- 1Department of Molecular Ecology, Max Planck Institute for Chemical Ecology, Jena, Germany
- 2Department of Evolutionary Neuroethology, Max Planck Institute for Chemical Ecology, Jena, Germany
- 3Laboratory of Entomology, Department of Plant Sciences, Wageningen University, Wageningen, Netherlands
- 4Grupo Entomología, Instituto de Medicina Tropical, Universidad Peruana Cayetano Heredia, Lima, Peru
Most flowering plants depend on animal pollination for successful sexual reproduction. Floral signals such as color, shape, and odor are crucial in establishing this (often mutualistic) interaction. Plant and pollinator phenotypes can vary temporally but also spatially, thus creating mosaic-like patterns of local adaptations. Here, we investigated natural variation in floral morphology, flower volatile emission, and phenology in four accessions of a self-compatible wild tobacco, Nicotiana attenuata, to assess how these traits match the sensory perception of a known pollinator, the hawkmoth Manduca sexta. These accessions differ in floral traits and also in their habitat altitudes. Based on habitat temperatures, the accession occurring at the highest altitude (California) is less likely to be visited by M. sexta, while the others (Arizona, Utah 1, and Utah 2) are known to receive M. sexta pollinations. The accessions varied significantly in flower morphologies, volatile emissions, flower opening, and phenology, traits likely important for M. sexta perception and floral handling. In wind tunnel assays, we assessed the seed set of emasculated flowers after M. sexta visitation and of natural selfed and hand-pollinated selfed flowers. After moth visitations, plants of two accessions (Arizona and Utah 2) produced more capsules than the other two, consistent with predictions that accessions co-occurring with M. sexta would benefit more from the pollination services of this moth. We quantified flower and capsule production in four accessions in a glasshouse assay without pollinators to assess the potential for self-pollination. The two Utah accessions set significantly more seeds after pollen supplementation compared with those of autonomous selfing flowers, suggesting a greater opportunistic benefit from efficient pollinators than the other two. Moreover, emasculated flowers of the accession with the most exposed stigma (Utah 2) produced the greatest seed set after M. sexta visitation. This study reveals intraspecific variation in pollination syndromes that illuminate the potential of a plant species to adapt to local pollinator communities, changing environments, and altered pollination networks.
Introduction
Flowers exploit the sensory bias of insect pollinators to improve their chance of receiving outcrossed (Balamurali et al., 2015) or at least geitonogamous pollinations (Vaughton and Ramsey, 2010; Sukumaran et al., 2020), the opportunities for which differ considerably depending on plant densities, interpopulation distances, and phenologies (Carvalheiro et al., 2014; Kantsa et al., 2018). Attracting (or luring) pollinators is achieved via different types of rewards or attractants offered by the plant. In some cases, these signals can vary among plants from different habitats and populations in response to the specific needs of pollinators that can maximize the reproductive output of the plant (Gómez et al., 2009a).
Most species are assemblages of different genotypic and phenotypic populations within an ecologically complex landscape. The geographic mosaic theory of coevolution incorporates this fact and postulates that populations are under different evolutionary selective pressures over large geographic distances due to spatial variation (Thomson, 2005). In a pollinator community context that would mean that due to spatial variations over the habitats of its range, a plant species can be either adapted or maladapted to certain pollinator(s). This suggests that when a plant species with a specialized pollination syndrome occurs outside the range of the optimal pollinator, many of the adaptations might become maladaptations as they could hinder the attraction of other potential pollinators. In contrast, if a plant has a generalist pollinator community, certain floral traits can be considered as local adaptations over its range to maximize the service of the most optimal local pollinators (Gómez et al., 2009a).
Scent is an important factor for speciation or pollinator fidelity to a certain plant species (Balamurali et al., 2015; Kantsa et al., 2018; Souto-Vilarós et al., 2018) in relation to the pollinator community. The loss of a specific major scent compound can be interpreted as a release from a main pollinator, particularly if the scent at high concentrations acts also as a repellent to herbivores (Baldwin et al., 1997; Kessler et al., 2019). Furthermore, pollinators can also act as herbivores for the same plant species. For example, besides searching for nectar, female moths are also in the search of oviposition sites. Both nectaring and oviposition behavior have been shown to be linked in the hawkmoth Manduca sexta, bearing the risk for the plant to be consumed by the caterpillars of its pollinator (Kessler et al., 2012; Smith et al., 2017).
Other studies addressing outcrossing versus selfing point out that self-compatible populations at edges of a native range will have a larger proportion of selfing compared with outcrossed. Plants growing at the edges of the native range may have fewer pollinators available that can perceive and interact successfully with the flowers, this being even more so for specialist plant–pollinator systems. Thus, in order to colonize new habitats and avoid Allee effects (Morgan et al., 2011), plants in edge populations would have to rely more on selfing rather than outcrossing. Over time, different floral traits can be (de)selected as those are no longer exploiting a specialized perception of a pollinator to increase the reproductive output of the plants. The reduction of investment on floral advertisement can lead to a shorter flower presentation time and faster self-pollination.
Additionally, there are physical–mechanical–morphological floral traits that can facilitate the visualization of certain traits such as the timing of flower opening and flower movement (Aizen, 2003; van Doorn and Van Meeteren, 2003; Hodges et al., 2004; Yon et al., 2016). Flower opening conditions the perception of a corolla display by a pollinator, as the pigments located in the front and back of the petals can be different, as well as allowing the visualization of anthers, pollen, and pistil. As studies have shown on Aquilegia and artificial flowers (Hodges et al., 2004; Sprayberry and Suver, 2011), the orientation of flowers can render the visual organs of many pollinators useless. In these cases, the obverse of the corolla and its features are not exposed to the pollinator, due to particular flying and foraging approaches to the flowers (Hodges et al., 2004; Ushimaru and Hyodo, 2005; Haverkamp et al., 2019).
Nicotiana attenuata is a solanaceous plant species mainly occurring in the Southwest United States, where it inhabits a broad range of elevations at least between 800 and 2,300 meters above sea level (masl) (Haverkamp et al., 2018). This represents a temperature variation between locations at the warmest month July of 7.5°C at high elevation to 19.9°C at lower elevation during the night (WorldClim database). N. attenuata is assumed to have a hawkmoth pollination syndrome, with white long tubular flowers, night opening, night upright orientation, and strong night scent that exploit the sensory bias of the hawkmoth M. sexta. Previous studies examining the interaction between M. sexta and N. attenuata have shown the importance of one particular floral volatile compound, benzyl acetone (BA; Kessler et al., 2015; Haverkamp et al., 2016b). The presence of this compound increases the foraging success of the moth, as well as the reproductive output of the plant (Haverkamp et al., 2016b).
At the same time, the larvae of this hawkmoth is a voracious herbivore on N. attenuata, and its attraction as pollinator therefore brings a risk of damage (Kessler et al., 2010, 2015; Reisenman et al., 2010; Kessler, 2012). This might select against exploiting M. sexta sensory bias and pollination service in some natural populations under less beneficial growth conditions (Gómez et al., 2009b). Besides M. sexta, also other hawkmoths such as Hyles lineata or Manduca quinquemaculata, but also day-active hummingbirds (e.g., Archilochus alexandri) and bee species (e.g., Apis mellifera, Lasioglossum spp.) have been observed to visit flowers of N. attenuata, which feed on nectar and pollen, respectively. In field experiments, emasculated flowers open during the day produced capsules, thus showing the capability of day-active pollinators to provide the plant with pollen (Kessler et al., 2015), especially when plants are attacked by M. sexta caterpillars (Kessler et al., 2010). N. attenuata plants that are under attack of M. sexta caterpillars produce flowers with reduced BA emissions that open in the morning and are preferred by day-active hummingbirds (Kessler et al., 2010). Thus, plants reduce herbivore pressure by switching from M. sexta pollination, which involves the risk of caterpillar-feeding damage, to hummingbird pollination.
We recently showed that N. attenuata originating from different populations are differentially attractive to the pollinator M. sexta (Haverkamp et al., 2018). In this follow-up study, we investigate how this relates to the reproductive success of four N. attenuata accessions. Moreover, we propose that some accessions are more specialized on M. sexta pollination than others, following the idea of local specialization.
Materials and Methods
Seed Germination and Plant Cultivation
The N. attenuata Torr. (Solanaceae) seeds for all experiments were sterilized and germinated on Petri dishes with Gamborg’s B5 media as described in Krügel et al. (2002). The seeds were maintained under 16 h:8 h light:dark conditions in a growth chamber with temperature set to 28°C in light and 26°C in dark (Percival, Perry, IA, United States) for 10 days. Afterwards, seedlings were transferred to TEKU pots (TEKU JP 3050 104 pots, Poppelmann, Lohne, Germany) with Klasmann plug soil (Klasmann-Deilmann, Saterland, Germany) in a glasshouse [16 h:8 h light:dark, humidity: 50–60%, temperature: 23–25°C (light), and 19–21°C (dark)]. After 10 days, plants were transferred from TEKUs to 1 L pots for glasshouse and wind tunnel experiments at the Max Planck Institute for Chemical Ecology (MPI CE), Jena, Germany.
In this study, we focused on genetically fixed differences among different accessions rather than phenotypic plasticity. Therefore, plants were inbred for several generations and raised under uniform growth conditions in the glasshouse. Plants originated from four different wild-type N. attenuata native populations: “Ut1” from the D.I. Ranch (Santa Clara, UT, United States; inbred for 31 generations), “Az” from Townsend Winona Road (east Flagstaff, AZ, United States; inbred for five generations), “Ca” from Benton Crossing Road (west Benton Hot Springs, CA, United States; inbred for five generations), and “Ut2” from Lytle Ranch Preserve (Lytle Ranch Preserve Rd, UT, United States; inbred for seven generations). The locations from which the seeds of the different accessions originated are shown in Figure 2.
Manduca sexta Rearing
Manduca sexta moths used for wind tunnel experiments were obtained from a colony maintained at the MPI CE. Moths were reared as previously described in Koenig et al. (2015). Eggs for the colony were collected from female moths, which were allowed to freely oviposit on N. attenuata plants. Caterpillars were fed on artificial diet at 27°C ambient temperature, 70% relative humidity, and 16:8 light:dark regime. As soon as caterpillars reached the wandering stage and stopped feeding, they were transferred into wooden blocks for pupation. Pupae were sexed 1 week before hatching, and male and female pupae were transferred in separate flight cages (15.5 h daylight with 25°C and 70% relative humidity, 7.5 h dim light at 0.5 lx with 20°C and 60% relative humidity). Between both phases (daylight and dim phase), a transition time of 30 min each was used. For experiments, male moths were used 3 days after hatching. Since M. sexta females frequently lay eggs while foraging on N. attenuata (Kessler et al., 2012), we used male moths to exclude the oviposition behavior from our experiments.
Flower Morphology and Opening
Flower morphology and opening of four N. attenuata accessions was investigated by measuring corolla limb diameter and area, pistil and corolla tube length, and flower opening of single freshly open flowers from seven independent plants per accession. The corolla limb diameter was measured between the most outer tips after flowers fully opened, and the area was measured from scanned calibrated images of the surfaces of corollas.
Flowers from seven independent plants per accession were cut open using forceps to measure the pistil and corolla tube length by hand with a ruler. The length of the pistil was measured from the flower base to the stigma. The corolla tube length was measured from the flower base to the tip of the open corolla petals. The length ratio of the corolla tube and the pistil within a flower was calculated by dividing the first over the second, where values above 1 means the pistil is shorter and the stigma is within the corolla tube and values below 1 means that the stigma is protruding from the corolla tube.
Flower opening (flower aperture) was recorded at 1/h acquisition intervals using a time-lapse imaging setup, composed of a webcam (Logitech Europe S.A., Lausanne, Vaud, Switzerland) connected to a laptop to automatically acquire and store the photos. To quantify the opening, the inner distance between opposite lobes was measured in pixels and converted to millimeter using the software IMAGE TOOLS v.3.0 (UTHSCSA, San Antonio, TX, United States). Later, the aperture values were converted to percentage of opening by taking the maximum value in millimeters from the fully open flower as denominator for all other intermediate values.
Pollen Count
For evaluating the number of pollen grains for four different natural accessions, anther heads were collected separately before anthesis (8–10 a.m.) into 2 ml reagent tubes and stored in a desiccator for 24 h until opening. For each accession, all five anthers from nine flowers of five plants were collected. After anthers opened, 250 μl of 2% sodium chloride solution was added to each tube and vortexed for approximately 1 min. The number of pollen grains per anther was counted under a microscope using a Neubauer cell counting chamber with a depth of 0.1 mm (Neubauer improved, Superior Marienfeld, Lauda-Königshofen, Germany). To ensure an equal distribution of pollen within the sample, we shortly vortexed each tube again directly before adding 10 μl to the chamber. For each sample, five large squares (5 × 1 mm2) were counted for estimation of the total number of pollen grains per anther following the Neubauer chamber formula:
Floral Benzyl Acetone Emissions
Benzyl acetone was measured as described in Haverkamp et al. (2018) using polydimethylsiloxane (PDMS) as traps and GC-MS for quantification. Here, we compare total overnight emissions of four flowers from different plants of the Ut2 accession with data for Ut1, Az, and Ca accessions taken from Haverkamp et al. (2018).
Nectar and Sugar Measurements
The amount of nectar was measured by carefully removing the corolla tube and collecting the nectar with a capillary (Brand, Wertheim, Germany) and measuring the length and dividing by its conversion factor 3.2. We used six plants per accessions and one flower per plant. The composition of sugars in the nectar was measured on a LC-Triple Quadrupole-MS instrument, Bruker EVOQ Elite (Bruker, Billerica, MA, United States), employing an HESI ion source as described in Schäfer et al. (2016). The quantification of glucose, fructose, and sucrose was done relative to the internal standard sorbitol.
Phenology Measurements
To examine the total number of flowers and capsules produced over the lifespan of the plants, we used five plants of each wild-type accession in the glasshouse. Since flowers remain open for approximately 3 days, the number of open flowers was recorded every 3 days in the morning (8–10 a.m.) to avoid multiple counting of the same flower. Additionally, all capsules were counted at each time point. At the end, the number of flowers from all time points was summed to estimate the total number of flowers and capsules as well as the total flower to total capsule ratio produced for each plant. Flower and capsule counts were recorded until plant senescence.
Map of Accessions and M. sexta Presence Prediction
The map of accessions was generated using the WorldClim database, referenced location of the accessions, M. sexta referential presence data in Utah (Yon et al., 2017b), and environmental temperature required for flying of M. sexta (Heinrich, 1971). The digital elevation model was used to generate altitude contour lines at 500 masl intervals using QGIS v.2.18. The predicted distribution map of M. sexta was generated using temperature as the proxy parameter for determining the likeliness of presence in southwestern United States using the function Bioclim modeling of the software DIVA-GIS v.7.5.
Wind Tunnel Bioassays
Behavioral assays with M. sexta moths were performed in a plexiglass wind tunnel (250 × 90 × 90 cm) at the MPI CE. The laminar flow of charcoal-filtered air was set to 0.37 m/s, which is similar to those conditions that hawkmoths commonly experience while foraging (Riffell et al., 2008). Climate conditions were adjusted to 25°C air temperature and 70% relative humidity. At the latest 1 h before the experiment, plants and moths were transferred to separate chambers with similar climate conditions as the wind tunnel.
To measure pollination rates of flowers of four natural accessions after M. sexta visitation, one flower per plant was emasculated before anthesis (daylight morning cycle) to exclude self-pollination (Kessler et al., 2008), and all other flowers were removed. Pollen of fresh flowers from plants not used in the wind tunnel was collected in the corresponding morning. In order to measure the output of pollen delivery, pollen was gently rubbed on the hawkmoth proboscis using a fine brush prior to its release in the wind tunnel (Haverkamp et al., 2016b). For each trial, another plant was placed at one side of the wind tunnel with the flower at a position 25 cm from the front end, 45 cm distant from both side walls, and approximately 70 cm from the ground of the wind tunnel. Moths were kept in individual mesh cages (15 cm × Ø13 cm) until being placed on a platform at the rear end of the wind tunnel opposite of the plant (10 cm from the rear end, 45 cm from both side walls, and 30 cm from the wind tunnel floor). Moths, which did not initiate wing fanning within 5 min, were excluded from the experiment. After the moths were taking off, they were allowed to fly freely for 4 min in the wind tunnel. The foraging behavior was observed via a video camera situated behind the flower at the beginning of the wind tunnel (Logitech C615, United States, infrared filter removed). The camera was recording at 30 Hz with a resolution of 800 × 600 pixels.
Flower approach was scored as flower contact of the moth with its proboscis or front legs. Flower handling time was counted from the first contact until the moth had no more contact with the flower for more than 1 s. Only the first flower approach was used for statistical analyses in order to exclude learning effects. Foraging success was evaluated by measuring the amount of residual nectar after an apparent successful moth contact with the flower, where a foraging event was scored as successful when nectar was fully removed as described in Haverkamp et al. (2018, 2019).
Pollination Experiments
All hand pollinations were performed in the evening between 7 and 9 p.m. with freshly open flowers, and the pollen was collected immediately before the start of the experiment from freshly open flowers. Hand pollinations with pollen of the same accession (self) and a pollen mixture of all four accessions (mix) were performed using emasculated flowers on five plants per accession. Therefore, anthers have been removed before anthesis in the morning between 5 and 6 a.m. of the day of flower opening as described in Kessler et al. (2008). For self hand pollination, we collected freshly open anthers of the accessions in separate 0.5 ml PCR reaction tubes. For mix hand pollination, the same number of freshly open anthers from the different accessions was collected with clean forceps into a 0.5-ml PCR reaction tube and mixed by tapping multiple times against the walls. Anther heads were carefully removed from the reaction tube prior to avoid any damage when applying pollen to the stigma. For each hand pollination treatment (self and mix), we used 20 flowers on five plants per accession. The same plants were used for pollen collection, self and mix hand pollination. Only capsules that produced seeds have been used for statistical analysis.
Additionally, we used nonemasculated flowers to evaluate if the seed set of autogamously selfing flowers (autogamous selfing) differs from that of flowers supplemented with pollen from the same flowers (selfing hand). Therefore, we removed all open flowers in the morning of the experiment to ensure the use of fresh flowers. For both treatments, 10 flowers on five plants per accession were used, whereas only flowers that produced capsules containing seeds have been considered for statistical analysis.
In all hand pollination experiments, pollen was applied to experimental flowers by homogeneously covering the stigma using a wooden tooth pick. Afterwards, the flowers’ pedicels were carefully labeled using a colored string with each treatment being assigned to a different color. Capsules of all pollination experiments were collected shortly before opening approximately 14–20 days after pollination and dried in a desiccator for 2 days before being further processed. The number of seeds from capsules obtained from hand and wind tunnel pollinations have been automatically counted using ImageJ2 (Fiji; Schindelin et al., 2012; Rueden et al., 2017).
Statistical Analysis
The data were analyzed using the software R version 4.0.4 (The R Foundation for Statistical Computing, 2021) and, additionally, the following R packages “pairwiseCI” v.0.1-27, “lsmeans” v.2.3, “multcomp” v.1.4-16, and “agricolae” v.1.3-3. Data normality was evaluated with Shapiro tests and additionally visualized with Q-Q plots. When data were normally distributed, we employed an analysis of variance (ANOVA) model; when data were not normally distributed, we employed a generalized linear model, with family adjustments depending on data type (Gaussian for continuous values and quasi-binomial for proportions). Direct pairwise comparisons, when not required one of the previous models, were analyzed with a Holm-corrected Fisher’s exact test for normally distributed data and a Wilcoxon test for non-normally distributed data. Additionally, in order to evaluate difference in floral trait variance among accessions, we used the Fligner test for pairwise variance analysis with Holm adjustment.
Results
Flower Morphology and Opening
The corolla limb area was analyzed using the “Momocs” R package and compared between accessions using a generalized linear model followed by post hoc Tukey with Holm adjustment for multiple comparison showing the greatest corolla limb area (Figure 1A and Supplementary Table 1) and corolla tube length with an ANOVA followed by the honestly significant difference (HSD) test (Figure 1B and Supplementary Table 1) for flowers of the accession Ut2. To evaluate differences in the position of the stigma relative to the corolla tube, the ratio of tube/pistil length was compared between the accessions (GLM followed by pairwise Wilcoxon test), whereas values <1 indicate a stigma located outside of the corolla tube (Ut2) and values >1 a stigma located inside the corolla tube (Ut1, Az, and Ca). The tube/pistil length ratio differed significantly between Ca and Ut2, whereas Ut1 and Az did not differ from any accession (Figure 1C and Supplementary Table 1). For these three morphological traits, the size of their variance was not significantly different when tested with the Fligner tests (corolla limb area p = 0.5315, tube length p = 0.9891, tube/pistil length p = 0.2999).
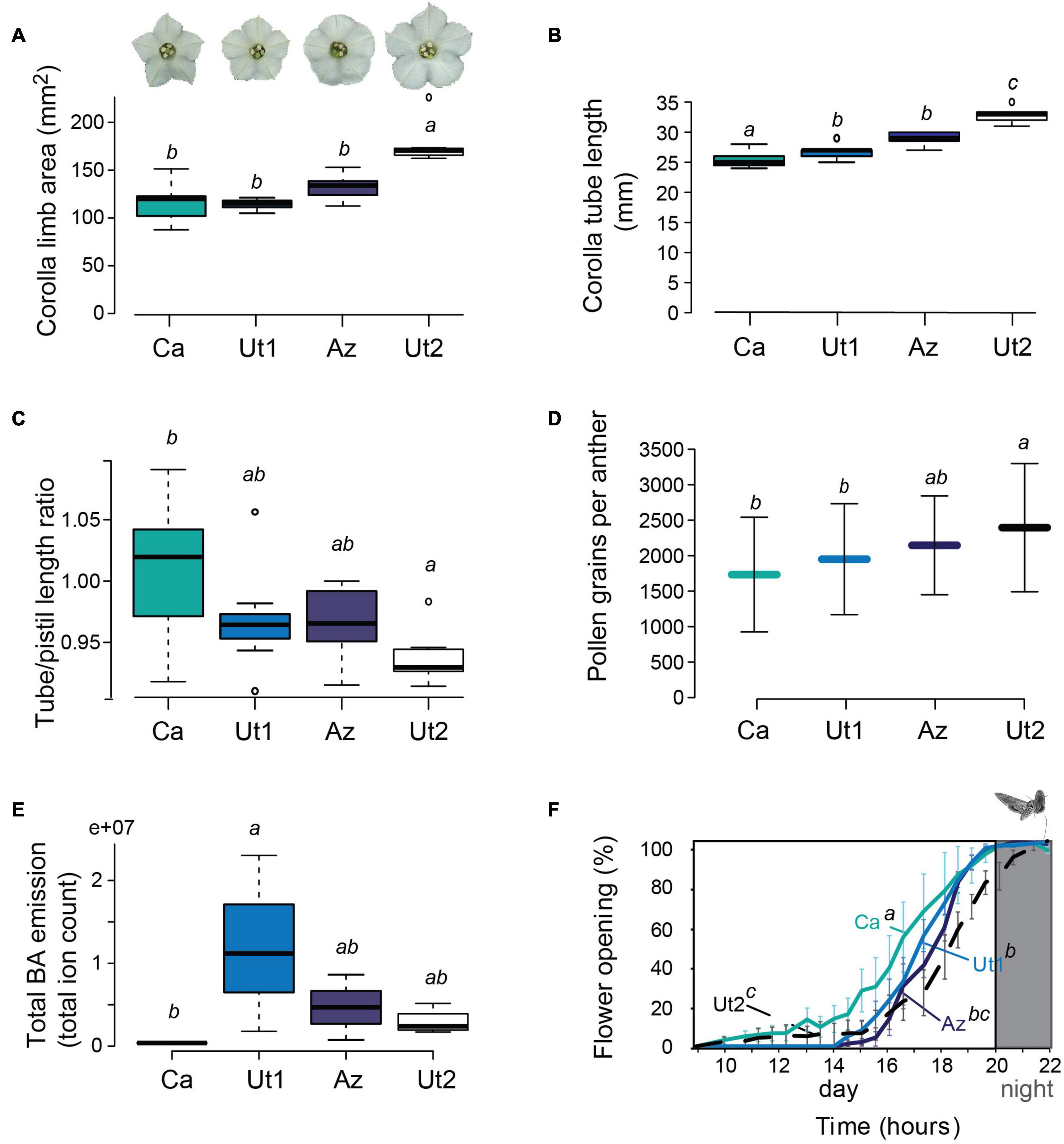
Figure 1. Floral morphology and pollen number of four native Nicotiana attenuata accessions. (A) Boxplot showing the corolla limb area analyzed using the R package “Momocs” [N = 7, generalized linear model followed by post hoc Tukey with Holm adjustment, different letters indicate significant differences (p < 0.001)]. (B) Boxplot of corolla tube length [N = 7, ANOVA model followed by HSD test with Holm adjustment, different letters indicate significant differences (p < 0.005)]. (C) Boxplot showing the corolla tube/pistil length ratio. Values above 1 indicate a stigma which is shorter than the corolla tube, whereas a value below 1 indicates a stigma located outside of the corolla tube [N = 7, generalized linear model followed by pairwise Wilcoxon test with Holm adjustment, different letters indicate significant differences (p < 0.05)]. (D) Number of pollen grains (mean ± SD) per anther [N = 45, ANOVA followed by HSD test, different letters indicate significant differences (p < 0.05)]. (E) Boxplot showing the total BA emission of flowers [N = 4, generalized linear model followed by HSD test, different letters indicate significant differences (p < 0.05)]. (F) Flower opening (mean ± SEM) between 9 and 22 h [N = 4, generalized linear model followed by HSD test with Holm adjustment, different letters indicate significant differences (p < 0.05)]. The flower opening refers to the flower aperture measured in millimeters and expressed as percentage of opening. The gray bar represents the activity period of M. sexta within the time span measured. Boxplots show the median (thick bar), the first and the third quartiles [box and 1.5 times the interquartile range (whiskers)]. Abbreviations for accessions: Az, Arizona; Ca, California; Ut1, Utah 1; Ut2, Utah 2.

Figure 2. Map of Manduca sexta predictions based on temperature range and pollination output of four natural accessions. (A) Predicted distribution map of M. sexta with temperature as the proxy parameter in the Great Basin Desert, United States. Red circles show the seed collection sites. (B) Total percentage of emasculated flowers of N. attenuata approached by M. sexta moths in a wind tunnel assay [p = 0.1326, Holm-corrected Fisher’s exact test, data published for Ca, Az, and Ut1 (Haverkamp et al., 2019)]. (C) Flower contact time (mean ± SD) for first flower approached [ANOVA followed by Holm-corrected least significant differences, data published for Ca, Az, and Ut1 (Haverkamp et al., 2019)]. (D) Total percentage of capsules containing seeds formed after M. sexta visitation (ANOVA followed by pairwise HSD test with Holm adjustment). (E) Seed output of emasculated flowers resulting from moth pollination (generalized linear model followed by least squares pairwise comparison). Numbers below the x-axis represent replicate numbers. Different letters indicate significant differences (p < 0.05). n.s., non-significant difference. Whiskers of the boxplots in panels (C,E) indicate 1.5 times the interquartile range; boxes depict first and third quartiles.
Flowers of Ut2 and Ca started opening earlier but slower than Ut1 and Az, which results in a similar flower opening when M. sexta activity period starts at 20 h (Figure 1F). The progressive opening of the corolla limb (millimeters open expressed as percentage of its full opening) was analyzed with a GLM [accessions F = 16.736, p < 0.0001; time (as factor) F = 146.059, p < 0.0001] followed by post hoc least square means Tukey for pairwise comparison between accessions (Supplementary Table 1). We observed that flowers of Ca and Ut2 started opening significantly earlier than flowers of Az or Ut1, while the latter two were not different between themselves.
Pollen Count
The number of pollen grains per anther (Figure 1D and Supplementary Table 1) was compared between the accessions. Due to its normal distribution, ANOVA followed by the HSD test was performed, showing that Ut2 has significantly more pollen grains per anther than Ca (p = 0.0007) and Ut1 (p = 0.0445), but does not differ from Az (p = 0.4514). No significant difference was found between the variance size with the Fligner test (p = 0.752).
Floral Benzyl Acetone Emissions
Furthermore, analysis of total BA emission in flowers of the four accessions was performed using a GLM followed by a multicomparison HSD test (Figure 1E). Flowers of Ut1 emit the greatest amount of BA, being only significantly different from Ca (p = 0.0221), which is originating from a population outside of the predicted M. sexta range (Figure 2A). No significant difference was found between the variance size with the Fligner test (p = 0.3208).
Nectar and Sugar Measurements
Nectar volume differed significantly among the four accessions (GLM p < 0.0001) and was the highest in Ca, followed by Ut2. The other two accessions presented similar low nectar volumes (Supplementary Figure 1A). When analyzing the sugar composition, Az had the highest concentrations of glucose, fructose, and sucrose compared with the other accessions (GLM followed by a multicomparison HSD test, glucose: p = 0.0044, fructose: p = 0.0031, sucrose: p = 0.0139), but was not significantly different for each pairwise comparison (Supplementary Figures 1B–D).
Flower and Capsule Count
Five plants per accessions were observed regarding their flower and capsule kinetics. Az plants started flowering around 28 days after potting, which is 2.4 to 4.8 days earlier than the other accessions. Moreover, Az plants attained their flowering peak 7.8 to 9 days earlier than Ca, Ut1, and Ut2. The flower kinetic was analyzed with a GLM with factors accession and a four term polynomial fitting for days after potting (dap) (accession p = 0.0014 and dap p < 0.0001). Followed by a least square means analysis for pairwise comparison, overall, the flowering kinetic of Az differed significantly from all other accessions (Figure 3A and Supplementary Table 2).
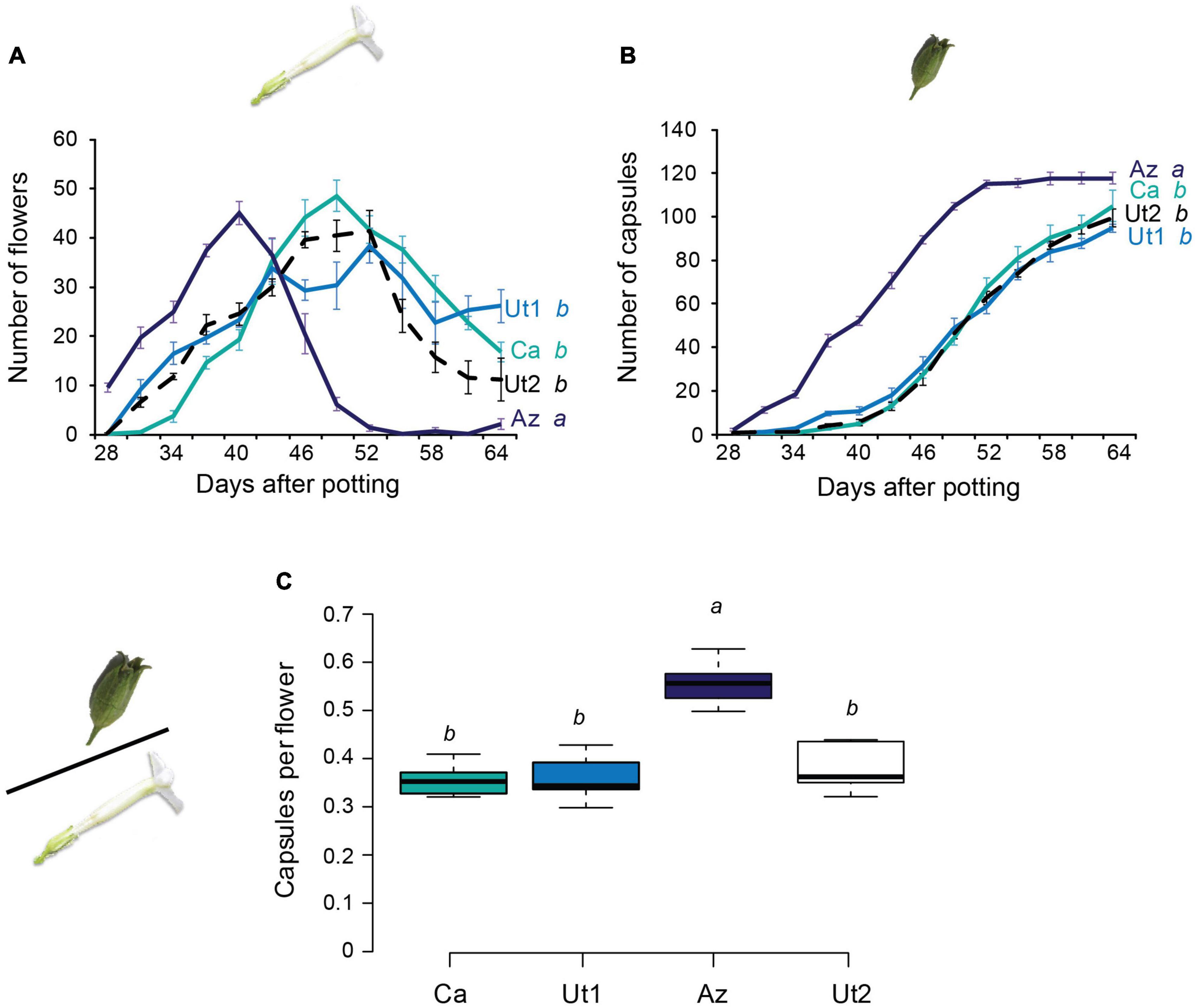
Figure 3. Flower and capsule count over the lifespan of four N. attenuata accessions. (A) Mean ± SEM number of flowers per plant (N = 5, generalized linear model with polynomial fitting with four terms, followed by least square means for pairwise comparison with Tukey adjustment). (B) Mean ± SEM accumulative number of capsules produced per plant (N = 5, generalized linear model with polynomial fitting with four terms, followed by least square means for pairwise comparison with Tukey adjustment). (C) Proportion of capsules formed per total number of flowers per plant [N = 5, generalized linear model (quasi-binominal family) followed by a least square mean pairwise comparison]. Error bars of the boxplot in panel (C) indicate 1.5 times the interquartile range; boxes depict first and third quartiles. Different letters indicate significant differences (p < 0.05).
Furthermore, the capsule kinetic, with the same statistical approach as the flower kinetic, showed significant differences between Az and all other accessions (Figure 3B and Supplementary Table 2), with Az starting to set capsules 4.2 to 8.4 days ahead of Ca, Ut1, and Ut2. The number of capsules produced per flower over the lifespan of the plant was compared as capsule-to-flower ratio between accessions (Figure 3C and Supplementary Table 1). Arizona plants showed a significantly greater capsule-to-flower ratio, being approximately 20% higher than those of the other accessions.
Manduca sexta Flower Handling and Pollination Output
The duration of flower contact was measured for the first flower approached by M. sexta. Solely, Ut1 and Ca showed a statistically significant difference in flower contact time, while Az and Ut2 did not differ from any accession (Figure 2C and Supplementary Table 1). When foraging on flowers of Ca, moths have the lowest foraging success (20%) compared with the other three accessions (Ut1: 66.67%, Az: 56.25%, and Ut2: 50%), although in a pairwise comparison, only Ca and Ut1 were significantly different (Supplementary Figure 2). The foraging success of the moth did not differ between Ut1, Ut2, and Az.Manduca sexta approached a similar percentage of emasculated flowers in the wind tunnel (p = 0.1326 Holm-corrected Fisher’s exact test, Figure 2B). We used the capsules formed by the approached flowers for seed counts to estimate the output of M. sexta pollination on emasculated flowers. Not all of the capsules contained fully formed seeds and the accessions differed in this percentage of capsules, where Az and Ut2 formed a significantly greater number of capsules containing seeds than Ca and Ut1 (Figure 2D and Supplementary Table 1; Ca: 15.91%, Ut1: 21.43%, Az: 47.73%, and Ut2: 53.06%).
To analyze the number of seeds per capsule that result from M. sexta pollinations, we only used capsules containing fully formed seeds. Capsules of Ut2 contained a greater number of seeds than Ca, Ut1, and Az, even though being only significantly different with Az (Figure 2E and Supplementary Table 1). The seed output presented a significant difference of the variance size with the Fligner test (p = 0.0061). Followed by a pairwise Fligner test comparison (with Holm adjustment) of the size of variances, a difference was found only between the pair Az–Ut2 (p = 0.022).
Hand Pollination Experiments
We compared the seed set of emasculated flowers hand pollinated either with self pollen or with mix pollen containing all four accessions (Figure 4A). The number of seeds produced per capsule did not differ significantly between both treatments in Ca (p = 0.096), Ut1 (p = 0.680), and Ut2 (p = 0.449). Only Az produced a significantly greater amount of seeds per capsule when pollinated with mix pollen (p = 0.024). No significant difference was found for the variance size between accessions either for self pollen or mix pollen with the Fligner tests (p = 0.6127 and p = 0.3775, respectively).
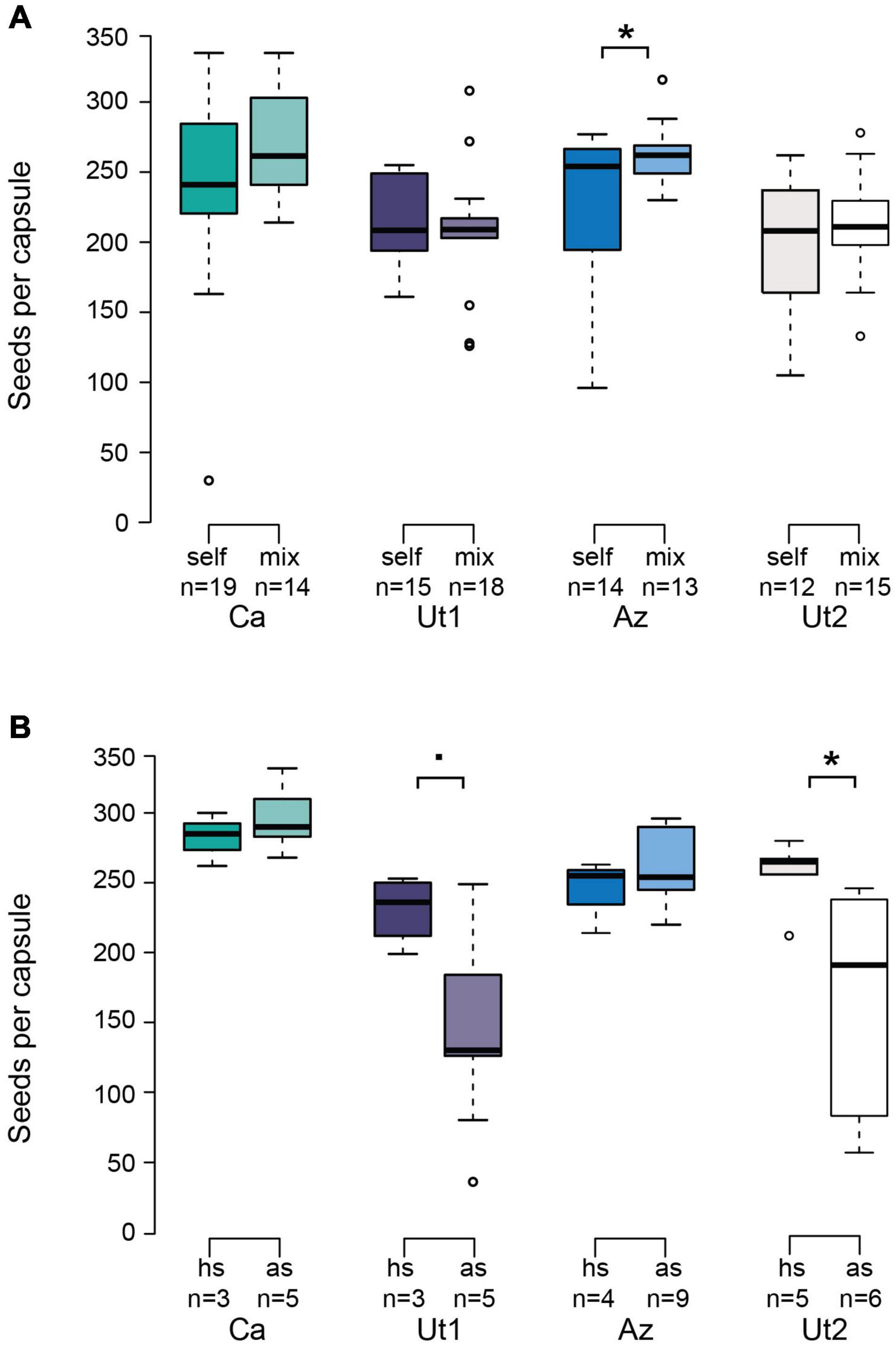
Figure 4. Seed output after natural selfing and hand pollination with self and mixed pollen. (A) Seed output of emasculated flowers hand pollinated with self pollen (“self,” left box) or a pollen mixture of all four accessions (“mix,” right box). (B) Seed output of natural selfing flowers (autogamous selfing “as,” right bars) in comparison with nonemasculated flowers hand pollinated with additional self pollen (selfing hand “hs,” left bars). Whiskers of the boxplots indicate 1.5 times the interquartile range; boxes depict first and third quartiles; centerline indicates median. Statistics for both data sets were performed using a generalized linear model followed by a pairwise Wilcoxon test to compare between both treatments of the same accession. Numbers below the x-axis represent the number of replicates. Asterisks indicate statistically significant differences (p < 0.05); dot indicates near statistically difference (p < 0.051).
To assess possible pollen limitation resulting from the lack of autogamous selfing, we used nonemasculated flowers. We compared the seed set between flowers allowed to naturally self (autogamous selfing) and flowers supplemented with pollen of the same plant (selfing hand, Figure 4B). In Ut2 (p = 0.0173), nonemasculated flowers additionally hand pollinated with self pollen (selfing hand) showed a significantly greater seed set compared with naturally selfing flowers (autogamous selfing), whereas no difference was detected for Ca (p = 0.5714) and Az (p = 0.7857). In Ut1, we observed the same tendency as in Ut2, even though the difference between both treatments was not significant (p = 0.0503). No significant difference was found for the variance size between accessions either with self pollen or mix pollen with the Fligner tests (p = 0.1699 and p = 0.9517, respectively).
Discussion
Specializing on a certain pollinator guild has been argued to restrict the range distribution of a plant due to limited pollination service outside the habitat of the optimal pollinators. Here, we investigated this hypothesis for N. attenuata by measuring flower traits and the ability for self-pollination and outcross pollination in plant populations with full access to one of their major pollinators and in a population at the edge of the habitat of pollinators.
Nicotiana attenuata is a plant species with high phenotypic plasticity of floral traits involved in pollinator attraction (Kessler et al., 2015; Zhou et al., 2017; Guo et al., 2020), and many of its flower characteristics seem to point toward a specialization for hawkmoth pollination. Previous studies have, for example, shown that flowers of N. attenuata are synchronized in flower opening, flower movement, and BA emission (Yon et al., 2017a; Haverkamp et al., 2019) with the activity of M. sexta. Therefore, the question can be raised if N. attenuata accessions varying in the prediction of M. sexta occurrence differ in floral traits that are playing a major role in the synchronization with the activity period of the moth and its ability to perceive the flowers.
Abiotic factors such as, for example, soil composition, temperature, water, and light availability can influence plant traits. Here, the accessions were grown under uniform conditions in the greenhouse to focus on genetically fixed differences and to avoid, e.g., herbivory, which has been shown to influence flower phenology (Kessler et al., 2010). Nevertheless, the growing conditions in the greenhouse differ from those in the natural habitat of accessions (e.g., temperature, soil, light). To what extent abiotic factors can alter traits that were measured in our study is not known for N. attenuata, and our conclusions were made based on the data collected from plants raised under the same conditions.
The floral volatile compound BA has been shown to be crucial to ensure a successful plant–pollinator interaction between N. attenuata and M. sexta, i.e., the presence of that compound is not only necessary to enable the moth to remove the nectar from the flowers, but it also increases the female reproduction success of the plant (Kessler et al., 2015; Haverkamp et al., 2016b). Interestingly, BA, which is a species-specific volatile compound of N. attenuata (Euler and Baldwin, 1996; Guo et al., 2020), can be perceived by the moth via olfactory receptors on its antennae but furthermore through specific chemical receptors on its proboscis (Haverkamp et al., 2016b). Thus, the presence of BA is not only important for long-distance attraction, but also increases flower handling time. Flower handling time directly correlated with the foraging success of the moth and the reproductive output of the plant in experiments were BA emissions had been genetically silenced, but other flower traits were left unaltered (Haverkamp et al., 2016b). Among the four N. attenuata accessions, BA emission ranged from high levels in Ut1 to no detectable emission for Ca (Figure 1E). Interestingly, Ca originated from a population at above 2,000 masl, where M. sexta is not expected to be present as a potential pollinator (Figure 2A). The lack of BA in Ca seems to be associated with a lower foraging success (Supplementary Figure 2) and is to a certain degree also associated with a decreased reproductive success of flowers visited by M. sexta (Figures 2D,E).
During foraging on N. attenuata flowers, M. sexta moths are hovering in front of the flower and have to navigate their approximately 7.5-cm-long proboscis (Haverkamp et al., 2016a) into the much shorter corolla tube for reaching the nectar at its base (Figure 1B). This challenging task might be facilitated by a greater corolla limb area, which offers more surface for the proboscis of the moth to land and, therefore, increases handling effectiveness (Deora et al., 2021). In our study, the flowers of Ut2 have the greatest corolla limb area (Figure 1A). Interestingly, Ut2 also had a relatively low flower handling time (Figure 2C), while maintaining a relatively high foraging success rate (Supplementary Figure 2). This suggests that the moths are able to handle these flowers rather effectively in spite of its low BA emissions (Figure 1E). In addition to the corolla limb size, also the corolla tube length affects foraging efficiency in M. sexta. Haverkamp et al. (2016a) tested the energy balance of M. sexta when foraging on flowers of different Nicotiana species varying in corolla tube length. The results of this study revealed a close correlation between the energetic foraging costs and the match between the proboscis length to the flower tube length, with better matching flowers leading to lower energetic costs. Consequently, it can be assumed that M. sexta would forage energetically and more efficiently on N. attenuata accessions with longer corolla tubes. In our study, flowers of the four accessions varied significantly in corolla tube length (Figure 1B) with the shortest flowers being measured for the accession originating from a population outside of the M. sexta range prediction (Ca, Figure 2A). The longest flowers were measured for Ut2, which does not only originate from a location with excellent prediction of M. sexta (Figure 2A) but also produced the highest seed set after M. sexta visitation in the wind tunnel (Figure 2E). In spite of its relatively low BA emissions, the Ut2 accession might therefore be an attractive flower for M. sexta due to its morphological properties, which likely facilitate a higher energy gain for the moths during foraging.
After having successfully reached the flower nectar, its quality and quantity are being evaluated by taste receptors on the proboscis and stretch receptors in the gut (Dethier, 1976). The outcome of this assessment will decide whether the moth will learn the particular flower and subsequently visit other flowers of the same type. Hawkmoth pollination has often been linked to high sucrose concentrations in the nectar followed by a preference for fructose but not for glucose (Kelber, 2003). Interestingly, sucrose amounts in the nectar of N. attenuata were very low for all accessions in comparison with glucose and fructose (Haverkamp et al., 2018). In spite of these low amounts, we did find a tendency for higher sucrose concentrations in those accessions that occur in the range of M. sexta (Supplementary Figure 1). Taste neurons on the moth proboscis respond to sucrose concentration as low as ≤5 mM, potentially enabling the moths to discriminate between the nectar of the different accessions tested here (Haverkamp et al., in prep.). Baker and Baker (1983) found sucrose contents of about 50% of the total sugars in hawkmoth-pollinated flowers. Nonetheless, later studies do not report such high proportions of sucrose for moth-pollinated flowers (Galetto et al., 1998; Perret et al., 2001). Differences in the perception of the nectar reward could then further impact the way moths learn and remember the flowers of a certain accession (Wright et al., 2009). In contrast to our prediction, the largest volume of nectar was found in the Ca accession (Supplementary Figure 1); however, this nectar was also the most diluted (Haverkamp et al., 2018), which might make these flowers less attractive to nectar-robbing carpenter bees, which often damage flowers during foraging (Kessler et al., 2008). Besides sugars, secondary metabolites are often found in floral nectar and have been argued as a mechanism to exclude unwanted flower visitors (van der Kooi et al., 2021). Nicotine is the most important defensive secondary metabolite in N. attenuata and is also found in the flower nectar; however, in a previous study, no differences in the nicotine concentration between N. attenuata accessions were found (Haverkamp et al., 2018). In addition to this, many more secondary compounds have been detected in the nectar of the Ut1 accession, some of which differentially attracted or repelled hawkmoth and hummingbird pollinators (Kessler and Baldwin, 2007), and it could be speculated that these compounds are also regulated as an adaption to the local pollinator community.
Assuming that N. attenuata is specialized on M. sexta (Haverkamp et al., 2016b), plant populations at higher elevations, where the nights are colder and the warm periods are shorter, face a dilemma concerning their reproductive strategy since M. sexta moths require temperatures not lower than 12.5°C to fly (Heinrich, 1971). Under these circumstances, switching to generalist pollinators available during day time would be a strategy to loosen the dependence on M. sexta. Alternative pollinators for N. attenuata are day-active bees (e.g., A. mellifera, “sweat bees” such as Lasioglossum spp.) or hummingbirds. When using transgenic N. attenuata lines altered in circadian clock or in BA emission, hummingbirds were identified as pollen vectors (Kessler et al., 2015; Yon et al., 2017b), showing the possibility of N. attenuata to switch to these generalist pollinators. Most of these day-active pollinators have been observed to be able to access the flowers as soon as the corolla starts opening. Additionally, H. lineata, a generalistic hawkmoth active at dusk, has been shown to pollinate N. attenuata flowers, although BA does not seem to be crucial in establishing the interaction with H. lineata or hummingbirds (Kessler et al., 2015).
The flowers of the Ca and Ut2 accessions open earlier during the day compared with those of Az and Ut1 (Figure 1F), which might allow for an early recruitment of day pollinators. N. attenuata flowers already contain nectar before they are fully open and keep producing it throughout the night until 4 a.m. (Kessler, 2012). Although the timing of nectar production seems to be synchronized with M. sexta activity period, due to the early nectar production, it is possible that hummingbirds visiting earlier open flowers, such as Ca and Ut2, would be rewarded. Furthermore, Bhattacharya and Baldwin (2012) showed that the stigma is already receptive to pollen before flowers are fully open in the evening. Thus, pollen deposited on the stigma during the day could result in successful fertilization. We observed that hummingbirds and sweat bees can visit flowers shortly after they start opening; subsequently, it could be assumed that flowers of Ca and Ut2 are accessible already in the morning hours, whereas flowers of Ut1 and Az could be accessed from around 15 h onwards (Figure 1F). However, it is unknown if flowers that are less open are pollinated as efficiently as fully open flowers by the different day-active pollinators. At the time when M. sexta is active, the flower opening is not different among the accessions (Figure 1F); therefore, we would rather expect differences in flower accessibility for day pollinators such as sweat bees and hummingbirds. Whether accessions whose flowers open earlier (Ut2, Ca) really benefit from early visitations during the day remains to be tested.
Plants of the Az accession start earlier to produce flowers and seed capsules and, furthermore, show the highest flower-to-capsule ratio in the absence of pollen vectors (Figure 3C). The flowers of Az invest more into seed production in the presence of outcross pollen, resulting in a higher seed set (Figure 4A). The same is observed for Ca flowers that show a similar tendency for investing into a higher seed production when outcross pollen is present (Figure 4A). This opportunistic behavior might optimize the reproduction cost of the plant by investing more only in the presence of outcross pollen. This could be beneficial, since not every pollinator visit might result in the deposition of outcross pollen but rather in the transfer of pollen from flowers within the same plant (geitonogamy). Especially if outcrossing occurs only occasionally, resources can be saved by investing not all energy in every flower, but rather in those that contribute to a greater genetic diversity of the offspring. Opposite to that, Ut1 and Ut2 seem to always invest in seed production (Figure 4A). This strategy would make sense, if an ample abundance of pollinators can be expected that provide the flower with outcross pollen. This might be true for Ut1 and Ut2 since previous studies performing field experiments with N. attenuata plants in Utah have identified outcrossing rates of above 30% (Sime and Baldwin, 2003; Kessler et al., 2008, 2012). The pollination service of hummingbirds might be restricted, for example by requirements such as nesting sites, and also most bees forage within a small radius, whereas hawkmoths are assumed to travel over long distances (Kawahara et al., 2013). It could be speculated that bees and hummingbirds might provide mainly pollination service within plants due to visiting multiple flowers on the same plant. Pollinators who visit multiple flowers within one plant might mainly contribute to geitonogamy instead of transferring pollen between conspecific plants (Vaughton and Ramsey, 2010; Sukumaran et al., 2020). In other words, they may be efficient pollinators from a quantity, but not necessarily from a quality perspective. Previous studies have shown that in N. attenuata, despite being a self-compatible species, not all pollen donors have the same chance of siring seeds and there are variations in mate selection preferences between accessions (Bhattacharya and Baldwin, 2012; Guo et al., 2019). Given that, some pollinators might transfer pollen loads of high intraspecific diversity and therefore contribute more to a diverse pool of pollen mates available for competition on the stigma. It still remains to be tested to which extent M. sexta and the other pollinators really contribute to outcrossing, and not only geitonogamy.
When testing for the capacity for self-pollination, the flowers of Ut1 and Ut2 produce less seeds in the absence of pollen supplementation (Figure 4B). One possible explanation for this could be that autogamous selfing flowers are pollen limited. In Ixiolirion songaricum, for example, the distance between the anther and stigma has been shown to negatively correlate with autonomous self-pollen deposition on the stigma (Jia and Tan, 2012). Here, Ut2 has a protruding stigma (Figure 2C), which may limit the ability to autogamously self-pollinate. An alternative hypothesis for the difference in seed set between autogamous selfing and pollen supplementation could be that flowers of Ut1 and Ut2 only invest in realizing their full reproductive potential when flowers have been visited. For example, flowers of Heliconia tortuosa use the capacity of tropical hummingbirds to extract nectar as a cue to turn on their reproduction (Betts et al., 2015).
When using emasculated flowers, subsequently disabling the possibility of flowers to autonomously self-pollinate, Ut2 produces the greatest number of seeds after visitation by a moth carrying pollen on the proboscis (Figure 2E). The protruding position of the stigma of Ut2 compared to a location inside the corolla tube (Ut1, Ca, Az; Figure 1C) might enhance the possibility of successful pollen deposition by pollinators, particularly with pollinators that do not dive inside the flower. Furthermore, this exposes the stigma to receive outcross pollen instead of self-pollen after the foraging interaction. The fact that flowers of Ut2 open earlier during the day (Figure 1F) could increase the chance to receive outcross pollen by both day and night pollinators.
Besides the differences found in floral traits that can indicate certain local adaptations, the variance range of the plant traits can give us information about the degeneration by drift of a particular trait. Higher trait variation will relate to either release from pollinator-mediated selection or a switch to generalist pollinators that do not necessarily impose selective pressure in a particular direction for a plant trait. In our analysis of variance range, we found almost no difference with the exception of the seed output after M. sexta interaction. Based on the presented results, we cannot conclude that the floral traits degenerate by drift of pollinator release or multidirectional generalist pollinator pressures.
Spatial (and temporal) variation in plant and pollinator traits as well as abundance can result in a geographic mosaic of coevolution, and previous studies have reported geographic covariation of different flower and pollinator traits (e.g., Thompson and Cunningham, 2002; Anderson and Johnson, 2008; Brown et al., 2011; Gross et al., 2016). In N. attenuata, variation in flower angle (Yon et al., 2017b; Haverkamp et al., 2019), flower opening (Figure 1F), and the specific volatile compound BA (Haverkamp et al., 2018, Figure 1E) might act as a pollinator filter, with the result that certain accessions are more specialized toward M. sexta pollination than others as a local adaptation to the pollinator community. Besides BA emission (Figure 1E), M. sexta foraging success (Supplementary Figure 2) and the reproductive success of the plant resulting from M. sexta visitation (Figures 2D,E) indicate specialization. Despite its interaction with a broad pollinator community and its self-pollination assurance, certain N. attenuata accessions (Ut1, Ut2, and Az) seem to be more specialized by exploiting the sensory abilities of M. sexta. Whether a specialization of N. attenuata accessions toward M. sexta pollination could also entail the possibility of receiving pollen loads of high intraspecific diversity, thus offering the stigma a broader pool of mates to choose from, remains to be tested in further studies.
Taken together, our study highlights how the ability of a plant species to adapt to the sensory bias of the local pollinator community or to resort to self-pollination might be crucial in determining the geographic range of a particular plant species. Gathering more knowledge of population-specific differences in the communication between flowers and pollinators might therefore be of the highest importance for the conservation of both plant and insect species across habitats.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AH, FY, JB, and XL performed the experiments and analyzed the results. JB and FY wrote the first draft of the manuscript. All authors contributed to the revision and experimental design of the study.
Funding
The funding was provided by the Max-Planck Society.
Conflict of Interest
XL performed the experiments while working exclusively for the MPI-CE and worked in the manuscript while employed by the Aura Optik GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Danny Kessler for fruitful discussions. Furthermore, we want to thank Sascha Bucks for his support in maintaining the insect colony and the glasshouse team of the MPICE for maintaining the different plant lines.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.680463/full#supplementary-material
Supplementary Figure 1 | Nectar properties of four Nicotiana attenuata accessions. (A) Boxplot showing the nectar amount (p < 0.0001). (B) Boxplot of the amount of glucose in the nectar (F = 5.9857, p = 0.0044). (C) Boxplot of fructose amount in the nectar (F = 6.4211, p = 0.0031). (D) Boxplot showing the amount of sucrose in the nectar (F = 4.6708, p = 0.0139). Numbers below plot D represent replicate numbers for all nectar property measurements. Boxplots show the median, the 3rd and 4th quartile as well as the interquartile range. All data was analyzed using GLM followed by post hoc HSD with Holm adjustment. Letters indicate significant differences (p < 0.05).
Supplementary Figure 2 | Manduca sexta foraging success on four N. attenuata accessions. Plot shows the percentage of flowers on which M. sexta foraged successfully. Data was analyzed with GLM with a binomial family logit type for binary data, followed by a pairwise comparison of marginal means. Asterisks indicate statistically significant differences (p < 0.05), dot indicates p < 0.1.
Supplementary Table 1 | P-values after multicomparison of flower morphology and opening, as well as flower and capsule production after Manduca sexta visitation and flower contact time. Asterisks indicate statistically significant differences (* = p < 0.05, ** = p < 0.01, *** = p < 0.001).
Supplementary Table 2 | P-values after multicomparison of phenology data on four N. attenuata accessions. Asterisks indicate statistically significant differences (* = p < 0.05, ** = p < 0.01, *** = p < 0.001).
References
Anderson, B., and Johnson, S. D. (2008). The geographical mosaic of coevolution in a plant-pollinator mutualism. Evolution 62, 220–225. doi: 10.1111/j.1558-5646.2007.00275.x
Baker, H. G., and Baker, I. (1983). “Floral nectar sugar constituents in relation to pollinator type,” in Handbook of Experimental Pollination Biology, eds C. E. Jones and R. J. Little (Chicago, IL: University of Chicago Press).
Balamurali, G., Krishna, S., and Somanathan, H. (2015). Senses and signals: evolution of floral signals, pollinator sensory systems and the structure of plant–pollinator interactions. Curr. Sci. 108, 1852–1861.
Baldwin, I. T., Preston, C., Euler, M., and Gorham, D. (1997). Patterns and consequences of benzyl acetone floral emissions from Nicotiana attenuata plants. J. Chem. Ecol. 23, 2327–2343. doi: 10.1023/B:JOEC.0000006677.56380.cd
Betts, M. G., Hadley, A. S., and Kress, W. J. (2015). Pollinator recognition by a keystone tropical plant. Proc. Natl. Acad. Sci. U.S.A. 112, 3433–3438. doi: 10.1073/pnas.1419522112
Bhattacharya, S., and Baldwin, I. T. (2012). The post-pollination ethylene burst and the continuation of floral advertisement are harbingers of non-random mate selection in Nicotiana attenuata. Plant J. 71, 587–601. doi: 10.1111/j.1365-313X.2012.05011.x
Brown, M., Downs, C. T., and Johnson, S. D. (2011). Covariation of flower trait and bird pollinator assamblages among populations of Kniphofia linearifolia (Asphodelaceae). Plant Syst. Evol. 294, 199–206. doi: 10.1007/s00606-011-0443-1
Carvalheiro, L. G., Biesmeijer, J. C., Benadi, G., Fründ, J., Stang, M., Bartomeus, I., et al. (2014). The potential for indirect effects between co-flowering plants via shared pollinators depends on resource abundance, accessibility and relatedness. Ecol. Lett. 17, 1389–1399. doi: 10.1111/ele.12342
Deora, T., Ahmed, M. A., Daniel, T. L., and Brunton, B. W. (2021). Tactile active sensing in an insect plant pollinator. J. Exp. Biol. 224, jeb.239442. doi: 10.1242/jeb.239442
Dethier, V. G. (1976). The Hungry Fly : A Physiological Study of The Behavior Associated With Feeding. Cambridge, MA: Harvard University Press.
Euler, M., and Baldwin, I. T. (1996). The chemistry of defense and apparency in the corollas of Nicotiana attenuata. Oecologia 107, 102–112. doi: 10.1007/BF00582240
Galetto, L., Bernardello, G., and Sosa, C. A. (1998). The relationship between floral nectar composition and visitors in Lycium (Solanaceae) from Argentina and Chile: what does it reflect? Flora 193, 303–314. doi: 10.1016/S0367-2530(17)30851-4
Gómez, J. M., Abdelaziz, M., Camacho, J. P. M., Muñoz-Pajares, A. J., and Perfectti, F. (2009a). Local adaptation and maladaptation to pollinators in a generalist geographic mosaic. Ecol. Lett. 12, 672–682. doi: 10.1111/j.1461-0248.2009.01324.x
Gómez, J. M., Perfectti, F., Bosch, J., and Camacho, J. P. M. (2009b). A geographic selection mosaic in a generalized plant – pollinator – herbivore system. Ecol. Monogr. 79, 245–263. doi: 10.1890/08-0511.1
Gross, K., Sun, M., and Schiestl, F. P. (2016). Why do floral perfumes become different? Region-specific selection on floral scent in a terrestrial orchid. PLoS One 11:e0147975. doi: 10.1371/journal.pone.0147975
Guo, H., Halitschke, R., Wielsch, N., Gase, K., and Baldwin, I. T. (2019). Mate selection in self-compatible wild tobacco results from coordinated variation in homologous self-incompatibility genes. Curr. Biol. 29, 2020–2030.e5. doi: 10.1016/j.cub.2019.05.042
Guo, H., Lackus, N. D., Köllner, T. G., Li, R., Bing, J., Wang, Y., et al. (2020). Evolution of a novel and adaptive floral scent in wild tobacco. Mol. Biol. Evol. 37, 1090–1099. doi: 10.1093/molbev/msz292
Haverkamp, A., Bing, J., Badeke, E., Hansson, B. S., and Knaden, M. (2016a). Innate olfactory preferences for flowers matching proboscis length ensure optimal energy gain in a hawkmoth. Nat. Commun. 7:11644. doi: 10.1038/ncomms11644
Haverkamp, A., Hansson, B. S., Baldwin, I. T., Knaden, M., and Yon, F. (2018). Floral trait variations among wild tobacco populations influence the foraging behavior of hawkmoth pollinators. Front. Ecol. Evol. 6:19. doi: 10.3389/fevo.2018.00019
Haverkamp, A., Li, X., Hansson, B. S., Baldwin, I. T., Knaden, M., and Yon, F. (2019). Flower movement balances pollinator needs and pollen protection. Ecology 100, 1–11. doi: 10.1002/ecy.2553
Haverkamp, A., Yon, F., Keesey, I. W., Mißbach, C., Koenig, C., Hansson, B. S., et al. (2016b). Hawkmoths evaluate scenting flowers with the tip of their proboscis. Elife 5:e15039. doi: 10.7554/eLife.15039.001
Heinrich, B. (1971). Temperature regulation of the sphinx moth, Manduca sexta: I. Flight energetics and body temperature during free and tethered fly. J. Exp. Biol. 54, 141–151. doi: 10.1242/jeb.54.1.141
Hodges, S. A., Fulton, M., Yang, J. Y., and Whittall, J. B. (2004). Verne grant and evolutionary studies of aquilegia. New Phytol. 161, 113–120. doi: 10.1046/j.1469-8137.2003.00950.x
Jia, J., and Tan, D. Y. (2012). Variation in style length and anther–stigma distance in Ixiolirion songaricum (Amaryllidaceae). South Afr. J. Bot. 81, 19–24. doi: 10.1016/j.sajb.2012.03.011
Kantsa, A., Raguso, R. A., Dyer, A. G., Olesen, J. M., Tscheulin, T., and Petanidou, T. (2018). Disentangling the role of floral sensory stimuli in pollination networks. Nat. Commun. 9:1041. doi: 10.1038/s41467-018-03448-w
Kawahara, A. Y., Breinholt, J. W., Ponce, F. V., Haxaire, J., Xiao, L., Lamarre, G. P., et al. (2013). Evolution of Manduca sexta hornworms and relatives: biogeographical analysis reveals an ancestral diversification in Central America. Mol. Phylogenet. Evol. 68, 381–386. doi: 10.1016/j.ympev.2013.04.017
Kelber, A. (2003). Sugar preferences and feeding strategies in the hawkmoth Macroglossum stellatarum. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 189, 661–666. doi: 10.1007/s00359-003-0440-0
Kessler, D. (2012). Context dependency of nectar reward-guided oviposition. Entomol. Exp. Appl. 144, 112–122. doi: 10.1111/j.1570-7458.2012.01270.x
Kessler, D., and Baldwin, I. T. (2007). Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. Plant J. 49, 840–854. doi: 10.1111/j.1365-313X.2006.02995.x
Kessler, D., Bhattacharya, S., Diezel, C., Rothe, E., Gase, K., Schöttner, M., et al. (2012). Unpredictability of nectar nicotine promotes outcrossing by hummingbirds in Nicotiana attenuata. Plant J. 71, 529–538. doi: 10.1111/j.1365-313X.2012.05008.x
Kessler, D., Bing, J., Haverkamp, A., and Baldwin, I. T. (2019). The defensive function of a pollinator−attracting floral volatile. Funct. Ecol. 33, 1223–1232. doi: 10.1111/1365-2435.13332
Kessler, D., Diezel, C., and Baldwin, I. T. (2010). Changing pollinators as a means of escaping herbivores. Curr. Biol. 20, 237–242. doi: 10.1016/j.cub.2009.11.071
Kessler, D., Gase, K., and Baldwin, I. T. (2008). Field experiments with transformed plants reveal the sense of floral scents. Science 321, 1200–1202. doi: 10.1126/science.1160072
Kessler, D., Kallenbach, M., Diezel, C., Rothe, E., Murdock, M., and Baldwin, I. T. (2015). How scent and nectar influence floral antagonists and mutualists. Elife 4:e07641. doi: 10.7554/eLife.07641
Koenig, C., Hirsh, A., Bucks, S., Klinner, C., Vogel, H., Shukla, A., et al. (2015). A reference gene set for chemosensory receptor genes of Manduca sexta. Insect Biochem. Mol. Biol. 66, 51–63. doi: 10.1016/j.ibmb.2015.09.007
Krügel, T., Lim, M., Gase, K., Halitschke, R., and Baldwin, I. T. (2002). Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12, 177–183. doi: 10.1007/pl00012666
Morgan, M. T., Wilson, W. G., Knight, T. M., Morgan, M. T., Wilson, W. G., and Knight, T. M. (2011). Plant population dynamics, pollinator foraging, and the selection of self-fertilization. Am. Nat. 166, 169–183. doi: 10.1086/431317
Perret, M., Chautems, A., Spichiger, R., Peixoto, M., and Savolainen, V. (2001). Nectar sugar composition in relation to pollination syndromes in Sinningieae (Gesneriaceae). Ann. Bot. 87, 267–273. doi: 10.1006/anbo.2000.1331
Reisenman, C. E., Riffell, J. A., Bernays, E. A., and Hildebrand, J. G. (2010). Antagonistic effects of floral scent in an insect-plant interaction. Proc. R. Soc. B Biol. Sci. 277, 2371–2379. doi: 10.1098/rspb.2010.0163
Riffell, J. A., Abrell, L., and Hildebrand, J. G. (2008). Physical processes and real-time chemical measurement of the insect olfactory environment. J. Chem. Ecol. 34, 837–853. doi: 10.1007/s10886-008-9490-7
Rueden, C. T., Schindelin, J., Hiner, M. C., DeZonia, B. E., Walter, A. E., Arena, E. T., et al. (2017). ImageJ2: imageJ for the next generation of scientific image data. BMC Bioinformatics 18:529. doi: 10.1186/s12859-017-1934-z
Schäfer, M., Brütting, C., Baldwin, I. T., and Kallenbach, M. (2016). High-throughput quantification of more than 100 primary- and secondary-metabolites, and phytohormones by a single solid-phase extraction based sample preparation with analysis by UHPLC–HESI–MS/MS. Plant Methods 12, 1–8. doi: 10.1186/s13007-016-0130-x
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. doi: 10.1038/nmeth.2019
Sime, K., and Baldwin, I. T. (2003). Opportunistic out-crossing in Nicotiana attenuata (Solanaceae), a predominantly self-fertilizing native tobacco. BMC Ecol. 3:6. doi: 10.1186/1472-6785-3-6
Smith, G., Johnson, C., Davidowitz, G., and Bronstein, J. (2017). Linkages between nectaring and oviposition preferences of Manduca sexta on two co−blooming Datura species in the Sonoran Desert. Ecol. Entomol. 43, 85–92. doi: 10.1111/een.12475
Souto-Vilarós, D., Proffit, M., Buatois, B., Rindos, M., Sisol, M., Kuyaiva, T., et al. (2018). Pollination along an elevational gradient mediated both by floral scent and pollinator compatibility in the fig and fig-wasp mutualism. J. Ecol. 106, 2256–2273. doi: 10.1111/1365-2745.12995
Sprayberry, J. D. H., and Suver, M. (2011). Hawkmoths’ innate flower preferences: a potential selective force on floral biomechanics. Arthropod. Plant Interact. 5, 263–268. doi: 10.1007/s11829-011-9150-7
Sukumaran, A., Khanduri, V. P., and Sharma, C. M. (2020). Pollinator-mediated self-pollination and reproductive assurance in an isolated tree of Magnolia grandiflora L. Ecol. Process. 9:45. doi: 10.1186/s13717-020-00254-5
Thompson, J. N., and Cunningham, B. M. (2002). Geographic structure and dynamics of coevolutionary selection. Nature 417, 735–738. doi: 10.1038/nature00810
Thomson, J. N. (2005). The Geographic Mosaic of Coevolution. Chicago, IL: University of Chicago Press.
Ushimaru, A., and Hyodo, F. (2005). Why do bilaterally symmetrical flowers orient vertically ? Flower orientation influences pollinator landing behaviour. Evol. Ecol. Res. 7, 151–160.
van der Kooi, C. J., Vallejo-Marín, M., and Leonhardt, S. D. (2021). Mutualism and (A)symmetry in plant-pollinator interactions. Curr. Biol. 31, R91–R99. doi: 10.1012/j.cub.2020.11.020
van Doorn, W. G., and Van Meeteren, U. (2003). Flower opening and closure: a review. J. Exp. Bot. 54, 1801–1812. doi: 10.1093/jxb/erg213
Vaughton, G., and Ramsey, M. (2010). Pollinator-mediated selfing erodes the flexibility of the best-of-both-worlds mating strategy in Bulbine vagans. Funct. Ecol. 24, 374–382. doi: 10.1111/j.1365-2435.2009.01648.x
Wright, G. A., Choudhary, A. F., and Bentley, M. A. (2009). Reward quality influences the development of learned olfactory biases in honeybees. Proc. Biol. Sci. 276, 2597–2604. doi: 10.1098/rspb.2009.0040
Yon, F., Joo, Y., Cortés Llorca, L., Rothe, E., Baldwin, I. T., and Kim, S.-G. (2016). Silencing Nicotiana attenuata LHY and ZTL alters circadian rhythms in flowers. New Phytol. 209, 1058–1066. doi: 10.1111/nph.13681
Yon, F., Kessler, D., Joo, Y., Cortés Llorca, L., Kim, S. G., and Baldwin, I. T. (2017a). Fitness consequences of altering floral circadian oscillations for Nicotiana attenuata. J. Integr. Plant Biol. 59, 180–189. doi: 10.1111/jipb.12511
Yon, F., Kessler, D., Joo, Y., Kim, S.-G., and Baldwin, I. T. (2017b). Fitness consequences of a clock pollinator filter in Nicotiana attenuata flowers in nature. J. Integr. Plant Biol. 59, 805–809. doi: 10.1111/jipb.12579
Keywords: Manduca sexta, Nicotiana attenuata, floral trait, pollination, plant reproduction, local adaptation
Citation: Bing J, Li X, Haverkamp A, Baldwin IT, Hansson BS, Knaden M and Yon F (2021) Variation in Manduca sexta Pollination-Related Floral Traits and Reproduction in a Wild Tobacco Plant. Front. Ecol. Evol. 9:680463. doi: 10.3389/fevo.2021.680463
Received: 14 March 2021; Accepted: 10 June 2021;
Published: 13 July 2021.
Edited by:
Casper J. Van Der Kooi, University of Groningen, NetherlandsReviewed by:
Anna Lisa Stöckl, Julius Maximilian University of Würzburg, GermanyKatarzyna Roguz, University of Warsaw, Poland
Copyright © 2021 Bing, Li, Haverkamp, Baldwin, Hansson, Knaden and Yon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Felipe Yon, ZmVsaXBlLnlvbi50QHVwY2gucGU=
†Present address: Julia Bing, Department for Systematic Botany, Institute for Ecology and Evolution, Friedrich Schiller University, Jena, Germany; Xiang Li, PartiQla GmbH, Jena, Germany
 Julia Bing
Julia Bing Xiang Li2†
Xiang Li2† Alexander Haverkamp
Alexander Haverkamp Ian T. Baldwin
Ian T. Baldwin Bill S. Hansson
Bill S. Hansson Markus Knaden
Markus Knaden Felipe Yon
Felipe Yon