
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 14 May 2021
Sec. Behavioral and Evolutionary Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.638039
This article is part of the Research Topic Determinants and Consequences of Perceived Predation Risk: From Individual Behavior to Transgenerational Effects View all 11 articles
Despite the wide recognition that strongly interacting species can influence distributions of other species, species interactions are often disregarded when assessing or projecting biodiversity distributions. In particular, it remains largely uncharted the extent to which the disappearance of a keystone species cast repercussions in the species composition of future communities. We tested whether an avian top predator can exert both positive and negative effects on spatial distribution of other species, and if these effects persist even after the predator disappeared. We acquired bird count data at different distances from occupied and non-occupied nests of Northern goshawks Accipiter gentilis. Using a Bayesian joint species distribution model, we found that large bird species (preferred prey) are less abundant in the proximity of nests occupied by goshawks, whereas smaller species –expected to get protection from subordinate predators displaced by goshawks– more often showed an opposite association. These spatial differences level off gradually, but still persist for years after the goshawks have disappeared. This indicates that the composition of local bird populations and communities might be conditional on past species interactions. Therefore, endeavors centered around species distributions could largely benefit from acknowledging the local extinction of keystone species.
Predators have had a central role in the concept of keystone species (Paine, 1966; Power et al., 1996) not only because of the direct negative pressure they impose on prey, but also due to the indirect effects they cause that may cascade through the entire community (Ripple et al., 2014). Although being a largely overlooked phenomena, keystone species can attract other species. Habitat selection theory predicts that animals prefer locations that maximize their fitness (Fretwell and Lucas, 1969; Rosenzweig, 1981; Morris, 2003). Predation risk is an important feature that defines habitat quality, sometimes driving prey to patches of lower relative quality but with lower predation risk (Lima and Dill, 1990; Lima and Bednekoff, 1999; Lind, 2005). Interestingly, because apex predators can also displace other competing predators (Sergio and Hiraldo, 2008), prey species that are hunted by subordinate predators should benefit from the presence of a top predator (Crooks and Soulé, 1999; Sergio et al., 2004; Ritchie and Johnson, 2009). Several studies have shown the presence of heterospecific attraction (Mönkkönen et al., 1999) for prey seeking shelter under protector species (see reviews by Caro, 2005; Quinn and Ueta, 2008; Lima, 2009). However, studies investigating attraction to protector species have, so far, mainly focused on pairs of species while not accounting for general consequences on the communities (but see Forsman et al., 2001). Additionally, the concept of keystone species has been considered mainly for mammalian carnivores (Sergio et al., 2008; Caro, 2010) while avian predators have been investigated but only to a smaller extent (Thomson et al., 2006; Mönkkönen et al., 2007).
It is well-established that effects of past land-use on ecosystems can persist for long periods of time (Koerner et al., 1997; Knick and Rotenberry, 2000; Hermy and Verheyen, 2007; Cuddington, 2011). Much less attention has been paid to how observed patterns of species distribution are a result of past species interactions, with no studies investigating how the extinction of a keystone species may leave a community footprint that persists in time. The capacity and speed by which single species, and species assemblages in general, adjust to new environmental conditions are associated with landscape connectivity, mobility and interactions among the species in the community (Pimm, 1984; Hanski, 1998; Peterson et al., 1998). It is thus expected that in a continuous landscape, highly mobile taxa (e.g., birds or large mammals) would rapidly respond to local perturbations. However, cues used to assess habitat quality may persist in time (Seppänen et al., 2007), arguably causing a “memory effect” in how animal assemblages react to changes. Being able to assess the relevance of past interactions is thus relevant for studies aiming to forecast ecosystem restoration or to better assess true habitat requirements of species.
In this study, we investigate the impact of an avian top predator, the northern goshawk Accipiter gentilis, on the bird community in space and time. We hypothesized that (i) the top predator can have both positive and negative effects on the spatial distribution of other species, and that (ii) these effects may persist even after the top predator has disappeared. We further hypothesized that these effects are driven by (iii) the displacement of prey species due to predator-prey interactions, and (iv) the attraction of species that benefit from the top predator displacing subordinate predators. We therefore expected that (i) bird assemblage composition is conditional on distance from goshawk nests and that (ii) this effect gradually diminishes in time after the goshawk’s disappearance. While (iii) prey species are expected to be less common nearby occupied nests, (iv) species that benefit from protection by the top predator are expected to be more common near the nest when the top predator is present.
To investigate the effect of top predators on avian assemblages, we used the widely distributed northern goshawk Accipiter gentilis (henceforth goshawk) as a top predator model species. The goshawk is a forest-dwelling species that primarily preys on middle-sized birds in Europe (Kenward, 2006). In Finland, the goshawk is usually found in mature Norwegian spruce Picea abies forest (Tornberg et al., 2006). During the breeding season, from March to August, goshawks concentrate their activity within a few kilometers of their nest and expand their range or move to other areas after the breeding season (Kenward, 2006; Tornberg et al., 2006).
Through an ongoing long-term goshawk population monitoring (e.g., Byholm and Nikula, 2007; Byholm et al., 2007, 2011; Burgas et al., 2016), we inspected goshawk territories on the west coast of Finland (latitude 62°00′–62°55′N, longitude 21°05′–22°40′E) from the second week of May to the first week of June in 2007. For each territory, we counted individuals of all bird species found at 12 sample sites around the goshawk nest; at 50, 250, and 500 m in each cardinal direction. In total, we surveyed 708 sample sites. The distances were chosen to investigate how distance from the goshawk nest influences the bird community. Because goshawk activity concentrates in forest habitats, we focused on forest bird species (sensu Solonen, 1994). Bird abundances were recorded for 7 min both from vocalizations and visual observations in every site within the first 4 h since sunrise. Birds flying over a site were not recorded. At the time of the bird surveys, goshawks were breeding in 29 of the 59 nests included in this study. Among the remaining 30 nests, goshawks were known to have been breeding in the nest the previous year in 14 nests, 2 years earlier in 6 nests, 3 years earlier in 6 nests, 4 years earlier in 2 nests, and 5 years earlier in 2 nests. All non-breeding territories were inspected to confirm that goshawks were not nesting in an alternative location within that same territory. The habitat composition of each sample site was classified in the field to the closest 10% among the following five categories: pine Pinus sylvestris forests, spruce forests, young forest plantations, pine fens, and open habitats.
We expected that while some species should be negatively associated with the goshawk (e.g., prey species, subordinate raptor species), other species that are not prevalent in the diet of the goshawk and/or that do not compete for resources should be either unaffected by the goshawk presence or show positive association if the goshawk offers shelter from other predators. To determine the diverging effect of the goshawk on the bird community, we categorized bird species into two groups according to body mass (Mönkkönen et al., 2007). We regarded birds with a body mass equal or higher than the redwing Turdus iliacus (i.e., 60 g) as a proxy for goshawk prey (hereafter large birds). Conversely, we presumptively denoted as non-susceptible to predation all bird species with body mass smaller than 60 g (hereafter small birds). This categorization is in line with the fact that goshawks rarely prey on small birds, even though these are the most abundant species in the forest (Møller et al., 2012).
To assess differences in habitat representation as a function of distance from the goshawk nests and time since the nests were last occupied and the interaction of distance and time, we carried out a Dirichlet regression model in R using function DirichletReg in package DiricReg (Maier, 2014). Dirichlet models are suited to analyze compositional data, where dependent variables are subject to a sum constrain.
We applied a joint distribution model (Warton et al., 2015; Ovaskainen et al., 2017; Ovaskainen and Abrego, 2020) to analyze the data simultaneously for all species. We model the abundance yijk of species i found at site k within territory j by assuming that yijk follows a Poisson distribution where the linear predictor is modeled as
Here Djk is the distance of site k in the goshawk territory j, Tj is the time since the goshawk left its nest in territory j, and Hjk is the forest habitat cover (i.e., proportion of pine and spruce forest together) of site k in territory j. The model involves a quadratic function of the distance from the nest to allow the model to fit species that peak in abundance at an intermediate distance from the goshawk nest (Mönkkönen et al., 2007). The model estimates one response curve (as a function of distance to the nest) for cases where the goshawk is presently at the nest, and another response curve for nests that have been occupied by the goshawk long ago, and assumes an exponential transition between these responses with a characteristic time scale αi for species i. The territory-level random effects (εij) were assumed to be distributed independently among the species and territories as , where the amount of random variation among territories for species i.
To facilitate the estimation of model parameters for rare species, we used the hierarchical modeling framework modified from that of Ovaskainen and Soininen (2011). We denoted by βi the vector of parameters to be estimated for species i, βi = (βi1, βi2, βi3, βi4, βi5, βi6, βi7, βi8), where βi8 = log(αi). We assumed that the parameter vectors βi are distributed (independently among the species) according to a multivariate normal distribution with mean μ (a vector of length 8) and a variance-covariance matrix Σ (a 8 × 8 matrix), βi∼N(μ,Σ). The vector μ models the responses of a “typical” species, whereas the matrix Σ measures how species vary in their responses to the explanatory variables (diagonal of Σ) and to pairs of explanatory variables (off-diagonal of Σ).
The parameters of the model were estimated using a Bayesian approach. As priors, we assumed for each component of μ a normal distribution with mean zero and variance one, for Σ an inverse Wishart distribution with 8 degree of freedom and an identity matrix as variance-covariance parameter, and for each a Gamma distribution with shape equaling 0.5 and rate equaling 0.5. We sampled the posterior distributions with a Monte Carlo Markov Chain (MCMC) method [modified from Ovaskainen and Soininen (2011) to account for count data], which we ran for 70,000 iterations, out of which the proposal distributions were adapted during the initial burn-in of 20,000 iterations. We thinned the samples by 10, resulting in 500 posterior samples, and assessed MCMC convergence by computing an effective number of samples (results shown in Supplementary Figure 1).
Habitat composition at the sample site level changed substantially when moving away from the goshawk nests, with spruce forest—the preferred nesting habitat of the goshawk—representing 75% of the land cover surrounding the nest while it covered approximately 30% of the sample sites at 500 m from the nests (Dirichlet regression P-value for spruce forest < 0.001, for other land cover types > 0.05; (Supplementary Table 1 and Supplementary Figure 2). Habitat composition did not vary as a function of the number of years since goshawks abandoned the nests, neither as the interaction between time and distance in any of the five habitat classes (Supplementary Table 1 and Supplementary Figure 2).
We recorded 25 large and 31 small bird species and included in the community-level model also those 15 large and 8 small bird species that were not detected in our surveys but that breed in the study area (Valkama et al., 2011). The average abundance at site level of a typical large species was eight times lower than that of a small-sized bird (0.02 vs. 0.16 individuals/site for large and small species, respectively).
Based on the community level parameters (μ) that represent the responses of a typical species, both small and large species were more common in the absence of goshawk than in its presence (Figures 1, 2A,D). However, while large species increased greatly in abundance with increasing distance from the goshawk nest (Figures 1, 2D), the abundance of small species varied only little with distance from the goshawk nest, the highest abundance being found at an intermediate distance (Figures 1, 2A). The posterior mean estimates for the community-level averages of the characteristic time scale parameter α was 6 years (95% credibility interval 1–21 years) for small birds and 4 years (95% credibility interval 1–18 years) for large birds. Thus, both small and large birds showed a substantial delay in their responses to goshawk abandoning the nest (Figures 2A,D). At the community level, habitat did not show an effect that would be either positive or negative with 95% posterior probability.
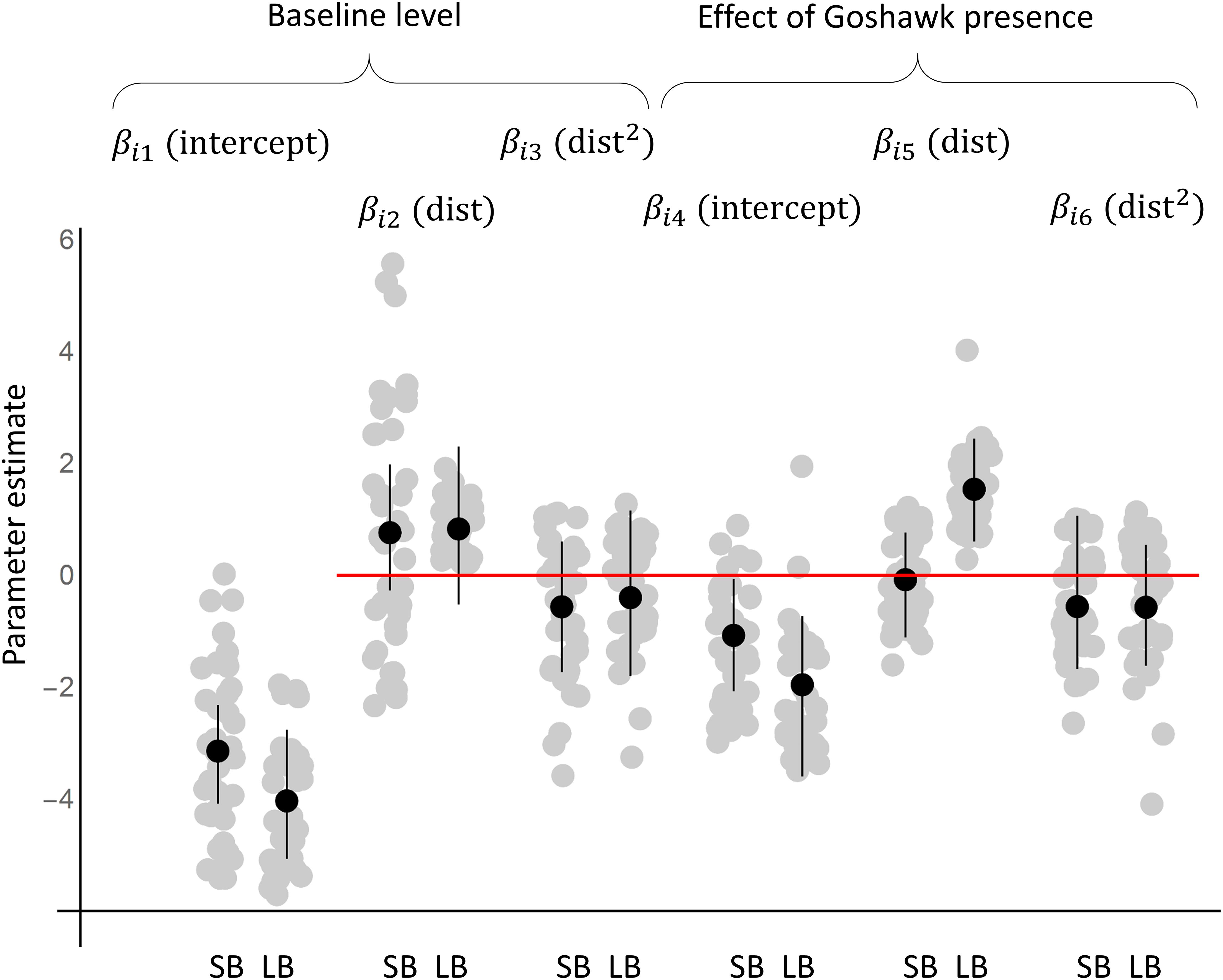
Figure 1. The responses of the species to goshawk presence based on the posterior distribution of the parameters of the joint species distribution model fitted to the data. The black dots and lines show the posterior means and the 95% credibility intervals for the community level parameters (μ), whereas the gray dots show the posterior means for the species level parameters (β). The results are shown separately for the models fitted with data on small bird species (SB) and with large bird species (LB).
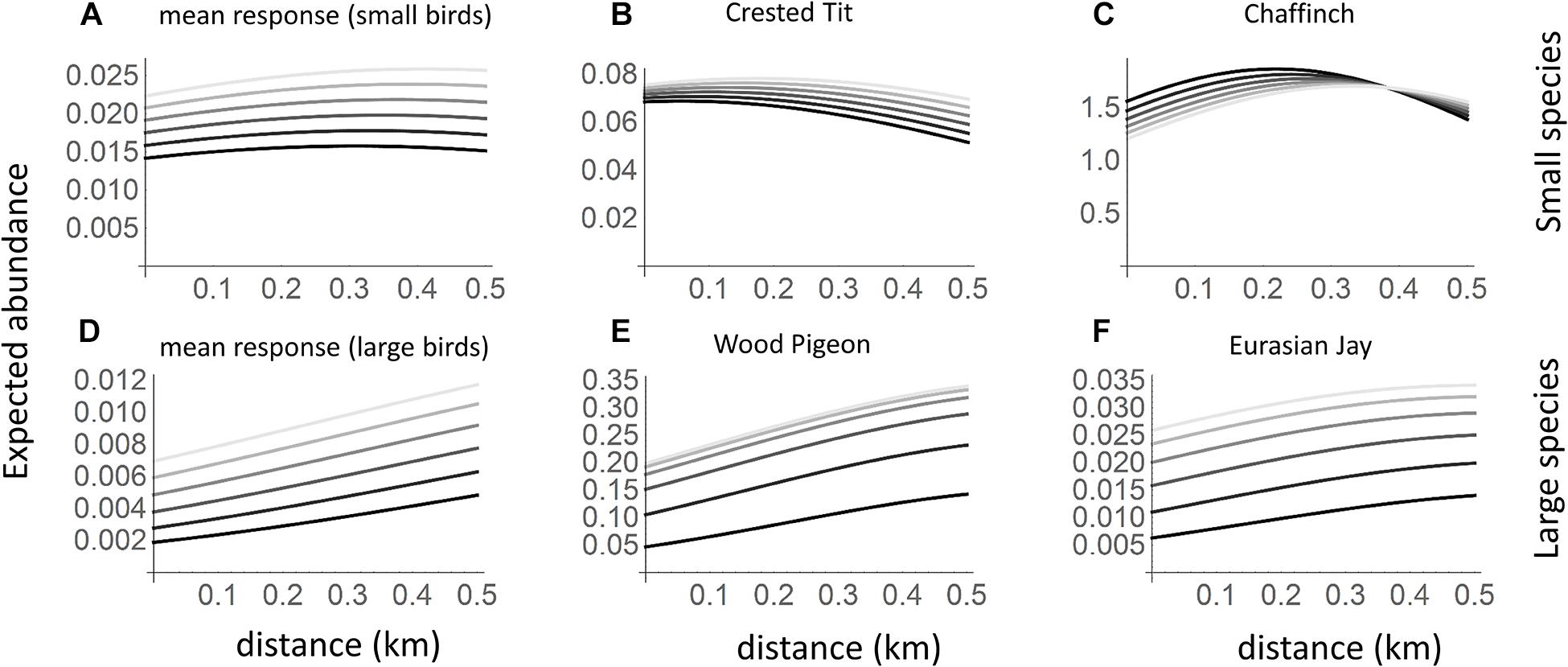
Figure 2. Expected abundances of small and large bird species in relation to goshawk nest proximity and time since the goshawk abandoned the nest. The black lines correspond to the current presence of the goshawk, and the different shades of gray to situations in which goshawk abandoned the nest from one (darkest gray) to five (lightest gray) years ago. The panels correspond to a typical small species (A; based on community level parameters), the crested Tit Parus cristatus (B), the chaffinch Fringilla coelebs (C), a typical large species (D), the wood pigeon Columba palumbus (E; a common prey of the goshawk) the Eurasian jay Garrulus glandarius (F; a common prey of the goshawk and an important avian nest predator). In the predictions, habitat was set to the average value over the sample sites.
The individual species showed marked variation around the expected community-level abundance (Figures 1, 2). Based on the posterior mean estimates obtained for the small species, in the presence of the goshawk, 11 species achieved the highest abundance near the goshawk nest (for example, see Figure 2B), 6 at an intermediate distance from the nest (for example, see Figure 2C), and 14 far away from the nest. Among the large species, in the presence of the goshawk none of the species achieved the highest abundance near the goshawk nest, 2 at an intermediate distance from the nest, and 23 far away from the nest (for examples, see Figures 2E,F).
Our results show that raptors, such as the goshawk, can have an important role in shaping the composition of the bird community both across space andacross time. Not surprisingly, such a keystone species-effect is strongest near occupied goshawk nests, but fades gradually with distance and time after the goshawk abandons its nest. Because top predators are usually territorial with uneven presence in space and time, they can be regarded as a structuring species that creates heterogeneity in the landscape (see Thomson et al., 2006; Mönkkönen et al., 2007). As such, it is expected that, if top predators affect large portion of the communities, they should also promote higher landscape-level diversity with the same importance as structural habitat features or small patches of scarce habitats (Davidar et al., 2001; Gibbons and Lindenmayer, 2008; Timonen et al., 2010). Further research should be pursued to validate or contrast this idea.
The goshawk drove stronger impact on the assemblage of large species than on the small species assemblage. This was anticipated as predators are expected to show stronger and more direct interactions with its pool of preferred preys. The causes of community turnover can be addressed by looking at the three main patterns observed across the community. Firstly, large birds were usually more abundant far from occupied goshawk nests and increased in numbers only years after the nest was abandoned. Even though, to our knowledge, a temporal effect of keystone species on local communities has not been tested in the past, this result is in line with other studies where predators were shown to alter their prey distribution spatially by actively predating them and through behavioral response of the prey actively avoiding the predator (Norrdahl and Korpimäki, 1998; Thomson et al., 2006; Lima, 2009). Some studies have found that prey species peaked at intermediate distances from the predator (Quinn and Kokorev, 2002; Mönkkönen et al., 2007) as a compromise between the higher direct predation risk near the raptor and the higher risk of nest predation far from the raptor. In our study, this behavior was found only for the fieldfare Turdus pilaris. However, it is possible that our distance range (up to 500 m) was too short to reveal this kind of pattern more generally. For instance, Mönkkönen et al. (2007) found that large bird abundance in goshawk territories peaked at approximately 2 km from goshawk nests.
Secondly, small species were more abundant near occupied goshawk nests while their abundances decreased after the nest was abandoned or even showed opposite patterns far away from the goshawk nest (Figures 2A–C). This suggests that some of the small-bodied species may actively choose to breed in the proximity of the hawk. This supports the heterospecific attraction hypothesis (Mönkkönen et al., 1999; Haemig, 2001; Caro, 2005; Lima, 2009), and is backed by other studies showing aggregation of birds to predators (e.g., Wiklund, 1982; Bogliani et al., 1999; Mönkkönen et al., 2007). This interpretation was also endorsed by abundances of the Eurasian jay Garrulus glandarius, an important nest predator for small forest birds and common prey of the goshawk. Eurasian jay was extremely rare in the proximity of active goshawk nests when the goshawk was present, while its abundance increased after the goshawk’s absence (Figure 2F).
Third, we found that several small species were more abundant at intermediate distances or even reacted in the same manner as a typical large species. Interestingly, this is against the general expectation that species outside the predator scope should, at least, not be negatively affected by it. In this respect, our results did not comply with previous findings showing that abundances of non-prey species were higher in the vicinity of predators (Norrdahl and Korpimäki, 1998; Mönkkönen et al., 2007). We see two possibilities explaining this discordancy. On the one hand, it might be that the presence of the goshawk attracts or allows the occurrence of species that have negative effects on the fitness of other species (see also Morosinotto et al., 2012). In this context, the great spotted woodpecker Dendrocopos major—a nest predator (Walankiewicz, 2002)—was more common in the close vicinity of the raptor nest than far away. On the other hand, because of different ecological and evolutionary history, one could expect variation in the species capability to grasp useful information. For example, given the fact that male goshawks closely resemble female Eurasian sparrowhawks Accipiter nisus, it is possible that some species preyed upon by sparrowhawks (which prefers small birds) are distressed by the goshawk presence if failing to clearly separate the two predators.
It took years to stabilize the community similarity and the abundances of several species after the goshawk abandoned the territory. Birds are not physically restricted in mobility, and therefore it could be expected that local abundances should adjust swiftly. There are a few reasons which can explain why the delay in the bird community was gradual. Firstly, birds are known to show breeding site fidelity (i.e., tendency of one individual to return to breed where it reproduced previously) and breeding philopatry (i.e., tendency of an individual to return to breed where it was born). This propensity may partly neutralize the importance of social cues. Because birds exhibit learning behavior and avoid returning to a location if a breeding attempt was unsuccessful (Lima and Dill, 1990), the increase of subordinated predators in subsequent years could cancel out site fidelity. Secondly, the goshawk nests are likely to remain as an indicator of the raptor’s presence even after the hawk has left. Individuals are known to use indirect cues to assess the presence of other species (e.g., Forsman et al., 2012). If so, the nest of a predator gives an insight into its presence. As the goshawk nests are large structures that can persist for several years and even decades after being abandoned, it is possible that the simple presence of large empty stick nests may affect the species composition. This temporal effect resembles the delay in response of communities to habitat perturbations (Knick and Rotenberry, 2000), with the novelty that in our case the response relates to a key species in the community. Our results suggest that studies on bird community turnover (i) should be monitored during a series of years even after environmental change in order to successfully assess its effects, and that (ii) the historical co-occurrence of keystone species should be considered. Moreover, it is possible that some species preemptively avoid patches with structural habitat properties preferred by the predator (here old forest stands with widely spaced trees). Such behavior would reduce the amount of community turnover found across space and time in this study, as prey would not occupy predator-suitable sites based on habitat cues to start with. Specific study designs to study such possibility are encouraged.
Our habitat measures were coarse as compared to the actual habitat selection done by different bird species. One strength of this study is the combination of distance from the nest, with time since the nest was last time occupied separately on small and large birds. Given that we did not find a change in habitat representation as function of time neither interaction of time and distance, we find it unlikely that there would be a strong bias in forest structure that would produce spurious effects of time since goshawk nest was occupied in a different manner between small birds and large birds.
Diversity in resource limitation, heterogeneity, and interspecific differences can explain the stable coexistence of numerous competing species (Tilman, 1982, 1994) while a higher number of interactions enhances ecosystem resilience (Peterson et al., 1998; Petchey and Gaston, 2009; Tylianakis et al., 2010). Given the ubiquity of predators and the fact that they present both negative and positive interactions, it is reasonable to suggest that top predators have a positive role in sustaining biodiversity. This can have direct practical implications in conservation endeavors that devote more efforts to charismatic species like predators.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethical review and approval was not required for the animal study because the data consists of bird counts in the field with minimal disturbance. Moreover, the field data collection was conducted in 2007, before the ethical committees for ecological field work was on place.
PB designed the study, secured funding, and coordinated the data collection. DB analyzed habitat composition. OO and FB run the community analyses with the input of DB and PB. DB made the first draft, on top of which all authors contributed significantly.
This study was financially supported by the Maj and Tor Nessling Foundation, Finnish Cultural Foundation, Societas pro Fauna et Flora Fennica, Svensk-Österbottniska Samfundet, and Swedish Cultural Foundation in Finland (all to PB and DB), the Academy of Finland (Grants 124242 and 284601 to OO), the Research Council of Norway (SFF-III Grant No. 223257), and the European Research Council (ERC Starting Grant 205905 to OO).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Johan Ekroos, Teppo Lehtola, Harry Lillandt, Ismo Nousiainen, Tuomas Seimola, and Turo Tuomikoski assisted with bird counts. We are grateful for the comments of Fabrizio Sergio and Oliver Krüger.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.638039/full#supplementary-material
Bogliani, G., Sergio, F., and Tavecchia, G. (1999). Woodpigeons nesting in association with hobby falcons: advantages and choice rules. Anim. Behav. 57, 125–131. doi: 10.1006/anbe.1998.0959
Burgas, D., Juutinen, A., and Byholm, P. (2016). The cost-effectiveness of using raptor nest sites to identify areas with high species richness of other taxa. Ecol. Indic. 70, 518–530. doi: 10.1016/j.ecolind.2016.06.052
Byholm, P., and Nikula, A. (2007). Nesting failure in Finnish Northern Goshawks Accipiter gentilis: incidence and cause. IBIS 149, 597–604. doi: 10.1111/j.1474-919X.2007.00687.x
Byholm, P., Nikula, A., Kentta, J., and Taivalmäki, J.-P. (2007). Interactions between habitat heterogeneity and food affect reproductive output in a top predator. J. Anim. Ecol. 76, 392–401. doi: 10.1111/j.1365-2656.2007.01211.x
Byholm, P., Rousi, H., and Sole, I. (2011). Parental care in nesting hawks: breeding experience and food availability influence the outcome. Behav. Ecol. 22, 609–615. doi: 10.1093/beheco/arr019
Caro, T. (2005). Antipredator Defenses in Birds and Mammals. Chicago, IL: University of Chicago Press.
Caro, T. (2010). Conservation by Proxy: Indicator, Umbrella, Keystone, Flagship, and Other Surrogate Species. Washington, DC: Island Press.
Crooks, K., and Soulé, M. (1999). Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563–566. doi: 10.1038/23028
Cuddington, K. (2011). Legacy effects: the persistent impact of ecological interactions. Biol. Theory 6, 203–210. doi: 10.1007/s13752-012-0027-5
Davidar, P., Yoganand, K., and Ganesh, T. (2001). Distribution of forest birds in the Andaman islands: importance of key habitats. J. Biogeogr. 28, 663–671. doi: 10.1046/j.1365-2699.2001.00584.x
Forsman, J. T., Mönkkönen, M., and Hukkanen, M. (2001). Effects of predation on community assembly and spatial dispersion of breeding forest birds. Ecology 82, 232–244.
Forsman, J. T., Monkkonen, M., Korpimaki, E., and Thomson, R. L. (2012). Mammalian nest predator feces as a cue in avian habitat selection decisions. Behav. Ecol. 24, 262–266. doi: 10.1093/beheco/ars162
Fretwell, S. D., and Lucas, H. L. (1969). On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 19, 16–36. doi: 10.1007/BF01601953
Gibbons, P., and Lindenmayer, D. (2008). The future of scattered trees in agricultural landscapes. Conserv. Biol. 22, 1309–1319. doi: 10.1111/j.1523-1739.2008.00997.x
Haemig, P. (2001). Symbiotic nesting of birds with formidable animals: a review with applications to biodiversity conservation. Biodivers. Conserv. 10, 527–540. doi: 10.1023/A:1016654326822
Hermy, M., and Verheyen, K. (2007). Legacies of the past in the present-day forest biodiversity: a review of past land-use effects on forest plant species composition and diversity. Ecol. Res. 22, 361–371. doi: 10.1007/s11284-007-0354-3
Knick, S., and Rotenberry, J. (2000). Ghosts of habitats past: contribution of landscape change to current habitats used by shrubland birds. Ecology 81, 220–227.
Koerner, W., Dupouey, J. L., Dambrine, E., and Benoit, M. (1997). Influence of past land use on the vegetation and soil of present day forest in the Vosge Mountains, France. J. Ecol. 85, 351–358. doi: 10.2307/2960507
Lima, S., and Bednekoff, P. (1999). Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659. doi: 10.1086/303202
Lima, S. L. (2009). Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol. Rev. 84, 485–513. doi: 10.1111/j.1469-185X.2009.00085.x
Lima, S. L., and Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. doi: 10.1139/z90-092
Lind, J. (2005). Determining the fitness consequences of antipredation behavior. Behav. Ecol. 16, 945–956. doi: 10.1093/beheco/ari075
Maier, M. J. (2014). DirichletReg: Dirichlet Regression for Compositional Data in R. Vienna: Institute for Statistics and Mathematics, Vienna University of Economics and Business, doi: 10.1111/j.1461-0248.2008.01182.x
Møller, A. P., Solonen, T., Byholm, P., Huhta, E., Nielsen, J. T., and Tornberg, R. (2012). Spatial consistency in susceptibility of prey species to predation by two Accipiter hawks. J. Avian Biol. 43, 390–396. doi: 10.1111/j.1600-048X.2012.05723.x
Mönkkönen, M., Härdling, R., Forsman, J. T., and Tuomi, J. (1999). Evolution of heterospecific attraction: using other species as cues in habitat selection. Evol. Ecol. 13, 93–106. doi: 10.1023/A:1006590215306
Mönkkönen, M., Husby, M., Tornberg, R., Helle, P., and Thomson, R. L. (2007). Predation as a landscape effect: the trading off by prey species between predation risks and protection benefits. J. Anim. Ecol. 76, 619–629. doi: 10.1111/j.1365-2656.2007.01233.x
Morosinotto, C., Thomson, R. L., Hänninen, M., and Korpimäki, E. (2012). Higher nest predation risk in association with a top predator: mesopredator attraction? Oecologia 170, 507–515. doi: 10.1007/s00442-012-2320-1
Morris, D. W. (2003). Toward an ecological synthesis: a case for habitat selection. Oecologia 136, 1–13. doi: 10.1007/s00442-003-1241-4
Norrdahl, K., and Korpimäki, E. (1998). Fear in farmlands: how much does predator avoidance affect bird community structure? J. Avian Biol. 29, 79–85. doi: 10.2307/3677344
Ovaskainen, O., and Abrego, N. (2020). Joint Species Distribution Modelling: With Applications in R. Cambridge University Press.
Ovaskainen, O., and Soininen, J. (2011). Making more out of sparse data: hierarchical modeling of species communities. Ecology 92, 289–295. doi: 10.1890/10-1251.1
Ovaskainen, O., Tikhonov, G., Norberg, A., Blanchet, F. G., Duan, L., Dunson, D., et al. (2017). How to make more out of community data? A conceptual framework and its implementation as models and software. Ecol. Lett. 20, 561–576. doi: 10.1111/ele.12757
Paine, R. (1966). Food web complexity and species diversity. Am. Nat. 100, 65–75. doi: 10.1086/282400
Petchey, O. L., and Gaston, K. J. (2009). Effects on ecosystem resilience of biodiversity, extinctions, and the structure of regional species pools. Theor. Ecol. 2, 177–187. doi: 10.1007/s12080-009-0041-9
Peterson, G., Allen, C., and Holling, C. (1998). Ecological resilience, biodiversity, and scale. Ecosystems 1, 6–18.
Pimm, S. (1984). The complexity and stability of ecosystems. Nature 307, 321–326. doi: 10.1038/307321a0
Power, M., Tilman, D., Estes, J., and Menge, B. (1996). Challenges in the quest for keystones. Bioscience 44, 609–620. doi: 10.2307/1312990
Quinn, J. L., and Kokorev, Y. (2002). Trading-off risks from predators and from aggressive hosts. Behav. Ecol. Sociobiol. 51, 455–460. doi: 10.1007/s00265-002-0466-2
Quinn, J. L., and Ueta, M. (2008). Protective nesting associations in birds. IBIS 150, 146–167. doi: 10.1111/j.1474-919X.2008.00823.x
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., et al. (2014). Status and ecological effects of the world’s largest carnivores. Science 343, 1241484–1241484. doi: 10.1126/science.1241484
Ritchie, E., and Johnson, C. (2009). Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998. doi: 10.1111/j.1461-0248.2009.01347.x
Seppänen, J., Forsman, J., Mönkkönen, M., and Thomson, R. L. (2007). Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 88, 1622–1633. doi: 10.1890/06-1757.1
Sergio, F., Caro, T., Brown, D., Clucas, B., Hunter, J., Ketchum, J., et al. (2008). Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Annu. Rev. Ecol. Evol. Syst. 39, 1–19. doi: 10.1146/annurev.ecolsys.39.110707.173545
Sergio, F., and Hiraldo, F. (2008). Intraguild predation in raptor assemblages: a review. IBIS 150, 132–145. doi: 10.1111/j.1474-919X.2008.00786.x
Sergio, F., Rizzolli, F., Marchesi, L., and Pedrini, P. (2004). The importance of interspecific interactions for breeding-site selection: peregrine falcons seek proximity to raven nests. Ecography 27, 818–826. doi: 10.1111/j.0906-7590.2004.04030.x
Solonen, T. (1994). Structure and dynamics of the Finnish avifauna. Memo. Soc. Pro Fauna Flora Fenn. 70, 1–22.
Thomson, R. L., Forsman, J. T., Sardà-Palomera, F., and Mönkkönen, M. (2006). Fear factor: prey habitat selection and its consequences in a predation risk landscape. Ecography 29, 507–514. doi: 10.1111/j.0906-7590.2006.04568.x
Tilman, D. (1982). Resource Competition and Community Structure. Princeton, NJ: Princeton Univ. Press.
Tilman, D. (1994). Competition and biodiversity in spatially structured habitats. Ecology 75, 2–16. doi: 10.2307/1939377
Timonen, J., Siitonen, J., Gustafsson, L., Kotiaho, J. S., Stokland, J. N., Sverdrup-Thygeson, A., et al. (2010). Woodland key habitats in northern Europe: concepts, inventory and protection. Scand. J. For. Res. 25, 309–324. doi: 10.1080/02827581.2010.497160
Tornberg, R., Korpimäki, E., and Byholm, P. (2006). Ecology of the northern goshawk in Fennoscandia. Stud. Avian Biol. 31, 141–157.
Tylianakis, J. M., Laliberté, E., Nielsen, A., and Bascompte, J. (2010). Conservation of species interaction networks. Biol. Conserv. 143, 2270–2279. doi: 10.1016/j.biocon.2009.12.004
Valkama, J., Vepsäläinen, V., and Lehikoinen, A. (2011). The Third Finnish Breeding Bird Atlas. Helsinki: Finnish Museum of Natural History and Ministry of Environment.
Walankiewicz, W. (2002). Breeding losses in the collared flycatcher ficedula albicollis caused by nest predators in the Białowieża National Park (Poland). Acta Ornithol. 37, 21–26. doi: 10.3161/068.037.0104
Warton, D. I., Blanchet, F. G., O’Hara, R. B., Ovaskainen, O., Taskinen, S., Walker, S. C., et al. (2015). So many variables: joint modeling in community ecology. Trends Ecol. Evol. 30, 766–779. doi: 10.1016/j.tree.2015.09.007
Keywords: Bayesian community-model, ecological legacy, species distribution, predator-prey interactions, keystone species, heterospecific attraction
Citation: Burgas D, Ovaskainen O, Blanchet FG and Byholm P (2021) The Ghost of the Hawk: Top Predator Shaping Bird Communities in Space and Time. Front. Ecol. Evol. 9:638039. doi: 10.3389/fevo.2021.638039
Received: 04 December 2020; Accepted: 21 April 2021;
Published: 14 May 2021.
Edited by:
Toni Laaksonen, University of Turku, FinlandReviewed by:
Fabrizio Sergio, Estación Biológica de Doñana (EBD), SpainCopyright © 2021 Burgas, Ovaskainen, Blanchet and Byholm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Burgas, ZGFuaWVsLmJ1cmdhc3JpZXJhQGhlbHNpbmtpLmZp
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.