS by PIF4 in the high-temperature response of stomatal development. Curr. Biol. 28, 1273–1280.e3. doi: 10.1016/j.cub.2018.02.054
Leghari, S. K., and Zaidi, M. A. (2013). Effect of air pollution on the leaf morphology of common plant species of Quetta city. Pak. J. Bot. 45, 447–454.
Lewsey, J. D., Gardiner, W. P., and Gettinby, G. (1997). A study of simple unbalanced factorial designs that use type II and type III sums of squares. Commun. Stat. Simul. Comput. 26, 1315–1328. doi: 10.1080/03610919708813442
Liu, X., Shi, L. J., Engel, B. A., Sun, S. K., Zhao, X. N., Wu, P. T., et al. (2020). New challenges of food security in Northwest China: water footprint and virtual water perspective. J. Clean. Prod. 245:118939. doi: 10.1016/j.jclepro.2019.118939
Liu, Y., Li, P., Wang, G., Liu, G., and Li, Z. (2016). Above-and below-ground biomass distribution and morphological characteristics respond to nitrogen addition in Pinus tabuliformis. N. Z. J. For. Sci. 46, 1–9.
Liu, Z., Hikosaka, K., Li, F., and Jin, G. (2020). Variations in leaf economics spectrum traits for an evergreen coniferous species: tree size dominates over environment factors. Funct. Ecol. 34, 458–467. doi: 10.1111/1365-2435.13498
Liu, Z., Jiang, F., Li, F., and Jin, G. (2019). Coordination of intra and inter-species leaf traits according to leaf phenology and plant age for three temperate broadleaf species with different shade tolerances. For. Ecol. Manage. 434, 63–75. doi: 10.1016/j.foreco.2018.12.008
Lovett, G. M., Traynor, M. M., Pouyat, R. V., Carreiro, M. M., Zhu, W.-X., and Baxter, J. W. (2000). Atmospheric deposition to oak forests along an urban- rural gradient. Environ. Sci. Technol. 34, 4294–4300. doi: 10.1021/es001077q
Lu, J., He, J., Zhou, X., Zhong, J., Li, J., and Liang, Y. K. (2019). Homologous genes of epidermal patterning factor regulate stomatal development in rice. J. Plant Physiol. 234–235, 18–27. doi: 10.1016/j.jplph.2019.01.010
Luomala, E.-M., Laitinen, K., Sutinen, S., Kellomäki, S., and Vapaavuori, E. (2005). Stomatal density, anatomy and nutrient concentrations of Scots pine needles are affected by elevated CO2 and temperature. Plant Cell Environ. 28, 733–749.
Maisto, G., Santorufo, L., and Arena, C. (2013). Heavy metal accumulation in leaves affects physiological performance and litter quality of Quercus ilex L. J. Plant Nutr. Soil Sci. 176, 776–784.
Mao, Q., Huang, G., Buyantuev, A., Wu, J., Luo, S., and Ma, K. (2014). Spatial heterogeneity of urban soils: the case of the Beijing metropolitan region, China. Ecol. Process. 3:23.
Mcdonald, P. G., Fonseca, C. R., and Westoby, J. M. O. (2003). Leaf-Size divergence along rainfall and soil-nutrient gradients: is the method of size reduction common among clades? Funct. Ecol. 17, 50–57. doi: 10.1046/j.1365-2435.2003.00698.x
Mukherjee, A., and Agrawal, M. (2018). Use of GLM approach to assess the responses of tropical trees to urban air pollution in relation to leaf functional traits and tree characteristics. Ecotoxicol. Environ. Saf. 152, 42–54. doi: 10.1016/j.ecoenv.2018.01.038
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed−effects models. Methods Ecol. Evol. 4, 133–142. doi: 10.1093/sysbio/syy060
Niinemets, Ü (2016). Leaf age dependent changes in within-canopy variation in leaf functional traits: a meta-analysis. J. Plant Res. 129, 313–338. doi: 10.1007/s10265-016-0815-2
Nikula, S., Vapaavuori, E., and Manninen, S. (2010). Urbanization-related changes in European aspen (Populus tremula L.): leaf traits and litter decomposition. Environ. Pollut. 158, 2132–2142. doi: 10.1016/j.envpol.2010.02.025
Oliva, S. R., Raitio, H., and Mingorance, M. D. (2003). Comparison of two wet digestion procedures for multi−element analysis of plant samples. Commun. Soil Sci. Plant Anal. 34, 2913–2923. doi: 10.1081/css-120025216
Osone, Y., Kawarasaki, S., Ishida, A., Kikuchi, S., Shimizu, A., Yazaki, K., et al. (2014). Responses of gas-exchange rates and water relations to annual fluctuations of weather in three species of urban street trees. Tree Physiol. 34, 1056–1068. doi: 10.1093/treephys/tpu086
Peng, J., Zhao, S., Liu, Y., and Tian, L. (2016). Identifying the urban-rural fringe using wavelet transform and kernel density estimation: a case study in Beijing City, China. Environ. Modell. Softw. 83, 286–302. doi: 10.1016/j.envsoft.2016.06.007
Pickett, S., Cadenasso, M., Rosi-Marshall, E. J., Belt, K. T., Groffman, P. M., Grove, J. M., et al. (2017). Dynamic heterogeneity: a framework to promote ecological integration and hypothesis generation in urban systems. Urban Ecosyst. 20, 1–14. doi: 10.1007/s11252-016-0574-9
Polsky, C., Grove, J. M., Knudson, C., Groffman, P. M., Bettez, N., Cavender-Bares, J., et al. (2014). Assessing the homogenization of urban land management with an application to US residential lawn care. Proc. Natl. Acad. Sci. U.S.A. 111, 4432–4437. doi: 10.1073/pnas.1323995111
Pourkhabbaz, A., Rastin, N., Olbrich, A., Langenfeld-Heyser, R., and Polle, A. (2010). Influence of Environmental pollution on leaf properties of urban plane trees, Platanus orientalis L. Bull. Environ. Contam. Toxicol. 85, 251–255. doi: 10.1007/s00128-010-0047-4
Pouyat, R. V., Yesilonis, I. D., Russell-Anelli, J., and Neerchal, N. K. (2007). Soil chemical and physical properties that differentiate urban land-use and cover types. Soil. Soc. Am. J. 71:1010.
Pretzsch, H., Biber, P., Uhl, E., Dahlhausen, J., Schütze, G., Perkins, D., et al. (2017). Climate change accelerates growth of urban trees in metropolises worldwide. Sci. Rep. 7:15403. doi: 10.1038/s41598-017-14831-w
Reich, P. B., and Oleksyn, J. (2004). Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. U.S.A. 101, 11001–11006. doi: 10.1073/pnas.0403588101
Reich, P. B., Wright, I., Cavender-Bares, J., Craine, J., Oleksyn, J., Westoby, M., et al. (2003). The evolution of plant functional variation: traits, spectra, and strategies. Int. J. Plant Sci. 164, S143–S164.
Römermann, C., Bucher, S. F., Hahn, M., and Bernhardt-Römermann, M. (2016). Plant functional traits–fixed facts or variable depending on the season? Folia Geobot. 51, 143–159. doi: 10.1007/s12224-016-9250-3
Rothe, A., Huber, C., Kreutzer, K., and Weis, W. (2002). Deposition and soil leaching in stands of Norway spruce and European beech: results from the Höglwald research in comparison with other European case studies. Plant Soil 240, 33–45.
Russell, L. (2018). emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.3.0. Available online at: https://CRAN.R-project.org/package=emmeans (accessed May 4, 2018).
Santangelo, J. S., Rivkin, L. R., Advenard, C., and Thompson, K. A. (2020). Multivariate phenotypic divergence along an urbanization gradient. Biol. Lett. 16:20200511. doi: 10.1098/rsbl.2020.0511
Sariyildiz, T., and Anderson, J. (2006). Intra-specific variation in cell wall constituents of needle age classes of Pinus sylvestris in relation to soil fertility status in Southwest England. Silva Fenn. 40:15.
Schreeg, L., Santiago, L., Wright, S. J., and Turner, B. L. (2014). Stem, root, and older leaf N: P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 95, 2062–2068. doi: 10.1890/13-1671.1
Searle, S. Y., Bitterman, D. S., Thomas, S., Griffin, K. L., Atkin, O. K., and Turnbull, M. H. (2011). Respiratory alternative oxidase responds to both low- and high-temperature stress in Quercus rubra leaves along an urban-rural gradient in New York. Funct. Ecol. 25, 1007–1017. doi: 10.1111/j.1365-2435.2011.01875.x
Searle, S. Y., Turnbull, M. H., Boelman, N. T., Schuster, W. S., Yakir, D., and Griffin, K. L. (2012). Urban environment of New York city promotes growth in northern red oak seedlings. Tree Physiol. 32, 389–400. doi: 10.1093/treephys/tps027
Shin, H. K., Lieth, J. H., and Kim, S. H. (2000). Effects of temperature on leaf area and flower size in rose. Acta Hortic. 547, 185–191. doi: 10.1603/0022-0493-94.1.129
Smith, W. K. (1978). Temperatures of desert plants: another perspective on the adaptability of leaf size. Science 201, 614–616. doi: 10.1126/science.201.4356.614
Song, G. M., Wang, J., Han, T. T., Wang, Q., Ren, H., Zhu, H. X., et al. (2019). Changes in plant functional traits and their relationships with environmental factors along an urban-rural gradient in Guangzhou, China. Ecol. Indic. 106:105558. doi: 10.1016/j.ecolind.2019.105558
Spilke, J., Piepho, H., and Hu, X. (2005). Analysis of unbalanced data by mixed linear models using the MIXED procedure of the SAS system. J. Agron. Crop Sci. 191, 47–54. doi: 10.1111/j.1439-037x.2004.00120.x
Tissue, D. T., Griffin, K. L., Turnbull, M. H., and Whitehead, D. (2001). Canopy position and needle age affect photosynthetic response in field-grown Pinus radiata after five years of exposure to elevated carbon dioxide partial pressure. Tree Physiol. 21, 915–923. doi: 10.1093/treephys/21.12-13.915
Turner, J., and Olson, P. (1976). Nitrogen relations in a Douglas-fir plantation. Ann. Bot. 40, 1185–1193. doi: 10.1093/oxfordjournals.aob.a085239
vanRensburg, L., Kruger, G. H. J., Ubbink, B., Scholes, M. C., and Peacock, J. (1997). A phytocentric perspective of Asterolecanium quercicola bouche infestation on Quercus robur L trees along an urbanization gradient. S. Afr. J. Bot. 63, 25–31. doi: 10.1016/s0254-6299(15)30688-8
Verma, R. B., Siddiqi, T. O., and Iqbal, M. (2006). Foliar response of Ipomea pes-tigridis L. to coal-smoke pollution. Turk. J. Bot. 30, 413–417.
Wang, C., Liu, J., Xiao, H., and Du, D. (2016). Response of leaf functional traits of Cerasus yedoensis (Mats.) yü li to serious insect attack. Pol. J. Environ. Stud. 25, 333–339. doi: 10.15244/pjoes/60328
Wang, F., Liu, Y., Li, X., and Liu, F. (2018). Effects of biochar amendment, CO2 elevation and drought on leaf gas exchange, biomass production and water use efficiency in maize. Paki. J. Bot. 50, 1347–1353.
Wang, H., Ouyang, Z., Chen, W., Wang, X., and Zheng, H. (2014). Transpiration Characteristics of Chinese Pines (Pinus tabulaeformis) in an Urban Environment, Designing Low Carbon Societies in Landscapes. Berlin: Springer, 57–71.
Wang, H., Sork, V. L., Wu, J., and Ge, J. (2010). Effect of patch size and isolation on mating patterns and seed production in an urban population of Chinese pine (Pinus tabulaeformis Carr.). For.Ecol. Manage. 260, 965–974. doi: 10.1016/j.foreco.2010.06.014
Wang, H.-F., Qureshi, S., Knapp, S., Friedman, C. R., and Hubacek, K. (2015). A basic assessment of residential plant diversity and its ecosystem services and disservices in Beijing, China. Appl. Geogr. 64, 121–131. doi: 10.1016/j.apgeog.2015.08.006
Williams, N. S. G., Hahs, A. K., and Vesk, P. A. (2015). Urbanisation, plant traits and the composition of urban floras. Perspect. Plant Ecol. Evol. Syst. 17, 78–86. doi: 10.1016/j.ppees.2014.10.002
Woodward, F., and Kelly, C. (1995). The influence of CO2 concentration on stomatal density. New Phytol. 131, 311–327. doi: 10.1111/j.1469-8137.1995.tb03067.x
Wright, I. J., Dong, N., Maire, V., Prentice, I. C., Westoby, M., Díaz, S., et al. (2017). Global climatic drivers of leaf size. Science 357, 917–921. doi: 10.1126/science.aal4760
Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., et al. (2004). The worldwide leaf economics spectrum. Nature 428, 821–827.
Xu, M., DeBiase, T. A., Qi, Y., Goldstein, A., and Liu, Z. (2001). Ecosystem respiration in a young ponderosa pine plantation in the Sierra Nevada Mountains, California. Tree Physiol. 21, 309–318. doi: 10.1093/treephys/21.5.309
Yang, N., Wang, X., Zheng, F., and Chen, Y. (2017). The response of marigold (Tagetes erecta Linn.) to ozone: impacts on plant growth and leaf physiology. Ecotoxicology 26, 151–164. doi: 10.1007/s10646-016-1750-7
Yin, X. (2002). Responses of leaf nitrogen concentration and specific leaf area to atmospheric CO2 enrichment: a retrospective synthesis across 62 species. Glob. Change Biol. 8, 631–642. doi: 10.1046/j.1365-2486.2002.00497.x
Yuan, Z., Shi, X., Jiao, F., and Han, F. (2018). N and P resorption as functions of the needle age class in two conifer trees. J. Plant Ecol. 11, 780–788. doi: 10.1093/jpe/rtx055
Zhao, J. (2010). Species Composition and Spatial Distribution of Urban Plants Within the Built-up Areas of Beijing, China. Ph.D. Thesis. Beijing: Chinese Academy of Sciences.
Zhao, S., Liu, S., and Zhou, D. (2016). Prevalent vegetation growth enhancement in urban environment. Proc. Natl. Acad. Sci. U.S.A. 113, 6313–6318. doi: 10.1073/pnas.1602312113
Zheng, L.-L., Zhao, Q., Yu, Z.-Y., Zhao, S.-Y., and Zeng, D.-H. (2017). Altered leaf functional traits by nitrogen addition in a nutrient-poor pine plantation: a consequence of decreased phosphorus availability. Sci. Rep. 7:7415. doi: 10.1038/s41598-017-07170-3
Ziska, L. H., Bunce, J. A., and Goins, E. W. (2004). Characterization of an urban-rural CO2/temperature gradient and associated changes in initial plant productivity during secondary succession. Oecologia 139, 454–458. doi: 10.1007/s00442-004-1526-2
Keywords: leaf morphological traits, leaf nutrient traits, urban-rural gradients, land-use type, Chinese pine
Citation: Su Y, Cui B, Luo Y, Wang J, Wang X, Ouyang Z and Wang X (2021) Leaf Functional Traits Vary in Urban Environments: Influences of Leaf Age, Land-Use Type, and Urban–Rural Gradient. Front. Ecol. Evol. 9:681959. doi: 10.3389/fevo.2021.681959
Received: 17 March 2021; Accepted: 17 November 2021;
Published: 09 December 2021.
Edited by:
J. D. Lewis, Fordham University, United StatesReviewed by:
Shan Yin, Shanghai Jiao Tong University, ChinaCopyright © 2021 Su, Cui, Luo, Wang, Wang, Ouyang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoke Wang, d2FuZ3hrQHJjZWVzLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.