- 1Evolutionary Ecology Laboratories, Department of Biology, Brigham Young University, Provo, UT, United States
- 2Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT, United States
Why bilaterally symmetrical organisms express handedness remains an important question in evolutionary biology. In some species, anatomical asymmetries have evolved that accompany behavioral handedness, yet we know remarkably little about causal links between asymmetric morphological traits and behavior. Here, we explore if a dextral or sinistral orientation of the male intromittent organ predicts side preferences in male behaviors. Our study addresses this question in the Costa Rican livebearing fish, Xenophallus umbratilis. This fish has a bilaterally symmetrical body plan, with one exception—the male anal fin (gonopodium), used to inseminate females, terminates with a distinct left- or right-handed corkscrew morphology. We used a detour assay to test males for side biases in approach behavior when exposed to four different stimuli (predator, potential mate, novel object, empty tank control). We found that left morph males preferred using their right eye to view potential mates, predators, and the control, and that right morph males preferred to use their left eye to view potential mates and predators, and their right eye to view the control. Males of both morphs displayed no eye bias when approaching the novel object. Our results suggest that there is a strong link between behavior and gonopodium orientation, with right and left morph males responding with opposite directional behaviors when presented with the same stimuli. This presents the intriguing possibility that mating preferences—in this case constrained by gonopodial morphology—could be driving lateralized decision making in a variety of non-mating behaviors.
Introduction
Body plan can have an important effect on behavior. For example, radially symmetrical species move differently than bilaterally symmetrical species (Hollo and Novak, 2012; Wakita et al., 2020). For example, bilateral symmetry typically results in behaviors that occur in an anterior-posterior orientation, including foraging (Jumars et al., 2015; Kane et al., 2017), locomotion (Hollo and Novak, 2012), and mating (Koshio et al., 2007), whereas radial symmetry leads to no such orientation (Wakita et al., 2020). An important property of bilateral symmetry is that it also allows an individual to express handedness, defined here as the propensity to use one side of the body preferentially over the other (Palmer, 2006; Bryden, 2016); we use the term “handed” in its broad sense as defined in previous work (Hata et al., 2011; Buchanan et al., 2015). Handedness is typically understood in a morphological context (Bock and Marsh, 1991), such as differences in the properties of structures [e.g., claw size in crabs (Spani et al., 2020)], or the orientation of structures [e.g., shell torsion in snails (Kurita and Wada, 2011)]. However, handedness can also be expressed behaviorally (Wiper, 2017). This occurs through decision-making, expressed where an individual moves to one side preferentially over the other, a phenomenon we refer to as behavioral handedness.
There is a growing body of research focused on behavioral handedness. For example, we know that such handedness can be expressed differently among closely related species (Bisazza et al., 1997b). We also know that in some cases, males and females differ from each other (Fitch et al., 1993). Further, we know that behavioral handedness can vary depending on environmental context, including factors such as individual posture or task complexity in primates, or neonatal handling in rats (Fitch et al., 1993; Fu et al., 2019). However, in each of these cases, the focal species studied exhibited morphological symmetry—that is, the morphologies on the left and right sides of the body were essentially mirror images. What happens in species that are bilaterally asymmetrical in some aspect of their morphology? We are still learning about behavioral handedness in these cases (Vallortigara and Rogers, 2005; Gunturkun et al., 2020). Clearly, such information may be critical to understanding why such behavioral biases evolved in the first place.
To address this gap in our understanding requires a species that shows a morphological asymmetry for some functional trait, and that also has the potential to show behavioral handedness. Here we present such a system. Xenophallus umbratilis is a livebearing fish native to Costa Rica; it shows a morphological asymmetry in the male mating structure, the gonopodium. We posit that this forces males to approach potential mates by turning their body to the left or right, in order to evaluate a female and to successfully copulate with her. Previous work in livebearing fishes shows that in some species, males have an eye-bias wherein they use one eye to evaluate mates and the other eye to evaluate risk, such as predators (de Andrade and de Sousa, 2018). Hence, in X. umbratilis, it may be that such eye-biases exist, but that they are expressed differently in left morph males vs. right morph males.
In this study, we address two questions. First, does X. umbratilis show behavioral handedness in response to different types of stimuli? To address this question, we tested if individuals have an eye preference as they approach potential mates, predators, and a novel object. We compared eye preferences across these different stimuli between left morph males and right morph males. Second, we asked if gonopodial morph had an effect on behavioral handedness? That is, does gonopodium morphology predict the direction of detour behavior? To address our second question, we compared preferences of right and left morph males when approaching each of the different stimuli. We found that behavioral handedness does indeed occur in this species, and more importantly, that it is associated with the orientation of the gonopodium.
Materials and Methods
Study System
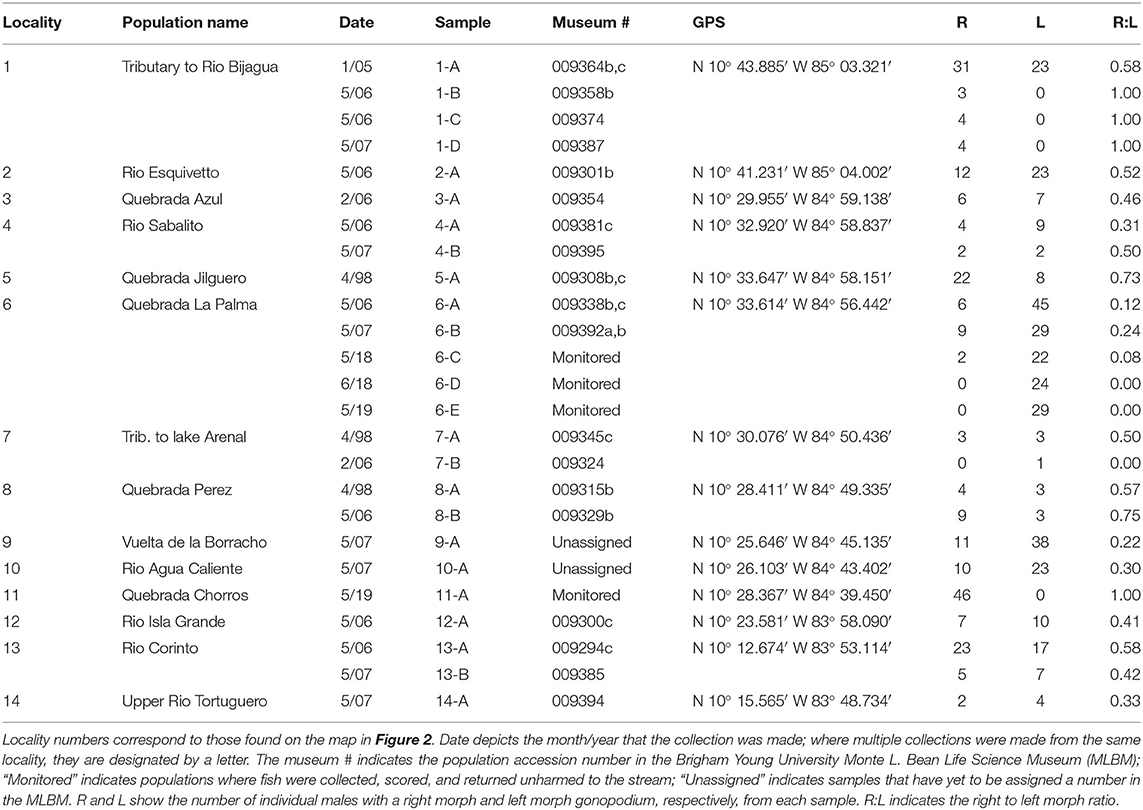
We addressed our questions using the livebearing fish species, X. umbratilis (hereafter referred to as Xenophallus). Xenophallus is the lone species in a monotypic genus (Parenti and Rauchenberger, 1989; Jones and Johnson, 2009). Its range includes several drainages in the Arenal and Guanacaste provinces of northern Costa Rica (Bussing, 1987). This species—like all members of the family Poeciliidae—give birth to live, free-swimming young that develop from eggs fertilized inside the female. Males transfer sperm directly to females using a modified anal fin called the gonopodium, which they bring in contact with the urogenital pore of the female. This structure varies among species, ranging from relatively simple rod-like morphs to rather complex forms with barbs and hooks (Langerhans, 2011). Yet, the gonopodium of Xenophallus is unlike other livebearers. It shows a unique form of asymmetry. At the tip of the shaft, the gonopodium turns in either a dextral or sinistral corkscrew (Figure 1). In the wild, both morphs are common within populations; in fact, in all populations that have been adequately sampled (n >5 males) to date, we found both male morphs (with one exception; see Table 1). However, the frequency of each morph appears to vary among populations at any given time; of 14 populations with both morphs that we surveyed between 1998 and 2019, we found dextral morph frequency ranged from 0.12 to 0.75 (see Results for details). Hence, gonopodium asymmetry in this species appears to be common and widespread.
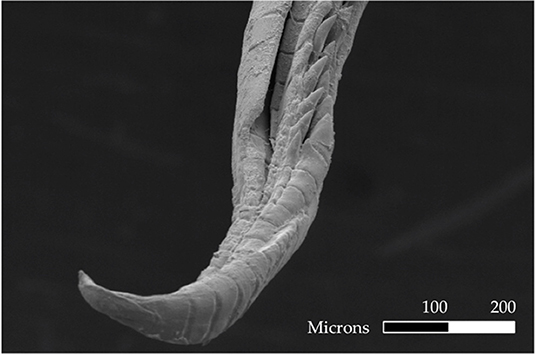
Figure 1. Electron micrograph of a male gonopodium with a sinistral orientation. Scale bar is 100 microns.
To test our hypotheses, we used live fish held in captivity that we collected from two locations in Costa Rica (Figure 2). In June 2018, we sampled Xenophallus from Quebrada La Palma, a small stream in the Lake Arenal drainage of northern Costa Rica. In April 2019, we sampled Xenophallus from Quebrada Chorros, a similar stream, also in the Lake Arenal drainage, near the city of Fortuna. We chose these two locations for several reasons: first, they are geographically close to each other, and therefore likely to experience similar micro-climate effects in the wild (Figure 2); second, the locations are ecologically similar for a variety of measures (Supplementary Table 1); and finally, at the time of collection, the two localities differed in the relative frequency of left- and right-morph males, allowing us to bring both morph types into the lab (Table 1). For both sampling events, we collected and transported 150 live fish to the fish breeding facility at Brigham Young University, where they were treated for parasites and held under common laboratory conditions (as described below). By chance, all adult males available for this study from Quebrada La Palma were left morph males and all adult males available from Quebrada Chorros were right morph males (see Table 1 for collection details). We recognize the potential confounding influence of locality with male morph type; however, our sampling was designed to minimize ecological differences between sites (see above). Hence, if locality effects exist, they are less likely to be ecological than genetic, a topic we will address in future work focused on the inheritance of male gonopodial morphs. Fish held in the lab, both males and females, were fed ad libitum twice daily and kept in 10-gallon tanks at 23°C on a 12L:12D light cycle. For both collections, individuals were kept in this common laboratory environment for 4 months prior to testing; in this way, we attempted to minimize any affect that condition might have on behavior during the trials.
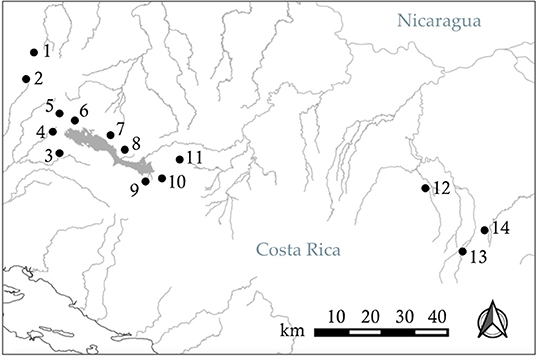
Figure 2. Distribution of 14 populations of Xenophallus across their range in northern Costa Rica, sampled or monitored from 1998 to 2019. See Table 1 for details.
Experimental Design
Our study was designed to determine if Xenophallus males show a bias with respect to the side that they use to approach different stimuli, and if this bias differs between left morph males and right morph individuals. We isolated 15 males of each morph (30 total fish) by placing them in individual tanks so each fish could be exposed to each stimulus (details explained below). We used a detour test approach similar to the one used by Bisazza et al. (1997a,b) to test for behavior handedness, a technique widely adopted in behavioral research (Kabadayi et al., 2018). This involved placing a single male in an arena built within a tank (Figure 3) and allowing him to swim back and forth from one end to the other. At each end of the raceway in the arena the male came to a fork where he was given a choice to proceed to the right or left. In a screened area behind the detour, we placed a visual stimulus. This enabled the individual in the trial to see the stimulus as it approached the detour fork, but in order to clearly view the stimulus, the focal male had to detour either to the right or the left. Individuals were allowed to move in the tank in both directions, making a choice each time they move from one end to the other. They were allowed to move freely without prodding so we could attribute their detour behavior directly to the stimulus. Each fish was given 20 min in the arena and we recorded the number of right and left detours. The male was then removed from the arena and the water was filtered for 10 min prior to the next trial. This was repeated for up to 30 individual males (15 dextral and 15 sinistral) for each visual stimulus, although for some treatments we tested slightly fewer males (n = 13–15 per male morph per treatment). Individuals were randomly assigned to each stimulus treatment (see below). We deemed this sample size sufficiently large based on similar studies in other species (Bisazza et al., 1997a; Torres-Dowdall et al., 2019), but not so large as to unnecessarily use live animals as per our approved IACUC protocol (see Acknowledgments). The entire arena was housed in a walk-in, sound-proof room, allowing all observations to be made remotely via a camera mounted over the tank.
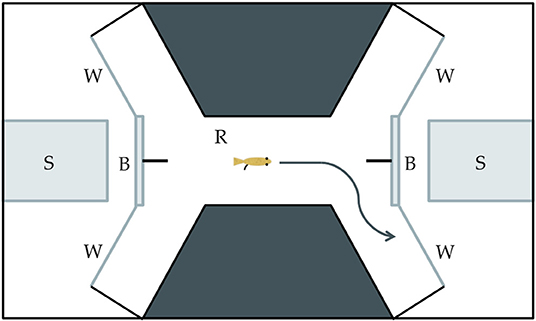
Figure 3. Drawing of the experimental tank used in the detour behavioral assay. (S) The stimulus insert is made of clear glass and used to house the live, physical stimulus. (B) The semi-opaque barrier partially obscures the stimulus from an individual in the raceway area. (W) The viewing window allows an individual to clearly see a stimulus through clear glass. (R) The raceway is where an individual moves toward a stimulus and has the opportunity to make a left or right detour.
We predicted that Xenophallus males might respond differently to different stimuli. Hence, we ran our detour assay with three different live stimuli as well as a control. The stimuli we tested were: (1) Xenophallus adult females (potential mates); (2), the fish Parachromis dovii (a known predator of Xenophallus); (3) a novel object (Lego, see explanation below) that Xenophallus would have never previously encountered; and (4) a negative control which consisted of an empty stimulus tank. These stimuli were chosen with the idea that males might specialize in the eye that they use to secure this visual information, perhaps as a function of their gonopodial anatomy. The stimuli were physically placed at either end of the tank. When viewed from straight on, the stimulus was partially obscured (see Figure 3), requiring the fish to make a lateral choice to view it clearly. Because teleost fish are unable to use both eyes to focus on the same object (Land, 2015), this test reveals which eye a fish actually uses to view the stimulus. We isolated six size-matched females to be used as potential mate stimuli, randomly selecting two of them to use in each trial. Predators were similarly selected for use from four different P. dovii individuals held in the lab. Finally, we chose a Lego stack, consisting of orange, yellow, blue, white, and red blocks in the shape of a “Y” as a novel object. This was an item that we thought would attract the interest of Xenophallus males, but that was completely foreign to this species. Overall, this design allowed us to determine if there is a behavioral bias in the way that male Xenophallus approach different stimuli, and also if this bias is at all affected by male gonopodial morph type.
Statistical Analyses
To determine if these fish show a bias in behavioral handedness, we followed the statistical approach of Torres-Dowdall et al. (2019). This required us to first calculate a laterality index (LI), which we used as a measure of the degree to which an individual shows a bias in right- or left-handed detours. We calculated this metric as follows:
Positive LI values indicate a right-handed detour bias, consistent with a preferred use of the left eye to view the stimulus. Negative LI values indicate a left-handed detour bias, consistent with a preferred use of the right eye to see the stimulus. A laterality score statistically indistinguishable from “0” indicates no bias in lateralized behavior. We calculated a mean laterality score for each treatment for both left morph and right morph males, resulting in eight tests. We analyzed these results using a two-tailed, one-sample t-test to determine if the observed LI in each treatment showed a significant departure from zero. All statistical tests were run in program R (R Core Team, 2020; version 3.6.3).
Results
Detour Tests
Xenophallus shows clear behavioral handedness in detour decisions when approaching various stimuli (Figure 4). Of the eight tests that we ran, six showed average LI scores that differed significantly from 0. Only the novel object treatment failed to elicit a significant response, which was true for both left morph (LI = −4.96, t13 = 0.72, p = 0.49) and right morph (LI = 3.92, t12 = 0.62, p = 0.54) males. Left morph males responded to the potential mate stimulus and the predator stimulus completely opposite of the right morph males. Left morph males detoured left in response to potential mates (LI = −34.22, t13 = 4.41, p < 0.001) and in response to predators (LI = −33.11, t13 = 7.39, p < 0.001). In contrast, right morph males detoured right in response to potential mates (LI = 20.16, t13 = 2.07, p = 0.05) and predators (LI = 21.65, t12 = 2.37, p = 0.03). Finally, in response to a no-stimulus control, we found the interesting pattern that both left morph males (LI = −23.71, t14 = 2.49, p = 0.03) and right morph males (-24.73, t14 = 2.35, p = 0.03) preferentially detoured to the left, rather than approaching the control at random.
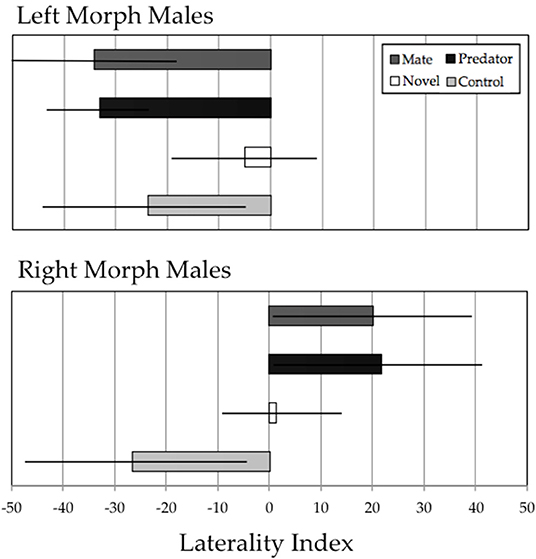
Figure 4. Results of the detour assay for left morph males (upper panel) and right morph males (lower panel). Each group of males was exposed to four experimental stimuli: potential mate, predator, novel object, and empty tank control. The Laterality Index (LI) is defined in the methods; briefly, negative LI scores indicate detour to the left, and positive LI scores indicate detour to the right. Figure shows a bar chart with error bars depicting ± 2 SE.
Male Morph Frequencies in the Wild
Of 14 populations of Xenophallus sampled between 1998 and 2019, almost all contained both left morph and right morph males (Figure 5; Table 1). In fact, we found no evidence that either male morph was fixed in a population where at least 5 males were sampled (see Table 1). This was true, regardless of collection locality, drainage, season of collection, or year of collection. The exception during our entire sampling were two samples taken at the La Palma site (sample 6-D, 6-E) that appeared to be fixed for the sinistral morph the last two times we sampled and one sample from Rio Chorros (sample 11-A) that appeared to be fixed for the dextral morph (Table 1). Moreover, we found considerable variation in the frequency of dextral and sinistral morphs among populations at the time they were surveyed, with some samples composed primarily of left morph males and others composed primarily of right morph males (Table 1). Interestinglym, one location, Rio Corinto, was sampled at two different times with the frequency of male morphs shifting from a right bias when we first sampled in 2006 to a left bias when we later sampled in 2007.
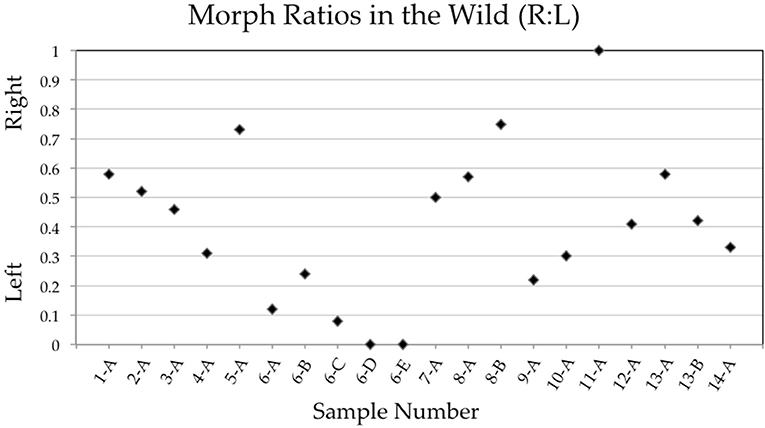
Figure 5. Plot showing gonopodial morph ratios (right to left) of samples taken from wild populations between 1998 and 2019. Sample numbers correspond to those found in Table 1. We only include samples here where the number of individuals scored was >5.
Discussion
Our study provides insight into the relationship between the anatomic asymmetry of the gonopodium and male behavior. It is perhaps not surprising to find that males approach potential mates in a non-random way—the gonopodium is used in copulation, and there is considerable evidence that the structural anatomy of the gonopodium in other poeciliids is important for successful sperm transfer (Langerhans, 2011). Specifically, in Xenophallus we found that males with gonopodia that terminate to the left approach females by detouring left, and the opposite was true for males with gonopodia that terminate to the right. Although we know little about how the gonopodium actually interacts with the female gonopore in Xenophallus, our results suggest that there may be some directionality to mating such that males with a particular morph might be more successful in mating on one side of the female than the other side. These results also set up the intriguing possibility that the success of males might depend to some extent on the frequency of each male morph in the population, with rare males having an advantage if females actively try to avoid forced copulations by more common males. Hence, this system may be well-suited for evaluating negative frequency dependent selection in the wild (Palmer, 1996), similar to the classic work on lateralized feeding on scales in cichlid fishes, where individuals with rare morphs had an advantage over their counterparts with the more common morph (Hori, 1993).
We also found that males detoured in a non-random way when evaluating predators. However, unlike previously published work which showed that some poeciliids evaluate predators and potential mates with opposite eyes (Torres-Dowdall et al., 2019), we found that Xenophallus males actually detour in the same direction in response to predators as they do to potential mates. Left morph males detour left in response to predators, and right morph males detour right in response to predators. These results are intriguing in two important ways. First, there appears to be an association between gonopodium morphology and predator inspection behavior. This is unexpected—there is no a priori reason that gonopodium morphology should favor viewing predators from one direction over another. Rather, previous work has suggested that side-bias in inspection behavior in fishes may have evolved to allow prey species to preferentially use one eye, and one side of the brain, to gather and process information about predation risk. If that is true in Xenophallus, then our data indicate that predator inspection behavior is not fixed to one eye or the other, and it appears to be associated with gonopodium morphology. Second, our results are inconsistent with previous work that argued that fish use one eye to specialize in gathering information about potential mates and the opposite eye to gather information about predation risk (Langerhans, 2011). We found that Xenophallus males use the same eye for both. Hence, there appears to be no division of labor among eyes and brain processing that some authors have suggested in other species.
Two other interesting patterns emerged from our detour trails. First, we found that neither left morph nor right morph males showed a side bias when approaching a novel object. There was simply no preference. This is important because it shows that Xenophallus are not fixed in their detour behaviors irrespective of the stimulus present. In other words, it tells us that males are deliberate in their response to potential mates and predators. Second, we found that in our control tests, where there was no stimulus present, that both left morph and right morph males consistently detoured to the left. That is, when faced with a barrier, and nothing present to inspect, males more often went around that barrier on the left side. At face value, this is a somewhat puzzling result. Why should individuals all show a detour preference in the absence of a stimulus? Our result, however, is consistent with an intriguing hypothesis that in the absence of a target stimulus, individuals in social species might move preferentially to one side to avoid collisions with other individuals (Vallortigara and Rogers, 2005). This so called “lateral locomotor bias” could result in social coordination resulting in the majority of individuals moving to the same side, even when that side is arbitrary. This type of lateralized social coordination has been found in a variety of other systems, including ants and bees (Frasnelli et al., 2012; Rogers et al., 2013), amphibians (Dadda et al., 2003), and fishes (Bisazza et al., 2000). In fact, a similar explanation is found for some lateralized human behaviors such as embracing, kissing, and cradling (Ocklenburg et al., 2018; Packheiser et al., 2019). Hence, our system with Xenophallus may also prove useful to test this more general hypothesis that social coordination can play a role in population-level lateralization.
Finally, our work might shed some light on the very fundamental question of why brain lateralization has evolved in so many vertebrate species. It is unclear why the gonopodium in Xenophallus is asymmetrical. It could simply have evolved as a randomly dimorphic trait. However, it appears that once this trait is present, it has potentially had an effect on lateralized behavior. We might imagine a scenario in which the evolution of anatomical asymmetries could lead to behavioral handedness, which in turn could lead to a specialization of the brain in processing information acquired from different sides of the body. Fishes have a general body form where the eyes are found on the sides of the head—not forward as in organisms with well-defined binocular vision—encouraging them to orient their body to one side or the other in order to obtain clear visual information. Hence, fishes, including systems like Xenophallus, are promising candidates for exploring different aspects of the origins of brain lateralization. Although future work will be necessary to evaluate these ideas, what is clear from our research is that there is a strong relationship between gonopodium morphology and lateralized behavior. Understanding the cause of this relationship may help reveal why so many forms of handedness exist in nature.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by Brigham Young University Institutional Animal Care and Use Committee.
Author Contributions
EJ and JJ conceived the idea for the study, designed the statistical analysis, and revised the manuscript in response to reviewer comments. EJ designed and built the test arena, collected the experimental data, and wrote the first draft of the manuscript. All authors reviewed and edited the manuscript, approved its final version, contributed to the preparation, research, writing of the manuscript, and were involved in the field collections.
Funding
This work was funded in part by ORCA grants from the BYU Office of Research and Creative Activities to EJ and M-EN, by a summer fellowship from the BYU Department of Biology to EJ, and by a grant from the US National Science Foundation (IOS 1045226) to JJ.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was conducted under IACUC protocol 18-0803, which established acceptable sample sizes for our trials. We appreciate the support of Javier Guevara Siquiera and Lourdes Vargas Fallas at the Vide Sylvestre, Ministrio del Ambiente y Energia (MINAE), Sistema Nacional de Áreas de Conservación (SINAC), Costa Rica, who processed our collecting permits. Trevor Williams, Alli Duffy, Megan Pew, and Becca White each provided help with different aspects of the project, including fieldwork, specimen processing, and fish care. Mark Belk consulted on possible statistical approaches to analyze these data. Finally, critical feedback provided by two peer reviewers greatly improved the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.606856/full#supplementary-material
References
Bisazza, A., Cantalupo, C., Capocchiano, M., and Vallortigara, G. (2000). Population lateralisation and social behaviour: a study with 16 species of fish. Laterality 5, 269–284. doi: 10.1080/713754381
Bisazza, A., Pignatti, R., and Vallortigara, G. (1997a). Detour tests reveal task- and stimulus-specific behavioural lateralization in mosquitofish (Gambusia holbrooki). Behav. Brain Res. 89, 237–242. doi: 10.1016/S0166-4328(97)00061-2
Bisazza, A., Pignatti, R., and Vallortigara, G. (1997b). Laterality in detour behaviour: interspecific variation in poeciliid fish. Anim. Behav. 54, 1273–1281. doi: 10.1006/anbe.1997.0522
Bock, G. R., and Marsh, J. (1991). Biological Asymmetry and Handedness. England: John Wiley and Sons.
Bryden, P. J. (2016). The influence of M. P. Bryden's work on lateralization of motor skill: Is the preferred hand selected for and better at tasks requiring a high degree of skill? Laterality 21, 312–328. doi: 10.1080/1357650X.2015.1099661
Buchanan, S. M., Kain, J. S., and de Bivort, B. L. (2015). Neuronal control of locomotor handedness in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 112, 6700–6705. doi: 10.1073/pnas.1500804112
Dadda, M., Sovrano, V. A., and Bisazza, A. (2003). Temporal pattern of social aggregation in tadpoles and its influence on the measurement of lateralised response to social stimuli. Physiol. Behav. 78, 337–341. doi: 10.1016/S0031-9384(02)01001-6
de Andrade, A. C., and de Sousa, A. B. (2018). Hand preferences and differences in extractive foraging in seven capuchin monkey species. Am. J. Primatol. 80:e22901. doi: 10.1002/ajp.22901
Fitch, R. H., Brown, C. P., Oconnor, K., and Tallal, P. (1993). Functional lateralization for auditory temporal processing in male and female rats. Behav. Neurosci. 107, 844–850. doi: 10.1037/0735-7044.107.5.844
Frasnelli, E., Iakovlev, I., and Reznikova, Z. (2012). Asymmetry in antennal contacts during trophallaxis in ants. Behav. Brain Res. 232, 7–12. doi: 10.1016/j.bbr.2012.03.014
Fu, W. W., Wang, X. W., Wang, C. L., Zhao, H. T., Ren, Y., and Li, B. G. (2019). Effects of age, sex and manual task on hand preference in wild Rhinopithecus roxellana. Zool. Res. 40, 129–138. doi: 10.24272/j.issn.2095-8137.2019.023
Gunturkun, O., Strockens, F., and Ocklenburg, S. (2020). Brain lateralization: a comparative perspective. Physiol. Rev. 100, 1019–1063. doi: 10.1152/physrev.00006.2019
Hata, H., Yasugi, M., and Hori, M. (2011). Jaw laterality and related handedness in the hunting behavior of a scale-eating characin, Exodon paradoxus. PLoS ONE 6:e29349. doi: 10.1371/journal.pone.0029349
Hollo, G., and Novak, M. (2012). The manoeuvrability hypothesis to explain the maintenance of bilateral symmetry in animal evolution. Biol. Direct 7:22. doi: 10.1186/1745-6150-7-22
Hori, M. (1993). Frequency-dependent natural-selection in the handedness of scale-eating cichlid fish. Science 260, 216–219. doi: 10.1126/science.260.5105.216
Jones, C. P., and Johnson, J. B. (2009). Phylogeography of the livebearer Xenophallus umbratilis (Teleostei: Poeciliidae): glacial cycles and sea level change predict diversification of a freshwater tropical fish. Mol. Ecol. 18, 1640–1653. doi: 10.1111/j.1365-294X.2009.04129.x
Jumars, P. A., Dorgan, K. M., and Lindsay, S. M. (2015). Diet of worms emended: an update of polychaete feeding guilds. Ann. Rev. Mar. Sci. 7, 497–520. doi: 10.1146/annurev-marine-010814-020007
Kabadayi, C., Bobrowicz, K., and Osvath, M. (2018). The detour paradigm in animal cognition. Anim. Cogn. 21, 21–35. doi: 10.1007/s10071-017-1152-0
Kane, A., Healy, K., Guillerme, T., Ruxton, G. D., and Jackson, A. L. (2017). A recipe for scavenging in vertebrates - the natural history of a behaviour. Ecography 40, 324–334. doi: 10.1111/ecog.02817
Koshio, C., Muraji, M., Tatsuta, H., and Kudo, S. (2007). Sexual selection in a moth: effect of symmetry on male mating success in the wild. Behav. Ecol. 18, 571–578. doi: 10.1093/beheco/arm017
Kurita, Y., and Wada, H. (2011). Evidence that gastropod torsion is driven by asymmetric cell proliferation activated by TGF-beta signalling. Biol. Lett. 7, 759–762. doi: 10.1098/rsbl.2011.0263
Land, M. F. (2015). Eye movements of vertebrates and their relation to eye form and function. J. Comp. Physiol. Neuroethol. Sens. Neural Behav. Phys. 201, 195–214. doi: 10.1007/s00359-014-0964-5
Langerhans, R. (2011). “Ecology and Evolution of Poeciliid Fishes,” in Ecology and Evolution of Poeciliid Fishes, eds J. Evans, A. Pilastro, and I. Schlupp (Chicago, IL: The University of Chicago Press).
Ocklenburg, S., Packheiser, J., Schmitz, J., Rook, N., Gunturkun, O., Peterburs, J., et al. (2018). Hugs and kisses - the role of motor preferences and emotional lateralization for hemispheric asymmetries in human social touch. Neurosci. Biobehav. Rev. 95, 353–360. doi: 10.1016/j.neubiorev.2018.10.007
Packheiser, J., Rook, N., Dursun, Z., Mesenholler, J., Wenglorz, A., Gunturkun, O., et al. (2019). Embracing your emotions: affective state impacts lateralisation of human embraces. Psychol. Res. 83, 26–36. doi: 10.1007/s00426-018-0985-8
Palmer, R. A. (1996). From symmetry to asymmetry: Phylogenetic patterns of asymmetry variation in animals and their evolutionary significance. Proc. Natl. Acad. Sci. U.S.A. 93, 14279–14286. doi: 10.1073/pnas.93.25.14279
Parenti, L., and Rauchenberger, M. (1989). “Systematic Overview of the Poeciliins,” in Ecology and Evolution of Livebearing Fishes, eds G. Meffe and F. Nelson (Englewood, NJ: Prentice Hall).
R Core Team (2020). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.r-project.org/index.htm
Rogers, L. J., Rigosi, E., Frasnelli, E., and Vallortigara, G. (2013). A right antenna for social behaviour in honeybees. Sci. Rep. 3:2045. doi: 10.1038/srep02045
Spani, F., Scalici, M., Crandall, K. A., and Piras, P. (2020). Claw asymmetry in crabs: approaching an old issue from a new point of view. Biol. J. Linn. Soc. 129, 162–176. doi: 10.1093/biolinnean/blz159
Torres-Dowdall, J., Rometsch, S. J., Aguilera, G. G., Oyenola, G., and Meyer, A. (2019). Asymmetry in genitalia is in sync with lateralized mating behavior but not with the lateralization of other behaviors. Curr. Zool. 66, 71–81. doi: 10.1093/cz/zoz019
Vallortigara, G., and Rogers, L. J. (2005). Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–89. doi: 10.1017/S0140525X05000105
Wakita, D., Kagaya, K., and Aonuma, H. (2020). A general model of locomotion of brittle stars with a variable number of arms. J. R. Soc. Interface 17:20190374. doi: 10.1098/rsif.2019.0374
Keywords: detour test, eye-bias, laterality, livebearing fishes, mate choice, novel object, Poeciliidae, predator-mediated behavior
Citation: Johnson ES, Nielsen M-E and Johnson JB (2020) Does Asymmetrical Gonopodium Morphology Predict Lateralized Behavior in the Fish Xenophallus umbratilis? Front. Ecol. Evol. 8:606856. doi: 10.3389/fevo.2020.606856
Received: 15 September 2020; Accepted: 06 November 2020;
Published: 27 November 2020.
Edited by:
Renoult P. Julien, UMR5175 Centre d'Ecologie Fonctionnelle et Evolutive (CEFE), FranceReviewed by:
Michel Raymond, UMR5554 Institut des Sciences de l'Evolution de Montpellier (ISEM), FranceFelix Ströckens, Ruhr University Bochum, Germany
Copyright © 2020 Johnson, Nielsen and Johnson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erik S. Johnson, c21lcmlram9obnNvbkBnbWFpbC5jb20=
 Erik S. Johnson
Erik S. Johnson Mary-Elise Nielsen
Mary-Elise Nielsen Jerald B. Johnson
Jerald B. Johnson