
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Ecol. Evol., 22 March 2021
Sec. Behavioral and Evolutionary Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.626869
This article is part of the Research TopicDeterminants and Consequences of Perceived Predation Risk: From Individual Behavior to Transgenerational EffectsView all 11 articles
Predators influence prey demography through consumption, but the mere presence of predators may trigger behavioural changes in prey that, if persistent or intense, may also influence prey demography. A tractable system to study such nonconsumptive effects (NCEs) of predators involves intertidal invertebrates. This mini review summarises recent research using barnacles and mussels as prey and dogwhelks as predators. The field manipulation of dogwhelk density revealed that pelagic barnacle larvae avoid benthic settlement near dogwhelks, which limits barnacle recruitment, a relevant outcome because recruitment is the only source of population replenishment for barnacles, as they are sessile. This avoidance behaviour is likely triggered by waterborne dogwhelk cues and may have evolved to limit future predation risk. Increasing densities of barnacle recruits and adults can prevent such NCEs from occurring, seemingly because benthic barnacles attract conspecific larvae through chemical cues. Barnacle recruit density increased with the abundance of coastal phytoplankton (food for barnacle larvae and recruits), so barnacle food supply seems to indirectly limit dogwhelk NCEs. By inhibiting barnacle feeding, dogwhelk cues also limited barnacle growth and reproductive output. Wave action weakens dogwhelk NCEs likely through hydrodynamic influences. Dogwhelk cues also limit mussel recruitment, as mussel larvae also exhibit predator avoidance behaviour. The NCEs on recruitment are weaker for mussels than for barnacles, possibly because mussel larvae can detach themselves after initial settlement, an ability that barnacle larvae lack. Overall, these field experiments provide evidence of predator NCEs on prey demography for coastal marine systems.
Predators influence the demography of prey through the consumption of organisms. The mere presence of predators, however, may trigger behavioural changes in prey that may ultimately also influence prey demography. For example, when detecting predator cues, prey can move away (Werner and Peacor, 2003; Keppel and Scrosati, 2004; Metaxas and Burdett-Coutts, 2006; Zanette and Clinchy, 2019) or reduce feeding activities (Peacor and Werner, 2000; Schmitz et al., 2008; Hermann and Thaler, 2014; Urban and Richardson, 2015; Boudreau et al., 2018) to limit predation risk. Depending on the magnitude and persistence of such behavioural responses, demographic consequences may result. These consequences can be referred to as nonconsumptive effects (NCEs) of predators on prey demography.
Predator effects on prey behaviour typically occur soon after predator cues are detected by prey, so they have been studied for many terrestrial and aquatic species (Peacor et al., 2020). However, due to their inherent complexity and longer times to be expressed, predator NCEs on prey demography have historically been less studied. As they are seemingly widespread, however (Peckarsky et al., 2008), their study has been gaining traction in recent years. Thus, for example, negative predator NCEs on prey reproduction (Creel et al., 2011; Zanette et al., 2011; Mukherjee et al., 2014; Dulude-de Broin et al., 2020), recruitment (Benkwitt, 2017), and survival (MacLeod et al., 2018) have been described for terrestrial and aquatic vertebrates and on prey survival for freshwater invertebrates (McCauley et al., 2011; Siepielski et al., 2014).
Given the large animal diversity on Earth, it is worth examining how predator NCEs on prey demography may take place in organisms with different life histories and living in different environments. Such an approach will enrich our understanding of the array of responses and underlying mechanisms that can be found in nature. This mini review focuses on intertidal sessile organisms as prey. In rocky intertidal habitats (those between the highest and lowest tides on marine rocky shores), sessile filter-feeders are often abundant, especially barnacles and mussels (Menge and Menge, 2013; Valdivia et al., 2015; Scrosati and Ellrich, 2018). Because sessile organisms remain attached to the substrate, monitoring their demography can be easily done, especially during low tides when such habitats can be safely accessed on foot. In addition, their main predators are often benthic invertebrates (e.g., snails) that move slowly across the substrate, which facilitates their field manipulation. Therefore, in recent years, studies have used these organisms to enrich our knowledge on how predator NCEs on prey demography can take place. This mini review summarises the main findings of such studies.
A convenient model prey species is the barnacle Semibalanus balanoides (Figure 1), which is often abundant in North Atlantic rocky intertidal communities (Jenkins et al., 2000; Scrosati and Heaven, 2007). Adults are benthic and live permanently attached to the rocky substrate. They reproduce through pelagic larvae that undergo various nauplius stages for 5–6 weeks in coastal waters (Bousfield, 1954) until reaching the final stage (cyprid), which lives on its own reserves without feeding and seeks benthic settlement (Minchinton and Scheibling, 1991). Soon after a cyprid settles on a substrate, it metamorphoses into a recruit, which looks as a typical barnacle but is small (Figure 1). For barnacles, then, settlement refers to the permanent contact that pelagic cyprid larvae establish with the substrate, while recruitment is the appearance of new benthic organisms on the substrate as a result of the metamorphosis of settled cyprid larvae.

Figure 1. (A) Barnacles (Semibalanus balanoides), including (a) adults and (r) recruits (recruits being 1–2 mm in shell diameter), (B) blue mussels (Mytilus spp.; shell length of up to a few cm), and (C) dogwhelk (Nucella lapillus; shell length typically of up to a few cm) from rocky intertidal habitats on the Nova Scotia coast, in Atlantic Canada. Photographs taken at low tide by the author.
Recruitment is a key demographic step for barnacles because it is the only source of population replenishment, as adult migration is impossible because of their sessile nature. To find suitable substrate for settlement, cyprids of S. balanoides follow chemical cues produced by benthic conspecifics (Gabbott and Larman, 1987; Crisp, 1990; Hills and Thomason, 1998). In contrast, cyprids of this barnacle species are repelled by chemical cues from its main benthic predator, the dogwhelk Nucella lapillus (Figure 1; Ellrich et al., 2015a). Thus, the field manipulation of dogwhelk density has been useful to understand dogwhelk NCEs on barnacle recruitment. NCE intensity is in turn modulated by biotic and abiotic factors. Ultimately, by inhibiting barnacle feeding, dogwhelk cues affect barnacle reproduction, another key demographic rate. These findings are discussed below.
Blue mussels (Mytilus spp.; Figure 1) are also convenient model prey species for NCE research, as they are also sessile organisms with pelagic larvae and also frequently occur on North Atlantic rocky intertidal communities (Hunt and Scheibling, 2002; Tam and Scrosati, 2011; Scrosati and Ellrich, 2018). Intertidal blue mussels are also commonly preyed upon by N. lapillus (Crothers, 1985; Sherker et al., 2017). Thus, dogwhelk NCEs on mussel recruitment have also been investigated, which is also discussed below.
Predator NCEs on barnacle settlement and recruitment were studied by manipulating dogwhelk density in rocky intertidal habitats in Nova Scotia, Canada, that experience a moderate degree of wave exposure. An experimental unit was a cage divided with mesh in a central compartment and a peripheral compartment (see photos in Ellrich et al., 2015a). The central compartment hosted a plate covered by a tape with a sandpaper texture (Permastik anti-skid safety tread, RCR International, Boucherville, QC, Canada) that provided a settlement substrate for cyprids similar to the natural rocky substrate (Ellrich et al., 2016b). Cyprids were free to access the central compartment during high tides through the cage’s mesh. The used mesh type was found not to alter water flow in caging experiments done in intertidal habitats (Beermann et al., 2013). The peripheral compartment surrounded the central compartment and had either no dogwhelks or dogwhelks at natural densities. The caged dogwhelks were unable to access the central compartment, but their waterborne chemical cues could reach it during high tides.
In Atlantic Canada, cyprids of S. balanoides settle on intertidal substrates in May and June, which is thus the recruitment season for this species (Scrosati, 2020). Thus, to investigate dogwhelk NCEs, cages of both treatments were installed at the intertidal zone in late April. Barnacle settlement (density of settled cyprids) was measured in mid-May, while barnacle recruitment (recruit density) was measured in late June, once new recruits no longer appeared on the substrate. All macroalgae and sessile invertebrates were previously removed from the vicinity of the cages to eliminate their possible influences on barnacle recruitment (Jenkins et al., 1999; Beermann et al., 2013). The caged dogwhelks were not fed during the experiments but, to prevent starvation, they were replaced every 2 weeks with new dogwhelks. More details on methods are provided in Ellrich et al. (2015a, 2016a).
Dogwhelk presence decreased barnacle larval settlement by an average of 69% (Ellrich et al., 2016a) and barnacle recruitment by experimentwise averages of 51–83% (Ellrich et al., 2015a). These results suggest that cyprids exhibited an avoidance behaviour in the presence of waterborne dogwhelk cues, ultimately decreasing benthic recruitment. Such a decrease should be demographically relevant because recruits are the only source of barnacle population replenishment, as these are sessile organisms. A lower recruitment might also limit reproduction because, barnacles being internal cross-fertilisers, reproductive success depends on the proximity to neighbours (Anderson, 1993). On the Japanese Pacific coast, dogwhelks (Nucella lima) were also found to exert negative NCEs on barnacle (Balanus glandula and Chthamalus dalli) recruitment (Yorisue et al., 2019).
The value of dogwhelk density used in the cages referred to above (3 dogwhelks dm–2) was common on the shore. A separate field experiment showed that lower densities cause either a weaker limitation of barnacle recruitment or, if too low, no limitation at all (Ellrich et al., 2015b). This finding is consistent with increases in predator density increasing levels of waterborne predator cues (Loose and Dawidowicz, 1994; von Elert and Ponert, 2000; Kesavaraju et al., 2007; Ferland-Raymond et al., 2010).
Chemical cues from adult barnacles attract conspecific cyprids that are seeking settlement (Gabbott and Larman, 1987; Prendergast et al., 2008). This is thought to allow cyprids to find suitable habitat for benthic development (Clare, 2011), a critical choice because recruits cannot move away after metamorphosis from a settled cyprid. Therefore, a field experiment found that the presence of adult barnacles can prevent the occurrence of dogwhelk NCEs on barnacle recruitment (Ellrich et al., 2016b). Barnacle recruit density has similar effects. Under recruit densities of up to experimentwise averages of 200 recruits dm–2, dogwhelk cues (from 3 dogwhelks dm–2) limited barnacle recruitment by 51–83% (Ellrich et al., 2015a), but no NCEs occurred under recruit densities averaging 300 recruits dm–2 (Ellrich et al., 2015a). The absence of NCEs at high recruit densities may have resulted from an abundance of cyprid settlement cues produced by the quickly accumulating recruits (Shanks, 2009) and by more abundant chemical footprints left by cyprids exploring the substrate for settlement, which also attract conspecific cyprids (Yule and Walker, 1985; Phang et al., 2008). Settling cyprids might also become less selective themselves under high densities. Ultimately, food supply may have been critical for the occurrence of the high recruit densities that prevented dogwhelk NCEs from happening. In barnacles, the pre-cyprid larval stages (nauplii) and the recruits feed on phytoplankton (Anderson, 1993). The high recruit densities noted above occurred under a high coastal phytoplankton abundance (Ellrich et al., 2015a), which may have enhanced the survival of larvae and recruits (Scrosati and Ellrich, 2016, 2018), thus increasing their density.
A laboratory experiment showed that waterborne cues from N. lapillus limit feeding activity in adult S. balanoides (Johnston et al., 2012), presumably because the cirral swipes that barnacles make to harvest plankton can also disperse metabolites that attract dogwhelks (Barnes, 1999). Correspondingly, a field experiment showed that dogwhelks have negative NCEs on barnacle growth from spring to fall. As body size is related to reproductive output in barnacles (Wethey, 1984), dogwhelk cues also limited egg production per barnacle in the fall (Ellrich et al., 2016a). Although not measured, such NCEs may have resulted in a lower larval production in the following spring.
The experiments discussed above were done in habitats subjected to a moderate wave action. A field experiment using the same cage design found that a higher degree of wave exposure (in habitats where dogwhelks also occur) prevented the occurrence of dogwhelk NCEs on barnacle recruitment (Ellrich and Scrosati, 2016). This result is consistent with a pattern of predator cue dilution under increased water velocities and with the notion that turbulent conditions decrease the ability of mobile organisms (such as cyprids) to locate cue sources (Finelli et al., 2000; Large et al., 2011; Robinson et al., 2011; Pruett and Weissburg, 2019, 2021).
Through an experiment done in the same sheltered habitats where dogwhelk NCEs on barnacle recruitment were revealed (Ellrich et al., 2015b, 2016b; Ellrich and Scrosati, 2016), dogwhelk NCEs on mussel recruitment were also evaluated (Ehlers et al., 2018). Two intertidal mussel species (Mytilus edulis and Mytilus trossulus) occur on the studied rocky shores (Tam and Scrosati, 2011, 2014), both of which are preyed upon by N. lapillus (Sherker et al., 2017). Because of morphological similarities (Innes and Bates, 1999) and hybridisation (Riginos and Cunningham, 2005), their visual identification is difficult, so mussel recruits were counted as Mytilus spp., as commonly done in field studies with these species (Cusson and Bourget, 2005; Le Corre et al., 2013). The same cage design was used to manipulate dogwhelk density but, instead of a plate, the central compartment of the cages hosted a plastic mesh scourer (Our Compliments Pot Scrubber, Mississauga, ON, Canada; see a picture in Ehlers et al., 2018). Mesh scourers are often used to quantify intertidal mussel recruitment because they resemble preferential habitat (filamentous algae or byssal mussel threads) for mussel larval settlement (Menge and Menge, 2013; South, 2016). This experiment ran between late May and late July and found that dogwhelk cues limited mussel recruitment, but only by 13% on average (Ehlers et al., 2018).
The weaker dogwhelk NCEs on mussel recruitment than on barnacle recruitment suggest that prey life history traits may help to predict NCE intensity. While barnacles cannot move away after recruitment, mussels can relocate, albeit limitedly, across the substrate after recruitment (Bayne, 1964; Hunt and Scheibling, 2002). Mussel adults can also immobilise dogwhelks with byssus (Farrell and Crowe, 2007). Overall, these abilities allow mussels to avoid predation through mechanisms that barnacles lack. Such differences might explain why mussel recruitment was less responsive to dogwhelk cues than barnacle recruitment. It will be interesting to evaluate if actively mobile benthic prey (e.g., herbivore snails) have even weaker responses to dogwhelk presence.
Overall, this mini review summarises recent studies with intertidal invertebrates that have revealed predator NCEs on prey demographic traits and external factors modulating such effects (Figure 2). Barnacles and mussels have demonstrated to be good model species to monitor demographic responses in prey, as counts can be accurately done because they are sessile organisms. The acquired body of knowledge is valuable because it resulted from field experiments, done under complex natural conditions that laboratory experiments cannot fully reproduce, as noted by other researchers (Weissburg et al., 2014; Babarro et al., 2016; Wiggins et al., 2018).
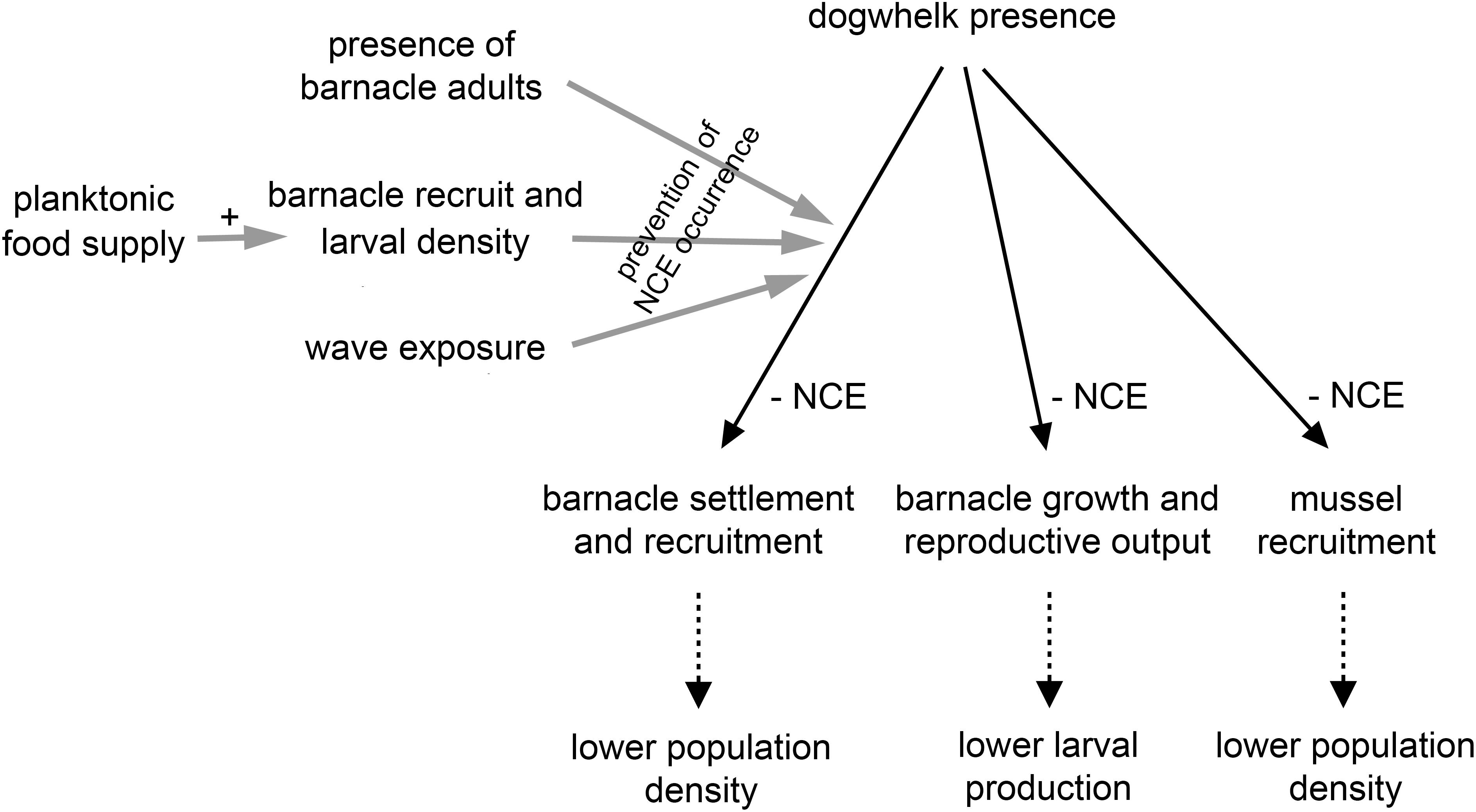
Figure 2. Diagram summarising the current evidence on dogwhelk NCEs on barnacle and mussel demography supported by field experiments (solid black lines). The factors listed on the left have experimentally been shown to prevent the occurrence of such NCEs (solid grey lines). The three items depicted at the bottom represent possible additional outcomes (dotted black lines).
The predator avoidance behaviour shown by barnacle and mussel larvae when seeking settlement may have evolved to limit predation risk for the subsequent benthic stages. This could be so because adult movements across the substrate are impossible for barnacles and limited for mussels. Although predators could eventually reach an area where they were absent at the time of prey settlement, the avoidance of predators by settling prey larvae does reduce future predation risk to an extent. Ultimately, the occurrence of negative predator NCEs on the recruitment of barnacles and mussels should locally limit population density for these organisms because of their sessile nature. For barnacles, this could be detrimental for reproduction because they need nearby neighbours to cross-fertilise.
The presence of barnacle adults and high recruit densities prevented the occurrence of dogwhelk NCEs on barnacle recruitment. Benthic barnacles attract conspecific cyprids through chemical cues, which is thought to aid cyprids find favourable habitats to settle. It appears that an abundance of conspecific settlement-inducing cues would thus neutralise the effects that dogwhelk cues would otherwise exert on cyprids seeking settlement. However, the occurrence of too many adult barnacles on the substrate might limit conspecific recruitment, as high adult densities may indicate cyprids the potential for strong intraspecific competition after recruitment (Scrosati and Ellrich, 2017). Therefore, for a given dogwhelk density, NCE intensity may have a non-linear dependence on adult barnacle density. On the other hand, a very high supply of cyprids from the water column (favoured by a high phytoplanktonic food supply) could swamp the shore with settlers, making benthic recruitment less responsive to dogwhelk cues. Factorial field experiments manipulating these variables could clarify these possible interactions. It could also be of interest to obtain more realistic estimates of NCE intensity given that dogwhelks move across the substrate (which cages do not allow to happen). The main goal of the field experiments hereby described was to demonstrate that NCEs on prey demographic traits can occur. Measures of NCEs on demography could thus be refined by manipulating dogwhelk density over time to mimic natural dogwhelk movements across the substrate.
The chemical nature of the dogwhelk cues that trigger the observed NCEs on barnacle and mussel demography is not known with certainty. Based on studies for other aquatic predator–prey systems (Poulin et al., 2018; Puglisi et al., 2019), such cues could be constitutive and/or related to the dogwhelks’ diet. Identifying their chemical nature should thus help to understand the physiological constraints affecting dogwhelks that can ultimately influence their remote detection by prey.
It is worth noting that predation risk can trigger morphological and physiological responses in prey besides behavioural responses (Hawlena and Schmitz, 2010). For brevity and consistency, this mini review has focussed on behavioural responses influencing prey demography. Through a field experiment, dogwhelk cues were also found to trigger shell thickening in mussels, which was experimentally shown to increase handling times of mussels by dogwhelks during attacks (Sherker et al., 2017). Whether that outcome decreases mortality rates in populations remains untested, but it is possible because longer handling times may limit predation success. Thus, investigating demographic influences of prey responses to predation risk other than behavioural might also be interesting using intertidal invertebrates.
It also worth emphasising that this mini review was aimed at summarising the current evidence of predator NCEs on prey demography using intertidal invertebrates. Its goals did not include aspects of NCE research that are more common in the literature, such as comparisons of consumptive versus nonconsumptive effects of predators (Peckarsky et al., 2008; Weissburg et al., 2014; Peacor et al., 2020). Such studies could be done using intertidal invertebrates by, for example, manipulating the ability of dogwhelks to consume barnacle and mussel recruits in addition to manipulating dogwhelk presence to evaluate their NCEs.
Finally, as for all interspecific interactions (Menge and Sutherland, 1987; Kondoh, 2001; Silliman and He, 2018), the intensity of predator NCEs on prey demography likely depends greatly on the abiotic context and food supply (Kimbro et al., 2020; Wirsing et al., 2021). In fact, as noted above, the intensity of dogwhelk NCEs on barnacle recruitment was found to depend on wave exposure and prey food supply. These factors, in turn, depend on coastal oceanography and climate (Ardhuin et al., 2019; Menge and Menge, 2019; Shanks and Morgan, 2019). Thus, for predator–prey systems in general, future research could aim to understand environmental influences on predator NCEs on prey demography.
RAS is the single author of this manuscript.
This mini review was funded by a Discovery Grant (#311624) awarded to the author by the Natural Sciences and Engineering Research Council of Canada (NSERC).
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
I am grateful to the two reviewers for their constructive comments on a previous version of this manuscript.
Anderson, D. T. (1993). Barnacles, Structure, Function, Development, and Evolution. London: Chapman & Hall, 357.
Ardhuin, F., Stopa, J. E., Chapron, B., Collard, F., Husson, R., Jensen, R. E., et al. (2019). Observing sea states. Front. Mar. Sci. 6:124. doi: 10.3389/fmars.2019.00124
Babarro, J. M. F., Vázquez, E., and Olabarria, C. (2016). Importance of phenotypic plastic traits on invasive success: response of Xenostrobus securis to the predatory dogwhelk Nucella lapillus. Mar. Ecol. Prog. Ser. 560, 185–198. doi: 10.3354/meps11904
Barnes, M. (1999). The mortality of intertidal cirripedes. Oceanogr. Mar. Biol. Annu. Rev. 37, 153–244.
Bayne, B. L. (1964). Primary and secondary settlement in Mytilus edulis L. (Mollusca). J. Anim. Ecol. 33, 513–523. doi: 10.2307/2569
Beermann, A. J., Ellrich, J. A., Molis, M., and Scrosati, R. A. (2013). Effects of seaweed canopies and adult barnacles on barnacle recruitment: the interplay of positive and negative influences. J. Exp. Mar. Biol. Ecol. 448, 162–170. doi: 10.1016/j.jembe.2013.07.001
Benkwitt, C. E. (2017). Predator effects on reef fish settlement depend on predator origin and recruit density. Ecology 98, 896–902. doi: 10.1002/ecy.1732
Boudreau, M. L., Scrosati, R. A., and Wong, M. C. (2018). Predator (Carcinus maenas) nonconsumptive limitation of prey (Nucella lapillus) feeding depends on prey density and predator cue type. J. Ethol. 36, 259–264. doi: 10.1007/s10164-018-0557-9
Bousfield, E. L. (1954). The distribution and spawning seasons of barnacles on the Atlantic coast of Canada. Bull. Natl. Mus. Canada 132, 112–154.
Clare, A. S. (2011). “Toward a characterization of the chemical cue to barnacle gregariousness,” in Chemical Communication in Crustaceans, eds T. Breithaupt and M. Thiel, (New York, NY: Springer Science), 431–450. doi: 10.1007/978-0-387-77101-4_22
Creel, A., Christianson, D., and Winney, J. A. (2011). A survey of the effects of wolf predation risk on pregnancy rates and calf recruitment in elk. Ecol. Appl. 21, 2847–2853. doi: 10.1890/11-0768.1
Crisp, D. J. (1990). Gregariousness and systematic affinity in some North Carolinian barnacles. Bull. Mar. Sci. 47, 516–525.
Crothers, J. H. (1985). Dog-whelks: an introduction to the biology of Nucella lapillus (L.). Field Stud. 6, 291–360.
Cusson, M., and Bourget, E. (2005). Small-scale variations in mussel (Mytilus spp.) dynamics and local production. J. Sea Res. 53, 255–268. doi: 10.1016/j.seares.2004.07.005
Dulude-de Broin, F., Hamel, S., Mastromonaco, G. F., and Côté, S. D. (2020). Predation risk and mountain goat reproduction: evidence for stress-induced breeding suppression in a wild ungulate. Funct. Ecol. 34, 1003–1014. doi: 10.1111/1365-2435.13514
Ehlers, S. M., Scrosati, R. A., and Ellrich, J. A. (2018). Nonconsumptive predator effects on prey demography: dogwhelk cues decrease benthic mussel recruitment. J. Zool. 305, 240–245. doi: 10.1111/jzo.12555
Ellrich, J. A., and Scrosati, R. A. (2016). Water motion modulates predator nonconsumptive limitation of prey recruitment. Ecosphere 7:e01402.
Ellrich, J. A., Scrosati, R. A., Bertolini, C., and Molis, M. (2016a). A predator has nonconsumptive effects on different life-history stages of a prey. Mar. Biol. 163:5.
Ellrich, J. A., Scrosati, R. A., Romoth, K., and Molis, M. (2016b). Adult prey neutralizes predator nonconsumptive limitation of prey recruitment. PLoS One 11:e0154572. doi: 10.1371/journal.pone.0154572
Ellrich, J. A., Scrosati, R. A., and Molis, M. (2015a). Predator nonconsumptive effects on prey recruitment weaken with recruit density. Ecology 96, 611–616. doi: 10.1890/14-1856.1
Ellrich, J. A., Scrosati, R. A., and Petzold, W. (2015b). Predator density affects nonconsumptive predator limitation of prey recruitment: field experimental evidence. J. Exp. Mar. Biol. Ecol. 472, 72–76. doi: 10.1016/j.jembe.2015.07.005
Farrell, E. D., and Crowe, T. P. (2007). The use of byssus threads by Mytilus edulis as an active defense against Nucella lapillus. J. Mar. Biol. Assoc. U.K. 87, 559–564. doi: 10.1017/s0025315407055622
Ferland-Raymond, B., March, R. E., Metcalfe, C. D., and Murray, D. L. (2010). Prey detection of aquatic predators: assessing the identity of chemical cues eliciting prey behavioral plasticity. Biochem. Syst. Ecol. 38, 169–177. doi: 10.1016/j.bse.2009.12.035
Finelli, C. M., Pentcheff, N. D., Zimmer, R. K., and Wethey, D. S. (2000). Physical constraints on ecological processes: a field test of odor-mediated foraging. Ecology 81, 784–797. doi: 10.1890/0012-9658(2000)081[0784:pcoepa]2.0.co;2
Gabbott, P. A., and Larman, V. N. (1987). “The chemical basis of gregariousness in cirripedes: a review (1953-1984),” in Barnacle Biology, eds R. Schram and A. J. Southward, (Rotterdam: A. A. Balkema), 377–388. doi: 10.1201/9781315138053-19
Hawlena, D., and Schmitz, O. J. (2010). Physiological stress as a fundamental mechanism linking predation to ecosystem functioning. Am. Nat. 176, 537–556. doi: 10.1086/656495
Hermann, S. L., and Thaler, J. S. (2014). Prey perception of predation risk: volatile chemical cues mediate non-consumptive effects of a predator on a herbivorous insect. Oecologia 176, 669–676. doi: 10.1007/s00442-014-3069-5
Hills, J. M., and Thomason, J. C. (1998). The effect of scales of surface roughness on the settlement of barnacle (Semibalanus balanoides) cyprids. Biofouling 12, 57–69. doi: 10.1080/08927019809378346
Hunt, H. L., and Scheibling, R. E. (2002). Movement and wave dislodgment of mussels on a wave-exposed rocky shore. Veliger 45, 273–277.
Innes, D. J., and Bates, J. A. (1999). Morphological variation of Mytilus edulis and Mytilus trossulus in eastern Newfoundland. Mar. Biol. 133, 691–699. doi: 10.1007/s002270050510
Jenkins, S. R., Åberg, P., Cervin, G., Coleman, R. A., Delany, J., Della Santina, P., et al. (2000). Spatial and temporal variation in settlement and recruitment of the intertidal barnacle Semibalanus balanoides (L.) (Crustacea: cirripedia) over a European scale. J. Exp. Mar. Biol. Ecol. 243, 209–225. doi: 10.1016/s0022-0981(99)00121-5
Jenkins, S. R., Norton, T. A., and Hawkins, S. J. (1999). Settlement and post-settlement interactions between Semibalanus balanoides (L.) (Crustacea: cirripedia) and three species of fucoid canopy algae. J. Exp. Mar. Biol. Ecol. 236, 49–67. doi: 10.1016/s0022-0981(98)00189-0
Johnston, B. R., Molis, M., and Scrosati, R. A. (2012). Predator chemical cues affect prey feeding activity differently in juveniles and adults. Can. J. Zool. 90, 128–132. doi: 10.1139/z11-113
Keppel, E., and Scrosati, R. (2004). Chemically mediated avoidance of Hemigrapsus nudus (Crustacea) by Littorina scutulata (Gastropoda): effects of species coexistence and variable cues. Anim. Behav. 68, 915–920. doi: 10.1016/j.anbehav.2003.11.020
Kesavaraju, B., Damal, K., and Juliano, S. A. (2007). Threat-sensitive behavioral responses to concentrations of water-borne cues from predation. Ethology 113, 199–206. doi: 10.1111/j.1439-0310.2006.01317.x
Kimbro, D. L., Scherer, A. E., Byers, J. E., Grabowski, J. H., Hughes, A. R., Piehler, M. F., et al. (2020). Environmental gradients influence biogeographic patterns of nonconsumptive predator effects on oysters. Ecosphere 11:e03260.
Kondoh, M. (2001). Unifying the relationships of species richness to productivity and disturbance. Proc. R. Soc. Lond. B 268, 269–271. doi: 10.1098/rspb.2000.1384
Large, S. I., Smee, D. L., and Trussell, G. C. (2011). Environmental conditions influence the frequency of prey responses to predation risk. Mar. Ecol. Prog. Ser. 422, 41–49. doi: 10.3354/meps08930
Le Corre, N., Martel, A. L., Guichard, F., and Johnson, L. E. (2013). Variation in recruitment: differentiating the roles of primary and secondary settlement of blue mussels Mytilus spp. Mar. Ecol. Prog. Ser. 481, 133–146. doi: 10.3354/meps10216
Loose, C. J., and Dawidowicz, P. (1994). Trade-offs in diel vertical migration by zooplankton: the costs of predator avoidance. Ecology 75, 2255–2263. doi: 10.2307/1940881
MacLeod, K. J., Krebs, C. J., Boonstra, R., and Sheriff, M. J. (2018). Fear and lethality in snowshoe hares: the deadly effects of non-consumptive predation risk. Oikos 127, 375–380. doi: 10.1111/oik.04890
McCauley, S. J., Rowe, L., and Fortin, M. J. (2011). The deadly effects of “nonlethal” predators. Ecology 92, 2043–2048. doi: 10.1890/11-0455.1
Menge, B. A., and Menge, D. N. L. (2013). Dynamics of coastal meta-ecosystems: the intermittent upwelling hypothesis and a test in rocky intertidal regions. Ecol. Monogr. 83, 283–310. doi: 10.1890/12-1706.1
Menge, B. A., and Menge, D. N. L. (2019). Testing the intermittent upwelling hypothesis: comment. Ecology 100:e02476.
Menge, B. A., and Sutherland, J. P. (1987). Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am. Nat. 130, 730–757. doi: 10.1086/284741
Metaxas, A., and Burdett-Coutts, V. (2006). Response of invertebrate larvae to the presence of the ctenophore Bolinopsis infundibulum, a potential predator. J. Exp. Mar. Biol. Ecol. 334, 187–195. doi: 10.1016/j.jembe.2006.01.025
Minchinton, T. E., and Scheibling, R. E. (1991). The influence of larval supply and settlement on the population structure of barnacles. Ecology 72, 1867–1879. doi: 10.2307/1940984
Mukherjee, S., Heithaus, M. R., Trexler, J. C., Ray-Mukherjee, J., and Vaudo, J. (2014). Perceived risk of predation affects reproductive life-history traits in Gambusia holbrooki, but not in Heterandria formosa. PLoS One 9:e88832. doi: 10.1371/journal.pone.0088832
Peacor, S. D., Barton, B. T., Kimbro, D. L., Sih, A., and Sheriff, M. J. (2020). A framework and standardized terminology to facilitate the study of predation-risk effects. Ecology 101:e03152.
Peacor, S. D., and Werner, E. E. (2000). Predator effects on an assemblage of consumers through induced changes in consumer foraging behavior. Ecology 81, 1998–2010. doi: 10.1890/0012-9658(2000)081[1998:peoaao]2.0.co;2
Peckarsky, B. L., Abrams, P. A., Bolnick, D. I., Dill, L. M., Grabowski, J. H., Luttberg, B., et al. (2008). Revisiting the classics: considering nonconsumptive effects in textbook examples of predator–prey interactions. Ecology 89, 2416–2425. doi: 10.1890/07-1131.1
Phang, I. Y., Aldred, N., Clare, A. S., and Vancso, G. J. (2008). Towards a nanomechanical basis for temporary adhesion in barnacle cyprids (Semibalanus balanoides). J. R. Soc. Interface 5, 397–401. doi: 10.1098/rsif.2007.1209
Poulin, R. X., Lavoie, S., Siegel, K., Gaul, D. A., Weissburg, M. J., and Kubanek, J. (2018). Chemical encoding of risk perception and predator detection among estuarine invertebrates. Proc. Natl. Acad. Sci. U.S.A. 115, 662–667. doi: 10.1073/pnas.1713901115
Prendergast, G. S., Zurn, C. M., Bers, A. V., Head, R. M., Hansson, L. J., and Thomason, J. C. (2008). Field-based video observations of wild barnacle cyprid behaviour in response to textural and chemical settlement cues. Biofouling 24, 449–459. doi: 10.1080/08927010802340135
Pruett, J. L., and Weissburg, M. J. (2019). Eastern oysters use predation risk cues in larval settlement decisions and juvenile inducible morphological defenses. Mar. Ecol. Prog. Ser. 621, 83–94. doi: 10.3354/meps12998
Pruett, J. L., and Weissburg, M. J. (2021). Environmental stress gradients regulate the relative importance of predator density- and trait-mediated indirect effects in oyster reef communities. Ecol. Evol. 11, 796–805. doi: 10.1002/ece3.7082
Puglisi, M. P., Sneed, J. M., Ritson-Williams, R., and Young, R. (2019). Marine chemical ecology in benthic environments. Nat. Prod. Rep. 36, 410–429. doi: 10.1039/c8np00061a
Riginos, C., and Cunningham, C. W. (2005). Local adaptation and species segregation in two mussel (Mytilus edulis x Mytilus trossulus) hybrid zones. Mol. Ecol. 14, 381–400. doi: 10.1111/j.1365-294x.2004.02379.x
Robinson, E. M., Smee, D. L., and Trussell, G. C. (2011). Green crab (Carcinus maenas) foraging efficiency reduced by fast flows. PLoS One 6:e21025. doi: 10.1371/journal.pone.0021025
Schmitz, O. J., Grabowski, J. H., Peckarsky, B. L., Preisser, E. L., Trussell, G. C., and Vonesh, J. R. (2008). From individuals to ecosystem function: toward an integration of evolutionary and ecosystem ecology. Ecology 89, 2436–2445. doi: 10.1890/07-1030.1
Scrosati, R., and Heaven, C. (2007). Spatial trends in community richness, diversity, and evenness across rocky intertidal environmental stress gradients in eastern Canada. Mar. Ecol. Prog. Ser. 342, 1–14. doi: 10.3354/meps342001
Scrosati, R. A. (2020). Effects of intertidal elevation on barnacle recruit density and size in wave-exposed habitats on the Atlantic Canadian coast. Northeast. Nat. 27, 186–194. doi: 10.1656/045.027.0201
Scrosati, R. A., and Ellrich, J. A. (2016). A 12-year record of intertidal barnacle recruitment in Atlantic Canada (2005-2016): relationships with sea surface temperature and phytoplankton abundance. PeerJ 4:e2623. doi: 10.7717/peerj.2623
Scrosati, R. A., and Ellrich, J. A. (2017). Unimodal relationship between small-scale barnacle recruitment and the density of pre-existing barnacle adults. PeerJ 5:e3444. doi: 10.7717/peerj.3444
Scrosati, R. A., and Ellrich, J. A. (2018). Benthic-pelagic coupling and bottom-up forcing in rocky intertidal communities along the Atlantic Canadian coast. Ecosphere 9:e02229. doi: 10.1002/ecs2.2229
Shanks, A. L. (2009). Barnacle settlement versus recruitment as indicators of larval delivery. I. Effects of post-settlement mortality and recruit density. Mar. Ecol. Prog. Ser. 385, 205–216. doi: 10.3354/meps08105
Shanks, A. L., and Morgan, S. G. (2019). Testing the intermittent upwelling hypothesis: reply. Ecology 100:e02516.
Sherker, Z. T., Ellrich, J. A., and Scrosati, R. A. (2017). Predator-induced shell plasticity in mussels hinders predation by drilling snails. Mar. Ecol. Prog. Ser. 573, 167–175. doi: 10.3354/meps12194
Siepielski, A. M., Wang, J., and Prince, G. (2014). Nonconsumptive predator-driven mortality causes natural selection on prey. Evolution 68, 696–704. doi: 10.1111/evo.12294
Silliman, B. R., and He, Q. (2018). Physical stress, consumer control, and new theory in ecology. Trends Ecol. Evol. 33, 492–503. doi: 10.1016/j.tree.2018.04.015
South, P. M. (2016). An experimental assessment of measures of mussel settlement: effects of temporal, procedural, and spatial variations. J. Exp. Mar. Biol. Ecol. 482, 64–74. doi: 10.1016/j.jembe.2016.05.002
Tam, J. C., and Scrosati, R. A. (2011). Mussel and dogwhelk distribution along the north-west Atlantic coast: testing predictions derived from the abundant-centre model. J. Biogeogr. 38, 1536–1545. doi: 10.1111/j.1365-2699.2011.02498.x
Tam, J. C., and Scrosati, R. A. (2014). Distribution of cryptic mussel species (Mytilus edulis and M. trossulus) along wave exposure gradients on northwest Atlantic rocky shores. Mar. Biol. Res. 10, 51–60. doi: 10.1080/17451000.2013.793809
Urban, M. C., and Richardson, J. L. (2015). The evolution of foraging rate across local and geographic gradients in predation risk and competition. Am. Nat. 186, E16–E32.
Valdivia, N., Aguilera, M. A., Navarrete, S. A., and Broitman, B. R. (2015). Disentangling the effects of propagule supply and environmental filtering on the spatial structure of a rocky shore metacommunity. Mar. Ecol. Prog. Ser. 538, 67–79. doi: 10.3354/meps11493
von Elert, E., and Ponert, G. (2000). Predator specificity of kairomones in diel vertical migration of Daphnia: a chemical approach. Oikos 88, 119–128. doi: 10.1034/j.1600-0706.2000.880114.x
Weissburg, M., Smee, D. L., and Ferner, M. C. (2014). The sensory ecology of nonconsumptive predator effects. Am. Nat. 184, 141–157. doi: 10.1086/676644
Werner, E. E., and Peacor, S. D. (2003). A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100. doi: 10.1890/0012-9658(2003)084[1083:arotii]2.0.co;2
Wethey, D. S. (1984). Effects of crowding on fecundity in barnacles: Semibalanus (Balanus) balanoides, Balanus glandula, and Chthamalus dalli. Can. J. Zool. 62, 1788–1795. doi: 10.1139/z84-261
Wiggins, W. D., Bounds, S., and Wilder, S. M. (2018). Laboratory-reared and field-collected predators respond differently to same experimental treatments. Behav. Ecol. Sociobiol. 72:19.
Wirsing, A. J., Heithaus, M. R., Brown, J. S., Kotler, B. P., and Schmitz, O. J. (2021). The context dependence of non-consumptive predator effects. Ecol. Lett. 24, 113–129.
Yorisue, T., Ellrich, J. A., and Momota, K. (2019). Mechanisms underlying predator-driven biotic resistance against introduced barnacles on the Pacific coast of Hokkaido, Japan. Biol. Invasions 21, 2345–2356. doi: 10.1007/s10530-019-01980-4
Yule, A. B., and Walker, G. (1985). Settlement of Balanus balanoides: the effect of cyprid antennular secretion. J. Mar. Biol. Assoc. U.K. 65, 707–712. doi: 10.1017/s0025315400052541
Keywords: barnacle, demography, mussel, Mytilus, Nucella, predation risk, Semibalanus, whelk
Citation: Scrosati RA (2021) Nonconsumptive Predator Effects on Prey Demography: Recent Advances Using Intertidal Invertebrates. Front. Ecol. Evol. 9:626869. doi: 10.3389/fevo.2021.626869
Received: 07 November 2020; Accepted: 24 February 2021;
Published: 22 March 2021.
Edited by:
Julien Terraube, University of the Sunshine Coast, AustraliaReviewed by:
Michael Sitvarin, Clayton State University, United StatesCopyright © 2021 Scrosati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ricardo A. Scrosati, cnNjcm9zYXRAc3RmeC5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.