
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 08 March 2021
Sec. Biogeography and Macroecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.640121
This article is part of the Research TopicAdaptation of Invasive Species to Islands and the Puerto Rican Honey BeeView all 14 articles
Native flora and fauna of Puerto Rico have a long biogeographic connection to South America. Theory and empirical evidence suggest that islands, particularly those distantly isolated from the mainland, should be more susceptible to naturalizations and invasions of non-native species than continental areas. Anthropogenic disturbances can facilitate accidental and deliberate introductions of non-native species. In this study, we asked: What is the current status of introduced species within El Yunque National Forest (EYNF), the largest and most well-conserved forest area of Puerto Rico? To address this question, we reviewed the literature and surveyed local experts to identify introduced plant and animal taxa that are behaving as invaders within EYNF. We hypothesized that well-conserved forest areas within EYNF would be more resistant to invasions than disturbed areas along roads and ruderal areas with a long history of human activity. We found that there is only partial evidence that supports our hypothesis and this evidence is strongest in vascular plants, but not for the other taxonomic groups analyzed. Our combined results showed that currently the more ubiquitous invasive species in EYNF include some mammals (feral cat, rat, and mongoose) and some invertebrates (earthworms, mosquito, and Africanized honeybee). For many taxa, there is little information to thoroughly test our hypothesis, and thus more detailed surveys of the status of non-native and invasive species in EYNF are needed.
Islands are isolated land masses that frequently exhibit simplified ecological systems containing locally adapted and endemic species, often with small population sizes, low reproductive rates, and a lack of predator defenses compared with mainland counterparts (Wilson and MacArthur, 1967; Moser et al., 2018). These attributes make island ecosystems more susceptible than mainland ecosystems to human-related impacts, such as the introduction and establishment of non-native species. The theory of island biogeography (Wilson and MacArthur, 1967) states that isolation and island size are critical factors determining the number of species that can colonize and establish on island ecosystems. Depending on species’ vagility, islands near the mainland may have similar native species composition and ecological characteristics and higher species richness than remote islands. Thus, islands with a biogeographic history of lying near continental areas may be more resistant to the establishment of new species than those isolated far from mainland (Moser et al., 2018). Islands across the Caribbean show strong species affinities with both North and South America because of their shared biogeographic history, which largely results from their proximity to these two continental regions (Roncal et al., 2020). Therefore, we might expect Caribbean islands to be more diverse and therefore more resistant to the establishment of introduced species, including those that are potentially invasive (i.e., species spreading rapidly into new areas; terminology follows Blackburn et al., 2011) than more isolated islands in ocean basins.
Humans are the ultimate ecosystem engineers, altering habitats for shelter, provision, and resource acquisition. Via their activities, they often introduce new species to areas they colonize, either intentionally for provision or ornamentation or unintentionally by bringing along parasites or commensals. Thus, isolation is effectively reduced. Studies have shown that in addition to isolation and island area (typical factors of the island biogeography theory), factors such as levels of anthropogenic disturbance, human activities (e.g., economic development, human population size, trade and transportation rates and pathways), and propagule pressure are key drivers explaining the diversity of introduced species and invasion success on islands (e.g., Gallardo et al., 2015). Human-mediated introductions of non-native species to islands may lead not only to increases in the number and distribution of species that may colonize islands but also to the displacement and/or extinction of native species. In this regard, human-related activities may lead to a breakdown of the “classical” biogeographic theory and may “redefine” species diversity on islands (Capinha et al., 2015; Blackburn et al., 2016; Rojas-Sandoval et al., 2020).
Caribbean islands have a long history of humans intentionally or accidentally introducing non-native species, and a subset of these have become invasive (e.g., Kairo et al., 2003; Reynolds and Niemiller, 2010; Wilson et al., 2011; Borroto-Páez and Mancina, 2017; Rojas-Sandoval et al., 2017; Shiels et al., 2020). Islands within this region have been exposed to pronounced anthropogenic disturbance, which has included ecosystem degradation and natural resource overexploitation, particularly since the beginning of the European colonization (Maunder et al., 2008; Acevedo-Rodríguez and Strong, 2008). These disturbances often facilitated the introduction and establishment of non-native and invasive species (Lugo, 2004; Rojas-Sandoval and Acevedo-Rodríguez, 2015; Rojas-Sandoval et al., 2017, 2020). On the other hand, biodiversity on Caribbean islands is so remarkable that it is considered one of the most important hotspots of diversity in the world (Mittermeier et al., 2004). Elton’s biotic resistance hypothesis proposes that areas of high diversity should be resistant to biological invasions (Elton, 1958). While this may be true at small scales, it is difficult to find evidence for it in larger regional scales (Stohlgren et al., 2003, 2006; Ackerman et al., 2017). Ackerman et al. (2017) evaluated Elton’s theory at different scales using a dataset of plant species in Puerto Rico. They found that there was a strong, positive correlation between invasive and native species richness at large scales (municipality scale), yet the relationship between native and invasive species was weak at local forest scales (forest reserves), suggesting some biotic resistance is present at local forest reserves in Puerto Rico.
The island of Puerto Rico is the smallest of the Greater Antilles, and at the whole-island scale, these dual processes of biotic resistance vs. direct or indirect anthropogenic facilitation of species introductions are present. Occupied first by the indigenous peoples beginning 4,000 years BP, then colonized by the Spanish and eventually annexed by the United States, 95% of the island of Puerto Rico was converted to agriculture, mining, and urban areas (Birdsey and Weaver, 1987). Human activities were absent at the summits of the Luquillo Mountains, and these mountains have remained as the largest forested area on the island (Figure 1). Located in the Luquillo Mountains, El Yunque National Forest (EYNF) is currently the largest and the most well-conserved forest area in Puerto Rico. The lower flanks are a mosaic of former human land use, mostly farming and forestry, where forest has recovered naturally or has been partly restored by humans. The summits and higher elevation areas are relatively well-conserved remnants of native forest, away from roads that extend to two of the three dominant peaks.
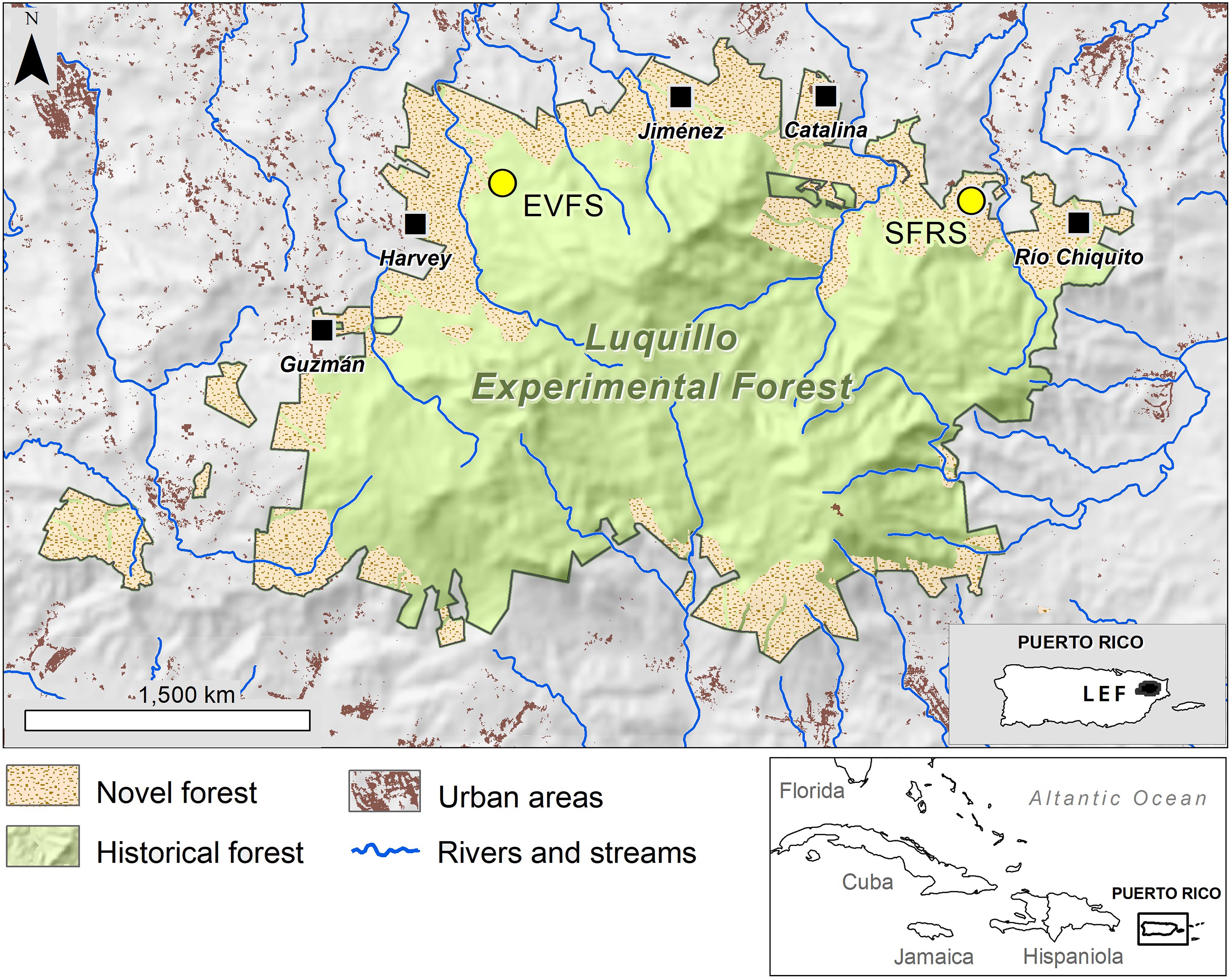
Figure 1. Map of the Luquillo Experimental Forest (El Yunque National Forest) showing the distribution of historical forest (well-conserved forest with relatively little history of human disturbance) and novel forests (secondary forest and former plantations). Major study areas include El Verde Field Station (EVFS) and Sabana Field Research Station (SFRS); filled squares mark locations of plantations.
In this study, we asked the following question: What is the current status of introduced species within El Yunque National Forest (EYNF), the largest and most well-conserved forest area of Puerto Rico? We intended to answer this question by first reviewing the literature on non-native species of vascular plants, invertebrates, and vertebrates that have been deliberately and/or accidentally introduced in EYNF, and then asking local experts to review the species list and identify and provide justification for those species that they considered to be currently behaving as invasive in EYNF. Based on previous studies showing that areas with higher anthropogenic disturbance often provide more opportunities for the introduction and establishment of non-native and invasive species (Lockwood et al., 2009; Blackburn et al., 2016; Dawson et al., 2017; Rojas-Sandoval et al., 2020), we hypothesized that areas within the EYNF with long-standing continuous native forest would have fewer non-native and invasive species than areas subject to high levels of anthropogenic disturbance such as those in the lowlands and areas near roads and other historical land-use sites.
For the purposes of this review, we used the term “introduced” to signify non-native species brought to Puerto Rico intentionally or accidentally via human activities and have already established wild populations. We reserved “invasive” for non-native species that have been shown to spread rapidly and widely and are suspected or shown to have negative ecological or economic effects in the EYNF (Blackburn et al., 2011). Data presented here are based on published literature, museum and herbarium collections, first-hand experience from local and academic experts, and personal field observations. Using all this information, we first compiled a list of all the introduced species occurring within the EYNF including vertebrates, invertebrates, and vascular plants. Then, we identified invasive and potentially invasive species based on the abundance and the risk of causing negative impact on native communities within the EYNF. We found that some groups are better studied (e.g., vascular plants, earthworms, and vertebrates) than others (e.g., arthropods) and few species have been thoroughly evaluated for their invasiveness (i.e., validated ecological or economic harm). Thus, our conclusions are preliminary in part and, where information is lacking, serve as a guide for future research.
In the EYNF, we identified 168 non-native plant species from 135 genera and 57 families indicating a very heterogeneous group in terms of their taxonomy, life forms, and ecological attributes (Supplementary Table S1). About 46% (77 species from the 168 non-native species) are species previously listed as invasive in Puerto Rico (O’Connor et al., 2000; Kairo et al., 2003; Brown et al., 2006; Cohen and Ackerman, 2009; Más and Lugo-Torres, 2013; Ackerman et al., 2014; Rojas-Sandoval and Acevedo-Rodríguez, 2015; Burman et al., 2017). When considering their invasive status within the EYNF, we identified a total of 37 species (Table 1). Most invaders in the EYNF are vines (14 species), herbs (9 species), and grasses (8 species) that are colonizing primarily disturbed areas (e.g., landslides), wastelands, river edges, and roadsides (Figure 2 and Table 1). Only three species of non-native trees (Schefflera actinophylla, Spathodea campanulate, and Syzygium jambos) are regarded as invasive within the EYNF. There are just two plant species regarded as invasive in mature forest, and these included the tree S. actinophylla and the orchid Oeceoclades maculata (Figure 2 and Table 1). We also found that many of the documented invaders in EYNF are species that were originally intentionally introduced into Puerto Rico as ornamentals (Valdés Pizzini et al., 2011; Areces-Berazain and Rojas-Sandoval, 2017) that have escaped cultivation (e.g., Calathea lutea, Hedychium coronarium, and Spathoglottis plicata). One example of this pathway of introduction is Selaginella willdenowii, a popular ornamental spikemoss that escaped cultivation and has become one of the most aggressive plant invaders in EYNF (EYNF, 2008), and it can be found forming dense patches along forest margins near El Verde Field Station and elsewhere (Figure 3).
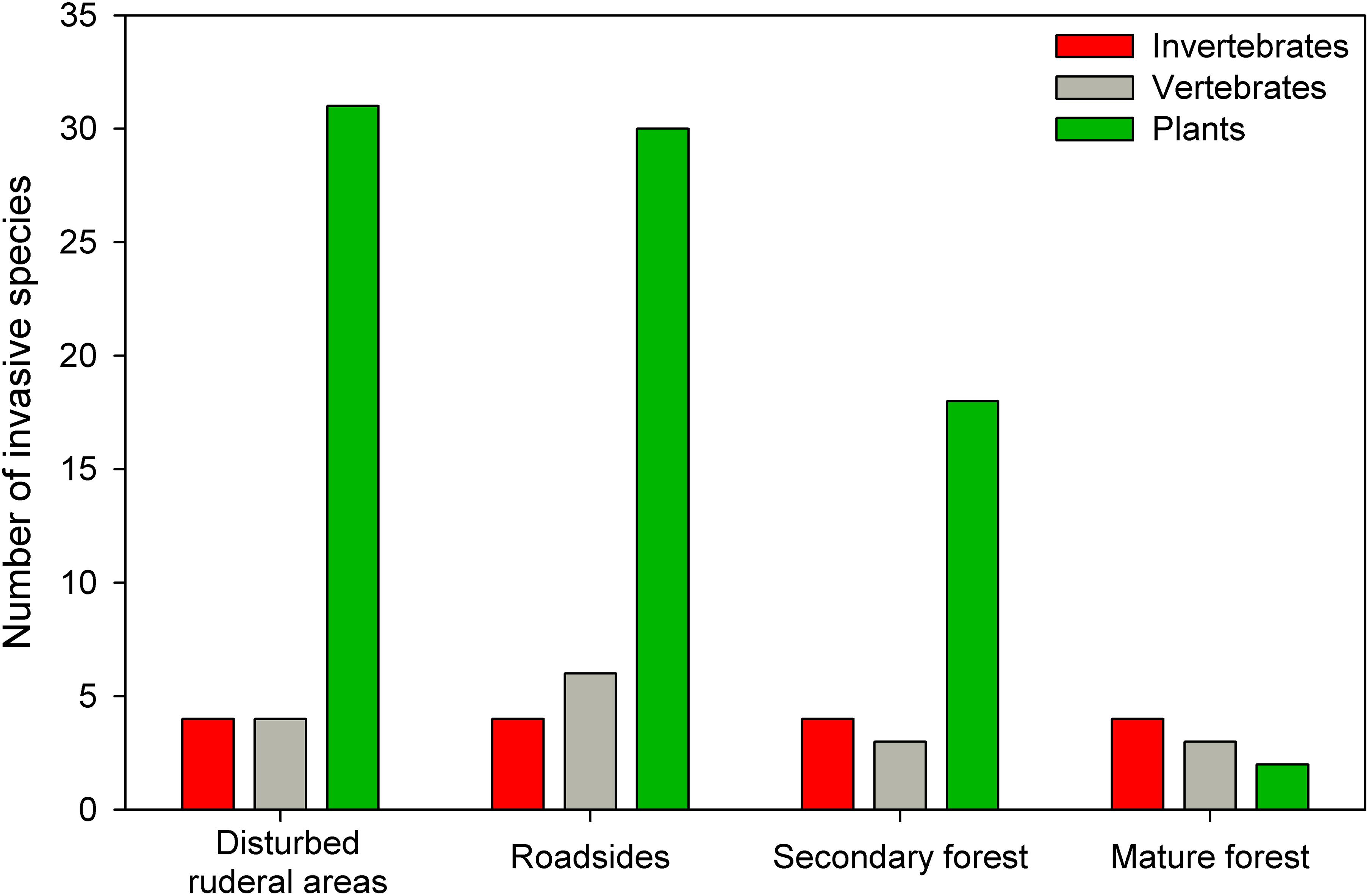
Figure 2. Number of invasive invertebrate (n = 4), vertebrate (n = 6), and plant (n = 37) species and the different habitat types that they are invading within El Yunque.

Figure 3. Selaginella willdenowii: one of the dominant invasive plant species that is rapidly spreading across El Yunque National Forest. This picture corresponds to a heavily invaded area along the PR-Highway 186. Photo: J. Zimmerman.
Our survey also revealed a prevalence of invasive vines (14 species) in the EYNF. Seven of the 14 species are morning-glory (Ipomoea spp.) that quickly respond to disturbances and have become a serious problem especially after hurricanes (Table 1). They display rapid growth and an ability to outcompete and smother native vegetation (J.D. Ackerman, personal communication). Moreover, vines such as Epipremnum pinnatum, Pueraria phaseoloides, Dioscorea alata, Thunbergia alata, and Thunbergia fragrans are also locally abundant across the EYNF and often can be found along roadsides colonizing forest margins.
In the case of grasses, three species were identified as the dominant invaders in the EYNF: Cenchrus purpureus, Megathyrsus maximus, and Paspalum fasciculatum. These three invaders are robust perennial C4 grasses that grow rapidly, colonizing new areas and forming dense monospecific stands that displace native plants and wildlife. Across the EYNF, these grasses are locally abundant in disturbed areas and along roadsides mainly at low and middle elevation, but C. purpureus has also been reported invading disturbed areas at upper elevations (Olander et al., 1998).
Comprehensive studies of the distribution of native vs. non-native plants for the EYNF are missing. Rather, perspectives come from individual studies that focus on research areas or conspicuous species. For example, Thompson et al. (2007) found that 12 agricultural, mostly woody species (e.g., coffee, mango, and breadfruit) occurred within mature forest but exclusively in the northern two-thirds of the 16-ha Luquillo Forest Dynamics Plot. This area was traced to a history of logging and agriculture dating to the 1920s (Thompson et al., 2002). The remaining one-third of the plot located in the southern end had species representative of the native forest and no non-native species. Thus, the distribution of native and non-native plant species appears to be demarked by the “anthropogenic ecotone.” None of these 12 introduced species were regarded as invasive by Thompson et al. (2007) since none of them were increasing strongly in abundance during the course of the 10-year study. One exception could be Simarouba amara, a tree species planted for timber across the Luquillo mountains, which has increased in numbers in areas of less intense land use since hurricane Georges passed through in 1998 (Thompson et al., 2007) and extensively since 2017 after hurricane Maria (Zimmerman, personal observation).
Brown et al. (2006) studied the ecology of S. jambos in the Luquillo Mountains with the goal of identifying whether this introduced ornamental was an invasive species. They concluded that where it occurs in areas of secondary forest within 30 m of stream beds, it is invasive and appears to outcompete other species due to its extreme shade tolerance. In their words: “After nearly 185 years since its introduction to the island, S. jambos is well-established” and “its presence does not appear limited,” suggesting “a new vegetation assemblage in the regenerating secondary forests in the Luquillo Mountains.” This case deserves further study as S. jambos is now the subject of an introduced pest, the guava rust, which appears to be negatively affecting its naturalized populations in Puerto Rico (Burman et al., 2017).
Another plant group that has been studied within the EYNF are bamboos. The planting of bamboo in this area started in the 1930s and has led to present-day bamboo monocultures in many riparian and roadside areas. Bamboos were originally introduced to stabilize recently constructed roadbeds (O’Connor et al., 2000; Blundell et al., 2003) and now there are about five species recognized as invasive in the EYNF (four species of Bambusa, and Dendrocalamus strictus; Table 1). Invasive bamboos are spreading along rivers and colonizing areas along roadsides, and they currently occupy about 2% of the forest area (O’Connor et al., 2000).
In the EYNF, our survey for introduced arthropods was not as exhaustive as for plants, partly because of the bias of fewer studies and infrequent reporting of non-native arthropods. However, four species are regarded as invasive in this area: the Africanized honeybee (Apis mellifera of European origin hybridized with A. mellifera scutella of African origin; Rivera-Marchand et al., 2008), the mosquito, Aedes aegypti, and two species of introduced earthworms, Pontoscolex corethrurus and Ocnerodrilus occidentalis (González et al., 2007). We also found that these four invasive species are occurring ubiquitously across all habitat types in EYNF: from well-conserved mature forests to highly disturbed ruderal areas (Figure 2 and Table 2).
In Puerto Rico, Africanized honeybee hybrids were first reported in 1994 (Cox, 1994), which was also the first report of these bee hybrids on an oceanic island. In the EYNF, Africanized honeybee hybrids have occupied 80% of tree cavities and threatened the endangered Puerto Rican Parrot (Amazona vittata) and other cavity nesting bird species (Blundell et al., 2003). Although it was several years ago, attacks to field researchers have been reported (Zimmerman, personal observation). As documented elsewhere on the island, the Africanized honeybees eventually evolved gentle behavioral characteristics and have ceased to be a great threat. The one persistent introduced insect in EYNF is the mosquito, A. aegypti (Weinbren and Weinbren, 1970). This mosquito, which originated in Africa and can be easily recognized by its white markings on the legs, is known as a vector of dengue fever, chikungunya, and Zika fever, and other human-disease agents. It is likely that A. aegypti is not reproducing in the elevations encompassed by EYNF, but instead the adult mosquitos observed within the EYNF could be transported by cars from San Juan and other low-elevation urban areas (Yee, pers. comm.).
González et al. (2007) studied the earthworm communities along the elevational gradient in the Luquillo Mountains, identifying eight different forest types from elfin forest at the summits to mangroves at the coast. Along this gradient, they found three introduced species of earthworms, P. corethrurus, O. occidentalis, and Drawida barwelli, and all of them are regarded as invasive species; however, D. barwelli was only found in lower-elevation forest areas located outside EYNF. P. corethrurus, a species introduced from Europe, was found in four forest types (i.e., elfin, palm, Colorado, and tabonuco) in the EYNF, and in one low-elevation forest type outside the EYNF. O. occidentalis, which is native to Central and South America (Shen et al., 2015), was found in all but one forest type (Colorado forest) in EYNF and in two additional lower-elevation forest types outside EYNF. These earthworm species are considered invasive because they alter biogeochemical cycling in the soil, which may further influence plant community dynamics (González et al., 2007). Additional invertebrates that may be of concern in the future, especially due to their ephemeral expansion after hurricanes, are non-native slugs and snails (e.g., Allopeas gracile; Bloch, unpubl. data). However, such invaders do not appear to be spreading rapidly or causing plant damage like they do on other islands such as Hawaii (e.g., Shiels et al., 2014).
Our survey for introduced vertebrates identified a total of six species in the EYNF (Table 2), and all of them are regarded as invasive despite only some species occurring across all habitat types and others are confined to disturbed areas along roadsides and ruderal areas (Figure 2 and Table 2). Out of the six, there are three dominant invasive mammals: black rats (Rattus rattus), mongoose (Herpestes auropunctatus), and feral cats (Felis catus). These three mammal species are widespread through disturbed and undisturbed mature forest (Engeman et al., 2006; Shiels et al., 2018), and each represents a threat to many native species, including the endangered Puerto Rican Parrot (A. vittata) (Engeman et al., 2006). In fact, some lethal trapping is regularly performed during the parrot nesting season to reduce local populations of these invasive mammals to mitigate their impact on the population of the endangered parrot (Engeman et al., 2006). Whereas rats and cats are acclaimed climbers, mongooses are not, but apparently, they consume birds that fall from the nest upon first flights (Engeman et al., 2006). Beyond parrots, diet studies of these three invasive mammals reveal their omnivorous behavior, as many species of native birds, reptiles, and invertebrates are susceptible to their consumption. Furthermore, a recent study of the black rat diet demonstrated that several native tree species (e.g., Guarea guidonia, Buchenavia capitata, and Tetragastris balsamifera) are at risk from seed removal and predation by rats in the EYNF (Shiels et al., 2019).
Another very common invasive species in EYNF is the house mouse (Mus musculus), which is a frequent invasive rodent of island ecosystems and it often coexists with invasive rats. While mice are typically less problematic for insular biodiversity than rats, their diet suggests that some native herbs and invertebrates are frequently consumed (Shiels et al., 2018). During a survey of small mammals along the elevation gradient of the PR-Highway 191 through EYNF, Shiels et al. (2018) found the presence of invasive back rats (R. rattus) at all elevations and habitat types, whereas house mice were also present at most elevations but were restricted to roadside habitats. Therefore, based on distribution and the range of dietary impact, it has been suggested that the black rat is a more damaging rodent in EYNF than the house mouse.
Two species of invasive herpetofauna have been reported in EYNF (Table 2), particularly near roadsides, and these include the cane toad (Rhinella marina) and green iguana (Iguana iguana). Whereas green iguanas are a relatively recent addition to the invasive fauna of EYNF (Lugo, 2005) and have been rarely observed in EYNF, cane toads have been established much longer and they are frequently seen nocturnally after heavy rains (Stewart, 1995).
For birds, Wunderle and Arendt (2011) provide a list of 99 bird species found in EYNF, including 23 species that breed there. Twelve breeding species are endemic. No introduced bird species are found in the EYNF, even in areas with a history of anthropogenic disturbance. Naturalized species of finches and psittacines are otherwise common on the forest’s periphery, mostly in anthropogenic-disturbed habitats (Vázquez Plass and Wunderle, 2013). Similarly, no introduced species of fish are found in streams of the EYNF except anecdotal findings of aquarium species in recreation areas. These non-native species apparently do not survive frequent flood events (Ramirez, pers. comm.). Non-native fish species are found in urban streams in areas nearby the EYNF and increases in their abundances are facilitated by drought events (Ramírez et al., 2018). Domestic dogs (Canis familiaris) are occasionally abandoned along lowland roads of EYNF, but they rarely establish and are not spreading to our knowledge.
As the theory of island biogeography predicts, the island of Puerto Rico is relatively poor in species richness compared to mainland tropical areas, with concomitantly reduced biological complexity. For example, among comparable-sized areas (16–25 ha), in a forest dynamics plot (FDP) in Puerto Rico (in wet forest), there are as many as 150 freestanding woody species, while one plot in central Panama has ∼300 species and one in Ecuador has > 1,000 species (Condit et al., 2005; Ostertag et al., 2014). Similarly, the food web at El Verde, a field station located within the EYNF, shows a complete absence of large herbivores and predators, low faunal richness compared to the tropical mainland, and an abundance of frogs and lizards (Reagan and Waide, 1996).
Nevertheless, Puerto Rico is much more diverse than other more isolated islands from other regions. For example, the 4-ha FDP in Palamanui, Hawaii has only 15 tree species (Ostertag et al., 2014). Does this roughly 10-fold difference in plant species richness between EYNF and Palamanui impart any resistance to species introductions for EYNF? A widely recognized pattern in island biogeography is the species–isolation relationship (SIR), in which a decrease in the number of native species on oceanic islands will occur with increasing island isolation, linked to lower rates of natural dispersion and colonization on the remotest islands (Wilson and MacArthur, 1967). While the negative SIR has been well-documented for native species, the response of non-native species to geographic isolation is less clear and remains an open question. In this regard, Moser et al. (2018) tested the SIR for a large dataset of native and non-native species on islands worldwide and they found that the number of introduced species increased with island isolation for all taxa studied except for birds, which is a pattern that is opposite to the widely recognized negative SIR for native species. Moser et al. (2018) argue that this pattern is due to reduced native diversity and greater ecological naiveté of native biota on more remote islands. Furthermore, their analyses removed the influence of factors such as island size, climatic and topographic heterogeneity, and socioeconomic development [using per capita gross domestic product (GDP) as a proxy]. Thus, the expectation is that, all else being equal, the close biogeographic history of Puerto Rico and nearby mainland areas should provide a relative degree of biotic resistance to invasions of new species into the island’s biotic communities.
Among the important elements set aside by Moser et al. (2018) was anthropogenic disturbance, which has factored considerably in Puerto Rico, and the Caribbean in general (Kueffer et al., 2010; Rojas-Sandoval et al., 2017, 2020). Puerto Rico currently has one of the highest GDPs in the Caribbean and a high population density (Kueffer et al., 2010; Rojas-Sandoval et al., 2017) relative to many other islands, and its original forest cover was reduced to ∼5% (Birdsey and Weaver, 1987), all indicating a high degree of human disturbance. All of this has led to a high rate of introduction and naturalization of non-native and invasive species. For example, Rojas-Sandoval and Acevedo-Rodríguez (2015) found that about 32% of the total flora in Puerto Rico is introduced, a percentage relatively high when compared to other islands in the Greater Antilles such as Cuba (12%), Dominican Republic (18%), and Jamaica (21%). For Puerto Rico and other Caribbean islands, previous studies have shown that successful establishment of non-native plant species is more likely to occur in human-modified habitats than in pristine habitats. On these islands, disturbance and human-related activities seem to be major drivers influencing and facilitating the introduction and establishment of non-native plant species (Kueffer et al., 2010; Rojas-Sandoval and Acevedo-Rodríguez, 2015; Ackerman et al., 2017; Rojas-Sandoval et al., 2017, 2020).
To understand the diversity and richness of invasive species in EYNF, it is necessary to take into account the socioeconomic history of human-modified areas where farmland and logging occurred until the 1930s (e.g., Thompson et al., 2002). Our initial hypothesis was that undisturbed natural areas within EYNF would harbor fewer non-native species and be more resistant to invasion than disturbed areas away from protected areas. The information we garnered in our literature reviews and surveys of experts lends only partial support of this hypothesis. In general, vascular plants seem to hold to the pattern quite well, and a large number of the non-native plant species were more common in disturbed areas with a history of agriculture or other human land use. We found no evidence from managers suggesting that those species are rapidly spreading into better-conserved forest areas (Thompson et al., 2007; EYNF, 2008). On the other hand, the non-native vertebrates and invertebrates currently invading EYNF were not restricted in their distributions, for the most part. For example, introduced earthworms were found in both disturbed and undisturbed forests and at elevations covering all forest types described for EYNF. Similarly, invasive mammals such as black rats, cats, and mongoose roam freely throughout EYNF, and at least, the rats do not appear to be more active or abundant in disturbed habitats such as roads, landslides, riparian areas, or treefall and hurricane gaps relative to the interior mature forest (Shiels et al., 2018, 2019). Birds and fish were exceptions, with introduced species not found even in areas with long histories of human disturbance. One outcome that is clear from our review is the lack of detailed information on the distribution and abundance of non-native species within EYNF, as well as the concomitant lack of information on ecological or economic impacts of non-native species and their classification as invasive species. One of the caveats of this work is that the information that we were able to obtain was for the most part too coarse in both space and time to fully evaluate our hypothesis.
Few species have been fully studied in EYNF for their invasiveness as we have defined it. Six plant species, S. jambos and five bamboos (four Bambusa spp. and D. strictus), appear to ecologically dominate riparian areas where they were initially planted and are slowly spreading across the area (O’Connor et al., 2000; Brown et al., 2006). Additional plant species such as S. wildenowii, Ipomoea vines, and the grasses C. purpureus, M. maximus, and P. fasciculatum are behaving as invaders and forming dense patches that outcompete and smother native vegetation, and thus, they have been on the radar of managers (EYNF, 2008). Introduced earthworms are altering soil processes (González et al., 2007) and could potentially influence other ecological processes. Africanized honeybees and A. aegypti mosquitos threaten human visitors to EYNF. Introduced black rats are a nuisance and may cause significant harm to native vegetation (Shiels et al., 2019) and birds, while mongooses, rats, and feral cats may prey on critically endangered wildlife (Engeman et al., 2006). In sum, we find some worrisome trends in the effects of invasive species but no strong evidence that the EYNF ecosystem as a whole is challenged by these invaders such that a novel ecosystem will take over.
Our combined results showed that many non-native and invasive species in the EYNF are yet confined to the lowlands and to areas with high levels of anthropogenic disturbance. However, accurate data on their distribution, abundance, and impacts are very limited. Therefore, systematic surveys and detailed studies monitoring non-native and invasive species are needed to draw conclusions on the invasive potential and social, economic, and/or ecological impacts caused by non-native invasive species within this protected area. Considering that EYNF is one of the few (and the most important) remnants of original native forest in Puerto Rico, the potential of significant impacts by invasive species on its unique native biodiversity is high. The control and management of the current and potentially invasive species within EYNF should remain a high priority.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
JZ initiated the study, provided guidance, wrote much of an initial draft, and finalized the text. JR-S analyzed plant distribution data and contributed to synthesis and writing. AS synthesized information on animals and contributed to the writing. All authors contributed to the article and approved the submitted version.
Support provided by the National Science Foundation (DEB-1831952) to the Luquillo Long-term Ecological Research Program, the University of Puerto Rico, and the International Institute of Tropical Forestry, USDA Forest Service.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the organizers of the conference “Puerto Rico Honey Bee and Evolution of Invasive Species on Islands” for their efforts. We thank J. D. Ackerman, M. Caraballo-Ortiz, and A. Lugo for contributing their expert knowledge and unpublished data on the presence, abundance, and impact of invasive plant species in EYNF. Financial support was provided by National Science Foundation grant DEB-1831952 to the University of Puerto Rico. Additional support was provided by the International Institute of Tropical Forestry, USDA Forest Service, USDA National Wildlife Research Center, and the University of Puerto Rico.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.640121/full#supplementary-material
Acevedo-Rodríguez, P. (2005). Vines and climbing plants of Puerto Rico and the Virgin Islands. Contrib. U S Natl. Herb. 51:483.
Acevedo-Rodríguez, P., and Strong, M. T. (2008). Floristic richness and affinities in the West Indies. Bot. Rev. 74, 5–36. doi: 10.1007/s12229-008-9000-9001
Ackerman, J. D., Falcón, W., Molinari, J., Vega, C., Espino, I., and Cuevas, A. A. (2014). Biotic resistance and invasional meltdown: consequences of acquired interspecific interactions for an invasive orchid, Spathoglottis plicata in Puerto Rico. Biol. Invasions 16, 2435–2447. doi: 10.1007/s10530-014-0676-3
Ackerman, J. D., Tremblay, R. L., Rojas-Sandoval, J., and Hernández-Figueroa, E. (2017). Biotic resistance in the tropics: patterns of seed plant invasions within an island. Biol. Invasions 19, 315–328. doi: 10.1007/s10530-016-1281-4
Areces-Berazain, F., and Rojas-Sandoval, J. (2017). “Coix lacryma-jobi” in Invasive Species Compendium. Wallingford, UK: CAB International. Available online at: www.cabi.org/isc.
Areces-Berazain, F., Vega López, V. J., and Ackerman, J. D. (2014). “Annotated list of the vascular plants at el verde field station, el yunque national forest,” Puerto Rico. Caribbean Naturalist, 1–51.
Birdsey, R. A., and Weaver, P. L. (1987). Forest Area Trends in Puerto Rico. Res. Note SO-331. New Orleans, LA: US Department of Agriculture, Forest Service, Southern Forest Experiment Station.
Blackburn, T. M., Delean, S., Pyšek, P., and Cassey, P. (2016). On the island biogeography of aliens: a global analysis of the richness of plant and bird species on oceanic islands. Global Ecol. Biogeography 25, 859–868. doi: 10.1111/geb.12339
Blackburn, T. M., Pyšek, P., Bacher, S., Carlton, J. T., Duncan, R. P., Jarošík, V., et al. (2011). A proposed unified framework for biological invasions. Trends Ecol. Evol. 26, 333–339. doi: 10.1016/j.tree.2011.03.023
Blundell, A. G., Scatena, F. N., Wentsel, R., and Sommers, W. (2003). Ecorisk assessment using indicators of sustainability: invasive species in the Caribbean National Forest of Puerto Rico. J. Forestry 101, 14–19.
Borroto-Páez, R., and Mancina, C. A. (2017). Biodiversity and conservation of Cuban mammals: past, present, and invasive species. J. Mammal. 98, 964–985. doi: 10.1093/jmammal/gyx017
Brown, K. A., Scatena, F. N., and Gurevitch, J. (2006). Effects of an invasive tree on community structure and diversity in a tropical forest in Puerto Rico. Forest Ecol. Management 226, 145–152. doi: 10.1016/j.foreco.2006.01.031
Burman, E., Ackerman, J. D., and Tremblay, R. L. (2017). Invasive Syzygium jambos trees in Puerto Rico: no refuge from guava rust. J. Trop. Ecol. 33:205. doi: 10.1017/s026646741700013x
Capinha, C., Essl, F., Seebens, H., Moser, D., and Pereira, H. M. (2015). The dispersal of alien species redefines biogeography in the Anthropocene. Science 348, 1248–1251. doi: 10.1126/science.aaa8913
Cohen, I. M., and Ackerman, J. D. (2009). Oeceoclades maculata, an alien tropical orchid in a Caribbean rainforest. Ann. Bot. 104, 557–563. doi: 10.1093/aob/mcn191
Condit, R. S., Ashton, M. S., Balslev, H., Brokaw, N. V., Bunyavejchewin, S., Chuyong, G. B., et al. (2005). Tropical tree a-diversity: results from a worldwide network of large plots. Biologiske Skrifter 55, 565–582.
Dawson, W., Moser, D., Van Kleunen, M., Kreft, H., Pergl, J., Pyšek, P., et al. (2017). Global hotspots and correlates of alien species richness across taxonomic groups. Nat. Ecol. Evol. 1:186.
Engeman, R., Whisson, D., Quinn, J., Cano, F., Quiñones, P., and White, T. H. Jr. (2006). Monitoring invasive mammalian predator populations sharing habitat with critically endangered Puerto Rican parrot Amazona vittata. Oryx 40, 95–102. doi: 10.1017/s0030605305001286
EYNF (2008). El Yunque National Forest Fiscal Year 2008 Monitoring and Evaluation Report. https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5304902.pdf (accessed May 6, 2020)
Gallardo, B., Zieritz, A., and Aldridge, D. C. (2015). The importance of the human footprint in shaping the global distribution of terrestrial, freshwater and marine invaders. PLoS One 10:e0125801. doi: 10.1371/journal.pone.0125801
González, G., García, E., Cruz, V., Borges, S., Zalamea, M., and Rivera, M. M. (2007). Earthworm communities along an elevational gradient in northeastern Puerto Rico. Eur. J. Soil Biol. 43, S24–S32.
Kairo, M., Ali, B., Cheesman, O., Haysom, K., and Murphy, S. (2003). Invasive Species Threats in the Caribbean Region. Arlington, TX: Report to the Nature Conservancy.
Kueffer, C., Daehler, C. C., Torres-Santana, C. W., Lavergne, C., Meyer, J. Y., Otto, R., et al. (2010). A global comparison of plant invasions on oceanic islands. Perspect. Plant Ecol. Evol. Syst. 12, 145–161. doi: 10.1016/j.ppees.2009.06.002
Lockwood, J. L., Cassey, P., and Blackburn, T. M. (2009). The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Divers. Distrib. 15, 904–910. doi: 10.1111/j.1472-4642.2009.00594.x
Lugo, A. E. (2004). The outcome of alien tree invasions in Puerto Rico. Front. Ecol. Environ. 5:265–273. doi: 10.2307/3868267
Más, E. G., and Lugo-Torres, M. D. L. (2013). Common Weeds in Puerto Rico and US Virgin Islands. Puerto Rico: University of Puerto Rico and the USDA Natural Resources Conservation Service.
Maunder, M., Leiva, A., and Santiago-Valentin, E. (2008). Plant conservation in the Caribbean island biodiversity hotspot. Bot. Rev. 74, 197–207. doi: 10.1007/s12229-008-9007-7
Mittermeier, R. A., Gil, R. R., and Hoffman, M. (2004). Hotspots Revisited: Earth’s Biologically Richest and Most Threatened Terrestrial Ecoregions. Mexico, DF: CEMEX.
Moser, D., Lenzner, B., Weigelt, P., Dawson, W., Kreft, H., Pergl, J., et al. (2018). Remoteness promotes biological invasions on islands worldwide. Proc. Natl. Acad. Sci. U S A. 115, 9270–9275. doi: 10.1073/pnas.1804179115
O’Connor, P. J., Covich, A. P., Scatena, F. N., and Loope, L. L. (2000). Non-indigenous bamboo along headwater streams of the Luquillo Mountains, Puerto Rico: leaf fall, aquatic leaf decay and patterns of invasion. J. Trop. Ecol. 16, 499–516. doi: 10.1017/s0266467400001541
Olander, L. P., Scatena, F. N., and Silver, W. L. (1998). Impacts of disturbance initiated by road construction in a subtropical cloud forest in the Luquillo Experimental Forest, Puerto Rico. Forest Ecol. Management 109, 33–49. doi: 10.1016/s0378-1127(98)00261-8
Ostertag, R., Inman-Narahari, F., Cordell, S., Giardina, C. P., and Sack, L. (2014). Forest structure in low-diversity tropical forests. PLoS One 9:e103268. doi: 10.1371/journal.pone.0103268
Ramírez, A., Gutiérrez-Fonseca, P. E., Kelly, S. P., Engman, A. C., Wagner, K., Rosas, K. G., et al. (2018). Drought facilitates species invasions in an urban stream: results from a long-term study of tropical island fish assemblage structure. Front. Ecol. Evol. 6:115. doi: 10.3389/fevo.2018.00115
Reagan, D. P., and Waide, R. B. (eds) (1996). The Food Web of a Tropical Rain Forest. Chicago, IL: University of Chicago Press.
Reynolds, R. G., and Niemiller, M. L. (2010). Island invaders: introduced amphibians and reptiles in the Turks and Caicos Islands. Reptiles Amphibians 17, 117–121.
Rivera-Marchand, B., Giray, T., and Guzmán-Novoa, E. (2008). The cost of defense in social insects: insights from the honeybee. Entomol. Experiment. Appl. 129, 1–10. doi: 10.1111/j.1570-7458.2008.00747.x
Rojas-Sandoval, J., and Acevedo-Rodríguez, P. (2015). Naturalization and invasion of alien plants in Puerto Rico and the Virgin Islands. Biol. Invasions 17, 149–163. doi: 10.1007/s10530-014-0712-3
Rojas-Sandoval, J., Ackerman, J. D., and Tremblay, R. L. (2020). Island biogeography of native and alien plant species: contrasting drivers of diversity across the Lesser Antilles. Diversity Distrib. 26, 1539–1550. doi: 10.1111/ddi.13139
Rojas-Sandoval, J., Tremblay, R. L., Acevedo-Rodríguez, P., and Díaz-Soltero, H. (2017). Invasive plant species in the West Indies: geographical, ecological, and floristic insights. Ecol. Evol. 7, 4522–4533. doi: 10.1002/ece3.2984
Roncal, J., Nieto-Blázquez, M. E., Cardona, A., and Bacon, C. D. (2020). “Historical Biogeography of Caribbean Plants Revises Regional Paleogeography,” in Neotropical Diversification: Patterns and Processes, (Berlin: Springer), 521–546. doi: 10.1007/978-3-030-31167-4_20
Sharpe, J. M., and Shiels, A. B. (2014). Understory fern community structure, growth and spore production responses to a large-scale hurricane experiment in a Puerto Rico rainforest. Forest Ecol. Manag. 332, 75–86. doi: 10.1016/j.foreco.2014.01.023
Shen, H. P., Chang, C. H., and Chih, W. J. (2015). Earthworms from Matsu, Taiwan with descriptions of new species of the genera Amynthas (Oligochaeta: Megascolecidae) and Drawida (Oligochaeta: Moniligastridae). Zootaxa 3973:425. doi: 10.11646/zootaxa.3973.3.2
Shiels, A. B., Ennis, M. K., and Shiels, L. (2014). Trait-based plant mortality and preference for native vs. non-native seedlings by invasive slug and snail herbivores in Hawaii. Biol. Invasions 16, 1929–1940. doi: 10.1007/s10530-013-0636-3
Shiels, A. B., Lombard, C. D., Shiels, L., and Hillis-Starr, Z. (2020). Invasive rat establishment and changes in small mammal populations on Caribbean islands following two hurricanes. Global Ecol. Conserv. 22:e00986. doi: 10.1016/j.gecco.2020.e00986
Shiels, A. B., and Ramírez de Arellano, G. E. (2018). Invasive rats (Rattus sp.), but not always mice (Mus musculus), are ubiquitous at all elevations and habitats within the Caribbean National Forest, Puerto Rico. Caribbean Nat. 48, 1–14.
Shiels, A. B., and Ramírez de Arellano, G. E. (2019). Habitat use and seed removal by invasive rats (Rattus rattus) in disturbed and undisturbed rainforest, Puerto Rico. Biotropica 51, 378–386. doi: 10.1111/btp.12640
Stewart, M. M. (1995). Climate driven population fluctuations in rain forest frogs. J. Herpetol. 29, 437–446. doi: 10.2307/1564995
Stohlgren, T. J., Barnett, D. T., and Kartesz, J. T. (2003). The rich get richer: patterns of plant invasions in the United States. Front. Ecol. Environ. 1:11–14. doi: 10.2307/3867959
Stohlgren, T. J., Jarnevich, C., Chong, G. W., and Evangelista, P. H. (2006). Scale and plant invasions: a theory of biotic acceptance. Preslia 78, 405–426.
Thompson, J., Brokaw, N., Zimmerman, J. K., Waide, R. B., Everham, E. M. I. I. I., Jean Lodge, D., et al. (2002). Land use history, environment, and tree composition in a tropical forest. Ecol. Appl. 12, 1344–1363. doi: 10.1890/1051-0761(2002)012[1344:luheat]2.0.co;2
Thompson, J., Lugo, A. E., and Thomlinson, J. (2007). Land use history, hurricane disturbance, and the fate of introduced species in a subtropical wet forest in Puerto Rico. Plant Ecol. 192, 289–301. doi: 10.1007/s11258-007-9318-5
Torres, J. A. (1992). Lepidoptera outbreaks in response to successional changes after the passage of hurricane hugo in puerto rico. J. Trop. Ecol. 8, 285–298.
Valdés Pizzini, M., González Cruz, M., and Martínez Reyes, J. E. (2011). La Transformación del Paisaje Puertorriqueño y la Disciplina del Cuerpo Civil de Conservación, 1933–1942. San Juan: CIS-Universidad de Puerto Rico.
Vázquez Plass, E. O., and Wunderle, J. M. Jr. (2013). Avian distribution along a gradient of urbanization in northeastern Puerto Rico. Ecol. Bull. 54, 141–156.
Weinbren, M. P., and Weinbren, B. M. (1970). “Observations on the mosquito population in the irradiated forest at El Verde,” in A Tropical Rain Forest: a Study of Irradiation and Ecology at El Verde, Puerto Rico, eds H. T. Odum and R. F. Pigeon (Springfield, VA: U.S. Atomic Energy Commission Division of Technical Information, National Technical Information Service), E159—-E167.
Wilson, B. S., Koenig, S. E., van Veen, R., Miersma, E., and Rudolph, D. C. (2011). Cane toads a threat to West Indian wildlife: mortality of Jamaican boas attributable to toad ingestion. Biol. Invasions 13, 55–60. doi: 10.1007/s10530-010-9787-7
Wilson, E. O., and MacArthur, R. H. (1967). The Theory of Island Biogeography. Princeton, NJ: Princeton University Press.
Keywords: alien biodiversity, biotic resistance, introduced animals, island biogeography, Luquillo Experimental Forest, non-native plants, tropical montane forest
Citation: Zimmerman JK, Rojas-Sandoval J and Shiels AB (2021) Invasive Species in Puerto Rico: The View From El Yunque. Front. Ecol. Evol. 9:640121. doi: 10.3389/fevo.2021.640121
Received: 10 December 2020; Accepted: 25 January 2021;
Published: 08 March 2021.
Edited by:
Shu-Ching Chen, Florida International University, United StatesReviewed by:
Lloyd W. Morrison, Missouri State University, United StatesCopyright © 2021 Zimmerman, Rojas-Sandoval and Shiels. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jess K. Zimmerman, amVzc2t6QGl0ZXMudXByLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.