
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 12 January 2021
Sec. Paleoecology
Volume 8 - 2020 | https://doi.org/10.3389/fevo.2020.589154
This article is part of the Research Topic A Golden Age for Strontium Isotope Research? Current Advances in Paleoecological and Archaeological Research View all 15 articles
 Christophe Snoeck1,2,3*
Christophe Snoeck1,2,3* Rick J. Schulting4
Rick J. Schulting4 Fiona Brock5
Fiona Brock5 Alexandra S. Rodler1
Alexandra S. Rodler1 Alicia Van Ham-Meert6,7
Alicia Van Ham-Meert6,7 Nadine Mattielli2
Nadine Mattielli2 Joanna Ostapkowicz4
Joanna Ostapkowicz4Strontium isotope ratios (87Sr/86Sr) are commonly used in archeological and forensic studies to assess if humans and fauna are local to the place they were found or not. This approach is largely unexplored for wooden artifacts recovered in archeological contexts, as wood – in the rare instances it does survive – is often poorly preserved. One of the most common ways wood is preserved is through the anoxic conditions found in waterlogged contexts. A more unusual form of preservation is through submergence in natural pitch. These depositional media contribute their own strontium values to the in vivo 87Sr/86Sr wood values, which needs to be removed prior to analysis. Here we test several pre-treatment methods to remove potential strontium contamination from wood samples that were artificially immersed in seawater and pitch from Trinidad’s Pitch Lake. Water rinses and acid-leaching tests were carried out with hydrochloric acid and acetic acid to remove exogenous strontium from experimentally waterlogged wood. These tests removed large amounts of strontium from the samples and did not enable the recovery of the endogenous 87Sr/86Sr signal. For samples artificially immersed in pitch, the pre-treatments tested were based on radiocarbon dating procedures and carried out with and without the aqueous-based acid-base-acid (ABA) step. The use of organic solvents alone (methanol and toluene) removed exogenous strontium originating from the pitch. However, the ABA step eliminates large amounts of in vivo strontium from the samples. These tests show that 87Sr/86Sr values of wood are altered by the presence of pitch and water. With adequate pre-treatment using exclusively organic solvents, it may be possible to remove this contamination for samples immersed in pitch. However, the aqueous-based ABA pre-treatment should be avoided. The removal of contamination from waterlogged samples was unsuccessful with the current pre-treatment protocols and more research is needed. More importantly, and unexpectedly, 87Sr/86Sr values may extend outside of the mixing line between the wood’s endogenous strontium and the water. These results indicate the need for extreme caution when attempting to determine the provenance of waterlogged wood.
Wood artifacts are rarely encountered in the archeological record, as their permeable, organic nature quickly deteriorates in typical depositional contexts. However, wood does survive when charred, desiccated or waterlogged. An additional, though very rare, means of preservation occurs in the anoxic conditions of natural asphalt or pitch deposits. Such artifacts, and particularly the materials from which they were carved, offer valuable insights on a variety of issues – from resource and landscape utilization to potential exchange links. It is vital to access as much of this information as possible, including radiocarbon dating of variously preserved objects (Ostapkowicz et al., 2012, 2013; Brock et al., 2017) and exploring possibilities for carrying out strontium (Sr) isotope analyses to assess the geographical origin of these artifacts.
Strontium-87 is the product of the radioactive decay of Rubidium-87 (87Rb), so Sr isotope ratios (87Sr/86Sr) vary between different types of bedrock, depending on the initial Rb-Sr ratio and time since deposition: the amount of 87Sr in relation to 86Sr increases with bedrock age as 87Rb decays to 87Sr (Faure and Powell, 1972). 87Sr/86Sr values as high as 0.9000 may be observed in some granites, for instance in the Mourne Mountains in Northern Ireland (Meighan et al., 1988) and in South Africa (Sillen et al., 1998). Younger geological formations often have values below 0.7060, and those with very low initial Rb/Sr ratios, such as basalt, typically have values of 0.7027–0.7040 (GEOROC, 2014). Modern ocean water has a 87Sr/86Sr ratio of 0.7092 (Hess et al., 1986), an important value as it contributes to world-wide precipitation and to geologically recent calcareous marine deposits. These general trends provide a first idea of the type and age of a deposit based on the Sr isotopic composition, however, to be confident in the interpretation appropriate baselines are needed.
Previous research has shown that, in plants, Sr follows similar pathways to calcium (Rediske and Selders, 1953; Storey and Leigh, 2004). Soluble Sr is taken up by plants from the soil and hence reflects the geology on which the plants are growing. While a large number of studies have focussed on measuring Sr isotopes in archeological human and animal remains (e.g., Price et al., 2006; Bentley, 2013; Laffoon et al., 2014) or charred/carbonized grains (e.g., Benson et al., 2010; though not always successfully – see Styring et al., 2019), as well as on modern plants to establish a biologically available Sr baseline (e.g., Evans et al., 2010; Snoeck et al., 2016, 2020), less has been done with archeological wood remains. Exceptions include a study of the well-preserved desiccated structural timbers at Chaco Canyon (English et al., 2001), and of desiccated prehistoric willow and tule textiles in the Great Basin (Benson et al., 2006), as well as some more recent work on pre-Columbian wood sculptures from Florida (Ostapkowicz et al., 2017a) and Trinidad (Ostapkowicz et al., 2017b), waterlogged shipwrecks (Rich et al., 2016; Hajj et al., 2017; Van Ham-Meert et al., 2020), and South American/Lesser Antillean wooden clubs from museum collections (Ostapkowicz et al., 2018). Little targeted research has focused on pre-treatments for archeological wood. Desiccated remains should not present a problem, as long as dust particles are removed prior to analysis, as they have not been exposed to Sr in solution. For all other cases, however, pre-treatments are required, as it is clear from studies carried out on human and animal remains as well as charred grains that pre-treatment is required to remove depositional Sr contamination (Sillen and LeGeros, 1991; Budd et al., 2000; Benson et al., 2010; Snoeck et al., 2015; Styring et al., 2019).
In trees Sr does not play a physiological role, but it is taken-up through the root system via the same pathways as calcium. Since it does not have a physiological role, there is no metabolic regulation of its concentration in (different parts of) the trees (Blum et al., 2012). Calcium is present as whewellite in oaks (Ca-oxalate monohydrate: CaC2O4.H2O). A Sr isomorph of this crystal exists, and it is probable Sr is present in those (water-soluble crystals) mostly in cell walls, particularly in pectin, cellulose, and lignin (Serdar and Demiray, 2012; Hajj et al., 2017). Strontium can also be present adsorbed on active groups in cell-walls, but probably not cellulose, while results on lignin as a candidate are mixed (Chen, 1997; Schmitt et al., 2017; Boyer et al., 2018). A spatially resolved chemical analysis (e.g., FTIR, Raman or XRD) of wood would help understand where Sr is present, and which type of chemical bonds are involved.
In waterlogged contexts and waterlogging experiments, 87Sr/86Sr measured on shipwrecks showed the impact of water on the Sr isotopic composition of the wood (Rich et al., 2016; Hajj et al., 2017; Van Ham-Meert et al., 2020). Two Mediterranean shipwrecks were investigated by Rich et al. (2016): the Bronze Age Uluburun shipwreck and the Athlit Ram galley from the Hellenistic period. Samples were washed to remove precipitates such as NaCl because biostratinomic and diagenetic Sr sources were a concern in waterlogged wood, but no other pre-treatment seems to have been undertaken. Similarly, no pre-treatment was carried out by Hajj et al. (2017) prior to analysis of the Ribadeo, a sixteenth century Spanish shipwreck. The few studies that have so far investigated pre-treatments for Sr isotope analysis of wood recovered from natural pitch (Ostapkowicz et al., 2017b) or on which consolidants have been applied (Ostapkowicz et al., 2017a, 2018) have used the pre-treatments commonly used in radiocarbon dating to remove carbon contamination (Brock et al., 2010, 2017, 2018). These pre-treatments showed that such carbon contamination can be successfully removed adequately using different organic solvents and acid-base-acid (ABA) steps (Brock et al., 2010, 2017, 2018; Dee et al., 2011; Ostapkowicz et al., 2012, 2013). Recent stable carbon and nitrogen isotope analyses of charred grain, however, highlighted possible issues with the use of ABA as it removed large amounts of material (Vaiglova et al., 2014), which is problematic when only small samples are available, as is often the case with archeological materials. Further work using HCl (6 M at room temperature) to remove exogenous soil Sr from charred grain was only partially successful (Styring et al., 2019). Recently, various pre-treatments including successive MilliQTM washes, a combination of HF and MilliQTM and alpha-cellulose extraction (which is used in oxygen isotope studies) were tested as means to remove exogeneous Sr from experimentally waterlogged oaks (Van Ham-Meert et al., 2020). However, none of these methods were successful, the different procedures removed both endogenous and exogeneous Sr and did not succeed in retrieving the original Sr isotopic composition. A sample from a shipwreck was also included in this study and behaved similarly (Van Ham-Meert et al., 2020).
In this paper, several artificially waterlogged wood and pitch-contaminated samples are studied using 87Sr/86Sr analyses. The effect of various pre-treatments on the Sr isotope ratio of these samples is investigated to (1) assess to what extent water and/or pitch have an impact on the original Sr isotope ratio of wood and wood artifacts; (2) determine which, if any, pre-treatment is the most effective in removing these sources of exogenous Sr prior to analyses.
The waterlogging experiments were carried out on (1) two modern wood samples from Trinidad (T69 and T88 – Ostapkowicz et al., 2017b; Table 1) and (2) six modern pine and cypress samples from Florida (FR2, FS3, GOE3, RC1, SW1, and TR4 – Schulting and Snoeck, unpublished data; Table 1). The samples weighing approximately 5 g were placed in individual 50 mL metal-free, sterile polypropylene (PP) tubes and immersed for 1 month in unfiltered seawater obtained from the North Sea in Knokke (Belgium) that has a 87Sr/86Sr isotope ratio of 0.7092 and a Sr concentration of 7.1 mg/L (similar to the range of 7.2 to 7.8 mg/L reported by Angino et al., 1966). Two fractions of samples FR2 and GOE3 were placed in separate tubes to assess the reproducibility of the pre-treatments. Samples T69 and T88 were also placed for 1 month in a Sr-87 enriched solution (11 mg/L of SrCO3 – MSR87C from Euriso-top, Saint-Aubin, France – of which 89.7% is composed of 87Sr) with a 87Sr/86Sr of 91.5306 (see Snoeck et al., 2015). All samples sank to the bottom of the tubes before the end of the month. The samples were then removed from the tubes and left to airdry at room temperature for a few days.
To test the impact of pitch on the 87Sr/86Sr isotope composition of wood samples, three modern wood samples from Trinidad (T34, T71, and T81 – Ostapkowicz et al., 2017b) were selected and about 5 g of each were placed in 50 mL pitch obtained from southwest Trinidad’s Pitch Lake for 3 months (Table 1). Two aliquots of the pitch (ca. 50 mg) were also analyzed to assess its Sr isotope ratio.
After drying, the waterlogged samples of the first experiment (T69 and T88) were ultrasonicated for 10 min in ultrapure MilliQTM and before being split into four fractions: (1) no further pre-treatment; (2) ultrasonication for 10 min in 1 M acetic acid and; (3) ultrasonication for 10 min in 1 M acetic acid, crushed to <5 mm using a coffee grinder and ultrasonicated again for 10 min in 1 M acetic acid; and (4) ultrasonication for 10 min in 1 M HCl. After pre-treatments 2, 3, and 4, the samples were ultrasonicated for 10 min again in MilliQTM. All experiments were at room temperature.
In the second waterlogging contamination experiment, several modern pine and cypress samples (FR2, FS3, GOE3, RC1, SW1, and TR4 – Table 1) were immersed in seawater from the Belgian coast (Knokke) until they sank. They were then crushed using a coffee grinder and separated into two fractions by sieving: between 2 and 5 mm (G – gross) and less than 2 mm (F – fine). All fractions were ultrasonicated in MilliQTM for 10 min. All fractions were then split in two: (1) no further pre-treatment; (2) ultrasonication for 10 min in 1 M acetic acid. For FS3 and SW1, large amount of materials were available from the less than 2 mm fraction and were separated in two aliquots that were pre-treated separately (allowing assessment of repeatability).
For samples contaminated with pitch from Trinidad’s Pitch Lake (T34, T71, and T81), even though Sr and carbon are different elements, present in different parts of the plant, the contamination source in both cases is the pitch in which they are immersed. Hence, radiocarbon pre-treatments were used as a first approach, using methanol and toluene (Table 2; Brock et al., 2017). Prior observations on charred grains showed that ABA pre-treatments can, in some cases, remove large amounts of sample, mostly in the form of humic acids (Vaiglova et al., 2014). Therefore, the pre-treatments were tested with (pre-treatment 5) and without ABA (pre-treatment 6) at the end of the sequence. Some samples were also pre-treated only with the ABA procedure (pre-treatment 7) to assess its impact on the 87Sr/86Sr of wood. All ABA procedures followed the protocol outlined for non-woody plant remains (WW) in Brock et al. (2010) rather than the one outlined for wood (UW) as the bleach step was not carried out. Each pre-treatment started with ca. 50 mg of sample – the standard amount usually available for 87Sr/86Sr analyses. The pre-treatment of the wood samples was carried out directly on uncontaminated wood as well as wood samples that had been contaminated with pitch to assess whether the observed 87Sr/86Sr variations are linked to the presence of pitch or to the pre-treatment method used. For both the waterlogging and pitch contamination experiments, 87Sr/86Sr were measured before and after pre-treatment directly on the wood material (not on the leachates).
The entire acid digestion process and subsequent Sr purification were achieved under a class 100 laminar flow hood in a class 1000 clean room (Université Libre de Bruxelles, Belgium, hereafter ULB). One gram from each of the untreated wood samples (Table 1) was ashed in porcelain crucibles and a muffle furnace by step heating to 650°C prior to digestion. For these samples, about 50 mg of ashed sample was dissolved in closed ® Savillex containers (LabAS, Brussels, Belgium) overnight using 14 M HNO3 on a heating plate at 110°C. For all the waterlogged and pitch-contaminated samples, about 50 mg of non-ashed sample was dissolved in closed ® Savillex containers overnight using 14 M HNO3 and 23 M HF (2:1) on a heating plate at 110°C. The samples were then dried and dissolved in a mixture of 14 M HNO3 and 6 M HCl (1:1) and left overnight on a heating plate at 110°C. If the solution was completely clear, the samples were dried awaiting column separation. If not, the dried samples were repeatedly dissolved in 8 M HCl until a clear solution was obtained.
The dried samples were re-dissolved in 2.5 mL of 2 M sub-boiled HNO3. 0.5 mL were extracted for Sr concentration measurements (see below). The Sr of the remaining 2 mL was then extracted, and purified following the protocol described in Snoeck et al. (2015), and measured on a Nu Plasma MC-ICP Mass Spectrometer (Nu015 from Nu Instruments, Wrexham, United Kingdom) at a Sr concentration ULB using a spray chamber and at a Sr concentration of ca. 300 ng/g. NIST SRM987 (87Sr/86Sr = 0.710248 – Weis et al., 2006) was used as reference material and measured following the samples-standard bracketing method, every two samples being bracketed by the NIST SRM987 standard solution. Nine additional NIST SRM987 measurements were carried out and returned an average 87Sr/86Sr value of 0.710253 ± 0.000042 (2 SD) consistent with measurements carried out on TIMS (Weis et al., 2006). Procedural blanks were considered negligible [total Sr (V) of max 0.02 V versus 7–8 V for sample analyses; i.e., ≈0.3%]. For each sample, the 87Sr/86Sr value is reported with ±2 SE representing the analytical uncertainty on each individual sample calculated from the 60 measurements within each run. To ensure that the various chemicals used in the pre-treatment do not contribute any Sr to the system, several blanks were measured. These showed that the various chemicals did not contain enough Sr to affect the results (i.e., the Sr beam intensity was at least 100x smaller than any of the analyzed samples).
Strontium concentrations in a fraction of the sample digests (see above) were determined using a Thermo Scientific Element 2 sector field ICP mass spectrometer at the Vrije Universiteit Brussel (VUB), Belgium, in low (88Sr) resolution using Indium (In) as an internal standard and external calibration versus various reference materials (SRM1400, CCB01). Accuracy was evaluated by the simultaneous analysis of two internal bioapatite standards (ENF and CBA). Based on repeated digestion and measurement of these reference materials, the analytical precision of the procedure outlined above is estimated to be better than 5% (1 SD, n = 33 for CBA and n = 5 for ENF).
The results of the first contamination experiment (Table 3) show that uncontaminated (i.e., untreated) samples (T69 and T88) are not affected by rinsing with MilliQ water and ultrasonication. The 87Sr/86Sr results of wood samples immersed in both seawater (Sea) and the Sr-87 enriched solution (Sr) are heavily affected in the direction of the solution in which they were immersed. Furthermore, there is a small decrease in Sr concentration in the samples immersed in seawater. The Sr concentrations in the samples immersed in the Sr-87 enriched solution, however, are much higher compared to the uncontaminated sample rinsed with MilliQ.
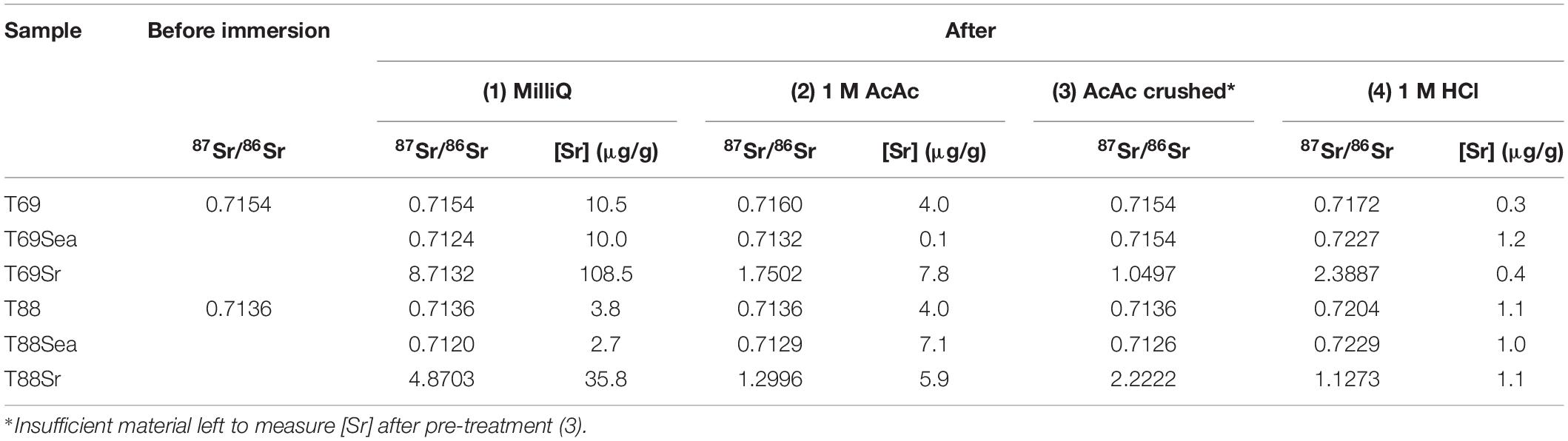
Table 3. Strontium isotope and concentration results for the first waterlogging experiment (full data available in Supplementary Table 1); Sea = immersed in seawater; and Sr = immersed in a Sr-87 enriched solution.
The use of acid on uncontaminated samples can have an effect on the measured 87Sr/86Sr. Acetic acid has a minor effect (Δ87Sr/86Sr of 0.0004; Δ87Sr/86Sr represents the difference between two 87Sr/86Sr measurements) on one of the uncrushed samples but no effect at all on the other untreated samples. Hydrochloric acid, however, has a large impact on the Sr isotope ratios of uncontaminated samples with Δ87Sr/86Sr up to 0.0068. In the samples immersed in seawater and the Sr-87 enriched solution, some of the contamination is removed after pre-treatment with acetic acid, and all of it in the case of T69Sea after crushing and pre-treatment with acetic acid (3). Pre-treatment with 1 M HCl, however, causes dramatic shifts in the Sr isotope ratios, probably due to the fact that it is too strong and removes endogenous Sr as also suggested by the very low Sr concentrations ([Sr] < 1.5 μg/g).
Following the results from the first contamination experiments, several modern pine and cypress wood samples from Florida with known (i.e., previously measured) 87Sr86Sr values were immersed in seawater until they sank, and were subsequently dried, crushed and rinsed with MilliQ. Some were then also pre-treated with acetic acid and ultrasonication. Two pieces (labeled “A” or “B”) of both FR2 and GOE3 were immersed separately to assess the variability in contamination. For FS3 and SW1, the fine fraction was split in two (labeled “1” or “2”) to test the reproducibility of the pre-treatment method (Table 4). The results show that all samples were heavily contaminated with seawater Sr and that rinsing with MilliQ water is ineffective in removing that contamination. Indeed, the samples have a mean 87Sr/86Sr value of 0.7092 ± 0.0001 (1 SD) after immersion in seawater and rinsing with MilliQ (Figure 1), equivalent at four decimals to the value of seawater (0.7092 – Hess et al., 1986). The Sr concentrations in 15 out of 18 cases are lower after immersion in seawater and rinsing. After pre-treatment with acetic acid, the Sr concentrations are even lower with values ranging from 0.1 to 4.9 μg/g (Figure 1). Additionally, none of the samples show a complete removal of contamination based on their 87Sr/86Sr. On the contrary, after pre-treatment some samples have ratios further away from their original value than before pre-treatment (e.g., RC1SeaG) while others retain the seawater value (e.g., FR2SeaAF). Comparing the results obtained for the fractions of different size, the 87Sr/86Sr values of the larger fraction (G; between 2 and 5 mm) after pre-treatment with acetic acid are generally further away from the original value than the smaller fraction (F; <2 mm). Most strikingly, and contrary to mass balance expectations, many of the acetic acid pre-treatments resulted in 87Sr/86Sr well above both the original wood value and the value of the contaminant seawater (Figure 1).
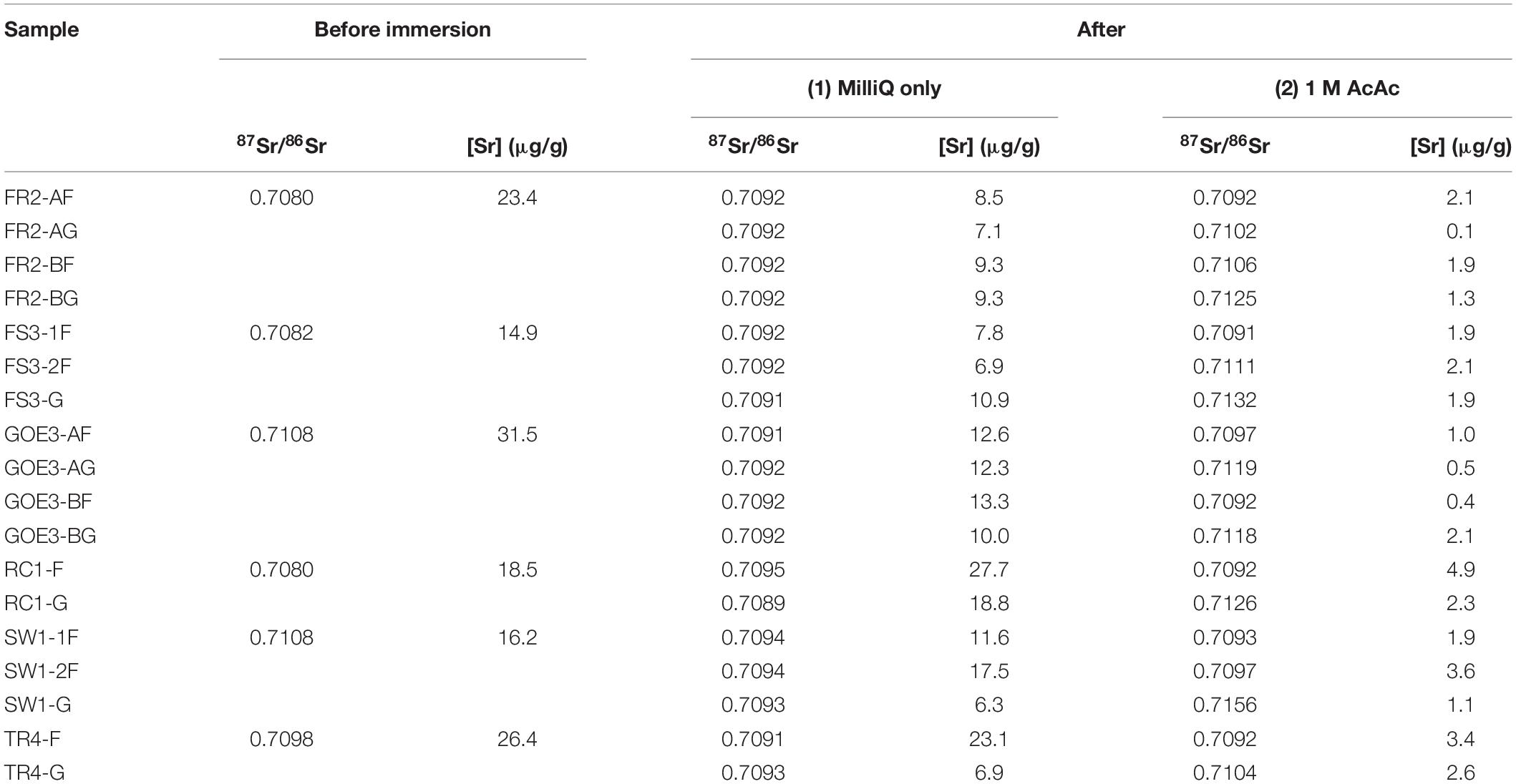
Table 4. Strontium concentration and isotope results from the second waterlogging experiment using seawater (full data available in Supplementary Table 2); F = fine fraction < 2 mm; G = gross fraction between 2 and 5 mm; A/B = two different immersions; and 1/2 = two aliquots of the same immersion pre-treated separately.
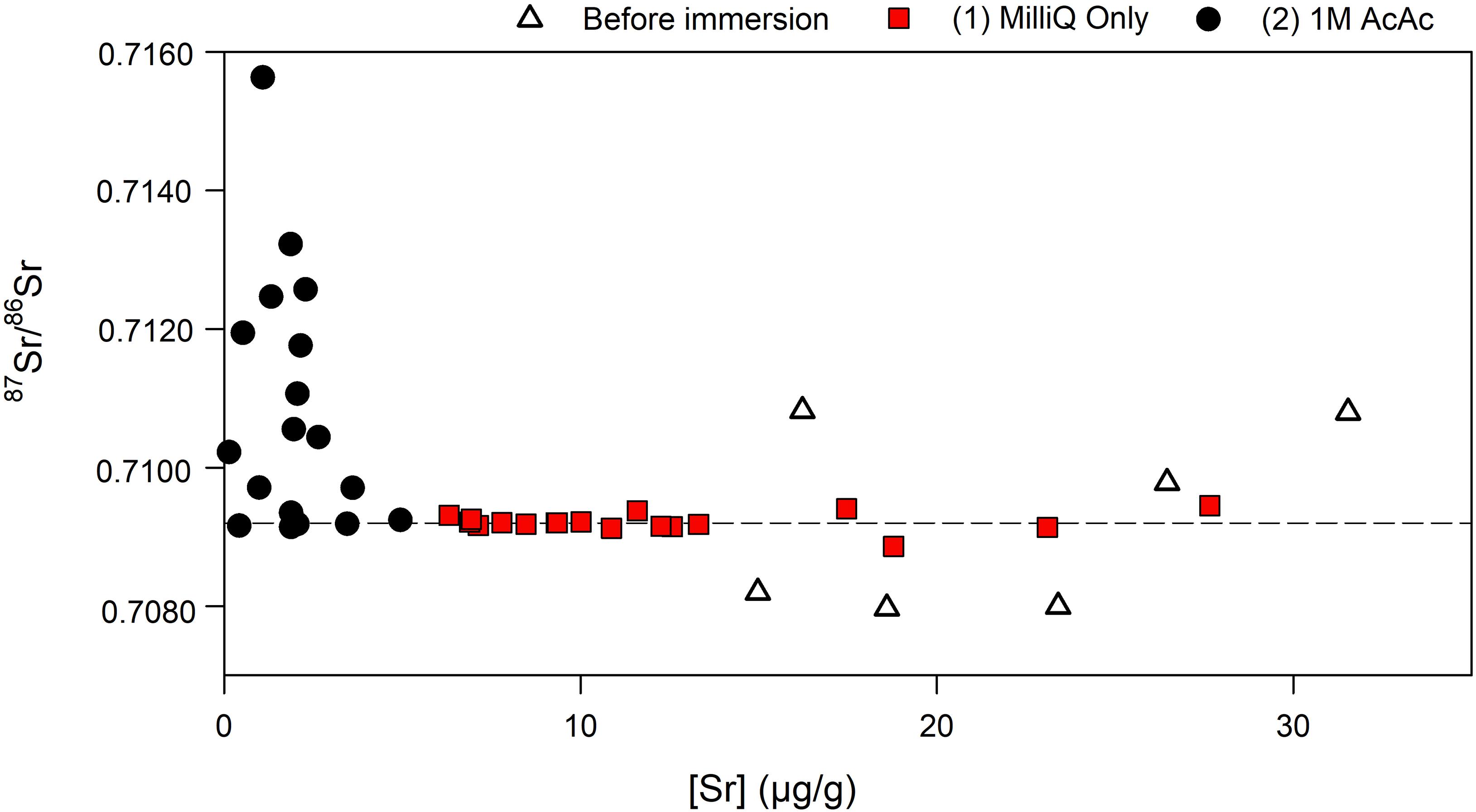
Figure 1. Strontium concentrations and isotope ratios of wood samples before immersion, after immersion and rinsed with MilliQ only, and pre-treated with acetic acid; the dashed line represents the Sr isotope ratio of seawater (0.7092), the analytical uncertainty is smaller than the marker.
Two fraction of pitch from Trinidad’s Pitch Lake used in this experiments returned 87Sr/86Sr (±2 SE) values of 0.712275 ± 0.000039 and 0.712251 ± 0.000039 which is distinct from the values of the modern trees sampled from around the lake [0.7107 ± 0.0013 (1 SD; n = 13); Ostapkowicz et al., 2017b]. The pitch 87Sr/86Sr value is identical to that of T34 and close to that of T81 (0.7130) but very distinct from the 87Sr/86Sr of T71 (0.7093). The first part of the experiment was to evaluate the impact of the pre-treatment [with (5) and without (6) ABA] on the 87Sr/86Sr of uncontaminated wood samples (Table 5). Several procedural blanks were measured and confirmed that the various chemicals used in these pre-treatments did not significantly add Sr to the system. Still, the results clearly show that the complete pre-treatment highly affects the uncontaminated samples leading to variation in Sr isotope ratios (Δ87Sr/86Sr between 0.0009 and 0.0023 – Figure 2). ABA alone (7) also impacts the Sr isotope ratios of the wood samples but to a lesser extent (Δ87Sr/86Sr up to 0.0004). Visually, it was clear during the pre-treatments that large amounts of material were removed from the wood samples (whether it had been immersed in pitch or not) during the ABA steps (mainly the base step), with the solutions turning light to dark brown (especially for sample T81). However, when this pre-treatment is used without the ABA step (pre-treatment 6, i.e., just using organic solvents), the wood samples are unaffected by the pre-treatment (Δ87Sr/86Sr ≤ 0.0001). The 87Sr/86Sr results for the pitch-contamination experiments (Table 5) show some variations between uncontaminated and pitch-contaminated samples as well as a variable effect of the different pre-treatments applied. However, when only organic solvents are used (6), the original uncontaminated value is reached, ±0.0002, which for the purpose of interpretation may be adequate (Figure 2).
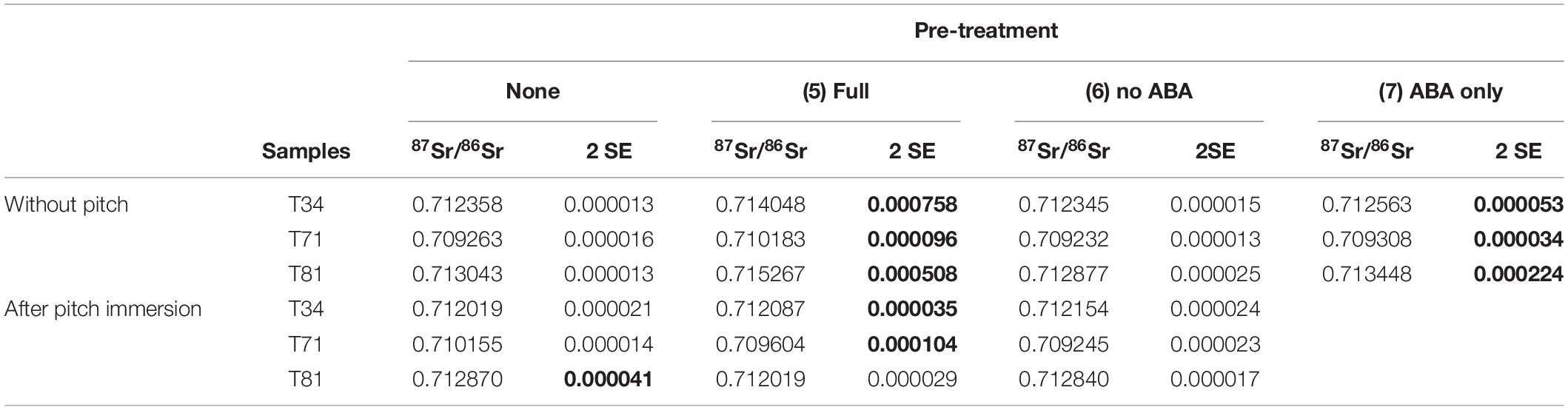
Table 5. Strontium isotope ratios (±2 SE) for the pitch-contamination experiments; see Table 2 for details about the pre-treatments; bold values show measurements with particularly large uncertainties resulting from low amounts of Sr still present after pre-treatment.
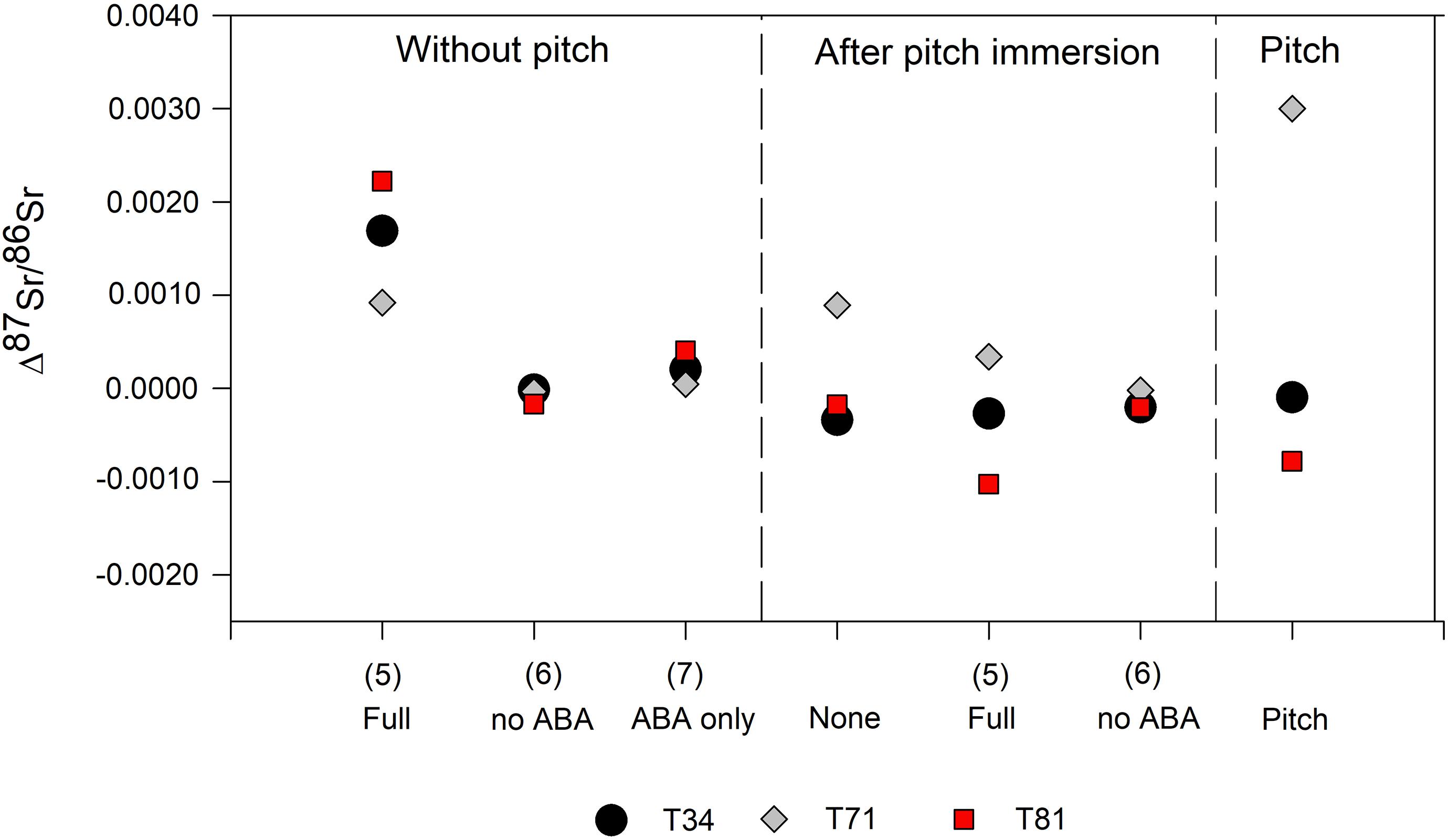
Figure 2. Strontium isotope ratios of the contaminated and/or pre-treated wood samples compared to the 87Sr/86Sr values of the respective untreated and uncontaminated sample (Δ87Sr/86Sr represents the difference between two 87Sr/86Sr measurements).
The 87Sr/86Sr values of wood placed in water are heavily impacted by the Sr present in that water, confirming the observations of Hajj et al. (2017). Indeed, the samples immersed in seawater (even after only 1 month) have 87Sr/86Sr values closer to that of seawater (0.7092 – Hess et al., 1986) with all samples from the second water contamination experiment having values between 0.7089 and 0.7095, with a mean of 0.7092 ± 0.0001 (1 SD). While this could be due to the absorption/incorporation of exogenous Sr from water, the Sr concentrations are in all but three cases lower after immersion and rinsing with MilliQ than before. This strongly suggests that large amounts of endogenous Sr are actually leached during the waterlogging experiments and replaced by Sr from the water and/or removed during rinsing with MilliQ as Sr is highly water-soluble. It is likely that this results from an equilibrium exchange between the wood and water, especially since Sr is likely present as Sr2+ in water-soluble chemical compounds such as Sr oxalate (Serdar and Demiray, 2012). In both cases, the Sr that remains in waterlogged wood samples after rinsing seems to originate mainly from the water in which it was immersed. The question then remains whether or not there is any endogenous Sr left and, if so, whether it is possible to retain this for provenance studies while selectively removing exogenous Sr (cf., Van Ham-Meert et al., 2020).
The results obtained after the various pre-treatments show that returning to the original value of wood was possible in only one case. This sample (T69Sea) was crushed and pre-treated with acetic acid. Why this particular specimen should have been successfully cleaned is not clear, since both this and T88 were moderately dense tropical hardwoods, though of different genera. Thus, it may in fact be coincidental. The samples pre-treated with acetic acid without prior crushing or with HCl provide unsatisfactory results. The alpha-cellulose extraction method applied to experimentally water-logged oak samples by Van Ham-Meert et al. (2020), modified from Andreu-Hayles et al. (2019) to avoid equilibration between wood samples, also involves treatment with acetic acid. This method led to what the authors termed “enigmatic results,” proposing a possible explanation the presence of various Sr reservoirs within the wood (in alpha-cellulose, lignin, and beta-cellulose, etc.) with distinct isotopic compositions. The present study returned a number of results departing even more strongly from mass balance expectations, but in the opposite direction to that seen in Van Ham-Meert et al. (2020), i.e., higher rather than lower, making the situation even more puzzling. This might reflect Sr present in oxalate crystals as opposed to adsorbed Sr and/or different parts of the tree being fed through different root systems (shallow versus deep) tapping into different Sr reservoirs (Schmitt et al., 2017). All of this remains speculative and leaves room for new research venues to be explored where combining radiogenic (87Sr/86Sr) with stable Sr isotope ratios (δ88Sr) might provide new insights in the biogeochemistry of Sr in wood (e.g., Andrews et al., 2016). Overall, these experiments, along with the work of Hajj et al. (2017), show that wood becomes contaminated with exogenous Sr within a few weeks when immersed in water and that the various pre-treatments tested here are inadequate for its removal. There is also a possibility that most of the endogenous Sr is removed during immersion in water.
HCl should be avoided as after pre-treatment almost no Sr remains in the samples thus removing both endogenous and exogenous Sr. The results also suggest that pre-treatment of waterlogged wood with acetic acid on bulk material (crushed or not) led to variable results even on the same samples (e.g., the four fractions of FR2 give very different results after pre-treatment with acetic acid with 87Sr/86Sr values ranging from 0.7092 to 0.7125) and is not a viable method. Future research should investigate other alternatives. Moreover, the fact that 87Sr/86Sr values may be altered beyond any mixing line between the original, endogenous bulk value and that of the source of exogenous contamination means that it is not possible to rely on values that appear to differ significantly from those expected even assuming full contamination with a known source (i.e., seawater). This in turn calls into question published results for waterlogged wood samples such as Mediterranean shipwrecks (Rich et al., 2016) and the pre-Columbian wood carvings from Thursby Island, Florida (Ostapkowicz et al., 2017a). This matter needs to be resolved in future work.
From the results of the pitch contamination experiment, it appears that when comparing uncontaminated wood with pitch-contaminated wood, some but not all samples show a shift in their 87Sr/86Sr. Those that show no difference (T34 and T81) had original values close to that of the pitch with which they were contaminated. After immersion, the 87Sr/86Sr value of T71 shifts closer to that of the pitch compared to its original, less radiogenic value, suggesting that samples recovered from pitch should be pre-treated to remove potential contamination. Nevertheless, the tests carried out here used Angelin (Andira sp.) and pitch from Trinidad’s Pitch Lake; different wood species (i.e., with different levels of resin) as well as different asphalt deposits (i.e., with different compositions) should be further tested.
The pre-treatment tests on uncontaminated wood show the large impact of the combination of organic solvents and aqueous-based pre-treatment (i.e., ABA) on their 87Sr/86Sr values. Using only organic solvents (i.e., without ABA), however, has little to no effect on the 87Sr/86Sr of uncontaminated wood. Together with the visual observation that large amounts of material are lost, this suggests that ABA is not only removing exogenous but also endogenous Sr and as such should be avoided. This is confirmed by the results obtained for the pitch-contaminated samples. Indeed, only when organic solvents are used without ABA are the original 87Sr/86Sr values of the pitch-contaminated samples regained. This pre-treatment, consisting of several washes with a mixture of methanol and toluene (Table 2), seems, therefore, efficient at removing pitch contamination. Radiocarbon dating results, however, have shown that pitch from different locations (e.g., Rancho La Brea, CA, United States) can have different chemical compositions and require different protocols (Brock et al., 2017) and specific protocols should be tested for each pitch/asphalt. These results also suggest that in contrast to waterlogged samples where endogenous Sr is replaced by Sr from the water, due to its higher viscosity and the apparently lower solubility of Sr in pitch, pitch from Trinidad’s Pitch Lake does not exchange Sr with the wood, but instead, protects it from external contamination.
These experiments show that, depending on the conditions under which wood materials and artifacts survive through time, they may or may not preserve their initial 87Sr/86Sr values. Due to its high solubility in aqueous solution, it is not altogether surprising that waterlogged wood samples are more affected than those immersed in pitch. Conversely, archeological wood preserved in dry desiccated environments and/or ethnographic wooden artifacts collected directly at the source and stored in museum collections (e.g., Ostapkowicz et al., 2018) face a different set of issues, relating to their post-collection histories such as the application of surface treatments and consolidants. Their removal must be dealt with on a case-by-case basis, as different materials will be involved, and penetrating to different depths on different woods, etc. A discussion of this issue is well beyond the scope of this paper. Nevertheless, such objects represent a valuable source of information for provenance studies, though it is critical to first assess and discount any conservation interventions that might have impacted on their Sr isotope ratios.
Our results identify potential issues linked to (sea)water and pitch contamination on wood and wooden artifacts when investigating their origin using Sr isotope analyses. The various aqueous-based pre-treatments were not successful at removing Sr contamination from waterlogged wood and more research is needed before 87Sr/86Sr from such materials can be used for provenance studies. Furthermore, these results show that even when a 87Sr/86Sr value deviates from the value of water in which the sample was immersed (before or after pre-treatment) it cannot be assumed the sample preserves an endogenous signal and thus that is it “non-local.” In the case of pitch, it is difficult to predict if the 87Sr/86Sr ratios will be affected by contamination. Therefore, all wood and wood artifacts believed to be contaminated with pitch should be pre-treated adequately by using, for example, organic solvents pre-treatments developed for radiocarbon dating. However, it is observed that, as for the waterlogging experiments, aqueous-based pre-treatments (in this case ABA) lead to inaccurate and/or imprecise results as it probably removes large amounts of endogenous Sr, and should, therefore, be avoided.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
CS, RS, FB, and JO: conceptualization. CS: formal analysis. JO, CS, and NM: funding acquisition. CS, RS, FB, AR, AV, and NM: methodology. CS, RS, FB, and JO: writing – original draft. CS, RS, FB, AR, AV, NM, and JO: writing – review and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The work undertaken on Trinidad’s Pitch Lake material was supported by the United Kingdom’s Arts and Humanities Research Council (AHRC) as part of the Black Pitch, Carved Histories project (AH/L00268X/1). The Trinidad herbarium collections that were part of the pitch contamination experiments were collected in partnership with colleagues from the National Herbarium of Trinidad and Tobago and Trinidad’s Forestry Division, including Yasmin Baksh-Comeu, Harris Sooklal, Jason Mungalsingh, K. Manaure, and N. Falby-Peters. Cyril Billy assisted in acquiring the samples of pitch from Pitch Lake. The collection of the Florida wood samples was supported by a grant from the Wenner-Gren Foundation to RS, with the permission from Florida State Parks and the Florida Forest Service.
The VUB and VUB Strategic Research fund are thanked for their financial support for analyses; the Research Foundation–Flanders (FWO) is thanked for CS’s postdoctoral fellowship. Wendy Debouge and Jeroen de Jong from the Laboratoire G-Time (Geochemistry: Tracing by Isotope, Mineral, and Element), Université Libre de Bruxelles (Belgium) are acknowledged for their help with the strontium isotope analyses by MC-ICP-MS. Philippe Claeys, Steven Goderis and Martine Leermakers from the Vrije Universiteit Brussel (VUB – Belgium) are thanked for their help with the strontium concentrations measurements by ICP-MS.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.589154/full#supplementary-material
Andreu-Hayles, L., Levesque, M., Martin-Benito, D., Huang, W., Harris, R., Oelkers, R., et al. (2019). A high yield cellulose extraction system for small whole wood samples and dual measurement of carbon and oxygen stable isotopes. Chem. Geol. 504, 53–65. doi: 10.1016/j.chemgeo.2018.09.007
Andrews, M. G., Jacobson, A. D., Lehn, G. O., Horton, T. W., and Craw, D. (2016). Radiogenic and stable Sr isotope ratios (87Sr/86Sr, δ88/86Sr) as tracers of riverine cation sources and biogeochemical cycling in the Milford Sound region of Fiordland, New Zealand. Geochim. Cosmochim. Acta 173, 284–303. doi: 10.1016/j.gca.2015.10.005
Angino, E. E., Billings, G. K., and Andersen, N. (1966). Observed variations in the strontium concentration of seawater. Chem. Geol. 1, 145–153. doi: 10.1016/0009-2541(66)90013-1
Benson, L. V., Hattori, E. M., Taylor, H. E., Poulson, S. R., and Jolie, E. A. (2006). Isotope sourcing of prehistoric willow and tule textiles recovered from western Great Basin rock shelters and caves - proof of concept. J. Arch. Sci. 33, 1588–1599. doi: 10.1016/j.jas.2006.02.012
Benson, L. V., Taylor, H. E., Plowman, T. I., Roth, D. A., and Antweiler, R. C. (2010). The cleaning of burned and contaminated archaeological maize prior to 87Sr/86Sr analysis. J. Arch. Sci. 37, 84–91. doi: 10.1016/j.jas.2009.09.005
Bentley, R. A. (2013). Mobility and the diversity of early Neolithic lives: isotopic evidence from skeletons. J. Anthropol. Archaeol. 32, 303–312. doi: 10.1016/j.jaa.2012.01.009
Blum, J. D., Hamburg, S. P., Yanai, R. D., and Artur, M. A. (2012). Determination of foliar Ca/Sr discrimination factors for six tree species and implications for Ca sources in northern hardwood forests. Plant Soil 356, 303–314. doi: 10.1007/s11104-011-1122-2
Boyer, A., Ning, P., Killey, D., Klukas, M., Rowan, D., Simpson, A. J., et al. (2018). Strontium adsorption and desorption in wetlands: role of organic matter functional groups and environmental implications. Water Res. 133, 27–36. doi: 10.1016/j.watres.2018.01.026
Brock, F., Dee, M., Hughes, A., Snoeck, C., Staff, R., and Bronk Ramsey, C. (2018). Testing the effectiveness of protocols for removal of common conservation treatments for radiocarbon dating. Radiocarbon 60, 35–50. doi: 10.1017/rdc.2017.68
Brock, F., Higham, T., Ditchfield, P., and Bronk Ramsey, C. (2010). Current pretreatment methods for AMS radiocarbon dating at the Oxford Radiocarbon Accelerator Unit (ORAU). Radiocarbon 52, 103–112. doi: 10.1017/s0033822200045069
Brock, F., Ostapkowicz, J., Wiedenhoeft, A. C., and Bull, I. D. (2017). Radiocarbon dating wooden carvings and skeletal remains from Pitch Lake, Trinidad. Radiocarbon 59, 1447–1461. doi: 10.1017/rdc.2017.78
Budd, P., Montgomery, J., Barreiro, B., and Thomas, R. G. (2000). Differential diagenesis of strontium in archaeological human dental tissues. Appl. Geochem. 15, 687–694. doi: 10.1016/s0883-2927(99)00069-4
Chen, J.-P. (1997). Batch and continuous adsorption of strontium by plant root tissues. Bioresour. Technol. 60, 185–189. doi: 10.1016/s0960-8524(97)00021-7
Dee, M. W., Brock, F., Bowles, A., and Bronk Ramsey, C. (2011). Using a silica substrate to monitor the effectiveness of radiocarbon pretreatment. Radiocarbon 53, 705–711. doi: 10.1017/s0033822200039151
English, N., Betancourt, J., Dean, J., and Quade, J. (2001). Strontium isotopes reveal distant sources of architectural timber in Chaco Canyon, New Mexico. Proc. Natl. Acad. Sci. U.S.A. 98, 11891–11896. doi: 10.1073/pnas.211305498
Evans, J. A., Montgomery, J., Wildman, G., and Boulton, N. (2010). Spatial variations in biosphere 87Sr/86Sr in Britain. J. Geol. Soc. Lond. 167, 1–4.
GEOROC (2014). Geochemistry of Rocks of the Oceans and Continents. Available online at: http://georoc.mpch-mainz.gwdg.de/georoc/ (accessed July 8, 2014).
Hajj, F., Poszwa, A., Bouchez, J., and Guérold, F. (2017). Radiogenic and “stable” strontium isotopes in provenance studies: a review and first results on archaeological wood from shipwrecks. J. Archaeol. Sci. 86, 24–49. doi: 10.1016/j.jas.2017.09.005
Hess, J., Bender, M. L., and Schilling, J. G. (1986). Evolution of the ratio of strontium-87 to strontium-86 in -water from Cretaceous to present. Science 231, 979–984. doi: 10.1126/science.231.4741.979
Laffoon, J. E., Rodríguez Ramos, R., Chanlatte Baik, L., Storde, Y. N., Rodríguez Lopez, M., Davies, G. R., et al. (2014). Long-distance exchange in the precolonial Circum-Caribbean: a multi-isotope study of animal tooth pendants from Puerto Rico. J. Anthropol. Archaeol. 35, 220–233. doi: 10.1016/j.jaa.2014.06.004
Meighan, I. G., McCormick, A. G., Gibson, D., Gamble, J. A., and Graham, I. J. (1988). Rb-Sr isotopic determinations and the timing of Tertiary central complex magmatism in NE Ireland. Geol. Soc. Lond. Special Publ. 39, 349–360. doi: 10.1144/gsl.sp.1988.039.01.30
Ostapkowicz, J., Brock, F., Wiedenhoeft, A. C., Snoeck, C., Pouncett, J., Baksh-Comeau, Y., et al. (2017a). Black pitch, carved histories: radiocarbon dating, wood species identification and strontium isotope analysis of prehistoric wood carvings from Trinidad’s Pitch Lake. J. Archaeol. Sci. Reports 16, 341–358. doi: 10.1016/j.jasrep.2017.08.018
Ostapkowicz, J., Ramsey, C. B., Brock, F., Cartwright, C., Stacey, R., and Richards, M. (2013). Birdmen, cemís and duhos: material studies and AMS 14C dating of Pre-Hispanic Caribbean wood sculptures in the British Museum. J. Archaeol. Sci. 40, 4675–4687. doi: 10.1016/j.jas.2013.07.015
Ostapkowicz, J., Ramsey, C. B., Brock, F., Higham, T., Wiedenhoeft, A. C., Ribechini, E., et al. (2012). Chronologies in wood and resin: AMS 14C dating of pre-Hispanic Caribbean wood sculpture. J. Archaeol. Sci. 39, 2238–2251. doi: 10.1016/j.jas.2012.01.035
Ostapkowicz, J., Roberts, A., Thistlewood, J., Brock, F., Wiedenhoeft, A. C., Snoeck, C., et al. (2018). The origins of Tradescant’s ‘India occidentali’ wooden clubs: 14C dating, material identification and strontium isotope studies. Antiquaries J. 98, 1–32.
Ostapkowicz, J., Schulting, R. J., Wheeler, R., Newsom, L., Brock, F., Bull, I., et al. (2017b). East-central Florida Pre-Columbian wood sculpture: radiocarbon dating, wood identification and strontium isotope studies. J. Archaeol. Sci. Reports 13, 595–608. doi: 10.1016/j.jasrep.2017.03.035
Price, T. D., Tiesler, V., and Burton, J. H. (2006). Early African diaspora in colonial Campeche, Mexico: strontium isotopic evidence. Am. J. Phys. Anthropol. 130, 485–490. doi: 10.1002/ajpa.20390
Rediske, J. H., and Selders, A. A. (1953). The absorption and translocation of strontium by plants. Plant Physiol. 28, 594–605. doi: 10.1104/pp.28.4.594
Rich, S., Manning, S. W., Degryse, P., Vanhaecke, F., Latruwe, K., and Van Lerberghe, K. (2016). To put a cedar ship in a bottle: dendroprovenancing three ancient East Mediterranean watercraft with the 87Sr/86Sr isotope ratio. J. Archaeol. Sci. Reports 9, 514–521. doi: 10.1016/j.jasrep.2016.08.034
Schmitt, A.-D., Gangloff, S., Labolle, F., Chabaux, F., and Stille, P. (2017). Calcium biogeochemical cycle at the beech tree-soil solution interface from the Strengbach CZO (NE France): insights from stable Ca and radiogenic Sr isotopes. Geochim. Cosmochim. Acta 213, 91–109. doi: 10.1016/j.gca.2017.06.039
Serdar, B., and Demiray, H. (2012). Calcium oxalate crystal types in three oak species (Quercus L.) in Turkey. Turkish J. Biol. 36, 386–393.
Sillen, A., Hall, G., Richardson, S., and Armstrong, R. (1998). 87Sr/86Sr ratios in modern and fossil food-webs of the Sterkfontein Valley: implications for early hominid habitat preferences. Geochim. Cosmochim. Acta 62:2463. doi: 10.1016/s0016-7037(98)00182-3
Sillen, A., and LeGeros, R. (1991). Solubility profiles of synthetic apatites and of modern and fossil bones. J. Archaeol. Sci. 18, 385–397. doi: 10.1016/0305-4403(91)90073-x
Snoeck, C., Pouncett, J., Ramsey, G., Meighan, I., Mattielli, N., Goderis, S., et al. (2016). Mobility during the Neolithic and Bronze Age in Northern Ireland explored using strontium isotope analysis of cremated human bone. Am. J. Phys. Anthropol. 160, 397–413. doi: 10.1002/ajpa.22977
Snoeck, C., Ryan, S., Pouncett, J., Pellegrini, M., Claeys, Ph, Wainwright, A. N., et al. (2020). Towards a biologically available strontium isotope baseline for Ireland. Sci. Total Environ. 712:136248. doi: 10.1016/j.scitotenv.2019.136248
Snoeck, C., Schulting, R. J., Lee-Thorp, J. A., de Jong, J., Debouge, W., and Mattielli, N. (2015). Calcined bone provides a reliable substrate for strontium isotope ratios as shown by an enrichment experiment. Rapid Commun. Mass Spectr. 29, 107–114. doi: 10.1002/rcm.7078
Storey, R., and Leigh, R. A. (2004). Processes modulating calcium distribution in citrus leaves. An investigation using X-Ray microanalysis with strontium as a tracer. Plant Physiol. 136, 3838–3848. doi: 10.1104/pp.104.045674
Styring, A. K., Evans, J. A., Nitsch, E. K., Lee−Thorp, J. A., and Bogaard, A. (2019). Revisiting the potential of carbonized grain to preserve biogenic 87Sr/86Sr signatures within the burial environment. Archaeometry 61, 179–193. doi: 10.1111/arcm.12398
Vaiglova, P., Snoeck, C., Nitsch, E., Bogaard, A., and Lee-Thorp, J. A. (2014). Impact of contamination and pre-treatment on stable carbon and nitrogen isotopic composition of charred plant remains. Rapid Commun. Mass Spectr. 28, 2497–2510. doi: 10.1002/rcm.7044
Van Ham-Meert, A., Rodler, A. S., Waight, T. E., and Daly, A. (2020). Determining the Sr isotopic composition of waterlogged wood – cleaning more is not always better. J. Archaeol. Sci. 124:105261. doi: 10.1016/j.jas.2020.105261
Keywords: strontium isotope (87Sr/86Sr), waterlogged wood, provenance, pitch, pre-treatment
Citation: Snoeck C, Schulting RJ, Brock F, Rodler AS, Van Ham-Meert A, Mattielli N and Ostapkowicz J (2021) Testing Various Pre-treatments on Artificially Waterlogged and Pitch-Contaminated Wood for Strontium Isotope Analyses. Front. Ecol. Evol. 8:589154. doi: 10.3389/fevo.2020.589154
Received: 30 July 2020; Accepted: 11 December 2020;
Published: 12 January 2021.
Edited by:
Kate Britton, University of Aberdeen, United KingdomReviewed by:
Michael Weber, Johannes Gutenberg University Mainz, GermanyCopyright © 2021 Snoeck, Schulting, Brock, Rodler, Van Ham-Meert, Mattielli and Ostapkowicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christophe Snoeck, Y2hyaXN0b3BoZS5zbm9lY2tAdnViLmJl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.