
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol. , 13 April 2021
Sec. Conservation and Restoration Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.613172
This article is part of the Research Topic Avian Biodiversity Collapse in the Anthropocene: Drivers and Consequences View all 17 articles
 Ding Li Yong1*
Ding Li Yong1* Wieland Heim2
Wieland Heim2 Sayam U. Chowdhury3,4
Sayam U. Chowdhury3,4 Chang-Yong Choi5
Chang-Yong Choi5 Pavel Ktitorov6
Pavel Ktitorov6 Olga Kulikova6
Olga Kulikova6 Alexander Kondratyev6
Alexander Kondratyev6 Philip D. Round7
Philip D. Round7 Desmond Allen8
Desmond Allen8 Colin R. Trainor9
Colin R. Trainor9 Luke Gibson10
Luke Gibson10 Judit K. Szabo9,11
Judit K. Szabo9,11With nearly 400 migratory landbird species, the East Asian Flyway is the most diverse of the world’s flyways. This diversity is a consequence of the varied ecological niches provided by biomes ranging from broadleaf forests to arctic tundra and accentuated by complex biogeographic processes. The distribution and migration ecology of East Asian landbirds is still inadequately known, but a recent explosion in the number of studies tracking the migration of raptors, cuckoos, kingfishers and passerines has greatly increased our knowledge about the stopover and wintering ecology of many species, and the migratory routes that link northeast Eurasia and the Asian tropics. Yet the East Asian Flyway also supports the highest number of threatened species among flyways. Strong declines have been detected in buntings (Emberizidae) and other long-distance migrants. While the conservation of migratory landbirds in this region has largely focused on unsustainable hunting, there are other threats, such as habitat loss and increased agro-chemical use driven directly by land cover change and climate-related processes. Important knowledge gaps to be addressed include (1) threats affecting species in different parts of their annual cycle, (2) range-wide population trends, (3) ecological requirements and habitat use during the non-breeding season, and (4) the conservation status of critical wintering sites (including understudied farming landscapes, such as rice fields) and migration bottlenecks along the flyway.
The decline of migratory species is a global conservation concern (Wilcove and Wikelski, 2008) across the world’s major flyways (Lloyd-Evans and Atwood, 2004; Galbraith et al., 2014; Beresford et al., 2019). Migratory species declines and the associated drivers are well documented for birds that migrate between North America and the Neotropics (Robinson and Wilcove, 1994; Lloyd-Evans and Atwood, 2004; Bennett et al., 2018) and for species that breed in Europe and winter in Sub-Saharan Africa (Bibby, 1992; Sanderson et al., 2006; Vickery et al., 2014; Beresford et al., 2019), but less so for other regions. The decline of many species has been linked to anthropogenic changes in habitat conditions, such as habitat loss and fragmentation (Robinson and Wilcove, 1994) intensified by climate change (Lloyd-Evans and Atwood, 2004; Sanderson et al., 2006; Vickery et al., 2014; Gilroy et al., 2016). Over time, climate change is expected to disrupt ecological and environmental processes that may affect migration and reproduction, for instance, by driving spatio-temporal mismatches in food resources (Carey, 2009). Given that migratory species have geographically complex life cycles that connect breeding, stopover and non-breeding sites over large areas, they are especially vulnerable to threats along their migratory routes (Runge et al., 2014, 2015).
The East Asian Flyway (EAF) as defined here overlaps with the eastern hemisphere (east of the 90th meridian), including the western Pacific Ocean (McClure, 1974, 1984; Nisbet, 1976; Yong et al., 2015), and spans arctic, boreal, temperate and tropical biomes (Figure 1). Compared to the African-Eurasian and the American flyways, the EAF is geographically more varied with an uneven distribution of landmass across boreal-temperate and tropical latitudes (Nisbet, 1976). While there are few ecological barriers in the west in the form of mountain ranges, the EAF contains extensive island chains at its eastern fringe and the vast Indo-Australian archipelago spanning the tropical belt. These island chains collectively pose complex barriers in the form of sea-crossings for migrating landbirds (Kuroda, 1961; Ellis et al., 1990; Yamazaki et al., 2012; Nourani et al., 2018) unmatched in other flyways.
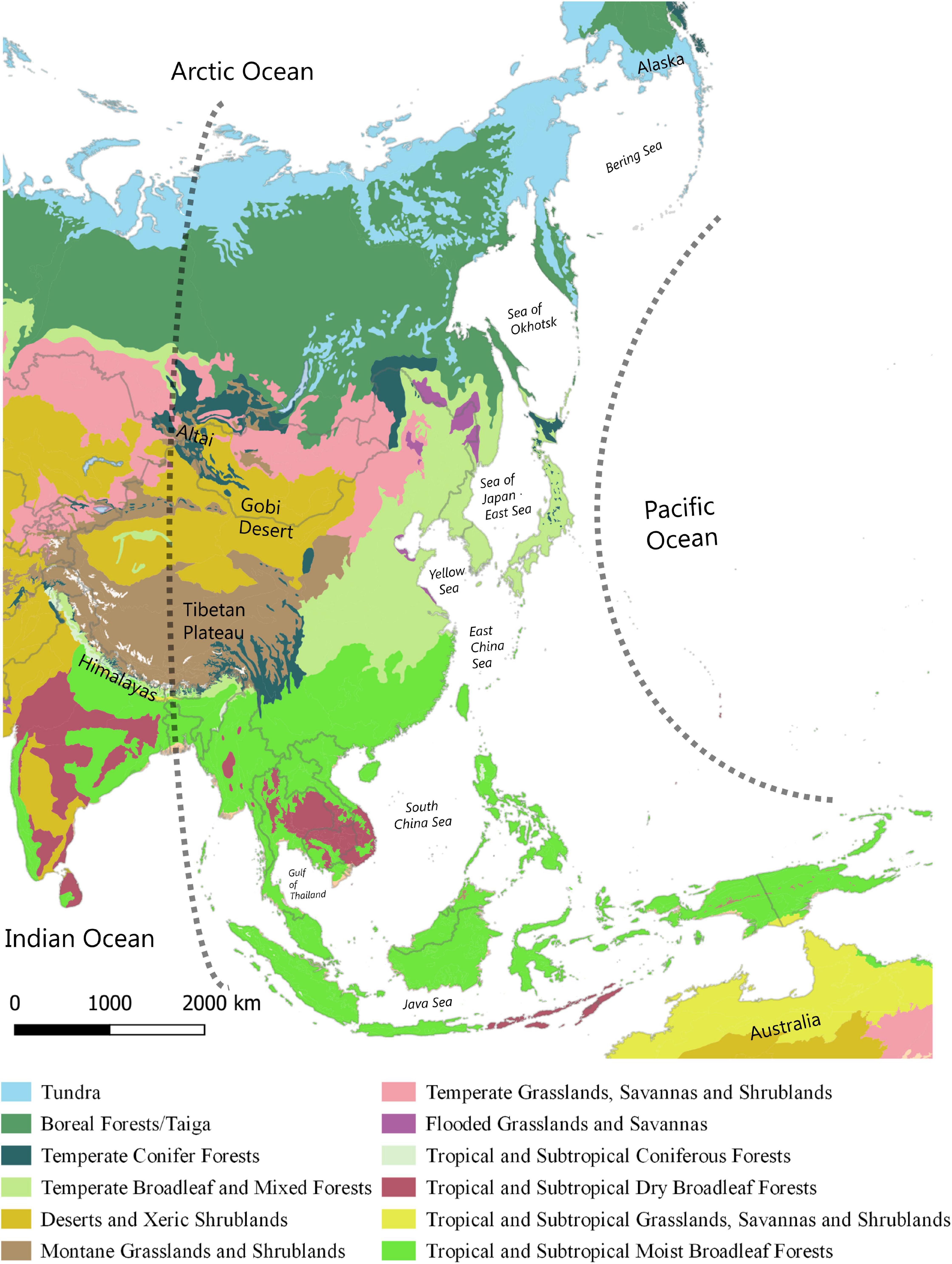
Figure 1. The extent of the East Asian Flyway (EAF) bounded by the 90th meridian on the west and the Pacific Ocean on the east including boreal, temperate and tropical biomes. Data from The Nature Conservancy. Terrestrial ecoregions (2020). https://tnc.maps.arcgis.com/home/item.html?id=7b7fb9d945544d41b3e7a914 94c42930 (accessed May 10, 2020).
With almost 400 species (387 species [63% of all migratory species]; see also Appendix I for full list), the bulk of EAF migratory species are in fact landbirds, with passerines and diurnal raptors among the most species-rich groups (McClure, 1974; Yamazaki et al., 2012; Yong et al., 2015). In this flyway, migratory landbirds fulfill diverse ecological roles in boreal, temperate and tropical biomes in terms of their trophic positions (e.g., herbivory, insectivory and predation) and transport processes (e.g., nutrient and parasite transfer, seed and pollen dispersal; see Şekercioğlu et al., 2004; Bauer and Hoye, 2014; López-Hoffman et al., 2017). Across East Asia, migratory landbirds consume and disperse the fruits and seeds of varied tree species, as evidenced by studies in Japan, China and South Korea (Nakanishi, 1996; Corlett, 1998; Choi and Chae, 2007; Cho et al., 2011; Choi et al., 2011; Jeong et al., 2014). Insectivorous species provide significant pest-control services to agroecosystems (Kim et al., 2012b) and directly reduce disease transmission through predation and scavenging (Kim et al., 2012a). Yet, despite their contribution to ecosystems at a large scale in East and Southeast Asia, landbirds are the least understood among the diverse migratory bird assemblages in Asia (Yong et al., 2015; Heim et al., 2020).
The declines in populations of many migratory waterbirds, and especially shorebirds, across East Asia and Australasia have been well documented (Szabo et al., 2016; Studds et al., 2017) and are far better understood than landbird declines. However, Emberiza buntings and many other East Asian landbirds have suffered dramatic population declines in recent years (Kamp et al., 2015; Edenius et al., 2017; Choi et al., 2020a), driven in part by habitat loss (Higuchi and Morishita, 1999) and unsustainable hunting (Kamp et al., 2015). Meanwhile, the lack of understanding of regional population trends and status of many East and Southeast Asian species continues to hamper conservation actions targeting landbird species. They are similarly affected by inconsistent and incomplete levels of protection offered by national and international legal frameworks for wildlife conservation (Runge et al., 2015; Yong et al., 2015, 2018).
Since 2010, growing interest in the ecology and conservation of migratory landbirds in the EAF has yielded fresh insights on their migration ecology and migratory connectivity (Yamaura et al., 2017; Heim et al., 2018b, 2020; Choi et al., 2020b; Uemura et al., 2020), species-specific population declines (Tamada et al., 2014, 2017; Kamp et al., 2015; Edenius et al., 2017; Choi et al., 2020a), and threats (Amano and Yamaura, 2007; Iqbal et al., 2014; Kamp et al., 2015). While large-bodied hawks and eagles (Accipitriformes) have traditionally been easier to study during migration (Higuchi et al., 2005; Reading et al., 2020) and at key bottleneck sites (Zalles and Bildstein, 2000; Yamazaki et al., 2012; DeCandido et al., 2015; Limparungpatthanakij et al., 2019), studies on migratory passerines and other landbirds are fast catching up thanks to emerging tracking technology and methods.
Against this ‘migratory landbird renaissance,’ we aim to synthesize existing knowledge on the conservation status of migratory landbirds (see Table 1 for definitions) in the EAF. We also identify key knowledge gaps for further work in this traditionally data-poor migratory flyway. We define the taxonomic scope of landbirds in our review based on the widely accepted definitions used in the literature (Kirby et al., 2008; Faaborg et al., 2010), which encompasses passerines, raptors and several near-passerine families (see Supplementary Data for full taxonomic scope). We (1) summarize recent advances in landbird migration ecology research in the EAF, (2) review emerging evidence of declines and associated drivers in key biomes, (3) discuss the ecological implications of these changes on Asian ecosystems in the wider context of land use change and unsustainable hunting, and (4) identify knowledge gaps and describe how addressing them can translate into conservation actions.
Understanding the different migration strategies used by landbirds is crucial to conservation at sites used over the annual cycle of migratory birds. Building on long-term bird ringing work in Japan, the Russian Far East, China and South Korea (reviewed in McClure, 1974; Yong et al., 2015), several new large-scale ringing projects have been established in this region, for instance in India and Mongolia (Buner et al., 2015; Davaasuren, 2018).
In general, the migration strategy of a species is fundamentally shaped by its habitat requirements and physiology. Many species that cover long distances need to refuel several times to replenish fat reserves before reaching their wintering grounds (Bairlein, 2002), and thus the availability of suitable stopover habitat is critical (Warnock, 2010). Ongoing studies at key stopover sites for landbirds at Muravioka Park in southeast Russia, in China, on various islands off South Korea and elsewhere in the region are beginning to unveil the diverse migration strategies (Nam et al., 2011; Heim et al., 2018b). Sander et al. (2017, 2020) describe the different strategies adopted by warblers to increase their fat stores during autumn migration: Pallas’s leaf warbler Phylloscopus proregulus carries more fat, fuelling longer flight bouts with fewer stops. Others, such as the thick-billed warbler Arundinax aedon, carry less fat, necessitating more frequent stopovers on migration (Bozó et al., 2019; Sander et al., 2020).
Stopover habitats utilized by landbirds range from old-growth, deciduous forests (Tojo, 2015) to a variety of human-modified landscapes and habitats (Kim et al., 2010). While many migratory landbird species may occupy a broader or different niche away from the breeding areas, some rely on similar environmental conditions throughout their annual cycle (Zurell et al., 2018). Phylloscopus, Acrocephalus and Locustella warbler species are among the most abundant landbirds in East Asia, and show species-specific habitat preferences at both breeding and stopover sites (Bozó et al., 2018b). On the other hand, Emberiza buntings often show a large overlap in habitat use at stopover sites (Heim et al., 2018a).
While migration strategies undoubtedly vary widely across taxa, temporal differences in migration within a species over different seasons have also been described. For instance, stopovers for many species (e.g., yellow-browed warbler Phylloscopus inornatus and red-flanked bluetail Tarsiger cyanurus) have been found to be considerably shorter in the spring than in the autumn, suggesting faster spring migration (Wang et al., 2006; Bozó et al., 2021), a generally well-described pattern for many migratory species (Kokko, 1999; Smith and Moore, 2005; Nilsson et al., 2013). This may be explained by competition to arrive at the breeding grounds before conspecifics (Nilsson et al., 2013). On the other hand, no significant differences between spring and autumn migration speed/duration are evident in non-passerines, e.g., in satellite-tracked Oriental honey buzzard Pernis ptilorhynchus (Yamaguchi et al., 2008) and great bustard Otis tarda (Kessler et al., 2013). In addition, specific morphological and behavioral adaptations have been found across various model species in the EAF that improve the efficiency of long migratory flights (Bozó et al., 2018a; Wang et al., 2020).
Analyses of the arrival and departure times of landbirds at key stopover sites have shown that migration distance and the relative locations of the breeding and wintering grounds determine the migratory phenology of Emberiza and Phylloscopus species and Siberian rubythroat Calliope calliope in the Russian Far East (Nam et al., 2011; Maslovsky et al., 2014, 2018; Bozó and Heim, 2015, 2016; Bozó et al., 2017, 2019; Heim et al., 2018a; Park et al., 2020; Wobker et al., 2021). Long-distance migrants generally arrive at stopover sites later in spring, while departing earlier in autumn, and of these, species breeding at higher latitudes tend to pass earlier during spring migration (Wobker et al., 2021). Additionally, biological factors, such as sex, age and the timing of molt are all known to influence migration phenology in many passerines (Maslovsky et al., 2018; Park et al., 2020; Wobker et al., 2021), with protandry being a common pattern (Nam et al., 2011; Bozó and Heim, 2016; Park et al., 2020).
Monitoring migration phenology is important, given that the ability of a species to adjust its migration timing to events along its annual cycle can indicate its vulnerability to climate change (Møller et al., 2008). Valchuk et al. (2017) suggested that links between the West Pacific climate index and the abundance of migrating landbirds at a stopover site in the Russian Far East can indicate the effects of climatic shifts on migration phenology at a broad scale. Indeed, long-term studies have revealed shifts in spring arrival times for several species in Japan, linked to warmer temperatures (Deguchi et al., 2015), consistent with observations in Europe and the Americas (Thorup et al., 2007; Hurlbert and Liang, 2012).
Studying the migratory connectivity of a species is necessary to understand its migration ecology (Marra et al., 2011), and holds important implications for its conservation. Data from ringing recoveries, increasingly complemented with data obtained from various tracking technologies (e.g., light-level geolocators and satellite telemetry) have traditionally been used to map migration routes, and infer migratory connectivity for species and populations (Finch et al., 2015; Heim et al., 2020; Figure 2A). As smaller devices become increasingly available, various small-bodied landbirds can now be effectively tracked. Over the past decade, the number of landbird species tracked in the EAF has steadily risen to at least 26 species, with an increasing number of small passerines (Figures 2A,B and Table 2).
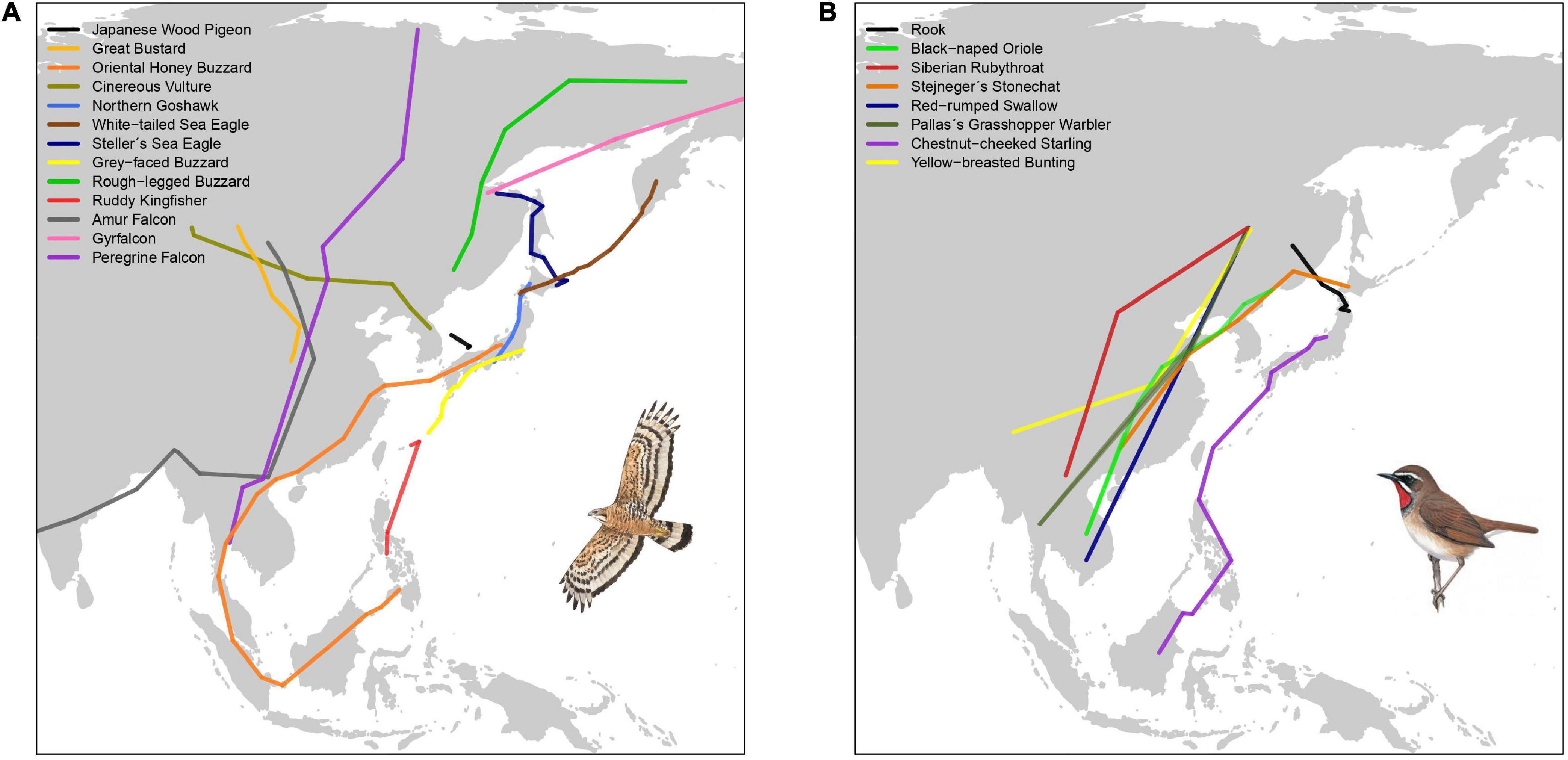
Figure 2. Autumn migration routes of landbirds in the EAF, including one individual track for each all species for which published data is available (n = 21). (A) Non-passerines (illustration of Oriental honey buzzard Pernis ptilorhynchus), (B) Passerines (illustration of Siberian rubythroat Calliope calliope). Based on Ueta et al. (1998, 2000), Higuchi et al. (2005), Shiu et al. (2006), Kim et al. (2007), Kudo (2008), McIntyre et al. (2009), Dixon et al. (2011, 2012), Kessler et al. (2013), Takagi et al. (2014), Koike et al. (2016), Yamaura et al. (2017), Yamaguchi et al. (2017), Heim et al. (2018a, 2020), Choi et al. (2019), and Uemura et al. (2020). Bird illustrations reproduced with permission of Lynx Edicions/Handbook of the Birds of the World.
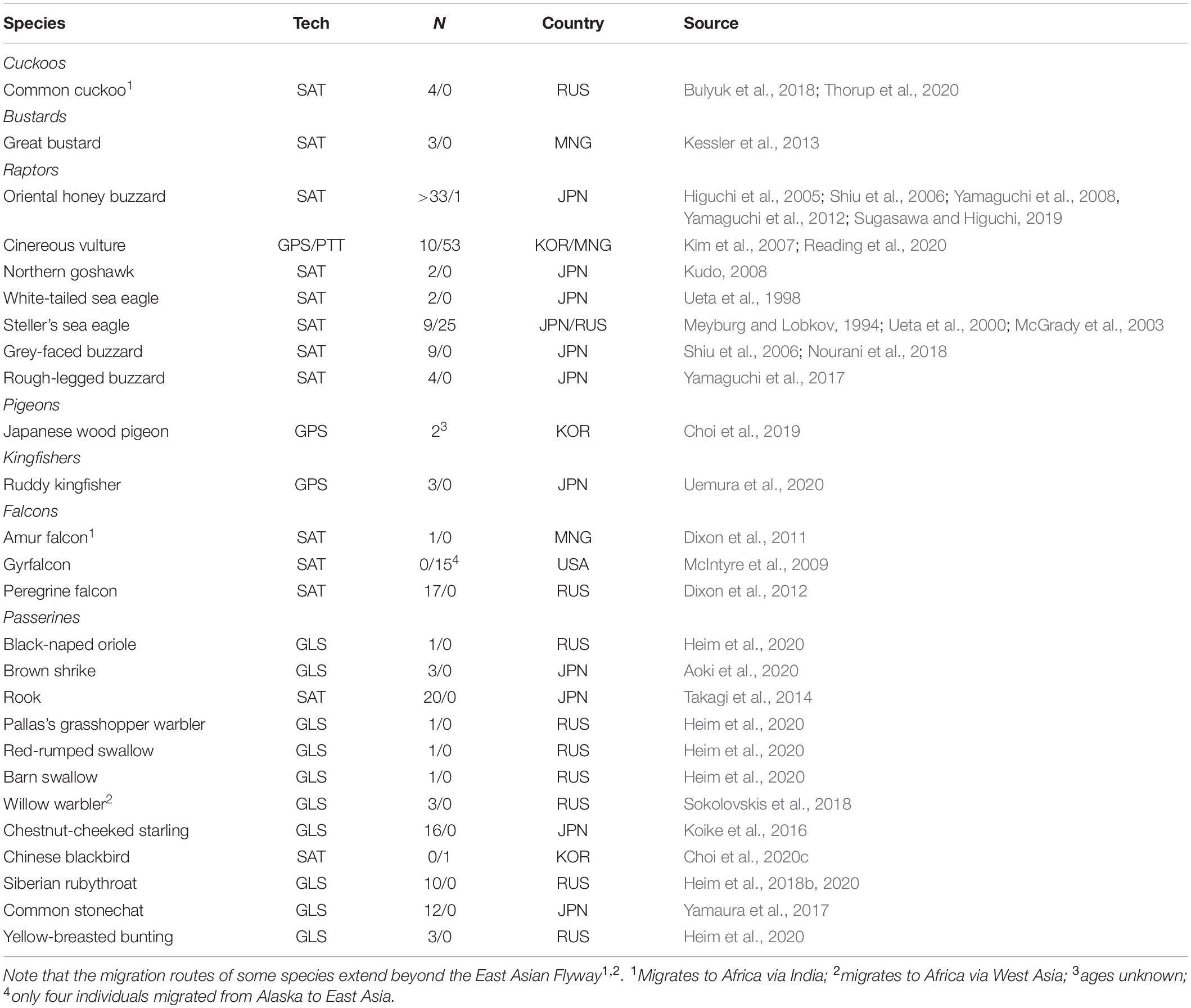
Table 2. East Asian landbird migrants for which tracking data have been published in the peer-reviewed literature (n = 23) with the tracking technique (Tech) used for each species (GLS = light-level geolocation, GPS = Global Positioning System data logging and SAT = satellite tracking), the number of individuals tracked (N, adults/juveniles), the country where the birds were tagged (RUS = Russia, MNG = Mongolia, JPN = Japan, USA = United States of America), and the source(s) where the original data were published.
Two main migratory corridors in the EAF are now well established, (1) the ‘island’ or ‘oceanic’ route linking eastern Russia (Kamchatka and Sakhalin) and Japan to the Philippines and eastern Indonesia, and (2) the ‘mainland’ route, linking eastern Russia, China and continental Southeast Asia (Higuchi, 2005; Yamazaki et al., 2012; Concepcion et al., 2017; Heim et al., 2020). Data from recent tracking studies (Figure 2) further illustrate the use of the ‘island route’ by passerines and raptors breeding in Japan (Koike et al., 2016; Nourani et al., 2018; Uemura et al., 2020) and species shared with Kamchatka (e.g., barn swallow Hirundo rustica and Siberian rubythroat) (Heim et al., 2020). These results corroborate direct observations in the Philippines and eastern Indonesia (Germi et al., 2009, 2013; Concepcion et al., 2017).
The specific island route(s) used remain poorly studied. Observational data show that two raptors, grey-faced buzzard Butastur indicus and Chinese sparrowhawk Accipiter soloensis, pass south through the Japanese archipelago, Taiwan and the Philippines (Ellis et al., 1990; Yamazaki et al., 2012; Concepcion et al., 2017). Similarly, some passerines, including wagtails, pipits and yellow bunting Emberiza sulphurata, also follow this least energetically costly route (Nourani et al., 2018). Certain species may take a more direct route from south Japan passing east of Taiwan, most likely aided by seasonal weather conditions, as suggested by tracking data of ruddy kingfisher Halcyon coromanda, chestnut-cheeked starlings Agropsar philippensis and grey-faced buzzards (Koike et al., 2016; Uemura et al., 2020). The northward migration route(s) in spring is less well known, with some species supposedly leaving the northern Philippines heading for southern China (Welch, 2011). The low number of observations of species such as Gray’s grasshopper warbler Locustella fasciolatus in Taiwan and the Philippines is unusual (Severinghaus et al., 2017; Allen, 2020) – either they largely overfly or bypass these islands, or are simply overlooked.
Meanwhile, recent tracking data from Russia, Mongolia and northern China are providing insights on precise migration paths along the ‘mainland route’ for small passerines (e.g., Siberian rubythroat, Pallas’s grasshopper warbler Locustella certhiola and yellow-breasted bunting Emberiza aureola) (Heim et al., 2018b, 2020), as well as for great bustard (Kessler et al., 2013) and cinereous vulture Aegypius monachus (Kim et al., 2007; Reading et al., 2020). Although there are no major geographical barriers, species using the ‘mainland route’ may cross uplands and mountains in central and southern China, northern Indochina (Wang et al., 2000; Tordoff, 2002; Fei et al., 2015) and the Malay Peninsula (Medway and Wells, 1976).
Based on satellite tracking studies, Oriental honey buzzards breeding in Japan (Higuchi et al., 2005; Yamaguchi et al., 2008, 2012; Sugasawa and Higuchi, 2019) and probably yellow-breasted buntings from Kamchatka (Heim et al., 2020) make major sea crossings to the mainland before migrating to southern China and Southeast Asia (Yamaura et al., 2017), potentially avoiding longer sea crossings further south. Such migratory patterns may suggest that these species colonized Japan from the mainland relatively recently (Agostini and Mellone, 2007). Satellite telemetry has demonstrated strong migratory linkages for large (soaring) species, particularly rough-legged buzzards Buteo lagopus moving between eastern Russia and Japan (Yamaguchi et al., 2017), while highlighting the importance of Japan for wintering Steller’s sea eagles Haliaeetus pelagicus and white-tailed sea eagles H. albicilla breeding in eastern Russia (Ueta et al., 1998; McGrady et al., 2003). Similarly, tracking studies and field observations at key watch sites have demonstrated the role of the Japanese archipelago as a land corridor for bird migration into tropical Asia (Shiu et al., 2006; Yamazaki et al., 2012; Koike et al., 2016; Nourani et al., 2018).
Nevertheless, whilst the majority of EAF landbird species migrate largely along latitudinal gradients into temperate and tropical Asia, a few species deviate from these two major migratory corridors to reach non-breeding grounds in India or Africa. These landbirds have migratory routes that partially overlap with the EAF; they eventually migrate westward toward Africa crossing the large ecological barrier of the Indian Ocean. Two of the best studied examples are Amur falcon Falco amurensis and common cuckoo Cuculus canorus, both known to cover the longest migratory distances among East Asian landbirds (Dixon et al., 2011; Townshend, 2016a; Bulyuk et al., 2018; Figure 2A).
The varied ecosystems of northeast Asia form the core breeding grounds of most migratory landbirds in the EAF (McClure, 1974; Nisbet, 1976; Yong et al., 2015). These include temperate broadleaf forests in China, Japan and the Korean Peninsula, which support the highest diversity of (migratory) breeding landbirds, as well as boreal (taiga) forests and Arctic tundra, which support a more depauperate breeding landbird fauna (Ganter and Gaston, 2013; Figure 3). There are fewer than 20 landbird species regularly breeding in the Arctic, only five of which are strictly associated with this biome (Vaughan, 2010; Ganter and Gaston, 2013). The migration patterns of Arctic-breeding landbirds are not well known, as the movements of only one species, the snow bunting Plectrophenax nivalis, have been tracked and only in an American flyway (McKinnon et al., 2016). The natural history of the landbirds of eastern Russia, which forms the largest part of the EAF, has, however, been relatively well studied for over a century (Dementiev and Gladkov, 1951–1954). Extensive regional reviews are also available for northeast Russia (Kistchinski, 1968, 1988; Krechmar et al., 1991), Sakhalin Island (Nechaev, 1991) and the diverse broadleaf temperate forest of Primorsky Krai (Gluschenko et al., 2016). Similarly, reviews have been published on the Chinese, Korean and Japanese breeding bird fauna since the 1960s (Cheng and Li, 1955; Yamashina, 1961; Won et al., 1966; Won and Gore, 1971), whilst natural history studies of many species exist.
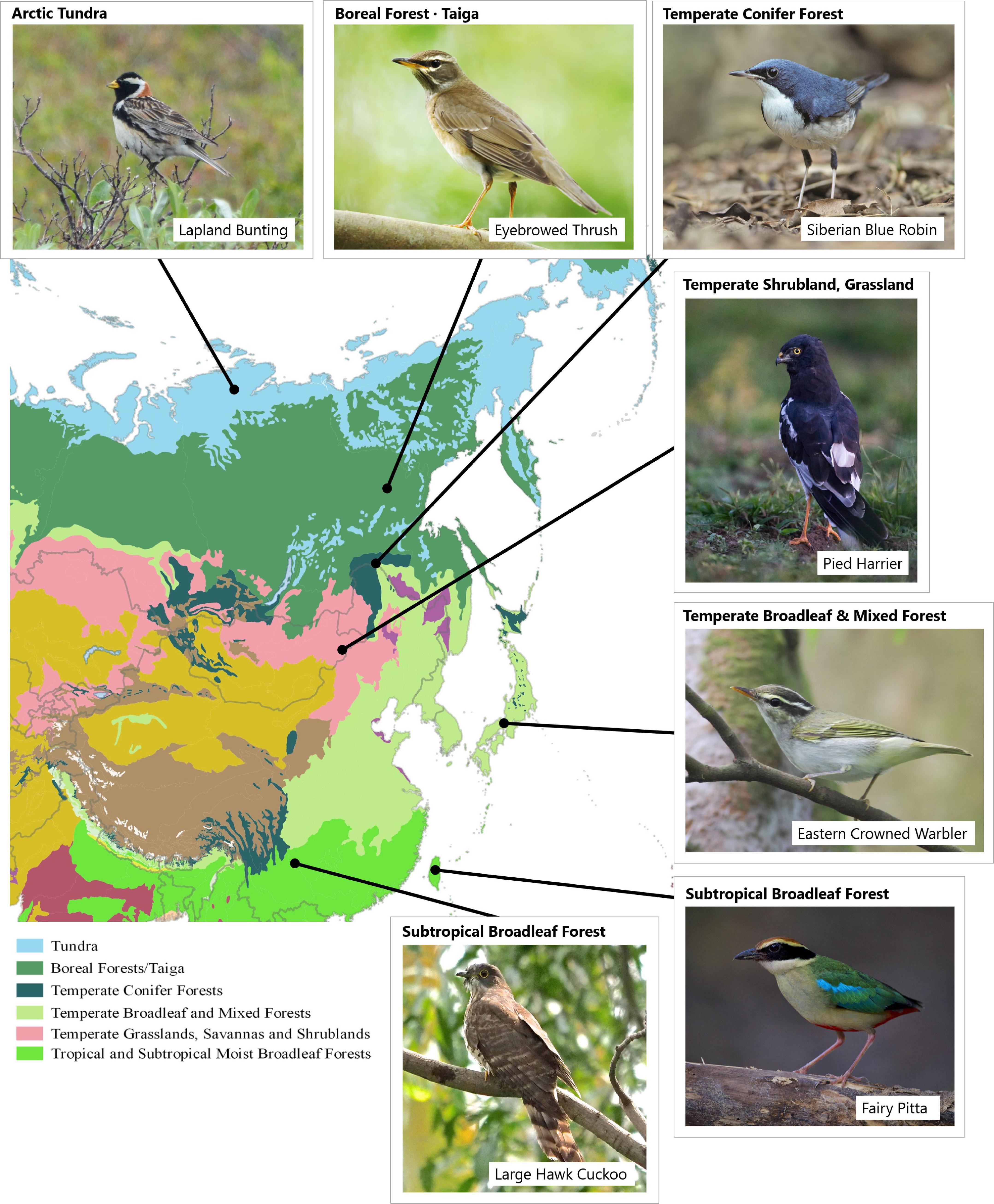
Figure 3. Six major biomes in northeast Asia form the breeding range of most migratory landbirds in the EAF (Image credits: Pavel Ktitorov, Tiah Khee Lee, Heng Yee Cheng, Yann Muzika, Bjorn Olesen, Geoff Lim).
The broad distribution of birds in the eastern Palearctic (which overlaps in its entirety with the northern EAF) was first described by Shtegman (1938), who defined seven major habitat-associated groups across the wider Palearctic region. Based on this classification, most migratory landbird species in the EAF (finches, chats, thrushes, buntings and Phylloscopus warblers) belong to the Siberian (usually boreal forest or taiga species) or the Chinese faunal group (usually species of mixed broadleaf forests; see Shtegman, 1938). The ratio of migratory species progressively increases with latitude: the handful of Arctic-breeding species are almost all migratory, while the proportion of migratory species drops to 60–80% in the boreal and temperate biomes (Ganter and Gaston, 2013; Somveille et al., 2013), and even lower in the subtropics (Yong et al., 2015).
The broad patterns associated with wintering landbird diversity and distributions across tropical Asia are relatively well described (Nisbet, 1976; White and Bruce, 1986). The diversity of wintering landbirds is highest in mainland Southeast Asia, followed by southern China and the Greater Sundas (McClure, 1974; Nisbet, 1976; Yong et al., 2015; Table 3), declining progressively through the Philippines and Wallacea toward Australia (White and Bruce, 1986). Unlike other Eurasian migratory systems, the EAF hosts a large number of insectivorous species that overwinter in forests, many with putative and/or narrow distributions (e.g., rufous-headed robin, blackthroat Calliope obscura, Gansu leaf-warbler Phylloscopus kansuensis and Ijima’s leaf-warbler Phylloscopus ijimae; see Yong et al., 2015). A few species, such as Gray’s grasshopper warbler Locustella fasciolata may be considered ‘extreme eastern’ landbird migrants that largely overwinter in eastern Indonesia (White and Bruce, 1986; Coates and Bishop, 1997; Allen, 2020). A handful of taxa have their core wintering ranges in New Guinea or continental Australia (e.g., white-throated needletail Hirundapus caudacutus) (White and Bruce, 1986; Tarburton, 2014; Menkhorst et al., 2017).
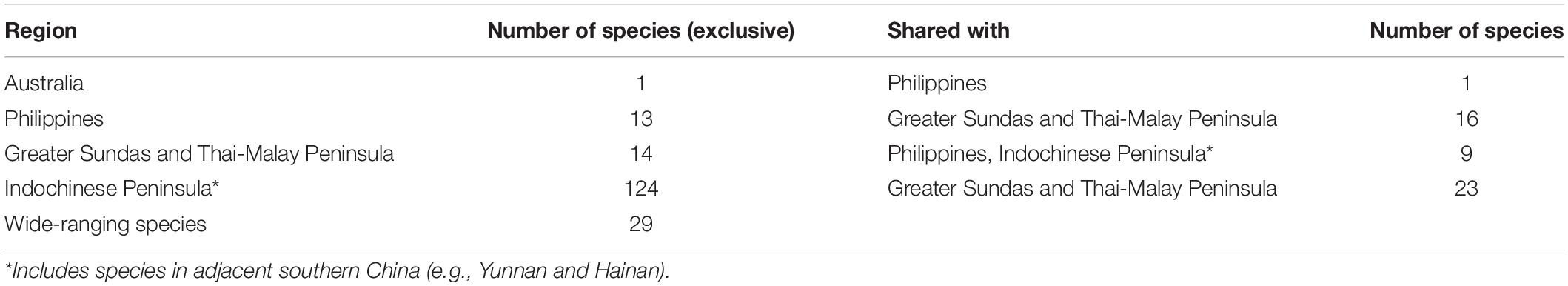
Table 3. Non-breeding distribution of migratory landbirds in different Southeast Asian regions and Australia.
Tropical broadleaf forests form the dominant wintering habitat for at least 90 migratory landbirds in the Southeast Asian tropics (Medway and Wells, 1976; Wells, 2007; Yong et al., 2015; Figure 4). An additional 60 species that rely on this habitat are Sino-Himalayan and/or short-distance migrants that breed at higher (subtropical and tropical) latitudes. The ecological niches occupied by these forest-dependent species vary considerably. Many occupy seasonal deciduous and evergreen forest mosaics, species wintering in the Greater Sundas mostly use evergreen (lowland) rainforests, while the few species reaching southern Wallacea are largely eurytopic (Schellekens et al., 2011). The elevational distribution of most forest-wintering taxa has been poorly defined, although some seem to be confined to lowlands or mountains (15 and 23 species, respectively). At least 36 species use inland freshwater wetlands, opportunistically exploiting wetland-like rice paddies (Figure 4) that represent the dominant anthropogenic land cover in many parts of tropical Asia (Round, 2008). As the region was formerly dominated by forests, the historical distribution of species assemblages currently associated with open anthropogenic landscapes is unclear. Nevertheless, some almost certainly have expanded their distribution to colonize new habitats created by the clearance of the climax vegetation (Yamaura et al., 2017). In Indonesia, starlings and other wintering species are now reported from urban areas and heavily modified mosaics of coastal vegetation (Diniarsih et al., 2016), suggesting some level of adaptability.
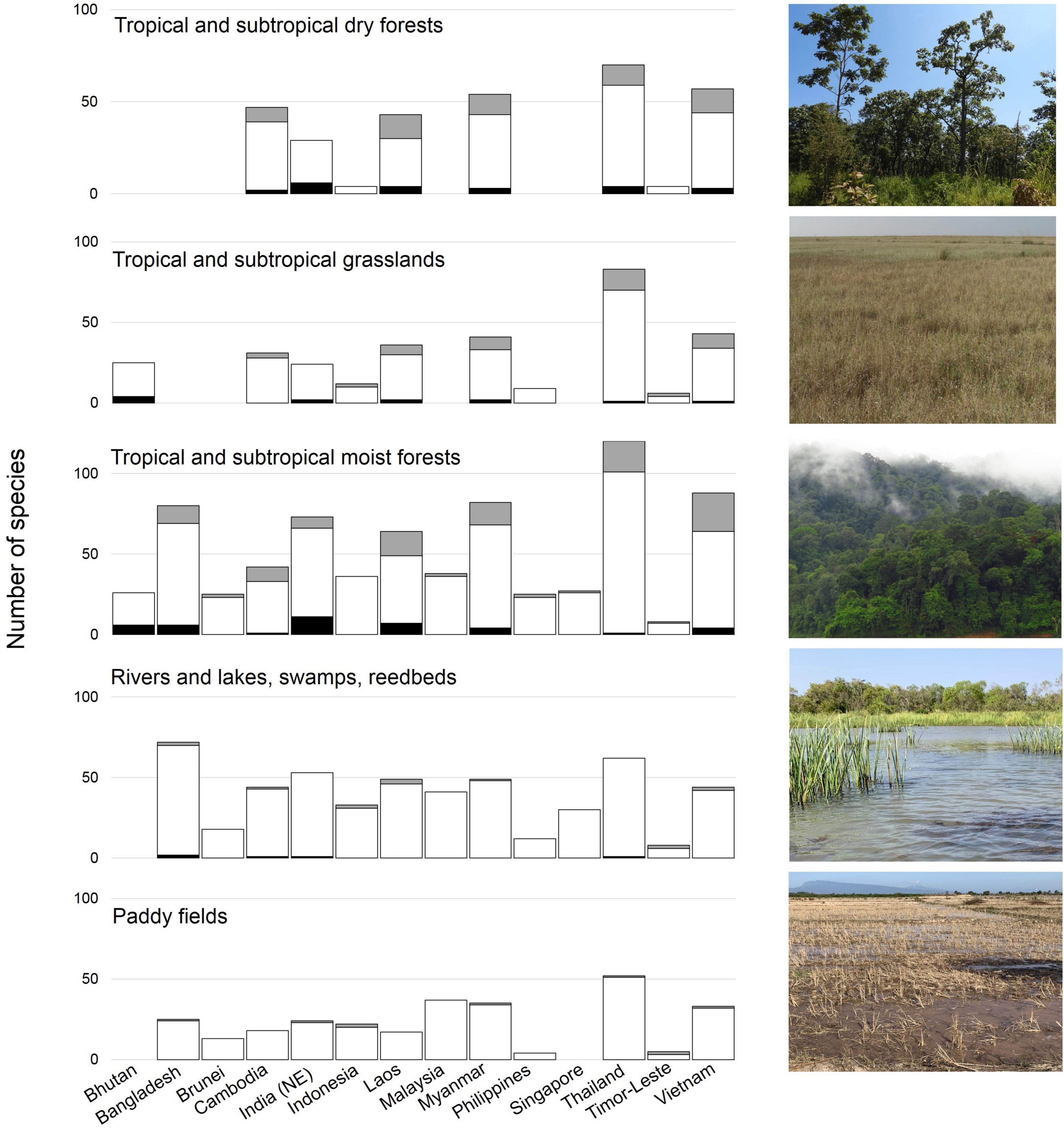
Figure 4. Relative importance of biomes in tropical Asia for wintering migratory landbirds. Cultivated land, such as rice paddies, is among the most important of the human-modified landscapes for migratory landbirds (Image credits: Yong Ding Li). Grey bars (breeding species), white bars (wintering species), black bars (species on stopover).
Ever since Silent Spring (Carlson, 1962) raised the alarm on bird declines globally, landbird population declines have been widely reported in Europe and North America (Terborgh, 1992; Vickery et al., 2014; Rosenberg et al., 2019). Long-term population trends for European species became available based on the large datasets collected by various large-scale bird monitoring and ringing schemes (Vorisek et al., 2008). In East Asia, the first worrying trends were detected in Japan, with declines found to be associated with forest-dependent and long-distance migrant species (Higuchi and Morishita, 1999; Amano and Yamaura, 2007), and further evidenced in Japan’s national-level bird atlas (Japan Bird Research Association, 2021). Wells (2007) reported larger declines for landbirds that rely on tropical forests as wintering habitat in the Thai-Malay Peninsula and data from Thailand also suggest a decline of at least some species (e.g., tiger shrike Lanius tigrinus; Round, 2010).
In the past decade, drastic declines in the yellow-breasted bunting (Figure 5) have raised major conservation concerns (Kamp et al., 2015). This species used to be one of the most abundant Palaearctic birds with a vast breeding distribution from Fennoscandia to eastern Russia and Japan (Dementiev and Gladkov, 1951–1954; McClure, 1974). However, since the 1990s, its global population has declined by almost 90% (Kamp et al., 2015), with national-level declines reported from Russia, Japan and Korea (Tamada et al., 2014, 2017; Choi et al., 2020a; Park et al., 2020; Heim et al., in press) and the species was uplisted to Critically Endangered in 2017 (BirdLife International, 2020).

Figure 5. Yellow-breasted bunting Emberiza aureola is the best example of an Asian landbird that has suffered steep declines in the recent decade (Kamp et al., 2015; Image credits: Michelle and Peter Wong).
A similar trend has been documented for the closely related rustic bunting Emberiza rustica, based on data from the breeding grounds in Russia (Yakovleva and Sukhov, 2017; Gerasimov and Lobkov, 2019), Fennoscandia and from stopover sites in China and Japan (Edenius et al., 2017). Buntings are typically widely distributed and thus data on their status and trends are available from multiple sites across their breeding range. Yet, for the majority of East Asian species with narrower distributions, little or no long-term monitoring data are available and it is nearly impossible to confidently elucidate population trends or conservation status (Yong et al., 2015).
Across the region, evidence for the decline of migratory landbirds has been growing steadily based on studies at stopover sites and the breeding grounds (see Supplementary Table 1). Based on diverse data sources, six breeding and migrating bunting species, including the once abundant rustic and chestnut buntings Emberiza rutila, were found to have declined in South Korea (Choi et al., 2020a). In Russia, significant declines have been detected for Pallas’s E. pallasi (Heim, 2017) and chestnut bunting (Valchuk et al., 2017) on migration and at breeding sites (Ananin, 2011). These patterns are consistent with the declining trends of passerine landbirds reported at ringing stations in mainland China (Jiao et al., 2016; Xu et al., 2017) and at breeding sites surveyed in Japan and Taiwan (Tamada et al., 2014, 2017; Kitazawa et al., 2020; Japan Bird Research Association, 2021), including the threatened fairy pitta Pitta nympha (Lin and Pursner, 2020). Meanwhile, population trends of landbirds on their wintering grounds remain largely unknown, even though the available evidence suggests declines for species that winter in India (SoIB, 2020) and Australia (Tarburton, 2014; see also Supplementary Table 1).
While many long-distance Asian migrants including those wintering in the tropics have suffered larger declines (Amano and Yamaura, 2007; Bourski, 2009; Ananin, 2011), consistent with patterns observed in the Western Palearctic (Vickery et al., 2014), the trends are inconsistent, even among species that share apparently similar migration strategies. The yellow-browed warbler has declined at both its breeding and wintering grounds (Ananin, 2010, 2011; Xu et al., 2017; SoIB, 2020), while increases have been documented for the closely related dusky warbler Phylloscopus fuscatus (Ananin, 2011; Gerasimov and Lobkov, 2019). There is an urgent need to understand overall population trends for migratory landbird species, drawing from monitoring work across the region. This will also help elucidate the specific threats and drivers of decline.
Some observed changes in species population trends and densities of landbirds can be explained by shifts in breeding distributions and range limits. Many European and North American species show recent range shifts likely driven by changes in habitat availability accelerated by climate change (Virkkala and Lehikoinen, 2014; Whitaker, 2017). Similarly, in the EAF, northward and eastward shifts have been detected in the breeding distribution and range limits of several species across eastern Asia. Some Chinese breeding species (e.g., red-billed starling Sturnus sericeus, light-vented bulbul Pycnonotus sinensis and yellow-bellied tit Parus venustulus) were not recorded in the Korean Peninsula until 2000. All three have since expanded their distribution ranges northeast across the Yellow Sea and became locally common breeding species in South Korea (Choi et al., 2011). The range of the yellow-bellied tit has expanded even further north and it has colonized the Russian Far East (Fetting et al., 2016; Redkin et al., 2020). The widespread Palearctic-breeding fieldfare Turdus pilaris has shown a recent eastward expansion across Siberia. At the end of the 20th century, fieldfares bred as far east as Yakutia, west of the Aldan River, with no records east of the Verkhoyansky Mountains. Since 2009, the species has rapidly spread eastward, reaching Magadan in 2014 (Kondratyev, 2014), Kamchatka (Lobkov, 2015) and the Anadyr River by 2020, a range extension of more than 1,000 km.
There are other examples of range shifts at smaller spatial scales, as well as longitudinal shifts along the tundra-forest-tundra gradient, and in the northern taiga transition zones. Little bunting Emberiza pusilla, Arctic warbler Phylloscopus borealis and Common stonechat Saxicola torquatus, all characteristic boreal species, have shifted northwards and breed more regularly in the tundra and at more diverse locations than previously known (Andreev et al., 2015; Syroechkovskiy et al., 2019). On Sakhalin Island, black-browed reed warbler Acrocephalus bistrigiceps (Ktitorov and Zdorikov, unpublished data), and White’s thrush Zoothera aurea (Ktitorov et al., 2019) have recently colonized the northern parts of the island. In temperate and subtropical areas of China, several migratory cuckoo species have exhibited northward and eastward shifts (Sun et al., 2018).
Shifts in distributions and range limits of many species may be explained by changes in vegetation cover and associated habitat dynamics, often anthropogenically caused, or may arise due to the broader effects of warming driven by climate change (Virkkala and Lehikoinen, 2014; Box 1). According to modeled climate change scenarios, the distributions of most East Asian species are expected to be affected, including many that are already threatened (Hu et al., 2020). These distribution shifts need to be considered when planning conservation actions for migratory species. While there is a risk that observed distribution shifts may arise from geographical biases in sampling, they need to be monitored and carefully documented, as they may manifest in precipitous local declines, as seen in the near-endemic Japanese breeding subspecies of brown shrike (Kitazawa et al., 2020).
BOX 1. Land cover change in eastern Siberia and the Russian Far East: impacts on breeding birds.
The varied and complex ecosystems of eastern Siberia and the Russian Far East provide breeding grounds for most long-distance migratory landbirds in the EAF (Figure 3). Much of eastern Russia has a sparse human population and thus appears little disturbed, based on the limited extent of built environments, population density and infrastructure (Venter et al., 2016). Not surprisingly, this understudied, but ecologically complex region is frequently perceived as being covered by vast tracts of boreal forests. However, recent global assessments of intact forested landscapes reveal that the extent of old-growth forest cover is less than expected and is declining even in the most remote areas of eastern Russia (Potapov et al., 2008, Potapov et al., 2017; Global Forest Watch, 2014).
The main drivers of deforestation and land use change in eastern Russia include legal and illegal logging, both which are unsustainable (Vandergert and Newell, 2003), and fire. While most forest fires (65%) are anthropogenic, their prevalence has increased as a result of the earlier spring and summer thaw, presumably exacerbated by climate change (Achard et al., 2008). Logging and forest fires mostly affect the more developed, southern regions of the Russian Far East (Kurdykov and Volkovskaya-Kurdyukova, 2016; Heim et al., 2019), but forest fire hotspots are becoming increasingly prevalent further north, particularly in central Yakutia and have even penetrated the forest-tundra zone of southern Chukotka (Achard et al., 2006).
Land cover changes associated with shifting agricultural policies have attracted less attention, but have a discernible effect in shaping Siberian ecosystems. The collapse of the Soviet Union in 1991 triggered the abandonment of vast areas of cropland and pasture, especially in southern Siberia and in the Far East. Since 2016, some areas have reverted to agriculture (Prishchepov et al., 2020), but these remain negligible in eastern Russia, mostly concentrated along the Chinese border (Teluguntla et al., 2015: Global Cropland Database). This synergy of anthropogenic drivers and natural ecological processes is re-shaping landscapes in eastern Russia and has resulted in the replacement of the mosaic of old-growth forests and cultivated land by more uniform early- and mid-successional shrub or forest habitat (Heim et al., 2019), although some areas of old-growth forest show signs of recovery (Potapov et al., 2008, Potapov et al., 2017).
In the Arctic and sub-Arctic regions of eastern Russia, changes in the vegetation cover, particularly in the tundra and taiga-tundra ecotone have received little attention from ecologists. One of the most visible features of the Arctic landscape associated with climate change is ‘shrubification,’ characterized by an increase in cover, height and thickness of shrub vegetation (Tape et al., 2006; Forbes et al., 2010). In the eastern Russian tundra, larch Larix spp. and willow Salix spp. are growing taller, leading to gradual transformation of the taiga-tundra transition zone into true taiga and the encroachment of shrubland into tundra (Forbes et al., 2010; Frost and Epstein, 2014; Heim et al., 2021). Remote sensing analyses show hotspots of tundra greening in many areas of the Russian Arctic (Frost and Epstein, 2014).
The large-scale consequences of land cover change for migratory landbirds have not been studied, partly due to the lack of a nation-wide bird monitoring scheme in Russia. However, these changes are presumably complex and multi-directional, affecting species dependent on old-growth forests negatively, but favoring species that exploit post-fire successional vegetation and forest edges (Osipov and Biserov, 2017). Based on the observed directionality of vegetation change, it is possible to make some broad predictions: (1) forest edge and shrub-dwelling species (i.e., most boreal-breeding migratory passerines) should benefit from increased habitat availability, while (2) old-growth forest species (e.g., large diurnal and nocturnal raptors) and (3) open habitat species (e.g., various bunting species) may suffer from a reduction of habitat.
The threats faced by migratory landbirds in the EAF are poorly understood compared with those faced by waterbirds, which have been extensively studied in the past decade (Studds et al., 2017; Gallo-Cajiao et al., 2020). Given that most landbirds in the region (Figure 4) are somewhat dependent on forests as stopover and non-breeding habitat, forest loss and degradation are predicted to be major threats. Intensive land management, especially intensive agriculture, often with heavy pesticide use, presumably also affects species stopping over or wintering in rice paddies or other cultivated land. It remains unclear how complex land use changes and land management regimes will impact bird populations across Asia under different climate change scenarios. Meanwhile, the intense harvesting of wildlife for use as food or pets has been directly implicated in the decline of several migratory landbirds in the EAF, the best known being the yellow-breasted bunting Emberiza aureola (Chan, 2004).
Forest loss and degradation affect many migratory landbirds throughout their distributions in the EAF, particularly at the tropical wintering grounds (Amano and Yamaura, 2007; Yong et al., 2015). Asian tropical lowlands have suffered the highest rates of forest loss globally (Corlett, 2014; Stibig et al., 2014; Namkhan et al., 2021). Extensive deforestation throughout the lowlands of the Greater Sundas is predicted to affect both globally threatened and more common species that winter predominantly in lowland forest (Wells, 2007; Yong and Liu, 2015). In one of few assessments of wintering forest landbird assemblages, Wells (2007) observed that the densities of Siberian blue robin Larvivora cyane were substantially lower in Malaysian hill-slope forests and in disturbed or agricultural habitats compared to old-growth lowland rainforests, suggesting that this common wintering species is sensitive to lowland deforestation.
Montane forest cover in Southeast Asia has been relatively less affected compared to the lowlands (Soh et al., 2019), and therefore species wintering in montane forests have been presumed to face lower risk. Yet, montane forests are also under increasing pressure (Peh et al., 2011; Zeng et al., 2018). In northeast India and Bangladesh, deforestation on mountains is driven by illegal logging and clearing for tea plantations and other fast-growing plantation species, exacerbated by ongoing shifting cultivation (Rasul, 2007; Lele and Joshi, 2009); in upland parts of Southeast Asia, expansion of vegetable farms is known to drive deforestation (Poudel et al., 1998). In mainland Southeast Asia and southern China, shifting cultivation has affected large tracts of montane forest landscapes (Fox et al., 2014). It remains unclear how such extensive land use change (perhaps in synergy with climate-change shifts) may impact species using tropical montane habitats. Anecdotal evidence suggests that montane species, including wintering landbirds, may be better adapted to exploit habitat mosaics and successional growth (e.g., Diamond and Lovejoy, 1985) and are therefore less sensitive to habitat degradation compared to lowland-dependent species.
The impact of wetland conversion on migratory landbird communities is another major knowledge gap. Until recently, only anecdotal observations were available, along with a few studies from coastal marshes in China and Japan (Ma et al., 2007). Across the floodplains of the major rivers in southern China, Southeast Asia, Bangladesh and northeast India, most climax wetland vegetation (e.g., seasonally inundated grasslands and marshland on floodplains) has been heavily modified and largely replaced by extensive areas of rice paddies, tea gardens and other agriculture (Round, 2008; Prokop, 2018). Rice paddies can support a subset of the migratory bird assemblages associated with freshwater wetlands (Round, 2008; Wong et al., 2009; Fujioka et al., 2010; Wood et al., 2010). For instance, on the Chao Phraya floodplains in central Thailand, the rice harvest period coincides with the arrival of migratory species. The leftover stubble is used by raptors, insectivores, such as bluethroat Luscinia svecica and eastern yellow wagtail Motacilla tschutschensis, and granivores, such as yellow-breasted bunting (McClure, 1974; Round, 2008). Similar examples have been documented in Indochina, Malaysia and southern China (Wong et al., 2009; Azman et al., 2019). In Timor in Wallacea, heavily grazed and modified floodplains support a few migratory species, such as eastern yellow wagtail (Trainor et al., 2007).
Widespread land-use change, in particular agricultural intensification with the associated loss of fallow and increased pesticide use, may have contributed to the declines of wintering buntings in Southeast Asia, and species that feed on large insects, such as shrikes and drongos (Round, 2008; Kitazawa et al., 2019). Meanwhile, changing water management and cropping regimes in rice paddies may affect wintering landbird assemblages to the detriment of species with specialised ecological needs. Across Southeast Asia, formerly rain-fed, single-crop rice (including floating rice) has now been extensively replaced by multi-cropped rice that demands intensive irrigation (Round, 2008). An associated reduction in fallow duration and the extent of fallow fields and paddy stubble may reduce food resources and habitat available to wintering birds. In the floodplain wetlands of Candaba in Luzon (the Philippines), which was the only known regular wintering site for the Endangered streaked reed-warbler Acrocephalus sorghophilus, the original natural wetlands have been converted to rice paddies, while intensive land management practices limit natural succession, reducing possibly essential habitat diversity (Round and Allen, 2010).
The hunting or trapping of wild birds in East and Southeast Asia is driven by diverse socioeconomic forces, with considerable variation across the region. Bird trapping to supply the pet trade is undoubtedly the leading driver of take in Southeast Asia and parts of China (Dai and Hu, 2017; Harris et al., 2017). It has been implicated in the fast deteriorating status of robins, thrushes, white-eyes, bulbuls, starlings and many other species (Eaton et al., 2015). Bird trapping has been most severe in Indonesia, where the practice of keeping pet birds is a well-established local tradition (Jepson and Ladle, 2005; Nijman, 2010) and it is estimated that 66–84 million individuals of songbirds are kept in Java alone (Marshall et al., 2020). Although surveys have revealed migratory songbirds, such as Siberian thrush Geokichla sibirica and Siberian rubythroat, in the Southeast Asian pet trade (Yong et al., 2015; Chng and Eaton, 2016; Chng et al., 2018), the degree to which trapping pressures have impacted their populations remains unclear.
Even less is known about the extent to which migratory raptor populations have been impacted by trapping in northern Asia. There is robust evidence that illegal capture in Mongolia, China and Russia to supply falconry and taxidermy markets outside the region has decimated saker Falco cherrug and other migratory falcon populations (Gombobaatar et al., 2004; Lobkov et al., 2011; Stretesky et al., 2018). In Russia, several thousand falcons were illegally caught for export in 2012–2016 and trade in falcons increased between 2006 and 2017 (Lobkov et al., 2011; Wyatt, 2011; Krever and Ivannikova, 2020), despite law enforcement efforts. In Southeast Asia, raptors including various migratory species are commonly traded in pet shops or on social media platforms (Iqbal, 2016; Paridi and Noske, 2017).
Hunting of wildlife for subsistence and the trade in wild meat and traditional medicines directly threaten a wide range of vertebrate species in much of Southeast Asia, the Philippines and parts of southern China (Nijman, 2010; Lee et al., 2014; Harrison et al., 2016). Unsustainably high levels of exploitation to supply local, domestic and international demand for wild meat have decimated vertebrate fauna and hunting is now recognized to be a more immediate threat to Southeast Asian vertebrate fauna than deforestation and logging (Tilker et al., 2019). Recent work is beginning to unravel the scale and impact of hunting on wild bird populations in Asia (Kamp et al., 2015; Gallo-Cajiao et al., 2020), particularly for small migratory songbirds (Box 2).
BOX 2. The role of trade in the decline of migratory passerines.
The global demand for wildlife for human consumption and the pet trade affects much of the world’s vertebrate biodiversity (Scheffers et al., 2019) and has driven the unsustainable take of many birds, bringing several species in Asia close to extinction (Sodhi et al., 2004; Eaton et al., 2015; McEvoy et al., 2019). Given that long-distance migratory passerines tend to build up significant fat stores in preparation for migration (Bairlein, 2002), it is not surprising that such species are targeted as food by humans. In parts of China and in certain Southeast Asian countries, the illegal capture and trade of small passerines for food occurs at a very large scale (Kamp et al., 2015). For instance, an estimated 120,000 songbirds were confiscated in Dongli, Tianjin in September 2018 alone (Anon, 2018). In another incident, poachers made a profit of over 140,000 USD from 510,000 songbirds trapped illegally within 40 days (Wen, 2018). In many instances, birds are kept in flat cages and are fed chemical agents to be fattened (Li, 2016). Barn swallows are trapped on an industrial scale in northern Laos, exceeding an estimated 100,000 individuals per year (Evans et al., 2000; Figure 6A). Similarly, thousands of eyebrowed thrushes Turdus obscurus are harvested in parts of Sumatra, Indonesia (Iqbal et al., 2014).

Figure 6. Migratory landbirds are heavily hunted in Southeast Asia and parts of China for the pet trade, and for food. (A) Grey-faced buzzard Butastur indicus for sale at a pet shop, (B) various swallow species are caught in large numbers for human consumption in northern Laos (Image credits: Yong Ding Li).
Until the 2000s, the species most traded for food in China was believed to be the yellow-breasted bunting. Since this species has declined catastrophically (Kamp et al., 2015), poachers appear to have shifted their attention to other species (Heim et al., in press). For example, 33,000 chestnut buntings and 2,000 chestnut-eared buntings Emberiza fucata were confiscated in a single raid in November 2019 in Pingle, Guangxi. Other species often found in ‘fattening centers’ include yellow-browed E. chrysophrys and black-faced bunting E. spodocephala and common rosefinch Carpodacus erythrinus (Let Wild Birds Fly, 2016).
Meanwhile, the pet trade also drives the harvest of large numbers of migratory passerines and raptors. Siberian rubythroats, long-tailed shrikes Lanius schach, and black-naped orioles Oriolus chinensis are among the more commonly traded species. Surveillance work has revealed a diversity of passerines, including thrushes, starlings, flycatchers and raptors being trapped in the wild and sold in pet bird shops from Vietnam to Indonesia (Iqbal, 2016; Chng et al., 2018; Figure 6B).
Amidst increasing efforts to tackle bird hunting, better monitoring of the vast trade in migratory birds across Asian countries is urgently required. There is a need, (1) to analyze whether the levels of illegal (and legal) hunting are sustainable and, (2) to assess if ongoing hunting could lead to further population declines, as already shown for some migratory taxa, such as waders (Gallo-Cajiao et al., 2020).
In some parts of Southeast Asia, the number of passerines taken to supply domestic demand for wild meat is potentially unsustainably high (Evans et al., 2000; Davenport and Heatwole, 2013), but surprisingly little monitoring exists. Wild meat markets selling high volumes of birds have been documented in Myanmar, Laos, Indonesia and northeast India (Brickle et al., 2008; Bhupathy et al., 2013). Studies of traditional hunting activities by local people at migration hotspots in China and the Philippines have also warned of the high number and diversity of species taken (McClure, 1974; Alonzo-Pasicolan, 1992; Aquino, 1997; Wang et al., 2000). Commercially produced mistnets, which are now widely available, and improvised fishing nets are extensively used in paddy fields and aquaculture areas for both bird hunting and crop protection (Evans et al., 2000; Gallo-Cajiao et al., 2020). Nets have also been used to reduce bird-strike at airfields (Townshend, 2016b).
In many parts of Southeast and East Asia, the deliberate release of captive wild animals for religious purposes or ‘merit release’ is well established in Buddhist tradition (McClure, 1974; Severinghaus and Chi, 1999; Chan, 2004). Gilbert et al. (2012) reported 57 species from bird sellers from Phnom Penh, Cambodia alone, with an annual turnover reaching 700,000 individuals, including large numbers of migratory passerines, such as swallows. In Thailand, Cambodia, and Myanmar, countries where religious releases are ubiquitous, the cumulative impact of merit release on migratory landbird populations is likely to exceed many millions of swallows, wagtails, reed warblers and other small passerines. While most individuals will be released back to the wild, release often occurs in unsuitable habitat and high mortalities during transport and capture can be expected (Clewley et al., 2018).
Drawing from diverse sources, we found evidence of trapping or trade for at least 180 of the over 380 migratory landbird species, representing nearly half (47%) of all species in the EAF (see Supplementary Table 2). The actual number of species involved is likely to be much higher, given that market and community surveys typically fail to identify many individuals to species, especially leaf-warblers, white-eyes and other difficult groups (Rentschlar et al., 2018). In several families (cuckoo-shrikes, pigeons and doves, drongos, starlings and buntings) almost all migratory species are affected by some level of trapping or trade in the region, whereas other families (swifts and accentors) seem to be unaffected.
Various knowledge gaps hinder our present understanding of many aspects of landbird migration in the EAF. The basic ecology of many landbirds, their migratory routes and conservation status, as well as the key threats and causes of decline still require study (re-worded due to poor structure). The increasing availability of novel tools to track migration is rapidly improving the situation. Unlike in Europe and North America, the lack of systematic, large-scale monitoring schemes with coordinated data collection across most parts of Asia means that it is difficult to determine broad population trends for migratory species. There is, however, a potential for citizen science to fill such gaps in the coming decades (Tulloch et al., 2013a, b), and there are already good precedents from the region, especially in Japan and Taiwan (Lin and Pursner, 2020; Japan Bird Research Association, 2021).
Data on migratory routes are now available for some raptors and larger songbirds (Figure 2 and Table 2). Apart from the studies of the eastern populations of some wide-ranging species, conducted by Japanese and Korean researchers, surprisingly few small Siberian passerines have been tracked (Heim et al., 2020). Large knowledge gaps remain for owls, nightjars, rollers, doves, and many passerines, including most thrushes, flycatchers and leaf-warblers. Tracking the migratory routes of particular species and populations has been instrumental in improving our understanding the migration ecology and survival of landbirds. There are ample opportunities for continued work to identify routes under threat, geographical connectivity, habitat use and key stopover areas, all of which have direct implications for conservation (Runge et al., 2014; McKinnon and Love, 2018). Indeed, several projects have been established to track the migration of some species (Townshend, 2019) aided by novel field techniques, such as the use of sound (Senda et al., 2018) and blue light (Zhao et al., 2020) to attract birds, which have increased the number of birds ringed during migration.
Light-level geolocators (Aoki et al., 2020; Heim et al., 2020), collaborative tracking network systems, such as the Motus automated radio telemetry arrays (Taylor et al., 2017) and GPS-based tracking systems may all offer cost-effective means to fill knowledge gaps on the migration and distributions of landbird migrants in the EAF. These methods may be complemented by molecular approaches (Seki and Ogura, 2007; Aoki et al., 2020) and intrinsic markers, such as stable isotope signatures in tissues (Choi and Nam, 2011; Choi et al., 2020b). For instance, genetic markers have been used to infer the breeding areas of migrating Ryukyu robins Larvivora komadori (Seki and Ogura, 2007). Stable isotopes from feathers have been used to link breeding and non-breeding areas (Choi and Nam, 2011; De Jong et al., 2019) and to determine migration patterns (e.g., leap-frog versus chain migration; Weng et al., 2014; Choi et al., 2020b). Stable isotope analysis can also provide missing data on the diets and molting patterns of migratory birds during stopover. Despite this, some challenges remain. For instance, no calibration algorithm between precipitation and feather isotope values is available for Asian landbirds (Choi et al., 2020b). Broad longitudinal similarities of hydrogen isotope ratios across Siberia make it difficult to pinpoint precise breeding areas for some species (Choi and Nam, 2011; De Jong et al., 2019) and stable isotope ratios may also be influenced by feeding habitats and environmental pollutants (Sun et al., 2012).
Compared to other flyways, the ecology of migratory landbirds in non-breeding areas in the Asian tropics remains poorly understood (Newton, 2010; McKinnon and Love, 2018). For instance, the rufous-headed robin Larvivora ruficeps is relatively well-documented at its breeding ground in central China (Zhao et al., 2017), but its wintering distribution in the tropics was entirely putative (Medway and Wells, 1976; Nisbet, 1976) until recent observations (Lim et al., 2020). In another example, the streaked reed warbler is on the verge of extinction, yet its breeding distribution remains largely undetermined (Round and Allen, 2010; Allen, 2020). Similarly, until recently, movements of most migratory raptors in Southeast Asia were known only through direct observations at bottleneck sites in northern Vietnam, the Thai-Malay Peninsula, the Greater Sundas and the Philippines (Tordoff, 2002; Nijman et al., 2006; Germi et al., 2009). Tracking studies (Higuchi et al., 2005; Shiu et al., 2006) have substantially improved understanding of the movements and ecology of some species, such as the Oriental honey buzzard. The wintering ecology of most raptors, however, remains scarcely studied (but see Syartinilia et al., 2015).
Furthermore, there are significant gaps in knowledge on how habitat conversion, loss and degradation impact assemblages of migratory landbirds in forests, cultivated areas and freshwater wetlands, especially at their wintering grounds. A better understanding of the ecological traits of a species, such as age at first breeding, clutch size, survival and life history, migration patterns, population size and trends, and the threats it is facing can all inform conservation actions that may reduce the risk of extinction (Soulé and Orians, 2001). Generation length, migratory habits, habitat preference and diet have all been shown to influence the sensitivity of bird species to habitat loss (Newbold et al., 2013). Studying spatial and temporal changes in life history can help to identify specific threats (Bibby, 1992). Stage-based life histories could provide insight into the likelihood of decline toward extinction for species facing anthropogenic threats (Van Allen et al., 2012).
Obtaining such information is not only costly and labour intensive, but also presents major challenges, particularly for rare and cryptic species (Soulé and Orians, 2001). Citizen science data now occupies a more prominent place in modern ornithology and can help fill many knowledge gaps (Tulloch et al., 2013b; Hu et al., 2020). Besides popular platforms such as ebird.org (1.1 million bird checklists for India, 84,000 for Thailand, 62,000 for China and 23,000 for Russia by December 2020) and iNaturalist.org (81,000 observations for India, 14,000 for Thailand, 18,000 for China and 230,000 for Russia by December 2020), species-specific or threat-based citizen science projects are also becoming more ubiquitous. A bird-window collision study in South Korea received over 16,000 case records in two years, as of December 2020; see Kim, 2018) and citizen science datasets are increasingly informing studies in East Asia (Heim et al., 2020; Tsai et al., 2021). Over time, more structured and focused citizen science effort can complement and enhance studies by professional researchers (Tulloch et al., 2013a).
Birds are among the most abundant and widely distributed group of terrestrial vertebrates in Asian ecosystems. Birds provide key ecosystem services, including the maintenance of food webs, and ecosystems functioning as foragers, predators, prey, seed dispersers, and pollinators (Şekercioğlu et al., 2004; Whelan et al., 2008). Specifically, migratory bird species play important roles in linking ecosystem processes and fluxes over great distances and times (Whelan et al., 2008) by supporting, regulating, and transferring ecosystem processes and the associated benefits along their migratory routes (Bauer and Hoye, 2014; López-Hoffman et al., 2017). Nutrient cycling and flow, and energy transfer across ecosystems and biomes at large spatial scales are key ecological contributions of migratory species (López-Hoffman et al., 2017), yet the specific roles played by migratory landbirds are largely unstudied. Meanwhile, common species play prominent roles in natural and anthropogenic ecosystems by contributing provisioning, regulating, supporting and cultural ecosystem services (Whelan et al., 2008) and by driving biotic interactions (Gaston, 2010). Inevitably, the decline of common migratory landbirds can thus be expected to drive a disproportionate loss in ecological services, with consequences on ecosystem functioning and processes, but the mechanisms of how these services may be impacted remain unstudied.
While there is limited evidence of disease transmission by migratory birds (McClure, 1974; Kim et al., 2012a; Moriguchi et al., 2016), their role as vectors and transporters of recently identified pathogens is largely unclear (Choi et al., 2015). More research on invasive species, known pathogens and other microorganisms carried by migratory landbirds will increase our knowledge of emerging diseases and protect animal and human health (World Health Organisation, 2017).
The EAF connects at least 20 countries, each with its unique culture and traditions. Migratory birds link these countries by providing cultural services, as they inspire art, local customs and recreation (Higuchi, 2005; Whelan et al., 2008; López-Hoffman et al., 2017). Aggregations of migratory birds often generate direct economic opportunities through ecotourism activities, such as birdwatching, which is rapidly growing in mainland China (Ma et al., 2013) and parts of Southeast Asia and Russia. Clearly, improved knowledge of the ecosystem roles and services provided by migratory landbirds has the potential to increase public awareness, and thus provide a stronger rationale to support conservation efforts targeted at migratory species.
The study of migratory landbirds in the EAF has advanced greatly since the 1950–1960s (Dementiev and Gladkov, 1951–1954; McClure, 1974). Novel methods and tools in tracking technology (Higuchi, 2005; Yamaura et al., 2017; McKinnon and Love, 2018; Heim et al., 2020), molecular/stable isotope analyses, (Choi et al., 2020b), and the advent of citizen science platforms offer exciting new sources of data to better infer the migration patterns and ecology of East Asian landbirds (Supp et al., 2015; Heim et al., 2020). Together, these complement other, more traditional approaches in the bird migration ‘toolbox’, such as ringing (Fiedler, 2009). These novel approaches have accelerated efforts to study landbird movements at the EAF continental scale and yielded new insights on the ecology of migrants. The newest tracking technologies, such as geolocators and ICARUS (Curry, 2018) enable the tracking of ever smaller animals to elucidate previously unknown migratory routes and patterns, further demonstrating the connectivity of bird populations, especially for widely distributed species and those with disjunct subpopulations. When contextualized against environmental datasets and ecological theory, the tracking of migratory species can play a major role in informing conservation strategies.
Despite recent advances, large knowledge gaps remain with regard to the migration ecology of EAF landbirds. We still lack an adequate understanding of the life histories and distribution of many landbird species, and their population trends in much of the region. There is an urgency to better understand the ecology of migratory species in tropical Asia, where species are most impacted by hunting and other forms of exploitation, and by habitat loss and land use conversion arising from human population pressure. This is particularly so as these drivers of decline are likely to be exacerbated by climate change, as observed in other migratory flyways (Sanderson et al., 2006; Vickery et al., 2014).
Specifically, there is a need to better understand, (1) the life histories and community ecology of migratory landbirds across different landscapes at non-breeding grounds, especially in tropical forests and cultivated landscapes, (2) the migration routes and connectivity of different populations, (3) the threats affecting species during their annual cycle, (4) the ecological roles played by migratory landbirds, such as the long-distance dispersal of plants and microorganisms, and (5) the conservation importance of sites used during the non-breeding period and migration bottlenecks.
Last, there is a need to improve monitoring frameworks to assess the population trends of migratory landbirds at broad spatial scales. Monitoring programs are crucial for identifying declines, population shifts and specific threats that underpin conservation actions. Currently, robust country-wide monitoring frameworks are established in South Korea and Japan (e.g., Choi et al., 2020a; Japan Bird Research Association, 2021), but are lacking elsewhere in the countries of the EAF. Going forward, there is much potential for strengthened regional collaboration between countries in the EAF to establish standardized monitoring programs for migratory landbirds.
DY, JS, SC, and WH conceptualized the manuscript. DY, PR, DA, SC, CT, and WH collected and analyzed the data. All authors participated in the writing and editing of the manuscript.
DY thanks Mark and Mo Constantine for their support to BirdLife International. C-YC was supported by the National Research Foundation of Korea (NRF 2018R1D1A1B07050135) grant funded by the Korean Government (Ministry of Education). PK, OK, and AK were supported by the Institute of Biological Problems of the North FEB RAS (registered research project AAAA-A17-117122790002-8).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge the hard work of current and past researchers, whose published studies have enabled us to prepare this review, especially the pioneering work on tracking by Hiroyoshi Higuchi. We are grateful to Yuichi Yamaura, Yang Liu, Mike Crosby, David Bishop, and many of our colleagues for their useful input during the preparation of the manuscript. We are grateful to Lynx Edicions for granting permission to use illustrations in Figure 2. We acknowledge the constructive input of the editor and two reviewers on our manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.613172/full#supplementary-material
Achard, F., Eva, H. D., Mollicone, D., and Beuchle, R. (2008). The effect of climate anomalies and human ignition factor on wildfires in Russian boreal forests. Philos. Trans. R. Soc. B Biol. Sci. 363, 2329–2337. doi: 10.1098/rstb.2007.2203
Achard, F., Mollicone, D., Stibig, H. J., Aksenov, D., Laestadius, L., Li, Z., et al. (2006). Areas of rapid forest-cover change in boreal Eurasia. For. Ecol. Manage 237, 322–334. doi: 10.1016/j.foreco.2006.09.080
Agostini, N., and Mellone, U. (2007). Migration strategies of Oriental Honey-buzzards Pernis ptilorhyncus breeding in Japan. Forktail 23, 182–183.
Amano, T., and Yamaura, Y. (2007). Ecological and life-history traits related to range contractions among breeding birds in Japan. Biol. Conserv. 137, 271–282. doi: 10.1016/j.biocon.2007.02.010
Ananin, A. A. (2010). Semicenturial changes of the birds population in a southern part of Vitim plateau. Ornitologia 1, 20–25.
Ananin, A. A. (2011). Long-term dynamics of abundance of background species birds of the Barguzinsky mountain ridge during the nesting period. Bull. Burjat State Univ. 4, 93–99.
Andreev, A. V., Kondratyev, A. V., and Potapov, E. R. (2015). Avifauna of the Kolima river lower ridges: interannual dynamics in the times of climate change. Part 1. Changing of the species composition in XX and the first decade of the XXI centuries. [In Russian with English summary]. Proc. North Eastern Sci. Cent. Far East Branch Russ. Acad. Sci. 2, 49–59.
Anon (2018). Wildlife Protection Volunteers Report 120,000 Migratory Birds in Fattening Centres in Dongli, Tianjin. Huanbao World. Available online at: https://www.huanbao-world.com/NGO/46085.html (accessed September 27, 2020).
Aoki, D., Sakamoto, H., Kitazawa, M., Kryukov, A., and Takagi, M. (2020). Migration-tracking integrated phylogeography supports long-distance dispersal-driven divergence for a migratory bird species in the Japanese archipelago. Authorea. [Preprint]. doi: 10.22541/au.160373183.39716449/v1
Aquino, C. P. (1997). The practice of ikik in Mt. Pulog National Park: implications to conservation. Sylviatrop 7, 96–100.
Azman, N. M., Sah, S. A. M., Ahmad, A., and Rosely, N. F. (2019). Contribution of rice fields to bird diversity in Peninsular Malaysia. Sains Malays. 48, 1811–1821. doi: 10.17576/jsm-2019-4809-02
Bairlein, F. (2002). How to get fat: nutritional mechanisms of seasonal fat accumulation in migratory songbirds. Naturwissenschaften 89, 1–10. doi: 10.1007/s00114-001-0279-6
Bauer, S., and Hoye, B. J. (2014). Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344:1242552. doi: 10.1126/science.1242552
Bennett, R. E., Leuenberger, W., Bosarreyes Leja, B. B., Cáceres, A. S., Johnson, K., and Larkin, J. (2018). Conservation of Neotropical migratory birds in tropical hardwood and oil palm plantations. PLoS One 13:e0210293. doi: 10.1371/journal.pone.0210293
Beresford, A. E., Sanderson, F. J., Donald, P. F., Burfield, I. J., Butler, A., Vickery, J. A., et al. (2019). Phenology and climate change in Africa and the decline of Afro-Palearctic migratory bird populations. Remote Sens. Ecol. Conserv. 5, 55–69. doi: 10.1002/rse2.89
Bhupathy, S., Kumar, S. R., Thirumalainathan, P., Paramanandham, J., and Lemba, C. (2013). Wildlife exploitation: a market survey in Nagaland, North-eastern India. Trop. Conserv. Sci. 6, 241–253. doi: 10.1177/194008291300600206
Bibby, C. J. (1992). Conservation of migrants on their breeding grounds. Ibis 134, 29–34. doi: 10.1111/j.1474-919X.1992.tb04748.x
BirdLife International (2020). Birdlife Data Zone. Available online at: http://datazone.birdlife.org/ (accessed October 1, 2020).
Bourski, O. V. (2009). Effect of remote spatial connections on the population dynamics of passerine birds. Dokl. Biol. Sci. 424, 45–48. doi: 10.1134/S0012496609010141
Bozó, L., Csörgö, T., and Heim, W. (2021). Factors controlling the migration phenology of Siberian Phylloscopus species. J. Ornithol. 162, 53–59. doi: 10.1007/s10336-020-01805-5
Bozó, L., and Heim, W. (2015). Trapping and ringing Pale-legged Leaf Warbler Phylloscopus tenellipes, Muraviovka Park, Amur region, Far East Russia. BirdingAsia 23, 118–120.
Bozó, L., and Heim, W. (2016). Sex-specific migration of Phylloscopus warblers at a stopover site in Far Eastern Russia. Ring. Migr. 31, 41–46. doi: 10.1080/03078698.2016.1195213
Bozó, L., Heim, W., and Csörgõ, T. (2017). Trapping and ringing Pallas’s Leaf Warbler Phylloscopus proregulus at the Muraviovka Park, Amur region, Far East Russia. BirdingAsia 28, 67–70.
Bozó, L., Csörgõ, T., and Heim, W. (2018a). Weather conditions affect spring and autumn migration of Siberian leaf warblers. Avian Res. 9:33. doi: 10.1186/s40657-018-0126-5
Bozó, L., Heim, W., and Csörgõ, T. (2018b). Habitat use by Siberian warbler species at a stopover site in Far Eastern Russia. Ring. Migr. 33, 31–35. doi: 10.1080/03078698.2018.1446889
Bozó, L., Heim, W., Trense, D., Fetting, P., Eilts, H.-J., Wobker, J., et al. (2019). Migration timing of Pallas’s Grasshopper-warbler Locustella certhiola and Lanceolated Warbler L. lanceolata at a stopover site in the Russian Far East. Ornithol. Sci. 18, 177–181. doi: 10.2326/osj.18.181
Brickle, N. W., Duckworth, J. W., Tordoff, A. W., Poole, C. M., Timmins, R., and McGowan, P. J. (2008). The status and conservation of Galliformes in Cambodia, Laos and Vietnam. Biodivers. Conserv. 17, 1393–1427. doi: 10.1007/s10531-008-9346-z
Bulyuk, V. N., Sokolov L. V., Markovets, M. Y., and Lubkovskaya, R. S. (2018). “Study of migratory strategies of common cuckoos (Cuculus canorus) using satelite transmitters,” in Ornithology: History, Traditions, Problems and Prospects (Moscow: KMK Scientific Press Ltd.), 61–67.
Buner, F., Dhiman, S., Walker, T., and Dhadwal, D. (2015). Pioneering bird ringing-capacity building in Sairopa, Great Himalayan National Park, Himachal Pradesh, India. BirdingAsia 23, 102–107.
Carey, C. (2009). The impacts of climate change on the annual cycles of birds. Philos. Trans. R. Soc. B Biol. Sci. 364, 3321–3330. doi: 10.1098/rstb.2009.0182
Cheng, T.-H., and Li, K.-Y. (1955). The migration of seasonal birds in China. Biol. Bull. 11, 23–26.
Chng, S. C., Shepherd, C. R., and Eaton, J. A. (2018). In the market for extinction: birds for sale at selected outlets in Sumatra. Traffic Bull. 30, 15–22.
Chng, S. C. L., and Eaton, J. A. (2016). In the Market for Extinction: Eastern and Central Java. Cambridge: TRAFFIC.
Cho, S. Y., Nam, H.-Y., Choi, C.-Y., and Chae, H.-Y. (2011). Effects of Seed Ingestion by Brown-eared Bulbuls (Microscelis amaurotis) on the Ligustrum japonicum seed germination. Korean J. Ornithol. 18, 241–247.
Choi, C.-Y., and Chae, H.-Y. (2007). Effects of bird ingestion on seed dispersal and germination of the Elaeagnus macrophylla. J. Korean For. Soc. 96, 633–638.
Choi, C.-Y., Kang, C.-W., Yun, Y.-M., and Nam, H.-Y. (2015). Can we blame migratory birds for transmission of the emerging Severe Fever with Thrombocytopenia Syndrome Virus in East Asia? Am. J. Trop. Med. Hyg. 93, 1391–1392. doi: 10.4269/ajtmh.15-0524a
Choi, C.-Y., and Nam, H.-Y. (2011). Breeding range estimation of wintering rooks in Korea by stableisotope analysis. Korean J. Nat. Conserv. 9, 129–137. doi: 10.30960/kjnc.2011.9.3_4.129
Choi, C.-Y., Nam, H.-Y., Kim, H.-K., Park, S.-Y., and Park, J.-G. (2020a). Changes in Emberiza bunting communities and populations spanning 100 years in Korea. PLoS One 15:e0233121. doi: 10.1371/journal.pone.0233121
Choi, C.-Y., Nam, H.-Y., Park, J.-G., and Bing, G.-C. (2020b). Migration pattern of Yellow-throated buntings revealed by isotope-based geographic assignment. Int. J. Geogr. Inf. Sci. 34, 504–519. doi: 10.1080/13658816.2019.1670832
Choi, C.-Y., Nam, H.-Y., Park, S.-Y., and Kang, H.-Y. (2020c). A case study on the spring migration of a young Chinese Blackbird (Turdus mandarinus). Korean J. Ornithol. 27, 116–119. doi: 10.30980/kjo.2020.12.27.2.116
Choi, C.-Y., Park, J. G., Moores, N., Kim, E. M., Kang, C. W., Nam, H.-Y., et al. (2011). The recent increase of the Red-billed Starling Sturnus sericeus in the Republic of Korea. Forktail 27, 89–91.
Choi, S. K., Park, Y. C., Park, J. C., Bing, G. C., and Kim, W. Y. (2019). Migration by the Japanese Wood Pigeon (Columba janthina) across the islands of East Asia: direct tracking by satellite telemetry. Pac. Sci. 73, 225–230. doi: 10.2984/73.2.4
Clewley, G. D., Robinson, R. A., and Clark, J. A. (2018). Estimating mortality rates among passerines caught for ringing with mist nets using data from previously ringed birds. Ecol. Evol. 8, 5164–5172. doi: 10.1002/ece3.4032
Coates, B. J., and Bishop, K. D. (1997). A Field Guide to the Birds of Wallacea. Alderley: Dove Publications.
Concepcion, C. B., Dumandan, P. T., Silvosa, M. R., Bildstein, K. L., and Katzner, T. E. (2017). Species composition, timing and meteorological correlates of autumn open-water crossings by raptors migrating along the East-Asian Oceanic Flyway. J. Raptor Res. 51, 25–37. doi: 10.3356/JRR-16-00001.1
Corlett, R. T. (1998). Frugivory and seed dispersal by birds in Hong Kong shrubland. Forktail 13, 23–27.
Corlett, R. T. (2014). The Ecology of Tropical East Asia. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780199681341.001.0001
Curry, A. (2018). The internet of animals that could help to save vanishing wildlife. Nature 562, 322–326. doi: 10.1038/d41586-018-07036-2
Dai, C., and Hu, W. (2017). Hunting strategies employed by bird hunters with economic pursuit in the city of Guiyang, Southwest China. J. Nat. Conserv. 40, 33–41. doi: 10.1016/j.jnc.2017.09.005
Davaasuren, B. (2018). Khurkh Bird Ringing Station. Annual Report. Ulaanbaatar: Wildlife Science and Conservation Centre of Mongolia.
Davenport, D., and Heatwole, H. (2013). Wild terrestrial vertebrates sold as food in open markets in Laos. Pac. Conserv. Biol. 19, 379–393. doi: 10.1071/PC130379
De Jong, A., Torniainen, J., Bourski, O. V., Heim, W., and Edenius, L. (2019). Tracing the origin of vagrant Siberian songbirds with stable isotopes: the case of Yellow-browed Warbler (Abrornis inornatus) in Fennoscandia. Ornis Fenn. 96, 90–99.
DeCandido, R., Siponen, M., Smit, H., Pierce, A., and Allen, D. (2015). Flight identification and migration pattern of the Oriental Honey Buzzard Pernis ptilorhynchus orientalis in southern Thailand, 2007–2014. BirdingAsia 23, 27–33.
Deguchi, T., Yoshiyasu, K., Ozaki, K., Sato, F., Shigeta, Y., Komeda, S., et al. (2015). Long-term changes in migration and breeding periods of avian summer visitors to Japan. Jap. J. Ornithol. 64, 39–51. doi: 10.3838/jjo.64.39
Dementiev, G. P., and Gladkov, N. A. (eds) (1951–1954). Birds of the Soviet Union. Volume I-VI. Moskva: Sovetskaya Nauka.
Diamond, A. W., and Lovejoy, T. E. (eds) (1985). Conservation of Tropical Forest Birds. Technical Publication No. 4. Cambridge: International Council for Bird Preservation.
Diniarsih, S., Jones, S., and Noske, R. N. (2016). Rosy Starling Pastor roseus: a first record for Indonesia. Kukila 19, 60–64.
Dixon, A., Batbayar, N., and Purev-Ochir, G. (2011). Autumn migration of an Amur Falcon Falco amurensis from Mongolia to the Indian Ocean tracked by satellite. Forktail 27, 86–89.
Dixon, A., Sokolov, A., and Sokolov, V. (2012). The subspecies and migration of breeding Peregrines in northern Eurasia. Falco 39, 4–9.
Eaton, J. A., Shepherd, C. R., Rheindt, F. E., Harris, J. B. C., van Balen, S. B., Wilcove, D. S., et al. (2015). Trade-driven extinctions and near-extinctions of avian taxa in Sundaic Indonesia. Forktail 31, 1–12.
Edenius, L., Choi, C.-Y., Heim, W., Jaakkonen, T., De Jong, A., Ozaki, K., et al. (2017). The next common and widespread bunting to go? Global population decline in the Rustic Bunting Emberiza rustica. Bird Conserv. Int. 27, 35–44. doi: 10.1017/S0959270916000046
Ellis, D. H., Kepler, A. K., and Kepler, C. B. (1990). Evidence for a fall raptor migration pathway across the South China Sea. J. Raptor Res. 24, 12–18.
Evans, T. D., Watson, L. G., and Duckworth, J. W. (2000). Large-scale swallow trapping in Xiangkhouang province, North Laos. Orient. Bird Club Bull. 32, 59–62.
Faaborg, J., Holmes, R. T., Anders, A. D., Bildstein, K. L., Dugger, K. M., and Gauthreaux, S. A. Jr., et al. (2010). Conserving migratory land birds in the New World: Do we know enough? Ecol. Appl. 20, 398–418. doi: 10.1890/09-0397.1
Fei, W., Luming, L., Jianyun, G., Dao, Y., Wanzhao, H., Ting, Y., et al. (2015). Birds of the Ailao Mountains, Yunnan province, China. Forktail 31, 47–54.
Fetting, P., Thorn, S., Päckert, M., and Heim, W. (2016). First record of Yellow-bellied Tit Pardaliparus venustulus in Russia suggests a significant range extension of a species formerly endemic to China. Forktail 32, 88–90.
Fiedler, W. (2009). New technologies for monitoring bird migration and behaviour. Ring. Migr. 24, 175–179. doi: 10.1080/03078698.2009.9674389
Finch, T., Saunders, P., Avilés, J. M., Bermejo, A., Catry, I., de la Puente, J., et al. (2015). A pan-European, multipopulation assessment of migratory connectivity in a near-threatened migrant bird. Divers. Distrib. 21, 1051–1062. doi: 10.1111/ddi.12345
Forbes, B., Fauria, M. M., and Zetterberg, P. (2010). Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Glob. Chang. Biol. 16, 1542–1554. doi: 10.1111/j.1365-2486.2009.02047.x
Fox, J., Castella, J. C., and Ziegler, A. D. (2014). Swidden, rubber and carbon: Can REDD+ work for people and the environment in Montane Mainland Southeast Asia? Glob. Environ. Chang. 29, 318–326. doi: 10.1016/j.gloenvcha.2013.05.011
Frost, G. V., and Epstein, H. E. (2014). Tall shrub and tree expansion in Siberian tundra ecotones since the 1960s. Glob. Chang. Biol. 20, 1264–1277. doi: 10.1111/gcb.12406
Fujioka, M., Lee, S. D., Kurechi, M., and Yoshida, H. (2010). Bird use of rice fields in Korea and Japan. Waterbirds 33, 8–29. doi: 10.1675/063.033.s102
Galbraith, C. A., Jones, T., Kirby, J., and Mundkur, T. (2014). A Review of Migratory Bird Flyways and Priorities for Management. Bonn: UNEP/CMS Secretariat.
Gallo-Cajiao, E., Morrison, T. H., Woodworth, B. K., Lees, A. C., Naves, L. C., Yong, D. L., et al. (2020). Extent and potential impact of hunting on migratory shorebirds in the Asia-Pacific. Biol. Conserv. 246:108582. doi: 10.1016/j.biocon.2020.108582
Ganter, B., and Gaston, A. J. (2013). “Birds” in Arctic Biodiversity Assessment, ed. H. Meltofte. Akureyri: Conservation of Arctic Flora and Fauna.
Gerasimov, Y., and Lobkov, E. (2019). Long-term trends of passerine birds’ numbers in Kamchatka. Vestnik TvGU 1, 54–59. doi: 10.26456/vtbio49
Germi, F., Salim, A., and Minganti, A. (2013). First records of Chinese Sparrowhawk Accipiter soloensis wintering in Papua (Indonesian New Guinea). Forktail 29, 43–47.
Germi, F., Young, G. S., Salim, A., Pangimangen, W., and Schellekens, M. (2009). Over-ocean raptor migration in a monsoon regime: spring and autumn 2007 on Sangihe, North Sulawesi, Indonesia. Forktail 25, 104–116.
Gilbert, M., Sokha, C., Joyner, P. H., Thomson, R. L., and Poole, C. (2012). Characterizing the trade of wild birds for merit release in Phnom Penh, Cambodia and associated risks to health and ecology. Biol. Conserv. 153, 10–16. doi: 10.1016/j.biocon.2012.04.024
Gilroy, J. J., Gill, J. A., Butchart, S. H., Jones, V. R., and Franco, A. M. (2016). Migratory diversity predicts population declines in birds. Ecol. Lett. 19, 308–317. doi: 10.1111/ele.12569
Global Forest Watch (2014). World Resources Institute. Available online at: www.globalforestwatch.org (accessed September 27, 2020).
Gluschenko, Y. N., Nechaev, V. A., and Red’kin, Y. A. (2016). Birds of Primorsky Krai: Brief Review of the Fauna. Moscow: KMK Scientific Press.
Gombobaatar, S., Sumiya, D., Shagdarsuren, O., Potapov, E., and Fox, N. (2004). Saker Falcon (Falco cherrug milvipes Jerdon) mortality in Central Mongolia and population threats. Mongolian J. Biol. Sci. 2, 13–21. doi: 10.22353/mjbs.2004.02.13
Harris, J. B. C., Tingley, M. W., Hua, F., Yong, D. L., Adeney, J. M., Lee, T. M., et al. (2017). Measuring the impact of the pet trade on Indonesian birds. Conserv. Biol. 31, 394–405. doi: 10.1111/cobi.12729
Harrison, R. D., Sreekar, R., Brodie, J. F., Brook, S., Luskin, M., O’Kelly, H., et al. (2016). Impacts of hunting on tropical forests in Southeast Asia. Conserv. Biol. 30, 972–981. doi: 10.1111/cobi.12785
Heim, R. J., Bucharova, A., Brodt, L., Kamp, J., Rieker, D., Soromotin, A. V., et al. (2021). Post-fire vegetation succession in the Siberian subarctic tundra over 45 years. Sci. Total Environ. 760:143425. doi: 10.1016/j.scitotenv.2020.143425
Heim, R. J., Hölzel, N., Heinken, T., Kamp, J., Thomas, A., Darman, G. F., et al. (2019). Post-burn and long-term fire effects on plants and birds in floodplain wetlands of the Russian Far East. Biodivers. Conserv. 28, 1611–1628. doi: 10.1007/s10531-019-01746-3
Heim, W., Chan, S., Ktitorov, P., Mischenko, A., Hölzel, N., and Kamp, J. (in press). East Asian buntings: ongoing illegal trade and encouraging conservation responses. Conserv. Sci. Pract.
Heim, W., Eccard, J. A., and Bairlein, F. (2018a). Migration phenology determines niche use of East Asian buntings (Emberizidae) during stopover. Curr. Zool. 64, 681–692. doi: 10.1093/cz/zoy016
Heim, W., Pedersen, L., Heim, R. J., Kamp, J., Smirenski, S. M., Thomas, A., et al. (2018b). Full annual cycle tracking of a small songbird, the Siberian Rubythroat Calliope calliope, along the East Asian flyway. J. Ornithol. 159, 893–899. doi: 10.1007/s10336-018-1562-z
Heim, W., Heim, R. J., Beermann, I., Burkovskiy, O., Gerasimov, Y., Ktitorov, P., et al. (2020). Using geolocator tracking data and ringing archives to validate citizen-science based seasonal predictions of songbird distribution in a data-poor region. Glob. Ecol. Conserv. 24:e01215. doi: 10.1016/j.gecco.2020.e01215
Higuchi, H., and Morishita, E. (1999). Population declines of tropical migratory birds in Japan. Actinia 12, 51–59.
Higuchi, H., Shiu, H.-J., Nakamura, H., Uematsu, A., Kuno, K., Saeki, M., et al. (2005). Migration of Honey-buzzards Pernis apivorus based on satellite tracking. Ornithol. Sci. 4, 109–115. doi: 10.2326/osj.4.109
Hu, R., Gu, Y., Luo, M., Lu, Z., Wei, M., and Zhong, J. (2020). Shifts in bird ranges and conservation priorities in China under climate change. PLoS One 15:e0240225. doi: 10.1371/journal.pone.0240225
Hurlbert, A. H., and Liang, Z. (2012). Spatiotemporal variation in avian migration phenology: citizen science reveals effects of climate change. PLoS One 7:e31662. doi: 10.1371/journal.pone.0031662
Iqbal, M., Noske, R., and Setiawan, D. (2014). Hunting of a very large aggregation of Eyebrowed Thrushes Turdus obscurus in Sumatra. Kukila 17, 148–151.
Japan Bird Research Association (2021). National Breeding Bird Distribution Survey. Available online at: http://bird-atlas.jp/ (accessed January 25, 2021).
Jeong, S. C., Kim, E. M., Hyun, H. J., Won, H. K., Kim, J. S., and Kim, C. S. (2014). Birds and fruits in Jeju Island. Seoul: Korea Forest Research Institute.
Jepson, P., and Ladle, R. J. (2005). Bird-keeping in Indonesia: conservation impacts and the potential for substitution-based conservation responses. Oryx 39, 442–448. doi: 10.1017/S0030605305001110
Jiao, S., Huettmann, F., Guo, Y., Li, X., and Ouyang, Y. (2016). Advanced long-term bird banding and climate data mining in spring confirm passerine population declines for the Northeast Chinese-Russian flyway. Glob. Planet. Change 144, 17–33. doi: 10.1016/j.gloplacha.2016.06.015
Kamp, J., Oppel, S., Ananin, A. A., Durnev, Y. A., Gashev, S. N., Hölzel, N., et al. (2015). Global population collapse in a superabundant migratory bird and illegal trapping in China. Conserv. Biol. 29, 1684–1694. doi: 10.1111/cobi.12537
Kessler, A. E., Batbayar, N., Natsagdorj, T., Batsuur’, D., and Smith, A. T. (2013). Satellite telemetry reveals long-distance migration in the Asian great bustard Otis tarda dybowskii. J. Avian Biol. 44, 311–320. doi: 10.1111/j.1600-048X.2013.00072.x
Kim, H. R., Lee, Y. J., Park, C. K., Oem, J. K., Lee, O. S., Kang, H. M., et al. (2012a). Highly pathogenic avian influenza (H5N1) outbreaks in wild birds and poultry, South Korea. Emerg. Infect. Dis. 18, 480–483. doi: 10.3201/1803.111490
Kim, J. H., Kim, K. H., Youn, H. J., Lee, S. W., Choi, H. T., Kim, J., et al. (2012b). Valuation of nonmarket forest resources. J. Korean Inst. For. Recreation 16, 9–18. doi: 10.34272/forest.2012.16.4.002
Kim, J. H., Chung, O.-S., Lee, W. S., and Kanai, Y. (2007). Migration routes of Cinereous Vultures (Aegypius monachus) in Northeast Asia. J. Raptor Res. 41, 161–165. doi: 10.3356/0892-1016(2007)41[161:MROCVA]2.0.CO;2
Kim, K. H., Chae, H. Y., and Yoo, J. C. (2010). Habitat use of passerine birds at Hongdo Island, a stopover site. Korean J. Environ. Ecol. 24, 325–333. doi: 10.5141/JEFB.2010.33.4.325
Kim, Y. J. (2018). Investigation on Bird Window Collisions. Available online at: https://www.naturing.net/m/2137/summary (accessed December 5, 2020).
Kirby, J. S., Stattersfield, A. J., Butchart, S. H., Evans, M. I., Grimmett, R. F., Jones, V. R., et al. (2008). Key conservation issues for migratory land-and waterbird species on the world’s major flyways. Bird Conserv. Int. 18, S49–S73. doi: 10.1017/S0959270908000439
Kitazawa, M., Senzaki, M., Matsumiya, H., Hara, S., and Mizumura, H. (2020). Drastic decline in the endemic brown shrike subspecies Lanius cristatus superciliosus in Japan. Bird Conserv. Int. [First view]. doi: 10.1017/S095927092556
Kitazawa, M., Yamaura, Y., Senzaki, M., Kawamura, K., Hanioka, M., and Nakamura, F. (2019). An evaluation of five agricultural habitat types for openland birds: abandoned farmland can have comparative values to undisturbed wetland. Ornithol. Sci. 18, 3–16. doi: 10.2326/osj.18.3
Koike, S., Hijikata, N., and Higuchi, H. (2016). Migration and wintering of Chestnut-Cheeked Starlings Agropsar philippensis. Ornithol. Sci. 15, 63–74. doi: 10.2326/osj.15.63
Kokko, H. (1999). Competition for early arrival in migratory birds. J. Anim. Ecol. 68, 940–950. doi: 10.1046/j.1365-2656.1999.00343.x
Kondratyev, A. V. (2014). First breeding record of Fieldfare Turdus pilaris in the Magadan Region. Russ. Ornithol. J. 23, 2785–2787.
Krechmar, A. V., Andreev, A. V., and Kondratyev, A. Y. (1991). Birds of Northern Plains. Leningrad: Nauka.
Krever, V. G., and Ivannikova, T. O. (eds) (2020). Wildlife trade in the Russian Federation. Moscow: WWF Russia.
Ktitorov, P. S., Dolinin, V. V., Golub, A. M., and Zhukov, A. Y. (2019). Annotated list of bird species of the Schmidt Peninsula, Northern Sakhalin, based on the 2016 survey. Proc. Sakh. Mus. 4, 186–202.
Kudo, T. (2008). Migration route and wintering area of Northern Goshawk Accipiter gentilis breeding in Hokkaido, northern Japan. Ornithol. Sci. 7, 99–102. doi: 10.2326/1347-0558(2008)7[99:MRAWAO]2.0.CO;2
Kurdykov, A. B., and Volkovskaya-Kurdyukova, E. A. (2016). The effect of grass fires on the bird population in the open landscapes of South Primorye. J. Russ. Ornithol. 25, 5143–5147.
Kuroda, N. (1961). The over-sea crossing of land birds in the western Pacific. Misc. Rep. Yamashina Inst. Ornithol. Zool. 3, 47–53. doi: 10.3312/jyio1952.3.47
Lee, T. M., Sigouin, A., Pinedo-Vasquez, M., and Nasi, R. (2014). The Harvest of Wildlife for Bushmeat and Traditional Medicine in East, South and Southeast Asia: Current Knowledge Base, Challenges, Opportunities and Areas for Future Research. Occasional Paper 115. Bogor: CIFOR. doi: 10.17528/cifor/005135
Lele, N., and Joshi, P. K. (2009). Analyzing deforestation rates, spatial forest cover changes and identifying critical areas of forest cover changes in North-East India during 1972–1999. Environ. Monitor. Assess. 156:159. doi: 10.1007/s10661-008-0472-6
Let Wild Birds Fly (2016). Replacements of Yellow-Breasted Bunting: Chestnut and Little Buntings, Common Rosefinch Needs to be Protected. Let Wild Birds Fly. Available online at: https://www.weibo.com/ttarticle/p/show?id=2309404522956008325323 (accessed September 27, 2020).
Li, X. (2016). Bird Syndicate Fattens and Sells 30,000 Birds in Guangdong. Guanjia News. Available online at: https://news.qq.com/a/20160907/046305.htm (accessed September 27, 2020).
Lim, K. S., Yong, D. L., Lim, K. C., and Gardner, D. (2020). Field Guide to the Birds of Malaysia and Singapore. Oxford: John Beaufoy Publishing Ltd.
Limparungpatthanakij, W., Nualsri, C., Jearwattanakanok, A., Hansasuta, C., Sutasha, K., Angkaew, R., et al. (2019). Abundance and timing of migratory raptors passing through Khao Dinsor, southern Thailand, in autumn 2015-2016. Forktail 35, 18–37.
Lin, D.-L., and Pursner, S. (eds) (2020). State of Taiwan’s Birds. Taiwan: Endemic Species Research Institute and Taiwan Wild Bird Federation.
Lloyd-Evans, T. L., and Atwood, J. L. (2004). 32 years of changes in passerine numbers during spring and fall migrations in coastal Massachusetts. Wilson J. Ornithol. 116, 1–16. doi: 10.1676/0043-5643(2004)116[0001:YOCIPN]2.0.CO;2
Lobkov, E. (2015). Status of Fieldfare Turdus pilaris in the Russian Far East: progressive range expansion. Russ. Ornithol. J. 24, 789–791.
Lobkov, E., Gerasimov, Y., and Gorovenko, A. (2011). “Status of the Kamchatka Gyrfalcon (Falco rusticolus) population and factors affecting it,” in Gyrfalcons and Ptarmigan in a Changing World, Vol. II, eds R. T. Watson, T. J. Cade, M. Fuller, G. Hunt, and E. Potapov (Boise, ID: The Peregrine Fund), 280–290. doi: 10.4080/gpcw.2011.0127
López-Hoffman, L., Chester, C. C., Semmens, D. J., Thogmartin, W. E., Rodríguez-McGoffin, M. S., Merideth, R., et al. (2017). Ecosystem Services from Transborder Migratory Species: implications for conservation governance. Annu. Rev. Environ. Resour. 42, 509–539. doi: 10.1146/annurev-environ-110615-090119
Ma, Z. J., Cheng, Y., Wang, J., and Fu, X. (2013). The rapid development of birdwatching in mainland China: a new force for bird study and conservation. Bird Conserv. Int. 23, 259–269. doi: 10.1017/S0959270912000378
Ma, Z. J., Gan, X., Choi, C. Y., Jing, K., Tang, S., Li, C., et al. (2007). Wintering bird communities in newly formed wetland in the Yangtze River estuary. Ecol. Res. 22, 115–124. doi: 10.1007/s11284-006-0193-7
Marra, P. P., Hunter, D., and Perrault, A. M. (2011). Migratory connectivity and the conservation of migratory animals. Environ. Law 41, 317–354.
Marshall, H., Collar, N. J., Lees, A. C., Moss, A., Yuda, P., and Marsden, S. J. (2020). Spatio-temporal dynamics of consumer demand driving the Asian Songbird Crisis. Biol. Conserv. 241:108237. doi: 10.1016/j.biocon.2019.108237
Maslovsky, K. S., Valchuk, O. P., and Leliuchina, E. V. (2018). Differential migration and dynamic of state of the transit population of Siberian rubythroat. Bull. Far East. Br. Russ. Acad. Sci. 2, 19–28.
Maslovsky, K. S., Valchuk, O. P., and Spiridonova, L. N. (2014). “The complex study of autumn migration of the siberian rubythroat (Luscinia calliope),” in Southern Primorye: Data Analyses on Banding and Sequencing of Cytochrome b Gene of Mitochondrial DNA, eds A. P. Saveljev and I. V. Seryodkin (Vladivostok: Reia), 181–189.
McClure, H. E. (1974). Migration and Survival of the Birds of Asia. Bangkok: US Army Component, South-East Asia Treaty Organization Medical Project.
McClure, H. E. (1984). Survival among the birds of Eastern and Southeastern Asia. J. Yamashina Inst. Ornithol. 16, 13–62. doi: 10.3312/jyio1952.16.13
McEvoy, J. F., Connette, G., Huang, Q., Soe, P., Htet, K., Pyone, K. H. H., et al. (2019). Two sides of the same coin–Wildmeat consumption and illegal wildlife trade at the crossroads of Asia. Biol. Conserv. 238:108197. doi: 10.1016/j.biocon.2019.108197
McGrady, M. J., Ueta, M., Potapov, E. R., Utekhina, I., Masterov, V., Ladyguin, A., et al. (2003). Movements by juvenile and immature Steller’s Sea Eagles Haliaeetus pelagicus tracked by satellite. Ibis 145, 318–328. doi: 10.1046/j.1474-919X.2003.00153.x
McIntyre, C. L., Douglas, D. C., and Adams, L. G. (2009). Movements of juvenile Gyrfalcons from western and interior Alaska following departure from their natal areas. J. Raptor Res. 43, 99–109. doi: 10.3356/JRR-08-43.1
McKinnon, E. A., and Love, O. P. (2018). Ten years tracking the migrations of small landbirds: lessons learned in the golden age of bio-logging. Auk Ornithol. Adv. 135, 834–856. doi: 10.1642/AUK-17-202.1
McKinnon, E. A., Macdonald, C. M., Gilchrist, H. G., and Love, O. P. (2016). Spring and fall migration phenology of an Arctic-breeding passerine. J. Ornithol. 157, 681–693. doi: 10.1007/s10336-016-1333-7
Medway, L., and Wells, D. R. (1976). The Birds of the Thai-Malay Peninsula. Volume V, Conclusion and Survey of Every Species. London: H.F. and G. Witherby Ltd.
Menkhorst, P., Rogers, D., Clarke, R., Davies, J., Marsack, P., and Franklin, K. (2017). The Australian Bird Guide. Melbourne: CSIRO Publishing.
Meyburg, B. U., and Lobkov, E. G. (1994). Satellite tracking of a juvenile Steller’s Sea Eagle Haliaeetus pelagicus. Ibis 136, 105–106. doi: 10.1111/j.1474-919X.1994.tb08138.x
Møller, A. P., Rubolini, D., and Lehikoinen, E. (2008). Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Nat. Acad. Sci. U.S.A. 105, 16195–16200. doi: 10.1073/pnas.0803825105
Moriguchi, S., Onuma, M., and Goka, K. (2016). Spatial assessment of the potential risk of avian influenza A virus infection in three raptor species in Japan. J. Vet. Med. Sci. 78, 1107–1115. doi: 10.1292/jvms.15-0551
Nakanishi, H. (1996). Fruit color and fruit size of bird-disseminated plants in Japan. Vegetatio 123, 207–218. doi: 10.1007/BF00118272
Nam, H.-Y., Choi, C.-Y., Park, J. G., Hong, G. P., Won, I. J., Kim, S., et al. (2011). Protandrous migration and variation in morphological characters in Emberiza buntings at an East Asian stopover site. Ibis 153, 494–501. doi: 10.1111/j.1474-919X.2011.01134.x
Namkhan, M., Gale, G. A., Savini, T., and Tantipisanuh, N. (2021). Loss and vulnerability of lowland forests in mainland Southeast Asia. Conserv. Biol. 35, 206–215 doi: 10.1111/cobi.13538
Newbold, T., Scharlemann, J. P. W., Butchart, S. H. M., Sekercioglu, Ç. H., Alkemade, R., Booth, H., et al. (2013). Ecological traits affect the response of tropical forest bird species to land-use intensity. Proc. R. Soc. Lond. B 280, 20122131.1–20122131.8. doi: 10.1098/rspb.2012.2131
Nijman, V. (2010). An overview of international wildlife trade from Southeast Asia. Biodivers. Conserv. 19, 1101–1114. doi: 10.1007/s10531-009-9758-4
Nijman, V., Germi, F., and van Balen, S. B. (2006). Relative status of two species of migrant sparrowhawks on Java and Bali, Indonesia. Emu 106, 157–162. doi: 10.1071/MU05016
Nilsson, C., Klaassen, R. H., and Alerstam, T. (2013). Differences in speed and duration of bird migration between spring and autumn. Am. Nat. 181, 837–845. doi: 10.1086/670335
Nisbet, I. C. T. (1976). “The Eastern Palearctic migration system in operation,” in The Birds of the Malay Peninsula, eds L. Medway and D. R. Wells (London: H.F. and G. Witherby Ltd), 57–69.
Nourani, E., Safi, K., Yamaguchi, N. M., and Higuchi, H. (2018). Raptor migration in an oceanic flyway: wind and geography shape the migratory route of grey-faced buzzards in East Asia. R. Soc. Open Sci. 5, 171555. doi: 10.1098/rsos.171555
Osipov, S. V., and Biserov, M. F. (2017). Pyrogenic dynamics of vegetation cover and bird population in a mountain taiga landscape (A case study at the Bureya mountains). Biol. Bull. 44, 449–459. doi: 10.1134/S1062359017040094
Paridi, A., and Noske, R. A. (2017). The illegal trade of Indonesian raptors through social media. Kukila 20, 1–11.
Park, J.-G., Park, C.-U., Jin, K.-S., Kim, Y.-M., and Nam, D.-H. (2020). Population trends and migration patterns in the critically endangered Yellow-breasted Bunting (Emberiza aureola) in the Republic of Korea. Korean J. Ornithol. 27, 56–63. doi: 10.30980/KJO.2020.6.27.1.56
Peh, K. S. H., Soh, M. C., Sodhi, N. S., Laurance, W. F., Ong, D. J., and Clements, R. (2011). Up in the clouds: Is sustainable use of tropical montane cloud forests possible in Malaysia? Bioscience 61, 27–38. doi: 10.1525/bio.2011.61.1.8
Potapov, P., Hansen, M. C., Laestadius, L., Turubanova, S., Yaroshenko, A., Thies, C., et al. (2017). The last frontiers of wilderness: tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 3:e1600821. doi: 10.1126/sciadv.1600821
Potapov, P., Yaroshenko, A., Turubanova, S., Dubinin, M., Laestadius, L., Thies, C., et al. (2008). Mapping the world’s intact forest landscapes by remote sensing. Ecol. Soc. 13:51. doi: 10.5751/ES-02670-130251
Poudel, D. D., Midmore, D. J., and Hargrove, W. L. (1998). An analysis of commercial vegetable farms in relation to sustainability in the uplands of Southeast Asia. Agric. Syst. 58, 107–128. doi: 10.1016/S0308-521X(98)00052-3
Prishchepov, A. V., Schierhorn, F., Dronin, N., Ponkina, E. V., and Müller, D. (2020). “800 years of agricultural land-use change in Asian (Eastern) Russia,” in KULUNDA: Climate Smart Agriculture, eds M. Frühauf, G. Guggenberger, T. Meinel, I. Theesfeld, and S. Lentz (Cham: Springer), 67–87. doi: 10.1007/978-3-030-15927-6_6
Prokop, P. (2018). Tea plantations as a driving force of long-term land use and population changes in the Eastern Himalayan piedmont. Land Use Policy 77, 51–62. doi: 10.1016/j.landusepol.2018.05.035
Rasul, G. (2007). Political ecology of the degradation of forest commons in the Chittagong Hill Tracts of Bangladesh. Environ. Conserv. 34, 153–163. doi: 10.1017/S0376892907003888
Reading, R. P., Azua, J., Garrett, T., Kenny, D., Lee, H., Paek, W. K., et al. (2020). Differential movement of adult and juvenile Cinereous Vultures (Aegypius monachus) in Northeast Asia. J. Asia Pac. Biodivers. 13, 156–161. doi: 10.1016/j.japb.2020.01.004
Redkin, Y. A., Glushenko, Y. N., Korobov, D. V., Murashev, I. A., and Kupriyanov, A. A. (2020). Short-tailed (yellow-bellied) tit Pardaliparus venustulus is a new nesting species of the fauna of Russia. Russ. Ornithol. J. 29, 141–145.
Rentschlar, K. A., Miller, A. E., Lauck, K. S., Rodiansyah, M., Bobby Muflihati et al. (2018). A silent morning: the songbird trade in Kalimantan, Indonesia. Trop. Conserv. Sci. 11:1940082917753909. doi: 10.1177/1940082917753909
Robinson, S. K., and Wilcove, D. S. (1994). Forest fragmentation in the temperate zone and its effects on migratory songbirds. Bird Conserv. Int. 4, 233–249. doi: 10.1017/S0959270900002793
Rosenberg, K. V., Dokter, A. M., Blancher, P. J., Sauer, J. R., Smith, A. C., Smith, P. A., et al. (2019). Decline of the North American avifauna. Science 366, 120–124. doi: 10.1126/science.aaw1313
Round, P. D. (2010). An analysis of records of three passage migrants in Thailand: Tiger Shrike Lanius tigrinus, Yellow-rumped Flycatcher Ficedula zanthopygia and Mugimaki Flycatcher F. mugimaki. Forktail 26, 24–31.
Round, P. D., and Allen, D. (2010). A record of active moult in the Streaked Reed Warbler Acrocephalus sorghophilus. Bull. Brit. Ornithol. Club 130, 145–146.
Runge, C. A., Martin, T. G., Possingham, H. P., Willis, S. G., and Fuller, R. A. (2014). Conserving mobile species. Front. Ecol. Environ. 12, 395–402. doi: 10.1890/130237
Runge, C. A., Watson, J. E., Butchart, S. H., Hanson, J. O., Possingham, H. P., and Fuller, R. A. (2015). Protected areas and global conservation of migratory birds. Science 350, 1255–1258. doi: 10.1126/science.aac9180
Sander, M. M., Eccard, J. A., and Heim, W. (2017). Flight range estimation of migrant Yellow-browed Warblers Phylloscopus inornatus on the East Asian Flyway. Bird Study 64, 1–4. doi: 10.1080/00063657.2017.1409696
Sander, M. M., Heim, W., and Schmaljohann, H. (2020). Seasonal and diurnal increases in energy stores in migratory warblers at an autumn stopover site along the Asian–Australasian flyway. J. Ornithol. 161, 73–87. doi: 10.1007/s10336-019-01701-7
Sanderson, F. J., Donald, P. F., Pain, D. J., Burfield, I. J., and Van Bommel, F. P. (2006). Long-term population declines in Afro-Palearctic migrant birds. Biol. Conserv. 131, 93–105. doi: 10.1016/j.biocon.2006.02.008
Scheffers, B. R., Oliveira, B. F., Lamb, I., and Edwards, D. P. (2019). Global wildlife trade across the tree of life. Science 366, 71–76. doi: 10.1126/science.aav5327
Schellekens, M., Trainor, C. R., and Duhan, G. U. U. (2011). New and significant bird records for Solor, Adonara, and Lembata (Lomblen) islands, Lesser Sundas. Kukila 15, 31–49.
Şekercioğlu, Ç. H., Daily, G. C., and Ehrlich, P. R. (2004). Ecosystem consequences of bird declines. Proc. Nat. Acad. Sci. U.S.A. 101, 18042–18047. doi: 10.1073/pnas.0408049101
Seki, S.-I., and Ogura, T. (2007). Breeding origins of migrating Ryukyu Robins Erithacus komadori inferred from mitochondrial control region sequences. Ornithol. Sci. 6, 21–27. doi: 10.2326/1347-0558(2007)6[21:BOOMRR]2.0.CO;2
Senda, M., Deguchi, T., Komeda, S., Shigeta, Y., Sato, F., Yoshiyasu, K., et al. (2018). Increasing the number of captured migrant buntings at an autumn stopover site using sound lures. Ornithol. Sci. 17, 103–108. doi: 10.2326/osj.17.103
Severinghaus, L. L., and Chi, L. (1999). Prayer animal release in Taiwan. Biol. Conserv. 89, 301–304. doi: 10.1016/S0006-3207(98)00155-4
Severinghaus, L. L., Ding, T.-S., Fang, W.-H., Lin, W.-H., Tsai, M.-C., and Yen, C.-W. (2017). The Avifauna of Taiwan, Vol. 1. Taipei: Forestry Bureau, Council of Agriculture.
Shiu, H.-J., Tokita, K., Morishita, E., Hiraoka, E., Wu, Y., Nakamura, H., et al. (2006). Route and site fidelity of two migratory raptors: Grey-faced Buzzards Butastur indicus and Honey-buzzards Pernis apivorus. Ornithol. Sci. 5, 151–156. doi: 10.2326/1347-0558(2006)5[151:RASFOT]2.0.CO;2
Shtegman, B. K. (1938). “Fundamentals of the Ornithogeographic Division of the Palearctic,” in Fauna of the Soviet Union: Birds, eds S. A. Zernov and A. A. Schtakelberg (Moscow: AN SSSR).
Smith, R. J., and Moore, F. R. (2005). Arrival timing and seasonal reproductive performance in a long-distance migratory landbird. Behav. Ecol. Sociobiol. 57, 231–239. doi: 10.1007/s00265-004-0855-9
Sodhi, N. S., Koh, L. P., Brook, B. W., and Ng, P. K. (2004). Southeast Asian biodiversity: an impending disaster. Trends Ecol. Evol. 19, 654–660. doi: 10.1016/j.tree.2004.09.006
Soh, M. C., Mitchell, N. J., Ridley, A. R., Butler, C. W., Puan, C. L., and Peh, K. S. H. (2019). Impacts of habitat degradation on tropical montane biodiversity and ecosystem services: a systematic map for identifying future research priorities. Front. For. Glob. Chang. 2:83. doi: 10.3389/ffgc.2019.00083
Sokolovskis, K., Bianco, G., Willemoes, M., Solovyeva, D., Bensch, S., and Åkesson, S. (2018). Ten grams and 13,000 km on the wing–route choice in willow warblers Phylloscopus trochilus yakutensis migrating from Far East Russia to East Africa. Move. Ecol. 6, 1–10. doi: 10.1186/s40462-018-0138-0
Somveille, M., Manica, A., Butchart, S. H. M., and Rodrigues, A. S. L. (2013). Mapping global diversity patterns for migratory birds. PLoS One 8:e70907. doi: 10.1371/journal.pone.0070907
Soulé, M. E., and Orians, G. H. (2001). Conservation Biology: Research Priorities For The Next Decade. Washington, DC: Island Press.
Stibig, H. J., Achard, F., Carboni, S., Rasi, R., and Miettinen, J. (2014). Change in tropical forest cover of Southeast Asia from 1990 to 2010. Biogeosciences 11, 247–258. doi: 10.5194/bg-11-247-2014
Stretesky, P. B., McKie, R. E., Lynch, M. J., Long, M. A., and Barrett, K. L. (2018). Where have all the falcons gone? Saker falcon (Falco cherrug) exports in a global economy. Glob. Ecol. Conserv. 13:e00372. doi: 10.1016/j.gecco.2017.e00372
Studds, C. E., Kendall, B. E., Murray, N. J., Wilson, H. B., Rogers, D. I., Clemens, R. S., et al. (2017). Rapid population decline in migratory shorebirds relying on Yellow Sea tidal mudflats as stopover sites. Nat. Commun. 8:14895. doi: 10.1038/ncomms14895
Sugasawa, S., and Higuchi, H. (2019). Seasonal contrasts in individual consistency of oriental honey buzzards’ migration. Biol. Lett. 15:20190131. doi: 10.1098/rsbl.2019.0131
Sun, Y., Cui, P., Li, J., Li, M., Wu, Y., and Li, D. (2018). The Common Koel Eudynamys scolopaceus in northern China, new distributional information, and a brief review of range extensions of cuckoos in China. Ornithol. Sci. 17, 217–221. doi: 10.2326/osj.17.217
Sun, Y., Luo, X., Mo, L., Zhang, Q., Wu, J., Chen, S., et al. (2012). Brominated flame retardants in three terrestrial passerine birds from South China: geographical pattern and implication for potential sources. Environ. Pollut. 162, 381–388. doi: 10.1016/j.envpol.2011.12.013
Supp, S. R., Sorte, F. A. L., Cormier, T. A., Lim, M. C., Powers, D. R., Wethington, S. M., et al. (2015). Citizen-science data provides new insight into annual and seasonal variation in migration patterns. Ecosphere 6, 1–19. doi: 10.1890/ES14-00290.1
Syartinilia, Makalew, A. D., Mulyani, Y. A., and Higuchi, H. (2015). Landscape characteristics derived from satellite-tracking data of wintering habitats used by oriental honey buzzards in Borneo. Landsc. Ecol. Eng. 11, 61–71. doi: 10.1007/s11355-013-0237-4
Syroechkovskiy, E. E., Morozov, V. V., Tomkovich, P. S., Golub, E. V., Kondratyev, A. V., Kuzmich, A. A., et al. (2019). Bird species new for Southern Chukotka. Ornitologia 43, 45–73.
Szabo, J. K., Choi, C. Y., Clemens, R., and Hansen, B. (2016). Conservation without borders–solutions to declines of migratory shorebirds in the East Asian–Australasian Flyway. Emu 116, 215–221. doi: 10.1071/MU15133
Takagi, K., Tokita, K., Hiraoka, E. N., Uchida, K., Tsutsumi, A., Hijikata, N., et al. (2014). Migration routes and breeding sites of Rooks wintering in the Hachiro-gata polder, northern Japan. Jpn. J. Ornithol. 63, 317–322. doi: 10.3838/jjo.63.317
Tamada, K., Hayama, S., Umeki, M., Takada, M., and Tomizawa, M. (2017). Drastic declines in Brown Shrike and Yellow-breasted Bunting at the Lake Utonai Bird Sanctuary, Hokkaido. Ornithol. Sci. 16, 51–57. doi: 10.2326/osj.16.51
Tamada, K., Tomizawa, M., Umeki, M., and Takada, M. (2014). Population trends of grassland birds in Hokkaido, focussing on the drastic decline of the Yellow-breasted Bunting. Ornithol. Sci. 13, 29–40. doi: 10.2326/osj.13.29
Tape, K. E. N., Sturm, M., and Racine, C. (2006). The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Glob. Chang. Biol. 12, 686–702. doi: 10.1111/j.1365-2486.2006.01128.x
Tarburton, M. K. (2014). Status of the White-throated Needletail Hirundapus caudacutus in Australia: evidence for a marked decline. Aus. Field Ornithol. 31, 122–140.
Taylor, P., Crewe, T., Mackenzie, S., Lepage, D., Aubry, Y., Crysler, Z., et al. (2017). The Motus Wildlife Tracking System: a collaborative research network to enhance the understanding of wildlife movement. Avian Conserv. Ecol. 12:8. doi: 10.5751/ACE-00953-120108
Teluguntla, P., Thenkabail, P. S., Xiong, J., Gumma, M. K., Giri, C., Milesi, C., et al. (2015). “Global Cropland Area Database (GCAD) derived from remote sensing in support of food security in the twenty-first century: current achievements and future possibilities,” in Land Resources: Monitoring, Modelling, and Mapping, Remote Sensing Handbook, ed. P. S. Thenkabail (Boca Raton, FL: Taylor and Francis).
Terborgh, J. (1992). Why American songbirds are vanishing. Sci. Am. 266, 98–105. doi: 10.1038/scientificamerican0592-98
Thorup, K., Tøttrup, A. P., and Rahbek, C. (2007). Patterns of phenological changes in migratory birds. Oecologia 151, 697–703. doi: 10.1007/s00442-006-0608-8
Thorup, K., Vega, M. L., Snell, K. R. S., Lubkovskaia, R., Willemoes, M., Sjöberg, S., et al. (2020). Flying on their own wings: young and adult cuckoos respond similarly to long-distance displacement during migration. Sci. Rep. 10:7698. doi: 10.1038/s41598-020-64230-x
Tilker, A., Abrams, J. F., Mohamed, A., Nguyen, A., Wong, S. T., Sollmann, R., et al. (2019). Habitat degradation and indiscriminate hunting differentially impact faunal communities in the Southeast Asian tropical biodiversity hotspot. Commun. Biol. 2, 1–11. doi: 10.1038/s42003-019-0640-y
Tojo, H. (2015). Seasonality of the bird community in the Ogawa Forest Reserve, an old-growth deciduous forest in central Japan. Ornithol. Sci. 14, 79–88. doi: 10.2326/osj.14.79
Tordoff, A. W. (2002). Raptor migration at Hoang Lien Nature Reserve, northern Vietnam. Forktail 18, 45–48.
Townshend, T. (2016a). The Beijing Cuckoo Project. Available online at: https://birdingbeijing.com/beijing-cuckoo-project/ (accessed October 1, 2020).
Townshend, T. (2016b). The Invisible Killers: Mist Nets at Chinese Airports. Available online at: https://birdingbeijing.com/2016/03/02/the-invisible-killer-mist-nets-at-chinese-airports/ (accessed October 1, 2020).
Townshend, T. (2019). The Mongolia Cuckoo Project. Available online at: https://birdingbeijing.com/the-mongolia-cuckoo-project/ (accessed October 1, 2020).
Trainor, C. R., Coates, B. J., and Bishop, K. D. (2007). As Aves de Timor-Leste. Melbourne: Dove Publications.
Tsai, P. Y., Ko, C. J., Chia, S. Y., Lu, Y. J., and Tuanmu, M. N. (2021). New insights into the patterns and drivers of avian altitudinal migration from a growing crowdsourcing data source. Ecography 44, 75–86. doi: 10.1111/ecog.05196
Tulloch, A. I. T., Mustin, K., Possingham, H. P., Szabo, J. K., and Wilson, K. A. (2013a). To boldly go where no volunteer has gone before: predicting volunteer activity to prioritize surveys at the landscape scale. Divers. Distrib. 19, 465–480. doi: 10.1111/j.1472-4642.2012.00947.x
Tulloch, A. I. T., Possingham, H. P., Joseph, L. N., Szabo, J. K., and Martin, T. G. (2013b). Realising the full potential of citizen science monitoring programs. Biol. Conserv. 165, 128–138. doi: 10.1016/j.biocon.2013.05.025
Uemura, S., Hamachi, A., Nakachi, K., and Takagi, M. (2020). First tracking of post-breeding migration of the Ruddy Kingfisher Halcyon coromanda by GPS data logger. Ornithol. Sci. 18, 215–219. doi: 10.2326/osj.18.219
Ueta, M., Sato, F., Lobkov, E. G., and Mita, N. (1998). Migration route of White-tailed Sea Eagles Haliaeetus albicilla in northeastern Asia. Ibis 140, 684–686. doi: 10.1111/j.1474-919X.1998.tb04715.x
Ueta, M., Sato, F., Nakagawa, H., and Mita, N. (2000). Migration routes and differences of migration schedule between adult and young Steller’s Sea Eagles Haliaeetus pelagicus. Ibis 142, 35–39. doi: 10.1111/j.1474-919X.2000.tb07681.x
Valchuk, O., Maslovsky, K. S., Leliukhina, E., Irinjakov, D., Gordienko, I., Prokopenko, O., et al. (2017). Long-Term Dynamics and Trends in the Number of Passerine Birds during Migrations in Southern Primorye. In Population Dynamics of Birds in Terrestrial Landscapes. Dedicated to the 30th Anniversary of Wintering Bird Monitoring Programs of Russia and Neighboring Regions. Moscow: KMK Scientific Press, 100–108.
Van Allen, B. G., Dunham, A. E., Asquith, C. M., and Rudolf, V. H. W. (2012). Life history predicts risk of species decline in a stochastic world. Proc. R. Soc. Lond. B 279, 2691–2697. doi: 10.1098/rspb.2012.0185
Vandergert, P., and Newell, J. (2003). Illegal logging in the Russian Far East and Siberia. Int. Forest. Rev. 5, 303–306. doi: 10.1505/IFOR.5.3.303.19150
Venter, O., Sanderson, E., Magrach, A., Allan, J. R., Beher, J., Jones, K. R., et al. (2016). Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 7:12558. doi: 10.1038/ncomms12558
Vickery, J. A., Ewing, S. R., Smith, K. W., Pain, D. J., Bairlein, F., Škorpilová, J., et al. (2014). The decline of Afro-Palaearctic migrants and an assessment of potential causes. Ibis 156, 1–22. doi: 10.1111/ibi.12118
Virkkala, R., and Lehikoinen, A. (2014). Patterns of climate-induced density shifts of species: poleward shifts faster in northern boreal birds than in southern birds. Glob. Chang Biol. 20, 2995–3003. doi: 10.1111/gcb.12573
Vorisek, P., Gregory, R. D., Van Strien, A. J., and Meyling, A. G. (2008). Population trends of 48 common terrestrial bird species in Europe: results from the Pan-European Common Bird Monitoring Scheme. Rev. Catal. d’Ornitol. 24, 4–14.
Wang, Y., Chang, J., Moore, F. R., Su, L., Cui, L., and Yang, X. (2006). Stopover ecology of red-flanked bush robin (Tarsiger cyanurus) at Maoershan, Northeast China. Acta Ecol. Sin. 26, 638–646. doi: 10.1016/S1872-2032(06)60011-5
Wang, Y., Yin, Y., Ren, Z., Jiang, C., Sun, Y., Li, J., et al. (2020). A comparison of flight energetics and kinematics of migratory Brambling and residential Eurasian Tree Sparrow. Avian Res. 11, 1–9. doi: 10.1186/s40657-020-00211-y
Wang, Z., Carpenter, C., and Young, S. S. (2000). Bird distribution and conservation in the Ailao Mountains, Yunnan, China. Biol. Conserv. 92, 45–57. doi: 10.1016/S0006-3207(99)00058-0
Warnock, N. (2010). Stopping vs. staging: the difference between a hop and a jump. J. Avian Biol. 41, 621–626. doi: 10.1111/j.1600-048X.2010.05155.x
Welch, G. (2011). Bird migration on Po Toi Island, Hong Kong. Hong Kong Bird Rep. 2007–2008, 310–346.
Wells, D. R. (2007). The Birds of the Thai-Malay Peninsula. Volume 2: Passerines. London: Christopher Helm.
Wen, C. L. (ed.) (2018). Over 510,000 Wild Birds Caught and Sold in 40 Days, with 34 Suspects Were Arrested in Linxiang, Hunan. The Paper. Available online at: https://www.thepaper.cn/newsDetail_forward_2051565 (accessed September 27, 2020).
Weng, G.-J., Lin, H.-S., Sun, Y.-H., and Walther, B. A. (2014). Molecular sexing and stable isotope analyses reveal incomplete sexual dimorphism and potential breeding range of Siberian Rubythroats Luscinia calliope captured in Taiwan. Forktail 30, 96–103.
Whelan, C. J., Wenny, D. G., and Marquis, R. J. (2008). Ecosystem services provided by birds. Ann. N.Y. Acad. Sci. 1134, 25–60. doi: 10.1196/annals.1439.003
Whitaker, D. (2017). Expanded range limits of boreal birds in the Torngat Mountains of Northern Labrador. Can. Field Nat. 131, 55–62. doi: 10.22621/cfn.v131i1.1957
White, C. M. N., and Bruce, M. D. (1986). The Birds of Wallacea. B.O.U. Checklist No. 7. London: British Ornithologist’s Union.
Wilcove, D. S., and Wikelski, M. (2008). Going, going, gone: is animal migration disappearing. PLoS Biol. 6:e188. doi: 10.1371/journal.pbio.0060188
Wobker, J., Heim, W., and Schmaljohann, H. (2021). Sex, age, molt strategy and approximated migration distance explain the variation in phenology of songbirds at a stopover along the East Asian flyway in spring and autumn. Behav. Ecol. Sociobiol. 75, 1–14. doi: 10.1007/s00265-020-02957-3
Won, P.-O., Woo, H.-C., Ham, K.-W., and Yoon, M.-B. (1966). Seasonal distribution and ecology of migrant bird populations by mistnetting and banding in Korea (I). J. Yamashina Inst. Ornithol. 8, 405–444. doi: 10.3312/jyio1952.4.405
Wong, C. L. C., Lam, V. W. Y., and Ades, G. W. J. (2009). Ecology of the Birds of Hong Kong. Hong Kong: Kaddoorie Farm and Botanical Gardens.
Wood, C., Qiao, Y., Li, P., Ding, P., Lu, B., and Xi, Y. (2010). Implications of rice agriculture for wild birds in China. Waterbirds 33, 30–43. doi: 10.1675/063.033.s103
World Health Organisation (2017). One Health. Available online at: https://www.who.int/news-room/q-a-detail/one-health (accessed September 28, 2020).
Wyatt, T. (2011). The illegal trade of raptors in the Russian Federation. Contemp. Justice Rev. 14, 103–123. doi: 10.1080/10282580.2011.565969
Xu, Y., Lin, S., He, J., Xin, Y., Zhang, L., Jiang, H., et al. (2017). Tropical birds are declining in the Hainan Island of China. Biol. Conserv. 210, 9–18. doi: 10.1016/j.biocon.2016.05.029
Yakovleva, M. V., and Sukhov, A. V. (2017). Rustic Bunting Ocyris rusticus at Kivach nature reserve. Russ. Ornithol. J. 6, 726–731.
Yamaguchi, N., Tokita, K., Uematsu, A., Kuno, K., Saeki, M., Hiraoka, E., et al. (2008). The large-scale detoured migration route and the shifting pattern of migration in Oriental honey-buzzards breeding in Japan. J. Zool. 276, 54–62. doi: 10.1111/j.1469-7998.2008.00466.x
Yamaguchi, N. M., Arisawa, Y., Shimada, Y., and Higuchi, H. (2012). Real-time weather analysis reveals the adaptability of direct sea-crossing by raptors. J. Ethol. 30, 1–10. doi: 10.1007/s10164-011-0301-1
Yamaguchi, N. M., Hiraoka, E., Hijikata, N., and Higuchi, H. (2017). Migration routes of satellite-tracked Rough-legged Buzzards from Japan: the relationship between movement patterns and snow cover. Ornithol. Sci. 16, 33–41. doi: 10.2326/osj.16.33
Yamaura, Y., Schmaljohann, H., Lisovski, S., Senzaki, M., Kawamura, K., et al. (2017). Tracking the Stejneger’s stonechat Saxicola stejnegeri along the East Asian–Australian Flyway from Japan via China to southeast Asia. J. Avian Biol. 48, 197–202. doi: 10.1111/jav.01054
Yamazaki, T., Nitani, Y., Murate, T., Lim, K. C., Kasorndorkbua, C., Rakhman, Z., et al. (2012). Field Guide to the Raptors of Asia, Vol. 1: Migratory Raptors of Oriental Asia. Asian Raptor Research and Conservation Network. Ulaanbaatar: Mongolica Tourism, Consulting & Publishing.
Yong, D. L., Jain, A., Liu, Y., Iqbal, M., Choi, C. Y., Crockford, N. J., et al. (2018). Challenges and opportunities for transboundary conservation of migratory birds in the East Asian-Australasian flyway. Conserv. Biol. 32, 740–743. doi: 10.1111/cobi.13041
Yong, D. L., and Liu, Y. (2015). Passage of Brown-chested Jungle Flycatcher Rhinomyias brunneatus through Singapore, with notes on wintering status in South-East Asia. Forktail 31, 43–46.
Yong, D. L., Liu, Y., Low, B. W., Española, C. P., Choi, C.-Y., and Kawakami, K. (2015). Migratory songbirds in the East Asian-Australasian Flyway: a review from a conservation perspective. Bird Conserv. Int. 25, 1–37. doi: 10.1017/S0959270914000276
Zalles, J. I., and Bildstein, K. L. (eds) (2000). Raptor Watch – A Global Directory Of Raptor Migration Sites. Cambridge: BirdLife International and USA.
Zeng, Z., Estes, L., Ziegler, A. D., Chen, A., Searchinger, T., Hua, F., et al. (2018). Highland cropland expansion and forest loss in Southeast Asia in the twenty-first century. Nat. Geosci. 11, 556–562. doi: 10.1038/s41561-018-0166-9
Zhao, M., Alström, P., Hu, R., Zhao, C., Hao, Y., Lei, F., et al. (2017). Phylogenetic relationships, song and distribution of the endangered Rufous-headed Robin Larvivora ruficeps. Ibis 159, 204–216. doi: 10.1111/ibi.12426
Zhao, X., Zhang, M., Che, X., and Zou, F. (2020). Blue light attracts nocturnally migrating birds. Condor 122:duaa002. doi: 10.1093/condor/duaa002
Keywords: biome, climate change, ecosystem role, habitat loss, hunting, land use change, migration, tracking
Citation: Yong DL, Heim W, Chowdhury SU, Choi C-Y, Ktitorov P, Kulikova O, Kondratyev A, Round PD, Allen D, Trainor CR, Gibson L and Szabo JK (2021) The State of Migratory Landbirds in the East Asian Flyway: Distributions, Threats, and Conservation Needs. Front. Ecol. Evol. 9:613172. doi: 10.3389/fevo.2021.613172
Received: 01 October 2020; Accepted: 08 February 2021;
Published: 13 April 2021.
Edited by:
Binbin Li, Duke Kunshan University, ChinaReviewed by:
Lei Cao, Research Center for Eco-Environmental Sciences (CAS), ChinaCopyright © 2021 Yong, Heim, Chowdhury, Choi, Ktitorov, Kulikova, Kondratyev, Round, Allen, Trainor, Gibson and Szabo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ding Li Yong, ZGluZ2xpLnlvbmdAYmlyZGxpZmUub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.