
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 18 March 2021
Sec. Behavioral and Evolutionary Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.619193
This article is part of the Research TopicEcology, Evolution, and Behavior of Viviparous FishesView all 19 articles
 Juliane Lukas1,2*
Juliane Lukas1,2* Felix Auer3
Felix Auer3 Tobias Goldhammer3
Tobias Goldhammer3 Jens Krause1,2,4
Jens Krause1,2,4 Pawel Romanczuk4,5,6
Pawel Romanczuk4,5,6 Pascal Klamser5,6
Pascal Klamser5,6 Lenin Arias-Rodriguez7
Lenin Arias-Rodriguez7 David Bierbach1,2,4
David Bierbach1,2,4Animals often face changing environments, and behavioral flexibility allows them to rapidly and adaptively respond to abiotic factors that vary more or less regularly. However, abiotic factors that affect prey species do not necessarily affect their predators. Still, the prey’s response might affect the predator indirectly, yet evidence from the wild for such a classical bottom-up effect of abiotic factors shaping several trophic levels remains sparse. In many aquatic environments, daily changes in oxygen concentrations occur frequently. When oxygen levels drop to hypoxic levels, many fishes respond with aquatic surface respiration (ASR), during which they obtain oxygen by skimming the upper, oxygenated surface layer. By increasing time at the surface, fish become more vulnerable to fish-eating birds. We explored these cascading effects in a sulfidic spring system that harbors the endemic sulphur molly (Poecilia sulphuraria) as prey species and several fish-eating bird species. Sulfide-rich springs pose harsh conditions as hydrogen sulfide (H2S) is lethal to most metazoans and reduces dissolved oxygen (DO). Field sampling during three daytimes indicated that water temperatures rose from morning to (after)noon, resulting in the already low DO levels to decrease further, while H2S levels showed no diurnal changes. The drop in DO levels was associated with a decrease in time spent diving in sulphur mollies, which corresponded with an increase in ASR. Interestingly, the laboratory-estimated threshold at which the majority of sulphur mollies initiate ASR (ASR50: <1.7 mg/L DO) was independent of temperature and this value was exceeded daily when hypoxic stress became more severe toward noon. As fish performed ASR, large aggregations built up at the water surface over the course of the day. As a possible consequence of fish spending more time at the surface, we found high activity levels of fish-eating birds at noon and in the afternoon. Our study reveals that daily fluctuations in water’s oxygen levels have the potential to alter predator-prey interactions profoundly and thus highlights the joined actions of abiotic and biotic factors shaping the evolution of a prey species.
Almost all organisms have to cope with changing environments during their lifetime (Bernhardt et al., 2020). The rate of change can occur at different temporal scales including diel and seasonal (e.g., light, temperature, oxygen, precipitation, wind, and water regime), as well as multiannual cycles (e.g., El Niño events), or can be completely unpredictable (aperiodic such as some human disturbances). Many environmental variables are correlated with selective conditions (e.g., resource availability or predation) and thus animals have evolved a suite of traits and strategies that allow them to detect and exploit environmental fluctuations to maximize their fitness (Bernhardt et al., 2020). For many animals, the first response to altered conditions is often behavioral (as opposed to physiological; Wong and Candolin, 2015), allowing for a rapid and (often) reversible response, which can be especially crucial in highly fluctuating environments.
Environmental fluctuations have the potential to affect not only individuals and populations, but also higher (community) levels through species interactions such as competition and predator-prey relationships (Nilsen et al., 2009; Cherry and Barton, 2017; Turner et al., 2017). For one, abiotic changes alter species distribution and consequently species overlap due to habitats becoming more or less accessible or favorable for survival. As a consequence, existing biotic interactions may cease or new ones may arise. For example, during periods of unfavorable water conditions, mobile species will seek out habitat refuges, but therein experience increased pressure of competition and predation (e.g., demersal species such as blue crab, croaker and spot in a river estuary with spatially and temporally dynamic hypoxia; Eby and Crowder, 2002). For another, the nature of the species interaction may change. Two species may respond differently to the same environmental condition, which can drastically shift interaction outcomes in favor of the one with greater tolerance or exploitation capacity [e.g., water turbidity affecting damselfly–fish interactions (Van de Meutter et al., 2005), temperature and oxygen affecting prey selection of an predatory insect (Cockrell, 1984), hypoxia increasing susceptibility of benthic prey to fish predation (Long and Seitz, 2008) or decreasing a fish predator’s foraging efficiency (Hedges and Abrahams, 2015)].
In aquatic ecosystems, oxygen is often a limiting factor and the lack of oxygen can cause detrimental stress to some animals (Wannamaker and Rice, 2000; Pollock et al., 2007; Galic et al., 2019). Many species leave oxygen-depleted (hypoxic) areas and move to normoxic regions when necessary (Pihl et al., 1991; Wannamaker and Rice, 2000; Eby and Crowder, 2002; Brady and Targett, 2013). Others are able to remain in the hypoxic areas due to specialized physiological, morphological, and behavioral adaptations that help minimize the effects of severe hypoxia (Pihl et al., 1991; Timmerman and Chapman, 2004). In fish, a common response to severe hypoxia is a compensatory behavior termed “aquatic surface respiration” (ASR), during which they utilize dissolved oxygen diffusing through the air-water interface (Kramer and Mehegan, 1981; Kramer and McClure, 1982; Chapman et al., 1995; Timmerman and Chapman, 2004; Tobler et al., 2009). Time allocated at the surface increases as oxygen decreases and while there are high costs associated with this behavior (both opportunity and predation; Kramer, 1983; Poulin et al., 1987), it is highly efficient against hypoxia-induced mortality (Kramer and Mehegan, 1981).
As a consequence of ASR, fish undergo a habitat compression, which often leads to dense aggregations at the surface and thus greater overlap with competitors and potential predators. Predators in the same medium (i.e., aquatic piscivores) will be exposed to the same hypoxic conditions. Depending on physiological tolerances of the specific interaction pair, this can hinder (Poulin et al., 1987) or favor the predator (Wolf and Kramer, 1987), yet, when predators cross ecosystem boundaries (e.g., aerial or terrestrial to aquatic), conditions experienced by one species may not be experienced by or even affect the other. Piscivorous birds, for example, will have advantages during periods of aquatic hypoxia. As prey becomes more clustered within surface waters (vertically, horizontally or both; Kramer, 1987; Eby and Crowder, 2002), it is easily accessible for avian predators. Also, hypoxia affects swimming activity and numerous anti-predator behaviors in fish (Domenici et al., 2000, 2007), thus shifting predation outcomes in favor of the birds. Empirical evidence for a link between hypoxia-induced ASR in fishes and aerial predation by birds was established in a laboratory setting, where an increase of hypoxia-induced ASR significantly reduced the survival of six species preyed on by a heron (Kramer et al., 1983). However, to date, evidence for such a classical bottom-up control of abiotic factors shaping predator-prey interactions in the wild remains sparse (but see Kersten et al., 1991). This is not least owing to the fact that predator-prey interactions are often complex (e.g., include multiple aquatic and aerial predators) and it is not always feasible to capture them at relevant temporal and spatial scales.
Here we report on a freshwater system that circumvents many of the above-mentioned observational shortcomings. In southern Mexico, poeciliids colonized multiple hydrogen-sulfide rich springs (Tobler et al., 2006; Palacios et al., 2013; Culumber et al., 2016). Hydrogen sulfide (H2S) is toxic to most metazoans and often contributes to hypoxic conditions (Bagarinao, 1992). Consequently, respiratory adaptations are essential for survival in this environment. Evidence suggests that sulfide-adapted ecotypes can evolve in as little as 250 years (Brown et al., 2018). In the case of our study species, the sulphur molly Poecilia sulphuraria, sulfidic and non-sulfidic lineages genetically diverged between 15 and 30 ky ago (Greenway, 2019). While several morphological and physiological adaptations allow this species to persist in these conditions (e.g., mouth and gill area enlargement for enhanced oxygen uptake, modified toxicity targets and detoxification pathways; Tobler and Hastings, 2011; Tobler et al., 2011; Greenway et al., 2020), they are still dependent on aquatic surface respiration (ASR) for survival. A previous study estimated that sulphur mollies spend up to 84% of their time performing ASR (Tobler et al., 2009), which was three times higher than a closely related species (Poecilia mexicana) from a nearby (slightly less) sulfidic spring system. As a result of the species’ high ASR rates, these fish frequently form large aggregations, likely to offset some of the risks associated with surfacing (equivalent to synchronous air-breathing; Kramer and Graham, 1976; Chapman and Chapman, 1994). These aggregations likely attract non-aquatic predators, and bird predation rates are estimated to be 20-fold increased compared to surrounding clearwater habitats (Riesch et al., 2010a). As such, this system provides a unique natural laboratory to investigate how changes in oxygen can affect ASR in fish and upscale to influence the interaction with fish-eating aerial predators.
With hypoxia and high avian predation being ubiquitous features of this sulfide spring habitat (albeit some spatial variation in DO and H2S along the 2.5 km long stream; Culumber et al., 2016), sulphur mollies need to respond behaviorally to mitigate the risks of hypoxia and predation, which will likely vary also temporally. Our objective was thus to examine the role of diurnal environmental variation in altering the bird-fish predator-prey interactions at the Baños del Azufre sulfidic springs. Our first aim was to observe the extent to which water conditions (especially temperature, DO and H2S) varied during daytime. On the basis of the inverse relationship between temperature and DO, we predicted that daytime warming of the water would likely reduce DO levels and possibly affect H2S throughout the day. We measured physico-chemical water parameters using standard methods for a period of 6 days at three times each day to capture diurnal variation of the system. Our second aim was to determine the role of hypoxia in influencing fish’s ASR tendency and the concomitant surface use. Fish perform ASR to compensate for oxygen shortages in the water. However, H2S also requires high amounts of oxygen for detoxification, and thus both may affect fish’s ASR rates (and accompanied times at the surface). We predicted the highest ASR rates during the most unfavorable water conditions (low DO, high H2S). Using a similar sampling regime (6 × 3), we quantified fish’s ASR tendency by observing the duration of fish’s voluntarily dives into the water column (with long dives equating to low ASR tendency). To further pinpoint how hypoxia levels affect ASR rates in this system, we established the threshold DO level at which fish initiate ASR in H2S-rich water in a laboratory experiment. Our third and last aim was to examine how hypoxia-related variation in fish’s ASR (surface) behavior affects bird predators. We hypothesized that high ASR rates would be associated with high bird activity, because birds are attracted by the easily accessible prey. We predicted piscivorous birds would exploit times when fish only dove for short periods (and thus spent most of their time at the surface) by increasing foraging efforts through staying longer and attacking more. To test this prediction, we quantified piscivorous bird activity whenever we measured the fish’s ASR tendencies. To our knowledge, the present study is the first to investigate temporal variation in abiotic and biotic stressors and their implications for the surface behavior of a sulfide-adapted freshwater fish.
Several springs in southern Mexico (states of Tabasco and Chiapas) are fed by sulfidic groundwater aquifers with high concentrations of hydrogen-sulfide (H2S) generated from volcanic deposits and bacterial sulfate reduction (e.g., drainages Pichucalco, Tacotalpa and Puyacatengo; Rosales Lagarde et al., 2014). The Baños del Azufre (17°33′ N, 93°00′ W) describes an approximately 2.5 km section of the Rio El Azufre, which is associated with multiple sulfidic springs. A previous study characterized it as a freshwater habitat with high temperatures and sulfide content, low oxygen and pH, and high conductivity, which showed little temporal variation across years (T: 31.9 ± 0.7°C, H2S: 190.4 ± 119.7 μmol/L, DO: 1.06 ± 0.92 mg/L, pH: 6.9 ± 0.1, EC: 2.7 ± 0.2 mS/cm; mean ± SD of 4 years; Tobler et al., 2011), but some spatial variation due to differences in habitat structure and spring discharge (Culumber et al., 2016).
The Baños del Azufre spring complex is inhabited by the endemic sulphur molly (P. sulphuraria) that shows distinct adaptations to these severe hypoxic and sulfidic conditions (for details see Plath et al., 2007; Riesch et al., 2010b; Tobler and Hastings, 2011; Tobler et al., 2011, 2018; Pfenninger et al., 2014; Kelley et al., 2016; Brown et al., 2017; Barts et al., 2018; Camarillo et al., 2020; Greenway et al., 2020). Most notably, fish spend a majority of their time performing aquatic surface respiration (ASR), but frequently dive to engage in benthic foraging as well as aggressive or reproductive activities under water (Tobler et al., 2009). Fish also dive in response to predation (Lukas et al., in review). Main predators in this system consist of fish-eating birds such as kingfishers, herons and egrets (Supplementary Table 1, Riesch et al., 2010a), while aquatic predators are mostly absent (see Riesch et al., 2009 for rare exceptions in less-sulfidic up- or downstream parts).
Preliminary observations revealed considerable temporal variation in fish’s tendency to perform ASR during daytime hours, with large aggregations of fish building at the surface throughout the day (Supplementary Video). This pattern was reliably observable at various sites of the Baños del Azufre (pers. observations of the authors, 2016–2019). To allow for higher temporal resolution we based our investigations at a single, representative site exposed to moderate to high levels of sulfidic and hypoxic stress (refer to site 1 in Culumber et al., 2016). Additionally, this site allowed for access during all sampling times and fish and birds were already habituated to a degree of human presence.
To explore whether the observed diurnal differences in fish behavior are driven by physicochemical water conditions and how they link to predator activity, we conducted two field surveys and one laboratory experiment. During one field season (subsequently termed field survey I), we first quantified how variable physicochemical water conditions were throughout the day. In another field season (subsequently termed field survey II), we investigated the link between fish’s behavior and predatory bird activity.
To link observations from both surveys, they were carried out at the same location and followed the same regimen by sampling each morning (07:00 – 09:30), midday (12:00 – 14:30) and afternoon (16:00 – 18:30) for six subsequent days. Surveys were matched for season (i.e., end of dry season; I: 12–17 April 2019, II: 05–10 May 2016) and we subsequently verified that no major deviations from the temperature trend occurred between years (compare Figure 1A and Supplementary Figure 1). Due to the spatial dynamics of predator-prey interactions, observations on water chemistry, fish and bird behavior were performed on slightly different spatial scales. While fish were clearly clustered in shoals with very little movement between, avian predators were much more mobile. Fish observations were based on a focal shoal, which reliably built up at the same location every day (crossing the Survey I transect; Supplementary Video). At its largest dimension, the focal shoal spanned completely across the stream’s width and was estimated to reach about 15 m in length. For bird observations, we considered a slightly longer stretch of the stream (50 m with focal shoal in the upper half) and included predators along the stream’s bench (∼2 m on each side), which were walking or perching close by (∼400 m2 total study area). Ultimately, this approach did not allow for abiotic and biotic observations to be directly linked, nor for the behavior of individual prey to be correlated with a single predator. Still, it does provide first useful insights into the overall trend of the relationship between water chemistry, prey’s surface aggregations and predator activity (see Statistical analyses on how we approached this).
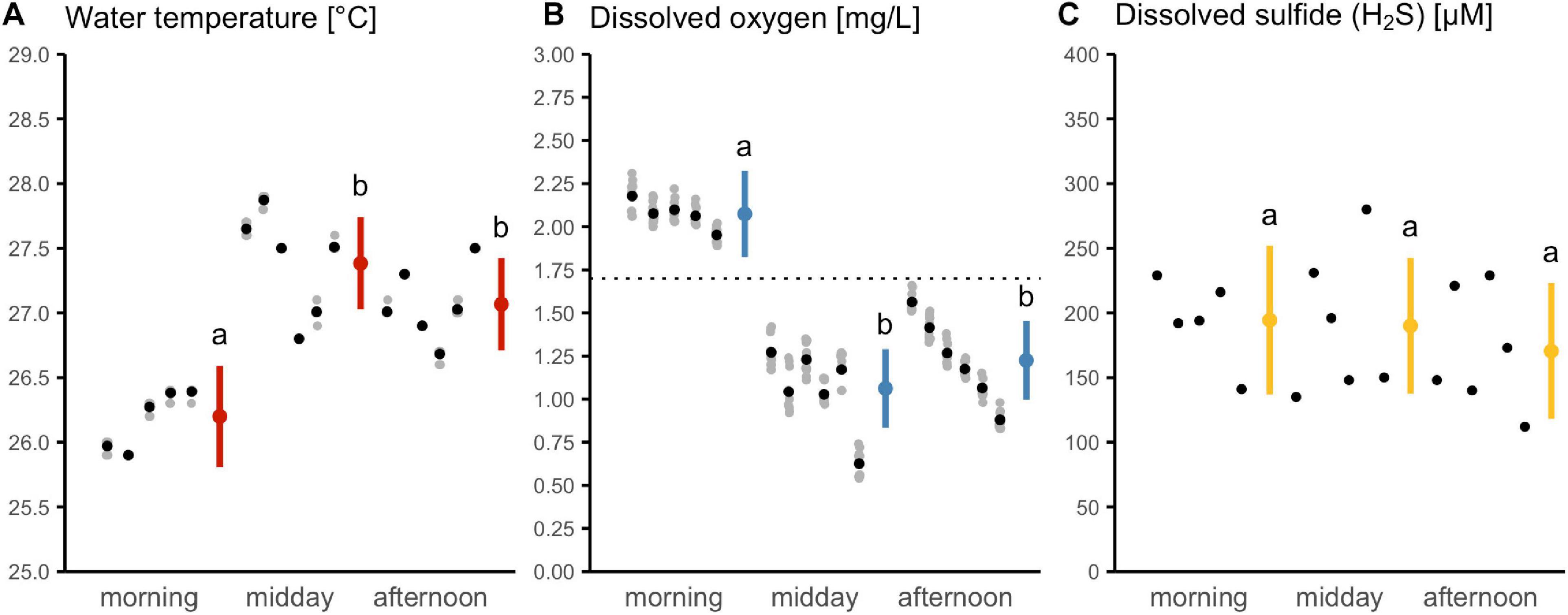
Figure 1. Diurnal variation in water parameters of a sulfidic stream at Baños del Azufre, Mexico. A cross section of the stream was repeatedly sampled during three daytime periods on 6 consecutive days (n = 17 sampling occasions; morning Day 1 excluded). Shown are multiple measurements (gray), which were sample-pooled for analysis (black). Time of day affected (A) water temperature and (B) dissolved oxygen concentration, but not (C) dissolved sulfide concentration (see model-estimated marginal means with 95% CI in red, blue, yellow). Letters indicate results from post hoc pairwise comparisons. The dashed line indicates a laboratory-established threshold at which Poecilia sulphuraria predominantly performed aquatic surface respiration (ASR50; see results below).
Extremely high fish densities at times prevented us from reliably scoring fish’s ASR (surface time) under field conditions as one would lose focus of individual fish at the surface. However, fish would interrupt ASR and drop below the surface for voluntary dives [i.e., without external disturbance; see description of the behavioral repertoire of P. sulphuraria above and similar observation on Poecilia reticulata (Kramer and Mehegan, 1981)]. To the human eye, these individuals were more conspicuous and their trajectory through the water column could be easily followed until they resurfaced again. While dive duration can merely present an inverse proxy of ASR tendency, we argue that it is a conservative assessment, considering that many individuals did not leave the surface at all when DO levels were low (see also Supplementary Video).
Lastly, to corroborate some of the above observations in a controlled laboratory experiment, we explored the proportion of time sulphur mollies spent performing ASR as well as the number and duration of dives at different dissolved oxygen levels without the confounding effects of variation in water chemistry or predator activity. It also allowed us to validate our ASR proxy by verifying that fish did not compensate shorter lasting dives by diving more often.
In their natural habitats sulphur mollies perform ASR in large groups. Such high social tendency has also been reported for sulfidic surface ecotypes of the closely related P. mexicana from a high predation site similar to ours (Bierbach et al., 2018, 2020). We therefore chose to test fish in groups. In nature, some individuals likely have to make compromises by surfacing earlier or later than they need to conform with group behavior. It is conceivable that a similar effect occurred in our experiment, so that some individuals (possibly due to differences in personality or metabolic demand) may have triggered the response of the entire group (Kramer and Graham, 1976; Chapman and Chapman, 1994; Borowiec et al., 2018; Killen et al., 2018). Nonetheless, when naturally shoaling species are tested in isolation, they are stressed and consume significantly more oxygen (see evidence for group’s “calming effect”: Queiroz and Magurran, 2005; Schleuter et al., 2007; Nadler et al., 2016). We are thus confident that our setup enabled us to gain biologically most relevant data on the DO thresholds for ASR behavior.
At each sampling, we took multiple measurements of water temperature, dissolved oxygen (DO), pH and electrical conductivity (EC), and – due to logistical constraints – one sulfide sample along a cross section of the sulfidic stream. Temperature, DO, pH and EC were measured using a multiparameter probe (WTW Multi 3630 IDS) fixed to a height-adjustable pole facing upstream. Calibration and measurements were carried out according to the manufacturer’s recommendations. To capture variation introduced through flow regime and/or stratification within the water column, measurements were taken according to a transect grid (i.e., one sample each meter starting at 0.5 m away from the stream bench and at depths of 0.05, 0.25, and 0.5 m as water levels would allow). With a width of 5.6 m and a depth of less than 0.8 m at the site, this resulted in 10–11 subsamples per sampling.
For the quantification of total free sulfides (i.e., sum of concentrations of H2S, HS– and acid-soluble metallic sulfides), we collected a water sample from the stream center (width: 2.5 m, depth: 0.25 m) with a syringe. The first 1 ml sample was discarded to clear the tubing and the subsequent 0.2 ml sample was diluted with 3.8 ml distilled water to obtain a concentration within the desired measurement range. The total sulfide concentration was immediately analyzed by cuvette test (Hach Lange LCK 653) using a spectrophotometer (Hach Lange DR 2800) with automated recognition of the measurement program and internal calibration. We calculated the pH-dependent speciation of measured total free sulfide into H2S and HS– using a pKa1 of 6.9 and pKa2 of 17 (Stumm and Morgan, 1996).
We assessed fish behavior using a focal animal sampling approach (see Tobler et al., 2009 for a similar approach to quantify fish’s time budgets on site). An observer (i.e., field assistant with no prior knowledge of the hypothesis or its predictions) sat quiescent on the stream bench, and within a few seconds of arrival fish resumed normal swimming activity.
Twenty diving fish of similar size were chosen randomly from a focal shoal and observed from the moment they initiated diving until they resurfaced to calculate the mean dive duration. In rare cases, observations had to be terminated and repeated because a disturbance occurred during the diving period (e.g., shadow of an overflying bird) or because a fish swam out of sight.
We assessed bird activity prior to any fish observations to minimize disturbances caused by human activity. We made observations from a natural hide alongside the stream using binoculars. Three observers recorded all sightings of piscivorous birds in the predefined study area within a 30-min period. Observers were versed in identifying the bird species previously described to predate on P. sulphuraria (refer to Riesch et al., 2010a).
For each bird, we determined species (to lowest feasible taxonomical level), entry and exit times, as well as the number of attacks launched. Given the highly stochastic nature of predation in time and space, we selected two measures of bird activity that we deemed robust against differences in predator abundance and species differences in foraging styles. We calculated presence time as the mean time piscivorous birds spent in the study area (excluding mere fly-throughs, i.e., flying through the transect without landing or attacking) and attacks as the total number of bird attacks launched in a sampling period.
We exposed fish to dissolved oxygen concentrations ranging from near-anoxic to normoxic conditions (0.6 – 5.1 mg/L DO). Due to logistical restraints (field lab in tropical climate), water temperature could not be controlled completely. As a consequence, we assigned fish post hoc to one of two temperature regimes (26.5°C or 28°C). Both temperatures are ecologically relevant as they represent the daily variations commonly experienced by the species between morning and afternoon.
We collected fish and water for the experiment from the same site previously used in Survey I and II. Fish were left to habituate in insulated coolers for at least 1 h without external disturbance. During habituation, fish were held in aerated water from the nearest freshwater source (5.5 mg/L DO). Treatment water was aerated to the desired DO level under constant mixing. DO and temperature were monitored with a multiprobe (OxyGuard Polaris 2) directly prior and after each trial to calculate a mean treatment value. Water was exchanged after each trial and testing was done under natural light.
We tested fish in groups of five adults visually matched for size. Under consideration of the sulphur molly’s ecology, we deemed group testing both ecologically relevant and necessary to avoid isolation stress. We tested a total of 27 groups (n = 135) with 12 groups experiencing the morning (26.5°C; mean ± SD: 26.6 ± 0.3°C) and 15 groups the afternoon temperature regime (28°C; 27.9 ± 0.1°C). Fish were netted haphazardly and introduced into the front third of a test tank (30 × 20 × 30 cm, water depth of 20 cm). A trial lasted 10 min, in which experimenters left the area and fish’s position in the water column was recorded with a camera (GoPro Hero6) facing the tanks’ front. As part of the acclimation protocol, we did not analyze the first 5 min of each trial to ensue fish had recovered from handling and resumed swimming. We quantified the cumulative time spent at the surface by all five fish (see Video analysis) and calculated a percentage surface time. To link laboratory and field observations of fish’s behavior (Survey II), we also assessed the total number of dives and mean dive duration performed by each group.
We analyzed videos obtained from the lab experiment using EthoVision 12 (Noldus Information Technology). The front view of the water column allowed for good monitoring of fish’s depth position, but the view of individual fish was sometimes occluded as they moved slowly along the surface and thus did not allow for trajectories with individual identity. As a prerequisite for ASR, fish need to have direct surface contact, but experiments on another poeciliid (P. reticulata) showed that these fish rarely spent time near the surface without being in surface contact (Kramer and Mehegan, 1981). Based on fish’ body depth, we defined the area less than 1 cm below the water surface as zone of interest, which was tracked for presence/absence of fish and corrected manually. We note that this approach may slightly overestimate ASR duration (e.g., due to brief transitions during up- and downward diving), nonetheless, we deem this negligible considering the overall observation period of 5 min.
In order to make both field surveys comparable, it was necessary to reduce some information of the obtained variables for statistical analyses. Multiple measurements taken during a single sampling were pooled into one mean score (sample pooling). Data from the morning of day 1 was excluded in both surveys due to increased human activity affecting the surveys (Sunday morning church), resulting in n = 17 observations for each parameter. We tested for temporal variation in all parameters of interest by performing separate (generalized) linear regressions (package lme4; Bates et al., 2015) with time (morning/midday/afternoon) as a fixed effect. Regressions of dive duration and predator presence time were performed with Gaussian error structure. Both variables were log(y)/log(y + 1)-transformed to avoid negative predictions and normalize model residuals. We assumed predator attacks to be (approximately) Poisson distributed. Models were validated by visual inspection of the residuals. Significance of coefficients was evaluated via Likelihood-Ratio tests.
We further explored the association between parameters (cor.test in package stats; R Core Team., 2020). Observations of temperature, oxygen and H2S were directly paired and fulfilled the assumption of a Pearson’s correlation. For prey and predator behavior, we used Mann-Kendall rank correlations, which are robust against outliers and appropriate for small sample sizes.
To explore the relationship between oxygen and aquatic surface respiration of P. sulphuraria, we used a model selection approach. Eight hypothesis-driven candidate models were considered, allowing for linear and non-linear effects of DO on fish’s surface time (Table 2). We did not include higher degree effects (>2) as we did not consider them biologically plausible. To account for an effect of test temperature, half of the models included the parameter but did no interactions due to the sample size. We fitted all models with binomial logit-normal error structure (glmer function in package lme4; Bates et al., 2015) and added an observation-level random effect to account for overdispersion (Harrison, 2015). Models were evaluated by using Akaike’s information criterion for finite sample sizes (AICc; package MuMIn; Bartoñ, 2020). With this approach, the model with the lowest AICc as well as all models within 7 ΔAICc units are considered equally supported (Burnham et al., 2011). In addition, we used multimodel inference to compare the relative importance of main effects (i.e., sum of the relative evidence weights for all models in which the parameter appeared). Estimations of half-maximal DO concentration (ASR50) for both temperature regimes were done on the basis of the global model.
Lastly, the more controlled laboratory experiment allowed us to validate our proxy of dive duration (Survey II) using direct measurements of ASR surface time. We first tested for differences in both number and duration of dives tested either above or below the established ASR50 threshold using Wilcoxon Signed-Ranks tests. We then estimated a Spearman correlation between surface time and dive duration (cor.test in package stats; R Core Team., 2020). All analyses were performed in R (see R script; R Core Team., 2020, version 4.0.2).
Overall, the study site exhibited high temperatures, high concentrations of sulfides (especially H2S), high specific conductivity, as well as low dissolved oxygen concentrations and low pH (Table 1). Temperature and DO varied significantly throughout the day (effect of time: temperature F2,14 = 19.2, p < 0.0001, DO F2,14 = 36.8, p < 0.0001), but were inversely related (Pearson’s rho = −0.82, t = −5, p < 0.0001, n = 17). While mornings were associated with temperatures below 26.5°C and DO levels around 2 mg/L, temperatures rose and DO decreased to severely hypoxic levels toward later parts of the day (i.e., <1.7 mg/L DO; Figures 1A,B). Dissolved sulfide concentrations showed considerable variation between samplings (Figure 1C). We found no evidence for a diurnal cycle (F2,14 = 0.4, p = 0.67) or an association with temperature (Pearson’s rho = −0.18, t = −0.7, p = 0.5, n = 17) or DO (Pearson’s rho = 0.23, t = 0.9, p = 0.4, n = 17).
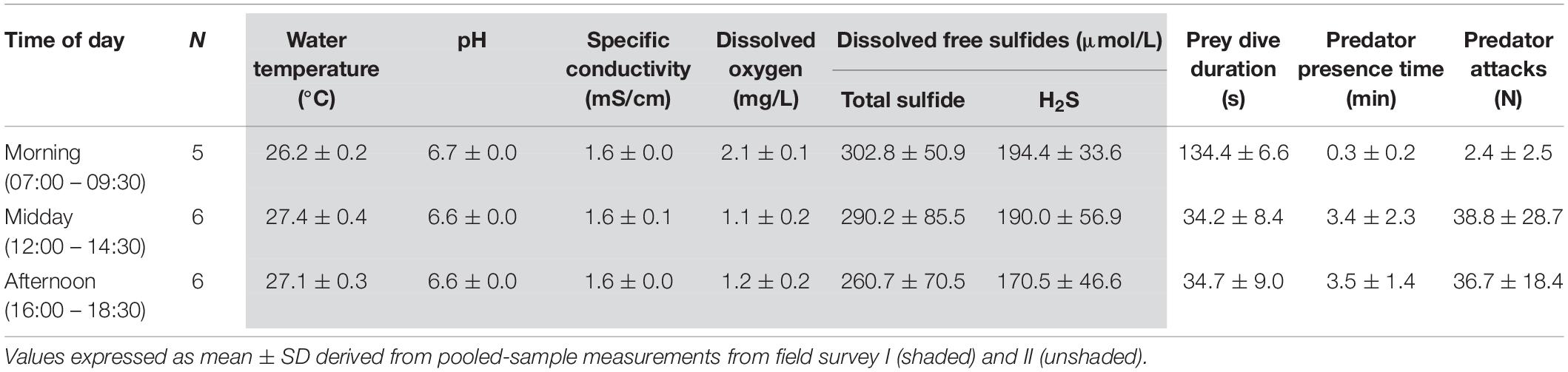
Table 1. Descriptive statistics of diurnal variation in abiotic and biotic parameters at the Baños del Azufre sulfur spring complex.
Fish frequently surfaced during mornings, middays and afternoons, but continuously aggregated at the surface toward the later parts of the day (Figure 2A and Supplementary Video). Fish performed occasional voluntary dives (i.e., without predatory or human disturbance) into the water column during all three periods. The duration of these dives varied throughout the day (effect of time: F2,14 = 75.8, p < 0.0001). Dives lasted significantly longer during mornings than later in the day (Figure 2B).
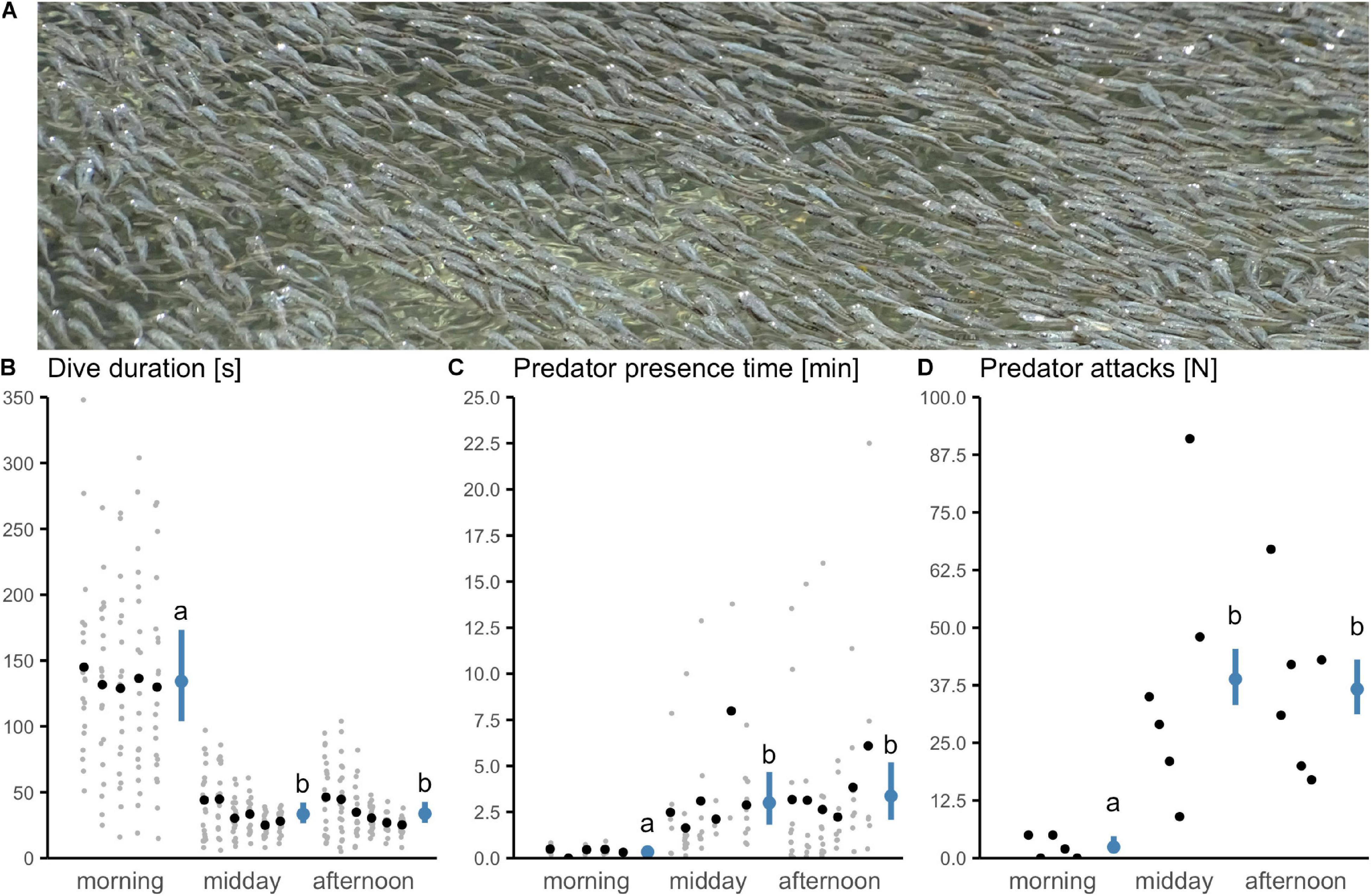
Figure 2. Diurnal variation in sulphur mollies’ diving behavior and predatory birds’ activity. (A) During daytime hours, sulphur mollies spent large amounts of time at the surface performing aquatic surface respiration, often resulting in densely-packed shoals. A stream section was repeatedly sampled during three daytime periods on six consecutive days (n = 17 sampling occasions; morning Day 1 excluded). Shown are measurements (gray), which were sample-pooled for analysis (black). Time of day affected (B) prey’s behavior (mean dive duration) as well as predator’s activity [(C): mean presence time and (D): total sum of attack; see model-estimated marginal means with 95% CI in blue]. Letters indicate results from post hoc pairwise comparisons.
Activity of avian predators was generally high. Within 8.5 h over 6 days, we observed nine different piscivorous bird species (Supplementary Table 1) and witnessed 465 attacks (Figure 2D). The time birds spent in the transect as well as the number of attacks peaked during middays and afternoons (effect of time: presence time F2,14 = 23.1, p < 0.0001; attacks χ2(2) = 234, p < 0.0001; Figures 2C,D). Periods of short dives (and an associated extension of surface time) were associated with high activity of avian predators. When fish were able to dive for longer periods, predators spent less time near the prey (Kendall’s tau = −0.62, T = 26, p = 0.0003, n = 17; Figure 3A) and launched fewer attacks (tau = −0.47, z = −3, p = 0.008, n = 17; Figure 3B).
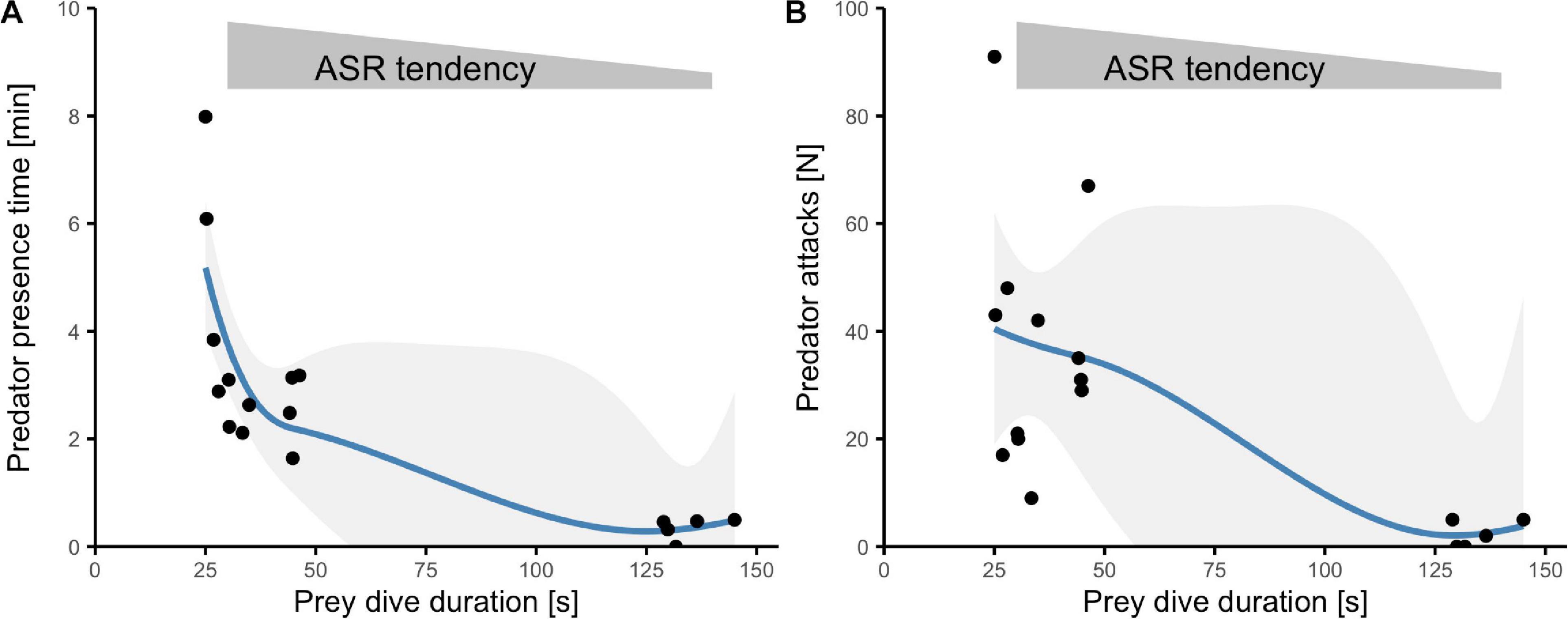
Figure 3. Interplay among prey behavior and predator activity. Relationship between dive duration and either (A) predator presence time (mean) or (B) predator attacks (total sum) at different daytime periods. Each point represents data for one sampling occasion (sample-pooled measurements). Blue lines represent loess-smoothed curve fits with 95% CI for significant Kendall rank correlations.
Time spent at the surface performing ASR varied as fish were exposed to dissolved oxygen concentrations ranging from near anoxia to normoxia (0.6 – 5.1 mg/L). We observed a non-linear (sigmoidal) relationship between surface-time and DO (Figure 4). This was supported by model selection (all top models included quadratic or logarithmic effects of DO; Table 2A) and multimodel interference, as a linear and quadratic effect of DO showed the highest relative importance (Table 2B). Under severe hypoxia, fish spent more than 90% of their time at the surface performing ASR, while fish in more normoxic conditions surfaced only rarely. The point at which fish switched from utilizing the water column to mainly perform ASR (50% of the time spent at the surface; ASR50) was at 1.7 mg/L DO. This behavioral switch was observable for fish tested at 26.5°C and 28°C (Figures 4A,B). Temperature exhibited low relative importance on surface time (Table 2B), although a more fine-scaled investigation of intermediate DO levels will be necessary to eliminate a possible shift of the behavioral threshold with temperature.
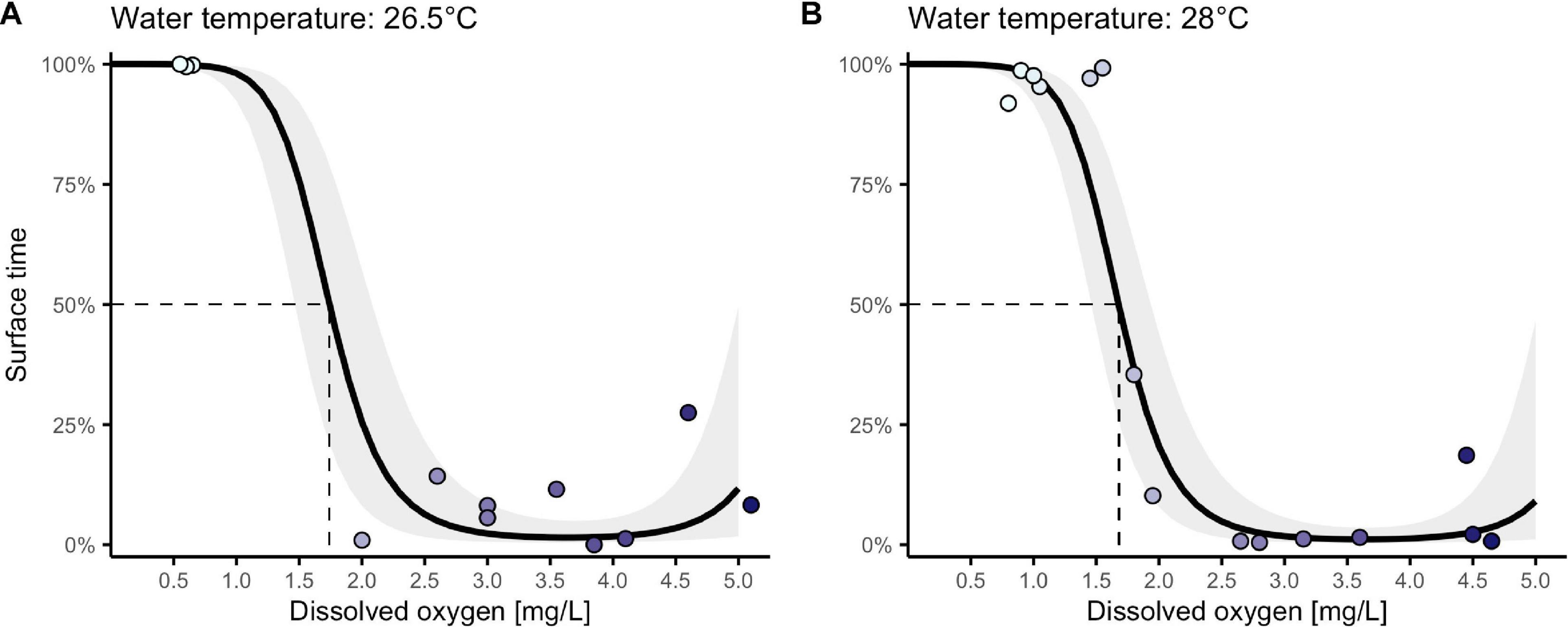
Figure 4. Hypoxia-driven ASR tendency of Poecilia sulphuraria for a morning (A) and midday temperature scenario (B). Time spent at the water surface performing ASR increased with decreasing oxygen concentrations at both test temperatures in a laboratory setting. Each data point equals the cumulative time spent at the surface for one group of 5 adult fish (n = 27 groups). Darker points represent data collected at higher levels of aquatic oxygen. The half-maximal concentration at which fish spend 50% of their time at the surface (ASR50) was estimated at 1.68 mg/L DO for 28°C and 1.74 mg/L DO for 26.5°C (M2-estimated trend lines in black).
In accordance with field observations, dive durations were shorter under severe hypoxia (i.e., <1.7 mg/L DO; mean ± SD: 7.1 ± 6.7s, n = 9) than under more favorable oxygen conditions (105.1 ± 83.1 s, n = 18; W = 0, p < 0.0001; Supplementary Figure 2A). This reduction in dive time was inversely proportional to surface time (Spearman’s rho = −0.86, S = 6089, p < 0.0001, n = 27), as fish also dove less with hypoxic stress (<1.7 mg/L DO: 10.4 ± 10.5, >1.7 mg/L DO: 23.7 ± 17.1; W = 38, p = 0.01; Supplementary Figure 2B).
This study revealed that predator-prey activity patterns in a bird-fish system from a sulfidic habitat are subject to a diurnal cycle, matching that of water temperature and dissolved oxygen. In a laboratory experiment, we disentangled that variation in DO drove changes in sulphur mollies’ surfacing behavior in a way that when oxygen levels dropped below 1.7 mg/L (i.e., daytime hypoxia) fish significantly increased the time proportion for aquatic surface respiration (ASR) and consequently reduced time in the water column (i.e., shorter-lasting but not more dives). Coinciding with predictions, activity of piscivorous birds was highest during times of high ASR tendency, suggesting that birds spent more time at the springs and launched more attacks when fish were concentrated at the surface. Despite some constraints with such correlative studies, our results provide first empirical evidence for a potential link between physicochemical water conditions, aquatic prey’s behavior and the activity of aerial predators.
The Baños del Azufre spring system is characterized by oscillations of temperature and dissolved oxygen (i.e., magnitude of ∼1°C and ∼1 mg/L DO). Many shallow waterbodies commonly experience periodic fluctuations which are predominately driven by biological processes, where submerged vegetation or algae blooms typically produce oxygen through photosynthesis during daytime and consume oxygen at night (i.e., daytime supersaturation and nighttime hypoxia; see French Camargue marshlands: Kersten et al., 1991; tidal creeks of Chesapeake Bay, United States: Shen et al., 2008). In the Baños system, where aquatic vegetation is absent, oxygen supply seems to be strongly driven by temperature-dependent solubility. Daytime hypoxia may be further aggravated by the presence of dense mats of sulfide-oxidizing bacteria, which increase their metabolic rate with temperature (Hotaling et al., 2019), yet the presence of dissolved hydrogen sulfide did not follow a diurnal rhythm suggesting that, at least during the day, the influx of H2S likely exceeded its consumption by bacterial activity.
Hypoxia and H2S elicit similar physiological and behavioral responses in fishes (Bagarinao, 1992) and through interactions with temperature induce even greater stress than reported for either stressor separately (Gee et al., 1978; Kramer and Mehegan, 1981; Skandalis et al., 2020). Our observations suggest diurnal fluctuations in DO concentration are a dominant factor driving the observed changes in fish’s diving behavior and associated ASR tendency. However, the relative importance of H2S, temperature or other factors such as food availability (which was not considered here), is still uncertain and will require further study. Our laboratory test indicates that the intensity with which sulphur mollies perform ASR is oxygen-driven with temperature having little effect. We attribute this to the relatively small temperature range tested here (26.5 versus 28°C), compared to the studies that found diurnal temperature effects on aquatic surface respiration in minnows (6–31°C; Gee et al., 1978) and guppies (25–32°C; Kramer and Mehegan, 1981). Nonetheless, for temperatures commonly experienced by sulphur mollies in the morning (26.5°C) or later parts of the day (28°C), responses were very similar and fish of either treatment spent the majority of their time at the surface below 1.7 mg/L DO (ASR50). Interestingly, similar thresholds have been established for the poeciliid Gambusia affinis both in the lab and in the field (i.e., ASR became obligatory below 1 and 2 mg/L DO, respectively; Cech et al., 1985; Kersten et al., 1991), but this species at least partially relied on ASR until values exceeded 3 mg/L (lab at 20°C) or even 6 mg/L DO (field during June/July in temperate climate). If temperature conditions were as high as in the present study, due to an increase in oxygen demand we would expect this species to rely on ASR at even higher DO concentrations. Hence, it is intriguing that, despite warm water temperatures, sulphur mollies do not seem to rely much on ASR at values above 2 mg/L and may in fact convey that many adaptations to tolerate hydrogen sulfide also improve hypoxic tolerance [especially morphological adaptations facilitating oxygen acquisition (Tobler and Hastings, 2011; Tobler et al., 2011), upregulation of oxygen transport genes (Barts et al., 2018), shifts toward anaerobic metabolism (Kelley et al., 2016) and reduced energy demand (Passow et al., 2017; Camarillo et al., 2020) as well as modulations of pathways maintaining mitochondrial function and aerobic ATP production (Pfenninger et al., 2014; Tobler et al., 2018; Greenway et al., 2020)].
Piscivorous bird activity peaked at times when fish showed reduced diving and hence were mostly aggregated at the surface. Many birds are easily disturbed by human-related presence and may leave an area or trade off foraging for increased vigilance (Burger and Gochfeld, 1998). Similarly, during periods of intense heat, bird’s foraging activity is often reduced or can cease completely (Edwards et al., 2015; Funghi et al., 2019). Nonetheless, we observed peak predator activity during the parts of the day with highest temperatures and increased human activity (noon and afternoon), rendering both unlikely explanations for the observed bird behavior in our system. While our study was not suitable to disentangle whether increased bird activity at the Baños del Azufre is due to a circadian rhythm or an exploitation of vulnerable prey, we argue in favor of the latter explanation. Herons, egrets, and kingfishers are the main predators in this system (Supplementary Table 1), all of which are visual hunters. As such, these species will maximize their foraging efforts during periods of good visibility and light conditions (mainly around midday). For avian predators, large surface aggregations are not only more easily accessible but also more conspicuous compared to times when these fish are less clustered (morning). On top, fish’s escape performance is likely limited during later parts of the day. Within an optimum range, swimming performance usually increases with temperature (Colchen et al., 2017), but hypoxia and high temperatures often act synergistically in hampering escape performance (Domenici et al., 2000, 2007; Lefrançois et al., 2005). This effect might be especially pronounced in sulphide-adapted fish, which are selected for more energy efficient swimming, resulting in a lower burst start performance compared to non-sulfidic populations (Camarillo et al., 2020). This line of evidence clearly suggests an interaction shift in favor of the avian predators with more severe hypoxia and as such is similar to observations made on little egrets (Egretta garzetta) in the Camargue that showed highest capture rates when mosquitofish performed ASR during morning hypoxia (Kersten et al., 1991). Through individual identification, future studies could examine whether birds frequently assess the quality of different foraging sites and only initiate foraging when profitability is high or if they have learned to predict when fish aggregate.
With an average of about 1 attack per minute, especially when DO levels dropped during the later parts of the day, our study confirmed the extreme predation pressure that sulphur mollies experience (see also Riesch et al., 2010a; Lukas et al., in review). This selection pressure likely promotes the formation of the dense, synchronized fish aggregations (Supplementary Video; Riesch et al., 2010a), which may help to reduce individual sulphur mollies’ predation risk through risk dilution, improved predator detection and predator confusion (Kramer and Graham, 1976; Krause and Ruxton, 2002). However, during times of reduced predation pressure (morning), costs of grouping may outweigh its benefits (e.g., foraging costs; Sogard and Olla, 1997) and thus favor solitary strategies. In fact, this balance might be further modulated by severe hypoxia rendering some solitary strategies (e.g., predator avoidance, escape and crypsis) less effective. As a result, sulphur mollies should flexibly adjust shoal density and anti-predator behavior during the day to match local conditions of predation intensity and hypoxia, which is an aspect of our research that will deserve further attention.
We note that all the information gathered here comes from the dry season (April–May), and it is essential to also understand the ecological pressures of P. sulphuraria during the rainy season. For example, sustained rain could majorly influence water chemistry and likely reduce hypoxic events, thus profoundly changing the system dynamics. On the other hand, rain also increases spring discharge so that the system would likely (with some lag) return to its former state. Anecdotal observations by one of the authors (DB) suggest sulphur mollies perform similar ASR behavior within dense shoals also in the rainy seasons (July to September), leading us to concur that this pattern is observable year-round in this species.
In summary, this study showcases a predator-prey system that is influenced by diurnal fluctuations in abiotic factors. Sulphur mollies face different selection pressures, namely hypoxia and predation, and adaptations to both have been found, highlighting their complex evolutionary trajectories. In this context, the sulfide spring systems of the Mexican states of Tabasco and Chiapas with their endemic fish fauna represent interesting natural laboratories to study periodicity, which is often not feasible in other ecosystems as they involve complex interactions and occur on much harder to capture temporal and spatial scales (e.g., population density cycles of wolf, elk and aspen (Fortin et al., 2005; Hebblewhite et al., 2005; Vucetich et al., 2005) or lynx and roe deer or hare (Nilsen et al., 2009; Lavergne et al., 2019)).
The raw data and analysis script supporting the conclusions of this article are available at: https://doi.org/10.6084/m9.figshare.14135045.
The animal study was reviewed and approved by the Mexican “Comisión Nacional de Acuacultura y Pesca” (CONAPESCA; DGOPA.09004.041111.3088, PRMN/DGOPA-003/2014, PRMN/DGOPA-009/2015, and PRMN/DGOPA-012/2017).
JL, DB, JK, PR, PK, and LA-R conducted the predator-prey survey. JL and DB performed the hypoxia experiment. JL and FA collected the water samples. FA and TG performed the physicochemical analyses of water samples. JL performed the statistical analysis and wrote the manuscript with input from all authors. All authors approved the final version of this manuscript.
This work was supported by the Elsa-Neumann-Scholarship of the state of Berlin (JL) and the German Research Foundation [DFG; BI 1828/3-1 (DB), RO 4766/2-1 (PR), EXC 2002/1 “Science of Intelligence” project 390523135 (JK, PR, and DB)]. We acknowledge support by the Open Access Publication Fund of the Humboldt-Universität zu Berlin.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the director and staff at the CIIEA Centro de Investigación e Innovación para la Enseñanza y el Aprendizaje field station for hosting our multiple research stays. We thank Marie Habedank, Carolina Doran, Joe Bak-Coleman, Alex Jourdan, David Lewis, Geoffrey Mazué, Marlene Sroka, Arabella Träger, Colin Twomey, Eloise Altmikus, and Kaya Martens for their assistance with pilot experiments in the field. Finally, we thank the editor and the two reviewers for their valuable comments and effort to improve this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.619193/full#supplementary-material
Bagarinao, T. (1992). Sulfide as an environmental factor and toxicant: tolerance and adaptations in aquatic organisms. Aquat. Toxicol. 24, 21–62. doi: 10.1016/0166-445x(92)90015-f
Bartoñ, K. (2020). MuMIn: Multi-Model Inference. Available online at: https://CRAN.R-project.org/package=MuMIn. (accessed March 1, 2021).
Barts, N., Greenway, R., Passow, C. N., Arias-Rodriguez, L., Kelley, J. L., and Tobler, M. (2018). Molecular evolution and expression of oxygen transport genes in livebearing fishes (Poeciliidae) from hydrogen sulfide rich springs. Genome 61, 273–286. doi: 10.1139/gen-2017-0051
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48.
Bernhardt, J. R., O’Connor, M. I., Sunday, J. M., and Gonzalez, A. (2020). Life in fluctuating environments. Phil. Trans. R Soc. B 375:20190454.
Bierbach, D., Krause, S., Romanczuk, P., Lukas, J., Arias-Rodriguez, L., and Krause, J. (2020). An interaction mechanism for the maintenance of fission–fusion dynamics under different individual densities. PeerJ 8:e8974. doi: 10.7717/peerj.8974
Bierbach, D., Lukas, J., Bergmann, A., Elsner, K., Höhne, L., Weber, C., et al. (2018). Insights into the social behavior of surface and cave-dwelling fish (Poecilia mexicana) in light and darkness through the use of a biomimetic robot. Front. Robot. AI 5:3.
Borowiec, B. G., O’Connor, C. M., Goodick, K., Scott, G. R., and Balshine, S. (2018). The preference for social affiliation renders fish willing to accept lower O2 levels. Physiol. Biochem. Zool. 91, 716–724. doi: 10.1086/695566
Brady, D. C., and Targett, T. E. (2013). Movement of juvenile weakfish cynoscion regalis and spot Leiostomus xanthurus in relation to diel-cycling hypoxia in an estuarine tidal tributary. Mar. Ecol. Prog. Ser. 491, 199–219. doi: 10.3354/meps10466
Brown, A. P., Arias-Rodriguez, L., Yee, M.-C., Tobler, M., and Kelley, J. L. (2018). Concordant changes in gene expression and nucleotides underlie independent adaptation to hydrogen-sulfide-rich environments. Genome Biol. Evol. 10, 2867–2881.
Brown, A. P., Greenway, R., Morgan, S., Quackenbush, C. R., Giordani, L., Arias-Rodriguez, L., et al. (2017). Genome-scale data reveal that endemic Poecilia populations from small sulphidic springs display no evidence of inbreeding. Mol. Ecol. 26, 4920–4934. doi: 10.1111/mec.14249
Burger, J., and Gochfeld, M. (1998). Effects of ecotourists on bird behaviour at loxahatchee national wildlife refuge, Florida. Envir. Conserv. 25, 13–21. doi: 10.1017/s0376892998000058
Burnham, K. P., Anderson, D. R., and Huyvaert, K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35. doi: 10.1007/s00265-010-1029-6
Camarillo, H., Rodriguez, L. A., and Tobler, M. (2020). Functional consequences of phenotypic variation between locally adapted populations: swimming performance and ventilation in extremophile fish. J. Evol. Biol. 33, 512–523. doi: 10.1111/jeb.13586
Cech, J. J., Massingill, M. J., Vondracek, B., and Linden, A. L. (1985). Respiratory metabolism of mosquitofish, Gambusia affinis: effects of temperature, dissolved oxygen, and sex difference. Environ. Biol. Fish. 13, 297–307. doi: 10.1007/bf00002914
Chapman, L. J., and Chapman, C. A. (1994). Observations on synchronous air breathing in Clarias liocephalus. Copeia 1994, 246–249. doi: 10.2307/1446696
Chapman, L. J., Kaufman, L. S., Chapman, C. A., and Mckenzie, F. E. (1995). Hypoxia tolerance in twelve species of east african cichlids: potential for low oxygen refugia in lake victoria. Conserv. Biol. 9, 1274–1288. doi: 10.1046/j.1523-1739.1995.9051262.x-i1
Cherry, M. J., and Barton, B. T. (2017). Effects of wind on predator-prey interactions. Food Webs 13, 92–9705. doi: 10.1016/j.fooweb.2017.02.005
Cockrell, B. J. (1984). Effects of temperature and oxygenation on predator-prey overlap and prey choice of notonecta glauca. J. Anim. Ecol. 53, 519–532. doi: 10.2307/4531
Colchen, T., Teletchea, F., Fontaine, P., and Pasquet, A. (2017). Temperature modifies activity, inter-individual relationships and group structure in a fish. Curr. Zool. 63, 175–183.
Culumber, Z. W., Hopper, G. W., Barts, N., Passow, C. N., Morgan, S., Brown, A., et al. (2016). Habitat use by two extremophile, highly endemic, and critically endangered fish species (Gambusia eurystoma and Poecilia sulphuraria. Poeciliidae). Aquat. Conserv. 26, 1155–1167. doi: 10.1002/aqc.2640
Domenici, P., Lefrançois, C., and Shingles, A. (2007). Hypoxia and the antipredator behaviours of fishes. Phil. Trans. R. Soc. B 362, 2105–2121. doi: 10.1098/rstb.2007.2103
Domenici, P., Steffensen, J. F., and Batty, R. S. (2000). The effect of progressive hypoxia on swimming activity and schooling in Atlantic herring. J. Fish. Biol. 57, 1526–1538. doi: 10.1111/j.1095-8649.2000.tb02229.x
Eby, L. A., and Crowder, L. B. (2002). Hypoxia-based habitat compression in the neuse river estuary: context-dependent shifts in behavioral avoidance thresholds. Can. J. Fish. Aquat. Sci. 59, 952–965. doi: 10.1139/f02-067
Edwards, E. K., Mitchell, N. J., and Ridley, A. R. (2015). The impact of high temperatures on foraging behaviour and body condition in the Western Australian Magpie Cracticus tibicen dorsalis. Ostrich 86, 137–144. doi: 10.2989/00306525.2015.1034219
Fortin, D., Beyer, H. L., Boyce, M. S., Smith, D. W., Duchesne, T., and Mao, J. S. (2005). Wolves influence elk movements: behavior shapes a trophic cascade in yellowstone national park. Ecology 86, 1320–1330. doi: 10.1890/04-0953
Funghi, C., McCowan, L. S. C., Schuett, W., and Griffith, S. C. (2019). High air temperatures induce temporal, spatial and social changes in the foraging behaviour of wild zebra finches. Anim. Behav. 149, 33–43. doi: 10.1016/j.anbehav.2019.01.004
Galic, N., Hawkins, T., and Forbes, V. E. (2019). Adverse impacts of hypoxia on aquatic invertebrates: a meta-analysis. Sci. Total Environ. 652, 736–743. doi: 10.1016/j.scitotenv.2018.10.225
Gee, J. H., Tallman, R. F., and Smart, H. J. (1978). Reactions of some great plains fishes to progressive hypoxia. Can. J. Zool. 56, 1962–1966. doi: 10.1139/z78-263
Greenway, R. (2019). From Genomes to Genitalia: Ecological Speciation in Sulfide Spring Fishes. Available online at: https://krex.k-state.edu/dspace/handle/2097/39817 (accessed March 1, 2021).
Greenway, R., Barts, N., Henpita, C., Brown, A. P., Rodriguez, L. A., Rodríguez Peña, C. M., et al. (2020). Convergent evolution of conserved mitochondrial pathways underlies repeated adaptation to extreme environments. PNAS 117, 16424–16430. doi: 10.1073/pnas.2004223117
Harrison, X. A. (2015). A comparison of observation-level random effect and Beta-binomial models for modelling overdispersion in binomial data in ecology & evolution. PeerJ 3:e1114. doi: 10.7717/peerj.1114
Hebblewhite, M., White, C. A., Nietvelt, C. G., McKenzie, J. A., Hurd, T. E., Fryxell, J. M., et al. (2005). Human activity mediates a trophic cascade caused by wolves. Ecology 86, 2135–2144. doi: 10.1890/04-1269
Hedges, K. J., and Abrahams, M. V. (2015). Hypoxic refuges, predator-prey interactions and habitat selection by fishes: hypoxia-induced refuges. J. Fish. Biol. 86, 288–303. doi: 10.1111/jfb.12585
Hotaling, S., Quackenbush, C. R., Bennett-Ponsford, J., New, D. D., Arias-Rodriguez, L., Tobler, M., et al. (2019). Bacterial diversity in replicated hydrogen sulfide-rich streams. Microb. Ecol. 77, 559–573. doi: 10.1007/s00248-018-1237-6
Kelley, J. L., Arias-Rodriguez, L., Patacsil Martin, D., Yee, M.-C., Bustamante, C. D., and Tobler, M. (2016). Mechanisms underlying adaptation to life in hydrogen sulfide–rich environments. Mol. Biol. Evol. 33, 1419–1434. doi: 10.1093/molbev/msw020
Kersten, M., Britton, R. H., Dugan, P. J., and Hafner, H. (1991). Flock feeding and food intake in little egrets: the effects of prey distribution and behaviour. J. Anim. Ecol. 60, 241–252. doi: 10.2307/5457
Killen, S. S., Esbaugh, A. J., Martins, N. F., Tadeu Rantin, F., and McKenzie, D. J. (2018). Aggression supersedes individual oxygen demand to drive group air-breathing in a social catfish. J. Anim. Ecol. 87, 223–234. doi: 10.1111/1365-2656.12758
Kramer, D. L. (1983). The evolutionary ecology of respiratory mode in fishes: an analysis based on the costs of breathing. Environ. Biol. Fish. 9, 145–158. doi: 10.1007/bf00690859
Kramer, D. L. (1987). Dissolved oxygen and fish behavior. Environ. Biol. Fish. 18, 81–92. doi: 10.1007/bf00002597
Kramer, D. L., and Graham, J. B. (1976). Synchronous air breathing, a social component of respiration in fishes. Copeia 1976, 689–697. doi: 10.2307/1443450
Kramer, D. L., and McClure, M. (1982). Aquatic surface respiration, a widespread adaptation to hypoxia in tropical freshwater fishes. Environ. Biol. Fish. 7, 47–55. doi: 10.1007/bf00011822
Kramer, D. L., and Mehegan, J. P. (1981). Aquatic surface respiration, an adaptive response to hypoxia in the guppy, Poecilia reticulata (Pisces, Poeciliidae). Environ. Biol. Fish. 6, 299–313. doi: 10.1007/bf00005759
Kramer, D. L., Manley, D., and Bourgeois, R. (1983). The effect of respiratory mode and oxygen concentration on the risk of aerial predation in fishes. Can. J. Zool. 61, 653–665. doi: 10.1139/z83-087
Lavergne, S. G., Smith, K., Kenney, A., Krebs, C. J., Palme, R., and Boonstra, R. (2019). Physiology and behaviour of juvenile snowshoe hares at the start of the 10-year cycle. Anim. Behav. 157, 141–152. doi: 10.1016/j.anbehav.2019.09.003
Lefrançois, C., Shingles, A., and Domenici, P. (2005). The effect of hypoxia on locomotor performance and behaviour during escape in Liza aurata. J. Fish. Biol. 67, 1711–1729. doi: 10.1111/j.1095-8649.2005.00884.x
Long, W. C., and Seitz, R. D. (2008). Trophic interactions under stress: hypoxia enhances foraging in an estuarine food web. Mar. Ecol. Prog. Ser. 362, 59–68. doi: 10.3354/meps07395
Nadler, L. E., Killen, S. S., McClure, E. C., Munday, P. L., and McCormick, M. I. (2016). Shoaling reduces metabolic rate in a gregarious coral reef fish species. J. Exp. Biol. 219, 2802–2805. doi: 10.1242/jeb.139493
Nilsen, E. B., Linnell, J. D. C., Odden, J., and Andersen, R. (2009). Climate, season, and social status modulate the functional response of an efficient stalking predator: the Eurasian lynx. J. Anim. Ecol. 78, 741–751. doi: 10.1111/j.1365-2656.2009.01547.x
Palacios, M., Arias-Rodriguez, L., Plath, M., Eifert, C., Lerp, H., Lamboj, A., et al. (2013). The rediscovery of a long described species reveals additional complexity in speciation patterns of poeciliid fishes in sulfide springs. PLoS One 8:e71069. doi: 10.1371/journal.pone.0071069
Passow, C. N., Arias-Rodriguez, L., and Tobler, M. (2017). Convergent evolution of reduced energy demands in extremophile fish. PLoS One 12:e0186935. doi: 10.1371/journal.pone.0186935
Pfenninger, M., Lerp, H., Tobler, M., Passow, C., Kelley, J. L., Funke, E., et al. (2014). Parallel evolution of cox genes in H2S-tolerant fish as key adaptation to a toxic environment. Nat. Comm. 5:3873.
Pihl, L., Baden, S. P., and Diaz, R. J. (1991). Effects of periodic hypoxia on distribution of demersal fish and crustaceans. Mar. Biol. 108, 349–360. doi: 10.1007/bf01313644
Plath, M., Tobler, M., Riesch, R., García, de León, F. J., Giere, O., et al. (2007). Survival in an extreme habitat: the roles of behaviour and energy limitation. Naturwissenschaften 94, 991–996. doi: 10.1007/s00114-007-0279-2
Pollock, M. S., Clarke, L. M. J., and Dubé, M. G. (2007). The effects of hypoxia on fishes: from ecological relevance to physiological effects. Envrion. Rev. 15, 1–14. doi: 10.1139/a06-006
Poulin, R., Wolf, N. G., and Kramer, D. L. (1987). The effect of hypoxia on the vulnerability of guppies (Poecilia reticulata, Poeciliidae) to an aquatic predator (Astronotus ocellatus, Cichlidae). Environ. Biol. Fish. 20, 285–292. doi: 10.1007/bf00005299
Queiroz, H., and Magurran, A. E. (2005). Safety in numbers? shoaling behaviour of the amazonian red-bellied piranha. Biol. Lett. 1, 155–157. doi: 10.1098/rsbl.2004.0267
R Core Team. (2020). R: A Language and Environment for Statistical Computing. Vienna: Austria: R Foundation for Statistical Computing.
Riesch, R., Duwe, V., Herrmann, N., Padur, L., Ramm, A., Scharnweber, K., et al. (2009). Variation along the shy–bold continuum in extremophile fishes (Poecilia mexicana, Poecilia sulphuraria). Behav. Ecol. Sociobiol. 63, 1515–1526. doi: 10.1007/s00265-009-0780-z
Riesch, R., Oranth, A., Dzienko, J., Karau, N., Schießl, A., Stadler, S., et al. (2010a). Extreme habitats are not refuges: poeciliids suffer from increased aerial predation risk in sulphidic southern Mexican habitats. Biol. J. Linn. Soc. 101, 417–426. doi: 10.1111/j.1095-8312.2010.01522.x
Riesch, R., Plath, M., García, de León, F. J., and Schlupp, I. (2010b). Convergent life-history shifts: toxic environments result in big babies in two clades of poeciliids. Naturwissenschaften 97, 133–141. doi: 10.1007/s00114-009-0613-y
Rosales Lagarde, L., Boston, P. J., Campbell, A. R., Hose, L. D., Axen, G., and Stafford, K. W. (2014). Hydrogeology of northern Sierra de Chiapas, Mexico: a conceptual model based on a geochemical characterization of sulfide-rich karst brackish springs. Hydrogeol. J. 22, 1447–1467. doi: 10.1007/s10040-014-1135-z
Schleuter, D., Haertel-Borer, S., Fischer, P., and Eckmann, R. (2007). Respiration rates of eurasian perch perca fluviatilis and ruffe: lower energy costs in groups. Trans. Am. Fish. Soc. 136, 43–55. doi: 10.1577/t06-123.1
Shen, J., Wang, T., Herman, J., Mason, P., and Arnold, G. L. (2008). Hypoxia in a coastal embayment of the chesapeake bay: a model diagnostic study of oxygen dynamics. Estuaries Coast 31, 652–663. doi: 10.1007/s12237-008-9066-3
Skandalis, D. A., Dobell, C. D., Shaw, J. C., and Tattersall, G. J. (2020). Hydrogen sulfide exposure reduces thermal set point in zebrafish. Roy. Soc. Open Sci. 7:200416. doi: 10.1098/rsos.200416
Sogard, S. M., and Olla, B. L. (1997). The influence of hunger and predation risk on group cohesion in a pelagic fish, walleye pollock Theragra chalcogramma. Environ. Biol. Fish. 50, 405–413. doi: 10.1023/a:1007393307007
Stumm, W., and Morgan, J. J. (1996). Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd Edn. New York: John Wiley & Sons.
Timmerman, C. M., and Chapman, L. J. (2004). Behavioral and physiological compensation for chronic hypoxia in the sailfin molly (Poecilia latipinna). Physiol. Biochem. Zool. 77, 601–610. doi: 10.1086/421754
Tobler, M., and Hastings, L. (2011). Convergent patterns of body shape differentiation in four different clades of poeciliid fishes inhabiting sulfide springs. Evol. Biol. 38, 412–42124. doi: 10.1007/s11692-011-9129-4
Tobler, M., Kelley, J. L., Plath, M., and Riesch, R. (2018). Extreme environments and the origins of biodiversity: adaptation and speciation in sulfide spring fishes. Mol. Ecol. 27, 843–859. doi: 10.1111/mec.14497
Tobler, M., Palacios, M., Chapman, L. J., Mitrofanov, I., Bierbach, D., Plath, M., et al. (2011). Evolution in extreme environments: replicated phenotypic differentiation in livebearing fish inhabiting sulfidic springs. Evolution 65, 2213–2228. doi: 10.1111/j.1558-5646.2011.01298.x
Tobler, M., Riesch, R. W., Tobler, C. M., and Plath, M. (2009). Compensatory behaviour in response to sulphide-induced hypoxia affects time budgets, feeding efficiency, and predation risk. Evol. Ecol. Res. 11, 935–948.
Tobler, M., Schlupp, I., Heubel, K. U., Riesch, R., De León, F. J. G., Giere, O., et al. (2006). Life on the edge: hydrogen sulfide and the fish communities of a Mexican cave and surrounding waters. Extremophiles 10, 577–585. doi: 10.1007/s00792-006-0531-2
Turner, K. L., Abernethy, E. F., Conner, L. M., Rhodes, O. E., and Beasley, J. C. (2017). Abiotic and biotic factors modulate carrion fate and vertebrate scavenging communities. Ecology 98, 2413–2424. doi: 10.1002/ecy.1930
Van de Meutter, F., Meester, L. D., and Stoks, R. (2005). Water turbidity affects predator–prey interactions in a fish–damselfly system. Oecologia 144, 327–336. doi: 10.1007/s00442-005-0050-3
Vucetich, J. A., Smith, D. W., and Stahler, D. R. (2005). Influence of harvest, climate and wolf predation on Yellowstone elk, 1961-2004. Oikos 111, 259–270. doi: 10.1111/j.0030-1299.2005.14180.x
Wannamaker, C. M., and Rice, J. A. (2000). Effects of hypoxia on movements and behavior of selected estuarine organisms from the southeastern United States. J. Exp. Mar. Biol. Ecol. 249, 145–163. doi: 10.1016/s0022-0981(00)00160-x
Wolf, N. G., and Kramer, D. L. (1987). Use of cover and the need to breathe: the effects of hypoxia on vulnerability of dwarf gouramis to predatory snakeheads. Oecologia 73, 127–132. doi: 10.1007/bf00376988
Keywords: predator-prey interactions, bird predation, poeciliidae, hypoxia, hydrogen-sulfide
Citation: Lukas J, Auer F, Goldhammer T, Krause J, Romanczuk P, Klamser P, Arias-Rodriguez L and Bierbach D (2021) Diurnal Changes in Hypoxia Shape Predator-Prey Interaction in a Bird-Fish System. Front. Ecol. Evol. 9:619193. doi: 10.3389/fevo.2021.619193
Received: 19 October 2020; Accepted: 23 February 2021;
Published: 18 March 2021.
Edited by:
Andrea S. Aspbury, Texas State University, United StatesReviewed by:
Michael Tobler, Kansas State University, United StatesCopyright © 2021 Lukas, Auer, Goldhammer, Krause, Romanczuk, Klamser, Arias-Rodriguez and Bierbach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juliane Lukas, Y29udGFjdEBqdWxpYW5lbHVrYXMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.