
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 10 June 2021
Sec. Paleontology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.636110
This article is part of the Research TopicRecent Advances in the Evolution of EuarchontogliresView all 15 articles
Palaeolagus, a late Eocene to early Miocene North American lagomorph genus, represented by numerous and well-preserved specimens, has been long considered a basal leporid, although it is currently understood as a stem lagomorph. Based on micro-computed tomography (μCT) data and 3D reconstructions, here we present the first description of intracranial structures of the nasal and auditory regions of a complete skull of Palaeolagus haydeni from the early Oligocene of Nebraska. Although Palaeolagus haydeni shows a puzzling mixture of extant leporid and ochotonid characters, it helps to polarize and re-evaluate already known lagomorph intracranial characters based on outgroup comparison with Rodentia and Scandentia. Common derived features of Palaeolagus haydeni and extant Lagomorpha are the dendritic maxilloturbinal and the excavated nasoturbinal that contacts the lamina semicircularis. Generally, Palaeolagus haydeni and Leporidae have several characters in common, some of which are certainly plesiomorphic (e.g., thin wall of bulla tympani and flat conic cochlea). Palaeolagus haydeni resembles Leporidae in having an interturbinal between the two frontoturbinals, and three ethmoturbinals plus one interturbinal between ethmoturbinal I and II. Now, this should also be regarded as a plesiomorphic grundplan pattern for Leporidae whereas ochotonids are derived from the lagomorph grundplan as concerns the number of frontoturbinals. Concerning the middle ear, Palaeolagus haydeni significantly contributes to the polarization of the anterior anchoring of the malleus in extant lagomorphs. Palaeolagus haydeni resembles the pattern observed in early ontogenetic stages of Ochotonidae, i.e., the attachment of the malleus to the ectotympanic via a short processus anterior. The patterns in adult ochotonids and leporids now can be regarded as two different and apomorphic character states. Autapomorphic characters of Palaeolagus haydeni are the reduced frontoturbinal 2 and the additional anterolaterally oriented process of the lamina semicircularis. Interestingly, among the investigated intracranial structures the loss of the secondary crus commune is the only apomorphic grundplan character of crown Lagomorpha.
Intracranial bony structures of the mammalian nasal and auditory regions provide proxies for sense organs of smell (the olfactory turbinals), balance (vestibular part of the inner ear bony labyrinth) and hearing (the auditory ossicles and cochlear part of the inner ear bony labyrinth) as well as for thermoregulation (the respiratory turbinals). Thus, they can significantly contribute to a deeper understanding of morphofunctional and ecological adaptations as demonstrated in extant and fossil mammal species (e.g., Spoor et al., 2007; Pfaff et al., 2017; Martinez et al., 2018, 2020; Wagner and Ruf, 2019). Furthermore, characters, especially those related to the auditory region, are frequently used in mammalian phylogeny to reconstruct relationships among major clades (Meng et al., 2003; Wible et al., 2009; Mennecart et al., 2016).
Lagomorpha have been rarely subject to detailed studies of these structures and the existing contributions concern almost exclusively extant taxa, particularly the domesticated form of Oryctolagus cuniculus (Voit, 1909; Frick and Heckmann, 1955; Hoyte, 1961; King et al., 2007). However, recent studies on extant Lagomorpha revealed phylogenetically relevant characters of the nasal and ear regions. Ochotonidae and Leporidae can clearly be distinguished by specific characters of the turbinal skeleton and of the middle ear such as the pattern of the processus anterior and the processus praearticularis internus of the malleus (Ruf, 2014; Maier et al., 2018).
When fossils are considered, the synapomorphy schemes for lagomorphs and their related groups (Duplicidentata as a whole) may be contradicted; a derived feature shared by living taxa may turn out to be secondary or convergent if fossil relatives do not possess that feature. Since Lagomorpha has been generally considered as a monophyletic (e.g., Asher et al., 2005; Fostowicz-Frelik and Meng, 2013) and morphologically conservative group (Fostowicz-Frelik, 2017; López-Torres et al., 2020), characters that we collect from fossil lagomorphs may help us to evaluate characters and their polarity obtained from the living lagomorph relatives.
The subject of this study is Palaeolagus haydeni, the most common and widespread fossil lagomorph of North America (Dawson, 1958, 2008) and arguably the best known fossil lagomorph to date. For decades, the phylogenetic position of Palaeolagus has remained somewhat disputed. Traditionally it has been considered a basal leporid (Dawson, 2008). In contrast, recent phylogenetic analyses consequently place this genus outside of the crown Lagomorpha (Asher et al., 2005; Wible, 2007; Fostowicz-Frelik, 2013; Fostowicz-Frelik and Meng, 2013; but see Asher et al., 2019). Nevertheless, Palaeolagus with its eight species, spanning from the late Eocene to the early Miocene is a key genus for a deeper understanding of lagomorph evolution. Palaeolagus haydeni, the type species of Palaeolagus, is represented by a large number of well-preserved specimens available for us to study, as compared to other fossil taxa. Recently, a detailed study of the skull anatomy of Palaeolagus haydeni supported a basal position based on a mixed pattern of ochotonid and leporid characters (Wolniewicz and Fostowicz-Frelik, 2021).
Here we present the first description of intracranial structures of the nasal and auditory regions of Palaeolagus haydeni and compare our observations to the pattern in extant Lagomorpha. We also discuss some character transformations based on new evidence, and consider the plausible ancestral character set for extant Lagomorpha. Our study complements the work by Wolniewicz and Fostowicz-Frelik (2021) that is based on the same specimen.
The study is based on μCT data obtained from the skull of an adult Palaeolagus haydeni (FMNH PM9476) housed in the Field Museum of Natural History (FMNH, Chicago, IL, United States). The skull was μCT scanned using a high-resolution GE phoenix| x-ray v|tome|x L 240 scanner (GE Measurement & Control Solutions) at the American Museum of Natural History (AMNH, New York, NY, United States). The parameters of the scan of the entire skull were as follows: voltage 170 kV, current 170 mA, and 0.1 mm Cu filter. The total of 1401 images were acquired at a resolution of 50.69 μm (isotropic voxels). A second scan of the posterior part of the skull (orbitotemporal and otical regions) has the following scan parameters: voltage 170 kV, current 170 mA, and 0.1 mm Cu filter, resolution 36.49 μm. Raw data were further reconstructed with GE phoenix| x-ray datos| x 2.0 software resulting in 16-bit TIFFs (1900 × 1000 pixel in size). Based on the μCT data virtual 3D reconstructions were performed with Avizo 9.0.1 (Thermo Fisher Scientific). Turbinals were segmented with the manual segmentation tool; hereby the entire turbinal structure up to the contact with the lateral wall of the nasal cavity, the lamina horizontalis and the lamina cribrosa was considered. The bony labyrinth and the auditory ossicles were segmented with the manual and automatic (magic wand) segmentation tools. The first scan was used for reconstruction of the selected structures (in Figures 1–3). The μCT image in Figure 4 as well as the auditory ossicles in Figure 5 are based on the second scan. The μCT data (image stacks) and the 3D models are deposited in the Dryad Digital Repository (Fostowicz-Frelik et al., 2021).
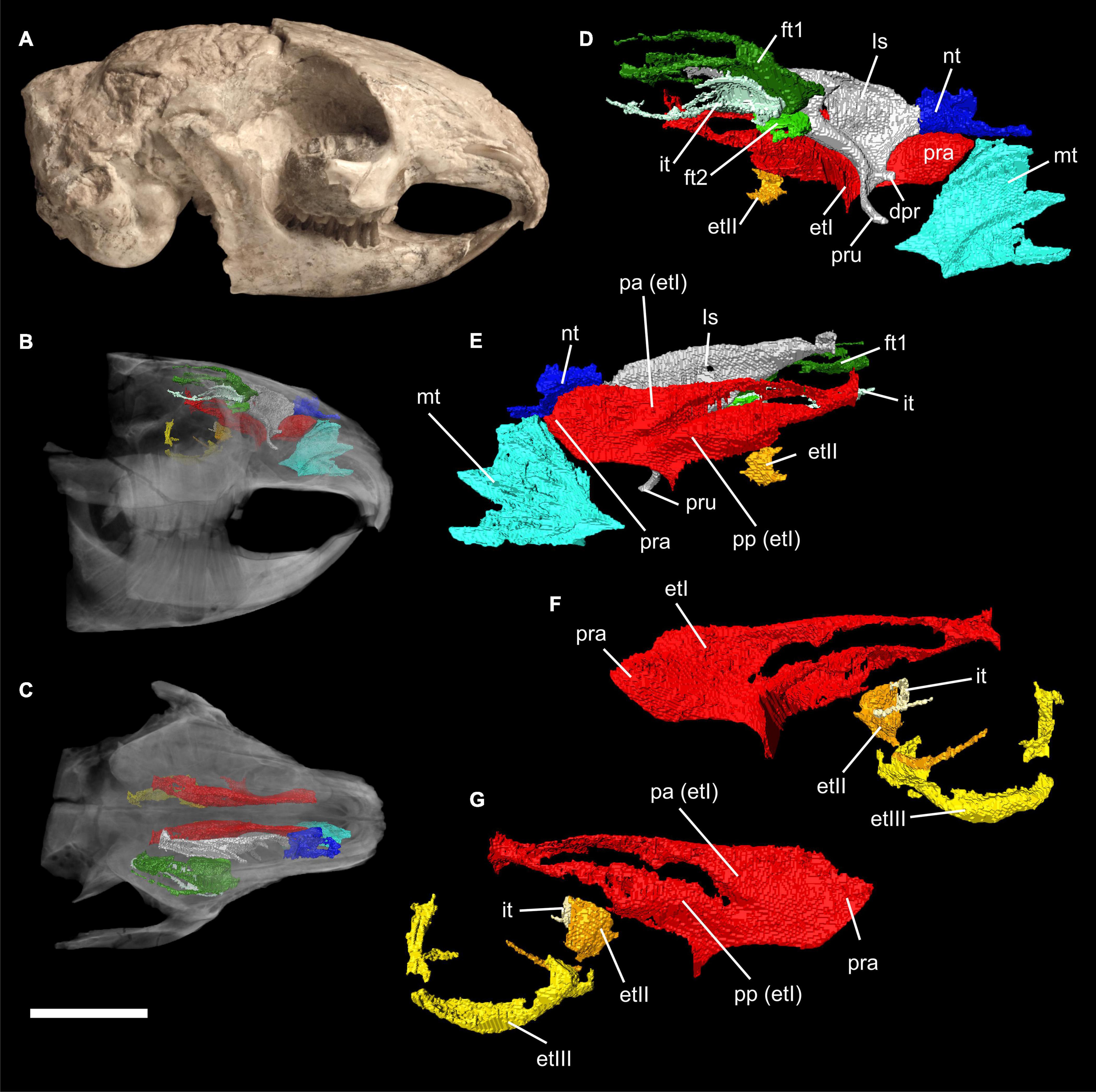
Figure 1. Virtual 3D reconstructions of the turbinal skeleton of Palaeolagus haydeni (FMNH PM9476). Actual skull in mirrored lateral view (A). Rostrum with highlighted turbinal skeleton in lateral (B) and dorsal (C) views. The skull bones are transparent. Right turbinal skeleton in lateral (D) and medial (E) views. The ethmoturbinals are incomplete. Left turbinal skeleton in lateral (F) and medial (G) views. Only the fronto- ethmo-, and interturbinals are reconstructed. Abbreviations: et I-III, ethmoturbinal I-III; dpr, dorsal process; ft 1-2, frontoturbinal 1-2; it, interturbinal; ls, lamina semicircularis; mt, maxilloturbinal; nt, nasoturbinal; pa, pars anterior; pp, pars posterior; pra, processus anterior; pru, processus uncinatus. Scale bar = 10 mm refers to (A–C); (D–G) not to scale.
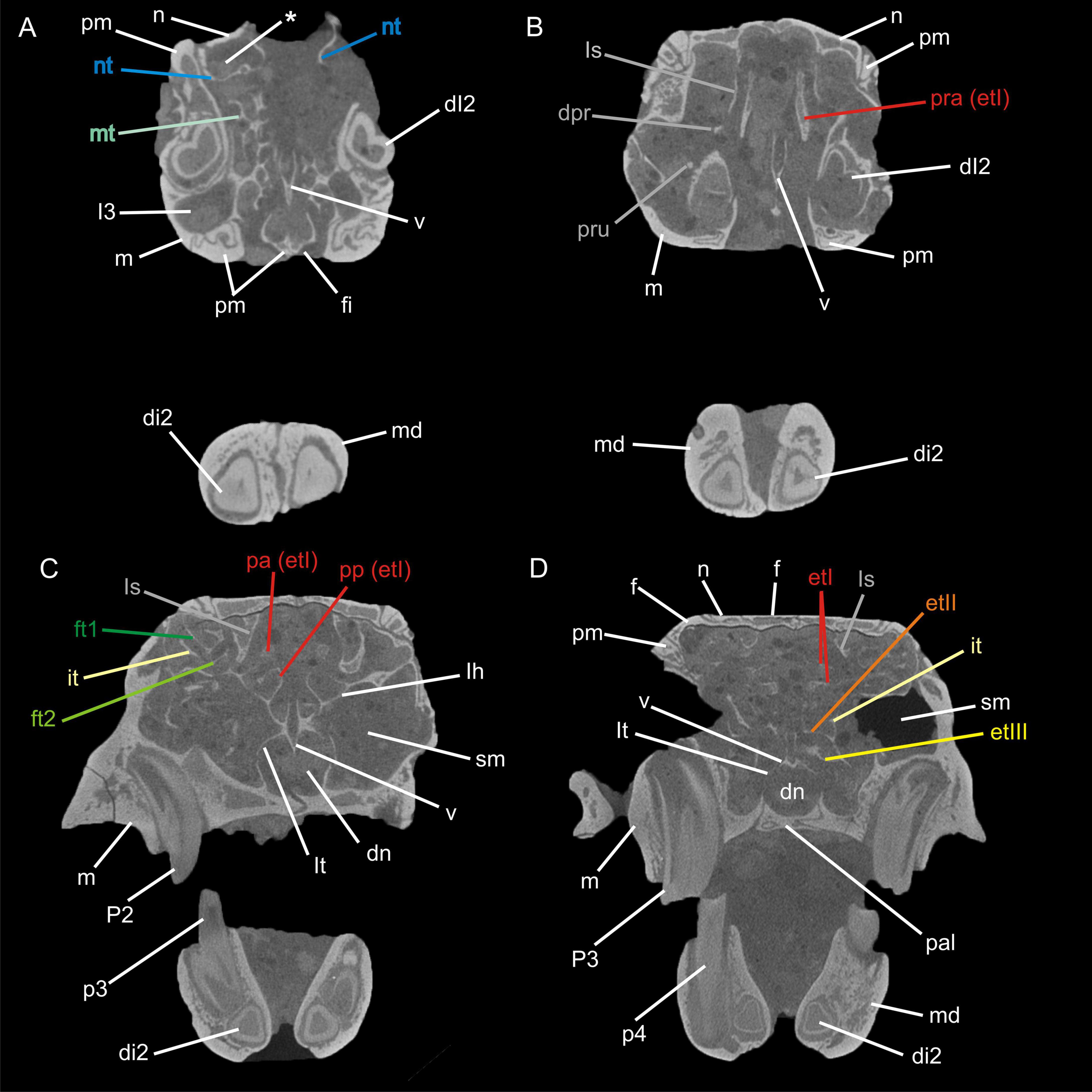
Figure 2. Transversal μCT images through the nasal cavity of Palaeolagus haydeni (FMNH PM9476) from anterior (A) to posterior (D). The asterisk (*) indicates the cavity formed by the nasoturbinal and the nasal. Abbreviations: dI2, deciduous upper incisor 2; di2, deciduous lower incisor 2; dn, ductus nasopharyngeus; dpr, dorsal process; et I-III, ethmoturbinal I–III; ft1-2, frontoturbinal 1–2; f, frontal; fi, foramen incisivum; I3, upper incisor 3; it, interturbinal; lh, lamina horizontalis; ls, lamina semicircularis; lt, lamina terminalis; m, maxilla; md mandible; mt, maxilloturbinal; n, nasal; nt, nasoturbinal; p3–4, lower premolars 3 and 4; P2–3, upper premolars 2 and 3; pa, pars anterior; pal, palatine; pm, premaxilla; pp, pars posterior; pra, processus anterior; pru, processus uncinatus; sm, sinus maxillaris; v, vomer. Not to scale.
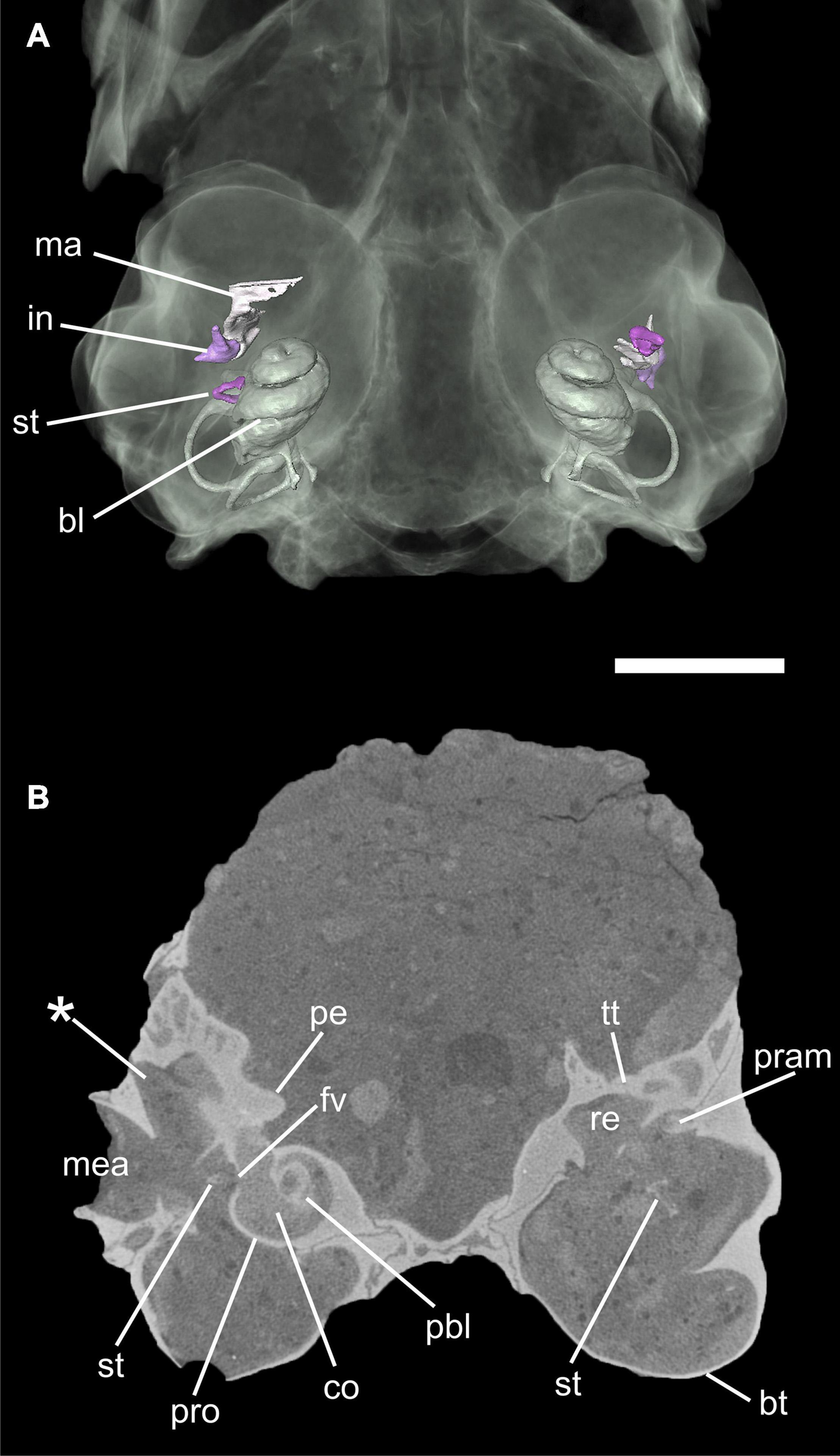
Figure 3. Virtual 3D reconstruction of the auditory region of Palaeolagus haydeni (FMNH PM9476) in ventral views (A). The skull bones are transparent in order to show the auditory ossicles and bony labyrinth. (B) transversal μCT image through the auditory region. Note the small processus anterior (pram) of the malleus below the tegmen tympani in the left ear. The asterisk (*) indicates the lateral expansion of the epitympanic recess. Abbreviations: bl, bony labyrinth; bt, bulla tympani; co, cochlea; re, epitympanic recess; fv, fenestra vestibuli; in, incus; ma, malleus; mea; meatus acusticus externus; pbl, primary bony lamina; pe, petrosal; pram, processus anterior of malleus; pro, promontorium; st, stapes; tt, tegmen tympani. Scale bar = 5 mm.
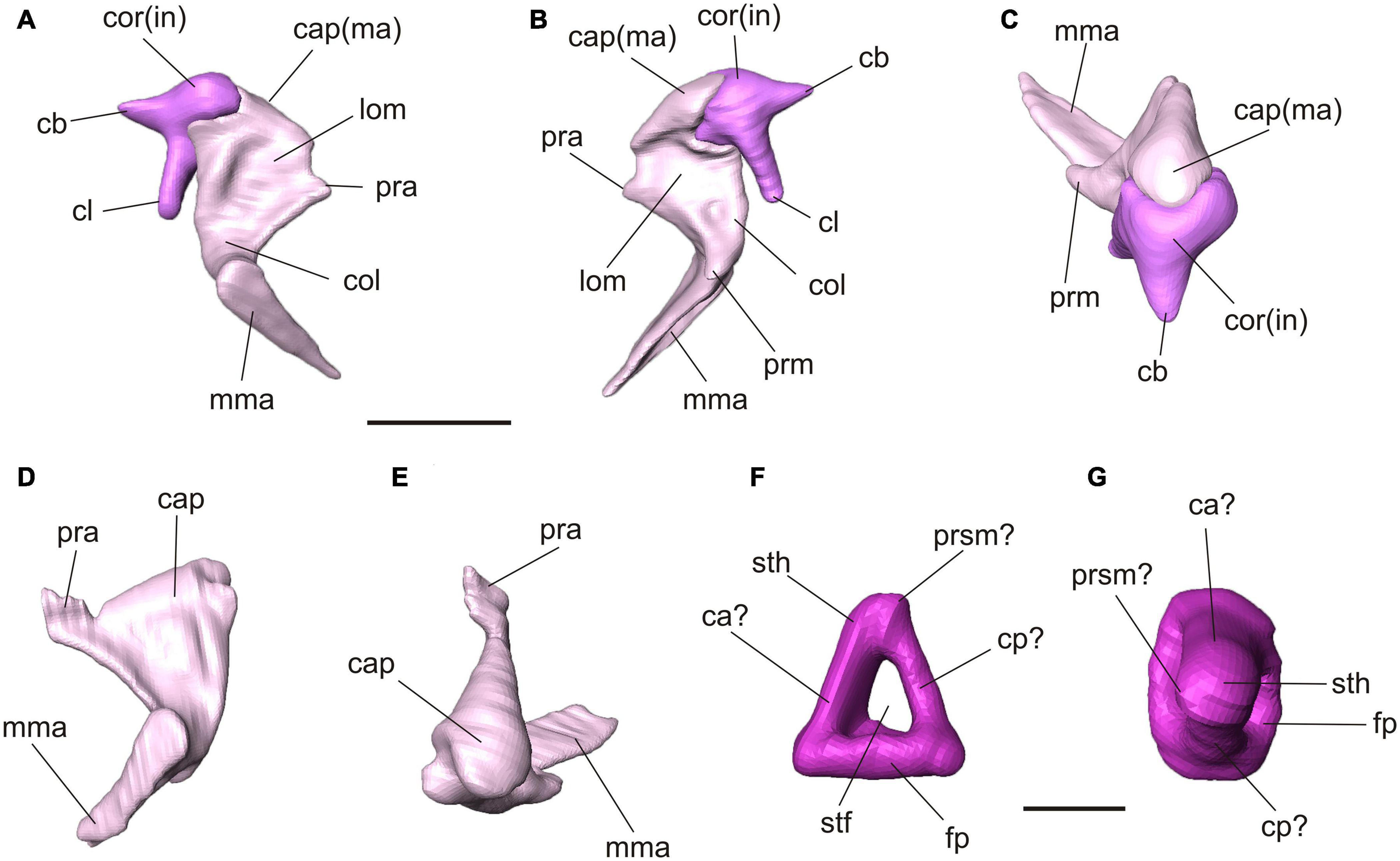
Figure 4. Virtual 3D reconstructions of the auditory ossicles of Palaeolagus haydeni (FMNH PM9476). Color code refers to Figure 3. Articulated right malleus and incus in lateral (A), medial (B) and dorsal (C) views. Note the incomplete processus anterior of the malleus. Left malleus in lateral (D) and dorsal (E) views. Ventral (F) and medial (G) views of the left stapes. Interpretation of orientation is tentative due to displacement and weaker preservation of the right stapes. Abbreviations: ca, crus anterior; cap, caput; cb, crus breve; cl, crus longum; cp, crus posterior; col, collum; cor, corpus; fp, footplate; in, incus; lom, lamina ossea mallei; ma, malleus; mma, manubrium mallei; pra, processus anterior; prm, processus muscularis; prsm, process for stapedial muscle; stf, stapedial foramen; sth, stapedial head. Scale bar for (A–E) = 2 mm, (F–G) = 1mm.
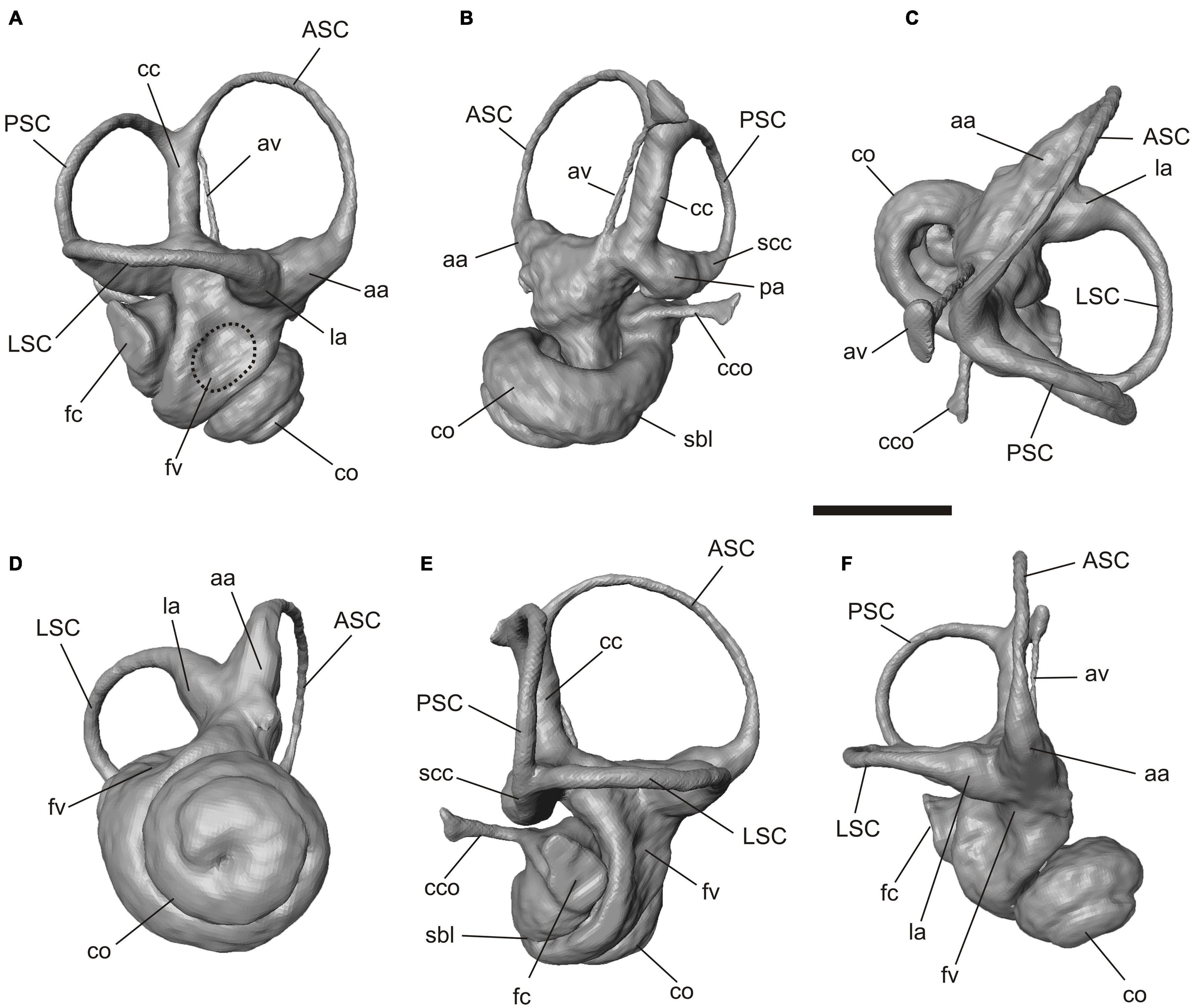
Figure 5. Virtual 3D reconstruction of the right bony labyrinth of Palaeolagus haydeni (FMNH PM9476). (A) lateral, (B) medial, (C) dorsal, (D) ventral, (E) posterolateral and (F) anterolateral views. Abbreviations: aa, anterior ampulla; ASC, anterior semicircular canal; av, aquaeductus vestibuli; cc, crus commune; cco, canaliculus cochleae; co, cochlea; la, lateral ampulla; fc, fenestra cochleae; fv, fenestra vestibuli; LSC, lateral semicircular canal; pa, posterior ampulla; PSC, posterior semicircular canal; sbl, secondary bony lamina; scc, secondary crus commune. Scale bar = 2 mm.
Terminology of the described structures follows Ekdale (2013, 2016), Ruf (2014), Maier and Ruf (2014), and Maier et al. (2018). The results concerning Palaeolagus haydeni are discussed in comparison to extant Lagomorpha based on data from literature (e.g., Ruf, 2014; Maier et al., 2018) and against the background of combined phylogeny from Matthee et al. (2004), and Fostowicz-Frelik and Meng (2013). For grundplan reconstruction that allows defining all characters (apomorphic and plesiomorphic) of the last common ancestor of a respective taxon we follow the definition by Hennig (1984). The same scans of adult extant Lagomorpha included in the study on the auditory ossicles in Maier et al. (2018) are used to discuss characters of the middle ear and the bony labyrinth.
For outgroup comparison recent studies on the nasal region of further members of Euarchontoglires are considered: Rodentia (Ruf, 2014, 2020; Martinez et al., 2018), Scandentia (Ruf et al., 2015; Lundeen and Kirk, 2019), Dermoptera (Maier and Ruf, 2014; Lundeen and Kirk, 2019), and Primates (Maier and Ruf, 2014; Smith et al., 2016; Lundeen and Kirk, 2019).
Palaeolagus haydeni FMHN PM9476 comprises a complete skull including the articulated mandible. The cavities inside the skull are completely filled by sediment. The specimen is mostly intact, although some parts are missing or have been damaged. These are the anterior nasal roof, the left zygomatic arch, the orbital wall of the nasal cavity, and the roof of the braincase (Figure 1A; see also Wolniewicz and Fostowicz-Frelik, 2021). In addition, the auditory bullae show some defects, resulting in their fenestration. Thus, the following anatomical description refers to the better preserved side, left or right depending on the completeness of the respective structure.
The turbinal skeleton of FMHN PM9476 is incomplete because all turbinals show defects or are broken. This affects especially the structures located in the most anterior and posterior parts of the nasal cavity. However, the number of turbinals can be clearly deduced from both sides of the nasal cavity, although the detailed anatomy remains uncertain in some parts. Ethmoturbinals II and III as well as the interturbinal of the pars posterior are much better preserved on the left side (Figures 1B–G).
The pars anterior of the nasal cavity houses a large and dendritic maxilloturbinal whose anterior portion is not preserved (Figures 1B–E, 2A). It covers the canalis nasolacrimalis that houses the ductus nasolacrimalis medially and ends ventral to the processus anterior of ethmoturbinal I. The anterior part of the nasoturbinal is also not preserved. The remnant of the nasoturbinal is a short straight lamella. Posteriorly, the nasoturbinal forms the floor of a deep cavity whose roof is the nasal bone (Figures 1B–E, 2A). The lamina semicircularis separates the pars posterior of the nasal cavity from the pars lateralis. It is a long lamella projecting from the nasal roof that ends at the anterior rim of the lamina cribrosa. This lamella corresponds to the dorsal lamella or flank of a typical sickle-shaped lamina semicircularis. Ventrally, the lamina semicircularis projects into the sinus maxillaris via the hiatus semilunaris. This ventral projection that might comprise a vestigial ventral lamina, shows two anterolaterally oriented processus. The dorsal process is short. The ventral one is more prominent and interpreted as the processus uncinatus (Figures 1D,E, 2B). The posterior margin of the lamina semicircularis is rolled-up laterally. The lamina semicircularis and the nasoturbinal are not fused but together form a continuous lamella and therefore, a functional unit attached to the nasal roof.
The recessus frontoturbinalis of the pars lateralis houses three turbinals, which are not completely preserved (Figures 1B–D, 2C). Of the three turbinals, the dorsal one can be identified as frontoturbinal 1. It is attached to the nasal roof (mostly the frontal bone) and merges posteriorly into the lamina cribrosa. The shape can be reconstructed as a double-scroll. The second turbinal is almost as long as frontoturbinal 1 and is similar in shape. It starts at the lateral rim of the lamina horizontalis and attaches to the lateral wall of the nasal cavity in its further course; the posterior end merges into the lamina cribrosa. We interpret this turbinal as the interturbinal. The third turbinal, most likely frontoturbinal 2, is very short and small. It is attached to the lamina horizontalis and its posterior end is in contact with the root of ethmoturbinal I. Frontoturbinal 2 probably shows only a lateral scroll but the exact shape is not preserved. The homology of the interturbinal and frontoturbinal is discussed below.
The pars posterior of the nasal cavity houses four turbinals that are only partly preserved except for ethmoturbinal I (Figures 1B–G, 2B–D). Ethmoturbinal I is attached to the lamina horizontalis and has a broad massive processus anterior that projects far into the pars anterior of the nasal cavity (Figures 1C–G, 2B). The two major lamellae of ethmoturbinal I, pars anterior and pars posterior, can be clearly separated. While the pars anterior is a straight lamella, the pars posterior is rolled-up laterally (Figures 1E,G, 2C). Posteriorly, both parts fuse to form a funnel-shaped recess that is attached to the lamina cribrosa. The following turbinals all arise from the lamina horizontalis and more posterior from the lateral sidewall of the nasal cavity. Ethmoturbinal II and the interturbinal between the former and ethmoturbinal I are represented only by their anteriormost part (Figures 1B,D–G, 2D). Their length and morphology cannot be determined from these remnants. However, it is visible that the interturbinal forms at least partly a double-scroll. Ethmoturbinal III extends far into the posterior nasal cavity and forms a prominent ventrally convex lamella. However, only the distal part of ethmoturbinal III is preserved and thus its detailed shape and number of lamellae remain unclear (Figures 1B,F,G, 2D).
The nasal septum is only partly preserved with its dorsoposterior part being attached to the lamina cribrosa as well as the very most end inside the cupula nasi posterior.
Palaeolagus haydeni shows a well-developed bulla tympani that completely encloses the tympanic cavity (Figure 3). In ventral view, the bulla is oval, with its long axis oriented anteromedially. The bulla is built exclusively by the ectotympanic, which laterally forms a short external auditory canal (meatus acusticus externus), projecting and opening dorsolaterally (Figure 3B). Anteriorly, a horizontal septum divides the cavum tympani into a dorsal and a ventral chamber. This septum continues into the bony ring that supports the tympanic membrane and forms the entrance of the meatus acusticus externus. There is no additional septum inside the bulla. On the dorsal side of the tympanic cavity, the epitympanic recess is situated posterolateral to the fenestra cochleae. The medial part of the epitympanic recess is a small, shallow fossa in the petrosal whereas, in contrast, its lateral part is expanded into a large chamber, extending anteriorly, laterally and dorsally (Figure 3B). This chamber is laterally bounded by the ectotympanic so that the squamosal is entirely excluded from the tympanic cavity. In ventral view, most of the lateral tympanic recess is covered by a bony lamina of the ectotympanic, which forms the dorsal wall of the external auditory canal and continuous anteriorly into the lamina anterior (Figure 3B). Anterolateral to the fossa for the tensor tympani muscle and anterior to the expanded epitympanic recess there is a large spherical chamber that develops entirely within the ectotympanic. This chamber is identified as the epitympanic sinus. On the intracranial side of the skull, the dorsal wall of the epitympanic sinus is exposed as an oval, convex patch between the petrosal and alisphenoid; thus, the ectotympanic contributes to the floor of the braincase.
The three auditory ossicles are preserved on both sides, mostly intact but displaced (Figures 3, 4). The malleus and incus are still in articulation on both sides. They are embracing each other with their criss-cross articulation facets (Figures 4A–C). The malleus has a distinct caput that shows a smooth convex dorsal surface. Anteroventrally, it continues into a broad lamina ossea mallei. The collum and manubrium mallei form an angle about 90°; the manubrium is bent anteromedially and its lateral surface, which was attached to the tympanic membrane, is broadened. On the medial side, a prominent processus muscularis for attachment of the musculus tensor tympani is present (Figures 4A–C). Dorsal to this process the neck bears a distinct fossa (Figure 4B). The manubrium mallei of the left malleus is incomplete distally. The processus anterior is incomplete in the right malleus but intact in the left one (Figures 4A–E). In the higher resolution μCT scan, the attachment of the processus anterior to the roof of the middle ear cavity can be traced. The processus anterior is short but bends dorsolaterally and ends as a free rod in a small cavity between the tegmen tympani and ectotympanic (Figures 3B, 4D,E). This cavity opens medially. A processus internus praearticularis is lacking. The incus shows a distinct crus breve and a significantly longer crus longum. A processus lenticularis is not visible because it may be broken (Figures 4A–C).
The right stapes is in situ but not well preserved and slightly projecting through the foramen vestibuli (Figure 3). Its footplate is incomplete. The left stapes is complete but displaced. The stapedial footplate is oval and flat. Both crura are prominent, positioned quite symmetrically at the rim of the footplate and embracing a distinct stapedial foramen. We tentatively interpret a small process next to the head of the left stapes as the process for the stapedial muscle. In the left stapes one crus is stronger and forms a half tube (Figures 4F,G). Although the right stapes is in situ, it can hardly be used for orientation as both crura show the same thickness and no stapedial muscle process is visible.
The ventral surface of the promontorium is gently rounded (Figure 3B). At its posterior end a nearly circular fenestra cochleae (sive rotunda) is present, not concealed by any bony outgrowth of the petrosal. On the lateral side of the promontorium there is a small, oval fenestra vestibuli (sive ovalis), facing lateroventrally (Figure 3B). The petrosal houses the bony labyrinth, a hollow space that is filled with the soft tissue structures and perilymphatics and endolymphatic fluids of the inner ear in the living animal.
The bony labyrinth is complete on both sides. The cochlea is tightlycoiled, conic but relatively flat; it has 2.25 turns (Figures 3A, 5). The secondary bony lamina that supports the basilar membrane together with the primary bony lamina extends up to a quarter of the basal turn (Figures 5B,E). The canaliculus cochleae is thin and relatively short. The pathways of the cochlear nerves that enter the internal auditory meatus and spread through the primary lamina can be traced. The three semicircular canals are thin but show a well pronounced curvature. In perpendicular view to their planes the anterior and lateral semicircular canals show an oval shape whereas the posterior semicircular canal is almost circular (Figure 5). The anterior semicircular canal is by far the largest in diameter (curvature) and its dorsalmost point is significantly higher than the crus commune, the joint section of the anterior and posterior semicircular canal (Figures 5A,E). This canal borders the entrance into a large and deeply excavated fossa subarcuata. The lateral semicircular canal forms a secondary crus commune together with the posterior semicircular canal (Figures 5B,D). This joint canal does only comprise the bony structures, but not the soft tissue ducts. While the anterior and lateral semicircular canal show almost no planar deviation the posterior semicircular canal is slightly undulating (Figures 5C,E). The ampullae of the canals are distinct but not significantly inflated. The aquaeductus vestibuli leaves the vestibulum anterior to the crus commune and runs as a thin canal in a gentle curve next to the latter (Figure 5B).
The turbinal pattern of Palaeolagus haydeni generally resembles that of extant Lagomorpha. Key characters of Lagomorpha, as revealed by extant species, are a dendritic maxilloturbinal, an excavated nasoturbinal that is continuous with the lamina semicircularis, two frontoturbinals, three ethmoturbinals and one interturbinal between ethmoturbinal I and II (see Ruf, 2014). All these characters are also present in Palaeolagus haydeni.
The highly dendritic maxilloturbinal observed in Palaeolagus haydeni and extant lagomorphs can be tentatively regarded as an apomorphic character. Some rodents (e.g., Sciurus vulgaris, Castor canadensis, Cricetus cricetus, and Myocastor coypus) also show a complex and up to highly dendritic maxilloturbinal that is constrained by functional adaptations at least in semiaquatic species (Ruf, 2014, 2020; Martinez et al., 2020). However, the grundplan pattern in Rodentia remains ambiguous. In Scandentia, Primates and Dermoptera the maxilloturbinal is a straight, scrolled or double-scrolled structure that can show further epiturbinals (additional lamellae) in the former (Maier and Ruf, 2014; Ruf, 2014; Ruf et al., 2015; Lundeen and Kirk, 2019) although such morphological complexity as in Palaeolagus haydeni and extant Lagomorpha is not achieved.
The lamina semicircularis of Palaeolagus haydeni shows a puzzling pattern. Similar to all extant Leporidae, the posterior margin of the lamina semicircularis is scrolled. Based on the present phylogeny, the straight lamina semicircularis of Ochotonidae has to be regarded a derived feature (Figure 6). However, a distinct ventral lamella is lacking in Palaeolagus haydeni, a trait shared with Ochotonidae. Because this pattern is also present in Sciurus vulgaris and muroid rodents, it can be regarded as a plesiomorphic feature for extant Lagomorpha as well as Palaeolagus haydeni (Ruf, 2014, 2020). In Scandentia, the lamina semicircularis has a distinct ventral lamella and a pronounced processus uncinatus (Ruf, 2014, 2020; Ruf et al., 2015; Lundeen and Kirk, 2019: lamina semicircularis labeled as nasoturbinal). A ventral lamina as well as processus uncinatus are also present at least in some primates (Maier, 1993; Maier and Ruf, 2014; Lundeen and Kirk, 2019: lamina semicircularis labeled as nasoturbinal). Unfortunately, detailed descriptions of the lamina semicircularis in adult stages are generally scarce and thus, information needs to be mostly drawn from the published figures. The processus uncinatus is proportionally longer in Palaeolagus haydeni than in Ochotonidae. The second processus of the lamina semicircularis as well as the anteroventrally orientation can be regarded as an apomorphic feature of Palaeolagus haydeni, as they are not known in any extant lagomorph species or outgroup member investigated so far (Maier and Ruf, 2014; Ruf, 2014, 2020; Ruf et al., 2015).
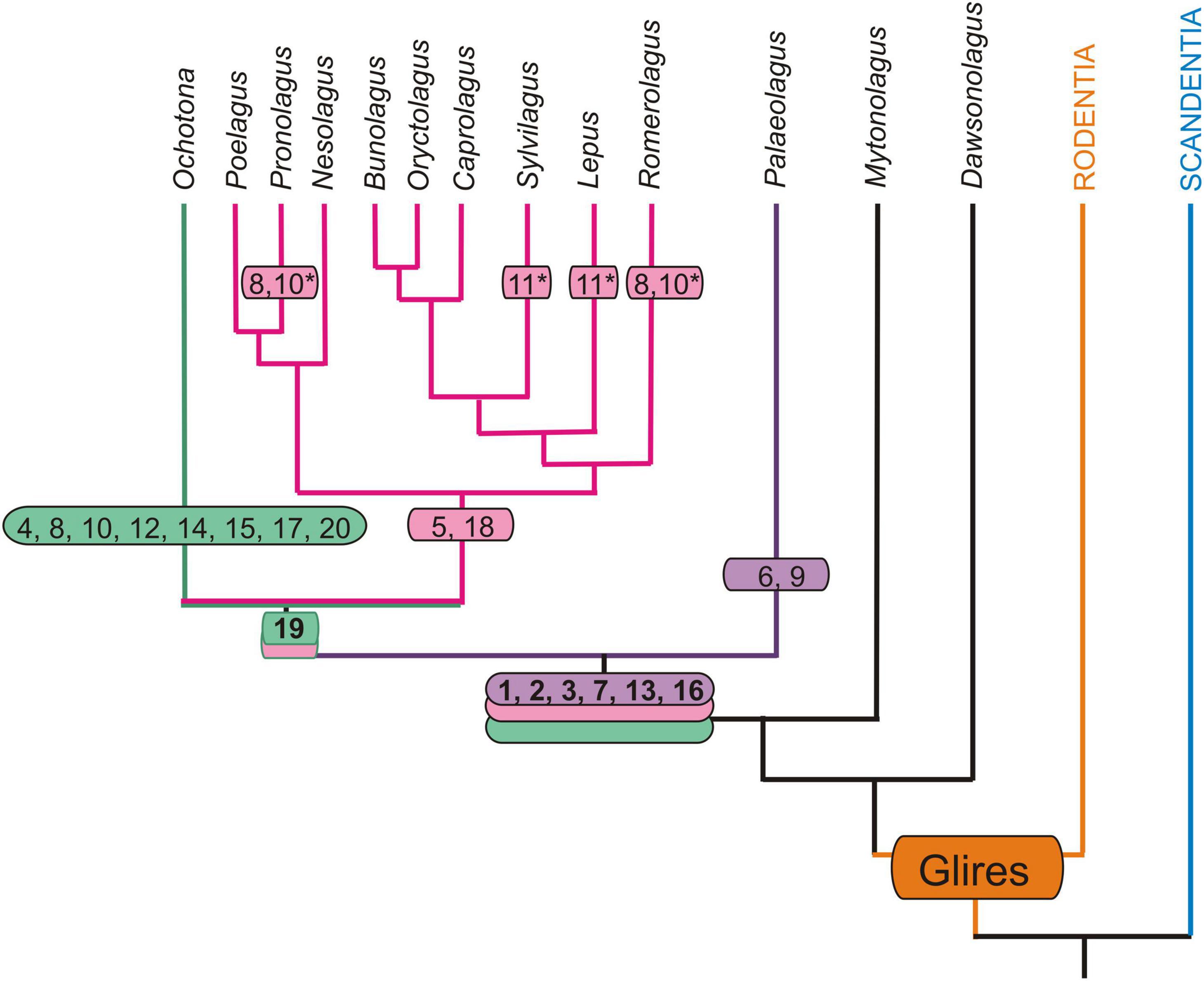
Figure 6. Autapomorphic characters of the nasal and auditory regions in Palaeolagus haydeni and extant Lagomorpha mapped onto phylogeny of Matthee et al. (2004) and Fostowicz-Frelik and Meng (2013). Only those extant genera that have been previously investigated are considered (Ruf, 2014; Maier et al., 2018). Note that for Nesolagus data on the turbinal skeleton are not available. Apomorphic characters based on outgroup comparison (Rodentia + Scandentia): 1, dendritic maxilloturbinal; 2, excavated nasoturbinal contacts lamina semicircularis; 3, scrolled posterior margin of lamina semicircularis; 4, straight posterior margin of lamina semicircularis; 5, lamina semicircularis has a ventral lamina; 6, lamina semicircularis with anterolaterally projecting additional process; 7, interturbinal between frontoturbinal 1 and 2; 8, loss of interturbinal between frontoturbinal 1 and 2; 9, reduced frontoturbinal 2; 10, loss of ethmoturbinal III; 11, additional interturbinals; 12, bulla tympani strongly pneumatized; 13, contribution of ectotympanic to cranial floor (unclear in Ochotonidae); 14, caput mallei with distinct cristae; 15, loss of musculus tensor tympani; 16, anchoring of malleus via short processus anterior that ends in cavity formed by the ectotympanic and tegmen tympani; 17, anchoring of malleus via short processus anterior fused to bony trabeculae of pneumatized tegmen tympani (adult stages); 18, anchoring of malleus via extensive processus internus praearticularis fused to ectotympanic; 19, loss of secondary crus commune; 20, high cylindric cochlea; asterisk (*) denotes characters restricted to certain species.
Similar to all extant species of Leporidae (except for Pronolagus spp. and Romerolagus diazi) Palaeolagus haydeni has three turbinals inside the frontoturbinal recess (two frontoturbinals and one large interturbinal in between), which is regarded as an apomorphic feature of this family. Ochotonids resemble the lagomorph grundplan in having only two frontoturbinals and lacking an interturbinal (Ruf, 2014).
Observations on prenatal stages of Oryctolagus cuniculus clearly show that after the development of the two frontoturbinals an additional turbinal, the interturbinal, occurs in-between in later fetal stages (Voit, 1909; Frick and Heckmann, 1955). In leporids both frontoturbinals are quite similar in size and the interturbinal is shorter than frontoturbinal 2 (Ruf, 2014). Palaeolagus haydeni shows a different pattern in that frontoturbinal 2 is very small. However, based on the comparison of the attachment and location of the three turbinals we conclude that Palaeolagus haydeni shows the leporid pattern with a highly reduced frontoturbinal 2 as an apomorphic character. Furthermore, given the likely systematic position of Palaeolagus outside crown Lagomorpha, three turbinals in the frontoturbinal recess could resemble the plesiomorphic grundplan pattern for Leporidae and instead the ochotonid pattern needs to be regarded apomorphic (Figure 6), although convergent evolution of the interturbinal in Palaeolagus and Leporidae would be equally parsimonious. Among Rodentia, Scandentia and Dermoptera two frontoturbinals are a common feature (Maier and Ruf, 2014; Ruf et al., 2015; Martinez et al., 2018; Lundeen and Kirk, 2019; Ruf, 2020) and likely represent the grundplan pattern of Euarchontoglires. In some species the recessus frontoturbinalis houses additional small interturbinals that tend to be variable (e.g., Maier and Ruf, 2014; Ruf, 2014). In primates the recessus frontoturbinalis becomes reduced in concert with the reduction and loss of the frontoturbinals; some strepsirrhines still show two or more frontoturbinals (Maier, 1993; Maier and Ruf, 2014; Smith et al., 2016; Lundeen and Kirk, 2019). Further investigation of stem lagomorphs would help elucidate the polarization of this character.
At first glance, it is tempting to interpret the small frontoturbinal 2 of Palaeolagus haydeni as an evolving third turbinal within the recessus frontoturbinalis. Regardless of whether this structure is interpreted as the frontoturbinal or interturbinal, this hypothesis would have considerable consequences for the homology of the respective turbinals. Interturbinals develop later in ontogeny and are hidden by the fronto- and ethmoturbinals in medial view; in many mammals they are smaller than the adjacent turbinals (Paulli, 1900; Reinbach, 1952a,b; Ruf, 2020). This pattern is supported by the prenatal ontogeny of Oryctolagus cuniculus as described above. Furthermore, in Ochotonidae both frontoturbinals are also of the same size (Ruf, 2014). Thus, evolutionary reduction of frontoturbinal 2 in Palaeolagus haydeni would be the most parsimonious hypothesis.
Although the turbinals of the ethmoturbinal recess are not fully preserved in Palaeolagus haydeni, their number (three ethmoturbinals and one interturbinal between ethmoturbinals I and II) resembles a common pattern observed in many Lagomorpha and Rodentia, all Scandentia, and some strepsirrhine primates, e.g., Eulemur collaris and Galago senegalensis (Ruf, 2014, 2020; Martinez et al., 2018; Lundeen and Kirk, 2019). At least for Glires this pattern might represent the grundplan character state. Four ethmoturbinals are present in Dermoptera and some Strepsirrhini, e.g., Daubentonia madagascariensis and Indri indri (Maier and Ruf, 2014; Lundeen and Kirk, 2019), and may reflect a basal pattern for Euarchontoglires. Haplorhini show a reduced number of ethmo- and interturbinals (Maier, 1993; Maier and Ruf, 2014; Lundeen and Kirk, 2019).
The bulla tympani of Palaeolagus haydeni has a thin wall, similar to that generally present in extant Leporidae, but differs from the heavily pneumatized bulla in extant Ochotonidae (Cockerell et al., 1914; Maier et al., 2018). Oryctolagus cuniculus has also a thin wall of the bulla tympani but the lateral part is only slightly pneumatized (this study). The pattern observed in Palaeolagus haydeni and Leporidae can be regarded as plesiomorphic for Lagomorpha because it is a common pattern in mammals and also present in sciuromorph rodents and scandentians (Wible, 2009; Pfaff et al., 2015b). However, the sciuromorph rodents show a specific pattern of septa not comparable to that of the lagomorphs under study. The anterodorsal exposure of the ectotympanic at the floor of the braincase is also present in Romerolagus diazi and Oryctolagus cuniculus (this study). As this pattern is uncommon in mammals, it might be regarded as an apomorphic feature of Palaeolagus haydeni and Leporidae (Novacek, 1977). However, the contribution of the ectotympanic to the cranial floor has also been observed in Desmodillus auricularis, a small desert murid with heavily expanded auditory bullae (Mason, 2016: fig. 3B). In Ochotonidae, the dorsal extension of the ectotympanic cannot be discerned as the petrosal bone is also mostly pneumatized and no sutures can be traced (see figures in Maier et al., 2018). Investigation of perinatal stages could help to elucidate the origin of the roof of the cavum tympani. However, for the given phylogeny we tentatively regard this character as apomorphic for the clade comprising Palaeolagus and crown Lagomorpha (Figure 6).
In general, the auditory ossicles of Paleolagus haydeni resemble those of extant Lagomorpha, especially Leporidae (Doran, 1878; Cockerell et al., 1914; Fleischer, 1973; Maier et al., 2018). Similar to leporids, the stapes shows a flat footplate and no stapedial muscle process and the dorsal surface of the mallear head is smooth. The musculus tensor tympani was certainly also present in Palaeolagus haydeni as this species shows a prominent processus muscularis. The auditory ossicles of Ochotonidae differ in several respects: the stapes has a convex footplate and a small process for the stapedial muscle, a processus muscularis of the malleus is missing due to the lack of the musculus tensor tympani, and the mallear head shows distinct cristae (Cockerell et al., 1914; Ruf et al., 2009; Maier et al., 2018). The cristae are most probably a derived character of the family. In this respect, Palaeolagus haydeni shows the plesiomorphic lagomorph pattern.
The most striking feature of the lagomorph auditory ossicles in terms of phylogenetic and morphofunctional implications is the processus anterior of the malleus, especially the processus internus praearticularis of Leporidae (Maier et al., 2018). According to Fleischer (1978), the auditory ossicles of Lagomorpha resemble the “freely mobile type” that is common in terrestrial mammals with medium-sized to large tympanic membrane. Here, the anterior process of the malleus is short and loosely attached to the roof of the tympanic cavity via the anterior ligament; the incus is also attached to the roof of the fossa incudis with a short ligament. Maier et al. (2018) clearly demonstrated that Lagomorpha show a much more complicated anatomy of the processus anterior of the malleus. In both living families a specific independently evolved pattern has been described. In Leporidae the thin processus internus praearticularis of the processus anterior is distally inflated (already in prenatal stages) and fused to the ectotympanic inside a bony cavity of the tympanic roof. In contrast, the processus internus praearticularis of Ochotonidae is very small and only present in prenatal stages; in adults the processus anterior is fused to bony trabeculae of the tegmen tympani (Maier et al., 2018). Until now, the two patterns in Lagomorpha could not have been polarized. Palaeolagus haydeni resembles the pattern in adult Ochotonidae in having a short processus anterior and no processus internus praearticularis and that of prenatal stages in having a laterally oriented tip of the processus anterior approaching the ectotympanic (see Maier et al., 2018: fig. 7i). The anterior attachment of the malleus in Palaeolagus haydeni resembles the pattern in Leporidae in certain aspects, although achieved with different parts of the malleus: a processus projecting into a small cavity formed by the ectotympanic bone. Furthermore, the processus anterior of Palaeolagus haydeni shows a different orientation than in extant Lagomorpha. It is clearly attached more laterally to the tympanic roof; a distortion of the delicate process during decomposition of the body cannot be excluded as this connection is very flexible (Mason, 2016; Maier et al., 2018). In certain rodents, the processus anterior is also a short tapering lamella attached to the roof of the tympanic cavity but no details on the attachment site are given (Mason, 2016). Thus, Palaeolagus haydeni resembles the plesiomorphic lagomorph pattern still present in early ontogenetic stages of Ochotonidae. In adult pikas the attachment would be adapted to the derived pattern of the highly pneumatized ectotympanic and petrosal. The extensive processus internus praearticularis of Leporidae is obviously derived from the lagomorph grundplan (see Figure 6). As proposed by Maier et al. (2018) for the auditory ossicles of Lagomorpha, Palaeolagus haydeni also resembles the “bone elasticity type.”
The bony labyrinth of Palaeolagus haydeni shows some plesiomorphic features compared to the extant lagomorph species. It still has a secondary crus commune, a character absent in extant Lagomorpha and regarded to be plesiomorphic for Theria (Ekdale, 2013, 2016; Ruf et al., 2016). In Romerolagus diazi, Oryctolagus cuniculus, Lepus californicus, Sylvilagus floridanus and Ochotona alpina, the lateral semicircular canal has a separate entrance into the vestibule resulting in a plane of the canal dorsal to the ampulla of the posterior semicircular canal (Ekdale, 2013; this study). Among the investigated structures of the present study this is the only apomorphy of crown Lagomorpha (Figure 6).
Furthermore, the extant leporids have relatively thin semicircular canals with great curvatures, as observed in Palaeolagus haydeni, whereas the pikas show more eliptical semicircular canals (Ekdale, 2013; this study). Shape and size of the semicircular canals are constrained by the type of locomotion and agility of the respective species (e.g., Spoor et al., 2007; Billet et al., 2012; Malinzak et al., 2012; Berlin et al., 2013; Pfaff et al., 2015a, 2017). Thus, the thin and prominently arcuated semicircular canals of Palaeolagus haydeni indicate a more agile locomotion compared to leporids but different from ochotonids. In that regard, a detailed morphometric study of the semicircular canals of living and fossil Lagomorpha is needed to elucidate their locomotory adaptations and allow reconstructions of the behavior for fossil species. The flat and conic cochlea of Palaeolagus haydeni resembles the flat and conic shape observed in living Leporidae but differs from the high and cylindric cochlea in Ochotonidae (Ekdale, 2013; this study).
In conclusion, the intracranial structures of the nasal and auditory regions of Palaeolagus haydeni contribute to our understanding of early lagomorph evolution and reveal a closer affinity to Leporidae than to Ochotonidae or members of the outgroup. In several characters, some of which are certainly plesiomorphic in lagomorphs, this fossil species resembles modern Leporidae. Common characters are the number of olfactory turbinals, the general morphology of the bulla tympani, shape and proportions of the semicircular canals and cochlea. However, Palaeolagus haydeni differs from modern Leporidae by plesiomorphic characters (e.g., the attachment of the malleus via its processus anterior, secondary crus commune) that still could justify a basal position within the lagomorph clade as proposed by Wolniewicz and Fostowicz-Frelik (2021). Apomorphic features of Palaeolagus haydeni are the reduced frontoturbinal 2 and the dorsal process at the lamina semicircularis. Future phylogenetic analyses including the presented herein characters of the nasal and auditory regions as well as data from further well-preserved fossil Lagomorpha could finally decide the phylogenetic position of Palaeolagus haydeni and its relatives.
The original contributions presented in the study are included in the article and the Dryad Digital Repository (https://doi.org/10.5061/dryad.kh189325r), further inquiries can be directed to the corresponding author/s.
ŁF-F and JM made the μCT scans. IR made the virtual 3D reconstructions. IR and ŁF-F wrote the manuscript and prepared the figures. All authors designed the project, analyzed data, discussed the results, and read and approved the final version.
This research was funded by National Science Centre (Cracow, Poland), Grant No. 2015/18/E/NZ8/00637 and an AMNH Roosevelt Research Fellowship to ŁF-F, and Deutsche Forschungsgemeinschaft DFG (German Research Foundation) Grant No. DFG RU 1496/4-1 to IR.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank W. Simpson (FMNH) for access to the Palaeolagus FMNH PM9476 specimen and M. Hill (AMNH) for μCT-scanning of the skull, and the following colleagues for access to extant lagomorph specimens: R. Hutterer (Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany), D. Möricke (Staatliches Museum für Naturkunde Stuttgart, Germany), F. Mayer (Museum für Naturkunde Berlin), A.A. Cohen (NCB Naturalis, Leiden, Netherlands), L. Costeur (Naturhistorisches Museum Basel, Switzerland), and D. Kalthoff and U. Johansson (Naturhistoriska riksmuseet, Stockholm, Sweden). We are grateful to J. Eberhardt (Senckenberg Forschungsinstitut und Naturmuseum Frankfurt) for support with segmentation of the μCT scans. Finally, we thank both reviewers who helped to improve the manuscript.
Asher, R. J., Meng, J., Wible, J. R., McKenna, M. C., Rougier, G. W., Dashzeveg, D., et al. (2005). Stem Lagomorpha and the antiquity of Glires. Science 307, 1091–1094. doi: 10.1126/science.1107808
Asher, R. J., Smith, M. R., Rankin, A., and Emry, R. J. (2019). Congruence, fossils and the evolutionary tree of rodents and lagomorphs. R. Soc. Open Sci. 6:190387. doi: 10.1098/rsos.190387
Berlin, J. C., Kirk, E. C., and Rowe, T. B. (2013). Functional implications of ubiquitous semicircular canal non-orthogonality in mammals. PLoS One 8:e79585. doi: 10.1371/journal.pone.0079585
Billet, G., Hautier, L., Asher, R. J., Schwarz, C., Crumpton, N., Martin, T., et al. (2012). High morphological variation of vestibular system accompanies slow and infrequent locomotion in three-toed sloths. Proc. R. Soc. B 279, 3932–3939. doi: 10.1098/rspb.2012.1212
Cockerell, T. D. A., Miller, L. I., and Printz, M. (1914). The auditory ossicles of American rodents. Bull. Am. Mus. Nat. Hist. 33, 347–380.
Dawson, M. R. (1958). Later Tertiary Leporidae of North America. Univ. Kansas Paleontol. Contr. Vertebr. 6, 1–75.
Dawson, M. R. (2008). “Lagomorpha,” in Evolution of Tertiary Mammals of North America, eds C. M. Janis, G. F. Gunnell, and M. D. Uhen (Cambridge: Cambridge University Press), 293–310. doi: 10.1206/0003-00902003275<0001:TOORMG<2.0.CO;2
Doran, A. H. G. (1878). Morphology of the mammalian ossicula auditus. Trans. Linn. Soc. Lond. Zool. 1, 371–497. doi: 10.1111/j.1096-3642.1878.tb00663.x
Ekdale, E. G. (2013). Comparative anatomy of the bony labyrinth (inner ear) of placental mammals. PLoS One 8:e66624. doi: 10.1371/journal.pone.0066624
Ekdale, E. G. (2016). Form and function of the mammalian inner ear. J. Anat. 228, 324–337. doi: 10.1111/joa.12308
Fleischer, G. (1973). Studien am Skelett des Gehörorgans der Säugetiere, einschließlich des Menschen. Säugetierkdl. Mitt. 21, 131–239.
Fleischer, G. (1978). Evolutionary principles of the mammalian middle ear. Adv. Anat. Embr. Cell Biol. 55, 1–70.
Fostowicz-Frelik, Ł (2013). Reassessment of Chadrolagus and Litolagus (Mammalia: Lagomorpha) and a new genus of North American Eocene lagomorph from Wyoming. Am. Mus. Novit. 3773, 1–76. doi: 10.1206/3773.2
Fostowicz-Frelik, Ł (2017). “Convergent and parallel evolution in early Glires (Mammalia),” in Evolutionary Biology: Self/Nonself Evolution, Species and Complex Traits Evolution, Methods and Concepts, ed. P. Pontarotti (Berlin: Springer), 199–216. doi: 10.1007/978-3-319-61569-1_11
Fostowicz-Frelik, Ł, and Meng, J. (2013). Comparative morphology of premolar foramen in lagomorphs (Mammalia: Glires) and its functional and phylogenetic implications. PLoS One 8:e79794. doi: 10.1371/journal.pone.0079794
Fostowicz-Frelik, Ł, Ruf, I., and Meng, J. (2021). Anatomy of the nasal and auditory regions of the fossil lagomorph Palaeolagus haydeni: systematic and evolutionary implications. Dryad Dataset doi: 10.5061/dryad.kh189325r
Frick, H., and Heckmann, U. (1955). Ein Beitrag zur Morphogenese des Kaninchenschädels. Cells Tissues Organs 24, 268–314. doi: 10.1159/000141047
Hennig, W. (1984). Taschenbuch der Speziellen Zoologie Teil 1, Wirbellose I, Ausgenommen Gliedertiere. Frankfurt: Verlag Harri Deutsch.
Hoyte, D. A. N. (1961). The postnatal growth of the ear capsule in the rabbit. Am. J. Anat. 108, 1–16. doi: 10.1002/aja.1001080102
King, A. M., Hall, J., Cranfield, F., and Sullivan, M. (2007). Anatomy and ultrasonographic appearance of the tympanic bulla and associated structures in the rabbit. Vet. J. 173, 512–521. doi: 10.1016/j.tvjl.2006.09.002
López-Torres, S., Bertrand, O. C., Lang, M. M., Silcox, M. T., and Fostowicz-Frelik, Ł (2020). Cranial endocast of the stem lagomorph Megalagus and brain structure of basal Euarchontoglires. Proc. R. Soc. B 287:20200665. doi: 10.1098/rspb.2020.0665
Lundeen, I. K., and Kirk, E. C. (2019). Internal nasal morphology of the Eocene primate Rooneyia viejaensis and extant Euarchonta: using μCT scan data to understand and infer patterns of nasal fossa evolution in primates. J. Hum. Evol. 132, 137–173. doi: 10.1016/j.jhevol.2019.04.009
Maier, W. (1993). Zur evolutiven und funktionellen Morphologie des Gesichtsschädels der Primaten. Z. Morph. Anthrop. 79, 279–299.
Maier, W., and Ruf, I. (2014). Morphology of the nasal capsule of Primates – with special reference to Daubentonia and Homo. Anat. Rec. 297, 1985–2006. doi: 10.1002/ar.23023
Maier, W., Tröscher, A., and Ruf, I. (2018). The anterior process of the malleus in extant Lagomorpha (Mammalia). J. Morphol. 279, 132–146. doi: 10.1002/jmor.20759
Malinzak, M. D., Kay, R. F., and Hullar, T. E. (2012). Locomotor head movements and semicircular canal morphology in primates. Proc. Natl. Acad. Sci. U.S.A. 109, 17914–17919. doi: 10.1073/pnas.1206139109
Martinez, Q., Clavel, J., Esselstyn, J. A., Achmadi, A. S., Grohé, C., Pirot, N., et al. (2020). Convergent evolution of olfactory and thermoregulatory capacities in small amphibious mammals. Proc. Natl. Acad. Sci. U.S.A. 117, 8958–8965. doi: 10.1073/pnas.1917836117
Martinez, Q., Lebrun, R., Achmadi, A. S., Esselstyn, J. A., Evans, A. R., Heaney, L. R., et al. (2018). Convergent evolution of an extreme dietary specialisation, the olfactory system of worm-eating rodents. Sci. Rep. 8:17806. doi: 10.1038/s41598-018-35827-0
Mason, M. J. (2016). Structure and function of the mammalian middle ear. I: large middle ears in small desert mammals. J. Anat. 228, 284–299. doi: 10.1111/joa.12313
Matthee, C. A., Vuuren van, B. J., Bell, D., and Robinson, T. J. (2004). A molecular supermatrix of the rabbits and hares (Leporidae) allows for the identification of five intercontinental exchanges during the Miocene. Syst. Biol. 53, 433–447. doi: 10.1080/10635150490445715
Meng, J., Hu, Y., and Li, C. (2003). The osteology of Rhombomylus (Mammalia, Glires): implications for phylogeny and evolution of Glires. Bull. Am. Mus. Nat. Hist. 275, 1–247. doi: 10.1206/0003-0090(2003)275<0001:toormg>2.0.co;2
Mennecart, B., Rössner, G. E., Métais, G., DeMiguel, D., Schulz, G., Müller, B., et al. (2016). The petrosal bone and bony labyrinth of early to middle Miocene European deer (Mammalia, Cervidae) reveal their phylogeny. J. Morphol. 277, 1329–1338. doi: 10.1002/jmor.20579
Novacek, M. J. (1977). Aspects of the problem of variation, origin and evolution of the eutherian auditory bulla. Mammal Rev. 7, 131–150. doi: 10.1111/j.1365-2907.1977.tb00366.x
Paulli, S. (1900). Über die Pneumaticität des Schädels bei Den Säugetieren. Eine morphologische Studie. III. Über die Morphologie des Siebbeins und die der Pneumaticität bei den Insectivoren, Hyracoideen, Chiropteren, Carnivoren, Pinnipedien, Edentaten, Rodentiern, Prosimiern und Primaten, nebsteiner zusammenfassenden Übersicht über die Morphologie des Siebbeins und die der Pneumaticität des Schädels bei den Säugethieren. Morphol. Jb. 28, 483–564.
Pfaff, C., Martin, T., and Ruf, I. (2015a). Bony labyrinth morphometry indicates locomotor adaptations in the squirrel-related clade (Rodentia, Mammalia). Proc. R. Soc. B 282:20150744. doi: 10.1098/rspb.2015.0744
Pfaff, C., Martin, T., and Ruf, I. (2015b). “Septal compass” and “septal formula”: a new method for phylogenetic investigations of the middle ear region in the squirrel-related clade (Rodentia: Mammalia). Org. Divers. Evol. 15, 721–730. doi: 10.1007/s13127-015-0222-x
Pfaff, C., Nagel, D., Gunnell, G., Weber, G. W., Kriwet, J., Morlo, M., et al. (2017). Palaeobiology of Hyaenodon exiguus (Hyaenodonta, Mammalia) based on morphometric analysis of the bony labyrinth. J. Anat. 230, 282–289. doi: 10.1111/joa.12545
Reinbach, W. (1952a). Zur Entwicklung des Primordialcraniums von Dasypus novemcinctus Linné (Tatusia novemcincta Lesson). Teil 1. Z. Morphol. Anthropol. 44, 375–444.
Reinbach, W. (1952b). Zur Entwicklung des Primordialcraniums von Dasypus novemcinctus Linné (Tatusia novemcincta Lesson). Teil 2. Z. Morphol. Anthropol. 45, 1–72. doi: 10.1007/978-3-319-23534-9_1
Ruf, I. (2014). Comparative anatomy and systematic implications of the turbinal skeleton in Lagomorpha (Mammalia). Anat. Rec. 297, 2031–2046. doi: 10.1002/ar.23027
Ruf, I. (2020). Ontogenetic transformations of the ethmoidal region in Muroidea (Rodentia, Mammalia): new insights from perinatal stages. Vert. Zool. 70, 383–415. doi: 10.26049/VZ70-3-2020-10
Ruf, I., Frahnert, S., and Maier, W. (2009). The chorda tympani and its significance for rodent phylogeny. Mamm. Biol. 74, 100–113. doi: 10.1016/j.mambio.2008.01.002
Ruf, I., Janßen, S., and Zeller, U. (2015). The ethmoidal region of the skull of Ptilocercus lowii (Ptilocercidae, Scandentia, Mammalia) – a contribution to the reconstruction of the cranial morphotype of primates. Primate Biol. 2:89. doi: 10.5194/pb-2-89-2015
Ruf, I., Volpato, V., Rose, K. D., Billet, G., de Muizon, C., and Lehmann, T. (2016). Digital reconstruction of the inner ear of Leptictidium auderiense (Leptictida, Mammalia) and North American leptictids reveals new insight into leptictidan locomotor agility. Pal. Z. 90, 153–171. doi: 10.1007/s12542-015-0276-2
Smith, T. D., Martell, M. C., Rossie, J. B., Bonar, C. J., and Deleon, V. B. (2016). Ontogeny and microanatomy of the nasal turbinals in Lemuriformes. Anat. Rec. 299, 1492–1510. doi: 10.1002/ar.23465
Spoor, F., Garland, T., Krovitz, G., Ryan, T. M., Silcox, M. T., and Walker, A. (2007). The primate semicircular canal system and locomotion. Proc. Natl. Acad. Sci. U.S.A. 104, 10808–10812. doi: 10.1073/pnas.0704250104
Voit, M. (1909). Das Primordialcranium des Kaninchens. Anat. Hefte 38, 425–616. doi: 10.1007/BF02214638
Wagner, F., and Ruf, I. (2019). Who nose the borzoi? Turbinal skeleton in a dolichocephalic dog breed (Canis lupus familiaris). Mamm. Biol. 94, 106–119. doi: 10.1016/j.mambio.2018.06.005
Wible, J. R. (2007). On the cranial osteology of the Lagomorpha. Bull. Carnegie Mus. Nat. Hist. 39, 213–234. doi: 10.2992/0145-9058(2007)39[213:otcoot]2.0.co;2
Wible, J. R. (2009). The ear region of the pen-tailed treeshrew, Ptilocercus lowii Gray, 1848 (Placentalia, Scandentia, Ptilocercidae). J. Mammal. Evol. 16:199. doi: 10.1007/s10914-009-9116-z
Wible, J. R., Rougier, G. W., Novacek, M. J., and Asher, R. J. (2009). The eutherian mammal Maelestes gobiensis from the late Cretaceous of Mongolia and the phylogeny of Cretaceous Eutheria. Bull. Am. Mus. Nat. Hist. 327, 1–123. doi: 10.1206/623.1
Keywords: computed tomography, lagomorph skull, auditory ossicles, bony labyrinth, bulla tympani, malleus, petrosal, turbinals
Citation: Ruf I, Meng J and Fostowicz-Frelik Ł (2021) Anatomy of the Nasal and Auditory Regions of the Fossil Lagomorph Palaeolagus haydeni: Systematic and Evolutionary Implications. Front. Ecol. Evol. 9:636110. doi: 10.3389/fevo.2021.636110
Received: 09 December 2020; Accepted: 11 May 2021;
Published: 10 June 2021.
Edited by:
Nathalie Bardet, UMR 7207 Centre de Recherche sur la Paléobiodiversité et les Paléoenvironnements (CR2P), FranceReviewed by:
Timothy B. Rowe, University of Texas at Austin, United StatesCopyright © 2021 Ruf, Meng and Fostowicz-Frelik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Łucja Fostowicz-Frelik, bGZvc3RAdHdhcmRhLnBhbi5wbA==
†ORCID: Irina Ruf, orcid.org/0000-0002-9728-1210; Jin Meng, orcid.org/0000-0002-3385-8383; Łucja Fostowicz-Frelik, orcid.org/0000-0002-1266-1178
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.