
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol. , 08 September 2020
Sec. Behavioral and Evolutionary Ecology
Volume 8 - 2020 | https://doi.org/10.3389/fevo.2020.568140
This article is part of the Research Topic Factors Affecting Host Selection by Mosquitoes: Implications for the Transmission of Vector-Borne Pathogens View all 12 articles
A large diversity of parasites manipulates their hosts in various ways to complete their own life cycle. Enhancing the attractiveness of their host to vectors has been suggested as a strategy allowing vector-borne parasites to increase their transmission. Indeed, a higher attraction of hematophagous, arthropod vectors to infected vertebrates compared to uninfected individuals has been found in many systems (e.g., Trypanosoma-tsetse flies, Leishmania-sand flies, Borrelia-ticks) but was most often verified in the Plasmodium-mosquitoes model. However, a number of studies found no difference in attractiveness, or a higher attractiveness of uninfected hosts. In this review, we present studies reporting a comparison of the attractiveness and/or the biting rate of infected and uninfected vertebrates. We then discuss several biological factors and experimental design aspects that can explain discrepancies between studies. Finally, we stress the importance of investigating the mechanisms of parasite-induced increased attractiveness of infected hosts to conclude that such observations are cases of adaptive manipulation.
Host manipulation by parasites has been fascinating parasitologists for decades (Thomas et al., 2005), especially when this manipulation results in dramatic changes in host’s physiology (Cordaux et al., 2011; Kageyama et al., 2012; Fayard et al., 2020), morphology (Bakker et al., 1997; Yanoviak et al., 2008; Fayard et al., 2020) or behavior (Berdoy et al., 2000; Thomas et al., 2002; Weinersmith, 2019; Fayard et al., 2020). This host exploitation strategy, described in many phylogenetically distant host-parasite systems, usually involves phenotypic changes in the infected host (extended phenotype, Dawkins, 1982). These alterations can be mediated by direct or indirect mechanisms (Thomas et al., 2005). Parasites can produce, store and release active manipulation factors that act directly on host tissues such as the nervous system or muscles. These manipulative factors are part of a complex mixture of molecules called the secretome (Adamo, 2013; Biron and Loxdale, 2013; Berger and Aubin-Horth, 2020). On the other hand, the mere physical presence of parasites in particular tissues or simply the pathological by-products of infection can influence the development and/or the metabolism of the host, secondarily leading to an alteration of its phenotype (Dingemanse et al., 2009; Thomas et al., 2012).
Vector-borne parasites have evolved different angles of attack to increase their own transmission by manipulating their vectors and hosts (Lefèvre and Thomas, 2008). Indeed, infected vectors show alterations in host-seeking and feeding behaviors, fecundity and longevity [reviewed in Hurd (2003), Cator et al. (2012), Murdock et al. (2017), Stanczyk et al. (2017)], microhabitat preference (Fialho and Schall, 1995) and selection of host species (Vantaux et al., 2018 but see Nguyen et al., 2017; Vogels et al., 2017), that all seem beneficial for the transmission of the parasites they harbor. For example, impaired ingestion forcing the vector to bite several times to achieve a complete blood-meal has been reported in Trypanosoma-infected tsetse flies and kissing bugs, Leishmania-infected sand-flies, Plasmodium-infected mosquitoes and plagued fleas [reviewed in Hurd (2003)]. Although this example can be considered as a simple pathological consequence of the infection with coincidental benefit for transmission, the convergent appearance of this phenomenon was hypothesized to reflect adaptive manipulation (Poulin, 1995).
In addition, parasites seem able to make their vertebrate hosts more attractive to their vectors. From this point of view, the vectors can be considered as “exploited”, because their host-seeking behavior is indirectly influenced by an alteration of the infected vertebrate’s phenotype. Although increased attractiveness of infected hosts has been reported in various host/parasite systems, many studies have obtained conflicting results. Here, we first propose a review of the current knowledge on this topic by gathering all the studies that have empirically investigated the impact of infection of vertebrate hosts on their attractiveness to arthropod vectors. We also take stock of current knowledge on the mechanisms potentially involved in attraction bias. Then, based on a review of the materials and methods used to study attraction bias, we seek to identify crucial aspects of the experimental designs that could explain the contradictory results frequently observed.
For over a century, the epidemiology of vector-borne diseases was addressed using mathematical models describing the life cycle of pathogens in vertebrate and invertebrate (vector) hosts. Although the early models included several strong assumptions, which greatly simplified the complex host-vector interactions (Ross, 1916; Macdonald, 1956, 1957; Bailey, 1975), they highlighted the impact of different host and vector life-cycle parameters on parasite transmission and, in particular, they pointed out that the vector’s biting rate could have major consequences on transmission dynamics. Then, epidemiological models became more and more complex by integrating new parameters such as vector feeding bias. Whereas early models assumed that vectors chose hosts and fed on them randomly, and therefore independently of the absence or presence of infection, empirical data acquired in the early 1980s have overturned this paradigm. It was observed that mosquitoes preferentially fed on malaria-infected rather than uninfected mice (Day and Edman, 1983; Day et al., 1983). Based on this observation, Kingslover was the first to develop and analyze a model for the dynamics of parasite transmission that incorporates nonrandom feeding behavior by the vector (Kingsolver, 1987). He demonstrated that increasing the preference of vectors for infected hosts leads to an easier maintenance of a stable infection, compared to the model where vectors feed on their host in a random manner. Subsequent studies confirmed this result and showed that the preference of vectors for infected hosts can strongly reinforce the transmission of the parasite at the beginning of the epidemic (McElhany et al., 1995; Hosack et al., 2008; Sisterson, 2008; Chamchod and Britton, 2011; Zeilinger and Daugherty, 2014; Gandon, 2018). Parasite that are able to manipulate their vertebrate hosts to make them more attractive to vectors should be therefore favored by natural selection. However, an extreme preference of vectors for infected hosts may also limit or stop the transmission of parasites (Kingsolver, 1987; McElhany et al., 1995; Sisterson, 2008; Zeilinger and Daugherty, 2014; Gandon, 2018). Indeed, when levels of host infection in a population are very high, vector feeding bias results in most of the bites occurring on already infected hosts. Natural selection should then favor parasites that induce either an intermediate level of attractiveness or a conditional change in the behavior of the vector depending on its own infection status (see Gandon, 2018).
We gathered the studies that investigated the manipulation of host attractiveness to vector-borne parasites, by searching Google Scholar and Web of Science databases with combinations of the terms “vector” and “parasite” or “virus” or “bacteria” and “attraction” or “attractiveness” or “choice” or “host selection” or “olfactometer” or “preference.” All studies that report an observational or experimental comparison of the numbers of arthropod vectors that were attracted to and/or bit infected and uninfected vertebrates were selected. We also included experiments using only the odor of the vertebrates. These studies are summarized in Table 1 (Culicidae) and Table 2 (other invertebrate vectors). The majority of studies focused on Culicidae vectors and Plasmodium infection, finding in most cases, but not always, that mosquitoes are more attracted to Plasmodium-infected hosts (12/18; Table 1, Figure 1).
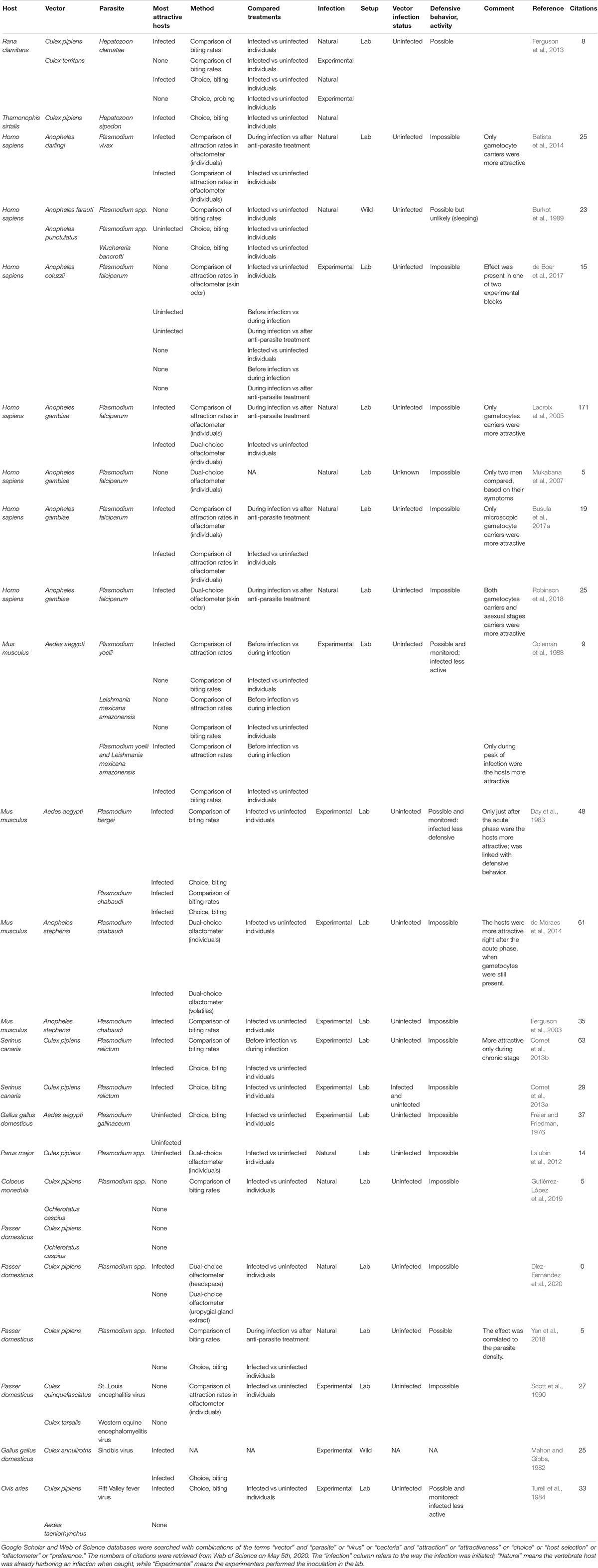
Table 1. Summary of studies comparing attractiveness of infected and uninfected hosts to mosquitoes.
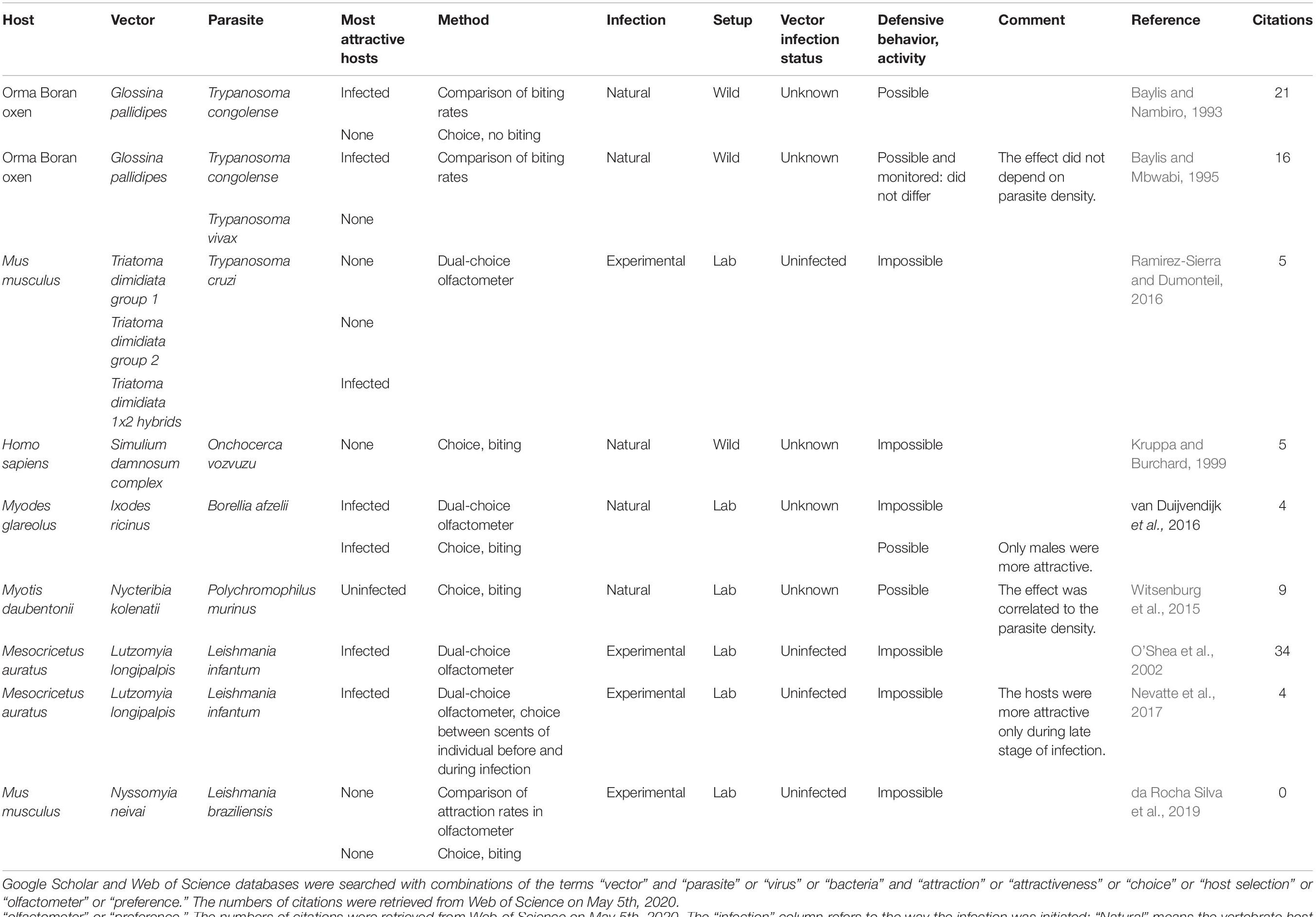
Table 2. Summary of studies comparing attractiveness of infected and uninfected hosts to non-mosquito vectors.
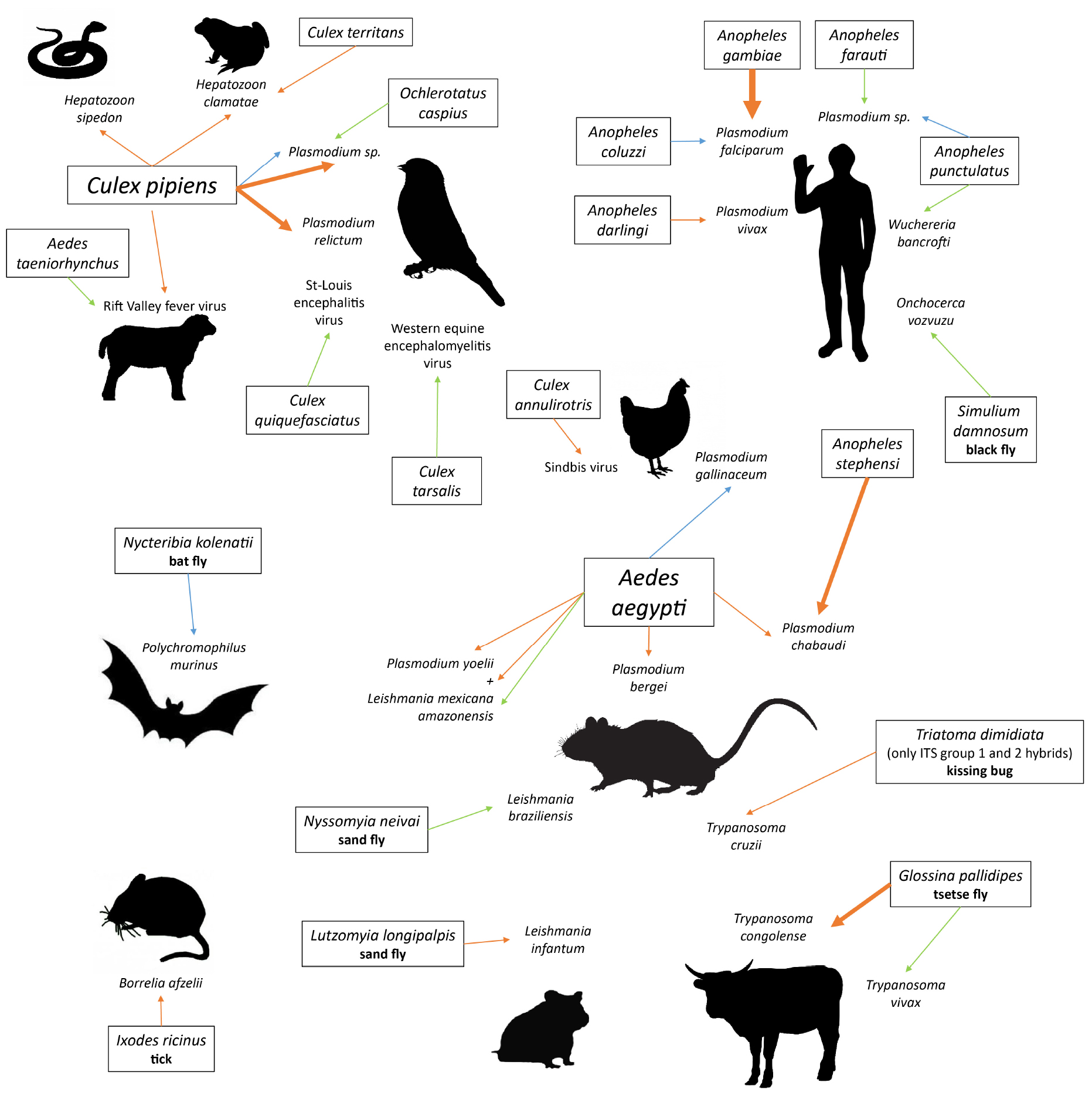
Figure 1. Pairs of vector-parasite species that have been tested for differential attractiveness of infected versus uninfected hosts. Line width is proportional to the number of studies finding this result. Orange lines: higher attractiveness of infected hosts; blue lines: higher attractiveness of uninfected hosts; green lines: no preference found. Species names in boxes are vectors; latin names that are not followed by a vernacular name are mosquitoes (Culicidae).
Only two studies looked for a biased attractiveness of hosts infected with filarial worms and found none (Burkot et al., 1989; Kruppa and Burchard, 1999). More tsetse flies (Glossina pallidipes) bit the Orma Boran oxen infected with Trypanosoma congolense, but not those infected by Trypanosoma vivax (Baylis and Nambiro, 1993; Baylis and Mbwabi, 1995), and only hybrids of the Triatoma dimidiata complex preferred Trypanosoma cruzi-infected mice over uninfected ones (Ramirez-Sierra and Dumonteil, 2016). Leishmania infantum made their hamster hosts more attractive to Lutzomyia longipalpis (O’Shea et al., 2002; Nevatte et al., 2017), but Leishmania braziliensis did not enhance attractiveness of mice for Nyssomyia neivai (da Rocha Silva et al., 2019). The probability that a bat fly chose a host was negatively correlated with Polychromophilus murinus parasitaemia in the Daubenton bat (Myotis daubentonii) (Witsenburg et al., 2015). The only non-insect study that we found showed that male bank voles infected with the bacteria Borrelia afzellii harbored more ticks (van Duijvendijk et al., 2016). Finally, a few early studies investigated the attractiveness of virus-infected animals and found mitigated results (Mahon and Gibbs, 1982; Turell et al., 1984; Scott et al., 1990).
Vectors of endoparasites infecting vertebrates are usually hematophagous arthropods, mostly insects and ticks. Insects perceive olfactory molecules thanks to receptor neurons in sensilla, distributed on their antennae, maxillary palps and labia (Keil, 2012; Suh et al., 2014). Ticks sense volatile organic compounds (VOC) through a specific organ called Haller’s organ located on their first pair of legs. Although not well known yet, it seems that chemoreceptors on Haller’s organ are not similar to those on insects’ sensilla (Carr et al., 2017). Molecules found to trigger responses in hematophagous arthropods mostly fall into the following categories: short chain carboxylic acids, aldehydes or low molecular weight nitrogenous compounds like ammonia (Syed, 2015). Indole and 1-octen-3-ol are often identified as attractant among the studied insects, and carbon dioxide attracts all vectors (triatomines, cimicidae, ticks, tsetse flies, sand flies and mosquitoes; Syed, 2015). Carbon dioxide is also used in long-distance detection by host-seeking insects (Gillies, 1980). Mosquitoes can perceive very small variations of CO2 concentration, like for instance Aedes aegypti, which has a detection threshold between 150 and 300 ppm and can perceive increments of 50 ppm (Grant et al., 1995). Cues used by vectors to select hosts are scattered in the air through the breath, the skin, and the excretions (Takken, 1991).
Recent studies have identified how VOC profiles differ between infected and uninfected hosts, and which compounds are involved in influencing attractiveness. Profiles of VOCs produced by humans and mice infected by Plasmodium falciparum and Plasmodium chabaudi, respectively, show they produce some compounds in higher quantities, while other compounds are suppressed in infected hosts (de Moraes et al., 2014; de Boer et al., 2017; Robinson et al., 2018). Some of these compounds (e.g., hexanoic acid, 2- and 3-methyl butanoic acid and tridecane in mice, heptanal, tetradecanoic acid, 3-methyl-1-butanol, and butan-1-amine in humans) have been shown to elicit a preference when added to the scent of a non-infected host (de Moraes et al., 2014; Robinson et al., 2018). Octanal, nonanal and decanal are among VOCs that showed significant variations between healthy dogs and dogs infected with Leishmania infantum (Magalhães-Junior et al., 2014), and were attractive to male sand fly vectors Lutzomyia longipalpis (Magalhães-Junior et al., 2019).
These attractive molecules can be produced by the infected host, by the parasite itself, or by the host microbiota. There is evidence that Plasmodium can synthesize terpenes, to which mosquitoes respond (Kelly et al., 2015), although they were not identified from infected mice or infected human skin and breath emissions (de Moraes et al., 2014; Berna et al., 2015; Robinson et al., 2018). Some malaria-associated VOCs are known to be produced by skin bacteria (de Moraes et al., 2014; de Boer et al., 2017). Plasmodium infection could cause a change in host microbial species composition, possibly mediated by immunological or endocrine systems (Busula et al., 2017b). Emami et al. (2017) have shown that a metabolite produced by Plasmodium falciparum ((E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate; HMBPP) induces red blood cells to produce molecules involved in mosquito attraction (CO2, aldehydes, and monoterpenes). The mechanisms involved in these potential parasite-induced phenotypic alteration (Poulin, 1995) are yet not all identified and several unknowns remain to be discovered (Busula et al., 2017b; Joice Cordy, 2020).
Although most of the investigated vector-borne parasite genera are featured in at least one study finding that infected hosts attract more vectors or are more often bitten than uninfected hosts (Figure 1), it is difficult to determine whether this is a widespread phenomenon. One of the reasons for this is that the methods used to study vector attraction bias vary considerably from one study to another, making it impossible to draw an overall picture. In this second part, we have sought to identify aspects of experimental designs that may explain why, for the same parasite/vector pair, studies come to different conclusions.
A source of discrepancies between conclusions of studies might come from the parasite developmental stages in the timeframe of the experiments. Enhanced attractiveness of the infected vertebrate host should coincide with stages when the parasites are transmissible to vectors. This is confirmed by most studies checking for parasite developmental stages, which found that only Plasmodium gametocytes (sexual, transmissible stage) carriers were more attractive to Anopheles mosquitoes [Lacroix et al., 2005; Batista et al., 2014; de Moraes et al., 2014; Busula et al., 2017a, reviewed in Busula et al. (2017b), but see Robinson et al. (2018)]. Most studies finding a preference for the uninfected hosts did not verify whether the infected host exhibited transmissible stages (Freier and Friedman, 1976; Burkot et al., 1989; Lalubin et al., 2012). de Boer et al. (2017) did control but found no gametocytes in the tested individuals. The exception comes from Witsenburg et al. (2015) who found that bat flies that switched hosts ended up more often on the one with the lowest density of Polychromophilus murinus gametocytes, a malaria-like parasite. While attracting vectors when harboring transmissible stages seems like a selective advantage for parasites, it would bring no benefit during non-transmissible stages. Premature blood meals might even be deleterious, for example if they bring new parasites that may compete for resources, trigger a stronger immune response or kill their host before their own transmission.
Plasmodium infection dynamics in the blood is in general composed of two stages: the acute phase, a few days after infection, characterized by a peak in parasite density, followed by the chronic phase, with low parasite density (Garnham, 1966). Hosts harboring high parasite densities during the acute stage of Plasmodium infections were sometimes found to be less or equally attractive than uninfected hosts, and hosts became more attractive once the peak passed (Cornet et al., 2013b; de Moraes et al., 2014). Authors suggested that, as blood characteristics linked to immune response suppress infectivity (Mendis et al., 1987; Naotunne et al., 1993; Ramiro et al., 2011), it makes it useless for the parasite to attract vectors when the proportion of gametocytes relative to asexual stages is low, even if the absolute quantity of gametocytes may be higher than during other infection stages. In addition, the strong reduction in red blood cells counts following erythrocytic meronts rupture makes the blood less nutritious, and thus potentially less attractive for hematophagous arthropods. This hypothesis could be tested by examining the attraction of hematophagous species to individuals infected with parasites that they do not transmit. A recent study suggests that at least Amblyomma tick nymphs do not avoid birds infected with haemosporidian parasites (Fecchio et al., 2020). However, ectoparasites that are relatively less mobile, such as ticks, might not be the best models to test this, as the cost of being too selective might be high, especially if the chances to encounter several potential hosts are low.
Conversely, Day et al. (1983) found that peak parasitaemia of P. chabaudi was associated with a peak of biting rate by Aedes aegypti, probably because infected mice exhibited fewer defensive behaviors due to illness. Similarly, P. berghei-infected mice were more bitten in the end of infection before mice died (but also right before the increase in parasitaemia), which can also be associated with the decrease in anti-mosquito behavior. Sickness behavior-linked reduction in anti-mosquito behavior could also explain Coleman et al. ‘s results (1988), who found that Plasmodium yoelii and Leishmania mexicana amazonensis co-infected mice were more bitten by Aedes aegypti than single- or un-infected mice, especially when the infection symptoms were the most severe.
Successful transmission to vectors is required to conclude that an increased attractiveness of infected hosts is an adaptive strategy of the parasite. Three main steps lead to transmission and could be influenced by parasites: attraction of vectors, vectors’ decision to bite, and vectors’ ability to bite, determined mostly by hosts’ defensive behavior. Experiments aiming at determining whether parasites make their host more attractive to vectors use diverse methods, which sometimes does not allow addressing, or differentiating between, each step of this process. Supplementary Figure S1 summarizes study main results in function of experimental characteristics.
A major difference between studies is whether uninfected and infected hosts attractiveness is compared through dual-choice experiments or through comparisons of biting/attraction rates. In dual-choice experiments, vectors are offered equal numbers of infected and uninfected hosts (in general, one of each) simultaneously, either in a dual-choice olfactometer (e.g., Lalubin et al., 2012), where vectors are not allowed to bite, or in a cage (e.g., Cornet et al., 2013a,b) or arena (e.g., van Duijvendijk et al., 2016). Dual-choice olfactometers have the advantage of allowing a distinction between attraction and vectors’ ability to bite and providing direct evidence that one category of hosts is more attractive than the other. They also allow using only host odor, carbon dioxide emission or individual volatile compounds and test the relative attractiveness of each molecule. Nevertheless, dual-choice olfactometer experiments should, when possible, be coupled with dual-choice experiments where vectors are able to bite. Indeed, a higher attraction does not necessarily translate into more blood meals. We can conclude that the apparent manipulation is adaptive only by showing that infected hosts get more bites and transmit their parasites.
Allowing animals to defend themselves against vectors during experiments admittedly provides a better representation of the process in the way it would happen in the wild. However, when infected hosts differ in their behavior due to sickness, they become in general less active and less defensive, which facilitates vector attacks (Day et al., 1983; Turell et al., 1984; Coleman et al., 1988). Thus, in this setting, a higher biting rate of infected host cannot be conclusively assigned to either sickness behavior, which may be associated with a decrease in defensive behavior, or parasite manipulation of host attractiveness. For example, Plasmodium yoelii-infected mice and P. yoelii + Leishmania mexicana – infected mice were less active during the peak infection and this coincided with a peak in the number of mosquitoes able to take a blood-meal (Coleman et al., 1988). Day et al. (1983) found an association between gametocyte density, defensive behavior and mosquito biting success in P. chabaudi- and P. berghei-infected mice. This type of change in behavior of infected animals is sometimes regarded as a type of parasite manipulation (Lefèvre and Thomas, 2008). While it can be coincidentally advantageous for parasite transmission, sickness behavior may have evolved as an adaptive strategy in hosts, increasing the probability to eliminate or recover from the infection (Hart, 1988; Johnson, 2002), rather than a part of parasite extended phenotype. Indeed, sickness behavior is triggered by the immune response, in particular cytokines, and can be induced by the injection of lipopolysaccharide without the actual presence of parasites (Dantzer, 2001). Thus, this response is not specific to any parasite, which supports the idea that this consequence of infection has not evolved as a parasite adaptation but is under the genetic control of the host. Adamo (2014) has formulated the hypothesis that sexually transmitted pathogens suppress sickness behavior, which generally includes loss of sexual motivation, thus favoring their transmission. It would seem that inhibition of sickness behavior is more likely to result from parasite manipulation than the opposite.
When trying to determine whether a parasite adaptively manipulates the attractiveness of its host, experiments should as much as possible decompose each step of the process to properly identify whether infected hosts are really more attractive, whether they really get more bites and whether bites really result in transmission. One way to test whether infected hosts get more bites, while avoiding the confounding factor of defensive behavior, is to anesthetize the tested animals (da Rocha Silva et al., 2019) or to mechanically immobilize them (Cornet et al., 2013a,b).
The possibility of observing parasitic manipulation of host attractiveness may depend on the level of co-adaptation in the host-vector-parasite trio. If being infected bears a fitness cost for vectors, they might evolve parasite avoidance strategies. Evidence for impacts of parasites on their arthropod vector reproductive parameters exist, especially in mosquitoes, although they are sometimes contradictory. Using lab-bred Culex pipiens and Plasmodium relictum, infection was shown to decrease mosquito fecundity (Vézilier et al., 2012; Pigeault and Villa, 2018) and to increase their survival under ad libitum food conditions (Vézilier et al., 2012). On the opposite, using mosquitoes reared from egg rafts collected in the field, the same infection was shown to slightly increase fecundity (Delhaye et al., 2016) and to decrease survival under a food restriction regime (Lalubin et al., 2014). Independently of the systems used, most of these studies did not find an effect of the infection on host longevity when food was given ad libitum (Lalubin et al., 2014; Delhaye et al., 2016; Pigeault and Villa, 2018). Moreover, Plasmodium falciparum increases fecundity and do not reduce survival in Anopheles gambiae (Alout et al., 2016). Evidence for cost of infection in other vector groups are less numerous; Trypanosoma cruzii infection decreases fecundity and survival of the triatomine bugs Meccus pallidipennis (Cordero-Montoya et al., 2019). Conflicting results regarding the manipulation of host choice by vector-borne parasites could result from differences in the associations of species tested (natural or not), as well as in the origin of the tested individuals, wild or laboratory-bred (Ferguson and Read, 2002; Tripet, 2009).
It may thus be possible that vectors and parasites face off in an evolutionary arms race between parasite avoidance and deceptive signaling. If this is the case, when we observe interactions between populations that are co-evolving, vectors’ potential anti-infection mechanisms might counteract parasite manipulation, depending on what stage of this arms race they are experiencing. In addition, we need to consider the release from selective pressure allowed to lab strains as potentially enhancing or reducing the effect of parasite on attractiveness. Indeed, lab-bred mosquito populations that have not been under selective pressure for parasite resistance/avoidance for hundreds of generations – as they are usually fed on uninfected blood sources – might show exacerbated attraction to hosts whose attractiveness has been manipulated by parasites. Alternatively, they might have lost some host-seeking skills by being constantly offered effortless food. Similarly, parasite strains that have been passaged across several years without the contribution of vectors (e.g., by intraperitoneal injections of infected host blood into another host) might have lost their capacity to make their hosts attractive to vectors. This might explain the discordant results of Lalubin et al. (2012) and Cornet et al. (2013b), who found a lower attraction of Culex pipiens toward wild great tits (Parus major) naturally infected by Plasmodium sp. and a higher biting rate in canaries infected with a lab strain of Plasmodium relictum (SGS1), respectively. In addition to the infection origin and the variable measured, these experiments differ with regards to the mosquito origins, as Lalubin et al. (2012) used mosquitoes emerged from freshly collected egg rafts, present at one of the sites where the tested great tits were caught, while Cornet et al. (2013b) used a lab colony initiated 10 years before the experiment. The generally reduced phenotypic variation of lab strains and the release from co-evolutionary selection pressures (Tripet, 2009) can explain the diverging results of these two experiments.
The expectation that mosquitoes should experience selection for parasite avoidance needs to be mitigated by two considerations. First, if the cost of seeking a new – hopefully uninfected – host exceeds the cost of being infected, evolution for parasite avoidance is unlikely. This could depend, among other factors, on host density and availability, and on parasite prevalence. Secondly, if the only effects of infection perceivable by vectors are quantitative variations of existing cues always used when host seeking, for instance the increased production of some VOCs (de Moraes et al., 2014; de Boer et al., 2017; Robinson et al., 2018), it might be more difficult for vectors to evolve parasite avoidance. They could rely on other cues, for instance the detection of a particular skin microbiota composition after landing on host, however, the mechanisms of this hypothetic detection is unknown to the best of our knowledge.
When comparing the attractiveness of uninfected and infected hosts, it is crucial to take into account the possibility of co-infection with other parasites. It is especially important when using naturally infected individuals, in particular if several parasites are locally transmitted by the same vector species. For example, none of the studies examining the attractiveness of wild Plasmodium-infected birds to Culex pipiens shown in Table 1 tried to determine whether the birds were harboring West Nile Virus (WNV), which is also transmitted by Culex pipiens. This could be a concern especially if these parasites co-occur in a non-random way, as it was found to be the case in Chicago, where there was a negative association between Plasmodium sp. infections and WNV serostatus (Medeiros et al., 2014). By ignoring other potential infections, we might under- or overestimate the capacity of the focal species to increase its hosts’ attractiveness.
Most studies comparing infected individuals and individuals after anti-parasite treatments found a preference for non-treated individuals (Lacroix et al., 2005; Batista et al., 2014; Busula et al., 2017a; Robinson et al., 2018; Yan et al., 2018; but see de Boer et al., 2017). Using treated individuals as uninfected controls is convenient for assessing the attractiveness of naturally infected hosts when uninfected hosts are difficult to find. It also allows avoiding a pre-existing bias, in the sense that naturally uninfected individuals might be so because they are, for other causes unrelated to the focal infection, less attractive to vectors (Mukabana et al., 2002). However, anti-parasitic drugs can have an effect on biting insects, independently of their infection status, either through a direct effect or through an alteration of blood characteristics. A preference for infected hosts in this type of experiments might actually indicate an avoidance of drug-treated hosts. In a recent study, de Boer et al. (2019) evaluated the effect of artemether-lumefantrine (AL) on mosquito host-seeking behavior and fitness. Using traps baited with socks worn by AL-treated men (before, during and after treatment) they found more Anopheles coluzzi attracted to the socks worn after treatment, but no difference between before and during treatments. In a semi-natural setting, Anopheles gambiae did not show a preference for either AL treatment time-points. However, a comparison of the attractiveness of socks worn the same day by AL-treated and untreated humans would have been useful to avoid an effect of “time since odor collection”. Membrane-feeding with human blood containing a high concentration of dissolved AL resulted in no difference in survival, oviposition rate, timing before oviposition and number of eggs. This tends to suggest that it is appropriate to use AL-treated individuals as “uninfected” hosts in mosquito choice trials. Nevertheless, studies allowing mosquitoes to feed on infected+treated, uninfected+treated, infected+untreated and uninfected+untreated humans are necessary to conclude, because metabolization of AL might induce changes in blood characteristics that would not occur in membrane-feeders. Lacroix et al. (2005) found that former gametocyte carriers treated with sulfadoxine pyrimethamine (SP) seemed to repel mosquitoes, although it was not the case of treated individuals that were not gametocyte-carriers. As high concentrations of SP can strongly reduce Anopheles stephensi survival (Kone et al., 2010), mosquitoes might evolve the capacity to detect and avoid SP-treated humans.
Parasitic manipulation of host attractiveness is a seducing hypothesis and publication bias is likely to occur, as was suggested by Poulin (2000). Among the studies presented here, those that found a preference for infected hosts were on average more cited than the studies finding a preference for the uninfected host, and both were more cited than studies finding no preference (Supplementary Figure S2). This might discourage researchers to submit (and publishers to publish) manuscripts reporting results that do not support the hypothesis of increased attractiveness of infected hosts.
Future work on this topic could use the selection of studies reviewed here to conduct a meta-regression aiming to quantify the magnitude of the effects. Indeed, while we discussed the potential influence of different experimental designs on effect directionality – i.e., the preference of vectors for infected versus uninfected hosts – a meta-regression would provide a formal testing of their impacts on the strength of the observed effect in a quantitative way. This would also allow a more accurate identification of possible publication bias. Finally, a meta-regression could be used to assess to what extent different transmission cycle characteristics or parasite life history strategies affect vector-borne parasite manipulation of host.
This review highlights that manipulation of host attractiveness is widespread among different groups of vector-borne parasites. However, contradictory results show that it cannot be considered as a rule. Manipulation of host attractiveness might be one side of an evolutionary arms race against parasite avoidance, and different pairs of vector and parasite populations could find themselves at one or the other stage of a co-evolutionary timeline. In addition, parasites other than Plasmodium sp. deserve that we put more effort into investigating their ability to trigger attractant molecules production in their hosts, especially vector-borne viruses and filarial worms. Experiments trying to assess the generality of this phenomenon should ideally design their experiment in a way that (1) verifies the presence of transmissible stages in host blood, (2) separates attraction from capacity to bite, and (3) uses parasite and vector strains that have not been released from co-evolutionary selective pressures for many generations. Finally, we stress the importance to investigate the proximate mechanisms responsible for a higher attractiveness of infected hosts in order to determine which partner has the genetic control of the situation, as well as its impact on parasite fitness, before concluding on adaptive manipulation (Herbison et al., 2018).
C-SC, OG, PC, and RP contributed to the original idea and the final version of this manuscript. C-SC and RP collected the data and contributed to the first draft of this manuscript. C-SC conceived the figures and analyzed the data. All authors contributed to the article and approved the submitted version.
This project was funded by the Swiss National Science Foundation (grant 31003A_179378).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the three reviewers who gave interesting comments on the earlier version of the manuscript and greatly helped in improving its quality.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.568140/full#supplementary-material
FIGURE S1 | Study main results in function of experimental characteristics. This figure summarizes the number of studies that found a greater attraction of infected vertebrate hosts according to different experimental characteristics (natural versus experimental infection, host defensive behavior possible or not). This figure does not account for effect sizes.
FIGURE S2 | Number of citations of the reviewed studies in function of the year of their publication. The regression lines were plotted using the “ggplot” package (Wickham, 2016), using values predicted by a negative binomial generalized linear model, using the number of citations as the response variable, and the year of publication and the main result as predictors (orange: at least one result of the study shows a higher attractiveness of infected hosts; blue: at least one result of the study shows a higher attractiveness of uninfected hosts; green: no difference found). We used the function glm.nb in the “MASS” package (Venables and Ripley, 2002) to fit the model. The likelihood ratio test was used to assess the significance of both predictors: “year of publication”: χ21 = 3.73, p = 0.053; “main result”: χ22 = 6.69, p = 0.035. We also fitted a model with an interaction between the year of publication and the main result, and the interaction was not significant (χ22 = 2.15, p = 0.34). Analyses were performed on R v.3.5.1 (R Core Team, 2018).
Adamo, S. A. (2013). Parasites: evolution’s neurobiologists. J. Exp. Biol. 216, 3–10. doi: 10.1242/jeb.073601
Adamo, S. A. (2014). Parasitic aphrodisiacs: manipulation of the hosts’ behavioral defenses by sexually transmitted parasites. Integr. Comp. Biol. 54, 159–165. doi: 10.1093/icb/icu036
Alout, H., Dabiré, R. K., Djogbénou, L. S., Abate, L., Corbel, V., Chandre, F., et al. (2016). Interactive cost of Plasmodium infection and insecticide resistance in the malaria vector Anopheles gambiae. Sci. Rep. 6, 1–11.
Bailey, N. T. (1975). The Mathematical Theory of Infectious Diseases and Its Applications. Glasgow: Charles Griffin and Company Ltd.
Bakker, T. C. M., Mazzi, D., and Zala, S. (1997). Parasite-induced changes in behavior and color make Gammarus pulex more prone to fish predation. Ecology 78, 1098–1104. doi: 10.2307/2265861
Batista, E. P., Costa, E. F., and Silva, A. A. (2014). Anopheles darlingi (Diptera: Culicidae) displays increased attractiveness to infected individuals with Plasmodium vivax gametocytes. Parasites Vect. 7:251. doi: 10.1186/1756-3305-7-251
Baylis, M., and Mbwabi, A. L. (1995). Feeding behaviour of tsetse flies (Glossina pallidipes Austen) on trypanosoma-infected oxen in Kenya. Parasitology 110, 297–305. doi: 10.1017/s0031182000080884
Baylis, M., and Nambiro, C. O. (1993). The effect of cattle infection by Trypanosoma congolense on the attraction, and feeding success, of the tsetse fly Glossina pallidipes. Parasitology 106, 357–361. doi: 10.1017/s0031182000067093
Berdoy, M., Webster, J. P., and Macdonald, D. W. (2000). Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. B 267, 1591–1594. doi: 10.1098/rspb.2000.1182
Berger, C. S., and Aubin-Horth, N. (2020). The secretome of a parasite alters its host’s behaviour but does not recapitulate the behavioural response to infection. Proc. R. Soc. B 287, 20200412. doi: 10.1098/rspb.2020.0412
Berna, A. Z., McCarthy, J. S., Wang, R. X., Saliba, K. J., Bravo, F. G., Cassells, J., et al. (2015). Analysis of breath specimens for biomarkers of Plasmodium falciparum infection. J. Infect. Dis. 212, 1120–1128.
Biron, D. G., and Loxdale, H. D. (2013). Host–parasite molecular cross-talk during the manipulative process of a host by its parasite. J. Exp. Biol. 216, 148–160. doi: 10.1242/jeb.073825
Burkot, T. R., Narara, A., Paru, R., Graves, P. M., and Garner, P. (1989). Human host selection by anophelines: no evidence for preferential selection of malaria or microfilariae-infected individuals in a hyperendemic area. Parasitol 98, 337–342.
Busula, A. O., Bousema, T., Mweresa, C. K., Masiga, D., Logan, J. G., Sauerwein, R. W., et al. (2017a). Gametocytemia and attractiveness of Plasmodium falciparum–infected Kenyan children to Anopheles gambiae mosquitoes. J. Infect. Dis. 216, 291–295. doi: 10.1093/infdis/jix214
Busula, A. O., Verhulst, N. O., Bousema, T., Takken, W., and de Boer, J. G. (2017b). Mechanisms of Plasmodium-enhanced attraction of mosquito vectors. Trends Parasitol. 33, 961–973. doi: 10.1016/j.pt.2017.08.010
Carr, A. L., Mitchell, R. D. Jr., Dhammi, A., Bissinger, B. W., Sonenshine, D. E., and Roe, R. M. (2017). Tick Haller’s organ, a new paradigm for arthropod olfaction: how ticks differ from insects. Int. J. Mol. Sci. 18:1563. doi: 10.3390/ijms18071563
Cator, L. J., Lynch, P. A., Read, A. F., and Thomas, M. B. (2012). Do malaria parasites manipulate mosquitoes? Trends Parasitol. 28, 466–470. doi: 10.1016/j.pt.2012.08.004
Chamchod, F., and Britton, N. F. (2011). Analysis of a vector-bias model on malaria transmission. Bull. Math. Biol. 73, 639–657. doi: 10.1007/s11538-010-9545-0
Coleman, R. E., Edman, J. D., and Semprevivo, L. H. (1988). Interactions between malaria (Plasmodium yoelii) and leishmaniasis (Leishmania mexicana amazonensis): effect of concomitant infection on host activity, host body temperature and vector engorgement success. J. Med. Entomol. 25, 467–471. doi: 10.1093/jmedent/25.6.467
Cordaux, R., Bouchon, D., and Grève, P. (2011). The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends Genet. 27, 332–341. doi: 10.1016/j.tig.2011.05.002
Cordero-Montoya, G., Flores-Villegas, A. L., Salazar-Schettino, P. M., Vences-Blanco, M. O., Rocha-Ortega, M., Gutiérrez-Cabrera, A. E., et al. (2019). The cost of being a killer’s accomplice: Trypanosoma cruzi impairs the fitness of kissing bugs. Parasitol. Res. 118, 2523–2529. doi: 10.1007/s00436-019-06413-8
Cornet, S., Nicot, A., Rivero, A., and Gandon, S. (2013a). Both infected and uninfected mosquitoes are attracted toward malaria infected birds. Malar. J. 12, 179.
Cornet, S., Nicot, A., Rivero, A., and Gandon, S. (2013b). Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol. Let. 16, 323–329. doi: 10.1111/ele.12041
da Rocha Silva, F. B., Miguel, D. C., Machado, V. E., Oliveira, W. H. C., Goulart, T. M., Tosta, C. D., et al. (2019). Influence of Leishmania (Viannia) braziliensis infection on the attractiveness of BALB/c mice to Nyssomyia neivai (Diptera: Psychodidae). PloS One 14:e0214574. doi: 10.1371/journal.pone.0214574
Dantzer, R. (2001). Cytokine-induced sickness behavior: where do we stand? Brain Behav. Immun. 15, 7–24. doi: 10.1006/brbi.2000.0613
Day, J. F., and Edman, J. D. (1983). Malaria renders mice susceptible to mosquito feeding when gametocytes are most infective. J. Parasitol. 69, 163–170.
Day, J. F., Ebert, K. M., and Edman, J. D. (1983). Feeding patterns of mosquitoes (Diptera: Culicidae). simultaneously exposed to malarious and healthy mice, including a method for separating blood meals from conspecific hosts. J. Med. Entomol. 20, 120–127. doi: 10.1093/jmedent/20.2.120
de Boer, J. G., Busula, A. O., Ten Berge, J., van Dijk, T. S., and Takken, W. (2019). Does artemether–lumefantrine administration affect mosquito olfactory behaviour and fitness? Malar. J. 18, 28.
de Boer, J. G., Robinson, A., Powers, S. J., Burgers, S. L. G. E., Caulfield, J. C., Birkett, M. A., et al. (2017). Odours of Plasmodium falciparum-infected participants influence mosquito-host interactions. Sci. Rep. 7, 1–9.
de Moraes, C. M., Stanczyk, N. M., Betz, H. S., Pulido, H., Sim, D. G., Read, A. F., et al. (2014). Malaria-induced changes in host odors enhance mosquito attraction. Proc. Nat. Acad. Sci. U.S.A. 111, 11079–11084. doi: 10.1073/pnas.1405617111
Delhaye, J., Aletti, C., Glaizot, O., and Christe, P. (2016). Exposure of the mosquito vector Culex pipiens to the malaria parasite Plasmodium relictum: effect of infected blood intake on immune and antioxidant defences, fecundity and survival. Parasites Vect. 9:616.
Díez-Fernández, A., Martínez-de la Puente, J., Gangoso, L., López, P., Soriguer, R., Martín, J., et al. (2020). Mosquitoes are attracted by the odour of Plasmodiuminfected birds. Int. J. Parasitol. 50, 569–575. doi: 10.1016/j.ijpara.2020.03.013
Dingemanse, N. J., Oosterhof, C., Van Der Plas, F., and Barber, I. (2009). Variation in stickleback head morphology associated with parasite infection. Biol. J. Linn. Soc. Lond. 96, 759–768.
Emami, S. N., Lindberg, B. G., Hua, S., Hill, S. R., Mozuraitis, R., Lehmann, P., et al. (2017). A key malaria metabolite modulates vector blood seeking, feeding, and susceptibility to infection. Science 355, 1076–1080. doi: 10.1126/science.aah4563
Fayard, M., Dechaume-Moncharmont, F. X., Wattier, R., and Perrot-Minnot, M. J. (2020). Magnitude and direction of parasite-induced phenotypic alterations: a meta-analysis in acanthocephalans. Biol. Rev. 000–000. doi: 10.1111/brv.12606
Fecchio, A., Martins, T. F., Bell, J. A., De La Torre, G. M., Pinho, J. B., Weckstein, J. D., et al. (2020). Low host specificity and lack of parasite avoidance by immature ticks in Brazilian birds. Parasitol. Res. 119, 1–7.
Ferguson, H. M., and Read, A. F. (2002). Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 18, 256–261. doi: 10.1016/s1471-4922(02)02281-x
Ferguson, H. M., Rivero, A., and Read, A. F. (2003). The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology 127, 9–19. doi: 10.1017/s0031182003003287
Ferguson, L. V., Hillier, N. K., and Smith, T. G. (2013). Influence of Hepatozoon parasites on host-seeking and host-choice behaviour of the mosquitoes Culex territans and Culex pipiens. Int. J. Parasitol. Parasites Wildl. 2, 69–76. doi: 10.1016/j.ijppaw.2012.11.006
Fialho, R. F., and Schall, J. J. (1995). Thermal ecology of a malarial parasite and its insect vector: consequences for the parasite’s transmission success. J. Anim. Ecol. 64, 553–562. doi: 10.2307/5799
Freier, J. E., and Friedman, S. (1976). Effect of host infection with Plasmodium gallinaceum on the reproductive capacity of Aedes aegypti. J. Invertebr. Pathol. 28, 161–166. doi: 10.1016/0022-2011(76)90117-8
Gandon, S. (2018). Evolution and manipulation of vector host choice. Am. Nat. 192, 23–34. doi: 10.1086/697575
Gillies, M. T. (1980). The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bull. Entomol. Res. 70, 525–532. doi: 10.1017/s0007485300007811
Garnham, P. C. C. (1966). Malaria Parasites and Other Haemosporidia. Oxford: Blackwell Scientific Publications Ltd.
Grant, A. J., Aghajanian, J. G., O’Connell, R. J., and Wigton, B. E. (1995). Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J. Comp. Physiol. A 177, 389–396.
Gutiérrez-López, R., Martínez-de la Puente, J., Gangoso, L., Soriguer, R., and Figuerola, J. (2019). Effects of host sex, body mass and infection by avian Plasmodium on the biting rate of two mosquito species with different feeding preferences. Parasites Vect. 12:87.
Hart, B. L. (1988). Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137. doi: 10.1016/s0149-7634(88)80004-6
Herbison, R., Lagrue, C., and Poulin, R. (2018). The missing link in parasite manipulation of host behaviour. Parasites Vect. 11:222.
Hosack, G. R., Rossignol, P. A., and van den Driessche, P. (2008). The control of vector-borne disease epidemics. J. Theor. Biol. 255, 16–25. doi: 10.1016/j.jtbi.2008.07.033
Hurd, H. (2003). Manipulation of medically important insect vectors by their parasites. Annu. Rev. Entomol. 48, 141–161.
Johnson, R. W. (2002). The concept of sickness behavior: a brief chronological account of four key discoveries. Vet. Immunol. Immunopathol. 87, 443–450. doi: 10.1016/s0165-2427(02)00069-7
Joice Cordy, R. (2020). Mining the human host metabolome toward an improved understanding of malaria transmission. Front. Microbiol. 11:164. doi: 10.3389/fmicb.2020.00164
Kageyama, D., Narita, S., and Watanabe, M. (2012). Insect sex determination manipulated by their endosymbionts: incidences, mechanisms and implications. Insects 3, 161–199. doi: 10.3390/insects3010161
Keil, T. A. (2012). Sensory cilia in arthropods. Arthropod Struct. Dev. 41, 515–534. doi: 10.1016/j.asd.2012.07.001
Kelly, M., Su, C. Y., Schaber, C., Crowley, J. R., Hsu, F. F., Carlson, J. R., et al. (2015). Malaria parasites produce volatile mosquito attractants. MBio 6:e235-15.
Kingsolver, J. G. (1987). Mosquito host choice and the epidemiology of malaria. Am. Nat. 130, 811–827.
Kone, A., Van de Vegte-Bolmer, M., Siebelink-Stoter, R., Van Gemert, G. J., Dara, A., Niangaly, H., et al. (2010). Sulfadoxine–pyrimethamine impairs Plasmodium falciparum gametocyte infectivity and Anopheles mosquito survival. Int. J. Parasitol. 40, 1221–1228. doi: 10.1016/j.ijpara.2010.05.004
Kruppa, T. F., and Burchard, G. D. (1999). Similar blackfly attraction by onchocerciasis patients and individuals putatively immune to. Trans. R. Soc. Trop. Med. Hyg. 93, 365–367. doi: 10.1016/s0035-9203(99)90117-7
Lacroix, R., Mukabana, W. R., Gouagna, L. C., and Koella, J. C. (2005). Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 3:2298. doi: 10.1371/journal.pbio.0030298
Lalubin, F., Bize, P., van Rooyen, J., Christe, P., and Glaizot, O. (2012). Potential evidence of parasite avoidance in an avian malarial vector. Anim. Behav. 84, 539–545. doi: 10.1016/j.anbehav.2012.06.004
Lalubin, F., Delédevant, A., Glaizot, O., and Christe, P. (2014). Natural malaria infection reduces starvation resistance of nutritionally stressed mosquitoes. J. Anim. Ecol. 83, 850–857. doi: 10.1111/1365-2656.12190
Lefèvre, T., and Thomas, F. (2008). Behind the scene, something else is pulling the strings: emphasizing parasitic manipulation in vector-borne diseases. Infect. Genet. Evol. 8, 504–519. doi: 10.1016/j.meegid.2007.05.008
Mahon, R., and Gibbs, A. (1982). “Arbovirus-infected hens attract more mosquitoes” in Viral Diseases in Southeast Asia and the western Pacific, ed. J. D. Mackenzie (Sydney: Academic Press), 502–504.
Macdonald, G. (1956). Epidemiological basis of malaria control. Bull. World Health Organ. 15, 613–626.
Magalhães-Junior, J. T., Mesquita, P. R. R., dos Santos, Oliveira, W. F., Oliveira, F. S., Franke, C. R., et al. (2014). Identification of biomarkers in the hair of dogs: new diagnostic possibilities in the study and control of visceral leishmaniasis. Anal. Bioanal. Chem. 406, 6691–6700. doi: 10.1007/s00216-014-8103-2
Magalhães-Junior, J. T., Oliva-Filho, A. D. A., Novais, H. O., Mesquita, P. R. R., Rodrigues, F. M., Pinto, M. C., et al. (2019). Attraction of the sandfly Lutzomyia longipalpis to possible biomarker compounds from dogs infected with Leishmania infantum. Med. Vet. Entomol. 33, 322–325. doi: 10.1111/mve.12357
McElhany, P., Real, L. A., and Power, A. G. (1995). Vector preference and disease dynamics: a study of Barley Yellow Dwarf Virus. Ecology 76, 444–457. doi: 10.2307/1941203
Medeiros, M. C., Anderson, T. K., Higashiguchi, J. M., Kitron, U. D., Walker, E. D., Brawn, J. D., et al. (2014). An inverse association between West Nile virus serostatus and avian malaria infection status. Parasites Vect. 7:415. doi: 10.1186/1756-3305-7-415
Mendis, K. N., Munesinghe, Y. D., De Silva, Y. N., Keragalla, I., and Carter, R. (1987). Malaria transmission-blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect. Immun. 55, 369–372. doi: 10.1128/iai.55.2.369-372.1987
Mukabana, W. R., Takken, W., Coe, R., and Knols, B. G. (2002). Host-specific cues cause differential attractiveness of Kenyan men to the African malaria vector Anopheles gambiae. Malar. J. 1:17.
Mukabana, W. R., Takken, W., Killeen, G. F., and Knols, B. G. (2007). Clinical malaria reduces human attractiveness to mosquitoes. Proc. Neth. Entomol. Soc. Meet. 18, 125–129.
Murdock, C. C., Luckhart, S., and Cator, L. J. (2017). Immunity, host physiology and behavior in infected vectors. Curr. Opin. Insect. Sci. 20, 28–33. doi: 10.1016/j.cois.2017.03.001
Naotunne, T. D. S., Karunaweera, N. D., Mendis, K. N., and Carter, R. (1993). Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology 78:555.
Nevatte, T. M., Ward, R. D., Sedda, L., and Hamilton, J. G. C. (2017). After infection with Leishmania infantum, Golden Hamsters (Mesocricetus auratus) become more attractive to female sand flies (Lutzomyia longipalpis). Sci. Rep. 7, 1–9. doi: 10.1007/978-3-319-23534-9_1
Nguyen, P. L., Vantaux, A., Hien, D. F., Dabiré, K. R., Yameogo, B. K., Gouagna, L. C., et al. (2017). No evidence for manipulation of Anopheles gambiae, An. coluzzii and An. arabiensis host preference by Plasmodium falciparum. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-09821-x
O’Shea, B., Rebollar-Tellez, E., Ward, R. D., Hamilton, J. G. C., El Naiem, D., and Polwart, A. (2002). Enhanced sandfly attraction to Leishmania-infected hosts. Trans. R Soc. Trop. Med. Hyg. 96, 117–118. doi: 10.1016/s0035-9203(02)90273-7
Pigeault, R., and Villa, M. (2018). Long-term pathogenic response to Plasmodium relictum infection in Culex pipiens mosquito. PLoS ONE 13:e0192315. doi: 10.1371/journal.pone.0192315
Poulin, R. (1995). “Adaptive” changes in the behaviour of parasitized animals: a critical review. Int. J. Parasitol. 25, 1371–1383. doi: 10.1016/0020-7519(95)00100-x
Poulin, R. (2000). Manipulation of host behaviour by parasites: a weakening paradigm? Proc. R. Soc. Lond. B 267, 787–792. doi: 10.1098/rspb.2000.1072
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available at: https://www.R-project.org/
Ramirez-Sierra, M. J., and Dumonteil, E. (2016). Infection rate by Trypanosoma cruzi and biased vertebrate host selection in the Triatoma dimidiata (Hemiptera: Reduvidae) species complex. J. Med. Entomol. 53, 20–25. doi: 10.1093/jme/tjv157
Ramiro, R. S., Alpedrinha, J., Carter, L., Gardner, A., and Reece, S. E. (2011). Sex and death: the effects of innate immune factors on the sexual reproduction of malaria parasites. PLoS Path. 7:e1001309. doi: 10.1371/journal.ppat.1001309
Robinson, A., Busula, A. O., Voets, M. A., Beshir, K. B., Caulfield, J. C., Powers, S. J., et al. (2018). Plasmodium-associated changes in human odor attract mosquitoes. Proc. Nat. Acad. Sci. U.S.A. 115, E4209–E4218.
Ross, R. (1916). An application of the theory of probabilities to the study of a priori pathometry. Lond. Ser. Contain. Pap. Math. Phys. Character 92, 204–230. doi: 10.1098/rspa.1916.0007
Scott, T. W., Lorenz, L. H., and Edman, J. D. (1990). Effects of house sparrow age and arbovirus infection on attraction of mosquitoes. J. Med. Entomol. 27, 856–863. doi: 10.1093/jmedent/27.5.856
Sisterson, M. S. (2008). Effects of insect-vector preference for healthy or infected plants on pathogen spread: insights from a model. J. Econ. Entomol. 101, 1–8. doi: 10.1093/jee/101.1.1
Stanczyk, N. M., Mescher, M. C., and de Moraes, C. M. (2017). Effects of malaria infection on mosquito olfaction and behavior: extrapolating data to the field. Curr. Opin. Insect Sci. 20, 7–12. doi: 10.1016/j.cois.2017.02.002
Suh, E., Bohbot, J. D., and Zwiebel, L. J. (2014). Peripheral olfactory signaling in insects. Curr. Opin. Insect Sci. 6, 86–92. doi: 10.1016/j.cois.2014.10.006
Syed, Z. (2015). Chemical ecology and olfaction in arthropod vectors of diseases. Curr. Opin. Insect Sci. 10, 83–89. doi: 10.1016/j.cois.2015.04.011
Takken, W. (1991). The role of olfaction in host-seeking of mosquitoes: a review. Int. J. Trop. Insect Sci. 12, 287–295. doi: 10.1017/s1742758400020816
Thomas, F., Adamo, S., and Moore, J. (2005). Parasitic manipulation: where are we and where should we go? Behav. Proc. 68, 185–199. doi: 10.1016/j.beproc.2004.06.010
Thomas, F., Rigaud, T., and Brodeur, J. (2012). “Evolutionary routes leading to host manipulation by parasites” in Host Manipulation by Parasites, eds D. P. Hughes, J. Brodeur, and F. Thomas (Oxford: Oxford University Press), 16–33.
Thomas, F., Schmidt-Rhaesa, A., Martin, G., Manu, C., Durand, P., and Renaud, F. (2002). Do hairworms (Nematomorpha) manipulate the water seeking behaviour of their terrestrial hosts? J. Evol. Biol. 15, 356–361. doi: 10.1046/j.1420-9101.2002.00410.x
Tripet, F. (2009). Ecological immunology of mosquito-malaria interactions: of non-natural versus natural model systems and their inferences. Parasitology 136, 1935–1942. doi: 10.1017/s0031182009006234
Turell, M. J., Bailey, C. L., and Rossi, C. A. (1984). Increased mosquito feeding on Rift Valley fever virus-infected lambs. Am. J. Trop. Med. Hyg. 33, 1232–1238. doi: 10.4269/ajtmh.1984.33.1232
van Duijvendijk, G., van Andel, W., Fonville, M., Gort, G., Hovius, J. W., Sprong, H., et al. (2016). A Borrelia afzelii infection increases larval tick burden on Myodes glareolus (Rodentia: Cricetidae) and nymphal body weight of Ixodes ricinus (Acari: Ixodidae). J. Med. Entomol. 54, 422–428.
Vantaux, A., Yao, F., Hien, D. F., Guissou, E., Yameogo, B. K., Gouagna, L. C., et al. (2018). Field evidence for manipulation of mosquito host selection by the human malaria parasite, Plasmodium falciparum. Biorxiv [preprint] 207183. doi: 10.1101/207183
Venables, W. N., and Ripley, B. D. (2002). Modern Applied Statistics with S. Fourth Edn. New York: Springer.
Vézilier, J., Nicot, A., Gandon, S., and Rivero, A. (2012). Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc. R. Soc. B 279, 4033–4041. doi: 10.1098/rspb.2012.1394
Vogels, C. B., Fros, J. J., Pijlman, G. P., van Loon, J. J., Gort, G., and Koenraadt, C. J. (2017). Virus interferes with host-seeking behaviour of mosquito. J. Exp. Biol. 220, 3598–3603. doi: 10.1242/jeb.164186
Weinersmith, K. L. (2019). What’s gotten into you?: a review of recent research on parasitoid manipulation of host behavior. Curr. Opin. Insect Sci. 33, 37–42. doi: 10.1016/j.cois.2018.11.011
Witsenburg, F., Schneider, F., and Christe, P. (2015). Signs of a vector’s adaptive choice: on the evasion of infectious hosts and parasite-induced mortality. Oikos 124, 668–676. doi: 10.1111/oik.01785
Yan, J., Martínez-de la Puente, J., Gangoso, L., Gutiérrez-López, R., Soriguer, R., and Figuerola, J. (2018). Avian malaria infection intensity influences mosquito feeding patterns. Int. J. Parasitol. 48, 257–264. doi: 10.1016/j.ijpara.2017.09.005
Yanoviak, S. P., Kaspari, M., Dudley, R., and Poinar, G. (2008). Parasite-induced fruit mimicry in a tropical canopy ant. Am. Nat. 171, 536–544. doi: 10.1086/528968
Keywords: attractiveness, extended phenotype, hematophagous arthropods, host-choice, host-seeking, manipulation, vector-borne parasites
Citation: Cozzarolo C-S, Glaizot O, Christe P and Pigeault R (2020) Enhanced Attraction of Arthropod Vectors to Infected Vertebrates: A Review of Empirical Evidence. Front. Ecol. Evol. 8:568140. doi: 10.3389/fevo.2020.568140
Received: 31 May 2020; Accepted: 17 August 2020;
Published: 08 September 2020.
Edited by:
Laura Gangoso, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Robert Poulin, University of Otago, New ZealandCopyright © 2020 Cozzarolo, Glaizot, Christe and Pigeault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Camille-Sophie Cozzarolo, Y2FtaWxsZS5zb3BoaWUuY296emFyb2xvQGdtYWlsLmNvbQ==
†ORCID: Camille-Sophie Cozzarolo, orcid.org/0000-0002-9056-8622; Olivier Glaizot, orcid.org/0000-0001-9116-3355; Philippe Christe, orcid.org/0000-0002-8605-7002; Romain Pigeault, orcid.org/0000-0002-8011-4600
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.