- 1Animal Ecology, Institute for Biochemistry and Biology, University of Potsdam, Potsdam, Germany
- 2Berlin-Brandenburg Institute of Advanced Biodiversity Research (BBIB), Berlin, Germany
The Anthropocene is the era of urbanization. The accelerating expansion of cities occurs at the expense of natural reservoirs of biodiversity and presents animals with challenges for which their evolutionary past might not have prepared them. Cognitive and behavioral adjustments to novelty could promote animals’ persistence under these altered conditions. We investigated the structure of, and covariance between, different aspects of responses to novelty in rural and urban small mammals of two non-commensal rodent species. We ran replicated experiments testing responses to three novelty types (object, food, or space) of 47 individual common voles (Microtus arvalis) and 41 individual striped field mice (Apodemus agrarius). We found partial support for the hypothesis that responses to novelty are structured, clustering (i) speed of responses, (ii) intensity of responses, and (iii) responses to food into separate dimensions. Rural and urban small mammals did not differ in most responses to novelty, suggesting that urban habitats do not reduce neophobia in these species. Further studies investigating whether comparable response patters are found throughout different stages of colonization, and along synurbanization processes of different duration, will help illuminate the dynamics of animals’ cognitive adjustments to urban life.
Introduction
Urbanization is a defining process of the Anthropocene, stemming from the conversion of natural areas into land covered by impervious artificial surfaces (e.g., Møller, 2009; Biermann et al., 2016; Munshi-South et al., 2016). Urbanized areas are characterized by elevated habitat fragmentation, strong edge effects, high levels of disturbance and environmental heterogeneity (e.g., Van Ham et al., 2013). As the global human population rises, the expansion of urbanized areas is accelerating at the expense of natural reservoirs of biodiversity (e.g., McKinney, 2002; Grimm et al., 2008; Seto et al., 2012; Gao and O’Neill, 2020). The loss of vulnerable species intensifies biological homogenization, and results in separate city landscapes that are more similar to each other than to the natural ecosystems that they replace (e.g., McKinney, 2002; Barnosky et al., 2012; Groffman et al., 2014; Alberti, 2015; Alberti et al., 2017).
These urbanized habitats present animals with specific challenges for which their evolutionary past might not have prepared them (e.g., McDonnell and Hahs, 2015). For instance, urban dwellers have to cope with novel or altered levels of human disturbance, habitat fragmentation, resource availability, predators, and competitor and parasite communities (e.g., Sih et al., 2011). Wildlife responses to novelty and urbanization are thus a key focus of current behavioral and cognitive research (e.g., Baxter-Gilbert and Whiting, 2019; Crane et al., 2020; Mettke-Hofmann et al., 2020; Sol et al., 2020; Lee and Thornton, 2021). Since behavior and cognition largely determine how individuals interact with their surroundings, behavioral and cognitive adaptations are expected to play a major role in coping with human-induced rapid environmental change (HIREC, sensu Sih et al., 2011) (e.g., Greggor et al., 2014; Barrett et al., 2019; Goumas et al., 2020).
“Neophobia is an ecologically relevant fear behavior that arises through a cognitive assessment of novel stimuli” (Greggor et al., 2015, p. 82). Responses to novelty are classically categorized into responses to novel objects, foods, and space (e.g., Greggor et al., 2015). Responses to novelty are often inferred to be interchangeable, and responses in one context are frequently considered predictive of responses in the others (e.g., Greenberg, 2003). However, it is still not well understood whether responses to different novelty dimensions correlate across contexts, and whether they are indicative of the same underlying mechanism (e.g., Greggor et al., 2015; Crane et al., 2020). Different selective pressures might have shaped responses to novelty dimensions independently, as well as the specific type of responses (e.g., Greenberg and Mettke-Hofmann, 2001; Mettke-Hofmann, 2014; Greggor et al., 2015). High predation risk might have selected for heightened fear responses to unfamiliar objects and stimuli, while high dispersal propensity might come with elevated exploration tendency of novel spaces (e.g., Ferrari et al., 2007; Brown et al., 2014; Mettke-Hofmann, 2014). Neophobic responses toward food could have evolved in species with frequent encounters with dangerous or poisoned food (e.g., Galef, 1993; Thornton, 2008). Classifying the types of responses themselves is not always straightforward, because novelty elicits two opposite tendencies: aversion (neophobia) but also attraction (neophilia), which are often seen as a continuum (e.g., Greenberg, 2003). In many bird species neophobia and neophilia are governed by independent motivations and can vary independently (e.g., Greenberg and Mettke-Hofmann, 2001; Mettke-Hofmann et al., 2002; Mettke-Hofmann, 2014). Neophilia facilitates information gathering, whereas neophobia mainly protects the animal from unknown potential dangers by heightened caution (e.g., Greenberg, 2003; Griffin and Guez, 2014).
Responses to novelty are often found to vary consistently among individuals (e.g., Herborn et al., 2010; Yuen et al., 2016; but see e.g., Brown et al., 2013). They are commonly used as a marker of animal personality or temperament, since they are considered a stable response to challenges or risks across times or situations (e.g., Réale et al., 2007; Greggor et al., 2016a). In free-living animals, responses to novelty are associated with reactions to other novel situations, for example innovation propensity in a novel foraging task or dietary conservatism toward new food types (e.g., Webster and Lefebvre, 2001; Thomas et al., 2004). Since positive responses to novelty facilitate interacting with aspects of the environment, they provide opportunities for learning (e.g., Herborn et al., 2010). Therefore, the propensity and intensity of novelty responses should matter in the process of adjustment to altered environmental conditions (e.g., Sol et al., 2013; McDonnell and Hahs, 2015). Neophobia has been proposed as a major predictor for the successful establishment in novel or altered habitats, and indeed several studies on urban birds report lower neophobic and/or higher neophilic responses in urban dwellers compared to conspecifics living under more natural conditions (e.g., Sol et al., 2011, 2013; Ducatez et al., 2017; but see e.g., Audet et al., 2016; Greggor et al., 2016b). In contrast, we know very little about the role of neophobia in coping with urban environments in species more limited in dispersal. These species might be precluded from easily moving away in response to intense human disturbance. Also, the colonization of human-altered environments could be hindered by pronounced aversion to novel stimuli in species with high predation pressure, in which individuals’ avoidance of dangers, and hence survival, strongly impacts ultimate fitness (e.g., Lima and Dill, 1990; Norrdahl and Korpimäki, 1995; Ceballos, 2002; Baxter-Gilbert et al., 2019). The dangerous niche hypothesis proposes that the primary benefit of aversive responses to novelty (neophobia) is protection against danger. Therefore, neophobia should be adaptive when novel stimuli are likely dangerous (e.g., if toxic foods, traps or a high level of predation risk characterize an individual’s environment; Greenberg, 2003). Novelty avoidance could then be a measure of general risk-aversion, particularly relevant in species that are heavily preyed upon, and are common (target and non-target) victims of pest management (e.g., Brakes and Smith, 2005; Herborn et al., 2010; Crane et al., 2020).
Here, we aimed to explore the potential role of responses to novelty in coping with urban environments for small mammals. We explored the behavioral responses to novelty of wild-caught, captive-held, rural and urban small mammals, in a battery of replicated tests designed to assess responses to single novelty types. Specifically, we investigated: (i) the temporal consistency and the structure of the neophobic responses to three novelty types: object, food, space, and (ii) differences in the responses to novelty between individuals of rural and urban origin. As study species, we selected common voles (Microtus arvalis, Arvicolidae) and striped field mice (Apodemus agrarius, Muridae). These are non-commensal rodents commonly found both in rural and urban areas of northern and central Europe (e.g., Riegert et al., 2009; Łopucki et al., 2019). Common voles are fossorial, mostly herbivorous, and inhabit open grasslands (e.g., Jacob et al., 2014). Striped field mice are omnivorous, and inhabit tall grasses and bushy underwood (e.g., Babińska-Werka, 1981). Both species are known to display between-individual variation in behavioral traits under both laboratory and natural conditions (e.g., Lantová et al., 2011; Pieniążek et al., 2017; Łopucki et al., 2020). Both species also show behavioral and cognitive adjustments to human-altered environments (e.g., Dammhahn et al., 2020; Mazza et al., 2020; Mazza and Guenther, 2021). We asked (i) whether there is one latent variable linking all the responses in the different tests; (ii) whether there is covariance between responses to the three novelty types; (iii) whether there is covariance between responses that express ‘speed’ (that may be read as latency to approach or neophobia) and ‘intensity’ (extent of the exploration or neophilia), independently of the novelty type; and (iv) whether there are common factors that underlie the specific covariance structures (‘general neophobia’). We tested these alternative scenarios of phenotypic integration at the among-individual level and, thus, asked whether specific responses of individuals to novelty are correlated across contexts. Such correlations are indicative of potential genetic or permanent environmental effects on novelty responses, in contrast to correlations at the within-individual level. Specific predictions on how behavioral responses to novelty cluster or vary across novelty types are reported in Table 1. Additionally, we asked whether the behavioral responses to novelty are repeatable, and if they differ between rural and urban individuals. Based on the notions reported above we predicted that animals would show consistent between-individual differences in responses to novelty, and that urban individuals would show lower levels of neophobia and higher levels of neophilia than their rural conspecifics.
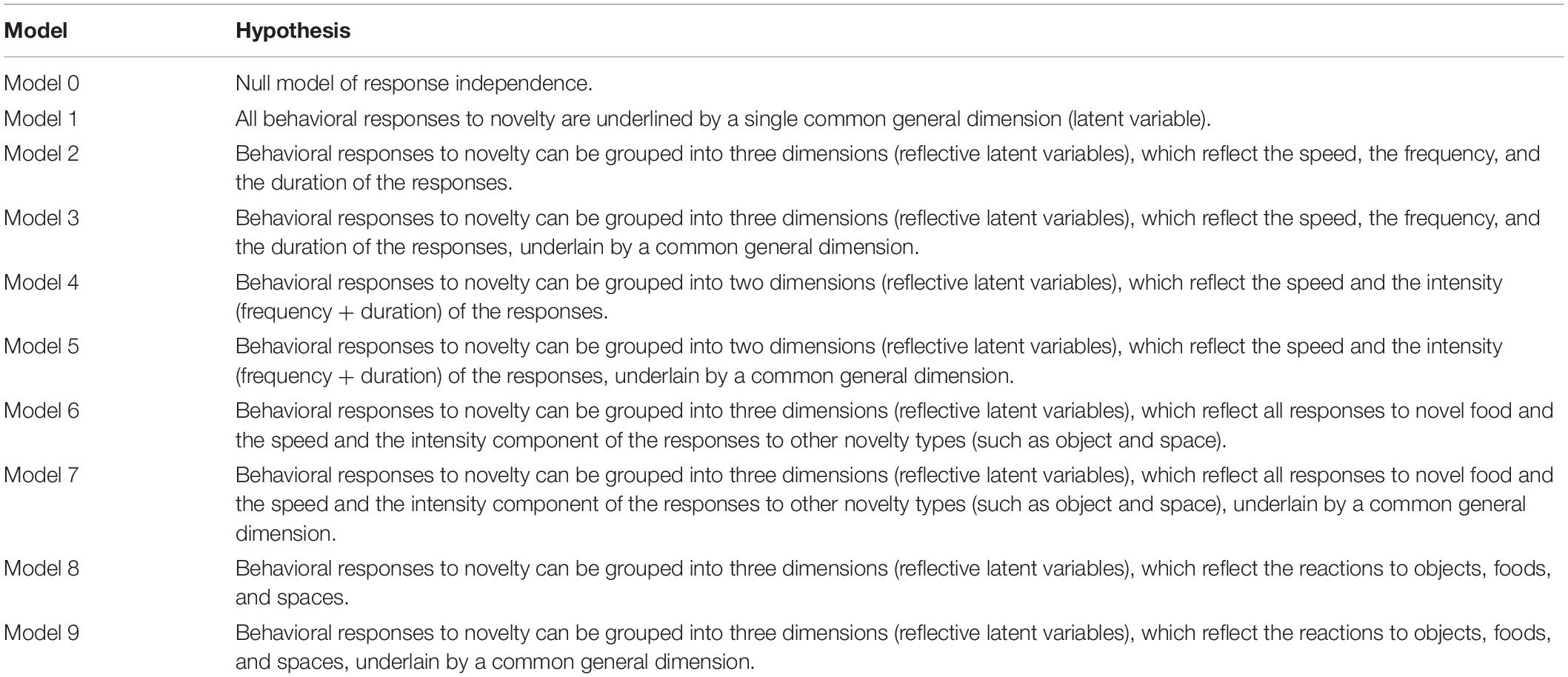
Table 1. A priori hypotheses on the structure of behavioral responses to novelty in common voles and striped field mice.
Materials and Methods
Trapping Sites
In total 48 common voles and 42 striped field mice were included in this study, and we report data for 47 common voles and 41 striped field mice. Animals originated from rural and urban wild populations of northeastern Germany. Rural common voles (N = 24; 7 females, 17 males) and striped field mice (N = 27; 13 females, 14 males) were trapped in three different sites in the region of Uckermark in NW-Brandenburg (53°35′ N, 13°71′ E, area 3,058 km2, Supplementary Figure 1). The area is characterized by agricultural landscapes and comparatively low human population density (for further details see Schirmer et al., 2019). Rural trapping sites were fallow lands between arable fields, composed of grassy areas streaked with nettles, bushes, and trees (ca. 0.85–1.66 ha). Urban common voles (N = 24; 11 females, 13 males) originated from four different sites, urban striped field mice (N = 15; 8 females, 7 males) from three different sites in the city of Berlin (52°31′ N, 13°24′ E, area 891 km2, Supplementary Figure 1). Urban sites were characterized by heterogeneous vegetation including grassy areas, bushes, and small trees (ca. 0.75–1.16 ha). Rural and urban trapping sites were on average 102.6 ± 3.7 km apart. Rural sites were on average 9.2 ± 6.7 km apart from each other, while urban sites were on average 13.3 ± 5.9 km apart. Common voles have an average home range size of 30–180 m2, an average core area of 4–25 m2, a daily range length of 9–49 m, and are estimated to disperse between 76 and 110 m per generation depending on sex (e.g., Jacob and Hempel, 2003; Briner et al., 2005; Gauffre et al., 2008, 2009; Roos et al., 2019). Striped field mice have an average home range size of 2,737 ± 2,046 m2, an average core area of 600 ± 446 m2, and an average daily distance traveled of 697 ± 312 m (Schirmer et al., 2019, 2020). In previous capture-mark-recapture studies conducted in the same sites, it never happened that individuals trapped in one site were found in another (Dammhahn et al., 2020). We therefore considered the sampled populations to be independent replicates. All sites were part of the CityScapeLabs or AgroScapeLabs experimental platforms (von der Lippe et al., 2020; https://www.bbib.org). We estimated a sealing index, i.e., the proportion of coverage of natural soil with artificial impervious surface (e.g., buildings and paved roads), as a proxy of the degree of urbanization in the animal’s original environment (Dammhahn et al., 2020; Mazza et al., 2020). The index was calculated in a 5-km buffer around each study site within the CityScapeLabs project for the urban sites, on the basis of the biotope mapping of Berlin (Seress et al., 2014; Buchholz et al., 2018). We used the same method for the rural sites. In brief, this method scores the abundance of paved roads, buildings, and vegetation within each 100 m × 100 m cell in a defined buffer area around the focal study sites, using a GIS (v. 10/ESRI; http://www.esri.com/; Buchholz et al., 2018). Sealed surface closely corresponds to other urbanization indices, such as human population density, disturbance by humans and pets, noise, and light pollution (e.g., Corsini et al., 2017, 2019; Buchholz et al., 2018). Sealing indexes ranged from 2.4 to 3.7 for rural and 32.0 – 54.9 for urban sites, indicating a marked contrast between the level of anthropogenic disturbances the animals were exposed to in their habitats (Mazza et al., 2020).
Animals and Housing
We captured animals during the same season, between July and September 2018, using multiple-capture live-traps (Ugglan Special Traps n. 1-2, Grahnab AB, Hillerstorp, Sweden). After a pre-baiting period of two nights, traps were activated every night between 18:00 and 19:00, and checked and de-activated every morning between 06:00 and 07:00. Trapping was performed on each site for 7–12 days and was discontinued once the capture success was <2 individuals/grid night. We are confident that pre-baiting and capturing over several subsequent days minimized potential bias concerning trap avoidance. Captured animals were sexed, weighed, and checked for reproductive status. Lactating females were immediately released at the site of capture. All other animals were given additional food and transported to our animal holding facility, where they were housed individually in standard polycarbonate cages (Ehret GmbH, Germany, Type III; dimensions: 42 cm × 27 cm × 16 cm) equipped with hay, bedding, and carton shelters. Food (Ssniff NM/V1244-0) and water were provided ad libitum. The animal holding facility receives natural light from windows, and has no thermal insulation, so light, temperature and humidity follow the natural conditions occurring outside the laboratory. Testing took place between October and November 2018. All animals had been in captivity for approximately 3 months when the test battery began (common voles: 91.1 ± 16.2; striped field mice for 109.6 ± 24.1 days). The sample comprised males (28 common voles and 19 striped field mice) and females (19 common voles and 22 striped field mice), all adult and all non-reproductive. After the conclusion of the present experiment, animals were kept in captivity, involved in further behavioral tests (Mazza and Guenther, 2021), and then released at their original trapping sites with a soft-release protocol.
Experimental Procedure
At the start of each testing session, animals were taken from their cage, moved into a separate testing room, and transferred into the experimental apparatus without direct handling. Here, after a 24 h habituation period, animals were presented with a sequence of tests involving a single novelty type: novel object, novel food, and novel space, in randomized order. The habituation phase started no later than 18:00 h and lasted no less than 24 h. A test phase started between 6:00–7:00 and lasted until 18:00–19:00, when the second phase began. Each test lasted 12 h and was immediately followed by the next test of a different novelty type (either food or object or space). Light conditions in the testing room were LD 12:12 (12 h light-12 h dark), and the change was concurrent with the change in novelty type. Voles alternate several independent short activity bouts (typically 2–3 h) and rest periods, both during daytime and at night, resulting in a polyphasic pattern (e.g., Halle and Lehmann, 1987; Halle, 2000; Wolff, 2007). Apodemus mice seem to be able to adjust their activity rhythms and phases to different factors (e.g., Wróbel and Bogdziewicz, 2015; Łopucki and Kiersztyn, 2020). Our own observations in the course of a previous field study confirm that striped field mice had activity periods distributed along the 24 h (i.e., they entered the traps during both day and night) in rural as in urban habitats (Dammhahn et al., 2020). Having 12-h-long testing phases allowed us to wait for each animal to be in an active phase, and to approach the novelty in its own time. All animals had activity bouts well within this limit, and all approached the novelty within the first 4.5 h (see below for further details). State differences while testing may account for large variation in behavioral measures, which is why we opted for a protocol that allowed animals to follow their own activity rhythms and provided enough time to habituate to the new set-up and housing conditions, as well as enough time to approach novelty on their own terms.
A video-recording system on the ceiling allowed simultaneous monitoring and recording of all tests. Groups of 20 conspecifics were tested simultaneously, i.e., they were in the experimental room at the same time but invisible to each other. From these videos, behavioral parameters (see below for each test) were extracted and analyzed by one observer (IC) with the program BORIS (Friard and Gamba, 2016). The overall testing session lasted 60 h (24 h of habituation and 36 h with access to the three novelty types). This allowed all animals to approach the novel stimuli in their own time, and allowed us to record their responses from the moment they made the first approach (see below). At the conclusion of the third test, animals were weighed and returned to their home cage. Tests were repeated after 15–24 days with a different object, food, space setting.
We removed all observations (N = 17) from the dataset when technical issues in any part of the test battery made the precise quantification of behaviors impossible for the observer (IC) (e.g., condensation on one of the camera lenses). The final data set comprised a total of 158 tests for 47 individual common voles and 41 individual striped field mice (mean number of test rounds per individual ±SD: 1.8 ± 0.4).
Novelty Test Battery Set-Up
The test battery was conducted in a modular set-up, consisting of two plastic boxes (IKEA “Samla” 39 cm × 28 cm × 28 cm) connected by a short tube (8 cm × 5 cm Ø) closed by a non-transparent door (Figure 1). Both boxes contained a thin layer of bedding and were closed by a lid with drilled holes to ensure ventilation. One box was used as a ‘familiar space’ or home box where the novel object and food were presented; the second was used for the novel space test (see below). The home box was equipped with a dark plastic tube as shelter (8 cm × 5 cm Ø). Food (Ssniff NM/V1244-0) and water were available ad libitum at all times throughout all experimental phases, including the ‘novel food’ test described below. At the end of each testing session, all equipment was cleaned with either ethanol or soap and water, and left to dry for 48 h.
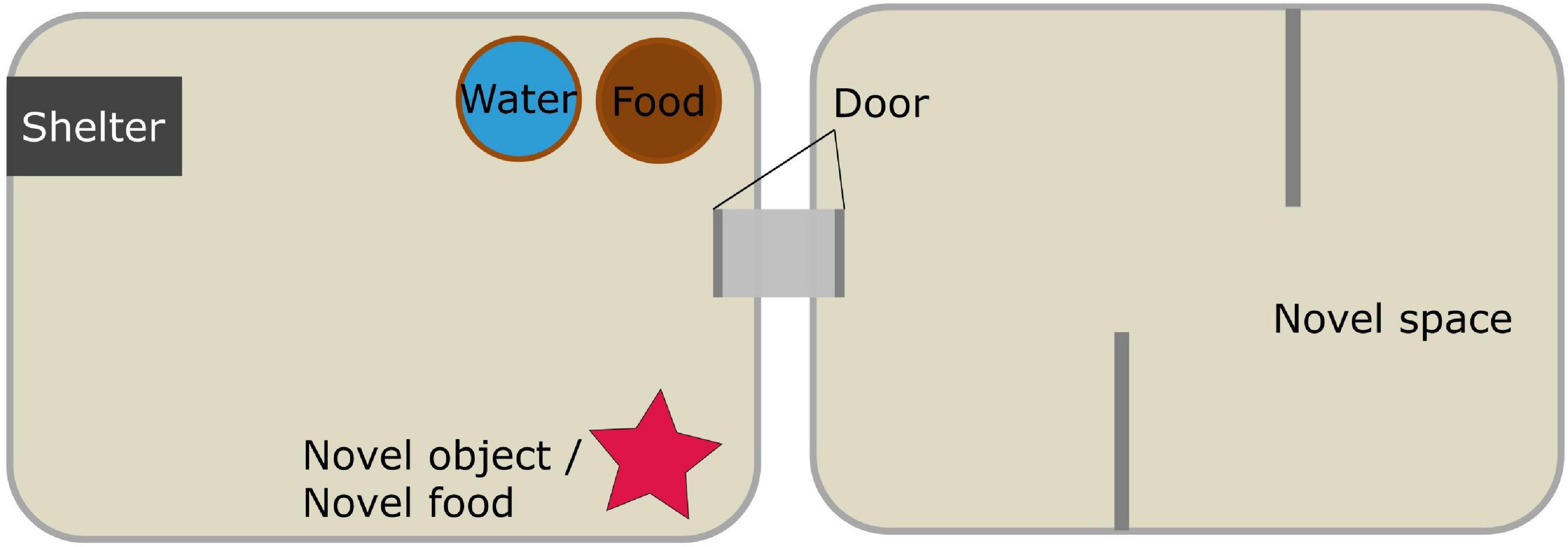
Figure 1. Schematic representation of the experimental set-up used to test one novelty type at a time. After each test session the respective novelty type was removed from the box or, in the case of the novel space, the doors leading to it were closed again.
Novel Object Test
The test started when a novel object was introduced in the home box, on the side opposite to the shelter. Different types of plastic toy animals were used as novel objects: a duck, a horse, a wolf, a shark, and a fish (each approximately 8 cm × 5 cm × 7 cm). The type of object was randomized across species and sexes. On the second round of testing, each individual was presented with a different type of object. If the animals were not in the shelter when the novel object was introduced in the box, they were gently guided to it without direct handling. The following parameters were measured over the following 12 h: (i) latency to approach the object, defined as coming within 2 cm of it, with the head turned toward it, after leaving the shelter for the first time; (ii) number of approaches to the novel object (defined as i) during the first hour after the first approach; (iii) overall duration of approaches (defined as i). Recording latencies from the moment the novel object was placed in the cage would have confounded activity levels with responses to novelty, in case an animal had a shorter latency to approach just because it was already active when the object was placed in the cage compared to a conspecific whose activity phase started hours later. To avoid confounding effects of higher activity levels or individual differences in reaction to disturbances (e.g., experimenter interacting with the home box) or slight variations in disturbance strength, with responses to novelty, we recorded and analyzed latency to approach from the moment the animals left their shelter, i.e., when they started an activity phase. Similarly, number and duration of approaches were recorded from the moment of the first approach.
All animals participated in the test, and approached the novel object within 4 h (for further details, see the “Results” section). At the end of the test, the novel object was removed from the home box.
Novel Food Test
The test started when a tray containing an unfamiliar food was introduced in the home box, on the side opposite to the shelter. Animals were presented with two food types in randomized order in the two repeats. We used millet flakes and quinoa seeds for common voles, and standard food pellets flavored with grapefruit or “tropical” fruit syrups for striped field mice. We checked that the novel foods and flavors would be approached and eaten by animals of both species during a short pilot, conducted with animals that were not included in the present study. If the animals were not in the shelter when the novel food was introduced in the box, they were gently guided to it. The following parameters were measured over the following 12 h: (i) latency to touch the novel food, i.e., to make physical contact (touching with nose or paws, nibbling, and sniffing), after leaving the shelter for the first time; (ii) number of contacts to the novel food (defined as i) during the first hour after the first contact, (iii) overall duration of contacts with the novel food (defined as i) during the first hour after the first contact. To avoid confounding effects of higher activity levels or individual differences in reaction to disturbances (e.g., experimenter interacting with the home box) or slight variations in disturbance strength, with responses to novelty, we recorded and analyzed latency to touch the food from the moment the animals left their shelter, i.e., when they started an activity phase. Similarly, number and duration of contacts were recorded from the moment of the first contact. Given the distance between the video cameras (on the room ceiling) and the food trays, we could not distinguish between contact and actual food consumption. All animals participated in the test and approached the novel food within 4.5 h (for further details, see the “Results” section). Familiar food (standard pellet described above in section “Animals and Housing”) and water were available ad libitum throughout the experiment. At the end of the test, the tray containing the novel food was removed from the home box.
Novel Space Test
The test started when the door to the second box was opened. To provide a novel space in both rounds of testing, we could not present an empty box, so we attempted to change the conformation of the available space by placing additional structures. The second box was therefore equipped with a round terracotta vase and a plastic closed pipe in the first round, and with vertical walls during the second round, creating a maze-like space (Figure 1). If the animals were not in the shelter when the door to the second box was opened, they were gently guided to it. The following parameters were measured over the following 12 h: (i) latency to visit the novel space for the first time, i.e., to enter the second box with the whole body without the tail, after leaving the shelter for the first time; (ii) number of visits to the novel space (defined as i) during the first hour after the first visit; (iii) overall duration of visits (defined as i). To avoid confounding effects of higher activity levels or individual differences in reaction to disturbances (e.g., experimenter interacting with the home box) or slight variations in disturbance strength, with responses to novelty, we recorded and analyzed latency to visit from the moment the animals left their shelter, i.e., when they started an activity phase. Similarly, number and duration of visits were recorded from the moment of the first visit.
All animals participated in the test and visited the novel space within 4 h (for further details, see the “Results” section). At the end of the test, the door leading to the novel space was closed.
Statistical Analyses
We conducted three sets of statistical analyses (described in detail below). First, we estimated adjusted repeatabilities and patterns of (co)variance among behavioral responses using a Bayesian multivariate mixed-effect model (e.g., Dingemanse et al., 2010; Moiron et al., 2019). As a second step, we fitted structural equation models (SEMs) to compare potential underlying structures of among-individual responses to novelty. We achieved this by testing the relative fit of 10 a priori hypothesized structures of phenotypic integration (Figure 2 and Table 1) (e.g., Dingemanse et al., 2010; Moiron et al., 2019). As a third step, we reduced the number of dependent variables and summarized the behaviors using a principal component analyses (PCA). We then used the summarized scores to compare rural and urban individuals. We scaled all nine variables to standard deviation units (i.e., mean centered and variance standardized) to facilitate comparison of the relative magnitudes of variance components across variables. To meet the normality requirement, latencies and durations were log-transformed prior to analyses. Data from the two species was analyzed separately, following the same protocol. Based on previous studies on small mammals’ personality and cognition (e.g., Herde and Eccard, 2013; Mazza et al., 2018), we did not expect differences between the sexes, and we did not include sex in the models to avoid overfitting. The accepted significance level was α ≤ 0.05. All data analyses were conducted with R version 3.6.1 (R Core Team, 2015).
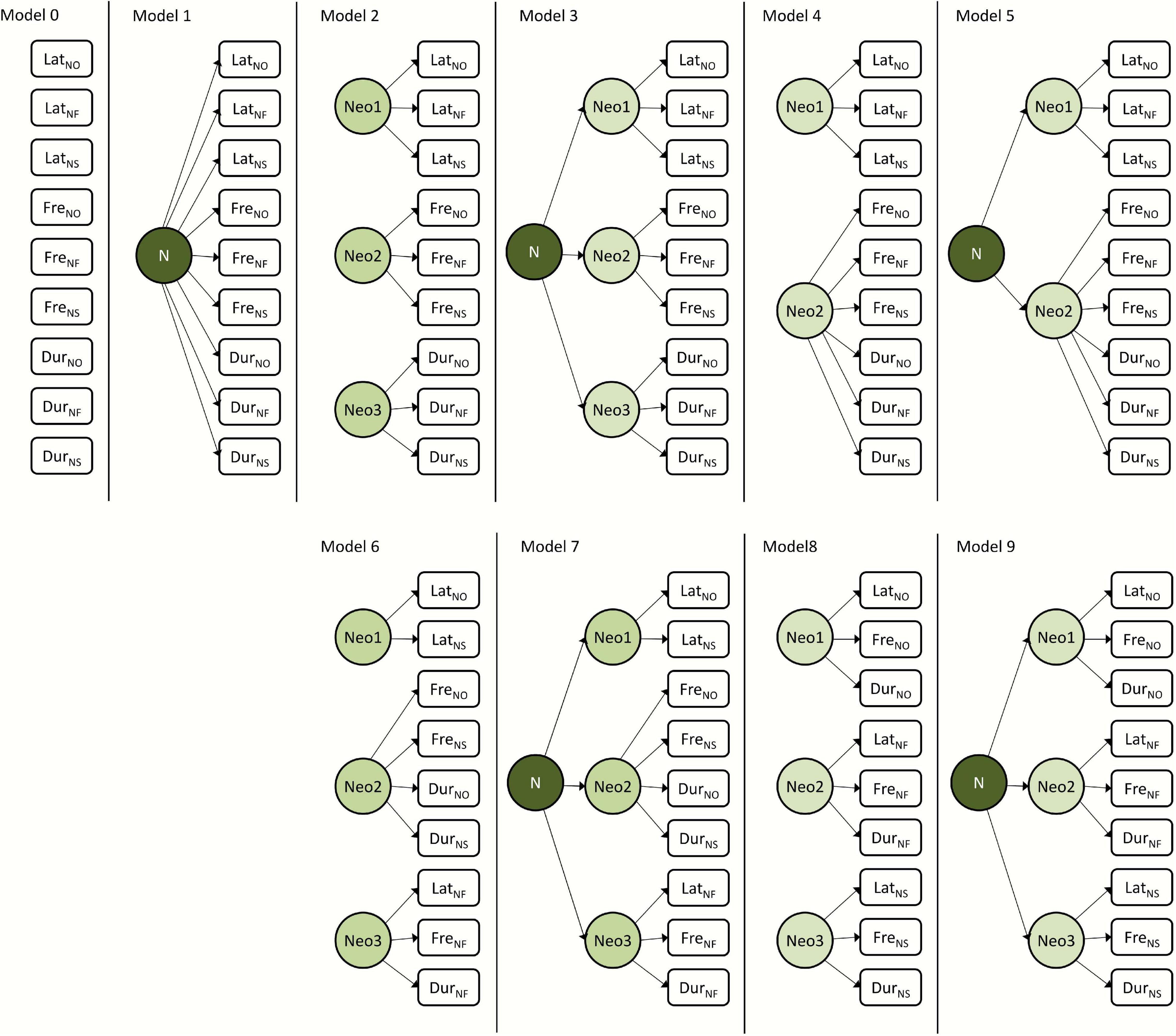
Figure 2. Schematic representation of the a priori hypotheses on the structure of behavioral responses to novelty in common voles (Microtus arvalis) and striped field mice (Apodemus agrarius) tested with SEMs for each species separately.
Multivariate Mixed-Effects Modeling
We estimated among-trait (co)variance at the within- and among-individual level using a Bayesian multivariate mixed-effects model [R package MCMCglmm, Markov chain Monte Carlo generalized linear mixed models (Hadfield, 2010)]. To do so, we built a random intercept model where we fitted all nine behavioral variables as response variables, and the trial number (round 1 vs. 2) and the presentation order of the tests as fixed effects. Individual identity was added as a random factor, specified as a random intercept, in each model to control for non-independent repeated measures of the same individual. We used an uninformative prior and ran 220,000 iterations, with a burn-in of 20,000 and a thinning interval of 100. These parameter settings resulted in low temporal autocorrelation between estimates of subsequent models, which were assessed by graphical diagnostics. The resulting among-individual covariance matrices were used to test our SEM hypotheses. We also calculated adjusted individual repeatability for each behavioral variable as the proportion of the total phenotypic variance not attributable to fixed effects that was explained by individual identity and its 95% credibility intervals based on the posterior distribution (Nakagawa and Schielzeth, 2010; Dingemanse and Dochtermann, 2013).
Structural Equation Modeling
To test the relative fit of alternative hypotheses on the structure of the novelty responses, we applied SEM to 10 a priori conceived scenarios assessing how the different responses were associated among individuals (Figure 2 and Table 1). SEM provides a powerful method for syndrome structure analysis, assessing how well alternative hypotheses fit the available behavioral data. SEMs allow the detection of patterns of phenotypic trait covariance which could be missed using more classical approaches (Dochtermann and Jenkins, 2011; Dingemanse and Dochtermann, 2013). Of the 10 models, model 0 represents a statistical “null” expectation where all responses are independent from each other; model 1 represents a scenario in which all responses were underlain by a single common general dimension (N, general neophobia). Models 2–6 represent different scenarios where behavioral responses to novelty can be grouped into two or three dimensions (reflective latent variables), which reflect the speed, the frequency, and the duration of responses to novelty, and where these dimensions can be underlain by a common general dimension (N). In these models, the dimension intensity combines duration and frequency. Models 7–8 represent hypotheses of behavioral responses grouped into dimensions (reflective latent variables), which reflect the responses to novelty in the form of object, food or potential resource, and space, related to the testing set-ups and where these three dimensions can be underlain by a common general dimension (N). Models were fitted with the package lavaan (Rosseel, 2012). We then compared the models’ fit and evaluated the relative support based on several indices, including the Akaike information criterion (AIC), the minimum fit function (FMIN), the normed fit index (NFI), and the parsimonious normed fit index (PNFI).
Principal Component Analysis
Since our sample sizes did not allow us to compare between rural and urban populations via SEMs, we used principal component analyses (PCA) followed by oblimin rotation to reduce the number of dependent variables (Tabachnick and Fidell, 2001). All variables were checked for suitability for a PCA by examination of the determinant of the correlation matrix, the Bartlett test, and the Kaiser–Mayer–Olkin (KMO) criterion (Field, 2012). We retained principal components with Eigenvalues > 1 (Kaiser–Guttman criterion, Kaiser, 1991). The PCA returned three main components that accounted for 62% of the variance in both species (Table 2). We then assessed the correlation between the three components by Spearman rank correlations. We assessed repeatability of the composite scores using linear mixed effects models (LMM) with individual identity as random factor (Nakagawa and Schielzeth, 2010). We calculated 95% confidence intervals (CI) of repeatabilities for each variable by parametric bootstrapping (N = 1,000 simulation iterations) (Faraway, 2006) using the R package rptR (Stoffel et al., 2017). P-values were calculated based on 1,000 permutations. We compared each component across rural and urban populations using linear mixed effects models, fitted by restricted maximum likelihood, run with the R package lme4 (Bates et al., 2015), with individual identity as random factor and test round as a fixed factor. Time in captivity was never significant and was removed in order to select the minimum parsimonious models. More complex structures with individual identity nested in trapping site did not have a better fit (Supplementary Table 4), so we chose a simpler structure as better suited to the sample size of observations.
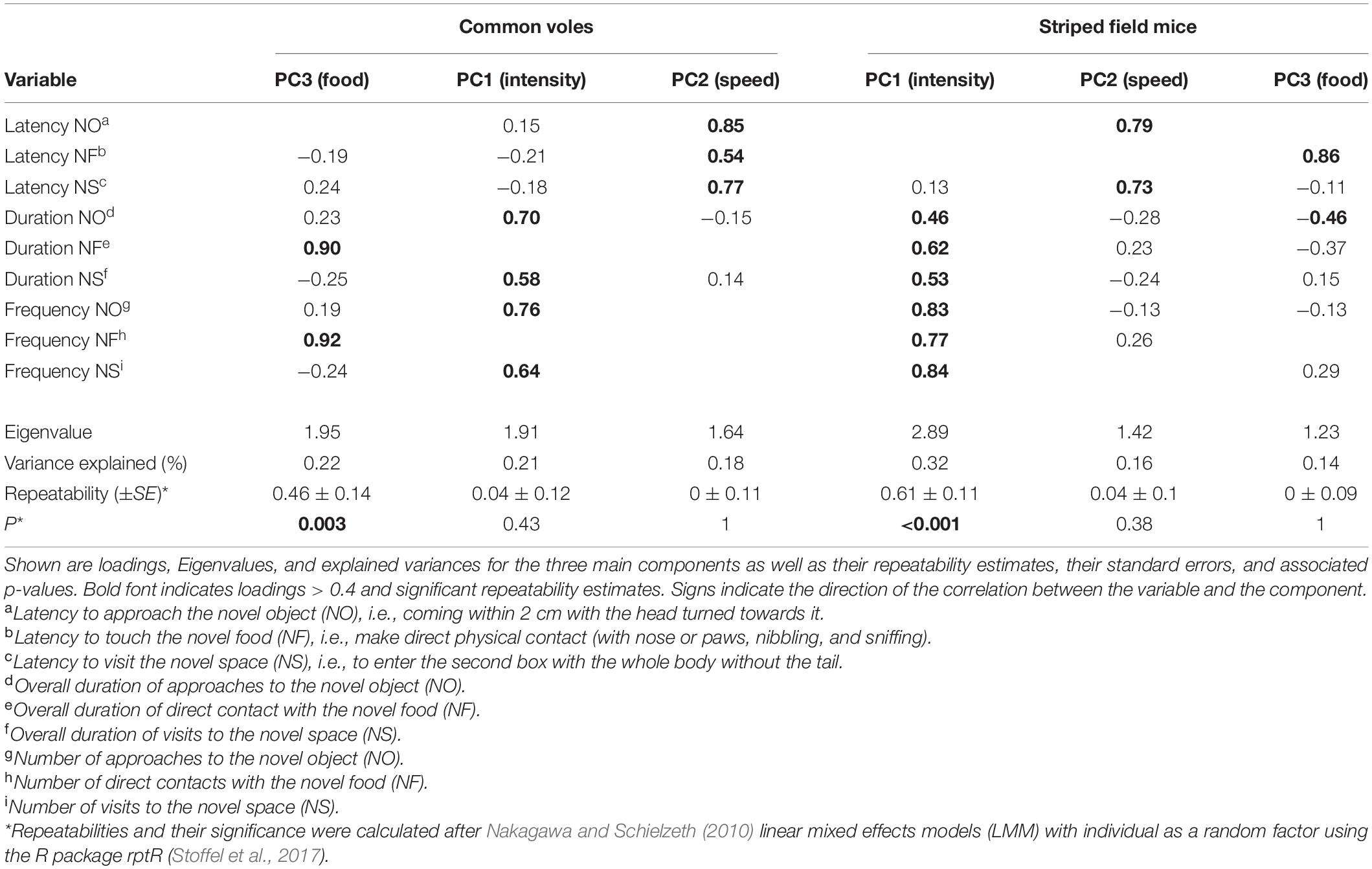
Table 2. Results of principal component analyses run separately for common voles (Microtus arvalis) and striped field mice (Apodemus agrarius).
Ethical Note
Animal capture and behavioral tests were conducted under the permission of the “Landesamt für Umwelt, Gesundheit und Verbraucherschutz Brandenburg” (reference number: RO7/SOB-0998A-C), the “Landesamt für Arbeitsschutz, Verbraucherschutz und Gesundheit, Brandenburg” (reference number: 2347-7-2019), the “Landeshauptstadt Potsdam, Fachbereich Soziales und Gesundheit, Veterinär- und Lebensmittelüberwachung” (reference number: 386-1), the “Senatsverwaltung für Stadtentwicklung und Umwelt” (reference number: IIIB2/OA/AS/G1394), and the “Landesamt für Gesundheit und Soziales” (reference number: G 0072/16). Experiments were performed in accordance with all applicable international, national, and/or institutional guidelines for the use of animals, including the ASAB/ABS guidelines for the Use of Animals in Research. We took great care in ensuring the animals’ welfare throughout the experimental procedure and afterward.
Trapping was conducted using multiple-capture live-traps (Ugglan Special Traps n. 1-2, Grahnab AB, Hillerstorp, Sweden), equipped with plenty of food and apples as water sources. We covered the traps with grass, branches, and leaves to provide shade and avoid overheating. In case of rainy nights, trapping was suspended. Lactating females were immediately released at the site of capture. All other animals were given additional oat flakes, apples, and cucumbers to provide food and water sources, and transported to our animal holding facility. If two or more animals were captured in the same trap, they were separated to avoid potentially stressful interactions during transport. Traps were firmly secured to avoid shaking and accidental opening. Temperature was kept constant at 20°C to avoid overheating.
Animals were housed individually in the animal holding facility in standard polycarbonate cages (Ehret GmbH, Germany, Type III; dimensions: 42 cm × 27 cm × 16 cm), that incorporated biologically relevant enrichment features such as natural material and refuges. Food (Ssniff NM/V1244-0) and water were provided ad libitum. Light, temperature, and humidity mirrored the natural conditions occurring outside the laboratory. We ensured that the animals learned to recognize the bottles as water sources by placing fresh apples and cucumbers directly above the bottles for the first week of captivity. Animals were never food-deprived or subjected to any form of potentially harmful manipulation. Disturbance by the experimenter was minimized by video-recording the tests, so that the experimenters’ presence in the room was not required. After the conclusion of this experiment, animals were kept in captivity and involved in further behavioral tests, and later were released at the capture sites in a waterproof shelter with hay and food to allow them to slowly re-acclimatize to their former habitat.
Results
All animals approached the novel object within 12,000 s, the novel food within 16,200 s and visited the novel space within 14,400 s. Descriptive statistics for measured variables are reported in Supplementary Table 5.
All behavioral variables were repeatable over time (Figure 3 and Supplementary Table 1) and showed considerable variation among individuals (Table 3). For the common voles, it was not possible to identify one model as best explaining the among-individual covariance structure (Supplementary Table 2). While some indices to assess model fit (FMIN, NFI, and PNFI) had best values for models 8–9, support based on AIC (ΔAIC < 2) was similarly high also for models 1, 4, and 5. Models 8–9 postulated the integration of the behavioral variables into three dimensions (reflective latent variables), reflecting the reactions to novel objects, foods, and spaces. Model 9 posited that these three dimensions were further underlain by a common general dimension N (Table 1). Models 1, 4–5 assumed the integration of behavioral variables into two dimensions, reflecting speed and intensity of the reactions, and the presence of a single common general dimension N.
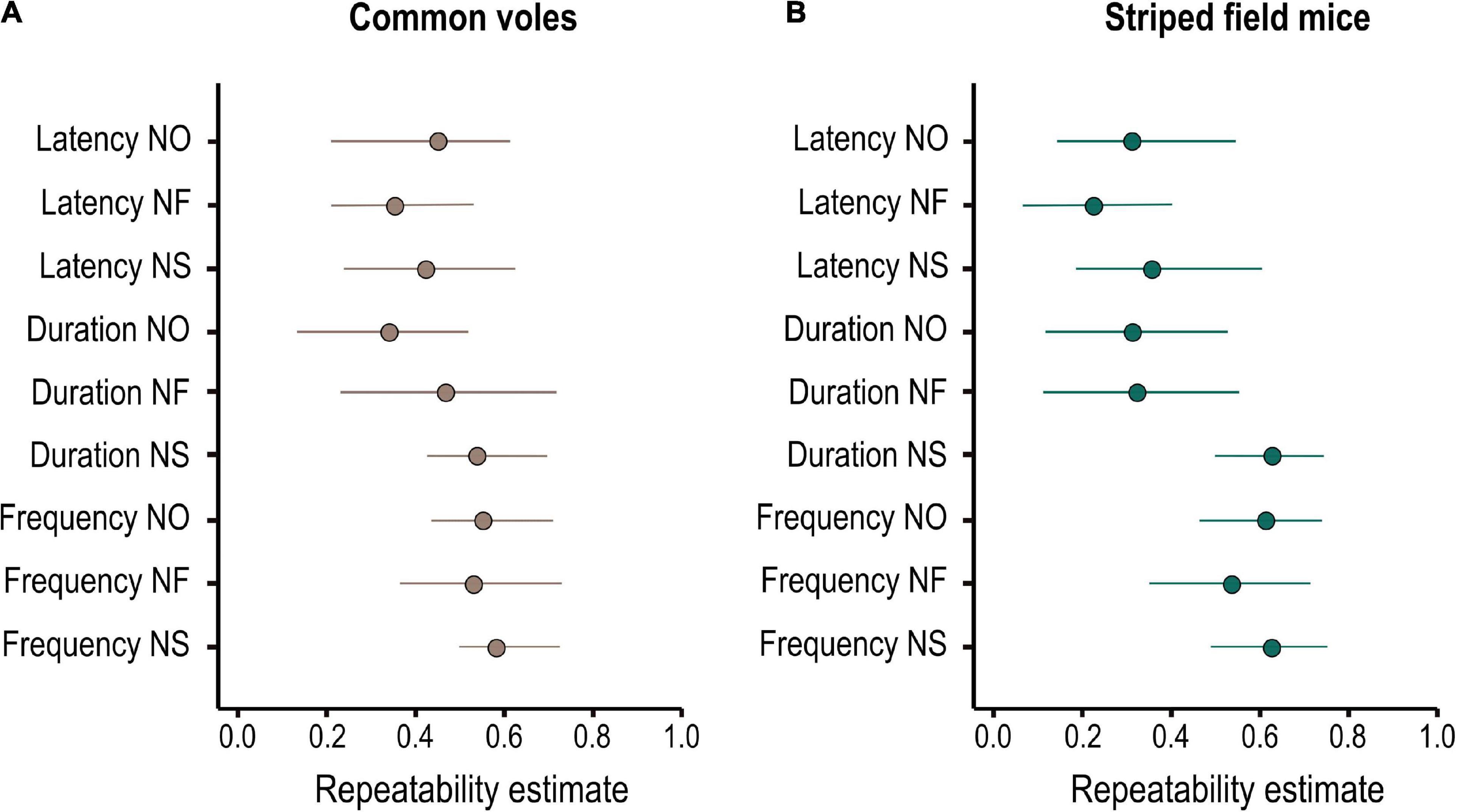
Figure 3. Adjusted individual repeatabilities of behavioral responses to novelty for (A) 47 common voles (M. arvalis) and (B) 41 striped field mice (A. agrarius) quantified in a battery of three tests (novel object, novel food, and novel space). Shown are mean and 95% credibility intervals of the posterior distribution of Bayesian mixed effects models.
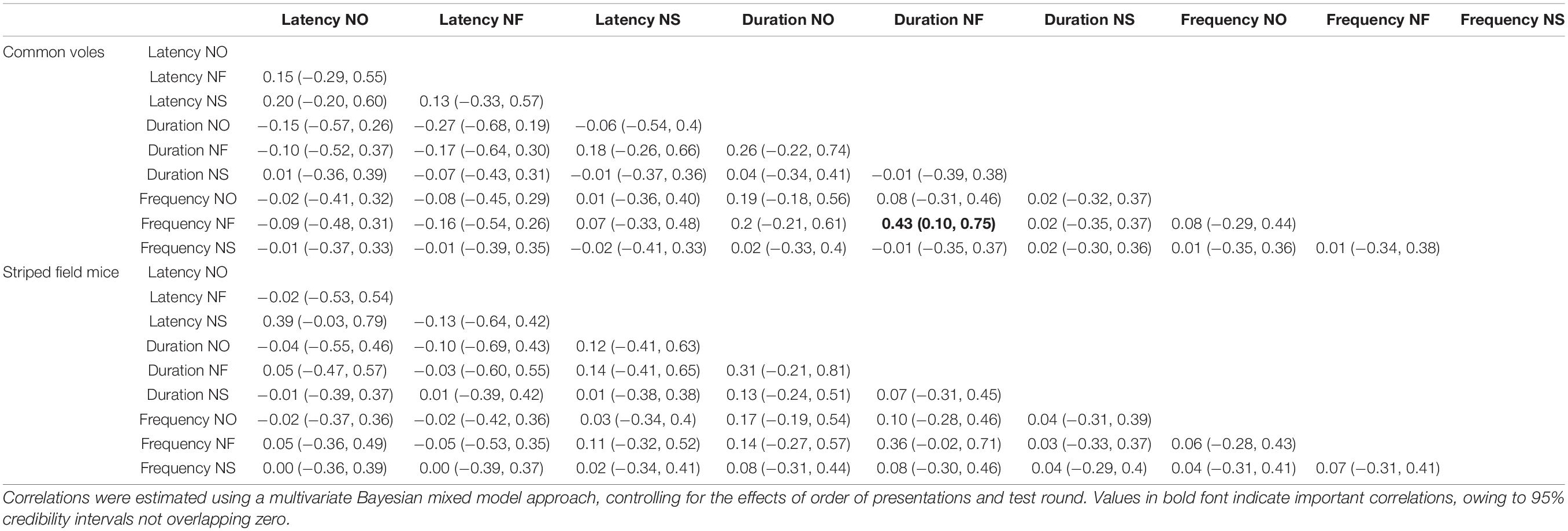
Table 3. Parameter estimates with associated 95% credibility intervals for among-individual correlation for nine behavioral variables for 47 common voles (Microtus arvalis) and 41 striped field mice (Apodemus agrarius) quantified in a repeated battery of three novelty tests (novel object, novel food, and novel space).
Likewise, for the striped field mice, SEM comparisons identified models 6 and 7 as best explaining the among-individual covariance matrix based on FMIN and NFI, but model 4 was best supported based on PFNI and AIC (Supplementary Table 2). Models 6–7 posited the integration of the behavioral variables into three dimensions (reflective latent variables), reflecting responses to novel food and the speed and the intensity component of the responses to other novelty types (such as object and space). Model 7 postulated that these two dimensions were underlain by a common general dimension N. It might have been too complex, though, and warning messages indicated that “a solution could not be found.” Model 4 posited the integration of the behavioral variables into two dimensions (reflective latent variables), reflecting the speed and the intensity (frequency and duration) of the responses to novelty. In both species, the null model was clearly unsupported, and so were the models grouping behavioral responses into separate clusters of speed, frequency, and duration (2–3, Supplementary Table 1).
The PCA returned three main components that accounted for 62% of the variance in both species (Table 2). For both species, the first component best explained the variance associated with the intensity of the response, i.e., the number and duration of the interaction with the novelty type (object and space) and the second component was associated with the latencies to interact with the novel stimulus. The third component was associated with the intensity (number and duration) of interaction with the novel food in the common voles, and with the latency to approach the food and the duration of interactions with the novel object for the striped field mice. We will refer to these components as PC1(intensity), PC2(speed), and PC3(food) because their categorization, especially for the third, could be controversial. We refer to the corresponding aspect of our hypotheses in the SEM in brackets only for purposes of clarity and brevity. Repeatabilities and correlations between the three components are shown in Table 2 and Supplementary Figure 2. Urban common voles approached novelty faster (β = −0.46 ± 0.19, t = −2.36, P = 0.018 — Supplementary Table 3) and tended to inspect the food less (β = −0.44 ± 0.26, t = −1.69, P = 0.09 — Supplementary Table 3) compared to rural conspecifics. The difference in speed of approach (PC2) was only present in the second round of testing (LM second round subset: β = −0.57 ± 0.23, t = −2.49, P = 0.018). No difference was detected in any of the components for rural and urban striped field mice. PC2 (speed of approach) increased with test round in both species, while PC1 (intensity of the responses) increased for common voles and decreased for striped field mice (Supplementary Table 3).
Discussion
How animals react to novelty might be an important predictor of their capability to adjust to human-altered environments. Using a battery of repeated tests involving novel object, food, and space, we found that measured behavioral responses were moderately to highly repeatable over time. Across the three novelty types and at the among-individual level, we found that responses are linked but the most likely structure of these links remains somewhat ambiguous. Although the null model was clearly unsupported, a priori hypothesized structures resembling a more general neophobic response and those of more specific response types were similarly well supported. Therefore, deciphering the overall structure of responses to novelty requires further testing. Notably, urban and rural individuals did not differ in their responses concerning intensity, speed, and reaction to novel food, with the exception of common voles being faster in approaching novelty in the second round of testing compared to rural conspecifics.
Structure of the Responses to Novelty
To analyze the structure of responses to novelty, we focused on three ecologically important novelty contexts and on the among-individual level, which should reflect individual intrinsic characteristics and permanent environmental effects (e.g., Dochtermann et al., 2015). For these novelty types and at this level, we could not single out a specific a priori hypothesis as being better supported than all others, although the null model was clearly unsupported in both species, indicating the presence of a structure in the measured behavioral responses. For common voles the best supported model with most, but not all, indices indicated a structuring of the behavioral responses to novelty into three dimensions, reflecting the reactions to objects, foods, and spaces. This would be in line with the idea that the three tests illuminate different aspects of the responses to novelty in different contexts, supporting the idea that different selective pressures act on these axes separately (e.g., Greenberg and Mettke-Hofmann, 2001; Mettke-Hofmann et al., 2002; Mettke-Hofmann, 2014). Similarly, there was not one single scenario that could be selected as unequivocally better at describing the data for striped field mice. Here the a priori hypotheses that appeared better supported pointed toward the integration of the behavioral variables into three dimensions, reflecting responses to novel food, and the speed and the intensity component of the responses to objects and spaces. Specifically, the models identified a pattern where the latencies to approach the novel object and space co-varied, the frequencies and durations of approaches to these novelty types – which we summarized as describing the intensity of the interaction – co-varied on a separate axis, and responses to food grouped together in a third. Notably, the model hypothesizing a latent variable reflecting a general response, that could not be excluded for common voles, was not supported.
However, in both species, some of the models did not converge, and a proper comparison among all models could not be made. Since the SEM analysis is not inferring ‘causality’ but rather helps to reject some a priori hypothesized structures and, thus, to provisionally accept a (set of) given models it always entails some uncertainty and we offer it as a ‘work in progress result.’ SEMs proved to be particularly sensitive to sample size, and although our sample sizes of observations followed the suggestion of Bentler and Chou (1987) with ca. five observations per estimated parameter, it might have limited our power to resolve structure with SEMs (e.g., Bókony et al., 2012; Wolf et al., 2013). Therefore, we supplemented our analyses with principal component analyses of behavioral variables at the phenotypic level. For both species, these analyses revealed a structure combining the most likely scenarios based on the SEMs, identifying (broadly speaking) a slow-fast continuum to approach the novelty (PC2 speed), one that described the intensity of the responses to object and space (PC1 intensity), and a third on which different aspects of the response to food were mainly represented (PC3 food), with a few differences between species.
Taken together, our results suggest that there are two common underlying factors that drive and structure different aspects of the behavioral responses to novelty. First, the speed of interaction, i.e., how long individuals will take until approaching novelty, and second its extent or intensity. This is in line with previous studies reporting an approach-avoidance conflict stemming from neophilic and neophobic reactions both being elicited by the presentation of novelty (e.g., Murphy, 1978; Mettke-Hofmann et al., 2002; Stieb et al., 2005; Stöwe et al., 2006). Here, however, we cannot tell if more intense interactions are due to higher neophilia or to the amount of information individuals need to collect on any aspect of their surroundings, therefore reflecting individual strategies of fast and shallow versus slow and thorough exploration (e.g., Carere and Locurto, 2011; Reader, 2015; Rojas-Ferrer et al., 2020). In both species the responses to food stood apart from the others. This could be interpreted in terms of behavioral reactions to novel food constituting a separate dimension and requiring a separate set of responses, that are not captured in the avoidance/attraction paradigm (e.g., Marples and Kelly, 1999).
The very weak correlations between behavioral responses at the among-individual level also seem to indicate a high degree of independence between these responses, or at least that they are reflecting separate aspects of the responses to novelty. From these results, we derive a tentative conclusion of responses to novelty being underlain by common factors expressing both the tendency to avoid novelty (neophobia) and drive to explore it (neophilia), but context-specific responses were also present. Specific responses to objects, foods, and spaces might not necessarily offer reliable predictions on the responses of small mammals in the other contexts, and while responses to objects and spaces seem to be underlain by the same factors, novel food appeared to be perceived and reacted to in a markedly different manner, perhaps indicating responses to potential unknown dangers in the first case and to potential resources in the other (e.g., Šlipogor et al., 2016).
Responses to Novelty in Rural and Urban Individuals
Urban areas differ from rural ones in spatial (e.g., habitat configurations, plant composition, and frequency of alterations), object (e.g., rubbish in the park, nesting material, or opportunities), and food (e.g., presence of human-waste, or different seed and arthropod communities) features. The types of novelty considered in this work (space, object, and food) thus encompass some of the main aspects which animals can be faced with in their environment. We did not find reduced neophobia in urban common voles and striped field mice, as most composite scores were similar across habitats. This contrasts with some previous studies reporting lower levels of neophobia in urban animals, and the general predictions made on behavioral adjustments to urban environments (e.g., Sol et al., 2011, 2013; Lowry et al., 2013; Ducatez et al., 2017; Jarjour et al., 2019). While further work is necessary to illuminate the mechanisms maintaining comparable levels of neophobia and neophilia in rural and urban small mammal populations, these results suggest that living in the city has not altered the pressures shaping these responses in the two species. Particularly, since small mammals’ fitness is strongly determined by the survival component, maintaining comparatively high life-preserving fear of novel stimuli might be the most appropriate response. Previous studies also reported comparable responses to novelty in rural and urban dwellers (e.g., Echeverría and Vassallo, 2008; Bókony et al., 2012). Although colonization and establishment in novel environments are commonly considered to promote flexible adjustment of behavioral responses to both information gathering and risk, it is still unclear whether plasticity in responses to novelty is due to a general or specific modification of fear (Greggor et al., 2016b). Neophobic responses likely evolved to protect the individual from potential harm in unknown situations (e.g., Greenberg, 2003). We have previously shown that rural and urban individuals of both species differ in consistent behavioral traits related to risk taking, with urban individuals being both bolder (i.e., more risk-prone) and more plastic in the way they express their behavioral responses (Dammhahn et al., 2020; Mazza et al., 2020). In this study, we report evidence of responses to novelty also being stable individual traits, as well as little to no support for differences between rural and urban populations. Taken together, these findings support the view that what is usually referred to as “boldness” indicates consistent individual differences in risk-taking behavior (Réale et al., 2007) in response to a familiar, known risk, such as being in an open unprotected empty space for rodent prey species, while neophobic responses may specifically target unknown potential dangers and be strongly maintained across populations, irrespective of habitat characteristics. The dangerous niche hypothesis posits that heightened aversion to novelty is adaptive when novel stimuli are likely dangerous, e.g., if toxic foods, traps or a high level of predation risk characterize an individual’s environment (Greenberg and Mettke-Hofmann, 2001; Greenberg, 2003; Bókony et al., 2012). Differential selective pressures in different stages of colonization might explain the contrasting findings between studies. Depending on the species-specific conditions, it could prove more advantageous to express higher cautiousness during the first stages of colonization, followed by reduced neophobia and increased neophilia, allowing for higher chances of encountering new resources (e.g., Greenberg, 2003; Bókony et al., 2012). For others, especially those that are targeted by deterrents and culling efforts by humans, there might be reasons to increase aversion to novel stimuli even further (e.g., Cowan, 1977; Frid and Dill, 2002; Barrett et al., 2019; Goumas et al., 2020). This is what Greggor et al. (2016b) suggest regarding corvids, whose neophobic responses were as strong as those of their rural counterparts and much stronger than non-corvid urban birds. Similar findings were reported by Bókony et al. (2012) for house sparrows (Passer domesticus). Common voles and striped field mice are non-commensal and not the target of any management program in urban areas. Small mammals are, however, among the most common non-target victims of rodenticides intended for other species (e.g., Brakes and Smith, 2005). Urban green areas also see high densities of predators (e.g., Fischer et al., 2012). We have no exact information on how long the two species have lived in the city of Berlin, but until ca. 250 years ago Berlin consisted mainly of villages separated by wide rural areas and therefore the process of its urbanization is relatively recent (e.g., Reulecke and Reuleke, 1977; Arandelovic and Bogunovich, 2014; http://atlasofurbanexpansion.org/cities/view/Berlin). Urban animals in our study therefore might not have been urbanized long enough to really develop major differences to their rural counterparts in this respect. Also, green spaces across the city might represent functional corridors for small mammals, eroding any local behavioral adaptations in responses to novelty. Long-term and population genetics data alongside behavioral assessments are needed to assess if and how responses to novelty vary with colonization stage, and in which direction. Since small mammals appear to avoid the road surface itself rather than traffic noise or other emissions, attention should also be given to the specific urban design, where the configuration of green areas that might be used as corridors could impact inter-generational dispersal (e.g., McGregor et al., 2008; Ascensão et al., 2016). To further illuminate the significance of responses to novelty, future work should focus on whether the novelty elicits a physiological stress response, as well as comparing responses to novelty with measures of risk responsiveness toward different potential stressors, and to the presence of humans specifically (e.g., Herborn et al., 2010; Barrett et al., 2019; Goumas et al., 2020).
Given the lack of information on individual experiences, we regard our results as rather conservative because this heterogeneity in experiences might actually erode some patterns visible only under controlled developmental conditions. Longer term studies that allow the tracking of the history of individual experiences and other developmental aspects are a necessary next step. These studies are only possible with captive-born individuals under laboratory conditions, which also have their drawbacks, for example due to reduced stimuli experiences compared to wild conditions. The present study – using wild-caught individuals of unknown developmental and experience background – is a first step toward understanding the structure of novelty responses, particularly since we combine individuals from different populations.
Since animals of both origins were kept in captivity for ca. 3 months before testing, it might be argued that their responses to novelty, as measured, are a product of habituation to the laboratory environment. Interpreting lab-obtained data to investigate evolutionary patterns can be problematic; at the same time, a shorter delay between capture and test might not have prevented a non-natural response, because the living conditions were different, and the stress of adjusting to captivity could have been a bigger confounding factor (e.g., Niemelä and Dingemanse, 2014). Several studies on various long-lived taxa report consistency in responses to novelty in captivity and in the wild (e.g., Herborn et al., 2010; Minderman et al., 2010; Pellegrini et al., 2010; van Overveld and Matthysen, 2010), throughout the lifespan (e.g., Payne et al., 2021) or across contexts (e.g., Stöwe et al., 2006; Tebbich et al., 2012; Castanheira et al., 2013). The few studies that, to our knowledge, address this issue in small mammals also report consistent responses to novelty across time and contexts in general, and across the laboratory-field context in particular, showing that neophobia measured in captivity reflected differences in neophobia in the wild (e.g., Yuen et al., 2016; Guenther and Brust, 2017; Schuster et al., 2017). Similarly, while a previous study of our group shows that urban animals were more likely to adjust their behavioral responses to the testing environment (Mazza et al., 2020), we also showed that cognitive aspects such as innovation propensity still differed between urban and rural populations after a much longer time in captivity (approx. 1 year) (Mazza and Guenther, 2021). Further studies measuring novelty responses directly in wild populations, challenging as they are, might shed light on this aspect.
The one aspect in which we could detect differences between rural and urban common voles was that urban voles approached novelty faster compared to rural common voles. Considering the importance of survival for prey animals, we think this result could indeed be explained by the amount of different stimuli animals are exposed to in different habitats, and not to a general lower aversion to novel stimuli. Notably, these differences did not emerge until the second round of testing. We repeated the tests to assess repeatability of the response and we used variation of the stimuli to maintain the aspect of novelty. Results indicate that we could not completely avoid habituation to the test, though, as the speed of the response increased for all animals in the second round, and the intensity varied for both species: voles increased the intensity of the interaction with the novelty while striped field mice decreased it. Responses to food did not show any habituation effects, further suggesting that they form a separate axis of responses to novelty. Urban common voles habituated faster than rural conspecifics, approaching the novelty at higher speed in the second round of testing (e.g., Vincze et al., 2016). Faster habituation rates are often interpreted as a sign of higher behavioral plasticity and/or a simple form of learning (e.g., Martin and Réale, 2008; Snell-Rood, 2013). This finding is in line with a wealth of literature reporting higher levels of behavioral plasticity in urban dwellers, both at the inter- and intra-specific levels (e.g., Sol et al., 2002, 2013; Lowry et al., 2013). In a previous study involving these individual common voles, we also found higher levels of behavioral plasticity (reversible phenotypic plasticity) in boldness and activity of urban individuals (Mazza et al., 2020). No such difference was detected for striped field mice, although here too we could have expected higher levels of behavioral plasticity, as these individuals were also tested in a battery of problem-solving tests, often considered as an indicator of behavioral plasticity (e.g., Reader and Laland, 2003; Stamps, 2016; Audet and Lefebvre, 2017), and urban mice outperformed their rural conspecifics (Mazza and Guenther, 2021). In that case, rural and urban striped field mice alike required an extensive habituation phase (up to 5 weeks) before they could be presented with the problem-solving set-ups. In that study, our aim was to test problem solving performance unhindered by among-individual variation in neophobia (Mazza and Guenther, 2021). The long time it took to reach the habituation criterion suggests that two repeats of the test 15–24 days apart might not be enough to detect potential differences in habituation rates between rural and urban striped field mice.
Conclusion
We offer this study as a first step toward understanding the structure of the responses to novelty in common voles and striped field mice. Results support the idea that responses are structured by common elements that could be broadly described as neophobia and neophilia toward objects and spaces, while reactions to novel food seem to belong to a separate dimension. Additionally, urban small mammals seem to maintain levels of neophobia and neophilia comparable to those of their rural counterparts, which could indicate that urban environments represent a dangerous niche for small mammals to live in, which rewards comparable behavioral responses to novelty. Further studies are needed to investigate the mechanisms maintaining similar levels of neophobia and neophilia in rural and urban small mammal populations, and the degree to which the constraints faced by very diverse taxa in each habitat type are comparable.
Data Availability Statement
The data that supports the findings of this study are available as Supplementary Material of this article.
Ethics Statement
Animal capture and behavioral tests were conducted under the permission of the “Landesamt für Umwelt, Gesundheit und Verbraucherschutz Brandenburg” (reference number: RO7/SOB-0998A-C), the “Landesamt für Arbeitsschutz, Verbraucherschutz und Gesundheit, Brandenburg” (reference number: 2347-7-2019), the “Landeshauptstadt Potsdam, Fachbereich Soziales und Gesundheit, Veterinär– und Lebensmittelüberwachung” (reference number: 386-1), the “Senatsverwaltung für Stadtentwicklung und Umwelt” (reference number: IIIB2/OA/AS/G1394), and the “Landesamt für Gesundheit und Soziales” (reference number: G 0072/16).
Author Contributions
VM and MD conceived and designed the study. IC performed the data collection. VM analyzed the data, with help from MD and JE. VM wrote the manuscript. All authors contributed to the final draft and gave final approval for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This work was made possible by funding from the German Federal Ministry of Education and Research (BMBF) within the Collaborative Project ‘Bridging in Biodiversity Science (BIBS)’ (funding number 01LC1501A), supporting prior steps of the project. During manuscript preparation, MD was supported by a grant of the German Science Foundation (DA 1377/4-1). We acknowledge the support of the Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Potsdam. We thank Angelika Beck for preparation of the city project and Elisa L sche and Stephanie Thiele for their help on the field during trapping. Thanks to Angela Puschmann and Elisa Lösche for helping to care for the animals in the laboratory. We thank the reviewers and handling editor for their constructive comments and suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.661971/full#supplementary-material
References
Alberti, M. (2015). Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 30, 114–126. doi: 10.1016/j.tree.2014.11.007
Alberti, M., Marzluff, J., and Hunt, V. M. (2017). Urban driven phenotypic changes: empirical observations and theoretical implications for eco-evolutionary feedback. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 372:20160029. doi: 10.1098/rstb.2016.0029
Arandelovic, B., and Bogunovich, D. (2014). City profile: Berlin. Cities 37, 1–26. doi: 10.1016/j.cities.2013.10.007
Ascensão, F., Mata, C., Malo, J. E., Ruiz-Capillas, P., Silva, C., Silva, A. P., et al. (2016). Disentangle the causes of the road barrier effect in small mammals through genetic patterns. PLoS One 11: e0151500.
Audet, J.-N., Ducatez, S., and Lefebvre, L. (2016). The town bird and the country bird: problem solving and immunocompetence vary with urbanization. Behav. Ecol. 27, 637–644. doi: 10.1093/beheco/arv201
Audet, J.-N., and Lefebvre, L. (2017). What’s flexible in behavioral flexibility? Behav. Ecol. 28, 943–947. doi: 10.1093/beheco/arx007
Babińska-Werka, J. (1981). Food of the striped field mouse in different types of urban green areas. Acta Theriol. 26, 285–299. doi: 10.4098/AT.arch.81-24
Barnosky, A. D., Hadly, E. A., Bascompte, J., Berlow, E. L., Brown, J. H., Fortelius, M., et al. (2012). Approaching a state shift in Earth’s biosphere. Nature 486, 52–58. doi: 10.1038/nature11018
Barrett, L. P., Stanton, L. A., and Benson-Amram, S. (2019). The cognition of ‘nuisance’ species. Animal Behav. 147, 167–177. doi: 10.1016/j.anbehav.2018.05.005
Bates, D., Maechler, M., Bolker, B., Walker, S., Christensen, R. H. B., Singmann, H., et al. (2015). Package ‘lme4.’. Convergence 12:2.
Baxter-Gilbert, J., Riley, J. L., and Whiting, M. J. (2019). Bold New World: urbanization promotes an innate behavioral trait in a lizard. Behav. Ecol. Sociobiol. 73:105. doi: 10.1007/s00265-019-2713-9
Baxter-Gilbert, J. H., and Whiting, M. J. (2019). Street fighters: Bite force, injury rates, and density of urban Australian water dragons (Intellagama lesueurii). Austral. Ecol. 44, 255–264. doi: 10.1111/aec.12670
Bentler, P. M., and Chou, C. P. (1987). Practical issues in structural equation modeling. Sociol. Methods Res. 16, 78–117. doi: 10.1177/0049124187016001004
Biermann, F., Bai, X., Bondre, N., Broadgate, W., Arthur Chen, C.-T., Dube, O. P., et al. (2016). Down to Earth: Contextualizing the Anthropocene. Glob. Environ. Change 39, 341–350. doi: 10.1016/j.gloenvcha.2015.11.004
Bókony, V., Kulcsár, A., Tóth, Z., and Liker, A. (2012). Personality traits and behavioral syndromes in differently urbanized populations of house sparrows (Passer domesticus). PLoS One 7:e36639. doi: 10.1371/journal.pone.0036639
Brakes, C. R., and Smith, R. H. (2005). Exposure of non-target small mammals to rodenticides: short-term effects, recovery and implications for secondary poisoning. J. Appl. Ecol. 42, 118–128. doi: 10.1111/j.1365-2664.2005.00997.x
Briner, T., Nentwig, W., and Airoldi, J.-P. (2005). Habitat quality of wildflower strips for common voles (Microtus arvalis) and its relevance for agriculture. Agri. Ecosyst. Environ. 105, 173–179. doi: 10.1016/j.agee.2004.04.007
Brown, G. E., Chivers, D. P., Elvidge, C. K., Jackson, C. D., and Ferrari, M. C. O. (2014). Background level of risk determines the intensity of predator neophobia in juvenile convict cichlids. Behav. Ecol. Sociobiol. 68, 127–133. doi: 10.1007/s00265-013-1629-z
Brown, G. E., Ferrari, M. C. O., Elvidge, C. K., Ramnarine, I., and Chivers, D. P. (2013). Phenotypically plastic neophobia: a response to variable predation risk. Proc. R. Soc. B. 280:20122712. doi: 10.1098/rspb.2012.2712
Buchholz, S., Hannig, K., Moller, M., and Schirmel, J. (2018). Reducing management intensity and isolation as promising tools to enhance ground-dwelling arthropod diversity in urban grasslands. Urban Ecosyst. 21, 1139–1149. doi: 10.1007/s11252-018-0786-2
Carere, C., and Locurto, C. (2011). Interaction between animal personality and animal cognition. Curr. Zool. 57, 491–498. doi: 10.1093/czoolo/57.4.491
Castanheira, M. F., Herrera, M., Costas, B., Conceicao, L. E. C., and Martins, C. I. M. (2013). Can we predict personality in fish? searching for consistency over time and across contexts. PLoS One 8:e62037. doi: 10.1371/journal.pone.0062037
Ceballos, G. (2002). Mammal population losses and the extinction crisis. Science 296, 904–907. doi: 10.1126/science.1069349
Corsini, M., Dubiec, A., Marrot, P., and Szulkin, M. (2017). Humans and tits in the city: quantifying the effects of human presence on great tit and blue tit reproductive trait variation. Front. Ecol. Evol. 5:82. doi: 10.3389/fevo.2017.00082
Corsini, M., Marrot, P., and Szulkin, M. (2019). Quantifying human presence in a heterogeneous urban landscape. Behav. Ecol. 30, 1632–1641. doi: 10.1093/beheco/arz128
Cowan, P. E. (1977). Neophobia and neophilia: New-object and new-place reactions of three Rattus species. J. Comp. Physiol. Psychol. 91, 63–71. doi: 10.1037/h0077297
Crane, A. L., Brown, G. E., Chivers, D. P., and Ferrari, M. C. O. (2020). An ecological framework of neophobia: from cells to organisms to populations. Biol. Rev. 95, 218–231. doi: 10.1111/brv.12560
Dammhahn, M., Mazza, V., Schirmer, A., Gottsche, C., and Eccard, J. A. (2020). Of city and village mice: behavioural adjustments of striped field mice to urban environments. Sci. Rep. 10:13056. doi: 10.1038/s41598-020-69998-6
Dingemanse, N. J., Dochtermann, N., and Wright, J. (2010). A method for exploring the structure of behavioural syndromes to allow formal comparison within and between data sets. Animal Behav. 79, 439–450. doi: 10.1016/j.anbehav.2009.11.024
Dingemanse, N. J., and Dochtermann, N. A. (2013). Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. doi: 10.1111/1365-2656.12013
Dochtermann, N. A., and Jenkins, S. H. (2011). Developing multiple hypotheses in behavioral ecology. Behav. Ecol. Sociobiol. 65, 37–45. doi: 10.1007/s00265-010-1039-4
Dochtermann, N. A., Schwab, T., and Sih, A. (2015). The contribution of additive genetic variation to personality variation: heritability of personality. Proc. R. Soc. B 282:20142201. doi: 10.1098/rspb.2014.2201
Ducatez, S., Audet, J. N., Rodriguez, J. R., Kayello, L., and Lefebvre, L. (2017). Innovativeness and the effects of urbanization on risk-taking behaviors in wild Barbados birds. Anim. Cogn. 20, 33–42. doi: 10.1007/s10071-016-1007-0
Echeverría, A. I., and Vassallo, A. I. (2008). Novelty responses in a bird assemblage inhabiting an urban area. Ethology 114, 616–624. doi: 10.1111/j.1439-0310.2008.01512.x
Ferrari, M. C. O., Gonzalo, A., Messier, F., and Chivers, D. P. (2007). Generalization of learned predator recognition: an experimental test and framework for future studies. Proc. R Soc. B. 274, 1853–1859. doi: 10.1098/rspb.2007.0297
Field, A. P. (2012). Discovering statistics using IBM SPSS statistics: and sex and drugs and rock “n” roll, 4th Edn. Los Angeles: Sage.
Fischer, J. D., Cleeton, S. H., Lyons, T. P., and Miller, J. R. (2012). Urbanization and the predation paradox: the role of trophic dynamics in structuring vertebrate communities. BioScience 62, 809–818. doi: 10.1525/bio.2012.62.9.6
Friard, O., and Gamba, M. (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. doi: 10.1111/2041-210X.12584
Frid, A., and Dill, L. (2002). Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 6:111.
Galef, B. G. (1993). Functions of social learning about food: a causal analysis of effects of diet novelty on preference transmission. Animal Behav. 46, 257–265. doi: 10.1006/anbe.1993.1187
Gao, J., and O’Neill, B. C. (2020). Mapping global urban land for the 21st century with data-driven simulations and Shared Socioeconomic Pathways. Nat. Commun. 11:2302. doi: 10.1038/s41467-020-15788-7
Gauffre, B., Estoup, A., Bretagnolle, V., and Cosson, J. F. (2008). Spatial genetic structure of a small rodent in a heterogeneous landscape. Mol. Ecol. 17, 4619–4629. doi: 10.1111/j.1365-294X.2008.03950.x
Gauffre, B., Petit, E., Brodier, S., Bretagnolle, V., and Cosson, J. F. (2009). Sex-biased dispersal patterns depend on the spatial scale in a social rodent. Proc. R Soc. B 276, 3487–3494. doi: 10.1098/rspb.2009.0881
Goumas, M., Lee, V. E., Boogert, N. J., Kelley, L. A., and Thornton, A. (2020). The Role of animal cognition in human-wildlife Interactions. Front. Psychol. 11:589978. doi: 10.3389/fpsyg.2020.589978
Greenberg, R. (2003). “The Role of Neophobia and Neophilia in the Development of Innovative Behaviour of Birds,” in Animal Innovation, eds S. M. Reader and K. N. Laland (Oxford: Oxford University Press), 175–196. doi: 10.1093/acprof:oso/9780198526223.003.0008
Greenberg, R., and Mettke-Hofmann, C. (2001). “Ecological Aspects of Neophobia and neophilia in birds,” in Current Ornithology, Volume 16, eds V. Nolan and C. F. Thompson (Boston, MA: Springer), 119–178. doi: 10.1007/978-1-4615-1211-0_3
Greggor, A. L., Jolles, J. W., Thornton, A., and Clayton, N. S. (2016a). Seasonal changes in neophobia and its consistency in rooks: the effect of novelty type and dominance position. Animal Behav. 121, 11–20. doi: 10.1016/j.anbehav.2016.08.010
Greggor, A. L., Clayton, N. S., Fulford, A. J. C., and Thornton, A. (2016b). Street smart: faster approach towards litter in urban areas by highly neophobic corvids and less fearful birds. Animal Behav. 117, 123–133. doi: 10.1016/j.anbehav.2016.03.029
Greggor, A. L., Clayton, N. S., Phalan, B., and Thornton, A. (2014). Comparative cognition for conservationists. Trends Ecol. Evol. 29, 489–495. doi: 10.1016/j.tree.2014.06.004
Greggor, A. L., Thornton, A., and Clayton, N. S. (2015). Neophobia is not only avoidance: improving neophobia tests by combining cognition and ecology. Curr. Opin. Behav. Sci. 6, 82–89. doi: 10.1016/j.cobeha.2015.10.007
Griffin, A. S., and Guez, D. (2014). Innovation and problem solving: A review of common mechanisms. Behav. Proc. 109, 121–134. doi: 10.1016/j.beproc.2014.08.027
Grimm, N. B., Faeth, S. H., Golubiewski, N. E., Redman, C. L., Wu, J., Bai, X., et al. (2008). Global change and the ecology of cities. Science 319, 756–760. doi: 10.1126/science.1150195
Groffman, P. M., Cavender-Bares, J., Bettez, N. D., Grove, J. M., Hall, S. J., Heffernan, J. B., et al. (2014). Ecological homogenization of urban USA. Front. Ecol. Environ. 12:74. doi: 10.1890/120374
Guenther, A., and Brust, V. (2017). Individual consistency in multiple cognitive performance: behavioural versus cognitive syndromes. Animal Behav. 130, 119–131. doi: 10.1016/j.anbehav.2017.06.011
Hadfield, J. D. (2010). MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R Package. J. Stat. Soft. 33, 1–22. doi: 10.18637/jss.v033.i02
Halle, S. (2000). “Ecological Relevance of Daily Activity Patterns,” in Activity Patterns in Small Mammals Ecological Studies, eds S. Halle and N. C. Stenseth (Berlin: Springer), 67–90. doi: 10.1007/978-3-642-18264-8_5
Halle, S., and Lehmann, U. (1987). Circadian activity patterns, photoperiodic responses and population cycles in voles: I. Long-term variations in circadian activity patterns. Oecologia 71, 568–572. doi: 10.1007/BF00379299
Herborn, K. A., Macleod, R., Miles, W. T. S., Schofield, A. N. B., Alexander, L., and Arnold, K. E. (2010). Personality in captivity reflects personality in the wild. Animal Behav. 79, 835–843. doi: 10.1016/j.anbehav.2009.12.026
Herde, A., and Eccard, J. A. (2013). Consistency in boldness, activity and exploration at different stages of life. BMC Ecol. 13, 1–10. doi: 10.1186/1472-6785-13-49
Jacob, J., and Hempel, N. (2003). Effects of farming practices on spatial behaviour of common voles. J. Ethol. 21, 45–50. doi: 10.1007/s10164-002-0073-8
Jacob, J., Manson, P., Barfknecht, R., and Fredricks, T. (2014). Common vole (Microtus arvalis) ecology and management: implications for risk assessment of plant protection products: Common voles in the risk assessment of plant protection products. Pest. Manag. Sci. 70, 869–878. doi: 10.1002/ps.3695
Jarjour, C., Evans, J. C., Routh, M., and Morand-Ferron, J. (2019). Does city life reduce neophobia? A study on wild black-capped chickadees. Behav. Ecol. 2019:arz167. doi: 10.1093/beheco/arz167
Kaiser, H. F. (1991). Unity as the universal upper bound for reliability. Percept Mot. Skills 72, 218–218. doi: 10.2466/pms.1991.72.1.218
Lantová, P., Šíchová, K., Sedláček, F., and Lanta, V. (2011). Determining behavioural syndromes in voles - the effects of social environment: personality and behavioural syndromes in the common vole. Ethology 117, 124–132. doi: 10.1111/j.1439-0310.2010.01860.x
Lee, V. E., and Thornton, A. (2021). Animal Cognition in an Urbanised World. Front. Ecol. Evol. 9:633947. doi: 10.3389/fevo.2021.633947
Lima, S. L., and Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. doi: 10.1139/z90-092
Łopucki, R., and Kiersztyn, A. (2020). The city changes the daily activity of urban adapters: Camera-traps study of Apodemus agrarius behaviour and new approaches to data analysis. Ecol. Indicat. 110:105957. doi: 10.1016/j.ecolind.2019.105957
Łopucki, R., Klich, D., and Kiersztyn, A. (2020). Changes in the social behavior of urban animals: more aggression or tolerance? Mamm. Biol. 2020:75. doi: 10.1007/s42991-020-00075-1
Łopucki, R., Klich, D., Ścibior, A., and Gołębiowska, D. (2019). Hormonal adjustments to urban conditions: stress hormone levels in urban and rural populations of Apodemus agrarius. Urban Ecosyst. 22, 435–442. doi: 10.1007/s11252-019-0832-8
Lowry, H., Lill, A., and Wong, B. B. M. (2013). Behavioural responses of wildlife to urban environments: Behavioural responses to urban environments. Biol. Rev. 88, 537–549. doi: 10.1111/brv.12012
Marples, N. M., and Kelly, D. J. (1999). Neophobia and dietary conservatism:two distinct processes? Evol. Ecol. 13, 641–653. doi: 10.1023/A:1011077731153
Martin, J. G. A., and Réale, D. (2008). Temperament, risk assessment and habituation to novelty in eastern chipmunks. Tamias striatus. Animal Behav. 75, 309–318. doi: 10.1016/j.anbehav.2007.05.026
Mazza, V., Dammhahn, M., Lösche, E., and Eccard, J. A. (2020). Small mammals in the big city: Behavioural adjustments of non-commensal rodents to urban environments. Glob. Change Biol. 2020:15304. doi: 10.1111/gcb.15304
Mazza, V., and Guenther, A. (2021). City mice and country mice: innovative problem solving in rural and urban noncommensal rodents. Animal Behav. 172, 197–210. doi: 10.1016/j.anbehav.2020.12.007
Mazza, V., Eccard, J. A., Zaccaroni, M., Jacob, J., and Dammhahn, M. (2018). The fast and the flexible: cognitive style drives individual variation in cognition in a small mammal. Anim. Behav. 137, 119–132. doi: 10.1016/j.anbehav.2018.01.011
McDonnell, M. J., and Hahs, A. K. (2015). Adaptation and adaptedness of organisms to urban environments. Annu. Rev. Ecol. Evol. Syst. 46, 261–280. doi: 10.1146/annurev-ecolsys-112414-054258
McGregor, R. L., Bender, D. J., and Fahrig, L. (2008). Do small mammals avoid roads because of the traffic? J. Appl. Ecol. 45, 117–123.
McKinney, M. L. (2002). Urbanization, Biodiversity, and Conservation. BioScience 52:883. doi: 10.1641/0006-35682002052
Mettke-Hofmann, C. (2014). Cognitive ecology: ecological factors, life-styles, and cognition: Cognitive ecology. WIREs Cogn. Sci. 5, 345–360. doi: 10.1002/wcs.1289
Mettke-Hofmann, C., Eccles, G. R., Greggor, A. L., and Bethell, E. J. (2020). Cognition in a changing world: red-headed gouldian finches enter spatially unfamiliar habitats more readily than do black-headed birds. Front. Ecol. Evol. 8:498347. doi: 10.3389/fevo.2020.498347
Mettke-Hofmann, C., Winkler, H., and Leisler, B. (2002). The significance of ecological factors for exploration and neophobia in parrots. Ethology 108, 249–272. doi: 10.1046/j.1439-0310.2002.00773.x
Minderman, J., Reid, J. M., Hughes, M., Denny, M. J. H., Hogg, S., Evans, P. G. H., et al. (2010). Novel environment exploration and home range size in starlings Sturnus vulgaris. Behav. Ecol. 21, 1321–1329. doi: 10.1093/beheco/arq151
Moiron, M., Araya-Ajoy, Y. G., Mathot, K. J., Mouchet, A., and Dingemanse, N. J. (2019). Functional relations between body mass and risk-taking behavior in wild great tits. Behav. Ecol. 30, 617–623. doi: 10.1093/beheco/ary199
Møller, A. P. (2009). Successful city dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159, 849–858. doi: 10.1007/s00442-008-1259-8
Munshi-South, J., Zolnik, C. P., and Harris, S. E. (2016). Population genomics of the Anthropocene: urbanization is negatively associated with genome-wide variation in white-footed mouse populations. Evol. Appl. 9, 546–564. doi: 10.1111/eva.12357
Murphy, L. B. (1978). The practical problems of recognizing and measuring fear and exploration behaviour in the domestic fowl. Animal Behav. 26, 422–431. doi: 10.1016/0003-3472(78)90059-3
Nakagawa, S., and Schielzeth, H. (2010). Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. doi: 10.1111/j.1469-185X.2010.00141.x
Niemelä, P. T., and Dingemanse, N. J. (2014). Artificial environments and the study of ‘adaptive’ personalities. Trends Ecol. Evol. 29, 245–247. doi: 10.1016/j.tree.2014.02.007
Norrdahl, K., and Korpimäki, E. (1995). Mortality factors in a cyclic vole population. Proc. R. Soc. Lond. B 261, 49–53. doi: 10.1098/rspb.1995.0116
Payne, E., Sinn, D. L., Spiegel, O., Leu, S. T., Gardner, M. G., Godfrey, S. S., et al. (2021). Consistent after all: behavioural repeatability in a long-lived lizard across a 6-year field study. Animal Behav. 174, 263–277. doi: 10.1016/j.anbehav.2021.01.025
Pellegrini, A. F. A., Wisenden, B. D., and Sorensen, P. W. (2010). Bold minnows consistently approach danger in the field and lab in response to either chemical or visual indicators of predation risk. Behav. Ecol. Sociobiol. 64, 381–387. doi: 10.1007/s00265-009-0854-y
Pieniążek, A., Boguszewski, P. M., and Meronka, R. A. (2017). The impact of urban noise on the behavior of two mouse species belonging to the genus apodemus. Sci. Res. 8, 55–68. doi: 10.4236/nr.2017.82004
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-proje ct.org/
Reader, S. M. (2015). Causes of individual differences in animal exploration and search. Top Cogn. Sci. 7, 451–468. doi: 10.1111/tops.12148
Réale, D., Reader, S. M., Sol, D., McDougall, P. T., and Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. doi: 10.1111/j.1469-185X.2007.00010.x
Reulecke, J., and Reuleke, J. (1977). Population growth and urbanization in germany in the 19th Century. Urbanism Past & Present 1977, 21–32. Available online at: http://www.jstor.org/stable/44403540
Riegert, J., Lövy, M., and Fainová, D. (2009). Diet composition of common kestrels Falco tinniculus and long-eared owls Asio otus coexisting in an urban environment. Ornis Fennica 86:123-130.
Rojas-Ferrer, I., Thompson, M. J., and Morand-Ferron, J. (2020). Is exploration a metric for information gathering? Attraction to novelty and plasticity in black-capped chickadees. Ethology 126, 383–392. doi: 10.1111/eth.12982
Roos, D., Caminero Saldaña, C., Arroyo, B., Mougeot, F., Luque-Larena, J. J., and Lambin, X. (2019). Unintentional effects of environmentally-friendly farming practices: Arising conflicts between zero-tillage and a crop pest, the common vole (Microtus arvalis). Agri. Ecosyst. Environ. 272, 105–113. doi: 10.1016/j.agee.2018.11.013
Rosseel, Y. (2012). Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). J. Stat. Softw. 48, 1–36.
Schirmer, A., Herde, A., Eccard, J. A., and Dammhahn, M. (2019). Individuals in space: personality-dependent space use, movement and microhabitat use facilitate individual spatial niche specialization. Oecologia 189, 647–660. doi: 10.1007/s00442-019-04365-5
Schirmer, A., Hoffmann, J., Eccard, J. A., and Dammhahn, M. (2020). My niche: individual spatial niche specialization affects within- and between-species interactions. Proc. R. Soc. B 287:20192211. doi: 10.1098/rspb.2019.2211
Schuster, A. C., Zimmermann, U., Hauer, C., and Foerster, K. (2017). A behavioural syndrome, but less evidence for a relationship with cognitive traits in a spatial orientation context. Front. Zool. 14:19. doi: 10.1186/s12983-017-0204-2
Seress, G., Lipovits, A., Bokony, V., and Czuni, L. (2014). Quantifying the urban gradient: A practical method for broad measurements. Landscape Urban Plan. 131, 42–50. doi: 10.1016/j.landurbplan.2014.07.010
Seto, K. C., Guneralp, B., and Hutyra, L. R. (2012). Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Nat. Acad. Sci. 109, 16083–16088. doi: 10.1073/pnas.1211658109
Sih, A., Ferrari, M. C. O., and Harris, D. J. (2011). Evolution and behavioural responses to human-induced rapid environmental change: Behaviour and evolution. Evol. Appl. 4, 367–387. doi: 10.1111/j.1752-4571.2010.00166.x
Šlipogor, V., Gunhold de Oliveira, T., Tadic, Z., Massen, J. J. M., and Bugnyar, T. (2016). Consistent inter-individual differences in common marmosets (Callithrix jacchus) in Boldness-Shyness, Stress-Activity, and Exploration-Avoidance. Am. J. Primatol. 78, 961–973. doi: 10.1002/ajp.22566
Snell-Rood, E. C. (2013). An overview of the evolutionary causes and consequences of behavioural plasticity. Animal Behav. 85, 1004–1011. doi: 10.1016/j.anbehav.2012.12.031
Sol, D., Griffin, A. S., Bartomeus, I., and Boyce, H. (2011). Exploring or avoiding novel food resources? the novelty conflict in an invasive bird. PLoS ONE 6:e19535. doi: 10.1371/journal.pone.0019535
Sol, D., Lapiedra, O., and Ducatez, S. (2020). Cognition and Adaptation to Urban Environments in Urban Evolutionary Biology. Oxford: Oxford University Press, 253–267.
Sol, D., Lapiedra, O., and González-Lagos, C. (2013). Behavioural adjustments for a life in the city. Animal Behav. 85, 1101–1112. doi: 10.1016/j.anbehav.2013.01.023
Sol, D., Timmermans, S., and Lefebvre, L. (2002). Behavioural flexibility and invasion success in birds. Animal Behav. 63, 495–502. doi: 10.1006/anbe.2001.1953
Stamps, J. A. (2016). Individual differences in behavioural plasticities: Behavioural plasticities. Biol Rev 91, 534–567. doi: 10.1111/brv.12186
Stieb, S., Schmidt, T., Ebert, C., Mettke-Hofmann, C., and Steiger, S. (2005). Personality traits in resident and migratory warbler species. Behav 142, 1357–1375. doi: 10.1163/156853905774539427
Stoffel, M. A., Nakagawa, S., and Schielzeth, H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. doi: 10.1111/2041-210X.12797
Stöwe, M., Bugnyar, T., Heinrich, B., and Kotrschal, K. (2006). Effects of group size on approach to novel objects in ravens (Corvus corax). Ethology 112, 1079–1088. doi: 10.1111/j.1439-0310.2006.01273.x
Tabachnick, B. G., and Fidell, L. S. (2001). Principal components and factor analysis. Using Multivar. Stat. 4, 582–633.
Tebbich, S., Stankewitz, S., and Teschke, I. (2012). The relationship between foraging, learning abilities and neophobia in two species of darwin’s finches: the relationship between foraging and learning abilities in darwin’s finches. Ethology 118, 135–146. doi: 10.1111/j.1439-0310.2011.02001.x
Thomas, R. J., Bartlett, L. A., Marples, N. M., Kelly, D. J., and Cuthill, I. C. (2004). Prey selection by wild birds can allow novel and conspicuous colour morphs to spread in prey populations. Oikos 106, 285–294. doi: 10.1111/j.0030-1299.2004.13089.x
Thornton, A. (2008). Social learning about novel foods in young meerkats. Animal Behav. 76, 1411–1421. doi: 10.1016/j.anbehav.2008.07.007
Van Ham, C., Genovesi, P., and Scalera, R. (2013). Invasive alien species: the urban dimension. Case studies. Gland: IUCN. 1621–1625.
van Overveld, T., and Matthysen, E. (2010). Personality predicts spatial responses to food manipulations in free-ranging great tits (Parus major). Biol. Lett. 6, 187–190. doi: 10.1098/rsbl.2009.0764
Vincze, E., Papp, S., Preiszner, B., Seress, G., Bókony, V., and Liker, A. (2016). Habituation to human disturbance is faster in urban than rural house sparrows. Behav. Ecol. 27, 1304–1313. doi: 10.1093/beheco/arw047
von der Lippe, M., Buchholz, S., Hiller, A., Seitz, B., and Kowarik, I. (2020). CityScapeLab berlin: a research platform for untangling urbanization effects on biodiversity. Sustainability 12:2565. doi: 10.3390/su12062565
Webster, S. J., and Lefebvre, L. (2001). Problem solving and neophobia in a columbiform–passeriform assemblage in Barbados. Animal Behav. 62, 23–32. doi: 10.1006/anbe.2000.1725
Wolf, E. J., Harrington, K. M., Clark, S. L., and Miller, M. W. (2013). Sample size requirements for structural equation models: an evaluation of power, bias, and solution propriety. Educat. Psychol. Measure. 73, 913–934. doi: 10.1177/0013164413495237
Wolff, J. O. (2007). Social biology of rodents. Integrat. Zool. 2, 193–204. doi: 10.1111/j.1749-4877.2007.00062.x
Wróbel, A., and Bogdziewicz, M. (2015). It is raining mice and voles: which weather conditions influence the activity of Apodemus flavicollis and Myodes glareolus? Eur. J. Wildl. Res. 61, 475–478. doi: 10.1007/s10344-014-0892-2
Keywords: animal cognition, anthropogenic environment, HIREC, novelty, neophobia, neophilia, rodents, urbanization
Citation: Mazza V, Czyperreck I, Eccard JA and Dammhahn M (2021) Cross-Context Responses to Novelty in Rural and Urban Small Mammals. Front. Ecol. Evol. 9:661971. doi: 10.3389/fevo.2021.661971
Received: 31 January 2021; Accepted: 27 September 2021;
Published: 28 October 2021.
Edited by:
Martin J. Whiting, Macquarie University, AustraliaReviewed by:
Andrew Sih, University of California, Davis, United StatesJodie Gruber, University of Exeter, United Kingdom
Copyright © 2021 Mazza, Czyperreck, Eccard and Dammhahn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Mazza, dmFsZXJpYS5tYXp6YTg1QGdtYWlsLmNvbQ==
 Valeria Mazza
Valeria Mazza Inken Czyperreck1
Inken Czyperreck1 Jana A. Eccard
Jana A. Eccard