
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 16 March 2021
Sec. Biogeography and Macroecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.619215
This article is part of the Research Topic Habitat Modification and Landscape Fragmentation in Agricultural Ecosystems: Implications for Biodiversity and Landscape Multi-Functionality View all 14 articles
Natural and seminatural habitat remnants play a crucial ecological role in intensified agroecosystems. Assumptions on the conservation value of small and poorly connected fragments in a hostile matrix come from generalization obtained from a limited number of taxa, mostly plants, and vertebrates. To date, few studies have analyzed the effect of fragmentation on ant communities in Mediterranean agroecosystems, despite the importance of this group of animals on several key ecosystem functions and services. Here, we analyze the effects of fragment area and connectivity on ant communities in gypsum outcrops in a large cereal agroecosystem of Central Spain. Ant communities were described by their species composition, abundance (total number of occurrences), and number of species, standardized both by area (species density), and abundance (species richness). Observed number of species was relatively high in comparison with other studies in the Mediterranean, and we found no effects of fragment characteristics on species density, species richness and species composition, which implies that even small and isolated patches do have a value for ant conservation. Moreover, total number of occurrences were higher for smaller and more isolated fragments. This finding contrasts with the results reported for other taxa in similar gypsum habitats and suggests that certain ant traits and strategies make them particularly resistant to fragmentation and capable to take advantage of small habitat patches. Given the important ecological role played by ants, we recommend the preservation of these small habitat fragments in the management plans of agroecosystems in these drylands, especially in those cases in which intensification of agricultural practices greatly diminish natural habitat availability.
There is consensus on the necessity of maintaining a certain amount of remnants of natural or semi-natural habitats in extensive agroecosystems, which act as reservoirs of biodiversity and provide key ecosystem services (Altieri, 1999; Tscharntke et al., 2008; Kennedy et al., 2013). It is not clear, however, to what extent small, isolated fragments can fulfill these functions. According to the most classical view of fragmentation, there is a threshold in which remnants are too small and isolated from each other, inducing population declines, increasing extinction risk, and exacerbating species loss (Andrén, 1994; Ewers and Didham, 2006). Fragmentation is therefore considered by many authors one of the most pervasive drivers of biodiversity loss (Fletcher et al., 2018). Some of the mechanisms proposed to explain these negative processes are related to the higher vulnerability to environmental and demographic stochasticity for small and poorly connected populations, or the reduction in colonization and rescue events, under a metapopulation perspective, which can become too infrequent to overcome local extinctions (Andrén, 1994; Ewers and Didham, 2006). These negative effects would emerge as a function of the specific characteristics of the landscapes, the habitat configuration and the biological groups involved (Villard and Metzger, 2014). Thus, for example, megafauna requires large patches to maintain viable populations, while in the case of invertebrates very small patches could be enough to host viable populations (Ewers and Didham, 2006).
Recently, however, it has been questioned whether fragmentation necessarily means a real loss of species. Fahrig (2003, 2017) considers that we should only attribute to the so-called fragmentation per se, those effects on populations and communities independent of the effects of habitat amount and, definitively, resource availability. The controversy is significant for conservation, since the lower conservation value assigned to small patches have derived in the protection of few large rather than many small fragments (“single large vs. several small,” or SLOSS debate; Fahrig et al., 2019). Much of the controversy has to do with the difficulties of distinguishing between the effects expected by simple species—area relationships or sampling effects, and those produced by mechanisms of another nature (Gotelli and Colwell, 2001; Whittaker et al., 2001). To face these confounding effects, Whittaker et al. (2001) propose to fix by design the sampling area, and recommend the use of species density to make comparisons, while (Gotelli and Colwell, 2001, 2011) propose the use of rarefaction curves to standardize datasets to a common number of samples, individuals or occurrences to assess species richness. Both species density and species richness are worth to be explicitly considered in this context, since while species density is of interest for conservation purposes, species richness is appropriated for testing models and theoretical predictions, and along with species abundance, is critical for determining species density (Gotelli and Colwell, 2001).
Agriculture intensification and maintenance of large and homogeneous agricultural fields has induced a critical and massive destruction of habitat and biodiversity loss (Emmerson et al., 2016). In this context, the value in conservation terms, but also as providers of ecosystem services of habitat remnants, is critical (Haddad et al., 2015). Although it exists a wide consensus that agroecosystems should maintain and extend the number and extension of natural and seminatural habitats fragments for maximizing the services provided, there is no information of the role these fragments have for many biological groups.
Despite their enormous biomass and importance in the functioning of terrestrial ecosystems (Folgarait, 1998; Del Toro et al., 2012), ants as a group have been rarely studied in relation to fragmentation. This is significant since ground dwelling ants, as invertebrates living in sessile colonies, probably respond to fine-scale effects better than larger or more mobile organisms. To our knowledge, however, there are no published studies specifically aimed to evaluate the effect of fragmentation on ants in Mediterranean agroecosystems, despite the ecological importance of ants in Mediterranean regions (Baraibar et al., 2009; Silvestre et al., 2019) where they provide important ecosystem services (Comas et al., 2016; Baraibar et al., 2017). Most of the existing studies on fragmentation and ants have been conducted in tropical regions (Armbrecht et al., 2001; Assis et al., 2018; Santos et al., 2018), forests (Melliger et al., 2018), or grasslands (Golden and Crist, 2000; Brascheler and Baur, 2003; Dauber et al., 2006). Few generalizations can be obtained from them, since apart from their variability in the target ecosystem, they also differ greatly in the spatial scale, ranging from <1 m (Brascheler and Baur, 2003) to several thousand square meters (Melliger et al., 2018).
Here, we aim to evaluate the effects of patch size and connectivity on ant biodiversity in an extensive cereal agroecosystem in the drylands of Central Spain. We assume that larger patches have a higher total number of species because of a simple random sampling effect, therefore this variable will not be measured in this study. Instead, we fix sampling area in each patch, and focus on species density, species richness and total abundance. If small or isolated patches add negative effects for ant populations other than simple random sampling effects, then we expect to find lower values of species density, richness and abundance. Conversely, if low and isolated patches are not affected by negative affects additional to random sampling effects, ant species richness, abundance and density should be similar to larger and better connected patches.
Our approach will provide additional conservation criteria for these valuable ecosystems, contributing to preserve their diversity and therefore their functionality and ecosystem services.
The study area is in central Spain, near the locality of Belinchón (Cuenca, Spain), at an altitude of ~700 a.s.l (Figure 1). The climate is continental Mediterranean, with an average annual temperature of ca 14°C, rainfall of ca 500 mm per year, severe summer drought and cold winters. The lithology of the area is mostly composed of tertiary evaporites, and the relief is gentle, with alternating hills and plains. Soils are gypseous (i.e. soils which main component is gypsum; Herrero and Porta, 2000), which imposes harsh conditions for the development of vegetation (Escudero et al., 2015). In spite of this, and especially from the mid-20th century (Matesanz et al., 2009), the expansion of rain-fed agriculture has progressively fragmented the landscape, which now consists of a mosaic of natural vegetation remnants interspersed in a matrix of dry herbaceous croplands, mostly cereal (Figure 1). Natural vegetation in these remnants is composed by dwarf shrublands dominated by creeping and cushion-like specialized gypsophytes (e.g., Helianthemum squamatum (L.) Dum.-Cours., Lepidium subulatum L., Centaurea hyssopifolia Vahl., Gypsophila struthium L) and the tussock-forming grass Stipa tenacissima L. Perennials cover is around 30% and alternates with clearings partially covered by a biological soil crust, dominated by crustose lichens and fairly diverse annual plant communities (Luzuriaga et al., 2018).
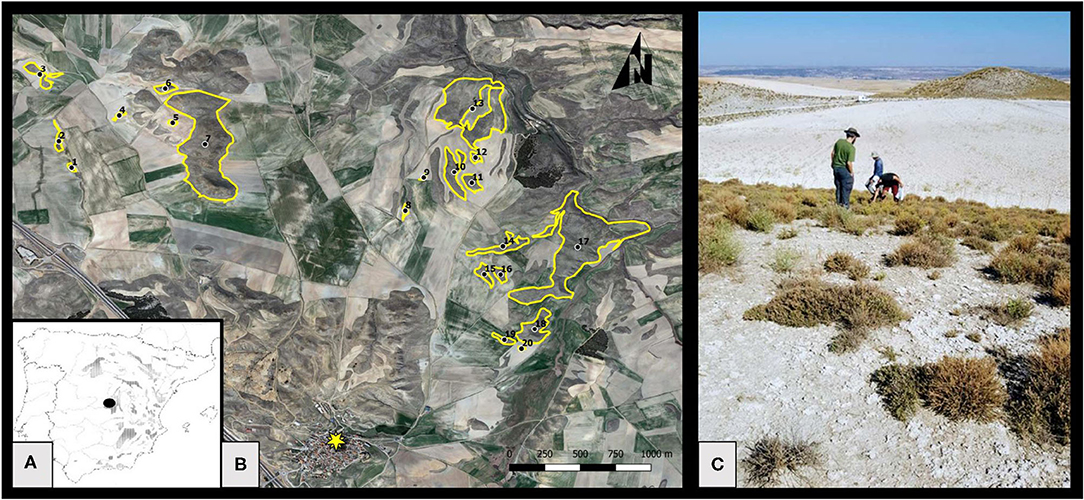
Figure 1. (A) Map showing the distribution of gypsum outcrops of the Iberian Peninsula, re-drawn from Escavy et al. (2012), and the study area location (black dot). (B) Studied habitat remnants. The black dots indicate the centroid of each remnant, and the yellow star indicates the village of Belinchón. Note that the area includes other habitat remnants that were not sampled. However, all remnants were considered for the calculation of the connectivity index of the sampled ones. (C) General view of the study area showing a habitat remnant on the forefront with typical gypsum shrubby vegetation, another habitat remnant in the background, and a fallow field in the middle.
Twenty natural habitat remnants were selected, separated from each other by a continuous cropland matrix (Figure 1, Table 1) and including a representative sample of existent range of size and connectivity in the study area. Fragment characteristics (area, connectivity) were measured on orthophotos from 2018, using the software QGIS 3.2.1. Bonn (QGIS.org, 2018). Fragment area ranged from 1,065 to 290,828 m2, and was log-transformed for all the analysis, given the strong skewness of its distribution. Log (area) ranged from 3.03 to 5.46 (mean = 4.07, s.d. = 0.72).
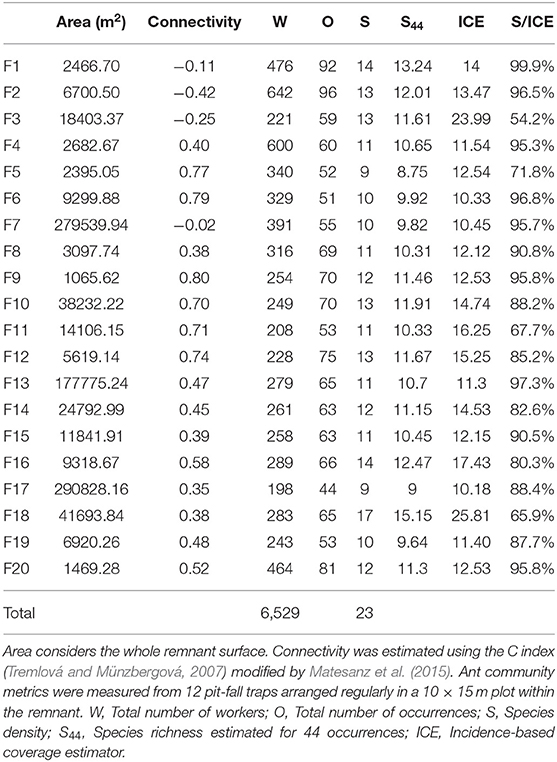
Table 1. Fragment characteristics and ant community metrics obtained for the 20 sampling units considered in this study.
Connectivity was estimated using the index C proposed by Tremlová and Münzbergová (2007), which accounts for the number of surrounding fragments, considering their area weighted by their distance to the target fragment. Because of the highly dispersed values obtained, we log10 transformed the index, following a modification by Matesanz et al. (2015). The modified index can adopt negative values for lowly connected remnants, and is calculated as follows:
where Cj is fragment j connectivity, k the fragments surrounding fragment j (in our case, within a radius of 1 km from the center of fragment j), Pk is fragment k area (in square meters), and djk is the distance between the centroids of fragments j and k (in meters). Connectivity index ranged from −0.42 to 0.80 (mean = 0.41, s.d. = 0.35).
Ant sampling was carried out in July 2018. In each habitat remnant a 10 × 15 m plot was located in a homogeneous vegetation area at a distance of 10 m from the cultivated area, so fixing the possible edge effect (Golden and Crist, 2000; Brascheler and Baur, 2003). On each plot, we placed 12 pitfall traps of 2.5 cm in diameter and 5 cm deep, forming a grid of 3 × 4 rows, with a separation of 5 m between traps. The traps contained a solution composed of 70% ethanol and 30% ethylene glycol (Azcárate and Peco, 2012; Silvestre et al., 2019). The traps were kept in the field for 7 days, and after their collection, all ant workers were sorted in the laboratory, and identified to the species level.
We characterized each plot by its species density, species richness and overall abundance, following Gotelli and Colwell (2011). Species density per plot was assimilated to the total number of species captured in the set of 12 pitfall traps. Total number of occurrences was used as an indicator of overall abundance (Longino et al., 2002). Each species present in one trap was counted as one occurrence, regardless the number of workers captured in the trap, and total number of occurrences was calculated as the sum of the occurrences of all the species present in one plot. We preferred the total number of occurrences instead of the total number of individuals, because in ants the number of workers captured by pitfall traps is extremely influenced by slight differences in the distance to foraging trails or nests, which results in a strong spatial clumping of individuals within traps. Anyhow, some analyses were replicated using total number of individuals, obtaining similar results to those reported in this study (Supplementary Table 1). Species richness was estimated as the expected number of species for a given number of randomly sampled occurrences. To assess it, we built occurrence-based rarefaction curves (Gotelli and Colwell, 2011), averaging 500 resamples with replacement, and then estimated for each plot the mean species richness expected for the lowest number of occurrences observed in any of the 20 plots. As a complement to these variables, and to check the capacity of our sampling to cover a representative proportion of the assemblage richness, we calculated the incidence-based coverage estimator (ICE), an asymptotic estimator of species richness based on incidence data (Gotelli and Colwell, 2011). Rarefaction analysis and ICE estimation were done using the software EstimateS v. 9.1.0 (Colwell, 2013).
We built generalized linear models for species density, total number of occurrences and species richness, as function of log (area) and connectivity, including their interaction. We used Poisson errors and log link functions for species density and total number of occurrences, and Gaussian errors and identity link function for species richness. This latter variable is non-integer, due to the fact that it is estimated from the rarefaction curves. We identified the best descriptive models using the Akaike information criterion corrected for small samples (AICc). Models were estimated with package MuMIn (Bartoń, 2009) of R 4.0.0.
We performed a PERMANOVA analysis in order to check whether there were differences in species composition related to area, connectivity and/or their interaction. We used frequency data to estimate species composition in each habitat fragment and calculated dissimilarity matrices based on Bray Curtis distance. Significance of the model was tested using a Monte-Carlo test with 9,999 permutations. Finally, we performed non-metric multidimensional scaling (NMDS) to visualize the differences in the species compositions. PERMANOVA and NMDS analyses were performed with the vegan package in R 4.0.0 (Oksanen et al., 2015).
We collected 6,529 ant workers belonging to 23 species, all of them native (Table 2). The recorded number of species per plot (species density) was, on average, 86% of the species richness expected by the ICE estimator (Table 1). The ratio between the number of detected and expected species showed no correlation with connectivity (Pearson r = 0.01) or with the area of the remnant (Pearson r = 0.17), so we can assume no sampling bias associated with the fragmentation condition of the remnants.
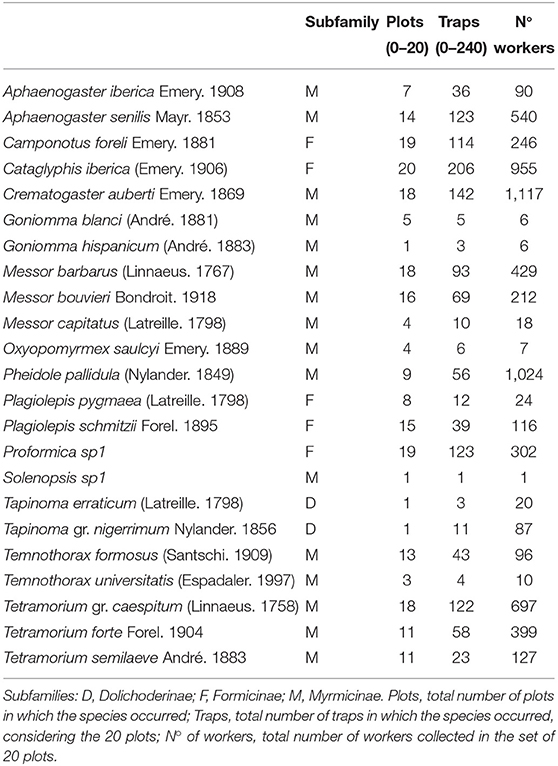
Table 2. Ant species identified in this study, with information on their subfamily, and general data on the frequency & abundance observed in this study.
Species density ranged from 9 to 17 species per plot (average 11.80, s.d. = 1.94), and did not respond to any of the predictors (Table 3). None of the combinations of the predictors produced a plausible model, according to the AICc (Table 4).
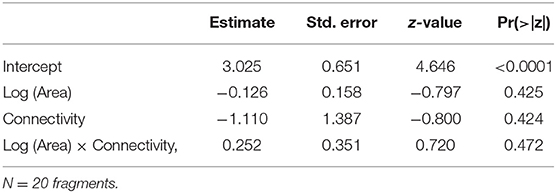
Table 3. Parameter estimates and P-values for the GLM (Poisson distribution, log link function) built for species density as a function of Log (Area), Connectivity and the interaction Log (Area) x Connectivity.
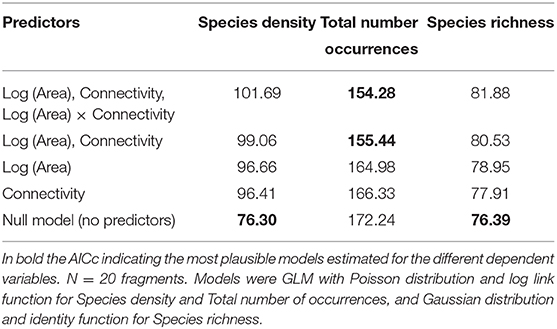
Table 4. Akaike Information Criterion corrected for small sample sizes (AICc) assessed for the models estimated for species density, total number of occurrences and species richness (for 44 occurrences) as function of the different combinations of the predictors Log (Area) Connectivity and Log (Area) x Connectivity.
The total number of occurrences per plot ranged from 44 to 96 (mean = 65.10, s.d. = 13.33), and responded significantly to log (Area) and connectivity both in in the complete model (Table 5) and in the model without interaction. AICc was very similar for the two models (Table 4). The percentage of explained residual deviance (D2) was 52.7% for the complete model and 44.1% for the model without interaction. Opposite to our expectations, the effects of both connectivity and Log (Area) were negative (Figure 2), suggesting that neither the reduction of fragment size nor the increase in isolation imply a loss of habitat quality for ants. Moreover, the significant interaction suggested that the increase in total number of occurrences observed for small remnants was greater if they were more isolated.
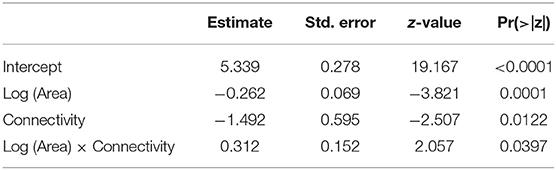
Table 5. Parameter estimates and P-values for the GLM (Poisson distribution, log link function) built for total number of occurrences as a function of Log (Area), Connectivity and the interaction Log (Area) x Connectivity.
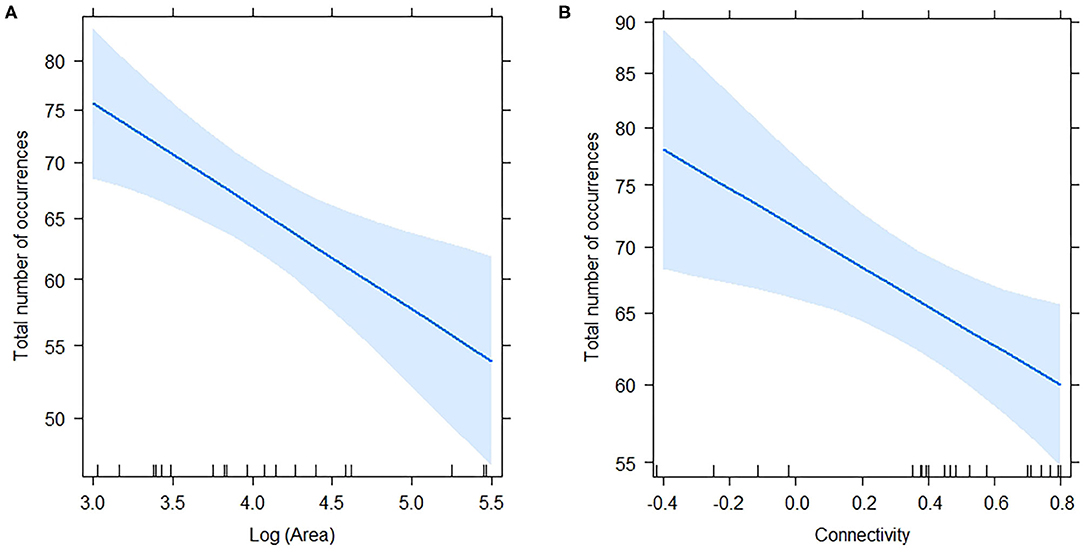
Figure 2. Effects of Log (Area) (A) and Connectivity (B) on Total number of occurrences estimated by the complete model (including the interaction; see Table 5). Shaded regions represent 95% confidence intervals. The model was a GLM with Poisson distribution and log link function.
Species richness was estimated for a sample size rarefied down to 44 occurrences (the lowest observed value for any of the plots), using individual-based rarefaction curves (Figure 3). Estimated values ranged between 8.75 and 15.15 species (mean = 11.08, s.d. = 1.49). Similarly to species density, rarefied species richness did not respond to any of the considered predictors (Table 6), and the null model was the one that showed the smallest AICc (Table 4)
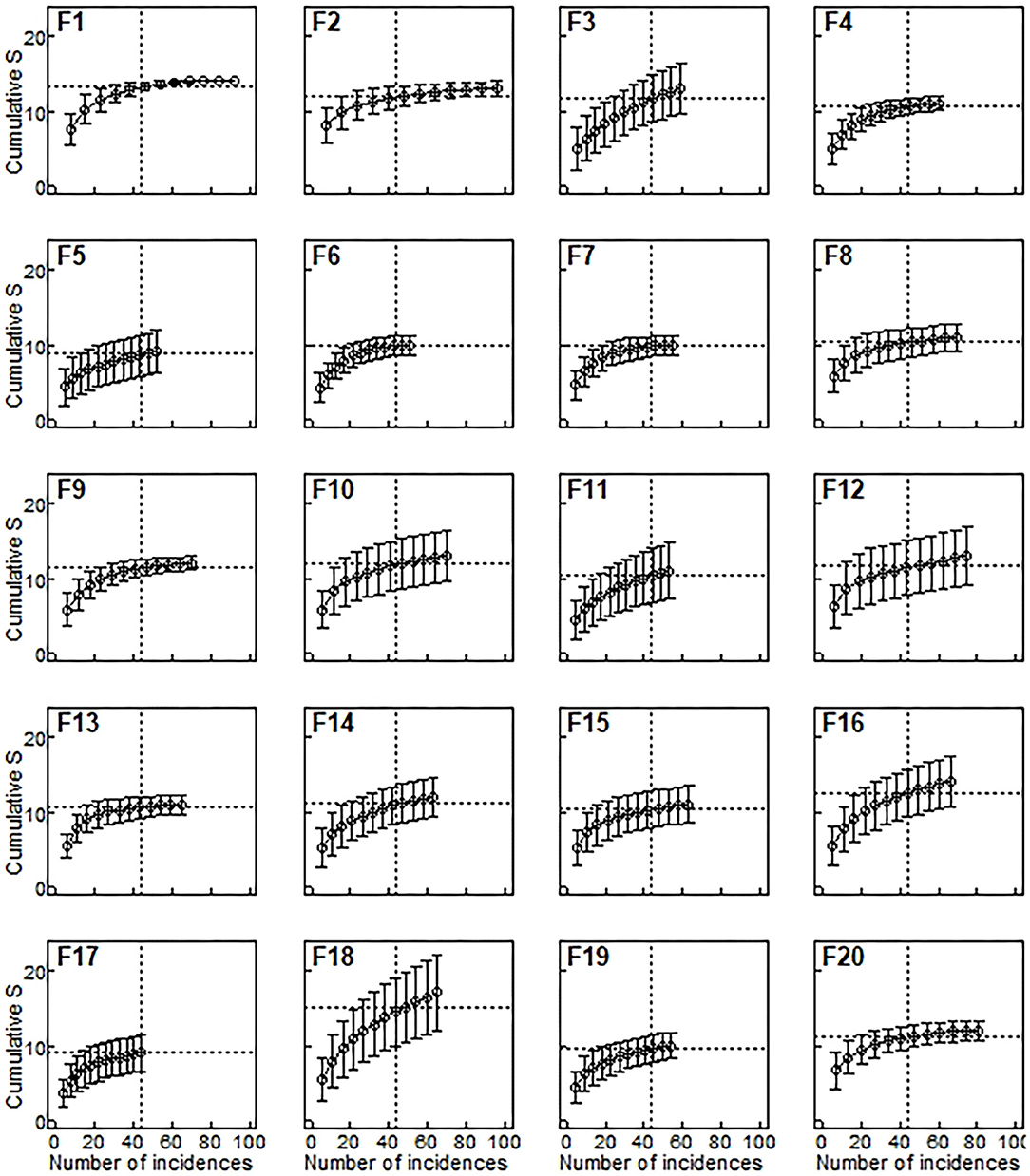
Figure 3. Species richness estimated from rarefaction curves for each remnant. Species richness (horizontal lines) was estimated as the expected number of species for 44 occurrences (vertical lines), which was the minimum number of occurrences observed in any of the 20 plots (F17). Occurrence-based rarefaction curves (Gotelli and Colwell, 2011) were estimated by averaging 500 resamples with replacement. Bars are ±95%confidence intervals.
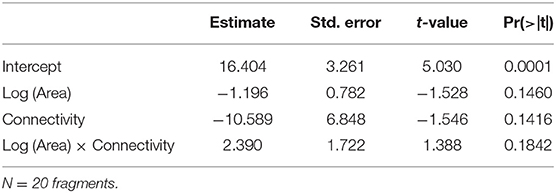
Table 6. Parameter estimates and P-values for the GLM (Gaussian distribution, identity link function) built for species richness rarefied to 44 occurrences as a function of Log (Area), Connectivity and the interaction Log (Area) x Connectivity.
The NMDS/PERMANOVA approaches also did not reveal any trend in relation to patch size or connectivity (Table 7, Supplementary Figure 1).
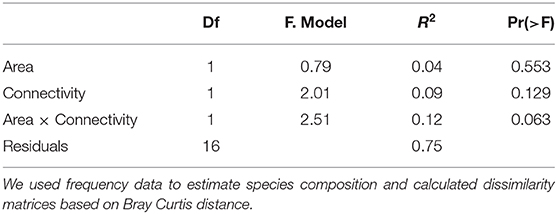
Table 7. Parameter estimates and P-values for the Permanova analysis built to test the effect of area and connectivity on ant species community composition in 20 habitat remnants.
According to the expectations of the classical theory of ecological fragmentation, too small and isolated habitat remnants should undergo an accelerated species loss (Andrén, 1994; Ewers and Didham, 2006). In our case study, however, neither ant species density, nor richness or community composition, responded to fragment size or connectivity. A possible first explanation could be related to the fact that processes causing species loss in habitat remnants (Levins, 1969; Andrén, 1994; Ewers and Didham, 2006) are not necessarily linear, and, even more, they could appear below certain thresholds of size or isolation or combination (Villard and Metzger, 2014). Therefore, the hypothetical negative effects of fragmentation on ant communities would only be detectable in remnants smaller and more isolated than those here considered. Furthermore, the total number of occurrences not only did not decrease but tended to increase in small and less connected patches, making clear that these fragments do not undergo any loss of quality as ant habitats.
These results contrast with those obtained for other organisms in the same region and type of agroecosystem. For example, Matesanz et al. (2009) and Matesanz et al. (2015) observed clear population-level effects for plants, and at the community level, other studies have reported reductions in species density for plants (Luzuriaga et al., 2018) and lichens (Concostrina-Zubiri et al., 2018). Fragmentation in gypsum agroecosystems also seems to negatively affect species richness of pollinator communities, as well as the frequency, diversity and topology of pollination interactions and network metrics (Santamaría et al., 2018). Fragmentation in this type of agroecosystem in drylands also influenced the risk of seed predation, although its effect was not straightforward and it appeared as highly dependent on each plant-animal interaction (Rabasa et al., 2005, 2009; Moncalvillo et al., 2021). Although we do not have studies specifically aimed at analyzing the effects of fragmentation on ants in Mediterranean agroecosystems, it has been observed that the presence of certain keystone structures is sufficient to provide a high number of ant species (Hevia et al., 2013; e.g., grassland corridors in agricultural landscapes: Azcárate et al., 2013). In other environments, such as forest remains in urban matrices (Melliger et al., 2018) or grassland remnants in forest matrices (Dauber et al., 2006), the capacity of ants to maintain relatively rich communities in small fragments has also been highlighted. Ants, therefore, would show less vulnerability to fragmentation than other groups.
This apparently surprising ability to resist in small, relatively isolated patches may be related to some ant features. Possibly, the eusocial organization provides mechanisms to manage the greater environmental stochasticity of small patches. For example, most ant colonies are long-lived, and can maintain food reserves that buffer fluctuations in resource availability (Davidson, 1977), as well as regulate the size of their colonies, or modify the task allocation of workers depending on conditions (Gordon and Mehdiabadi, 1999). Ants are in general central-place foragers (Harkness and Maroudas, 1985), so their resource-catching strategy is based on the use of a particular territory, not necessarily a large one (Azcárate and Peco, 2003). It is likely, therefore, that the patch size threshold below which negative population effects appear will be lower for ants than for other animal groups such as bees, whose foraging depends on spatial scales that can be very large (Greenleaf et al., 2007). Moreover, most ant species produce winged sexuals, which gives them a dispersal ability that may be enough to minimize isolation between fragments (Dauber et al., 2006). Dispersal ability is often complemented by other effective strategies to deal with habitat patchiness, like poligyny or multiple queening (e.g., Plagiolepis, Proformica, Solenopsis, Tapinoma) or flexible modalities of nuptial flights (Bourke and Heinze, 1994). In addition, foraging strategies may be completed in the apparently hostile matrix (especially in the case of granivores) were weeds and crops can provide some valuable resources (Baraibar et al., 2009, 2017). Matrices in fragmented landscapes are not necessarily hostile for all species, and in fact, management of the matrix has often been pointed out as a key factor for the conservation of the biodiversity within fragments (e.g., Armbrecht et al., 2001). Lastly, resistance to habitat loss can be higher for generalist species (Armbrecht et al., 2001; Marvier et al., 2004), and we should not discard that the regional species pool is impoverished since long ago, so that today it is dominated by generalist ants, more tolerant to fragmentation. We think, however, that this explanation is unlikely, given the elevated species densities found in this study (higher than those observed in nearby locations, Flores et al., 2018), and the fact that some of the species recorded in our samplings are not so common in the Iberian context (Temnothorax formosus, T. universitatis, and Goniomma blanci).
Particularly noteworthy is the unexpected negative effect exerted by fragment area and connectivity on the total number of occurrences per plot, a result that was also observed when repeating the analysis with the total number of workers per plot (Supplementary Table 1). Interestingly, this increase in abundance for small and poorly connected patches was not linked to any change in species composition, suggesting a general effect favoring ants as a group. One possible explanation could be that these remnants have a higher edge effect, which may have more positive than negative consequences on ants (e.g., possibility of foraging in matrix habitats, higher productivity of edges especially in agroecosystems where farmlands are fertilized, etc.; Brascheler and Baur, 2003; Fahrig, 2017; Fahrig et al., 2019). However, this explanation should be taken with caution since plots were located at the same distance from the field edge (10 m) regardless of fragment size. Other plausible explanation would be related to the existence of refuge effects of small and isolated patches against predators or parasites (Fahrig, 2017). In any case, the observed effect would be limited to the range of sizes analyzed here, and we cannot exclude a hypothetical unimodal response if smaller and more isolated remnants were included in the study. Finally, it is also interesting that the increase in ant abundance in small and isolated patches did not translate into an increase in species density, suggesting that the rise in abundance is experienced mostly by common species.
In the context of a generalized loss of habitat and fragmentation exacerbation, protection of small and isolated habitat remnants has traditionally been rarely considered in favor of large patches (SLOSS debate), even though the same amount of habitat spread over many small patches can result in more biodiversity than when it is grouped into one or very few large areas (Fahrig, 2017; Fahrig et al., 2019). Recently, however, several studies have highlighted the important role of small reserves and habitats in harboring biodiversity (Wintle et al., 2019; Volenec and Dobson, 2020), which advises revisiting the SLOSS debate. While there is no doubt that larger patches host higher numbers of species in absolute terms, this effect is at least in part a consequence of random sampling effects and species—area relationships, and does not necessarily imply negative effects attributable to fragmentation per se (Fahrig, 2017; Fahrig et al., 2019), nor a higher conservation value for the area gathered in large patches. There are also other mechanisms by which habitat distribution in many smaller patches can provide positive effects for biodiversity conservation and ecosystem services. These mechanisms include the provision of spatial subsidies by the matrix (Ewers and Didham, 2006), positive edge effects (Ewers and Didham, 2006; Fletcher et al., 2018), landscape or habitat complementarity (Fahrig, 2017) or a general increase of beta diversity in heterogeneous landscapes (Andrén, 1994), which ultimately means that fragmented landscapes often have more diversity than non-fragmented ones when considered as a whole. A higher fragmentation (therefore, a higher habitat patchiness) can also produce positive effects at the population level, by spreading the risk of extinction over a large number of sites, thus reducing the risk of simultaneous extinction of all local populations, stabilizing predator—prey or host—parasitoid systems, or by reducing intraspecific and interspecific competition (Fahrig, 2017). In addition, the existence of a high number of small fragments in the territory can contribute to overall landscape connectivity by acting as key “stepping stones” for species able to move long distances (Herrera et al., 2017).
Finally, it should be noted that the small, poorly connected patches of the dry agroecosystem studied here were not only effective in maintaining ant communities, but also showed high diversity values when compared to geographically close areas. Thus, for example, Flores et al. (2018) analyzed ant communities in grassland ecosystems of central Spain along a wide environmental gradient using the same protocol as the one followed here, and found that species density ranged from 2 to 15 species per plot, well below the 9–17 species per plot found in our area. The cited study estimated the expected species density as a function of altitude, obtaining values from 7 to 8 species per plot for the altitude corresponding to our study area (700 m), also below the mean value of 11.8 found here. Therefore, as with other groups (Escudero et al., 2015), the high conservation value of gypsum ecosystems must also be highlighted in the case of ants. Furthermore, this group plays a central role in the functioning of terrestrial ecosystems, and participates in the delivery of a number of ecological services (Del Toro et al., 2012), so maintaining an appropriate mix of species rich fragments interspersed with croplands is of particular interest. In Mediterranean agroecosystems, the service of weed control stands out, and is mainly performed by ants of the genus Messor (Baraibar et al., 2009), which are very present in the communities studied here. Other important services of direct interest to agriculture are pest control, soil movement, decomposition and nutrient cycling (Del Toro et al., 2012, 2015).
In summary, this study corroborates that the ecological phenomena related to landscape configuration are complex and can give rise to very different outcomes depending on the group and the type of environment. It is therefore necessary to pay attention to the particularities of each case, and to be aware of the possible importance for biodiversity conservation of small ecological fragments or structures, even though at first sight they may not seem important, and especially when we are talking about organisms that are valuable for the provision of ecosystem services. This seems to be the case of ants in Mediterranean gypsum agroecosystems, where we have observed that small and weakly connected patches do not present losses of diversity with respect to larger patches and higher connectivity. As a take home message, we also want to recommend the maintenance of the smallest remnants of natural habitats because they can shelter important levels of biodiversity completing the list of services of any agroecosystem.
The original contributions generated for the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
FA, AE, and AS planned and designed the research. AA-M, FA, and AS conducted field work. AA-M and FA conducted laboratory work. FA and AS conducted data statistical analyses. FA wrote the manuscript with extensive input from the rest of the authors. All authors contributed to the article and approved the submitted version.
This study was supported by the Rey Juan Carlos University (GYPVIFUN URJC, M2148), by the European Social Fund managed by the Regional Government of Madrid (Remedinal TE-CM: S2018/EMT-4338), and by the Horizon 2020 initiative from the European Comission (Gypworld H2020-MSCA-RISE-2007-777803).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Roberto López Rubio, Rufino Alameda Sánchez, and Eugenio López Martínez for their help in fieldwork.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.619215/full#supplementary-material
Altieri, M. A. (1999). The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 74, 19–31. doi: 10.1016/S0167-8809(99)00028-6
Andrén, H. (1994). Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71, 355–366. doi: 10.2307/3545823
Armbrecht, I., Tischer, I., and Chacón, P. (2001). Nested subsets and partition patterns in ant assemblages (Hymenoptera, Formicidae) of Colombian dry forest fragments. Pan-Pac. Entomol. 77, 196–209.
Assis, I. A., Dos Santos, F. N., Ramos, K. E., Barrios-Rojas, J. D., Majer, J. D., and Vilela, E. F. (2018). Agricultural matrices affect ground ant assemblage composition inside forest fragments. PLoS ONE 13:e0197697. doi: 10.1371/journal.pone.0197697
Azcárate, F. M., and Peco, B. (2003). Spatial patterns of seed predation by harvester ants (Messor Forel) in Mediterranean grassland and scrubland. Insect. Soc. 50, 120–126 doi: 10.1007/s00040-003-0635-y
Azcárate, F. M., and Peco, B. (2012). Abandonment of grazing in a Mediterranean grassland area: consequences for ant assemblages. Insect Conserv. Divers. 5, 279–288. doi: 10.1111/j.1752-4598.2011.00165.x
Azcárate, F. M., Seoane, J., Castro, S., and Peco, B. (2013). Drove roads: keystone structures that promote ant diversity in Mediterranean forest landscapes. Acta Oecol. 49, 107–115. doi: 10.1016/j.actao.2013.03.011
Baraibar, B., Canadell, C., Torra, J., Royo-Esnal, A., and Recasens, J. (2017). Weed seed fate during summer fallow: the importance of seed predation and seed burial. Weed Sci. 65, 515–524. doi: 10.1614/WS-D-16-00031.1
Baraibar, B., Westerman, P. R., Carrión, E., and Recasens, J. (2009). Effects of tillage and irrigation in cereal fields on weed seed removal by seed predators. J. Appl. Ecol. 46, 380–387. doi: 10.1111/j.1365-2664.2009.01614.x
Bourke, A. F. G., and Heinze, J. (1994). The ecology of communal breeding: the case of multiple-queen Leptothoracine ants. Philos. Trans. Biol. Sci. 345, 359–372 doi: 10.1098/rstb.1994.0115
Brascheler, B., and Baur, B. (2003). Effects of experimental small-scale grassland fragmentation on spatial distribution, density, and persistence of ant nests. Ecol. Entomol. 28, 651–658 doi: 10.1111/j.1365-2311.2003.00549.x
Colwell, R. K. (2013). EstimateS: statistical estimation of species richness and shared species from samples. Version 9.1.0.
Comas, C., Royo-Esnal, A., Recasens, J., and Torra, J. (2016). Analysing spatial correlation of weeds and harvester ants in cereal fields using point processes. Arthropod Plant Interact. 10, 197–205. doi: 10.1007/s11829-016-9425-0
Concostrina-Zubiri, L., Martínez, I., and Escudero, A. (2018). Lichen-biocrust diversity in a fragmented dryland: fine scale factors are better predictors than landscape structure. Sci. Total Environ. 628–629, 882–892. doi: 10.1016/j.scitotenv.2018.02.090
Dauber, J., Bengtsson, J. A. N., and Lenoir, L. (2006). Evaluating effects of habitat loss and land-use continuity on ant species richness in seminatural grassland remnants. Conserv. Biol. 20, 1150–1160. doi: 10.1111/j.1523-1739.2006.00373.x
Davidson, D. W. (1977). Ecology and community organization in desert seed-eating ants. Ecology 58, 725–737. doi: 10.2307/1936209
Del Toro, I., Ribbons, R. R., and Ellison, A. M. (2015). Ant-mediated ecosystem functions on a warmer planet: effects on soil movement, decomposition and nutrient cycling. J. Anim. Ecol. 84, 1233–1241. doi: 10.1111/1365-2656.12367
Del Toro, I., Ribbons, R. R., and Pelini, S. L. (2012). The little things that run the world revisited: a review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 17, 133–146.
Emmerson, M., Morales, M. B., Oñate, J. J., Batary, P., Berendse, F., Liira, J., et al. (2016). How agricultural intensification affects biodiversity and ecosystem services. Adv. Ecol. Res. 55, 43–97. doi: 10.1016/bs.aecr.2016.08.005
Escavy, J. I., Herrero, M. J., and Arribas, M. E. (2012). Gypsum resources of Spain: temporal and spatial distribution. Ore geol. Rev. 49, 72–84. doi: 10.1016/j.oregeorev.2012.09.001
Escudero, A., Palacio, S., Maestre, F. T., and Luzuriaga, A. L. (2015). Plant life on gypsum: a review of its multiple facets. Biol. Rev. 90, 1–18. doi: 10.1111/brv.12092
Ewers, R. M., and Didham, R. K. (2006). Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 81, 117–142. doi: 10.1017/S1464793105006949
Fahrig, L. (2003). Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 34:487–515. doi: 10.1146/annurev.ecolsys.34.011802.132419
Fahrig, L. (2017). Ecological responses to habitat fragmentation per se. Annu. Rev. Ecol. Evol. Syst. 48, 1–23. doi: 10.1146/annurev-ecolsys-110316-022612
Fahrig, L., Arroyo-Rodríguez, V., Bennett, J. R., Boucher-Lalonde, V., Cazetta, E., Currie, D. J., et al. (2019). Is habitat fragmentation bad for biodiversity? Biol. Conserv. 230, 179–186. doi: 10.1016/j.biocon.2018.12.026
Fletcher, R. J., Didham, R. K., Banks-Leite, C., Barlow, J., Ewers, R. M., Rosindell, J., et al. (2018). Is habitat fragmentation good for biodiversity? Biol. Conserv. 226, 9–15. doi: 10.1016/j.biocon.2018.07.022
Flores, O., Seoane, J., Hevia, V., and Azcárate, F. M. (2018). Spatial patterns of species richness and nestedness in ant assemblages along an elevational gradient in a Mediterranean mountain range. PLoS ONE 13:e0204787. doi: 10.1371/journal.pone.0204787
Folgarait, P. (1998). Ant biodiversity and its relationship to ecosystem functioning: a review. Biodivers. Conserv. 7, 1221–1244. doi: 10.1023/A:1008891901953
Golden, D. M., and Crist, T. O. (2000). Experimental effects of habitat fragmentation on rove beetles and ants: patch area or edge? OIKOS 90, 525–538. doi: 10.1034/j.1600-0706.2000.900311.x
Gordon, D. M., and Mehdiabadi, N. J. (1999). Encounter rate and task allocation in harvester ants. Behav. Ecol. Sociobiol. 45, 370–377. doi: 10.1007/s002650050573
Gotelli, N. J., and Colwell, R. K. (2001). Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391. doi: 10.1046/j.1461-0248.2001.00230.x
Gotelli, N. J., and Colwell, R. K. (2011). “Estimating species richness,” in Biological Diversity: Frontiers in Measurement and Assessment, eds A. E. Magurran and B. J. McGill (Oxford: Oxford University Press), 39–54.
Greenleaf, S. S., Williams, N. M., Winfree, R., and Kremen, C. (2007). Bee foraging ranges and their relationship to body size. Oecologia 153, 589–596. doi: 10.1007/s00442-007-0752-9
Haddad, N. M., Brudvig, L. A., Clobert, J., Davies, K. F., Gonzalez, A., Holt, R. D., et al. (2015). Habitat fragmentation and its lasting impact on Earth's ecosystems. Appl. Ecol. 1, 1–9. doi: 10.1126/sciadv.1500052
Harkness, R. D., and Maroudas, N. G. (1985). Central place foraging by an ant (Cataglyphis bicolor Fab.): a model of searching. Anim. Behav. 33, 916–928. doi: 10.1016/S0003-3472(85)80026-9
Herrera, L. P., Sabatino, M. C., Jaimes, F. R., and Saura, S. (2017). Landscape connectivity and the role of small habitat patches as stepping stones: an assessment of the grassland biome in South America. Biodivers. Conserv. 26, 3465–3479. doi: 10.1007/s10531-017-1416-7
Herrero, J., and Porta, J. (2000). The terminology and the concepts of gypsum-rich soils. Geoderma 96, 47–61. doi: 10.1016/S0016-7061(00)00003-3
Hevia, V., Azcárate, F. M., Oteros-Rozas, E., and González, J. A. (2013). Exploring the role of transhumance drove roads on the conservation of ant diversity in Mediterranean agroecosystems. Biodivers. Conserv. 22, 2567–2581. doi: 10.1007/s10531-013-0539-8
Kennedy, C. M., Lonsdorf, E., Neel, M. C., Williams, N. M., Ricketts, T. H., Winfree, R., et al. (2013). A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 16, 584–599. doi: 10.1111/ele.12082
Levins, R. (1969). Some demographic and genetic consequences of environmental heterogeneity for biological control. Bulletin of the Entomol. Soc. Am. 15, 237–240. doi: 10.1093/besa/15.3.237
Longino, J. T., Coddington, J., and Colwell, R. K. (2002). The ant fauna of a tropical rain forest: estimating species richness three different ways. Ecology 83, 689–702 doi: 10.1890/0012-9658(2002)083[0689:TAFOAT]2.0.CO;2
Luzuriaga, A. L., Sánchez, A. M., López-Angulo, J., and Escudero, A. (2018). Habitat fragmentation determines diversity of annual plant communities at landscape and fine spatial scales. Basic Appl. Ecol. 29, 12–19. doi: 10.1016/j.baae.2018.03.008
Marvier, M., Kareiva, P., and Neubert, M. G. (2004). Habitat destruction, fragmentation, and disturbance promote invasion by habitat generalists in a multispecies metapopulation. Risk Anal. 24, 869–878. doi: 10.1111/j.0272-4332.2004.00485.x
Matesanz, S., Escudero, A., and Valladares, F. (2009). Impact of three global change drivers on a Mediterranean shrub. Ecology 90, 2609–2621. doi: 10.1890/08-1558.1
Matesanz, S., Gómez-Fernández, A., Alcocer, I., and Escudero, A. (2015). Fragment size does not matter when you are well connected: effects of fragmentation on fitness of coexisting gypsophiles. Plant Biol. 17, 1047–1056. doi: 10.1111/plb.12329
Melliger, R. L., Braschler, B., Rusterholz, H.-P., and Baur, B. (2018). Diverse effects of degree of urbanisation and forest size on species richness and functional diversity of plants, and ground surface-active ants and spiders. PLoS ONE 13:e0199245. doi: 10.1371/journal.pone.0199245
Moncalvillo, B., Matesanz, S., Escudero, A., and Sánchez, A. M. (2021). Habitat fragmentation and population features differently affect fruit predation, fecundity and offspring performance in a non-specialist gypsum plant. Plant Biol. 23, 184–192. doi: 10.1111/plb.13183
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O'Hara, R., et al. (2015). Vegan: community ecology package. R Package Version 2.3-2. Available onlibe at: http://CRAN.R-project.org/package=vegan
QGIS.org (2018). QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available onlibe at: http://qgis.org
Rabasa, S., Gutiérrez, D., Escudero, A., and Spence, J. (2005). Egg laying by a butterfly on a fragmented host-plant: a multi-level approach. Ecography 28, 629–639. doi: 10.1111/j.2005.0906-7590.04229.x
Rabasa, S. G., Gutiérrez, D., and Escudero, A. (2009). Temporal variation in the effects of habitat fragmentation on reproduction of the Mediterranean shrub Colutea hispanica. Plant Ecol. 200, 241–254. doi: 10.1007/s11258-008-9448-4
Santamaría, S., Sánchez, A. M., López-Angulo, J., Ornosa, C., and Escudero, A. (2018). Landscape effects on pollination networks in Mediterranean gypsum islands. Plant Biol. 20, 184–194. doi: 10.1111/plb.12602
Santos, L. A. O., Bischoff, A., and Fernandes, O. A. (2018). The effect of forest fragments on abundance, diversity and species composition of predatory ants in sugarcane fields. Basic Appl. Ecol. 33, 58–65. doi: 10.1016/j.baae.2018.08.009
Silvestre, M., Aguilar, A., Seoane, J., and Azcárate, F. M. (2019). Responses of seed size, ant worker size, and seed removal rate to elevation in Mediterranean grasslands. Oecologia 189, 781–793. doi: 10.1007/s00442-019-04356-6
Tremlová, K., and Münzbergová, Z. (2007). Importance of species traits for species distribution in fragmented landscape. Ecology 88, 965–977. doi: 10.1890/06-0924
Tscharntke, T., Sekercioglu, C. H., Dietsch, T. V., Sodhi, N. S, Hoehn, P., and Tylianakis, J. M. (2008). Landscape constraints on functional diversity of birds and insects in tropical agroecosystems. Ecology 89, 944–951. doi: 10.1890/07-0455.1
Villard, M. A., and Metzger, J. P. (2014). Beyond the fragmentation debate: a conceptual model to predict when habitat configuration really matters. J. Appl. Ecol. 51, 309–318. doi: 10.1111/1365-2664.12190
Volenec, Z. M., and Dobson, A. P. (2020). Conservation value of small reserves. Conserv. Biol. 34, 66–79. doi: 10.1111/cobi.13308
Whittaker, R. J., Willis, K. J., and Field, R. (2001). Scale and species richness: towards a general, hierarchical theory of species diversity. J. Biogeogr. 28, 453–470. doi: 10.1046/j.1365-2699.2001.00563.x
Keywords: agroecosystems, ants, biodiversity conservation, drylands, fragmentation, gypsum habitats
Citation: Azcárate FM, Alameda-Martín A, Escudero A and Sánchez AM (2021) Ant Communities Resist Even in Small and Isolated Gypsum Habitat Remnants in a Mediterranean Agroecosystem. Front. Ecol. Evol. 9:619215. doi: 10.3389/fevo.2021.619215
Received: 19 October 2020; Accepted: 14 January 2021;
Published: 16 March 2021.
Edited by:
Anna Sofie Persson, Lund University, SwedenReviewed by:
Inge Armbrecht, University of Valle, ColombiaCopyright © 2021 Azcárate, Alameda-Martín, Escudero and Sánchez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisco M. Azcárate, Zm0uYXpjYXJhdGVAdWFtLmVz
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.