
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Ecol. Evol. , 14 August 2020
Sec. Behavioral and Evolutionary Ecology
Volume 8 - 2020 | https://doi.org/10.3389/fevo.2020.00265
This article is part of the Research Topic Social Functions of Bat Vocalizations View all 15 articles
Social feedback plays an important role in human language development and in the vocal ontogeny of non-human animals. A special form of vocal feedback in humans, infant-directed speech – or motherese – facilitates language learning and is socially beneficial by increasing attention and arousal in the child. It is characterized by high pitch, expanded intonation contours and slower speech tempo. Furthermore, the vocal timbre (i.e., “color” of voice) of motherese differs from the timbre of adult-directed speech. In animals, pup-directed vocalizations are very common, especially in females. But so far there is hardly any research on whether there is a similar phenomenon as motherese in animal vocalizations. The greater sac-winged bat, Saccopteryx bilineata, is a vocal production learner with a large vocal repertoire that is acquired during ontogeny. We compared acoustic features between female pup-directed and adult-directed vocalizations and demonstrated that they differed in timbre and peak frequency. Furthermore, we described pup-directed vocalizations of adult males. During the ontogenetic period when pups’ isolation calls (ICs) (used to solicit maternal care) are converging toward each other to form a group signature, adult males also produce ICs. Pups’ ICs are acoustically more similar to those of males from the same social group than to other males. In conclusion, our novel findings indicate that parent-offspring communication in bats is more complex and multifaceted than previously thought, with female pup-directed vocalizations reminiscent of human motherese and male pup-directed vocalizations that may facilitate the transmission of a vocal signature across generations.
The social environment influences both speech acquisition in infants and vocal ontogeny in non-human animals. In animals, the vocal ontogeny can be influenced by (unrelated) group members (bats: Prat et al., 2015; songbirds: reviewed in Doupe and Kuhl, 1999) and parents (bats: Esser and Schmidt, 1989; parrots: Berg et al., 2011). Parental influence includes passively provided auditory input (i.e., song production in songbirds) and infant-directed vocalizations. Infant-directed vocalizations are produced in many birds and mammals, for example in primates (Whitham et al., 2007), bats (Esser and Schmidt, 1989), seals (Charrier et al., 2001), cliff swallows (Beecher et al., 1985), and king penguins (Jouventin et al., 1999). The function of these vocalizations is to mediate social interactions between adults and young (parent-offspring reunions) and to influence the vocal ontogeny of offspring (Balcombe and McCracken, 1992; Charrier et al., 2001; Whitham et al., 2007; Takahashi et al., 2015). In non-vocal learning species, they can influence vocal repertoire maturation (Takahashi et al., 2015; Gultekin and Hage, 2017) or turn-taking (Chow et al., 2015) whereas in vocal learning species, they can influence vocal signatures (Berg et al., 2011). In humans, the use of infant-directed speech by which adults address the child is a well-known phenomenon (Fernald and Kuhl, 1987; Kuhl et al., 1997). This infant-directed speech – or motherese – is characterized by unique universal prosodic features such as higher pitch, increased frequency range and slow tempo and is significantly different from adult-directed speech (Grieser and Kuhl, 1988; Broesch and Bryant, 2015). These prosodic attributes support linguist learning (Kuhl et al., 1997; Thiessen et al., 2005) and motherese also includes social benefits (Grieser and Kuhl, 1988). Besides the differences in general acoustic features, a recent study reported that the timbre (i.e., the unique tone “color” of a voice) of motherese is significantly different from the timbre of adult directed speech timbre (Piazza et al., 2017). Studies on a similar phenomenon as motherese with regard to acoustic characteristics in non-human animals are extremely rare. To our knowledge there are only two studies comparing the acoustic parameters between infant-directed vocalizations and other adult vocalizations and discussing the results in relation to motherese in human infants (Biben et al., 1989; Chen et al., 2016). Moreover, differences in timbre between infant-directed and adult-directed vocalizations in non-human animals have never been addressed before.
In this study, we wanted to investigate if we can detect a phenomenon reminiscent of motherese in infant-directed female vocalizations of the greater sac-winged bat, Saccopteryx bilineata. This highly social bat species lives in stable perennial groups (i.e., colonies) and possesses a large vocal repertoire (reviewed in Voigt et al., 2008). S. bilineata is a vocal production learner (Knörnschild et al., 2010, 2012) and exhibits a distinct vocal practice phase during ontogeny (Knörnschild et al., 2006). Parental care is restricted to the female. During vocal ontogeny, mothers produce a so-called maternal directive call (MD, Figure 1A) to communicate with their single pups (i.e., maternal care is restricted to the own pup; Knörnschild and von Helversen, 2008). This is the only pup-directed female vocalization. We wanted to investigate (1) whether pup-directed and adult-directed female vocalizations differ in their acoustic characteristics. We hypothesized that the acoustic characteristics of female MDs, including timbre, would differ from those of adult-directed vocalizations produced by the same females. This MD call often occurs during mother-pup reunions and during pups’ daily vocal practice bouts (see Supplementary Material). Vocal signatures facilitate parent-offspring reunions (Esser and Schmidt, 1989; Charrier et al., 2001), therefore we additionally investigated (2) if MDs contain an individual signature.
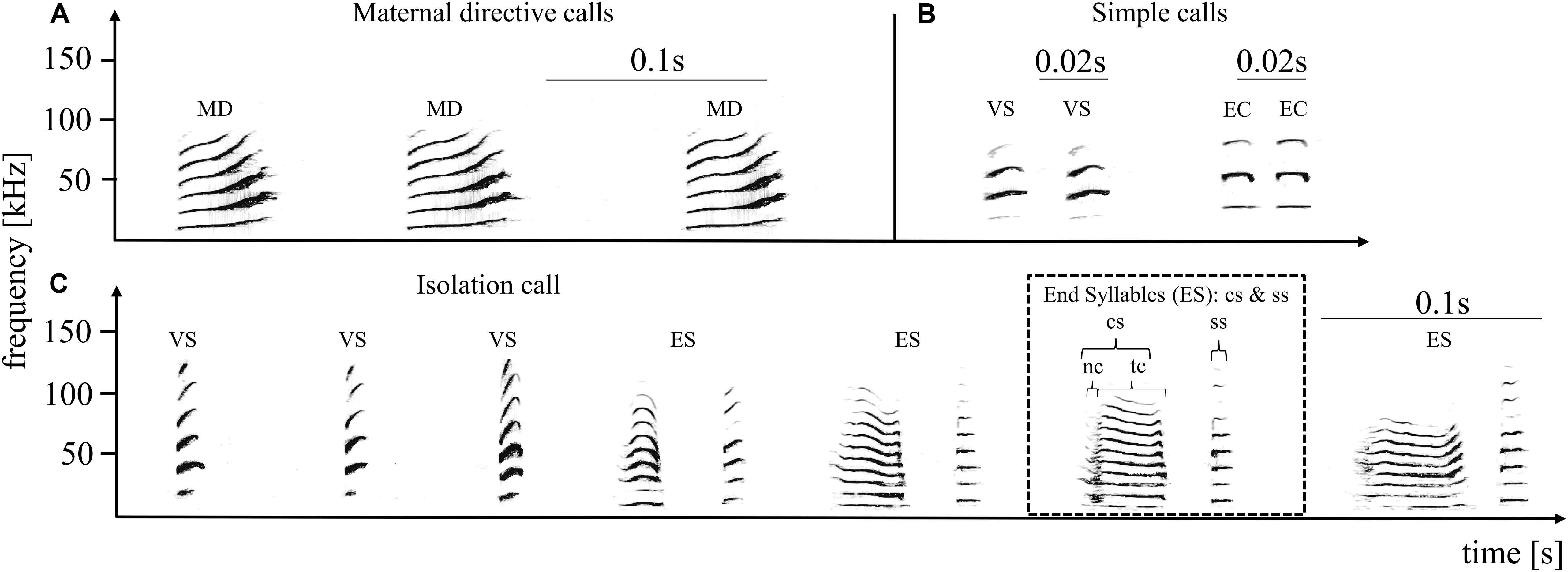
Figure 1. Pup-directed – (A) and adult-directed vocalizations (B) from an adult female and the pup-directed vocalization of an adult male (C). The upper panel shows three successive directive calls produced by an adult female (directed toward the own pup), and four adult-directed calls produced by an adult female. Adult-directed female vocalizations included variable short syllables (VS) and echolocation calls (EC), summarized as simple calls. The lower panel shows one multisyllabic isolation call produced by an adult male. Maternal directive calls (A) are always produced in monosyllabic series, from three up to 15 syllables. An isolation call (C) is composed of simple tonal calls (variable short syllable: VS) followed by the end syllables (ES). End syllables are composed of two syllable types, the composite (cs) and the stereotyped short syllable (ss). The cs part is further composed of a facultative noisy part (nc) succeeded by a tonal part (tc). Several simple frequency modulated syllables followed by several end syllables result in a typical isolation call. The spectrograms depict frequency (in kHz) as a function over time (in seconds) and were generated using a 1042 point fast Fourier transform and a Hamming window with 87.5% overlap.
Pup-directed vocalizations are either produced by a single parent (cats; Szenczi et al., 2016) or by both (parrots: Berg et al., 2011), depending on parental investment, whereby to our knowledge these are exclusively produced by females in bats (reviewed in Kunz and Hood, 2000). Like all bat pups studied to date, S. bilineata pups produce isolation calls (ICs; Figure 1C) to solicit maternal care (Knörnschild and von Helversen, 2008). Pup ICs encode information about individual identity, age and social group affiliation (Knörnschild and von Helversen, 2008; Knörnschild et al., 2012; Fernandez and Knörnschild, 2017). During ontogeny, ICs of pups from the same social group become progressively more similar to one another, i.e., develop a group signature based on social modification (Knörnschild et al., 2012). Recent new observations suggest that adult males also produce pup-directed vocalizations that resemble pup ICs. So far, studies investigating the influence of adult vocal input on the formation of group signatures in juvenile vocalizations are restricted to songbirds (for review see Boughman and Moss, 2003) and two parrot species (Farabaugh et al., 1994; Berg et al., 2011). We wanted to investigate (3) whether pup-directed vocalizations of adult males have the potential to influence the pups’ vocal ontogeny. We hypothesized that ICs of pups are more similar to ICs of adult males from the same social group than to ICs of adult males from different social groups.
We conducted sound recordings at three different locations in Central America during three consecutive field seasons (May–September in 2015–2017). We recorded the vocal and social behaviors of pups and pup-directed vocalizations of adult males and females at libitum throughout the pups’ ontogeny (i.e., from birth until weaning at 10–12 weeks of age; recording sessions occurred in the day-roosts, at least twice per week and colony, see Supplementary Material). In 2015, we conducted sound recordings at Barro Colorado Island, a field station of the Smithsonian Tropical Research Institute located in the Gatún lake of the Panamá Canal. We recorded vocalizations of six females belonging to four colonies. In 2016, we conducted sound recordings in the natural reserve Curú in Costa Rica and obtained vocalizations from seven females belonging to three colonies. Moreover, we recorded pup-directed vocalizations of 11 adult males in four colonies. In 2017, we conducted sound recordings in Gamboa, a field station of the Smithsonian Tropical Research Institute which is located at the Panamá Canal. We recorded pup-directed vocalizations of 11 adult males from three colonies. In total, we recorded pup-directed vocalizations from 13 females (maternal directive calls) and for six females we obtained additional recordings of adult-directed calls (see Supplementary Material for more information). We recorded pup-directed vocalizations from 22 males (ICs). We also recorded ICs of 14 pups. For subsequent acoustic analyses we only included recordings with good signal-to-noise ratio and if possible from multiple recording sessions. Male IC recordings with good signal-to-noise ratio were not obtained from all males and our sample size was therefore reduced to eight males.
All recordings were performed throughout the day in day-roosts which were located in tree cavities or on the outside of man-made structures. Focal recordings were feasible because the bats were individually banded with colored plastic rings on their forearms (see Supplementary Material). Furthermore, the colonies are part of a long-term project and bats are well habituated to human observers allowing close-range (2–4 m) recordings and observations. Vocalizations were recorded using a high-quality ultrasonic sound recording equipment (500 kHz sampling rate, 16-bit depth resolution, for details see Supplementary Material). The recording set-up consisted of a microphone (Avisoft UltraSoundGate 116 Hm, with condenser microphone CM16, frequency range 1–200 kHz ± 3 dB) connected to a laptop (Lenovo S21e) running the software Avisoft RECORDER (v4.2.05 R. Specht, Avisoft Bioacoustics, Glienecke, Germany).
We recorded both pup-directed calls (MDs) and two frequently occurring adult-directed calls (short variable calls and echolocation calls, from here on, these two adult-directed vocalizations are summarized and referred to as simple calls (SI), Figure 1B). Otherwise, females produced so-called screech calls, which are directed at other adults and are very noisy without clear tonal structure. Therefore, we decided to not include screeches in our analyses but to focus on tonal adult-directed vocalizations for comparison with (tonal) pup-directed vocalizations. MD calls consist of frequency modulated tonal syllables (i.e., smallest acoustic unit surrounded by silence) which are produced in sequences of up to 15 calls (min: three calls; Figure 1A). To investigate whether the acoustic characteristics of pup-directed versus adult-directed female vocalizations differed, we analyzed MD and SI sequences from the same females. In total, we analyzed 26 MD- and 26 SI call sequences from six females (the number of MD and SI call sequences per female was balanced; i.e., either 4-4 or 5-5).
To investigate if MDs encoded an individual signature we analyzed 120 MD sequences composed of at least three syllables from 13 females (range: 7–12 MD sequences per female). Additionally, we investigated the temporal relation between MD sequences and the pups’ vocal practice (N = 13 females, see Supplementary Material for details).
We analyzed 120 ICs of 14 pups (range: 6–10 calls per pup) and 39 ICs of eight males from six colonies (range: 4–9 calls per male). The sound recordings of adult males were challenging to obtain because it was not predictable whether a male would produce an IC after a pup ceased its IC production. Moreover, males did not direct their ICs toward a specific pup (see Supplementary Material). Hence, recording ICs from males required a fast change of microphone orientation (i.e., from pup to male) which resulted in fewer recordings with sufficient quality for subsequent acoustic analyses compared to ICs produced by pups.
Each sound file was prepared in Cool Edit (Cool Edit 2000 Inc., Syntrillium Software Corporation P. O. Box 62255, Phoenix, AZ, United States) for subsequent acoustic measurements (see Supplementary Material). The acoustic analyses of ICs and MD calls were conducted using the software Avisoft-SASLab Pro (v.5.2.09; R. Specht, Avisoft Bioacoustics, Glienicke, Germany).
For the pup-directed and adult-directed female vocalizations we extracted acoustic features that were based on linear-frequency cepstral coefficients (LFCCs) since those capture important acoustic characteristics of bat vocalizations (Knörnschild et al., 2017). Each LFCC describes the spectral properties of an entire acoustic signal, comprising its most important features in a compact form. LFCC extraction is comparable to the MFCC extraction (mel frequency cepstral coefficient) used in human voice recognition (reviewed in Jain and Sharma, 2013) but it uses a linear scale instead of the mel scale to account for the bats’ high frequency hearing. Extracted acoustic features summarize not only common acoustic parameters such as peak frequency but also the timbre in a voice (Piazza et al., 2017). We used a customized MATLAB script in the toolbox “voicebox” (v. R2014a) for the feature extraction. Each vocalization sequence (i.e., MD sequence and SI sequence) was composed of three syllables containing the first three harmonics (F0–F2). Because we compared different call types with different durations (i.e., average simple call duration: 0.01 s versus average MD call duration: 0.03 s) we adapted the frame length of the feature extraction accordingly (i.e., MD calls: 24 ms, SI calls: 8 ms) to obtain comparable amounts of information. We extracted 20 LFCCs from each sequence and used them for subsequent statistical analyses. Furthermore, we measured the minimum, maximum and peak frequencies for each call type (MD, EC, VS).
To test for an individual signature in MDs we measured several temporal and spectral parameters for each syllable (n = 120 MD sequences, see Supplementary Material). Principal component analyses (PCAs) were performed on the original acoustic parameters and derived acoustic parameters were used for subsequent statistical analyses (see Supplementary Material).
In the case of ICs we focused our analyses on the end syllables because former studies found that both the individual and the group signature are encoded in the end syllables (Knörnschild and von Helversen, 2008; Knörnschild et al., 2012; Fernandez and Knörnschild, 2017). For each syllable type or part (Figure 1C), we measured several temporal and spectral parameters (see Supplementary Material). We measured at least three end syllables per IC and subsequently averaged measurements per syllable type and part to minimize temporal dependence among syllable produced in direct succession. PCAs were performed to reduce multicollinearity between original parameters and to obtain uncorrelated derived acoustic parameters (see Supplementary Material). Additionally, we extracted LFCCs of each IC. To obtain comparable acoustic features for each IC we extracted features from the first three harmonics (F0–F2) of the end syllables (without the noisy part since it was not always present). For each end syllable sequence we extracted 5 LFCCs using overlapping 6 ms frames. A set of original acoustic parameters, derived parameters from the PCA and extracted LFCCs was used for subsequent multivariate analyses (see Supplementary Material).
We first conducted a multivariate GLM (with female ID, call type and their interaction as fixed factors) in which all acoustic features (LFCC1-20) and three original parameters (peak frequency, minimum and maximum frequency of the entire signal) were included. Subsequently, we selected the dependent variables which showed the same pattern for all females (no overlapping estimated marginal means for ID and call type, i.e., the differences between call types were all either de- or increasing) to calculate a second multivariate GLM with the same fixed factors as the first GLM. Six features (LFCC 2, 5, 6, 7, 9, and 12) and peak frequency were included as dependent variables in our second GLM. Minimum and maximum frequencies were not included because they were strongly correlated with peak frequency.
To test for the existence of an individual signature in maternal directive calls, we performed a discriminant function analysis (DFA; n = 120 MD sequences from 13 females). We adjusted the DFA to the unequal number of analyzed call sequences per female by computing group sizes based on prior probabilities. We used a cross-validation procedure to estimate the correct classification success (n-1 cross-validation procedure), which classified each sequence based on discriminant functions established with all sequences except the one being classified. We selected one original acoustic parameter, namely duration, and five derived parameters, namely frequency curvature 1–3 and entropy curvature 1–2 (see Supplementary Material). All parameters were checked for multicollinearity and included simultaneously into the DFA.
To assess the acoustic similarity between ICs of pups and males we performed a DFA and subsequently calculated the Euclidean distances between individual centroids in the DFA signal space (see Supplementary Material). For each pup, we calculated the distance between itself and the male from the same colony and the average distance to all other males. Distances were compared with a paired Wilcoxon test. Because population affiliation could influence the acoustic similarity between pups and males, we additionally calculated the Euclidean distances between individual centroids separated by population (see Supplementary Material). All statistical analyses were conducted in SPSS (v.20; IBM SPSS Statistics, Chicago, IL, United States) and R (RStudio 2018, version 3.5.2).
Pup-directed and adult-directed female vocalizations differed significantly in their acoustic parameters [F(1,40) = 9.73, p < 0.001, η2 = 0.66, Figure 2A] whereas female ID had no significant effect [ID: F(5,40) = 0.93, p = 0.57, η2 = 0.15; call type∗ID: F(5,40) = 1.30, p = 0.14, η2 = 0.20]. Pup-directed vocalizations had a lower peak frequency and higher LFCC values than adult-directed vocalizations from the same females (Table 1). Details on the GLMs (Supplementary Tables S1, S2) and additional paired Wilcoxon tests can be found in the Supplementary Material.
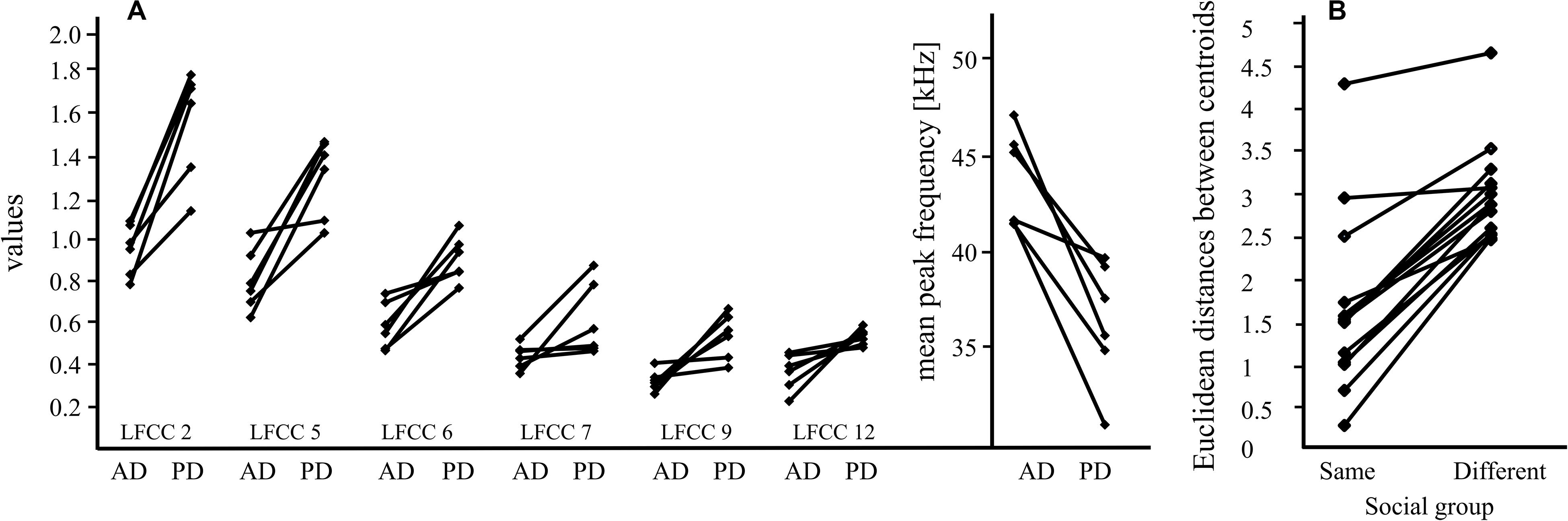
Figure 2. Main results for pup-directed vocalizations of females and males. (A) Pup-directed (PD) and adult-directed (AD) vocalizations of six females differ significantly for six LFCCs (linear frequency cepstral coefficients; LFCC 2, 5, 6, 7, 9, and 12); this suggests that pup-directed vocalizations have different acoustic properties (e.g., timbre) than adult-directed vocalizations of the same individual. Means for each call category (PD, AD) and female are shown. The differences in peak frequency of PD and AD vocalizations (mean: PD vocalizations: 36.5 kHz, AD vocalizations: 44 kHz) are shown next to the LFCC results. (B) The Euclidean distance, a proxy for acoustic similarity, between isolation calls of pups and males from their social group is smaller than the distance between isolation calls of pups and males from a different social group; this indicates that isolation calls from males and pups of the same social group share a group signature. The data includes calls from 8 males and 14 pups.
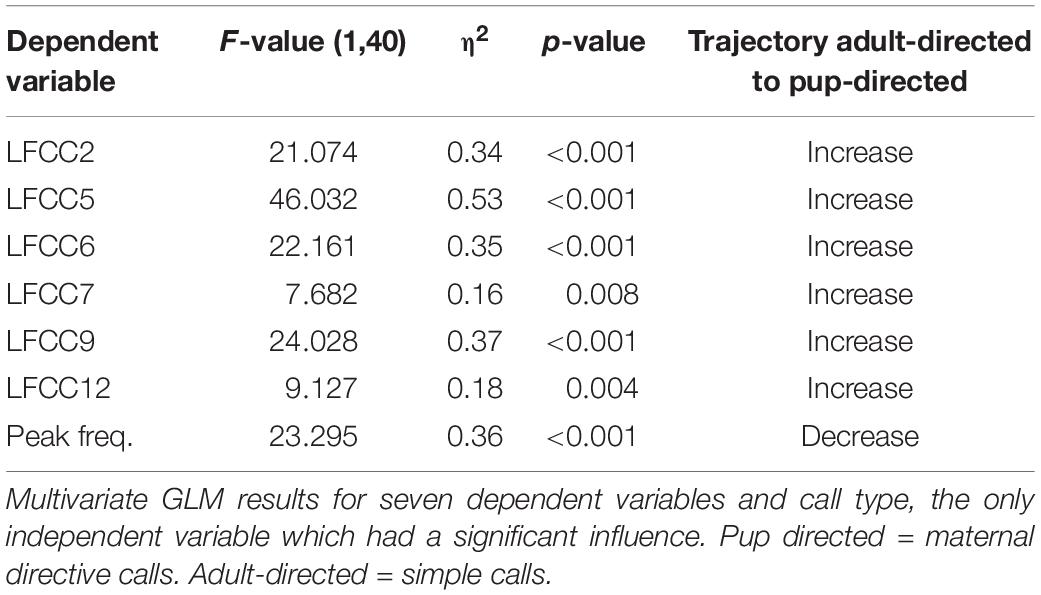
Table 1. Difference in acoustic parameters between pup-directed and adult-directed vocalizations of females.
Although the overall classification success (25%) of the DFA was higher than expected by chance (7.7%), most MD sequences were not correctly classified to the respective female (N = 13 females; Supplementary Table S3). The overall mean classification success resulted from a few females that had a classification success of 50% or higher (three females), whereas in many females the classification success was 0% (six females). Therefore, MDs do not seem to encode sufficient interindividual variation to allow for reliable individual discrimination.
In each monitored colony, both harem males and peripheral males produced complete ICs in response to pup ICs (Table 2, columns 5 and 6). Male IC production was usually restricted to a single IC, only in a few cases males produced several successive ICs. Male IC production was observed when pups were between 10 and 30 days old (observed during 5 weeks, at least once up to three times per week in the same colony). In most cases (78%), males produced ICs after a pup emitted ICs. Male IC production seemed not to be directed to a specific pup. In 11% of cases, males produced ICs after a pup uttered a short vocal practice bout (i.e., multisyllabic vocal sequence; see Knörnschild et al., 2006) which contained mainly IC end syllables. In the remaining 11% of cases, male IC production could not be related to any preceding pup vocalization, but was sometimes followed by pup ICs or vocal practice sequences. During IC production males and pups never engaged in any behavioral activity with one another.
Pup ICs had a higher acoustic similarity to the ICs of males that belonged to their colony than to ICs of males from other colonies (paired Wilcoxon Test: V = 105, p = 0.0001, effect size: r = 0.881, Figure 2B). For all 14 pups, the Euclidean distances to the male from the same colony was smaller than to the mean value for the males from the other colonies. When investigating the Euclidean distances between pups and males separated for populations the result is not significant anymore, but shows a trend (paired Wilcoxon Test: V = 78, p = 0.1; see Supplementary Material).
We detected pronounced acoustic differences between pup-directed and adult-directed female vocalizations which were consistent for all tested females. The values for all six LFCCs increased from adult-directed to pup-directed vocalizations (Figure 2A). Thus, our data indicates that the timbre of female vocalizations differed between adult-directed and pup directed calls. Pup-directed and adult directed calls are different vocalization types, so differences in peak frequency are not surprising (average peak frequency of pup-directed vocalizations: 36.5 kHz, adult-directed vocalizations: 44 kHz). However, the large and consistent differences in LFCCs, which encode information on both pitch and timbre (De Poli and Prandoni, 1997; Piazza et al., 2017), suggest that the sound of the females’ voice changed depending whether they were addressing their pups or adult conspecifics. This is similar to findings from human mothers which, irrespective of language, consistently shifted the timbre between adult-directed speech and motherese (Piazza et al., 2017). Our study describes for the first time a phenomenon that could be interpreted as reminiscent to motherese in bats. However, since our data set is very small, further investigations are needed before any final conclusions can be drawn.
In humans, motherese facilitates language learning (Kuhl et al., 1997) and its prosodic salience draws the infants’ attention toward the linguistic input (Grieser and Kuhl, 1988).
Despite the seemingly effortless language acquisition by infants, language learning is a complex and challenging task. Infants must learn the phonetic repertoire; they have to learn which speech subunits mark word boundaries (i.e., meaningful units) and which syllabic compositions occur in their native language. Motherese supports language learning by exaggerating lexical and grammatical structures (e.g., exaggeration of formant frequencies is crucial for vowel discrimination) (Kuhl et al., 1997; Thiessen et al., 2005). Furthermore, motherese also provides social benefits; it promotes turn taking enhances the infants’ attention toward the speech input and increases arousal (Fernald, 1985; Fernald and Kuhl, 1987; Grieser and Kuhl, 1988). The latter two are known to play an important role in memory and associative learning, two cognitive skills that influence language learning (Werker et al., 1994; Frick and Richards, 2001). Therefore, it is suggested that motherese might also function as a general positive feedback for the vocalizing child, promoting further speech production (Fernald, 1985; Grieser and Kuhl, 1988). However, childlike vocalizations (e.g., cries) are themselves a trigger for parental responses. Parents are even able to infer the level of distress based on the acoustic structure (Lingle et al., 2012). Also, playful vocal behavior such as babbling elicits motherese (Gros-Louis et al., 2014; Albert et al., 2018) which in turn promotes further babbling, thus leading to a positive feedback loop.
The function of female MDs in our focal bat species is not yet fully conclusive. The onset of MD call production coincides with increased pup independence, increased vocal practice behavior (Knörnschild et al., 2006) and increased behavioral activity (e.g., short flights within the day-roost). The production of MDs was observed in two contexts, during mother-pup reunions and during vocal practice bouts of the pup. Contrary to our expectation, we did not detect an individual signature in MDs, suggesting that they do not support mother-pup reunions as is the case in other bats (Brown, 1976; Esser and Schmidt, 1989; Balcombe and McCracken, 1992). In S. bilineata, mothers are able to discriminate between own and alien pups based on an individual signature encoded in ICs (Knörnschild and von Helversen, 2008) and females do not react aggressively toward alien pups, even when pups persistently and unsuccessfully solicit for maternal care from an alien female (personal observation A.A.F). Hence, pups may not need to discriminate between females because unidirectional recognition is sufficient. As aforementioned, MDs were also observed during vocal practice bouts of pups, in which pups learn to sing by imitating adult tutors (Knörnschild et al., 2010). Usually, infant-directed vocalizations are frequently produced in response to ICs (Esser and Schmidt, 1989). ICs can encode different types of information such as identity information (e.g., vocal signatures; Knörnschild and von Helversen, 2008; Knörnschild et al., 2012) and motivational state (Scheumann et al., 2007; Konerding et al., 2016). A few studies show that parents adjust their response according to the acoustic structure conveying the level of arousal (Lingle and Riede, 2014; Konerding et al., 2016). However, in our case the MD was emitted in relation to vocal practice bouts (see Supplementary Material). So far, we did not detect any temporal relation between MD sequences and pup vocalizations (Supplementary Table S4) but we need further investigations and a larger sample size to be sure whether our suggestion has to be rejected or can be confirmed. We suggest that MDs serve as a general positive feedback to pups during vocal practice and provide similar social benefits as discussed previously for motherese in infants.
Furthermore, we describe a pup-directed adult male vocalization (adult IC) which seems strongly related to IC production in pups. Pups’ ICs were acoustically more similar to ICs of males from their own social group than to ICs of males from other groups (Figure 2B). But we also found that population affiliation affects the acoustic similarity between males and pups (see Supplementary Material). However, this influence is small and, with an adequate sample size, most likely no longer significant. In most cases, pup IC bouts triggered the IC production of adult males. Considering these findings, we hypothesize that ICs of adult males may serve as guidance for the formation of the group signature in pup ICs, which is observed after the onset of flight in pups (Knörnschild et al., 2012; Fernandez and Knörnschild, 2017). Thus, call convergence toward an already existing IC group signature could occur which would render vocal group signatures stable over time. The function of the group signature in ICs of S. bilineata remains to be investigated experimentally. Observations suggest that it may be of use when ICs of adult males are directed toward other adults. Two scenarios have been observed so far: (1) During agonistic interactions, submissive males produced ICs after which the dominant males ceased to be aggressive (Knörnschild et al., 2012). (2) Philopatric harem males produce ICs when courting newly immigrated females for the first time (Knörnschild et al., 2012). These observations suggest that adult-directed ICs are used for appeasement and to signal natal group affiliation; in both cases, the observed group signature would be beneficial. Thus, our new finding that adult males may influence the group signature of pups’ ICs by producing ICs themselves is intriguing but we need further investigations with a considerably higher sample size (i.e., calls per individual and individual males per colony) to conclusively confirm our hypothesis.
To conclude, our study indicates that parent-offspring communication in bats is more complex than was anticipated. Female pup-directed vocalizations seem to be reminiscent of human motherese, an interesting phenomenon that warrants further detailed studies. Moreover, male pup-directed vocalizations may facilitate the transmission of a vocal signature across generations, thus adding a new aspect to the study of social influences on vocal development.
The datasets of this study will be made available by the authors, without undue reservation, to any qualified researcher.
The animal study was reviewed and approved by STRI Animal Care and Use Committee (ACUC), Smithsonian Tropical Research Institute.
AF and MK designed the study and wrote the manuscript. AF collected the data, conducted the acoustic analyses, and performed data analyses. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was financed by a stipend from the Elsa-Neumann Foundation to AF. Fernandez and a Heisenberg Fellowship (DFG KN935 3−1) and a research grant (DFG KN935 4−1) from the German Research Foundation to MK. Moreover, the research leading to these results has received funding from the European Research Council under the European Union’s Horizon 2020 Programme (2014–2020)/ERC GA 804352. Open Access Funding provided by the Freie Universität Berlin.
We thank the Smithsonian Tropical Research Institute, the field station BCI, and the wildlife nature reserve Curú for providing excellent research conditions. This study includes content which first appeared in the thesis of Fernandez (2020).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.00265/full#supplementary-material
Albert, R. R., Schwade, J. A., and Goldstein, M. H. (2018). The social functions of babbling: acoustic and contextual characteristics that facilitate maternal responsiveness. Dev. Sci. 21:e12641. doi: 10.1111/desc.12641
Balcombe, J. P., and McCracken, G. F. (1992). Vocal recognition in Mexican free-tailed bats: do pups recognize mothers? Anim. Behav. 43, 79–87. doi: 10.1016/S0003-3472(05)80073-9
Beecher, M. D., Stoddard, P. K., and Loesche, P. (1985). Recognition of parents’ voices by young cliff swallows. Auk 102, 600–605. doi: 10.1093/auk/102.3.600
Berg, K. S., Delgado, S., Cortopassi, K. A., Beissinger, S. R., and Bradbury, J. W. (2011). Vertical transmission of learned signatures in a wild parrot. Proc. Ry. Soc. B Biol. Sci. 279, 585–591. doi: 10.1098/rspb.2011.0932
Biben, M., Symmes, D., and Bernhards, D. (1989). Contour variables in vocal communication between squirrel monkey mothers and infants. Dev. Psychobiol. 22, 617–631. doi: 10.1002/dev.420220607
Boughman, J. W., and Moss, C. F. (2003). “Social sounds: vocal learning and development of mammal and bird calls,” in Acoustic Communication, eds A. Megela-Simmons, A. N. Popper, and R. Fay (New York, NY: Springer-Verlag), 138–224. doi: 10.1007/0-387-22762-8_4
Broesch, T. L., and Bryant, G. A. (2015). Prosody in infant-directed speech is similar across western and traditional cultures. J. Cogn. Dev. 16, 31–43. doi: 10.1080/15248372.2013.833923
Brown, P. (1976). Vocal communication in the pallid bat, Antrozous pallidus. Z. Tierpsychol. 41, 34–54. doi: 10.1111/j.1439-0310.1976.tb00469.x
Charrier, I., Mathevon, N., and Jouventin, P. (2001). Mother’s voice recognition by seal pups. Nature 412:873. doi: 10.1038/35091136
Chen, Y., Matheson, L. E., and Sakata, J. T. (2016). Mechanisms underlying the social enhancement of vocal learning in songbirds. Proc. Natl. Acad. Sci. U.S.A. 113, 6641–6646. doi: 10.1073/pnas.1522306113
Chow, C. P., Mitchell, J. F., and Miller, C. T. (2015). Vocal turn-taking in a non-human primate is learned during ontogeny. Proc. R. Soc. Lond. B 282:20150069. doi: 10.1098/rspb.2015.0069
De Poli, G., and Prandoni, P. (1997). Sonological models for timbre characterization. J. New Music Res. 26, 170–197. doi: 10.1080/09298219708570724
Doupe, A. J., and Kuhl, P. K. (1999). Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci. 22, 567–631. doi: 10.1146/annurev.neuro.22.1.567
Esser, K. H., and Schmidt, U. (1989). Mother-infant communication in the lesser spear-nosed bat phyllostomus-discolor (Chiroptera, Phyllostomidae) - evidence for acoustic learning. Ethology 82, 156–168. doi: 10.1111/j.1439-0310.1989.tb00496.x
Farabaugh, S. M., Linzenbold, A., and Dooling, R. J. (1994). Vocal plasticity in budgerigars (Melopsittacus undulatus): evidence for social factors in the learning of contact calls. J. Comp. Psychol. 108:81. doi: 10.1037/0735-7036.108.1.81
Fernald, A. (1985). Four-month-old infants prefer to listen to motherese. Infant Behav. Dev. 8, 181–195. doi: 10.1016/S0163-6383(85)80005-9
Fernald, A., and Kuhl, P. (1987). Acoustic determinants of infant preference for motherese speech. Infant Behav. Dev. 10, 279–293. doi: 10.1016/0163-6383(87)90017-8
Fernandez, A. A. (2020). Vocal Ontogenetic Processes in Bat Pups From Babbling Behavior to the Interplay of Social and Vocal Complexity. Dissertation, Free University of Berlin, Berlin, GE.
Fernandez, A. A., and Knörnschild, M. (2017). Isolation calls of the bat Saccopteryx bilineata encode multiple messages. Anim. Behav. Cogn. 4, 169–186. doi: 10.12966/abc.04.05.2017
Frick, J. E., and Richards, J. E. (2001). Individual differences in infants’ recognition of briefly presented visual stimuli. Infancy 2, 331–352. doi: 10.1207/s15327078in0203_3
Grieser, D. L., and Kuhl, P. K. (1988). Maternal speech to infants in a tonal language: support for universal prosodic features in motherese. Dev. Psychol. 24:14. doi: 10.1037/0012-1649.24.1.14
Gros-Louis, J., West, M. J., and King, A. P. (2014). Maternal responsiveness and the development of directed vocalizing in social interactions. Infancy 19, 385–408. doi: 10.1111/infa.12054
Gultekin, Y. B., and Hage, S. R. (2017). Limiting parental feedback disrupts vocal development in marmoset monkeys. Nat. Commun. 8, 1–9. doi: 10.1038/ncomms14046
Jain, A., and Sharma, O. (2013). A vector quantization approach for voice recognition using mel frequency cepstral coefficient (MFCC): a review. IJECT 4, 26–29.
Jouventin, P., Aubin, T., and Lengagne, T. (1999). Finding a parent in a king penguin colony: the acoustic system of individual recognition. Anim. Behav. 57, 1175–1183. doi: 10.1006/anbe.1999.1086
Knörnschild, M., Behr, O., and von Helversen, O. (2006). Babbling behavior in the sac-winged bat (Saccopteryx bilineata). Naturwissenschaften 93, 451–454. doi: 10.1007/s00114-006-0127-9
Knörnschild, M., Blüml, S., Steidl, P., Eckenweber, M., and Nagy, M. (2017). Bat songs as acoustic beacons - male territorial songs attract dispersing females. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-14434-5
Knörnschild, M., Nagy, M., Metz, M., Mayer, F., and von Helversen, O. (2010). Complex vocal imitation during ontogeny in a bat. Biol. Lett. 6, 156–159. doi: 10.1098/rsbl.2009.0685
Knörnschild, M., Nagy, M., Metz, M., Mayer, F., and von Helversen, O. (2012). Learned vocal group signatures in the polygynous bat Saccopteryx bilineata. Anim. Behav. 84, 761–769. doi: 10.1016/j.anbehav.2012.06.029
Knörnschild, M., and von Helversen, O. (2008). Nonmutual vocal mother-pup recognition in the greater sac-winged bat. Anim. Behav. 76, 1001–1009. doi: 10.1016/j.anbehav.2008.05.018
Konerding, W. S., Zimmermann, E., Bleich, E., Hedrich, H. J., and Scheumann, M. (2016). Female cats, but not males, adjust responsiveness to arousal in the voice of kittens. BMC Evol. Biol. 16:157. doi: 10.1186/s12862-016-0718-9
Kuhl, P. K., Andruski, J. E., Chistovich, I. A., Chistovich, L. A., Kozhevnikova, E. V., Ryskina, V. L., et al. (1997). Cross-language analysis of phonetic units in language addressed to infants. Science 277, 684–686. doi: 10.1126/science.277.5326.684
Kunz, T. H., and Hood, W. R. (2000). “Parental care and postnatal growth in the Chiroptera,” in Reproductive Biology of Bats, eds E. G. Crichton and P. H. Krutzsch (Cambridge, MA: Academic Press), 415–468. doi: 10.1016/b978-012195670-7/50011-4
Lingle, S., and Riede, T. (2014). Deer mothers are sensitive to infant distress vocalizations of diverse mammalian species. Am. Nat. 184, 510–522. doi: 10.1086/677677
Lingle, S., Wyman, M. T., Kotrba, R., Teichroeb, L. J., and Romanow, C. A. (2012). What makes a cry a cry? A review of infant distress vocalizations. Curr. Zool. 58, 698–726. doi: 10.1093/czoolo/58.5.698
Piazza, E. A., Iordan, M. C., and Lew-Williams, C. (2017). Mothers consistently alter their unique vocal fingerprints when communicating with infants. Curr. Biol. 27, 3162–3167. doi: 10.1016/j.cub.2017.08.074
Prat, Y., Taub, M., and Yovel, Y. (2015). Vocal learning in a social mammal: Demonstrated by isolation and playback experiments in bats. Sci. Adv. 1:e1500019. doi: 10.1126/sciadv.1500019
Scheumann, M., Zimmermann, E., and Deichsel, G. (2007). Context-specific calls signal infants’ needs in a strepsirrhine primate, the gray mouse lemur (Microcebus murinus). Dev. Psychobiol. 49, 708–718. doi: 10.1002/dev.20234
Szenczi, P., Bánszegi, O., Urrutia, A., Faragó, T., and Hudson, R. (2016). Mother-offspring recognition in the domestic cat: Kittens recognize their own mother’s call. Dev. Psychobiol. 58, 568–577. doi: 10.1002/dev.21402
Takahashi, D. Y., Fenley, A. R., Teramoto, Y., Narayanan, D., Borjon, J. I., Holmes, P., et al. (2015). The developmental dynamics of marmoset monkey vocal production. Science 349, 734–738. doi: 10.1126/science.aab1058
Thiessen, E. D., Hill, E. A., and Saffran, J. R. (2005). Infant-directed speech facilitates word segmentation. Infancy 7, 53–71. doi: 10.1207/s15327078in0701_5
Voigt, C. C., Behr, O., Caspers, B., von Helversen, O., Knörnschild, M., Mayer, F., et al. (2008). Songs, scents, and senses: sexual selection in the greater sac-winged bat, Saccopteryx bilineata. J. Mammal. 89, 1401–1410. doi: 10.1644/08-mamm-s-060.1
Werker, J. F., Pegg, J. E., and McLeod, P. J. (1994). A cross-language investigation of infant preference for infant-directed communication. Infant Behav. Dev. 17, 323–333. doi: 10.1016/0163-6383(94)90012-4
Keywords: motherese, vocal ontogeny, timbre, maternal directive call, pup-directed male vocalization
Citation: Fernandez AA and Knörnschild M (2020) Pup Directed Vocalizations of Adult Females and Males in a Vocal Learning Bat. Front. Ecol. Evol. 8:265. doi: 10.3389/fevo.2020.00265
Received: 19 March 2020; Accepted: 27 July 2020;
Published: 14 August 2020.
Edited by:
Claudia Fichtel, Deutsches Primatenzentrum, GermanyReviewed by:
Yossi Yovel, Tel Aviv University, IsraelCopyright © 2020 Fernandez and Knörnschild. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahana Aurora Fernandez, YWEuZmVybmFuZGV6QGZ1LWJlcmxpbi5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.