
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 16 March 2021
Sec. Behavioral and Evolutionary Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.624245
This article is part of the Research Topic Ecology, Evolution, and Behavior of Viviparous Fishes View all 19 articles
Invasive species are one of the greatest threats to biodiversity. Behavioral traits are recognized as key to promote individual’s survival in changing conditions. For social species being part of a group is key to carry out vital activities. Heterospecific social environments could provide exotic species with the opportunity to join groups and gain the advantages of being part of a larger population. Short latency to exit a refuge is a behavioral response that could be linked to invasion success as it increases the chances of individuals to locate food sources and other resources in novel environments. The guppy (Poecilia reticulata), a successful invader, has been found to take advantage of the presence of native species to reduce its refuge emergence latency and acquire information. The research was carried out in Mexico, we investigated the effect of heterospecific social contexts that include natives and other invasive viviparous fishes on guppies’ refuge emergence latency. We found that guppies’ emergence latency was shorter when accompanied by another guppy than when alone. Their latency was also shorter when with other invaders and when with native goodeids, but with one of the invaders (Pseudoxiphophorus bimaculatus) and with goodeids (Skiffia bilineata) latency reduction was not as high as when with conspecifics or with the invader Poecilia gracilis. Our experiment supports both the idea that already established invaders could provide benefits to new ones, and that native species also provide benefits but less than invaders. Increasing our knowledge about conspecific and heterospecific social interactions that could make an exotic species become invasive is key to assess the invasion risk of a community.
Accelerated changes in the world threaten biodiversity and favor the spread of invasive species that modify the structure of the communities they invade (Early et al., 2016). Behavior is important at each stage of the invasion process (i.e., movement, introduction, establishment, and spread) and understanding the mechanisms that promote or decrease individual’s fitness at each stage is crucial when trying to predict the success or failure of unintentional introductions of species (Chapple et al., 2012). Invasive species are considered among the greatest threats to biodiversity, the main cause for extinction for birds and the second main cause for mammals and fish (Clavero and Garcia-Berthou, 2005; Simberloff et al., 2013). During the first stages of invasion, the low availability of conspecifics could decrease the chances for a species to establish due to the shortage of conspecifics to effectively perform vital tasks like foraging or avoiding predators (Liebhold and Bascompte, 2003). Even though animals can still forage and evade predators, they often do it better when in the company of others (Snijders et al., 2020).
Social animals tend to become part of groups when the advantages of doing so outweigh the disadvantages (Krause et al., 2002; Ward, 2012). When facing a low availability of conspecifics exotic individuals might choose to join heterospecific groups. Indeed, the “meltdown theory” states that previously established invaders promote invasion of new species as a result of interactions between them that enhance survival or population size, which could be through habitat modification, seed dispersal or any social interaction in which species benefit from each other (Simberloff and Von Holle, 1999; Simberloff, 2006; Rennó Braga et al., 2018). However, little research has been done on whether native species could act as facilitators as well. There are some examples in fish (Camacho-Cervantes et al., 2014a) and lizards (Damas-Moreira et al., 2018), but to the best of our knowledge not a comparison has been made on how an invasive species could act as a facilitator compared to a native one.
Risk-taking behavior has been recognized as valuable when establishing in a novel environment, as it could enhance individuals’ response to changing conditions by enabling them to explore novel objects or areas, which might lead to the opportunity to find food, new refuges, partners, among others (Rehage and Sih, 2004; Reale et al., 2007; Sol et al., 2018). Risk-taking behavior is part of the shy-bold continuum and some of the ways in which variation of risk-taking behavior in fish is quantified are: novel object test (inspect-bold, do not inspect-shy), latency to feed under risk of predation (feed-bold, don’t feed-shy) and the novel environment test (emerge quickly bold, emerge slowly shy) (Huntingford et al., 2013). Individuals must constantly assess costs and benefits of risk-taking behavior, and benefits (such as reproduction enhancement and food acquisition) should outweigh the risk of encountering predators or competition so that risk-taking behaviors actually results in benefits (Reale et al., 2007). An animal then must continually decide whether to remain sheltered or emerge into an open habitat depending on current factors such as its energy status and vulnerability to predation (Sih, 1992; Dowling and Godin, 2002).
The guppy (Poecilia reticulata) is an invasive species with a natural distribution range in Trinidad, Guyana, Venezuela and Suriname. They inhabit shallow rivers and ponds (Seghers et al., 1995; Magurran, 2005). As invaders, guppies are reported present in every continent except Antarctica (Deacon et al., 2011), including the Central Mexican Plateau where they are expanding its invasive range (Contreras-MacBeath et al., 1998; Gesundheit and Macías Garcia, 2018). They manage to survive and settle at temperatures (Chung, 2001; Reeve et al., 2014) and salinities (Chervinski, 1984) that are distant from those of their native environment. For example, guppies can be found in unusual locations such as the Moscow sewer (Zhuikov, 1993) and the River Lee in Essex, England (Wheeler, 1998); or in Germany where temperature can drop to 12°C (Lukas et al., 2017), in these places artificial heating effluents keep the water temperature high enough for them to survive. The guppy is also a very social species that performs most of its vital tasks in groups (Magurran, 1999, 2005) and one of the characteristics that is thought to have favored guppies’ invasion in the Mexican Central Plateau (MCP) is their tendency to associate with native Goodeids, which translates into benefits such as locating food faster or acquiring information on food availability (Camacho-Cervantes et al., 2014a, 2015). The guppy is not the only invasive poeciliid present in the MCP, porthole livebearers (Poeciliopsis gracilis) and twospot livebearers (Pseudoxiphophorus bimaculatus) have also managed to establish populations in the area (Contreras-MacBeath et al., 1998; Gesundheit and Macías Garcia, 2018).
Given that guppies derive benefits from native species, and that this could be one of the tools they use during the first stages of invasion when conspecifics availability is low (Camacho-Cervantes et al., 2014a, 2015), the present investigation aims to research if the latency to exit a refuge when shoaling with conspecifics, other invasive or natives species changes. We hypothesize that guppies will show a lower latency to leave the refuge to an unknown area when they are accompanied by other invaders than when they are in the company of native Mexican goodeid twoline skiffia (Skiffia bilineata), given that poeciliid species are more associated with risk taking behaviors than goodeids (Valero et al., 2008; Brown et al., 2009). If so, then by being more prone to take risks invasive guppies could gain some of the benefits associated with it, such as locating food faster, however they might be under higher predation risk too, as this is also related to risk taking behavior. This information could contribute to the idea that poeciliids might be part of an invasive meltdown mechanism in the Mexican rivers.
The guppy (Poecilia reticulata)—is a freshwater invasive species that belongs to the Poeciliidae family. It is one of the most popular aquarium fish with many standardized varieties in the world for its easy maintenance. Guppies are able to establish populations in a wide range of conditions (Gibson and Hirst, 1955; Chung, 2001); in the wild, guppies can occupy aquatic habitats that are highly turbid as well as pristine ponds, canals or lakes (Rodriguez, 1997). They belong to a group of ovoviviparous fish whose females are able to store sperm for up to 6 months and their offspring can be from different males (Meffe and Snelson, 1989; Hain and Neff, 2007). Thus the release of even one sexually mature female can result in the establishment of viable invasive populations in the wild (Deacon et al., 2011). Guppies are omnivorous and have a strong sexual dimorphism. Females are light grayish in color and continue to grow throughout their lives, reaching a body length of around 4 cm but up to 6 cm. Males grow to sexual maturity and do not usually measure more than 4 cm, they present a color pattern composed mainly of yellow, orange and black spots (Magurran, 2005). Guppies are carriers of exotic parasites, and are considered a threat to native fishes, as the decline of several endangered species have been linked to its invasion (Magellan and Magurran, 2009; Global Invasive Species Database, 2020).
The Porthole livebearer (Poeciliopsis gracilis)—is a freshwater species from the Poeciliidae family. Native to central America found from Southern Mexico to Honduras. The species has been introduced in the MCP in the Balsas river basin, in the states of Guerrero and Michoacán (Miller et al., 2009). This species inhabits quiet water bodies of streams, flood-water ponds, lagoons, micro reservoirs and lakes, and is normally found within waters that can range from clear to muddy (Meffe and Snelson, 1989; Miller et al., 2009). Males and females are only differentiated by the modified anal fin of males (gonopodium). Males grow up to 5 cm while females are usually larger reaching up to 6.6 cm (MCC Pers. Obs.). However, little is known about the biology and particular mechanisms of invasion of this species, particularly what is related to their behavior because its study is a relatively new approach to elaborate managing plans and to identify potential invaders (Carere and Gherardi, 2013). To the best of our knowledge, there are no published records of the particular way in which porthole livebearers pose a threat to native species, but Gesundheit and Macías Garcia (2018) recognized invasive poeciliids in general as a threat to native species in the MCP.
The Twospot livebearer (Pseudoxyphophorus bimaculatus)—is a freshwater species belonging to de Poeciliidae family. Is native to the Atlantic slope drainages of Central America, occurring from the Misantla River in central Veracruz, Mexico, southwards to the Prinzapolka River in Nicaragua (Miller et al., 2009). This species has also been translocated into a number of drainages in Mexico, including the Teuchitlan drainage in Jalisco and the upper Balsas drainage (Ayotac River) in Guerrero (Ramírez-García et al., 2018). Is an habitat generalist that can be found in lotic and lentic systems, lakes and seasonal wetlands (Pérez Alvarado et al., 2004; Escalera-Vázquez et al., 2017). The twospot livebearer has the ability to change its diet according to the alterations in the habitat structure, water quality, and biotic integrity of places it inhabits, it is recognized as omnivore (Carbajal-Becerra et al., 2020). As the porthole livebearer, the twospot livebearer has not been as thoroughly studied as the guppy in its invasion contexts. However, this species is more aggressive than the guppy and grows up to 6.7 cm (MCC Pers. Obs.). They are also carriers of fish parasites that are exotic to the MCP (Salgado-Maldonado et al., 2014).
The Twoline skiffia (Skiffia bilineata)—the native fish in this study, is a freshwater species, belonging to the Goodeidae family. This species is endemic to the MCP, where it is found in the Rio Lerma Grande de Santiago basin and the Rio Grande de Morelia basin within the States of Michoacán and Guanajuato. This species can be found in freshwater river systems, in quiet, shallow, muddy, and typically slow waters (depths of less than 1 m over mud), with occasional dense vegetation (Koeck, 2019). The species is threatened by a continuing decline in the quality of its habitat, as a result of water pollution from human activities, predation and competition from introduced invasive alien species (De La Vega-Salazar et al., 2003a.). Because of this it is considered an endangered species in the IUCN Red List (Koeck, 2019). Twoline skiffias are also omnivores and are amongst the smallest goodeid species. They are sexually dimorphic, females reach up to 5 cm while males reach up to 6 cm. Its reported optimum temperature range is 19–22°C (Ornelas-Garcia et al., 2012).
Experiments were carried out in the Institute of Marine Sciences and Limnology of the Universidad Nacional Autónoma de México in Mexico City from the 4th to the 26th of June 2019. Twoline skiffias used in this experiment were collected from outdoor ponds in the Institute of Ecology (19°18′44″N 99°11′46″O); guppies, porthole livebearers and twospot livebearers were collected from Mixquiahuala in the Tula River (20° 30′ 25″ N, 99° 14′ 44″ W), each species was collected at different ponds created by trees’ roots on the river side. The ponds were apart from each other by 10–15 m. The fish were collected with scoop nets and traps on the riverbank, in periods of 15 min. After collection, all fishes were carefully transported to the aquarium at the Institute of Marine Sciences and Limnology in breathable bags, with Pentabiocare®, salt and zeolite, which is a filter substrate. Fish were kept in the aquarium for 3 weeks prior to the trials in separate stock tanks (40 L) for each species that contained between 25 and 30 fish per tank. Tanks were set up with aged tap water—which is tap water left in a container with an aerator for at least 8 days to evaporate any trace of chlorine. Water was also treated with a solution called Pentabiocare®, which is a high-performance colloidal conditioner with a high concentration of Thiamine (Vitamin B1, also known as antineurotic vitamin). This solution has the property of helping fish to better withstand stress conditions in the different stages of collection, such as transport, capture and during fish adaptation period to the conditions of a new aquarium. Each tank contained a filter, gravel at the bottom, a water pump and plastic plants. Photoperiod was 12L:12D. Water daily temperature ranged between 19 and 22°C. Tanks were visually isolated from one another and individuals used in each trial were kept separated for at least 2 weeks prior to observations to avoid familiarity effects (Griffiths and Magurran, 1997). Fish were fed with commercial flake food daily. To avoid sexually motivated behavior we only used female fish of all species in our experiment, at least in the case of guppies females have been found to allocate more time to shoaling and foraging behavior than males (Sievers et al., 2012).
Our experiment had an independent measurement approach, as all observations were made independently from each other; each fish (focal guppy or companion) were observed only once and discarded to a tank in a different section of the aquarium to avoid confusions. We observed focal guppies that were randomly selected prior to each observation in five different treatments: (1) alone, (2) focal guppy accompanied by a conspecific, (3) by a porthole livebearer, (4) by a twospot livebearer and (5) by a twoline skiffia. Observations for each treatment were made randomly but ensuring that at least one of each was made each day. The observation tank was 50 × 30 × 30 cm and was filled with water up to 26 cm. In addition, it contained gravel on the bottom and plastic plants that formed a 15 cm long refuge on one side of the tank (Figure 1).
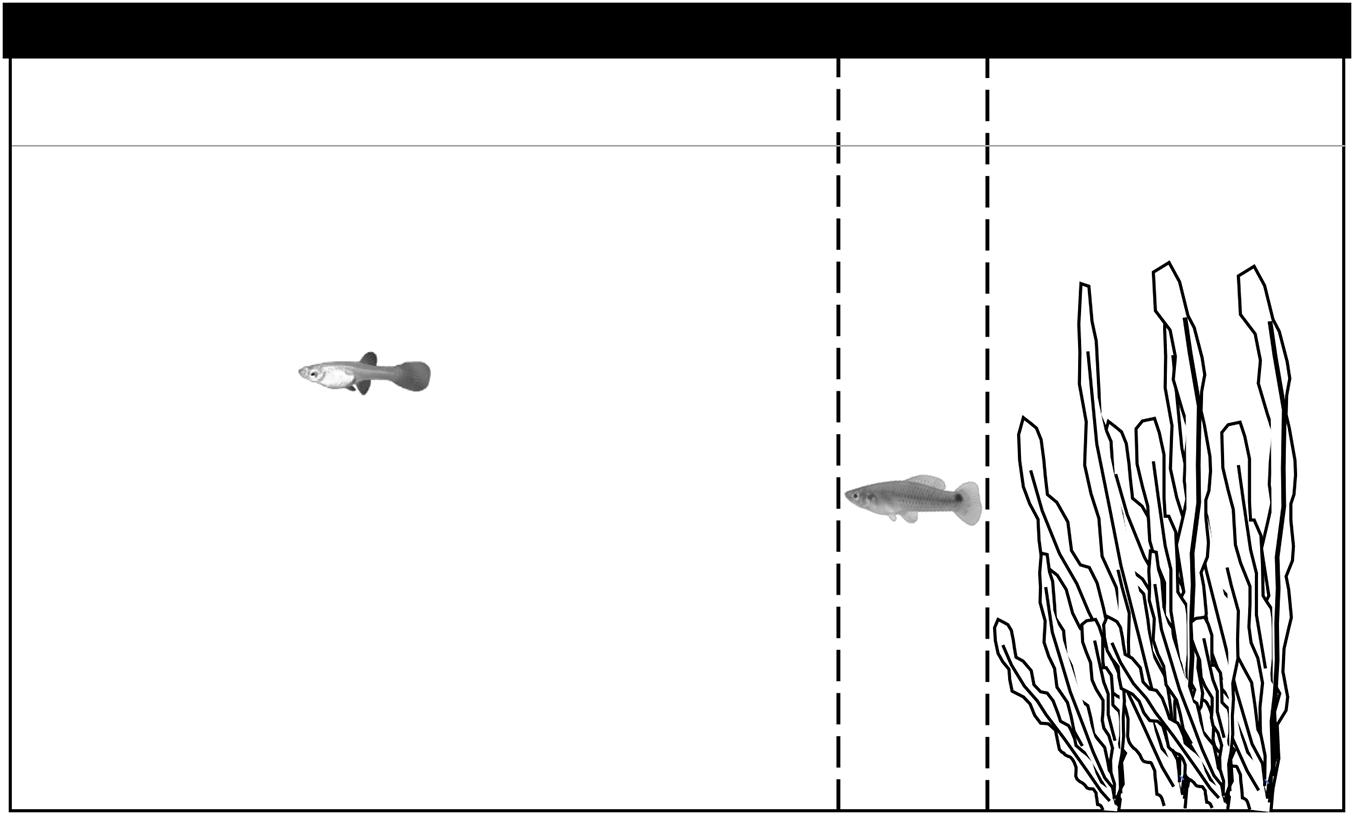
Figure 1. Diagram of the experimental tank set up. Plastic plants were used to simulate a refuge, the refuge was identical for all observations. Observations started when fish were gently released in the upper right corner and lasted until both fish left the refuge, we considered fish had left the refuge when they were one body length (distance between dashed lines) away from the end of the refuge (dashed line closest to plants). We averaged the standard length of 10 fish randomly selected from the stock to calculate the distance between dashed lines, these lines were indicated for the observer and invisible to the fish.
Observations started when the focal guppy or couple were gently released in the refuge section of the tank by submerging the edge of the transporting container (100 ml) and letting the fish switch to the observation tank, which reduced stress (i.e., shaking, freezing, nervous swimming patterns). Both fish left the transporting container almost immediately to swim into the plants refuge. Observations lasted until both fish left the refuge. We recorded fish (both focal and companion) had exited the refuge when they were at least one body length away from the edge (this distance was calculated after averaging the standard length of 10 randomly selected fish from the stock). To ensure exiting the refuge was considered equally in each observation, we drew an indicating mark at the end of the refuge followed by another mark separated by the average standard length of fish, both marks were invisible to the fish. In none of the observations made fish displayed antagonistic behaviors. A total of 17 replicates for each treatment were made, we thus used 120 guppies, 20 porthole livebearers, 20 twospot livebearers and 20 twoline skiffias. Observations were made between 9:00 and 13:00 h. Focal guppy fish and companions were paired in size but still photographed after the end of each observation to measure their standard length. Average sizes of individuals used for each species were: guppies 49.4 mm (SD = 10.3 mm), porthole livebearers 41.8 mm (SD = 12.7 mm), twospot livebearers 50.5 mm (SD = 5.6 mm), and twoline skiffias 51.1 mm (SD = 9.7 mm). The average size difference between focals and companions was 2.5 mm (SD = 1.7 mm). Size difference between focals and companions was not normally distributed thus we ln-transformed it to achieve normality (Shapiro-Wilk: W = 0.96, p = 0.053). We tested for differences between treatments and since we did not find any [lm: t(3,68) < −0.38, p > 0.71] we removed the difference in size between focals and companions from the analysis to improve clarity. Still, we incorporated size of the focal fish to explore its effect on emergence latency. Variables recorded and analyzed were: frequency of focal guppies exiting the refuge first, latency of the focal guppy to exit the refuge (s) and latency of the companion to exit the refuge (s). In addition, using exit latencies of focals and companions we calculated the difference between the time one and the other fish left the refuge by subtracting the time when the focal left from the time when the companion left, thus positive numbers mean focal exited first and negative companions left first.
We performed a Z-test to compare the proportion of times focal guppies were the first to exit the refuge in each treatment against the 0.5 null hypothesis proportion—focal and companions exiting first an equal number of times. Latency to exit the refuge of focal guppies, of the companion and difference between their emergence latencies were not normally distributed. Given that time is a continuous variable, we performed generalized linear models (glm) specifying Inverse Gaussian and Gamma distributions to later select the best model through the distribution of their residuals and their Akaike information criterion (AIC), models with the lowest AIC value score indicates the most parsimonious model to explain variation in data (Burnham and Anderson, 2002; Johnson and Omland, 2004). We included treatment, fish size, as well as the interaction between treatment and fish size as explanatory variables in all our models. However, in all models, the interaction between treatment and fish size was not significant, and thus, it was removed from the analysis to increase clarity. Since neither Gamma nor Inverse Gaussian distributions work with negative values we added a fixed number (200) to differences between emergence latencies of focals and companions in order to shift all values to positive. Finally, for variables that had a significant p-value we carried out a Tukey post hoc (glht) analysis from the “multcomp” R package to identify the treatments that were significantly different (Bretz et al., 2016). The more parsimonious model to test focal’s emergence latency was the one that specified Gamma distribution, for partner’s emergence latency was the one that specified Inverse Gaussian and for the difference between focal’s and partner’s emergence latencies was Gamma; please refer to our Supplementary Material 1 file for the complete output of our models plus their Residuals vs. Fitted, Normal Q-Q, Scale-Location and Residuals vs. Leverage plots. All our analysis were carried out in the R Studio statistical software (R Core Team., 2020).
All fish from all treatments left the refuge before reaching 6 min after release. In the four treatments that included a companion, guppies and the companion exited the refuge a similar number of times as the proportion of times guppies exited the refuge first was not significantly different from the 0.5 null hypothesis proportion (Z17 < 1.2, p = 0.2). Focal guppies’ emergence latencies were different between treatments (glm: |t| 4,85 > 2.4, p < 0.02, Figure 2A). Focals took longer when alone than when accompanied by conspecifics or porthole livebearers (glht: |Z| > 2.7, p < 0.05), and as long when with twospot livebearers or twoline skiffias (glht: |Z| < 2.4, p > 0.1). Size had no effect in the emergency latency of focal guppies (glm: |t| 4,85 < 0.1, p > 0.91). Emergence latency of companions was similar between treatments and size did not had an effect on it [glm: t(3,68) < 1.27, p > 0.21]. Regarding the difference between the emergence latencies of focals and companions, we found no difference between treatments and no effect of focals’ size [glm: t(3,68) < 2, p > 0.054, Figure 2B].
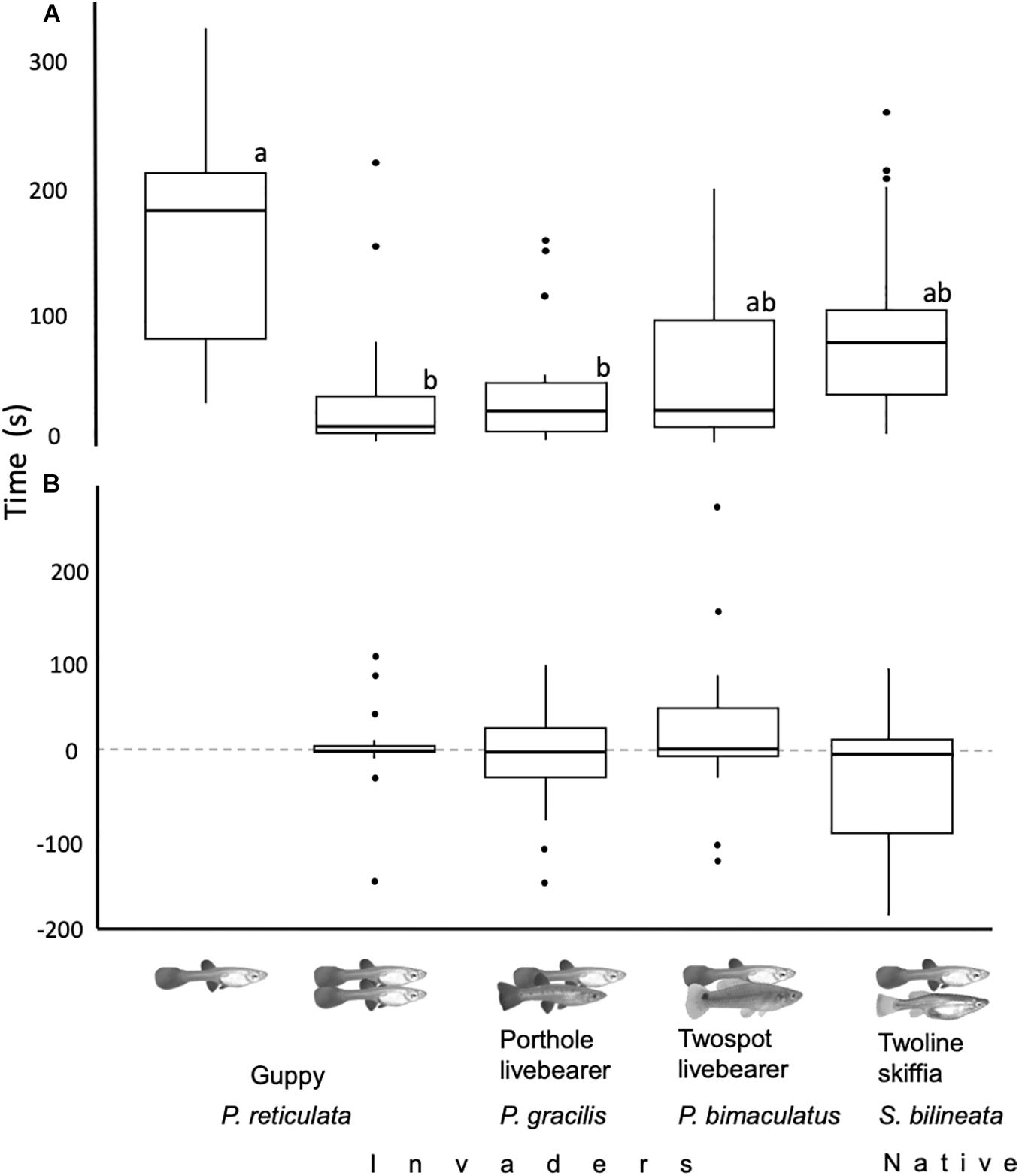
Figure 2. (A) Time it took focal guppies to exit the refuge between treatments was different. They took longer to exit the refuge when alone than when accompanied, lower case letters represent significant differences between treatments. (B) Differences between the emergence latency of the first and the second fish were similar between treatments; positive numbers mean focal exited first and negative companions exited first. For both panels centerlines of boxplots represent the median, top and bottom of boxes represent the 25th and 75th percentile, while dots outliers.
Behavior of an individual is influenced by the changing presence or absence of conspecifics and/or heterospecifics in the environment (Ward, 2012). Traits of temperament, such as the tendency to take risks, affects how an individual interacts with conspecifics and heterospecifics they socialize with, as well as their reactions to predators, food sources, and habitat (Reale et al., 2007). In our study, guppies emerged quicker from a refuge when with a conspecific or an invasive porthole livebearer than when alone. When accompanied by a twospot livebearer or a goodeid, focal guppies emergence latencies were intermediate, similar as when accompanied by conspecifics and other species but not significantly different from when alone. We hypothesize that when with twospot livebearers guppies might not feel as secure as when with porthole livebearers being that twospot livebearers are generally more aggressive. However, we did not record any agonistic behavior in any of our observations, thus more research on the topic should be made before reaching any conclusion. Regarding focals emergence latency when with goodeids, the fact that the native twoline skiffias used in our experiment are less related and not sympatric to guppies might contribute to the decreased facilitation effect compared to the other (invasive) species.
Previous studies have found that size has an effect in fish risk taking behavior, Bierbach et al. (2020) found a pattern of “the bigger the better” in the leadership in schools of guppies, they proved that fish more easily followed a larger leader regardless of the size of the follower, while Lukas et al. (2021) found smaller individuals tend to be bolder. Nevertheless, in our experiment, we did not find evidence that body size influenced the emergence latency of guppies. Our results are in accordance with those of Harris et al. (2010). They found the variables that influence emergence latency of guppies the most are sex (males showed a higher tendency to risk taking behaviors) and predation risk in their origin locations (guppies from high predation risk locations were more prompt to risk taking behaviors) while size had no effect. This is also the case for Brachyrhaphis roseni and Brachyrhaphis terrabensis, Ingley et al. (2014) found these species’ tendency to risk related behaviors are related to their sex (males are more eager to engage in risk taking behaviors than females) and the presence of predators in their origin locations (fish from high predation risk locations were more prompt to risk taking behaviors) while size had no effect. However this is not the case for all poeciliid species, Brown and Braithwaite (2004) examined the relationship between size and risk taking in eight populations of the Poeciliid Brachyraphis episcopi and found size showed influence in this species since larger fish took longer to exit the refuge.
Previous research has shown that when accompanied by others, fish spend longer in open (higher risk) areas and resume faster their foraging activities after a simulated predation attack (Webster et al., 2007; Magnhagen and Bunnefeld, 2009). Similarly, in our experiment, focal guppies showed a shorter emergence latency from a refuge when accompanied, by conspecifics or heterospecifics, than when alone. We hypothesize this could be due to the fact that social individuals, such as guppies, form groups to increase their safety from predators via collective detection and dilution effect (Krause et al., 2002). We acknowledge that our results are valid only for females as we only tested them, thus the behavior of males is still to be investigated. Generally speaking, guppy males have been found to be bolder than females (Godin and Dugatkin, 1996; Harris et al., 2010), and that females tend to be more at risk when accompanied by males (Piyapong et al., 2010). However, females could be more important than males in the dispersion of the species and colonization of further areas as a single sexually mature female could found a viable population (Deacon et al., 2011). Still, in the case of both sexes being present, males could even enhance females’ tendency to take risks, but this idea would also need further testing before making any assumption.
When established invaders and exotic individuals have mutualistic associations or simply benefit from being close, there could be a facilitation mechanism for invasion of exotics, known as invasional meltdown (Simberloff, 2006; Green et al., 2011). This phenomenon remains controversial as some studies demonstrate empirical evidence for positive interactions that confirm the invasional meltdown hypothesis (Green et al., 2011). While other studies propose that non-native species have additive effects at ecosystem, community and population level, which does not support the invasional meltdown hypothesis (Rennó Braga et al., 2018).
Risk taking behaviors could aid fish populations to better cope with novel conditions as it might contribute to their spread into novel areas; individuals with a higher tendency to take risks could be at a better chance to find food sources, mating partners or shelter, which in turn could influence their invasion success (Rehage and Sih, 2004; Chapple et al., 2012). Our results suggest that, when conspecific availability is low, guppies could be as quick to exit a refuge to an unknown environment when associating with other invasive poeciliids as when they associate with conspecifics. This could represent a tool to take advantage from in their invasion context if conspecific individuals to associate with are scarce.
Although we only researched one behavioral response, emergence latency from a refuge, our results could be evidence to build on the idea of an invasion meltdown mechanism when certain other invaders are present, while still a facilitation mechanism when only natives are present. Previous research has found that guppies do prefer to associate with conspecifics than with native heterospecifics (Camacho-Cervantes et al., 2014b, 2018) and they derive more benefits from conspecifics than from native individuals (Camacho-Cervantes et al., 2014a). Which has been discussed as a native facilitation mechanism at least for food finding, transmission of information on food availability and boldness (Camacho-Cervantes et al., 2014a, 2015). We found a similar trend in our experiment and this could suggest that deriving benefits from either other invaders or natives is a tool that would be useful only during the initial stages of their invasion process, while conspecifics availability is low. Furthermore, given that species from which they derive benefits have similar ecological requirements, they could become competitors for space and food creating an invasional interference after settling down (Rauschert and Shea, 2017). This possibly suggests that the invasional meltdown facilitation is only a short stage until offsetting the disadvantages of being part of small populations in the early invasional stages (Drake, 2004; Tobin et al., 2011). However, in the case of our experiment, this would only prove true if indeed a higher tendency to take risks is advantageous (e.g., through finding food sources or novel areas to disperse and establish). More research is needed to test for different behavioral traits (e.g., predator avoidance, food finding) and population dynamics in time to be able to fully support this.
Our results build on the idea that sites that are already invaded may be at increased risk for new invasions, as natives and established invaders could aid exotic individuals to engage in risk taking behaviors (such as abandoning a refuge to explore an unknown area), which have been linked with invasion success (Sol and Maspons, 2016). Since we only researched one behavioral response, we acknowledge that more research on the positive interactions between invaders, other invaders and natives is needed to fully understand how social exotic species become successful invaders. Specially because species with similar ecological requirements, as the ones tested here, would be expected to be competitors and not allies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the animal study because the experiments for the purposes of this article were conducted at the Universidad Nacional Autónoma de México (UNAM) in Mexico City using fish of four different species, three of which are invasive and considered pests in the site of collection and thus the Mexican Ministry of Environmental and Natural Resources (SEMARNAT) resolved we did not need a permit from them to collect these species. The native species we used has been kept for over 10 years in an outdoors pond in the Institute of Ecology (UNAM) for research purposes, we were granted a verbal permit to use these fish to perform our experiment by the researcher responsible of the population, Prof Constantino Macías-Garcia. Fish were transported to the laboratory following the Official Mexican Norm NOM-051-ZOO-1995 for humanitarian treatment in the mobilization of animals. Field and laboratory protocols followed all guidelines provided by the Mexican Official Norm NOM-062-ZOO-1999 for the use and maintenance of vertebrates for research purposes. Given that the experimental design involved behavioral observations in glass aquarium tanks, which did not include any surgery, anesthesia, or other invasive procedure that could have produced distress in the fish we were not required to get a permit from the Ethics Committee of the Institute of Marine Sciences and Limnology.
AS-A and MC-C designed the experiment. VP-H and AS-A collected the data. MC-C obtained the funding to carry out this research. All authors analyzed, discussed, and prepared this manuscript.
This research was carried out thanks to a grant provided by the PAPIIT-DGAPA-UNAM (IA202419).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Constantino Macías Garcia for letting us fish in his pond and Edgar Avila Luna for helping us with the fishing. Miguel Angel Mosqueda Cabrera for his valuable comments on the discussion of our results. Isabel Salazar-Rueda, Sebastian Gomez-Maldonado, and Yannire Vazquez-Benitez for their help in the field collection and maintenance of fish in the aquarium.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.624245/full#supplementary-material
Bierbach, D., Mönck, H. J., Lukas, J., Habedank, M., Romanczuk, P., Landgraf, T., et al. (2020). Guppies prefer to follow large (robot) leaders irrespective of own size. Front. Bioengin. Biotechnol. 8:441. doi: 10.3389/fbioe.2020.00441
Bretz, F., Hothorn, T., and Westfall, P. (2016). Multiple comparisons using R.. Boca Raton, FL: CRC Press.
Brown, C., and Braithwaite, V. A. (2004). Size matters: a test of boldness in eight populations of the poeciliid Brachyraphis episcopi. Anim. Behav. 68, 1325–1329. doi: 10.1016/j.anbehav.2004.04.004
Brown, G. E., Macnaughton, C. J., Elvidge, C. K., Ramnarine, I., and Godin, J.-G. J. (2009). Provenance and threat-sensitive predator avoidance patterns in wild-caught trinidadian guppies. Behav. Ecol. Sociobiol. 63, 699–706. doi: 10.1007/s00265-008-0703-4
Burnham, K. P., and Anderson, D. R. (2002). A practical information-theoretic approach. Model selection and multimodel inference, 2nd Edn. New York: Springer. 2.
Camacho-Cervantes, M., Garcia, C. M., Ojanguren, A. F., and Magurran, A. E. (2014a). Exotic invaders gain foraging benefits by shoaling with native fish. Royal Soc. Open Sci. 1, 1–9. doi: 10.1098/rsos.140101
Camacho-Cervantes, M., Ojanguren, A. F., Deacon, A. E., Ramnarine, I. W., and Magurran, A. E. (2014b). Association tendency and preference for heterospecifics in an invasive species. Behaviour 151, 769–780. doi: 10.1163/1568539X-00003169
Camacho-Cervantes, M., Ojanguren, A. F., Domínguez-Domínguez, O., and Magurran, A. E. (2018). Sociability between invasive guppies and native topminnows. PLoS One 13:e0192539. doi: 10.1371/journal.pone.0192539
Camacho-Cervantes, M., Ojanguren, A. F., and Magurran, A. E. (2015). Exploratory behaviour and transmission of information between the invasive guppy and native mexican topminnows. Anim. Behav. 106, 115–120. doi: 10.1016/j.anbehav.2015.05.012
Carbajal-Becerra, O., Olvera-Rodríguez, K. J., Souza, G. M., de Durán-Rodríguez, O. Y., Ramírez-García, A., and Ramírez-Herrejón, J. P. (2020). Trophic strategies of the invasive twospot livebearer (pseudoxiphophorus bimaculatus, teleostei: poeciliidae) in a gradient of environmental quality in central mexico. Neotrop. Ichthyol. 18:e190080. doi: 10.1590/1982-0224-2019-0080
Carere, C., and Gherardi, F. (2013). Animal personalities matter for biological invasions. Trends Ecol. Evol. 28, 5–6. doi: 10.1016/j.tree.2012.10.006
Chapple, D. G., Simmonds, S. M., and Wong, B. B. M. (2012). Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol. Evol. 27, 57–64. doi: 10.1016/j.tree.2011.09.010
Chervinski, J. (1984). Salinity tolerance of the guppy, Poecilia reticulata Peters. J. Fish Biol. 24, 449–452. doi: 10.1111/j.1095-8649.1984.tb04815.x
Chung, K. (2001). Critical thermal maxima and acclimation rate of the tropical guppy Poecilla reticulata. Hydrobiologia 462, 253–257. doi: 10.1023/A:1013158904036
Clavero, M., and Garcia-Berthou, E. (2005). Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 20:110. doi: 10.1016/j.tree.2005.01.003
Contreras-MacBeath, T., Mojica, H. M., and Wilson, R. C. (1998). Negative impact on the aquatic ecosystems of the state of morelos, mexico from introduced aquarium and other commercial fish. Aquarium Sci. Conserv. 2, 67–78.
Damas-Moreira, I., Oliveira, D., Santos, J. L., Riley, J. L., Harris, D. J., and Whiting, M. J. (2018). Learning from others: an invasive lizard uses social information from both conspecifics and heterospecifics. Biol. Lett. 14:20180532. doi: 10.1098/rsbl.2018.0532
De La Vega-Salazar, M. Y., Avila-Luna, E., and Macías-Garcia, C. (2003a). Ecological evaluation of local extinction: the case of two genera of endemic mexican fish, Zoogoneticus and Skiffia. Biodiv. Conserv. 12, 2043–2056.
Deacon, A. E., Ramnarine, I. W., and Magurran, A. E. (2011). How reproductive ecology contributes to the spread of a globally invasive fish. PLoS One 6:e24416. doi: 10.1371/journal.pone.0024416
Dowling, L. M., and Godin, J.-G. J. (2002). Refuge use in a killifish: influence of body size and nutritional state. Can. J. Zool. 80, 782–788. doi: 10.1139/z02-036
Drake, J. M. (2004). Allee effects and the risk of biological invasion. Risk Analysis Int. J. 24, 795–802. doi: 10.1111/j.0272-4332.2004.00479.x
Early, R., Bradley, B. A., Dukes, J. S., Lawler, J. J., Olden, J. D., Blumenthal, D. M., et al. (2016). Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 7, 1–9.
Escalera-Vázquez, L. H., Calderón-Cortés, N., and Zambrano-González, L. (2017). Fish population responses to hydrological variation in a seasonal wetland in southeast méxico. Neotrop. Ichthyol. 15:20160129. doi: 10.1590/1982-0224-20160129
Gesundheit, P., and Macías Garcia, C. (2018). The role of introduced species in the decline of a highly endemic fish fauna in central mexico. Aquatic Conserv. Mar. Freshwater Ecosyst. 28, 1384–1395. doi: 10.1002/aqc.2927
Gibson, M., and Hirst, B. (1955). The effect of salinity and temperature on the pre-adult growth of guppies. Copeia 1955, 241–243. doi: 10.2307/1440474
Global Invasive Species Database. (2020). Global Invasive Species. Available Online at: www.iucngisd.org/gisd/search.php on 04-09-2020
Godin, J.-G., and Dugatkin, L. A. (1996). Female mating preference for bold males in the guppy, Poecilia reticulata. Proc. Natl. Acad. Sci. 93, 10262–10267. doi: 10.1073/pnas.93.19.10262
Green, P. T., O’Dowd, D. J., Abbott, K. L., Jeffery, M., Retallick, K., and Mac Nally, R. (2011). Invasional meltdown: Invader–invader mutualism facilitates a secondary invasion. Ecology 92, 1758–1768. doi: 10.1890/11-0050.1
Griffiths, S. W., and Magurran, A. E. (1997). Familiarity in schooling fish: how long does it take to acquire? Anim. Behav. 53, 945–949. doi: 10.1006/anbe.1996.0315
Hain, T. J., and Neff, B. D. (2007). Multiple paternity and kin recognition mechanisms in a guppy population. Mol. Ecol. 16, 3938–3946. doi: 10.1111/j.1365-294x.2007.03443.x
Harris, S., Ramnarine, I. W., Smith, H. G., and Pettersson, L. B. (2010). Picking personalities apart: estimating the influence of predation, sex and body size on boldness in the guppy poecilia reticulata. Oikos 119, 1711–1718. doi: 10.1111/j.1600-0706.2010.18028.x
Huntingford, F., Mesquita, F., and Kadri, S. (2013). Personality variation in cultured fish: implications for production and welfare. Animal personalities: behavior, physiology, and evolution. Chicago: University of Chicago Press.
Ingley, S. J., Rehm, J., and Johnson, J. B. (2014). Size doesn’t matter, sex does: a test for boldness in sister species of brachyrhaphis fishes. Ecol. Evol. 4, 4361–4369. doi: 10.1002/ece3.1304
Johnson, J. B., and Omland, K. S. (2004). Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108. doi: 10.1016/j.tree.2003.10.013
Koeck, M. (2019). Neotoca bilineata. IUCN Red List Threatened Species 2019:e.T191713A2000012. doi: 10.2305/IUCN.UK.2019-2.RLTS.T191713A2000012.en
Krause, J., Ruxton, G. D., Ruxton, G. D., and Ruxton, I. G. (2002). Living in groups. Oxford: Oxford University Press.
Liebhold, A., and Bascompte, J. (2003). The allee effect, stochastic dynamics and the eradication of alien species. Ecol. Lett. 6, 133–140. doi: 10.1046/j.1461-0248.2003.00405.x
Lukas, J., Kalinkat, G., Kempkes, M., Rose, U., and Bierbach, D. (2017). Feral guppies in germany–a critical evaluation of a citizen science approach as biomonitoring tool. Bull. Fish Biol. 17, 13–27.
Lukas, J., Kalinkat, G., Miesen, F. W., Landgraf, T., Krause, J., and Bierbach, D. (2021). Consistent behavioral syndrome across seasons in an invasive freshwater fish. Front. Ecol. Evol. 8:583670. doi: 10.3389/fevo.2020.583670
Magellan, K., and Magurran, A. E. (2009). The effect of social environment during ontogeny on life history expression in the guppy Poecilia reticulata. J. Fish Biol. 74, 2329–2337. doi: 10.1111/j.1095-8649.2009.02245.x
Magnhagen, C., and Bunnefeld, N. (2009). Express your personality or go along with the group: what determines the behaviour of shoaling perch? Proc. R. Soc. B 276, 3369–3375. doi: 10.1098/rspb.2009.0851
Magurran, A. (1999). “The causes and consequences of geographic variation in antipredator behavior: perspectives from fish populations,” in Geographic variation in behavior: Perspectives on evolutionary mechanisms, eds S. A. Foster and J. A. Endler (Oxford: Oxford University Press), 139–163.
Magurran, A. E. (2005). Evolutionary ecology: the Trinidadian guppy. Oxford: Oxford University Press on Demand.
Meffe, G. K., and Snelson, F. (1989). “An ecological overview of poeciliid fishes,” in Ecology and evolution of livebearing fishes (Poeciliidae), eds G. K. Meffe and F. F. Snelson (Englewood Cliffs, NJ: Prentice Hall), 13–31.
Miller, R. R., Minckley, W. L., Norris, S. M., and Gach, M. H. (2009). Peces dulceacuícolas de México, Primera edición Edn. Tlalpan, México, D.F: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Ornelas-Garcia, C. P., Alda, F., Diaz-Pardo, E., Gutierrez-Hernandez, A., and Doadrio, I. (2012). Genetic diversity shaped by historical and recent factors in the live-bearing twoline skiffia neotoca bilineata. J. Fish Biol. 81, 1963–1984. doi: 10.1111/j.1095-8649.2012.03456.x
Pérez Alvarado, L. C., Álvarez, M. R., and Dix, M. (2004). La ictiofauna del Refugio de Vida Silvestre Bocas del Polochic y la cuenca del lago de Izabal: composición, distribución y ecología. Organización de las Naciones Unidas para la Educación, la Ciencia y la Cultura. Guatemala: Universidad del Valle de Guatemala.
Piyapong, C., Krause, J., Chapman, B. B., Ramnarine, I. W., Louca, V., and Croft, D. P. (2010). Sex matters: a social context to boldness in guppies (Poecilia reticulata). Behav. Ecol. 21, 3–8. doi: 10.1093/beheco/arp142
R Core Team. (2020). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available Online at: https://www.R-project.org/
Ramírez-García, A., Ramírez-Herrejón, J. P., Medina-Nava, M., Hernández-Morales, R., and Domínguez-Domínguez, O. (2018). Reproductive biology of the invasive species Pseudoxiphophorus bimaculatus and Poecilia sphenops in the teuchitlán river, méxico. J. Appl. Ichthyol. 34, 81–90. doi: 10.1111/jai.13543
Rauschert, E. S. J., and Shea, K. (2017). Competition between similar invasive species: modeling invasional interference across a landscape. Popul. Ecol. 59, 79–88. doi: 10.1007/s10144-016-0569-7
Reale, D., Reader, S. M., Sol, D., McDougall, P. T., and Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. doi: 10.1111/j.1469-185X.2007.00010.x
Reeve, A. J., Ojanguren, A. F., Deacon, A. E., Shimadzu, H., Ramnarine, I. W., and Magurran, A. E. (2014). Interplay of temperature and light influences wild guppy (Poecilia reticulata) daily reproductive activity. Biol. J. Linnean Soc. 111, 511–520. doi: 10.1111/bij.12217
Rehage, J. S., and Sih, A. (2004). dispersal behavior, boldness, and the link to invasiveness: a comparison of four gambusia species. Biol. Invasions 6, 379–391. doi: 10.1023/B:BINV.0000034618.93140.a5
Rennó Braga, R., Gómez-Aparicio, L., Heger, T., Vitule, J. R. S., and Jeschke, J. (2018). Structuring evidence for invasional meltdown: broad support but with biases and gaps. Biol. Invasions 20, 923–936. doi: 10.1007/s10530-017-1582-2
Rodriguez, C. M. (1997). Phylogenetic Analysis of the Tribe Poeciliini (Cyprinodontiformes: Poeciliidae). Copeia 4, 663–679. doi: 10.2307/1447285
Salgado-Maldonado, G., Novelo-Turcotte, M. T., Vazquez, G., Caspeta-Mandujano, J. M., Quiroz-Martínez, B., and Favila, M. (2014). The communities of helminth parasites of heterandria bimaculata (teleostei: poeciliidae) from the upper Río La Antigua basin, east-central Mexico show a predictable structure. Parasitology 141, 970–980. doi: 10.1017/S0031182014000122
Seghers, B., Shaw, P., and Carvalho, G. (1995). The behavioral diversity and evolution of guppy, poecilia reticulata, populations in trinidad. Adv. Study Behav. 24, 155–202. doi: 10.1016/s0065-3454(08)60394-0
Sievers, C., Willing, E.-M., Hoffmann, M., Dreyer, C., Ramnarine, I., and Magurran, A. (2012). Reasons for the invasive success of a guppy (Poecilia reticulata) population in Trinidad. PloS One 7:e38404. doi: 10.1371/journal.pone.0038404
Sih, A. (1992). Prey uncertainty and the balancing of antipredator and feeding needs. Am. Nat. 139, 1052–1069. doi: 10.1086/285372
Simberloff, D. (2006). Invasional meltdown 6 years later: important phenomenon, unfortunate metaphor, or both? Ecol. Lett. 9, 912–919. doi: 10.1111/j.1461-0248.2006.00939.x
Simberloff, D., Martin, J. L., Genovesi, P., Maris, V., Wardle, D. A., Aronson, J., et al. (2013). Impacts of biological invasions: what’s what and the way forward. Trends Ecol. Evol. 28, 58–66. doi: 10.1016/j.tree.2012.07.013
Simberloff, D., and Von Holle, B. (1999). Positive interactions of nonindigenous species: invasional meltdown? Biol. Invasions 1, 21–32. doi: 10.1023/A:1010086329619
Snijders, L., Krause, S., Tump, A. N., Breuker, M., Ortiz, C., Rizzi, S., et al. (2020). Causal evidence for the adaptive benefits of social foraging in the wild. Commun. Biol. 4:94.
Sol, D., and Maspons, J. (2016). “Life history, behaviour and invasion success,” in Biological invasions and animal behaviour, ed. J. S. Weis (Cambridge: Cambridge University Press), 63–81. doi: 10.1017/cbo9781139939492.006
Sol, D., Maspons, J., Gonzalez-Voyer, A., Morales-Castilla, I., Garamszegi, L. Z., and Møller, A. P. (2018). Risk-taking behavior, urbanization and the pace of life in birds. Behav. Ecol. Sociobiol. 72:59.
Tobin, P. C., Berec, L., and Liebhold, A. M. (2011). Exploiting allee effects for managing biological invasions. Ecol. Lett. 14, 615–624. doi: 10.1111/j.1461-0248.2011.01614.x
Valero, A., Macías Garcia, C., and Magurran, A. E. (2008). Heterospecific harassment of native endangered fishes by invasive guppies in mexico. Biol. Lett. 4, 149–152. doi: 10.1098/rsbl.2007.0604
Ward, A. J. W. (2012). Social facilitation of exploration in mosquitofish (Gambusia holbrooki). Behav. Ecol. Sociobiol. 66, 223–230. doi: 10.1007/s00265-011-1270-7
Webster, M. M., Ward, A. J. W., and Hart, P. J. B. (2007). Boldness is influenced by social context in threespine sticklebacks (Gasterosteus aculeatus). Behaviour 144, 351–371. doi: 10.1163/156853907780425721
Keywords: social context, risk-taking behavior, heterospecific interactions, poeciliids, goodeids
Citation: Santiago-Arellano A, Palomera-Hernandez V and Camacho-Cervantes M (2021) Con- and Heterospecific Shoaling Makes Invasive Guppies More Risk Taking. Front. Ecol. Evol. 9:624245. doi: 10.3389/fevo.2021.624245
Received: 30 October 2020; Accepted: 24 February 2021;
Published: 16 March 2021.
Edited by:
Bart Pollux, Wageningen University and Research, NetherlandsReviewed by:
David Bierbach, Leibniz-Institute of Freshwater Ecology and Inland Fisheries (IGB), GermanyCopyright © 2021 Santiago-Arellano, Palomera-Hernandez and Camacho-Cervantes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Morelia Camacho-Cervantes, bWNjQGNtYXJsLnVuYW0ubXg=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.