
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 17 September 2020
Sec. Behavioral and Evolutionary Ecology
Volume 8 - 2020 | https://doi.org/10.3389/fevo.2020.498347
This article is part of the Research Topic Links Between Cognition and Fitness: Mechanisms and Constraints in the Wild View all 16 articles
Human activities are increasingly confronting animals with unfamiliar environmental conditions. For example, habitat change and loss often lead to habitat fragmentation, which can create barriers of unsuitable and unfamiliar habitat affecting animal movements and survival. When confronted with habitat changes, animals’ cognitive abilities play an important, but often neglected part in dealing with such change. Animals must decide whether to approach and investigate novel habitats (spatial neophilia) or whether to avoid them (spatial neophobia) due to potential danger. For species with strict habitat preferences, such as the Gouldian finch (Erythrura gouldiae), which is an open habitat specialist, understanding these novelty responses may be especially important for predicting responses to habitat changes. The Gouldian finch is a polymorphic species, with primarily red or black head colors, which are linked to differing behavioral phenotypes, including novelty reactions. Here we investigate responses to novel habitats (open, dense) in the Gouldian finch, manipulating the color composition of same-sex pairs. Two experiments, each consisting of novel open and novel dense habitat, tested birds in opposite head color combinations in the two experiments. We measured the number of approaches birds made (demonstrating conflict between approach and avoidance), and their entry latency to novel habitats. Gouldian finches showed more approach attempts (stronger approach-avoidance conflict) toward the dense as compared to the open habitat, confirming their open habitat preferences. Black-headed birds also hesitated longer to enter the dense habitat as compared to the open habitat, particularly in experiment 1, appearing less neophilic than red-headed birds, which showed similar entry latencies into both habitat types. This is surprising as black-headed birds were more neophilic in other contexts. Moreover, there was some indication that pairings including at least one black-headed bird had a stronger approach-avoidance conflict than pairings of pure red-headed birds. Results suggest that the black-headed birds use a cognitive strategy typical for residents, whereas red-headed birds use a cognitive strategy known for migrants/nomads, which may cognitively complement each other. However, as 70% of the population in the wild are black-headed, the spatial wariness we document could be widespread, which may negatively affect population persistence as habitats change.
Most species experience environmental variation to some degree, but organisms are increasingly exposed to climatic and human-induced environmental change at a rate much higher than evolutionary time scales. For example, habitats are altered, fragmented and/or lost, which can affect movement patterns (Riotte-Lambert and Matthiopoulos, 2019), such as dispersal, migration, and movements for foraging and breeding (e.g., Norris and Stutchburry, 2001; Shadbolt and Ragai, 2010; Stouffer et al., 2011; Amos et al., 2014). The ability of individuals to respond to such changes is paramount for their survival and long-term population persistence. Therefore, an understanding of animal behavior can help predict responses toward varying types of environmental change (Wong and Candolin, 2015), including movement in response to habitat-related changes (Knowlton and Graham, 2010). In fact, a better understanding of impediments to animal movement and their influence on the functional connectivity of habitat has been flagged as a high priority for conservation behavior research (Greggor et al., 2016a).
Movement decisions inherently involve cognition. In moving into a habitat, animals must perceive a given space and assess available predator and foraging cues, relative to experience or an ingrained bias; all of which involve cognitive mechanisms (Shettleworth, 2010). Cognitive biases consistently guide how animals make often imperfect assessments of their environment (Marshall et al., 2013) and are, therefore, instrumental in understanding responses to habitat change and the downstream effects on survival. This is especially true when animals are faced with evolutionarily novel conditions because their responses and decisions may not be easy to predict without considering underlying perceptual abilities and learning tendencies (Greggor et al., 2019).
Organisms confronted with habitat change, such as a newly fragmented landscape, must decide whether to approach and investigate the new habitat or whether to avoid it. The decision to approach or avoid the novelty associated with change is governed by two independent motivations, both of which are cognitive by nature. Neophobia, the fear of any novelty, leads to avoidance and protects an animal from potentially dangerous situations. Neophilia, the attraction to novelty, results in approach, investigation and information gathering (Mettke-Hofmann et al., 2002). The two motivations are independent of each other (Wood-Gush and Vestergaard, 1993) and have different gene expressions (Powell et al., 2003; Weisstaub et al., 2006). However, they are both elicited when an animal is confronted with novelty, resulting in four possible extreme combinations of neophobia and neophilia (2-Factor model, Greenberg and Mettke-Hofmann, 2001). (A) An individual can show high neophilia and low neophobia, resulting in a fast approach of the novel situation without hesitation, followed by information gathering. (B) Likewise, low neophilia (no interest in novelty) can be combined with high neophobia, leading to a delayed approach and little information gathering. (C) However, a lack of approach can also result from a combination of low neophilia (no interest) and low neophobia. Accordingly, the delayed approach is driven by the low interest in the novel situation rather than fear. (D) Finally, a high level of neophilia and neophobia can be in conflict with each other, in which there are repeated approach attempts (reflecting the conflict between the two motivations; Mettke-Hofmann et al., 2009). Approach attempts in this scenario will result in information gathering (to some extent) and in later exploration. The relative expression of neophobia versus neophilia is a species level trait, with considerable individual variation, and has evolved in relation to the costs and benefits of approaching or avoiding a certain type of novelty (e.g., novel spaces, objects or foods, Greggor et al., 2015). Accordingly, neophobia and neophilia are often correlated with other traits such as learning speed and problem solving (e.g., Seferta et al., 2001; Benson-Amram and Holekamp, 2012).
Spatial neophobia and neophilia have been shown to differ between habitat generalists and specialist species. For example, butterfly species with a more local distribution were less likely to explore new habitats, showed greater avoidance of unfamiliar habitats (i.e., low spatial neophilia and high spatial neophobia) and consequently hesitated longer to enter unfamiliar and deviating habitats than butterflies with a large distributional range (Norberg et al., 2002; Leimar et al., 2003). The authors concluded that spatial neophobia and neophilia can have consequences on the distribution of populations. Similar patterns appear in songbirds, where diet and habitat specialists have shown greater spatial neophobia to feed from novel micro habitats than closely related generalist species (Greenberg, 1983, 1989).
Spatial novelty reactions also vary with species’ movement patterns. Migratory bird species are often confronted with unfamiliar environments and readily enter new habitats (high spatial neophilia) with few approach attempts (indicative of a low approach-avoidance conflict). However, as they only stay for short periods in each area, they only superficially explore unfamiliar areas (Mettke-Hofmann et al., 2009). In contrast, residents are more hesitant to enter new areas, and demonstrate high approach-avoidance conflict (Mettke-Hofmann et al., 2009) due to the potential danger of the novel environment. Residents thoroughly explore once they are in an environment as they can use information in the long-term (Mettke-Hofmann and Gwinner, 2004; Mettke-Hofmann et al., 2005, 2012). Generally, residents are more flexible in their responses (e.g., innovations, Sol et al., 2005) and positive population trends have been linked to this higher flexibility (Mettke-Hofmann, 2017). Finally, dispersal decisions have been linked to the amount of spatial exploration (spatial neophilia, Selonen and Hanski, 2006).
Although novelty reactions are species-specific traits, they can also vary considerably intraspecifically, and are often linked to existing polymorphisms. Individuals can differ in their novelty responses due to individual coping styles or personalities (consistent individual differences), which can affect the genetic composition of populations (Dingemanse et al., 2003). Variation in novelty reactions can also be linked to other polymorphisms such as distinct differences in coloration in the same population. For instance, melanin-based polymorphism in siskins (Carduelis spinus) was linked to variation in the speed to approach a novel object with faster approach in individuals with a larger black bib (Mateos-Gonzalez and Senar, 2012). Polymorphism in general has been proposed to enhance ecological success, particularly in the light of environmental change, due to individuals utilizing different environmental resources and behavioral strategies (Forsman et al., 2008). Accordingly, a mix of personalities has been found to facilitate earlier entry into novel environments or better patch exploration in combination with improved group cohesion as compared to groups consisting of single personalities (Dyer et al., 2009; Aplin et al., 2014). However, other studies have challenged the adaptational advantage of polymorphic species due to constraints of correlational evolution of traits and interdependence of morphs (Bolton et al., 2015).
Overall, the interspecific and intraspecific variation in spatial novelty responses and the link to habitat specialization and movement suggest a potential mechanism for the maintenance of avoidance traits affecting decision-making and information gathering. Such persistent avoidance should therefore be useful for predicting how space use patterns will carry over into novel habitats.
The current study aimed to investigate how decision-making about engaging with unfamiliar environments is affected by spatial neophobia and neophilia in the ecologically highly specialized Gouldian finch (Erythrura gouldiae). Gouldian finches are color polymorphic in both sexes consisting of three distinct head colors (Brush and Seifried, 1968). Head colors signal personality, including responses to novelty. Black-headed birds were more explorative when facing environmental changes (object neophilia) and risk-prone in dangerous environments but less aggressive than red-headed birds (Mettke-Hofmann, 2012; Williams et al., 2012). Gouldian finches are classed as endangered by the Australian Government (EPBC, 2018) and as near threatened in the IUCN Red List (BirdLife International, 2016) due to habitat change (Legge et al., 2015; Weier et al., 2016). Knowledge about how the species responds to unfamiliar habitats in an increasingly fragmented landscape is important to understand population persistence, particularly as populations are far apart and an estimated number of only approximately 2,500 individuals remain in the wild (Legge et al., 2015; Weier et al., 2016; EPBC, 2018).
While the species shows low site-fidelity (Bolton et al., 2016) and is nomadic during the wet season, tracking resources over extensive areas (Dostine et al., 2001), little is known about its responses to habitat change and habitat fragmentation. However, from its ecology one would expect little hesitation to enter unfamiliar environments due to its nomadic nature, but this may only apply for suitable habitats due to its high habitat specialization. If Gouldian finches only readily explore habitats that they are specialized to use, their movements may be increasingly compromised or hindered by continuing habitat fragmentation. The role that cognitive and behavioral differences between the color morphs may play in orchestrating novelty reactions is currently unknown. We aimed to investigate (a) whether morphs differ in their spatial neophobia/neophilia affecting information gathering and decision-making to enter unfamiliar environments that differ in their ecological suitability and (b) whether the morph combination (same head color or different head colors) in a group affects these decisions. Considering morphs will provide a more nuanced picture of the species’ cognitive ability to respond to habitat change and its ability to overcome gaps in suitable habitat availability.
We exposed the black-headed and red-headed morphs to two unfamiliar environments – one simulating an open habitat in correspondence with their habitat preference, the other one a dense habitat. We measured each bird’s number of attempts to approach the novel habitat before entering (revealing their level of approach-avoidance conflict, Mettke-Hofmann et al., 2009), and their latency to enter the novel habitat. Due to the highly social nature of the species and to address our second aim, birds were tested in pairs of same sex birds. The following predictions were made.
(1) As an open habitat specialist, we expected Gouldian finches to enter the open habitats earlier than the dense habitats and to show a lower approach-avoidance conflict for the open habitat (due to less spatial neophobia).
(2) Morphs often differ in behavior and cognition. We therefore expected black-headed birds to enter the novel habitats earlier than red-headed birds as the former are more neophilic toward changes in their familiar environment (Mettke-Hofmann, 2012; Williams et al., 2012) which may translate to novel spaces. Consequently, the number of approach attempts before entering may differ as a function of differences in spatial neophilia, despite similar neophobia levels (Mettke-Hofmann, 2012).
(3) Group composition can affect behavioral responses (Dyer et al., 2009). We expected head color combination to effect decision-making with mixed head colors entering faster than pure head color combinations. If black-headed birds are faster to enter, they may facilitate faster entry in red-headed birds with or without reducing the number of approach attempts in red-headed birds.
Gouldian finches are diet and habitat specialists preferring open savannah woodlands with suitable breeding trees and understory dominated by annual grasses for foraging on seeds (Dostine et al., 2001; Dostine and Franklin, 2002; Weier et al., 2016). Their color polymorphism consists of about 70% black-headed, 30% red-headed and less than 1% yellow-headed birds in both sexes in the same population (Brush and Seifried, 1968). The red/black polymorphism is located on the z chromosome with the red allele being genetically dominant (Toomey et al., 2018). Population declines have been attributed to habitat change caused by current fire regimes and cattle grazing affecting resource availability (Legge et al., 2015; Weier et al., 2016).
Experiments were conducted under controlled conditions in the laboratory. Thirty-two wild type, parent-reared Gouldian finches originating from 12 private breeders took part in the study. Ages ranged from 1 to 6 years and the sex ratio was equal with 16 males (eight of each head color) and 16 females (seven red-headed and nine black-headed). Birds were housed in flight cages (1.20 m × 0.80 m × 1.00 m) in groups of five to six birds of mixed age, sex and head color. The only exception were ten 1-year old birds, which were housed in same sex groups to avoid harassment. All birds were purchased when 1 year old and knew each other from changing group compositions linked to experiments and moving birds between holding cages. Cages consisted of a mixture of natural branches and perches. Food was offered in feeders consisting of a mixture of Astrilden Spezial, Amadinen-Zucht Spezial, and Red Sibirica millet (all products from Blattner-Heimtierfutter, Ermengerst, Germany). Additional feeders contained grit (Blattner-Heimtierfutter) and egg shells. Water was available ad libitum. Birds were kept at 24°C with full spectrum light at a light:dark cycle of 13:11 h. All birds took part in a food neophobia test (Eccles et al., unpublished) which ran the week preceding the spatial neophobia/neophilia testing and novel object experiments conducted in the morning before the spatial experiments.
Experiments were conducted in six experimental cages (1.20 m × 0.70 m × 1.00 m) located in a separate room from the holding cages. Each experimental cage had three wooden walls with wire mesh on the front and the ceiling. Cages were arranged in two rows of three cages, each. Birds from the two rows could not see each other. The outer two cages in a row were used as the home cage during the experiment. Home cages consisted of two perches left and right in the cage 30 cm away from the side wall and a perch running parallel to the front wire. Food and water were offered at the front of the cage. Each home cage had a movable partition (15.5 cm × 19 cm) in one sidewall providing access to the middle cage with the new spatial environment. The new spatial environment simulated an open or dense habitat. For both habitat types, the novel room had three perches, the two outer ones at the same height as the lower border of the partition and the middle one about 10 cm higher (Figure 1). Furthermore, six cardboard tubes (4 cm × 45 cm) were attached to the ceiling. In experiment 1 for the open habitat, these tubes were decorated with green eucalyptus leave garlands made of soft plastic tightly woven around the tube. They covered relatively little area in the novel spatial environment (Figure 1A) simulating an open habitat. For the dense habitat, silken Daisies were used consisting of green leaves and yellow/white flowers. Daisies were loosely woven around the tube occupying much more space in the novel spatial environment representing a denser habitat (Figure 1B). The dense habitat deviated from the birds’ preferred habitat, potentially increasing neophobia, and was also more complex than the open habitat, potentially hiding more threats (Mettke-Hofmann et al., 2006). In experiment 2, the open habitat consisted of silken green vine leave garlands (Figure 1C) tightly woven around the tube and the dense habitat of silken roses consisting of dark green leaves and red flowers loosely woven around the tube (Figure 1D). All birds experienced all four habitats.

Figure 1. Novel environments representing open and dense habitats. In the first experiment, pairs of Gouldian finches got access to an open habitat simulated by soft plastic eucalyptus leaves tightly woven around cardboard tubes (A) and a dense habitat simulated by silken daisies (B). The pictures show the habitats from outside with access for the birds from either the right or left side. Experiments were repeated with different pair compositions and silken vines for the open habitat (C) and silken roses for the dense habitat (D). The pictures show the view for the birds through the opened partition.
Pairs were strategically formed for the experiments to help control for variables such as age and sex, and to test our hypothesis about the influence of partner head color. Pairs were formed with same sex individuals, matching partners for size (average tarsus length 15.02 mm, mean difference 0.64 ± SE 0.10 mm) and body mass (average mass 20.11 g, mean difference 2.4 ± SE 0.35 g) as much as possible. Age has been shown to affect object neophobia (Mettke-Hofmann, 2012). As we could not match same-sexed birds of the same age due to unequal age distribution, we decided to have all pairs of different age for consistency. Birds in a pair were at least 2 years apart.
To address our hypothesis about group composition, all birds were tested with a partner of the same head color and a partner of the different head color in separate experiments. Half of the birds were first paired with a partner of the same head color and half with a partner of the different head color (experiment 1). This was reversed in the second experiment which started once all birds had been through experiment 1. The interval between experiment 1 and 2 ranged from 3 to 18 weeks for individual birds. In each experiment, half of the pairs were exposed to the open habitat first, whereas the other half experienced the dense habitat first balanced for head color combination and sex. The two open and dense habitats could not be balanced across experiments due to the re-pairing of the birds. As we had more black-headed than red-headed females and also uneven numbers within head colors, two black-headed females were tested with partner birds in both experiments that had gone through their own testing already (hereafter named experienced partner bird). In experiment 2, two additional black-headed females and one red-headed female were tested with experienced partner birds. Only the responses of the focal birds were included from these pairings, whereas for all other pairings both individuals in a pairing were considered.
Four pairs could be tested simultaneously with head color combination and sex balanced across cages. Overall, four batches with four pairs each were tested over a period of 8 weeks. Pairs were moved to the experimental cages for 2 weeks, first undergoing food neophobia testing (week 1) as part of a separate experiment. Spatial neophobia experiments commenced on day 11 or 12 as two pairs each had access to the same novel environment from different sides. On day 13 or 14 birds got access to the other habitat than experienced before. Birds were given access to the novel habitats for 3 h from 12:00 to 15:00, by temporarily removing the partition separating the two cages. Behaviors were video recorded with digital video cameras using GeoVision 1480 for later analysis.
Data preparation and statistical analyses were performed in R version 3.6.0. (R Core Team, 2019). Raw data can be found in the Supplementary Materials (S1). We extracted two response variables: (1) number of approach attempts before entering the novel environment and (2) latency to enter the novel environment. An approach attempt was recorded when the bird landed either on the perch closest to the open partition (30 cm from the opening) or on the lower part of the opening without flying into the novel environment. The number of approaches provided a measure of the approach-avoidance conflict between the motivation to enter and explore the novel environment (neophilia) and the motivation to avoid the novel environment due to potential danger (neophobia; Mettke-Hofmann et al., 2009). Latency to enter the novel environment was measured as the time between removing the partition and the bird flying into the novel environment. Birds that did not enter a habitat within the 3 h were given the maximum time of 10,800 s. The two response variables were not correlated (Pearson correlation: r = 0.314, df = 29, P = 0.09). Due to unrelated circumstances, one bird died after the first experiment and was not included in the analyses resulting in a sample size of 31 birds.
Initially, we fitted linear mixed models using the R package ‘lme4’ version 1.1-20 (Bates et al., 2015) to analyze our two response variables: number of approaches and entry latency. For number of approaches, transformation did not improve distribution and therefore we specified a Poisson family error distribution with log-link function in a generalized linear mixed model (GLMM) for the untransformed data. For entry latency we log transformed data to improve the distribution and used the default family error structure (Gaussian) in a linear mixed model (LMM).
For each response variable, we built two full models: one model to address hypotheses 1 and 2 regarding novelty responses to the two habitat types and the effect of morph on these reactions, the other model to address hypothesis 3 about social effects of morph composition. The analyses were separated into these two models for each response variable to avoid inclusion of too many variables in any single model. All explanatory variables were factors with two levels. To test our hypothesis about the relationship between habitat type and morph response (model 1), we entered into each model two predictor variables: habitat type (dense and open) and head color (black and red); and two control variables: age (1 year old, older than 1 year; to control for age effects linked to experience; Langham, 2006; Benson-Amram and Holekamp, 2012; Mettke-Hofmann, 2012; Biondi et al., 2013) and experiments (1, 2; to account for the repeated testing). We included the three-way interaction between habitat type, head color and experiment because novelty responses to the open and dense habitat may differ between morphs (stronger differences toward the more deviating and complex dense habitat) and these differences may be particularly prevalent during the first experiment as the entire situation was new. Sample sizes in the three-way interaction for all comparisons were n = 31 birds (124 rows of data) as all birds were tested in both head color combinations and both habitat types. Where the three-way interaction was not significant, its component two-way interactions were tested and retained in the final model only when significant: habitat type × head color, experiment × head color and habitat × experiment. To test our third hypothesis about effects of social factors (model 2), we entered into each model two predictor variables: head color (black and red) and partner head color (black and red) and one control variable: relative age within each pairing (younger or older to account for age effects within pairings as found in earlier studies; Mettke-Hofmann, 2012). We included the two-way interaction between head color and partner head color because the combination of morphs may matter (e.g., Dyer et al., 2009). Bird identity, partner identity and cage number were entered as crossed random effects in all models (crossed rather than nested because assigning birds to new pairings for experiment 2 precluded birds being tested in the same cage as in experiment 1). To account for using the same data in both models we used sequential Bonferroni adjustments were necessary (Rice, 1989; Chandler, 1995).
We inspected interaction terms, retaining all interactions that were P < 0.05 and excluding all others, in a stepwise model simplification, following Crawley (2012). Orthogonal data are robust to stepwise removal of interaction terms as variation attributable to each factor is constant at each stage of the stepwise simplification (Crawley, 2012). All main predictor and control variables were retained as fixed effects in all final models. Retaining fixed effects in final models minimizes repeated testing and hence concern about the risk of type I errors (e.g., Steel et al., 2013) and increased our ability to interpret model output and effect size calculations in a biologically meaningful way (e.g., Nakagawa and Cuthill, 2007). We adjusted convergence tolerance using the arguments ‘allFit’ and ‘control’ to specify the optimizer to ‘bobyqa’ and increased the number of iterations to 100,000, a practice considered ‘gold standard’ for ensuring stable model fit (Bates et al., 2019). Model fit was assessed by visually inspecting plots of fitted model residuals to ensure an even spread of residuals, which we found in all cases. We assessed each final model by comparing it against the null model (an identical model except for the removal of the predictor and control variables, with an intercept of 1 specified) using the anova command in R. The final model was only accepted where it was a significantly better fit than the null model (Burnham and Anderson, 2002). To aid model interpretation significant interactions were explored using appropriate planned post hoc comparisons.
We checked for evidence of collinearity within models using the function ‘vif’ (variance inflation factor) in the package ‘car,’ and extracted effect sizes using the r.squaredGLMM command in the package MuMIn (Barton, 2015). To facilitate future meta-analyses, we report both marginal and conditional effect sizes, r2m and r2c, respectively, where r2m explains variance due to fixed effects and r2c explains variance due to fixed and random effects (Nakagawa and Cuthill, 2007). We assessed repeatability (R) of behavior by accounting for the degree of variation attributable to bird identity using the rptR package (Stoffel et al., 2017). Repeatability can highlight persistent differences in novelty reactions between individuals (Dingemanse et al., 2003; Nakagawa and Schielzeth, 2010).
Finally, we re-ran all models with a restricted data set (n = 25 birds; 100 rows of data) excluding all focal birds that had been tested with an experienced partner bird to control for possible influences of these experienced partners on the focal birds’ behavior.
We conducted all experiments in accordance with published guidelines for the treatment of animals in behavioral research (ASAB/ABS guidelines, ASAB, 2018; ARRIVE guidelines; Kilkenny et al., 2010). Holding and experimental aviaries conformed to Home Office codes of practice (Home Office, 2013) and were carried out in approved facilities within Liverpool John Moores University. All experiments were non-regulated by the Home Office and complied with the ethical and welfare guidelines for animals and the legal requirements of the University (CMH_GE/2016-5) and the United Kingdom.
Responses to the novel environments differed between individual birds: In experiment 1, all birds entered the open habitat and 26 out of 31 birds entered the dense habitat. Birds that did not enter were three black-headed birds and two red-headed birds. In experiment 2, all but one bird (black-headed) entered the open habitat and 27 out of 31 birds the dense habitat. Birds that did not enter were three black-headed birds and one red-headed bird. Overall, six birds (four black-headed and two red-headed) failed to enter a particular habitat (all but one dense) of which two (one red and one black) never entered any dense habitat and one black-headed bird only entered the first open habitat.
There was no significant three-way interaction between habitat type, head color and experiment number on number of approaches (model 1). Removal of this term revealed a significant two-way interaction between head color and experiment (GLMM: LRT = 5.848, P = 0.016; Table 1A). Planned post hoc comparisons revealed black headed birds made significantly more approaches prior to entering in experiment 1 than they did in experiment 2 (Wilcoxon signed rank test: V = 117, P = 0.001; Figure 2). All other planned post hoc comparisons were non-significant (all Ps > 0.16). There was a main effect of habitat type (LRT = 45.935, df = 1, P < 0.001). Birds made more approaches before entering dense habitat (mean = 5.47 ± SE = 0.64) compared to open habitat (2.98 ± SE = 0.30). There was no effect of age (1 year vs. older) on number of approaches. Effect size for number of approaches was larger when random effects were included (r2m = 0.22; r2c = 0.65). Repeatability of number of approaches approached significance (R = 0.14, P = 0.068). Repeatability of number of approaches was significant for black-headed birds (R = 0.29, P = 0.019) but not for red-headed birds (R = 0, P = 1).
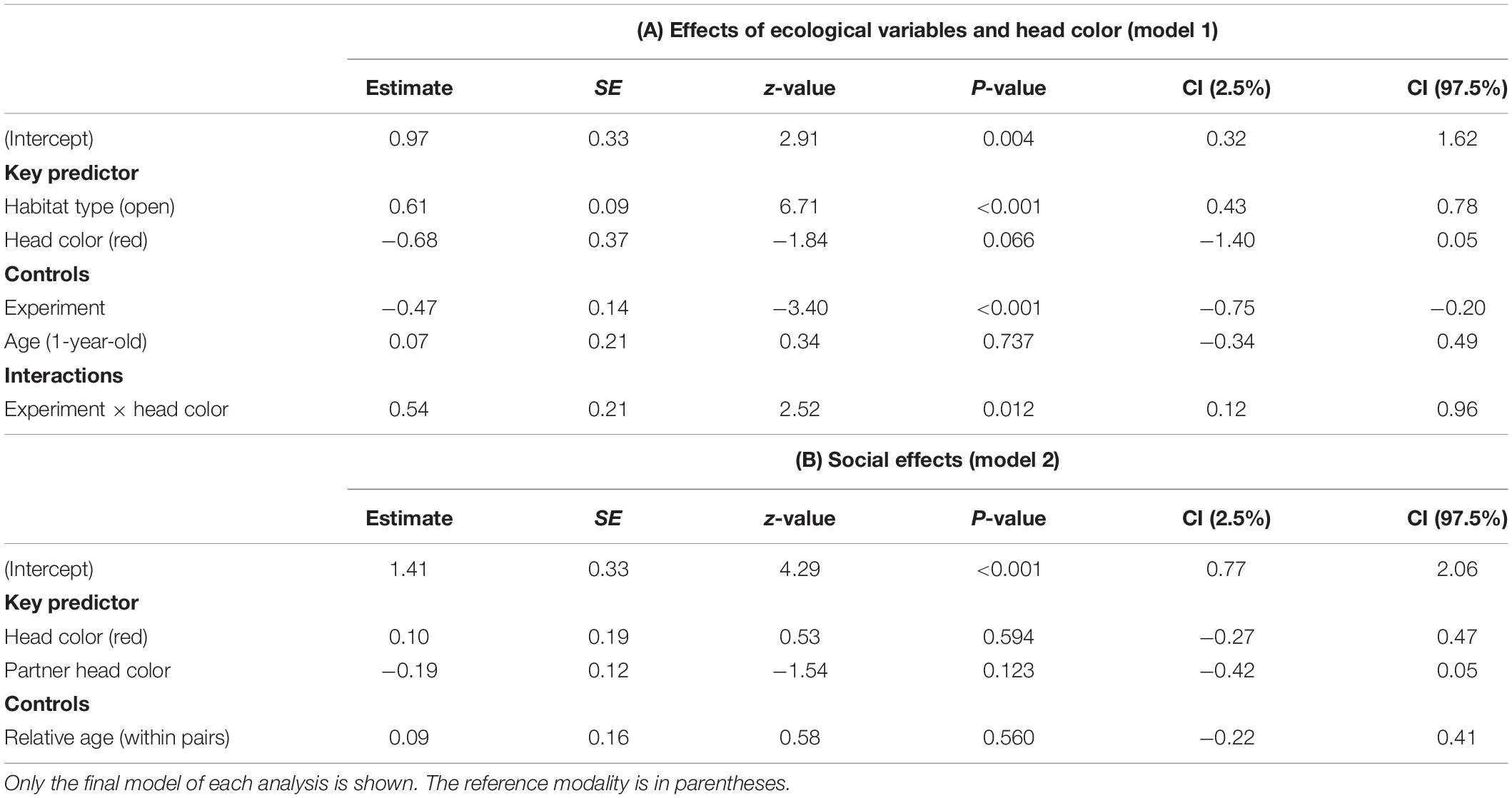
Table 1. Results of the general linear mixed effects model on the number of approaches before entering the novel open and dense habitats of Gouldian finches addressing (A) the effect of ecological variables and color morphs and (B) social effects.
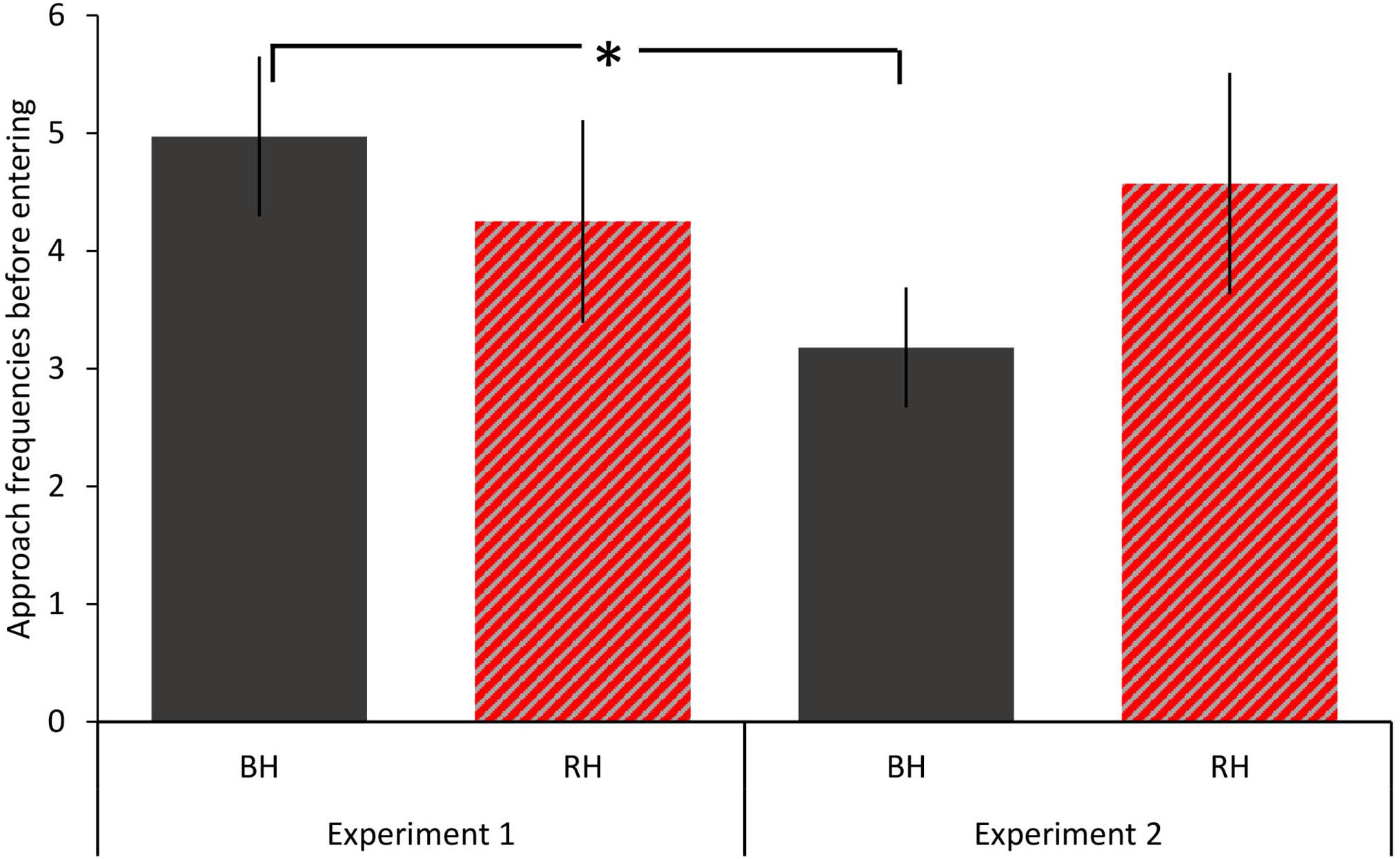
Figure 2. Effects of head color and experiment on number of approaches before entering the novel habitats. In experiment 1, same sex pairs of either same or different head color were tested on their response to enter an artificial open habitat (their preferred habitat type) and an artificial dense habitat. In experiment 2, birds were tested in the opposite head color composition with a new open and dense habitat. Mean and SE of number of approaches to novel habitats in the first and second experiment for each head color. *P < 0.05. Black bars: black-headed birds (BH), striped red and gray bars: red-headed birds (RH).
When we re-ran the analysis using the restricted dataset the three-way interaction between head color, habitat and experiment was significant (GLMM: n = 25; LRT = 5.064, P = 0.024; S2) including all associated two-way interactions between head color and experiment (z = 2.857, P = 0.004), head color and habitat (z = 1.990, P = 0.047) and habitat and experiment (z = 2.074, P = 0.038; S2, Supplementary Table S1). Planned post hoc comparisons revealed that black-headed birds made significantly more approaches in experiment 1 than they did in experiment 2 (Wilcoxon signed-rank test: V = 54, P = 0.008), that during experiment 1 black headed birds made more approaches to dense habitat than open habitat (Wilcoxon rank sum test: W = 547, P = 0.009), and they did so significantly more than did red-headed birds (Mann–Whitney test: U = 140, P = 0.051; S2, Supplementary Figures S1, S2). All other planned post hoc comparisons were non-significant (all Ps > 0.06). There was no significant effect of age (S2, Supplementary Table S1). This largely confirms the findings from the full data set but also reveals some additional effect of head color.
The GLMM to test for social factors (model 2) did not retain any significant variables and the variables did not explain the data any better than the null model (Table 1B). The restricted dataset resulted in a significant two-way interaction between head color and partner head color (GLMM: n = 25; LRT = 5.820, P = 0.016; S2, Supplementary Table S1). Planned post hoc comparisons revealed that red-headed birds paired with another red-head made significantly fewer approaches than they did when paired with a black-headed bird (Wilcoxon signed rank test: V = 33, P = 0.042; S2, Supplementary Figure S2). All other head color combinations were non-significant (all Ps > 0.235). There was no significant effect of relative age within pairs. Results of the two models remained significant after sequential Bonferroni correction.
There was a significant three-way interaction between habitat type, head color and experiment on latency to enter the two habitat types (model 1; LMM: n = 31; LRT = 5.967, P = 0.015; Table 2A) including the associated two-way interactions between head color and habitat type (t = 2.509, P = 0.012, Table 2A) and between head color and experiment (t = 2.603, P = 0.009; Table 2A). Planned post hoc comparisons revealed the interaction was driven by the significantly longer entry latency of black-headed birds to dense habitat in experiment 1 compared to red-headed birds (Mann–Whitney test: U = 176, P = 0.025, Figure 3). All other planned post hoc comparisons were non-significant (all Ps > 0.077). Age did not affect entry latencies. Effect size for entry latencies was larger when random effects were included (r2m = 0.12; r2c = 0.46). Entry latencies across all birds were repeatable (0.33, P < 0.001). Entry latencies across head colors were not repeatable but showed a trend in black-headed birds, (R = 0.26, P = 0.087) but not in red-headed ones (R = 0.15, P = 0.195). The restricted data set model (n = 25) confirmed the findings from the full data set model (S2, Supplementary Table S2).
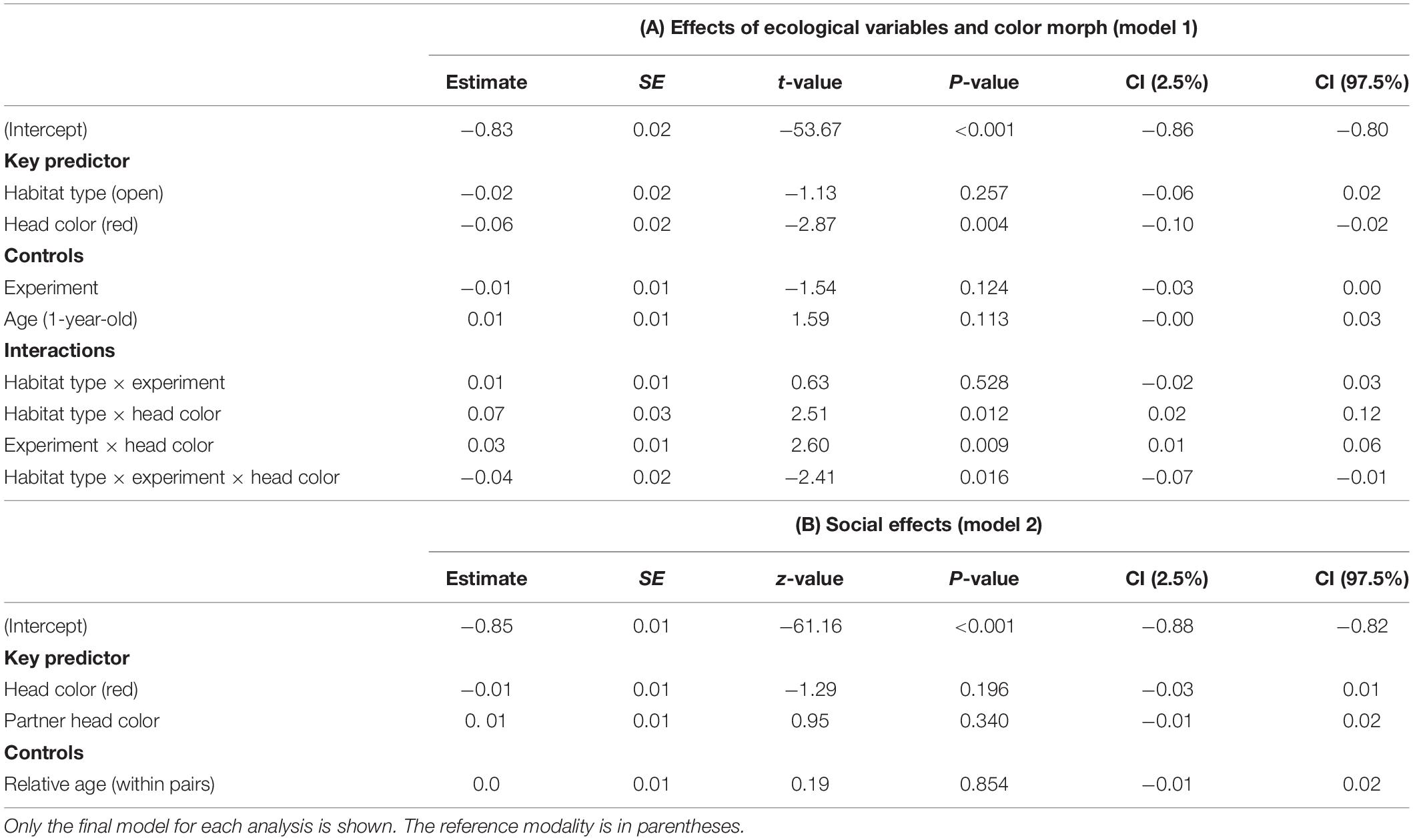
Table 2. Results of the linear mixed effects models on the entry latencies of Gouldian finches into open and dense habitats addressing (A) the relationship between ecological variables and color morph and (B) social effects.

Figure 3. Effects of habitat type, head color and experiment on entry latencies of Gouldian finches to unfamiliar habitats. The experimental setup was the same as in Figure 2. Mean and SE for entry latencies into open and dense habitats for experiments 1 and 2 and the two head colors. Cut-off points for entry latencies were 10,800 s (3 h). Black bars: black-headed birds (BH), striped red and gray bars: red-headed birds (RH). *p < 0.05.
The LMM output to test for social effects (model 2) did not retain any significant variables and the variables did not explain the data any better than the null model (Table 2B). Similarly, the restricted data set model (n = 25) did not retain any significant variables (S2, Supplementary Table S2).
We investigated novelty responses of the color-polymorphic Gouldian finch toward unfamiliar habitats that deviated to different degrees from their preferred habitat. Decisions to enter unfamiliar habitats differed between open and dense habitats and were affected by head color. All birds showed more approach-avoidance conflict before entering the dense as compared to the open habitat. Additionally, black-headed birds entered the dense habitat later, particularly during the first experiment.
As an open habitat specialist (Brazill-Boast et al., 2013), we expected Gouldian finches to take longer to enter the dense habitat and show a stronger approach-avoidance conflict for this habitat (hypothesis 1). Nearly all Gouldian finches entered the novel open habitat quickly, on average within half an hour, and demonstrated a low approach-avoidance conflict; both of which indicate low spatial neophobia and high spatial neophilia toward their open habitat preference. This mirrors similar novelty reactions in migratory birds (Mettke-Hofmann et al., 2009) facilitating swift entry into unfamiliar but suitable habitats. These novelty reactions seem to be well suited for the nomadic lifestyle of the Gouldian finch.
The picture changed when confronted with dense habitats, particularly for black-headed birds. Almost a third of the black-headed birds refused to enter one of the dense habitats, their entry latencies in the first experiment nearly doubled compared to the open habitats, and they made more approach attempts before entering. Their behavior suggests considerable avoidance of a habitat type that deviates from their preferred habitat, supporting hypothesis 1. Higher neophobia toward novelty that deviates stronger from what has been experienced before or from innate preferences has been shown in other species (Grünberger and Leisler, 1993; Greenberg and Mettke-Hofmann, 2001; Mettke-Hofmann et al., 2006). Moreover, the dense habitats were more complex than the open ones, potentially hiding more threats (Mettke-Hofmann et al., 2006). The black-headed birds’ repeated approaches allowed them to collect information about the novel habitat, thereby reducing uncertainty (Inglis et al., 2001) and subsequently neophobia. In Sardinian warblers, spatially neophobic individuals not only had a higher frequency of approaches to but also spent more time in front of a novel environment supporting the idea of information gathering (Mettke-Hofmann et al., 2009). Overall, black-headed birds seem to be repelled by deviating habitats, which may affect decisions about small-scale as well as large scale movements in fragmented landscapes.
In contrast, red-headed birds showed a lesser response to dense habitats, supporting hypothesis 1 only partly. While they appeared more afraid of the dense than the open habitat (more approach attempts), they also seemed to be motivated to explore this unfamiliar habitat because they showed similar entry latencies as compared to the open habitat. Consequently, unsuitable habitats may be less of a barrier for red-headed than black-headed birds. This is an unexpected finding and clearly rejects hypothesis 2 that predicted black-headed birds to enter unfamiliar habitats faster. In other contexts, red-headed birds were known to be less neophilic than black-headed birds (Mettke-Hofmann, 2012; Williams et al., 2012). The finding contributes to the growing evidence that novelty reactions are context dependent (e.g., Greggor et al., 2016b).
Interestingly, similar opposing novelty responses to changes in the familiar environment and novel spatial environments have been found in resident and migratory warbler species (Mettke-Hofmann et al., 2005, 2009). The black-headed birds’ novelty responses resemble a resident response (Mettke-Hofmann et al., 2005) with early approach to and investigation of any change in their familiar environment (Mettke-Hofmann, 2012; Williams et al., 2012) but reduced interest to enter unsuitable novel environments. This is further supported by an increased approach-avoidance conflict to enter unsuitable habitat in experiment 1 as found in the restricted model (note that this was not the case in the model with the full data set). Red-headed birds’ novelty responses are like a migrant’s response with a low propensity to explore changes in the familiar environment (Mettke-Hofmann, 2012; Williams et al., 2012) but fast entry into unfamiliar environments (Mettke-Hofmann et al., 2009). Consequently, the two morphs may cognitively complement each other in different situations. While black-headed birds thoroughly assess changes in their familiar environment and may be able to find new resources from which red-headed birds can benefit (Williams et al., 2012), red-headed birds are more prone to venture into new and potentially unsuitable habitats facilitating movements across fragmented landscapes. As a gregarious species, individuals of both morphs may benefit from their diverging cognitive strategies and make them better prepared for environmental change, which could improve survival and population persistence. Moreover, since the polymorphism exists across the entire species’ distribution, i.e., is not restricted to a mixing zone where the two morphs meet as in many other polymorphic species (e.g., Roulin, 2004; Holderby et al., 2014), individuals may benefit from associating with different morphs. This supports Forsman et al. (2008) who proposed that polymorphic species are better prepared for environmental change due to the existence of different behavioral strategies.
Red-headed birds are usually more aggressive (Pryke and Griffith, 2006; Pryke, 2007; Williams et al., 2012) and their higher willingness to move into new habitats may help them find populations with fewer red-headed birds. This corresponds to similar findings in Great tits (Parus major). More aggressive individuals explored novel environments faster than less aggressive individuals (Verbeek et al., 1996). Fast explorers also dispersed further than slow explorers (Dingemanse et al., 2003). The former had also more problems coping with defeat (Verbeek et al., 1999) and their higher propensity to emigrate allowed them to settle into populations where they were subjected to less social stress (Dingemanse et al., 2003). In the Gouldian finch, red-headed birds are also more prone to social stress than black-headed birds when densities of red-headed birds increase (Pryke et al., 2007). In bluebirds, the more aggressive Western bluebird (Sialia mexicana) is the more successful colonizer as compared to the Mountain bluebird (Sialia currucoides; Duckworth and Badyaev, 2007) indicating that a combination of aggression and movement seem to be beneficial. Indeed, Duckworth and Kruuk (2009) showed that aggression and dispersal were genetically correlated in the Western bluebird. Besides a potential role of aggressiveness, our study shows that the willingness to enter and explore unfamiliar environments is another important component to initiate movement into the unknown.
Individual responses were in part repeatable across the two experiments, indicating that some individuals consistently refuse to interact with unsuitable habitats. Repeatability was higher and more often significant in black-headed than red-headed birds. Spatial novelty responses may be part of a larger personality syndrome characterizing an individual’s strategy to cope with environmental challenges. Novelty responses to changes in the familiar environment in this species have been identified as being part of personality traits linked to their head color (Mettke-Hofmann, 2012; Williams et al., 2012). As the black morph accounts for about 70% of the population (Brush and Seifried, 1968) this could negatively affect decision making on the group level to move into unfamiliar habitats, particularly unsuitable ones, ultimately affecting movement patterns and gene flow.
Many of the differences linked to head color only occurred during the first experiment when the situation was entirely new. Most differences disappeared when presented with new environments a second time. This indicates that birds became more familiar with the general procedure and may have generalized from one experiment (and one habitat type) to the other. The ability to generalize to similar but unsuitable habitats may facilitate faster engagement with similar but unfamiliar and unsuitable habitats. Again, this would suit a nomadic lifestyle.
Morph composition did not affect the number of approaches in the full data set that included the experienced partner bird data but was significant in the restricted data set. As the experienced partner birds had experienced the situation before, they may have responded differently which could have affected the focal bird’s responses. The restricted data set indicates that whenever a pairing included a black-headed bird, the number of approaches before entering increased. This rejects hypothesis 3 that predicted mixed pairs would have shorter entry latencies and potentially fewer approach attempts. Black-headed focal birds or focal birds of any head color partnered with a black-headed bird reacted more cautiously than pure red-headed pairs. This means that black-headed birds induce more hesitation and avoidance in other black-headed birds as well as red-headed birds. This shows that Gouldian finches pay attention to responses of others, particularly black-headed birds, resulting in social conformity (Frost et al., 2007; Magnhagen and Bunnefeld, 2009; Hellstroem et al., 2011). Conformity has also been found in Gouldian finches with respect to risky situations when risk-prone birds became slower when paired with a risk-averse partner and risk-averse birds became faster with a risk-prone partner (King et al., 2015). The only exception to this was when black-headed birds were tested with red-headed birds; black-headed birds did not conform to red-headed birds (King et al., 2015). Interestingly, in our experiment red-headed birds did not affect responses in other birds either. Nonetheless, the effect of black-headed birds on others may improve group cohesion as has been found in species with mixed personalities (Aplin et al., 2014). An effect of group composition with respect to head color was only found in the restricted data set and only for the number of approach attempts. More research with a larger data set is needed to substantiate these findings.
It is currently unclear whether the increased cautiousness in black-headed birds would translate into delayed entry latencies in black-headed dominated groups as they occur in the wild (Brush and Seifried, 1968), but is worth further investigation. Currently, habitat fragmentation does not pose a major barrier for Gouldian finches as there is no evidence of genetic differentiation between populations (Bolton et al., 2016). Nonetheless, fragmentation may affect behaviors during more stationary periods such as breeding. For example, the distance birds flew for extra-pair copulations in Hooded warblers (Wilsonia citrina) was restricted by habitat fragmentation, with excursions not exceeding 500 m in fragmented habitats, despite otherwise moving up to 2.5 km (Norris and Stutchburry, 2001) indicating that perception of habitat suitability rather than physical abilities affected movement decisions. If habitat fragmentation restricts decisions about foraging movements in Gouldian finches during breeding or molting before they become nomadic during the wet season (Bolton et al., 2016), then this can negatively affect breeding success and individual condition, particularly in the black-headed morph. Indeed, Gouldian finches living in areas with extreme fire regimes and therefore low availability of suitable seeds at the end of the dry season have lower body condition and higher stress levels than populations with less severe fire regimes (Legge et al., 2015). Unfortunately, the study did not distinguish between red-headed and black-headed birds. The current study would predict that black-headed birds are more affected by food shortage in fragmented habitats as they may be less willing to move into unsuitable habitats than red-headed birds. Over the long-term, this could change morph numbers. Moreover, the higher willingness of red-headed birds to cross unsuitable habitats may have consequences for dispersal as red-headed birds may disperse further than black-headed ones. Again gene flow would be affected and maintained by the more dispersing morph as has been found in several woodland bird species where the more dispersive sex maintained genetic connectivity across fragmented landscapes (Amos et al., 2014). Likewise, novelty responses may affect site faithfulness. While Gouldian finches are nomadic during the non-breeding season, the cognitively more resident-like black-headed birds may decide to return to known sites for breeding, whereas the red-headed birds that cognitively resemble a more migratory type may be more willing to settle in new areas. However, current conservation oriented research with the Gouldian finch (e.g., Brazill-Boast et al., 2011a,b, 2013; Legge et al., 2015; Maute et al., 2015; Weier et al., 2016) rarely considers morph-specific differences in responses.
The current study contributes to the growing evidence that morphs differ in their decision-making and may follow different cognitive strategies when encountering unfamiliar situations. While black-headed morphs invest in local exploration and information gathering, which helps them to update information and keep track of newly emerging resources in their familiar environment, red-headed morphs are better cognitively equipped for movements as they have a high motivation to enter unsuitable habitats and which may allow them exploiting a larger area despite habitat fragmentation. Therefore, the two morphs may cognitively complement each other in different novel situations providing an advantage in rapidly changing environments. Interestingly, whenever black-headed birds were involved in pairings, focal birds showed more cautious spatial behavior, which may help group cohesion. More research is needed regarding the effect of the existing morph ratios on novelty responses as the majority of birds in the wild are black-headed, which may facilitate local exploration and adaptation but hinder larger scale movements in fragmented landscapes.
All datasets generated for this study are included in the article/Supplementary Material.
The animal study was reviewed and approved by Liverpool John Moores Ethical Team (CMH_GE/2016-5).
GE conducted all experiments, transcribed all data, did initial analyses, and provided the general part of the Methods section. EB analyzed the data for the manuscript and wrote the Methods and Results section. AG contributed to the experimental design and initial analyses and gave important intellectual feedback on the manuscript. CM-H came up with the design, advised on data collection and analyses, and wrote the Abstract, Introduction, and Discussion. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Blattner Heimtierfutter, Ermengerst, Germany, for sponsoring the bird food, the bird breeders for providing the birds, and the animal facility technicians for assistance and support throughout data collection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.498347/full#supplementary-material
Amos, J. N., Harrisson, K. A., Radford, J. Q., White, M., Newell, G., Mac Nally, R., et al. (2014). Species- and sex-specific connectivity effects of habitat fragmentation in a suite of woodland birds. Ecology 95, 1556–1568. doi: 10.1890/13-1328.1
Aplin, L. M., Farine, D. R., Mann, R. P., and Sheldon, B. C. (2014). Individual-level personality influences social foraging and collective behaviour in wild birds. Proc. R. Soc. B 281:20141016. doi: 10.1098/rspb.2014.1016
ASAB (2018). Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 135, 1–5.
Barton, K. (2015). MuMIn: Multi-Model Inference. R Package Version 1.43.6. Available online at: http://CRAN.R-project.org/package = MuMIn (accessed April 09, 2019).
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effect models using lme4. J. Stat. Softw. 67, 1–48.
Bates, D., Maechler, M., Bolker, B. M., Walker, S. C., Christensen, R. H. B., Singmann, H., et al. (2019). Package ‘lme4’: Linear Mixed-Effects Models Using ‘Eigen’ and S4. Available online at: https://cran.r-project.org/web/packages/lme4/lme4.pdf (accessed March 05, 2019).
Benson-Amram, S., and Holekamp, K. E. (2012). Innovative problem solving by wild spotted hyenas. Proc. R. Soc. B 279, 4087–4095. doi: 10.1098/rspb.2012.1450
Biondi, L. M., Guido, J., Madrid, E., Bó, M. S., and Vassallo, A. I. (2013). The effect of age and sex on object exploration and manipulative behavior in a Neotropical raptor, the Chimango Caracara, Milvago chimango. Ethology 119, 221–232. doi: 10.1111/eth.12056
BirdLife International (2016). Chloebia gouldiae. The IUCN Red List of Threatened Species 2016: e.T22719744A94642482. Available online at: http://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22719744A94642482.en (accessed August 26, 2019).
Bolton, P. E., Rollins, L. A., and Griffith, S. C. (2015). The danger within: the role of genetic, behavioural and ecological factors in population persistence of colour polymorphic species. Mol. Ecol. 24, 2907–2915. doi: 10.1111/mec.13201
Bolton, P. E., West, A. J., Cardilini, A. P. A., Clark, J. A., Maute, K. L., Legge, S., et al. (2016). Three molecular markers show no evidence of population genetic structure in the gouldian finch (Erythrura gouldiae). PLoS One 11:e0167723. doi: 10.1371/journal.pone.0167723
Brazill-Boast, J., Dessmann, J. K., Davies, G. T. O., Pryke, S. R., and Griffith, S. C. (2011a). Selection of breeding habitat by the endangered Gouldian finch (Erythrura gouldiae) at two spatial scales. EMU 111, 304–311.
Brazill-Boast, J., van Rooij, E., Pryke, S. R., and Griffith, S. C. (2011b). Interference from long-tailed finches constrains reproduction in the endangered Gouldian finch. J. Anim. Ecol. 80, 39–48. doi: 10.1111/j.1365-2656.2010.01756.x
Brazill-Boast, J., Pryke, S. R., and Griffith, S. C. (2013). Provisioning habitat with custom-designed nest-boxes increases reproductive success in an endangered finch. Austr. Ecol. 38, 405–412.
Brush, A. H., and Seifried, H. (1968). Pigmentation and feather structure in genetic variants of the Gouldian finch, Poephila gouldiae. Auk 85, 416–430. doi: 10.2307/4083290
Burnham, K. P., and Anderson, D. R. (2002). “A practical information-theoretic approach,” in Model Selection and Multimodel Inference, 2nd Edn (New York: Springer).
Chandler, C. R. (1995). Practical considerations in the use of simultaneous inference for multiple tests. Anim. Behav. 49, 524–527. doi: 10.1006/anbe.1995.0069
Dingemanse, N. J., Both, C., van Noordwijk, A. J., Rutten, A. L., and Drent, P. J. (2003). Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747. doi: 10.1098/rspb.2002.2300
Dostine, P. L., and Franklin, D. C. (2002). A comparison of the diet of three finch species in the Yinberrie Hills area, Northern Territory. EMU 102, 159–164. doi: 10.1071/mu01034
Dostine, P. L., Johnson, G. C., Franklin, D. C., Zhang, Y., and Hempel, C. (2001). Seasonal use of savanna landscapes by the Gouldian finch, Erythrura gouldiae, in the Yinberrie Hills area, Northern territory. Wildl. Res. 28, 445–458. doi: 10.1071/wr00049
Duckworth, R. A., and Badyaev, A. V. (2007). Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl. Acad. Sci. U.S.A. 104, 15017–15022. doi: 10.1073/pnas.0706174104
Duckworth, R. A., and Kruuk, L. E. B. (2009). Evolution of genetic integration between dispersal and colonization ability in a bird. Evolution 63, 968–977. doi: 10.1111/j.1558-5646.2009.00625.x
Dyer, J. R. G., Croft, D. P., Morrell, L. J., and Krause, J. (2009). Shoal composition determines foraging success in the gupy. Behav. Ecol. 20, 165–171. doi: 10.1093/beheco/arn129
EPBC (2018). Species Profile and Threats Data Base: Erythrura gouldiae, Gouldian Finch. Available online at: http://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id = 413 (accessed August 15, 2019).
Forsman, A., Ahnesjoe, J., Caesar, S., and Karlsson, M. (2008). A model of ecological and evolutionary consequences of color polymorphism. Ecology 89, 34–40. doi: 10.1890/07-0572.1
Frost, A. J., Winrow-Giffen, A., Ashley, P. J., and Sneddon, L. U. (2007). Plasticity in animal personality traits: does prior experience alter the degree of boldness? Proc. R. Soc. Lond. B 274, 333–339. doi: 10.1098/rspb.2006.3751
Greenberg, R. (1983). The role of neophobia in determining the degree of foraging-specialisation in some migrant warblers. Am. Nat. 122, 444–453. doi: 10.1086/284148
Greenberg, R. (1989). Neophobia, aversion to open space, and ecological plasticity in Song and Swamp Sparrows. Can. J. Zool. 67, 1194–1199. doi: 10.1139/z89-172
Greenberg, R., and Mettke-Hofmann, C. (2001). Ecological aspects of neophobia and neophilia in birds. Cur. Ornithol. 16, 119–178. doi: 10.1007/978-1-4615-1211-0_3
Greggor, A. L., Berger-Tal, O., Blumstein, D. T., Angeloni, L., Bessa-Gomes, C., Blackwell, B. F., et al. (2016a). Research priorities from animal behaviour for maximising conservation progress. Tree 31, 953–964. doi: 10.1016/j.tree.2016.09.001
Greggor, A. L., McIvor, G. E., Clayton, N., and Thornton, A. (2016b). Contagious risk taking: social information and context influence wild jackdaws’ responses to novelty and risk. Sci. Rep. 6:27764. doi: 10.1038/srep27764
Greggor, A. L., Thornton, A., and Clayton, N. S. (2015). Neophobia is not only avoidance: improving neophobia tests by combining cognition and ecology. Curr. Opin. Behav. Sci. 6, 82–89. doi: 10.1016/j.cobeha.2015.10.007
Greggor, A. L., Trimmer, P. C., Barrett, B. J., and Sih, A. (2019). Challenges of learning to escape evolutionary traps. Front. Ecol. Evol. 7:408. doi: 10.3389/fevo.2019.00408
Grünberger, S., and Leisler, B. (1993). Auswirkung der Umwelterfahrung auf die Neophobie der Tannenmeise (Parus ater). J. Ornithol. 134, 352–355. doi: 10.1007/bf01640433
Hellstroem, G., Heynen, M., Oosten, J., Borcherding, J., and Magnhagen, C. (2011). The effect of group size on risk taking and social conformity in Eurasian perch. Ecol. Freshw. Fish. 20, 499–502. doi: 10.1111/j.1600-0633.2011.00506.x
Holderby, Z., Hill, A., Palacios, E., Green, M. C., Amador, E., and De Dios, C. (2014). Comparisons of reddish egret (Egretta rufescens) diet during the breeding season across its geographic range. Waterbirds 37, 136–143. doi: 10.1675/063.037.0202
Home Office (2013). Guidance on the Operation of the UK Legislation on Animals Used in Research and Codes of Practice. Available online at: https://www.gov.uk/guidance/research-and-testing-using-animals (accessed September 6, 2019).
Inglis, I. R., Langton, S., Forkman, B., and Lazarus, J. (2001). An information primacy model of exploratory and foraging behaviour. Anim. Behav. 62, 543–557. doi: 10.1006/anbe.2001.1780
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., and Altman, D. G. (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8:e1000412. doi: 10.1371/journal.pbio.1000412
King, A. J., Williams, L. J., and Mettke-Hofmann, C. (2015). The effects of social conformity on Gouldian finch personality. Anim. Behav. 99, 25–31. doi: 10.1016/j.anbehav.2014.10.016
Knowlton, J. L., and Graham, C. H. (2010). Using behavioral landscape ecology to predict species’ responses to land-use and climate change. Biol. Conserv. 143, 1342–1354. doi: 10.1016/j.biocon.2010.03.011
Langham, G. (2006). Rufous-tailed jacamars and aposematic butterflies: do older birds attack novel prey? Behav. Ecol. 17, 285–290. doi: 10.1093/beheco/arj027
Legge, S., Garnett, S., Maute, K., Heathcote, J., Murphy, S., Woinarski, J. C. Z., et al. (2015). A landscape-scale, applied fire management experiment promotes recovery of a population of the threatened Gouldian finch, Erythrura goudiae, in Australia’s Tropical Savannas. PLoS One 10:e0137997. doi: 10.1371/journal.pone.0137997
Leimar, O., Norberg, U., and Wiklund, C. (2003). Habitat preference and habitat exploration in two species of satyrine butterflies. Ecography 26, 474–480. doi: 10.1034/j.1600-0587.2003.03466.x
Magnhagen, C., and Bunnefeld, N. (2009). Express your personality or go along with the group: what determines the behaviour of shoaling perch? Proc. R. Soc. Lond. B 276, 3369–3375. doi: 10.1098/rspb.2009.0851
Marshall, J. A. R., Trimmer, P. C., Houston, A. I., and McNamara, J. M. (2013). On evolutionary explanations of cognitive biases. Trends Ecol. Evol. 28, 469–473. doi: 10.1016/j.tree.2013.05.013
Mateos-Gonzalez, F., and Senar, J. C. (2012). Melanin-based trait predicts individual exploratory behaviour in siskins, Carduelis spinus. Anim. Behav. 83, 229–232. doi: 10.1016/j.anbehav.2011.10.030
Maute, K., French, K., Legge, S., Astheimer, L., and Garnett, S. (2015). Condition index monitoring supports conservation priorities for the protection of threatened grass-finch populations. Conserv. Physiol. 3:cov025. doi: 10.1093/conphys/cov025
Mettke-Hofmann, C. (2012). Head colour and age relate to personality traits in Gouldian finches. Ethology 118, 906–916. doi: 10.1111/j.1439-0310.2012.02079.x
Mettke-Hofmann, C. (2017). Avian movements in a modern world - cognitive challenges. Anim. Cogn. 20, 77–86. doi: 10.1007/s10071-016-1006-1
Mettke-Hofmann, C., and Gwinner, E. (2004). Differential assessment of environmental information in a migratory and a non-migratory passerine. Anim. Behav. 68, 1079–1086. doi: 10.1016/j.anbehav.2004.02.012
Mettke-Hofmann, C., Lorentzen, S., Schlicht, E., Schneider, J., and Werner, F. (2009). Spatial neophilia and spatial neophobia in resident and migratory warblers (Sylvia). Ethology 115, 482–492. doi: 10.1111/j.1439-0310.2009.01632.x
Mettke-Hofmann, C., Rowe, K. C., Hayden, T. J., and Canoine, V. (2006). Effects of experience and object complexity on exploration in garden wearblers (Sylvia borin). J. Zool. 268, 405–413. doi: 10.1111/j.1469-7998.2005.00037.x
Mettke-Hofmann, C., Wink, M., Braun, M., and Winkler, H. (2012). Residency and a broad feeding spectrum are related to extensive spatial exploration in parrots. Behav. Ecol. 23, 1365–1371. doi: 10.1093/beheco/ars130
Mettke-Hofmann, C., Wink, M., Winkler, H., and Leisler, B. (2005). Exploration of environmental changes relates to lifestyle. Behav. Ecol. 16, 247–254. doi: 10.1093/beheco/arh159
Mettke-Hofmann, C., Winkler, H., and Leisler, B. (2002). The significance of ecological factors for exploration and neophobia in parrots. Ethology 108, 249–272. doi: 10.1046/j.1439-0310.2002.00773.x
Nakagawa, S., and Cuthill, I. C. (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605. doi: 10.1111/j.1469-185x.2007.00027.x
Nakagawa, S., and Schielzeth, H. (2010). Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956.
Norberg, U., Enfjaell, K., and Leimar, O. (2002). Habitat exploration in butterflies - an outdoor cage experiment. Evol. Ecol. 16, 1–14. doi: 10.1023/a:1016007521178
Norris, D. R., and Stutchburry, B. J. M. (2001). Extraterritorial movements of a forest songbird in a fragmented landscape. Conserv. Biol. 15, 729–736. doi: 10.1046/j.1523-1739.2001.015003729.x
Powell, S. B., Paulus, M. P., Hartman, D. S., Godel, T., and Geyer, M. A. (2003). RO-10-5824 is a selective dopamine D4 receptor agonist that increases novel object exploration in C57 mice. Neuropharmacy 44, 473–481. doi: 10.1016/s0028-3908(02)00412-4
Pryke, S. R. (2007). Fiery red heads: female dominance among head colour morphs in the Gouldian finch. Behav. Ecol. 18, 621–627. doi: 10.1093/beheco/arm020
Pryke, S. R., Astheimer, L. B., Buttemer, W. A., and Griffith, S. C. (2007). Frequency-dependent physiological trade-offs between competing colour morphs. Biol. Lett. 3, 494–497. doi: 10.1098/rsbl.2007.0213
Pryke, S. R., and Griffith, S. C. (2006). Red dominates black: agonistic signalling among head morphs in the colour polymorphic Gouldian finch. Proc. R. Soc. B 273, 949–957. doi: 10.1098/rspb.2005.3362
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Riotte-Lambert, L., and Matthiopoulos, J. (2019). Environmental predictability as a cause and consequence of animal movement. Tree 35, 163–174. doi: 10.1016/j.tree.2019.09.009
Roulin, A. (2004). Covariation between plumage colour polymorphism and diet in the Barn Owl Tyto alba. Ibis 146, 509–517. doi: 10.1111/j.1474-919x.2004.00292.x
Seferta, A., Guay, P.-J., Marzinotto, E., and Lefebvre, L. (2001). Learning differences between feral pigeons and Zenaida doves: the role of neophobia and human proximity. Ethology 107, 281–293. doi: 10.1046/j.1439-0310.2001.00658.x
Selonen, V., and Hanski, I. K. (2006). Habitat exploration and use in dispersing juvenile flying squirrels. J. Anim. Ecol. 75, 1440–1449. doi: 10.1111/j.1365-2656.2006.01168.x
Shadbolt, A. B., and Ragai, R. (2010). Effects of habitat fragmentation on the movement patterns and dispersal ability of the brown spiny rat (Maxomys rajah) in the planted forest Zone of Sarawak, Eastern Malaysia. Biodiv. Conserv. 19, 531–541. doi: 10.1007/s10531-009-9729-9
Sol, D., Lefebrvre, L., and Rodriguez-Teijeiro, J. D. (2005). Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proc. R. Soc. B 272, 1433–1441. doi: 10.1098/rspb.2005.3099
Steel, E. A., Kennedy, M. C., Cunningham, P. G., and Stanovick, J. S. (2013). Applied statistics in ecology: common pitfalls and simple solutions. Ecosphere 4:115.
Stoffel, M. A., Nakagawa, S., and Schielzeth, H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Meth. Ecol. Evol. 8, 1639–1644. doi: 10.1111/2041-210x.12797
Stouffer, P. C., Johnson, E. I., Bierregaard, R. O., and Lovejoy, T. E. (2011). Understory bird communities in Amazonian rainforest fragments: species turnover through 25 years post-isolation in recovering landscapes. PLoS One 6:e020543. doi: 10.1371/journal.pone.020543
Toomey, M. B., Marques, C. I., Andrade, P., Araujo, P. M., Sabatino, S., Gazda, M. A., et al. (2018). A non-coding region near Follistatin controls head colour polymorphism in the Gouldian finch. Proc. R. Soc. B 285:20181788. doi: 10.1098/rspb.2018.1788
Verbeek, M. E. M., Boon, A., and Drent, P. J. (1996). Exploration, aggressive behaviour and dominance in pair-wise confrontations of juvenile male Great tits. Behaviour 133, 945–963. doi: 10.1163/156853996x00314
Verbeek, M. E. M., De Goede, P., Drent, P. J., and Wiepkema, P. R. (1999). Individual behavioural characteristics and dominance in aviary groups of Great Tits. Behaviour 136, 23–48. doi: 10.1163/156853999500659
Weier, A., Radford, I. J., Oliveira, S. L. J., and Lawes, M. J. (2016). Recently but infrequently burnt breeding sites are favoured by threatened Gouldian finches (Erythrura gouldiae). Int. J. Wildl. Fire 25, 1281–1290. doi: 10.1071/wf16105
Weisstaub, N. V., Zhou, M., Lira, A., Lambe, E., González-Maeso, J., Hornung, J. P., et al. (2006). Cortical 5-HT2A receptor signalling modulates anxiety-like behaviours in mice. Science 313, 536–540. doi: 10.1126/science.1123432
Williams, L. J., King, A. J., and Mettke-Hofmann, C. (2012). Colourful characters: head colour reflects personality in a social bird, the Gouldian finch, Erythrura gouldiae. Anim. Behav. 84, 159–165. doi: 10.1016/j.anbehav.2012.04.025
Wong, B. B. M., and Candolin, U. (2015). Behavioral responses to changing environments. Behav. Ecol. 26, 665–673. doi: 10.1093/beheco/aru183
Keywords: exploration, fear, specialist, decision-making, color polymorphism, nomad, conformity, conservation
Citation: Mettke-Hofmann C, Eccles GR, Greggor AL and Bethell EJ (2020) Cognition in a Changing World: Red-Headed Gouldian Finches Enter Spatially Unfamiliar Habitats More Readily Than Do Black-Headed Birds. Front. Ecol. Evol. 8:498347. doi: 10.3389/fevo.2020.498347
Received: 16 September 2019; Accepted: 26 August 2020;
Published: 17 September 2020.
Edited by:
Blandine Françoise Doligez, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Wiebke Schuett, University of Sussex, United KingdomCopyright © 2020 Mettke-Hofmann, Eccles, Greggor and Bethell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia Mettke-Hofmann, Yy5jLm1ldHRrZS1ob2ZtYW5uQGxqbXUuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.