
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 04 March 2021
Sec. Behavioral and Evolutionary Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.631417
This article is part of the Research Topic Recent Advances in the Evolution of Euarchontoglires View all 15 articles
 Dong-Po Xia1,2*†
Dong-Po Xia1,2*† Xi Wang2,3†
Xi Wang2,3† Paul A. Garber4,5
Paul A. Garber4,5 Bing-Hua Sun2,3
Bing-Hua Sun2,3 Lori K. Sheeran6
Lori K. Sheeran6 Lixing Sun7
Lixing Sun7 Jin-Hua Li2,3*
Jin-Hua Li2,3*Hierarchical steepness, defined as status asymmetries among conspecifics living in the same group, is not only used as a main characteristic of animal social relationships, but also represents the degree of discrepancy between supply and demand within the framework of biological market theory. During September and December 2011, we studied hierarchical steepness by comparing variation in grooming patterns in two groups of Tibetan macaques (Macaca thibetana), a primate species characterized by a linear dominance hierarchy. Using a focal sampling method, we collected behavioral data from two provisioned, free-ranging groups (YA1 and YA2) at Mt. Huangshan, China. We found that female dominance hierarchies were steeper in the YA1 group (0.81 based on the proportion of wins-losses and 0.66 based on dyadic dominance indices) than among members of the YA2 group (0.76 based on the proportion of wins-losses and 0.56 based on dyadic dominance indices). Females in the YA1 group groomed more frequently and for longer duration than females in YA2. Further analysis showed that grooming patterns of high- and low-ranking females did not differ between the two groups. However, middle-ranking females in YA1 groomed conspecifics more frequently and for longer duration than middle-ranking females in YA2. Our results suggest that the steepness of a dominance hierarchy plays an important role in the set of social strategies used by middle-ranking females to avoid a reduction in rank, as well as to increase their rank (the dilemma of middle class hypothesis). We suggest that future studies focus on individuals of middle-rank in order to better understand how the dynamics of rank stability and rank changes influence social relationships, and affiliative and competitive interactions in non-human primates.
In many species of gregarious animals, individuals form strong social bonds and alliances based on a dynamic set of reciprocal and mutualistic interactions with conspecifics as well competitive interactions associated with access to limited resources (van Schaik, 1989; Aureli et al., 2002; Silk, 2007). These affiliative relationships can enhance the collective benefits of social group living to individuals and their offspring (Sussman and Garber, 2011), (e.g., baboon, Papio cynocephalus, Silk et al., 2003, 2009, 2010).
Social grooming (hereafter grooming), defined as an individual cleaning another individual’s fur with hand or mouth, has been considered a fundamental component of primate social interactions (see Sussman and Garber, 2011). Given that grooming is a common and widespread affiliative behavior present in all primate species and relatively easy to observe and measure, grooming relationships have traditionally been used as an index of social and sexual bonds among members of the same social group (Henzi and Barrett, 1999). In non-human primates, grooming accounts for 10–20% of an individual’s daily activity budget and is the most extensively studied affiliative behavior (Dunbar, 2010; Schino and Aureli, 2010; Sussman and Garber, 2011). In addition to a hygienic function (Zamma, 2002), grooming plays important role in establishing and maintaining social bonds and promoting social cohesion among partners (Lehmann et al., 2007; Schino and Aureli, 2008).
Reciprocity and biological market theory provide alternative explanation for primate grooming patterns (Trivers, 1971; Noë and Hammerstein, 1994, 1995). Several studies have shown that grooming, especially among kin and individuals of similar social rank, is reciprocal, both across individual grooming bouts and over time (Schino and Aureli, 2008). In other studies, grooming was found to be interchanged for rank-related benefits (tolerance at feeding sites: Gumert and Ho, 2008; Carne et al., 2011; Tiddi et al., 2011; Kurihara, 2016; access to infants in response to aunting behavior: Henzi and Barrett, 2002; Tiddi et al., 2010; Fruteau et al., 2011; agonistic support: Hemelrijk and Ek, 1991; Carne et al., 2011; or increased mating opportunities: Gumert, 2007; Sonnweber et al., 2015).
Over the past two decades, several primate researchers has focused on the mutual benefits gained from exchange/interchange with group mates (Noë, 2001, 2016). Given that across many primate species individuals reside in the same social group for months, years, and decades, individuals are expected to alter their social interactions with individual group members based on changes in age, group size and composition, reproductive condition, resource availability, and social rank (Noë and Hammerstein, 1995; de Waal, 2000). In this regard, the degree to which a species is characterized by a rigid or relaxed dominance hierarchy is considered an important factor in balancing the frequency and context of cooperative interactions and competitive interactions among group members. For example, in species characterized by a strict or linear hierarchy, individuals may target grooming partners of similar social rank or attempt to groom up the hierarchy in an attempt to form alliances that enhance their social rank within the group (Xia et al., 2013; Kurihara, 2016). Within such a system, higher-ranking individuals typically receive more grooming than they give (e.g., mandrills, Mandrillus sphinx, Schino and Pellegrini, 2011; Tibetan macaques, M. thibetana, Xia et al., 2012, 2013). Individuals of higher-rank also may direct grooming down the hierarchy (captive Cebus apella; Parr et al., 1997). However, low-ranking individuals may have little opportunity to groom the group’s highest-ranked individuals because of competition for access to these preferred grooming partners (Seyfarth, 1977).
Biological market principles offer a productive framework for proposing and testing hypotheses to explain variation in grooming interactions in non-human primates. In primate societies characterized by high levels of within-group contest competition and linear or steep dominance hierarchies, higher-ranking individuals are expected to maintain access to monopolizable commodities. Social strategies used by lower-ranking individuals are expected to include the interchange of different services. For example, lower-ranking individuals might groom higher-ranking individuals in exchange for agonistic support at feeding sites. In contrast, when within-group competition is low and rank gradients shallow, grooming relationships are expected to be more time-matched and reciprocal.
It has been proposed, that as the steepness of a hierarchy increases, investment patterns change so that the interchange of grooming for agonistic support increases (Balasubramaniam et al., 2012). This added benefit (increased grooming frequency and/or increased duration of grooming bouts) for the individuals being groomed, in response to higher demand, should provide an incentive for all market participants to increase their supply of grooming. Such behavioral sensitivity to social circumstance in macaques can be viewed as an adaptive reaction to different market conditions in the trading of social grooming (Dunbar, 2010). Because an increment in the steepness of a hierarchy drives up the levels of both top-down (from higher-ranking individuals to lower-ranking individuals) and bottom-up (from lower-ranking individuals to higher-ranking individuals), the demand for grooming as a social tool is expected to increase as well. Accordingly, biological market theory integrates competitive regimes, dominance gradients, and grooming relationships (Barrett et al., 1999). Here we examine the predictive strength of biological market theory by comparing individual social strategies in two groups of Tibetan macaques (Macaca thibetana) that differ in hierarchical steepness.
Tibetan macaques offer an instructive model for investigating the relationships between biological markets, hierarchical steepness, and grooming relationships. Tibetan macaques are endemic to China and live in multi-male and multi-female social groups [number of adult males: 8.52 ± 0.67, number of adult females: 9.35 ± 0.51 (data from 1987 to 2017) (Li et al., 2020)]. They are characterized by male dispersal and female philopatry (Li et al., 2020). Males typically disperse from their natal group after reaching puberty (about 6–7 years of age), whereas females remain in their natal groups (Li et al., 2020). Although initial studies proposed that they showed a tolerant or relaxed dominance style based on the presence of ritualized greetings (Ogawa, 1995), more recent studies provide strong evidence that Tibetan macaque groups maintain a more despotic dominance style, a strict linear dominance hierarchy, with low levels of reconciliation and counter-aggression (Berman et al., 2004, 2006; Zhu et al., 2013; Li et al., 2020). Berman et al. (2006) report that among female-female dyads there was no evidence that tolerant interaction was disrupted after conflicts suggesting that dominance hierarchies may be relatively stable or that after disputes females are able to re-establish social relationships without the need for reconciliatory behavior. Moreover, females prefer to groom members of their matriline (Berman et al., 2008), and grooming up the hierarchy serves to reduce aggression from higher-ranking females (Xia et al., 2012).
The aim of this study is to examine the effects of hierarchical steepness on grooming interactions in two groups of free-ranging Tibetan macaques. We expect that with increased hierarchical steepness, females would groom more frequently or for longer duration. Moreover, we also expected that low-ranking individuals use grooming (for high- and middle-rankings) to promote upward social mobility, whereas high-ranking individuals use grooming to consolidate their current social relationships with middle- and low-rankings. In contrast, mid-ranking individuals are expected to use a broader range of social grooming tactics, use the biological market condition to gain favor with individuals above them in the hierarchy (high-rankings) and to maintain relationships with individuals below them in the hierarchy (low-rankings). Consequently, middle-ranking females might invest more grooming than both high- and low-ranking females within group. In addition, the steeper the social hierarchy, the more grooming the mid-ranking individuals would invest.
Our study took place in the Huangshan Scenic District, Anhui Province, China. Our data were collected using non-invasive, observational methods, so no review by an Institutional Animal Care and Use Committee was required according to the Chinese wildlife law. Our methods of data collection complied with the Wildlife Protection Law of the People’s Republic of China and the regulatory requirements of Huangshan Garden Forest Bureau.
This study was conducted at Mt. Huangshan National Reserve located in Anhui Province, China. Mt. Huangshan (118.3 E, 30.2 N, elevation 1841 m) is a scenic area and tourist destination in east-central China. It consists of steep, sparsely treed peaks at high elevations and mixed deciduous and evergreen forests in middle and lower elevations. The study site is adjacent to Mt. Huangshan. Additional details of our study site can be found in Li et al. (2020). Several groups of Tibetan macaques are found throughout the reserve (Berman and Li, 2002). Groups appear to have non-overlapping home ranges (Wada et al., 1987).
Our two study groups are the Yulinkeng A1 group (YA1) and the Yulinkeng A2 group (YA2). Researchers began monitoring and studying YA1 in 1986. The local government provisioned YA1 at the beginning of our study in order to open the reserve to ecotourism (Li et al., 1996; Berman and Li, 2002). YA2 naturally fissioned from YA1 in 1996 (Li et al., 1996). In October 2010, YA2 experienced a second natural fissioning event and group size decreased from 74 to 41. Each group is provisioned in virtually the same manner; with a total of ca. 6 kg of dried corn per day at feeding sites set up by the reserve. The monkeys are provisioned four times per day, and feeding duration usually lasted approximately 30 min per provisioning event. After feeding, the monkeys leave the provisioned area and continue their natural and undisturbed activities in the forest.
All individuals of the two subject groups were individually recognizable based on distinct physical features such as facial or body characteristics (Li, 1999; Li et al., 2020). The compositions of two subject social groups can be found in Table 1. Matrilineal kin relationships are known for all female members of both the YA1 and YA2 groups as demographic data have been collected on a daily basis since 1986 (for YA1) and 2004 (for YA2) (Li, 1999; Li et al., 2020).
We collected behavioral data from all adult females in each group (≥5 years old; N = 8 in YA1; N = 11 in YA2). To avoid the possibility of rank changes during our study, we collected data over a limited period (108 days from September to December 2011) during which the social hierarchy remained stable. We alternated and followed the two groups from dawn to dusk. We began observations at approximately 0700–0800 and ended at 1700–1800 each day (depending on the season of year). We used focal animal sampling (with 20 min for each sample duration) and continuous recording (with a digital voice recorder, model Newsmy RV50) to collect the following behavioral data (Altmann, 1974; Xia et al., 2012): the identities of individuals in a grooming dyad, and the frequency and duration of grooming given and grooming received by identity.
Prior to daily recording, we randomly selected an ordered list of focal females for observation (Xia et al., 2012). If a preselected focal female was lost from view during the sampling interval, we observed the next female from the randomized list (Shutt et al., 2007; Xia et al., 2012). To minimize the potential influence of tourists on macaque behavior, we collected all focal animal data in the forest, where tourists were absent. In total, we recorded 133.7 h of data from group YA1 and 184 h from YA2 with approximately equal distribution of time among all adult females (16.7 ± 0.12, Mean ± SE hours per female, n = 19 females).
We defined to grooming as any act in which a macaque (groomer) uses its hand or mouth to touch, clean, or manipulate the fur of another individual (groomee) for a continuous period lasting at least 5 s (Berman et al., 2008; Xia et al., 2012, 2013). We recorded a grooming bout when an individual initiated grooming a conspecific. When the groomer and the groomee separated or if no grooming was exchanged for more than 30 s, and then grooming resumed, this was scored as a new grooming bout. Within a grooming bout, when the groomer and groomee reversed their roles, we scored this as new grooming bout (Wei et al., 2012). If individual A groomed individual B and then B groomed A grooming bout, we considered this to be two bouts (Chancellor and Isbell, 2009). For each grooming bout, we recorded the identities of the participants and the time spent grooming and being groomed.
We also recorded aggressive and submissive interactions ad libitum and used the frequency and direction of these among identified female dyads to determine dominance relationships. Aggressive behavior was defined as one individual threatening, chasing, slapping, grabbing, or biting another individual (Berman et al., 2007). Submissive behavior was defined as an individual showing fearful behaviors, such as a fear grin, cower, mock leave, avoid, flee, or scream as defined in Berman et al. (2004).
We calculated hierarchical steepness in each group. This was accomplished by building an aggressive/submission matrix according to the direction of agonistic interactions given and received. Each aggression matrix generated a matrix of Dyadic Dominance Index (DDI) values corrected for chance (Dij scores) (de Vries et al., 2006). From these scores, we generated a David’s Score for each individual as a measure of relative aggressive success. We defined hierarchical steepness as the absolute slopes of plots of NDS (Normalized David’s Score) and the rank of each resident female (Gammell et al., 2003; de Vries et al., 2006). The greater the NDS, the higher the individual’s social rank. We also calculated hierarchical steepness values from wins-losses matrices containing the dyadic proportion of wins (Pij) (scores can be found in Supplementary Table S1) (see de Vries et al., 2006). We used both Dij- and Pij-based scores to quantify and compare the steepness of the dominance hierarchy for both YA1 and YA2 social groups, according to Balasubramaniam et al. (2012). Based on Pij and Dij of the YA1 and YA2, we calculated a best-fit equation line for each. Following Barrett et al. (2002), the greater the absolute slope, the higher degree of steepness in the dominance hierarchy.
In order to analyze the effects of social rank on grooming, we assigned all adult female subjects to high-ranking, middle-ranking, and low-ranking categories. According to Zhang et al. (2010) and Xia et al. (2012), we used hierarchical cluster analysis to assign these categorize. Each females was assigned to one of the following rank classes: high-ranking (TR, YZ in YA1; HON, HM, HT in YA2), middle-ranking (TT, HHU, YH, YM in YA1; HPG, BLN, HHA, GZ, HMG in YA2), and low-ranking (TH, HH in YA1; HZ, HY, LAN in YA2) (see Table 2).
We reported data as mean (±SE) grooming frequency (episodes/h within bouts) and grooming duration (min/h). We used Wilcoxon ranked test to analyze the difference between groups, among ranks within groups, and the variation of the same rank-classes between groups. All analyses, unless otherwise specified, were two-tailed with alpha set at 0.05 a priori. We used SPSS 13.0 software (SPSS Inc., Chicago, IL, United States; see Norusis, 2005) to perform all tests. Finally, to prevent false positive results due to multiple pairwise comparisons, we calculated adjusted P-values (q-values) based on the “Graphically Sharpened” false discovery rate method (FDR-adjust) in R (version 3.2.4 for windows; TUNA Team, 2016). The use of q-values was to avoid biases using the Bonferroni correction, which tends to be too conservative (Pike, 2011).
The equations for hierarchical steepness in group YA1 were Y = −0.81X + 7.16 (based on Pij) and Y = −0.66X + 6.45 (based on Dij). For females in the YA2 group, the equations were Y = −0.76X + 9.56 (based on Pij) and Y = −0.56X + 8.37 (based on Dij) (see Figure 1). Because the slope for YA1 (0.66) was higher than that for YA2 (0.56), the female dominance hierarchy in YA1 was judged to be steeper than in YA2.

Figure 1. Plots between NDS and ranks of members for YA1 and YA2 of hierarchical stability based on Dij and Pij values, respectively. Steepness for each group was measured as the absolute slopes of these regression lines.
At the group level, adult females in YA1 groomed at a rate of 0.30 ± 0.09 bouts/h and 0.96 ± 0.15 min/h in duration. For females in YA2, these values were 0.12 ± 0.06 bouts/h and 0.48 ± 0.09 min/h. Overall, females in YA1 invested more effort in grooming than did females in YA2 (frequency: Z = 7.684, N1 = 8, N2 = 12, P < 0.01; duration: Z = 6.459, N1 = 8, N2 = 12, P < 0.01; see Figure 2).
In YA1, middle-ranking females groomed more frequently (0.38 ± 0.09 bouts/h) and for a longer duration (1.30 ± 0.09 min/h) than both high-ranking (frequency: 0.30 ± 0.07 bouts/h, Z = −5.332, adjusted P-values: q < 0.05; duration: 0.78 ± 0.12 min/h, Z = −8.301, adjusted P-values: q < 0.01) and low-ranking females (frequency: 0.13 ± 0.07 bouts/h, Z = −6.445, adjusted P-values: q < 0.05; duration: 0.46 ± 0.08 min/h, Z = −7.101, adjusted P-values: q < 0.05). In YA2, middle-ranking females groomed less frequently and for a shorter duration (frequency: 0.12 ± 0.09 bouts/h; duration: 0.55 ± 0.08 min/h) than high-ranking females (frequency: 0.17 ± 0.08 bouts/h, Z = 3.442, adjusted P-values: q < 0.05; duration: 0.60 ± 0.08 min/h, Z = 4.011, adjusted P-values: q < 0.05). However, in the group, mid-ranking females groomed others more frequently and for longer duration than did low-ranking females (frequency: 0.07 ± 0.02 bouts/h, Z = 6.703, adjusted P-values: q < 0.05; 0.30 ± 0.06 min/h, Z = 6.559, adjusted P-values: q < 0.05).
A comparison between groups indicates no statistical difference in either the frequency or duration of grooming bouts among high-ranking females (frequency: Z = 1.103, adjusted P-values: q > 0.05; duration: Z = 0.981, adjusted P-values: q > 0.05) or among low-ranking females (frequency: Z = 1.005, adjusted P-values: q > 0.05; duration: Z = 1.014, adjusted P-values: q > 0.05). However, middle-ranking females in YA1 groomed other females more frequently (Z = 8.216, adjusted P-values: q < 0.01, see Figure 3A) and for a greater duration (Z = 8.409, adjusted P-values: q < 0.05; see Figure 3B) compared to middle-ranking females in YA2.
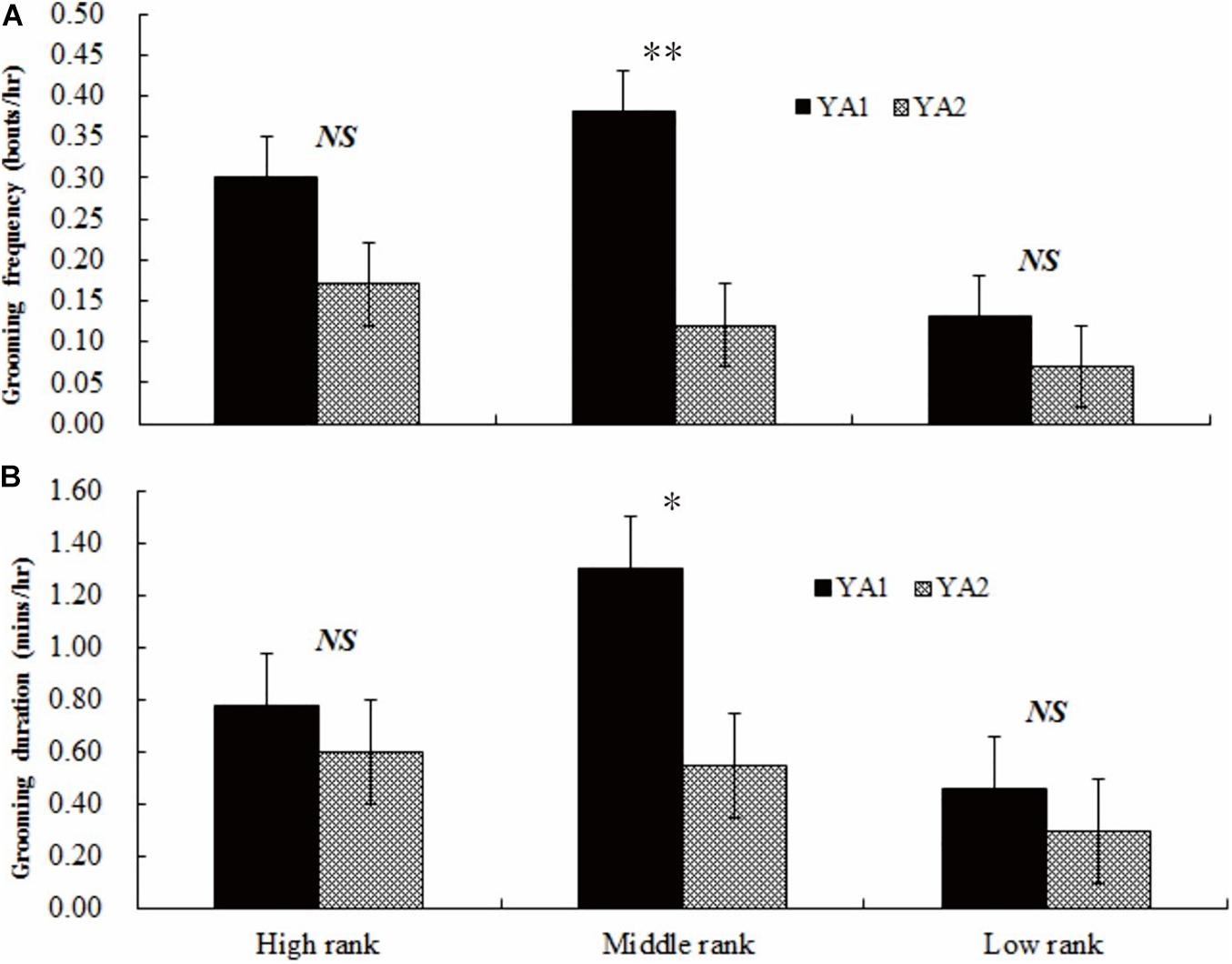
Figure 3. Grooming frequency (A) and duration (B) of individuals of similar ranks between the two groups. High rank, middle rank, and low rank represents high-ranking, middle-ranking, and low-ranking females. NS, no significant difference; ∗P < 0.05; ∗∗P < 0.01.
To our knowledge, this is the first study to investigate variation in female grooming patterns in the context of hierarchical steepness in Tibetan macaques. Our results provide evidence that Tibetan macaque females residing in social groups characterized by increased hierarchical steepness groomed others more frequently and for longer duration than females residing in a neighboring group characterized by a shallower hierarchy. Moreover, middle-ranking individuals invested more time in grooming than both high- and low-ranking individuals, and the steeper the group’s social hierarchy, the more grooming the middle-ranking females invested. This study offers new insights into the set of social strategies used by Tibetan macaques to form social bonds and social alliances within the context of a linear dominance hierarchy.
Among female primates living in matrilineal groups, social grooming has been used extensively to enhance and maintain social relationships (Dunbar, 2010). Previous studies have shown that factors such as group size and kinship influence grooming relationships (Chapais, 2006; Lehmann et al., 2007). In this regard, Dunbar (1991) who tested the group cohesion hypothesis and Lehmann et al. (2007) using a meta-analysis found that grooming tends to increase with group size across primate species. However, in this study, we found that females in smaller social group (YA1: 27 individuals) groomed more frequently and for longer duration than females in larger social group (YA2: 43 individuals). It indicated that effect of group size might be offset by the steepness of dominance hierarchy, which is one of the main variables we examined in this study. Moreover, although kinship was considered as an important factor to influence grooming patterns, reciprocity and interchange for other behavioral services (such as increasing tolerance from higher rankings) appeared to play a more critical role than kinship in explaining social grooming relationships in primates with linear dominance hierarchy (Schino and Aureli, 2010; Xia et al., 2012). Thus, multiple lines of evidence indicate that primates use social grooming as an effective tool to form, maintain, and alter social relationships. The results of our study add to our understanding of primate sociality and by providing evidence that female Tibetan macaques alter their grooming behavior based on the hierarchical steepness of their group in an attempt to improve their social rank.
In Tibetan macaques, grooming is a valuable behavioral service used for strengthening bonds among adult females (Xia et al., 2012). Assuming hierarchical steepness is a response to increased females competition for access to limited food resources, resident females are expected to select high-ranking grooming partners who will provide coalitionary support or be more socially tolerant at feeding sites (van Schaik, 1989; Sterck et al., 1997). Although two study groups are provisioned and therefore food could not be important limited factor, we found that females in YA1 (with steeper linear dominance hierarchy) groomed more frequently and for longer duration than females in YA2 (with shallower linear dominance hierarchy). A similar relationship between dominance style and grooming patterns have been reported in lion-tailed macaques (Macaca silenus) (Singh et al., 2006) and bonobos (Pan paniscus) (Stevens et al., 2005). Singh et al. (2006) found that female lion-tailed macaques residing in a group characterized by a despotic social hierarchy groomed each other more than female lion-tailed macaques living in an egalitarian group. Stevens et al. (2005) showed that grooming among female bonobos was more reciprocal in groups characterized by a shallow dominance hierarchy, compared to groups exhibiting a more steep dominance hierarchy. As such, hierarchical steepness could be considered as one of the alternative indicators to explain social grooming and social relationships between groups.
Importantly, we found that in Tibetan macaques, middle-ranking females invested more in grooming than both higher- and lower-ranking females. The steeper the group’s social hierarchy, the more frequent and the longer middle-ranking females invested in grooming. As suggested by Parr et al.(1997:336) for captive brown capuchins, middle-ranking females “…play the pivotal role by grooming both up and down the hierarchy, and may provide an affiliative link between the top and bottom divisions of the female hierarchy.” In the case of wild chacma baboons (Papio hamadryas), middle-ranking female have been observed to trade increased minutes of grooming with both high- and low-ranking group members (Henzi et al., 2003). Thus, middle-ranking females may increase their effort and groom their preferred partners to maintain affiliative bonds with both high- and low-ranking females. These females may groom low ranking females as a strategy to maintain their current social rank or groom higher ranking females in an attempt to improve their position in the hierarchy (Sun, 2013).
Furthermore, Tibetan macaques is described as a seasonal breeder, with the mating season lasting from July to January (see Li et al., 2020). Our study period was a peak of the mating season, both males and females competed for mating partners during this period from September to December. Female intrasexual competitions for access to adult males was higher, especially for high- and middle-rankings with high or middle fecundity with the effects of male mate choice (Zhang et al., 2010). Previous study has demonstrated that, to reduce aggression from higher-ranking females, lower-ranking females groomed more frequently and for longer duration to high-rankings than vice versa (Seyfarth, 1977). Empirical study showed that these patterns occurred between high- and middle-ranking, and middle- and low-ranking dyads (Xia et al., 2012). The grooming interactions of middle-ranking females lies in contrast to both high- and low-ranking females, who are found to direct grooming at either low- or high-ranking females, but not both. Berghaenel et al. (2010) noted a higher level of grooming in middle-rankings in Macaca sylvanus. Our study provides evidence that grooming frequency and duration of middle-ranking female Tibetan macaques increases with hierarchical steepness of the group, and that these females might strategically adjust their supply of grooming services to the demands of their group’s biological market. That is, if a steeper hierarchy increases the demand for grooming as a tool to reduce social anxiety, increase tolerance from high-rankings, or re-establish social bonds, middle-ranking females who most readily adjusted their behavior. In groups characterized by a shallower hierarchy, and presumed lower for grooming as a social tool, middle-ranking females supplied less grooming. Recent study at the same study site showed that middle-rankings displayed higher cortisol levels than both high- and low-ranking individuals (Wu et al., unpublished data). It provided supportive evidence that middle-rankings might devote more grooming to reduce social anxiety.
Social grooming is not only the indicator to represent dyadic social relationships, but also a process to establish and maintain dyadic social relationships. Complex factors might be involved, and thus there is no simple model to describe. Based on our results, although sample size limited, we provided an alternative explanation for grooming patterns between social groups within the framework of hierarchical steepness. We also hypothesize that middle-ranking females across a number of primate species, and possibly in other social mammals, alter their grooming behavior in response to the steepness of a social hierarchy. We suggest that in response to the dilemma faced by the middle class, these females adjust their grooming behavior in order to form and maintain social bonds with particular conspecifics. Future studies, with a larger number of sample size and a longer study period, will need pay more attention to conspecifics occupying middle social rank among more primate species in order to better understand the joint effects of rank, dominance style, social affiliation, grooming relationships and fitness in non-human primates.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the animal study because our data were collected using non-invasive, observational methods, so no review by an Institutional Animal Care and Use Committee was required according to the Chinese wildlife law. In our data collection we complied with the Wildlife Protection Law of the People’s Republic of China and the regulatory requirements of Huangshan Garden Forest Bureau.
DPX and JHL designed the study. DPX and XW performed the experiments. DPX, XW, BHS, and LKS contributed to analysis. DPX, XW, PG, LS, and JHL contributed to writing and editing the manuscript. All authors contributed to the article and approved the submitted version.
DPX acknowledges support from the National Natural Science Foundation of China (Nos. 32070455 and 31772475). JHL acknowledges support from the National Natural Science Foundation of China (No. 31672307). XW acknowledges support from the National Natural Science Foundation of China (No. 31801983). LKS acknowledges support from National Science Foundation-IRES (#1065589).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the Huangshan Garden Forest Bureau for their permission to and support our research. We acknowledge Mr. H. B. Cheng’s family for logistic support. PG wishes to thank Chrissie, Sara, Jenni, and Dax for their love and support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.631417/full#supplementary-material
Altmann, J. (1974). Observational study of behavior: sampling methods. Behaviour 49, 227–267. doi: 10.1163/156853974x00534
Aureli, F., Cords, M., and van Schaik, C. P. (2002). Conflict resolution following aggression in gregarious animals: a predictive framework. Anim. Behav. 64, 325–343. doi: 10.1006/anbe.2002.3071
Balasubramaniam, K. N., Berman, C. M., Ogawa, H., and Li, J. H. (2012). Using biological markets principles to examine patterns of grooming exchange in Macaca thibetana. Am. J. Primatol. 73, 1269–1279. doi: 10.1002/ajp.20999
Barrett, L., Gaynor, D., and Henzi, S. P. (2002). A dynamic interaction between aggression and grooming reiciprocity among female chacma baboons. Animal Behaviour 63, 1047–1053. doi: 10.1006/anbe.2002.3008
Barrett, L., Henzi, S. P., Weingrill, T., Lycett, J. E., and Hill, R. A. (1999). Market forces predict grooming reciprocity in female baboons. Proc. R. B. Biol. Sci. 266, 665–670. doi: 10.1098/rspb.1999.0687
Berghaenel, A., Schülke, O., and Ostner, J. (2010). Coalition formation among Barbary macaque males: the influence of scramble competition. Anim. Behav. 80, 675–682. doi: 10.1016/j.anbehav.2010.07.002
Berman, C. M., Ionica, C., and Li, J. H. (2004). Dominance style among Macaca thibetana on Mt. Huangshan, China. Int. J. Primatol. 25, 1283–1312. doi: 10.1023/b:ijop.0000043963.77801.c3
Berman, C. M., Ionica, C., and Li, J. H. (2007). Supportive and tolerant relationships among male Tibetan macaques at Huangshan. China. Behav. 144, 631–661. doi: 10.1163/156853907781347790
Berman, C. M., Ionica, C. S., Dorner, M., and Li, J. H. (2006). Postconflict affiliation between former opponents in Macaca thibetana on Mt. Huangshan, China. Int. J. Primatol. 27, 827–854. doi: 10.1007/s10764-006-9039-y
Berman, C. M., and Li, J. H. (2002). Impact of translocation, provisioning and range restriction on a group of Macaca thibetana. Int. J. Primatol. 23, 283–297.
Berman, C. M., Ogawa, H., Ionica, C., Yin, H., and Li, J. H. (2008). Variation in kin bias over time in a group of Tibetan macaques at Huangshan. China: contest competition, time constraints or risk reponse? Behaviour 145, 863–896. doi: 10.1163/156853908784089252
Carne, C., Wiper, S., and Semple, S. (2011). Reciprocation and interchange of grooming, agonistic support, feeding tolerance, and aggression in semi-free-ranging barbary macaques. Am. J. Primatol. 73, 1127–1133. doi: 10.1002/ajp.20979
Chancellor, R. L., and Isbell, L. A. (2009). Female grooming markets in a polulation of gray-cheeked mangabeys (Lophocebus albigena). Behav. Ecol. 20, 79–86. doi: 10.1093/beheco/arn117
Chapais, B. (2006). “Kinship, competence and cooperation in primates,” in Cooperation in primates and humans: Mechanisms and evolutions, eds P. M. Kappeler and C. P. van Schaik (New York: Springer), 47–64. doi: 10.1007/3-540-28277-7_3
de Vries, H., Stevens, J. G., and Vervaecke, H. (2006). Measuring and testing the steepness of domiance hierarchies. Anim. Behav. 71, 585–592. doi: 10.1016/j.anbehav.2005.05.015
de Waal, F. B. M. (2000). Primates: a natural heritage of conflict resolution. Science 289, 586–590. doi: 10.1126/science.289.5479.586
Dunbar, R. I. M. (1991). Functional significance of social grooming in primates. Folia Primatologica 57, 121–131. doi: 10.1159/000156574
Dunbar, R. I. M. (2010). The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neurosci. Biobehav. Rev. 34, 260–268. doi: 10.1016/j.neubiorev.2008.07.001
Fruteau, C., van de Waal, E., van Damme, E., and Noë, R. (2011). Infant access and handling in sooty mangabeys and vervet monkeys. Anim. Behav. 81, 153–161. doi: 10.1016/j.anbehav.2010.09.028
Gammell, M. P., De Vries, H., Jennings, D. J., Carlin, C. M., and Hayden, T. J. (2003). David’s score: a more appropriate dominance ranking method than Clutton-Brock et al.’s index. Anim. Behav. 66, 601–605. doi: 10.1006/anbe.2003.2226
Gumert, M. D. (2007). Payment for sex in a macaque mating market. Anim. Behav. 74, 1655–1667. doi: 10.1016/j.anbehav.2007.03.009
Gumert, M. D., and Ho, M. R. (2008). The trade balance of grooming and its coordination of reciprocation and tolerance in Indonesian long-tailed macaques (Macaca fascicularis). Primates 49, 176–185. doi: 10.1007/s10329-008-0089-y
Hemelrijk, C. K., and Ek, A. (1991). Reciprocity and interchange of grooming and ‘support’ in captive chimpanzees. Anim. Behav. 41, 923–935. doi: 10.1016/s0003-3472(05)80630-x
Henzi, S. P., and Barrett, L. (1999). The value of grooming to female primates. Primates 40, 47–59. doi: 10.1007/bf02557701
Henzi, S. P., and Barrett, L. (2002). Infants as a commodity in a baboon market. Anim. Behav. 63, 915–921. doi: 10.1006/anbe.2001.1986
Henzi, S. P., Barrett, L., Gaynor, D., Greeff, J., Weingrill, T., et al. (2003). Effect of resource competition on the long-term allocation of grooming by female baboons: evaluating Seyfarth’s model. Anim. Behav. 66, 931–938. doi: 10.1006/anbe.2003.2244
Kurihara, Y. (2016). Low-ranking female Japanese macaques make efforts for social grooming. Curr. Zool. 62, 99–108. doi: 10.1093/cz/zow006
Lehmann, J., Korstjens, A. H., and Dunbar, R. I. M. (2007). Group size, grooming and social cohesion in primates. Anim. Behav. 74, 1617–1629. doi: 10.1016/j.anbehav.2006.10.025
Li, J. H., Sun, L., and Kappeler, P. M. (2020). The behavioral ecology of the Tibetan macaque. Berlin: Springer.
Li, J. H., Wang, Q. S., and Li, M. (1996). Migration of male Tibetan macaques (Macaca thibetana) at Mt. Huangshan, Anhui province, China. Acta Theriolog. Sinca 16, 1–6. doi: 10.1007/s10329-011-0276-0
Norusis, M. (2005). SPSS 13.0 Advanced Statistical Procedures Companion. Upper Saddle River, NJ: Prentice Hall.
Noë, R. (2001). “Biological markets: partner choice as the driving force behind the evolution of mutualisms,” in Nature: Social Dilemmas, Mate Choice and Biological Markets, eds R. Noë, J. van Hooff, and P. Hammerstein, and Economics (Cambridge: Cambridge University Press).
Noë, R. (2016). How do biological markets compare to the markets of economics? Mpra Paper. France: Université de Strasbourg. 72509.
Noë, R., and Hammerstein, P. (1994). Biological market: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 35, 1–11. doi: 10.1007/s002650050063
Ogawa, H. (1995). Recognition of social relationships in bridging behavior among Tibetan macaques (Macaca thibetana). Am. J. Primatol. 35, 305–310. doi: 10.1002/ajp.1350350406
Parr, L. A., Matheson, M. D., Bernstein, I. S., and de Waal, F. B. M. (1997). Grooming down the hierarchy: Allogrooming in captive brown capuchin monkeys. Cebus paella. Anim. Behav. 54, 361–367. doi: 10.1006/anbe.1996.0419
Pike, N. (2011). Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol. Evol. 2, 278–282. doi: 10.1111/j.2041-210x.2010.00061.x
Schino, G., and Aureli, F. (2008). Tradeoffs in primate grooming reciprocity: testing behavioral flexibility and correlated evolution. Biol. J. Linnean Soc. 95, 439–446. doi: 10.1111/j.1095-8312.2008.01067.x
Schino, G., and Aureli, F. (2010). The relative roles of kinship and reciprocity in explaining primate altruism. Ecol. Lett. 13, 45–50. doi: 10.1111/j.1461-0248.2009.01396.x
Schino, G., and Pellegrini, B. (2011). Grooming and the expectation of reciprocation in mandrills (Mandrillus sphinx). Int. J. Primatol. 32, 406–414. doi: 10.1007/s10764-010-9477-4
Seyfarth, R. M. (1977). A model of social grooming among adult female monkeys. J. Theor. Biol. 65, 671–698. doi: 10.1016/0022-5193(77)90015-7
Shutt, K., MacLarnon, A., Heistermann, M., and Semple, S. (2007). Grooming in Barbary macaques: better to give than to receive? Biol. Lett. 3, 231–233. doi: 10.1098/rsbl.2007.0052
Silk, J. B. (2007). The adaptive value of sociality in mammalian groups. Philosop. Transac. R Soc. London B 362, 539–559. doi: 10.1098/rstb.2006.1994
Silk, J. B., Alberts, S. C., and Altmann, J. (2003). Social bonds of female baboons enhance infant survival. Science 302, 1231–1234. doi: 10.1126/science.1088580
Silk, J. B., Beehner, J. C., Bergman, T. J., Crockford, C., Engh, A. L., Moscovice, L. R., et al. (2009). The benefits of social captital: Close social bonds among female baboons enhance offspring survival. Proc. R Soc. B 276, 3099–3104. doi: 10.1098/rspb.2009.0681
Silk, J. B., Beehner, J. C., Bergman, T. J., Crockford, C., Engh, A. L., Moscovice, L. R., et al. (2010). Female chacma baboons form strong, equitable, and enduring social bonds. Behav. Ecol. Sociobiol. 64, 1733–1747. doi: 10.1007/s00265-010-0986-0
Singh, M., Krishna, B. A., and Singh, M. (2006). Dominance hierarchy and social grooming in female lion-tailed macaques (Macaca silenus) in the Western Ghats, India. J. Biosci. 31, 369–377. doi: 10.1007/bf02704110
Sonnweber, R. S., Massen, J. J. M., and Fitch, W. T. (2015). Post-copulatory grooming: a conditional mating strategy? Behav. Ecol. Sociobiol. 69, 1749–1759. doi: 10.1007/s00265-015-1987-9
Sterck, E. H. M., Watts, D. P., and van Schaik, C. P. (1997). The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291–309.
Stevens, J. M. G., Vervaeche, H., de Vries, H., and Van Elsacher, L. (2005). The influence of the steepness of dominance hierarchies on reciprocity and interchange in captive groups of bonobos (Pan paniscus). Behaviour 142, 941–960. doi: 10.1163/1568539055010075
Sun, L. (2013). The fairness instinct: the robin hood mentality and our biological nature. New York. Amherst: Prometheus Books.
Sussman, R. W., and Garber, P. A. (2011). “Cooperation, collective action, and competition in primate social interactions,” in Primates in Perspective, Vol. 2, eds C. J. Campbell, A. Fuentes, K. C. MacKinno, S. Bearder, and R. Stumpf (New York: Oxford University Press), 587–599.
Tiddi, B., Aureli, F., di Sorrentino, E. P., Janson, C. H., and Schino, G. (2011). Grooming for tolerance? Two mechanisms of exchange in wild tufted capuchin monkeys. Behav. Ecol. 22, 663–669. doi: 10.1093/beheco/arr028
Tiddi, B., Aureli, F., and Schino, G. (2010). Grooming for infant handling in tufted capuchin monkeys: a reappraisal of the primate infant market. Anim. Behav. 79, 1115–1123. doi: 10.1016/j.anbehav.2010.02.008
Trivers, R. L. (1971). The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57. doi: 10.1086/406755
van Schaik, C. P. (1989). “The ecology of social relationships amongst female primates,” in Comparative socioecology: the behavioural ecology of humans and other animals, eds V. Standen and R. A. Foley (Oxford: Blackwell Scientific), 195–218.
Wada, K., Xiong, C. P., and Wang, Q. S. (1987). On the distribution of Tibetan and rhesus monkeys in Southern Anhui province. China. Acta Theriolog. Sinica 7, 148–176.
Wei, W., Qi, X. G., Guo, S. T., Zhao, D. P., Zhang, P., et al. (2012). Market powers predict reciprocal grooming in Golden Snub-nosed monkeys (Rhinopithecus roxellana). PLoS One 7:e36802. doi: 10.1371/journal.pone.0036802
Xia, D. P., Li, J. H., Garber, P. A., Matheson, M. D., Sun, B. H., et al. (2013). Grooming reciprocity in male Tibetan macaques. Am. J. Primatol. 75, 1009–1020. doi: 10.1002/ajp.22165
Xia, D. P., Li, J. H., Garber, P. A., Sun, L., Zhu, Y., et al. (2012). Grooming reciprocity in female Tibetan macaques Macaca thibetana. Am. J. Primatol. 74, 569–579. doi: 10.1002/ajp.21985
Zamma, K. (2002). Grooming site preferences determined by lice infection among Japanese macaques in Arashiyama. Primates 43, 41–49. doi: 10.1007/bf02629575
Zhang, M., Li, J. H., Zhu, Y., Wang, X., and Wang, S. (2010). Male mate choice in Tibetan macaques Macaca thibetana at Mt. Huangshan, China. Curr. Zool. 56, 213–221.
Keywords: hierarchical steepness, grooming, biological market, dilemma of the middle class, primates
Citation: Xia DP, Wang X, Garber PA, Sun BH, Sheeran LK, Sun L and Li JH (2021) Effects of Hierarchical Steepness on Grooming Patterns in Female Tibetan Macaques (Macaca thibetana). Front. Ecol. Evol. 9:631417. doi: 10.3389/fevo.2021.631417
Received: 20 November 2020; Accepted: 16 February 2021;
Published: 04 March 2021.
Edited by:
Lucja A. Fostowicz-Frelik, Institute of Paleobiology (PAN), PolandReviewed by:
Małgorzata Arlet, Adam Mickiewicz University, PolandCopyright © 2021 Xia, Wang, Garber, Sun, Sheeran, Sun and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Po Xia, ZHB4aWFAYWh1LmVkdS5jbg==; Jin-Hua Li, amhsaUBhaHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.