
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 03 December 2021
Sec. Population, Community, and Ecosystem Dynamics
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.637452
This article is part of the Research Topic Vagrancy, Exploratory Behavior and Colonization by Birds: Escape from Extinction? View all 5 articles
 Lucinda C. Zawadzki1*
Lucinda C. Zawadzki1* Gunnar T. Hallgrimsson2
Gunnar T. Hallgrimsson2 Richard R. Veit3,4
Richard R. Veit3,4 Lars M. Rasmussen5
Lars M. Rasmussen5 David Boertmann6
David Boertmann6 Natasha Gillies1
Natasha Gillies1 Tim Guilford1
Tim Guilford1Vagrancy is critical in facilitating range expansion and colonization through exploration and occupation of potentially suitable habitat. Uncovering origins of vagrants will help us better understand not only species-specific vagrant movements, but how the dynamics of a naturally growing population influence vagrancy, and potentially lead to range expansion. Under the premise that occurrence of vagrants is linked to increasing population growth in the core of the breeding range, we assessed the utility of breeding population survey data to predict source populations of vagrants. Lesser Black-backed Gulls (LBBG) (Larus fuscus) served as our focal species due to their dramatic and well-documented history of vagrancy to North America in the last 30 years. We related annual occurrence of vagrants to indices of breeding population size and growth rate of breeding populations. We propose that the fastest growing population is the most likely source of recent vagrants to North America. Our study shows that it is possible to predict potential source populations of vagrants with breeding population data, but breeding surveys require increased standardization across years to improve models. For the Lesser Black-backed Gull, Iceland’s breeding population likely influenced vagrancy during the early years of colonization, but the major increase in vagrants occurred during a period of growth of Greenland’s population, suggesting that Greenland is the source population of the most recent pulse of vagrant LBBG to North America.
Anthropogenic climate change continues to threaten species’ survival (Román-Palacios and Wiens, 2020), and species must remain flexible if they are to escape extinction driven by climate change. Occupation of potentially suitable habitat through range expansion and colonization is one way in which species can survive climatic effects. Vagrancy, a process by which organisms engage in long-distance dispersal movements outside of their known species range (Grinnell, 1922; Baker, 1978), may provide the mechanism through which individuals can explore and occupy potentially suitable habitat. Though these movements have often been attributed to internal errors in navigation (Rabøl, 1969; DeSante, 1973; Cottridge and Vinicombe, 1996), or passive displacement by wind or weather systems (Williamson, 1959; Elkins, 1979; McLaren, 1981; McLaren et al., 2006), vagrancy is a natural part of mobile populations (Baker, 1978; Hengeveld, 1989), and can result in range expansion (e.g., Veit and Lewis, 1996; Massa et al., 2014) and colonization of new habitat (e.g., Veit and Lewis, 1996; Veit et al., 2016). Vagrants, however, are often difficult to study directly due to their inherent rarity and unpredictable occurrence. In order to investigate the role that vagrancy plays in range expansion and colonization, we therefore need to study variability in this behavior through a variety of methods.
It has been suggested that in order to naturally colonize new habitat, a species must have a growing source population (Hengeveld, 1989; Szűcs et al., 2014). Of the studies that have examined factors that influence vagrancy (DeBenedictis, 1971; DeSante, 1973; Hampton, 1997; Veit, 1990, 1997, 2000; Elkins, 1999; Thorup, 2004; McLaren et al., 2006; Pfeifer et al., 2007; Jiguet et al., 2008; De Juana and Garcia, 2010; Farnsworth et al., 2015; Ralph and Wolfe, 2018; Zawadzki et al., 2019), the majority have found that vagrancy is strongly correlated with population, either of the species’ overall population size (DeBenedictis, 1971; DeSante, 1983; Thorup, 2004; Pfeifer et al., 2007; Ralph and Wolfe, 2018), or annual variation in population size (Veit, 1990, 1997, 2000; McLaren et al., 2006; De Juana and Garcia, 2010; Jiguet et al., 2008; Zawadzki et al., 2019). It has also been found that increased incidence of vagrancy is linked to annual population growth in the core of a species’ breeding range (Veit, 1997; McLaren et al., 2006; Zawadzki et al., 2019). Population size and growth are therefore highly influential predictors when estimating vagrant occurrence. Vagrancy is likely a density-dependent phenomenon whereby increased productivity in a given year leads to the production of more individuals than the current habitat can support, leading to increased dispersal (Southwood et al., 1974) and vagrancy (DeBenedictis, 1971; DeSante, 1983; Patten and Marantz, 1996) to find newly suitable habitat. Data on a species’ population size and growth will be important in understanding range expansions, as colonizers may be coming from regions that have experienced the most rapid population growth, and may therefore be predisposed to vagrancy (Phillips et al., 2010).
Using this information, we may be able to track colonization of species undergoing range expansion, to determine where vagrants are coming from, and may even be able to predict which species are likely to occur as vagrants in the future. Though tagging of individuals at known breeding sites through either field-readable bands or GPS devices may provide more direct evidence on individual movement, the likelihood that any one bird tagged or banded will occur as a vagrant is low. Recovery rates of banded passerines within their normal range are already as low as 1.5% (North, 2020). Modeling with long-term population data is thus vital in understanding range expansion and colonization of vagrants.
Breeding population surveys of birds provide information on annual breeding pairs at colonies or nesting-sites where species are known to breed. Though protocol often vary by country, data from these surveys provide long-term estimates of population size and growth that may be useful for studying how population dynamics in the breeding range influence vagrancy. To examine the potential use of breeding population survey data in determining source populations of vagrants, we investigated the relationship between annual breeding population size and growth rate of three known breeding populations of Lesser Black-backed Gulls (L. fuscus; hereafter LBBG) in western Europe, and the occurrence of vagrant LBBG in North America during the years 1986–2018. LBBG are a unique case since they have a well-documented history of vagrancy to North America, yet, as with most vagrants, it is unknown where they are arriving from. We predict that Greenland is the source population of vagrant LBBGs to North America based on Greenland’s increasing population after colonization, and proximity to North America.
Lesser Black-backed Gulls are a polytypic species whose breeding range extends from Greenland to western Asia (Olsen and Larsson, 2004). During the mid- to late-twentieth century, LBBGs experienced a rapid increase in global population (Liebers and Helbig, 2002; Olsen and Larsson, 2004), mainly attributed to an increase in population growth of the Atlantic subspecies: (1) intermedius, whose breeding range extends from Belgium and Netherlands, to Norway, and (2) graellsii, which breeds in Greenland and Iceland, along western Europe, south to the Iberian Peninsula (Olsen and Larsson, 2004; Wetlands International, 2020). As their populations increased, LBBGs expanded their range westward across the Atlantic (Post and Lewis, 1995), moving from breeding grounds in western Europe to Iceland in the 1920s (Olsen and Larsson, 2004), and subsequently colonizing Greenland between 1985 and 1990 (Boertmann, 2008). Iceland’s breeding population increased from a 1,250 breeding pairs in 1974 to over 40,000 breeding pairs in 2004 (Hallgrimsson et al., 2011). The colonization of Greenland also rose rapidly, increasing from 13 records of non-breeding individuals by 1984 (Boertmann, 2008), to an estimated 2,060 breeding pairs in 2016, the majority of which nest in southwestern Greenland (Boertmann and Frederiksen, 2016; Boertmann et al., 2020).
This rapid expansion shifted the LBBG breeding range closer to North America, and was accompanied by increased numbers of vagrant LBBGs on the continent. The first LBBG was recorded in North America in 1934 (Edwards, 1935), and LBBG have now been seen in every state and Canadian province (Figure 1; eBird, 2017), with over 1,000 individuals recorded annually since 2005 (National Audubon Society, 2020). LBBGs are mainly seen in North America from September to March (Figure 2), though sightings do occur year-round. Despite increasing occurrence of LBBGs each year, they have yet to breed in North America (with the exception of two hybrid pairs with Herring Gulls (Larus argentatus); vanVliet et al., 1993; Ellis et al., 2007; Ellis et al., 2014). This means that colonization of North America by LBBGs is a result of repeated vagrancy by gulls arriving from outside the continent.

Figure 1. eBird map of LBBG sightings in part of North America. The frequency is the percentage of submitted checklists from that region in which a LBBG was recorded. Image provided by eBird (www.ebird.org) and created November 10, 2020.
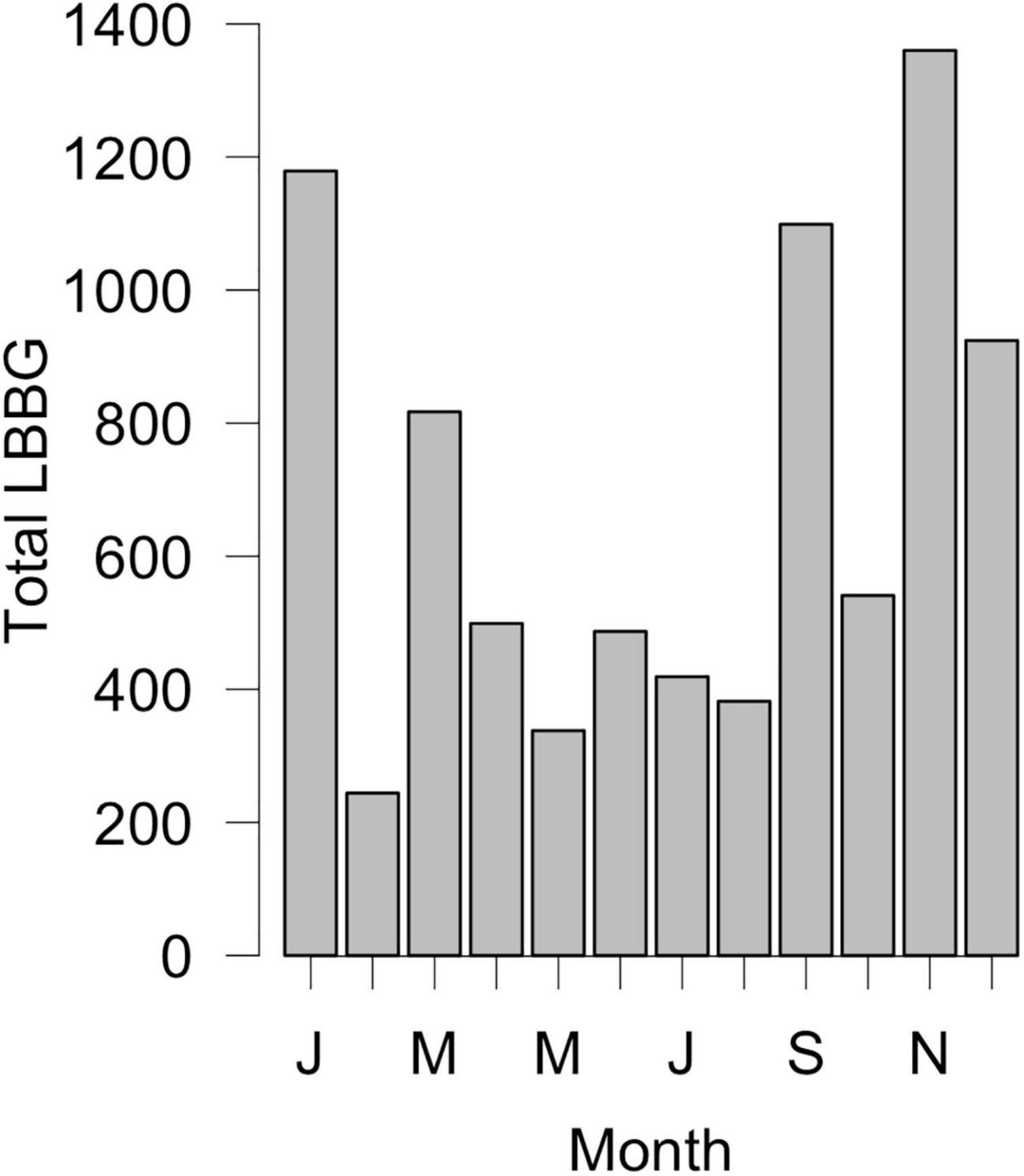
Figure 2. Monthly totals of LBBG recorded in Massachusetts from the journal Bird Observer between the years 1971–2018. LBBG are more commonly seen from September to March.
Our study focuses on the Atlantic subspecies graellsii, which is predominantly responsible for the westward range expansion of LBBG across the Atlantic that coincided with the rapid increase in global population during the mid- to late-twentieth century (Olsen and Larsson, 2004; Sibley, 2014; Burger et al., 2020). The other Atlantic subspecies intermedius was not involved in this range expansion, and the other three recognized subspecies, fuscus, heuglini, and taimyrensis (Liebers and Helbig, 2002; Collinson et al., 2008), are restricted to breeding and wintering sites outside the Atlantic area (Cramp and Simmons, 1983; Olsen and Larsson, 2004), and are very unlikely to have contributed to these movements (Sibley, 2014).
We collected records of vagrant LBBGs in North America from two sources. Numbers of wintering gulls (14 December to 5 January) were taken from Christmas Bird Count (CBC) data (Figure 3; National Audubon Society, 2020) from 1986 to 2018. All count circles with available data within the North American continent were included, covering the contiguous United States, all Canadian provinces, Central America (Mexico and Panama) and the Caribbean (Bahamas, Bermuda, the Dominican Republic, Haiti, and Puerto Rico). To correct for variation in observer effort across years, data were extracted as CBC trend estimates of median abundance (T. Meehan, pers. comm. 2020, Meehan et al., 2020), which were calculated using Bayesian hierarchical models (Soykan et al., 2016).
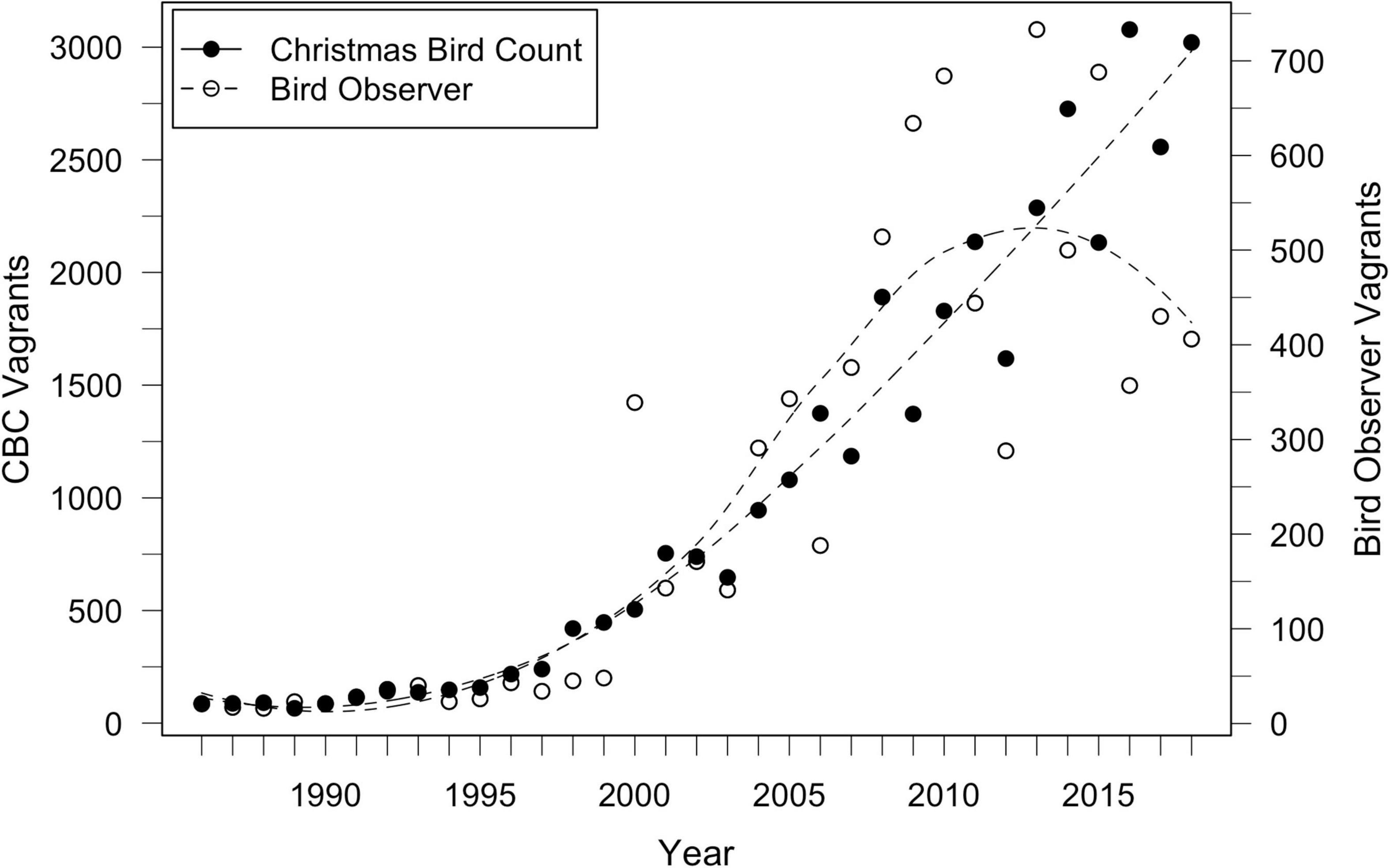
Figure 3. Records of individual LBBG seen in North America during yearly Christmas Bird Counts (CBC) and from Bird Observer records from 1986 to 2018. LBBG sightings increased rapidly after the early 1990s, and in the last decade, over 1,000 individuals have been present each year. CBC and Bird Observer records are positively correlated with each other (r = 0.82, p < 0.001).
Additional year-round sighting records of gulls were extracted from Bird Observer1, a Massachusetts-based journal, and used to analyze ages of vagrant LBBG. Data were available from 1973 to 2018. We chose this publication due to its intensive and consistent record-keeping of birds sighted in the state throughout the year (Veit, 1997), often with individuals identified to age. Additionally, Massachusetts has a high concentration of LBBGs each year (Veit and Petersen, 1993; Nisbet et al., 2013), and is likely reflective of the pattern of sightings throughout North America (Figure 3; correlation between CBC and Bird Observer (see footnote 1) data: r = 0.82, p < 0.001).
While eBird has a large collection of sighting records of vagrant LBBG, we chose to use CBC and Bird Observer (see footnote 1) data since data from these sources are available for the entire time series of our study, and records are ensured to be single individuals (Zawadzki et al., 2019). eBird was not founded until 2002, therefore any sightings prior to this year may not be available on the platform. Additionally, eBird often reports multiple sightings for the same individual, and there are no consistent methods to distinguish single birds from multiple records. Further, standardization of protocol used during CBCs ensures that vagrant data are consistent across years.
We refrain from drawing an arbitrary line over whether each particular LBBG in North America is a vagrant, but rather define vagrancy as the process of birds moving outside of the core of their species’ range, driven by growing populations (DeBenedictis, 1971; DeSante, 1983; Patten and Marantz, 1996; Veit, 1997, 2000; Zawadzki et al., 2019) and exploratory movements (Grinnell, 1922; Baker, 1978, Baker, 1980). This definition incorporates vagrants that become recurrent seasonal visitors, and those vagrant individuals that travel to an area and stay for their lifetime. Accordingly, all sightings of LBBG in North America have been included in our analyses, including individuals that may return annually each winter.
Breeding population data were extracted from countries where graellsii breed, i.e., the United Kingdom (hereafter, UK), Iceland, and Greenland (Figure 4). UK data were taken from the Seabird Monitoring Programme (SMP) (JNCC, 2020), which annually monitors breeding seabird colonies in Britain and Ireland. Data are recorded as numbers of apparently occupied nests (AON), and an estimate of AON for each year is derived from these counts (I. Win, pers. comm. 2020, JNCC, 2020). Data were available from 1986 to 2018.
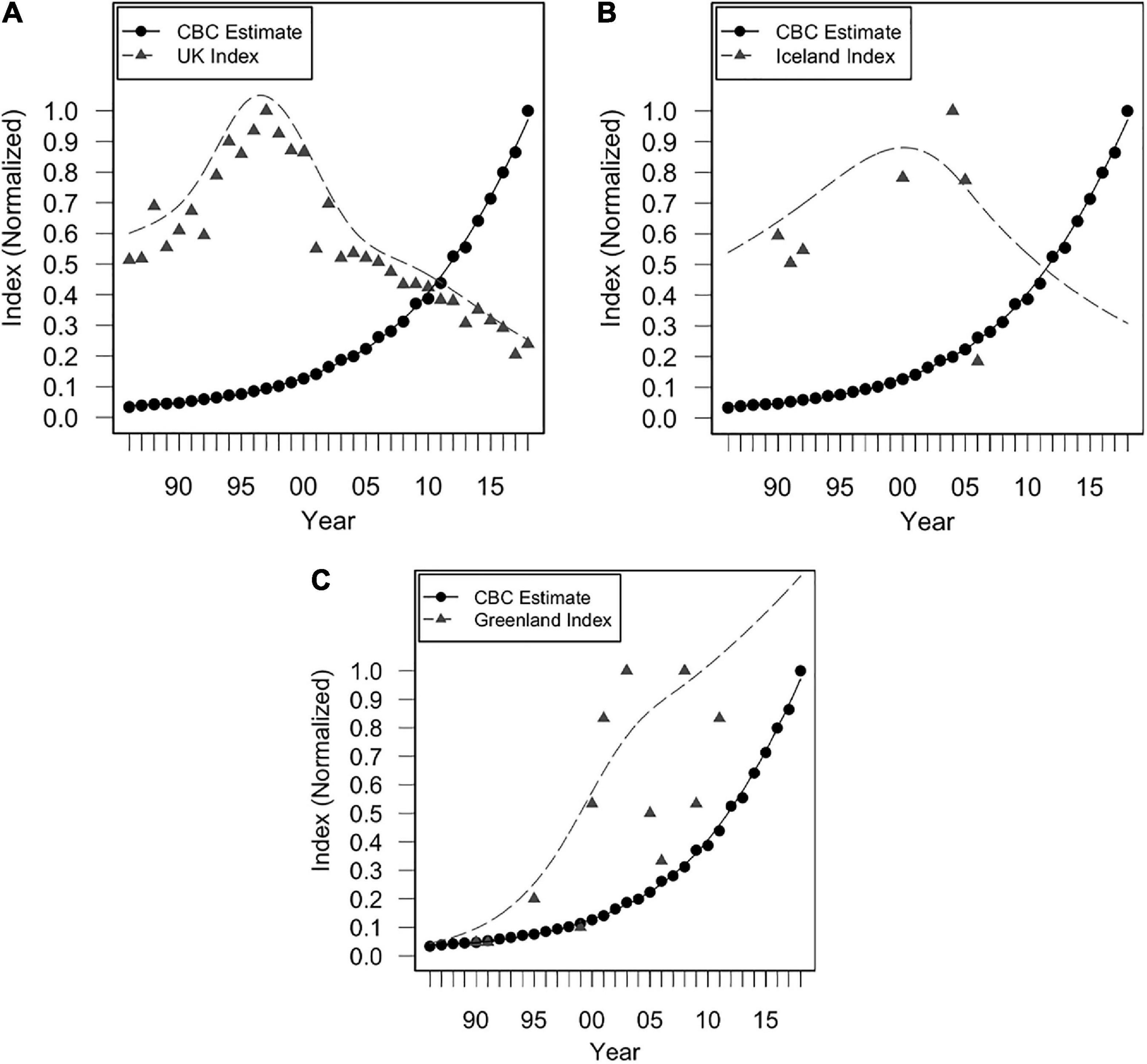
Figure 4. Indices of annual breeding population size from 1986 to 2018 for (A) the UK, (B) Iceland (colony of Midnesheidi), and (C) Greenland (colony of Eqaluit), and Christmas Bird Count (CBC) trend estimates of vagrants. Trend estimates are calculated from CBC surveys as estimates of median abundance, using Bayesian hierarchical methods (Soykan et al., 2016; Meehan et al., 2020). Normalized values were obtained by dividing each value in a given dataset by the maximum value of that dataset.
Iceland and Greenland do not have routine monitoring programs, therefore information on breeding colonies came from other sources. Population estimates from Iceland were obtained by estimating the number of active nests at the breeding colony of Midnesheidi, Reykjanes Peninsula in southwest Iceland, the largest breeding colony in Iceland. We chose to only use data from Midnesheidi because it is the only LBBG colony that has been consistently surveyed in Iceland, and population trends at this colony are likely representative of the overall breeding population in the country (Hallgrimsson et al., 2006). The addition of limited surveys of other colonies around Iceland would only increase the estimates by a few hundred nests per year (Hallgrimsson et al., 2006), which is within the 95% confidence interval of the nest estimates at Midnesheidi. Estimates were taken between the years 1990 and 2006. Nests were counted within a sample of 4,000 m2 plots within the colony, and the number of plots surveyed each year ranged from 28 to 34. Outlines of the whole colony were observed and marked on a map, and the colony edge was observed from high points using a telescope and by walking with a GPS unit. The edge was determined through observations of nest defense by adults, a standard means of determining breeding status of birds (e.g., Mitchell et al., 2004). The colony area was then calculated using global information system (GIS; Esri Inc, 2008, ArcMap™ 9.3). To calculate the mean density, and 95% confidence limits of active nests, we used the program NEGBINOM (Krebs, 1999). The size of the breeding population was found by multiplying the density by the area.
Greenland data were collected from the Greenland Seabird Colony Register. These data consisted of a series of sightings and surveys conducted across Greenland between 1986 and 2018. Data prior to 1990 were excluded from our analysis since the first confirmed case of breeding was not until 1990. Due to inconsistent survey efforts across Greenland, we used data from the most consistently surveyed colony of Eqaluit in southwest Greenland to represent breeding populations across Greenland. The first evidence of breeding LBBG was at Eqaluit in 1990, and Eqaluit has been surveyed 12 times between 1990 and 2018. Counts of LBBG were recorded as either individuals, pairs, or nests. A total pair count was calculated for each year, using the formula: pairs in year t + nests in year t + (individuals in year t) × 0.7, where 0.7 is the k value used to convert individuals to pairs (Harris et al., 2015).
Breeding data from all populations were converted to indices for model analysis. Indices were calculated as percentages relative to the base-year (first year of monitoring), which was set to 100% (Thomas, 1993). Percentages were divided by 100 to derive an index value. Growth rates (r) for each population were calculated as r = ln(λ).
We constructed generalized linear models (GLMs) for each breeding population to estimate the relationship between numbers of vagrants in North America (as trend estimates from CBC data), and indices of annual breeding population size and annual growth rate (r). We constrained our models to 1986 to 2018, when range expansion to Greenland and North America occurred. We formulated four competitive GLMs, including a null (no predictors). Models were ranked using Akaike information criterion (AIC) comparison, with AIC values corrected for small sample sizes (AICc) using function aictab from the package “AICcmodavg” (Mazerolle, 2020). The model with the lowest AICc value was chosen as the best model if it was at least two AICc values lower than the next most competitive model (Burnham and Anderson, 2003). All analyses were conducted using R (R Core Team, 2019).
Due to the non-linear trajectory of population count data, we also constructed generalized additive models (GAMs) for each breeding population to estimate the relationship between numbers of vagrants in North America (as trend estimates from CBC data), and indices of annual breeding population size. Growth rates (r) were not included in these models due to insufficient sample sizes to include additional smoothing parameters. GAMs are much better at dealing with variability in count data due to the non-linear estimates calculated (Wood, 2006; Knape, 2016), and are often used to estimate changes in population over time (Knape, 2016). Our GAMs were constrained to the same timeframe as our GLMs. A total of four models were constructed, including a null (no explanatory variables), using the package “mgcv” in R (Wood, 2017). We used a negative binomial distribution of errors to correct for overdispersion in our data (Lindén and Mäntyniemi, 2011). Models were ranked using AIC comparison using the function model.sel from package “MuMIn” (Bartoń, 2020) to determine which model best fit our data. All analyses were conducted using R (R Core Team, 2019).
Our most competitive model was the model for Greenland’s breeding population (Table 1). Occurrence of vagrancy was positively correlated with both Greenland’s index of population size and growth rate, albeit not significantly (Table 1). The model for Iceland competed with the model for Greenland as the most competitive model, differing in AICc value by only 1.25. Iceland’s index of population size and growth rate were positively correlated with vagrancy, of which the index of population size was significantly correlated with vagrancy. UK breeding populations were significantly inversely related with vagrant occurrence (Table 1), and were the least competitive models alongside the null models.
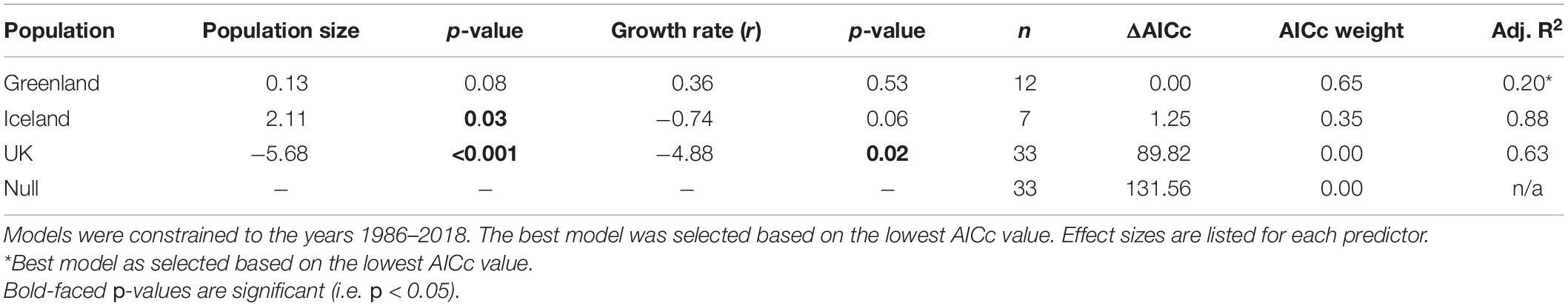
Table 1. Parameter estimates for univariate generalized linear models (GLMs) of the relationship between trend estimates of vagrant LBBG occurrence in North America from CBC data and indices of annual breeding population size and growth rate (r) of the breeding population in the UK, Iceland, and Greenland.
Our most competitive GAM was the model for Iceland’s breeding population (Table 2). Plots of this relationship indicate that Icelandic breeding populations seem to influence vagrant occurrence at two extremes (Figure 5B). When Icelandic populations are low or high, vagrants are abundant in North America. However, when Icelandic populations are steady, numbers of vagrants in North America are low. On the other hand, our GAM for Greenland’s breeding population showed a direct positive relationship between breeding population size and occurrence of vagrants in North America (Figure 5C), indicating occurrence of vagrants increases with increasing breeding population size in Greenland. UK breeding populations were inversely correlated with vagrancy (Figure 5A), and were the least competitive models alongside the null models.
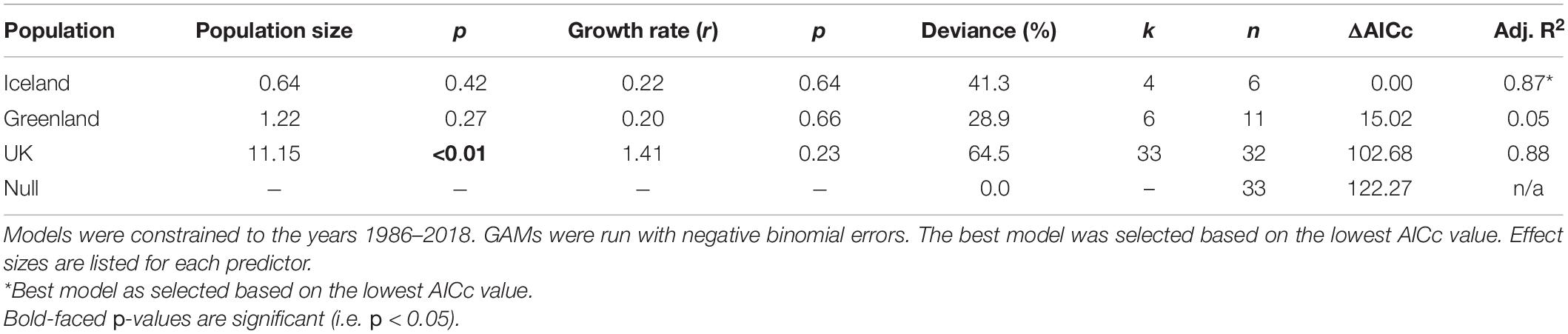
Table 2. Model selection for generalized additive models (GAMs) of the relationship between trend estimates of vagrant LBBG occurrence in North America from CBC data and indices of annual breeding population size and growth rate (r) of the breeding population in the UK, Iceland, and Greenland.
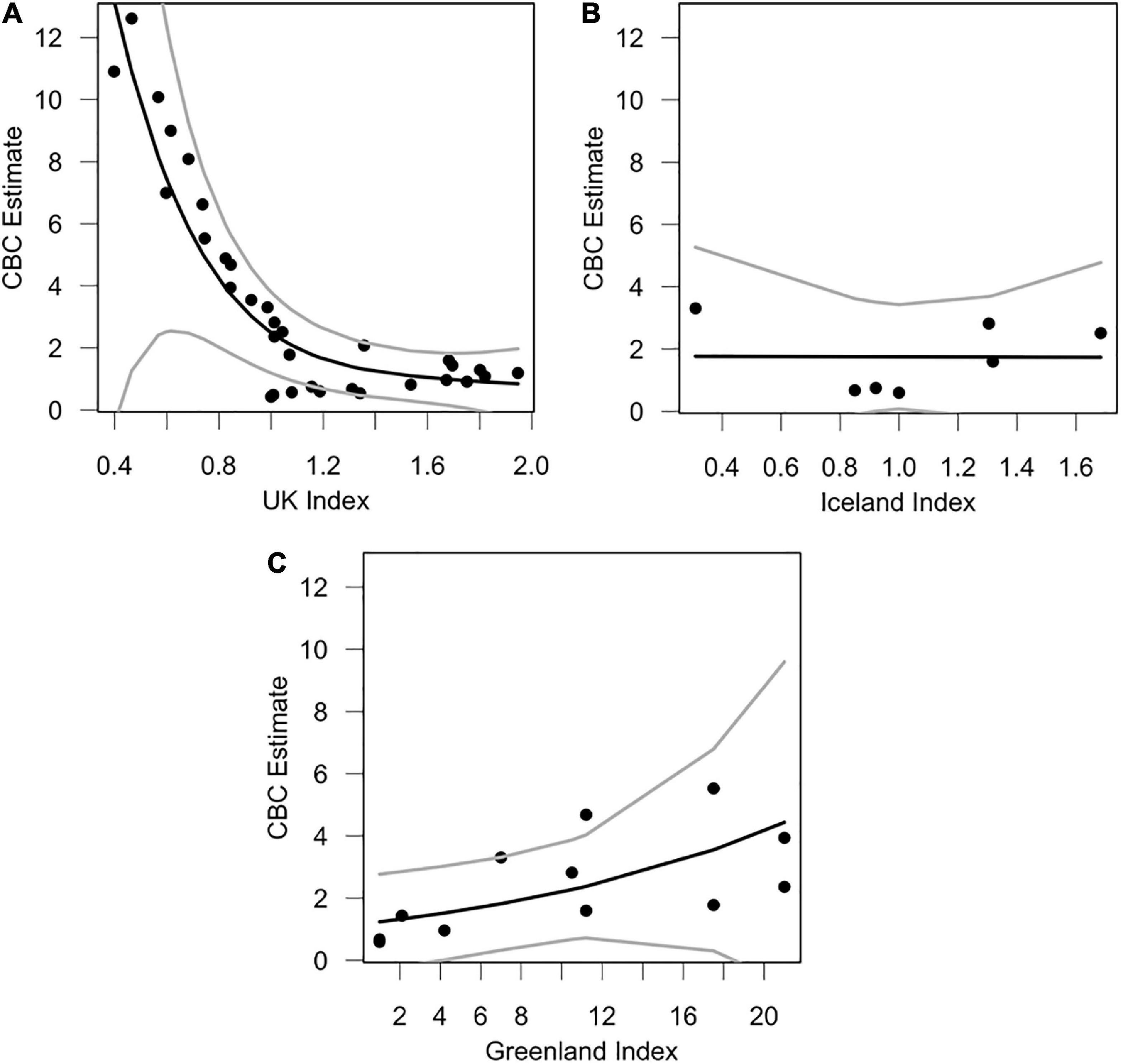
Figure 5. Trends between annual breeding population size and numbers of vagrants using GAMs. The black line indicates the trend of the model, and the gray lines represent the lower and upper 95% confidence intervals. The graphs are allocated as follows: (A) CBC vagrants ∼ UK pairs, (B) CBC vagrants ∼ Iceland pairs, (C) CBC vagrants ∼ Greenland pairs. CBC vagrants are calculated as trend estimates from CBC surveys, which are an estimate of median abundance, using Bayesian hierarchical methods (Soykan et al., 2016; Meehan et al., 2020). Breeding pair indices were calculated as the percent change in total breeding pairs in that year relative to the base-year (first year of monitoring), which was set to 100%. Percentages were divided by 100 to create an index.
We conclude that it is possible to predict potential source populations of vagrants using breeding population data. Our GLMs and GAMs for each breeding population modeled occurrence of vagrants better than the null model (Tables 1, 2). However, there are limitations present in this type of analysis if survey efforts are inconsistent or incomplete during the breeding season. For LBBG, the source population was likely derived from breeding populations in both Greenland and Iceland, though Greenland is most likely the source of the recent (since 2000) surge of vagrants to North America. The model that related Greenlandic populations to occurrence of vagrants was our most competitive model for our GLMs, while the model that related Icelandic populations to occurrence of vagrants was our most competitive model for our GAMs. While we acknowledge that other unknown or untested factors could also explain vagrancy, these are secondary to our study since we are focused on testing whether breeding population data can be used to predict a plausible source population of vagrants under the premise that population growth drives vagrancy (Patten and Marantz, 1996; Ralph and Wolfe, 2018; Zawadzki et al., 2019).
The ability to predict source populations of vagrants through breeding data alone is invaluable to studies on vagrancy, and will be widely applicable to a variety of vagrant species. Vagrants are difficult to study directly due to their inherent rarity, and have been seldom studied as a result. Having a methodology available to be able to study vagrants indirectly will increase the possibility for research in this area. This is particularly true of short-lived passerines, which are often too small for direct studies on their movement, such as through GPS-tracking. Further, this strong relationship between population and occurrence of vagrants has important implications for the role that vagrancy plays in both range expansion and colonization of species. Range expansion and/or colonization are a likely result of vagrancy, and studies on population dynamics of species can provide inference into species that are more likely to expand their range in the future (e.g., Jiguet and Barbet-Massin, 2013). Since population dynamics are also tied to a species’ habitat, it is imperative that we explore how habitat change influences vagrancy through its continued effect on population (DeBenedictis, 1971; DeSante, 1983; Patten and Marantz, 1996).
Iceland’s breeding population was likely important during early years of colonization in North America (either directly or indirectly; Figure 5B), prior to its stark decline in 2005 (82% decline between 2004 and 2006). Early colonization of North America coincided with an increase in both the Icelandic and Greenlandic breeding populations (Figure 4). It has been proposed that Greenland’s breeding population was founded by Icelandic gulls between 1986 and 1990 (Boertmann, 2008). It is therefore possible that some vagrant LBBG in North America originated from Iceland prior to their decline in 2005, either directly from Iceland, or through colonization of Greenland and subsequent migration to North America, though any records of LBBG in North America prior to 1990 must have derived from other populations since a breeding population was not yet established in Greenland. Nevertheless, it is unambiguous that the major increase of vagrant LBBG to North America occurred during a period of growth of the Greenland population (Figure 4). Greenland’s population has been significantly influential across the entire time series (Figure 5C).
Recent tracking efforts by Pennsylvania’s (PA) Game Commission confirm this link (Barber et al., in press). Barber et al. (in press) affixed 9 wintering vagrant LBBG in Pennsylvania with GPS devices, and discovered that five of these individuals traveled to Greenland in the summer for two consecutive migrations. Furthermore, a noticeable “jump” in the arrival of first calendar year to third calendar year birds occurred in Massachusetts and Rhode Island in 2005–2010 [Bird Observer (see footnote 1), eBird, 2017], and again in 2018–2021, including 500 immatures (<2 years old) at Fire Island, New York. Due to absence of breeding in North America, these birds must derive from a rapidly growing colony elsewhere. Therefore, our premise that increasing source populations are producing large numbers of vagrants is supported. Peaks of immature gulls in North America also coincide with peaks in breeding population size of Greenland LBBG (Supplementary Figure 1).
The large number of unbanded gulls in North America also indicates that they are arriving from locations where graellsii remain unbanded, such as colonies in western Iceland and Greenland. In spite of considerable color-banding effort in Iceland, Netherlands, and the British Isles, only two banded gulls have been re-sighted in North America: (1) a juvenile gull banded in Netherlands that was spotted as an adult in Long Island, New York on 7 October 1997, and (2) a first-winter gull from southwestern Iceland, that was seen during its first winter in Puerto Rico on 16 and 20 November 2002 (Hallgrimsson et al., 2011). No other re-sightings have been reported even though thousands of LBBG are seen in North America each year.
Our models also suggest that vagrant LBBG do not originate from the UK, and vagrancy is inversely correlated with UK LBBG populations (Table 1 and Figure 5A). UK breeding populations have declined rapidly since 2000 (Figure 4A), as a result of increased culling practices, and changes to landfill and fishing practices (Ross-Smith et al., 2014a,b; Nager and O’Hanlon, 2016). This rapid decline in UK breeders at a time when vagrants were increasing makes it unlikely that vagrant gulls originate from UK colonies. Additionally, decadal censuses of wintering gulls have found that UK-breeding LBBG are wintering more frequently in the UK (Burton et al., 2003, 2013; Banks et al., 2009; Ross-Smith et al., 2015), rather than migrating to southwest Europe and northwest Africa (Hallgrimsson et al., 2012). Since UK LBBG show high wintering site fidelity (Thaxter et al., 2012), increased residency of UK LBBG further decreases the likelihood that North American vagrants originate from UK colonies.
It has often been speculated that the recent influx of LBBG to North America is a result of breeding on the continent, rather than repeated instances of vagrancy. These breeding claims lack support. Only two instances of breeding have ever been confirmed in North America, and both cases were hybridizations between a LBBG and a Herring Gull (Larus argentatus)—one nesting pair in Juneau, Alaska in 1993 (vanVliet et al., 1993), and another pair on Appledore Island, Maine from 2007 to present, which has since fledged five hybrid chicks (Ellis et al., 2007, 2014). Additionally, despite the presence of some breeding plumaged adults during summer for nearly two decades, they have not been seen visiting breeding habitats, even though LBBG are known to breed in mixed gull colonies (Camphuysen, 2011). The majority of vagrant LBBG seen in North America are juveniles [Bird Observer (see footnote 1); Supplementary Figure 2A], particularly first calendar year birds (Supplementary Figure 2B), and adults are mainly seen outside of the breeding season (Supplementary Figure 3), making breeding unlikely.
Baker (1980) proposed that LBBG follow an exploratory migration model, whereby young birds explore during the first 18 months after fledging, and areas that are found to be more suitable for overwintering will facilitate a change in their migratory behavior as adults. Tracking studies of immature LBBG also show that pre-breeding LBBG are more prone to these pre-migratory and post-migratory dispersal movements (Pütz et al., 2007, 2008; Camphuysen, 2011). This coincides with the age profile we see in North America, where younger birds are seen throughout the year, while adult birds are mainly present during the winter season.
Our analysis did pose limitations due to inconsistent survey efforts during breeding. For LBBG, while surveys in the UK are annually consistent (JNCC, 2020), survey efforts in Iceland and Greenland vary widely from year to year, and many years are missing from the dataset. Furthermore, in Greenland, the same colonies are not surveyed each year, creating between-year variation that is not representative of the actual size of the breeding population (Boertmann and Frederiksen, 2016; Boertmann et al., 2020), and could only be resolved by limiting analyses to one colony (which itself was under-surveyed; n = 12; Supplementary Tables 1, 2). These irregularities constrained our models since we only had small sample sizes to work with. Despite these limitations, we feel that the data available faithfully identify the periods of maximum growth for each of these populations, though there is room for improvement in collecting future datasets.
Long-term, consistent, and systematic breeding bird surveys are necessary to more accurately assess patterns of vagrant occurrence and establishment of vagrant colonies. Such surveys do exist in a number of countries for other species [e.g., North American Breeding Bird Survey (Pardieck et al., 2020), SMP (JNCC, 2020), Netherlands Ecological Monitoring Network (Sovon, 2019), and many others], but this is not the case globally, particularly in areas of the globe that are less accessible. Furthermore, species that are secretive or classified as pests (many gulls) may be overlooked, even in countries where robust breeding survey efforts are in place. Annual study plots with standardized protocol for each species (where possible) should be the aim, and would benefit not only future studies on LBBG vagrancy, but on vagrancy and population dynamics as a whole. For species in which frequent surveys are not possible, modeling could be supplemented by other field methods, such as tracking of vagrant individuals to their breeding/summering grounds with GPS devices, or stable isotope analysis to determine the natal origin of vagrant individuals (De Jong et al., 2019), but both of these methods pose additional problems themselves since vagrants are hard to catch, and isoscapes (for stable isotope analysis) are not as well-defined across Europe (Hobson et al., 2004). Improving survey efforts of native breeders would be the best way to improve studies of vagrancy since these studies often require indirect means of analysis. Furthermore, from an analytical perspective, statistical approaches must be carefully considered to improve our understanding of population dynamics of vagrants. For example, GAMs may provide a more robust alternative to GLMs when accounting for temporal variation in count data, and are increasingly becoming the norm when modeling changes in populations over time (Knape, 2016).
Our study shows that data on breeding populations can be used to determine plausible source populations of vagrants, but the fit of these models will be greatly improved with standardized survey efforts at breeding sites, and implementation of GAMs over GLMs for count data. For LBBG, Greenland and Iceland have influenced the increase in vagrant LBBG to North America. While Iceland may have contributed to vagrancy in early years of colonization, Greenland populations have consistently increased alongside numbers of vagrants, suggesting Greenland is the source population of vagrant LBBG. We predict that within the next few decades LBBG will occur regularly enough in North America to be considered an established wintering population, and, following Boertmann’s (2008) predictions, may even establish a small breeding population. Future studies of vagrant LBBG should incorporate GPS-tracking of wintering gulls in North America to their breeding/summering grounds to verify the results of our model analysis, and uncover the migratory routes of North American LBBG. Stable isotope analyses and mitochondrial DNA studies (Liebers and Helbig, 2002) may also help to directly elucidate the origin of vagrant LBBG.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
LZ compiled available breeding population and vagrant data, designed the models and analyzed the data, and wrote the manuscript with input from all authors (GH, RV, LM, DB, NG, TG). GH collected Iceland data in the field. LR and DB collected Greenland data in the field. RV suggested the topic, and assisted LZ with collection and processing of the data. NG, TG, and RV aided LZ in analysis and interpretation of the models. TG supervised the project. All authors contributed to the article and approved the submitted version.
Monitoring at Midnesheidi was financially supported by the ISAVIA Keflavik Airport authorities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank all that have helped with the completion of this project. Flemming Merkel answered many of our questions about Greenland data during the early stages of analyses, Ilka Win at the JNCC provided data for UK LBBG and helped us understand the dataset, and Tim Meehan and Geoff LeBaron at Audubon assisted with interpretation of CBC data. We thank Agnar Ingolfsson and Pall Hersteinsson who started the count of Icelandic gulls at Midnesheidi, and introduced the survey to GH. We would also like to thank Karin Harding and four reviewers whose constructive comments and input helped greatly improve our manuscript. Furthermore, many thanks to the various nature conservations, research organizations, and volunteers who collected data on LBBG in the UK (through the SMP), Iceland and Greenland, as well as the compilers for both Christmas Bird Counts and Bird Observer (see footnote 1). And the utmost thanks to the dedicated birders across North America whose participation in Christmas Bird Counts and lone efforts continue to help us document vagrancy in action.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.637452/full#supplementary-material
Baker, R. R. (1978). The Evolutionary Ecology of Animal Migration. New York, NY: Holmes and Meier Publishers.
Baker, R. R. (1980). The significance of the Lesser black-backed gull to models of bird migration. Bird Study 27, 41–50. doi: 10.1080/00063658009476655
Banks, A. N., Burton, N. H. K., Calladine, J. R., and Austin, G. E. (2009). Indexing winter gull numbers in great britain using data from the 1953 to 2004 winter gull roost surveys. Bird Study. 56, 103–109. doi: 10.1080/00063650802681623
Bartoń, K. (2020). MuMIn: Multi-Model Inference. R Package Version 1.43.17. Available online at: https://CRAN.R-project.org/package=MuMIn.
Boertmann, D. (2008). The lesser black-backed gull, Larus fuscus, in Greenland. Arctic 61, 129–133. doi: 10.14430/arctic17
Boertmann, D., and Frederiksen, M. (2016). Status of Greenland populations of great black-backed gull (Larus marinus), lesser black-backed gull (Larus fuscus) and herring gull (Larus argentatus). Waterbirds 39, 29–35. doi: 10.1675/063.039.sp109
Boertmann, D., Merkel, F., and Gilg, O. (2020). Seabird breeding colonies in East and North Greenland: a baseline. Arctic 73, 20–39. doi: 10.14430/arctic69885
Burger, J., Gochfeld, M., Kirwan, G. M., Christie, D. A., and de Juana, E. (2020). “Lesser black-backed gull (Larus fuscus), version 1.0,” in Birds of the World, eds J. del Hoyo, A. Elliott, J. Sargatal, D. A. Christie, and E. de Juana (Ithaca, NY: Cornell Lab of Ornithology). doi: 10.2173/bow.lbbgul.01
Burnham, K. P., and Anderson, D. R. (2003). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd Edn. New York, NY: Springer-Verlag.
Burton, N. H. K., Banks, A. N., Calladine, J., and Austin, G. E. (2013). The importance of the United Kingdom for wintering gulls: population estimates and conservation requirements. Bird Study 60, 87–101. doi: 10.1080/00063657.2012.748716
Burton, N. H. K., Musgrove, A., Rehfisch, M. M., Sutcliffe, A., and Waters, R. (2003). Numbers of wintering gulls in the United Kingdom, Channel Islands, and Isle of man: a review of the 1993 and previous winter gull roost surveys. Br. Birds 96, 376–401.
Camphuysen, C. J. (2011). Lesser Black-Backed Gulls Nesting at Texel: Foraging Distribution, Diet, Survival, Recruitment and Breeding Biology of Birds Carrying Advanced GPS Loggers. NIOZ-Report 2011-05. Texel: Royal Netherlands Institute for Sea Research.
Collinson, J. M., Parkin, D. T., Knox, A. G., Sangster, G., and Svensson, L. (2008). Species boundaries in the herring and lesser black-backed gull complex. Br. Birds. 101, 340–363.
Cottridge, D., and Vinicombe, K. (1996). Rare Birds in Britain and Ireland - A Photographgic Record. London: Harper Collins.
Cramp, S., and Simmons, K. E. L. (eds) (1983). The Birds of the Western Palearctic, Vol. 3. Oxford: Oxford University Press.
De Jong, A., Torniainen, J., Bourski, O. V., Heim, W., and Edenius, L. (2019). Tracing the origin of vagrant Siberian songbirds with stable isotopes: the case of the yellow-browed warbler (Abrornis inornatus) in fennoscandia. Ornis Fenn 96, 90–99.
De Juana, E., and Garcia, E. F. J. (2010). Vagrancy or migration: why do american teals cross the atlantic? Ardeola 57, 417–430.
DeBenedictis, P. (1971). Wood warblers and vireos in California: the nature of the accidental. Calif. Birds 2, 111–128.
DeSante, D. F. (1973). An Analysis of the Fall Occurrences and Nocturnal Orientations of Vagrant Wood Warblers (Parulidae) in California. PhD thesis. Stanford, CA: Stanford University.
DeSante, D. F. (1983). Annual variability in the abundance of migrant landbirds on Southeast Farallon Island. California. Auk 100, 826–852. doi: 10.1093/auk/100.4.826
eBird (2017). eBird: An Online Database of Bird Distribution and Abundance [Web Application]. Ithaca, NY: Cornell Lab of Ornithology.
Elkins, N. (1979). Nearctic landbirds in Britain and Ireland: a meterological analysis. Br. Birds. 72, 417–433.
Elkins, N. (1999). Recent records of Nearctic landbirds in Britain and Ireland. Br. Birds 92, 83–95. Available online at: https://britishbirds.co.uk/content/recent-records-nearctic-landbirds-britain-and-ireland
Ellis, J. C., Bogdanowicz, S. M., Stoddard, M. C., and Clark, L. W. (2014). Hybridization of a lesser black-backed gull and herring gulls in eastern North America. Wilson J. Ornithol. 126, 338–345. doi: 10.1676/13-095.1
Ellis, J. C., Stoddard, M. C., and Clark, L. W. (2007). Breeding by a lesser black-backed gull (Larus fuscus) on the Atlantic coast of North America. North Am. Birds 61, 546–548.
Esri Inc (2008). ArcMap (Version 9.3). Esri Inc. Available online at: https://desktop.arcgis.com/en/arcmap/ (accessed 2018).
Farnsworth, A., La Sorte, F. A., and Iliff, M. J. (2015). Warmer summers and drier winters correlate with more winter vagrant Purple Gallinules (Porphyrio martinicus) in the North Atlantic Region. Wilson J. Ornithol. 127, 582–592. doi: 10.1676/14-086.1
Hallgrimsson, G. T., Gunnarsson, H. V., Torfason, O., Buijs, R.-J., and Camphuysen, K. C. J. (2012). Migration pattern of Icelandic lesser black-backed gulls Larus fuscus graellsii: indications of a leap-frog system. J. Ornithol. 153, 603–609. doi: 10.1007/s10336-012-0816-4
Hallgrimsson, G. T., Gunnarsson, H., and Hersteinsson, P. (2006). Stærð sílamáfsvarps á Álftanesi á Mýrum [Size of the Lesser Black-backed Gull colony at Álftanes on Mýrar (W-Iceland) in 2005]. Bliki 27, 55–57.
Hallgrimsson, G. T., van Swelm, N. D., Gunnarsson, H. V., Johnson, T. B., and Rutt, C. L. (2011). First two records of Europe-banded lesser black-backed gulls Larus fuscus in America. Mar. Ornithol. 39, 137–139.
Hampton, S. (1997). Rare migrants in California: the determinants of their frequency. West. Birds 28, 30–42.
Harris, M. P., Heubeck, M., Newell, M. A., and Wanless, S. (2015). The need for year-specific correction factors (k values) when converting counts of individual common guillemots uria aalge to breeding pairs. Bird Study 62, 276–279. doi: 10.1080/00063657.2015.1017444
Hobson, K. A., Bowen, G. J., Wassenaar, L. I., Ferrand, Y., and Lormee, H. (2004). Using stable hydrogen and oxygen isotope measurements of feathers to infer geographical origins of migrating European birds. Oecologia 141, 477–488. doi: 10.1007/s00442-004-1671-7
Jiguet, F., and Barbet-Massin, M. (2013). Climate change and rates of vagrancy of Siberian bird species to Europe. IBIS 155, 194–198. doi: 10.1111/ibi.12001
Jiguet, F., Doxa, A., and Robert, A. (2008). The origin of out-of-range pelicans in Europe: wild bird dispersal or zoo escapes? Ibis 150, 606–618. doi: 10.1111/j.1474-919X.2008.00830.x
JNCC (2020). Seabird Population Trends and Causes of Change: 1986-2018 Report.[Online]. Peterborough: Joint Nature Conservation Committee.
Knape, J. (2016). Decomposing trends in Swedish bird populations using generalized additive mixed models. J. Appl. Ecol. 53, 1852–1861. doi: 10.1111/1365-2664.12720
Liebers, D., and Helbig, A. J. (2002). Phylogeography and colonization history of lesser black-backed gulls (Larus fuscus) as revealed by mtDNA sequences. J. Evol. Biol. 15, 1021–1033. doi: 10.1046/j.1420-9101.2002.00454.x
Lindén, A., and Mäntyniemi, S. (2011). Using the negative binomial distribution to model overdispersion in ecological count data. Ecoloy 92, 1414–1421. doi: 10.1890/10-1831.1
Massa, C., Doyle, M., and Fortunato, R. C. (2014). On how cattle egret (Bubulcus ibis) spread to the Americas: meteorological tools to assess probable colonization trajectories. Int. J. Biometeorol. 58, 1879–1891. doi: 10.1007/s00484-014-0790-z
Mazerolle, M. J. (2020). AICcmodavg: Model Selection and Multimodel Inference Based On (Q)AIC(c). R Package Version 2.3-1.
McLaren, I. A., Lees, A. C., Field, C., and Collins, K. J. (2006). Origins and characteristics of Nearctic landbirds in Britain and Ireland in autumn: a statistical analysis. IBIS 148, 1–20. doi: 10.1111/j.1474-919X.2006.00574.x
Meehan, T. D., LeBaron, G. S., Dale, K., Krump, A., Michel, N. L., and Wilsey, C. B. (2020). Abundance Trends of Birds Wintering in the USA and Canada, From Audubon Christmas Bird Counts, 1966-2019, Version 3.0. New York, NY: National Audubon Society.
Mitchell, P. I., Newton, S. F., Ratcliffe, N., and Dunn, T. E. (2004). Seabird Populations of Britain and Ireland. London: T. & AD Poyser.
Nager, R. G., and O’Hanlon, N. J. (2016). Changing numbers of the three gull species in the British Isles. Waterbirds. 39, 15–28. doi: 10.1186/s12862-015-0484-0
National Audubon Society (2020). The Christmas Bird Count Historical Results[Online]. Available online at: http://www.christmasbirdcount.org (accessed July 14, 2020).
Nisbet, I. C., Veit, R. R., Auer, S. A., and White, T. P. (2013). Marine birds of the eastern United States and the bay of fundy. Nuttall Ornithol. Monogr. 29:188. doi: 10.1371/journal.pone.0194389
Olsen, K. M., and Larsson, H. (2004). Gulls of North America, Europe and Asia. Princeton: Princeton University Press.
Pardieck, K. L., Ziolkowski, D. J. Jr., Lutmerding, M., Aponte, V. I., and Hudson, M.-A. R. (2020). North American Breeding Bird Survey Dataset 1966 - 2019: U.S. Geological Survey Data Release. Reston, VA: U.S. Geological Survey, doi: 10.5066/P9J6QUF6
Patten, M. A., and Marantz, C. A. (1996). Implications of vagrant southeastern vireos and warblers in California. Auk 113, 911–923. doi: 10.2307/4088868
Pfeifer, R., Stadler, J., and Brandl, R. (2007). Birds from the Far East in Central Europe: a test of the reverse migration hypothesis. J. Ornithol. 148, 379–385. doi: 10.1007/s10336-007-0140-6
Phillips, B. L., Brown, G. P., and Shine, R. (2010). Evolutionarily accelerated invasions: the rate of dispersal evolves upwards during the range advance of cane toads. J. Evol. Biol. 23, 2595–2601. doi: 10.1111/j.1420-9101.2010.02118.x
Post, P. W., and Lewis, R. H. (1995). The lesser black-backed gull in the americas: occurrence and subspecific identity. Part I: Taxonomy, distribution, and migration. Birding 27, 282–290.
Pütz, K., Helbig, A. J., Pedersen, K. T., Rahbek, C., Saurola, P., and Juvaste, R. (2008). From fledging to breeding: long-term satellite tracking of the migratory behaviour of a Lesser Black-backed Gull Larus fuscus intermedius. Ringing Migr. 24, 7–10. doi: 10.1080/03078698.2008.9674376
Pütz, K., Rahbek, K. C., Saurola, P. L., Pedersen, K. T., Juvaste, R., and Helbig, A. J. (2007). Satellite tracking of the migration pathways of first-year LBBGs Larus fuscus departing from the breeding grounds of different subspecies. Vogelwelt 128, 141–146.
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rabøl, J. (1969). Reversed migration as a cause of westward vagrancy by four Phylloscopus warblers. Br. Birds 62, 89–92.
Ralph, C. J., and Wolfe, J. D. (2018). Factors affecting the distribution and abundance of autumn vagrant New World warblers in northwestern California and southern Oregon. PeerJ. 6:e5881. doi: 10.7717/peerj.5881
Robbins, C. S. (1980). Predictions of future Nearctic landbird vagrants to Europe. Br. Birds 73, 448–457.
Román-Palacios, C., and Wiens, J. J. (2020). Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. U. S. A. 117, 4211–4217. doi: 10.1073/pnas.1913007117
Ross-Smith, V. H., Grantham, M. J., Robinson, R. A., and Clark, J. A. (2014a). Analysis of Lesser Black-Backed Gull Data to Inform Meta-Population Studies. BTO Research Report No. 654. Norfolk: Thetford.
Ross-Smith, V. H., Robinson, R. A., Banks, A. N., Frayling, T. D., Gibson, C. C., and Clark, J. A. (2014b). The lesser black-backed gull Larus fuscus in England: how to resolve a conservation conundrum. Seabird 27, 41–61.
Ross-Smith, V. H., Robinson, R. A., and Clark, J. A. (2015). Dispersal and Movements of Lesser Black-Backed Gull in Europe. BTO Research Report No. 671. Norfolk: Thetford.
Southwood, T. R. E., May, R. M., Hassell, M. P., and Conway, G. R. (1974). Ecological strategies and population parameters. Am. Nat. 108, 791–804. doi: 10.1086/282955
Sovon (2019). Netwerk Ecologische Monitoring (Network Ecological Monitoring). Provincies & CBS. Available online at: https://www.sovon.nl/ (accessed July 15, 2020).
Soykan, C. U., Sauer, J., Schuetz, J. G., LeBaron, G. S., Dale, K., and Langham, G. M. (2016). Population trends for North American winter birds based on hierarchical models. Ecosphere 7:e01351. doi: 10.1002/ecs2.1351
Szűcs, M., Melbourne, B. A., Tuff, T., and Hufbauer, R. A. (2014). The roles of demography and genetics in the early stages of colonization. Proc. R. Soc. B. 281:20141073. doi: 10.1098/rspb.2014.1073
Thaxter, C. B., Ross-Smith, V. H., Clark, N. A., Conway, G. J., Wade, H., Masden, E. A., et al. (2012). Measuring the Interaction Between Marine Features of Special Protection Areas with Offshore Wind Farm Development Zones Through Telemetry: Second Year Report. BTO Research Report No. 610. Norfolk: Thetford.
Thomas, G. E. (1993). Estimating annual total heron population counts. Appl. Stat. 42, 473–486. doi: 10.2307/2986326
Thorup, K. (2004). Reverse migration as a cause of vagrancy. Bird Study 51, 228–238. doi: 10.1080/00063650409461358
vanVliet, G., Marshall, B., Craig, D., and Egolf, J. (1993). First record of nesting activity by a Lesser Black-backed Gull (Larus fuscus) in North America. Bull. Pac. Seabirds Group 20:21.
Veit, R. R. (1997). Long-distance dispersal and population growth of the yellow-headed blackbird Xanthocephalus xanthocephalus. Ardea 85, 135–143.
Veit, R. R. (1990). Do vagrant birds in Massachusetts reflect population growth and dispersal rather than weather patterns? Bird Obs. 18, 86–91.
Veit, R. R. (2000). Vagrants as the expanding fringe of a growing population. Auk 117, 242–246. doi: 10.1093/auk/117.1.242
Veit, R. R., and Lewis, M. A. (1996). Dispersal, population growth, and the Allee effect: dynamics of the house finch invasion of eastern North America. Am. Nat. 148, 255–274. doi: 10.1086/285924
Veit, R. R., and Petersen, W. R. (1993). Birds of Massachusetts. Massachusetts, MA: Massachusetts Audubon Society.
Veit, R. R., Zawadzki, L. C., Manne, L. L., Cales, P., Fibikar, D., Curley, S., et al. (2016). Vagrancy and colonization of St. Thomas and St. John, U.S. Virgin Islands, by Adelaide’s Warblers (Setophaga adelaidae). J. Caribb. Ornithol. 29, 47–50.
Wetlands International (2020). Waterbird Population Estimates. Available online at: http://wpe.wetlands.org/ (accessed July 25 2020).
Wood, S. (2006). Generalized Additive Models: An Introduction With R. Boca Raton, FL: CRC Press. doi: 10.1201/9781420010404
Wood, S. N. (2017). Generalized Additive Models: An Introduction With R, 2nd Edn. Boca Raton, FL: Chapman and Hall/CRC.
Keywords: vagrancy, range expansion, colonization, long-distance dispersal (LDD), source population, Lesser black-backed gull
Citation: Zawadzki LC, Hallgrimsson GT, Veit RR, Rasmussen LM, Boertmann D, Gillies N and Guilford T (2021) Predicting Source Populations of Vagrants Using Breeding Population Data: A Case Study of the Lesser Black-Backed Gull (Larus fuscus). Front. Ecol. Evol. 9:637452. doi: 10.3389/fevo.2021.637452
Received: 03 December 2020; Accepted: 12 November 2021;
Published: 03 December 2021.
Edited by:
Karin Charlotta Harding, University of Gothenburg, SwedenReviewed by:
Steven Carl Latta, National Aviary, United StatesCopyright © 2021 Zawadzki, Hallgrimsson, Veit, Rasmussen, Boertmann, Gillies and Guilford. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucinda C. Zawadzki, bHVjaW5kYS56YXdhZHpraUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.